Abstract
“Tropheryma whippelii”-associated infections are usually confirmed histopathologically by using light microscopy. PCR assays targeting the 16S rRNA gene (16S rDNA) of “T. whippelii” are increasingly being applied for this purpose. Compared to microscopic analysis, PCR seems to be more sensitive, as indicated by the fact that several cases of Whipple's disease with negative histopathological findings but positive PCR results have been reported. Considering the lack of pathognomonic clinical features for this disease and the fact that “T. whippelii” DNA has repeatedly been found in patients without clinical Whipple's disease, such PCR results should be confirmed by additional tests. We have, therefore, evaluated a “T. whippelii”-specific nested PCR targeting domain III of the 23S rDNA with 41 clinical specimens known to contain “T. whippelii” 16S rDNA. All of these specimens were also positive for “T. whippelii” 23S rDNA. The specificity of the test was shown by sequencing of the amplicons and by the absence of amplicons in 38 negative controls. We consider this PCR test to be a suitable tool for confirming the presence of “T. whippelii” DNA in specimens with inconclusive histopathological findings. The information derived from sequencing of the partial “T. whippelii” 23S rDNA was then combined with our recent data of the 16S-23S rDNA spacer region of this organism. Overall, four different rDNA types are recognized in our proposed classification system for molecular variants of “T. whippelii.” This preliminary scheme may provide a basis for further epidemiological and clinical studies with “T. whippelii” and associated diseases.
Patients suffering from Whipple's disease often present with malabsorption and other gastrointestinal symptoms. However, articular, cardiac, and central nervous system involvement is not uncommon and may be more prominent clinically (5). The various manifestations reflect the systemic nature of a chronic infection associated with rod-shaped organisms which have so far never been reproducibly cultivated on artificial media (5, 19). Thus, the traditional laboratory diagnosis of Whipple's disease is based on light microscopy with the demonstration of diastase-resistant, periodic-acid-Schiff (PAS)-positive, non-acid-fast rods in macrophages of intestinal biopsies (5, 6). According to recent comparative sequence analysis of the 16S ribosomal RNA gene (16S rDNA), the putative agent was identified as an actinobacterium (17, 23) constituting the novel, not-yet-validated taxon “Tropheryma whippelii” (17). Based on this information, several diagnostic PCR assays targeting various parts of the 16S rDNA of “T. whippelii” were established (1, 2, 4, 17, 18, 21).
Compared to the traditional histopathological examination, DNA amplification methods are considerably more sensitive, thus facilitating the (early) laboratory diagnosis and monitoring of both typical and atypical cases of Whipple's disease (1–4, 14–18, 21, 22). However, “T. whippelii” DNA has also been found in a significant number of persons without clinical evidence of Whipple's disease (7). Thus, positive PCR results in PAS-negative specimens should be interpreted with a view of the clinical features and, in addition, be confirmed by alternative laboratory tests, e.g., PCR assays with independent target sequences. Although hybridization and sequencing of PCR products may provide further evidence for the presence of “T. whippelii” DNA, these techniques are rather tedious and time-consuming and do not reliably exclude the possibility of amplicon carryover contamination. In contrast, an additional species-specific PCR with an independent target region of the Whipple's disease bacterium might provide the necessary confirmation within a reasonable time frame. An essential prerequisite for such a diagnostic strategy is, of course, that the PCR assays used for screening and confirmation, respectively, have been shown to be of comparable sensitivity as well as specificity.
In this study, we have evaluated a recently established nested PCR targeting a part of the 23S rDNA domain III of the Whipple's disease bacterium (12a) with an extensive collection of clinical specimens, all of which had previously been tested by using other “T. whippelii”-specific PCR assays. Performances of the different methods were compared to assess the suitability of the new amplification system as a tool to confirm the presence of “T. whippelii” DNA irrespective of histopathological findings. In addition, sequence data of the 23S rDNA domain III amplicons were included in a proposed classification system for molecular variants of uncultivated “T. whippelii.”
