Abstract
Simple Summary
Poly (ADP-ribose) polymerases (PARPs) are DNA damage repair proteins that are involved in various biological activities ranges from cell proliferation to cell death. The prognostic significance of PARPs is not fully clarified in various cancers. This systematic review aims to reveal the prognostic value of PARP expression in solid cancers and to further correlate with clinicopathological and immunohistochemical markers. Lastly, the inhibition of this pathway through its specific inhibitors may increase the survival of patients with high PARP expression.
Abstract
Poly (ADP-ribose) polymerase (PARP) is a DNA damage repair protein, and its inhibitors have shown promising results in clinical trials. The prognostic significance of PARP is inconsistent in studies of various cancers. In the present study, we conducted a systematic review and meta-analysis to reveal the prognostic and clinicopathological significance of PARP expression in multiple solid cancers. We searched the MEDLINE, EMBASE, and Cochrane databases for relevant research articles published from 2005 to 2021. The pooled hazard ratio (HR) with confidence interval (CI) was calculated to investigate the relationship between PARP expression and survival in multiple solid cancers. In total, 10,667 patients from 31 studies were included. A significant association was found between higher PARP expression and overall survival (OS) (HR = 1.54, 95% CI = 1.34–1.76, p < 0.001), disease-free survival (DFS) (HR = 1.15, 95% CI = 1.10–1.21, p < 0.001), and progression-free survival (PFS) (HR = 1.05, 95% CI = 1.03–1.08, p < 0.001). Subgroup analyses showed that PARP overexpression was significantly related to poor OS in patients with breast cancers (HR = 1.38, 95% CI = 1.28–1.49, p < 0.001), ovary cancers (HR = 1.21, 95% CI = 1.10–1.33, p = 0.001), lung cancers (HR = 2.11, 95% CI = 1.29–3.45, p = 0.003), and liver cancers (HR = 3.29, 95% CI = 1.94–5.58, p < 0.001). Regarding ethnicity, Asian people have almost twice their worst survival rate compared to Caucasians. The pooled odds ratio analysis showed a significant relationship between higher PARP expression and larger tumour size, poor tumour differentiation, lymph node metastasis, distant metastasis, higher TNM stage and lymphovascular invasion, and positive immunoreactivity for Ki-67, BRCA1, and BRCA2. In addition, nuclear expression assessed by the QS system using Abcam and Santa Cruz Biotechnology seems to be the most commonly used and reproducible IHC method for assessing PARP expression. This meta-analysis revealed that higher PARP expression was associated with a worse OS, DFS, and PFS in patients with solid cancers. Moreover, inhibition of this pathway through its specific inhibitors may extend the survival of patients with higher PARP expression.
Keywords: poly(ADP-ribose) polymerases, neoplasm, BRCA1 and BRCA2, prognosis, meta-analysis
1. Introduction
Poly (ADP-ribose) polymerases (PARPs) are DNA damage repair proteins, and their inhibitors have received great attention from researchers owing to their promising results in clinical trials [1,2]. PARPs are generally involved in many biological activities, including cell proliferation and cell death, mRNA transcription, DNA replication and repair, inflammation, cell apoptosis, and the maintenance of genomic integrity [1,2]. Upon DNA damage, PARPs bind to the damaged site and produce a poly-ADP chain from the NAD+ substrate that recruits the DNA repair protein through single-stranded break (SSB) or double-stranded break (DSB) repair pathways [1,2]. The overexpression of PARPs has been examined in various cancers and is linked with a resistance to DNA-damaging therapeutic agents [2,3]. The blockade of this pathway by specific PARP inhibitors inhibits the recruitment of DNA repair proteins and causes cell death, which may extend the long-term survival of patients with cancer [2,4]. The efficacy of PARP inhibitors is currently being investigated in clinical trials. Olaparib is the first US Food and Drug Administration (FDA)-approved PARP inhibitor for use in treating advanced ovarian cancer with germline BRCA1/2 mutations [5,6]. In a phase 2 clinical trial, Olaparib treatment at a dose of 400 mg twice/day in platinum-sensitive and high-grade ovarian cancer patients significantly improved their median progression-free survival (PFS) by 4 months [6].
Based on this background, many studies have investigated the prognostic significance of PARP expression in various cancers, and their results were inconsistent because of their limited sample sizes and suboptimal study designs [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. The prognostic significance of PARPs has been studied in multiple cancers, such as breast [10], ovarian [24], lung [26], glial [32], oesophageal [33], and pancreatic cancers [35]. Most studies in these cancers have shown that a higher expression of PARP-1 is associated with poor outcomes [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,36,37]. However, a better prognosis has been reported in a few studies on pancreatic [35] and breast cancers [7]. The discrepancy between these studies may be due to the study design, sample size, organ type, ethnicity, and other secondary factors. Hence, it is indispensable to evaluate the prognostic significance of PARPs in various solid cancers.
In the present study, we conducted a systematic review and meta-analysis to reveal the prognostic value of PARP expression in solid cancers and to further correlate with the clinicopathological and immunohistochemical (IHC) markers. Additionally, we analysed the implications of the antibody clones, scoring systems, and the localisation of the immunoreactivity in IHC for the PARPs.
2. Materials and Methods
We performed a systematic review and meta-analysis of the literature according to the following guidelines set out by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) statement [38]. The detailed protocol for this systematic review is registered in PROSPERO (280990).
2.1. Search Strategy
The Institutional Review Board of the Catholic University of Korea, College of Medicine approved this meta-analysis-based study (SC20ZISE0050). For this study, we retrieved articles between January 2005 and January 2021 from major electronic databases such as PubMed (MEDLINE), EMBASE, and the Cochrane Library using the following search keywords: “poly(ADP-ribose) polymerases”, “neoplasms”, “PARP-1 prognostic significance”, “immunohistochemistry”, and “PARP malignant neoplasm”. We also manually obtained articles to identify relevant papers. These records were managed using EndNote 20 (ver. 20.0.1, Bld. 15043, Thomson Reuters, New York, NY, USA).
2.2. Inclusion and Exclusion Criteria
Studies were included if they met the following criteria: (1) studies that included solid cancers, (2) studies that measured the PARP expression in tumour tissues via IHC and polymerase chain reaction (PCR), and (3) studies that showed the association between PARP expression and the clinicopathological parameters and survival outcomes, such as overall survival (OS) and disease-free survival (DFS). In the exclusion process, the following criteria were used: (1) meta-analyses, reviews, duplicate reports, commentaries, ongoing studies, editorials, non-English papers, or conference abstracts and (2) articles whose experimental designs and research methods were not similar and were mainly conducted on cell lines and animals.
2.3. Data Extraction and Quality Assessment
The author NT independently extracted the relevant data from the included studies and noted any discrepancies, which were resolved by consulting YC. The following data were extracted from each eligible study: name of the first author, year of publication, country, study period, organ, patient age range, median age, median follow-up period, PARP phenotype, chemotherapy regimen used, detection method used, PARP cut-off value, results of the survival analysis, and HRs. The information about IHC such as antibody vendors and clones, scoring systems, and localisation of the immunoreactivity (nuclear/cytoplasmic) were also documented. Furthermore, we used the Newcastle–Ottawa quality assessment scale (NOS) to evaluate the quality of all the eligible articles. The NOS consists of three parameters: selection, comparability, and outcome, with the total scores ranging from 0 to 9.
2.4. Statistical Methods
For the statistical analysis, we used Review Manager (version 5.3; Cochrane Collaboration, Oxford, UK) to calculate the pooled hazard ratios (HRs) with a 95% confidence interval (95% CI) by making a forest plot and investigated the relationship between PARP expression and patient survival (direct method). A subgroup analysis was conducted to determine the heterogeneity using a fixed-effects model, with HRs >1 denoting adverse outcomes. In the studies with Kaplan–Meier (K–M) curves but no HRs, the method used by Parmar et al. was performed to extract the data from the K–M curves and calculate the HR (indirect method) [39]. Mantel–Haenszel pooled odds ratios (ORs) with 95% CIs were used to evaluate the relationship between the PARP expression and clinicopathological and other IHC markers. An I2 statistic greater than 50% indicated the existence of heterogeneity between the studies. Begg and Egger’s tests were used to evaluate the publication bias quantitatively. Statistical significance was set at p < 0.05.
3. Results
3.1. Search Results
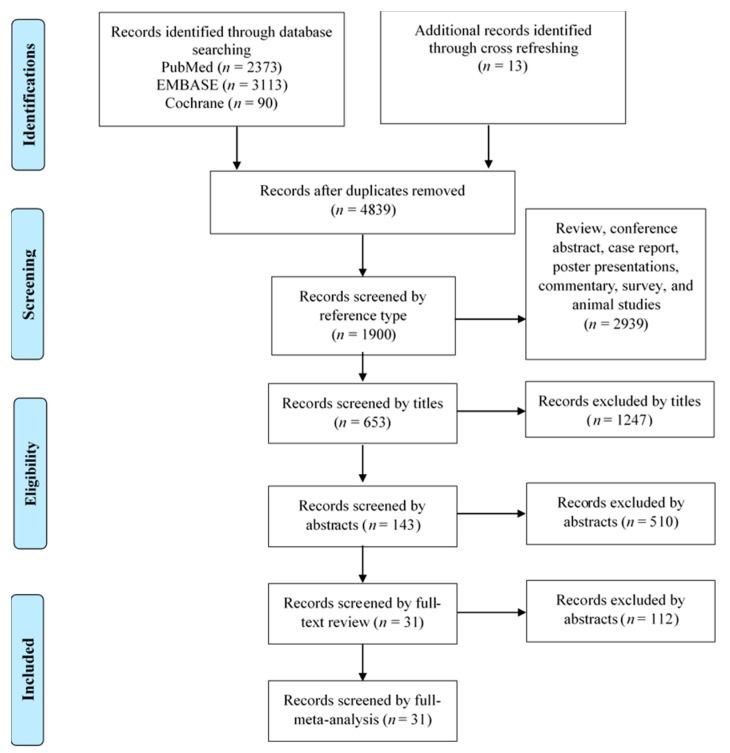
Figure 1 summarises the flowchart of the article selection process for the meta-analysis and review. The initial database searches identified a total of 5589 records (2373 in MEDLINE, 3113 in EMBASE, and 90 in the Cochrane library), and 13 supplementary records were identified from forward and backward searches, all of which were then imported to Endnote. After removing 780 duplicate records, 2939 records were removed because of irrelevant reference types. Next, 1247 records were excluded by titles, and the abstracts from 653 records were reviewed. After the exclusion of 510 records by abstract reviewing, a full-text review was performed for 143 records. After excluding 112 records, only 31 records (consisting of 33 cohorts) met the inclusion criteria for qualitative and meta-analytic synthesis.
