Abstract
Polymer nanofibers have exceptionally high surface area. This is advantageous compared to bulk polymeric structures, as nanofibrils increase the area over which materials can be transported into and out of a system, via diffusion and active transport. On the other hand, since hydrogels possess a degree of flexibility very similar to natural tissue, due to their significant water content, hydrogels made from natural or biodegradable macromolecular systems can even be injectable into the human body. Due to unique interactions with water, hydrogel transport properties can be easily modified and tailored. As a result, combining nanofibers with hydrogels would truly advance biomedical applications of hydrogels, particularly in the area of sustained drug delivery. In fact, certain nanofiber networks can be transformed into hydrogels directly without the need for a hydrogel enclosure. This review discusses recent advances in the fabrication and application of biomedical nanofiber hydrogels with a strong emphasis on drug release. Most of the drug release studies and recent advances have so far focused on self-gelling nanofiber systems made from peptides or other natural proteins loaded with cancer drugs. Secondly, polysaccharide nanofiber hydrogels are being investigated, and thirdly, electrospun biodegradable polymer networks embedded in polysaccharide-based hydrogels are becoming increasingly popular. This review shows that a major outcome from these works is that nanofiber hydrogels can maintain drug release rates exceeding a few days, even extending into months, which is an extremely difficult task to achieve without the nanofiber texture. This review also demonstrates that some publications still lack careful rheological studies on nanofiber hydrogels; however, rheological properties of hydrogels can influence cell function, mechano-transduction, and cellular interactions such as growth, migration, adhesion, proliferation, differentiation, and morphology. Nanofiber hydrogel rheology becomes even more critical for 3D or 4D printable systems that should maintain sustained drug delivery rates.
Keywords: nanofiber, hydrogel, nanofiber hydrogel, drug release, gel rheology
1. Introduction
Definition of Hydrogels and Their Applications
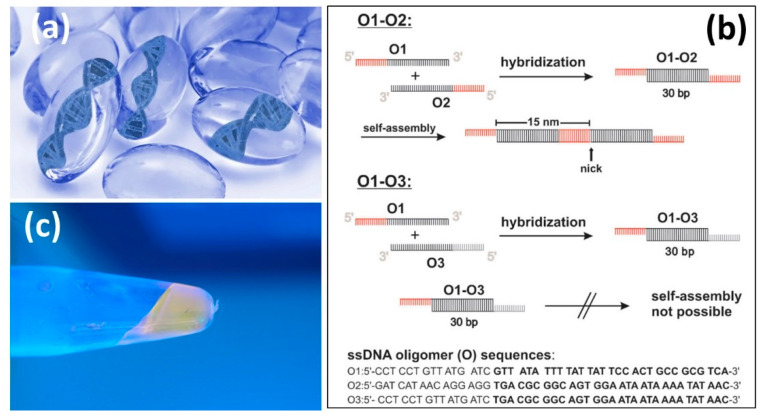
A hydrogel is defined as a three-dimensional (3D) network of hydrophilic molecules or polymers that are able to swell in water and retain large amounts of water in their bulk. In such a state, hydrogels can maintain their structure and stability due to chemical or physical cross-linking of individual polymer chains. The first reported hydrogels in the literature were due to Wichterle and Lím (1960) [1]. It is generally accepted that water must constitute at least 10% of the total weight or volume of the polymeric network to be labeled as a hydrogel. Hydrogels should feature a good degree of flexibility identical to natural tissue. The hydrophilicity of the polymer-water network is maintained by the presence of hydrophilic groups such as -NH2, -COOH, -OH, -CONH2, -CONH-, and -SO3H. Hydrogel technologies are rapidly evolving, and new examples are emerging, such as DNA hydrogels (see Figure 1) that are known as swollen networks of DNA molecules cross-linked in an aqueous solution [2,3,4]. DNA is a biological polymer that is hydrophilic, biocompatible, and highly programmable by Watson–Crick base pairing (i.e., adenine (A) forms a base pair with thymine (T) using two hydrogen bonds, and guanine (G) forms a base pair with cytosine (C) using three hydrogen bonds). DNA can be transformed into a hydrogel by itself under certain conditions, and it can also be integrated into synthetic polymers to form DNA-hybrid hydrogels. Functional DNAs, such as aptamers and DNAzymes, offer additional molecular recognition capabilities and versatility [5].
Figure 1.
(a) DNA based hydrogels. DNA can be used as the sole component of a hydrogel. It can act as the backbone or a cross-linking agent connecting active molecules to the hydrogel via chemical reactions or physical entanglement. (b) A typical DNA hydrogel formation mechanism known as oligomer hybridization. By hybridization of O1 with O2, a double-stranded DNA monomer (O1-O2, 30 base pairs and 15 base sticky ends) is generated, which can self-assemble. The double-stranded DNA monomer O1-O3 (30 base pairs) comprises non-complementary 15-base overhangs and cannot further self-assemble. (c) Optical micrograph of a DNA hydrogel produced from the oligomers O1 and O2. The DNA hydrogel was stained with CybrSafe® DNA dye and illuminated with UV light (λmax = 366 nm). Reproduced from [2,6] with permission.
In Figure 1b,c, a schematic and a photograph show a three-dimensional DNA hydrogel that was made by self-assembly of short linear double-stranded DNA building blocks furnished with sticky ends. The resulting DNA hydrogel was thermo-responsive, and the length of the supramolecular double-stranded DNA structures varied with temperature [6]. Proper selection of oligomer pairs is critical to ensure the formation of hydrogels; for instance, pairing O1 and O3 oligomers does not result in self-assembly and gelation (Figure 1b). A recent review [7] studied the existing data and methods related to the mechanical design of pristine DNA and hybrid hydrogels and their use in cell culture. The focus of the article was to advance further studies toward designing multifunctional DNA hydrogels with programmable and controlled mechanical properties.
In medicine, hydrogels are used as scaffolds for tissue engineering, sustained drug release media, optical and microfluidic actuation, and as model extracellular matrices in biotechnology. Applications of hydrogels can be limited by their mechanical response. Most hydrogels are not elastomeric networks with low stretching, such as an alginate hydrogel that ruptures upon elongation to about 1.2 times its original length [8]. Most hydrogels are still classified as brittle, with fracture energies of about 10 Jm−2 compared to ∼1000 Jm−2 for cartilage and ∼10,000 Jm−2 for natural rubbers [8].
The flexibility and stretching and elastic recovery of hydrogels are very important sought-after properties, as shown in Figure 2, because poor mechanical stability of hydrogels used for cell encapsulation can cause involuntary cell release and death [9], and low toughness severely restricts the durability of contact lenses [10]. In Figure 2, a highly stretchable, tough hydrogel is demonstrated by mixing two types of cross-linked polymers, namely, ionic cross-linking of alginates and covalently cross-linked polyacrylamide [8].
Figure 2.
(a) The non-perturbed hydrogel between two rigid clamps. (b) The gel was stretched 21 times its initial length in a tensile machine. (c) The gel was damaged by forming razor blade notch. (d) Due to the damage, the stretching was 17 times the initial length instead, but still significant. Reproduced from [8] with permission.
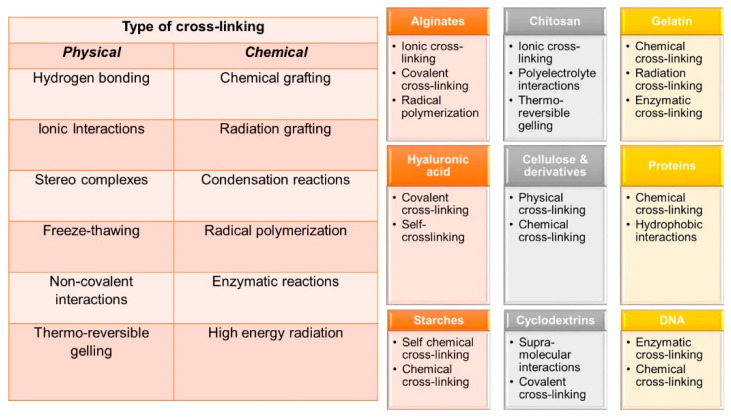
As mentioned earlier, hydrogels are three-dimensional networks in which hydrophilic polymers cross-link together. Figure 3 summarizes typical physical and chemical cross-linking reactions leading to hydrogels, as well as a list of common natural polymers and relevant cross-linking pathways used in transforming them into hydrogels.
Figure 3.
Typical hydrogel forming interactions and cross-linking pathways. The figure also displays widely used hydrogel-forming reactions of natural polymers including DNA.
There are several dedicated reviews on each of these natural polymer based hydrogels and more and the readers should consult to them for further details on cross-linking and applications. For instance, alginate based hydrogels have been reviewed and discussed in [11,12,13,14,15]. Chitosan based hydrogels have been presented and discussed in [16,17,18,19,20]. Gelatin and protein based hydrogels have been extensively studied and classified in [21,22,23,24,25,26,27,28,29,30]. Cellulose based hydrogels are also very common and reviewed several times in the literature with relevant applications [31,32,33,34,35,36,37,38]. Similarly, starch based hydrogels are also common and have been analyzed carefully in [39,40,41,42,43,44,45,46]. Cyclodextrins are a family of cyclic oligosaccharides, consisting of a macrocyclic ring of glucose subunits joined by α-1,4 glycosidic bonds. Cyclodextrins are produced from starch by enzymatic conversion. They are used in food, pharmaceutical, drug delivery, and chemical industries, as well as agriculture and environmental engineering. Hydrogels based on cyclodextrins are extensively reviewed in [47,48,49,50,51,52,53]. DNA based hydrogels are rapidly gaining in popularity and can find a wide range of applications in the biomedical field. Hyaluronic acid (HA), also called hyaluronan, is an anionic, non-sulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. It is unique among glycosaminoglycans as it is non-sulfated, forms in the plasma membrane, and can have very high molecular weight; for instance, human synovial HA averages about 7 million Da per molecule, or about 20,000 disaccharide monomers, and some HA molecules extracted from tissues can reach 3–4 million Da [54]. HA based hydrogels are extremely biocompatible materials that can be rendered functional by various approaches including cross-linking in the presence of drugs and particularly for cancer treatment [55,56,57,58,59,60,61]. Chemical and physical properties of various DNA based hydrogels as well as their properties can be found in [62,63,64,65,66,67,68]. There is even a much larger body of literature on hydrogels based solely on synthetic polymers and/or hydrogel interpenetrating networks between natural and synthetic polymers, as well as their successful applications, and readers can refer to a number of reviews in [67,68,69,70,71,72,73,74,75,76,77,78,79]. Figure 4 shows typical general applications of hydrogels in the biomedical field, and the figure shows a timeline of review articles that have been published on each application field. Table 1 further lists those specific review articles that readers can refer to for the advances made in those specific periods. In particular, it can be seen that drug delivery from hydrogels is the oldest subject for review articles; however, there appear to be significant differences in the total number of reviews published on each application (Table 1). Since the general principles and applications of hydrogels are beyond the scope of this review article, readers can refer to a number of works cited herein [80]. In the following sections, we will discuss the process of electrospinning briefly and review recent advances in hydrogels made from electrospun materials while specifically focusing on controlled drug-release works from such materials.
Figure 4.
Schematic display of common hydrogel applications and the publication years of comprehensive reviews relevant to each application.
Table 1.
Number of reviews and publication periods on different hydrogel applications in biomedical field.
| Hydrogel Application | Number of Reviews | Publication Period | Reference |
|---|---|---|---|
| Tissue engineering | 4 | 2011–2020 | [81,82,83,84] |
| Drug delivery | 6 | 1993–2021 | [85,86,87,88,89,90] |
| Wound dressing | 6 | 2017–2020 | [91,92,93,94,95,96] |
| Contact lenses | 7 | 2017–2021 | [97,98,99,100,101,102,103] |
| Bio-sensors | 5 | 2012–2020 | [104,105,106,107,108] |
2. Principles of Electrospinning, and Hydrogels from Electrospun Materials
The technology of electrospinning is rapidly evolving, as it is a unique way to produce polymers, membranes, textiles, composites, and hydrogels based on hierarchically structured fibers with diameters typically being hundreds of nanometers [109]. The electrospinning process encompasses a complex combination of fluid mechanics, polymer science, and electrostatics. Electrostatic aerosol spraying of organic monomers can be considered as the launching pad for electrospinning and has been studied for a long time. In contrast, electrospinning applies to macromolecules or sol-gels rather than small molecules or monomers. It is generally argued that the morphology and physical entanglement of macromolecular chains can avert the capillary breakup of the electrospinning jet and result in the formation of nanofibers (Figure 5). In fact, electrospinning has been known for almost a century; with the literature including reports dating back even to the 18th century [110]. However, a real boost in electrospinning came about due to the development of nanoscience and nanotechnology [111]. Architecturally, an electrospun polymeric jet resembles a tree, as shown in Figure 5. The “roots” evolve from an exceedingly thin charged surface layer, called Debye’s layer, of the polymer solution that acts as one of the electrodes that is connected to a high voltage supply. Further downstream, a stable part of the jet is formed that looks like a tree stem. The following, larger section is the whipping zone or bending instability within the jet that resembles the branches [112].
Figure 5.
Schematic representation of electrospinning system: (1) syringe and metering pump, (2) needle/capillary acting as the electrode, (3) stable part of the jet, (4) whipping/coiling zone, (5) collector, (6) ground, and (7) high voltage supply. Reproduced from [112] with permission.
