Abstract
BPA is identified as an endocrine-disrupting chemical that deteriorates the physiological function of the hormones of the male reproductive system. Bisphenol F (BPF), bisphenol S (BPS), and bisphenol AF (BPAF) are actively explored as substitutes for BPA and are known as BPA analogues in most manufacturing industries. These analogues may demonstrate the same adverse effects as BPA on the male reproductive system; however, toxicological data explaining the male reproductive hormones’ physiological functions are still limited. Hence, this mini-review discusses the effects of BPA and its analogues on the physiological functions of hormones in the male reproductive system, focusing on the hypothalamus-pituitary-gonad (HPG) axis, steroidogenesis, and spermatogenesis outcomes. The BPA analogues mainly show a similar negative effect on the hormones’ physiological functions, proven by alterations in the HPG axis and steroidogenesis via activation of the aromatase activity and reduction of spermatogenesis outcomes when compared to BPA in in vitro and in vivo studies. Human biomonitoring studies also provide significant adverse effects on the physiological functions of hormones in the male reproductive system. In conclusion, BPA and its analogues deteriorate the physiological functions of hormones in the male reproductive system as per in vitro, in vivo, and human biomonitoring studies.
Keywords: aromatase, bisphenol F, bisphenol AF, bisphenol S, HPG axis, steroidogenesis, spermatogenesis
1. Introduction
Bisphenol A (BPA) (4,4′-dihydroxy-2,2-diphenyl propane) is an organic synthetic monomer, reported to be one of the most produced chemicals in the world. About 7 million tonnes of BPA was produced in 2013, and this figure is estimated to be growing annually [1,2]. BPA was initially synthesized by the scientist Aleksandr Pavlovich Dianin in 1891. BPA’s resistance to high temperatures, shatterproof nature, and electrical insulation capability has led to this chemical being predominantly used in most epoxy resin and polycarbonate plastic industries [3,4,5]. Epoxy resin is a precursor chemical of any product that requires resilience and high durability, such as a dental sealant, amalgams, tanks, sports equipment, roads, structural adhesives, and coating materials in cans [6,7,8,9]. Polycarbonate plastic is also used as packaging or as containers for many daily consumable products, such as vegetables, fruits, milk bottles, plastic bags, and food and beverage containers. Furthermore, these polycarbonate plastics can also be involved in the packaging of personal care products such as shampoo, body wash, and cosmetics [7,8,9,10,11,12,13].
The extensive use of BPA in various industries has resulted in environmental pollution: BPA has reportedly been found in rivers, lakes, soils, sediments, home dust, and air [14,15,16,17,18]. The most alarming concern is that BPA has also been detected in human biological samples, such as in the blood, urine, sweat, breast milk, and in the umbilical cord [19,20,21]. BPA in human biological samples is expected, as it can be found in their food and drinking water, indicating that the most common source of BPA exposure in humans is oral [22,23,24,25]. Furthermore, BPA can also be exposed to humans via the dermis, which mostly occurs when someone is in indirect contact with thermal receipt paper. Humans may also be exposed to BPA through inhalation [26,27,28,29]. Recently, the negative impact of BPA has been widely reported by scientists and has been identified as a well-known endocrine-disrupting chemical (EDC) [30,31,32]. Previous studies have reported that exposure to BPA causes many diseases, such as tumours, cardiovascular diseases, neurodegenerative diseases, metabolic diseases, such as diabetes mellitus, autoimmune diseases, and male and female infertility, and can disturb the development of children, babies, and foetuses [29,33,34,35,36,37,38]. BPA increases the risk of transgenerational toxicity effects in the offspring, proven by an increase in epigenetic markers such as H3K9Ac and H3K27Ac in the spermatozoa due to parental BPA exposure [39,40].
The extensive adverse effects of BPA on human health have raised alarming awareness in society. Therefore, this chemical is being replaced with its analogues. Among the commonly used BPA analogues are bisphenol F (BPF), bisphenol S (BPS), and bisphenol AF (BPAF) [18,41,42,43,44]. These analogues share the same basic structure as BPA, where the two benzene rings are attached either with the short carbon or with other chemical chains [45,46]. However, these BPA analogues did not undergo proper toxicity evaluation testing before being used in the industry [47]. In 2015, the Environmental Protection Agency (EPA) reported a negative impact of BPA analogues on the aquatic system, environment, and human health [48]. Like BPA, its analogues also result in carcinogenicity, genotoxicity, neurotoxicity, reproductive toxicity, and developmental disorders [48]. Moreover, these analogues are also being categorized as EDCs.
Evidence showing that BPA and its analogues cause male infertility is growing. The most common mechanisms involved in toxicant-induced male infertility are oxidative stress and reproductive hormonal imbalance [49,50]. BPA and its analogues are also reported to exert oxidative stress in the plasma, testis, and sperm, resulting in spermatogenesis disturbance [51,52]. This disturbance leads to a reduction in sperm quality and testicular abnormalities, as shown by Leydig cell (LC), Sertoli cell (SC), and germ cell dysfunction [53]. BPA and its analogues also exhibited endocrine-disrupting activities in the male reproductive system, shown by the disturbance in the hypothalamus-pituitary-gonad (HPG) axis and steroidogenesis [54,55,56]. BPF, BPS, and BPAF share a property with oestradiol (E2), that is, these analogues possess the ability to activate the oestrogenic pathway in the human body system [57,58]. Their capability to mimic oestrogen-like properties causes a deterioration in the physiological function of the male reproductive system. Like other toxicants, BPA and its analogues display their affinity to bind to the oestrogen receptor (ER), such as ERα, ERβ, and oestrogen-related receptor gamma (ERR-γ) [56,59,60,61,62]. Furthermore, these chemicals also have the ability to disturb gene expression, such as Kiss1 and CYP19A1, which are involved in the HPG axis and steroidogenesis, respectively [63]. Recently, the effects of BPA and its analogues on these three significant pathways, in the form of the HPG axis [56,59,62,64,65,66,67,68,69,70,71,72,73,74,75,76,77], steroidogenesis [55,56,59,64,65,66,67,68,69,70,74,75,76,77,78,79], and spermatogenesis outcomes [64,65,66,67,68,69,70,75,76,77,80,81,82,83,84,85], have been well reported. To the best of our knowledge, the link between these three pathways in the exposures of BPA and its analogue is limited. Hence, this review was performed to connect the HPG axis, testicular steroidogenesis, and spermatogenesis outcomes after exposure to BPA and its analogues, leading to male reproductive toxicity
2. Bisphenols and Hypothalamus-Pituitary-Gonadal Axis in Male Reproductive System
The hypothalamus-pituitary axis is the main centre regulating endocrine hormone production in the human body, including the male reproductive system [2]. The hypothalamus releases the hormones responsible for stimulating the neuroendocrine activity of the pituitary glands, either in the anterior or posterior gland. One of the neuroendocrine activities regulated by the anterior pituitary gland is the HPG axis [3]. The HPG axis encounters three levels of hormone production: the hypothalamus releases the gonadotropin-releasing hormone (GnRH), the anterior pituitary gland secretes the follicle-stimulating hormone (FSH) and luteinizing hormone (LH), and the testis, specifically the LC, synthesises testosterone. GnRH, which is released from the hypothalamus, stimulates the anterior pituitary gland to release FSH and LH. Both hormones act on the testes to release target hormones, such as testosterone, oestrogen, progesterone, and inhibin. The primary purpose of this mechanism is to achieve homeostasis balance and modulate the positive and negative feedback of hormone regulation [4,5]. GnRH, LH, and FSH secretion are controlled by the neuropeptide kisspeptin (KiSS1), which is regulated by gene kiss1 [6,7,8]. Generally, the kiSS1 and G protein-coupled receptor 54 (GPR54) complex are involved in HPG axis feedback regulations [86]. KiSS1 binds to GPR54, also known as the kiss1 receptor, to form a Kiss1/GPR54 complex. This complex regulates the neuroendocrine reproductive axis by targeting the GnRH neuron to stimulate GnRH release [87]. Subsequently, GnRH stimulates neuron transmission at the anterior pituitary gland to induce the secretion of gonadotropic hormones, specifically LH and FSH. Furthermore, ERα also plays a significant role in regulating reproductive and sexual behaviour [88,89]. Once E2 binds to ERα in the hypothalamus, it suppresses GnRH secretion [90].
Several experimental studies revealed that BPA and its analogues, such as BPF, BPS, and BPAF, disturbed the HPG axis via KiSS1 and ERα by targeting mRNA gene expression. However, BPA and its analogues have either direct or indirect effects and are still controversial. Previous studies have reported that laboratory animals, such as rats and zebrafish, exposed to BPA showed an increase in Kiss1 mRNA expression in the brain [62,71,72,73]. Exposure to BPA at a dose of 50 µg/kg/bw via drinking water caused an increase in Kiss 1 mRNA expression [71]. The same findings were also recorded in the offspring and pups of rats and the transgenic embryo of zebrafish [62,72,73]. Exposure of zebrafish embryos to 1000 μg/L of BPA and BPS at 120 h post-fertilization (hpf) revealed an increased expression of the Kiss1 gene. Moreover, the Kiss 1 receptor was highly expressed, leading to an increased number of GnRH3 neurons in the hypothalamus [62]. GnRH3 neuron is a neuromodulator that indirectly controls the pituitary gonadal axis of the reproductive system in zebrafish [91,92]. Furthermore, Yang et al. [56] found that male zebrafish in an aquarium containing 0.1 and 1 mg/L BPF showed an increase in the expression of GnRH receptors (GnRHR1 and GnRHR2), which influences the increase of GnRH neurons. Another BPA analogue, BPAF, has also been reported to disturb the HPG axis in the offspring of male zebrafish by increasing the mRNA expression of gnrh2, fshβ, and lhβ [74]. In zebrafish, gnrh2, fshβ, and lhβ are orthologous to human GNRH2, FSHβ, and LHβ, respectively.
BPA and its analogues also showed the ability to interfere with ERα in in vivo and in vitro studies. The BPA and its analogues, BPF and BPAF, increased the binding affinity towards ERα, while BPS has no effect on this receptor in zebrafish embryos [59]. This observation is supported by in vivo studies where subcutaneous exposure to BPA at a dose of 50 mg/kg/bw for two days increased the expression of ERα in the hypothalamus [73]. The same finding was also shown in the transgenic zebrafish embryo exposed to BPA and BPS at doses of 1000 μg/L and 100 μg/L, respectively. In contrast, perinatal exposure to BPA at a dose of 50 µg/kg/bw via drinking water caused a decrease in the expression of both ERα and β in the hypothalamus of male Wistar rats during adulthood [71]. The increase of Kiss1 expression in the brain increases GnRH secretion, stimulating LH and FSH secretion. A study by Bai et al. [72] found that perinatal exposure to BPA at a dose of 2 µg/kg/bw increased the GnRH neuron in the brain, leading to an increase in LH levels in the blood of male rat offspring.
In contrast, several previous studies have reported a decrease in the FSH and LH levels in the blood of adult male rats when exposed to various dosages of BPA ranging from 25 mg/kg/bw to 200 mg/kg/bw either via oral gavage or intraperitoneal injection [64,65,66,67,75,76,77,80]. A similar finding was also shown in rats who were exposed to BPA analogues. A study by Ullah et al. [68] showed that BPF at a dose of 1 mg/kg/bw via oral gavage significantly reduced the LH and FSH levels in the plasma of male rats. Therefore, from the previous findings, we may assume that BPA and its analogues at a lower dosage may increase LH and FSH levels via Kiss1 expression. However, a contrasting finding was noted when BPA was exposed at a higher dosage. LH in the blood binds to the LH receptor on the LC membrane to stimulate testosterone synthesis [93]. A previous study reported a decrease in the testosterone levels in the blood of adult male rats when exposed to BPA either during adulthood or exposure of offspring via oral gavage, subcutaneously, or intraperitoneal injection [64,65,66,75,76,77,80].
Furthermore, exposure to BPF and BPS for 28 days in male rats also decreased testicular and plasma testosterone [52,68,69,70]. BPA and its analogues, BPF and BPAF, also caused a decrease in testosterone levels in the plasma or testis of male rats and zebrafish [42,72,74,75,76]. In contrast, a study by Stoker et al. [71] reported that perinatal exposure to BPA at a dose of 50 µg/kg/bw via drinking water caused an increase in the testosterone level of male Wistar rats during their adulthood. The contradictory results might be due to the dose, route, and length of BPA exposure. The effects of BPA and its analogues on the correlation between Kiss1, ERα, and GnRH in the brain with the levels of FSH, LH, and testosterone in the plasma is still doubtful. The differences may be due to the different doses, way of exposure, types of animals, and study duration. There might also be other mechanisms involving the correlation; for instance, the bisphenols can disrupt the mechanism of negative/positive feedback system in regulating the reproductive hormones or may do so via competitive inhibition at the receptor level either in the brain or the testis. Table 1 shows a summary of the effect of BPA and its analogues on the HPG axis involving the male reproductive system.
Table 1.
