Abstract
Stem cells have been widely used for treating disease due to the various benefits they offer in the curing process. Several treatments using stem cells have undergone clinical trials, such as cell-based therapies for heart disease, sickle cell disease, thalassemia, etc. Adipose-derived stem cells are some of the many mesenchymal stem cells that exist in our body that can be harvested from the abdomen, thighs, etc. Adipose tissue is easy to harvest, and its stem cells can be obtained in higher volumes compared to stem cells harvested from bone marrow, for which a more invasive technique is required with a smaller volume obtained. Many scientists have expressed interest in investigating the role of adipose-derived stem cells in treating disease since their use was first described. This is due to these stem cells’ ability to differentiate into multiple lineages and secrete a variety of growth factors and proteins. Previous studies have found that the hormones, cytokines, and growth factors contained in adipose tissue play major roles in the metabolic regulation of adipose tissue, as well as in energy balance and whole-body homeostasis through their endocrine, autocrine, and paracrine functions. These are thought to be important contributors to the process of tissue repair and regeneration. However, it remains unclear how effective and safe ADSCs are in treating diseases. The research that has been carried out to date is in order to investigate the impact of ADSCs in disease treatment, as described in this review, to highlight its “trick or treat” effect in medical treatment.
Keywords: adipose tissue, adipose tissue stem cell, stem cells, cancer cells, ADSCs’ treatment, wound healing
1. Introduction
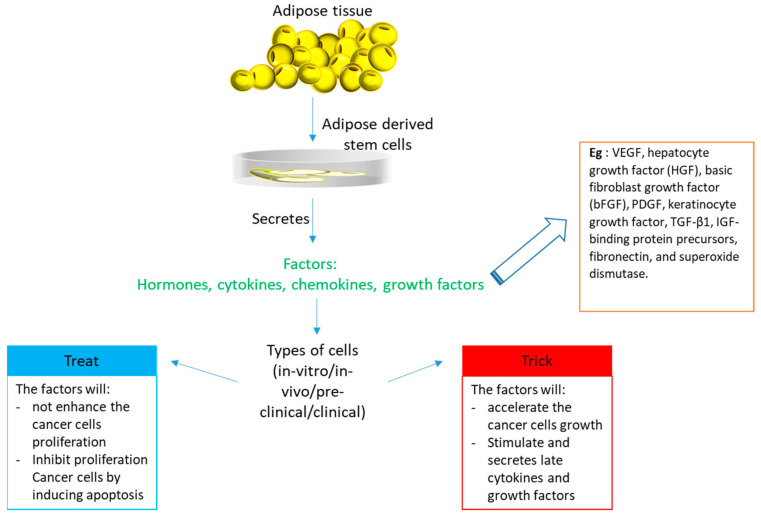
Adipose-derived stem cells (ADSCs) are mesenchymal stem cells that are known for their angiogenic properties, plasticity, and multipotent ability to differentiate into different cell lineages, such as chondrogenic, adipogenic, and osteogenic [1]. Mesenchymal stem cells can be obtained from various sites, such as bone marrow, the umbilical cord, and adipose tissue, but adipose tissue has become the optimal source for harvesting MSCs [2]. It was reported to be easier to harvest ADSCs with a high yield compared to mesenchymal stem cells harvested from bone marrow and cord blood [3]. It also reported that ADSCs have a longer life span, higher proliferative capacity, shorter doubling time, and later In-vitro senescence compared to mesenchymal stem cells derived from bone marrow [4]. Aside from that, ADSCs are multipotent and are able to differentiate into different lineages, such as neuron-like cells [5]. They can be used for the development of new blood vessels, and the induction of ADSCs into different cell types and lineages can be utilized for tissue engineering (chondrogenic, neuronal-like, osteogenic). ADSCs also contribute to the cellular turnover of adipose tissue [6]. A recent study by Hamid et al. [7] on differentiating ADSCs for chondrogenesis by co-culturing them with chondrocytes indicates that ADSCs could be used as a replacement for FBS to help grow a sufficient number of chondrogenic cells to repair cartilage injury in the near future [7]. In this review, we aimed to highlight the “trick or treat” aspect of the adipose-derived stem cells used in the treatment of diseases (Figure 1).
Figure 1.
An overview of the therapeutic potential of adipose-derived stem cells for various applications.
2. Part I—The Treat: Adipose Tissue Stem Cells’ Application as a Treatment
The study of cell-based treatments using adipose tissue-derived stem cells has been ongoing since Zuk et al., (2001) proposed the use of stem cells harvested from adipose tissue [8]. Their study explained the process used for the isolation of adipose-derived stem cells from lipoaspirate through a centrifugation process. The product obtained can either be applied directly as a treatment or can be further cultured and expanded in a flask for future use [8]. Since the technique was discovered, it has been used as a means for scientists to easily harvest ADSCs and use them for studies on disease treatments.
In a recent cell-based treatment study, ADSCs were used to improve bone regeneration after post-traumatic osteomyelitis due to the fact that they could differentiate into osteogenic materials. Post-traumatic osteomyelitis is a severe complication of open fractures that can be treated through surgical debridement, which is a process used to remove infected purulent bone tissue [9]. However, the surgical debridement process inhibits the bone regulation process while increasing bone resorption (osteoclastogenesis), hence affecting the healing process [9]. The process of bone resorption is mediated by an inflammatory reaction that regulates various cytokines from osteoblasts in infected bone [10]. In their study, Wagner and colleagues harvested ADSCs from the inguinal subcutaneous fat pads of C57B16 mice using an enzymatic isolation technique, before the ADSCs were transferred to bony defects right after the surgical treatment of their infected bones. The outcome of this study showed that ADSCs were able to improve bone healing via the elevation of osteoblastogenesis and downregulation of osteoclasts. In addition, stem cells were also found to downregulate the B-cell population, which is a potent activator of osteoclastogenesis activity [10].
In order to prolong rejection-free survival in an allogenic hind-limb transplantation (animal) model, an allograft was infused with ADSCs and hypoxia-primed ADSCs before being transfused via the vascular system, along with the administration of a short-term immunosuppressant treatment [11]. The result indicated that ex-vivo treatment using allografts with ADSCs prolonged the survival of the allografts, suppressed the proliferation and infiltration of T-lymphocytes, and improved the secretion of immunomodulatory cytokines, such as IL-10, through the potential induction of Treg expression in the allografts compared with the control [11].
