Abstract
Background: Functional magnetic resonance imaging (fMRI) is one of the most important neuroimaging techniques; nevertheless, the acoustic noise of the MR scanner is unavoidably linked to the process of data acquisition. We hypothesized that the auditory noise of the scanner has an effect on autonomic activity. Methods: We measured heart rate variability (HRV) while exposing 30 healthy subjects to fMRI noise. In doing so, we demonstrated an increase in parasympathetic nervous system (PNS) activity compared to silence and white noise and a decrease in sympathetic nervous system (SNS) activity compared to white noise. Conclusions: The influence of MR scanner noise on the autonomic nervous system should be taken into account when performing fMRI experiments.
Keywords: HRV, fMRI, auditory processing
1. Introduction
Resting-state functional magnetic resonance imaging (fMRI) is an invaluable tool for clinical and basic neuroscience [1]. The process of fMRI sequence acquisition is unavoidably linked to high intensity (mostly >80 dB) and rhythmic acoustic noise, typically lasting 5–10 min. Previous studies suggest that fMRI noise may affect brain activity and connectivity, but the potential impact on the autonomic system is poorly known. Interestingly, music and auditory stimuli influence autonomic activity and brain connectivity, as revealed by heart rate variability (HRV) [2,3]. The introduction of noise to the brain has long been shown to have an influence on signal processing, the effects of which have been termed stochastic and coherence resonance [4,5]. We designed an experiment where an electrocardiogram (EKG) was acquired under three conditions: (a) while listening to fMRI acoustic noise (hereafter fMRI), (b) during acoustic White noise exposure (hereafter White) and (c) at rest during silence (hereafter Silence). Our goal was to assess the influence of fMRI acoustic noise on HRV in a within-subject design.
2. Materials and Methods
Thirty healthy participants aged 20–50 were included (mean age = 28.57 ± 4.18 years; 24 female). Exclusion criteria were: (a) present or past history of neuropsychiatric disorders, (b) hearing deficits, (c) irregular wakefulness-sleep cycle, (d) medications that act on the central nervous system, and (e) medical conditions potentially affecting the autonomic activity. The study was approved by the Research Ethics Board of the Province of Venice, Italy, complied with the 1964 Declaration of Helsinki and its later amendments and was performed at IRCCS San Camillo Hospital in Venice, Italy. Subjects signed a written informed consent prior to participation.
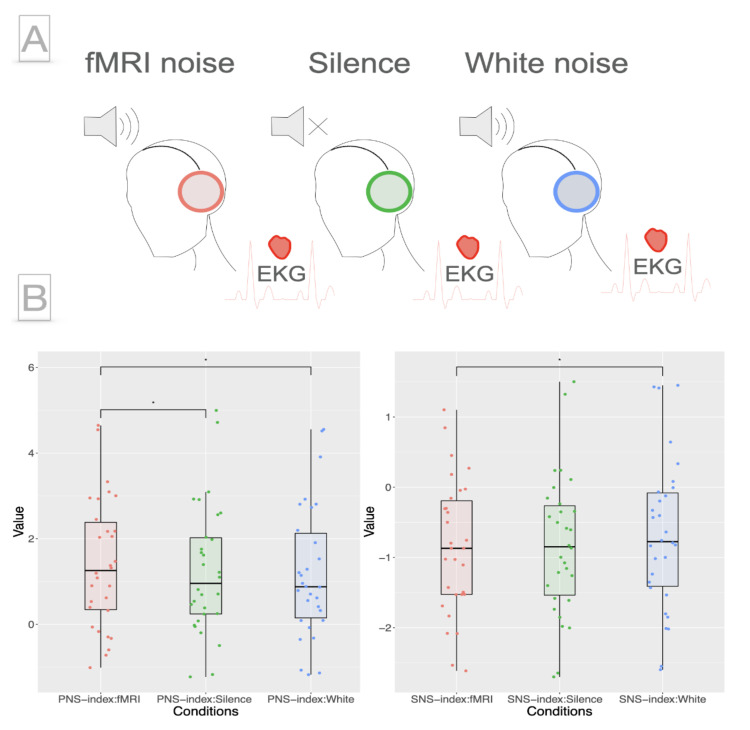
Participants were lying supine with eyes closed and wearing earplugs connected to the audio delivery system. They were instructed to relax during the experiment. An EKG was recorded with one bipolar electrode on the left and right upper part of the musculus pectoralis. The acoustic noise of the fMRI corresponded to a standard echo planar imaging (EPI) sequence with the following parameters: [TR] = 2000 ms, [TE] = 35 ms, voxel size = 3.3 mm3 isotropic, slices = 37, frequency = 0.5 Hz + 54 Hz + ~11 kHz (220 voxel in gradient direction x slices acquisition frequency = 220 × 54 Hz) of a 3T Ingenia CX Philips scanner. The sound pressure level was set to 85 dB during White and fMRI. A depiction of the fMRI noise spectrum can be found in Figure S1. The order of the conditions was counterbalanced across subjects. Each condition lasted 8 min (Figure 1, Panel A).
