Abstract
Epstein-Barr virus (EBV) DNA was quantitated in peripheral blood mononuclear cells (PBMC) from 25 healthy subjects, 105 asymptomatic solid-organ transplant (SOT) recipients, and 15 SOT recipients with symptomatic EBV infections by using a newly developed quantitative-PCR technique. Patients with symptomatic EBV infections had significantly higher (P < 0.001) median EBV DNA levels than asymptomatic SOT recipients and immunocompetent individuals. In SOT recipients, the positive predictive value of EBV DNA levels of >1,000 genome equivalents (GE)/0.5 μg of total PBMC DNA was 64.7% for symptomatic EBV infection, while the negative predictive value was 96.1%. In 19 of 32 (59.3%) asymptomatic SOT recipients, EBV DNA levels were consistently below 1,000 GE for as long as 18 months, while 10 of 32 (31.2%) patients had 1,000 to 5,000 EBV GE at least once during follow-up. In a minority of patients (3 of 32; 9.3%), ≥5,000 GE could be detected at least once during follow-up. Reduction of immunosuppressive treatment decreased EBV DNA levels by ≥1 log10 unit in patients with symptomatic EBV infections. Quantification of EBV DNA is valuable for the diagnosis and monitoring of symptomatic EBV infections in SOT recipients.
Posttransplant lymphoproliferative disorders (PTLD) consist of a wide spectrum of manifestations ranging from lymphoid hyperplasia to clonal malignancy that occur following solid-organ and bone marrow transplantation (1, 2, 4, 9, 15, 16, 19, 27).
A strong correlation between Epstein-Barr virus (EBV) infection and the development of PTLD has been recognized (14, 17, 20, 26, 31). In particular, uncontrolled EBV-induced B-cell proliferation in these immunosuppressed patients may play a central role in the pathogenesis of this disease (3, 11, 12, 28). In this respect, it is generally assumed that there is a correlation between the amount of immunosuppression and the risk of developing PTLD (14, 18, 29).
PTLD may be reversible in solid-organ transplant (SOT) recipients following reduction or discontinuation of immune suppression (27). Other therapeutic options include the administration of antiviral drugs (acyclovir, famcyclovir, or foscarnet), alpha interferon, anti-B-cell monoclonal antibodies, and cytotoxic chemotherapy (5, 7, 25). However, these approaches are associated with high mortality rates in patients who do not respond to treatment (15). Recently, the adoptive transfer of EBV-specific cytotoxic T-cell clones has proven highly effective for treatment of PTLD after bone marrow transplantation (BMT) with T-cell depletion (22). Thus, timely detection of PTLD in transplant recipients is necessary so that the appropriate therapy can be adopted. The detection and quantification of EBV DNA in the blood have been utilized as prognostic markers for the development of lymphoproliferative disorders (21, 24, 30), showing a correlation between high levels of EBV DNA in the blood and the development of PTLD (10, 21, 24). However, in two recent papers very different threshold values were indicated as predictive for PTLD, and large differences both in the prevalence and in the levels of EBV DNA in the blood of control populations were reported (13, 23).
In the present study, we describe a novel and simple quantitative PCR (Q-PCR) technique, developed according to an original design, which allowed for reproducible DNA quantification in the range of 101 to 106 EBV genome equivalents (GE)/0.5 μg of peripheral blood mononuclear cell (PBMC) DNA. EBV DNA was comparatively quantitated in immunocompetent individuals as well as in SOT recipients with asymptomatic and symptomatic EBV infections. Threshold values predictive for PTLD were determined in a prospective follow-up study.
MATERIALS AND METHODS
Study populations.
EBV DNA was quantitated in the blood of three groups of individuals: (i) 25 immunocompetent healthy subjects, (ii) 77 randomly selected asymptomatic adult SOT recipients (65 heart, 8 lung, and 4 liver transplant recipients), as well as 28 asymptomatic pediatric heart transplant recipients, and (iii) 12 adult SOT recipients (11 with heart transplants and 1 with a liver transplant) and 3 pediatric heart transplant recipients with symptomatic EBV infections.
The demographics of the study population were as follows. The median age of the 25 immunocompetent subjects (25 females) studied was 27.0 years (range, 23 to 31 years). The median age of the 31 pediatric heart recipients (17 males and 14 females) was 7.6 years (range, 0.2 to 17.3 years). Finally, for the 76 adult heart (64 males and 12 females), 8 lung (4 males and 4 females), and 5 liver (3 males and 2 females) transplant recipients, the median ages were 46.7, 42.3, and 50.1 years, respectively (ranges, 18.2 to 64.4, 22 to 60, and 28.3 to 60.5 years, respectively).