MATERIALS AND METHODS
Patients.
A total of 41 specimens from 28 patients with or without Whipple's disease (Table 1) previously shown to contain “T. whippelii” 16S rDNA by using either a species-specific seminested PCR (n = 39) (2) or a species-specific amplification with primer pair TW-1 and TW-3 (n = 2) (1) were evaluated. They included one EDTA-anticoagulated blood sample, heart valves (n = 3), cerebrospinal fluids (n = 2), synovial fluids (n = 5), intestinal biopsies (n = 18), gastric aspirates (n = 11), and an intraoperative swab from a joint. For 21 patients, the presence of “T. whippelii” DNA had been confirmed by direct sequencing of either species-specific or broad-range bacterial 16S rDNA amplicons as indicated in Table 1. For most of these patients, as well as for the remaining seven patients (patients 2, 4, 14, 16, 21, 22, and 23), comparable results had been obtained by sequence analysis of the 16S-23S rDNA spacer region (12). A total of 34 specimens from 26 patients had already been assigned to one of the three currently recognized “T. whippelii” spacer types by means of sequence analysis, single-strand conformation polymorphism (SSCP) analysis, and type-specific PCR assays (12). Some intestinal and extraintestinal biopsy specimens from patients with Whipple's disease were histopathologically negative (Table 1).
TABLE 1.
Source and characterization of “T. whippelii”-positive specimens
| Patient
|
Specimen(s)
|
Results of “T. whippelii” testing by PCR and sequencing
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | WDa | Origin | Code | Histologyb | 16S rDNA fragment
|
16S-23S rDNA spacer
|
23S rDNA domain IIIc
|
|||
| PCRd | Sequencinge | PCRf | Typeg | PCR | Type | |||||
| 1 | + | Heart valve | 724/98 | Gram+ | + | EUB | + | 1 | + | A |
| PEh heart valve | 838/98 | Gram+ | + | NDk | + | 1 | + | A | ||
| 2 | + | Duodenum (I) | 5713/96 | Normal | + | ND | + | 1 | + | A |
| Duodenum (II) | 5974/96 | Normal | +i | ND | − | ND | + | A | ||
| 3 | − | Duodenum | 735II/97 | PAS− | + | ND | − | 1 | + | A |
| Gastric aspirate | 736/97 | ND | + | TW | − | ND | + | A | ||
| 4 | − | Gastric aspirate (I) | 616/97 | ND | + | ND | + | 1 | + | A |
| Gastric aspirate (II) | 616II/97 | ND | + | ND | + | 1 | + | A | ||
| 5 | + | Small intestine | 368/97 | Normal | + | TW | + | 1 | + | A |
| 6 | + | PE Duodenum | 459-98 | ND | + | TWj | + | 1 | + | A |
| 7 | + | PE Duodenum | 836-96 | ND | + | TWj | + | 1 | + | A |
| 8 | + | PE Duodenum | 4549-94 | ND | + | TWj | + | 1 | + | A |
| 9 | − | Ileum | 168/97 | Normal | + | TW | + | 1 | + | A |
| Jejunum | 198/97 | Normal | + | ND | − | 1 | + | A | ||
| Duodenum | 995/98 | Normal | + | ND | − | ND | + | A | ||
| 10 | + | Cerebrospinal fluid | 141/97 | Normal | + | TW | + | 1 | + | A |
| 11 | + | Small intestine | 5766/96 | Normal | +i | ND | + | 1 | + | A |
| Synovial fluid | 5831/96 | Normal | + | EUB | + | 1 | + | A | ||
| 12 | − | Gastric aspirate | 1073/97 | ND | + | TW | + | ND | + | A |
| 13 | − | Gastric aspirate | 761/97 | ND | + | TW | − | ND | + | A |
| 14 | + | Ileum | 2249/96 | Normal | + | ND | + | 2 | + | A |
| 15 | + | Intraoperative swab | 875/98 | ND | + | ND | + | 2 | + | A |
