Abstract
Eclipta prostrata (L.) L. (Syn.: Eclipta alba (L.) Hassak, Family: Asteraceae) is an important medicinal plant in the tropical and subtropical regions. It is widely used in treating various diseases of skin, liver and stomach in India, Nepal, Bangladesh, and other countries. The main aim of this review was to collect and analyze the available information on traditional uses, phytoconstituents, and biological activities of E. prostrata. The scientific information was collected from the online bibliographic databases such as Scopus, MEDLINE/PubMed, Google Scholar, SciFinder, etc. and books and proceedings. The active phytochemicals were coumestan derivatives, phenolic acid derivatives, flavonoids, triterpenoid and steroid saponins, substituted thiophenes, etc. Various extracts and isolated compounds of E. prostrata showed a wide range of biological activities such as antimicrobial, anticancer, hepatoprotective, neuroprotective and hair growth promoting activities. Relatively a few studies have been performed to reveal the exact phytoconstituents responsible for their corresponding pharmacological activities. Future studies should focus on detailed mechanism based studies using animal models and clinical studies.
Keywords: Eclipta prostrata, Eclipta alba, ecalbasaponin, hepatoprotective, wedelolactone
1. Introduction
The use of plants in traditional medicines covers a wide range of therapeutic uses to treat the infection as well as many chronic diseases [1,2,3,4]. Many people still rely on the traditional medicine and healthcare because of their wider cultural acceptance and affordability [5]. The plant based bioactive compounds have been an important source of modern drugs discovery and development [6]. Hence, the medicinal value of various plants should be explored with their pharmacological significance and potential application in different products.
Eclipta prostrata (L.) L. (Syn.: Eclipta alba (L.) Hassak, Family: Asteraceae) is commonly known as False daisy or Ink plant in English and locally known as Bhringraj, Bhumiraj, Aali jhar, and Nash jhar in Nepali language (Figure 1) [7,8]. E. prostrata is a medium-sized, branched, annual herb-bearing white flower natively found in the tropical and subtropical regions of the world [9,10]. It grows mostly in moist sites such as swamp edges, river or lake banks and edge of rice-fields and easily propagated and spread throughout China, India, Nepal, Brazil and other parts of the world [8,11,12,13]. It is widely distributed in tropical and sub-tropical regions of Asia, Africa, and South America (Figure 2) [14,15]. Traditionally, it is used to treat different skin problems such as wounds, hair loss prevention, and dermatitis. The leaves are used to treat snakebite in India, China, and Brazil. The mixture of leaf juice and honey is used to cure catarrh in infants [16,17]. The juice of E. prostrata is taken orally or applied locally to promote hair growth [18].
Figure 1.
Photographs of Eclipta prostrata (Photos by Basu Dev Neupane, used with permission).
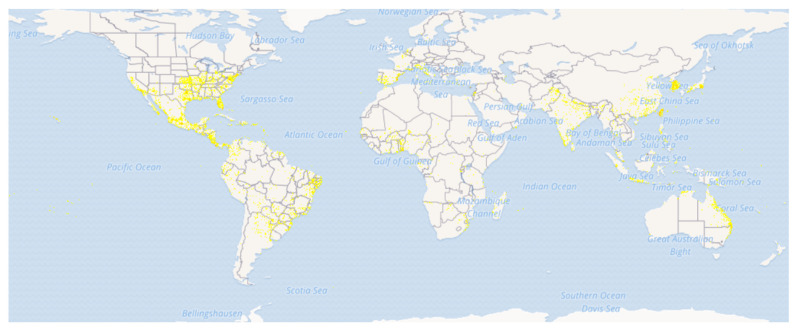
Figure 2.
Distribution map of Eclipta prostrata. (Source: GBIF, https://www.gbif.org/species/5384950 (accessed on 1 November 2021) [15]).
Various research articles have been published regarding the chemical constituents and biological activities of different plant parts of E. prostrata. Critical analysis of these published scientific studies would provide the detailed understanding about the potential use of E. prostrata as medicine, cosmetic, and other formulations along with highlighting the gaps in research. Hence, the main aim of this article was to collect the information about traditional uses, chemical constituents, and the biological activities of E. prostrata.
2. Methods
The scientific information on E. prostrata was retrieved from various online bibliographic databases such as ScienceDirect, PubMed, Google scholar, SciFinder, etc. and books and proceedings. The articles with rigorous quality were selected for the review. Relevant articles published before June 2021 were collected using the key words Eclipta prostrata, Eclipta alba, phytochemistry, traditional use, biological activity, pharmacological activity, etc.
3. Ethnomedicinal Uses
This plant is widely used in different regions of India for the treatment of skin problems, hepatic problems such as jaundice, gastrointestinal problems, respiratory problems such as asthma, and other symptoms such as fever, hair loss and whitening of hair, cuts, and wounds, spleen enlargement, etc. [19,20]. The leaf juice is used with honey to cure catarrh in infants, shoot juice and mustard oil is taken together for diarrhea and dysentery, and the whole plant is used for the treatment of symptoms related to hepatitis, itching, hemoptysis, bleeding, hematuria, diarrhea, and diphtheria [16]. The leaves and shoots are used in preventing infection in wounds and its treatment in Nepal [7,8,11,21]. Some ethnic groups in South American countries use it to treat snakebites [22]. In Ayurveda, it is used for its revitalizing and anti-aging properties [23]. Many ethnic groups of Bangladesh use it for the treatment of jaundice [24,25]. The plant juice has been used to control, kill, and inhibit the growth of diseases carrying vectors such as mosquito [26,27]. Additionally, it is also used to treat different types of symptoms such as acidity, alopecia [28], gingivitis, fever, body pain, asthma, bronchitis, burns, constipation, wounds, wrinkles, edema, pimples, and other skin diseases [29,30,31,32].
4. Bioactive Chemical Constituents
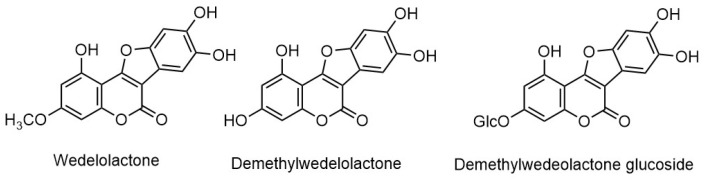
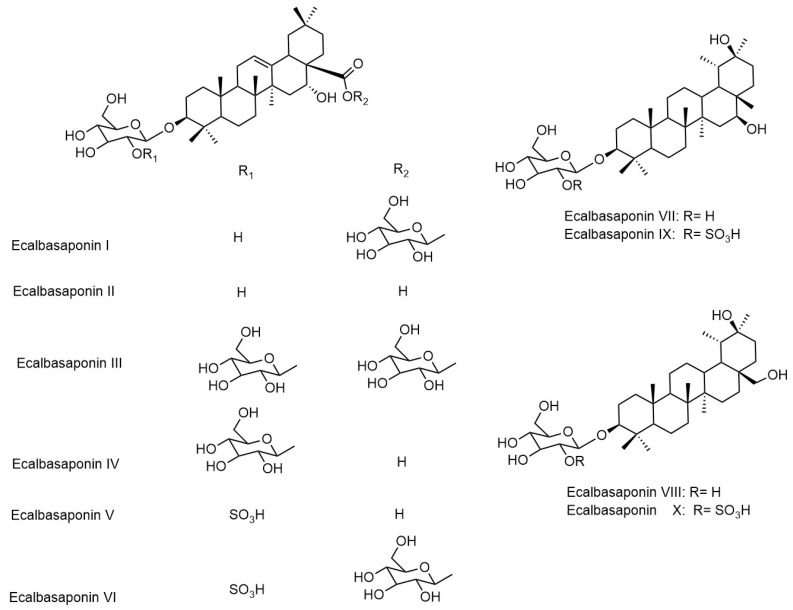
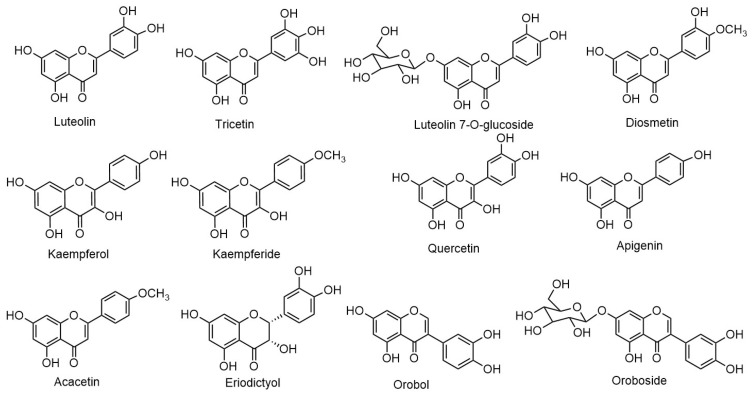
Eclipta prostrata contains a wide range of active phytoconstituents, which includes coumestan derivatives, triterpene saponins, steroidal saponins, triterpenes, steroids, steroidal alkaloids, flavonoids, phenolic acids, thiophene derivatives and many other compounds. Most of the chemical analysis are reported for whole plant or aerial parts. The detailed list of these compounds is given in Table 1 and Table 2. The structures of main coumestan derivatives, triterpene saponins and flavonoids are represented in Figure 3, Figure 4 and Figure 5, respectively.