Asymptomatic SOT recipients were randomly enrolled 1 year or more after transplantation concomitantly with a routine physical examination, which was performed every 3 to 6 months, and blood samples were taken and assayed by PCR for the presence of EBV DNA. EBV DNA-positive patients were prospectively monitored every 1 to 3 months for as long as 18 months. Given the very high prevalence of EBV seropositivity in Italy, routine EBV serology was not performed for asymptomatic patients. The virological follow-up of symptomatic patients was initiated in the presence of clinical symptoms suggestive of PTLD. Patients who experienced a febrile syndrome (body temperature of >37.5°C for ≥2 weeks) without lymphadenopathy and for whom rejection, human cytomegalovirus infection, and other causes of fever had been carefully ruled out, were defined as symptomatic. PTLD was diagnosed as reported elsewhere (4, 11, 15, 20, 28), by histologic examination of biopsy or autopsy tissue specimens from patients with lymphadenopathy or disseminated disease. Sections were evaluated for the degree of polymorphism, necrosis, plasmacytic differentiation, and number of large atypical cells. The cellular clonal status was determined by Southern blot analysis of lesional DNA. The presence of EBV within the tumor cells was established by in situ hybridization for EBV encoded RNA (EBER), performed on routinely processed paraffin sections by using a fluorescein-conjugated EBER DNA probe and a phytohemagglutinin in situ hybridization detection kit (DAKO A/S, Glostrup, Denmark) according to the manufacturer's instructions.
DNA extraction from Ficoll-separated PBMC samples.
Aliquots of 106 PBMC were lysed with proteinase K (25 mg/ml) for 1 h at 55°C. After enzyme inactivation at 94°C for 10 min, samples were centrifuged at 3,200 × g for 5 min. Supernatants were collected and precipitated at −80°C for 1 h in the presence of 0.2 M sodium acetate and 3 volumes of 100% ethanol. Then samples were centrifuged at 13,500 × g for 20 min at 4°C. The DNA pellets were washed three times with 70% ethanol and dried at 55°C. Finally, DNA was resuspended in H2O, and 0.5-μg DNA aliquots, roughly corresponding to 105 PBMC, were used in each PCR.
Construction of the internal control and external standards for quantification of EBV DNA by Q-PCR.
Viral DNA in PBMC was quantified by following a previously reported method (6) in principle. Two recombinant DNA molecules, referred to as pP3 and pRM, were constructed. pP3 was obtained by cloning into plasmid PCR 2000 (TA cloning system; Invitrogen, Carlsbad, Calif.) a 269-bp region of the EBV EBNA-1 gene (BamHI K region) which was amplified by the primer pair 1–2 (primer 1, 5′-GTCATCATCATCCGGGTCTC-3′; primer 2, 5′-TTCGGGTTGGAACCTCCTTG-3′). (30). pRM was obtained by cloning into plasmid PCR 2000 a 330-bp recombinant DNA molecule consisting of a pGEM 3Z plasmid (Promega, Madison, Wis.) sequence that was similar in its GC and AT contents to the EBNA-1 amplified region sequence and had the target sequences of primers specific for EBNA-1 artificially added to its ends according to a previously reported PCR-directed mutagenesis technique (32). Thus, pP3 and pRM were amplified by the same set of primers but were different in size. Competition experiments were performed in order to verify the PCR kinetics of the two molecules and to select the most appropriate conditions for the Q-PCR. EBV DNA quantification in PBMC samples was achieved by amplifying serial amounts of pP3 (104, 103, 102, 101, and 100 copies, which were used as external standards) in the presence of 0.5 μg of PBMC DNA from EBV DNA-negative donors and in parallel with PBMC samples. A fixed amount of pRM (100 copies) was added both to standards and to sample PCR mixtures and was used as an internal amplification control.
PCR was carried out for 45 cycles (each consisting of 94°C for 30 s, 58°C for 45 s, and 72°C for 1 min plus 1 additional second per cycle) in 1× PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 1.5 mM MgCl2, 0.001% gelatin) by using 25 pmol of each primer and 2 U of Taq polymerase (Perkin-Elmer, Foster City, Calif.) in a final volume of 50 μl. The pP3 and pRM amplification products were differentiated by ethidium bromide staining after agarose gel electrophoresis. Gel signals were then digitalized and submitted to densitometric analysis (6).
Statistical analysis.
Since the sensitivity of our PCR technique was 10 EBV DNA GE, median EBV DNA levels were calculated by arbitrarily assigning the EBV DNA-negative (<10 GE) samples the value of 5 EBV DNA GE. The prevalences of EBV DNA in immunocompetent individuals, asymptomatic SOT recipients, and SOT recipients with symptomatic EBV infections were compared by the Pearson chi-square test, while differences in EBV DNA levels among the three groups of individuals were analyzed by the Kolmogorov-Smirnov two-sample test for nonparametric sample distribution (Systat for Macintosh; Systat Inc., Evanston, Ill.).
RESULTS
Quantification of EBV DNA by Q-PCR.
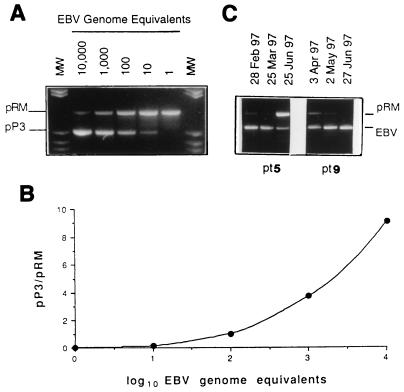
In repeated PCR amplifications of serial dilutions of pP3 (external standard) and pRM (internal control), it was shown that 10 copies of each molecule could be reproducibly detected. Thus, this was defined as the cutoff of our PCR method. In addition, by coamplifying serial concentrations of the two molecules as well as fixed amounts of pRM and serial concentrations of pP3, it was demonstrated that gel signals of identical intensity were consistently obtained following PCR amplification of the same pP3 and pRM copy numbers (Fig. 1). Thus, the two molecules showed comparable PCR kinetics and were amplified with similar efficiency. EBV DNA was quantified according to a previously reported method (6) by comparing the gel signals of the PCR products from the clinical samples with those obtained from the external standards. The use of a fixed amount of pRM (100 copies) as an internal control of amplification allowed for detection of PCR inhibitors in clinical samples and verification of PCR efficiency in each tube, thus allowing normalization of the EBV DNA results during densitometric analysis. The pP3/pRM (100 copies) gel signal ratios were used to plot a standard curve for each test run. Quantification of EBV DNA GE in clinical samples was obtained by interpolating EBV EBNA-1/pRM (100 copies) gel signal ratios (Fig. 1). PBMC samples containing ≥104 GE were diluted 1:10 or 1:100 and then quantitated by the same procedure. Thus, DNA quantification was also achieved in the range of 104 to 106 GE.