Furthermore, the ability of adipose-derived stem cells to increase the cell proliferation rate and upregulate cells has made them an interesting material for wound-healing studies. The potential of using ADSCs as a treatment was examined through an In-vivo wound-healing assay on the application of ADSCs on circular cutaneous wounds on the back dermal skin of mice [12]. The outcomes showed that the cutaneous wound-healing rate was increased 2 to 5 weeks after the application of ADSCs [12]. ADSCs were also applied as a hair growth treatment aiming to increase the generation of new hair. Dermal papillae, which are important factors in hair regeneration, have been used for hair regeneration therapy but are difficult to isolate. Thus, dermal papillae-like tissues (DPLT) were formed by culturing ADSCs in a papilla-forming medium. In this study, wounds were created on the backs of mice; these wounds were then injected with DPLTs to evaluate the formation of new hair growth. The new hair growth throughout the treatment area showed a significantly higher number of sebaceous glands [13]. Therefore, these data suggest that engineered canine DPLTs demonstrated the characteristics of dermal papillae and had a positive effect on hair regeneration [13]. This is possible because adipose tissues are multipotent and can differentiate into other cells.
ADSCs from lipoaspirate are able to promote the outgrowth of cells [9], repopulate chondrocytes [7], repair damaged cartilage [14], and have paracrine effects on resident chondrocytes [6]. They are, therefore, good options for advancing cell-based treatment studies. Cell-based treatments using ADSCs have been applied for various purposes, including wound healing [15], the 3D bioprinting of adipose tissue for breast reconstruction [16], hair loss treatment [13,17], allograft studies [10,18], and cancer treatment. ADSCs are able to secrete a high amount of growth factors such as vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), keratinocyte growth factor (KGF), transforming growth factor beta 1 (TGF-β1), IGF-binding protein precursors, fibronectin, and superoxide dismutase [19]. Thus, ADSCs can have both pro-angiogenetic and anti-apoptotic effects [14,20]. Moreover, TGF-β1 secretion promotes an immunomodulatory effect and increases extra-cellular matrix deposition and collagen organization [21].
For these reasons, ADSCs have been used as an alternative medium for various cancer treatments. They have shown beneficial effects in the treatment of oral tongue squamous carcinoma (OTSCC), pancreatic cancer cells, breast cancer cells, etc. In one cancer treatment study, ADSCs were shown to have the ability to induce apoptosis and inhibit the growth of gastric cells [22]. Zhao et al. observed the growth of tumor cells by injecting ADSCs and human gastric cancer-27 (HGC-27) cells together into nude mice. After 19 days, the mice were sacrificed, and their tumors were excised and weighed. Later, a decrease in tumor formation was shown. In an In-vitro study, HGC-27 cells were cultured in ADSCs-conditioned medium (CM), showing the ability of ADSCs to secrete cell factors that would indirectly act on GC cells, causing changes in the microenvironment of HGC-27 cells. Thus, the outcome showed that ADSCs had an inhibitory effect on the proliferation of HGC-27 cells [22]. It was suggested that ADSCs could inhibit the growth of HGC-27 cells, indicating the ability of ADSCs to treat gastric cancer [22]. Observations have shown both positive and negative results depending on the type of cancer cells involved.
The rising interest in the use of ADSCs for cancer treatment can be seen through the many studies carried out aiming to observe the proliferation rate, migration rate, or influence of stem cells on cancer cell progression. The application of ADSCs in medical treatment could be improved in the future. The dual roles played by ADSCs in cancer cells, in which they are either pro-tumor or tumor inhibiting, are still being investigated in studies using various types of cancer cells. Recently, the application of ADSCs was shown to have no effect on the progression of oral tongue cancer. A study carried out by Sinha et al. indicated that ADSCs did not induce the proliferation, migration, or invasion of human tongue squamous carcinoma cells in a co-culture of ADSCs and human tongue squamous carcinoma (HSC-3) cells [23]. The authors pointed out that ADSCs do not enhance the aggressiveness of oral dysplastic and cancer cells in vitro [23]. Along with the positive results from this study, more findings can be found in Table 1, which shows the benefits of this use of ADSCs for cancer treatment.
Table 1.
The application of adipose-derived stem cells as a treatment for certain disease.
| Source | Treatment | Type of Study | Method | Results | Ref(s) |
|---|---|---|---|---|---|
| ADSCs from subcutaneous fat pads harvested from C57Bl6 mice. | Bone regeneration after post-traumatic osteomyelitis. | In-vitro | ASCs were administered to debrided bony defects after bone infection | ASCs overcame the impairment of bone regeneration after osteomyelitis in a murine animal model. | [10] |
| ADSCs from lipoaspirates. | Oral tongue squamous carcinoma | In-vitro | IncuCyte wound-healing migration assay was used to study the effect of ADSCs on the cancer and dysplastic cells’ migration ability. | ADSCs did not enhance the aggressiveness of oral dysplastic and cancer cells in- vitro. | [23] |
| ADSC from omentum tissue | Breast cancer | In-vitro | Paracrine and contact co-culturing of breast cancer cells with ADSCs from the same donors | ADSCs did not increase the proliferation rate of the breast cancer cells either through paracrine- or contact-dependent interactions. Additionally, they inhibited MDA-MB-231 breast cancer cell contact-dependent interactions. Quantitative real-time PCR revealed no significant increase in the EMT-related genes in breast cancer cells upon co-culture with ADSCs. |
[24] |