Electrospun fiber networks can be transformed directly into hydrogels based on the type of polymer or polymers used, but also fiber networks can be combined with hydrogels, as in a composite, to design new functional biomedical materials [113]. In fact, hydrogel-electrospun fiber composites fortified with cells appear to be quite promising candidates for cartilage repair. In Figure 6, for instance, rabbit cartridge damage is shown to be repaired very effectively using a hydrogel-nanofiber composite. Specifically, the nanofiber hydrogel was used to deliver chondrocytes to promote the cartilage repair [114]. Self-assembled nanofiber hydrogels are considered to be utilized with collagen-like functions towards, for instance, cardiomyocyte culture in 2D and 3D [115]. Recent works showed that hydrophilic self-assembling nanofiber hydrogels can support the culture of both rat cardiomyocytes and human embryonic stem-cell-derived cardiomyocytes, which could lead to promising applications in cardiac tissue engineering [115]. Hydrogels were also fabricated from silk nanofibers by combining β-sheet-rich silk nanofibers with amorphous silk nanofibers. The composite nanofiber networks were transformed into hydrogels by horseradish peroxidase cross-linking in an electric field. Such nanofiber hydrogels demonstrated osteogenic differentiation and the aligned aggregation of stem cells in vitro, while also exhibiting osteoinductive capacity in vivo. Such improved tissue performance with nanofiber hydrogels supports encouraging applications in bone tissue engineering [116,117].
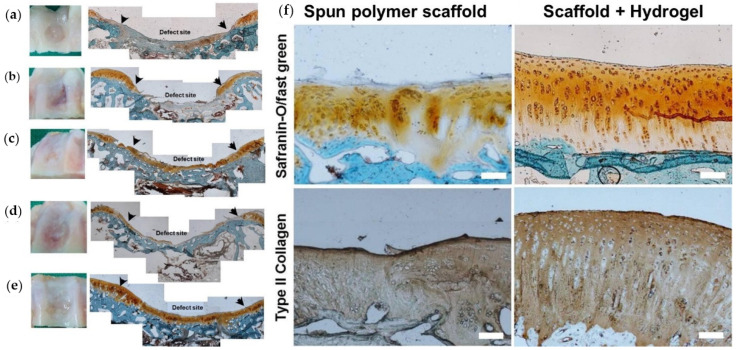
Figure 6.
Photographs of the defect site and histological staining (safranin-O/fast green) of cryo-sectioned samples in rabbit knee at 4 months. (a) Control partial defect site; (b) electrospun polymer scaffold with no cell impregnation; (c) the same scaffold with cells; (d) the polymer scaffold/hydrogel without cells; and (e) the scaffold–hydrogel composite with cells. The arrows point to the borders of initial defect site. Finally, (f) photographs of histological (safranin-O/fast green) and immune-histochemical (type II collagen) stained images of cryo-sectioned samples in rabbit knee at 4 month after implantation of electrospun scaffolds or scaffold–hydrogel composites are shown with scale bars: 100 μm. Reproduced from [114] with permission.
Nanofiber hydrogels also can be constructed from special blends of polymers that are usually immiscible with very diverse physical and chemical properties, as shown in Figure 7. Therein, polyimide and polyvinyl alcohol polymers were blended in solution and electrospun into hydrogels that demonstrate unique mechanical properties, particularly stretching ability suitable for abiotic soft tissue applications. Finally, we will discuss direct electrospinning of functional hydrogels into nanofiber networks. Direct hydrogel electrospinning is still quite challenging to be adapted to any hydrogel material system. However, several promising works have been reported, and this specific technology should be furthered possibly by utilizing the concepts of reactive electrospinning [118,119]. Very recent studies showed that co-electrospinning of biopolymers with gels or hydrogels is also possible, and that such products could be designed specifically for drug delivery [120]. For instance, polycaprolactone nanofibers were produced by co-electrospinning with vancomycin hydrochloride and simvastatin drugs that were already in gel form. The resultant hydrogel/polymer nanofiber composite could sustain drug release for as long as 14 days [120]. Another notable work [121] demonstrated synthesis of reactive macromers that contained protease-cleavable and fluorescent peptides that could form electrospun fibrous hydrogels through a photoinitiated polymerization. These nanofiber hydrogels could release hyaluronic acid for a period of 24 days near protease implants, ensuring effective implant-tissue interaction.
Figure 7.
Nanofiber stretchable hydrogels made from aramid nanofibers and polyvinyl alcohol polymer. (a) Photographs of the polymer solutions and their mixing before electrospinning. (b) A hydrogel under tension with 0% (left) and 300% (right) tensile strains. Scale bar: 10 mm. (c) A hydrogel containing much less polyvinyl alcohol with (right) and without (left) a compressive load of 10 N. Scale bar: 30 mm. (d) The same sample in (c) with (right) and without (left) a tensile load of 10 N. Scale bar: 50 mm. (e) An SEM image of the same sample in (c) after drying. Scale bar: 300 nm. Reproduced from [115] with permission.
The technology and applications of encapsulated electrospun nanofibers in hydrogel matrices have been reviewed by Bosworth et al. [122], including the formation of coatings from such composites. Impressive sustained drug release profiles (40 days) were reported from ultraviolet light-assisted electrospinning of core–shell fully cross-linked poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide) hydrogel-based nanofibers for thermally induced drug delivery [123]. Therein, a model drug (organic dye), Rhodamine B, was used instead of a medicinal compound for temperature-induced drug release. Likewise, Nakielski et al. [124] fabricated a poly(l-lactide)-Rhodamine B-loaded nanofibrous material, which encapsulated poly(Nisopropylacrylamideco-N-isopropylmethacrylamide) hydrogel containing gold nanorods that the authors indicated had resembled natural structures such as jellyfish or hydra. This nanofiber hydrogel could regulate drug release when subjected to near-infrared light stimulation. Remarkable sustained drug release profiles were obtained extending into 75 days. Furthermore, a key review article discussed diverse fabrication methods and extensive biomedical applications of nanofiber-based hydrogels ranging from bone tissue generation to sensing [125].
Table 2 summarizes recent advances in hydrogel nanofibers in terms of the types of polymer(s) used as well as resulting mechanical properties and the targeted applications. Inspection of Table 2 indicates that in a majority of these recent works, the required application target was successfully achieved without the inclusion of medicinal or other active ingredients. Only a small percentage of the studies did not measure and discuss the mechanical properties of the nanofiber hydrogels. However, careful studies on the mechanical properties of nanofiber hydrogels must be conducted regardless of the biomedical focus, particularly for cartilage repair but also for soft tissue engineering.
Table 2.
A summary of nanofibers in hydrogels, including types of polymers or polymer blends used and the resultant mechanical properties.
| Polymer Type | Spinning Conditions | Mechanical Properties | Biological Additives | Biomedical Applications | Reference |
|---|---|---|---|---|---|
| Poly(ε-caprolactone)-hyaluronic acid | Mechanical mixing of spun fibers | Storage moduli similar to skin | n/a | Soft tissue restoration | [131] |
| Supramolecular peptide | Naturally nanofibrous | Good viscoelastic properties | n/a | Cell delivery | [132] |
| Collagen/cellulose | Acetic acid | Good compressive strength | n/a | Tissue scaffold | [133] |
| Polyvinyl alcohol/alginate | Water solution | Good balance between strength and elasticity | n/a | Burn treatment | [134] |
| Poly(vinyl alcohol)/β-cyclodextrin | PBS solution with different pH levels | Close to 100% elongation | Au nanoparticles | Glucose sensor | [135] |
| Thiolated hyaluronic acid/polyethylene glycol diacrylate/polycaprolactone | Mixed solvent | Storage moduli similar to spinal cord nervous tissue | n/a | Spinal cord regeneration | [136] |
| Polycaprolactone/alginate | Mixed solvent | Strength/elasticity similar to peripheral nerve | n/a | Peripheral nerve regeneration | [137] |
| Polycaprolactone/chondrocyte/alginate | Mixed solvent | Close to 100% elongation | Dexamethasone (Dex) | Cartilage injury | [138] |
| Fibrin/poly(ethylene oxide) | Saline solution | n/a | Chitosan | Peripheral nerve regeneration | [139] |
| PuraMatrixTM Peptide Hydrogel | Acidic solution | n/a | Insulin | Subcutaneous injection | [140] |
| Poly(lactic-co-glycolic acid)/hydroxybutyl chitosan | Mixed solvent | High elastic modulus better than chitosan | n/a | Cartilage tissue engineering, 3D printing | [141] |
| Chitin | Mixed solvent | n/a | Nanoscale bone minerals | Bone regeneration | [142] |
| Chitin | Acidic solution | Storage moduli similar to cellulose nanofibers | Silver nanoparticles | Refractory wound management | [143] |
| Keratin/polyurethane | Acidic solution | Close to 200% elongation | n/a | Tissue engineering gel | [144] |
| Polycaprolactone-polyethylene glycol (PCL-PEG) | Mixed solvent | n/a | Nucleic acids | Axon regeneration | [145] |
Special attention should be devoted to poly(N-isopropyl acrylamide) and its copolymers, since they can form a variety of thermo-responsive hydrogels but can also be electrospun into nanostructured systems [126,127]. Nanofiber/hydrogel composites based on poly(N-isopropyl acrylamide) composites also have been fabricated recently [128]. Combining cellulose nanofibers with poly(N-isopropyl acrylamide) produced enhanced swelling and compressive properties compared to pure poly(N-isopropyl acrylamide) hydrogels [128]. In fact, a unique dual-responsive (pH and temperature) composite hydrogel based on cellulose nanofibril and poly (N-isopropyl acrylamide) was developed for model drug release [129]. A model drug (methylene blue) was released from these nanofiber hydrogels for up to 2 days, triggered by either pH or temperature. Another notable work was based on the development of chitinous nanofiber-based poly(N-isopropyl acrylamide) flexible composite hydrogels for controlling cell adhesion and detachment [130]. Using these types of thermo-responsive stretchable nanofiber hydrogels, the authors could control the growth direction of the cells [130].
As mentioned at the beginning of this review, increasing the surface area of hydrogels with nanofiber networks will have tremendous implications for biomedical applications, particularly for delivering special bioactive macromolecules or drugs in a sustained manner but also facilitating newly emerging nanotechnology-mediated RNA therapies [146].
3. Rheology of Nanofiber Hydrogels
The rheological properties of hydrogels are strongly dependent on the molecular structure of polymeric randomly cross-linked or supramolecular gel networks. Rheological studies of hydrogels in general are important in order to identify limits of their application range. Even though the assessment of the network construction of randomly cross-linked and supramolecular gels seems to be straightforward, there are several variables that affect the gel consistency and response to stimuli through physical and chemical reactions formed by the fractal nature of the bonding. A poor understanding of the rheological properties of hydrogels can cause significant problems for practical applications. For instance, the rheological properties of peptide-based hydrogels for biomedical applications have been extensively reviewed in [147]. To clarify this point, namely the general influence of hydrogel rheological properties on cell function, we can cite a number of notable works that specifically studied these interactions. For instance, extracellular matrix (ECM) hydrogels stimulate constructive tissue remodeling in various tissues. Minimally invasive delivery of such hydrogels to the central nervous system (CNS) must be conducted by injecting a liquid form of the gel at room temperature, while forming hydrogels at body temperature. This liquid–hydrogel transformation is strongly related to the rheological properties of the system and directly impacts tissue cell response at the cavity where injection is introduced [148]. More specifically, the work of Massensini et al. [148] proved that by studying and tuning the rheology (gelation parameters) of biodegradable hydrogels, optimum cell infiltration responses for the injected hydrogels were obtained. Similarly, Du et al. [149] studied the effect of rheological properties on cell growth in peptide-based multicomponent hydrogels.
They concluded that by studying the rheology of supramolecular hydrogels, one can tune the ability of the hydrogel to efficiently store the work of deformation during cell division and transform the cell-laden hydrogel into healthy tissue. Finally, other studies showed that modulus-regulated 3D-cell proliferation in an injectable self-healing hydrogel could lead to optimized cell response and higher therapeutic efficiency for cell therapy [150].
In this section, we reviewed rheological properties of peptide and polypeptide-based hydrogels by summarizing bulk mechanical properties, gelation mechanisms, and the behavior of hydrogels during and after flow. Although these rheological results alone can be informative and, with further modeling, yield a rational understanding, rheological studies should always be considered in conjunction with structural characterization data (e.g., microscopy and scattering) for a better understanding of the observed gel properties. To that end, the authors of the aforementioned studies reviewed rheological properties of peptide and polypeptide-based hydrogels by discussing bulk mechanical properties, gelation mechanisms, and hydrogel behavior during and after flow. They suggested that anytime a new hydrogel is constructed, detailed rheological studies should be accompanied by structural characterization data (e.g., microscopy and scattering) to achieve the best biomedical application outcome.
As indicated, both the flow properties of the solution and mechanical properties of the gels are critical for successful applications. Nevertheless, both properties depend on the polymer concentration, and solely increasing the concentration to improve the gel properties often leads to excessively high fluid viscosities. Thus, it was argued that hydrogels with a mixture of high molecular weight polymer and a lower molecular weight polymer would ensure decoupling the dependence of the two properties (viscosity and mechanical) from the overall concentration [151]. For instance, in the case of 3D or 4D printing of nanofiber gels, optimization of the rheological properties of the matrix is critical for high-fidelity matrix-assisted 3D printing that enables the free-form fabrication of fluidic soft materials. In Figure 8, for instance, to have a printable ink, Laponite (a type of clay) was used to induce shear-thinning behavior. As shown in Figure 8a, the rheological performance at 95 °C of the ink displayed a high viscosity (η ≈ 104 Pa·s) at low shear rate (0.01 s−1), while the viscosity reduced rapidly with an increase in shear rate. When the shear rate was 100 s−1, the viscosity was only about 1 Pa·s. The high viscosity ensures good shape stability after printing, whereas the low viscosity enables the ink to be extruded easily. The strain amplitude sweep of the ink is shown in Figure 8b, in which the storage modulus (G′) was about 5 kPa. It surpassed the loss modulus (G″) at oscillation strain below 30%, demonstrating solid-like behavior up to this strain level; at strain levels higher than 30%, G″ exceeded G′, and the ink changed from a solid-like state to a liquid-like state. Conducting continuous step strain measurements on the nanofiber hydrogels indicated that the ink was in solid state at 1% oscillation strain; after the oscillation strain was raised to 300%, it changed rapidly to a liquid state, and hence, the sol–gel transition time was sufficiently short (Figure 8c). This fast and repeatable transformation ensures good 3D or 4D printing of nanofiber hydrogels [152]. These sol–gel and gel–sol transformations of 4D ink were repeatable. Additionally, as shown in Figure 8d–g, the hydrogel ink could be solidified rapidly at ambient temperature after printing in a repeatable manner.