FIG. 1.
Construction of the standard curve. (A) pP3 copy number, ranging from 10,000 to 1 (external standards); pRM, 100 copies for each sample (internal control). MW, molecular weight markers (pBR322 cleaved with HaeIII). (B) Standard curve obtained by densitometric analysis of gel signals. (C) Example of EBV DNA quantification in sequential PBMC samples from patient (pt) 5 (from left to right, 7,420, 11,520, and 70 GE) and pt 9 (from left to right, 1,200, 3,980, and 38,900 GE) (see Table 3).
EBV DNA prevalence and quantification.
Significant differences in the prevalence of EBV DNA in the blood were found among the three groups of subjects studied (Table 1). All patients with symptomatic EBV infections (both adults and children) were EBV DNA positive, whereas about half of the asymptomatic SOT recipients had EBV DNA in their blood (P < 0.001). Among asymptomatic SOT recipients, pediatric patients showed a slightly higher prevalence of EBV DNA than adults. However, this difference was not significant. In addition, for asymptomatic patients, no difference was observed in either the prevalence of DNA positivity or EBV DNA levels with respect to time after transplantation. Specifically, at ≤2 years, 2 to 5 years, and >5 years after transplantation, 11 of 21 (52.3%), 21 of 36 (58.3%), and 27 of 48 (56.2%) patients were positive for EBV DNA, respectively. At the same times, median EBV DNA values were 10 (range, <10 to 1,500), 10 (range, <10 to 800), and 10 (range, <10 to 3,000), respectively. In contrast, the rate of EBV DNA positivity in the blood was very low in immunocompetent healthy individuals compared to transplant patients (P < 0.001).
TABLE 1.
Prevalences of EBV DNA-positive subjects in the three indicated population groups
| Population group | No. of EBV DNA-positive subjects/total no. tested (%)a |
|---|---|
| SOTRb with symptomatic EBV infection | |
| Adults | 12/12 (100) |
| Pediatric patients | 3/3 (100) |
| Total | 15/15 (100) |
| Asymptomatic SOTR | |
| Adults | 40/77 (51.9) |
| Pediatric patients | 19/28 (67.8) |
| Total | 59/105 (56.2) |
| Immunocompetent adults | 2/25 (8.0) |
By the Pearson chi-square test, P > 0.05 for adult versus pediatric patients with symptomatic EBV infection and for asymptomatic adult versus asymptomatic pediatric patients, while P < 0.001 for total symptomatic versus total asymptomatic SOT recipients, total symptomatic SOT recipients versus immunocompetent adults, and total asymptomatic SOT recipients versus immunocompetent adults.
SOTR, SOT recipients.
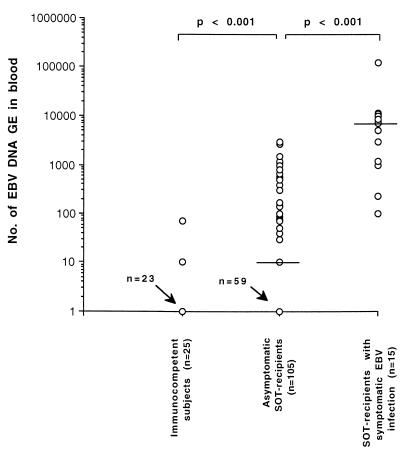
From a quantitative standpoint, marked differences in EBV DNA levels were observed among the three groups of subjects (Fig. 2): median EBV DNA values in immunocompetent individuals, asymptomatic SOT recipients, and SOT recipients with symptomatic EBV infections (values at time of first diagnosis) were <10 (range, <10 to 70), 10 (range, <10 to 3,000), and 5,000 (range, 100 to 126,000), respectively (P < 0.001). No significant difference between EBV DNA onset and peak levels (7,500; range, 100 to 126,000) was observed in symptomatic patients (P > 0.05). A detailed analysis of quantitative EBV DNA levels in the three groups of subjects is shown in Table 2. The large majority of immunocompetent individuals were negative for EBV DNA, while the two EBV DNA-positive subjects had very low EBV DNA levels (<100 EBV GE). Fewer than half of the asymptomatic SOT recipients were EBV DNA negative (46 of 105; 43.8%). However, the great majority of asymptomatic EBV DNA-positive patients had low EBV DNA levels in their blood (≤1,000 EBV GE). Only 6 of 105 (5.7%) asymptomatic SOT recipients had >1,000 EBV GE. In contrast, the majority of symptomatic patients (11 of 15; 73.3%) had >1,000 EBV GE in their blood at the onset of clinical symptoms. On the other hand, 4 of 15 (26.6%) symptomatic patients had low EBV DNA values (10 to 1,000 EBV GE). The positive predictive value (PPV), which was calculated by comparing patients with PTLD to asymptomatic transplant patients, increased with increasing EBV DNA levels (Table 2). By combining data from all patients with >1,000 EBV GE, the PPV was 64.7%, while the negative predictive value (NPV) was 96.1%. However, the NPV was always ≥84.5%, thus confirming the direct correlation between the presence of EBV DNA and the emergence of PTLD (Table 2).
