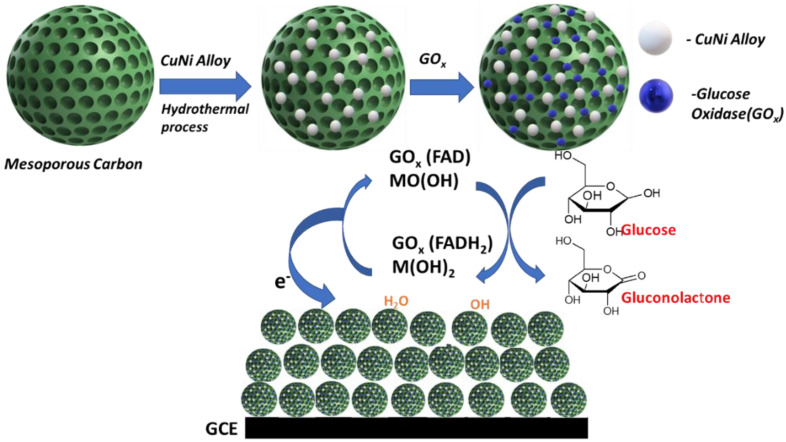
Abstract
The assessment of blood glucose levels is necessary for the diagnosis and management of diabetes. The accurate quantification of serum or plasma glucose relies on enzymatic and nonenzymatic methods utilizing electrochemical biosensors. Current research efforts are focused on enhancing the non-invasive detection of glucose in sweat with accuracy, high sensitivity, and stability. In this work, nanostructured mesoporous carbon coupled with glucose oxidase (GOx) increased the direct electron transfer to the electrode surface. A mixed alloy of CuNi nanoparticle-coated mesoporous carbon (CuNi-MC) was synthesized using a hydrothermal process followed by annealing at 700 °C under the flow of argon gas. The prepared catalyst’s crystal structure and morphology were explored using X-ray diffraction and high-resolution transmission electron microscopy. The electrocatalytic activity of the as-prepared catalyst was investigated using cyclic voltammetry (CV) and amperometry. The findings show an excellent response time of 4 s and linear range detection from 0.005 to 0.45 mM with a high electrode sensitivity of 11.7 ± 0.061 mA mM cm−2 in a selective medium.
Keywords: sweat sensor, glucose detection, non-invasive, mesoporous carbon, bimetallic nanomaterials
1. Introduction
The accurate quantification of glucose is key to the diagnosis and management of patients with diabetes. In 2019, the International Diabetes Federation estimated that diabetes affects 463 million people, constituting 9.3% of the adult population aged 20–79, but with 1 in 2 remaining undiagnosed. Chronic hyperglycemia is a major risk parameter for long-term microvascular and macrovascular complications leading to increased morbidity and mortality, with 11.3% of global deaths being attributed to diabetes [1,2]. Additionally, in patients with COVID-19, whilst long-term glycemic control has not been associated with adverse outcomes, the severity of hyperglycemia at admission has been allied to the prerequisite for mechanical ventilation, admission to the intensive care unit, and mortality, independent of age, diabetes, and hypertension [3,4].
There is increasing use of bioelectrode interfaces with wearable bioelectronics to enable the continuous non-invasive transfer of physiological information [5,6,7,8,9,10]. In addition, the detection of biochemical molecules to indicate physiology and metabolism can be achieved using wearable electrochemical biosensors [11,12,13]. These electrochemical bio-sensors are generally established on enzyme–electrochemical reactions with high specificity [14]. The key step for wearable electrochemical biosensors is the enzyme–electrode interface where the electron transfer takes (ET) place [15,16,17]. The ET between enzymes and electrodes is problematic, as the polypeptides are embedded with the enzymes at the redox-active center [18,19]. Accordingly, to overcome this issue, electron mediators have been used to enhance the enzyme–electrochemical reactions. However, cross-reaction and leakage of the electron mediators result in low selectivity and instability of the biosensors [20,21]. A wearable electrochemical biosensor with high sensitivity and selectivity for biochemical molecules requires direct ET at enzyme–electrode interfaces without electron mediators. For a wearable sensor, a sensing platform is also essential for reproducibility, real-time detection, and high sensitivity. In 2014, Google partnered with Novartis to develop a smart contact for real-time assessment of tear glucose levels but was subsequently abandoned as there was a poor correlation between blood glucose levels and tears.
Sensing glucose in sweat is easy and avoids penetrating the skin to access blood or interstitial fluid. The excretion of glucose in sweat primarily depends on blood glucose; for a healthy individual, the sweat glucose level is 0.02–0.6 mM and 0.01–1 mM for diabetic patients [22,23,24,25,26]. It has been suggested that the electrochemical analysis of glucose concentration in sweat could accurately reflect blood glucose levels [27,28]. Although a recent study showed no correlation between sweat and blood glucose concentrations after submaximal exercise, indicating that sweat composition is at least relatively independent of blood composition [29].
Enzymatic sensors relying on glucose oxidation have been developed to detect glucose from sweat with high sensitivity but have a short lifespan of 1–2 weeks [30]. Interfaces of biological components are not necessary for nonenzymatic glucose sensors, and, therefore, they are cheaper to fabricate and have a longer life span [31]. However, they are less efficient because of their high working potential, slow kinetics, and weak sensing parameters with poor selectivity and low sensitivity [32]. An alternative metal and metal oxide can be used at the interface of nanoporous gold to overcome some of these issues [33,34], but this is expensive and limits commercial usage since the fabrication of low-cost glucose sensors is a market prerequisite [35]. Prussian blue/hydrogen peroxide has been utilized to enhance glucose sensitivity and selectivity in sweat, but it lacks stability and selectivity. Nanomaterials have also shown excellent performance in sweat-based electrochemical glucose biosensing systems. However, nanomaterials such as ZnO nanowires, Cu NCs, CNTs cannot be used efficiently because of their high working potential, pH conditions, low kinetics, and sensing parameters [36]. Therefore, researchers have integrated nanomaterial having high electroconductive and large surface area with glucose oxidase (GOx) enzymes to enhance selectivity and sensitivity. It is worth mentioning that one research group synthesized hierarchical mesoporous CNTs to achieve a sensitivity of 270 ± 10 μA mM−1 cm−2. CuNi glucose-sensing includes good reproducibility, reusability, and stability given the controllable preparation of electrodes and the surface with a robust chemical state. The disadvantage is that glucose-sensing can only be achieved at higher pH.
Glucose levels have primarily been evaluated from blood samples, although there is now increasing use of continuous glucose monitoring, relying on subcutaneous interstitial glucose levels [37,38,39]. This is particularly relevant to assess hypoglycemia, which can occur during Ramadan due to glucose-lowering therapies, particularly insulin [40,41]. Other body fluids such as sweat, saliva, and tears may also be utilized to assess glucose levels; however, the glucose amount in these body fluids is very low, and the sensing devices require high sensitivity to detect glucose. In this context, biosensors have high sensitivity and better stability than traditional methods of glucose detection [42]. Non-invasive glucose monitoring helps in continuous glucose monitoring by providing real-time and dynamic information about the glucose concentration. This method of glucose monitoring measures glucose without puncturing the skin to withdraw blood and without causing pain or trauma [43].