Figure 8.
Rheological behaviors of the agarose/AM/Laponite 4D ink at 95 °C. Flow rheology pattern showing viscosity against shear rate (a). Oscillatory rheology pattern showing shear storage modulus (G′) and shear loss modulus (G″) evolution of inks used, increasing shear strain (b). Oscillatory rheology pattern showing modulus evolution of inks between 1% and 300% strain (c). The storage modulus variation of 4D ink during cooling (d). Four-dimensional printing product printed by 4D ink: a whale-like hydrogel was used as a model schematic for the 4D transition process (e), the softening and hardening cycles of an octopus-like gel (f), the storage modulus (e) variation of 4D gel during heating and cooling cycles measured by rheology (g). Reproduced from [152] with permission.
An optimum thermo-rheological characterization of different nanofiber hydrogels used in 3D printing is crucial to identify the best processing conditions and to extend the understanding framework to 4D printing for biomedicine. Consequently, the rheological features of printable nanofiber hydrogels, as summarized in Table 3, must be carefully monitored during processing. Although some publications demonstrated good printability of injection properties, some failed to report full thermo-rheological characteristics, as shown in Table 3. It should be noted that the nanofiber hydrogels should possess shear-thinning properties, allowing smooth extrusion from the nozzle under shear, and the extruded hydrogel must be strong enough to maintain its shape and withstand the weight of additional layers that need to be deposited.
Table 3.
Rheological studies on nanofiber hydrogels are extremely important for effective 3D and 4D printing applications. Oscillatory shear measurements as well as standard temperature dependence measurements are needed for thixotropic gel printing.
| Nanofiber Hydrogel | Oscillatory Shear Measurement | Standard Shear Measurement | Temperature Dependence Measurement | Production Method | Targeted Application | Reference |
|---|---|---|---|---|---|---|
| Agarose/acrylamide | Yes | Yes | Yes | 4D printing | Scaffolds, soft medical robots | [152] |
| Poly(vinyl alcohol)/β-cyclodextrin | No | Yes | No | Syringe injection | Glucose sensor | [135] |
| Cellulose nanofiber | Yes | Yes | no | 3D printing | Scaffold, drug release | [153] |
| Biphenyl-tripeptide | Yes | Yes | No | Solution gelation | Regenerative medicine | [154] |
| Spider silk | Yes | Yes | No | Solution gelation | Scaffold, drug release | [155] |
| Liposome-Peptide Nanofiber | Yes | Yes | No | Solution gelation | Regenerative medicine | [156] |
| Betamethasone phosphate | No | Yes | No | Solution gelation | Tissue injection | [157] |
| Collagen type I | Yes | Yes | Yes | Self-assembly | Regenerative medicine and drug delivery | [158] |
| Chitosan/acrylamide | Yes | Yes | No | Radical polymerization | Implants | [159] |
| Cellulose/polyvinyl alcohol/sodium alginate | No | Yes | No | 3D Printing | Scaffold | [160] |
| Peptide/gelatin | Yes | Yes | Yes | 3D Printing | Vascularization | [161] |
| Bacterial cellulose/silk | Yes | Yes | Yes | 3D Printing | Soft tissue repair | [162] |
| Bacterial cellulose/silk | Yes | Yes | No | 3D Printing | Lung tissue regeneration | [163] |
| Hydroxybutyl chitosan/polycaprolactone | Yes | Yes | No | 3D Printing | Cartilage differentiation | [141] |
| Alginate/gelatin/carbon nanofibers | Yes | Yes | No | 3D Printing | Cardiac and neuronal tissue engineering | [164] |
| Alginate/polylactic acid | Yes | Yes | No | 3D Printing | Cardiovascular stents | [165] |
| Poly(lactide-co-caprolactone)/collagen | No | Yes | No | 3D Printing | Nerve regeneration | [166] |
| Alginate/gelatin/bacterial nanocellulose | Yes | Yes | Yes | 3D Printing | Neural tissue engineering | [167] |
As we summarized in this section, improved understanding and reporting of rheological properties will also translate into better theoretical explanations of how the gel components affect rheological performance along with better prediction and design of the next generation of printable biomedical nanofiber hydrogels or nanofiber/hydrogel composites [168].
4. Advances in Sustained Drug Release from Nanofiber Hydrogels
4.1. A Brief Look at Controlled Drug Release
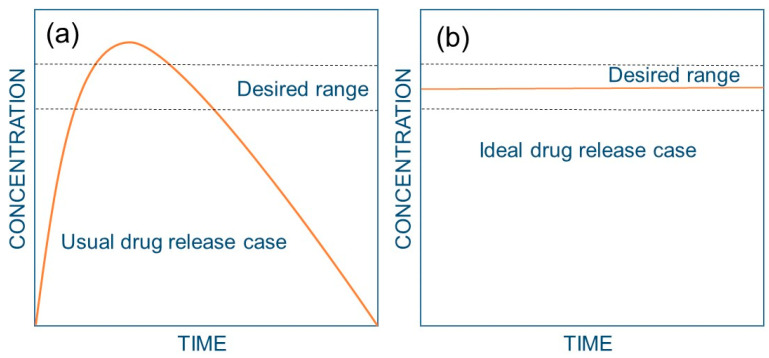
In order to have an efficient therapeutic effect, a drug should have high affinity and selectivity for the intended biological function. For instance, pure proteins or protein complexes should target certain cells, penetrate the membrane, and reach a sufficient concentration at that specific site. To achieve this, the drug needs to be released from the delivery system, transported from the site of application to the site of action, take part in biological reactions, and finally be eliminated via metabolic pathways. It is now generally accepted that rapidly released drugs, whether taken orally or administered in other ways, result in peaks and valleys in drug levels (Figure 9a), which may cause inefficient drug administration and, possibly, undesirable side effects [169]. Sustained drug delivery technology signifies one of the most rapidly evolving areas in human health care. These delivery systems (Figure 9b) have numerous advantages compared to conventional dosage forms, including better efficacy, reduced toxicity, and improved patient compliance and convenience. Controlled release often requires synthetic polymers as carriers for the drugs some of which were cited in [170]. A comprehensive study of drug release from hydrogels in general was conducted by Lee and Kim [171] in 1991. Therein, they conducted detailed studies on the sustained release of both hydrophilic and lipophilic model drugs. An earlier study by Lee [172] presented a detailed analysis of water-soluble drugs from initially dehydrated hydrogel matrices that generally involved the simultaneous absorption of water and desorption of drug via a swelling-controlled diffusion mechanism. The author developed a method to model constant-rate drug delivery from glassy hydrogel matrices via an immobilized non-uniform drug concentration distribution.
Figure 9.
Plasma drug levels as a function of time for the usual and ideal case for drug administration. (a) Usual case; i.e., injection or pill: the plasma drug level reaches a peak as the drug enters the bloodstream. However, the drug quickly diminishes and falls to a low or non-existent level, dictating repeated drug administration to enable re-entry into the therapeutic range, resulting in a saw-tooth pattern of plasma drug levels. When the drug is above the desired range, it may cause unwanted side effects; when the drug is below the desired range, it no longer has a therapeutic effect. (b) Ideal case: a controlled-release system causes the drug level to be within the desired range for specified periods (days or months).
Very recently, a new mathematical approach to predict the actual drug release from hydrogels was published [173], taking into account hydrogel swelling and drug diffusion within the hydrogel before release. An exclusive review article on pulsed and/or pulsatile release from hydrogels was published by Kikuchi and Okano [174]. The authors argued that hydrogels could be the ideal media for developing new drug delivery devices to achieve pulsed delivery of a certain amount of a drug in order to mimic the function of living systems while minimizing undesired side effects.
Table 4 lists a number of studies in which various stimuli were utilized. The table also shows a number of review articles that specifically focused on light, pH, and electro-induced drug release from hydrogels. In many cases listed in the table, cancer drugs such as doxorubicin (chemotherapy) were extensively used as model drugs.
Table 4.
A summary of stimuli responsive drug release studies of hydrogels in general.
| Type of Study | Hydrogel | Model Drugs | Release Mechanism | Reference |
|---|---|---|---|---|
| Experimental | Poly(L-lactide)-co-polyethyleneglycol-co-poly(L-lactide) dimethacrylates | Doxorubicin and tetracycline | pH-induced | [175] |
| Experimental | Poly(N-isopropylacrylamide) | Bovine serum albumin | Thermal-induced | [176] |
| Experimental | low molecular weight gelators (LMWGs) | 6-aminoquinoline (6-AQ) | Enzyme-induced | [177] |
| Experimental | Poly(N-isopropylacrylamide) | Vitamin B12 and methylene blue | Magnetic-induced | [178] |
| Experimental | Dextran | Dexamethasone and indomethacin | Electro-induced | [179] |
| Experimental | Polyacrylic acid/poly(vinyl alcohol) | Indomethacin | Electro-induced | [180] |
| Review | Several | Several | Electro-induced | [181] |
| Review | Several | Several | Electro-induced | [182] |
| Experimental | Low molecular weight gelators (LMWGs) | Doxorubicin | Light-induced | [183] |
| Experimental | Low molecular weight gelators (LMWGs) | Naproxen, diclofenac, ciprofloxacin, actinomycin D, cytochrome | Light-induced | [184] |
| Review | Several | Several | Light-induced | [185] |
| Experimental | Poly([6-bromo-7-hydroxycoumarin-4-yl]methyl methacrylate) | None | Light-induced | [186] |
| Experimental | Poly(N-isopropylacrylamide)/ poly(ethyl acrylate) | Daidzein | pH-induced | [187] |
| Experimental | Carboxymethyl chitosan/poloxamer | Nepafenac | pH-induced | [188] |
| Theoretical/experimental | Polyelectrolyte hydrogels | None | pH-induced | [189] |
| Review | Several | Several | pH-induced | [190] |
| Experimental | Gelatin | Vitamin B12 | Magnetic-induced | [191] |
| Experimental | Chitosan | Curcumin | Magnetic-induced | [192] |
| Experimental | Chitosan | Adriamycin, rifampicin | Magnetic-induced | [193] |
| Experimental | Poly(acrylamide–gelatin) | Doxorubicin | Magnetic-induced | [194] |
| Experimental | Experimental | FITC-dextran | Magnetic-induced | [195] |
| Review | Several | Several | pH-induced | [196] |
| Review | Several | Several | Electro-induced | [197] |
As can be seen in Table 4, drug release from hydrogels is generally triggered by various stimuli such as pH, magnetic fields, electricity, and light. Some of these stimuli, such as light and magnetic triggering, were also used together or in tandem. Some of the studies presented in Table 4 did not use or include any model drugs but investigated the response of the hydrogel to external stimuli in terms of changes in their viscoelastic properties.
4.2. Sustained Drug Release from Nanofiber Hydrogels
Nanofiber hydrogels can be fabricated by using various approaches, such as electrospinning certain polymers and swelling them in water under a controlled environment [198], embedding nanofibrillar materials (either natural or synthesized, including electrospinning) into hydrogels made by common procedures [199], or swelling nanofiber networks of natural polymers such as cellulose and chitin or chitosan in certain coagulation systems [200]. A recent review article primarily focused on recent advances in polyvinyl alcohol-polysaccharide based hydrogels and electrospun nanofibers in biomedical applications [201]. Therein, the authors argued that these systems can meet many requirements of wound management membranes in terms of surface area to volume ratio, high porosity, tolerable permeability, wound-exudates absorption capacity, architecture similarity with the extracellular matrix, and sustained release capabilities. They argued that several limitations related to scaling up electrospinning to industrial production levels and issues related to nanofiber quality reproducibility will still need attention before significant commercialization of nanofiber hydrogels made from polyvinyl alcohol and polysaccharide combinations.
A notable work was recently conducted by modulating sustained drug release from nanocellulose hydrogels by adjusting the inner geometry of implantable capsules [202]. The authors 3D printed capsules using PLA and inserted drug-loaded nanocellulose hydrogels inside them, so that the PLA would sustain the release from the nanofiber hydrogel as shown in Figure 10.
Figure 10.
Computer-aided designs and 3D printed PLA capsules (left). The tubes were filled with a hydrogel formulation that can sustain drug release after 3 weeks (right). The drugs were metoprolol (METO) and nadolol (NAD); small, tube, and large denote different PLA tube sizes. Reproduced from [202] with permission.
An important work on the fabrication and application of nanofiber hydrogels is worth mentioning here [203]. Therein, the authors reviewed the design and synthesis of nanofibers, as well as their self-assembly into supramolecular hydrogels that originate from small bioactive molecules. They demonstrated their efficient utility in applications such as molecular recognition, wound healing, toxin removal, and drug release. Their review article also described the use of enzymes as switches to modulate the self-assembly of small molecules for nanofiber generation and subsequent hydrogel formation. As mentioned earlier, their review was considered to be a highly motivating work for recent advances in supramolecular hydrogels for sustained drug release [203]. Table 5 lists a number of published sustained drug-release studies conducted by using self-gelling or composite systems carrying different drugs tailored for biomedical applications ranging from cancer treatment to wound healing. Interestingly, the maximum release period for all the systems presented in Table 5 was at least 1 day and in many cases extended into a week. This shows that most nanofiber hydrogels are designed for sustained release, indeed, and they can prolong the release for up to 2 months [204]. The table also revealed that peptides, silk (natural proteins), and cellulose and chitosan (i.e., polysaccharides) are highly popular as nanofiber materials. For composite systems, alginates and polyvinyl alcohol were actively used as the encapsulating gel.
Table 5.