| Human ADSC lines | Human gastric cancer (HGC-27) cells | In-vitro and in-vivo | HGC-27 cells cultured in ADSCs-conditioned medium (in-vitro). Nude mice injected with mixture of ADSCs and HGC-27 (In-vivo) |
ADSCs effectively inhibited the growth of HGC-27 cells by inducing apoptosis in-vitro and in vivo. | [22] |
3. Part II—The Trick: Side Effect of Treatment Using ADSCs
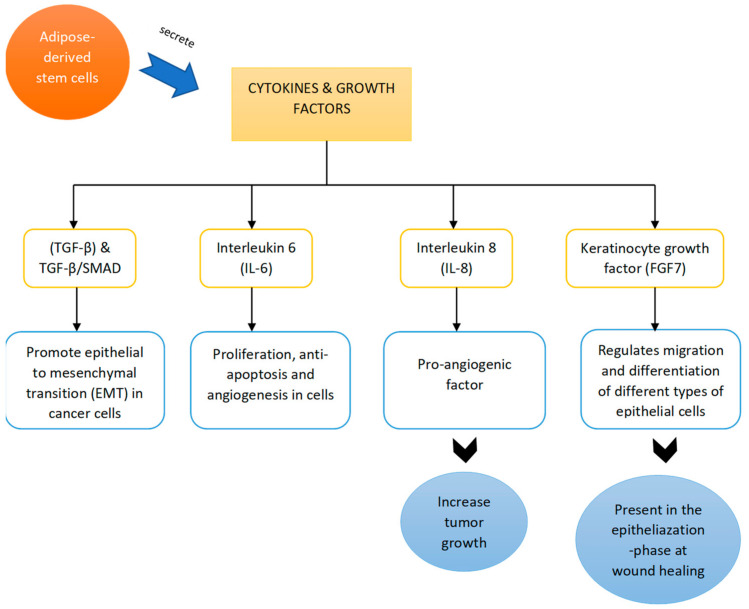
Despite the positive outcomes gained from using ADSCs in treatment, there have been some issues regarding the role of ADSCs in tumor progression. Some researchers discovered the presence of stem cell-like features in several cancers that were then identified as cancer stem cells (CSCs); it was speculated that these originated from transformed mesenchymal stem cells (MSCs) [25]. Some pre-clinical studies based on In-vitro and In-vivomodels have suggested that ADSCs may act as potential tumor promoters for different cancer cell types and support tumor progression and invasiveness through the activation of several intracellular signals [26]. Many cell-based therapy studies and observations using ADSCs have been carried out, and positive evidence has been generated, but it still remains unclear as to whether the use of ADSCs in treatment could give rise to cancer or not. In contrast, there has been much heated discussion regarding the role of adipose-derived stem cell in tumor progression. The growth factor secreted and induced by ADSCs is TGF-β, and the TGF-β/SMAD signaling pathway promotes epithelial-to-mesenchymal transition (EMT) in cancer cells [27]. In parallel, TGF-β signaling has been shown to be able to induce myofibroblastic differentiation in ADSCs exposed to breast cancer exosomes, thus promoting the desmoplastic transformation of the tumor microenvironment [28]. TGF-β is also among the main causes of the immunomodulatory effect of ADSCs. The immune-mediated response to tumors is impaired by TGF-β1, HGF, indoleamine-pyrrole 2,3-dioxygenase (IDO), and interferon gamma (IFN-γ), which are secreted by ADSCs [29]. ADSCs are also able to elicit drug resistance and cell proliferation in the breast cancer cell line MCF-7/ADR (a multidrug-resistant breast cancer cell model), mediated through the expression of C-terminal (Src) kinase (Csk)-binding protein (Cbp) [30].
The ability of ADSCs to promote tumors is mainly due to their active release of factors. In the study of Preisner et al., (2018), the team co-cultured a lower passage of primary melanoma cells and ADSCs to determine the effect of ADSCs in promoting tumors [29]. The main reason for the use of lower-passage primary melanoma cells instead of other passages is because they closely resemble cancer cells In vivo. The results obtained from this study showed an increment in HGF in melanoma cells after they were co-cultured together with ADSCs [29]. These findings showed that HGF plays an important role in affecting the growth of melanoma cells. HGF is a multifunctional cytokine that can activate various downstream pathways involved in angiogenesis, tumor growth, migration, and metastasis. Moreover, HGF has been shown to activate melanoma proliferation through MAPK signaling. This latter effect could, at least in part, be responsible for the increased proliferation of low-passage primary melanoma cell cultures. Furthermore, other regulating factors have also been detected, such as interleukin-6 (IL-6), which is responsible for proliferation, anti-apoptosis, and angiogenesis in cells [29].
There have been some reports on the impact of adipose-derived stem cells in breast cancer. There are possible interactions between primary human ADSCs and five human breast cancer cell line BRCAs (MCF-7, MDA-MB-231, SK-BR-3, ZR-75-30, and EVSA-T), pointing towards the potential increased oncological risk [31]. These results showed that ADSCs significantly affect multiple malignant features of BRCAs in vitro, such as gene expression, protein secretion, migration, and angiogenesis. Several tumor-associated proteins, such as cytokines and matrix metalloproteinases (MMPs), were found to be strongly increased in co-cultures of ADSCs and BRCAs. MMP is a protein that could possibly degrade the extracellular matrix and thereby enable invasion, as well as facilitating neo-angiogenesis and, thus, further supporting tumor growth. Altered MMP expression has already been linked to poor disease prognosis in different human cancers and enhanced cancer cell invasion [32]. In this case, ADSCs secrete high levels of various MMPs, such as MMP-1, -2, -3, -9, and -10. In addition, ADSCs promote tumor growth In-vivo by secreting interleukin-8 (IL-8), which stimulates angiogenesis and supplies nutrition, thus supporting angiogenesis [31]. A previous study found increased angiogenesis in all co-cultured BRCAs and almost all ADSC co-cultures, as well as an upregulated gene expression of IL-8 in MDA-MB-231 cell lines with ADSC co-cultures. In addition, a co-culture of ADSCs and primary BRCAs showed a strong total increase in IL-8 protein concentration, as well as a corresponding induction of angiogenesis [32].
However, in a recent study carried out by Ejaz et al., (2020), it was found that lipoaspirates and ADSCs did not increase the proliferation rate of breast cancer cells either through paracrine- or contact-dependent interactions [33]. They stated that a previous study on the effect of ADSCs in increasing tumor progression using co-culturing or an In-vivomodel method was inconsistent with the clinical scenario based on the injection of intact adipose parcels into a large tissue bed for breast reconstruction. Since most of the studies on this topic relied on In-vitro differentiated adipocytes or paracrine interactions between cells as experimental models, Ejaz et al., (2020) used a clinically relevant animal model and reported no increase in tumor size, proliferation, histological grade, or metastatic spread [33]. Even though their result contrasted with the conclusion of a previous study by Koellenspergers et al., (2017), its positive outcome might become something that scientists could build on in the future to create novel breast cancer treatments.