Figure 1.
Flow chart for the study selection process.
3.2. Study Characteristics
The major characteristics of all the studies included in this meta-analysis are summarised in Table 1. In total, 31 records comprising 33 cohorts and 10,667 patients were included. Most of the studies were conducted in China (n = 8), followed by the UK (n = 4); Hungary and South Korea (n = 3 each); Egypt, Italy, Germany, the USA, and France (n = 2 each); and Poland, Spain, Saudi Arabia, Japan, and Austria (n = 1 each) (Table 1) [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. The ages of the patients ranged from 21 to 89 years. The sample sizes of all the studies ranged from 50 to 2811, and the follow-up periods ranged from 1.9 to 15 years. The studies included in this meta-analysis were published between 2010 and 2021. The PARP phenotypes included in this study were mostly PARP-1 (n = 23) with the occasional PARP-2 (n = 1) and PARP-3 (n = 1), and the detection methods used to evaluate the PARP expression levels were IHC (n = 28) and PCR (n = 3). Moreover, 11 cohorts reported OS, five cohorts reported DFS, two cohorts reported PFS, nine cohorts reported both DFS and OS, and six cohorts reported both PFS and OS. The following cohorts were stratified according to organ types: breast (n = 14) [7,8,9,10,11,12,13,14,15,16,18,19], ovary (n = 7) [20,21,22,23,24,25], lung (n = 3) [26,27,28], liver (n = 2) [29,30], soft tissue (n = 2) [31,37], brain (n = 1) [32], oesophagus (n = 1) [33], stomach (n = 1) [34], pancreas (n = 1) [35], and skin (n = 1) [36]. The NOS scores of all the studies were higher than 7, which represents a relatively good quality (Table 1 and Supplementary Table S1).
Table 1.
Basic characteristics of all the included studies regarding PARPs for this meta-analysis.
| Organ | Authors | Year | Country | Ethnicity | Study Period | Cancer Type/TNM Stage | Patients No. |
Average Age (Range) |
Median f/u (Years) | PARP Phenotype | Chemo Regimen | Detection Method | Hazard Ratio (CI 95%) |
NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast | Gonclaves [10] |
2011 | France | Caucasian | NA | IDC/ILC/MC/ others Stage I-IIIB |
2485 | NA | 8 | PARP-1 | Adj. Ant/Tax/CMF |
PCR | OS: 1.16 (1.04–1.29) | 8 |
| Minckwitz [16] |
2011 | Germany | Caucasian | 2001–2005 | IDC/ILC/ others Stage IIA-IIIB |
638 | NA | 4.8 | PARP | Neoadj. Ant/Tax |
IHC | OS: 1.76 (0.87–3.50) DFS: 1.44 (1.88–2.36) |
8 | |
| Rojo [17] |
2012 | Spain | Caucasian | 1998–2000 | IDC/ILC/ others NA |
330 | 58 (26–90) |
8.5 | PARP-1 | Adj. CMF/horm |
IHC | OS: 1.82 (1.32–2.52) DFS: 10.05(5.42–18.66) |
8 | |
| Donizy [9] |
2014 | Poland | Caucasian | 1993–1994 | IDC Stage IIA-IIB |
83 | 55.2 | 15 | PARP-1 | Neoadj. CMF/Ant |
IHC | OS: 2.81 (1.12–7.03) | 7 | |
| Aiad (1) * [7] |
2015 | Egypt | Caucasian | 2008–2012 | IDC Stage IIIB |
84 | 53 (29–86) |
NA | PARP-1 | Neoadj.FEC | IHC | OS: 0.40 (0.08–2.05) | 7 | |
| Aiad (2) * [7] |
2015 | Egypt | Caucasian | 2008–2012 | IDC Stage IIIB |
84 | 53 (29–86) |
NA | PARP-1 | Neoadj.FEC | IHC | OS: 0.21 (0.04–1.00) | 7 | |
| Green [11] |
2015 | UK | Caucasian | 1989–2004 | OBC Stage I–III |
1269 | 55 | NA | PARP-1 | NA | IHC | DFS: 1.32 (0.99–1.77) | 7 | |
| Park [14] |
2015 | South Korea | Asian | 1997–2003 | IC/ILC Stage I–IV |
192 | 47 (22–73) |
11.2 | PARP-1 | Adj. Ant/Tax/CMF |
IHC | OS: 3.64 (1.88–7.05) DFS: 1.95 (1.44–3.34) |
8 | |
| Zhai [15] |
2015 | China | Asian | 2007–2012 | IDC/ILC Stage I-IIB |
198 | 53 (29–70) |
4 | PARP-1 | Neoadj. Ant/Tax |
IHC | OS: 4.30 (0.74–25.00) PFS: 1.18 (1.03–1.35) |
8 | |
| Mazzotta [13] |
2016 | Italy | Caucasian | 1998–2012 | IDC/others Stage I-IIIB |
114 | 53 | 4.8 | PARP-1 | Adj. Horm/chemo |
IHC | OS: 1.59 (1.40–181.19) DFS: 6.61 (1.52–28.80) |
8 | |
| Deng [8] |
2017 | China | Asian | 2000–2012 | TNBC Stage I-IIIB |
118 | 51.6 (25–81) |
6.2 | PARP-1 | Adj. Ant/Tax |
IHC | DFS: 2.23 (1.20–4.13) | 8 | |
| Mangia [12] |
2017 | Italy | Caucasian | 1996–2012 | IDC/ILC/ others NA |
308 | 51 (24–80) |
6.1 | PARP-1 | NA | IHC | OS: 1.71 (0.36–8.16) DFS: 0.78 (0.32–1.91) |
8 | |
| Song [19] |
2017 | China | Asian | 2005–2010 | IDC, LC, MC Stage I-IV |
547 | 51 (20–82) |
9.8 | PARP-3 | Adj. CAF/CEFD | IHC | OS: 1.71 (0.93–3.15) DFS: 1.94 (1.19–3.19) |
8 | |
| Siraj [18] |
2018 | Saudi Arabia | Caucasian | 1990–2011 | IDC, ILC, MC Stage I–IV |
1008 | 45 (39–54) |
4 | PARP | NA | IHC | OS: 1.43 (1.01–2.04) | 8 | |
| Ovary | Brustmann [22] |
2007 | Austria | Caucasian | 1985–1996 | SOC Stage I–III |
50 | 64 | NA | PARP | Adj. PBC | IHC | DFS: 1.16 (1.02–1.31) | 7 |
| Barnett [21] |
2010 | USA | Caucasian | 1995–2003 | SOC Stage I–IV |
186 | 61 (19–86) |
NA | PARP | Adj. PBC | IHC | OS: 0.71 (0.50–0.99) PFS: 0.36 (0.09–1.51) |
7 | |
| Gan (1) * [23] |
2013 | UK | Caucasian | 1991–2007 | SOC Stage I–IV |
174 | 61 (36–86) |
NA | PARP-1 | Adj. PBC | IHC | OS: 1.90 (1.10–3.20) PFS: 2.59 (1.12–6.00) |
7 | |
| Gan (2) * [23] |
2013 | UK | Caucasian | 1991–2007 | SOC Stage I–IV |
174 | 61 (36–86) |
NA | C-PARP-1 | Adj. PBC | IHC | OS: 1.63 (1.04–2.57) PFS: 1.20 (1.00–1.44) |
7 | |
| Ali [20] |
2019 | UK | Caucasian | 1997–2010 | SOC Stage I–IV |
525 | NA | NA | PARP-1 | Adj. PBC | IHC | PFS: 1.13 (0.83–1.54) | 7 | |
| Molnar [24] |
2020 | Hungary | Caucasian | 2011–2017 | SOC Stage IIIA-IIIB |
86 | 57 | 2.7 | PARP | Adj. Pac/Carbo | IHC | OS: 11.74 (1.30–105.63) PFS: 13.52 (1.86–98.04) |
8 | |
| Molnar [25] |
2021 | Hungary | Caucasian | 2011–2019 | SOC Stage IIIA-IIIB |
104 | 57.9 | 2.8 | PARP | Adj. Pac/Carbo | IHC | OS: 1.14 (1.03–1.27) PFS: 1.05 (1.02–1.07) |
8 | |
| Lung | Kim [27] |
2014 | South Korea | Asian | 2008–2012 | SCLC Stage I–III |
79 | 62 | 1.6 | PARP-1 | Neoadj. Eto/Cis/Carbo | IHC | PFS: 0.49 (0.26–0.91) | 8 |
| Xie [28] |
2014 | China | Asian | 2008–2010 | NSCLC Stage I–IV |
111 | 63 (43–81) |
NA | PARP-1 | NA | IHC | OS: 2.29 (1.08–4.85) | 7 | |
| Michels [26] |
2015 | France | Caucasian | 1994–2002 | NSCLC Stage I-II |
225 | 64 (40–82) |
10.03 | PARP | NA | IHC | OS: 1.99 (1.04–3.76) DFS: 1.71 (0.95–3.62) |
8 | |
| Liver | Lin [29] |
2016 | China | Asian | 2005–2008 | HCC Stage I–IV |
145 | 45.4 | NA | PARP-2 | NA | IHC | OS: 4.56 (2.12–9.79) | 7 |
| Yu [30] |
2019 | China | Asian | NA | HCC Stage I–IV |
298 | NA | NA | PARP-1 | NA | PCR | OS: 2.43 (1.17–5.07) DFS: 2.11 (1.46–3.04) |
7 | |
| Soft tissue |
Li [31] |
2016 | China | Asian | 1996–2012 | SC NA |
50 | 55.1 (24–90) |
5.4 | PARP-1 | NA | IHC | DFS: 1.07 (1.02–1.14) | 7 |
| Kim [37] |
2016 | South Korea | Asian | 1998–2013 | STS Stage I–IV |
105 | NA | 15 | PARP-1 | Adj. chemo | IHC | DFS: 2.78 (1.70–4.55) | 8 | |
| Brain | Murnyák [32] |
2017 | Hungary | Caucasian | 2006–2014 | Glioma Stage II-IV |
135 | 60.5 (21–89) |
NA | PARP-1 | NA | PCR | OS: 1.11 (1.01–1.22) | 7 |
| Oesophagus | Yamamoto [33] |
2017 | Japan | Asian | 1998–2011 | SCC IA-IVB |
86 | NA | 3.5 | PARP-1 | NA | IHC | OS: 2.39 (1.29–4.44) | 8 |
| Pancreas | Klauschen [35] |