The effects of BPA and its analogues on the hypothalamus-pituitary-gonadal axis in male reproductive system.
| Type of Bisphenol |
Purity (Manufacturer) |
Dose (Route) | Animal | Duration of Exposure | Findings | Author |
|---|---|---|---|---|---|---|
| BPA | 99% (Sigma-Aldrich, St. Louis, MO, USA) | 50 µg/kg/bw (drinking water) | Adult male Wistar rats | Perinatal exposure (From gestation day 9 until lactation day 20) | ↑ Kiss1 mRNA expression (hypothalamus) ↓ ERα, ERβ (hypothalamus) ↑ testosterone (blood) ↓ estradiol (blood) |
[71] |
| BPA | >99% (Sigma-Aldrich) | 2 µg/kg/bw (s.c) |
Offspring male SD | Perinatal exposure Day 10 of gestation until day 7 of lactation | ↑ Kiss1 mRNA expression (brain) ↑ GnRH neuron ↑ LH, estradiol (blood) ↓ testosterone (blood) |
[72] |
| BPA | - | 50 mg/kg/bw (s.c.) |
Male pup Long Evans rats | 2 days Postnatal day 0–2 | ↑ expression of Kiss1 (brain) ↑ expression of ERα (brain) |
[73] |
| BPA | MERCK, Kenilworth, NJ, USA | 50 mg/kg/bw (i.p.) | Adult male SD rats | Alternate day until 21 days | ↓ FSH, LH, testosterone (serum) ↑ estradiol (serum) |
[75] |
| BPA | Sigma-Aldrich | 5 or 25 mg/kg/bw (oral gavage) | Adult male Wistar rats | 40 days | ↑ expression of Gnrhr, Lhb, Fshb, ERβ, AR mRNA (pituitary) ↓ Gnrh, ERα (hypothalamus) ↓ FSH, LH, testosterone (blood) ↑ estradiol (blood) |
[76] |
| BPA | >99% (Sigma-Aldrich) | 50 mg/kg/bw (oral gavage) |
Adult male Wistar rats | 14 days | ↓ FSH, LH, testosterone (serum) | [77] |
| BPA | Sigma-Aldrich | 50 mg/kg/bw (oral gavage) |
Adult male Wistar rats | 30 days | ↓ FSH, LH, testosterone, E2 (plasma) | [64] |
| BPA | Sigma-Aldrich | 200 mg/kg (oral gavage) |
Adult male SD rats | 42 days | ↓ FSH, LH, testosterone (blood) | [65] |
| BPA | - | 25 mg/kg/bw (i.p.) |
Adult male SD rats | Alternate day for 30 days | ↓ FSH, LH, testosterone (plasma) | [66] |
| BPA | Gracia chengdu chemical technology co, Chengdu, Sichuan, China. | 200 mg/kg (oral gavage) |
Adult male SD rats | 28 days | ↓ FSH, LH, testosterone (blood) | [67] |
| BPF | 99% purity (Santa Cruz Biotechnologie, Dallas, TX, USA) |
1, 5, 25, 50, and 100 mg/kg/bw (Oral gavage) |
Adult male SD rats | 28 days | ↓ FSH, LH, testosterone (plasma) ↓ testicular testosterone |
[68] |
| BPA/BPS | >98% | 1000 μg/L (BPA) 100 μg/L (BPS) |
Transgenic zebrafish (embryo) |
2 h of post fertilization until 25 or 20 h of post fertilization | BPA: ↑ number of GnRH3 neuron at 25 hpf (hypothalamus) ↑ expression of Kiss1 mRNA, Kiss1 receptor, gnrh3, lhβ, and fshβ after 120 h post fertilization (embryo) ↑ expression of ERα at 25 hpf BPS: ↑ number of GnRH3 neuron at 25 hpf (hypothalamus) ↑ expression of mRNA Kiss1 and gnrh3 at 25 hpf (hypothalamus) ↑ expression of ERα 25 hpf |
[62] |
| BPF/BPS | 99% purity (Santa Cruz Biotechnologie) |
5, 50, and 500 mg/kg/bw (Oral gavage) |
Adult male SD rats | 28 days | BPF: ↓ testicular and plasma testosterone BPS: ↓ testicular and plasma testosterone |
[69] |
| BPF |
> 98% (J&K Scientific Ltd., Newark, DE, USA) |
0.1 and 1 mg/L in aquarium water Renewed 50% of water every 2 days |
Male Zebrafish | 21 days | ↑ expression of GnRH2, GnRH3, GnRHR1, and GnRHR2 (Brain) ↑ FSHR, LHR (testis) ↓ testosterone (testis) ↑ estradiol (testis) |
[56] |
| BPS/BPF/ BPAF |
98% (Sigma-Aldrich) |
In vitro: BPAF: 0.076 µm BPA: 2.8 µm BPF: 10.6 µm |
Zebrafish (embryo and larvae) | From day 1 of hdf until 7-dpf larva | BPAF, BPA, BPF: ↑ affinity toward binding of ERα (in vitro) BPS: No affinity toward the ERα receptor (in vitro) |
[59] |
| BPS | - | 1, 50 µg/kg/bw (oral gavage) | Adult male SD rats | 28 days | ↓ testicular and plasma testosterone | [70] |
| BPAF | 99% (Sigma-Aldrich) |
25 and 125 µg/L | Offspring male zebrafish (embryo) | 120 days (exposure during embryo and larva stages) | ↑ gnrh2, fshβ, lhβ, and cyp19b in 125 µg/L ↑ estradiol ↓ testosterone |
[74] |
Abbreviations: ↑ Increase; ↓ Decrease.
3. Bisphenols and Steroidogenesis
Testicular steroidogenesis is another important process in regulating the normal physiology of the male reproductive system. Steroidogenesis products such as testosterone, oestrogen, inhibin B, and progesterone play an essential role in maintaining the homeostasis of hormones in blood circulation. LC is a well-known site for steroidogenesis, particularly in the male reproductive system [94]. Steroidogenesis occurs in two different locations in the LC: the mitochondria and endoplasmic reticulum [93,94]. LH in the circulation binds to the LH receptor (LHR) at the LC membrane, thus activating the G protein groups to form the LHR/G protein complex. This complex activates two pathways by increasing cyclic adenosine monophosphate (cAMP) production and allowing the entry of arachidonic acid (AA) into the LC [95]. Next, cAMP activates protein kinase A (PKA) and mitogen-activated protein kinase (MAPK) for the stimulation of the steroidogenic acute regulatory (StAR) protein. This StAR protein is responsible for the transportation and movement of cholesterol from the outer membrane to the inner membrane of mitochondria [96,97].
Meanwhile, the presence of AA in the LC helps control testosterone production by inhibiting cholesterol movement to the mitochondria [98,99]. AA produces prostaglandin-E2 (PGE-2) via the activation of cyclooxygenase-2 (COX-2) and inhibits StAR functions [100,101,102]. Cholesterol is the primary substrate acting as a precursor in testicular steroidogenesis [94,96]. In mitochondria, cholesterol is converted to pregnenolone through the action of CYP11A1 [103]. The pregnenolone then moves into the endoplasmic reticulum, and the steroidogenic cascade of enzyme reaction that takes place involves the CYP450 enzyme (CYP17) and hydroxy steroid dehydrogenase (HSD) enzymes (3β-HSD and 17β-HSD). The conversion of pregnenolone into testosterone can be divided into two pathways (Δ4 and Δ5). These pathways can be alternated depending on the binding affinity of the CYP17 towards the substrates, 17α-hydroxy pregnenolone and 17α-hydroxyprogesterone, which activate the Δ5 and Δ4 pathways, respectively [103]. Humans mainly undertake this activity through the Δ5 pathway, while rats and mice mostly take the Δ4 pathway. In normal physiology, in testicular steroidogenesis, the Δ5 pathway is less prone to be converted to the alternative pathway synthesizing the E2 than the Δ4 pathway.
Nowadays, growing evidence has demonstrated the ability of bisphenols to disturb the steroidogenesis pathway. StAR, a protein responsible for transporting and moving cholesterol into the mitochondria, is among the proteins affected by exposure to BPA and its analogues. The gene and protein expression of StAR was decreased in adult male rats exposed to BPA for 28 days and 42 days, respectively, at a dose of 200 mg/kg by oral gavage [65,67]. Furthermore, BPA analogues such as BPF, BPAF, and BPS also caused a decrease in the expression of StAR mRNA. The expression of StAR mRNA was decreased in BPF and BPAF in the adults and offspring of male zebrafish, respectively [56,74]. Meanwhile, Eladak et al. [78] found that BPF and BPS also significantly decreased the expression of StAR mRNA in mouse foetal testicular cells (mFeTA) at a concentration of 10,000 nmol/L. The disturbance of the StAR mRNA expression may lead to the deterioration of testicular steroidogenesis due to the disturbance in cholesterol transportation and movement into the mitochondria in the LC.
Previous findings have also shown disturbance in the gene and protein expression of cytochrome P450 and HSD enzymes either in the mitochondrial or reticulum endoplasmic of LC, such as CYP11A1, CYP 17A1, 3β-HSD, and 17β-HSD. Exposure to BPA at a dose of 200 mg/kg for 28 days reduced the gene expression of CYP11A1 in the testicular mitochondria of male Sprague-Dawley rats [67]. Furthermore, exposure to BPA at the same dose for 42 days also decreased the protein expression of CYP11A1 [65]. In contrast, the expression of CYP11A1 was found to increase in adults and embryos of male zebrafish exposed to BPF and BPAF, respectively [56,74]. The disruption of CYP11A1 either involving the gene or protein expression decreases the conversion of cholesterol to pregnenolone in the mitochondria of LC. The CYP17A1 and 3β-HSD gene expression involved in the steroidogenic enzyme cascade in the endoplasmic reticulum also decreased in BPA-intoxicated rats [65]. The 17β-HSD, 3β-HSD, and CYP17A1 protein expression also decreased in the testis of male Sprague-Dawley rats exposed to BPA [80]. The same findings were also noted with exposure to BPA analogues in either in vivo or in vitro studies. BPF and BPAF exposure decreased CYP17 expression in the testis of zebrafish, while 17βHSD was found to be decreased in the testis of adult male zebrafish after 21 days of exposure to BPF [56,74]. An in vitro study conducted by Eladak et al. [78] found that BPF and BPS at the highest dose (10,000 nmol/L) caused a decrease in the expression of HSD3β1 and CYP17A1 in mFeTA after three days of exposure.
Furthermore, BPF and BPAF also decreased the gene expression of HSD3β2 and CYP17A1 in the human adrenocortical carcinoma cell line [79]. However, only BPS exposure has been reported to decrease the gene expression of CYP17A1 in the same cell line [79]. Hence, the interference of the steroidogenic enzymes, demonstrated by the changes in the gene and protein expression of 17β-HSD, 3β-HSD, and CYP17A1, may be the reason for the abnormal testosterone level in BPA, BPF, BPS, and BPAF exposure [56,65,74,78,79].
Most exposure to BPA and its analogues causes decreased testosterone levels due to the low level of LH and the disturbance of the steroidogenic enzyme cascade in the LC [52,56,64,65,66,67,68,69,72,74,75,76,77,78,79]. In contrast, Roelofs et al. [55] reported that exposure to BPF and BPS for 48 h caused an increase in the level of testosterone in the MA-10 LC culture. Moreover, Stoker et al. [71] also reported an increase in the testosterone level in male Wistar rats during their adulthood when exposed to BPA in their perinatal stage [71]. Decreasing testosterone synthesis activates the backdoor pathway, whereby dehydroepiandrosterone (DHEA) is converted into androstenedione (AD). AD is responsible for the Δ4 pathway, and this pathway poses a high risk of stimulating the overproduction of E2. This backdoor pathway is also known as the alternative pathway in testicular steroidogenesis involving the activation of p450 aromatase. The activation of aromatase is exhibited by the increased gene and protein expression of CYP19A1, which leads to the activation of cAMP. This mechanism causes the overproduction of E2 in the testis, which also presents as an effect of BPA and its analogues [56,59,72,74,75,76,79]. However, studies conducted by Stoker et al. [71] and Alboghobeish et al. [64] found a decrease in E2 levels in the blood circulation of adult male rats exposed to BPA. The disruption in this steroidogenesis pathway leads to a disturbance in sperm synthesis known as spermatogenesis. Table 2 shows the effects of BPA and its analogues on steroidogenesis in the male reproductive system.
Table 2.
