Abstract
Cancer is one of the most common causes of death worldwide, and its development is a result of the complex interaction of genetic factors, environmental cues, and aging. Hormone-sensitive cancers depend on the action of one or more hormones for their development and progression. Sex steroids and corticosteroids can regulate different physiological functions, including metabolism, growth, and proliferation, through their interaction with specific nuclear receptors, that can transcriptionally regulate target genes via their genomic actions. Therefore, interference with hormones’ activities, e.g., deregulation of their production and downstream pathways or the exposition to exogenous hormone-active substances such as endocrine-disrupting chemicals (EDCs), can affect the regulation of their correlated pathways and trigger the neoplastic transformation. Although nuclear receptors account for most hormone-related biologic effects and their slow genomic responses are well-studied, less-known membrane receptors are emerging for their ability to mediate steroid hormones effects through the activation of rapid non-genomic responses also involved in the development of hormone-sensitive cancers. This review aims to collect pre-clinical and clinical data on these extranuclear receptors not only to draw attention to their emerging role in cancer development and progression but also to highlight their dual role as tumor microenvironment players and potential candidate drug targets.
Keywords: ZIP9, OXER1, GPRC6A, TRPM8, GPER, mPR, PGRMC, breast cancer, prostate cancer, ovarian cancer, endometrial cancer
1. Introduction
Cancer is one of the leading causes of death worldwide, with nearly 10 million deaths reported in 2020 [1]. The transformation of normal cells in tumor cells is a multi-step process—from pre-cancerous lesions to the malignant tumor—that results from the interaction between genetic factors; environmental factors, such as chemical, physical, and biological carcinogens (as external agents); and aging. Among all types of tumors, those types that strictly depend on one or more hormones for growth, spread, and survival are defined hormone-sensitive cancers or hormone-dependent cancers. These include breast, ovarian, uterine (or endometrial), prostate, testis, and thyroid cancer [2].
Estrogen and progesterone appear to be the main sex hormones involved in growth of breast and uterine cancers, while ovarian and prostate cancers are mainly related to estrogens and androgens, respectively [2]. Breast and prostate cancers alone accounted for 2.26 and 1.41 million cases, respectively, in 2020. Although the type of tumor-initiating cues for hormone-sensitive cancers can be wide-ranging, the promotion event and subsequent growth and proliferation are driven by a hormone [2]. In addition to endogenous hormones, exogenous hormone-active substances, i.e., endocrine-active substances (EASs) and endocrine-disrupting chemicals (EDCs), can interact or interfere with the normal hormonal action and drive cell proliferation, thus increasing the cell division rate and, therefore, the consequent number of random genetic errors capable of triggering the cancerous transformation [3,4].
Steroid hormones that are derived from cholesterol are divided into sex steroids (estrogens, androgens, and progestogens) and corticosteroids (glucocorticoids and mineralocorticoids). These hormones, by interacting with specific nuclear Steroid Receptors (nSRs), can regulate different physiological functions, including growth, development, metabolism, and reproduction [5]. Therefore, their abnormal production, deregulation of their signaling pathways, and interference with their action all play a role in supporting the growth, proliferation, and spread of hormone-sensitive cancers. Indeed, treatments for these cancer types are mostly based on the interruption of the hormonal stimuli, e.g., with inhibitors for key enzymes involved in hormone synthesis and with nSR antagonists. In addition to nSRs that produce slow genomic responses, other extranuclear steroid receptors have also been reported to mediate the biological effects of steroids. These membrane steroid receptors (mSRs) are cell surface receptors capable of mediating rapid and non-genomic signaling by modulating different intracellular molecular pathways that, through distinct signaling cascades, contribute to the regulation of steroid hormone-correlated functions [6,7].
The aim of this review is to gather the existing scientific knowledge on mSRs to show their pivotal role in mediating the effects of steroid hormones and hormone-active substances in both physiologic and pathologic contexts; we also seek to elucidate their role as key players in shaping the cancer environment and, therefore, as potential drug targets.