2012 | Germany | Caucasian | NA | PDAC Stage I–IV |
178 | NA | NA | PARP | NA | IHC | OS: 0.63 (0.41–0.96) | 7 |
| Skin | Donizy [36] |
2020 | USA | Caucasian | 1989–2018 | MM Stage I–IV |
192 | 65 | 1.9 | PARP-1 | NA | IHC | OS:1.53 (1.01–2.33) | 8 |
| Stomach | Liu [34] |
2016 | China | Asian | NA | GC stage I–IV |
564 | 60 (29–82) |
5.5 | PARP-1 | NA | IHC | OS: 1.64 (0.99–2.71) DFS: 1.35 (0.86–2.12) |
8 |
Abbreviations: PARP: nuclear and cytoplasmic poly(ADP-ribose) polymerases; NOS: Newcastle–Ottawa scale; NA: not available; IHC: immunohistochemistry; PCR: polymerase chain reaction; HR: hazard ratio; OS: overall survival; DFS: disease free-survival; PFS: progression free-survival; IDC: invasive ductal carcinoma; IC: invasive carcinoma; ILC: invasive lobular carcinoma; MC: mucinous carcinoma; TNBC: triple-negative breast cancer; FEC: fluorouracil, epirubicin, and cyclophosphamide; Ant/Tax: anthracycline/taxane; Horm: hormonal therapy; CMF: cyclophosphamide, methotrexate, and 5-fluorouracil; Chemo: chemotherapy; CAF/CEFD: cyclophosphamide/doxorubicin or epirubicin/5-fluorouracil and docetaxel; C-PARP: cleaved PARP; SOC: serous ovarian carcinoma (include serous ovarian carcinoma, serous cystadenocarcinoma, endometrioid, clear cell carcinoma, mucinous cystadenocarcinoma, mixed, other, and unknown); Carbo/Cyclo: carboplatin and cyclophosphamide; Pac: Paclitaxel; PBC: platinum-based chemotherapy; SCLC: small cell lung cancer; Eto/Cis/Carbo: etoposide/cisplatin/carboplatin; NSCLC: non-small cell lung cancer; HCC: hepatocellular carcinoma; SCC: squamous cell carcinoma; PDAC: pancreatic ductal adenocarcinoma; MM: mucosal melanomas; SC: sacral chordoma; STS: soft tissue sarcoma; GC: gastric cancer; and Adj.: adjuvant. Note: * Aiad (1): target cytoplasmic PARP, Aiad (2): target nuclear PARP, Gan (1), Target Nuclear PARP-1, and Gan (2): target nuclear cleaved PARP-1.
Detailed information about the IHC scoring methods and the cut-off values used for PARP expression in the included studies are summarised in Table 2 and Supplementary Table S2. Tissue microarrays and whole tissue sections were commonly used (n = 14 each). Most studies used PARP antibodies from Abcam (n = 8; Cambridge, UK) and Santa Cruz Biotechnology (n = 7; Dallas, TX, USA). To interpret the IHC results, the H score (n = 10), immunoreactivity score (IRS, n = 6), and quick scoring system (QS, n = 5) were highly exploited. Nuclear location (n = 26) was mainly used for immunoreactivity (Table 2).
Table 2.
Immunohistochemistry detection methods used for the PARP expression in the included studies.
| Organ | Study, Year | Format of Sampling | IHC Evaluation Method | Antibody | No. of Involved Pathologists | IHC Cut-Off Value |
Localisation | Number of High PARP Cases (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Company | Source | Type | Clone | Dilution | ||||||||
| Breast | Minckwitz, 2011 [16] | TMA | IRS | BD Pharmingen | Mouse | mAb | 7D3-6 | 1:1500 | 2 * | 6 | Cytoplasm | 151 (23.7) |
| Rojo, 2012 [17] |
TMA | Computer based scoring | Abcam | Mouse | mAb | A6.4.12 | 1:300 | 1 | NA (29–133.094) |
Nuclear | 103 (31.2) | |
| Donizy, 2014 [9] | TMA | IRS | Abcam | Rabbit | pAb | ab6079 | 1:150 | 2 | 6 | Cytoplasm and Combined N&C |
48 (57.8) 35 (42.2). |
|
| Aiad (1), 2015 [7] | NCBs | H score | eBioscience | Mouse | mAb | C2-10 | 1:100 | 3 | 70 | Cytoplasm | 40 (48.0) | |
| Aiad (2), 2015 [7] | NCBs | H score | eBioscience | Mouse | mAb | C2-10 | 1:100 | 3 | 10 | Nuclear | 64 (76.0) | |
| Green, 2015 [11] | TMA | H score | BD Pharmingen | Mouse | mAb | 7D3-6 | 1:1000 | 2 * | 10 | Nuclear | 524 (41.2) | |
| Park, 2015 [14] |
TMA | Allred score | Santa Cruz Biotechnology |
Mouse | mAb | F-2, sc-8007 |
1:100 | 2 | 13 | Nuclear | 78 (41.0) | |
| Zhai, 2015 [15] |
WTS | QS | Santa Cruz Biotechnology |
Mouse | mAb | F-2, sc-8007 |
1:300 | 7 * | 10 | Nuclear | 59 (54.6) | |
| Mazzotta, 2016 [13] |
WTS | QS | Santa Cruz Biotechnology |
Mouse | mAb | F-2, sc-8007 |
1:500 | 2 | 10 | Nuclear | 68 (59.6) | |
| Deng, 2017 [8] |
WTS | QS | Abcam | Rabbit | pAb | ab6079 | NA | 3 * | 10 | Nuclear | 52 (44.1) | |
| Mangia, 2017 [12] |
TMA | QS | Santa Cruz Biotechnology |
Mouse | mAb | F-2, sc-8007 |
1:500 | 2 | 10 | Nuclear | 76 (28.9) | |
| Siraj, 2018 [18] |
TMA | QS | Cell signaling | Rabbit | mAb | D64E10 | NA | 2 * | 10 | Nuclear | 451 (44.7) | |
| Song, 2017 [19] | WTS | H score | Abcam | Rabbit | pAb | 96601 | 1:100 | 2 | 57.5 | Nuclear | 234 (47.5) | |
| Ovary | Brustmann, 2007 [22] | TMA | IRS | Novocastra | Mouse | mAb | NA | 1:30 | 1 | 8 | Nuclear | 38 (76.0) |
| Barnett, 2010 [21] | WTS | IRS | NeoMarkers Fremont | Mouse | mAb | A6.4.12 | 1:200 | 1 | 8 | Nuclear | 101 (54.0) | |
| Gan (1), 2013 [23] | TMA | H score | BD Pharmingen | Mouse | mAb | 7D3-6 | 1:600 | 1 | 180 | Nuclear | 33 (22.0) | |
| Gan (2), 2013 [23] | TMA | H score | Abnova | Mouse | mAb | A6.4.12 | 1:600 | 1 | 75 | Nuclear | 117 (79.0) | |
| Ali, 2019 [20] |
TMA | H score | Cell signaling | Rabbit | mAb | 46D11 | 1:600 | 3 * | 80 | Nuclear | 208 (51.9) | |
| Molnar, 2020 [24] | WTS | SI based scoring | Abcam | Rabbit | pAb | Ab6079330 | 1:500 | 1 * | 1 | Nuclear | 45 (52.3) | |
| Molnar, 2021 [25] |
WTS | SI based scoring | Abcam | Rabbit | pAb | Ab6079330 | 1:500 | 1 * | 1 | Nuclear | 70 (67.3) | |
| Lung | Kim, 2014 [27] |
WTS | SP based scoring | Bethyl Laboratories Inc. | Rabbit | pAb | 00279 | 1:200 | 1 * | 3 | Nuclear | 33 (41.8) |
| Xie, 2014 [28] |
WTS | IRS | Biorbyt | Rabbit | pAb | NA | 1:300 | 2 | 4 | Nuclear | 62 (55.9) | |
| Michels, 2015 [26] |
WTS | H score | Merck | Mouse | mAb | 10H | 1:1500 | 3 * | 145 * | Nuclear | 49 (53.3) | |
| Liver | Lin, 2016 [29] |
WTS | SI based scoring | Raybiotech, Inc | Goat | NA | Q9UGN5 | NA | NA | 2 | Nuclear | 75 (51.7) |
| Soft tissue |
Li, 2016 [31] |
WTS | Summation based scoring | Abcam | Rabbit | pAb | ab6079 | 1:50 | NA | 3 | Combined N&C | 39 (78.0) |
| Kim, 2016 [37] |
TMA | Allred score | Santa Cruz Biotechnology |
Mouse | mAb | F-2 sc-8007 | 1:100 | 3 | 10 | Nuclear | 64 (57.1) | |
| Oesophagus | Yamamoto, 2017 [33] | WTS | SI based scoring | Santa Cruz Biotechnology |
Mouse | mAb | F2, sc-8007 |
1:50 | 2 | 2 | Nuclear | 54 (62.8) |
| Pancreas | Klauschen, 2012 [35] | TMA | IRS | BD Pharmingen | Mouse | mAb | 7D3-6 | 1:1000 | 2 | 3 | Nuclear | 138 (77.5) |
| Skin | Donizy, 2020 [36] | WTS | H score | Santa Cruz Biotechnology |
Mouse | mAb | sc-74470 (B10) | 1:50 | 1 | 200 | Nuclear | 72 (43.0) |
| Stomach | Liu, 2016 [34] |
TMA | H score | Abcam | Rabbit | pAb | ab6079 | 1:200 | 2 | 175 | Nuclear | 266 (47.2) |
Abbreviations: mAb: monoclonal antibody, pAb; polyclonal antibody TMA: tissue microarray, WTS: whole tissue section, CNBs: core needle biopsies, IRS: immunoreactivity score, QS: quick score, SI: staining intensity, SP: staining percentage, N&C: nuclear–cytoplasmic expression, and HPA: Human Protein Atlas database. *: Signifies the number of pathologists from the articles list.