FIG. 2.
EBV DNA levels in the blood of the three population groups studied. Median levels are indicated by horizontal lines. Statistical differences between groups are given at the top.
TABLE 2.
EBV DNA levels in immunocompetent individuals, asymptomatic SOT recipients, and SOT recipients with symptomatic EBV infections
| EBV DNA level (GE/0.5 μg of DNA) | No. of subjects with the indicated level of EBV DNA/total no. tested (%)
|
PPV (%)b | NPV (%)b | ||
|---|---|---|---|---|---|
| Immunocompetent individuals | Asymptomatic SOT recipients | SOT recipients with symptomatic EBV infectionsa | |||
| <10 | 23/25 (92.0) | 46/105 (43.8) | 0/15 | 100.0 | |
| 10–100 | 2/25 (8.0) | 34/105 (32.3) | 2/15 (13.3) | 5.5 | 84.5 |
| 101–1,000 | 0/25 | 19/105 (18.1) | 2/15 (13.3) | 9.5 | 86.8 |
| 1,001–10,000 | 0/25 | 6/105 (5.7) | 8/15 (53.3) | 57.1 | 93.3 |
| >10,000 | 0/25 | 0/105 (0.0) | 3/15 (20.0) | 100.0 | 89.7 |
Onset values.
Calculated with respect to asymptomatic SOT recipients.
Clinical outcome and virologic follow-up.
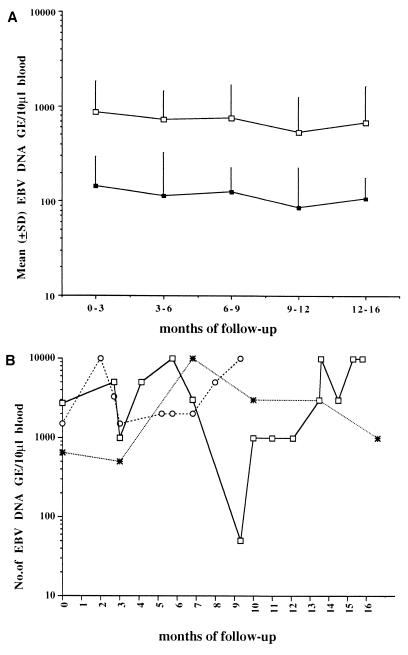
Virologic monitoring of 32 asymptomatic SOT recipients who were EBV DNA positive at the time of enrollment showed some fluctuations in EBV DNA levels over time (Fig. 3). Nineteen of these 32 patients (59.3%) showed EBV DNA levels consistently below 1,000 GE for as long as 16 months (Fig. 3A, lower curve), while 10 (31.2%) had at least one sample with EBV DNA values in the range of 1,000 to 5,000 GE (Fig. 3A, upper curve), and 3 (9.3%) showed ≥5,000 EBV GE in their blood at least once during follow-up (Fig. 3B). In two of these patients, DNA levels of ≥5,000 GE were often detected, and after the 1-year follow-up one of these patients developed a febrile syndrome concomitantly with the appearance of an EBV DNA level of 10,000 GE in the blood (Fig. 3B).
FIG. 3.
Follow-up of EBV DNA levels in 32 SOT recipients with asymptomatic EBV infections. (A) EBV DNA levels (means ± standard deviations) in 19 patients consistently showing <1,000 GE during follow-up (■) and in 10 patients with at least one blood sample in the range of 1,000 to 5,000 GE during follow-up (□). (B) Individual fluctuations of EBV DNA levels in three patients showing ≥5,000 GE in the blood at least once during follow-up.
A summary of the clinical and demographic characteristics of the 15 SOT recipients with symptomatic EBV infections is given in Table 3. Six heart transplant recipients developed febrile illness, and one liver transplant recipient experienced a mild elevation of liver function test results. Patients were diagnosed on the basis of EBV DNA positivity in the blood in the absence of other possible causative agents, as assessed after extensive clinical, radiological, and microbiological analyses. Among the eight patients with more-severe disease, five had non-Hodgkin's lymphoma (NHL), two had disseminated lymphoproliferative disease with multiple organ involvement, and one showed B-cell lymphoproliferation at multiple node sites. The mean time to development of EBV-associated clinical symptoms was 5.3 (range, 1.1 to 9.9) years after transplantation, and no significant difference was observed between patients with mild and severe disease (5.9 ± 3.2 versus 4.7 ± 1.9 years). Although the seven patients with mild EBV infections had lower mean levels of EBV DNA in their blood than the eight patients with more-severe presentations (4,885.7 ± 4,880.7 versus 19,636.6 ± 32,640.0), the difference was not significant (P > 0.05 by the Kolmogorov-Smirnov test).