Sustained drug release studies of nanofiber hydrogels.
| Nanofiber Material | Gelation Process | Composite System | Composite Components | Type of Drug | Release Medium | Maximum Release Period (Day) | Reference |
|---|---|---|---|---|---|---|---|
| Chitosan-graft-poly(N-isopropylacrylamide) (CTS-g-PNIPAAm) | Swelling | Yes | PEO | BSA | HCl-KCl pH 2.2; PBS, pH 7.4 | 2.5 | [203] |
| Polydopamine-intercalated silicate/ | Gelling in solution | Yes | Chitosan/gelatin | Tetracycline hydrochloride | PBS, pH 7.4 | 5–6 | [205] |
| Polyaniline | Gelling in solution | Yes | Polyacrylamide | Amoxicillin | PBS, pH 7.4 | 0.21 | [206] |
| Silk | Gelling in solution | No | n/a | Doxorubicin | PBS pH 4.5, 6.0, or 7.4 | 21–56 | [204] |
| Silk | Gelling in solution/thermal | No | n/a | Desferrioxamine | PBS, pH 7.4 | 40 | [207] |
| Peptide | Gelling/self-assembling in solution | No | n/a | Timolol Maleate | PBS, pH 7.4 | 1 | [208] |
| Peptide | Gelling/self-assembling in solution | No | n/a | Human antibodies | PBS, pH 7.4 | 12 | [209] |
| Peptide | Gelling/self-assembling in solution | No | n/a | Recombinant receptor activator of NF-kB ligand | PBS, pH 7.4 | 4 | [210] |
| Peptide amphiphiles | Gelling/self-assembling in solution | No | n/a | Doxorubicin | PBS, pH 7.4 | 7 | [211] |
| Coiled-coil protein, Q, | Gelling/self-assembling in solution | No | n/a | Curcumin | DMSO buffer | 18 | [212] |
| Peptide | Gelling/self-assembling in solution | No | n/a | Lysozyme, trypsin inhibitor, BSA, IgG | PBS, pH 7.4 | 2–3 | [213] |
| Peptide (RADA 16) | Gelling/self-assembling in solution | No | n/a | Pindolol, Quinine and Timolol | PBS, pH 7.4 | 7 | [214] |
| Cellulose | Cross-linking in solution | Yes | Polyvinyl alcohol | Cisplatin | PBS, pH 7.4 | 18 | [215] |
| Silk | Gelling in solution/thermal | Yes | Concentrated mesenchymal stem cells | Various growth factors | PBS, pH 7.4 | 2 | [216] |
| d-Amino Acid Dipeptide | Gelling/self-assembling in solution | No | n/a | Folic acid | HEPES buffer | 0.2–1 | [217] |
| Bacterial cellulose | Cross-linking in solution | Yes | Sodium alginate | Ibuprofen | PBS, pH 7.4 and other pHs | 1.5 | [218] |
| Cellulose | Commercial | No | n/a | FITC-dextrans, d-(+)-trehalose dehydrate, Metronidazole, Nadolol, Ketoprofen, Lysozyme | PBS, pH 7.4 | 6 | [219] |
| Peptide hydrosol | Gelling/self-assembling in solution | No | n/a | Insulin | PBS, pH 7.4 | 0.5 | [140] |
| Fibrin | Gelling in solution | Yes | Poly(DL-lactic-co-glycolic acid) | Albumin from bovine Serum, FITC conjugate | PBS, pH 7.4 | 83 | [220] |
| Polycaprolactone | Cross-linking in solution | Yes | Alginate | Adenosine triphosphate | PBS, pH 7.4 | 12 | [221] |
| Peptide | Gelling/self-assembling in solution | No | n/a | Olsalazine | Buffer, pH 5 | n/a | [222] |
| Poly(vinyl alcohol-co-ethylene) | Cross-linking in solution | Yes | Alginate | Vancomycin | PBS, pH 7.4 | 5 | [223] |
| Peptide amphiphile | Gelling/self-assembling in solution | No | n/a | Prodan | PBS, pH 7.4-DMSO | 40 | [224] |
| Polydopamine | Gelling/self-assembling in solution | Yes | Alginate | Bortezomib | PBS, pH 5–7.4 | 7 | [225] |
| Peptide | Gelling/self-assembling in solution | No | n/a | BSA-linked cisplatin | PBS, pH 7.4 | 1 | [226] |
| N-(9-fluorenylmethoxycarbonyl)-L-phenylalanine | Gelling/self-assembling in solution | Yes | Puerarin | Berberine hydrochloride | PBS, pH 5.8 | 1.5 | [227] |
| Betamethasone phosphate | Gelling/self-assembling in solution | No | No | PDL1 antibody | PBS, pH 7.4 | 7 | [157] |
| Polyvinylpyrrolidone | Gelling/self-assembling in solution | No | No | Hydroxycinnamic acids | PBS, pH 7.4 | 9 | [228] |
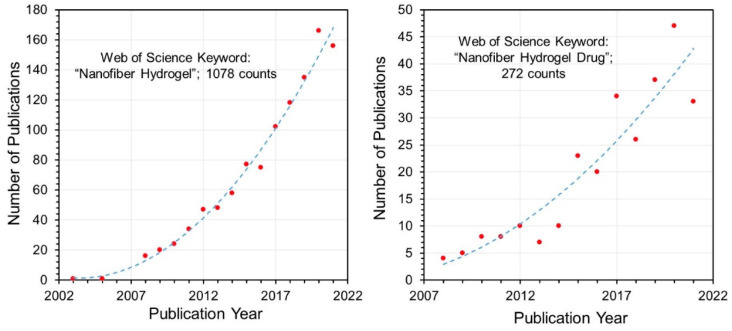
Using the Web of ScienceTM database with key words such as “Nanofiber Hydrogel” and “Nanofiber Hydrogel Drug”, it was possible to analyze the distribution of publications over the years from 2008 onwards. Figure 11 shows a polynomial increase in the number of publications on nanofiber hydrogel materials. About 25% of the publications use “drug” or “drug release” in their titles, indicating that a significant portion of nanofiber hydrogel research is focused on drug release.
Figure 11.
Web of Science keyword search results on “nanofiber hydrogel” and “nanofiber hydrogel drug” searches conducted on 18 October 2021. Number of publications as a function of publication year are plotted for both keywords. Both trends show a second-order polynomial increase.
Finally in closing, nanofiber networks can also be fabricated in core-shell format, and these fibers can be very effective as controlled drug delivery vehicles [229]. Furthermore, nanofibers can be fabricated as 3D elastic scaffolds with self-fitting capability and tailorable gradient formation from inorganic materials [230] that can be used for hard tissue generation. The combination of this new generation of electrospun materials with hydrogels is expected to occur soon, and such new nanofiber hydrogels will deliver new advances in biomedical materials science.
5. Conclusions
In this review, we showed that the majority of the published works on nanofiber hydrogels could maintain exceptional drug release rates exceeding a few days and even extending into months, which is an extremely difficult task to achieve in the absence of nanofibers. This review also established that some publications still lack vigilant rheological studies on nanofiber hydrogels, although the rheological properties of hydrogels can affect cell function, mechano-transduction, and cellular interactions such as growth, migration, adhesion, proliferation, differentiation, and morphology. This can even be more precarious for 3D or 4D printable gel systems while maintaining efficient drug delivery rates. Polymer nanofibers have extraordinarily high surface areas. This increases the area over which materials can be transported into and out of a system via diffusion and active transport. Similarly, since hydrogels retain a degree of flexibility very similar to natural tissue, due to their substantial water content, hydrogels made from natural or biodegradable macromolecular systems can even be injectable and kept in syringes. Hydrogel transport properties can be easily modified and tailored and can encapsulate nanofiber networks. It has been shown that certain nanofiber networks can be transformed into hydrogels directly, without the need for a hydrogel inclusion. This review compiled such recent advances in the fabrication and application of biomedical nanofiber hydrogels, with special attention to drug release performance. Most of the drug release studies and recent advances have focused on designing self-gelling nanofiber systems made from peptides or other natural proteins loaded with cancer drugs. Next in line are the polysaccharide nanofiber gels that are being developed and studied for drug release. Thirdly, electrospun biodegradable polymer networks embedded in polysaccharide-based hydrogels have been exceedingly popular. There appears to be no clear advantage of one system over the other. However, this will be determined by the practicality of the fabrication method, cost-effectiveness of the materials used, the longevity and controllability of the drug release action, and protection of the drug functionality over a long period within the nanofiber hydrogels.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wichterle O., Lím D. Hydrophilic Gels for Biological Use. Nat. Cell Biol. 1960;185:117–118. doi: 10.1038/185117a0. [DOI] [Google Scholar]
- 2.Gačanin J., Synatschke C.V., Weil T. Biomedical applications of DNA-based hydrogels. Adv. Funct. Mater. 2020;30:1906253. doi: 10.1002/adfm.201906253. [DOI] [Google Scholar]
- 3.Qi H., Ghodousi M., Du Y., Grun C., Bae H., Yin P., Khademhosseini A. DNA-directed self-assembly of shape-controlled hydrogels. Nat. Commun. 2013;4:1–10. doi: 10.1038/ncomms3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang H., Pan V., Vivek S., Weeks E.R., Ke Y. Programmable DNA Hydrogels Assembled from Multidomain DNA Strands. Chem. Bio. Chem. 2016;17:1156–1162. doi: 10.1002/cbic.201500686. [DOI] [PubMed] [Google Scholar]
- 5.Xiong X., Wu C., Zhou C., Zhu G., Chen Z., Tan W. Responsive DNA-Based Hydrogels and Their Applications. Macromol. Rapid Commun. 2013;34:1271–1283. doi: 10.1002/marc.201300411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nöll T., Schönherr H., Wesner D., Schopferer M., Paululat T., Nöll G. Construction of Three-Dimensional DNA Hydrogels from Linear Building Blocks. Angew. Chem. 2014;126:8468–8472. doi: 10.1002/ange.201402497. [DOI] [PubMed] [Google Scholar]
- 7.Bush J., Hu C.-H., Veneziano R. Mechanical Properties of DNA Hydrogels: Towards Highly Programmable Biomaterials. Appl. Sci. 2021;11:1885. doi: 10.3390/app11041885. [DOI] [Google Scholar]
- 8.Sun J.-Y., Zhao X., Illeperuma W.R.K., Chaudhuri O., Oh K.H., Mooney D., Vlassak J.J., Suo Z. Highly stretchable and tough hydrogels. Nature. 2012;489:133–136. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez R.M., Orive G., Murua A., Pedraz J.L. Microcapsules and microcarriers for in situ cell delivery. Adv. Drug Deliv. Rev. 2010;62:711–730. doi: 10.1016/j.addr.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Maldonado-Codina C., Efron N. Impact of Manufacturing Technology and Material Composition on the Clinical Performance of Hydrogel Lenses. Optom. Vis. Sci. 2004;81:442–454. doi: 10.1097/01.opx.0000135103.94039.40. [DOI] [PubMed] [Google Scholar]
- 11.Sarker B., Rompf J., Silva R., Lang N., Detsch R., Kaschta J., Fabry B., Boccaccini A.R. Alginate-based hydrogels with improved adhesive properties for cell encapsulation. Int. J. Biol. Macromol. 2015;78:72–78. doi: 10.1016/j.ijbiomac.2015.03.061. [DOI] [PubMed] [Google Scholar]
- 12.Reakasame S., Boccaccini A.R. Oxidized Alginate-Based Hydrogels for Tissue Engineering Applications: A Review. Biomacromolecules. 2018;19:3–21. doi: 10.1021/acs.biomac.7b01331. [DOI] [PubMed] [Google Scholar]
- 13.García-Astrain C., Avérous L. Synthesis and evaluation of functional alginate hydrogels based on click chemistry for drug delivery applications. Carbohydr. Polym. 2018;190:271–280. doi: 10.1016/j.carbpol.2018.02.086. [DOI] [PubMed] [Google Scholar]
- 14.Jeon O., Alt D.S., Ahmed S.M., Alsberg E. The effect of oxidation on the degradation of photocrosslinkable alginate hydrogels. Biomaterials. 2012;33:3503–3514. doi: 10.1016/j.biomaterials.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Augst A.D., Kong H.J., Mooney D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006;6:623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 16.Bhattarai N., Gunn J., Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010;62:83–99. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Ahmadi F., Oveisi Z., Samani S.M., Amoozgar Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015;10:1–16. [PMC free article] [PubMed] [Google Scholar]
- 18.Pellá M., Lima-Tenório M.K., Tenório-Neto E., Guilherme M.R., Muniz E.C., Rubira A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018;196:233–245. doi: 10.1016/j.carbpol.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 19.Shariatinia Z., Jalali A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018;115:194–220. doi: 10.1016/j.ijbiomac.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Tao L., Shuxi L., Wei Y. Synthesis of Multiresponsive and Dynamic Chitosan-Based Hydrogels for Controlled Release of Bioactive Molecules. Biomacromolecules. 2011;12:2894–2901. doi: 10.1021/bm200423f. [DOI] [PubMed] [Google Scholar]
- 21.Jonker A.M., Löwik D.W.P.M., van Hest J.C.M. Peptide- and Protein-Based Hydrogels. Chem. Mater. 2012;24:759–773. doi: 10.1021/cm202640w. [DOI] [Google Scholar]
- 22.Sui Z., King W.J., Murphy W.L. Protein-Based Hydrogels with Tunable Dynamic Responses. Adv. Funct. Mater. 2008;18:1824–1831. doi: 10.1002/adfm.200701288. [DOI] [Google Scholar]
- 23.Kapoor S., Kundu S.C. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016;31:17–32. doi: 10.1016/j.actbio.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 24.Katyal P., Mahmoudinobar F., Montclare J.K. Recent trends in peptide and protein-based hydrogels. Curr. Opin. Struct. Biol. 2020;63:97–105. doi: 10.1016/j.sbi.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Abaee A., Mohammadian M., Jafari S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017;70:69–81. doi: 10.1016/j.tifs.2017.10.011. [DOI] [Google Scholar]