3.3. Association between PARP Expression and OS
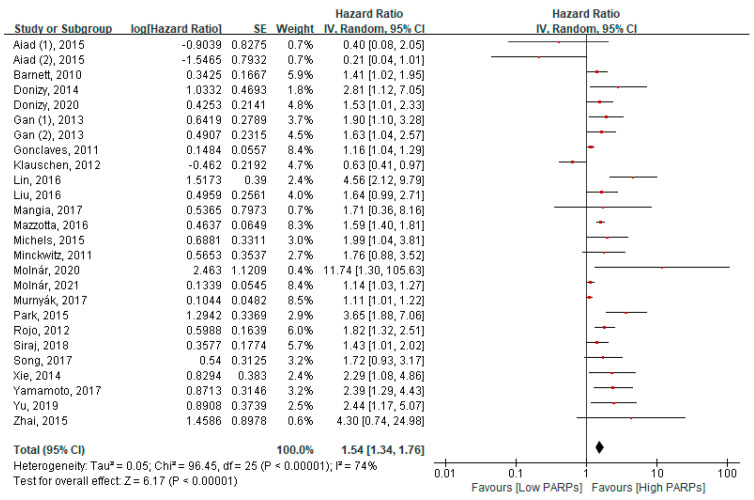
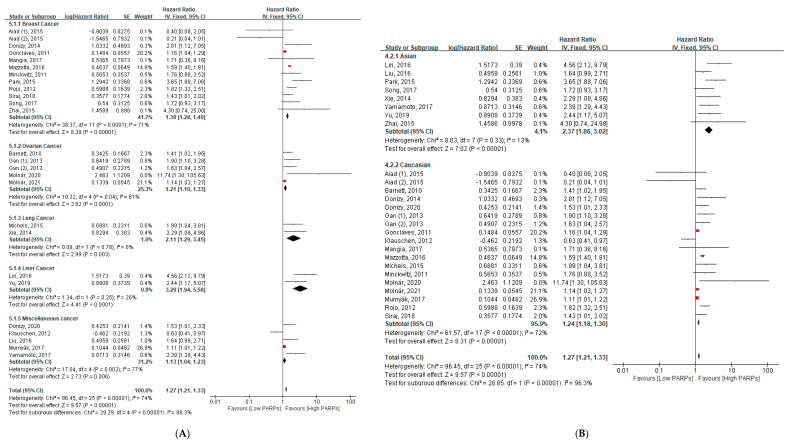
Twenty-four studies, including twenty-six cohorts, reported an association between PARP expression and OS in 8471 patients with cancer. In a pooled HR analysis using the fixed model, a higher expression of PARPs was significantly associated with reduced OS in all cancers (HR = 1.54, 95% CI: 1.34–1.76, p < 0.001) (Figure 2). In a subgroup analysis, a higher expression of PARPs was significantly associated with poor OS in breast cancers (HR = 1.38, 95% CI = 1.28–1.49, p < 0.001), ovary cancers (HR = 1.21, 95% CI = 1.10–1.33, p < 0.001), lung cancers (HR = 2.11, 95% CI = 1.29–3.45, p = 0.003), and liver cancers (HR = 3.29, 95% CI = 1.94–5.58, p < 0.001) (Figure 3A). A quantitative synthesis of two soft tissue studies was not done due to the differences of the histologic subtypes of both studies. No significant relationship was found in miscellaneous cancers (Figure 3A).
Figure 2.
Forest plot of the hazard ratio for the PARP expression and overall survival in solid cancers.
Figure 3.
The subgroup analysis between the PARP expression and overall survival (A) according to the types of cancers and (B) ethnicity.
Regarding ethnicity, poor survival was associated with a higher expression of PARPs across different groups with almost double the HR in Asians than Caucasians (Asian: HR = 2.37, 95% CI = 1.86–3.02, p < 0.001; Caucasian: HR = 1.24, 95% CI = 1.18–1.30, p < 0.001) (Figure 3B).
OS based on both univariate and multivariate analyses showed a significant association with adverse survival (univariate: HR = 1.10, 95% CI = 1.06–1.14, p < 0.001; multivariate: HR = 1.60, 95% CI = 1.46–1.76, p < 0.001) (Supplementary Figure S1). Regarding the sample size, only studies with more than 100 samples showed a significant association with poor OS (HR = 1.26, 95% CI = 1.20–1.33, p < 0.001) (Supplementary Figure S2A). Both direct and indirect methods (pooled HRs versus K–M curve data extraction) showed a correlation with poor OS (direct: HR = 1.58, 95% CI = 1.33–1.88, p < 0.001; indirect: HR = 1.41, 95% CI = 1.12–1.78, p < 0.001) (Supplementary Figure S2B).
Based on a chemotherapy regimen, a significantly poor OS was found with high PARP expression in breast cancer patients receiving neoadjuvant chemotherapy of anthracycline and taxane (HR = 1.98, 95% CI = 1.04–3.78 p = 0.04) and ovarian cancer patients receiving an adjuvant chemotherapy of paclitaxel and carboplatin (HR = 1.15, 95% CI = 1.03–1.28, p = 0.01) and platinum-based chemotherapy (agents not specified) (HR = 1.52, 95% CI = 1.15–2.02, p = 0.003) (Supplementary Figure S3A).
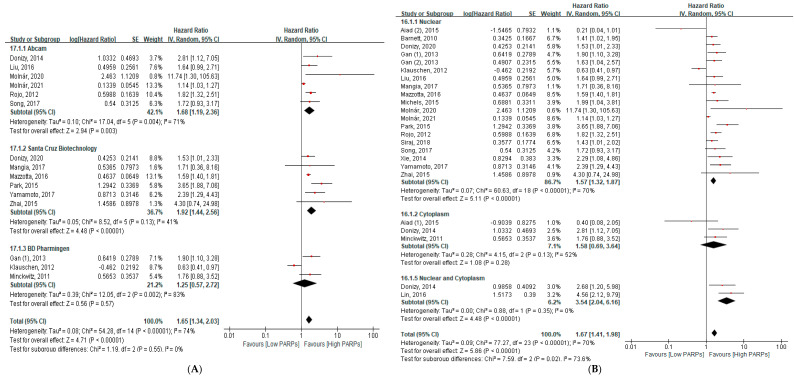
According to antibody types, only Abcam (HR = 1.68, 95% CI = 1.19–2.36, p = 0.003) and Santa Cruz Biotechnology (HR = 1.92, 95% CI = 1.44–2.56, p < 0.001) showed significantly poor OS with high PARP expression (Figure 4A). Both nuclear and combined nuclear and cytoplasm immunoreactivity showed a significant association with poor OS (nuclear: HR = 1.57, 95% CI = 1.32–1.87, p < 0.001; combined nuclear and cytoplasm: HR = 3.54, 95% CI = 2.04–6.16, p < 0.001) (Figure 4B).
Figure 4.
The subgroup analysis between the PARP expression and overall survival (A) according to the antibody clones and (B) immunoreactivity location-wise.
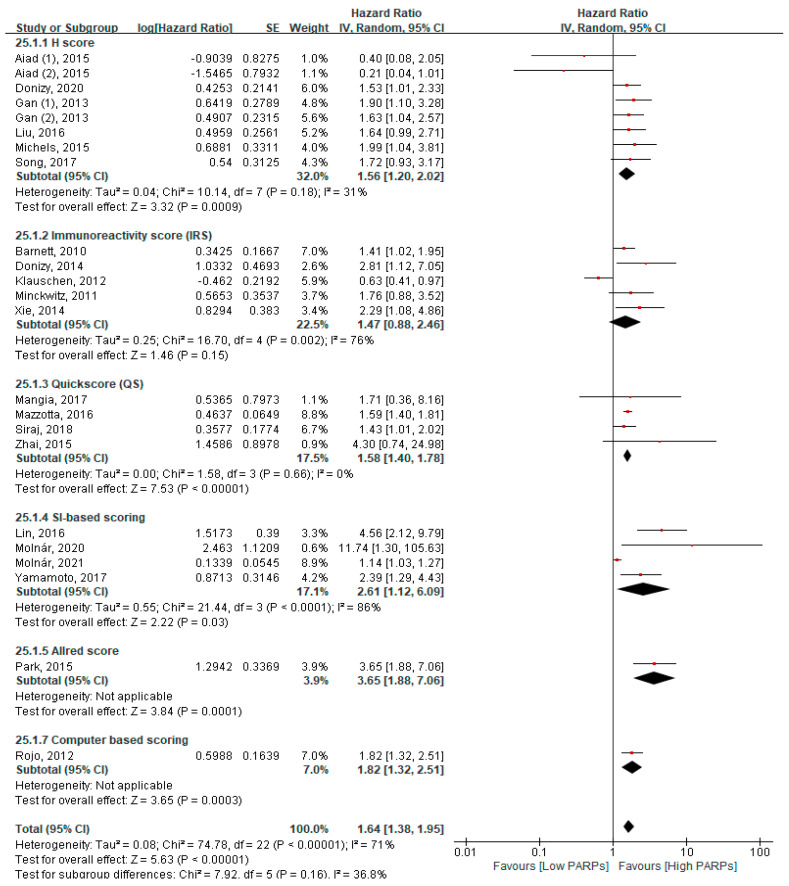
Finally, according to the scoring methods, a significant poor OS was associated with the H score system (HR = 1.56, 95% CI = 1.20–2.20, p < 0.001), QS (HR = 1.58, 95% CI = 1.40–1.78, p < 0.001), staining intensity (SI) (HR = 2.61, 95% CI = 1.12–6.09, p = 0.03), Allred score (HR = 3.65, 95% CI = 1.88–7.06, p = 0.0001), and computer-based scoring (HR = 1.82, 95% CI = 1.32–2.51, p < 0.001) (Figure 5).