TABLE 3.
Clinical and demographic characteristics of patients with symptomatic EBV infections
| Patient no. | Age (yr)/sexa | Type of transplant | No. of yrs posttransplantation | EBV DNA level (GE/0.5 μg of PBMC DNA) | Clinical symptom(s)b | Treatmentc | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 46/F | Heart | 5 | 100 | FI, LFTa, leukopenia | ↓ISR | Improved |
| 2 | 62/M | Heart | 10 | 3,000 | FI | ↓ISR | Improved |
| 3 | 62/M | Heart | 5 | 5,000 | NHL | ↓ISR, IFN-α | Dead |
| 4 | 60/F | Liver | 1 | 100 | LFTa | ↓ISR | Improved |
| 5 | 65/M | Heart | 9 | 11,520 | FI | Stop ISR | Improved |
| 6 | 59/M | Heart | 7 | 7,500 | FI | ↓ISR | Improved |
| 7 | 42/M | Heart | 5 | 126,000 | MOI | Stop ISR, ACV | Dead |
| 8 | 60/M | Heart | 8 | 1,000 | FI | ↓ISR | Improved |
| 9 | 51/M | Heart | 3 | 1,200 | FI | ↓ISR | Improved |
| 10 | 46/M | Heart | 8 | 11,200 | MOI | ↓ISR, IFN-α | Dead |
| 11 | 10/F | Heart | 2 | 10,000 | NHL | ↓ISR, ACV | Dead |
| 12 | 67/M | Heart | 6 | 7,000 | Multiple lymph node involvement | ↓ISR, ACV | Dead |
| 13 | 18/F | Heart | 4 | 223 | NHL | ↓ISR, ACV | Improved |
| 14 | 21/M | Heart | 2 | 8,300 | NHL | ↓ISR, ACV | Improved |
| 15 | 21/M | Heart | 7 | 8,570 | NHL | ↓ISR, ACV | Dead |
M, male, F, female.
FI, febrile illness; LFTa, liver function test abnormalities; MOI, multiple organ involvement.
↓ISR, immunosuppressive regimen reduction; IFN-α, alpha interferon; ACV, acyclovir.
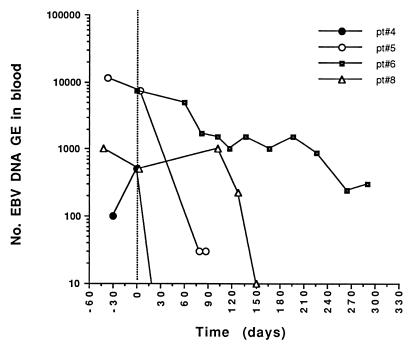
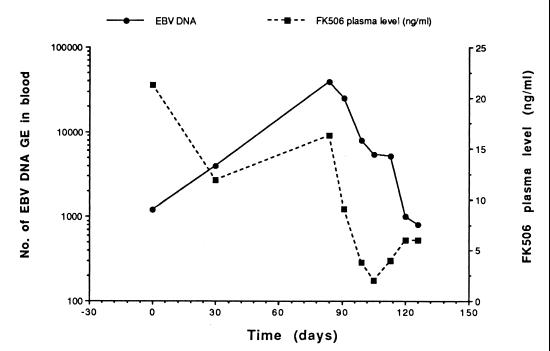
The six patients with febrile illness and the single patient with liver function test abnormalities were treated solely by reduction of their immunosuppressive regimens, and all the patients improved clinically. From the virological standpoint, modulation of immunosuppressive treatment was effective in drastically reducing EBV DNA levels in the blood of each patient. Four patients showed clearance of EBV DNA from the blood, while one patient had a 2-log10 decrease and one patient had a 1-log10 decrease in the level of EBV DNA in the blood. The median time to clearance or ≥1-log10 decrease in the EBV DNA load was 3.2 (range, 1.1 to 5.8) months following reduction of the immunosuppressive regimen. Virological follow-up data for four of the most representative patients are shown in Fig. 4 (patients 4, 5, 6, and 8). Determination of FK506 plasma levels for patient 9 documented parallel decreases in levels of the immunosuppressive drug and EBV DNA in the blood (Fig. 5).
FIG. 4.
Effect of modulation of immune-suppressive therapy on EBV DNA levels in the blood of four SOT recipients with symptomatic EBV infections (see Table 3). The vertical dotted line indicates the initial time of reduction of the immunosuppressive regimen. pt, patient.
FIG. 5.
Parallel reductions in FK506 plasma levels and the EBV DNA load in the blood of an SOT recipient (patient 9 [see Table 3]) with a symptomatic EBV infection.