| Synovial fluid (I) | 749/98 | ND | + | EUB | + | 2 | + | A | ||
| Synovial fluid (II) | 935/98 | ND | + | ND | + | 2 | + | A | ||
| Synovial fluid (III) | 936/98 | ND | + | ND | + | 2 | + | A | ||
| 16 | − | Gastric aspirate | 895/97 | ND | + | ND | + | 2 | + | A |
| 17 | − | Small intestine | 5996/96 | Normal | + | TW | + | 2 | + | A |
| Ileum | 135/97 | Normal | + | TW | + | ND | + | A | ||
| 18 | − | Duodenum | 604/97 | PAS− | + | TW | + | 2 | + | A |
| 19 | − | Gastric aspirate | 1092/97 | ND | + | TW | + | 2 | + | A |
| 20 | − | Gastric aspirate | 1037/97 | ND | + | TW | + | 2 | + | A |
| 21 | + | Cerebrospinal fluid | 20/98 | ND | + | ND | + | 2 | + | A |
| 22 | + | Synovial fluid | 21/98 | ND | + | ND | + | 2 | + | A |
| 23 | + | EDTA blood | 24/98 | ND | + | ND | + | 2 | + | A |
| 24 | + | Heart valve | 5194/95 | PAS+ | + | EUB | + | 2 | + | A |
| 25 | − | Duodenum | 771/97 | PAS− | + | TW | + | 2 | + | ND |
| Ileum | 905/97 | PAS− | + | TW | + | ND | + | A | ||
| 26 | + | Duodenum | 220/97 | Normal | + | TW | + | 3 | + | A |
| 27 | − | Gastric aspirate | 672/97 | ND | + | TW | + | 3 | + | A |
| 28 | − | Gastric aspirate (I) | 532/97 | ND | + | TW | + | 3 | + | B |
| Gastric aspirate (II) | 952/97 | ND | + | TW | + | 3 | + | B | ||
Presence (+) or absence (−) of Whipple's disease (WD) based on overall assessment of the patient including clinical and laboratory data. Most patients without Whipple's disease come from a prospective study (7).
Overall histologic assessment or result of Gram or PAS stain.
Products and sequence types obtained by species-specific nested PCR by using primer pairs HGC-23InsF and TW-23InsR1, followed by TW-23InsF and TW-23InsR2 and direct sequencing. Specimens 724/98 (patient 1), 368/97 (patient 5), 168/97 (patient 9), 5996/96 (patient 17), 1037/97 (patient 20), 5194/95 (patient 24), 220/97 (patient 26), 532/97 (patient 28), and 952/97 (patient 28) were previously investigated by using the same approach (Henrikson et al., submitted).
Species-specific seminested PCR (2).
Partial 16S rDNA sequence identical to “T. whippelii” reference sequence (18) for TW-4 and TW-2 (TW) fragment or broad-range (EUB) amplicon as described previously by Brändle et al. (2) and Goldenberger et al. (9), respectively.
Species-specific nested PCR (12).
Classification by means of “T. whippelii” spacer type-specific nested PCR assays (12).
PE, paraffin-embedded.
Species-specific PCR with primer pair TW-1 and TW-3 (1).
Partial 16S rDNA sequence identical to “T. whippelii” reference sequence (17) as determined via a species-specific approach (10).
ND, no data available.
Complete data sets (i.e., including PCR and sequencing results of the “T. whippelii” 23S rDNA domain III) have already been published in our related study (12a) for the following specimens: 724/98, patient 1; 368/97, patient 5; 168/97, patient 9; 5996/96, patient 17; 1037/97, patient 20; 5194/95, patient 24; 220/97, patient 26; 532/97, patient 28; and 952/97, patient 28.
Controls.
A DNA extract previously used to determine the original “T. whippelii” 16S-23S rDNA intergenic spacer sequence (13) (kindly provided by M. Maiwald, Heidelberg, Germany) was included as positive control.