Figure 5.
The subgroup analysis between the PARP expression and overall survival according to the scoring methods.
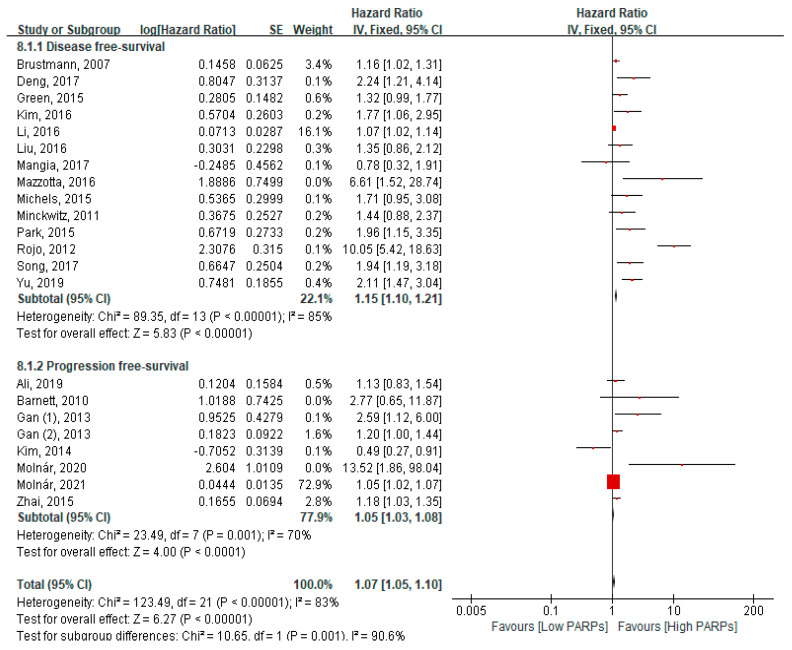
3.4. Association of High PARP Expression with DFS and PFS
Fourteen studies reported DFS, and seven studies, including eight cohorts reporting PFS, were included in the meta-analysis (Figure 6). High PARP expression was associated with poor DFS (HR = 1.15, 95% CI = 1.10–1.23, p < 0.001). Similarly, high PARP expression was associated with significantly worse PFS (HR = 1.05, 95% CI = 1.03–1.08, p < 0.001) (Figure 6). PARP expression was associated with poor DFS in breast cancers and poor PFS in ovary cancers (Supplementary Figure S4). A subgroup analysis according to chemotherapy regimen, univariate analysis and multivariate analysis, ethnicity (Asian vs. Caucasian), and direct/indirect methods (pooled HRs vs. K–M curve data extraction) showed a significant relationship with a poor outcome (Supplementary Figures S3B, S5 and S6).
Figure 6.
Forest plot of the hazard ratio for PARP expression and disease-free survival and progression-free survival in solid cancer patients.
3.5. Association of High PARP Expression with Clinicopathological Parameters and Immunohistochemical Markers
The association of PARPs with the clinicopathological parameters and IHC markers is summarised in Table 3. Higher PARP expression was significantly associated with larger tumours (OR = 1.53, 95% CI = 1.00–2.34, p = 0.048), a higher tumour grade (HR = 1.53, 95% CI = 1.00–2.34, p = 0.048), the presence of lymph node metastasis, the presence of distant metastases (OR = 1.53, 95% CI = 1.00–2.34, p = 0.048), a higher TNM stage (HR = 1.53, 95% CI = 1.00–2.34, p = 0.048), and the presence of lymphovascular invasion (Table 3 and Supplementary Figures S7 and S8). However, no significant relationship was found between age and T stage (Table 3 and Supplementary Figures S7 and S8).
Table 3.
The association of PARP expression with the clinicopathological and immunohistochemical parameters.
| Parameters | Number of Studies | Number of Patients | Pooled OR (95% CI) |
p-Value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I2 (%) | p-Value | Model | |||||
| Clinicopathological parameters | |||||||
| Age (<50 vs. >50) | 8 | 2977 | 0.96 (0.82–1.12) | 0.59 | 44 | 0.08 | Fixed |
| Tumour size (<5 cm vs. >5 cm) |
8 | 2453 | 1.60 (1.32–1.95) | <0.001 * | 80 | < 0.001 | Fixed |
| Histologic grade (1 + 2 vs. 3) | 16 | 4927 | 1.79 (1.58–2.04) | <0.001 * | 72 | < 0.001 | Fixed |
| T stage (1 + 2 vs. 3+4) | 4 | 1460 | 1.26 (0.91–1.75) | 0.16 | 14 | 0.32 | Fixed |
| Lymph node metastasis (absent vs. present) | 17 | 3743 | 1.27 (1.10–1.46) | 0.001 * | 42 | 0.03 | Fixed |
| Distant metastasis (absent vs. present) |
6 | 1668 | 2.40 (1.79–3.23) | <0.001 * | 61 | 0.03 | Fixed |
| TNM stage (I + II vs. III + IV) | 6 | 2260 | 1.49 (1.24–1.79) | <0.001 * | 0 | 0.94 | Fixed |
| Lympho-vascular invasion (absent vs. present) | 10 | 3141 | 1.22 (1.05–1.42) | 0.01 * | 62 | 0.004 | Fixed |
| Immunohistological markers | |||||||
| Ki-67 (negative vs. positive) | 5 | 2529 | 1.60 (1.35–1.90) | <0.001 * | 83% | < 0.001 | Fixed |
| BRCA1 (negative vs. positive) | 4 | 1546 | 1.63 (1.32–2.02) | <0.001 * | 89% | < 0.001 | Fixed |
| BRCA2 (negative vs. positive) | 3 | 1040 | 2.78 (1.94–3.98) | <0.001 * | 92% | < 0.001 | Fixed |
Abbreviations: CI: confidential interval, OR: odds ratio, and TNM: tumour node metastasis. *: Significant p-value.
The pooled results showed that high PARP expression was significantly associated with the positivity of Ki-67 (OR = 0.12, 95% CI = 0.08–0.19, p = 0.048), BRCA1 (OR 0.12, 95% CI 0.08, 0.19, p = 0.048), and BRCA2 (OR 2.78, 95% CI (1.94, 3.98, p = 0.048) (Table 3 and Supplementary Figure S8).
3.6. Publication Bias
Table 4 summarises the publication bias assessments. The funnel plot showed asymmetry in the OS, DFS, and PFS, and the trim-and-fill method was used to create a symmetrical funnel (Supplementary Figure S9). Publication bias was significantly associated with the OS, DFS, and age, according to the Egger test (p = 0.024, p = 0.001, and p = 0.017, respectively). In contrast, none of the parameters showed any publication bias according to the Begg test (Table 4).
Table 4.
The publication bias assessment of the included studies.
| Parameters | No. of Studies/Cohorts | Begg Test (p-Value) |
Egger Test (p-Value) |
Model |
|---|---|---|---|---|
| PARPs and survival outcomes | ||||
| Overall Survival | 24/26 | 0.133 | 0.024 * | Fixed |
| Disease-free survival | 14 | 0.062 | 0.001 * | Fixed |
| Progression-free survival | 7/8 | 0.536 | 0.455 | Fixed |
| PARPs and clinicopathological parameters | ||||
| Age (>50 vs. <50) | 8 | 0.063 | 0.017 * | Fixed |
| Tumour size (>5 cm vs. <5 cm) |
8 | 1.000 | 0.723 | Fixed |
| Histologic grade (1 + 2 vs. 3) | 4 | 0.308 | 0.345 | Fixed |
| T stage (1 + 2 vs. 3 + 4) | 17 | 0.964 | 0.532 | Fixed |
| Lymph node metastasis (absent vs. present) | 6 | 1.000 | 0.804 | Fixed |
| Distant metastasis (absent vs. present) |
6 | 0.259 | 0.354 | Fixed |
| TNM stage (I + II vs. III + IV) | 10 | 1.000 | 0.513 | Fixed |
| PARPs and Immunohistological markers | ||||
| Ki-67 (negative vs. positive) | 5 | 0.220 | 0.113 | Fixed |
| BRCA1 (negative vs. positive) | 4 | 0.308 | 0.368 | Fixed |
| BRCA2 (negative vs. positive) | 3 | 1.000 | 0.370 | Fixed |
*: Significant p-value.
4. Discussion
The present comprehensive systematic review and meta-analysis is the first to scrutinise the prognostic value and clinicopathological significance of PARP expression in patients with solid cancers. First, high PARP expression was associated with poor OS, DFS, and PFS in most cancers, as previously reported. Second, by ethnicity, the Asian population showed a higher HR than the Caucasian subgroup (HR = 2.37 vs. 1.24), which means a high susceptibility of the Asian population to high PARP expression. Third, high PARP expression was associated with clinicopathological prognostic markers and positive immunoreactivity for Ki-67, BRCA1, and BRCA2. Fourth, according to the IHC clones, localisation methods, and scoring systems, nuclear expression by Abcam and Santa Cruz Biotechnology antibody clones were most commonly used, and the QS method was most reproducible, with the lowest heterogeneity.