All five patients with B-cell NHL (Table 3) were treated by reduction of immunosuppressive medication in association with the administration of acyclovir (patients 11, 13, and 14), alpha interferon (patient 3), or chemotherapy (patient 15). Three of these patients died shortly after diagnosis (median survival, 5.4 months; range, 1.1 to 12.6 months), while two patients who showed reductions in EBV DNA levels in the blood were still alive 3.3 and 7.4 months after diagnosis, respectively, with clinical improvement. One patient who developed a PTLD presentation involving multiple lymph nodes (patient 12) was treated by reduction of the immunosuppressive regimen and intravenous (i.v.) administration of acyclovir (30 mg/kg of body weight/day) but eventually died 12 days after diagnosis. Finally, two patients (patients 7 and 10) developed disseminated disease which led to death 0.37 and 4.4 months after diagnosis, respectively, despite complete withdrawal of immunosuppression and administration of i.v. acyclovir for one (patient 7) and a marked decrease in immunosuppression and administration of alpha interferon for the other (patient 10). Although clinically useless (both patients died), administration of alpha interferon in association with modulation of the immunosuppressive regimen was followed by sharp decreases in EBV DNA levels (>1 log10 unit) in both patients.
DISCUSSION
PTLD is characterized by a wide spectrum of manifestations, and it appears particularly important to diagnose PTLD when it is still in the polyclonal phase. In fact, current therapeutic approaches include reduction of immunosuppressive therapy in order to allow the recipient's immune system to control EBV-induced proliferation episodes, as occurs in immunocompetent individuals (27). Treatment of EBV-induced clonal malignancies has proven to be more difficult, since both the greater tumor load and possible variations in the expression of EBV latency proteins may impair B-cell proliferation control and result in poor immune surveillance. Recently, EBV-specific cytotoxic T-cell lines expanded from pretransplant blood samples of SOT recipients have been shown to be an effective therapeutic tool in the posttransplant period (8). However, this treatment approach appears more feasible in the BMT setting (13, 22), given the high incidence of PTLD in T-cell-depleted BMT recipients in the early posttransplant period (13, 14, 22). Generation, storage, and utilization of EBV-specific cytotoxic T-cell lines for SOT recipients appear more problematic, given the lower incidence and the later appearance of PTLD in these patients (2, 4, 9, 15, 16, 19, 27). Thus, the availability of a simple and reliable assay for timely detection of PTLD in transplant recipients is a pressing need.
In a previous study, Riddler et al. found an association between high EBV DNA copy numbers and PTLD in SOT recipients (21). More recently, a cutoff of >500 EBV GE/105 lymphocytes was indicated as associated with PTLD in pediatric SOT recipients, while asymptomatic SOT recipients were mostly EBV DNA negative or showed very low (<200 GE) EBV DNA levels (23). In a third study, using a semiquantitative PCR, it was shown that not all BMT patients with elevated levels of EBV DNA developed PTLD, but all PTLD patients had high EBV DNA levels (13). Although there might be differences in the threshold values related to each method, the emerging pattern indicates that PTLD is associated with a high EBV DNA load in peripheral blood, as one would expect when considering the proposed pathogenesis of this disease. This finding is clinically relevant, and it appears most important to differentiate between patients showing self-limiting reactivations and patients progressing toward an aggressive disease in order to avoid unnecessary modification in the immune-suppressive regimen. However, to date no definite cutoff values predictive for the development of PTLD have been established for SOT and BMT recipients.
We developed a simple and reproducible assay allowing EBV DNA quantitation in the range of 101 to 106 EBV GE. Striking differences in the prevalence of EBV DNA positivity in the blood were observed among the three groups of subjects studied. In fact, all symptomatic patients were EBV DNA positive, whereas about half of the asymptomatic SOT recipients and nearly all the immunocompetent individuals were EBV DNA negative. These results confirm a close association between PTLD and the replication of EBV-infected cells in peripheral blood and, together with data presented by Rowe et al. (23), indicate a marked influence of long-term immune suppression on enhanced replication of EBV-infected cells. No difference was observed between adult and pediatric patients. Quantitation of EBV DNA confirmed the finding by Lucas et al. (13) that a few patients with elevated EBV GE counts did not develop PTLD. Moreover, it was shown that a minority of patients with symptomatic EBV infection had low EBV DNA copy numbers at the onset of symptoms. However, the data clearly demonstrated that the PPV increased with increasing levels of EBV DNA in the blood, reaching 64.7% with EBV DNA levels of >1,000 GE. In addition, the virological follow-up of a subset of asymptomatic EBV DNA-positive SOT recipients showed large fluctuations in EBV DNA levels over time. However, levels were consistently below 1,000 EBV GE in the majority of asymptomatic patients, while about one-third of them showed transiently elevated values. Finally, EBV DNA levels of >5,000 GE were detected repeatedly in only a few asymptomatic patients (3 of 32; 9.3%), and one of these patients developed a symptomatic EBV infection.
These results suggest that the extent of EBV replication in SOT recipients is greater than that in immunocompetent individuals due to long-term administration of immunosuppressive treatment. However, it appears that the enhanced EBV-induced B-cell replication can still be controlled by the patients' immune systems for a long time, at least below a threshold level. Our results also indicate that this threshold is apparently settled at 1,000 EBV GE and that a grey zone might be identified between 1,000 and 5,000 EBV GE. Thus, patients with EBV DNA values in this range may remain asymptomatic or, if the immune system is no longer able to control EBV replication, may progress to PTLD. Detection of these high-risk patients may be helpful for timely therapeutic intervention. In fact, in our series, the best results with immunosuppressive-regimen modulation were obtained for patients with lower EBV DNA counts, in the absence of severe manifestations of PTLD.