Negative controls consisted of 38 clinical specimens shown to be negative for “T. whippelii” 16S rDNA by seminested PCR as described above. They included intestinal biopsies (n = 21) and gastric aspirates (n = 17). These specimens were all from patients without clinical signs typical for Whipple's disease (7). All biopsies were found to be negative by histopathology with PAS staining.
Extraction of DNA.
EDTA-anticoagulated blood samples (100 μl each) were mixed with 300 μl of erythrocyte (EC)-lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) and incubated on ice for 30 min with gentle mixing every 5 min. Mixtures were centrifuged at 20,000 × g for 15 min at 4°C, and the resulting supernatants were carefully removed. The pellets were resuspended in 300 μl of EC-lysis buffer and centrifuged as described above. The pellets obtained were resuspended in 200 μl of digestion buffer (50 mM Tris-HCl [pH 8.5], 1 mM EDTA, 0.5% sodium dodecyl sulfate, 200 μg of proteinase K per ml) and further processed as described below.
Paraffin-embedded tissues (two to five 5- to 10-μm sections per specimen) were suspended in 200 μl of digestion buffer without proteinase K. Mixtures were incubated at 95°C for 10 min with moderate agitation in a thermomixer (Eppendorf, Hamburg, Germany), followed by centrifugation at 14,000 × g for 30 min at room temperature. The resulting paraffin layers were removed with sterile pipette tips. Proteinase K was then added to a final concentration of 200 μg/ml for further processing as described below.
The intraoperative swab was thoroughly washed in 2 ml of 0.85% NaCl. This suspension, all cerebrospinal fluids, synovial fluids, and gastric aspirates were then centrifuged at 14,000 × g for 10 min at room temperature. The resulting pellets as well as the native biopsies were suspended in 200 μl of digestion buffer and incubated at 55°C for 90 min with agitation in a thermomixer. DNA was extracted by using QIAamp DNA binding columns (Qiagen, Hilden, Germany) as described previously (12).
Amplification and direct sequencing of partial 23S rDNA domain III.
Composition of PCR reactions for amplification or reamplification on a GeneAmp PCR System 9600 (Perkin-Elmer, Norwalk, Conn.) was done as described previously (12). All PCRs included an initial activation-denaturation step at 95°C for 12 min and a final extension at 72°C for 10 min. Amplification with primers HGC-23InsF (5′-CGT AGT CGA TGG ACA ACG) and TW-23InsR1 (5′-TAG AAC CTT GTG TCG ATG C) consisted of 40 cycles at 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Nested reamplification with “T. whippelii”-specific primers TW-23InsF (5′-GGT TGA TAT TCC CGT ACC GGC AAA G) and TW-23InsR2 (5′-GCA TAG GAT CAC CAA TTT CGC GCC) was performed for 20 cycles at 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min. Amplicons were separated and visualized on ethidium bromide-stained agarose gels. Reamplification products were purified with the QIAquick PCR Purification Kit (Qiagen) prior to sequencing. Cycle sequencing was performed by using the Cy5-labeled primers TW-23InsF and TW-23InsR2 and the Thermo Sequenase Kit (Amersham Pharmacia Biotech, Little Chalfont, England) as described elsewhere (12a). Sequences were determined on an ALFexpress DNA Sequencer (Amersham Pharmacia Biotech) and compared to the corresponding “T. whippelii” reference sequences (GenBank accession numbers AF148136 and AF148137) (12a) by using the Genetics Computer Group software package (University of Wisconsin, Madison).
RESULTS
“T. whippelii”-specific nested PCR of partial 23S rDNA domain III.