Figure 1.
Experimental design and autonomic difference between Silence, fMRI and White noise. Panel A. Subjects were exposed to fMRI noise, Silence or White noise in a counterbalanced order during the course of one EKG recording session. Panel B. Parasympathetic activity was significantly higher during fMRI noise as compared to Silence and White noise. Sympathetic activity was significantly higher for White noise as compared to fMRI noise.
Kubios software [6] was used to extract metrics for the autonomic nervous system activity from EKG R-R intervals. We specifically focused on two comprehensive measures for sympathetic nervous system (SNS) and parasympathetic nervous system (PNS) activity [7], as defined in the Kubios software (v. 3.5, https://www.kubios.com/) (accessed on 25 October 2021). While the SNS index assumes an increase in heartrate and a decrease in heart rate variability, the PNS index takes into account the opposite pattern [8]. The levels of SNS and PNS were evaluated between the three conditions using repeated measures ANOVA.
3. Results
Kolmogorov-Smirnov tests for each condition indicated normal distribution for PNS and SNS variables. Mauchly’s tests indicated the sphericity of data.
Repeated measures ANOVA was significant for PNS (F2,58 = 4.129, p = 0.021) as well as SNS (F2,58 = 4.612, p = 0.014).
Consequently, post hoc pairwise paired t-tests were calculated in order to reveal significant differences between conditions.
PNS was significantly higher for fMRI compared to Silence (t29 = 2.33, pfdr = 0.04, mSilence = 1.25, mfMRI = 1.41) as well as compared to White (t29 = 2.54, pfdr = 0.04, mWhite = 1.19, mfMRI = 1.41; Figure 1, Panel B, left). There was no significant difference in PNS between Silence and White (t29 = 0.78, pfdr = 0.44). SNS was significantly higher for White than for fMRI (t29 = 2.62, pfdr = 0.04, mWhite = −0.68, mfMRI = −0.85; Figure 1, Panel B, right); there were no differences in the comparisons of fMRI–Silence (t29 = 0.63 pfdr = 0.54) and White–Silence (t29 = 2.18, pfdr = 0.057) for SNS.
4. Discussion
The significant enhancement of PNS and the decrease of SNS during fMRI might reflect the tendency to transition into a relaxed state and drowsiness favored by exposure to the monotonous rhythmic sound environment [9]. People in the MR scanner face difficulties in maintaining a state of wakefulness over the time of the scanning session, with sleep states contaminating resting-state connectivity patterns and 30% of the subjects unable to sustain wakefulness after 3 min [10]. On the other hand, we may speculate that the increase of PNS/decrease in SNS score might be independent of drowsiness and possibly due to the specific noise pattern produced during fMRI acquisition. This interpretation would be in agreement with previous studies, demonstrating the association between autonomic function and auditory processing [2,11]. In this respect, exposure to rhythmic noise might influence the autonomic nervous system via entrainment [5], compared to white noise that might influence the excitability of the brain [12,13].
The effect of fMRI acoustic noise on autonomic activity should therefore not be neglected. The acquisition of an EKG during fMRI should be considered a potential tool to unveil and account for such an effect.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/brainsci11111416/s1, Figure S1: Signal Spectrum of the fMRI Acoustic Noise Stimulus.