In conclusion, our Q-PCR assay appears useful for the diagnosis and monitoring of EBV-induced lymphoproliferative diseases in SOT recipients. In addition, on the basis of our results, it appears reasonable to suggest a routine prospective virologic follow-up of all SOT recipients in order to better understand the kinetics of EBV infection in these patients and to modulate the immunosuppressive regimens accordingly in a timely manner. Adoptive transfer of EBV-specific cytotoxic T lymphocytes may be attempted and may represent the basis for the adoption of specific therapeutic strategies.
ACKNOWLEDGMENTS
We thank Lucia Chezzi, Cinzia Zanello, and Luca Dossena for excellent technical assistance and Linda D'Arrigo for English revision.
This work was partially supported by IRCCS Policlinico S. Matteo, Ricerca Corrente grant 820RCR95/01, Ricerca Finalizzata grant 390 RFM 97/01, and Progetto Finalizzato CNR, grant 97.01256.PF49.
REFERENCES
- 1.Alfrey E J, Friedman A L, Grossman R A, Perloff L J, Naji A, Barker C F, Montone K T, Tomaszewski J E, Chmielewski C, Holland T, Zmijewski C, Dafoe D C. A recent decrease in the time to development of monomorphous and polymorphous post-transplant lymphoproliferative disorder. Transplantation. 1992;54:250–253. doi: 10.1097/00007890-199208000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Armitage J M, Kormos R L, Stuart R S, Fricker F J, Griffith B P, Nalesnik M, Hardesty R L, Dummer J S. Posttransplant lymphoproliferative disease in thoracic organ transplant patients: ten years of cyclosporine-based immunosuppression. J Heart Lung Transplant. 1991;10:877–886. [PubMed] [Google Scholar]
- 3.Cleary M L, Sklar J. Lymphoproliferative disorders in cardiac transplant recipients are multiclonal lymphomas. Lancet. 1984;2:489–493. doi: 10.1016/s0140-6736(84)92566-2. [DOI] [PubMed] [Google Scholar]
- 4.Craig F E, Gulley M L, Banks P M. Posttransplantation lymphoproliferative disorders. Am J Clin Pathol. 1993;99:265–276. doi: 10.1093/ajcp/99.3.265. [DOI] [PubMed] [Google Scholar]
- 5.Fischer A, Blanche S, Le Bidois J, Bordigoni P, Garnier J L, Niaudet P, Morinet F, Le Deist F, Fischer A M, Griscelli C, Hirn M. Anti-B-cell monoclonal antibodies in the treatment of severe B-cell lymphoproliferative syndrome following bone marrow and organ transplantation. N Engl J Med. 1991;324:1451–1456. doi: 10.1056/NEJM199105233242102. [DOI] [PubMed] [Google Scholar]
- 6.Gerna G, Furione M, Baldanti F, Sarasini A. Comparative quantitation of human cytomegalovirus DNA in blood leukocytes and plasma of transplant and AIDS patients. J Clin Microbiol. 1994;32:2709–2717. doi: 10.1128/jcm.32.11.2709-2717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanto D W, Frizzera G, Gajl-Peczalska K J, Sakamoto K, Purtilo D T, Balfour H H, Jr, Simmons R L, Najarian J S. Epstein-Barr virus-induced B-cell lymphoma after renal transplantation: acyclovir therapy and transition from polyclonal to monoclonal B-cell proliferation. N Engl J Med. 1982;306:913–918. doi: 10.1056/NEJM198204153061506. [DOI] [PubMed] [Google Scholar]
- 8.Haque T, Amlot P L, Helling N, Thomas J A, Sweny P, Rolles K, Burroughs A K, Prentice H G, Crawford D H. Reconstitution of EBV-specific T cell immunity in solid organ transplant recipients. J Immunol. 1998;160:6204–6209. [PubMed] [Google Scholar]
- 9.Ho M, Jaffe R, Miller G, Breinig M K, Dummer J S, Makowka L, Atchison R W, Karrer F, Nalesnik M A, Starzl T E. The frequency of Epstein-Barr virus infection and associated lymphoproliferative syndrome after transplantation and its manifestations in children. Transplantation. 1988;45:719–727. doi: 10.1097/00007890-198804000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenagy D N, Schleisinger Y, Weck K, Ritter J H, Gaudreault-Keener M M, Storch G A. Epstein-Barr virus DNA in peripheral blood leukocytes of patients with posttransplant lymphoproliferative disease. Transplantation. 1995;60:547–554. doi: 10.1097/00007890-199509270-00005. [DOI] [PubMed] [Google Scholar]
- 11.Knowles D M, Cesarman E, Chadburn A, Frizzera G, Chen J, Rose E A, Michler R E. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood. 1995;85:552–565. [PubMed] [Google Scholar]
- 12.Locker J, Nalesnik M. Molecular genetic analysis of lymphoid tumors arising after organ transplantation. Am J Pathol. 1989;135:977–987. [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas K G, Burton R L, Zimmerman S E, Wang J, Cornetta K G, Robertson K A, Lee C H, Emanuel D J. Semiquantitative Epstein-Barr virus (EBV) polymerase chain reaction for the determination of patients at risk for EBV-induced lymphoproliferative disease after stem cell transplantation. Blood. 1998;91:3654–3661. [PubMed] [Google Scholar]