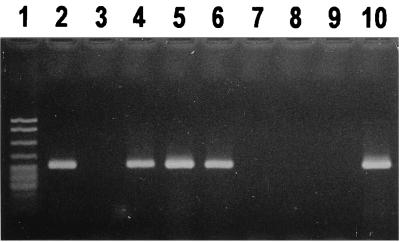
Amplification products (298 bp) with primer pair HGC-23InsF and TW-23InsR1 were visible on the agarose gel for 23 of the 41 patient specimens, while no amplicons were detected for the remaining samples, including the 38 negative controls (not shown). However, “T. whippelii”-specific reamplification with primer pair TW-23InsF and TW-23InsR2 resulted in the expected amplicons of 250 bp for all patients but in none of the negative controls (Fig. 1). For strongly positive specimens, reamplification products were detected together with trace amounts of first-round amplicons. Further, both amplification and reamplification did not generate any nonspecific products with the extracts investigated.
FIG. 1.
Detection of partial 23S rDNA domain III of the Whipple's disease bacterium directly from human clinical specimens. Products of “T. whippelii”-specific nested reamplification (250 bp) were analyzed on an ethidium bromide-stained agarose gel. Lane 1, pBR322 DNA digested with MspI (marker bands are of the following sizes from top to bottom: 622, 527, 404, 307, and ≤240 bp); lanes 2, 4 to 6, and 10, patients positive for “T. whippelii” 23S rDNA fragments with lanes 5 and 10 showing trace amounts of first-round amplicons (298 bp); lanes 3 and 7 to 9, patients negative for “T. whippelii” 23S rDNA fragments (controls).
Comparative sequence analysis of partial 23S rDNA domain III of “T. whippelii”.
Except for specimen 771/97 of patient 25 (Table 1), all amplicons derived from “T. whippelii”-specific reamplification were sequenced and aligned with the respective reference sequences containing the “T. whippelii” 23S rDNA actinobacterial insertion types A and B, respectively (12a). All sequences determined for patients 1 to 27 were found to be identical to the reference sequence with insertion type A (Table 1). The same result was obtained with the “T. whippelii” reference extract used as positive control (not shown). The sequences of the two independently collected and processed specimens of patient 28 have been shown to represent a different type B (differing at one single nucleotide position from the insertion sequence type A) (12a). These results were confirmed in this study.
Classification of molecular variants of “T. whippelii.”
Except for specimen 771/97 of patient 25 (Table 1), all samples previously assigned to one of the three “T. whippelii” 16S-23S rDNA spacer types (12) were further classified according to the two different “T. whippelii” actinobacterial insertion types found in the 23S rDNA domain III. Overall, four different molecular variants were recognized as defined in the proposed classification system for “T. whippelii” (Table 2). These four rDNA types designated according to the particular spacer type (number code) as well as the insertion type (letter code) were detected with the following frequencies: “T. whippelii” type 1A, 11 patients; type 2A, 12 patients; type 3A, 2 patients; and type 3B, 1 patient. No correlation was found between the “T. whippelii” types and the types of specimens investigated.
TABLE 2.
Proposed classification system for rDNA variants of uncultivated “T. whippelii” recognized in this study
| “T. whippelii” rDNA typea | No. of patients (no. of specimens) | Characteristic nucleotide positions
|
|||||
|---|---|---|---|---|---|---|---|
| 16S-23S rDNA spacerb, position:
|
23S rDNA domain III,c position 88 | ||||||
| 319 | 346–349 | 387 | 411 | 420 | |||
| 1A | 11 (15) | T | TTTT | C | T | A | T |
| 2A | 12 (14) | C | -TTT | C | T | A | T |
| 3A | 2 (2) | C | -TTT | T | C | G | T |
| 3B | 1 (2) | C | -TTT | T | C | G | C |
The proposed type designation indicates both the sequence types determined for the spacer (number code) as well as for domain III (letter code) (for future extensions of the proposed system, see Discussion).
GenBank accession number AF148136 (12a).