In short, a few genes and proteins have been discovered to be upregulated, such as those promoting angiogenesis and proneness to tumor production, but effects on the genes responsible for EMT, such as TWIST1, Snail1, Snail2, and CDH2, have not been found [32]. The upregulation of protein genes such as TNFSF-10 certainly induces apoptosis but does not kill normal cells [34]. Ejaz et al., (2020) mentioned in their study that the co-culture of ADSCs and MDA-MB-231 reduced the proliferation rate of MDA-MB-231 cell lines. Koellensperger et al., (2017) also stated that co-culturing did not significantly affect cellular proliferation [32,33]. Thus, it is possible that this was not a true reflection of the interaction of these cells with breast cancer cells, as the type of breast cancer cells used (either primary cells or lines) might influence the results. It is possible that the tested cancer cell lines might have lost some important features of cellular interactions with ADSCs throughout their process of immortalization or due to the different media conditions used for cell lines and primary cells [32]. Moreover, the level of upregulation of BRCA genes in primary breast cancer cell lines may cause differences, for example, angiogenesis factor is usually found in higher levels in primary cells than in cell lines. This is because monolayer cell lines that have not yet formed spheroids may deliver more oxygen to compact structures. Figure 2 shows the proposed signaling pathway involved in the activation of ADSCs to induce tumor progression. Meanwhile, a summary of studies showing the negative effects of ADSC treatments is provided in Table 2.
Figure 2.
Proposed signaling pathways involved in the activation of ADSCs to induce tumor progression.
Table 2.
The treatment using ADSCs and the negative side effect in In-vitro and In vivo.
| Source | Treatment | Type of Study | Method | Results | Ref(s) |
|---|---|---|---|---|---|
| ADSCs from mouse abdominal tissue | Colon and Breast Cancer | In-vitro and in-vivo | Co-culture (in vitro) Inoculated 4T1 or CT26 cells with or without ADSCs into BALB/c mice (In vivo) |
|
[35] |
| ADSCs’ cell | Cervical cancer cell | In-vitro and in-vivo |
Co-culture (In vitro) Injection on 6-week-old BALB/c nude mice. (In vivo) |
|
[36] |
| ADSC from omentum tissue | Epithelial Ovarian Cancer | In-vitro | Co-culture of cancel cells with ADSC |
|
[24] |
4. Updates and Development on Adipose-Derived Stem Cells for Clinical Applications
The current development of treatments using adipose-derived stem cells has proven their effectiveness in and benefits for curing disease. Recent updates regarding the application of ADSCs have included their use for cutaneous wound-healing treatments, as they release exosomes, which are important paracrine substances that can be used as extracellular vesicles to carry various bioactive molecules that mediate adjacent or distant intercellular communication [37]. Researchers have indicated that ADSCs-derived exosomes (ADSCs-Exos) promote skin wound healing by affecting all stages of wound healing, including regulating inflammatory response, promoting the proliferation and migration of fibroblasts and keratinocytes, facilitating angiogenesis, and regulating the remodeling of the extracellular matrix. Moreover, ADSCs-Exos are also able to improve graft retention in a manner comparable to that of their source cells, thereby achieving improved graft retention by up-regulating early inflammation [38]. The application of ADSCs-Exos has many benefits, such as their easy acquisition method, sustainable source, convenient storage and transportation, high long-term survival rate, quantitative usage, ability to avoid immune rejection, and lack of ethical problems [39]. These positive aspects show the bright side of the application of ADSCs for future stem cell-based treatments.
The emerging field of study on ADSCs has progressed beyond expectation; this research has expanded to improving the survival of ovarian tissue transplants. To overcome the problem of the massive follicle loss seen in transplanted tissue in the early post-grafting period, the benefits and properties described by ADSCs have been taken into consideration. ADSCs have been used as models of xenotransplantation, where they are embedded into a fibrin scaffold and transplanted to the peritoneum of immunodeficient mice. As expected, higher rates of the oxygenation and vascularization of ovary tissue were obtained in the early post-grafting period, along with increased follicle survival and reduced apoptosis [40]. This finding will be useful for future transplantation studies, proving that adipose stem cells can overcome the various issues if properly studied. In some cases, variations in the effectiveness of ADSCs may occur due to the differences in the method of study used, such as in vitro, In vivo, and others. It is important to determine how effective ADSCs are if administered directly to human beings. Recently, researchers tested a therapy for knee osteoarthritis based on the injection of adipose-derived stem cells to determine its efficacy and safety [41]. In this study, fat-derived cells showed a very low complication rate (16.15%), and all the complications observed were considered to be minor [41]. Additionally, ADSCs have produced promising to excellent clinical results for the treatment of knee osteoarthritis. It also appears that the use of adipose-derived stem cells is associated with clinical and radiological improvements and minimal complication rates [41].
The plastic and reconstructive surgery fields are leaders in the use of ADSCs; many practices, such as fat grafting [41,42], wound healing [43,44], and scar treatment [45], have been tested. It is overwhelming to see the growth of studies using stem cells derived from adipose tissue in other fields as well. This is promising for the health industry across the world and shows the great expansion in the knowledge of scholars and researchers. Further improvements in studies and research might lead to the discovery of many more benefits of adipose-derived tissue stem cells that could be utilized in treatments for various diseases; perhaps additional functions will be discovered. Table 3 shows some of the clinical trials that have made use of ADSCs. In the future, applications we cannot yet imagine might be discovered that will bring benefits for maintaining human health.
Table 3.
The treatment using ADSCs in clinical trials.