The effects of BPA and its analogues on the steroidogenesis in male reproductive system.
| Type of Bisphenol |
Purity (Manufacturer) |
Dose (Route) | Animal | Duration of Exposure | Findings | Author |
|---|---|---|---|---|---|---|
| BPA | 99% (Sigma-Aldrich) | 50 mg/kg/bw (oral gavage) |
Adult male Wistar rats | 14 days | ↓ testosterone (serum) | [80] |
| BPA | 99% (Sigma-Aldrich) | 50 µg/kg/bw (drinking water) | Adult male Wistar rats | Perinatal exposure (gestation day 9 until lactation day 20) | ↑ testosterone (blood) ↓ estradiol (blood) |
[71] |
| BPA | >99% (Sigma-Aldrich) |
2 µg/kg/bw (s.c) |
Offspring male SD rats | Perinatal exposure Day 10 of gestation until day 7 of lactation | ↑ estradiol (blood) ↓ testosterone (blood) |
[72] |
| BPA | Sigma-Aldrich | 5 or 25 mg/kg/bw (oral gavage) | Adult male Wistar rats | 40 days | ↓ testosterone (blood) ↑ estradiol (blood) |
[76] |
| BPA | MERCKS | 50 mg/kg/bw (i.p.) | Adult male SD rats | Alternate day until 21 days | ↓ testosterone (serum) ↑ estradiol (serum) |
[75] |
| BPA | Gracia chengdu chemical technology co. | 200 mg/kg (oral gavage) |
Adult male SD rats | 28 days | ↓ testosterone (blood) ↓ expression of mRNA StAR, CYP11A1, 3β-HSD, CYP17A1 (testis) |
[67] |
| BPA | Sigma-Aldrich | 200 mg/kg (oral gavage) |
Adult male SD rats | 42 days | ↓ testosterone (blood) ↓ expression of protein StAR, CYP11A1, 17β-HSD, 3β-HSD, CYP17A1 (testis) |
[65] |
| BPA | Sigma-Aldrich | 50 mg/kg/bw (oral gavage) |
Adult male Wistar rats | 30 days | ↓ testosterone, estradiol (plasma) | [64] |
| BPA | - | 25 mg/kg/bw (i.p.) | Adult male SD rats | Alternate day of 30 days | ↓ testosterone (plasma) | [66] |
| BPF | 99% (Santa Cruz Biotechnologie) |
1, 5, 25, 50, and 100 mg/kg/bw |
Adult male SD rats | 28 days | ↓ testosterone (plasma) ↓ testosterone (testis) |
[68] |
| BPF/BPS | 99% (Santa Cruz Biotechnologie) |
5, 50, and 500 mg/kg/bw | Adult male SD rats | 28 days | BPF: ↓ testicular and plasma testosterone BPS: ↓ testicular and plasma testosterone |
[69] |
| BPF |
> 98% (J&K Scientific Ltd) |
0.1 and 1 mg/L in aquarium water | Male Zebrafish | 21 days | ↓ testosterone (homogenate) ↑ estradiol (homogenate) ↑ expression of CYP19A1b (aromatase)(Brain) ↑ expression of mRNA CYP11A, CYP19A (testis) ↓ expression of mRNA StAR, CYP17, 17βHSD (testis) |
[56] |
| BPS/BPF/ BPAF |
98% (Sigma-Aldrich) |
In vivo: 1 µm In vitro: BPAF: 0.076 µm BPA:2.8 µm BPF: 10.6 µm |
Zebrafish (embryo and larvae) | From day 1 of hdf until 7-dpf larva | BPAF, BPF, BPS: ↑ expression of mRNA CYP19A1 gene in 7-dpf (embryo) |
[59] |
| BPS | - | 1, 50 µg/kg/bw (oral gavage) | Adult male SD rats | 28 days | ↓ testicular and plasma testosterone | [70] |
| BPAF | 99% (Sigma–Aldrich) |
25 and 125 µg/L | Offspring male zebrafish (embryo) | 120 days through exposure of embryo and larva stages - |
↑ estradiol ↓ testosterone ↑ CYP19b (brain) ↑ expression of mRNA CYP19A and CYP11A1 (testis) ↓ expression of mRNA StAR and CYP17 (testis) |
[74] |
| BPF/BPS | BPF (>99%) BPS (>98%) |
BPF: 0.01–100 µM BPS: 0.01–30 µM |
MA-10 Leydig cell culture | 48 h | BPF, BPS: ↑ testosterone secretion ↑ expression of 5αRed1 |
[55] |
| BPF/BPS | - | 10, 100, 1000, 10,000 nmol/L | Mouse fetal testis assay (mFeTA) | 1–3 days | BPF, BPS (10 000 nmol/L): ↓ testosterone secretion ↓ expression of mRNA StAR, HSD3β1 and CYP17A1 |
[78] |
| BPF/BPS/ BPAF |
BPF (99%) BPS (98%) BPAF (99%) |
0.1, 1, 10, 30, 50 and 70 µM | Human adrenocortical carcinoma cell line (H295R) | - | BPF: ↑ estradiol and progesterone secretion (dose-dependent manner) ↓ expression of mRNA HSD3β2 (50 µM) and CYP17A1 (dose-dependent manner) BPS: ↓ testosterone secretion (dose-dependent manner) ↓ expression of mRNA CYP17A1 BPAF: ↓ testosterone secretion (dose-dependent manner) ↑ progesterone secretion ↓ expression of mRNA CYP17A1, HSD3β2 |
[79] |
Abbreviations: ↑ Increase; ↓ Decrease.
4. Bisphenols and Spermatogenesis
Spermatogenesis occurs within the seminiferous tubules in the testis. The germ cells, such as spermatogonia, spermatocyte, and spermatid, undergo various stages of spermatogenesis to form sperm. Spermatogenesis occurs via specific processes, such as proliferation, differentiation, mitosis, meiosis, and spermiogenesis, to develop mature spermatozoa. Among these specific processes, proliferation, differentiation, and mitosis occur in the basement membrane, while the remaining processes occur in the adluminal compartment [104]. The blood–testis barrier (BTB) is formed after the basal membrane to protect the microenvironment of the adluminal compartment for the processes relevant to that area. Therefore, the germ cells found in the basement membrane are more vulnerable to any toxicants than the germ cells found in the adluminal compartment [93]. The integrity of the BTB is also crucial because changes in its structure may affect the production and morphological structure of the sperm [95]. Spermatogenesis involves not only different stages of germ cells but also SCs. These cells secrete pyruvate and lactate to nourish germ cells during their development and are responsible for the organization of the germ cells [103]. Therefore, any disturbance in the SC causes degeneration and disorganization of the germ cells. Testosterone plays a critical role in spermatogenesis owing to its ability for BTB maintenance, meiosis, Sertoli-spermatid adhesion, and the release of mature spermatozoa [104]. Testosterone maintains the remodelling of the BTB by binding with AR to form the protein involved in the integrity of tight junctions. Testosterone is needed in the completion of meiosis during the development of spermatocytes. Moreover, testosterone also plays an essential role in preventing the elongated spermatid from being released earlier. However, the testosterone hormone helps release the mature spermatozoa into the lumen of the seminiferous tubule, thus preventing spermatozoa from being engulfed by the SC [104].
BPA and its analogues were reported to disturb spermatogenesis by diminishing the BTB integrity, changing testicular histopathology, and causing sperm defects [52,64,65,66,67,68,69,70,75,76,77,80,81]. A study carried out by Li et al. [105] found a disturbance in the BTB of male Wistar rats, which was proven by the reduction of occludin and nectin-3 when exposed to BPA in a dose-dependent manner. The reduction of occludin was also found in the SCs, which were exposed to BPA in in vitro studies [82,83]. Furthermore, both studies also showed a decrease in the ZO-1 protein level [82,83]. The disturbances of these proteins lowered the integrity of BTB, which was proven by the reduction in cell viability and androgen receptor (AR) after 6 h of BPA exposure [82]. Moreover, Feng et al. [83] also found that the reduction of occludin and ZO-1 in SCs significantly perturb the tight junction barrier, lowering the integrity of the BTB. The disruption of BTB integrity may allow germ cells in the adluminal compartment to be exposed to toxicants, leading to the disturbance of spermatogenesis in the seminiferous tubules.
Spermatogenesis disruption after exposure to BPA and its analogues can be shown by histological observations, such as reduction in the diameter and epithelial height of germ cells, atrophy and separation of germinal epithelium, and irregular seminiferous tubule structure [66,67,80]. Previous studies reported that BPA exposure causes histopathological changes, proven by the vacuolation, degeneration, and disorganization of germ cells [66,67,80]. The vacuolation and degeneration of germ cells were reported after BPA exposure either via oral gavage for 52 days or intraperitoneal injection on alternate days for 30 days in adult male Sprague-Dawley rats [66,80]. The spermatogenesis process was found to be weak, arrested in the seminiferous tubule of adult male Wistar rats exposed to BPA at a dose of 50 mg/kg via oral gavage for 14 days [77]. Furthermore, the same study also found that spermatocytes are among the most affected germ cells in BPA-intoxicated rats [77]. Wang et al. [67] found that BPA at a dose of 200 mg/kg via oral gavage caused disorganization of germ cells [67]. However, these changes were not observed in BPA analogue-intoxicated rats. Moreover, BPA analogues such as BPF and BPS cause spermatids to become longer, and the absence of mature spermatozoa in the lumen of seminiferous tubules disrupts spermatogenesis in adult male Sprague-Dawley rats [65,68,69]. According to Liang et al. [81], BPA and its analogues (BPS and BPAF) decrease cell viability and increase the DNA damage of the spermatogonia cell line (C18-4). Among these bisphenols, BPAF causes significant outcomes at the lowest concentrations within 24 h of exposure [81].
BPA and its analogues disrupt spermatogenesis, leading to the deterioration of its outcome, which is proven by a reduction in sperm quality. Low sperm production induced by toxicants is usually associated with oxidative stress and the reduction of testosterone in the blood circulation [49,106]. Furthermore, the reduction in sperm development may also be due to abnormal SC causing insufficient nutrition, which is necessary for spermatogenesis [105]. According to previous studies, adult male rats’ exposure to BPA causes a decrease in sperm quality, proven by a reduction in sperm production, count, motility, viability, and the integrity of sperm acrosome and plasma membrane mitochondrial activity [64,65,66,67,75,76,77]. BPA at a dose of 50 mg/kg/bw for 14 days caused mild oedema in the LC, leading to a reduction in testosterone, thus lowering the sperm quality of adult male Wistar rats [77]. There is also an association reported between mitochondrial activity and motility in sperm because the mitochondria is the only source of ATP that enables the energy production necessary for sperm movement [76]. The sperm-specific ion calcium (Ca2+) channel (CatSper) is also crucial for sperm motility, hyperactivation, and acrosome reaction. This pH-sensitive channel is responsible for providing enough Ca2+ for sperm function [107]. Progesterone is a factor that influences the activation of the CatSper channel for sperm hyperactivation and acrosomal reaction to penetrate the oocyte [107]. Previous findings reported that the expression of the CatSper channel and charges were significantly downregulated and decreased after exposure to 10, 50, and 250 µg/kg/kg doses of BPA to the sperm mice orally [84]. These reductions parallel the finding where the motility and acrosome reaction in the presence of progesterone were significantly decreased as well. Exposure of healthy human sperm to 10 μM BPA analogues showed a similar effect on the CatSper channel’s ability. In a study, the scholars found that BPG, BPAF, BPBP, BPC, and BPB are potent chemicals that inhibit progesterone-induced Ca2+ [85]. These BPA analogues are shown to affect Ca2+ signaling, which can interfere with normal CatSper signaling and result in infertility [85]. Table 3 shows the effects of BPA and its analogues on spermatogenesis in the male reproductive system.
Table 3.
The effects of BPA and its analogues on spermatogenesis in the male reproductive system.