In this study, we confirmed a significantly poor OS of high PARP expression in breast, ovary, lung, and liver cancers. In a previous meta-analysis on breast cancers by Qiao et al., the authors reported a significantly poor OS of high PARP expression in early breast cancer but not in locally advanced breast cancers [40]. In contrast, through our systematic analysis, we found that many studies other than Aiad et al.’s included stage III or IV breast cancers (Table 1) [7]. However, Qiao et al. did not include these studies in the locally advanced breast cancer group in their subgroup analysis but included them in the nonlocally advanced breast cancer group [40]. The results of Aiad et al.’s study should be interpreted with caution, because it included only stage IIIB breast cancers, only 84 cases, which is relatively small, and only core needle biopsy samples (no other study used core needle biopsy samples), which could highly result in a sampling bias [7]. In addition, Qiao et al. somehow combined two different results from one cohort of Aiad et al.’s study to perform a subgroup analysis of a locally advanced breast cancer group [40]. The one result was targeting cytoplasmic immunoreactivity while another was targeting nuclear immunoreactivity, which finally misled to the wrong conclusion that high PARP expression is associated with better survival in locally advanced breast cancers and poor survival in early breast cancers. In this study, other than that, we included all results from the additional studies and found a higher statistical significance of high PARP expression to poor OS in overall breast cancers. Similarly, the same relationship between PARP expression and poor OS was found in ovary, lung, and liver cancers.
On the other hand, additional studies with more samples are still required in other cancers, such as pancreas, brain, soft tissues, skin, and stomach, to investigate the exact prognostic role of PARPs. Interestingly, a better survival of high PARP expression was reported in pancreatic cancers [35], even though it was only one study, which raises the possibility of the different roles of PARPs in different cancers. Therefore, more studies with sufficient samples are expected in these cancers.
According to the quality assessment, heterogeneity was high in the breast, ovary, and miscellaneous cancer groups (I2 = 71%, 61%, and 77%, respectively), although the NOS of the included studies were relatively good. The source of heterogeneity may be due to different cancer types, histological subtypes, stages, IHC scoring systems, cut-off values, antibody clones, target PARP phenotypes, etc. Additionally, there seemed to be a publication bias among the included studies. However, the subgroup analysis also consistently revealed a statistically significant relationship of PARP expression to a poor prognosis, regardless of the cancer types, ethnicity, statistical analysis methods (univariate vs. multivariate), direct/indirect methods for HR extraction, antibody clones, scoring systems, and localisation of immunoreactivity. In addition, similar findings were found in DFS and PFS in every subgroup showing an unfavourable impact of PARP expression in solid cancers. These are consistent findings reported in many hematologic disorders (nonsolid cancers) as well [41].
The subgroup analysis by ethnicity revealed that Asian patients have a worse survival rate compared to Caucasians. Our results were also consistent with the previous findings of two meta-analyses, which reported that the allele frequency of A in the PARP-1 V762A polymorphism is significantly higher in Asian patients compared to Caucasians and related to a higher risk of cancer development [42,43]. The difference between both ethnicities is due to their genetic backgrounds and environmental variables. These result in tumours with various biological characteristics [44]. Furthermore, the most common tumour sites within organs differ between Asian and Caucasian populations, resulting in variations in tumour activity and prognoses [45]. This could be the reason for the differences in HR between Asians and Caucasians. Overall, our results indicate that Asian patients have a higher risk of cancer with a higher PARP expression than Caucasians.
Next, a significant relationship was found between a higher PARP expression and various clinicopathological risk factors, such as tumour size, tumour differentiation, lymph node metastasis, distant metastasis, TNM stage, and lymphovascular invasion. These findings indicate that PARPs are involved in cancer development, proliferation, invasion, and metastasis, which are mainly related to a poor prognosis and cancer mortality in patients. At the cellular level, the main effect of PARPs is to regulate the proliferation, migration, and invasion of cancer cells [46,47,48]. A previous study reported that PARPs activate the metastasis-related genes integrin β-1, matrix metallopeptidase-2 (MMP-2), and MMP-9 through the nuclear factor kappa-light-chain pathway in colon cancer [46]. Another study showed that a loss of function in PARP-1 activated the epithelial–mesenchymal transition pathway through the increased expression of N-cadherin and ZEB-1 and the decreased expression of E-cadherin and β-catenin, which promote tumour progression through the TGF-β signalling pathway [47].
Furthermore, Ki-67, BRCA1, and BRCA2 expressions were also significantly associated with a higher PARP expression. In primary mucosal melanoma, it has been observed that a higher PARP-1 expression was significantly associated with higher mitotic activity, which showed that PARP-1 is involved in the regulation of mitosis. Moreover, a loss of function in BRCA1 and BRCA2 most commonly occurs in breast and ovarian cancers [49]. It has been observed that breast tumours with BRCA1/2 mutations lack homologous repair recombination capabilities, making it difficult to repair DNA damage, causing cell apoptosis. [50]. A previous study demonstrated that a higher expression of PARP-1, BRCA 1, and BRCA2 resulted in a shorter OS at 10 years in patients with breast cancer [14]. Hence, there may be the possibility that these patients might benefit from treatment with PARP inhibitors. Emerging evidence has revealed that BRCA1 mutations in breast and ovarian cancers are more sensitive to PARP inhibitors [51].
Interestingly, we found that only Abcam and Santa Cruz Biotechnology showed poor OS with high PARP expression in subgroup analyses by antibody clones. The heterogeneity in Abcam was higher than Santa Cruz Biotechnology (I2 = 71% vs. 41%) (Figure 4A). In terms of immunoreactivity location-wise, nuclear localisation was commonly targeted as compared to the cytoplasm and combined nuclear and cytoplasm. Although the heterogeneity in nuclear localisation was significantly higher than the combined nuclear and cytoplasm (I2 = 70% vs. 0%), both represent a poor OS with high PARP expression (Figure 4B). The primary reason for a higher heterogeneity may be different antibody clones, scoring systems, and cancer types. According to the scoring system, the QS method was more reproducible as compared to other scoring methods, such as the H score, IRS, and SI-based scoring methods (I2 = 0%, 31%, 76%, and 86%, respectively) (Figure 5). Based on this data, we concluded that the QS method is easier and uniformly applicable compared to other scoring methods. Further studies should be designed based on these findings.
PARP inhibitors have emerged as a promising target-based therapy owing to their excellent results in clinical trials [52,53,54,55]. Among 17 members of the PARP family, PARP-1 is the most well-studied and has been identified as a key player in the DNA repair pathway [52,53,54,55]. Generally, PARPs have three functional domains: a DNA-binding domain that comprises zinc finger motifs, an auto-modification domain, and a carboxyl catalytic domain [52,53,54,55]. Damaged DNA is often repaired via two pathways, SSB and DSB, which are critical for maintaining cell viability [52,53,54,55]. SSBs are repaired via the mismatch repair, base excision repair (BER), and nucleotide-excision repair pathways, while DSBs are repaired through the homologous recombination and nonhomologous end-joining repair pathways [52,53,54,55]. If any damage occurs on the DNA strand, PARPs bind to the DNA damage site and produce a poly-ADP chain from the NAD+ substrate that recruits the DNA repair protein through the SSB or DSB repair pathway [52,53,54,55].
In our study, a subgroup analysis according to the chemotherapy regimen in breast and ovarian cancer patients showed a poor OS and PFS in the high PARP expression group regardless of the chemotherapy regimen. Although this alone cannot be direct evidence of the adverse biologic role of PARP expression because we could not include chemotherapy-free cohorts, the results blend smoothly with the other findings from many other preclinical and clinical trial study results [52,53,54,55]. A previous preclinical study showed that treatment with PARP inhibitors leads to cell cycle arrest and cell death in cells with a homologous recombination deficiency (HRD) [54,56,57]. In the pancreas, a clinical trial treating metastatic pancreatic cancer patients with germline BRCA1/2 mutations with Olaparib extended the PFS [58]. In the ovaries, a clinical trial showed that using Olaparib, paclitaxel, and carboplatin increased the PFS in recurrent cancer patients [59]. All these lines of evidence indirectly support our results. To overcome the aforementioned limitations, more evidence on the effects of the treatment regimen is needed in the future.
Robust efforts have been made to perform this comprehensive meta-analysis. However, our study has some limitations, such as: (i) studies not published in the English language were excluded due to the hassle of obtaining the data more precisely, which may have caused a bias in our results; (ii) in the case of studies without HRs with 95% CI, the data were extracted using digitised software from the K–M curve (indirect method) before the pooled HR was calculated, which may have compromised the accuracy of the data; (iii) the cut-off values differentiating between high and low PARP expression observed via IHC varied among the included studies, which may be the source of heterogeneity in the overall results and subgroup results; and (iv) the different clones and sources of antibodies used for PARP expression between the studies may have also impacted the precise estimation of the prognoses. Therefore, a large multicentre study using the same cut-off values with the same clones and sources of antibodies is required to obtain more precise outcomes. Regardless of the above limitations, our meta-analysis revealed the prognostic and clinicopathological significance of PARPs in solid cancers.
5. Conclusions
The present meta-analysis revealed that a higher expression of PARPs could act as a risk factor for poor OS, DFS, and PFS in various solid cancers, such as breast, ovary, lung, and liver cancers. Asians with a high PARP expression are more susceptible to a worse survival diagnosis than Caucasians. Furthermore, a high PARP expression was significantly associated with aggressive clinicopathological parameters and positive immunoreactivity for Ki-67, BRCA1, and BRCA2. In addition, nuclear expression assessed by the QS system using Abcam and Santa Cruz Biotechnology seems to be the most commonly used and reproducible IHC method for assessing PARP expressions. Collectively, the inhibition of this pathway through its specific inhibitors may extend the survival of patients with high PARP expression. Well-designed multicentre studies with larger sample sizes are required in miscellaneous cancers, such as the pancreas.