- 26.Jaipan P., Nguyen A., Narayan R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017;7:416–426. doi: 10.1557/mrc.2017.92. [DOI] [Google Scholar]
- 27.Wang X., Ao Q., Tian X., Fan J., Tong H., Hou W., Bai S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymer. 2017;9:401. doi: 10.3390/polym9090401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Einerson N.J., Stevens K.R., Kao W.J. Synthesis and physicochemical analysis of gelatin-based hydrogels for drug carrier matrices. Biomaterials. 2003;24:509–523. doi: 10.1016/S0142-9612(02)00369-1. [DOI] [PubMed] [Google Scholar]
- 29.Crescenzi V., Francescangeli A., Taglienti A. New Gelatin-Based Hydrogels via Enzymatic Networking. Biomacromolecules. 2002;3:1384–1391. doi: 10.1021/bm025657m. [DOI] [PubMed] [Google Scholar]
- 30.Kang J.I., Park K.M. Advances in gelatin-based hydrogels for wound management. J. Mater. Chem. B. 2021;9:1503–1520. doi: 10.1039/D0TB02582H. [DOI] [PubMed] [Google Scholar]
- 31.Chang C., Zhang L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011;84:40–53. doi: 10.1016/j.carbpol.2010.12.023. [DOI] [Google Scholar]
- 32.Sannino A., Demitri C., Madaghiele M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials. 2009;2:353–373. doi: 10.3390/ma2020353. [DOI] [Google Scholar]
- 33.Fu L.-H., Qi C., Ma M.-G., Wan P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B. 2019;7:1541–1562. doi: 10.1039/C8TB02331J. [DOI] [PubMed] [Google Scholar]
- 34.Yang X., Liu G., Peng L., Guo J., Tao L., Yuan J., Chang C., Wei Y., Zhang L. Highly Efficient Self-Healable and Dual Responsive Cellulose-Based Hydrogels for Controlled Release and 3D Cell Culture. Adv. Funct. Mater. 2017;27:1703174. doi: 10.1002/adfm.201703174. [DOI] [Google Scholar]
- 35.Bashari A., Shirvan A.R., Shakeri M. Cellulose-based hydrogels for personal care products. Polym. Adv. Technol. 2018;29:2853–2867. doi: 10.1002/pat.4290. [DOI] [Google Scholar]
- 36.Thakur V.K., Thakur M.K. Recent advances in green hydrogels from lignin: A review. Int. J. Biol. Macromol. 2015;72:834–847. doi: 10.1016/j.ijbiomac.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 37.Kabir S.M.F., Sikdar P.P., Haque B., Bhuiyan M.A.R., Ali A., Islam M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018;7:153–174. doi: 10.1007/s40204-018-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du H., Liu W., Zhang M., Si C., Zhang X., Li B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019;209:130–144. doi: 10.1016/j.carbpol.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Ismail H., Irani M., Ahmad Z. Starch-Based Hydrogels: Present Status and Applications. Int. J. Polym. Mater. 2013;62:411–420. doi: 10.1080/00914037.2012.719141. [DOI] [Google Scholar]
- 40.Xiao C. Current advances of chemical and physical starch-based hydrogels. Starch-Starke. 2013;65:82–88. doi: 10.1002/star.201200113. [DOI] [Google Scholar]
- 41.Ali A.E.-H., AlArifi A. Characterization and in vitro evaluation of starch based hydrogels as carriers for colon specific drug delivery systems. Carbohydr. Polym. 2009;78:725–730. doi: 10.1016/j.carbpol.2009.06.009. [DOI] [Google Scholar]
- 42.Zhang L.-M., Yang C., Yan L. Perspectives on: Strategies to Fabricate Starch-based Hydrogels with Potential Biomedical Applications. J. Bioact. Compat. Polym. 2005;20:297–314. doi: 10.1177/0883911505053382. [DOI] [Google Scholar]
- 43.Sun T., Zhu C., Xu J. Multiple stimuli-responsive selenium-functionalized biodegradable starch-based hydrogels. Soft Matter. 2018;14:921–926. doi: 10.1039/C7SM02137B. [DOI] [PubMed] [Google Scholar]
- 44.Noè C., Tonda-Turo C., Chiappone A., Sangermano M., Hakkarainen M. Light Processable Starch Hydrogels. Polymers. 2020;12:1359. doi: 10.3390/polym12061359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elvira C., Mano J., Román J.S., Reis R.L. Starch-based biodegradable hydrogels with potential biomedical applications as drug delivery systems. Biomaterials. 2002;23:1955–1966. doi: 10.1016/S0142-9612(01)00322-2. [DOI] [PubMed] [Google Scholar]
- 46.Biduski B., Silva W.M.F., Colussi R., Halal S., Lim L.-T., Dias Álvaro R.G., Zavareze E.D.R. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018;113:443–449. doi: 10.1016/j.ijbiomac.2018.02.144. [DOI] [PubMed] [Google Scholar]
- 47.Daoud-Mahammed S., Grossiord J.L., Bergua T., Amiel C., Couvreur P., Gref R. Self-assembling cyclodextrin based hydrogels for the sustained delivery of hydrophobic drugs. J. Biomed. Mater. Res. Part A. 2008;86A:736–748. doi: 10.1002/jbm.a.31674. [DOI] [PubMed] [Google Scholar]
- 48.Pinho E., Grootveld M., Soares G., Henriques M. Cyclodextrin-based hydrogels toward improved wound dressings. Crit. Rev. Biotechnol. 2013;34:328–337. doi: 10.3109/07388551.2013.794413. [DOI] [PubMed] [Google Scholar]
- 49.Rey-Rico A., Cucchiarini M. Supramolecular Cyclodextrin-Based Hydrogels for Controlled Gene Delivery. Polymers. 2019;11:514. doi: 10.3390/polym11030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan S., Ladewig K., Fu Q., Blencowe A., Qiao G.G. Cyclodextrin-Based Supramolecular Assemblies and Hydrogels: Recent Advances and Future Perspectives. Macromol. Rapid Commun. 2014;35:1166–1184. doi: 10.1002/marc.201400080. [DOI] [PubMed] [Google Scholar]
- 51.Liu G., Yuan Q., Hollett G., Zhao W., Kang Y., Wu J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018;9:3436–3449. doi: 10.1039/C8PY00730F. [DOI] [Google Scholar]
- 52.Zhang Y.M., Liu Y.H., Liu Y. Cyclodextrin-based multistimuli-responsive supramolecular assemblies and their biological functions. Adv. Mater. 2020;32:1806158. doi: 10.1002/adma.201806158. [DOI] [PubMed] [Google Scholar]
- 53.Domiński A., Konieczny T., Kurcok P. α-Cyclodextrin-Based Polypseudorotaxane Hydrogels. Materials. 2019;13:133. doi: 10.3390/ma13010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayer I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules. 2020;25:2649. doi: 10.3390/molecules25112649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo Y., Kirker K.R., Prestwich G.D. Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. J. Control. Release. 2000;69:169–184. doi: 10.1016/S0168-3659(00)00300-X. [DOI] [PubMed] [Google Scholar]
- 56.Burdick J.A., Prestwich G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011;23:H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Highley C.B., Prestwich G.D., Burdick J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016;40:35–40. doi: 10.1016/j.copbio.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Walimbe T., Panitch A., Sivasankar P.M. A Review of Hyaluronic Acid and Hyaluronic Acid-based Hydrogels for Vocal Fold Tissue Engineering. J. Voice. 2017;31:416–423. doi: 10.1016/j.jvoice.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins M.N., Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013;92:1262–1279. doi: 10.1016/j.carbpol.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 60.Trombino S., Servidio C., Curcio F., Cassano R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics. 2019;11:407. doi: 10.3390/pharmaceutics11080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmadian E., Dizaj S.M., Eftekhari A., Dalir E., Vahedi P., Hasanzadeh A., Samiei M. The Potential Applications of Hyaluronic Acid Hydrogels in Biomedicine. Drug Res. 2020;70:6–11. doi: 10.1055/a-0991-7585. [DOI] [PubMed] [Google Scholar]
- 62.Zhou L., Jiao X., Liu S., Hao M., Cheng S., Zhang P., Wen Y. Functional DNA-based hydrogel intelligent materials for biomedical applications. J. Mater. Chem. B. 2020;8:1991–2009. doi: 10.1039/C9TB02716E. [DOI] [PubMed] [Google Scholar]
- 63.Kahn J.S., Hu Y., Willner I. Stimuli-Responsive DNA-Based Hydrogels: From Basic Principles to Applications. Accounts Chem. Res. 2017;50:680–690. doi: 10.1021/acs.accounts.6b00542. [DOI] [PubMed] [Google Scholar]
- 64.Lee J.B., Peng S., Yang D., Roh Y.H., Funabashi H., Park N., Rice E.J., Chen L., Long R., Wu M., et al. A mechanical metamaterial made from a DNA hydrogel. Nat. Nanotechnol. 2012;7:816–820. doi: 10.1038/nnano.2012.211. [DOI] [PubMed] [Google Scholar]
- 65.Cheng E., Xing Y., Chen P., Yang Y., Sun Y., Zhou D., Xu L., Fan Q., Liu D. A pH-Triggered, Fast-Responding DNA Hydrogel. Angew. Chem. Int. Ed. 2009;48:7660–7663. doi: 10.1002/anie.200902538. [DOI] [PubMed] [Google Scholar]
- 66.Li F., Tang J., Geng J., Luo D., Yang D. Polymeric DNA hydrogel: Design, synthesis and applications. Prog. Polym. Sci. 2019;98:101163. doi: 10.1016/j.progpolymsci.2019.101163. [DOI] [Google Scholar]
- 67.Shahbazi M.A., Bauleth-Ramos T., Santos H.A. DNA hydrogel assemblies: Bridging synthesis principles to biomedical applications. Adv. Ther. 2018;1:1800042. doi: 10.1002/adtp.201800042. [DOI] [Google Scholar]
- 68.Xu N., Ma N., Yang X., Ling G., Yu J., Zhang P. Preparation of intelligent DNA hydrogel and its applications in biosensing. Eur. Polym. J. 2020;137:109951. doi: 10.1016/j.eurpolymj.2020.109951. [DOI] [Google Scholar]
- 69.Keys K.B., Andreopoulos F.M., Peppas N.A. Poly(ethylene glycol) Star Polymer Hydrogels. Macromolecules. 1998;31:8149–8156. doi: 10.1021/ma980999z. [DOI] [Google Scholar]
- 70.Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hutson C.B., Nichol J.W., Aubin H., Bae H., Yamanlar S., Al-Haque M.S., Koshy S., Khademhosseini A. Synthesis and Characterization of Tunable Poly(Ethylene Glycol): Gelatin Methacrylate Composite Hydrogels. Tissue Eng. Part A. 2011;17:1713–1723. doi: 10.1089/ten.tea.2010.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rao K.S.V.K., Ha C.-S. pH Sensitive hydrogels based on acryl amides and their swelling and diffusion characteristics with drug delivery behavior. Polym. Bull. 2009;62:167–181. doi: 10.1007/s00289-008-0011-1. [DOI] [Google Scholar]
- 73.Athawale V.D., Lele V. Recent Trends in Hydrogels Based on Starchgraft-Acrylic Acid: A Review. Starch-Starke. 2001;53:7–13. doi: 10.1002/1521-379X(200101)53:1<7::AID-STAR7>3.0.CO;2-Q. [DOI] [Google Scholar]
- 74.Kim S.J., Shin S.R., Kim N.G., Kim S.I. Swelling Behavior of Semi-Interpenetrating Polymer Network Hydrogels Based on Chitosan and Poly (acryl amide) J. Macromol. Sci. Part A Pure Appl. Chem. 2005;42:1073–1083. doi: 10.1081/MA-200065934. [DOI] [Google Scholar]
- 75.Güven O., Şen M., Karadağ E., Saraydın D. A review on the radiation synthesis of copolymeric hydrogels for adsorption and separation purposes. Radiat. Phys. Chem. 1999;56:381–386. doi: 10.1016/S0969-806X(99)00326-6. [DOI] [Google Scholar]
- 76.Bayer G., Grasselli S., Malchiodi A., Bayer I.S. Antiseptic povidone-iodine encapsulating edible phospholipid gels. Colloids Surf. A Physicochem. Eng. Asp. 2021;619:126537. doi: 10.1016/j.colsurfa.2021.126537. [DOI] [Google Scholar]
- 77.Povea M.B., Monal W.A., Cauich-Rodríguez J.V., Pat A.M., Rivero N.B., Covas C.P. Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, Characterization and Sustained Protein Release Studies. Mater. Sci. Appl. 2011;2:509–520. doi: 10.4236/msa.2011.26069. [DOI] [Google Scholar]
- 78.Kobayashi M., Chang Y.-S., Oka M. A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials. 2005;26:3243–3248. doi: 10.1016/j.biomaterials.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 79.Pal K., Banthia A.K., Majumdar D.K. Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. AAPS PharmSciTech. 2007;8:E142–E146. doi: 10.1208/pt080121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bahram M., Mohseni N., Moghtader M. Emerging Concepts in Analysis and Applications of Hydrogels. IntechOpen; Rijeka, Croatia: 2016. An Introduction to Hydrogels and Some Recent Applications. [Google Scholar]
- 81.Van Vlierberghe S., Dubruel P., Schacht E. Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules. 2011;12:1387–1408. doi: 10.1021/bm200083n. [DOI] [PubMed] [Google Scholar]
- 82.Billiet T., Vandenhaute M., Schelfhout J., Van Vlierberghe S., Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33:6020–6041. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 83.Suntornnond R., An J., Chua C.K. Bioprinting of Thermoresponsive Hydrogels for Next Generation Tissue Engineering: A Review. Macromol. Mater. Eng. 2017;302:1600266. doi: 10.1002/mame.201600266. [DOI] [Google Scholar]