| Treatments | Sample Number | Randomization/ Blinding |
Source/Type of ADSCs | Application Method | Results | Ref(s) |
|---|---|---|---|---|---|---|
| Chronic ulcers | 16 cases 24 controls Total 40 |
Yes/No | e-PRP from 42 cm3 of peripheral blood combined with ADSC from 80 mL of abdominal fat vibrated at 600 vibrations/min for 6 min and centrifuged at 52× g for 6 min. 5 × 105 cells |
Same-day procedure. 5 mL injected in multiple injections around and under the ulcer using a 10-mL syringe Follow up: 18 months |
Similar healing rates. Wound-closure rates higher in case group. No adverse events |
[46] |
| 10 cases 0 controls Total 10 |
No/No | Fresh, non-fractioned, non-cultured. Enzymatic congestion using collagenase and centrifugation. Donor site: abdomen 250–350 cm3 fat. 19.1 to 157.8 × 106 cells |
3–4 mL administered using a 26-gauge needle into the plane between the gastrocnemius and soleus muscles in a pattern of injections (22 per muscle, 11 in the external and 11 in the internal gastrocnemius, each one 1.5 cm to 2 cm apart) of equal volume each (0.5 mL), on either side of the midline. Follow up: 18 months |
Four of six wounds closed within 9 months, one patient had a healing wound when she died at 4 months and one patient had a skin graft to close the wound at 5 months. Reduced pain in all patients. No adverse events |
[47] | |
| 10 cases 0 controls Total 10 |
No/No | LipoStructure®. Freshly purified fat using centrifugation at 3000 rpm for 3 min. | Same-day procedure. Multiple injections around and under the ulcer with 0.8-mm cannula. Follow up: 6 months |
73.2% median closure rate at 3 months, 93.1% at 6 months. Reduced fibrin, necrosis, and pain. Increased granulation. No adverse events | [48] | |
| 16 cases 0 controls Total 16 |
No/No | The Transpose RT™ Processing Unit (TPU) (InGeneron Inc., Houston, TX, USA) 30 mL lipoaspirate. Donor site: abdomen. 9–15 × 106 cells |
Same-day procedure. 4 mL injected 5 to 10 mm deep into the central and bordering ulcer area using a 1-mL Luer-Lock syringe and a 24-gauge needle. Additionally, 2.5 mL applied on a collagen sponge onto the wound. Follow up: 6 months |
All venous patients and four of nine arterial-venous patients had 100% wound closure within 9–26 weeks. Reduced wound pain in all patients within days of treatment. No adverse events |
[49] | |
| Breast reconstruction Breast reconstruction |
Preliminary 1 patient |
No/No | Tulip low-pressure syringe lipoaspiration system was used to obtain 520 cm3 of lipoaspirate from the hypogastrium and the thighs. | Cell-enriched fat graft was injected into and around the defect area in multiple planes through blunt-tipped 17-gauge cannulae. As for the right breast, 50 cm3 of cell-enriched fat grafting were injected into the hollowness over the NAC and another 100 cm3 in the upper pole, while 90 and 20 cm3 were used in the upper and the lower pole of the left breast, respectively. | Significant contour improvement in both breasts that remained stable at 3 and 22 months of follow-up. Two minor complications occurred in the left breast; one episode of cellulitis 4 months post grafting that resolved with IV antibiotics uneventfully, and the development of a slightly painful lump 10 months post grafting that turned out to be a liponecrotic cyst after excision biopsy. Both had no impact on the cosmetic effect. |
[50] |
| Preliminary 1 patient |
No/No | 580 cm3 of lipoaspirate from the hypogastrium and the thighs, 260 cm3 of which were processed and yielded 7 cm3 of ASCs that subsequently enriched the remaining fat. | Skin adherence to the lateral aspect of the left breast was initially released. Overall, 320 cm3 of enriched fat was grafted in the periphery of the reconstructed breast in order to improve the contour and correct the tethering of the skin | No complications occurred and a satisfying cosmetic result was retained at 3 and 19 months follow-up. | [50] |
The stromal vascular fraction cells (SVFs) and ADSCs alone are particularly good in treating some diseases. Both are present in Stromal Vasculasr Fraction (SVF) portion, which can be obtained from human adipose tissue. Recently, the allogenic use of SVF and decellularized extracellular matrices (ECM) went through a clinical study in advancing tissue regeneration in which it could be a useful treatment alongside autologous, if the results are positive [51]. Due to the higher availability of fat, it can be easily accessed and obtained with the implementation of a fat donor program. Despite that, the safety and efficacy of the treatment are still undetermined. Based on the study mentioned, the human allogenic use of ADSCs appears to be safe and effective and surely a lot of improvement should be done in the future to determine the safest therapy. At this point, it was shown that the use of ADSCs is really good and could be exploited for various studies instead of its tricks in In-vitro and In-vivo studies.
Aside from that, the combination of ADSCs with other materials to generate or increase the proliferation of other cells is a good alternative in expanding the medical field. The characteristic of ADSC itself, which could turn into different types of cells, make it useful to be used. The right combination was possible due to the factor releases by the ADSCs, as mentioned in a previous section in this article. The same condition could be seen in the implementation of platelet-rich plasma (PRP) from the blood for tissue repair treatment due to the properties of the platelets favoring wound healing [52]. Platelets hold about 50–80 alpha-granules that contain hundreds of bioactive proteins, including a wide range of growth factors (GFs), principally represented by PDGF, FGF, VEGF, epidermal growth factor (EGF), TGF-β1, insulin-like growth factor (IGF), connecting tissue growth factor (CTGF), and HGF [53]. The In-vitro studies here reviewed state almost consistently that PRP stimulates the proliferation of the human cell [52].
The combination practice of PRP was also used together with hyaluronic acid (HA) for a wound-healing study. The study concluded that HA acts as a scaffold for the PRP [54]. The speedy development of the granulation tissue can help to shorten the healing times and reduce the need for reconstructive surgery on supplementary soft tissue. Therefore, body repair mechanisms are stimulated to heal previously irreparable tissues. In addition, the study validated that combined treatment with PRP as a bio-stimulator and HA engaged in a bio-functionalized scaffold can reduce the healing period (p < 0.05) and patient pain, and is cost-effective compared to traditional treatment, increasing the patient’s quality of life. Both ADSCs and PRP have shown that they could proliferate other cells [54].
In a recent update, there was positive news with the usage of stem cells as a therapy to combat with the recent COVID-19 disease. COVID-19 is the recent viral disease that could cause multi-organ damage and for which there are no particular therapies, drugs, or vaccines available for now. With the COVID-19 pandemic, stem cell therapies and especially mesenchymal stem cell (MSC)-related therapies have demonstrated their therapeutic potential for newly emerging diseases with no available treatments. So far, 88 trials were found to be registered to investigate the safety and efficacy of the transplantation therapy of stem cells or stem cell-derived exosomes for COVID-19 patients. Indications under investigation include COVID-19 with critical pneumonia, respiratory failure, ARDS, and pulmonary fibrosis [55]. Another pilot clinical study also reported on aerosol inhalation of the exosomes derived from allogenic adipose mesenchymal stem cells in the treatment of severe patients with novel coronavirus pneumonia (NCT Number: NCT04276987). In the future, we hope any great outcome will be discovered by scientists through stem cell therapy, which will help people who suffered with COVID-19.