| Type of Bisphenol |
Purity (Manufacturer) |
Dose (Route) | Animal | Duration of Exposure | Findings | Author |
|---|---|---|---|---|---|---|
| BPA | MERCK | 50 mg/kg/bw (i.p.) | Adult male SD rats | Alternate day until 21 days | Sperm: ↓ sperm count, motility, viability |
[75] |
| BPA | Sigma-Aldrich | 50 mg/kg/bw (Oral gavage) |
Adult male SD rats | 52 days | Histopathology: Vacuolated and degeneration of germ cells |
[80] |
| BPA | Sigma-Aldrich | 5 or 25 mg/kg/bw (Oral gavage) |
Adult male Wistar rats | 40 days | Sperm: ↓ total and daily sperm production, integrity of acrosome, plasma membrane and mitochondria activity in sperm |
[76] |
| BPA | 99% (Sigma-Aldrich) |
50 mg/kg/bw | Adult male Wistar rats | 14 days | Histopathology: Leydig cells mild edema Spermatocyte depletion Spermatogenesis from weak to arrest. Sperm: ↓ daily sperm production, sperm count, sperm motility |
[77] |
| BPA | Sigma-Aldrich | 50 mg/kg/bw (Oral gavage) |
Adult male Wistar rats | 30 days | Histopathology: ↓ diameter and epithelial height of seminiferous tubule Atrophy and separation of germinal epithelium Sperm: ↓ sperm count |
[64] |
| BPA | Sigma-Aldrich | 200 mg/kg (Oral gavage) |
Adult male SD rats | 42 days | Sperm: ↓ sperm count, daily sperm production, motility |
[65] |
| BPA | - | 25 mg/kg/bw (i.p.) | Adult male SD rats | Alternate day of 30 days | Histopathology: Degeneration and vacuolation of germ cells Sperm: ↓ sperm count, motility, and viability |
[66] |
| BPA | Gracia chengdu chemical technology co. | 200 mg/kg (Oral gavage) |
Adult male SD rats | 28 days | Histopathology: ↓ quantity of mature sperm, Longer spermatid Disorganization of germ cells Sperm: ↓ sperm count, motility |
[67] |
| BPF | 99% (Santa Cruz Biotechnologie) |
1, 5, 25, 50, 100 mg/kg/bw |
Adult male SD rats | 28 days | Histopathology: ↓ germinal epithelial height Absence of sperm in lumen |
[68] |
| BPF/BPS | 99% (Santa Cruz Biotechnologie) |
5, 50, 500 mg/kg/bw | Adult male SD rats | 28 days | Histopathology: BPF: Seminiferous tubules irregular Longer spermatid BPS: Absence of sperm in lumen |
[69] |
| BPS | 99% (Santa Cruz Biotechnologie) |
25, 50 µg/kg/bw (Oral gavage) |
Adult male SD rats | 28 days | Histopathology: ↓ epithelial of seminiferous tubules Spermatid become longer. |
[70] |
| BPA/BPAF /BPS |
BPA (>99%) BPS (98%) BPAF (98%) |
25, 50, 100 µM | C18-4 spermatogonial cell line | 24–72 h | BPA: ↓ the cell viability after 24 h (100 µM) ↑ DNA damage after 48 h (50 µM) BPAF: ↓ the cell viability after 24 h (50 µM) ↑ DNA damage after 24 h (25 µM) BPS: ↓ the cell viability after 24 h (100 µM) ↑ DNA damage after 24 h (50 µM) |
[81] |
| BPA | Sigma-Aldrich | 10 and 50 mg/kg/bw | Adult male wistar rat | - | ↓ occludin (10 mg/kg for 11 weeks and 50 mg/kg for 4 weeks) ↓ nectin-3 (50 mg/kg for 2–4 weeks) |
[105] |
| BPA | - | 20 µM | Sertoli cells | - | ↓ occludin (after 48 h) ↓ Z0-1 (after 6 and 48 h) ↓ cells viability after 6 and 48 h ↓ androgen receptor after 6 and 48 h |
[82] |
| BPA | Sigma-Aldrich | 25 and 100 µM | Sertoli cells isolated from 20 days of wistar rats | - | ↓ occludin and ZO-1 (both doses) ↑ conexxin (both dosage) Significantly perturb the tight junction barrier at dosage 100 µM (p < 0.05) |
[105] |
| BPA | Sigma-aldrich (US) | 10, 50,250 µg/kg/D (Oral gavage) |
Sperm of C57BL/6 mice | 8 weeks | ↓ sperm motility (p < 0.05) ↓ Progesterone-induced acrosome reaction (p < 0.05) |
[84] |
| BPG, BPAF, BPC, BADGE, BPB | Sigma-aldrich (MO,US) | Ca2+ signal: 10 µM Progesterone-induced Ca2+ signal: BPF: 5 µM BPAF&BPBP: 10 µM BPC, BADGE, BPB: 50 µM |
Healthy human semen | - | ↑ Ca2+ signaling ↓ Progesterone-induced Ca2+ signal |
[85] |
Abbreviations: ↑ Increase; ↓ Decrease.
5. The Effects of BPA and Its Analogues on Male Reproductive Hormones: Human Biological Studies Evidence
Human epidemiology findings show a strengthened impact of BPA on the male reproductive system, involving the sexual hormones and sperm characteristics, through in vivo and in vitro studies. However, to the best of our knowledge, the number of human studies on the effects of BPA analogues on the male reproductive system focusing on the HPG axis is still limited. Most human studies have shown an association between the presence of BPA and its analogues in biological samples, such as urine and serum, with male sexual hormones and its effects on spermatogenesis outcomes, such as sperm characteristics parameters. A cross-sectional study was carried out between male children and adolescents in the United States of America. This study found a significant association between a high concentration of BPA in the urine and a low level of total testosterone in the serum of male adolescents only [108]. Researchers have estimated that 1 unit of BPA might lower the total testosterone levels by approximately 50% [108]. The finding prevailed for associations between BPA and other male reproductive hormones, such as AD and FSH, in men who work in the epoxy resin industry in Shanghai, China [109]. Liu et al. [109] found that increased BPA concentration in the urine indicated a significant decrease in AD and FSH levels in the worker’s serum [109]. Another study among men in the same industry in Guangdong, China, also presented the same findings [110]. The researcher found that BPA concentration and the duration of BPA exposure also influence hormone levels. Higher concentration and longer duration of BPA exposure decreased AD levels increasingly. Furthermore, Zhuang et al. [110] also found that a high concentration and a longer duration of BPA exposure also increased the sex hormone binding globulin (SHBG) in the serum of workers in the industry [110]. SHBG is a globulin protein that carries testosterone, dihydrotestosterone (DHT), and E2. Among these hormones, testosterone is carried the most by this protein. Therefore, an increase in SHBG level suggests that the testosterone level has decreased in the serum of the workers exposed to BPA. However, when comparing worker and non-worker groups, no association was found on the levels of SHBG, total testosterone, inhibin B, and AD [110].
Meanwhile, a study by Lassen et al. [111] found that increased BPA concentration in the urine significantly increased the total and free testosterone, LH, and E2 levels in the serum of the population of young men in Denmark [111]. Furthermore, a cross-sectional study conducted by Adoamnei et al. [112] found that increased concentrations of BPA in the urine also present a significant increase in the LH level among the population of young men in Spain. However, no association was found between BPA and FSH, free testosterone, SHBG, inhibin B, or E2 levels in the same population [112]. BPA acts as an anti-androgen agent by its ability to competitively bind to the AR, causing an increase in testosterone levels in circulation. However, even though BPA can bind to AR, it does not have any effects on testosterone, as a massive amount of BPA is required in serum to exhibit any effect as an antagonist towards AR. Therefore, the HPG axis is being induced to secrete LH for steroidogenesis activation in the LC, leading to increasing testosterone formation. This mechanism explains the increased levels of LH and free testosterone reported by Lessen et al. [111] and Adoamnei et al. [112].
BPA is known to raise oestrogenic activity either by stimulating or inhibiting the ER [113]. BPA has been reported to have a higher affinity towards ERβ than ERα in an in vitro study [112]. The action of BPA towards these receptors appears to be very complex, but the response depends on the presence of the ER subtypes (ERα and ERβ) and the co-regulatory protein, which either acts as a co-activator protein (stimulate) or the co-repressor protein (inhibit) [113]. The stimulation or inhibition of ER causes an increase or decrease of E2 in circulation, respectively [112]. Several studies have reported increasing E2 levels in the blood after exposure to BPA [108,111]. BPA can strongly bind to oestrogen-related receptors, such as ERR-γ, which interfere with the steroid synthetase functions and gene expression involved in steroidogenesis [110]. BPA has also been identified to bind with the ER at the anterior and posterior pituitary gland, leading to HPG axis disturbance and activation of prolactin secretion. The increased prolactin level in men who were occupationally exposed to BPA was evidence for this [109]. Furthermore, BPA can also increase the E2 level by its ability to activate an alternative steroidogenesis pathway. In humans, the Δ5 pathway is dominant; however, BPA may activate the alternative pathway where DHEA is converted into AD, which has a high incidence of stimulating the overproduction of E2 [109,110].
A retrospective study known as the ELEMENT project was carried out by Ferguson et al. [114], who reported no association between BPA levels found in mothers’ urine during pregnancy with SHBG, inhibin B, or free and total testosterone levels in the serum of male children aged between 8 and 14 years in Mexico [114]. Furthermore, the same study also found no association between BPA in the urine of the male children with SHBG, inhibin B, and the free and total testosterone levels in the serum [114]. However, a retrospective study done by Hart et al. [115] found a significant weak positive correlation between BPA in the serum of pregnant women and sperm motility and concentration in the semen of young men aged between 20 and 22 years old in Australia [115]. BPA was found in almost 90% of the maternal serum sample [115]. Furthermore, other epidemiological studies showed that a high concentration of BPA decreased sperm quality, proven by decreasing sperm concentrations, count, and motility [111,112,116,117]. A study by Lessen et al. [111] found that a high concentration of BPA present in the urine caused a decrease in the percentage of progressively motile sperm in the semen of young men in Denmark [111]. The same finding was reported between BPA and sperm count and concentration in young men aged 18–23 years in Spain. The high BPA concentration found in the urine of young men decreased the sperm count and concentration. However, the sperm motility and morphology in the same study population did not show any alterations [112]. Furthermore, there was no association between BPA and semen analysis, such as sperm morphology, sperm concentration, total sperm count, and semen volume, among fertile men aged over 18 years in Michigan and Texas [118]. Interestingly, BPA has also been identified to affect semen quality in IVF patients in Slovenia, where sperm count, concentration, vitality and motility decreased with the increasing concentration of BPA in the urine [116].
BPA analogues, such as BPS, also presented adverse effects in semen analysis among the population of young men involved in the FEPOS project in Denmark. The semen volume was lowered in subjects who had high BPA in their urine [119]. In contrast, Abou Ghayda et al. [117] found that infertile patients in Boston, MA, USA, with a high BPS concentration showed a high semen volume; however, this semen has a lower sperm concentration [117]. Low sperm concentration was found in infertile patients who were exposed to BPS. The researcher also reported that an increased level of BPS causes a significant decrease in sperm quality among obese or overweight patients who have a BMI >25 kg/m2. Therefore, BPS can increase the severity of sperm defects observed in obese or overweight infertile patients. Table 4 summarises human epidemiological studies of BPA and its analogues towards the male reproductive system.
Table 4.
The summary of human epidemiological studies of BPA and its analogues towards male reproductive system.