2. Membrane Steroid Receptors and Their Role in Hormone-Sensitive Cancers
Steroid hormones can regulate gene expression in the nucleus but can also exert non-genomic functions by binding at or near the plasma membrane to induce rapid changes in cell physiology. Genetic and pharmacologic studies demonstrate mSRs function in different cell types and tissues where they respond to steroid ligands independently of the corresponding nSR. Some nSRs can exert their function at the plasma membrane, e.g., through post-translational modifications via palmitoylation or association with membrane scaffold proteins, to trigger cellular responses independently of their function as transcription factors [8]. In contrast, mSRs are integral membrane steroid receptors that feature transmembrane protein domains and, in response to steroid hormone binding, can activate or inactivate other proteins and second messenger cascades. Here, we focus on mSRs—which are lesser known and studied compared to their corresponding nSR—to dissect their molecular function and their pharmacology in hormone-sensitive cancers.
2.1. Membrane Androgen Receptors
In addition to the nSR known as Androgen Receptor (AR), other molecular mediators of the androgenic action include different plasma membrane receptors, namely, the Zinc Transporter Member 9 (ZIP9), the Oxoeicosanoid Receptor 1 (OXER1), the G protein-coupled receptor family C group 6 member A (GPRC6A), the Ca2+ channel Transient Receptor Potential Cation Channel Subfamily M (Melastatin) Member 8 (TRPM8) [9], and the L-type Voltage-dependent Calcium Channel (CaV1.2) [10].
2.1.1. ZIP9
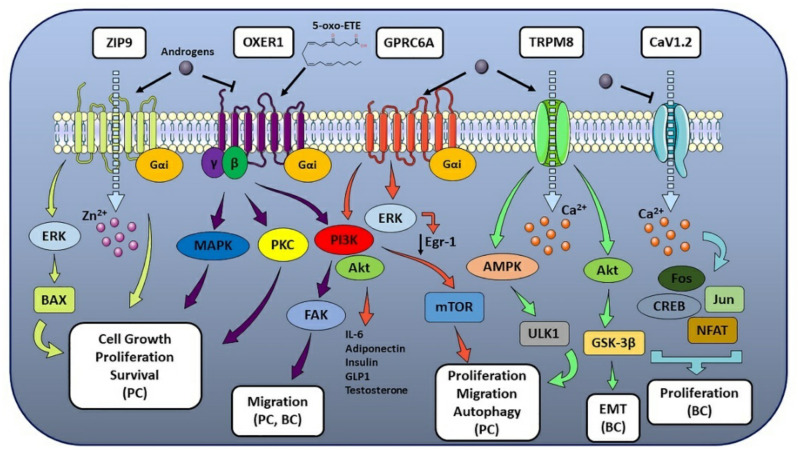
ZIP9 (also known as Zrt- and Irt-like Protein 9 and Solute Carrier family 39 member 9, SLC39A9) is a Zn2+ transporter protein involved in the Zn2+ influx from the extracellular space to the cytoplasm [11] and a membrane Androgen Receptor (mAR) coupled to G proteins [12]. ZIP9, encoded by the SLC39A9 gene in human [12], is member 9 out of 14 ZIP family proteins and is present in three isoforms with different lengths and molecular weights. Unlike other ZIP proteins that feature 8 Trans-Membrane (TM) domains and an extracellular C-terminus, ZIP9 has a 7TM structure with an intracellular C-terminal domain and is the only member of the family to signal via G protein [12]. In particular, ZIP9 is coupled to a stimulatory Gα protein (Gαs), and, upon activation, it mediates Mitogen-Activated Protein kinase (MAPK) and Zn2+ signaling [13]. This dual role of Zn2+ transporter and mAR is exerted through G proteins involved in apoptotic pathways activated by androgens [12]. Indeed, testosterone exhibits high affinity for ZIP9 and has been demonstrated to act as an agonist for this receptor, while other endogenous androgens such as androstenedione and dihydrotestosterone (DHT) display low affinity [12]. Since Zn2+ is required for the structure of different proteins (e.g., zinc-finger-containing transcriptional factors and Zn2+-dependent metalloenzymes [14]) and for several signaling pathways involved in cell growth, proliferation, and apoptosis [14], Zn2+ homeostasis is pivotal for human health. Therefore, its dysregulation has been associated with different pathologies, including inflammation, diabetes, and cancer [14]. In this regard, ZIP9 has been found highly expressed and bound by androgen hormones in ovarian, breast and prostate cancer cells [15], and testosterone has been demonstrated to increase intracellular Zn2+ levels in the same hormone-sensitive tumors, where it led to high Zn2+ concentration-mediated apoptosis [12,16]. ZIP9 has been shown to mediate a testosterone-induced, AR-independent increase of cell migration in metastatic prostate cancer cells [17]. In contrast to previous literature data on ZIP9, testosterone treatment in human breast and prostate cancer cell lines results in the activation of an inhibitory Gα protein (Gαi) [18]. Interestingly, ZIP9 was found significantly up-regulated in breast cancer tissues compared with normal breast tissues [19], the ZIP9-encoding SLC39A9 gene has been observed to form a fusion transcript with MAP3K9 gene (encoding for Mitogen-Activated Protein Kinase 9, MAP3K9), and the resulting fusion gene has been reported with repetitive incidence in different types of breast cancer [20] (Figure 1).