DISCUSSION
Since the implementation of molecular techniques into the laboratory diagnosis of Whipple's disease, several PCR-positive but histopathologically negative cases have been described (3, 4, 14, 16, 18). For some patients, this might be explained by a patchy distribution of the “Whipple bacilli” at the particular sampling sites (5). For others, e.g., our patient suffering from spondylodiscitis (1), such discrepancies are more likely to be due to an increased sensitivity of the current “T. whippelii” 16S rDNA-targeting PCRs as compared to the traditional PAS staining. However, in the absence of conclusive histopathological findings, positive PCR results should preferably be confirmed by an independent approach. This may be achieved by a second specific amplification with an alternative target sequence. Therefore, we have evaluated a recently established PCR assay targeting domain III of the 23S rDNA of the Whipple's disease bacterium (12a) with specimens which had been analyzed previously by other “T. whippelii”-specific amplification methods.
The evaluation included 41 specimens from 28 patients positive for “T. whippelii” as shown previously by either broad-range and/or “T. whippelii”-specific PCRs targeting 16S rDNA (Table 1). An alternative nested PCR with primers complementary to the flanking regions of the 16S-23S rDNA spacer of “T. whippelii” had been positive for only 35 of these specimens despite the use of 40 cycles each for amplification and reamplification (12). However, the reduced sensitivity (85.4%) of this alternative PCR may be explained by the relatively large spacer products (±490 bp) compared to the 16S rDNA fragments (229 and 141 bp) generated by the two “T. whippelii”-specific assays. Smaller amplicons have previously been shown to significantly increase PCR sensitivity for “T. whippelii” (1, 18). Thus, the spacer PCR may be false negative with “T. whippelii”-positive specimens and, consequently, cannot be used routinely to confirm results obtained with the 16S rDNA-based specific PCRs.
Unlike the spacer PCR, the nested approach targeting domain III of the 23S rDNA with 40 amplification and 20 reamplification cycles resulted in the expected amplicons for all 41 specimens, whereas all 38 controls lacking detectable amounts of “T. whippelii” 16S rDNA by PCR remained negative (Fig. 1, Table 1). The specificity of the 23S rDNA approach was further confirmed by direct sequencing of the reamplification products from almost all positive specimens (Table 1). The sequences determined for patients 1 to 27, as well as for the reference sample (positive control), were all found to be identical to the reference sequence containing the “T. whippelii” actinobacterial insertion type A (12a). For the remaining patient 28, a slightly altered sequence, i.e., insertion type B, had been determined previously with two independent specimens (12a) and was confirmed in this study. These findings show that the 23S rDNA amplification system is both sensitive and specific and that it is suitable as a primary or confirmatory test especially with specimens which remained inconclusive or negative histopathologically.
As discussed in detail in our related study with nine specimens from eight patients (12a), the virtual sequence homogeneity found for the partial domain III of the 23S rDNA of “T. whippelii” supports our recent concept of three different 16S-23S rDNA spacer types of this species as opposed to three closely related but different species (12). This conclusion is further supported by the present investigation with additional 32 specimens from 20 patients representing the three spacer types as shown previously by type-specific PCR assays, SSCP, and sequencing (Table 1). However, this concept still remains to be proven by DNA-DNA hybridization studies (8, 20), which are currently not feasible due to the lack of an efficient cultivation method for “T. whippelii” (19). Meanwhile, to facilitate concise reporting of uncultivated variants of “T. whippelii,” a preliminary molecular classification system is proposed (Table 2). The actual scheme delineates four different rDNA types of “T. whippelii” based on their characteristic nucleotides found in the 16S-23S rDNA spacer region and domain III of the 23S rDNA. The present type designation indicates both the sequence types determined for the spacer (number code) as well as for domain III (letter code). Possible additional spacer and/or domain III sequence types may be included by the appropriate adaption of the actual scheme and additional number-letter codes (e.g., an additional dimorphic site found in the spacer may constitute the novel “T. whippelii” rDNA type 4A). We believe that the proposed classification system provides a basis for coherent reporting of epidemiological as well as of clinical investigations of “T. whippelii” and associated diseases.