| Type of Bispheno |
Study Design |
Study Population (Age) (Project Name) |
Country (Sample Population) | Biological Sample | [Bisphenol] Detected in Biological Sample (Mean/ Median) |
Findings | Beta Coefficient |
Significant Values | Author |
|---|---|---|---|---|---|---|---|---|---|
| BPA | Cross sectional study | Male children (6–11 y.o) Male Adolescents (12–19 y.o) (NHANES Project) |
USA (n = 588) | Urine Serum | Mean: male children 1.74 ng/mL (urine) Mean: male adolescents 1.94 ng/mL (urine) |
Reproductive hormones: No association between BPA and reproductive hormones in male children across the quartiles. Increased BPA level caused a significant decrease in TT in male adolescents across the quartiles. |
Q2: β = −49.34% Q3: β = −36.87% Q4: β = −53.70% |
p < 0.05 | [108] |
| BPA | Cross sectional study | Male worker of epoxy resin manufacturer (16–63 y.o) |
Shanghai, China (n = 592) | Urine Serum | Median occupational exposure: 685.9 µg/g Cr (urine) Median non-occupational exposure: 4.2 µg/gCr (urine) |
Reproductive hormones: Increased level of BPA cause significant increase in:
Increased level of BPA cause significant decreased in levels:
|
[109] | ||
| β = 0.0589 ng/mL | p < 0.001 | ||||||||
| β = 0.0293 nmol/L | p < 0.01 | ||||||||
| β = 0.0362 pg/mL | p < 0.001 | ||||||||
| β = −0.0367 ng/mL | p < 0.001 | ||||||||
| β = −0.024 mIU/mL | p < 0.05 | ||||||||
| BPA | Cross sectional study | Male worker of epoxy resin manu-facturer | Guang-dong, China (n = 559) | Serum | Median workers: 8.75 ng/mL (serum) Median non-workers: 3.37 ng/mL (serum) |
Reproductive hormones: No association between workers and non-workers on the level of SHBG, TT, INB and AD |
[110] | ||
| Increased exposure time caused significant decreased in median AD level among workers. | - | p < 0.001 | |||||||
| Increased exposure time caused significant increase in median SHBG level among workers. | - | p < 0.05 | |||||||
| Increased BPA level caused significant increase in median SHBG level among workers. | β = 2.79 nmol/L | p < 0.05 | |||||||
| Increased of BPA level caused significant decreased in median AD level among workers. | β = −0.18 ng/mL | p < 0.001 | |||||||
| BPA | Cross sectional study | Young men | Denmark (n = 308) |
Urine Serum Semen |
Median: 3.74 ng/mL (Osm)(urine) |
Reproductive hormones: Increased level of BPA caused significant increase in:
|
[111] | ||
| β = 0.7 nmol/L | p < 0.01 | ||||||||
| β = 2.7% | p < 0.05 | ||||||||
| β = 3.5% | p < 0.05 | ||||||||
| β = 2.7% | p < 0.05 | ||||||||
| Sperm characteristics: Increased level of BPA caused a significant decreased in percentage of progressive motile spermatozoa across the quartiles. |
β = −1.82% | p < 0.01 | |||||||
| BPA | Cross sectional study | Young men (18–23 y.o) |
Spain (n = 215) |
Urine Serum Semen |
Mean: 1.8 µg/g (urine) |
Reproductive hormones: Increased level of BPA caused significant increase in LH level across the quartiles. No association between BPA and FSH, FT, SHBG, INB and E2 across the quartile. |
β = 0.07 IU/L | p < 0.01 | [112] |
| Sperm characteristics: Increased level of BPA caused significant decreased in sperm characteristic across the quartiles:
|
|||||||||
| β = −0.04 Mill./mL | p < 0.01 | ||||||||
| β = −0.05 Mill. | p < 0.01 | ||||||||
| BPA | Retro-spective cohort | Pregnant woman Male chil-dren (8–14 y.o) (ELEMENT project) |
Mexico (n = 118) |
Urine Urine Serum |
Mean: 0.7 ng/mL (urine) Mean: 1.1 ng/mL (urine) |
Reproductive hormones: No association between prenatal urinary BPA and the boy sex hormones in the level of SHBG, INB, TT, E2, DHEAS and FT |
- | - | [114] |
| No association between child-hood urinary BPA and the boy sex hormones in the level of SHBG, INB, TT, E2, DHEAS and FT | - | - | |||||||
| BPA | Retro-spective cohort | Pregnant woman (Week 18 & 34) Young men (20–22 y.o) |
Australia (n = 423) |
Serum (mother) Semen |
Median: 0.25 µg/L (serum) | Sperm characteristics: Maternal exposure of BPA caused significant changes in sperm characteristics of young men such as increased in the sperm concentration and motility. |
- | p < 0.05 | [115] |
| BPA | Pro-spective cohort |
Men IVF patient (34.05 y.o) |
Slovenia (n = 149) |
Semen | Mean: 1.33 ng/mg (urine) | Sperm characteristics: Increase concentration of BPA cause significant decrease in:
|
[116] | ||
| β = −0.219, R2 = 0.071 | p = 0.047 | ||||||||
| β = −0.241, R2 = 0.092 | p = 0.039 | ||||||||
| β = −0.273, R2 = 0.075 | p = 0.043 | ||||||||
| β = −0.266, R2 = 0.052 | p = 0.026 | ||||||||
| BPA | Pro-spective cohort | Fertile men (>18 y.o) |
Michigan and Texas (n = 418) |
Urine Semen |
Mean: 0.51 µg/g (urine) | Sperm characteristics: Increased level of BPA caused significant decreased in sperm DNA fragmentation. No association between BPA and semen analysis (sperm morphology, sperm concentration, total sperm count, semen volume). |
β = −0.0544 | p < 0.05 | [118] |
| BPA/ BPF/ BPS |
Cross sectional study |
Young men (18–20 y.o) FEPOS |
Denmark (n = 556) |
Urine Semen |
BPA (urine) Q1: <0.68 ng/mL Q3: 1.3–2.74 ng/mL BPF (urine) Q1: <0.06 ng/mL Q3: 0.14–0.34 ng/mL BPS Q1: <0.03 ng/mL Q3: 0.06–0.17 ng/mL (urine) |
Sperm characteristics: Percentage of motile spermatozoa in Q3 is significantly higher compared to Q1 in BPA and BPF exposures. Volume of semen per ejaculate in Q3 is significantly lower compared to Q1 in BPA and BPS exposures. No association between (BPA, BPF, and BPS) with the other semen analysis (sperm concentration, total sperm count, normal sperm morphology, motility and ejaculate volume). |
β = 1.07% β = −0.87 mL |
p < 0.05 p < 0.05 |
[119] |
| BPS | Cross sectional study |
Infertile patient (18–56 y.o) |
Boston, MA, USA (n = 158) |
Urine Semen |
Mean BPA: 0.77 µg/L (urine) Mean BPS: 0.37 µg/L (urine) |
Semen characteristics: Volume of semen per ejaculate in Q2 is significantly higher compared to Q1 in BPS exposure. Sperm concentration in Q3 is significantly lower compared to Q1 in BPS exposure. Increased level of BPS caused significant decreased in sperm quality among obese/overweight men (BMI >25 kg/m2):
|
β = 3.0 mL β = −29.2 mil/mL |
p < 0.05 p < 0.05 |
[117] |
Abbreviations: ↑: Increase; ↓ Decrease
6. Discussion
There is significant evidence that endocrine disrupting chemicals such as BPA and its analogues are an additional risk factor to be considered. Understanding the link between them and hormonal physiological function in the male reproductive system would aid in ensuring that suitable efforts are made to enhance public awareness and prevent their detrimental impacts on health.
BPA and its analogues have been the topic of extensive research and controversy in recent decades, and the amount of information available about them today is astounding. There are numerous papers that dealing with BPA and its analogues on human health, and the trend is for more to be published in the future. It is considered that the effects of BPA and its analogues on human health are a relatively new field with many unresolved problems. As previously stated, BPA and its analogues are commonly used substances in developing and underdeveloped countries. This means that scientists cannot dismiss the possibility that one of the functions of these compounds is to disturb the endocrine system. Numerous studies, both epidemiological and experimental, published in recent years have contributed to our understanding of some of the features and how they influence the hormonal physiological functions in the male reproductive system.
BPA and its analogues have a negative impact on hormonal physiological functions in the male reproductive system, which have been proven in vitro [55,78,79], in animals [56,64,65,66,67,68,69,70,72,74,75,76,77,80] and in human studies [108,109,110,111,116,118]. These hormonal physiological dysfunctions affect spermatogenesis outcomes. Some studies have shown decreased LH, FSH and testosterone levels when exposed to certain concentrations of BPA and its analogues as occurs in the HPG axis, specifically when this exposure occurs during adulthood [64,65,66,67,68,75,76,77]. The result of this review suggests that BPA and its analogues are capable of competitively binding to AR and ER and stimulating KiSS1 expression in the hypothalamus of the brain, causing the HPG axis feedback mechanism disturbance. These effects will alter GnRH secretion, resulting in variations in pituitary secretion of LH and FSH, with most studies reporting lower levels of these hormones. It is, therefore, logical that experimental animal studies showed low levels of testosterone as the negative feedback mechanism for these phenomena. Furthermore, BPA and 17-beta-oestrogen molecules are structurally similar; therefore, the former can adhere to the ER and mimic the oestrogenic effects in a human study [110,111], which is supported by the animal data [60,72,73]. Epidemiology studies have also shown that BPA and its analogues found in human urine are associated with the disturbance of male reproductive hormones via its effects as anti-androgenic and anti-oestrogenic agents [110,111,112,116].
Not only involving the HPG axis mechanism, BPA and its analogues could interfere with the steroidogenesis pathway via upregulation and downregulation of genes and proteins such as CYP and HSD, causing steroidogenesis disturbance [110,112]. BPA and its analogues can activate an alternative pathway known as the aromatase pathway, which alternates from testosterone synthesis to oestrogen synthesis [110,112]. This aromatase activity happens due to the upregulation of genes and protein expression of CYP19A1, which has been proven in in vitro and animal studies [56,59,74]. One of the primary results of this analysis is that BPA and its analogues have the potential to alter the steroidogenesis pathway, resulting in outcomes that are analogous to those of prostate cancer, such as high oestrogen levels in blood circulation. Therefore, BPA and its analogues exposure could be listed as one of the risk factors for prostate cancer.
In the Section 3 of the findings of this literature review, we examined articles that investigated the effects of BPA and its analogues on spermatogenesis outcomes. The disturbance of male reproductive hormones reduces spermatogenesis outcomes proven by sperm and semen quality defects. Testosterone and FSH play important roles in spermatogenesis; therefore, a decrease in these hormones will result in sperm quality defects, which were reported in animals and cell cultures exposed to BPA and its analogues [64,65,66,67,68,69,70,75,76,77,80,81,82]. The human findings also reported a decrease in sperm and semen quality when exposed to BPA and its analogues [111,112,116]. CatSper channel is another factor that influences sperm motility, hyperactivation and acrosomal reaction to penetrate the oocyte. As discussed previously, CatSper is one of the channels found in the sperm responsible for the influx of Ca2+ for sperm function. Therefore, downregulation and alteration in this channel after BPA and its analogues exposure could lead to immotile sperm and reduction in oocyte penetration ability even with the presence of progesterone. All these events in spermatogenesis disturbances lead to sperm quality defects resulting in infertility [85].
The significant drawback of this work is that most publications examine animal studies rather than human studies, owing to the logical and ethical challenges of doing this type of study in humans. Some findings are parallel between humans and animals; however, some findings showed differences, such as the doses used in animal and human studies. The low observed adverse effects level (LOAEL) dose for BPA in a mammalian animal is 50 mg/kg/bw. Even though the dose of BPA used in the previous studies was below the LOAEL, it reportedly caused changes in the reproductive hormone, sperm characteristic, and histological of the testis in laboratory animals [66,70,71,72,76]. Rats were more susceptible to BPA when compared to humans. Therefore, the adverse effect is more severe in rats, specifically the male reproductive system, proven by the disturbance of reproductive hormone, gene, and protein expression in steroidogenesis and reduced sperm quality.
Even though the BPA concentration found in human urine is lower than the tolerable daily intake (TDI), which is below than 4 µg/kg, significant negative effects were found in our literature findings proven by alterations in the reproductive hormones, sperm characteristics, and semen quality. However, the data from human biomonitoring studies are uncertain, which might be due to different metabolism rates in individuals influencing their ability to excrete the BPA [9]. Furthermore, the concentration of BPA and the duration of exposure might also be significant factors that must be considered in assessing the effect of BPA on the hormones’ physiological function in the male reproductive system. There are a few possible challenges from the previous literature findings which derive interpretation concern: subject population (fertile versus infertile, young adult versus children; BPA and its analogues exposure levels (due to non-linear effects of BPA); sampling frequency limitations (sample may not be representative of exposure such as retrospective study); and BPA exposure window which may not be related to the spermatogenesis outcomes because the spermatogenesis process in the human takes about three months to be complete [9]. Another problem is that the effects of BPA and its analogues on physiological hormonal functions were explored without taking into account probable interactions with other environmental factors.
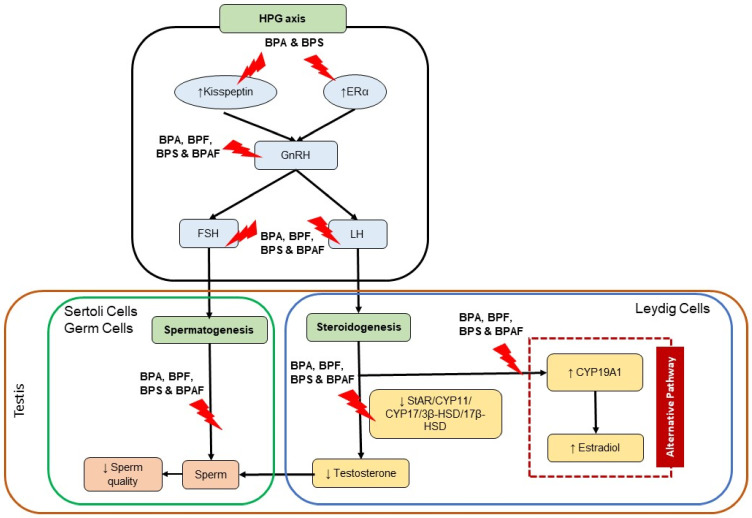
Figure 1 shows the possible mechanism of BPA and its analogues on the hormonal physiology pathway of the male reproductive system. BPA and its analogues negatively impact the physiological hormonal functions in the male reproductive system and its outcome, which were proven by sperm and semen quality defects. The BPA and its analogues can alter the HPG axis by increasing the expression of KiSS1 and competitively bind with ERα in the brain’s hypothalamus. These effects will influence GnRH secretion, leading to changes in the pituitary secretion of LH and FSH, which most of the studies reported decreased levels of these hormones. Decreased LH levels in the plasma could disturb the steroidogenesis process in Leydig cells, resulting in decreased testosterone levels. Furthermore, these endocrine disrupting chemicals were also reported to alter the StAR protein, HSD and CYP450 genes and enzymes in the mitochondrial and ER of Leydig cells involved in the steroidogenesis pathway. These alterations also lead to the decrease of testosterone synthesis. Therefore, once testosterone synthesis is inhibited, the alternative pathway will be activated. BPA and its analogues can activate this alternative pathway of steroidogenesis by activating aromatase activity, thus increasing the formation of E2. As the consequences of HPG axis disturbance and activation of the alternative steroidogenesis pathway specifically proven by the FSH and testosterone levels decreased, respectively, these phenomena lead to the disturbance of spermatogenesis reported in most studies. Therefore, the spermatogenesis outcome, such as sperm quality, will have deteriorated.
Figure 1.
Possible mechanism of BPA and its analogues on the hormonal physiology of male reproductive system. Abbreviations: ↓ inhibit/decrease, ↑ enhance/increase.
7. Conclusions
In conclusion, BPA and its analogues may be an additional risk factor to consider because they disrupt the hormonal physiological functions of the male reproductive system. The results of the experimental studies mostly point to BPA and its analogues having the ability to disrupt the endocrine system, resulting in HPG axis disturbances and activation of the steroidogenesis alternative pathway, which is why more experimental and epidemiological research will be required to establish the scale of the effects caused by these chemicals in large populations and its molecular mechanism to a better understanding of the connection between HPG axis, steroidogenesis, and spermatogenesis outcomes. Despite the fact that many nations have adopted policies to limit exposure to BPA and its analogues in their populations, epidemiological research on humans imply that the same abnormalities seen in experimental studies on animals may be detected. Understanding the association between BPA and its analogues and physiological hormonal activities can aid in raising public awareness and implementing public health campaigns to prevent exposure to these compounds, particularly among those attempting to conceive and elderly populations.