In this work, we have developed non-invasive glucose biosensing based on a Ni-Cu/mesoporous carbon/GOx nanocomposite. The synthesis method is cost-effective and straightforward without bulky and expensive instruments. The prepared composite was analyzed using XRD and TEM, and cyclic voltammetry was performed at various glucose concentrations in sweat samples. In addition, chronoamperometry was used to evaluate the sensitivity and interference (fructose, sucrose, uric acid, and dopamine) performance of the electrode in artificial sweat samples for glucose detection at pH 12.
2. Experimental
2.1. Materials
Sodium hydroxide was purchased from Scharlau, glucose and sucrose were purchased from Fisher scientific. Uric acid and fructose were bought from the Research lab; however, NaCl was purchased from Avonchem. Urea and lactic acid were bought from Alfa Aesar. Mesoporous carbon, copper acetate Cu(CH3COO)2·3H2O and nickel acetate Ni(CH3COO)2·6H2O, N, N-dimethylformamide (DMF) oleic acid, potassium chloride KCl, dopamine, and oleylamine were purchased from Sigma Aldrich.
2.2. Preparation of CNMC
The bimetallic Cu-Ni nanoparticles coated mesoporous carbon was obtained using the same method [11]. Briefly, Cu(CH3COO)2·3H2O and Ni(CH3COO)2·6H2O were placed in 20 mL N, N-dimethylformamide (DMF). The mesoporous carbon was added to the solution and left for 24 h until a homogenous mixture was obtained. Oleic acid and oleylamine were added to the solution and heated to 180 °C for 8 h using a 75 mL sealed Teflon-lined stainless autoclave. The acquired product was centrifuged at 10,000 rpm for 30 min and washed several times using ethanol and deionized water. Subsequently, the dried powder is heated up to 700 °C for 2 h at a heating rate of 5 °C per minute under an argon gas flow to obtain the final product of the mixed Cu-Ni nanoalloy decorated the mesoporous carbon (CuNi-MC).
2.3. Fabrication of Electrode and Sweat Solution
The catalyst ink was synthesized by adding 2 mL of ethanol to a mixture of 10 mg Ni/Cu-mesoporous composite, 2 mg GOx, and 40 μL nafion. The mixture was kept for sonication in an ice bath for 1 h. After that, 10 μL of the suspension was dropped on the GC electrode’s active surface, which was left to dry at room temperature for 1 h to obtain a uniform thin coating on the surface. The artificial sweat solution was synthesized using 5.5 mM lactic acid, 22 mM urea, 10 mM KCl, and 25 mM uric acid [13].
2.4. Characterization Techniques
The TEM analysis of the as-prepared catalyst was obtained by dispersing catalyst in IPA and a drop was left to dry on molybdenum grid to avoid interference with sample elements. A FEI-Thermo scientific-TALOS A FEG transmission electron microscope with a 200 kV beam voltage was used. Elemental analysis and mapping was performed using a FEI-Thermo scientific-SuperX EDS detector. The XRD analysis was carried using Rigaku, Miniflex2 Desktop, Tokyo, Japan, equipped with Cu Kα radiations. The and phase study structural of the as-synthesized catalyst was carried out at a scanning step of 0.02° in the 2θ range from 10° to 90°. Gamry-potentiostat/galvanostat (Ref. 600) was employed to perform the cyclic voltammetry (CV) and chronoamperometry experiments using a three-electrode cell configuration consisting of Ag/AgCl electrode as a reference electrode, the GC as the working electrode, and platinum wire as a counter electrode. The cyclic voltammetry was conducted at a potential range from −0.1 V to 0.8 V vs. Ag/AgCl at 25 mV/s, and the chronoamperometry was done at an applied potential of 0.6 V.
3. Results and Discussion
3.1. XRD and TEM
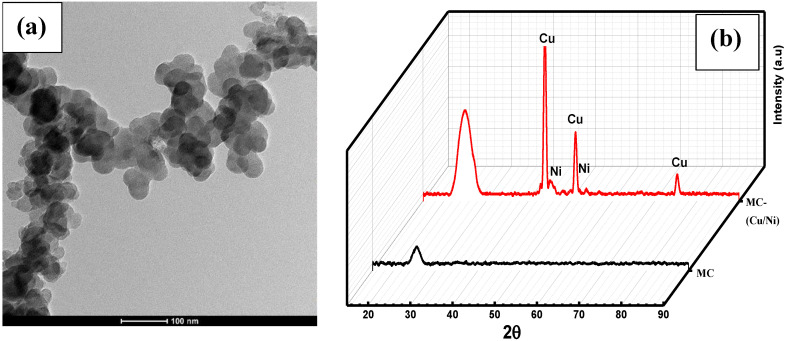
Figure 1a shows the TEM image of the used mesoporous carbon synthesizing the CuNi-MC catalyst with less than 100 nm particle size. The XRD pattern of CuNi-MC is presented in Figure 1b. The average crystal size was estimated from the XRD patterns using the Debye–Scherer equation.
| (1) |
where D is the crystallite size, λ is the wavelength of Cu-kα1 = 1.54060 Å, can be estimated from the experimental peak width (FWHM) of the most intense peak (002). The average crystal size of the bimetallic Ni-Cu-nanoparticles was calculated in the range of 10 ± 2 nm. The amorphous peak positioned at 24.8° was attributed to the mesoporous carbon. However, the peaks located at 43.9°, 51.1°, and 75.1° are related to d values of 2.06, 1.78, and 1.26 Å, which are accredited to the well-determined (111), (200), and (220) crystallographic planes of the bimetallic Cu-Ni nanoparticles, respectively [23,44].
Figure 1.
(a) TEM image of the used mesoporous carbon for catalyst synthesis and (b) XRD of bimetallic CuNi nanoparticle-coated mesoporous carbon.