In conclusion, the recently established nested PCR targeting domain III of the 23S rDNA may be used for the sensitive and specific detection of “T. whippelii” directly from human clinical specimens. This approach may also be helpful to confirm results obtained by other “T. whippelii”-specific PCRs and thus represents a suitable tool for molecular analysis of clinical specimens from suspected cases of Whipple's disease with inconclusive histopathological findings. Further, the proposed classification system for molecular variants of “T. whippelii” may facilitate concise communication on the currently recognized types of this species. Whether or not these types may be correlated with the various clinical manifestations found in the affected individuals with or without Whipple's disease (7) remains to be shown.
ACKNOWLEDGMENTS
This work was supported by a grant from the Swiss National Science Foundation (grant 32-50790.97) to M. Altwegg.
We thank the following colleagues for generous gifts of specimens from patients with Whipple's disease: D. H. Persing, Rochester, Minnesota (specimens 20/98, 21/98, and 24/98); D. Harmsen, Würzburg, Germany (specimens 459-98, 836-96, and 4549-94); and M. Maiwald, Heidelberg, Germany (“T. whippelii” reference extract). A. von Graevenitz is acknowledged for careful reading of the manuscript.
REFERENCES
- 1.Altwegg M, Fleisch-Marx A, Goldenberger D, Hailemariam S, Schaffner A, Kissling R. Spondylodiscitis caused by Tropheryma whippelii. Schweiz Med Wochenschr. 1996;126:1495–1499. [PubMed] [Google Scholar]
- 2.Brändle M, Ammann P, Spinas G A, Dutly F, Galeazzi R L, Schmid C, Altwegg M. Relapsing Whipple's disease presenting with hypopituitarism. Clin Endocrinol. 1999;50:399–403. [PubMed] [Google Scholar]
- 3.Cohen L, Berthet K, Dauga C, Thivart L, Pierrot-Deseilligny C. Polymerase chain reaction of cerebrospinal fluid to diagnose Whipple's disease. Lancet. 1996;347:329. doi: 10.1016/s0140-6736(96)90505-x. [DOI] [PubMed] [Google Scholar]
- 4.Dauga C, Miras I, Grimont P A D. Strategy for detection and identification of bacteria based on 16S rRNA genes in suspected cases of Whipple's disease. J Med Microbiol. 1997;46:340–347. doi: 10.1099/00222615-46-4-340. [DOI] [PubMed] [Google Scholar]
- 5.Dobbins W O., III . Whipple's disease. Springfield, Ill: Charles C. Thomas; 1987. [Google Scholar]
- 6.Dobbins W O., III The diagnosis of Whipple's disease. N Engl J Med. 1995;332:390–392. doi: 10.1056/NEJM199502093320611. [DOI] [PubMed] [Google Scholar]
- 7.Ehrbar H-U, Bauerfeind P, Dutly F, Koelz H-R, Altwegg M. PCR-positive tests for Tropheryma whippelii in patients without Whipple's disease. Lancet. 1999;353:2214. doi: 10.1016/S0140-6736(99)01776-6. [DOI] [PubMed] [Google Scholar]
- 8.Fox G E, Wisotzkey J D, Jurtshuk P. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberger D, Künzli A, Vogt P, Zbinden R, Altwegg M. Molecular diagnosis of bacterial endocarditis by broad-range PCR amplification and direct sequencing. J Clin Microbiol. 1997;35:2733–2739. doi: 10.1128/jcm.35.11.2733-2739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmsen D, Heesemann J, Brabletz T, Kirchner T, Müller-Hermelink H K. Heterogeneity among Whipple's disease-associated bacteria. Lancet. 1994;343:1288. doi: 10.1016/s0140-6736(94)92176-8. [DOI] [PubMed] [Google Scholar]