- 84.Hernández-González A.C., Téllez-Jurado L., Rodríguez-Lorenzo L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020;229:115514. doi: 10.1016/j.carbpol.2019.115514. [DOI] [PubMed] [Google Scholar]
- 85.Hoare T.R., Kohane D.S. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49:1993–2007. doi: 10.1016/j.polymer.2008.01.027. [DOI] [Google Scholar]
- 86.Kamath K.R., Park K. Biodegradable hydrogels in drug delivery. Adv. Drug Deliv. Rev. 1993;11:59–84. doi: 10.1016/0169-409X(93)90027-2. [DOI] [Google Scholar]
- 87.Vigata M., Meinert C., Hutmacher D.W., Bock N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics. 2020;12:1188. doi: 10.3390/pharmaceutics12121188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bernhard S., Tibbitt M.W. Supramolecular engineering of hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2021;171:240–256. doi: 10.1016/j.addr.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 89.Matricardi P., Di Meo C., Coviello T., Hennink W.E., Alhaique F. Interpenetrating Polymer Networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013;65:1172–1187. doi: 10.1016/j.addr.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 90.Kalshetti P.P., Rajendra V.B., Dixit D.N., Parekh P.P. Hydrogels as a drug delivery system and applications: A review. Int. J. Pharm. Pharm. Sci. 2012;4:1–7. [Google Scholar]
- 91.Gupta A., Kowalczuk M., Heaselgrave W., Britland S.T., Martin C., Radecka I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019;111:134–151. doi: 10.1016/j.eurpolymj.2018.12.019. [DOI] [Google Scholar]
- 92.Liang Y., He J., Guo B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano. 2021;15:12687–12722. doi: 10.1021/acsnano.1c04206. [DOI] [PubMed] [Google Scholar]
- 93.Kamoun E.A., Kenawy E.-R.S., Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017;8:217–233. doi: 10.1016/j.jare.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang A., Liu Y., Qin D., Sun M., Wang T., Chen X. Research status of self-healing hydrogel for wound management: A review. Int. J. Biol. Macromol. 2020;164:2108–2123. doi: 10.1016/j.ijbiomac.2020.08.109. [DOI] [PubMed] [Google Scholar]
- 95.Tavakoli S., Klar A.S. Advanced Hydrogels as Wound Dressings. Biomolecules. 2020;10:1169. doi: 10.3390/biom10081169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stan D., Tanase C., Avram M., Apetrei R., Mincu N.B., Mateescu A.L., Stan D. Wound healing applications of creams and “smart” hydrogels. Exp. Dermatol. 2021 doi: 10.1111/exd.14396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang D., Park K., Famili A. Hydrogels for sustained delivery of biologics to the back of the eye. Drug Discov. Today. 2019;24:1470–1482. doi: 10.1016/j.drudis.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 98.Lin Q., Lim J.Y., Xue K., Su X., Loh X.J. Polymeric hydrogels as a vitreous replacement strategy in the eye. Biomaterials. 2021;268:120547. doi: 10.1016/j.biomaterials.2020.120547. [DOI] [PubMed] [Google Scholar]
- 99.Kopecek J. Hydrogels: From soft contact lenses and implants to self-assembled nanomaterials. J. Polym. Sci. Part A Polym. Chem. 2009;47:5929–5946. doi: 10.1002/pola.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ribeiro A.M., Figueiras A., Veiga F. Improvements in Topical Ocular Drug Delivery Systems: Hydrogels and Contact Lenses. J. Pharm. Pharm. Sci. 2015;18:683–695. doi: 10.18433/J3H60P. [DOI] [PubMed] [Google Scholar]
- 101.Pimenta A., Ascenso J., Fernandes J.C.S., Colaco R., Serro A., Saramago B. Controlled drug release from hydrogels for contact lenses: Drug partitioning and diffusion. Int. J. Pharm. 2016;515:467–475. doi: 10.1016/j.ijpharm.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 102.Xinming L., Yingde C., Lloyd A.W., Mikhalovsky S.V., Sandeman S.R., Howel C.A., Liewen L. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Contact Lens Anterior Eye. 2008;31:57–64. doi: 10.1016/j.clae.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 103.Alvarez-Lorenzo C., Igea S.A., Varela-García A., Vivero-Lopez M., Concheiro A. Bioinspired hydrogels for drug-eluting contact lenses. Acta Biomater. 2019;84:49–62. doi: 10.1016/j.actbio.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 104.Dhanjai, Sinha A., Kalambate P.K., Mugo S.M., Kamau P., Chen J., Jain R. Polymer hydrogel interfaces in electrochemical sensing strategies: A review. TrAC Trends Anal. Chem. 2019;118:488–501. doi: 10.1016/j.trac.2019.06.014. [DOI] [Google Scholar]
- 105.Tavakoli J., Tang Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers. 2017;9:364. doi: 10.3390/polym9080364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mateescu A., Wang Y., Dostalek J., Jonas U. Thin Hydrogel Films for Optical Biosensor Applications. Membranes. 2012;2:40–69. doi: 10.3390/membranes2010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buenger D., Topuz F., Groll J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012;37:1678–1719. doi: 10.1016/j.progpolymsci.2012.09.001. [DOI] [Google Scholar]
- 108.Pinelli F., Magagnin L., Rossi F. Progress in hydrogels for sensing applications: A review. Mater. Today Chem. 2020;17:100317. doi: 10.1016/j.mtchem.2020.100317. [DOI] [Google Scholar]
- 109.Greiner A., Wendorff J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007;46:5670–5703. doi: 10.1002/anie.200604646. [DOI] [PubMed] [Google Scholar]
- 110.Kulkarni A., Bambole V.A., Mahanwar P.A. Electrospinning of Polymers, Their Modeling and Applications. Polym. Technol. Eng. 2010;49:427–441. doi: 10.1080/03602550903414019. [DOI] [Google Scholar]
- 111.Lukáš D., Sarkar A., Martinová L., Vodsed’álková K., Lubasová D., Chaloupek J., Komárek M. Physical principles of electrospinning (Electrospinning as a nano-scale technology of the twenty-first century) Text. Prog. 2009;41:59–140. doi: 10.1080/00405160902904641. [DOI] [Google Scholar]
- 112.Li Y., Zhu J., Cheng H., Li G., Cho H., Jiang M., Gao Q., Zhang X. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021:2100410. doi: 10.1002/admt.202100410. [DOI] [Google Scholar]
- 113.Xu S., Deng L., Zhang J., Yin L., Liandong D. Composites of electrospun-fibers and hydrogels: A potential solution to current challenges in biological and biomedical field. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016;104:640–656. doi: 10.1002/jbm.b.33420. [DOI] [PubMed] [Google Scholar]
- 114.Kim M., Hong B., Lee J., Kim S.E., Kang S.S., Kim Y.H., Tae G. Composite System of PLCL Scaffold and Heparin-Based Hydrogel for Regeneration of Partial-Thickness Cartilage Defects. Biomacromolecules. 2012;13:2287–2298. doi: 10.1021/bm3005353. [DOI] [PubMed] [Google Scholar]
- 115.Xu L., Zhao X., Xu C., Kotov N.A. Water-rich biomimetic composites with abiotic self-organizing nanofiber network. Adv. Mater. 2018;30:1703343. doi: 10.1002/adma.201703343. [DOI] [PubMed] [Google Scholar]
- 116.Ikonen L., Kerkelä E., Metselaar G., Stuart M., de Jong M.R., Aalto-Setälä K. 2D and 3D Self-Assembling Nanofiber Hydrogels for Cardiomyocyte Culture. BioMed Res. Int. 2013;2013:1–12. doi: 10.1155/2013/285678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ding Z., Lu G., Cheng W., Xu G., Zuo B., Lu Q., Kaplan D.L. Tough Anisotropic Silk Nanofiber Hydrogels with Osteoinductive Capacity. ACS Biomater. Sci. Eng. 2020;6:2357–2367. doi: 10.1021/acsbiomaterials.0c00143. [DOI] [PubMed] [Google Scholar]
- 118.Xu F., Sheardown H., Hoare T. Reactive electrospinning of degradable poly(oligoethylene glycol methacrylate)-based nanofibrous hydrogel networks. Chem. Commun. 2016;52:1451–1454. doi: 10.1039/C5CC08053C. [DOI] [PubMed] [Google Scholar]
- 119.Molnár K., Jedlovszky-Hajdu A., Zrinyi M., Jiang S., Agarwal S. Poly(amino acid)-Based Gel Fibers with pH Responsivity by Coaxial Reactive Electrospinning. Macromol. Rapid Commun. 2017;38:1700147. doi: 10.1002/marc.201700147. [DOI] [PubMed] [Google Scholar]
- 120.Al-Baadani M.A., Yie K.H.R., Al-Bishari A.M., Alshobi B.A., Zhou Z., Fang K., Dai B., Shen Y., Ma J., Liu J., et al. Co-electrospinning polycaprolactone/gelatin membrane as a tunable drug delivery system for bone tissue regeneration. Mater. Des. 2021;209:109962. doi: 10.1016/j.matdes.2021.109962. [DOI] [Google Scholar]
- 121.Wade R.J., Bassin E., Rodell C.B., Burdick J.A. Protease-degradable electrospun fibrous hydrogels. Nat. Commun. 2015;6:1–10. doi: 10.1038/ncomms7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bosworth L.A., Turner L.-A., Cartmell S. State of the art composites comprising electrospun fibres coupled with hydrogels: A review. Nanomed. Nanotechnol. Biol. Med. 2013;9:322–335. doi: 10.1016/j.nano.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 123.Pawłowska S., Rinoldi C., Nakielski P., Ziai Y., Urbanek O., Li X., Pierini F. Ultraviolet Light-Assisted Electrospinning of Core–Shell Fully Cross-Linked P (NIPAAm-co-NIPMAAm) Hydrogel-Based Nanofibers for Thermally Induced Drug Delivery Self-Regulation. Adv. Mater. Interfaces. 2020;7:2000247. doi: 10.1002/admi.202000247. [DOI] [Google Scholar]
- 124.Nakielski P., Pawłowska S., Rinoldi C., Ziai Y., De Sio L., Urbanek O., Zembrzycki K., Pruchniewski M., Lanzi M., Salatelli E., et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl. Mater. Interfaces. 2020;12:54328–54342. doi: 10.1021/acsami.0c13266. [DOI] [PubMed] [Google Scholar]
- 125.Fu Q., Duan C., Yan Z., Li Y., Si Y., Liu L., Yu J., Ding B. Nanofiber-Based Hydrogels: Controllable Synthesis and Multifunctional Applications. Macromol. Rapid Commun. 2018;39:e1800058. doi: 10.1002/marc.201800058. [DOI] [PubMed] [Google Scholar]
- 126.Young R.E., Graf J., Miserocchi I., Van Horn R.M., Gordon M.B., Anderson C.R., Sefcik L.S. Optimizing the alignment of thermoresponsive poly(N-isopropyl acrylamide) electrospun nanofibers for tissue engineering applications: A factorial design of experiments approach. PLoS ONE. 2019;14:e0219254. doi: 10.1371/journal.pone.0219254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Su C., Liu J., Yang Z., Jiang L., Liu X., Shao W. UV-mediated synthesis of carboxymethyl cellulose/poly-N-isopropylacrylamide composite hydrogels with triple stimuli-responsive swelling performances. Int. J. Biol. Macromol. 2020;161:1140–1148. doi: 10.1016/j.ijbiomac.2020.06.094. [DOI] [PubMed] [Google Scholar]
- 128.Wei J., Chen Y., Liu H., Du C., Yu H., Zhou Z. Thermo-responsive and compression properties of TEMPO-oxidized cellulose nanofiber-modified PNIPAm hydrogels. Carbohydr. Polym. 2016;147:201–207. doi: 10.1016/j.carbpol.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 129.Masruchin N., Park B.-D., Causin V. Dual-responsive composite hydrogels based on TEMPO-oxidized cellulose nanofibril and poly(N-isopropylacrylamide) for model drug release. Cellulose. 2018;25:485–502. doi: 10.1007/s10570-017-1585-2. [DOI] [Google Scholar]
- 130.Goto K., Teramoto Y. Development of chitinous nanofiber-based flexible composite hydrogels capable of cell adhesion and detachment. Polym. J. 2020;52:959–967. doi: 10.1038/s41428-020-0324-y. [DOI] [Google Scholar]
- 131.Li X., Cho B., Martin R., Seu M., Zhang C., Zhou Z., Choi J.S., Jiang X., Chen L., Walia G., et al. Nanofiber-hydrogel composite–mediated angiogenesis for soft tissue reconstruction. Sci. Transl. Med. 2019;11:eaau6210. doi: 10.1126/scitranslmed.aau6210. [DOI] [PubMed] [Google Scholar]
- 132.Tang J.D., Mura C., Lampe K.J. Stimuli-Responsive, Pentapeptide, Nanofiber Hydrogel for Tissue Engineering. J. Am. Chem. Soc. 2019;141:4886–4899. doi: 10.1021/jacs.8b13363. [DOI] [PubMed] [Google Scholar]
- 133.Lohrasbi S., Mirzaei E., Karimizade A., Takallu S., Rezaei A. Collagen/cellulose nanofiber hydrogel scaffold: Physical, mechanical and cell biocompatibility properties. Cellulose. 2019;27:927–940. doi: 10.1007/s10570-019-02841-y. [DOI] [Google Scholar]