Table 1.
Coumestan, steroid and triterpene derivatives and related compounds from various parts of E. prostrata.
| Chemical Compounds (Class/Constituents) | Plant Parts | References |
|---|---|---|
| Coumestan derivatives | ||
| Wedelolactone | Leaves | [21,33,34,35,36] |
| Demethylwedelolactone | Leaves | [35,36] |
| Isodemethylwedelolactone | Whole plant | [36] |
| Strychnolactone | Whole plant | [36] |
| Demethylwedelolactone glucoside | Aerial Parts | [37] |
| Steroidal and triterpene saponins, steroidal alkaloids, steroids and triterpenoids | ||
| Eclalbasaponins I | Whole plant | [34,38,39] |
| Eclalbasaponins II | Whole plant | [34,38,39] |
| Eclalbasaponins III | Whole plant | [34,38,39] |
| Eclalbasaponins IV | Whole plant | [38,39] |
| Eclalbasaponins V | Whole plant | [34,38,39] |
| Eclalbasaponins VI | Whole plant | [38,39] |
| Eclalbasaponins VII | Whole plant | [38] |
| Eclalbasaponins VIII | Whole plant | [38] |
| Eclalbasaponins IX | Whole plant | [38] |
| Eclalbasaponins X | Whole plant | [38] |
| Eclalbasaponin XI | Whole plant | [38] |
| Eclalbasaponin XII | Whole plant | [38] |
| Eclalbasaponin XIII | Whole plant | [38] |
| Eclalbasaponin A | Whole plant | [40] |
| Eclalbasaponin B | Whole plant | [40] |
| Eclalbasaponin C | Whole plant | [40] |
| Eclalbasaponin D | Whole plant | [40] |
| Echinocystic acid | Whole plant | [40] |
| Echinocystic acid-3-O-(6-O-acetyl)-β-D-glucopyranoside | Aerial parts | [41] |
| Eclalbatin | Aerial Parts | [42,43] |
| 3β,25-Dihydroxy-23E-lemmaphyll-8,23-diene | Whole plant | [44] |
| 16α-Hydroxy-olean-12-en-3-on-28,21β-olide | Whole plant | [44] |
| 3β-Hydroxy-17-epi-28-norolean-12-en-16-one 3-O-β-D-glucopyranoside | Whole plant | [44] |
| 3β-O-(6-O-Crotonyl-β-D-glucopyranosyl)-16α-hydroxy-olean-12-en-28-oic acid 28-O-β-D-glucopyranosyl ester | Whole plant | [44] |
| 3-O-(2-O-Acetyl-β-D-glucopyranosyl) oleanolic acid-28-O-(β-D-glucopyranosyl) ester | Aerial parts | [45] |
| 3-O-(6-O-Acetyl-β-D-glucopyranosyl) oleanolic acid-28-O-(β-D-glucopyranosyl) ester | Aerial parts | [45] |
| 3-O-(β-D-Glucopyranosyl) oleanolic acid-28-O-(6-O-acetyl-β-D-glucopyranosyl) ester | Aerial parts | [45] |
| 3β,16β,29-Trihydroxy oleanane-12-ene-3-O-β-D-glucopyranoside | Aerial parts | [46] |
| 3,28-di-O-β-D-Glucopyranosyl-3β,16β-dihydroxy oleanane-12-ene-28-oleanlic acid | Aerial parts | [46] |
| 3-O-β-D-Glucopyranosyl-(1-2)-β-D-glucopyranosyl oleanlic-18- ene acid-28-O-β-D-glucopyranoside | Aerial parts | [46] |
| (20S)(25S)-22,26-Imino-cholesta-5,22(N)-dien-3β-ol (Verazine) | Leaves | [47] |
| 20-epi-3-Dehydroxy-3-oxo-5,6-dihydro-4,5-dehydroverazine | Leaves | [32,47] |
| (20R)-20-Pyridyl-cholesta-5-ene-3β,23-diol (Ecliptalbine) | Leaves | [47] |
| (20R)-25β-Hydroxyverazine | Leaves | [47] |
| 20-epi-4β-Hydroxyverazine | Leaves | [47] |
| 20-epi-25β-Hydroxyverazin | Leaves | [47] |
| 4β-Hydroxyverazine | Leaves | [47] |
| 25β-Hydroxyverazine | Leaves | [47] |
| Lanost-5,24-dien-3β-ol-18, 21-olide -3β- yl tetradecanoate | Whole plant | [48] |
| α-Amyrin | Whole plant | [43] |
| Ursolic acid | Whole plant | [43] |
| Oleanolic acid | Whole plant | [43] |
| 3-Oxo-16α-hydroxy-olean-12-en-28-oic acid | Aerial parts | [49] |
| Machaeroceric acid | Aerial parts | [34] |
| Silphioside C | Whole plant | [50] |
| β-Sitosterol | Whole plant | [36] |
| Stigmasterol | Leaves/Stems | [40] |
| Stigmasterol-3-O-glucoside | Aerial parts/leaves/Stems | [19,34,40] |
| 3-O-(6′-O-Palmitoyl-β-D-glucopyranosyl) stigmasterol | Whole plant | [50] |
| Daucosterol | Leaves/Stems | [40] |
Table 2.
Flavonoids, phenolic acids, substituted thiophines, and other compounds present in the E. prostrata.
| Flavonoids | ||
| Luteolin | Aerial parts | [34,41,51] |
| Tricetin | Aerial parts | [34] |
| Luteolin-7-O-β-D-glucoside | Aerial parts | [34,41,51] |
| Diosmetin | Aerial parts | [52] |
| Skullcapflavone Ⅱ | Whole plant | [51] |
| Kaempferol | Whole plant | [51] |
| Kaempferol-7-O-α-D-rhamnoside | Aerial parts | [34] |
| Kaempferide | Whole plant | [51] |
| Quercetin | Aerial parts | [51,53] |
| Quercetin-3-O-β-D-glucoside | Aerial parts | [34] |
| Apigenin | Aerial parts | [34,41,51] |
| Acacetin | Whole plant | [51] |
| Acacetin-7-O-rutinoside | Whole plant | [51] |
| Eriodictyol | Whole plant | [50] |
| Pyracanthoside | Whole plant | [50] |
| Hesperetin-7-O-β-D-glucoside | Aerial parts | [34] |
| 3′-Hydroxybiochanin A | Aerial Parts | [37,49] |
| Orobol (isoluteolin) | Whole plant | [31,34] |
| 7-O-Methylorobol-4′-O-β-D-glucopyranoside | Aerial Parts | [34,49,50] |
| 7-Dihydroxyl-3′, 6′-dimethoxylisoflavone-7-O-glucoside | Whole plant | [51] |
| 3′-O-Methylorobol | Aerial parts | [50,52] |
| Pratensein | Aerial parts | [37,49,50] |
| Pratensein-7-O-β-D-glucopyranoside | Aerial parts | [41,50] |
| Oroboside (Orobol-7-O-β-D-glucoside) | Whole plant | [34,37,50,51] |
| Phenolic acids | ||
| Protocatechuic acid | Leaves/Steam/Whole plant | [34,36,40,43] |
| 4-Hydroxybenzoic acid | Leaves/Steam | [34,40,43] |
| Vanillic acid | Aerial parts | [34] |
| Syringic acid | Aerial parts | [34] |
| Chlorogenic acid | Aerial parts | [34] |
| Syringic acid | Aerial parts | [34] |
| Tachinoside | Whole plant | [50] |
| Coniferylaldehyde | Whole plant | [50] |
| Leonuriside A | Whole plant | [50] |
| Caffeic acid | Whole plant | [50] |
| Ferulic acid ethyl ester | Whole plant | [50] |
| Caffeic acid ethyl ester | Whole plant | [50] |
| Lignin | ||
| Ecliptalignin A | ||
| Coumarins | ||
| Psoralen | Whole plant | [51] |
| Isopsoralen | Whole plant | [51] |
| Polyacetylinic compounds | ||
| (5E)-Hendeca-1,5- dien-7,9-diyne-diol-4-O-β-D-glucopyranoside | Stem | [54] |
| (5E)-Trideca-1,5-dien-7,9,11-triyne-3,4-diol-4-O-β-D-glucopyranoside | Stem | [46,54] |
| 3-O-β-D-Glucopyranosyl1-hydroxy-4E,6E-tetradecene,8,10,12-triyne | Stem | [46,54] |
| 2-O-β-D-Glucosyltrideca-3E,11E-dien5,7,9-triyne-1,2,13-triol | Stem | [54] |
| 2-O-β-D-Glucosyltrideca-3E,11E-dien-5,7,9-triyne-1,2-diol | Stem | [54] |
| 2-O-β-D-Glucosyltrideca-3E,11Z-dien-5,7,9-triyne3–1,2-diol | Stem | [54] |
| Substituted thiophenes | ||
| 5-Hydroxymethyl-(2,2′:5′,2″)-terthienyl tiglate | Whole plant | [55] |
| 5-Hydroxymethyl-(2,2′:5′,2″)-terthienyl agelate | Whole plant | [55] |
| 5-Hydroxymethyl-(2,2′:5′,2″)-terthienyl acetate | Whole plant | [55] |
| 5-Formyl-(2, 2:5, 2″)-terthiophene (Ecliptal) | Whole plant | [56] |
| 5-Hydroxymethyl-(2, 2: 5, 2″)-terthiophene (α-terthienylmethanol) | Whole plant | [56] |
| 5-Methoxy-(2, 2:5, 2″)-terthiophene | Whole plant | [56] |
| 3′-Methoxy-2,2′:5′,2″-terthiophene | Aerial parts | [41] |
| 5-(3″,4″-Dihydroxy-1″-butynyl)-2,2′-bithiophene | Aerial parts | [41] |
| α-Terthienyl | Aerial parts | [41] |
| α-Formylterthienyl | Whole plant | [54] |
| α-Terthienyl methanol | Whole plant | [41,54,56] |