Abbreviations
| 3β-HSD | 3β-hydroxysteroid dehydrogenase |
| 5αRed1 | 5α- reductase type 1 |
| 17β-HSD | 17β-hydroxysteroid dehydrogenase |
| AA | arachidonic acid |
| AD | androstenedione |
| AR | androgen receptor |
| ATP | adenosine 5’-triphosphate |
| BPA | bisphenol A |
| BPAF | bisphenol AF |
| BPF | bisphenol F |
| BPS | bisphenol S |
| BTB | blood-testis-barrier |
| cAMP | cyclic adenosine monophosphate |
| CatSper | sperm-specific ion calcium (Ca2+) channel |
| CYP11A1 | cytochrome P450 isoform 1A1 |
| CYP17 | cytochrome P450 isoform 17 |
| CYP19A1 | cytochrome P450 isoform 19A1 |
| COX-2 | cyclooxygenase-2 |
| DHEA | dehydroepiandrosterone |
| E2 | estradiol |
| EDCs | endocrine disrupting chemicals |
| EPA | Environmental Protection Agency |
| ER | estrogen receptor |
| ER α/β | estrogen receptor α/β |
| ERR-γ | estrogen-related receptor gamma |
| FSH | follicle-stimulating hormone |
| FSHβ | follicle-stimulating hormone beta |
| FT | free testosterone |
| GnRH | gonadotropin-releasing hormone |
| Gnrh2 | gonadotropin-releasing hormone 2 |
| GnRH3 | gonadotropin-releasing hormone 3 |
| GnRHR | GnRH receptor |
| GnRHR 1/2 | GnRH receptor 1/2 |
| GPR54 | G-protein coupled receptor 54 |
| HPG | hypothalamic–pituitary–gonadal axis |
| HSD | hydroxysteroid dehydrogenase |
| INB | inhibin B |
| IVF | in vitro fertilization |
| LC | Leydig cells |
| LH | luteinizing hormone |
| LHβ | luteinizing hormone beta |
| LHR | luteinizing hormone receptor |
| MAPK | mitogen-activated protein kinase |
| mFeTA | mouse fetal testicular cell assay |
| mRNA | messenger RNA |
| PGE-2 | prostaglandin E2 |
| PKA | protein kinase A |
| PSA | prostate-specific antigen |
| Q | quartile |
| SC | Sertoli cell |
| SHBG | Sex hormone binding globulin |
| StAR | Steroidogenic acute regulatory |
| TT | total testosterone |
| YO | years old |
| ZO-1 | zona occludens-1 |
Author Contributions
Conceptualization, A.‘A.S.; N.J.S. and I.S.T.; methodology, A.‘A.S.; N.J.S. and I.S.T.; validation, I.S.T.; S.B.B. and Z.A.H.; writing—original draft preparation, A.‘A.S.; writing—review and editing, A.‘A.S. and I.S.T.; supervision, I.S.T.; S.B.B.; Z.A.H. and N.J.S.; funding acquisition, I.S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the research university grant, GUP-2019-086, from the National University of Malaysia (UKM). This work was also supported by the Centre for Diagnostic, Therapeutic and Investigative Studies (CODTIS), Faculty of Health Sciences, (UKM.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Michałowicz J., Bisphenol A. Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014;37:738–758. doi: 10.1016/j.etap.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Park B., Kwon J.E., Cho S.M., Kim C.W., Lee D.E., Koo Y.T., Lee S.H., Lee H.M., Kang S.C. Protective effect of Lespedeza cuneata ethanol extract on Bisphenol A-induced testicular dysfunction in vivo and in vitro. Biomed. Pharmacother. 2018;102:76–85. doi: 10.1016/j.biopha.2018.03.045. [DOI] [PubMed] [Google Scholar]
- 3.Abraham A., Chakraborty P. A review on sources and health impacts of bisphenol A. Rev. Environ. Health. 2020;35:201–210. doi: 10.1515/reveh-2019-0034. [DOI] [PubMed] [Google Scholar]
- 4.Castillo-Sanchez R., Ramirez-Ricardo J., Martinez-Baeza E., Cortes-Reynosa P., Candanedo-Gonzales F., Gomez R., Salazar E.P. Bisphenol A induces focal adhesions assembly and activation of FAK, Src and ERK2 via GPER in MDA-MB-231 breast cancer cells. Toxicol. Vitr. 2020;66:104871. doi: 10.1016/j.tiv.2020.104871. [DOI] [PubMed] [Google Scholar]
- 5.Talpade J., Shrman K., Sharma R.K., Gutham V., Singh R.P., Meena N.S. Bisphenol A: An endocrine disruptor. J. Entomol. Zool. Stud. 2018;6:394–397. [Google Scholar]
- 6.Bakar N.A., Salleh M.M., Umar A.A., Shapter J.G. Design and measurement technique of surface-enhanced Raman scattering for detection of bisphenol A. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017;8:025008. doi: 10.1088/2043-6254/aa5e22. [DOI] [Google Scholar]
- 7.Kang J.-H., Kondo F., Katayama Y. Human exposure to Bisphenol A. Toxicology. 2006;226:79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura Y., Etoh M., Hirakawa Y., Abe Y., Mutsuga M. Bisphenol A in domestic and imported canned foods in Japan. Food Addit. Contam. Part A Chem. 2014;31:330–340. doi: 10.1080/19440049.2013.874047. [DOI] [PubMed] [Google Scholar]
- 9.Pelch K., Wignall J.A., Goldstone A.E., Ross P.K., Blain R.B., Shapiro A.J., Holmgren S.D., Hsieh J.H., Svoboda D., Auerbach S.S., et al. A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology. 2019;424:152235. doi: 10.1016/j.tox.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Bakirhan N.K., Ozkan S.A. The recent electrochemical studies on bisphenol a detection in beverages. In: Grumezescu A.M., Holban A.M., editors. Safety Issues in Beverage Production. Volume 18. Academic Press; Cambridge, MA, USA: 2019. pp. 309–333. [DOI] [Google Scholar]
- 11.Bashir A.B., Audu A.A. Determination of Bisphenol A Released From Polycarbonate Infant Feeding Bottles By UV-Vis Spectrophotometry. J. Chem. Soc. Niger. 2020;45:1120–1127. doi: 10.46602/jcsn.v45i6.555. [DOI] [Google Scholar]
- 12.Ugboka U.G., Ihedioha J.N., Ekere N.R., Okechukwu F.O. Human health risk assessment of bisphenol A released from polycarbonate drinking water bottles and carbonated drinks exposed to sunlight in Nigeria. Int. J. Environ. Anal. Chem. 2020:1–11. doi: 10.1080/03067319.2020.1759572. [DOI] [Google Scholar]
- 13.Wang R., Huang Y., Dong S., Wang P., Su X. The occurrence of bisphenol compounds in animal feed plastic packaging and migration into feed. Chemosphere. 2021;265:129022. doi: 10.1016/j.chemosphere.2020.129022. [DOI] [PubMed] [Google Scholar]
- 14.Lalonde B., Garron C. Spatial and Temporal Distribution of BPA in the Canadian Freshwater Environment. Arch. Environ. Contam. Toxicol. 2020;78:568–578. doi: 10.1007/s00244-020-00721-2. [DOI] [PubMed] [Google Scholar]
- 15.Liao C., Liu F., Guo Y., Moon H.B., Nakata H., Wu Q., Kannan K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: Implications for human exposure. Environ. Sci. Technol. 2012;46:9138–9145. doi: 10.1021/es302004w. [DOI] [PubMed] [Google Scholar]
- 16.Liao C., Kannan K. A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Addit. Contam. Part A. 2014;31:319–329. doi: 10.1080/19440049.2013.868611. [DOI] [PubMed] [Google Scholar]
- 17.Rotimi O.A., Olawole T.D., De Campos O.C., Adelani I.B., Rotimi S.O. Bisphenol A in Africa: A review of environmental and biological levels. Sci. Total Environ. 2021;764:142854. doi: 10.1016/j.scitotenv.2020.142854. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X., Zhang H., Chen Z.-L., Wang X.-C., Shen J.-M. Spatial and temporal distributions of bisphenol analogues in water and sediment from the Lanzhou section of the Yellow River, China. Arab. J. Geosci. 2020;13:1–8. doi: 10.1007/s12517-020-06122-y. [DOI] [Google Scholar]
- 19.Derakhshan A., Philips E.M., Ghassabian A., Santos S., Asimakopoulos A.G., Kannan K., Kortenkamp A., Jaddoe V.W.V., Trasande L., Peeters R.P., et al. Association of urinary bisphenols during pregnancy with maternal, cord blood and childhood thyroid function. Environ. Int. 2021;146:106160. doi: 10.1016/j.envint.2020.106160. [DOI] [PubMed] [Google Scholar]
- 20.Jin H., Xie J., Mao L., Zhao M., Bai X., Wen J., Shen T., Wu P. Bisphenol analogue concentrations in human breast milk and their associations with postnatal infant growth. Environ. Pollut. 2020;259:113779. doi: 10.1016/j.envpol.2019.113779. [DOI] [PubMed] [Google Scholar]
- 21.Polydorou O., Schmidt O.C., Spraul M., Vach K., Schulz S.D., König A., Hellwig E., Gminski R. Detection of Bisphenol A in dental wastewater after grinding of dental resin composites. Dent. Mater. 2020;36:1009–1018. doi: 10.1016/j.dental.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Cao X.L., Kosarac I., Popovic S., Zhou S., Smith D., Dabeka R. LC-MS/MS analysis of bisphenol S and five other bisphenols in total diet food samples. Food Addit. Contam Part A. 2019;36:1740–1747. doi: 10.1080/19440049.2019.1643042. [DOI] [PubMed] [Google Scholar]
- 23.Haq M.E.U., Akash M.S.H., Sabir S., Mahmood M.H., Rehman K. Human exposure to bisphenol A through dietary sources and development of diabetes mellitus: A cross-sectional study in Pakistani population. Environ. Sci. Pollut. Res. 2020;27:26262–26275. doi: 10.1007/s11356-020-09044-0. [DOI] [PubMed] [Google Scholar]
- 24.Malaisé Y., Lencina C., Cartier C., Olier M., Ménard S., Guzylack-Piriou L. Perinatal oral exposure to low doses of bisphenol A, S or F impairs immune functions at intestinal and systemic levels in female offspring mice. Environ. Health. 2020;19:1–11. doi: 10.1186/s12940-020-00614-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolfo A., Nuzzo A.M., De Amicis R., Moretti L., Bertoli S., Leone A. Fetal–maternal exposure to endocrine disruptors: Correlation with diet intake and pregnancy outcomes. Nutrients. 2020;12:1744. doi: 10.3390/nu12061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adeyemi J.A., Gallimberti M., Olise C.C., Rocha B.A., Adedire C.O., Barbosa F. Evaluation of bisphenol A levels in Nigerian thermal receipts and estimation of daily dermal exposure. Environ. Sci. Pollut. Res. 2020;27:37645–37649. doi: 10.1007/s11356-020-09898-4. [DOI] [PubMed] [Google Scholar]
- 27.González N., Marquès M., Cunha S.C., Fernandes J.O., Domingo J.L., Nadal M. Biomonitoring of co-exposure to bisphenols by consumers of canned foodstuffs. Environ. Int. 2020;140:105760. doi: 10.1016/j.envint.2020.105760. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Piñero J., Bowerbank S.L., Moreda-Piñeiro J., López-Mahía P., Dean J.R. The occurrence and distribution of polycyclic aromatic hydrocarbons, bisphenol A and organophosphate flame retardants in indoor dust and soils from public open spaces: Implications for human exposure. Environ. Pollut. 2020;266:115372. doi: 10.1016/j.envpol.2020.115372. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H., Quan Q., Zhang M., Zhang N., Zhang W., Zhan M., Xu W., Lu L., Fan J., Wang Q. Occurrence of bisphenol A and its alternatives in paired urine and indoor dust from Chinese university students: Implications for human exposure. Chemosphere. 2020;247:125987. doi: 10.1016/j.chemosphere.2020.125987. [DOI] [PubMed] [Google Scholar]
- 30.Hart R.J. The Impact of Prenatal Exposure to Bisphenol A on Male Reproductive Function. Front. Endocrinol. 2020;11:320. doi: 10.3389/fendo.2020.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meli R., Monnolo A., Annunziata C., Pirozzi C., Ferrante M.C. Oxidative stress and BPA toxicity: An antioxidant approach for male and female reproductive dysfunction. Antioxidants. 2020;9:405. doi: 10.3390/antiox9050405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shokry D.A., Mohamed M.I., Abdel-Satar M.F., Selim N.E.S., Abd El Salam M.A. Correlation between urinary bisphenol a (BPA) levels and male reproductive functions among sample of Egyptian population. Indian J. Forensic Med. Toxicol. 2020;14:1821–1826. doi: 10.37506/ijfmt.v14i3.10688. [DOI] [Google Scholar]
- 33.Aljadeff G., Longhi E., Shoenfeld Y. Bisphenol A: A notorious player in the mosaic of autoimmunity. Autoimmunity. 2018;51:370–377. doi: 10.1080/08916934.2018.1551374. [DOI] [PubMed] [Google Scholar]