5. Conclusions
In conclusion, the use of ADSCs for treating certain diseases might be effective and might be harmful. Their use could either be a treat or a trick, depending on certain factors. These factors include the type of cancer involved, as some types might benefit from the use of ADSCs, such as pancreatic cancer, breast cancer, and oral tongue squamous carcinoma. However, the use of ADSCs in colon cancer, cervical cancer, and epithelial ovarian cancer may have negative effects. At present, the positive and negative effects of ADSC(s) seem to be based on the region of the cancer, e.g., the colon, breast, or cervix, as cells in different areas react differently when being treated or co-cultured with ADSCs. In some cases, the proliferation rate of cancer cells has been promoted, while in some cases, no effect on the cells has been shown. The reason behind these inconsistent results regarding the potency of adipose-derived stem cells for the treatment of different types of cancer remains undetermined. These inconsistent results might also be because of the in vitro/In vivo/ex-vivo microenvironment itself being different to that of a human. Therefore, the results produced in the laboratory are still questionable and further exploration is still needed. The way ADSCs are administered to patients, In-vivo or in vitro, the cell growing process, the technique involved, etc., could also influence the effectiveness of ADSC(s) as a component of medical-based treatment. However, the effectiveness of ADSCs in the area of wound healing and breast augmentation/reconstruction as well as other clinical applications has been proven promising and positive by previous research carried out by several scientists. Future studies could focus on their clinical use to observe whether these cells can legitimately be regarded as a novel treatment. Further studies need to be conducted to determine whether ADSC-based treatments (sole or combination treatments) will provide a positive impact on human beings.
Acknowledgments
We thank the staff of the Reconstructive Sciences Unit, USM, for their direct or indirect support.
Author Contributions
Conceptualization, N.A.M.N. and S.S.A.; investigation and data curation (literature search), S.S.A. and N.A.M.N.; writing—original draft preparation, S.S.A. and N.A.M.N.; writing—review and editing, R.K., A.S.H. and W.A.W.S.; supervision, N.A.M.N., A.S.H., W.A.W.S. and R.K.; project administration, N.A.M.N.; and funding acquisition, N.A.M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Fundamental Research Grant Scheme (Grant Number: 203/PPSP/6171291) from Ministry of Higher Education, Malaysia, and Short term grant from Universiti Sains Malaysia (Grant Number: 304/PPSP/6315501).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L., Redl H., Rubin J.P., Yoshimura K., Gimble J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International S. Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraser J.K., Wulur I., Alfonso Z., Hedrick M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Locke M., Windsor J., Dunbar P.R. Human adipose-derived stem cells: Isolation, characterization and applications in surgery. ANZ J. Surg. 2009;79:235–244. doi: 10.1111/j.1445-2197.2009.04852.x. [DOI] [PubMed] [Google Scholar]
- 4.Hass R., Otte A. Mesenchymal stem cells as all-round supporters in a normal and neoplastic microenvironment. Cell Commun. Signal. 2012;10:26. doi: 10.1186/1478-811X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardozo A.J., Gómez D.E., Argibay P.F. Neurogenic differentiation of human adipose-derived stem cells: Relevance of different signaling molecules, transcription factors, and key marker genes. Gene. 2012;511:427–436. doi: 10.1016/j.gene.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Thiagarajan P.S. Cancer Stem Cells. Elsevier Saunders; Amsterdam, The Netherlands: 2016. Adipose Tissue-Derived Stem Cells in Regenerative Medicine and Impact on Cancer; pp. 411–438. [Google Scholar]
- 7.Hamid A.A., Jeyabalan S.V., Omar A., Yasin N.Z.H.M., Lin W.T., Ling L.L., Ghani N.A.A., Hamizan A.K.W., Hui C.K. Chondrogenesis of adipose-derived stem cells with chondrocytes in low serum towards clinical application. Sains Malays. 2018;47:2369–2379. doi: 10.17576/jsm-2018-4710-13. [DOI] [Google Scholar]
- 8.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 9.Wang M., Yuan Z., Ma N., Hao C., Guo W., Zou G., Zhang Y., Chen M., Gao S., Peng J., et al. Advances and Prospects in Stem Cells for Cartilage Regeneration. Stem Cells Int. 2017;2017:4130607. doi: 10.1155/2017/4130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner J.M., Reinkemeier F., Wallner C., Dadras M., Huber J., Schmidt S.V., Drysch M., Dittfeld S., Jaurich H., Becerikli M., et al. Adipose-Derived Stromal Cells Are Capable of Restoring Bone Regeneration After Post-Traumatic Osteomyelitis and Modulate B-Cell Response. Stem Cells Transl. Med. 2019;8:1084–1091. doi: 10.1002/sctm.18-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Wang S., Gu C., Xiong Y., Shen H., Liu F., Yang J. Ex-vivo treatment of allografts using adipose-derived stem cells induced prolonged rejection-free survival in an allogenic hind-limb transplantation model. Ann. Transl. Med. 2020;8:867. doi: 10.21037/atm-19-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Na Y.K., Ban J.-J., Lee M., Im W., Kim M. Wound healing potential of adipose tissue stem cell extract. Biochem. Biophys. Res. Commun. 2017;485:30–34. doi: 10.1016/j.bbrc.2017.01.103. [DOI] [PubMed] [Google Scholar]