| 3′-Methoxy-2,2′:5′,2″-terthiophene | Aerial parts | [41] |
| 4-(2,2′-Bithiophen-5-yl)but-3-yne-1,2-diol | Aerial parts | [57] |
| Arctinol B | Aerial parts | [57] |
| 2-(Penta-1,3-diynyl)-5-(3,4-dihydroxy-but-1-ynyl)-thiophene | Aerial parts | [57] |
| 6-Methoxy-arctinol-b | Aerial parts | [57] |
| 5-[l-(4-Hydroxybut-l-ynyl)]-2,20 -bithiophene-50 -carbaldehyde | Aerial parts | [57] |
| 5-Hydroxymethyl- (2,2′:5′,2′’-terthienyl) | Aerial parts | [57] |
| 5′-Hydroxymethyl-5-(3-butene-1-ynyl)-2,2′ -bithiophene | Aerial parts | [46,57] |
| 3′-Hydroxy-2,2′:5′,2′’ terthiophene-3′-O-β-D-glucopyranoside | Aerial parts | [57] |
| Ecliprostin A | Aerial parts | [58] |
| Ecliprostin B | Aerial parts | [58] |
| Ecliprostin C | Aerial parts | [58] |
| Alkaloids | ||
| Crinumaquine | Whole plant | [51] |
| 2,3,9,12-Tetramethoxyprotoberberine | Whole plant | [51] |
| Lignans | ||
| Pinoresinol-4-O-β-D-glucopyranoside | Whole plant | [50] |
| 4,4′-Dimethoxy-3′-hydroxy-7,9′:7′,9-diepoxylignan-3-O-β-D-glucopyranoside | Whole plant | [50] |
| Syringaresinol-4′-O-β-D-glucopyroside | Whole plant | [50] |
| Lanicepside A | Whole plant | [50] |
| Longifloroside | Whole plant | [50] |
| Other compounds | ||
| 1-O-Octadecanoyl-2-O-(9Z,12Z-octadecadienoyl)-3-O-[α-D-galactopyranosyl- (1′′→6′)-O-β-D-galactopyranosyl]glycerol | Whole plant | [50] |
| (2S)-3-O-α-D-Galactopyranosyl-(1′′→6′)-β-D-galactopyranosyl-1,2-di-O-[(9Z,12Z,15Z)-octadeca-9,12,15-trienoyl]-sn-glycerol | Whole plant | [50] |
| 1-O-(9Z,12Z,15Z-Octadecatrienoyl)-2-O-hexadecanoyl-3-O-[α-D-galactopyranosyl-(1′′→6′)-O-β-D-galactopyranosyl]glycerol | Whole plant | [50] |
| 1-O-(β-D-glucopyranosyl)- (2S,3S,4R,8Z)-2N-[(2′R)-2′-hydroxytetracosanoyl]-8-(Z)-octadecene-1,3,4-triol | Whole plant | [50] |
| (2S,3S,4R,10E)-2-[(2′R)-2′- Hydroxytetracosanoylamino]-10-octadecene-1,3,4-triol | Whole plant | [50] |
| (3S,5R,6S,7E,9R)-3-Hydroxy-5,6-epoxy-β-ionyl-3-O-β-D-glucopyranoside | Whole plant | [50] |
| Euodionoside A | Whole plant | [50] |
| Junipeionoloside | Whole plant | [50] |
| Calaliukiuenoside | Whole plant | [50] |
| rel-(1S,2S,3S,4R,6R)-1,6-Epoxy-menthane-2,3-diol-3-O-β-D-glucopyranoside | Aerial parts | [46] |
| rel-(1S,2S,3S,4R,6R)-3-O-(6-O-caffeoyl-β-D-glucopyranosyl)-1,6-epoxy menthane-2,3-diol | Aerial parts | [46] |
| Siliphioside E | Aerial parts | [46] |
| (2E,6E)- 2,6,10-trimethyl-2,6,11-dodecatriene-1,10-diol-1-O-β-D-glucopyranoside | Aerial parts | [46] |
| (2S)-1-O-Stearoyl-3-O-β-D-galactopyranosyl-sn-glycerol | Aerial parts | [34] |
| (2S)-3-O-(9Z,12Z-Octadecadienoyl) glyceryl-O-β-D-galactopyranoside | Aerial parts | [34] |
| Bidensmenthoside A | Whole plant | [50] |
| Bidensmenthoside B | Whole plant | [50] |
| 11β,17-Dihydroxy-beyer-15-ene | Whole plant | [44] |
| 4β- Hydroxy-guai-10(14),11(13)-dien-12-oic acid | Whole plant | [44] |
Figure 3.
Structures of major coumestan derivatives.
Figure 4.
Structures of main triterpene saponins.
Figure 5.
Structures of main flavonoids.
5. Biological Activities
Due to the wide range of ethnomedicinal values and applications, several studies have been performed regarding the biological activities of extracts and compounds obtained from E. prostrata using both in vitro and in vivo models [59,60,61]. Some of them are discussed in detail in following sub-sections.
5.1. Antioxidant Activity
The antioxidant effects of E. prostrata were evaluated in Charles River Sprague-Dawley rats. The extract at 50 mg/kg and 100 mg/kg dose significantly reduced the oxidative biomarkers such as serum lipid peroxide, serum hydroxyl radical levels in [41].
In another study, the in vitro antioxidant activity was evaluated based on the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay. An IC50 value of extract was determined to be 45.68 µg/mL for the whole plant as compared to the IC50 of 3.26 µg/mL of standard ascorbic acid. When evaluated using hydrogen peroxide scavenging assay, the extract showed potent activity with the IC50 values of 1.34 µg/mL as compared to ascorbic acid (IC50: 1.03 µg/mL) [9]. The antioxidants present in the extract of E. prostrata showed the reduction of ferricyanide complex (Fe+3) to ferrous form (Fe+2) in a dose-dependent manner. The highest reducing ability (75.59%) for the whole plant of E. prostrata was reported at 250 µg/mL concentration. The IC50 values for reducing ability of the extract was 100 µg/mL [62]. The studies are mostly conducted using in vitro methods and detailed mechanism is yet to be established.
5.2. Antimicrobial Activity
The butanol and water extract, at a concentration of 3 mg/disc inhibited the growth of Bacillus cereus by 45% and 42%, respectively. The highest growth inhibition of 63% for butanol extract and 54% for ethyl acetate fraction were reported against B. subtilis at the concentrations of 3 mg/disc. The butanol extracted sample showed inhibition of 57%, 72%, and 89% at concentrations of 1, 2 and 3 mg/disc, respectively and that of ethyl acetate extract showed the growth inhibitions of 40, 57, and 83%, at concentrations of 1, 2, and 3 mg/disc, respectively. Similarly, methanol extract inhibited the growth of Candida albicans by 38, 48, and 59% at concentrations of 1, 2, and 3 mg/disc, respectively. [16].
A coumestan derivative, wedelolactone (10 µg/mL), isolated from the plant showed promising antibacterial properties against Staphylococcus epidermidis, Salmonella Typhimurium, Staphylococcus aureus, Pseudomonas aeruginosa, Shigella flexneri and Escherichia coli with the zone of inhibition (ZOI) of 10.24, 9.16, 9.14, 8.0, 7.60, 8.60 mm, respectively at 10 µg/mL and minimum inhibitory concentration (MIC) of 15, 25, 20, 1250, 1300, 1000 µg/mL respectively [21].
The percentage inhibition of 36.84, 38.94, and 47.36% was reported against Aspergillus niger by 2 mg, 3 mg, and 4 mg of ethanolic extract of E. prostrata, respectively as compared to the inhibition of 64.21% by 100 µg of standard fluconazole. Similarly, the percentage inhibitions of 20, and 28% were reported against Aspergillus ustus by 3 mg, and 4 mg of ethanolic extract, respectively as compared to inhibition of 40% by 100 µg of standard fluconazole. Similarly, against Aspergillus ochraceus, the percentage inhibition of 41.37, 44.82, and 51.52% in was reported for 2 mg, 3 mg, and 4 mg of ethanolic extract, respectively compared to the inhibition of 37.93% by 100 µg of standard fluconazole [9].
The alkaloids from the leaves were also studied for the antimicrobial properties against E. coli, P. aeruginosa, Shigella boydii, S. aureus and S. faecalis by agar-well diffusion and broth-microdilution methods. The ZOI ranged from 9.8–16.5 mm in 500 μg/mL and MIC ranged from 42–89 μg/mL for the sample which was found to be comparable to the positive control ciprofloxacin with MIC range of 0.8–1.3 μg/mL [63].