- 34.Gao X., Wang H.S. Impact of bisphenol A on the cardiovascular system—Epidemiological and experimental evidence and molecular mechanisms. Int. J. Environ. Res. Public Health. 2014;11:8399–8413. doi: 10.3390/ijerph110808399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kharrazian D. The potential roles of bisphenol A (BPA) pathogenesis in autoimmunity. Autoimmune Dis. 2014;2014:743616. doi: 10.1155/2014/743616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima Y., Goldblum R.M., Midoro-Horiuti T. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: An animal model study. Environ. Health. 2012;11:8. doi: 10.1186/1476-069X-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skakkebaek N.E., Toppari J., Söder O., Gordon C.M., Divall S., Draznin M. The exposure of fetuses and children to endocrine disrupting chemicals: A European Society for Paediatric Endocrinology (ESPE) and Pediatric Endocrine Society (PES) call to action statement. J. Clin. Endocrinol. Metab. 2011;96:3056–3058. doi: 10.1210/jc.2011-1269. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z., Liang H., Tu X., Yuan W., Zhou Z., Jin L., Miao M., Li D.K. Bisphenol A and pubertal height growth in school-aged children. J. Expo. Sci. Environ. Epidemiol. 2019;29:109–117. doi: 10.1038/s41370-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chianese R., Troisi J., Richards S., Scafuro M., Fasano S., Guida M., Pierantoni R., Meccariello R. Bisphenol A in Reproduction: Epigenetic Effects. Curr. Med. Chem. 2018;25:748–770. doi: 10.2174/0929867324666171009121001. [DOI] [PubMed] [Google Scholar]
- 40.Lombó M., Fernández-Díez C., González-Rojo S., Herráez M.P. Genetic and epigenetic alterations induced by bisphenol A exposure during different periods of spermatogenesis: From spermatozoa to the progeny. Sci. Rep. 2019;9:18029. doi: 10.1038/s41598-019-54368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gys C., Bastiaensen M., Malarvannan G., Ait Bamai Y., Araki A., Covaci A. Short-term variability of bisphenols in spot, morning void and 24-hour urine samples. Environ. Pollut. 2021;268:115747. doi: 10.1016/j.envpol.2020.115747. [DOI] [PubMed] [Google Scholar]
- 42.Huang M., Liu S., Fu L., Jiang X., Yang M. Bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF induce oxidative stress and biomacromolecular damage in human granulosa KGN cells. Chemosphere. 2020;253:126707. doi: 10.1016/j.chemosphere.2020.126707. [DOI] [PubMed] [Google Scholar]
- 43.Li A., Zhuang T., Shi W., Liang Y., Liao C., Song M., Jiang G. Serum concentration of bisphenol analogues in pregnant women in China. Sci. Total Environ. 2020;707:136100. doi: 10.1016/j.scitotenv.2019.136100. [DOI] [PubMed] [Google Scholar]
- 44.Sharma P., Gautam D.K., Bhagat P., Mandal M.B. Comparative assessment of impact of Bisphenol A and Bisphenol S on hematological parameters in rats after 4 weeks exposure. J. Crit. Rev. 2020;7:1250–1256. [Google Scholar]
- 45.Andújar N., Gálvez-Ontiveros Y., Zafra-Gómez A., Rodrigo L., Álvarez-Cubero M.J., Aguilera M., Monteagudo C., Rivas A. Bisphenol A analogues in food and their hormonal and obesogenic effects: A review. Nutrients. 2019;11:2136. doi: 10.3390/nu11092136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.García-Córcoles M.T., Cipa M., Rodríguez-Gómez R., Rivas A., Olea-Serrano F., Vílchez J.L., Zafra-Gómez A. Determination of bisphenols with estrogenic activity in plastic packaged baby food samples using solid-liquid extraction and clean-up with dispersive sorbents followed by gas chromatography tandem mass spectrometry analysis. Talanta. 2018;178:441–448. doi: 10.1016/j.talanta.2017.09.067. [DOI] [PubMed] [Google Scholar]
- 47.Harnett K.G., Chin A., Schuh S.M. BPA and BPA alternatives BPS, BPAF, and TMBPF, induce cytotoxicity and apoptosis in rat and human stem cells. Ecotoxicol. Environ. Saf. 2021;216:112210. doi: 10.1016/j.ecoenv.2021.112210. [DOI] [PubMed] [Google Scholar]
- 48.Environmental Protection Agency (EPA) Bisphenol A Alternatives in Thermal Paper Final Report. [(accessed on 10 June 2021)]; Available online: https://www.epa.gov/sites/default/files/2015-08/documents/bpa_final.pdf.
- 49.Jubaidi F.F., Mathialagan R.D., Noor M.M., Taib I.S., Budin S.B. Monosodium glutamate daily oral supplementation: Study of its effects on male reproductive system on rat model. Syst. Biol. Reprod. Med. 2019;65:194–204. doi: 10.1080/19396368.2019.1573274. [DOI] [PubMed] [Google Scholar]
- 50.Yusoff N.A., Mohamed M., Budin S.B., Taib I.S. Fenitrothion impaired sexual behaviour and reproductive performance in male sprague-Dawley rats. Sains Malays. 2020;49:1333–1344. doi: 10.17576/jsm-2020-4906-11. [DOI] [Google Scholar]
- 51.Gules O., Yildiz M., Naseer Z., Tatar M. Effects of folic acid on testicular toxicity induced by bisphenol-A in male Wistar rats. Biotech. Histochem. 2019;94:26–35. doi: 10.1080/10520295.2018.1493222. [DOI] [PubMed] [Google Scholar]
- 52.Ullah A., Pirzada M., Jahan S., Ullah H., Khan M.J. Bisphenol A analogues bisphenol B, bisphenol F, and bisphenol S induce oxidative stress, disrupt daily sperm production, and damage DNA in rat spermatozoa: A comparative in vitro and in vivo study. Toxicol. Ind. Health. 2019;35:294–303. doi: 10.1177/0748233719831528. [DOI] [PubMed] [Google Scholar]
- 53.Yusoff N.A., Budin S.B., Taib I.S. Pesticide Exposures Induce Male-Mediated Reproductive Toxicity: A Review. J. Agric. Sci. 2017;9:122–135. doi: 10.5539/jas.v9n13p122. [DOI] [Google Scholar]
- 54.Rahman M.S., Pang M.G. Understanding the molecular mechanisms of bisphenol A action in spermatozoa. Clin. Exp. Reprod. Med. 2019;46:99–106. doi: 10.5653/cerm.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roelofs M.J.E., van den Berg M., Bovee T.F.H., Piersma A.H., Duursen M.B.M. Structural bisphenol analogues differentially target steroidogenesis in murine MA-10 Leydig cells as well as the glucocorticoid receptor. Toxicology. 2015;329:10–20. doi: 10.1016/j.tox.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Yang Q., Yang X., Liu J., Ren W., Chen Y., Shen S. Effects of BPF on steroid hormone homeostasis and gene expression in the hypothalamic–pituitary–gonadal axis of zebrafish. Environ. Sci. Pollut. Res. 2017;24:21311–21322. doi: 10.1007/s11356-017-9773-z. [DOI] [PubMed] [Google Scholar]
- 57.Bruks S. Bachelor’s Thesis. Umeå University; Umeå, Sweden: Apr 24, 2020. Metabolism and Estrogenicity of Bisphenol A and Its Analogues: A Comparative Analysis of Experimental and Computational Data on Metabolism of Bisphenols. [Google Scholar]
- 58.Zühlke M.K., Schlüter R., Mikolasch A., Henning A.K., Giersberg M., Lalk M., Kunze G., Schweder T., Urich T., Schauer F. Biotransformation of bisphenol A analogues by the biphenyl-degrading bacterium Cupriavidus basilensis—A structure-biotransformation relationship. Appl. Microbiol. Biotechnol. 2020;104:3569–3583. doi: 10.1007/s00253-020-10406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cano-Nicolau J., Vaillant C., Pellegrini E., Charlier T.D., Kah O., Coumailleau P. Estrogenic effects of several BPA analogs in the developing zebrafish brain. Front. Neurosci. 2016;10:112. doi: 10.3389/fnins.2016.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.In S., Cho H., Lee K.W., Won E.J., Lee Y.M. Cloning and molecular characterization of estrogen-related receptor (ERR) and vitellogenin genes in the brackish water flea Diaphanosoma celebensis exposed to bisphenol A and its structural analogues. Mar. Pollut. Bull. 2020;154:111063. doi: 10.1016/j.marpolbul.2020.111063. [DOI] [PubMed] [Google Scholar]
- 61.Lina S., Eliza H., Hashida N.H., Ibrahim S.F., Osman K. Androgen receptor and ultrastructural features of nigella sativa oil and nicotine-treated male rat reproductive glands. Sains Malays. 2018;47:1827–1833. doi: 10.17576/jsm-2018-4708-22. [DOI] [Google Scholar]
- 62.Qiu W., Zhao Y., Yang M., Farajzadeh M., Pan C., Wayne N.L. Actions of bisphenol A and bisphenol S on the reproductive neuroendocrine system during early development in zebrafish. Endocrinology. 2016;157:636–647. doi: 10.1210/en.2015-1785. [DOI] [PubMed] [Google Scholar]
- 63.Matuszczak E., Komarowska M.D., Debek W., Hermanowicz A. The Impact of Bisphenol A on Fertility, Reproductive System, and Development: A Review of the Literature. Int. J. Endocrinol. 2019;2019:4068717. doi: 10.1155/2019/4068717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alboghobeish S., Mahdavinia M., Zeidooni L., Samimi A., Oroojan A.A., Alizadeh S., Dehghani M.A., Ahangarpour A., Khorsandi L. Efficiency of naringin against reproductive toxicity and testicular damages induced by bisphenol A in rats. Iran J. Basic Med. Sci. 2019;22:315–323. doi: 10.22038/ijbms.2019.29757.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang Z., Wang J., Li X., Zhang X. Echinacoside and Cistanche tubulosa (Schenk) R. wight ameliorate bisphenol A-induced testicular and sperm damage in rats through gonad axis regulated steroidogenic enzymes. J. Ethnopharmacol. 2016;193:321–328. doi: 10.1016/j.jep.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 66.Zahra Z., Khan M.R., Majid M., Maryam S., Sajid M. Gonadoprotective ability of Vincetoxicum arnottianum extract against bisphenol A-induced testicular toxicity and hormonal imbalance in male Sprague Dawley rats. Andrologia. 2020;52:e13590. doi: 10.1111/and.13590. [DOI] [PubMed] [Google Scholar]
- 67.Wang J., Chen C., Jiang Z., Wang M., Jiang H., Zhang X. Protective effect of Cordyceps militaris extract against bisphenol A induced reproductive damage. Syst. Biol. Reprod. Med. 2016;62:249–257. doi: 10.1080/19396368.2016.1182234. [DOI] [PubMed] [Google Scholar]
- 68.Ullah A., Pirzada M., Afsar T., Razak S., Almajwal A., Jahan S. Effect of bisphenol F, an analog of bisphenol A, on the reproductive functions of male rats. Environ. Health Prev. Med. 2019;24:41. doi: 10.1186/s12199-019-0797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ullah A., Pirzada M., Jahan S., Ullah H., Shaheen G., Rehman H., Siddiqui M.F., Butt M.A. Bisphenol A and its analogs bisphenol B, bisphenol F, and bisphenol S: Comparative in vitro and in vivo studies on the sperms and testicular tissues of rats. Chemosphere. 2018;209:508–516. doi: 10.1016/j.chemosphere.2018.06.089. [DOI] [PubMed] [Google Scholar]
- 70.Ullah H., Jahan S., Ain Q.U., Shaheen G., Ahsan N. Effect of bisphenol S exposure on male reproductive system of rats: A histological and biochemical study. Chemosphere. 2016;152:383–391. doi: 10.1016/j.chemosphere.2016.02.125. [DOI] [PubMed] [Google Scholar]
- 71.Stoker C., Andreoli M.F., Kass L., Bosquiazzo V.L., Rossetti M.F., Canesini G., Luque E.H., Ramos J.G. Perinatal exposure to bisphenol A (BPA) impairs neuroendocrine mechanisms regulating food intake and kisspetin system in adult male rats. Evidences of metabolic disruptor hypothesis. Mol. Cell. Endocrinol. 2020;499:110614. doi: 10.1016/j.mce.2019.110614. [DOI] [PubMed] [Google Scholar]
- 72.Bai Y., Chang F., Zhou R., Jin P.P., Matsumoto H., Sokabe M., Chen L. Increase of anteroventral periventricular kisspeptin neurons and generation of E2-induced LH-surge system in male rats exposed perinatally to environmental dose of bisphenol-A. Endocrinology. 2011;152:1562–1571. doi: 10.1210/en.2010-1042. [DOI] [PubMed] [Google Scholar]
- 73.Cao J., Mickens J.A., McCaffrey K.A., Leyrer S.M., Patisaul H.B. Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33:23–36. doi: 10.1016/j.neuro.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi J., Jiao Z., Zheng S., Li M., Zhang J., Feng Y., Yin J., Shao B. Long-term effects of Bisphenol AF (BPAF) on hormonal balance and genes of hypothalamus-pituitary-gonad axis and liver of zebrafish (Danio rerio), and the impact on offspring. Chemosphere. 2015;128:252–257. doi: 10.1016/j.chemosphere.2015.01.060. [DOI] [PubMed] [Google Scholar]