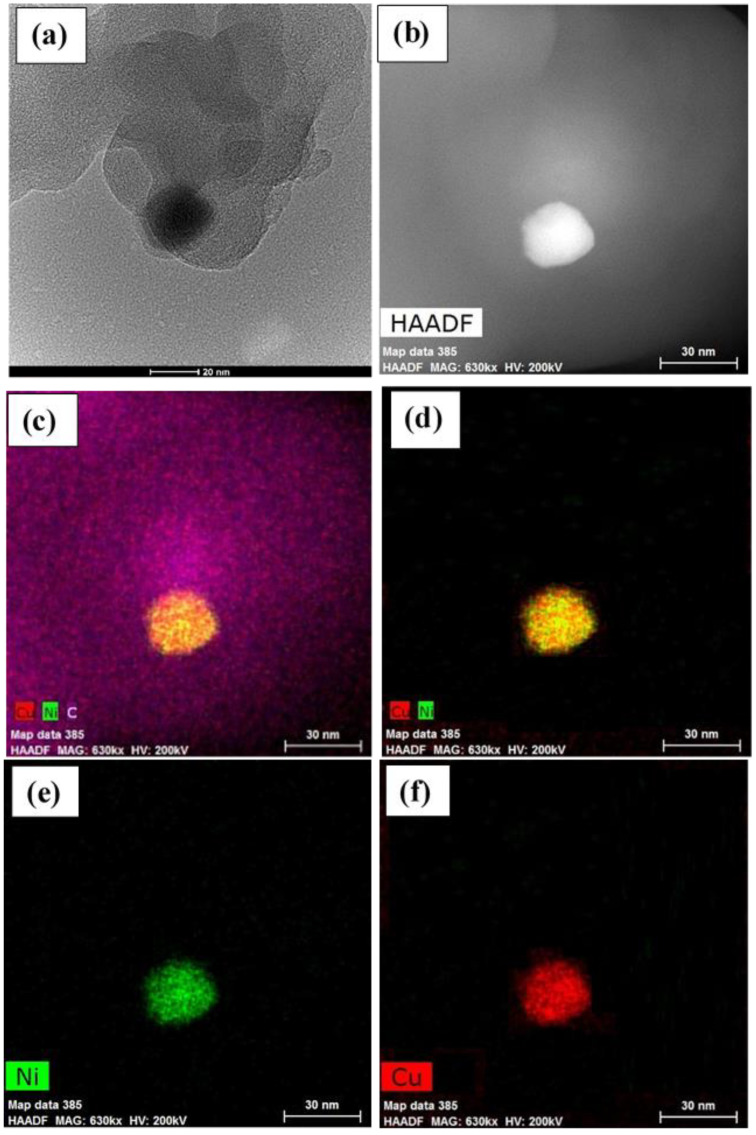
The morphological studies of the as-prepared catalyst were explored using TEM (Figure 2a). The bimetallic Cu/Ni nanoparticles have a spherical shape attached to the mesoporous carbon surface with an average size of 22 ± 5 nm. The STEM and elemental mapping analysis illustrated the construction of mixed Cu/Ni nanoalloy, see Figure 2b–f.
Figure 2.
(a) TEM BF image of the CuNi-MC and (b) High-angle annular dark-field (HAADF)-STEM image, (c–f) shows elemental mapping images of bimetallic CuNi nanoparticle-coated mesoporous carbon.
3.2. Electrochemical Studies
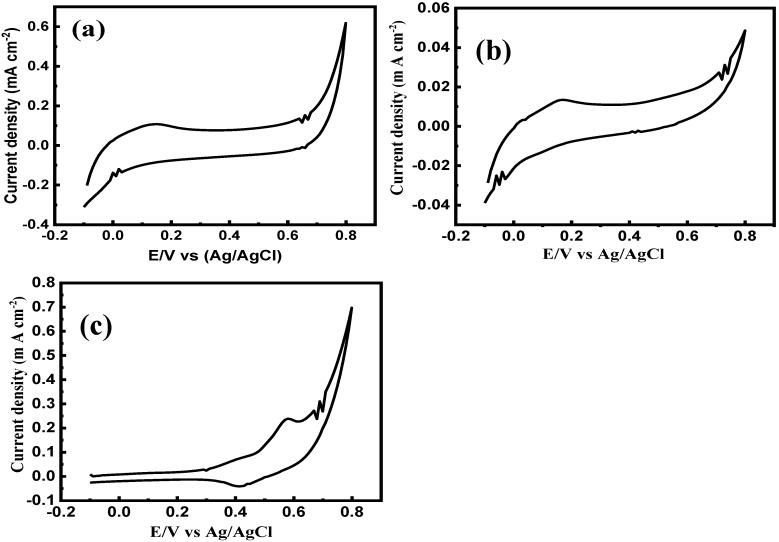
The glucose sensitivity of the as-prepared catalyst was evaluated at variable pH of 7 and 12. Figure 3a shows the CV of the glucose sensor at pH 7, exhibiting no oxidation-reduction peaks. Similar studies have been carried out exploring the effect of the pH on the electrode sensitivity, revealing that the catalyst sensitivity to glucose is boosted by increasing the pH [45,46,47]. The reduced species transferred the excess electrons to the Ni2+/Ni3+ redox couple leading to an enhancement in the redox current. It is worth mentioning that ion contamination limitation can be eliminated employing alkaline conditions on the electrode surface, as the hydroxyl groups (OH¯) suppress the chloride (Cl¯) adsorption to the electrode surface [45,47]. Accordingly, changing the real pH of the sweat solution in this work significantly enhanced the sensitivity of the as-prepared electrode. Figure 3b,c exhibited the CV of the as-synthesized catalyst in the absence and presence of glucose oxidase, showing a distinct variation in their response to glucose. This was ascribed to the glucose oxidation by GOx enzyme-generating H2O2, which was reduced to H2O + O2 at CuNi-MC surface and consequently increased the electrocatalytic currents.
Figure 3.
(a) CV of the enzymatic CuNi-MC in sweat solution after adding glucose of 0.11 mM at pH 7. The CV of CuNi-MC in sweat solution (b) before (c) after the addition of the glucose oxidase (0.11 mM) at pH 12. For all measurements, a scan rate was 50 mV s−1.