Acknowledgments
We appreciate Na Jin Kim from The Medical Library, The Catholic University of Korea for the strategic literature search.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13225594/s1: Table S1: Results of the quality assessment. Table S2: Scoring system of immunohistochemical staining for the PARPs used in the included studies. Figure S1: Forest plot for studies evaluating the hazard ratio (HR) for the PARP expression and overall survival (OS): (A) univariate analysis and (B) multivariate analysis in solid cancer patients. Figure S2: Subgroup analysis between the PARP expression and overall survival (OS): (A) according to the type of sample size and (B) source of the hazard ratio. Figure S3. Subgroup analysis between PARP expression and (A) overall survival (OS) and (B) progression-free survival (PFS) according to chemotherapy regimen. Figure S4: Subgroup analysis between the PARP expression and (A) disease-free survival (DFS) according to the cancer types and (B) progression-free survival (PFS) according to the cancer types. Figure S5: Forest plot for studies evaluating the hazard ratio (HR) for the PARP expression and disease-free survival (DFS) and progression-free survival (PFS): (A) univariate analysis and (B) multivariate analysis in solid cancer patients. Figure S6: Forest plot evaluating the subgroup analysis between the PARP expression and (A) disease-free survival (DFS) and progression-free survival (PFS) according to ethnicity (Asian vs. Caucasian) and (B) DFS and PFS of the direct and indirect methods (pooled HRs vs. K–M curve data extraction). Figure S7: Forest plot of the association between the PARP expression and clinicopathological characteristics: (A) age, (B) gender, (C) tumour size, (D) histological grade, (E) tumour stage, and (F) lymph node metastasis. Figure S8: Forest plot of the association between the PARP expression and clinicopathological characteristics: (A) TNM stage, (B) lymph vascular invasion, (C) Ki-67, (D) BRCA1, and (E) BRCA2. Figure S9: Funnel plot: (A) overall survival, (C) disease-free survival, (E) progression-free survival and the trim-and-fill funnel plot and (B) overall survival, (D) disease-free survival, and (F) progression-free survival.
Author Contributions
Conceptualization, N.T. and Y.C.; methodology, N.T. and Y.C.; software, N.T., Y.C. and K.Y.; validation, N.T. and Y.C.; formal analysis, N.T. and Y.C.; investigation, N.T. and Y.C.; resources, N.T. and Y.C.; data curation, N.T., Y.C. and K.Y.; writing—original draft preparation, N.T.; writing—review and editing, N.T., Y.C., K.Y., J.A.-G. and K.J.S., visualization, N.T. and Y.C.; supervision, N.T., Y.C., K.Y., J.A.-G. and K.J.S. and project administration, N.T., Y.C., K.Y., J.A.-G. and K.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of the Catholic University of Korea, College of Medicine (SC20ZISE0050) (11 December 2020).
Informed Consent Statement
Patient consent was waived, because this study used publicly available data only.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (https://www.researchgate.net/profile/Yosep-Chong, accessed on 8 November 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bai P. Biology of poly (ADP-ribose) polymerases: The factotums of cell maintenance. Mol. Cell. 2015;58:947–958. doi: 10.1016/j.molcel.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 2.Gupte R., Liu Z., Kraus W.L. PARPs and ADP-ribosylation: Recent advances linking molecular functions to biological outcomes. Genes Dev. 2017;31:101–126. doi: 10.1101/gad.291518.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou D., Liu Z., Xu X., Liu Q., Zhang X., Kong B., Wei J.-J., Gong Y., Shao C. Increased oxidative stress mediates the antitumor effect of PARP inhibition in ovarian cancer. Redox Biol. 2018;17:99–111. doi: 10.1016/j.redox.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu H., Wei M., Xu J., Hua J., Liang C., Meng Q., Zhang Y., Liu J., Zhang B., Yu X. PARP inhibitors in pancreatic cancer: Molecular mechanisms and clinical applications. Mol. Cancer. 2020;19:1–15. doi: 10.1186/s12943-020-01167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Washington C.R., Richardson D.L., Moore K.N. Olaparib in the treatment of ovarian cancer. Future Oncol. 2019;15:3435–3449. doi: 10.2217/fon-2019-0271. [DOI] [PubMed] [Google Scholar]
- 6.Ledermann J., Harter P., Gourley C., Friedlander M., Vergote I., Rustin G., Scott C., Meier W., Shapira-Frommer R., Safra T. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 7.Aiad H.A., Kandil M.A., El-Tahmody M.A., Abulkheir I.L., Abulkasem F.M., Elmansori A.A., Aleskandarany M.A. The prognostic and predictive significance of PARP-1 in locally advanced breast cancer of Egyptian patients receiving neoadjuvant chemotherapy. Appl. Immunohistochem. Mol. Morphol. 2015;23:571–579. doi: 10.1097/pai.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 8.Deng L., Lei Q., Wang Y., Wang Z., Xie G., Zhong X., Wang Y., Chen N., Qiu Y., Pu T. Downregulation of miR-221-3p and upregulation of its target gene PARP1 are prognostic biomarkers for triple negative breast cancer patients and associated with poor prognosis. Oncotarget. 2017;8:108712. doi: 10.18632/oncotarget.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donizy P., Pietrzyk G., Halon A., Kozyra C., Gansukh T., Lage H., Surowiak P., Matkowski R. Nuclear-cytoplasmic PARP-1 expression as an unfavorable prognostic marker in lymph node-negative early breast cancer: 15-year follow-up. Oncol. Rep. 2014;31:1777–1787. doi: 10.3892/or.2014.3024. [DOI] [PubMed] [Google Scholar]
- 10.Gonçalves A., Finetti P., Sabatier R., Gilabert M., Adelaide J., Borg J.-P., Chaffanet M., Viens P., Birnbaum D., Bertucci F. Poly(ADP-ribose) polymerase-1 mRNA expression in human breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2011;127:273–281. doi: 10.1007/s10549-010-1199-y. [DOI] [PubMed] [Google Scholar]
- 11.Green A.R., Caracappa D., Benhasouna A.A., Alshareeda A., Nolan C.C., Macmillan R.D., Madhusudan S., Ellis I.O., Rakha E.A. Biological and clinical significance of PARP1 protein expression in breast cancer. Breast Cancer Res. Treat. 2015;149:353–362. doi: 10.1007/s10549-014-3230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangia A., Scarpi E., Partipilo G., Schirosi L., Opinto G., Giotta F., Simone G. NHERF1 together with PARP1 and BRCA1 expression as a new potential biomarker to stratify breast cancer patients. Oncotarget. 2017;8:65730–65742. doi: 10.18632/oncotarget.19444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzotta A., Partipilo G., De Summa S., Giotta F., Simone G., Mangia A. Nuclear PARP1 expression and its prognostic significance in breast cancer patients. Tumour Biol. 2016;37:6143–6153. doi: 10.1007/s13277-015-4465-0. [DOI] [PubMed] [Google Scholar]
- 14.Park S.-H., Noh S.J., Kim K.M., Bae J.S., Kwon K.S., Jung S.H., Kim J.R., Lee H., Chung M.J., Moon W.S. Expression of DNA damage response molecules PARP1, γH2AX, BRCA1, and BRCA2 predicts poor survival of breast carcinoma patients. Transl. Oncol. 2015;8:239–249. doi: 10.1016/j.tranon.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhai L., Li S., Li X., Li H., Gu F., Guo X., Liu F., Zhang X., Fu L. The nuclear expression of poly (ADP-ribose) polymerase-1 (PARP1) in invasive primary breast tumors is associated with chemotherapy sensitivity. Pathol. Res. Pract. 2015;211:130–137. doi: 10.1016/j.prp.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Von Minckwitz G., Müller B.M., Loibl S., Budczies J., Hanusch C., Darb-Esfahani S., Hilfrich J., Weiss E., Huober J., Blohmer J.U., et al. Cytoplasmic poly(adenosine diphosphate-ribose) polymerase expression is predictive and prognostic in patients with breast cancer treated with neoadjuvant chemotherapy. J. Clin. Oncol. 2011;29:2150–2157. doi: 10.1200/jco.2010.31.9079. [DOI] [PubMed] [Google Scholar]
- 17.Rojo F., García-Parra J., Zazo S., Tusquets I., Ferrer-Lozano J., Menendez S., Eroles P., Chamizo C., Servitja S., Ramírez-Merino N., et al. Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann. Oncol. 2012;23:1156–1164. doi: 10.1093/annonc/mdr361. [DOI] [PubMed] [Google Scholar]
- 18.Siraj A.K., Pratheeshkumar P., Parvathareddy S.K., Divya S.P., Al-Dayel F., Tulbah A., Ajarim D., Al-Kuraya K.S. Overexpression of PARP is an independent prognostic marker for poor survival in Middle Eastern breast cancer and its inhibition can be enhanced with embelin co-treatment. Oncotarget. 2018;9:37319–37332. doi: 10.18632/oncotarget.26470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song Z., Wang Y., Xiao Q., Yu Z., Zhao L., Wu H., Sun M., Chai Z., Hou P., Geng X., et al. Poly(ADP-ribose) polymerase-3 overexpression is associated with poor prognosis in patients with breast cancer following chemotherapy. Oncol. Lett. 2018;16:5621–5630. doi: 10.3892/ol.2018.9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali R., Alabdullah M., Alblihy A., Miligy I., Mesquita K.A., Chan S.Y., Moseley P., Rakha E.A., Madhusudan S. PARP1 blockade is synthetically lethal in XRCC1 deficient sporadic epithelial ovarian cancers. Cancer Lett. 2020;469:124–133. doi: 10.1016/j.canlet.2019.10.035. [DOI] [PubMed] [Google Scholar]
- 21.Barnett J.C., Kondoh E., Whitaker R., Murphy S.K., Berchuck A. High poly (ADP-ribose) polymerase (PARP) expression is associated with poor survival in advanced-stage serous ovarian cancer. Gynecol. Oncol. 2009;112:S107. doi: 10.1016/j.ygyno.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Brustmann H. Poly(adenosine diphosphate-ribose) polymerase expression in serous ovarian carcinoma: Correlation with p53, MIB-1, and outcome. Int. J. Gynecol. Pathol. 2007;26:147–153. doi: 10.1097/01.pgp.0000235064.93182.ec. [DOI] [PubMed] [Google Scholar]