- 75.Majid M., Ijaz F., Baig M.W., Nasir B., Khan M.R., Haq I.U. Scientific Validation of Ethnomedicinal Use of Ipomoea batatas L. Lam. as Aphrodisiac and Gonadoprotective Agent against Bisphenol A Induced Testicular Toxicity in Male Sprague Dawley Rats. Biomed. Res. Int. 2019;2019:8939854. doi: 10.1155/2019/8939854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wisniewski P., Romano R.M., Kizys M.M.L., Oliveira K.C., Kasamatsu T., Giannocco G., Chiamolera M.I., Dias-da-Silva M.R., Romano M.A. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis. Toxicology. 2015;329:1–9. doi: 10.1016/j.tox.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 77.Akintunde J.K., Farouk A.A., Mogbojuri O. Metabolic treatment of syndrome linked with Parkinson’s disease and hypothalamus pituitary gonadal hormones by turmeric curcumin in Bisphenol-A induced neuro-testicular dysfunction of wistar rat. Biochem. Biophys. Rep. 2019;17:97–107. doi: 10.1016/j.bbrep.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eladak S., Grisin T., Moison D., Guerquin M.J., N’Tumba-Byn T., Pozzi-Gaudin S., Benachi A., Livera G., Rouiller-Fabre V., Habert R. A new chapter in the bisphenol a story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015;103:11–21. doi: 10.1016/j.fertnstert.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Feng Y., Jiao Z., Shi J., Li M., Guo Q., Shao B. Effects of bisphenol analogues on steroidogenic gene expression and hormone synthesis in H295R cells. Chemosphere. 2016;147:9–19. doi: 10.1016/j.chemosphere.2015.12.081. [DOI] [PubMed] [Google Scholar]
- 80.Jahan S., Ain Q.U., Ullah H. Therapeutic effects of quercetin against bisphenol A induced testicular damage in male Sprague Dawley rats. Syst. Biol. Reprod. Med. 2016;62:114–124. doi: 10.3109/19396368.2015.1115139. [DOI] [PubMed] [Google Scholar]
- 81.Liang S., Yin L., Yu K.S., Hofmann M.C., Yu X. High-content analysis provides mechanistic insights into the testicular toxicity of Bisphenol A and selected analogues in mouse spermatogonial cells. Toxicol. Sci. 2017;155:43–60. doi: 10.1093/toxsci/kfw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Freitas A.T.A.G., Ribeiro M.A., Pinho C.F., Peixoto A.R., Domeniconi R.F., Scarano W.R. Regulatory and junctional proteins of the blood-testis barrier in human Sertoli cells are modified by monobutyl phthalate (MBP) and bisphenol A (BPA) exposure. Toxicol. Vitr. 2016;34:1–7. doi: 10.1016/j.tiv.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 83.Feng Y.-L., Yin Y.-Y., Wang X.-S., Wang P.-T. BPA Disturb TJ-Permeability of Rat Sertoli Cells During Spermatogenesis in Vitro. Prog. Mod. Biomed. 2012;12:1430–1434. [Google Scholar]
- 84.Wang H.F., Liu M., Li N., Luo T., Zheng L.P., Zeng X.H. Bisphenol a impairs mature sperm functions by a CatSper-relevant mechanism. Toxicol. Sci. 2016;152:145–154. doi: 10.1093/toxsci/kfw070. [DOI] [PubMed] [Google Scholar]
- 85.Rehfeld A., Andersson A.M., Skakkebæk N.E. Bisphenol A Diglycidyl Ether (BADGE) and Bisphenol Analogs, but Not Bisphenol A (BPA), Activate the CatSper Ca2+ Channel in Human Sperm. Front. Endocrinol. 2020;11:324. doi: 10.3389/fendo.2020.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu N., Zhao M., Song Y., Ding L., Ni Y. The KiSS-1/GPR54 system: Essential roles in physiological homeostasis and cancer biology. Genes Dis. 2020 doi: 10.1016/j.gendis.2020.07.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dedes I. Kisspeptins and the control of gonadotrophin secretion. Syst. Biol. Reprod. Med. 2012;58:121–128. doi: 10.3109/19396368.2011.651555. [DOI] [PubMed] [Google Scholar]
- 88.Khbouz B., de Bournonville C., Court L., Taziaux M., Corona R., Arnal J.F., Lenfant F., Cornil C.A. Role for the membrane estrogen receptor alpha in the sexual differentiation of the brain. Eur. J. Neurosci. 2020;52:2627–2645. doi: 10.1111/ejn.14646. [DOI] [PubMed] [Google Scholar]
- 89.Weiser M.J., Foradori C.D., Handa R.J. Estrogen receptor beta in the brain: From form to function. Brain Res. Rev. 2008;57:309–320. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chimento A., Sirianni R., Casaburi I., Pezzi V. Role of estrogen receptors and G protein-coupled estrogen receptor in regulation of hypothalamus-pituitary-testis axis and spermatogenesis. Front. Endocrinol. 2014;5:1. doi: 10.3389/fendo.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeo S.H., Colledge W.H. The role of Kiss1 neurons as integrators of endocrine, metabolic, and environmental factors in the hypothalamic-pituitary-gonadal axis. Front. Endocrinol. 2018;9:188. doi: 10.3389/fendo.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao Y., Lin M.-C.A., Farajzadeh M., Wayne N.L. Early Development of the Gonadotropin-Releasing Hormone Neuronal Network in Transgenic Zebrafish. Front. Endocrinol. 2013;4:107. doi: 10.3389/fendo.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zirkin B.R., Papadopoulos V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018;99:101–111. doi: 10.1093/biolre/ioy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flück C.E., Pandey A.V. Testicular Steroidogenesis. In: Simoni M., Huhtaniemi I., editors. Endocrinology of the Testis and Male Reproduction. 1st ed. Springer; Cham, Switzerland: 2017. pp. 343–371. [DOI] [Google Scholar]
- 95.Wang Y., Chen F., Ye L., Zirkin B., Chen H. Steroidogenesis in leydig cells: Effects of aging and environmental factors. Reproduction. 2017;154:R111–R122. doi: 10.1530/REP-17-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jefcoate C.R., Lee J. Cholesterol signaling in single cells: Lessons from STAR and sm-FISH. J. Mol. Endocrinol. 2018;60:R213–R235. doi: 10.1530/JME-17-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Papadopoulos V., Aghazadeh Y., Fan J., Campioli E., Zirkin B., Midzak A. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol. Cell. Endocrinol. 2015;408:90–98. doi: 10.1016/j.mce.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ronco A.M., Moraga P.F., Llanos M.N. Arachidonic acid release from rat Leydig cells: The involvement of G protein, phospholipase A2 and regulation of cAMP production. J. Endocrinol. 2002;172:95–104. doi: 10.1677/joe.0.1720095. [DOI] [PubMed] [Google Scholar]
- 99.Hadley K.B., Ryan A.S., Forsyth S., Gautier S., Salem N. The essentiality of arachidonic acid in infant development. Nutrients. 2016;8:216. doi: 10.3390/nu8040216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rouzer C.A., Marnett L.J. Cyclooxygenases: Structural and functional insights. J. Lipid Res. 2009;50:S29–S34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perrotta I., Santoro M., Guido C., Avena P., Tripepi S., De Amicis F., Gervasi M.C., Aquila S. Expression of cyclooxygenase-1 (COX-1) and COX-2 in human male gametes from normal patients, and those with varicocele and diabetes: A potential molecular marker for diagnosing male infertility disorders. J. Anat. 2012;221:209–220. doi: 10.1111/j.1469-7580.2012.01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang X., Dyson M.T., Jo Y., Stocco D.M. Inhibition of cyclooxygenase-2 activity enhances steroidogenesis and steroidogenic acute regulatory gene expression in MA-10 mouse Leydig cells. Endocrinology. 2003;144:3368–3375. doi: 10.1210/en.2002-0081. [DOI] [PubMed] [Google Scholar]
- 103.Scott H.M., Mason J.I., Sharpe R.M. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr. Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- 104.Xiao X., Mruk D.D., Wong C.K.C., Yan Cheng C. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology. 2014;29:286–298. doi: 10.1152/physiol.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li M.W.M., Mruk D.D., Lee W.M., Cheng C.Y. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: Is this a suitable model for studying blood-testis barrier dynamics? Int. J. Biochem. Cell Biol. 2009;41:2302–2314. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kamaruzaman K.A., Mat Noor M. Reversible spermatoxic effect of Andrographis paniculata methanol extract in Sprague Dawley rats. Malays. Appl. Biol. 2012;46:225–232. [Google Scholar]
- 107.Sun H.X., Zhu Y., Wang L., Ling Liu H., Ling Y., Li Z.L., Sun L.B. The Catsper channel and its roles in male fertility: A systematic review. Reprod. Biol. Endocrinol. 2017;15:65. doi: 10.1186/s12958-017-0281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scinicariello F., Buser M.C. Serum testosterone concentrations and urinary bisphenol a, benzophenone-3, triclosan, and paraben levels in male and female children and adolescents: NHANES 2011–2012. Environ. Health Perspect. 2016;124:1898–1904. doi: 10.1289/EHP150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu X., Miao M., Zhou Z., Gao E., Chen J., Wang J., Sun F., Yuan W., Li D.K. Exposure to bisphenol-A and reproductive hormones among male adults. Environ. Toxicol. Pharmacol. 2015;39:934–941. doi: 10.1016/j.etap.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 110.Zhuang W., Wu K., Wang Y., Zhu H., Deng Z., Peng L., Zhu G. Association of Serum Bisphenol-A Concentration and Male Reproductive Function among Exposed Workers. Arch. Environ. Contam. Toxicol. 2015;68:38–45. doi: 10.1007/s00244-014-0078-7. [DOI] [PubMed] [Google Scholar]
- 111.Lassen T.H., Frederiksen H., Jensen T.K., Petersen J.H., Joensen U.N., Main K.M., Skakkebaek N.E., Juul A., Jørgensen N., Andersson A.M. Urinary bisphenol a level in young men: Association with reproductive hormones and semen quality. Environ. Health Perspect. 2014;122:478–484. doi: 10.1289/ehp.1307309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Adoamnei E., Mendiola J., Vela-Soria F., Fernández M.F., Olea N., Jørgensen N., Swan S.H., Torres-Cantero A.M. Urinary bisphenol A concentrations are associated with reproductive parameters in young men. Environ. Res. 2018;161:122–128. doi: 10.1016/j.envres.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 113.Taylor J.A., Richter C.A., Ruhlen R.L., Vom Saal F.S. Estrogenic environmental chemicals and drugs: Mechanisms for effects on the developing male urogenital system. J. Steroid Biochem. Mol. Biol. 2011;127:83–95. doi: 10.1016/j.jsbmb.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ferguson K.K., Peterson K.E., Lee J.M., Mercado-García A., Blank-Goldenberg C., Téllez-Rojo M.M., Meeker J.D. Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reprod. Toxicol. 2014;47:70–76. doi: 10.1016/j.reprotox.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hart R.J., Doherty D.A., Keelan J.A., Minaee N.S., Thorstensen E.B., Dickinson J.E., Pennell C.E., Newnham J.P., McLachlan R., Norman R.J., et al. The impact of antenatal Bisphenol A exposure on male reproductive function at 20–22 years of age. Reprod. Biomed. Online. 2018;36:340–347. doi: 10.1016/j.rbmo.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 116.Knez J., Kranvogl R., Breznik B.P., Vončina E., Vlaisavljević V. Are urinary bisphenol A levels in men related to semen quality and embryo development after medically assisted reproduction? Fertil. Steril. 2014;101:215–221.e5. doi: 10.1016/j.fertnstert.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 117.Ghayda R.A., Williams P.L., Chavarro J.E., Ford J.B., Souter I., Calafat A.M., Hauser R., Mínguez-Alarcón L. Urinary bisphenol S concentrations: Potential predictors of and associations with semen quality parameters among men attending a fertility center. Environ. Int. 2019;131:105050. doi: 10.1016/j.envint.2019.105050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goldstone A.E., Chen Z., Perry M.J., Kannan K., Louis G.M.B. Urinary bisphenol A and semen quality, the LIFE study. Reprod. Toxicol. 2015;51:7–13. doi: 10.1016/j.reprotox.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Benson T.E., Gaml-Sørensen A., Ernst A., Brix N., Hougaard K.S., Hærvig K.K., Ellekilde Bonde J.P., Tøttenborg S.S., Lindh C.H., Ramlau-Hansen C.H., et al. Urinary Bisphenol A, F and S Levels and Semen Quality in Young Adult Danish Men. Int. J. Environ. Res. Public Health. 2021;8:1742. doi: 10.3390/ijerph18041742. [DOI] [PMC free article] [PubMed] [Google Scholar]