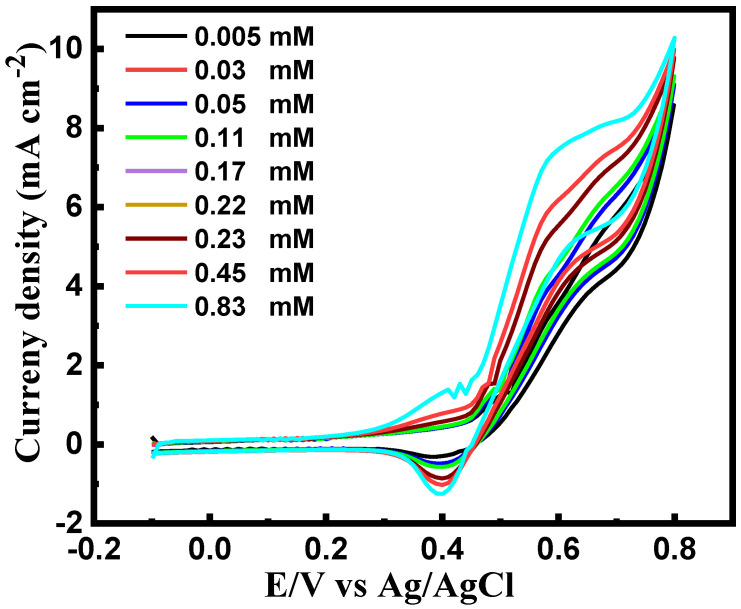
Figure 4 shows the CV of the electrode modified with CuNi-MC in sweat solution at different concentrations of glucose. The redox peak potential of CuNi-MC could be linked to the redox couple of Ni2+ to Ni3+ and Cu2+ to Cu3+ and then back to Ni2+ and Cu2+. This finding indicates that the direct electrochemical reaction of GOx occurs on the CuNi-MC/GOx electrode surface, with GOx maintaining its electroactivity on the CuNi-MC/GOx surface. The current density increased by adding a higher amount of glucose, which could be accredited to the existence of many active sites due to the porous structure of the mesoporous carbon resulting in a high electron transfer rate. A Ni(OH)2 (Equation (5)) film is first constructed on the bimetallic CuNi surface, leading to Cu enrichment on the metal surface followed by oxidation to CuO and Cu(OH)2 (Equations (2) and (3)). Consequently, the film surface is composed of Cu(OH)2 and NiOOH, providing high electrical conductivity for the reaction [48]. Moreover, some CuO and Cu(OH)2 can be further oxidized to Cu3+ oxide [49]. Mathew et al. [50] demonstrated that an electroactive species of Ni(OH)2 could be reconstructed with a predominant α-structure during the reaction of γ-NiO(OH) with glucose while testing their enzymatic nickel hydroxide sensor in an alkaline medium. The generated α-Ni(OH)2 will be oxidized to γ-NiO(OH) during the redox reaction, boosting the anodic current. Consequently, concentrated Ni3+ will be formed on the surface and reduced to Ni2+ during the glucose oxidation reaction, increasing the cathodic current. It is worth mentioning that few of γ-NiO(OH) species could be converted to β-Ni(OH)2, generating NiH or NiO as an irreversible reaction since the test was carried out in an alkaline medium where α-Ni(OH)2 was not stable [50]. Nonetheless, the presence of higher content of Ni2+ and Ni3+ ions on the surface, smaller conversions could slightly influence the redox reaction and current [50]. Nevertheless, the bimetallic CuNi nanoparticles can efficiently restrain the unfavorable γ-NiOOH species construction, stabilizing β-NiOOH forms at pH 12 [51]. The presence of β-NiOOH species is considered to be an improved electroactive phase for superior electrochemical performance in an alkaline medium [52]. The presence of the CuNi bimetallic nanospecies facilitates the e¯ transfer between the GC surface and the GOx, improving glucose detection.
| Cu+ 2OH− → CuO + H2O + 2e− | (2) |
| CuO + H2O → Cu(OH)2 | (3) |
| Cu(OH)2 + 2OH− → CuOOH + H2O + 2e− | (4) |
| Ni+ 2OH− → Ni(OH)2 + 2e− | (5) |
| Ni(OH)2 + 2OH− → NiOOH + H2O+ 2e− | (6) |
Figure 4.
The cyclic voltammetric response of Cu-Ni modified electrode on sweat solution at pH 12 and a scan rate of 50 mV s−1.
Scheme 1 describes the glucose-sensing mechanism of the enzymatic biosensor. GOx catalyzes the glucose oxidation to gluconolactone in the existence of oxygen while generating (H2O2) hydrogen peroxide and water as by-products. In addition, further carboxylic acid, gluconic acid are formed as a result of the reaction of gluconolactone and water. On the other hand, (FAD+) flavin adenine dinucleotide is employed as a redox cofactor for GOx to perform this oxidation reaction. FAD+ acts as an electron acceptor, which will be reduced to FADH2 during the redox process [53]. Subsequent reaction with (O2) to yield hydrogen peroxide revive the FAD+ cofactor. This process takes place at the anode surface, where the number of transferred electrons can be interrelated to the quantity of generated H2O2 and, therefore, the glucose content [53].
| GOx(FAD) + Glucose → GOx(FADH2) + Gluconolactone | (7) |
| GOx(FADH2) → GOx(FAD) + 2e− + 2H+ | (8) |
| NiOOH + Glucose → Ni(OH)2 + Gluconolactone | (9) |
| CuOOH + Glucose → Cu(OH) + Gluconolactone | (10) |
Scheme 1.
Schematic illustration for glucose sensing mechanism based on CuNi-MC/GOx catalyst.
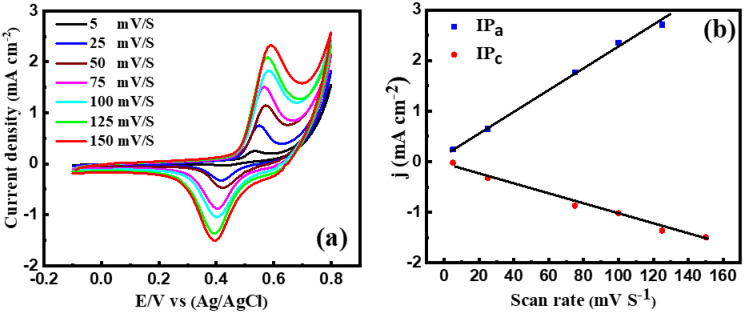
The electrochemical behavior of CuNi-MC was explored at a scan rate that varied from 5 to 150 mV/s in sweat solution (Figure 5a). It is noteworthy that by increasing the scan rate, the hydroxide ions (OH−) penetrate the generated Ni(OH)2 layer giving rise to thickening the NiOOH wrought layer [54]. Both cathodic and anodic peak currents increased in proportion to the scan rates with a linear regression formula of ipa = 0.186 − 0.02 v1/2 (n = 10, R = 0.9967) and ipc = −0.034 + 0.019 v1/2 (n = 10, R = 0.9943), see Figure 5b. These outcomes specify that the electrochemical process of Ni(OH)2/NiOOH transformation is diffusion-controlled [54,55]. The ipc shifted to higher negative values, and the ipa shifted positively by increasing the scan rates, leading to a larger peak-to-peak potential separation. The response of CuNi nanoalloys to glucose electro-oxidation is considerably better than that of pure Cu or Ni due to the synergistic electrochemical influence of the bimetallic nanoparticles since the unalloyed Ni is not robust under highly oxidizing conditions, and Cu has a stabilizing influence on the Ni surface [56].
Figure 5.
(a) Cyclic voltammograms (CVs) of CuNi-MC at different scan rates and (b) peak current density as a function of scan rates at pH 12.