- 13.Lee A., Bae S., Lee S.H., Kweon O.-K., Kim W.H. Hair growth promoting effect of dermal papilla like tissues from canine adipose-derived mesenchymal stem cells through vascular endothelial growth factor. J. Vet. Med. Sci. 2017;78:1811–1818. doi: 10.1292/jvms.16-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salem H.K., Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H., Hyun M.R., Kim S.W. The Effect of Adipose-Derived Stem Cells on Wound Healing: Comparison of Methods of Application. Stem Cells Int. 2019;2019:2745640. doi: 10.1155/2019/2745640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chae M.P., Hunter-Smith D.J., Murphy S.V., Findlay M.W. 15—3D Bioprinting Adipose Tissue for Breast Reconstruction. In: Thomas D.J., Jessop Z.M., editors. Whitaker ISBT-3D B for RS. Woodhead Publishing; Sawston, UK: 2018. pp. 305–353. [Google Scholar]
- 17.Fukuoka H., Narita K., Suga H. Hair Regeneration Therapy: Application of Adipose-Derived Stem Cells. Curr. Stem Cell Res. Ther. 2017;12:531–534. doi: 10.2174/1574888X12666170522114307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner J.M., Jaurich H., Wallner C., Abraham S., Becerikli M., Dadras M., Harati K., Duhan V., Khairnar V., Lehnhardt M., et al. Diminished bone regeneration after debridement of posttraumatic osteomyelitis is accompanied by altered cytokine levels, elevated B cell activity, and increased osteoclast activity. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2017;35:2425–2434. doi: 10.1002/jor.23555. [DOI] [PubMed] [Google Scholar]
- 19.Park B.-S., Jang K.A., Sung J.-H., Park J.-S., Kwon Y.H., Kim K.J., Kim W.-S. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatolog. Surg. Off. Publ. Am. Soc. Dermatolog. Surg. 2008;34:1323–1326. doi: 10.1111/j.1524-4725.2008.34283.x. [DOI] [PubMed] [Google Scholar]
- 20.Tolar J., Le Blanc K., Keating A., Blazar B.R. Concise review: Hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gadelkarim M., Abushouk A.I., Ghanem E., Hamaad A.M., Saad A.M., Abdel-Daim M.M. Adipose-derived stem cells: Effectiveness and advances in delivery in diabetic wound healing. Biomed. Pharmacother. 2018;107:625–633. doi: 10.1016/j.biopha.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Zhao J., Zhang Z., Cui Q., Zhao L., Hu Y., Zhao S. Human adipose-derived mesenchymal stem cells inhibit proliferation and induce apoptosis of human gastric cancer HGC-27 cells. 3 Biotech. 2020;10:129. doi: 10.1007/s13205-020-2090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha S., Narjus-Sterba M., Tuomainen K., Kaur S., Seppänen-Kaijansinkko R., Salo T., Mannerström B., Al-Samadi A. Adipose-Derived Mesenchymal Stem Cells do not Affect the Invasion and Migration Potential of Oral Squamous Carcinoma Cells. Int. J. Mol. Sci. 2020;21:6455. doi: 10.3390/ijms21186455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Y., Tang H., Guo Y., Guo J., Huang B., Fang F., Cai J., Wang Z. Adipose-derived mesenchymal stem cells promote cell proliferation and invasion of epithelial ovarian cancer. Exp. Cell Res. 2015;337:16–27. doi: 10.1016/j.yexcr.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Abarrategi A., Tornin J., Martinez-Cruzado L., Hamilton A., Martinez-Campos E., Rodrigo J.P., González M.V., Baldini N., Garcia-Castro J., Rodriguez R. Osteosarcoma: Cells-of-Origin, Cancer Stem Cells, and Targeted Therapies. Stem Cells Int. 2016;2016:3631764. doi: 10.1155/2016/3631764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scioli M.G., Storti G., D’Amico F., Gentile P., Kim B.-S., Cervelli V., Orlandi A. Adipose-Derived Stem Cells in Cancer Progression: New Perspectives and Opportunities. Int. J. Mol. Sci. 2019;20:3296. doi: 10.3390/ijms20133296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Y., Xiao C.-H., Tan L.-D., Wang Q.-S., Li X.-Q., Feng Y.-M. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer. 2014;110:724–732. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Y.H., Warncke C., Choi S.J., Choi S., Chiou A.E., Ling L., Liu H.-Y., Daniel S., Antonyak M.A., Cerione R.A., et al. Breast cancer-derived extracellular vesicles stimulate myofibroblast differentiation and pro-angiogenic behavior of adipose stem cells. Matrix Biol. 2017;60–61:190–205. doi: 10.1016/j.matbio.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preisner F., Leimer U., Sandmann S., Zoernig I., Germann G., Koellensperger E. Impact of Human Adipose Tissue-Derived Stem Cells on Malignant Melanoma Cells in An In Vitro Co-culture Model. Stem Cell Rev. Rep. 2018;14:125–140. doi: 10.1007/s12015-017-9772-y. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y., Yang Y., Liu Y., Hao Y., Zhang Y., Hu Y., Jiang L., Gong Y., Wu K., Liu Y. Upregulation of PAG1/Cbp contributes to adipose-derived mesenchymal stem cells promoted tumor progression and chemoresistance in breast cancer. Biochem. Biophys. Res. Commun. 2017;494:719–727. doi: 10.1016/j.bbrc.2017.10.118. [DOI] [PubMed] [Google Scholar]
- 31.Koellensperger E., Bonnert L.-C., Zoernig I., Marmé F., Sandmann S., Germann G., Gramley F., Leimer U. The impact of human adipose tissue-derived stem cells on breast cancer cells: Implications for cell-assisted lipotransfers in breast reconstruction. Stem Cell Res. Ther. 2017;8:121. doi: 10.1186/s13287-017-0579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi J., Wei P.-K. Interleukin-8: A potent promoter of angiogenesis in gastric cancer. Oncol. Lett. 2016;11:1043–1050. doi: 10.3892/ol.2015.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ejaz A., Yang K.S., Venkatesh K.P., Chinnapaka S., Kokai L.E., Rubin J.P. The Impact of Human Lipoaspirate and Adipose Tissue-Derived Stem Cells Contact Culture on Breast Cancer Cells: Implications in Breast Reconstruction. Int. J. Mol. Sci. 2020;21:9171. doi: 10.3390/ijms21239171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yenilmez E.N., Genc D., Farooqi A.A., Tunoglu S., Zeybek U., Akkoc T., Yaylim İ. Mesenchymal Stem Cells Combined With IFNγ Induce Apoptosis of Breast Cancer Cells Partially Through TRAIL. Anticancer Res. 2020;40:5641–5647. doi: 10.21873/anticanres.14577. [DOI] [PubMed] [Google Scholar]