5.3. Hepatoprotective Activities
In vivo hepatoprotective activity was evaluated by Thirumalai et al. [64]. The aqueous extract of leaves of E. prostrata was administered to carbon tetrachloride (CCl4)-induced hepatotoxicity in male albino rats. The extract (250 mg/kg b.w.) reduced the elevated levels of all the biochemical parameters such as glutamic-oxaloacetic transaminase (GOT), glutamine-pyruvate transaminase (GPT) and bilirubin.
The chloroform extract of the roots and methanol extract of the leaves of E. prostrata were investigated for hepatoprotective activity in CCl4-induced hepatotoxicity in male albino rats by measuring the levels of lysosomal enzymes. The chloroform extract of roots showed 47.96% reduction in the lysosomal enzyme whereas, methanol extract of leaves showed a 72.8% reduction. The triterpenoid and alkaloid fractions obtained from the methanol extract of showed the 78.78% and 60.65% reduction in lysosomal enzyme levels, respectively. Similarly, triterpenoid saponin fraction and coumestan fractions obtained from the chloroform extract of roots reduced the lysosomal enzyme level by 52.41% and 75.6%, respectively [65].
The ethanolic extract (50%) obtained from the whole plant of E. prostrata was studied for its hepatoprotective effect. The study was conducted in rats against CCl4-induced hepatic damage. The result revealed that E. prostrata extract significantly normalized the biochemical parameters by counteracting the hepatic drug-metabolizing enzyme inhibition. The reduction of a biochemical parameter such as hepatic lysosomal acid phosphatase and alkaline phosphatase by CCl4 was observed to be restored when treated with E. prostrata. This study shows that the hepatoprotective activity of this plant relies on the regulation of the levels of hepatic microsomal drug-metabolizing enzymes [66]. The echniocystic acid and eclabasaponin II from the aerial parts showed antiproliferative activity in hepatic stellate cells [67].
The experiments were performed mostly in vivo using only rats. Its clinical significance is not well studied. Some studies lack positive and negative controls. Further, studies on human and animal models along with the isolation of active compounds from this plant may lead to the discovery of potent therapeutic agents.
5.4. Anti Hyperlipidemic Activities
Diacylglycerol acyltransferase (DGAT) is a key enzyme of biosynthesis in the final step of the glycerol phosphate pathway. The triglycerides synthesized in excess causes various symptoms such as type II diabetes mellitus, hypertriglyceridemia, and obesity. The active polyacetylene constituents from the stem of E. prostrata was tested for the inhibition of the DGAT enzyme. Kuraridine was used as positive control, which is known as DGAT inhibitor. A total of 8 isolates showed potent activity and IC50 values was of 74.4 ± 1.3 to 101.1 ± 1.1 μM range while that of positive control was 10.4 ± 1.4 μM [54].
The effect of methanol extract of E. prostrata on non-alcoholic fatty liver in rats was evaluated after inducing fatty liver via high-fat diets with cholesterol and cholic acids. The biochemical and histopathological analysis revealed that high dose treatment of E. prostrata (200 mg/kg and 300 mg/kg) exhibited significant improvement in lipid profile and liver function [68].
5.5. Cerebroprotective and Nervous System Related Activities
The hydroalcoholic extract of E. prostrata was subjected to the study of cerebroprotective function in Wister albino rats [12]. The pretreatment of the hydroalcoholic extract global cerebral ischemia model resulted in a great difference in ischemia control. E. prostrata extract (250 and 500 mg/kg) administration gradually improved the antioxidant enzyme levels, decreased brain edema, and altered some histopathological status in mice after bilateral cerebral artery occlusion.
The aqueous extract obtained from leaves of E. prostrata at the dose of 100 and 200 mg/kg was evaluated for its potential application on transfer latency (TL) as a parameter of acquisition and retrieval learning in rats using an elevated plus-maze. The administration of extract was reported to significantly improve the retrieval memory [69].
The acetylcholine formation in the brain and oxidative stress inhibition in the brain and serum of rats were accessed before and after feeding the experimental diet. The rats were fed with 25, 50,100 mg/kg of butanol fraction of the aqueous extract of E. prostrata. The acetylcholine level was increased by 9.6–12.1% in 50 mg/kg and 100 mg/kg fed group as compared to control. Monoamine oxidase-B activity and superoxide radical levels were decreased by 10.5% and 9.4%, respectively in the 100 mg/kg treated group [70].
The in vivo anti-epileptic activities were studied by Tambe et al. in mice [71]. This study isolated the luteolin as a major constituent from the leaves of E. prostrata which was evaluated for its anticonvulsant and anti-epileptic activities. The study found that luteolin exhibited anticonvulsant activities and also ameliorated the oxidative level in the mice induced by kindling.
5.6. Anti-Diabetic/Anti-Hyperglycemic Activities
The in vitro α-amylase inhibition activity of methanol extract of the whole plant of E. prostrata was evaluated. The result revealed mild potency in α-amylase inhibition indicating the potential anti-diabetic property with the IC50 value of 322.138 ± 0.025 µg/mL [72].
In another experiment, the rats were injected with streptozotocin in the peritoneum at a dosage of 70 mg/kg of body weight to induce diabetics. With the administration of wedelolactone from E. prostrata to diabetic rats, HbA1c (%) level was recovered from 10.3 ± 0.72 in the untreated diabetic rats to 7.2 ± 0.52 in wedelolactone treated diabetic group. The change in a biochemical parameter such as an increase in urea and creatinine in the streptozotocin treated group was declined towards normal by treating with wedelolactone. The c-peptide and insulin secreted from β-cells were investigated to confirm the treatment by wedelolactone and these hepatic-parameters were shifted towards the normal values after 28 days of treatment of wedelolactone [73].
The ethanolic extract of E. prostrata showed inhibition of α-glucosidase in a dose-dependent manner. About 85% inhibition was observed at 100 µg/mL concentration while that of standard acarbose showed around 56% inhibitory at the same concentration. The IC50 value of the extract was 54 µg/mL. The aldose reductase activity was also found to be inhibited in a dose-dependent manner. The maximum inhibition was observed at the concentration of 10 µg/mL at which the enzyme activity was decreased by 88.6%. The IC50 values of the extract were calculated to be 4.5 µg/mL [74].
Many polyherbal formulations include E. prostrata as an essential ingredient. It is reported to act upon the pancreas via restoration and regeneration of β-cell and to possess antidiabetic activity [75].
The antidiabetic properties of E. prostrata in alloxan-induced diabetic rats were evaluated using the leaf suspension of the plant (2 & 4 g/kg) orally. The extract significantly normalized the biochemical parameters altered by diabetes. The activity of liver hexokinase was increased; blood glucose level and glycosylated hemoglobin were reduced due to the reduced activity of glucose-6 phosphatase and fructose-1,6-bisphosphatase which revealed the anti-hyperglycemic activity of E. prostrata in the rats [76]. However, the mechanism on the chemical model related to the antidiabetic properties has not been well studied.
5.7. Anticancer Activities
The methanol extract of E. prostrata was administered orally at the dose of 250 and 500 mg/kg to Ehrlich ascites carcinoma (EAC) bearing mice and it was found to increase life span. It also decreased the viable cell count and tumor volume of the tumor-bearing mice when compared to that of control. As compared to EAC control, E. prostrata extract restored the hematological parameters such as red blood cells (RBC) count and hemoglobin content. In the treated group, the percentage of lymphocytes was increased with a decreased level of neutrophils [77].
The in vitro and in vivo tumor growth inhibition of breast cancer cells by the chloroform fraction of methanol extract of E. prostrata was reported by Arya et al. [78] which resulted the marked inhibition of the breast tumor growth in vitro and in vivo by selective regulation of Hsp60 cell. The extract specifically activated the apoptotic pathway by the process of disruptions of mitochondrial membrane potential by upregulating and downregulating the Hsp60 and anti-apoptotic protein XIAP, respectively. The extract was also found to mitigate tumor-induced hepato-renal toxicity. Further, the LC-MS approach identified luteolin as a major contributor to the anti-cancer activities.
The antitumor activities of terthiophenes isolated from the n-hexane fraction of E. prostrata were evaluated against the endometrial cancer cells (Hec1A and Ishikawa cells) by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Among the five terthiophenes isolated, α-terthienylmethanol was found to be most potent to inhibit the Hec1 (IC50 = 0.38) and Ischikawa (IC50 = 0.35) cancer cells [56].
The cytotoxic activities of various compounds isolated from aerial parts of E. prostrata were evaluated against human ovarian cancer cell lines SKOV3. Cytotoxicity was assessed by MTT assay, and the IC50 values of more than ten compounds were less than 100 µM and that of positive control cisplatin was 11.25 ± 0.27 µM [79].
The antiproliferative potential of ethanolic extract of E. prostrata was evaluated using MTT assay in which the extract inhibited the cell proliferation in a dose-dependent manner. The IC50 value for MTT assay was calculated from the growth curve and was found to be 22.1 ± 2.9 g/mL for HepG2, 25.3 ± 3.6 g/mL for A498, and 50.2 ± 8.7 g/mL for C6 cell lines. This result showed that extract was much effective for HepG2 cells. When treated with different doses of the extract resulted in a decrease in cell density which was supported by the MTT assay. The cells in all three cell lines were found to be detached, round, and floating at higher concentrations of extract [80]. However, the effects on the biochemical pathway of healthy cells have not been well studied yet.