- 14.Martin P J, Shulman H M, Schubach W H, Hansen J A, Fefer A, Miller G, Thomas E D. Fatal Epstein-Barr virus-associated proliferation of donor B cells after treatment of acute graft-versus-host disease with a murine anti-T-cell antibody. Ann Intern Med. 1984;101:310–315. doi: 10.7326/0003-4819-101-3-310. [DOI] [PubMed] [Google Scholar]
- 15.Nalesnik M A. Posttransplantation lymphoproliferative disorders (PTLD): current perspectives. Semin Thorac Cardiovasc Surg. 1996;8:139–148. [PubMed] [Google Scholar]
- 16.Nalesnik M A, Jaffe R, Starzl T E, Demetris A J, Porter K, Burnham J A, Makowka L, Ho M, Locker J. The pathology of post-transplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133:173–192. [PMC free article] [PubMed] [Google Scholar]
- 17.Patel R, Paya C V. Infections in solid-organ transplant recipients. Clin Microbiol Rev. 1997;10:86–124. doi: 10.1128/cmr.10.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penn I. The changing pattern of posttransplant malignancies. Transplant Proc. 1991;23:1101–1113. [PubMed] [Google Scholar]
- 19.Penn I, Hammond W, Brettschneider L, Starzl T E. Malignant lymphomas in transplantation patients. Transplant Proc. 1969;1:106–112. [PMC free article] [PubMed] [Google Scholar]
- 20.Randhawa P S, Markin R S, Starzl T E, Demetris A J. Epstein-Barr virus-associated syndromes in immunosuppressed liver transplant recipients. Clinical profile and recognition on routine allograft biopsy. Am J Surg Pathol. 1990;14:538–547. doi: 10.1097/00000478-199006000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riddler S A, Breining M C, McKnight J L. Increased levels of circulating Epstein-Barr virus (EBV)-infected lymphocytes and decreased EBV nuclear antigen antibody responses are associated with the development of post-transplant lymphoproliferative disease in solid-organ transplant recipients. Blood. 1994;84:972–984. [PubMed] [Google Scholar]
- 22.Rooney C M, Smith C A, Ng C Y, Loftin S, Li C, Krance R A, Brenner M K, Heslop H E. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 23.Rowe D T, Qu L, Reynes J, Jabbour N, Yunis E, Putnam P, Todo S, Green M. Use of quantitative competitive PCR to measure Epstein-Barr virus genome load in the peripheral blood of pediatric transplant patients with lymphoproliferative disorders. J Clin Microbiol. 1997;35:1612–1615. doi: 10.1128/jcm.35.6.1612-1615.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savoie A, Perpete C, Carpentier L, Joncas J, Alfieri C. Direct correlation between the load of Epstein-Barr virus-infected lymphocytes in the peripheral blood of pediatric transplant patients and risk of lymphoproliferative disease. Blood. 1994;83:2712–2722. [PubMed] [Google Scholar]
- 25.Shapiro R S, Chauvenet A, McGuire W, Pearson A, Craft A W, McGlave P, Filipovich A. Treatment of B-cell lymphoproliferative disorders with interferon alfa and intravenous gamma globulin. N Engl J Med. 1988;318:1334. doi: 10.1056/NEJM198805193182013. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro R S, McClain K, Frizzera G, Gajl-Peczalska K J, Kersey J H, Blazar B R, Arthur D C, Patton D F, Greenberg J S, Burke B, Ramsay N K C, McGlave P, Filipovich A M. Epstein-Barr virus-associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood. 1988;71:1234–1243. [PubMed] [Google Scholar]
- 27.Starzl T E, Nalesnik M A, Porter K A, Ho M, Iwatsuki S, Griffith B P, Rosenthal J T, Hakala T R, Shaw B W, Jr, Hardesty R L, Atchinson R W, Jaffe R, Bahnson H T. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984;i:583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swerdlow S H. Post-transplant lymphoproliferative disorders: a morphologic, phenotypic and genotypic spectrum of disease. Histopathology. 1992;20:373–385. doi: 10.1111/j.1365-2559.1992.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 29.Swinnen L J, Costanzo-Nordin M R, Fisher S G, O'Sullivan E J, Johnson M R, Heroux A L, Dizikes G J, Pifarre R, Fisher R I. Increased incidence of lymphoproliferative disorders after immunosuppression with the monoclonal antibody OKT3 in cardiac transplant recipients. N Engl J Med. 1990;323:1723–1728. doi: 10.1056/NEJM199012203232502. [DOI] [PubMed] [Google Scholar]
- 30.Telenti A, Marshall W F, Smith T F. Detection of Epstein-Barr virus by polymerase chain reaction. J Clin Microbiol. 1990;28:2187–2190. doi: 10.1128/jcm.28.10.2187-2190.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss L M, Mohaved L A. In situ demonstration of Epstein-Barr viral genomes in viral-associated B cell lymphoproliferations. Am J Pathol. 1989;134:651–659. [PMC free article] [PubMed] [Google Scholar]
- 32.Zipeto D, Baldanti F, Zella D, Furione M, Cavicchini A, Milanesi G, Gerna G. Quantification of human cytomegalovirus DNA in peripheral blood polymorphonuclear leukocytes of immunocompromised patients by the polymerase chain reaction. J Virol Methods. 1993;44:45–51. doi: 10.1016/0166-0934(93)90006-d. [DOI] [PubMed] [Google Scholar]