- 35.Wei H.-J., Zeng R., Lu J.-H., Lai W.-F.T., Chen W.-H., Liu H.-Y., Chang Y.-T., Deng W.-P. Adipose-derived stem cells promote tumor initiation and accelerate tumor growth by interleukin-6 production. Oncotarget. 2015;6:7713–7726. doi: 10.18632/oncotarget.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhai Y., Wu W., Xi X., Yu R. Adipose-Derived Stem Cells Promote Proliferation and Invasion in Cervical Cancer by Targeting the HGF/c-MET Pathway. Cancer Manag. Res. 2020;12:11823–11832. doi: 10.2147/CMAR.S277130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiliang Z., Lili G. Research Advances in the Application of Adipose-Derived Stem Cells Derived Exosomes in Cutaneous Wound Healing. Ann. Dermatol. 2021;33:309–317. doi: 10.5021/ad.2021.33.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen B., Cai J., Wei Y., Jiang Z., Desjardins H.E., Adams A.E., Li S., Kao H.-K., Guo L. Exosomes Are Comparable to Source Adipose Stem Cells in Fat Graft Retention with Up-Regulating Early Inflammation and Angiogenesis. Plast. Reconstr. Surg. 2019;144:816e–827e. doi: 10.1097/PRS.0000000000006175. [DOI] [PubMed] [Google Scholar]
- 39.Lee B.-C., Kang I., Yu K.-R. Therapeutic Features and Updated Clinical Trials of Mesenchymal Stem Cell (MSC)-Derived Exosomes. J. Clin. Med. 2021;10:711. doi: 10.3390/jcm10040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolmans M.-M., Cacciottola L., Amorim C.A., Manavella D. Translational research aiming to improve survival of ovarian tissue transplants using adipose tissue-derived stem cells. Acta Obstet. Gynecol. Scand. 2019;98:665–671. doi: 10.1111/aogs.13610. [DOI] [PubMed] [Google Scholar]
- 41.Xiong B.-J., Tan Q.-W., Chen Y.-J., Zhang Y., Zhang D., Tang S.-L., Zhang S., Lv Q. The Effects of Platelet-Rich Plasma and Adipose-Derived Stem Cells on Neovascularization and Fat Graft Survival. Aesthetic Plast. Surg. 2018;42:1–8. doi: 10.1007/s00266-017-1062-1. [DOI] [PubMed] [Google Scholar]
- 42.Harris W.M., Plastini M., Kappy N., Ortiz T., Chang S., Brown S., Carpenter J.P., Zhang P. Endothelial Differentiated Adipose-Derived Stem Cells Improvement of Survival and Neovascularization in Fat Transplantation. Aesthetic Surg. J. 2019;39:220–232. doi: 10.1093/asj/sjy130. [DOI] [PubMed] [Google Scholar]
- 43.McCarthy M.E., Brown T.A., Bukowska J., Bunnell B.A., Frazier T., Wu X., Gimble J.M. Therapeutic applications for adipose-derived stem cells in wound healing and tissue engineering. Curr. Stem Cell Rep. 2018;4:127–137. doi: 10.1007/s40778-018-0125-9. [DOI] [Google Scholar]
- 44.Toyserkani N.M., Christensen M.L., Sheikh S.P., Sørensen J.A. Adipose-Derived Stem Cells: New Treatment for Wound Healing? Ann. Plast. Surg. 2015;75:117–123. doi: 10.1097/SAP.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 45.Deng J., Shi Y., Gao Z., Zhang W., Wu X., Cao W., Liu W. Inhibition of Pathological Phenotype of Hypertrophic Scar Fibroblasts Via Coculture with Adipose-Derived Stem Cells. Tissue Eng. Part A. 2017;24:382–393. doi: 10.1089/ten.tea.2016.0550. [DOI] [PubMed] [Google Scholar]
- 46.Raposio E., Bertozzi N., Bonomini S., Bernuzzi G., Formentini A., Grignaffini E., Pio Grieco M. Adipose-derived Stem Cells Added to Platelet-rich Plasma for Chronic Skin Ulcer Therapy. Wounds Compend. Clin. Res. Pract. 2016;28:126–131. [PubMed] [Google Scholar]
- 47.Carstens M.H., Gómez A., Cortés R., Turner E., Pérez C., Ocon M., Correa D. Non-reconstructable peripheral vascular disease of the lower extremity in ten patients treated with adipose-derived stromal vascular fraction cells. Stem Cell Res. 2017;18:14–21. doi: 10.1016/j.scr.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Chopinaud M., Labbé D., Creveuil C., Marc M., Bénateau H., Mourgeon B., Chopinaud E., Veyssière A., Dompmartin A. Autologous Adipose Tissue Graft to Treat Hypertensive Leg Ulcer: A Pilot Study. Dermatology. 2017;233:234–241. doi: 10.1159/000478009. [DOI] [PubMed] [Google Scholar]
- 49.Konstantinow A., Arnold A., Djabali K., Kempf W., Gutermuth J., Fischer T., Biedermann T. Therapy of ulcus cruris of venous and mixed venous arterial origin with autologous, adult, native progenitor cells from subcutaneous adipose tissue: A prospective clinical pilot study. J. Eur. Acad. Dermatol. Venereol. 2017;31:2104–2118. doi: 10.1111/jdv.14489. [DOI] [PubMed] [Google Scholar]
- 50.Tsekouras A., Mantas D., Tsilimigras D.I., Ntanasis-Stathopoulos I., Kontos M., Zografos G.C. Adipose-derived stem cells for breast reconstruction after breast surgery—Preliminary results. Case Rep. Plast. Surg. Hand Surg. 2017;4:35–41. doi: 10.1080/23320885.2017.1316201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gentile P., Sterodimas A., Pizzicannella J., Dionisi L., De Fazio D., Calabrese C., Garcovich S. Systematic Review: Allogenic Use of Stromal Vascular Fraction (SVF) and Decellularized Extracellular Matrices (ECM) as Advanced Therapy Medicinal Products (ATMP) in Tissue Regeneration. Int. J. Mol. Sci. 2020;21:4982. doi: 10.3390/ijms21144982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gentile P., Garcovich S. Systematic Review-The Potential Implications of Different Platelet-Rich Plasma (PRP) Concentrations in Regenerative Medicine for Tissue Repair. Int. J. Mol. Sci. 2020;21:5702. doi: 10.3390/ijms21165702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blair P., Flaumenhaft R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Angelis B., D’Autilio M.F.L.M., Orlandi F., Pepe G., Garcovich S., Scioli M.G., Orlandi A., Cervelli V., Gentile P. Wound Healing: In Vitro and In Vivo Evaluation of a Bio-Functionalized Scaffold Based on Hyaluronic Acid and Platelet-Rich Plasma in Chronic Ulcers. J. Clin. Med. 2019;8 doi: 10.3390/jcm8091486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z., Niu S., Guo B., Gao T., Wang L., Wang Y., Wang L., Tan Y., Wu J., Hao J. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif. 2020;53:e12939. doi: 10.1111/cpr.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.