5.8. Hair Growth Promoting Activity
The dorsal area was shaved and the effect on growth of hair was observed in Wister rats by Mondal et al. to evaluate hair growth promoting activity [81]. The time for the hair growth initiation was noted in days and the time required to complete the hair growth was 19.01 ± 0.51 days for ethanolic extract of the leaves of E. prostrata treated group compared to the time taken for 2% minoxidil (standard) treated group (16.05 ± 0.41 days). The hair growth in the extract-treated group observed at the 10th, 20th, and 30th day was 8.91 ± 0.03 mm, 16.01 ± 0.01 mm, and 21.08 ± 0.03 mm, respectively as compared to the length of hair increased in 2% minoxidil (standard) treated group as 9.23 ± 0.01 mm, 17.63 ± 0.02 mm and 22.13 ± 0.04 mm, respectively. Both the extract and standard treated groups showed a significant increase in as compared to the control group.
The effects of petroleum ether extract of E. prostrata on stimulation of the hair coverage area of the nude mice were evaluated after applying specific concentrations of petroleum ether extract vs. the vehicle and or 2% minoxidil. A score of 0 to 8 was given for each mouse to estimate the effects of petroleum ether extract on the hair coverage area of the mice in all treatment groups. From day 8 the mice treated with petroleum ether extract of E. prostrata showed maximum hair growth than in the mice of other groups and gradually covered the maximum area of the body on day 16. But rapid hair loss was observed in the minoxidil and other treatment groups in the case of the nude mice. In terms of hair density, the mice treated with petroleum ether extract exhibited a significant increase in hair density compared to the other groups on day 8 and 16. Although minoxidil had a significant effect on sustaining hair density on day 8, progressive hair loss occurred and hair density also decreased on day 16 [82].
The hair growth cycle was found to be significantly affected by minoxidil and petroleum ether extract of E. prostrata treatment. In the case of control, one to two hair follicles were in the catagenic phase, while most were in the telogen phase. A similar scenario was observed in the group treated by ethanolic extract with the absence of anagen hair follicle. The reverse scenario was observed in minoxidil and petroleum ether extract treated group; where most hair follicles were in anagenic phase, a few hair follicles in catagen phase and almost no hair follicles were in telogen phase. In the control group, there were low anagenic hair follicles, but in the case of the 2% and 5% petroleum ether extract group, it was about 68 ± 1.2% and 70 ± 1.6% anagenic hair follicles, respectively. The major contributors were wedelolactone and β- sitosterol in petroleum ether extract, which were responsible for hair growth promotion [28].
The study on hair growth promotion by a polyherbal formulation containing E. prostrata was reported by Roy et al. [83]. The time taken for complete hair growth was 18 d in the group receiving oil of 10% E. prostrata, 10% hibiscus, and 5% jatamasi (DF3) and 22 d in the group receiving 10% E. prostrata, 5% hibiscus and 10% jatamasi (DF2). On comparing the activity of DF3 and minoxidil, DF3 hair oil formulation showed a better result of hair growth. Mean hair length was 4.6 mm and 3.6 mm in DF3 and DF2 groups, respectively. The study revealed the fact that DF3 formulation resulted in significant increment in the number of hair follicles in the anagen phase of the hair growth cycle. The percentage of the population of anagen follicle was 67 in the standard group, while in DF3 and DF2 formulations, it was 82 and 65, respectively. The results revealed that DF3 formulation had more potent to hair growth activity [83]. However, the role of E. prostrata in the treatment of hair fall caused by other reasons such as diseases, aging, and genetics are not clear yet.
5.9. Immunomodulatory Activities
The immunostimulatory effects of E. prostrata in tilapia fish (Oreochromis mossambicus) was studied. A diet with 0.01, 0.1, and 1% of the aqueous extract of E. prostrata were fed to fish, and after successive weeks, various nonspecific humoral responses (complement, antiprotease, and lysozyme), cellular responses (reactive oxygen and nitrogen production, myeloperoxidase content) and disease resistance were observed against the activity of common pathogen of fish and human, Aeromonas hydrophila. After feeding aqueous extract for 1, 2, or 3 weeks, the activity of lysozyme was increased significantly. After 1 week of aqueous extract supplement, the enhancement of reactive oxygen species production and myeloperoxidase content was observed. The mortality rate in fish had decreased significantly when fed with the extract [84].
The wedelolactone obtained from the methanol extract of the whole plant of E. prostrata was reported to show immunomodulatory responses in mice at different dose ranges from 100 to 500 mg/kg. Various parameters such as carbon clearance, antibody titer, and cyclophosphamide immunosuppression were accessed, which showed the significant increase in the phagocytic index and antibody titer which resulted in a significant ratio of the phagocytic index and white blood cells (WBC) count [17].
5.10. Others Activities
The study using in vitro assay suggested that wedelolactone from E. prostrata extract exhibited anti-hepatitis C virus (HCV) activity. It was reported that the mechanism was due to the inhibition of HCV replicase activity in the cell culture systems treated with wedelolactone [33].
E. prostrata was reported to exhibit active inhibition activity against Bothrops jararacussu venom. The root extract was reported to have inhibition of the phospholipase A2 activity nearly by 50%. The highest result was obtained for the extract of aerial part as compared to other extracts, which showed approximately 70% inhibition of the phospholipase A2 activity [22].
Echniocystic acid, the triterpene component of E. prostrata was found to be effective in treatment of ovariectomy-induced osteoporosis in rats. Administration of 5–15 mg/kg per day for 12 weeks was reported to prevent the level of stress and Young’s modulus of the femur. The compound also restored the bone biomarkers level such as osteocalcin, alkaline phosphatase, deoxypyridinoline, phosphorus, and urinary calcium. Treatment from E. prostrata also prevented the altercation of the bone mineral density, improved trabecular architecture, trabecular number, and trabecular thickness [85].
The methanol extract of leaves of E. prostrata was reported to show nephroprotective activity in gentamycin-induced nephrotoxicity in rats. The activity of the extract was evaluated for its ability to decrease the gentamicin-induced elevations of biochemical parameter such as serum creatinine and histological changes in renal tissues. It was reported that the extract of could significantly act as a nephroprotective agent against gentamicin toxicity comparable to quercetin [86]. Similar studies were made on doxorubicin hydrochloride-induced nephrotic syndrome on mice. The significant improvement on biochemical parameter such as urine protein, triglyceride etc. was seen on the E. prostrata treated group. The pathological changes in kidney also supported the fact [87]. Another study revealed that the major constituents of E. prostrata i.e., wedelolactone could inhibit the abnormal proliferation of human renal mesangial cells (HRMCs) due to inflammation of renal tissues via the regulation of NF-κB signaling pathway [88].
6. Toxicity Evaluation against Brine Shrimp and Mosquito
Uddin et al. evaluated toxicity of the ethanol extract of E. prostrata by brine shrimp lethality assay. In this assay, the concentrations of 25, 31.25, 62.5, 125, 250, 500 µg/mL of extract was used to determine cytotoxicity against brine shrimp. The percentage lethality was 0, 10.0, 41.70, 61.70, 83.30, and 100% for the concentrations of 25, 31.25, 62.5, 125, 250, 500 µg/mL of extract, respectively. The lethality was observed in a dose-dependent manner [9].
The toxicity of crude extracts of E. prostrata extracted in different solvent was tested against Aedes aegypti. The lethality values were expressed in LC50 and LC90 values. The LC50 values of hexane, benzene, ethyl acetate, methanol, chloroform and methanol extracts of E. prostrata against larvae at the early stage were 151.38, 165.10, 151.38, 154.88, 146.28, and 127.64 ppm, while LC90 values were, 297.70, 274.34, 288.61, 274.42, and 245.73 ppm, respectively. The methanol extract was reported to have highest larvicidal activities followed by other solvents such as chloroform, benzene, ethyl acetate, and hexane extract. The relation of egg hatchability and that of concentration of leaf extract of E. prostrata were estimated. The methanol extract exerted 0% hatchability (100% mortality) at 300 ppm [26].
7. Conclusions
Eclipta prostrata is widely used as traditional medicine in various countries specially for skin, liver and stomach problems, and for promoting hair growth. Various compounds such as coumestan derivatives, steroidal and triterpenoid saponins, phenolic acids, flavonoids, and substituted thiophenes were isolated and identified from the extracts. Similarly, various biological activity evaluations were performed for extracts and isolated compounds such as antioxidative, antimicrobial, hepatoprotective, anticancer, hair growth promoting activities. Many of these activities were performed based on in vitro methods and mechanisms of action are not explored in detail using animal models. Properly designed clinical studies are necessary to evaluate the safety and efficacy E. prostrata in future.
Acknowledgments
Authors would like to thank Basu Dev Neupane for providing the photographs of plant used in this article and permission to use them.