3.3. Chronoamperometric Studies
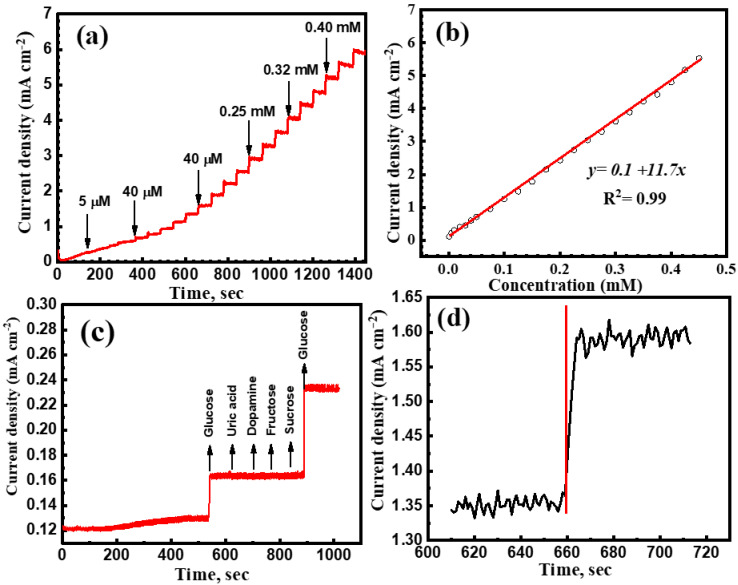
Figure 6a shows the corresponding amperometric response for the CuNi-MC/GOx based glucose sensor. With increasing the glucose content from 5 μm to 0.45 mM, the response of the modified electrode was enhanced due to the enhanced conductivity of the catalyst. Figure 6b exhibits a calibration plot between glucose and the current response of CuNi-MC/GOx modified electrode. The sensor showed a sensitivity of 11.7 ± 0.061 mA mM cm−2 with a linear range of 0.005 to 0.45 mM and a response time of 4 s. This can be explained by the high electrocatalytic activity of the as-prepared catalyst, which acquires the uniformity of the glucose oxidase (GOx) enzyme to perform the electrochemical reaction between the electrode and glucose analyte. Some easily oxidizable compounds such as fructose, sucrose, dopamine, and uric acid coexist with glucose in human sweat. The selectivity of these compounds was analyzed with amperometric measurements concerning glucose concentration. There is no substantial interference with uric acid, dopamine, fructose, and sucrose (Figure 6c). The molecular size of mesoporous carbon is 50 nm, whereas the molecular size of glucose is 0.9 nm. This might be the reason for enhancement in glucose sensitivity, as the glucose can be entrapped in the sites of the mesoporous carbon and interact with GOx enzyme with an increased surface area. Additionally, the molecular size of the other interacting species such as uric acid, dopamine, fructose, and sucrose is more or less similar to glucose. Nevertheless, the presence of GOx enzyme enhances the selectivity of glucose by interacting with the glucose molecules. Therefore, the mesoporous carbon coated on Cu-Ni is selective towards glucose rather than interfering species.
Figure 6.
The chronoamperometric response of the mesoporous carbon coated with CuNi-MC sensor to (a) successive additions of higher glucose concentration, (b) calibration curve including the linear range, (c) after the addition of 40 µM glucose, uric acid, dopamine, fructose, and sucrose at pH 12 and −0.6 V (Ag/AgCl) and (d) response time of the sensor to reach steady-state current density after the addition of 40 µM glucose.
The limit of detection (LOD = 5.2 µm L−1) is calculated employing the following formula.
| (11) |
where σ and m correspond to the standard deviation of the linear range produced after the addition of glucose and the slope of the fitted calibration curve, respectively.
Figure 6d displayed that a fast current response was attained upon adding 40 µM glucose, reaching the steady-state current in less than 4 s. The rapid response time exhibits competent activity of the as-prepared catalyst toward glucose sensing.
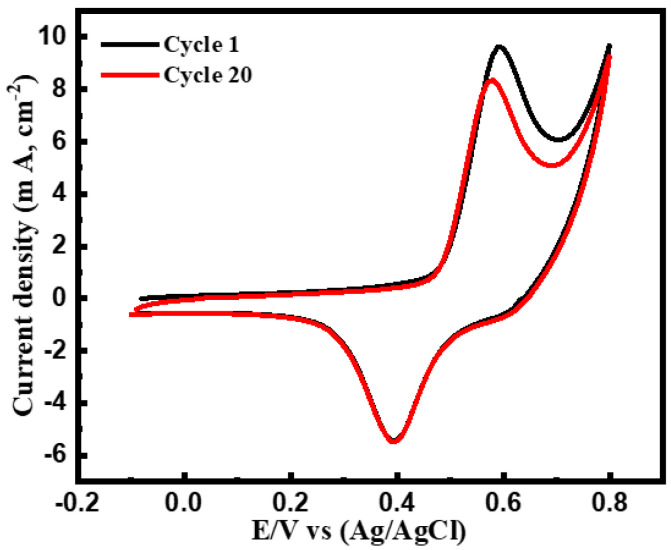
The stability of the as-prepared catalyst has been assessed by recording the current response of the redox couple in 1 mM glucose solution after 20 consecutive voltammetric cycles, see Figure 7. It is worth mentioning that the redox current response has insignificantly reduced after a continuous scan of 20 cycles, demonstrating that the as-prepared sensor is robust and stable.
Figure 7.
Cyclic voltammetric response of CuNi-MC electrode in 1 mM glucose solution at the first cycle and after 20 cycles at a scan rate of 50 mV s−1.
Table 1 shows a comparative study of the mesoporous carbon’s sensitivity with Ni-Cu on glucose with different modified electrodes. The electrocatalytic activity with glucose for the electrode is depicted in terms of the electrode’s high sensitivity. We introduced the mesoporous carbon along with CuNi, which enhanced the glucose sensitivity as the surface area for glucose interaction increased. The highly conductive mesoporous carbon increases the electron transport, which results in high sensitivity of glucose. The electrode modified with mesoporous carbon coated with Ni-Cu shows high sensitivity compared to other electrodes considered here.
Table 1.
Comparative study of the as-prepared electrode with enzymatic electrochemical glucose sensors.
| Sensor | Sensitivity (µA mM cm−2) |
Linear Range | LOD (µm/L) |
Ref. |
|---|---|---|---|---|
| CuNi/MC/GOx/Nafion/GCE | 11.7 × 103 | 5–450 μM | 5.2 | Current work |
| Au-NiO/Ni(OH)2-GOx | 1.95 | up to 30 mM | 1.54 × 103 | [57] |
| GOx/Cu-hemin | 22.77 | 9.1 μM–36 mM | 2.73 | [58] |
| MOFs | ||||
| GOx/Au-ZnO/ | 1.409 | 1–20 mM | 20 | [59] |
| GCE | ||||
| PtNWA/AuNP/ | 184 | 15 μM–2.5 mM | 15 | [60] |
| GOx | ||||
| GOx/CoS-MWCNTs/Nafion/GCE | 14.9 × 103 | 0.008–1.5 mM | 5 | [61] |
4. Conclusions
The composite mesoporous carbon coated with Ni-Cu nanoparticles can be used to fabricate wearable sensors to detect the amount of glucose in sweat. The as-synthesized catalyst’s structural and morphological properties were assessed using XRD and TEM techniques. The electrochemical analysis showed a high electroactivity of the as-synthesized catalyst to glucose electro-oxidation. The catalyst exhibited glucose sensitivity at a wide linear range from 0.005 to 0.45 mM, a low detection limit of 5.2 µM (S/N = 3), high sensitivity of 11.7 mA mM cm−2, and a fast response time of 4 s. The fabricated electrode’s sensitivity range was compared with other previously established electrodes suggesting that the composite is a promising material for the fabrication of wearable sensors for glucose detection. The high sensitivity and selectivity of the glucose sensor can be explained on the basis of the molecular structure and its integrations.