- 23.Gan A., Green A.R., Nolan C.C., Martin S., Deen S. Poly(adenosine diphosphate-ribose) polymerase expression in BRCA-proficient ovarian high-grade serous carcinoma; association with patient survival. Hum. Pathol. 2013;44:1638–1647. doi: 10.1016/j.humpath.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Molnár S., Beke L., Méhes G., Póka R. The Prognostic Value of PARP Expression in High-Grade Epithelial Ovarian Cancer. Pathol. Oncol. Res. 2020;26:2549–2555. doi: 10.1007/s12253-020-00856-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molnár S., Vida B., Beke L., Méhes G., Póka R. The Prognostic Relevance of Poly (ADP-Ribose) Polymerase Expression in Ovarian Cancer Tissue of Wild Type and BRCA-Mutation Carrier Patients. Diagnostics. 2021;11:144. doi: 10.3390/diagnostics11010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michels J., Adam J., Goubar A., Obrist F., Damotte D., Robin A., Alifano M., Vitale I., Olaussen K., Girard P. Negative prognostic value of high levels of intracellular poly (ADP-ribose) in non-small cell lung cancer. Ann. Oncol. 2015;26:2470–2477. doi: 10.1093/annonc/mdv393. [DOI] [PubMed] [Google Scholar]
- 27.Kim H.C., Song J.S., Lee J.C., Lee D.H., Kim S.W., Lee J.S., Kim W.S., Rho J.K., Kim S.Y., Choi C.M. Clinical significance of NQO1 polymorphism and expression of p53, SOD2, PARP1 in limited-stage small cell lung cancer. Int. J. Clin. Exp. Pathol. 2014;7:6743–6751. [PMC free article] [PubMed] [Google Scholar]
- 28.Xie K.J., He H.E., Sun A.J., Liu X.B., Sun L.P., Dong X.J. Expression of ERCC1, MSH2 and PARP1 in non-small cell lung cancer and prognostic value in patients treated with platinum-based chemotherapy. Asian Pac. J. Cancer Prev. 2014;15:2591–2596. doi: 10.7314/APJCP.2014.15.6.2591. [DOI] [PubMed] [Google Scholar]
- 29.Lin L., Zhang Y.D., Chen Z.Y., Chen Y., Ren C.P. The clinicopathological significance of miR-149 and PARP-2 in hepatocellular carcinoma and their roles in chemo/radiotherapy. Tumour Biol. 2016;37:12339–12346. doi: 10.1007/s13277-016-5106-y. [DOI] [PubMed] [Google Scholar]
- 30.Yu B., Ding Y., Liao X., Wang C., Wang B., Chen X. Overexpression of PARPBP Correlates with Tumor Progression and Poor Prognosis in Hepatocellular Carcinoma. Dig. Dis. Sci. 2019;64:2878–2892. doi: 10.1007/s10620-019-05608-4. [DOI] [PubMed] [Google Scholar]
- 31.Li Z., Lv T., Liu Y., Huang X., Qiu Z., Li J. PARP1 is a novel independent prognostic factor for the poor prognosis of chordoma. Cancer Biomark. 2016;16:633–639. doi: 10.3233/CBM-160605. [DOI] [PubMed] [Google Scholar]
- 32.Murnyák B., Kouhsari M.C., Hershkovitch R., Kálmán B., Marko-Varga G., Klekner Á., Hortobágyi T. PARP1 expression and its correlation with survival is tumour molecular subtype dependent in glioblastoma. Oncotarget. 2017;8:46348–46362. doi: 10.18632/oncotarget.18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto M., Yamasaki M., Tsukao Y., Tanaka K., Miyazaki Y., Makino T., Takahashi T., Kurokawa Y., Nakajima K., Takiguchi S., et al. Poly (ADP-ribose) polymerase-1 inhibition decreases proliferation through G2/M arrest in esophageal squamous cell carcinoma. Oncol. Lett. 2017;14:1581–1587. doi: 10.3892/ol.2017.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Zhang Y., Zhao Y., Gao D., Xing J., Liu H. High PARP-1 expression is associated with tumor invasion and poor prognosis in gastric cancer. Oncol. Lett. 2016;12:3825–3835. doi: 10.3892/ol.2016.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klauschen F., von Winterfeld M., Stenzinger A., Sinn B.V., Budczies J., Kamphues C., Bahra M., Wittschieber D., Weichert W., Striefler J., et al. High nuclear poly-(ADP-ribose)-polymerase expression is prognostic of improved survival in pancreatic cancer. Histopathology. 2012;61:409–416. doi: 10.1111/j.1365-2559.2012.04225.x. [DOI] [PubMed] [Google Scholar]
- 36.Donizy P., Wu C.L., Mull J., Fujimoto M., Chłopik A., Peng Y., Shalin S.C., Angelica Selim M., Puig S., Fernandez-Figueras M.T., et al. Up-regulation of PARP1 expression significantly correlated with poor survival in mucosal melanomas. Cells. 2020;9:1135. doi: 10.3390/cells9051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim K.M., Moon Y.J., Park S.H., Park H.J., Wang S.I., Park H.S., Lee H., Kwon K.S., Moon W.S., Lee D.G., et al. Individual and Combined Expression of DNA Damage Response Molecules PARP1, γH2AX, BRCA1, and BRCA2 Predict Shorter Survival of Soft Tissue Sarcoma Patients. PLoS ONE. 2016;11:e0163193. doi: 10.1371/journal.pone.0163193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 39.Parmar M.K., Torri V., Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:243.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Qiao W., Pan L., Kou C., Li K., Yang M. Prognostic and clinicopathological value of poly (adenosine diphosphate-ribose) polymerase expression in breast cancer: A meta-analysis. PLoS ONE. 2017;12:e0172413. doi: 10.1371/journal.pone.0172413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diamantopoulos P., Zervakis K., Zervakis P., Sofotasiou M., Vassilakopoulos T., Kotsianidis I., Symeonidis A., Pappa V., Galanopoulos A., Solomou E., et al. Poly (ADP-ribose) polymerase 1 mRNA levels strongly correlate with the prognosis of myelodysplastic syndromes. Blood Cancer J. 2017;7:e533. doi: 10.1038/bcj.2016.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu H., Ma H., Yin M., Wei Q. Association between PARP-1 V762A polymorphism and cancer susceptibility: A meta-analysis. Genet. Epidemiol. 2012;36:56–65. doi: 10.1002/gepi.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin Q., Lu J., Zhu H., Xu L., Cheng H., Zhan L., Yang X., Zhang C., Sun X. PARP-1 Val762Ala polymorphism and risk of cancer: A meta-analysis based on 39 case-control studies. PLoS ONE. 2014;9:e98022. doi: 10.1371/journal.pone.0098022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen W., Xi H., Wei B., Chen L. The prognostic role of matrix metalloproteinase 2 in gastric cancer: A systematic review with meta-analysis. J. Cancer Res. Clin. Oncol. 2014;140:1003–1009. doi: 10.1007/s00432-014-1630-6. [DOI] [PubMed] [Google Scholar]
- 45.Wen L., Chen X.Z., Yang K., Chen Z.X., Zhang B., Chen J.P., Zhou Z.G., Mo X.M., Hu J.K. Prognostic value of cancer stem cell marker CD133 expression in gastric cancer: A systematic review. PLoS ONE. 2013;8:e59154. doi: 10.1371/journal.pone.0059154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li M., Threadgill M.D., Wang Y., Cai L., Lin X. Poly(ADP-Ribose) Polymerase Inhibition Down-Regulates Expression of Metastasis-Related Genes in CT26 Colon Carcinoma Cells. Pathobiology. 2009;76:108–116. doi: 10.1159/000209388. [DOI] [PubMed] [Google Scholar]
- 47.Pu H., Horbinski C., Hensley P.J., Matuszak E.A., Atkinson T., Kyprianou N. PARP-1 regulates epithelial–mesenchymal transition (EMT) in prostate tumorigenesis. Carcinogenesis. 2014;35:2592–2601. doi: 10.1093/carcin/bgu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodríguez M.I., Peralta-Leal A., O’Valle F., Rodriguez-Vargas J.M., Gonzalez-Flores A., Majuelos-Melguizo J., López L., Serrano S., de Herreros A.G., Rodríguez-Manzaneque J.C. PARP-1 regulates metastatic melanoma through modulation of vimentin-induced malignant transformation. PLoS Genet. 2013;9:e1003531. doi: 10.1371/journal.pgen.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antoniou A., Pharoah P.D., Narod S., Risch H.A., Eyfjord J.E., Hopper J.L., Loman N., Olsson H., Johannsson O., Borg A., et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turk A.A., Wisinski K.B. PARP inhibitors in breast cancer: Bringing synthetic lethality to the bedside. Cancer. 2018;124:2498–2506. doi: 10.1002/cncr.31307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faraoni I., Graziani G. Role of BRCA Mutations in Cancer Treatment with Poly(ADP-ribose) Polymerase (PARP) Inhibitors. Cancers. 2018;10:487. doi: 10.3390/cancers10120487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krishnakumar R., Kraus W.L. The PARP Side of the Nucleus: Molecular Actions, Physiological Outcomes, and Clinical Targets. Mol. Cell. 2010;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ray Chaudhuri A., Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bryant H.E., Schultz N., Thomas H.D., Parker K.M., Flower D., Lopez E., Kyle S., Meuth M., Curtin N.J., Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 55.Slade D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020;34:360–394. doi: 10.1101/gad.334516.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashworth A. A synthetic lethal therapeutic approach: Poly (ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J. Clin. Oncol. 2008;26:3785–3790. doi: 10.1200/JCO.2008.16.0812. [DOI] [PubMed] [Google Scholar]
- 57.Tutt A., Tovey H., Cheang M.C.U., Kernaghan S., Kilburn L., Gazinska P., Owen J., Abraham J., Barrett S., Barrett-Lee P. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial. Nat. Med. 2018;24:628–637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golan T., Hammel P., Reni M., Van Cutsem E., Macarulla T., Hall M.J., Park J.O., Hochhauser D., Arnold D., Oh D.Y., et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: The ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–2106. doi: 10.1016/S0140-6736(03)13718-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author (https://www.researchgate.net/profile/Yosep-Chong, accessed on 8 November 2021).