Abbreviations
| CCl4 | Carbon Tetrachloride |
| DGAT | Diacylglycerol Acyltransferase |
| DPPH | 1,1-Diphenyl-2-Picrylhydrazyl |
| EAC | Ehrlich Ascites Carcinoma |
| GPT | Glutamine-Pyruvate Transaminase |
| GOT | Glutamic-Oxaloacetic Transaminase |
| HCV | Hepatitis C Virus |
| HRMCs | Human Renal Mesangial Cells |
| IC50 | Inhibition Concentration 50% |
| MBC | Minimum Bacterial Concentration |
| MIC | Minimum Inhibitory Concentration |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | Nuclear Factor κB |
| RBC | Red Blood Cells |
| TFC | Total Flavonoid Content |
| TG | Triglycerides |
| TPC | Total Phenolic Content |
| TBARS | Thiobarbituric Acid Reactive Substances |
| WBC | White Blood Cells |
Author Contributions
Both authors conceived the idea, prepared the first draft, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new datasets analyzed or generated during the study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ambu G., Chaudhary R.P., Mariotti M., Cornara L. Traditional Uses of Medicinal Plants by Ethnic People in the Kavrepalanchok District, Central Nepal. Plants. 2020;9:759. doi: 10.3390/plants9060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunwar R.M., Bussmann R.W. Ethnobotany in the Nepal Himalaya. J. Ethnobiol. Ethnomed. 2008;4:24. doi: 10.1186/1746-4269-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald M., Heinrich M., Booker A. Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Front. Pharmacol. 2020;10:1480. doi: 10.3389/fphar.2019.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David B., Wolfender J.L., Dias D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015;14:299–315. doi: 10.1007/s11101-014-9367-z. [DOI] [Google Scholar]
- 5.Khanal A., Devkota H.P., Kaundinnyayana S., Gyawali P., Ananda R., Adhikari R. Culinary Herbs and Spices in Nepal: A Review of Their Traditional Uses, Chemical Constituents, and Pharmacological Activities. Ethnobot. Res. Appl. 2021;21:1–18. doi: 10.32859/era.21.40.1-18. [DOI] [Google Scholar]
- 6.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manandhar N.P. Plants and People of Nepal. Timber Press; Portland, OR, USA: 2002. [Google Scholar]
- 8.Sherchan J., Poudel P., Sapkota B., Jan H.A., Bussmann R.W. Eclipta prostrata (L.) L. Asteraceae. In: Kunwar R.M., Sher H., Bussmann R.W., editors. Ethnobotany of the Himalayas. Springer International Publishing; Cham, Switzerland: 2020. pp. 1–19. [Google Scholar]
- 9.Uddin M.N., Rahman M.A., Ahmed N.U., Rana M.S., Akter R., Chowdhury A.M.A. Antioxidant, Cytotoxic and Antimicrobial Properties of Eclipta alba Ethanol Extract. Int. J. Biol. Med. Res. 2010;4:341–346. [Google Scholar]
- 10.Baskaran P., Jayabalan N. An Efficient Micropropagation System for Eclipta alba—A Valuable Medicinal Herb. In Vitro Cell. Dev. Biol. Plant. 2005;41:532–539. doi: 10.1079/IVP2005667. [DOI] [Google Scholar]
- 11.Adhikari M., Thapa R., Kunwar R.M., Devkota H.P., Poudel P. Ethnomedicinal Uses of Plant Resources in the Machhapuchchhre Rural Municipality of Kaski District, Nepal. Medicines. 2019;6:69. doi: 10.3390/medicines6020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansoorali K.P., Prakash T., Kotresha D., Prabhu K., Rama Rao N. Cerebroprotective Effect of Eclipta alba against Global Model of Cerebral Ischemia Induced Oxidative Stress in Rats. Phytomedicine. 2012;19:1108–1116. doi: 10.1016/j.phymed.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Gupta A., Kumar A., Kumar D., Nandan S., Shankar K., Varshney S., Rajan S., Srivastava A., Gupta S., Kanojiya S., et al. Ethyl Acetate Fraction of Eclipta alba: A Potential Phytopharmaceutical Targeting Adipocyte Differentiation. Biomed. Pharmacother. 2017;96:572–583. doi: 10.1016/j.biopha.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Hussain I., Khan N., Ullah R., Ahmed S., Khan F.A., Yaz S. Phytochemical, Physiochemical and Anti-Fungal Activity of Eclipta alba. Afr. J. Pharm. Pharmacol. 2011;5:2150–2155. doi: 10.5897/AJPP11.453. [DOI] [Google Scholar]
- 15.Global Biodiversity Information Facility Secretariat (GBIF). Eclipta prostrata (L.) L. [(accessed on 1 November 2021)]. Available online: https://www.gbif.org/species/5384950.
- 16.Bakht J., Islam A., Ali H., Tayyab M., Shafi M. Antimicrobial Potentials of Eclipta alba by Disc Diffusion Method. Afr. J. Biotechnol. 2011;10:7658–7667. doi: 10.4314/ajb.v10i39. [DOI] [Google Scholar]
- 17.Jayathirtha M.G., Mishra S.H. Preliminary Immunomodulatory Activities of Methanol Extracts of Eclipta alba and Centella asiatica. Phytomedicine. 2004;11:361–365. doi: 10.1078/0944711041495236. [DOI] [PubMed] [Google Scholar]
- 18.Datta K., Singh A.T., Mukherjee A., Bhat B., Ramesh B., Burman A.C. Eclipta alba Extract with Potential for Hair Growth Promoting Activity. J. Ethnopharmacol. 2009;124:450–456. doi: 10.1016/j.jep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Jahan R., Al-Nahain A., Majumder S., Rahmatullah M. Ethnopharmacological Significance of Eclipta alba (L.) Hassk. (Asteraceae) Int. Sch. Res. Not. 2014;2014:385969. doi: 10.1155/2014/385969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalal S., Kataria S., Sastry K., Rana S.V.S. Phytochemical Screening of Methanolic Extract and Antibacterial Activity of Active Principles of Hepatoprotective Herb, Eclipta alba. Ethnobot. Leafl. 2010;2010:3. [Google Scholar]
- 21.Gautam T.P. Indigenous Uses of Some Medicinal Plants in Panchthar District, Nepal. Nepal. J. Biosci. 2011;1:125–130. doi: 10.3126/njbs.v1i0.7479. [DOI] [Google Scholar]
- 22.Diogo L.C., Fernandes R.S., Marcussi S., Menaldo D.L., Roberto P.G., Matrangulo P.V.F., Pereira P.S., França S.C., Giuliatti S., Soares A.M., et al. Inhibition of Snake Venoms and Phospholipases A2 by Extracts from Native and Genetically Modified Eclipta alba: Isolation of Active Coumestans. Basic Clin. Pharmacol. Toxicol. 2009;104:293–299. doi: 10.1111/j.1742-7843.2008.00350.x. [DOI] [PubMed] [Google Scholar]
- 23.Puri H.S. Rasayana: Ayurvedic Herbs for Longevity and Rejuvenation. J. Altern. Complement. Med. 2003;9:331–332. doi: 10.1089/10755530360623446. [DOI] [Google Scholar]
- 24.Rai M.B. Medicinal Plants of Tehrathum District, Eastern Nepal. Our Nat. 2003;1:42–48. doi: 10.3126/on.v1i1.304. [DOI] [Google Scholar]
- 25.Badgujar S.B., Patil M.B. Ethnomedicines for Jaundice Used in Tribal Areas of North Maharashtra. Ind. J. Nat. Prod. Resour. 2008;7:79–81. [Google Scholar]
- 26.Govindarajan M., Karuppannan P. Mosquito Larvicidal and Ovicidal Properties of Eclipta alba (L.) Hassk (Asteraceae) against Chikungunya Vector, Aedes aegypti (Linn.) (Diptera: Culicidae) Asian Pac. J. Trop. Med. 2011;4:24–28. doi: 10.1016/S1995-7645(11)60026-6. [DOI] [PubMed] [Google Scholar]
- 27.Rajith N.P., Ramachandran V.S. Ethnomedicines of Kurichyas, Kannur District, Western Ghats, Kerala. Indian J. Nat. Prod. Resour. 2010;1:249–253. [Google Scholar]
- 28.Roy R.K., Thakur M., Dixit V.K. Hair Growth Promoting Activity of Eclipta alba in Male Albino Rats. Arch. Dermatol. Res. 2008;300:357–364. doi: 10.1007/s00403-008-0860-3. [DOI] [PubMed] [Google Scholar]
- 29.Khan A.V., Khan A.A. Ethnomedicinal Uses of Eclipta prostrata Linn. Indian J. Trad. Knowl. 2008;7:316–320. [Google Scholar]