Acknowledgments
This work was supported by an NPRP grant from the Qatar National Research Fund under the grant number NPRP11S-0110-180247. The statements made herein are solely the responsibility of the authors. The authors would like to acknowledge the support of Jana Ponraj and QEERI Core Labs., Hamad Bin Khalifa University, Qatar Foundation, Qatar, for TEM support.
Author Contributions
Conceptualization, A.B.R.; methodology, A.B.R.; validation, A.B.R., S.P. and K.K.S.; formal analysis, A.B.R. and S.P.; resources, J.-J.C., R.A.M., P.K., R.A.S. and K.K.S.; data curation, J.-J.C., A.K.A.-A., R.A.M. and K.K.S.; writing—original draft preparation, A.B.R. and S.P.; writing—review and editing, J.-J.C., A.K.A.-A., P.K., R.A.S., R.A.M., S.A.M. and K.K.S.; supervision, A.B.R. and K.K.S.; funding acquisition, R.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Qatar National Research Fund grant number NPRP11S-0110-180247.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lin X., Xu Y., Pan X., Xu J., Ding Y., Sun X., Song X., Ren Y., Shan P.-F. Global, Regional, and National Burden and Trend of Diabetes in 195 Countries and Territories: An Analysis from 1990 to 2025. Sci. Rep. 2020;10:14790. doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 3.Chen M., Zhou H., Liu X., Yuan T., Wang W., Zhao C., Zhao Y., Zhou F., Wang X., Xue Z., et al. Single Iron Site Nanozyme for Ultrasensitive Glucose Detection. Small. 2020;16:2002343. doi: 10.1002/smll.202002343. [DOI] [PubMed] [Google Scholar]
- 4.Carrasco-Sánchez F.J., López-Carmona M.D., Martínez-Marcos F.J., Pérez-Belmonte L.M., Hidalgo-Jiménez A., Buonaiuto V., Fernández C.S., Castro S.J.F., Luordo D., Fontan P.M.P., et al. Admission Hyperglycaemia as a Predictor of Mortality in Patients Hospitalized with COVID-19 Regardless of Diabetes Status: Data from the Spanish SEMI-COVID-19 Registry. Ann. Med. 2021;53:103–116. doi: 10.1080/07853890.2020.1836566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers J., Bao Z., Lee T.-W. Wearable Bioelectronics: Opportunities for Chemistry. Accounts Chem. Res. 2019;52:521–522. doi: 10.1021/acs.accounts.9b00048. [DOI] [PubMed] [Google Scholar]
- 6.Imani S., Bandodkar A.J., Mohan A.M.V., Kumar R., Yu S., Wang J., Mercier P.P. A Wearable Chemical–Electrophysiological Hybrid Biosensing System for Real-Time Health and Fitness Monitoring. Nat. Commun. 2016;7:11650. doi: 10.1038/ncomms11650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandodkar A.J., Wang J. Non-Invasive Wearable Electrochemical Sensors: A Review. Trends Biotechnol. 2014;32:363–371. doi: 10.1016/j.tibtech.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X. Nanowires Pin Neurons: A Nano “Moon Landing”. Matter. 2019;1:560–562. doi: 10.1016/j.matt.2019.08.011. [DOI] [Google Scholar]
- 9.Dong Q., Ryu H., Lei Y. Metal Oxide Based Non-Enzymatic Electrochemical Sensors for Glucose Detection. Electrochim. Acta. 2021;370:137744. doi: 10.1016/j.electacta.2021.137744. [DOI] [Google Scholar]
- 10.Bai J., Beyer S., Trau D. Conjugated Polymers for Biosensor Devices. In: Ducheyne P., editor. Comprehensive Biomaterials. Elsevier; Oxford, UK: 2011. pp. 529–556. [DOI] [Google Scholar]
- 11.Pankratov D., González-Arribas E., Blum Z., Shleev S. Tear Based Bioelectronics. Electroanalysis. 2016;28:1250–1266. doi: 10.1002/elan.201501116. [DOI] [Google Scholar]
- 12.Kim J., Imani S., de Araujo W.R., Warchall J., Valdés-Ramírez G., Paixao T.R.L.C., Mercier P.P., Wang J. Wearable Salivary Uric Acid Mouthguard Biosensor with Integrated Wireless Electronics. Biosens. Bioelectron. 2015;74:1061–1068. doi: 10.1016/j.bios.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao W., Emaminejad S., Nyein H.Y.Y., Challa S., Chen K., Peck A., Fahad H.M., Ota H., Shiraki H., Kiriya D., et al. Fully Integrated Wearable Sensor Arrays for Multiplexed In Situ Perspiration Analysis. Nature. 2016;529:509–514. doi: 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bollella P., Gorton L. Enzyme based Amperometric Biosensors. Curr. Opin. Electrochem. 2018;10:157–173. doi: 10.1016/j.coelec.2018.06.003. [DOI] [Google Scholar]
- 15.Kim J., Jeerapan I., Sempionatto J.R., Barfidokht A., Mishra R.K., Campbell A.S., Hubble L.J., Wang J. Wearable Bioelectronics: Enzyme-Based Body-Worn Electronic Devices. Accounts Chem. Res. 2018;51:2820–2828. doi: 10.1021/acs.accounts.8b00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Windmiller J.R., Wang J. Wearable Electrochemical Sensors and Biosensors: A Review. Electroanalysis. 2013;25:29–46. doi: 10.1002/elan.201200349. [DOI] [Google Scholar]
- 17.Wu F., Yu P., Mao L. Bioelectrochemistry for In Vivo Analysis: Interface Engineering toward Implantable Electrochemical Biosensors. Curr. Opin. Electrochem. 2017;5:152–157. doi: 10.1016/j.coelec.2017.08.008. [DOI] [Google Scholar]
- 18.Milton R.D., Minteer S.D. Direct Enzymatic Bioelectrocatalysis: Differentiating between Myth and Reality. J. R. Soc. Interface. 2017;14:20170253. doi: 10.1098/rsif.2017.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karyakin A.A. Principles of Direct (Mediator Free) Bioelectrocatalysis. Bioelectrochemistry. 2012;88:70–75. doi: 10.1016/j.bioelechem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Prévoteau A., Mano N. Oxygen Reduction on Redox Mediators May Affect Glucose Biosensors based on “Wired” Enzymes. Electrochim. Acta. 2012;68:128–133. doi: 10.1016/j.electacta.2012.02.053. [DOI] [Google Scholar]