- 134.Jirkovec R., Samkova A., Kalous T., Chaloupek J., Chvojka J. Preparation of a Hydrogel Nanofiber Wound Dressing. Nanomaterials. 2021;11:2178. doi: 10.3390/nano11092178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim G.J., Kim K.O. Novel glucose-responsive of the transparent nanofiber hydrogel patches as a wearable biosensor via electrospinning. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-75906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li X., Zhang C., Haggerty A.E., Yan J., Lan M., Seu M., Yang M., Marlow M.M., Maldonado-Lasunción I., Cho B., et al. The effect of a nanofiber-hydrogel composite on neural tissue repair and regeneration in the contused spinal cord. Biomaterials. 2020;245:119978. doi: 10.1016/j.biomaterials.2020.119978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shelke N.B., Lee P., Anderson M., Mistry N., Nagarale R.K., Ma X.-M., Yu X., Kumbar S.G. Neural tissue engineering: Nanofiber-hydrogel based composite scaffolds. Polym. Adv. Technol. 2016;27:42–51. doi: 10.1002/pat.3594. [DOI] [Google Scholar]
- 138.Formica F.A., Öztürk E., Hess S.C., Stark W.J., Maniura-Weber K., Rottmar M., Zenobi-Wong M. A Bioinspired Ultraporous Nanofiber-Hydrogel Mimic of the Cartilage Extracellular Matrix. Adv. Healthc. Mater. 2016;5:3129–3138. doi: 10.1002/adhm.201600867. [DOI] [PubMed] [Google Scholar]
- 139.Du J., Liu J., Yao S., Mao H.-Q., Peng J., Sun X., Cao Z., Yang Y., Xiao B., Wang Y., et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017;55:296–309. doi: 10.1016/j.actbio.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 140.Nishimura A., Hayakawa T., Yamamoto Y., Hamori M., Tabata K., Seto K., Shibata N. Controlled release of insulin from self-assembling nanofiber hydrogel, PuraMatrix™: Application for the subcutaneous injection in rats. Eur. J. Pharm. Sci. 2012;45:1–7. doi: 10.1016/j.ejps.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 141.Liu X., Song S., Huang J., Fu H., Ning X., He Y., Zhang Z. HBC-nanofiber hydrogel scaffolds with 3D printed internal microchannels for enhanced cartilage differentiation. J. Mater. Chem. B. 2020;8:6115–6127. doi: 10.1039/D0TB00616E. [DOI] [PubMed] [Google Scholar]
- 142.Kawata M., Azuma K., Izawa H., Morimoto M., Saimoto H., Ifuku S. Biomineralization of calcium phosphate crystals on chitin nanofiber hydrogel for bone regeneration material. Carbohydr. Polym. 2016;136:964–969. doi: 10.1016/j.carbpol.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 143.Song S., Zhao Y., Yuan X., Zhang J. β-Chitin nanofiber hydrogel as a scaffold to in situ fabricate monodispersed ultra-small silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019;574:36–43. doi: 10.1016/j.colsurfa.2019.04.047. [DOI] [Google Scholar]
- 144.Yang G., Yao Y., Wang X. Comparative study of kerateine and keratose based composite nanofibers for biomedical applications. Mater. Sci. Eng. C. 2018;83:1–8. doi: 10.1016/j.msec.2017.07.057. [DOI] [PubMed] [Google Scholar]
- 145.Nguyen L.H., Gao M., Lin J., Wu W., Wang J., Chew S.Y. Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci. Rep. 2017;7:srep42212. doi: 10.1038/srep42212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rinoldi C., Zargarian S.S., Nakielski P., Li X., Liguori A., Petronella F., Pierini F. Nanotechnology-Assisted RNA Delivery: From Nucleic Acid Therapeutics to COVID-19 Vaccines. Small Methods. 2021;5:2100402. doi: 10.1002/smtd.202100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yan C., Pochan D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010;39:3528–3540. doi: 10.1039/b919449p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Massensini A., Ghuman H., Saldin L.T., Medberry C.J., Keane T., Nicholls F., Velankar S.S., Badylak S.F., Modo M. Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. Acta Biomater. 2015;27:116–130. doi: 10.1016/j.actbio.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Du E.Y., Ziaee F., Wang L., Nordon R.E., Thordarson P. The correlations between structure, rheology, and cell growth in peptide-based multicomponent hydrogels. Polym. J. 2020;52:947–957. doi: 10.1038/s41428-020-0351-8. [DOI] [Google Scholar]
- 150.Li Y., Zhang Y., Shi F., Tao L., Wei Y., Wang X. Modulus-regulated 3D-cell proliferation in an injectable self-healing hydrogel. Colloids Surf. B Biointerfaces. 2017;149:168–173. doi: 10.1016/j.colsurfb.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 151.Kong H.-J., Lee K.Y., Mooney D. Decoupling the dependence of rheological/mechanical properties of hydrogels from solids concentration. Polymer. 2002;43:6239–6246. doi: 10.1016/S0032-3861(02)00559-1. [DOI] [Google Scholar]
- 152.Guo J., Zhang R., Zhang L., Cao X. 4D Printing of Robust Hydrogels Consisted of Agarose Nanofibers and Polyacrylamide. ACS Macro Lett. 2018;7:442–446. doi: 10.1021/acsmacrolett.7b00957. [DOI] [PubMed] [Google Scholar]
- 153.Shin S., Hyun J. Rheological properties of cellulose nanofiber hydrogel for high-fidelity 3D printing. Carbohydr. Polym. 2021;263:117976. doi: 10.1016/j.carbpol.2021.117976. [DOI] [PubMed] [Google Scholar]
- 154.Sun Y., Li X., Zhao M., Chen Y., Xu Y., Wang K., Bian S., Jiang Q., Fan Y., Zhang X. Bioinspired supramolecular nanofiber hydrogel through self-assembly of biphenyl-tripeptide for tissue engineering. Bioact. Mater. 2022;8:396–408. doi: 10.1016/j.bioactmat.2021.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rammensee S., Huemmerich D., Hermanson K., Scheibel T., Bausch A. Rheological characterization of hydrogels formed by recombinantly produced spider silk. Appl. Phys. A. 2006;82:261–264. doi: 10.1007/s00339-005-3431-x. [DOI] [Google Scholar]
- 156.Wickremasinghe N.C., Kumar V.A., Hartgerink J.D. Two-Step Self-Assembly of Liposome-Multidomain Peptide Nanofiber Hydrogel for Time-Controlled Release. Biomacromolecules. 2014;15:3587–3595. doi: 10.1021/bm500856c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen M., Tan Y., Dong Z., Lu J., Han X., Jin Q., Zhu W., Shen J., Cheng L., Liu Z., et al. Injectable Anti-inflammatory Nanofiber Hydrogel to Achieve Systemic Immunotherapy Post Local Administration. Nano Lett. 2020;20:6763–6773. doi: 10.1021/acs.nanolett.0c02684. [DOI] [PubMed] [Google Scholar]
- 158.O’Leary L.E.R., Fallas J.A., Bakota E.L., Kang M.K., Hartgerink J. Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat. Chem. 2011;3:821–828. doi: 10.1038/nchem.1123. [DOI] [PubMed] [Google Scholar]
- 159.Zhou C., Wu Q. A novel polyacrylamide nanocomposite hydrogel reinforced with natural chitosan nanofibers. Colloids Surf. B Biointerfaces. 2011;84:155–162. doi: 10.1016/j.colsurfb.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 160.Abouzeid R.E., Khiari R., Salama A., Diab M., Beneventi D., Dufresne A. In situ mineralization of nano-hydroxyapatite on bifunctional cellulose nanofiber/polyvinyl alcohol/sodium alginate hydrogel using 3D printing. Int. J. Biol. Macromol. 2020;160:538–547. doi: 10.1016/j.ijbiomac.2020.05.181. [DOI] [PubMed] [Google Scholar]
- 161.Chu B., He J.-M., Wang Z., Liu L.-L., Li X.-L., Wu C.-X., Chen C.-S., Tu M. Proangiogenic peptide nanofiber hydrogel/3D printed scaffold for dermal regeneration. Chem. Eng. J. 2020;424:128146. doi: 10.1016/j.cej.2020.128146. [DOI] [Google Scholar]
- 162.Huang L., Du X., Fan S., Yang G., Shao H., Li D., Cao C., Zhu Y., Zhu M., Zhang Y. Bacterial cellulose nanofibers promote stress and fidelity of 3D-printed silk based hydrogel scaffold with hierarchical pores. Carbohydr. Polym. 2019;221:146–156. doi: 10.1016/j.carbpol.2019.05.080. [DOI] [PubMed] [Google Scholar]
- 163.Huang L., Yuan W., Hong Y., Fan S., Yao X., Ren T., Song L., Yang G., Zhang Y. 3D printed hydrogels with oxidized cellulose nanofibers and silk fibroin for the proliferation of lung epithelial stem cells. Cellulose. 2021;28:241–257. doi: 10.1007/s10570-020-03526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Serafin A., Murphy C., Rubio M.C., Collins M.N. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater. Sci. Eng. C. 2021;122:111927. doi: 10.1016/j.msec.2021.111927. [DOI] [PubMed] [Google Scholar]
- 165.Veerubhotla K., Lee Y., Lee C.H. Parametric Optimization of 3D Printed Hydrogel-Based Cardiovascular Stent. Pharm. Res. 2021;38:1–16. doi: 10.1007/s11095-021-03049-1. [DOI] [PubMed] [Google Scholar]
- 166.Yoo J., Park J.H., Kwon Y.W., Chung J.J., Choi I.C., Nam J.J., Lee H.S., Jeon E.Y., Lee K., Kim S.H., et al. Augmented peripheral nerve regeneration through elastic nerve guidance conduits prepared using a porous PLCL membrane with a 3D printed collagen hydrogel. Biomater. Sci. 2020;8:6261–6271. doi: 10.1039/D0BM00847H. [DOI] [PubMed] [Google Scholar]
- 167.Wu Z., Xie S., Kang Y., Shan X., Li Q., Cai Z. Biocompatibility evaluation of a 3D-bioprinted alginate-GelMA-bacteria nanocellulose (BNC) scaffold laden with oriented-growth RSC96 cells. Mater. Sci. Eng. C. 2021;129:112393. doi: 10.1016/j.msec.2021.112393. [DOI] [PubMed] [Google Scholar]
- 168.Townsend J.M., Beck E.C., Gehrke S.H., Berkland C.J., Detamore M.S. Flow behavior prior to crosslinking: The need for precursor rheology for placement of hydrogels in medical applications and for 3D bioprinting. Prog. Polym. Sci. 2019;91:126–140. doi: 10.1016/j.progpolymsci.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Langer R. Invited Review Polymeric Delivery Systems for Controlled Drug Release. Chem. Eng. Commun. 1980;6:1–48. doi: 10.1080/00986448008912519. [DOI] [Google Scholar]
- 170.Uhrich K., Cannizzaro S.M., Langer R.S., Shakesheff K. Polymeric Systems for Controlled Drug Release. Chem. Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 171.Lee P.I., Kim C.-J. Probing the mechanisms of drug release from hydrogels. J. Control. Release. 1991;16:229–236. doi: 10.1016/0168-3659(91)90046-G. [DOI] [Google Scholar]
- 172.Lee P.I. Kinetics of drug release from hydrogel matrices. J. Control. Release. 1985;2:277–288. doi: 10.1016/0168-3659(85)90051-3. [DOI] [Google Scholar]
- 173.Kalkhoran A.H.Z., Vahidi O., Naghib S.M. A new mathematical approach to predict the actual drug release from hydrogels. Eur. J. Pharm. Sci. 2018;111:303–310. doi: 10.1016/j.ejps.2017.09.038. [DOI] [PubMed] [Google Scholar]
- 174.Kikuchi A., Okano T. Pulsatile drug release control using hydrogels. Adv. Drug Deliv. Rev. 2002;54:53–77. doi: 10.1016/S0169-409X(01)00243-5. [DOI] [PubMed] [Google Scholar]
- 175.Xu L., Qiu L., Sheng Y., Sun Y., Deng L., Li X., Zhang R. Biodegradable pH-responsive hydrogels for controlled dual-drug release. J. Mater. Chem. B. 2018;6:510–517. doi: 10.1039/C7TB01851G. [DOI] [PubMed] [Google Scholar]
- 176.Zhang X.-Z., Wu D.-Q., Chu C.-C. Synthesis, characterization and controlled drug release of thermosensitive IPN–PNIPAAm hydrogels. Biomaterials. 2004;25:3793–3805. doi: 10.1016/j.biomaterials.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 177.Van Bommel K.J., Stuart M.C., Feringa B.L., van Esch J. Two-stage enzyme mediated drug release from LMWG hydrogels. Org. Biomol. Chem. 2005;3:2917–2920. doi: 10.1039/b507157g. [DOI] [PubMed] [Google Scholar]
- 178.Satarkar N.S., Hilt J.Z. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. J. Control. Release. 2008;130:246–251. doi: 10.1016/j.jconrel.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 179.Qu J., Liang Y., Shi M., Guo B., Gao Y., Yin Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019;140:255–264. doi: 10.1016/j.ijbiomac.2019.08.120. [DOI] [PubMed] [Google Scholar]
- 180.Indermun S., Choonara Y., Kumar P., du Toit L., Modi G., Luttge R., Pillay V. An interfacially plasticized electro-responsive hydrogel for transdermal electro-activated and modulated (TEAM) drug delivery. Int. J. Pharm. 2014;462:52–65. doi: 10.1016/j.ijpharm.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 181.Carayon I., Gaubert A., Mousli Y., Philippe B. Electro-responsive hydrogels: Macromolecular and supramolecular approaches in the biomedical field. Biomater. Sci. 2020;8:5589–5600. doi: 10.1039/D0BM01268H. [DOI] [PubMed] [Google Scholar]
- 182.Murdan S. Electro-responsive drug delivery from hydrogels. J. Control. Release. 2003;92:1–17. doi: 10.1016/S0168-3659(03)00303-1. [DOI] [PubMed] [Google Scholar]
- 183.Pianowski Z.L., Karcher J., Schneider K. Photoresponsive self-healing supramolecular hydrogels for light-induced release of DNA and doxorubicin. Chem. Commun. 2016;52:3143–3146. doi: 10.1039/C5CC09633B. [DOI] [PubMed] [Google Scholar]
- 184.Karcher J., Pianowski Z.L. Photocontrol of Drug Release from Supramolecular Hydrogels with Green Light. Chem.-A Eur. J. 2018;24:11605–11610. doi: 10.1002/chem.201802205. [DOI] [PubMed] [Google Scholar]