- 30.Kumari C.S., Govindasamy S., Sukumar E. Lipid Lowering Activity of Eclipta prostrata in Experimental Hyperlipidemia. J. Ethnopharmacol. 2006;105:332–335. doi: 10.1016/j.jep.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 31.Tewtrakul S., Subhadhirasakul S., Cheenpracha S., Karalai C. HIV-1 Protease and HIV-1 Integrase Inhibitory Substances from Eclipta prostrata. Phytother. Res. 2007;21:1092–1095. doi: 10.1002/ptr.2252. [DOI] [PubMed] [Google Scholar]
- 32.Neeraja P.V., Margaret E. Eclipta alba (L.) Hassk: A Valuable Medicinal Herb. Int. J. Curr. Pharm. Rev. Res. 2011;2:188–197. [Google Scholar]
- 33.Kaushik-Basu N., Bopda-Waffo A., Talele T.T., Basu A., Costa P.R.R., da Silva A.J.M., Sarafianos S.G., Noël F. Identification and Characterization of Coumestans as Novel HCV NS5B Polymerase Inhibitors. Nucleic Acids Res. 2008;36:1482–1496. doi: 10.1093/nar/gkm1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le D.D., Nguyen D.H., Ma E.S., Lee J.H., Min B.S., Choi J.S., Woo M.H. PTP1B Inhibitory and Anti-Inflammatory Properties of Constituents from Eclipta prostrata L. Biol. Pharm. Bull. 2021;44:298–304. doi: 10.1248/bpb.b20-00994. [DOI] [PubMed] [Google Scholar]
- 35.Wagner H., Geyer B., Kiso Y., Hikino H., Rao G. Coumestans as the Main Active Principles of the Liver Drugs Eclipta alba and Wedelia calendulacea. Planta Med. 1986;52:370–374. doi: 10.1055/s-2007-969188. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J.S., Guo Q.M. Studies on the chemical constituents of Eclipta prostrata (L) Yao Xue Xue Bao. 2001;36:34–37. [PubMed] [Google Scholar]
- 37.Feng L., Zhai Y.-Y., Xu J., Yao W.-F., Cao Y.-D., Cheng F.-F., Bao B.-H., Zhang L. A Review on Traditional Uses, Phytochemistry and Pharmacology of Eclipta prostrata (L.) L. J. Ethnopharmacol. 2019;245:112109. doi: 10.1016/j.jep.2019.112109. [DOI] [PubMed] [Google Scholar]
- 38.Yahara S., Ding N., Nohara T., Masuda K., Ageta H. Taraxastane Glycosides from Eclipta alba. Phytochemistry. 1997;44:131–135. doi: 10.1016/S0031-9422(96)00473-6. [DOI] [Google Scholar]
- 39.Yahara S., Ding N., Nohara T. Oleanane Glycosides from Eclipta alba. Chem. Pharm. Bull. 1994;42:1336–1338. doi: 10.1248/cpb.42.1336. [DOI] [Google Scholar]
- 40.Zhang M., Chen Y.Y., Di X.H., Liu M. Isolation and identification of ecliptasaponin D from Eclipta alba (L.) Hassk. Yao Xue Xue Bao. 1997;32:633–634. [PubMed] [Google Scholar]
- 41.Kim D.-I., Lee S.-H., Choi J.-H., Lillehoj H.S., Yu M.-H., Lee G.-S. The Butanol Fraction of Eclipta prostrata (Linn) Effectively Reduces Serum Lipid Levels and Improves Antioxidant Activities in CD Rats. Nutr. Res. 2008;28:550–554. doi: 10.1016/j.nutres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Khanna V.G., Kannabiran K. Anticancer-Cytotoxic Activity of Saponins Isolated from the Leaves of Gymnema Sylvestre and Eclipta prostrata on HeLa Cells. Int. J. Green Pharm. 2009;3:227–229. doi: 10.22377/ijgp.v3i3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Upadhyay R.K., Pandey M.B., Jha R.N., Pandey V.B. Eclalbatin, a Triterpene Saponin from Eclipta alba. J. Asian Nat. Prod. Res. 2001;3:213–217. doi: 10.1080/10286020108041393. [DOI] [PubMed] [Google Scholar]
- 44.Yu S.-J., Yu J.-H., Yu Z.-P., Yan X., Zhang J.-S., Sun J., Zhang H. Bioactive Terpenoid Constituents from Eclipta prostrata. Phytochemistry. 2020;170:112192. doi: 10.1016/j.phytochem.2019.112192. [DOI] [PubMed] [Google Scholar]
- 45.Xi F.-M., Li C.-T., Mi J.-L., Wu Z.-J., Chen W.-S. Three New Olean-Type Triterpenoid Saponins from Aerial Parts of Eclipta prostrata (L.) Nat. Prod. Res. 2014;28:35–40. doi: 10.1080/14786419.2013.832674. [DOI] [PubMed] [Google Scholar]
- 46.Xi F.-M., Li C.-T., Han J., Yu S.-S., Wu Z.-J., Chen W.-S. Thiophenes, Polyacetylenes and Terpenes from the Aerial Parts of Eclipta prostrata. Bioorg. Med. Chem. 2014;22:6515–6522. doi: 10.1016/j.bmc.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 47.Abdel-Kader M.S., Bahler B.D., Malone S., Werkhoven M.C.M., van Troon F., David ⊥., II, Wisse J.H., Bursuker I., Neddermann K.M., Mamber S.W., et al. DNA-Damaging Steroidal Alkaloids from Eclipta alba from the Suriname Rainforest. J. Nat. Prod. 1998;61:1202–1208. doi: 10.1021/np970561c. [DOI] [PubMed] [Google Scholar]
- 48.Sethiya N., Tomer K., Singh V., Kumar M., Jaiswal D., Yadav I., Singh H., Chandra D., Jain D. Isolation and Characterization of New Lanosteriod from Ethanolic Extract of Eclipta alba Linn. J. Pharm. Res. 2009;2:1635–1637. [Google Scholar]
- 49.Han L., Zhao J., Zhang Y., Kojo A., Liu E., Wang T. Chemical Constituents from Dried Aerial Parts of Eclipta prostrata. Chin. Herb. Med. 2013;5:313–316. doi: 10.1016/S1674-6384(13)60047-7. [DOI] [Google Scholar]
- 50.Xiong H.-P., Xi F.-M., Chen W.-S., Lu W.-Q., Wu Z.-J. Chemical Constituents of Eclipta prostrata. Chem. Nat. Compd. 2021;57:166–168. doi: 10.1007/s10600-021-03308-y. [DOI] [Google Scholar]
- 51.Li W., Pang X., Han L.-F., Zhou Y., Cui Y.-M. Chemical constituents of Eclipta prostrata. China J. Chin. Mater. Medica. 2018;43:3498–3505. doi: 10.19540/j.cnki.cjcmm.20180625.001. [DOI] [PubMed] [Google Scholar]
- 52.Lee M.K., Ha N.R., Yang H., Sung S.H., Kim Y.C. Stimulatory Constituents of Eclipta prostrata on Mouse Osteoblast Differentiation. Phytother. Res. 2009;23:129–131. doi: 10.1002/ptr.2560. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y., Peng L., Lu W., Wang Y., Huang X., Gong C., He L., Hong J., Wu S., Jin X. Effect of Eclipta prostrata on Lipid Metabolism in Hyperlipidemic Animals. Exp. Gerontol. 2015;62:37–44. doi: 10.1016/j.exger.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 54.Meng X., Li B.-B., Lin X., Jiang Y.-Y., Zhang L., Li H.-Z., Cui L. New Polyacetylenes Glycoside from Eclipta Prostrate with DGAT Inhibitory Activity. J. Asian Nat. Prod. Res. 2019;21:501–506. doi: 10.1080/10286020.2018.1452914. [DOI] [PubMed] [Google Scholar]
- 55.Tewtrakul S., Subhadhirasakul S., Tansakul P., Cheenpracha S., Karalai C. Antiinflammatory Constituents from Eclipta prostrata Using RAW264.7 Macrophage Cells. Phytother. Res. 2011;25:1313–1316. doi: 10.1002/ptr.3383. [DOI] [PubMed] [Google Scholar]
- 56.Lee J.-S., Ahn J.-H., Cho Y.-J., Kim H.-Y., Yang Y.-I., Lee K.-T., Jang D.-S., Choi J.-H. α-Terthienylmethanol, Isolated from Eclipta prostrata, Induces Apoptosis by Generating Reactive Oxygen Species via NADPH Oxidase in Human Endometrial Cancer Cells. J. Ethnopharmacol. 2015;169:426–434. doi: 10.1016/j.jep.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 57.Yu S.-J., Zhang J.-S., He H., Yu J.-H., Bao J., Zhang H. Thiophene Enantiomers from the Aerial Parts of Eclipta prostrata. J. Asian Nat. Prod. Res. 2021;23:745–753. doi: 10.1080/10286020.2020.1769610. [DOI] [PubMed] [Google Scholar]
- 58.Yu S.-J., Yu J.-H., He F., Bao J., Zhang J.-S., Wang Y.-Y., Zhang H. New Antibacterial Thiophenes from Eclipta prostrata. Fitoterapia. 2020;142:104471. doi: 10.1016/j.fitote.2020.104471. [DOI] [PubMed] [Google Scholar]
- 59.Boregowda R.S., Murali N., Udayashankar A.C., Niranjana S.R., Lund O.S., Prakash H.S. Antifungal Activity of Eclipta alba Metabolites against Sorghum Pathogens. Plants. 2019;8:72. doi: 10.3390/plants8030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee H.Y. Enhancement of Skin Anti-Inflammatory Activities of Eclipta prostrata L. from the Ultrasonic Extraction Process. Appl. Sci. 2017;7:1227. doi: 10.3390/app7121227. [DOI] [Google Scholar]