- 21.Matsumoto R., Mochizuki M., Kano K., Ikeda T. Unusual Response in Mediated Biosensors with an Oxidase/Peroxidase Bienzyme System. Anal. Chem. 2002;74:3297–3303. doi: 10.1021/ac015684n. [DOI] [PubMed] [Google Scholar]
- 22.Lee H., Choi T.K., Lee Y.B., Cho H.R., Ghaffari R., Wang L., Choi H.J., Chung T.D., Lu N., Hyeon T., et al. A Graphene-based Electrochemical Device with Thermoresponsive Microneedles for Diabetes Monitoring and Therapy. Nat. Nanotechnol. 2016;11:566–572. doi: 10.1038/nnano.2016.38. [DOI] [PubMed] [Google Scholar]
- 23.Lee H., Song C., Hong Y.S., Kim M.S., Cho H.R., Kang T., Shin K., Choi S.H., Hyeon T., Kim D.-H. Wearable/Disposable Sweat-Based Glucose Monitoring Device with Multistage Transdermal Drug Delivery Module. Sci. Adv. 2017;3:e1601314. doi: 10.1126/sciadv.1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strakosas X., Selberg J., Pansodtee P., Yonas N., Manapongpun P., Teodorescu M., Rolandi M. A Non-Enzymatic Glucose Sensor Enabled by Bioelectronic pH Control. Sci. Rep. 2019;9:10844. doi: 10.1038/s41598-019-46302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nery E.W., Kundys M., Jeleń P.S., Jönsson-Niedziółka M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal. Chem. 2016;88:11271–11282. doi: 10.1021/acs.analchem.6b03151. [DOI] [PubMed] [Google Scholar]
- 26.Müsse A., La Malfa F., Brunetti V., Rizzi F., De Vittorio M. Flexible Enzymatic Glucose Electrochemical Sensor Based on Polystyrene-Gold Electrodes. Micromachines. 2021;12:805. doi: 10.3390/mi12070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyer J., Wilson D., Finkelshtein I., Wong B., Potts R. Correlation Between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther. 2012;14:398–402. doi: 10.1089/dia.2011.0262. [DOI] [PubMed] [Google Scholar]
- 28.Kim J., Campbell A.S., Wang J. Wearable Non-Invasive Epidermal Glucose Sensors: A Review. Talanta. 2018;177:163–170. doi: 10.1016/j.talanta.2017.08.077. [DOI] [PubMed] [Google Scholar]
- 29.Klous L., de Ruiter C.J., Scherrer S., Gerrett N., Daanen H.A.M. The (In)Dependency of Blood and Sweat Sodium, Chloride, Potassium, Ammonia, Lactate and Glucose Concentrations during Submaximal Exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020;121:803–816. doi: 10.1007/s00421-020-04562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falk M., Andoralov V., Silow M., Toscano M.D., Shleev S. Miniature Biofuel Cell as a Potential Power Source for Glucose-Sensing Contact Lenses. Anal. Chem. 2013;85:6342–6348. doi: 10.1021/ac4006793. [DOI] [PubMed] [Google Scholar]
- 31.Rahman M., Ahammad A.J.S., Jin J.-H., Ahn S.J., Lee J.-J. A Comprehensive Review of Glucose Biosensors Based on Nanostructured Metal-Oxides. Sensors. 2010;10:4855–4886. doi: 10.3390/s100504855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thatikayala D., Ponnamma D., Sadasivuni K.K., Cabibihan J.J., Al-Ali A.K., Malik R.A., Min B. Progress of Advanced Nanomaterials in the Non-Enzymatic Electrochemical Sensing of Glucose and H2O2. Biosensors. 2020;10:151. doi: 10.3390/bios10110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan J.H., Wang K., Xia X.H. Highly Ordered Platinum-Nanotubule Arrays for Amperometric Glucose Sensing. Adv. Funct. Mater. 2005;15:803–809. doi: 10.1002/adfm.200400321. [DOI] [Google Scholar]
- 34.Gong X., Gu Y., Zhang F., Liu Z., Li Y., Chen G., Wang B. High-Performance Non-Enzymatic Glucose Sensors Based on CoNiCu Alloy Nanotubes Arrays Prepared by Electrodeposition. Front. Mater. 2019;6 doi: 10.3389/fmats.2019.00003. [DOI] [Google Scholar]
- 35.Hoshino T., Sekiguchi S.-I., Muguruma H. Amperometric Biosensor Based on Multilayer Containing Carbon Nanotube, Plasma-Polymerized Film, Electron Transfer Mediator Phenothiazine, and Glucose Dehydrogenase. Bioelectrochemistry. 2012;84:1–5. doi: 10.1016/j.bioelechem.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Xia H.-Q., Tang H., Zhou B., Li Y., Zhang X., Shi Z., Deng L., Song R., Li L., Zhang Z., et al. Mediator-Free Electron-Transfer on Patternable Hierarchical Meso/Macro Porous Bienzyme Interface for Highly-Sensitive Sweat Glucose and Surface Electromyography Monitoring. Sens. Actuators B Chem. 2020;312:127962. doi: 10.1016/j.snb.2020.127962. [DOI] [Google Scholar]
- 37.Liu Q., Liu Y., Wu F., Cao X., Li Z., Alharbi M., Abbas A.N., Amer M.R., Zhou C. Highly Sensitive and Wearable In2O3 Nanoribbon Transistor Biosensors with Integrated On-Chip Gate for Glucose Monitoring in Body Fluids. ACS Nano. 2018;12:1170–1178. doi: 10.1021/acsnano.7b06823. [DOI] [PubMed] [Google Scholar]
- 38.Liu X., Huang D., Lai C., Qin L., Zeng G., Xu P., Li B., Yi H., Zhang M. Peroxidase-Like Activity of Smart Nanomaterials and Their Advanced Application in Colorimetric Glucose Biosensors. Small. 2019;15:e1900133. doi: 10.1002/smll.201900133. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X., Gu A., Wang G., Huang Y., Ji H., Fang B. Porous Cu–NiO Modified Glass Carbon Electrode Enhanced Nonenzymatic Glucose Electrochemical Sensors. Analyst. 2011;136:5175–5180. doi: 10.1039/c1an15784a. [DOI] [PubMed] [Google Scholar]
- 40.Tong C.V., Yow H.Y., Noor N.M., Hussein Z. Diabetes Emergencies around Ramadan Study (DEARS)—A Multi-Center Study of Diabetes Emergencies Admitted before, during and after Ramadan in Malaysia. Diabetes Res. Clin. Pr. 2021;175:108854. doi: 10.1016/j.diabres.2021.108854. [DOI] [PubMed] [Google Scholar]