Author Contributions
Conceptualization, G.P.; Methodology, G.P.; Validation, G.P. and A.-L.S.; Formal Analysis, G.P. and A.-L.S.; Investigation, G.P.; Resources, G.P.; Data Curation, G.P. and A.-L.S.; Writing—Original Draft Preparation, A.-L.S.; Writing—Review and Editing, G.P.; Visualization, A.-L.S.; Supervision, G.P.; Project Administration, G.P.; Funding Acquisition, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
Data acquisition was funded by an operating grant from Italian Ministry of Health to San Camillo Hospital IRCCS. G.P. has been funded by the Frederick Andermann Epilepsy and EEG fellowship.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Province of Venice, Italy (protocol code 2015.11 (31 March 2015)).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data will be made available on reasonable request to the authors.
Conflicts of Interest
None of the authors has potential conflict of interest to be disclosed.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biswal B.B. Resting State FMRI: A Personal History. Neuroimage. 2012;62:938–944. doi: 10.1016/j.neuroimage.2012.01.090. [DOI] [PubMed] [Google Scholar]
- 2.Regaçone S.F., Lima D.D., Banzato M.S., Gução A.C., Valenti V.E., Frizzo A.C. Association between Central Auditory Processing Mechanism and Cardiac Autonomic Regulation. Int. Arch. Med. 2014;7:1–4. doi: 10.1186/1755-7682-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Praag C.D.G., Garfinkel S.N., Sparasci O., Mees A., Philippides A.O., Ware M., Ottaviani C., Critchley H.D. Mind-Wandering and Alterations to Default Mode Network Connectivity When Listening to Naturalistic versus Artificial Sounds. Sci. Rep. 2017;7:1–12. doi: 10.1038/srep45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonnell M.D., Abbott D. What Is Stochastic Resonance? Definitions, Misconceptions, Debates, and Its Relevance to Biology. PLoS Comput. Biol. 2009;5:e1000348. doi: 10.1371/journal.pcbi.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sancristóbal B., Rebollo B., Boada P., Sanchez-Vives M.V., Garcia-Ojalvo J. Collective Stochastic Coherence in Recurrent Neuronal Networks. Nat. Phys. 2016;12:881–887. doi: 10.1038/nphys3739. [DOI] [Google Scholar]
- 6.Tarvainen M.P., Niskanen J.-P., Lipponen J.A., Ranta-Aho P.O., Karjalainen P.A. Kubios HRV–Heart Rate Variability Analysis Software. Comput. Methods Programs Biomed. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Assenza G., Mecarelli O., Tombini M., Pulitano P., Pellegrino G., Benvenga A., Assenza F., Campana C., Di Pino G., Di Lazzaro V. Hyperventilation Induces Sympathetic Overactivation in Mesial Temporal Epilepsy. Epilepsy Res. 2015;110:221–227. doi: 10.1016/j.eplepsyres.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Acharya U.R., Joseph K.P., Kannathal N., Lim C.M., Suri J.S. Heart Rate Variability: A Review. Med Biol. Eng. Comput. 2006;44:1031–1051. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 9.Stein P.K., Pu Y. Heart Rate Variability, Sleep and Sleep Disorders. Sleep Med. Rev. 2012;16:47–66. doi: 10.1016/j.smrv.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Tagliazucchi E., Laufs H. Decoding Wakefulness Levels from Typical FMRI Resting-State Data Reveals Reliable Drifts between Wakefulness and Sleep. Neuron. 2014;82:695–708. doi: 10.1016/j.neuron.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Viviane B., Frizzo A.C.F., Oliveira F.R., Garner D.M., Raimundo R.D., Valenti V.E. Interaction Between Cortical Auditory Processing and Vagal Regulation of Heart Rate in Language Tasks: A Randomized, Prospective, Observational, Analytical and Cross-Sectional Study. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-41014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutt A., Lefebvre J., Hight D., Sleigh J. Suppression of Underlying Neuronal Fluctuations Mediates EEG Slowing during General Anaesthesia. Neuroimage. 2018;179:414–428. doi: 10.1016/j.neuroimage.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 13.Moret B., Donato R., Nucci M., Cona G., Campana G. Transcranial Random Noise Stimulation (TRNS): A Wide Range of Frequencies Is Needed for Increasing Cortical Excitability. Sci. Rep. 2019;9:1–8. doi: 10.1038/s41598-019-51553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be made available on reasonable request to the authors.