- 61.Kang E.Y., Kim H.K., Jung J.Y., Kim J.H., Woo T.K., Choi J.I., Kim J.H., Ahn C., Lee H.G., Go G.-W. Combined Extract of Leonurus Japonicus Houtt, Eclipta prostrata L., and Pueraria Lobata Ohwi Improved Hot Flashes and Depression in an Ovariectomized Rat Model of Menopause. Foods. 2021;10:180. doi: 10.3390/foods10010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel M., Verma R., Srivastav P. Antioxidant Activity of Eclipta alba Extract. J. Med. Plants Stud. 2016;4:92–98. [Google Scholar]
- 63.Gurrapu S., Mamidala E. In Vitro Antibacterial Activity of Alkaloids Isolated from Leaves of Eclipta alba against Human Pathogenic Bacteria. Pharmacogn. J. 2017;9:573–577. doi: 10.5530/pj.2017.4.91. [DOI] [Google Scholar]
- 64.Thirumalai T., David E., Therasa S.V., Elumalai E. Restorative Effect of Eclipta alba in CCl4 Induced Hepatotoxicity in Male Albino Rats. Asian Pac. J. Trop. Dis. 2011;1:304–307. doi: 10.1016/S2222-1808(11)60072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lal V.K., Kumar A., Kumar P., Yadav K.S. Screening of Leaves and Roots of Eclipta alba for Hepatoprotective Activity. Arch. Appl. Sci. Res. 2010;2:86–94. [Google Scholar]
- 66.Saxena A.K., Singh B., Anand K.K. Hepatoprotective Effects of Eclipta alba on Subcellular Levels in Rats. J. Ethnopharmacol. 1993;40:155–161. doi: 10.1016/0378-8741(93)90063-B. [DOI] [PubMed] [Google Scholar]
- 67.Lee M.K., Ha N.R., Yang H., Sung S.H., Kim G.H., Kim Y.C. Antiproliferative Activity of Triterpenoids from Eclipta prostrata on Hepatic Stellate Cells. Phytomed. Int. J. Phytother. Phytopharm. 2008;15:775–780. doi: 10.1016/j.phymed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 68.Helmy A.S., Sherif N.M., Ghanem H.Z., Ibrahim N.A., El Gendy A.N.G., Hussein N.S., Abdel-Hamid A.-H.Z. Targeted Metabolomics Reveals the Therapeutic Impact of Eclipta prostrata on Diet-Induced Non-Alcoholic Fatty Liver Disease in Rats. J. Appl. Pharm. Sci. 2019;9:77–90. [Google Scholar]
- 69.Banji O., Banji D., Annamalai A., Manavalan R. Investigation on the Effect of Eclipta alba on Animal Models of Learning and Memory. Indian J. Physiol. Pharmacol. 2007;51:274–278. [PubMed] [Google Scholar]
- 70.Kim D.-I., Lee S.-H., Hong J.-H., Lillehoj H.S., Park H.-J., Rhie S.-G., Lee G.-S. The Butanol Fraction of Eclipta prostrata (Linn) Increases the Formation of Brain Acetylcholine and Decreases Oxidative Stress in the Brain and Serum of Cesarean-Derived Rats. Nutr. Res. 2010;30:579–584. doi: 10.1016/j.nutres.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 71.Tambe R., Patil A., Jain P., Sancheti J., Somani G., Sathaye S. Assessment of Luteolin Isolated from Eclipta alba Leaves in Animal Models of Epilepsy. Pharm. Biol. 2017;55:264–268. doi: 10.1080/13880209.2016.1260597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alam N., Sharma K.r. Estimation of Phenolic Content, Flavonoid Content, Antioxidant, and Alpha Amylase Inhibitiory Activity of Some Selected Plant from Siraha District of Nepal. Asian J. Pharm. Clin. Res. 2020;13:18–23. doi: 10.22159/ajpcr.2020.v13i4.36734. [DOI] [Google Scholar]
- 73.Shahab U., Faisal M., Alatar A.A., Ahmad S. Impact of Wedelolactone in the Anti-Glycation and Anti-Diabetic Activity in Experimental Diabetic Animals. IUBMB Life. 2018;70:547–552. doi: 10.1002/iub.1744. [DOI] [PubMed] [Google Scholar]
- 74.Jaiswal N., Bhatia V., Srivastava S.P., Srivastava A.K., Tamrakar A.K. Antidiabetic Effect of Eclipta alba Associated with the Inhibition of Alpha-Glucosidase and Aldose Reductase. Nat. Prod. Res. 2012;26:2363–2367. doi: 10.1080/14786419.2012.662648. [DOI] [PubMed] [Google Scholar]
- 75.Hemalatha S., Ayyappan T., Shanmugan S., Nagavalli D., Shrivijaya Kirubha T. Evaluation of Antidiabetic and Diuretic Activity of Polyherbal Formulation. Indian J. Tradit. Knowl. 2006;5:468–470. [Google Scholar]
- 76.Ananthi J., Prakasam A., Pugalendi K.V. Antihyperglycemic Activity of Eclipta alba Leaf on Alloxan-Induced Diabetic Rats. Yale J. Biol. Med. 2003;76:97–102. [PMC free article] [PubMed] [Google Scholar]
- 77.Singh A., Singh A., Dwivedi V. Screening of Hydro-Alcoholic Extract of Eclipta alba for Its Anticancerous Efficacy. Int. J. Sci. Res. 2017;6:488–491. [Google Scholar]
- 78.Arya R.K., Singh A., Yadav N.K., Cheruvu S.H., Hossain Z., Meena S., Maheshwari S., Singh A.K., Shahab U., Sharma C., et al. Anti-Breast Tumor Activity of Eclipta Extract in-Vitro and in-Vivo: Novel Evidence of Endoplasmic Reticulum Specific Localization of Hsp60 during Apoptosis. Sci. Rep. 2015;5:18457. doi: 10.1038/srep18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim H.-Y., Kim H.M., Ryu B., Lee J.-S., Choi J.-H., Jang D.S. Constituents of the Aerial Parts of Eclipta prostrata and Their Cytotoxicity on Human Ovarian Cancer Cells in Vitro. Arch. Pharm. Res. 2015;38:1963–1969. doi: 10.1007/s12272-015-0599-2. [DOI] [PubMed] [Google Scholar]
- 80.Chaudhary H., Dhuna V., Singh J., Kamboj S.S., Seshadri S. Evaluation of Hydro-Alcoholic Extract of Eclipta alba for Its Anticancer Potential: An in Vitro Study. J. Ethnopharmacol. 2011;136:363–367. doi: 10.1016/j.jep.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 81.Mondal S., Ghosh D., Ganapaty S., Sushrutha M. Preliminary Phytochemical Analysis and Evaluation of Hair Growth Stimulating Potential of Ethanol Extract from L. (Asteraceae) Leaves in Wistar Albino Eclipta alba Rats. Asian J. Pharm. Pharmacol. 2016;2:121–127. [Google Scholar]
- 82.Begum S., Lee M.R., Gu L.J., Hossain J., Sung C.K. Exogenous Stimulation with Eclipta alba Promotes Hair Matrix Keratinocyte Proliferation and Downregulates TGF-Β1 Expression in Nude Mice. Int. J. Mol. Med. 2015;35:496–502. doi: 10.3892/ijmm.2014.2022. [DOI] [PubMed] [Google Scholar]
- 83.Roy R.K., Thakur M., Dixit V.K. Development and Evaluation of Polyherbal Formulation for Hair Growth–Promoting Activity. J. Cosmet. Dermatol. 2007;6:108–112. doi: 10.1111/j.1473-2165.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 84.Christybapita D., Divyagnaneswari M., Michael R.D. Oral Administration of Eclipta alba Leaf Aqueous Extract Enhances the Non-Specific Immune Responses and Disease Resistance of Oreochromis mossambicus. Fish Shellfish Immunol. 2007;23:840–852. doi: 10.1016/j.fsi.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 85.Deng Y., Kang W., Zhao J., Liu G., Zhao M. Osteoprotective Effect of Echinocystic Acid, a Triterpone Component from Eclipta prostrata, in Ovariectomy-Induced Osteoporotic Rats. PLoS ONE. 2015;10:e0136572. doi: 10.1371/journal.pone.0136572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dungca N.T.P. Protective Effect of the Methanolic Leaf Extract of Eclipta alba (L.) Hassk. (Asteraceae) against Gentamicin-Induced Nephrotoxicity in Sprague Dawley Rats. J. Ethnopharmacol. 2016;184:18–21. doi: 10.1016/j.jep.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 87.Wang L., Huang B., Li C., Yang B., Jia X., Feng L. The Combination of HPLC and Biological Analysis to Determine the Quality Markers and Its Structural Composition of Eclipta prostrata L. Phytochem. Anal. 2020;31:968–981. doi: 10.1002/pca.2969. [DOI] [PubMed] [Google Scholar]
- 88.Shen P., Yang X., Jiang J., Wang X., Liang T., He L. Wedelolactone from Eclipta alba Inhibits Lipopolysaccharide-Enhanced Cell Proliferation of Human Renal Mesangial Cells via NF-ΚB Signaling Pathway. Am. J. Transl. Res. 2017;9:2132–2142. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new datasets analyzed or generated during the study.