- 41.Elhadd T., Bashir M., Baager K.A., Ali H.A., Almohannadi D.H., Dabbous Z., Malik R.A., Abou-Samra A.-B. Mitigation of Hypoglycemia During Ramadan Using the Flash Glucose Monitoring System Following Dose Adjustment of Insulin and Sulphonylurea in Patients Taking Multiple Glucose-Lowering Therapies (The PROFAST-IT Study) Diabetes Res. Clin. Pr. 2021;172:108589. doi: 10.1016/j.diabres.2020.108589. [DOI] [PubMed] [Google Scholar]
- 42.Mehrotra P. Biosensors and their Applications—A Review. J. Oral Biol. Craniofacial Res. 2016;6:153–159. doi: 10.1016/j.jobcr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung J.W., So C.-F., Choi K.-S., Wong T.K. Recent Advances in Noninvasive Glucose Monitoring. Med. Devices Évid. Res. 2012;5:45–52. doi: 10.2147/MDER.S28134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashemizadeh S.A., Biglari M. Cu:Ni Bimetallic Nanoparticles: Facile Synthesis, Characterization and its Application in Photodegradation of Organic Dyes. J. Mater. Sci. Mater. Electron. 2018;29:13025–13031. doi: 10.1007/s10854-018-9424-2. [DOI] [Google Scholar]
- 45.Hassan M., Vyas C., Grieve B., Bartolo P. Recent Advances in Enzymatic and Non-Enzymatic Electrochemical Glucose Sensing. Sensors. 2021;21:4672. doi: 10.3390/s21144672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Şavk A., Aydın H., Cellat K., Şen F. A Novel High Performance Non-Enzymatic Electrochemical Glucose Biosensor based on Activated Carbon-Supported Pt-Ni Nanocomposite. J. Mol. Liq. 2019;300:112355. doi: 10.1016/j.molliq.2019.112355. [DOI] [Google Scholar]
- 47.Gao X., Du X., Liu D., Gao H., Wang P., Yang J. Core-Shell Gold-Nickel Nanostructures as Highly Selective and Stable Nonenzymatic Glucose Sensor for Fermentation Process. Sci. Rep. 2020;10:1365. doi: 10.1038/s41598-020-58403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Druska P., Strehblow H.-H., Golledge S. A Surface Analytical Examination of Passive Layers on CuNi Alloys: Part I. Alkaline Solution. Corros. Sci. 1996;38:835–851. doi: 10.1016/0010-938X(96)00170-9. [DOI] [Google Scholar]
- 49.Luo P., Prabhu S.V., Baldwin R.P. Constant Potential Amperometric Detection at a Copper-Based Electrode: Electrode Formation and Operation. Anal. Chem. 1990;62:752–755. doi: 10.1021/ac00206a021. [DOI] [Google Scholar]
- 50.Mathew M., Sandhyarani N. A Highly Sensitive Electrochemical Glucose Sensor Structuring with Nickel Hydroxide and Enzyme Glucose Oxidase. Electrochim. Acta. 2013;108:274–280. doi: 10.1016/j.electacta.2013.07.010. [DOI] [Google Scholar]
- 51.Ding R., Liu J., Jiang J., Wu F., Zhu J., Huang X. Tailored Ni–Cu Alloy Hierarchical Porous Nanowire as a Potential Efficient Catalyst for DMFCs. Catal. Sci. Technol. 2011;1:1406–1411. doi: 10.1039/c1cy00164g. [DOI] [Google Scholar]
- 52.Jafarian M., Forouzandeh F., Danaee I., Gobal F., Mahjani M.G. Electrocatalytic Oxidation of Glucose on Ni and NiCu Alloy Modified Glassy Carbon Electrode. J. Solid State Electrochem. 2008;13:1171–1179. doi: 10.1007/s10008-008-0632-1. [DOI] [Google Scholar]
- 53.Bankar S.B., Bule M.V., Singhal R.S., Ananthanarayan L. Glucose Oxidase—An Overview. Biotechnol. Adv. 2009;27:489–501. doi: 10.1016/j.biotechadv.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Cheshideh H., Nasirpouri F. Cyclic Voltammetry Deposition of Nickel Nanoparticles on Tio2 Nanotubes and their Enhanced Properties for Electro-Oxidation of Methanol. J. Electroanal. Chem. 2017;797:121–133. doi: 10.1016/j.jelechem.2017.05.024. [DOI] [Google Scholar]
- 55.Pan W., Zheng Z., Wu X., Gao J., Liu Y., Yuan Q., Gan W. Facile synthesis of 2D/3D hierarchical NiCu bimetallic MOF for non-enzymatic glucose sensor. Microchem. J. 2021;170:106652. doi: 10.1016/j.microc.2021.106652. [DOI] [Google Scholar]
- 56.Yi W., Liu J., Chen H., Gao Y., Li H. Copper/Nickel Nanoparticle Decorated Carbon Nanotubes for Nonenzymatic Glucose Biosensor. J. Solid State Electrochem. 2015;19:1511–1521. doi: 10.1007/s10008-015-2766-2. [DOI] [Google Scholar]
- 57.Njoko N., Louzada M., Britton J., Khene S., Nyokong T., Mashazi P. Bioelectrocatalysis and Surface Analysis of Gold Coated with Nickel Oxide/Hydroxide and Glucose Oxidase towards Detection of Glucose. Colloids Surf. B Biointerfaces. 2020;190:110981. doi: 10.1016/j.colsurfb.2020.110981. [DOI] [PubMed] [Google Scholar]
- 58.He J., Yang H., Zhang Y., Yu J., Miao L., Song Y., Wang L. Smart Nanocomposites of Cu-Hemin Metal-Organic Frameworks for Electrochemical Glucose Biosensing. Sci. Rep. 2016;6:36637. doi: 10.1038/srep36637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fang L., Liu B., Liu L., Li Y., Huang K., Zhang Q. Direct Electrochemistry of Glucose Oxidase Immobilized on Au Nanoparticles-Functionalized 3D Hierarchically ZnO Nanostructures and its Application to Bioelectrochemical Glucose Sensor. Sens. Actuators B Chem. 2016;222:1096–1102. doi: 10.1016/j.snb.2015.08.032. [DOI] [Google Scholar]
- 60.Li Z., Gao F., Gu Z. Vertically aligned Pt Nanowire Array/Au Nanoparticle Hybrid Structure as Highly Sensitive Amperometric Biosensors. Sens. Actuators B Chem. 2017;243:1092–1101. doi: 10.1016/j.snb.2016.12.033. [DOI] [Google Scholar]
- 61.Li J., Liu Y., Tang X., Xu L., Min L., Xue Y., Hu X., Yang Z. Multiwalled Carbon Nanotubes Coated with Cobalt(II) Sulfide Nanoparticles for Electrochemical Sensing of Glucose via Direct Electron Transfer to Glucose Oxidase. Microchim. Acta. 2020;187:80. doi: 10.1007/s00604-019-4047-8. [DOI] [PubMed] [Google Scholar]