- 11.Hinrikson H P, Dutly F, Altwegg M. Homogeneity of 16S-23S ribosomal intergenic spacer regions of Tropheryma whippelii in Swiss patients with Whipple's disease. J Clin Microbiol. 1999;37:152–156. doi: 10.1128/jcm.37.1.152-156.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinrikson H P, Dutly F, Nair S, Altwegg M. Detection of three different types of “Tropheryma whippelii” directly from clinical specimens by sequencing, single-strand conformation polymorphism (SSCP) analysis and type-specific PCR of their 16S-23S ribosomal intergenic spacer region. Int J Syst Bacteriol. 1999;49:1701–1706. doi: 10.1099/00207713-49-4-1701. [DOI] [PubMed] [Google Scholar]
- 12a.Hinrikson, H. P., F. Dutly, and M. Altwegg. Analysis of the actinobacterial insertion in domain III of the 235 ribosomal RNA gene of uncultured variants of the bacterium associated with Whipple's disease using broad-range and “Tropheryma whippelii”-specific PCR. Int. J. Syst. Bacteriol., in press. [DOI] [PubMed]
- 13.Maiwald M, Ditton H-J, von Herbay A, Rainey F A, Stackebrandt E. Reassessment of the phylogenetic position of the bacterium associated with Whipple's disease and determination of the 16S-23S ribosomal intergenic spacer sequence. Int J Syst Bacteriol. 1996;46:1078–1082. doi: 10.1099/00207713-46-4-1078. [DOI] [PubMed] [Google Scholar]
- 14.Misbah S A, Ozols B, Franks A, Mapstone N. Whipple's disease without malabsorption: new atypical features. Q J Med. 1997;90:765–772. doi: 10.1093/qjmed/90.12.765. [DOI] [PubMed] [Google Scholar]
- 15.Müller C, Petermann D, Stain C, Riemer H, Vogelsang H, Schnider P, Zeiler K, Wrba F. Whipple's disease: comparison of histology with diagnosis based on polymerase chain reaction in four consecutive cases. Gut. 1997;40:425–427. doi: 10.1136/gut.40.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramzan N N, Loftus E, Burgart L J, Rooney M, Batts K P, Wiesner R H, Fredricks D N, Relman D A, Persing D H. Diagnosis and monitoring of Whipple's disease by polymerase chain reaction. Ann Intern Med. 1997;126:520–527. doi: 10.7326/0003-4819-126-7-199704010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Relman D A, Schmidt T M, MacDermott R P, Falkow S. Identification of the uncultured bacillus of Whipple's disease. N Engl J Med. 1992;327:293–301. doi: 10.1056/NEJM199207303270501. [DOI] [PubMed] [Google Scholar]
- 18.Rickman L S, Freeman W S, Green W S, Feldman S T, Sullivan J, Russack V, Relman D A. Uveitis caused by Tropheryma whippelii (Whipple's bacillus) N Engl J Med. 1995;322:363–366. doi: 10.1056/NEJM199502093320604. [DOI] [PubMed] [Google Scholar]
- 19.Schoedon G, Goldenberger D, Forrer R, Gunz A, Dutly F, Höchli M, Altwegg M, Schaffner A. Deactivation of macrophages with Interleukin-4 is the key to the isolation of Tropheryma whippelii. J Infect Dis. 1997;176:672–677. doi: 10.1086/514089. [DOI] [PubMed] [Google Scholar]
- 20.Stackebrandt E, Goebel B M. Taxonomic note: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 21.von Herbay A, Ditton H-J, Maiwald M. Diagnostic application of a polymerase chain reaction assay for the Whipple's disease bacterium to intestinal biopsies. Gastroenterology. 1996;110:1735–1743. doi: 10.1053/gast.1996.v110.pm8964398. [DOI] [PubMed] [Google Scholar]
- 22.von Herbay A, Ditton H-J, Schuhmacher F, Maiwald M. Whipple's disease: staging and monitoring by cytology and polymerase chain reaction analysis of cerebrospinal fluid. Gastroenterology. 1997;113:434–441. doi: 10.1053/gast.1997.v113.pm9247461. [DOI] [PubMed] [Google Scholar]
- 23.Wilson K H, Blitchington R, Frothingham R, Wilson J A P. Phylogeny of the Whipple's-disease-associated bacterium. Lancet. 1991;338:474–475. doi: 10.1016/0140-6736(91)90545-z. [DOI] [PubMed] [Google Scholar]