- 185.Samadian H., Maleki H., Allahyari Z., Jaymand M. Natural polymers-based light-induced hydrogels: Promising biomaterials for biomedical applications. Coord. Chem. Rev. 2020;420:213432. doi: 10.1016/j.ccr.2020.213432. [DOI] [Google Scholar]
- 186.Zhu C., Bettinger C.J. Light-induced remodeling of physically crosslinked hydrogels using near-IR wavelengths. J. Mater. Chem. B. 2014;2:1613–1618. doi: 10.1039/C3TB21689F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu Y.Y., Shao Y.H., Lü J. Preparation, properties and controlled release behaviors of pH-induced thermosensitive amphiphilic gels. Biomaterials. 2006;27:4016–4024. doi: 10.1016/j.biomaterials.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 188.Yu S., Zhang X., Tan G., Tian L., Liu D., Liu Y., Pan W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017;155:208–217. doi: 10.1016/j.carbpol.2016.08.073. [DOI] [PubMed] [Google Scholar]
- 189.Chu Y., Varanasi P.P., McGlade M.J., Varanasi S. pH-induced swelling kinetics of polyelectrolyte hydrogels. J. Appl. Polym. Sci. 1995;58:2161–2176. doi: 10.1002/app.1995.070581203. [DOI] [Google Scholar]
- 190.Rizwan M., Yahya R., Hassan A., Yar M., Azzahari A.D., Selvanathan V., Sonsudin F., Abouloula C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers. 2017;9:137. doi: 10.3390/polym9040137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu T.-Y., Hu S.-H., Liu K.-H., Liu D.-M., Chen S.-Y. Preparation and characterization of smart magnetic hydrogels and its use for drug release. J. Magn. Magn. Mater. 2006;304:e397–e399. doi: 10.1016/j.jmmm.2006.01.203. [DOI] [Google Scholar]
- 192.Paulino A.T., Pereira A.G., Fajardo A., Erickson K., Kipper M., Muniz E., Belfiore L.A., Tambourgi E.B. Natural polymer-based magnetic hydrogels: Potential vectors for remote-controlled drug release. Carbohydr. Polym. 2012;90:1216–1225. doi: 10.1016/j.carbpol.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 193.Wang Y., Li B., Xu F., Han Z., Wei D., Jia D., Zhou Y. Tough Magnetic Chitosan Hydrogel Nanocomposites for Remotely Stimulated Drug Release. Biomacromolecules. 2018;19:3351–3360. doi: 10.1021/acs.biomac.8b00636. [DOI] [PubMed] [Google Scholar]
- 194.Reddy N.N., Varaprasad K., Ravindra S., Reddy G.S., Reddy K., Raju K.M. Evaluation of blood compatibility and drug release studies of gelatin based magnetic hydrogel nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2011;385:20–27. doi: 10.1016/j.colsurfa.2011.05.006. [DOI] [Google Scholar]
- 195.Campbell S., Maitland D., Hoare T. Enhanced Pulsatile Drug Release from Injectable Magnetic Hydrogels with Embedded Thermosensitive Microgels. ACS Macro Lett. 2015;4:312–316. doi: 10.1021/acsmacrolett.5b00057. [DOI] [PubMed] [Google Scholar]
- 196.Hendi A., Hassan M.U., Elsherif M., Alqattan B., Park S., Yetisen A.K., Butt H. Healthcare applications of pH-Sensitive hydrogel-based devices: A review. Int. J. Nanomed. 2020;15:3887. doi: 10.2147/IJN.S245743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kulkarni R.V., Biswanath S. Electrically responsive smart hydrogels in drug delivery: A review. J. Appl. Biomater. Biomech. 2010;5:125–139. [PubMed] [Google Scholar]
- 198.Loh X.J., Peh P., Liao S., Sng C., Li J. Controlled drug release from biodegradable thermoresponsive physical hydrogel nanofibers. J. Control. Release. 2010;143:175–182. doi: 10.1016/j.jconrel.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 199.Fang Y., Wang C.-F., Zhang Z.-H., Shao H., Chen S. Robust Self-Healing Hydrogels Assisted by Cross-Linked Nanofiber Networks. Sci. Rep. 2013;3:2811. doi: 10.1038/srep02811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ma H., Yu J., Liu L., Fan Y. An optimized preparation of nanofiber hydrogels derived from natural carbohydrate polymers and their drug release capacity under different pH surroundings. Carbohydr. Polym. 2021;265:118008. doi: 10.1016/j.carbpol.2021.118008. [DOI] [PubMed] [Google Scholar]
- 201.Kamoun E.A., Loutfy S.A., Hussein Y., Kenawy E.-R.S. Recent advances in PVA-polysaccharide based hydrogels and electrospun nanofibers in biomedical applications: A review. Int. J. Biol. Macromol. 2021;187:755–768. doi: 10.1016/j.ijbiomac.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 202.Auvinen V.-V., Virtanen J., Merivaara A., Virtanen V., Laurén P., Tuukkanen S., Laaksonen T. Modulating sustained drug release from nanocellulose hydrogel by adjusting the inner geometry of implantable capsules. J. Drug Deliv. Sci. Technol. 2020;57:101625. doi: 10.1016/j.jddst.2020.101625. [DOI] [Google Scholar]
- 203.Yang Z., Xu B. Supramolecular hydrogels based on biofunctional nanofibers of self-assembled small molecules. J. Mater. Chem. 2007;17:2385–2393. doi: 10.1039/b702493b. [DOI] [Google Scholar]
- 204.Pérez-Martínez C., Chávez S.D.M., del Castillo-Castro T., Ceniceros T.E.L., Castillo-Ortega M., Rodríguez-Félix D., Ruiz J.C.G. Electroconductive nanocomposite hydrogel for pulsatile drug release. React. Funct. Polym. 2016;100:12–17. doi: 10.1016/j.reactfunctpolym.2015.12.017. [DOI] [Google Scholar]
- 205.Yuan H., Li B., Liang K., Lou X., Zhang Y. Regulating drug release from pH- and temperature-responsive electrospun CTS-g-PNIPAAm/poly(ethylene oxide) hydrogel nanofibers. Biomed. Mater. 2014;9:055001. doi: 10.1088/1748-6041/9/5/055001. [DOI] [PubMed] [Google Scholar]
- 206.Chen Y., Qiu Y., Wang Q., Li D., Hussain T., Ke H., Wei Q. Mussel-inspired sandwich-like nanofibers/hydrogel composite with super adhesive, sustained drug release and anti-infection capacity. Chem. Eng. J. 2020;399:125668. doi: 10.1016/j.cej.2020.125668. [DOI] [Google Scholar]
- 207.Wu H., Liu S., Xiao L., Dong X., Lu Q., Kaplan D.L. Injectable and pH-responsive silk nanofiber hydrogels for sustained anticancer drug delivery. ACS Appl. Mater. Interfaces. 2016;8:17118–17126. doi: 10.1021/acsami.6b04424. [DOI] [PubMed] [Google Scholar]
- 208.Ding Z., Zhou M., Zhou Z., Zhang W., Jiang X., Lu X., Zuo B., Lu Q., Kaplan D.L. Injectable Silk Nanofiber Hydrogels for Sustained Release of Small-Molecule Drugs and Vascularization. ACS Biomater. Sci. Eng. 2019;5:4077–4088. doi: 10.1021/acsbiomaterials.9b00621. [DOI] [PubMed] [Google Scholar]
- 209.Karavasili C., Komnenou A., Katsamenis O.L., Charalampidou G., Kofidou E., Andreadis D., Koutsopoulos S., Fatouros D.G. Self-Assembling Peptide Nanofiber Hydrogels for Controlled Ocular Delivery of Timolol Maleate. ACS Biomater. Sci. Eng. 2017;3:3386–3394. doi: 10.1021/acsbiomaterials.7b00706. [DOI] [PubMed] [Google Scholar]
- 210.Koutsopoulos S., Zhang S. Two-layered injectable self-assembling peptide scaffold hydrogels for long-term sustained release of human antibodies. J. Control. Release. 2012;160:451–458. doi: 10.1016/j.jconrel.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 211.Xing J.Z., Lu L., Unsworth L., Major P.W., Doschak M.R., Kaipatur N.R. RANKL release from self-assembling nanofiber hydrogels for inducing osteoclastogenesis in vitro. Acta Biomater. 2017;49:306–315. doi: 10.1016/j.actbio.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 212.Cinar G., Ozdemir A., Hamsici S., Gunay G., Dana A., Tekinay A.B., Guler M.O. Local delivery of doxorubicin through supramolecular peptide amphiphile nanofiber gels. Biomater. Sci. 2017;5:67–76. doi: 10.1039/C6BM00656F. [DOI] [PubMed] [Google Scholar]
- 213.Karavasili C., Panteris E., Vizirianakis I., Koutsopoulos S., Fatouros D.G. Chemotherapeutic Delivery from a Self-Assembling Peptide Nanofiber Hydrogel for the Management of Glioblastoma. Pharm. Res. 2018;35:166. doi: 10.1007/s11095-018-2442-1. [DOI] [PubMed] [Google Scholar]
- 214.Hill L.K., Meleties M., Katyal P., Xie X., Delgado-Fukushima E.G., Jihad T., Liu C.-F., O’Neill S.C., Tu R.S., Renfrew P.D., et al. Thermoresponsive Protein-Engineered Coiled-Coil Hydrogel for Sustained Small Molecule Release. Biomacromolecules. 2019;20:3340–3351. doi: 10.1021/acs.biomac.9b00107. [DOI] [PubMed] [Google Scholar]
- 215.Koutsopoulos S., Unsworth L.D., Nagai Y., Zhang S. Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc. Natl. Acad. Sci. USA. 2009;106:4623–4628. doi: 10.1073/pnas.0807506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Briuglia M.-L., Urquhart A.J., Lamprou D.A. Sustained and controlled release of lipophilic drugs from a self-assembling amphiphilic peptide hydrogel. Int. J. Pharm. 2014;474:103–111. doi: 10.1016/j.ijpharm.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 217.Liang G., Yang Z., Zhang R., Li L., Fan Y., Kuang Y., Gao Y., Wang T., Lu W.W., Xu B. Supramolecular Hydrogel of a d-Amino Acid Dipeptide for Controlled Drug Release in Vivo. Langmuir. 2009;25:8419–8422. doi: 10.1021/la804271d. [DOI] [PubMed] [Google Scholar]
- 218.Shi X., Zheng Y., Wang G., Lin Q., Fan J. pH- and electro-response characteristics of bacterial cellulose nanofiber/sodium alginate hybrid hydrogels for dual controlled drug delivery. RSC Adv. 2014;4:47056–47065. doi: 10.1039/C4RA09640A. [DOI] [Google Scholar]
- 219.Paukkonen H., Kunnari M., Laurén P., Hakkarainen T., Auvinen V.-V., Oksanen T., Koivuniemi R., Yliperttula M., Laaksonen T. Nanofibrillar cellulose hydrogels and reconstructed hydrogels as matrices for controlled drug release. Int. J. Pharm. 2017;532:269–280. doi: 10.1016/j.ijpharm.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 220.Yao S., Yang Y., Wang X., Wang L. Fabrication and characterization of aligned fibrin nanofiber hydrogel loaded with PLGA microspheres. Macromol. Res. 2017;25:528–533. doi: 10.1007/s13233-017-5121-x. [DOI] [Google Scholar]
- 221.Wu R., Niamat R.A., Sansbury B., Borjigin M. Fabrication and Evaluation of Multilayer Nanofiber-Hydrogel Meshes with a Controlled Release Property. Fibers. 2015;3:296–308. doi: 10.3390/fib3030296. [DOI] [Google Scholar]
- 222.Li X., Li J., Gao Y., Kuang Y., Shi J., Xu B. Molecular Nanofibers of Olsalazine Form Supramolecular Hydrogels for Reductive Release of an Anti-inflammatory Agent. J. Am. Chem. Soc. 2010;132:17707–17709. doi: 10.1021/ja109269v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Huang Y., Li X., Lu Z., Zhang H., Huang J., Yan K., Wang D. Nanofiber-reinforced bulk hydrogel: Preparation and structural, mechanical, and biological properties. J. Mater. Chem. B. 2020;8:9794–9803. doi: 10.1039/D0TB01948H. [DOI] [PubMed] [Google Scholar]
- 224.Matson J., Newcomb C.J., Bitton R., Stupp S.I. Nanostructure-templated control of drug release from peptide amphiphile nanofiber gels. Soft Matter. 2012;8:3586–3595. doi: 10.1039/c2sm07420f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rezk A.I., Obiweluozor F.O., Choukrani G., Park C.H., Kim C.S. Drug release and kinetic models of anticancer drug (BTZ) from a pH-responsive alginate polydopamine hydrogel: Towards cancer chemotherapy. Int. J. Biol. Macromol. 2019;141:388–400. doi: 10.1016/j.ijbiomac.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 226.Liang J., Liu G., Wang J., Sun X.S. Controlled release of BSA-linked cisplatin through a PepGel self-assembling peptide nanofiber hydrogel scaffold. Amino Acids. 2017;49:2015–2021. doi: 10.1007/s00726-017-2444-z. [DOI] [PubMed] [Google Scholar]
- 227.Li W. Supramolecular nanofiber-reinforced Puerarin hydrogels as drug carriers with synergistic controlled release and antibacterial properties. J. Mater. Sci. 2020;55:6669–6677. doi: 10.1007/s10853-020-04455-3. [DOI] [Google Scholar]
- 228.Contardi M., Kossyvaki D., Picone P., Summa M., Guo X., Heredia-Guerrero J.A., Giacomazza D., Carzino R., Goldoni L., Scoponi G., et al. Electrospun polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 2021;409:128144. doi: 10.1016/j.cej.2020.128144. [DOI] [Google Scholar]
- 229.Zupančič Š., Sinha-Ray S., Sinha-Ray S., Kristl J., Yarin A.L. Controlled Release of Ciprofloxacin from Core–Shell Nanofibers with Monolithic or Blended Core. Mol. Pharm. 2016;13:1393–1404. doi: 10.1021/acs.molpharmaceut.6b00039. [DOI] [PubMed] [Google Scholar]
- 230.Wang L., Qiu Y., Lv H., Si Y., Liu L., Zhang Q., Cao J., Yu J., Li X., Ding B. 3D Superelastic Scaffolds Constructed from Flexible Inorganic Nanofibers with Self-Fitting Capability and Tailorable Gradient for Bone Regeneration. Adv. Funct. Mater. 2019;29:1901407. doi: 10.1002/adfm.201901407. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.