Figure 1.
mAR-associated pathways and their effect on hormone-sensitive cancer progression. The figure illustrates the molecular pathways correlated to the different mARs and their biological effects on multiple hormone-sensitive cancer types. When one or more tumor-related processes were reported only for a specific cancer type, the latter was made explicit in the figure and put in parentheses (e.g., breast cancer as BC, prostate cancer as PC) (see text for details).
2.1.2. OXER1
OXER1 (previously known as G Protein-coupled Receptor 170 (GPR170), hGPCR48, HGPCR48, TG1019 or R527) is a G protein-coupled receptor (GPCR) coupled to a Gαi [21] and activated by 5-oxoeicosatretraenoic acid (5-oxo-ETE), 5-lipoxygenase (5-LOX) and peroxidase metabolite of arachidonic acid [22]. OXER1 mediates several intracellular actions, including steroidogenesis stimulation, immune and inflammatory responses, cell proliferation and survival [23]. Upon 5-oxo-ETE binding, OXER1 activation results in the Gαi-mediated inhibition of cAMP production and Gβγ-mediated induction of Ca2+ mobilization, although Gβγ is responsible for mediating several other signaling pathways [22,24]. These include chemotactic response and actin polymerization in different leukocyte types (e.g., neutrophils, eosinophils, and monocytes) [24,25], activation of PI3K and Akt (also known as Protein Kinase B, PKB), focal adhesion kinase (FAK), extracellular signal-regulated kinases 1/2 (ERK1/2), Phospholipase A2 (PLA2) and modulation of different Protein Kinase C (PKC) isoforms [26,27]. Most of these pathways are involved, even in different cellular contexts, in proinflammatory responses, infiltration and cell migration [9]. Despite the fact that most literature data are focused on OXER1 role in immune context, their role in the progression of different hormone-related cancers has also been investigated. Literature data confirmed that OXER1 is highly expressed in cancer cells and tissues, including prostate and breast [28,29], where it has been observed to mediate survival-promoting effects and inhibition of cell apoptosis, when activated by its endogenous ligand 5-oxo-ETE [23,29]. Cancer cells display high levels of 5-hydroxy-eicosatetraenoic acid dehydrogenase (5-HEDH) that can convert inflammatory cell-derived 5-hydroxyeicosatetraenoic acid (5S-HETE) to 5-oxo-ETE, especially when the cells are stressed [30]. 5-oxo-ETE produced within the tumor microenvironment (TME) can support OXER1-mediated cancer cell growth and promote further infiltration of inflammatory cells. Both prostate cancer-derived cell lines and prostate cancer tissue from patients contain high levels of OXER1 at both mRNA and protein levels [23,29]. In addition, membrane staining for OXER1 has been found to be significantly increased in prostate cancerous tissues compared to non-cancerous ones [18], supporting OXER1’s role in prostate cancer cell growth and metastasis [23,31]. Indeed, OXER1 mediates cell proliferation, survival-promoting effects, and inhibition of cell apoptosis in prostate cancer cells, as well as adhesion, migration and invasion through its major signaling pathways involving p38α, PI3K/Akt and FAK [31]. OXER1 has also been identified as a specific mAR, and testosterone acts as an antagonist on OXER1-mediated PI3K, FAK and p38α signaling pathways, resulting in the inhibition of cell migration and metastasis [31,32] (Figure 1). OXER1 can also mediate androgen actions that antagonize the effects of 5-oxo-ETE, providing a novel link between steroid and lipid actions and an interesting target for therapeutic intervention [31]. In addition, since rapid androgen actions have been reported in classical AR-lacking breast cancer models [33] and membrane androgen binding sites have been observed in breast carcinoma cells [34], OXER1 has been recently proposed as a potential drug target in breast cancer [35]. Indeed, OXER1 role in malignant cell growth was firstly reported in MDA-MB-231 and MCF-7 breast cancer cell lines [28], which express high levels of OXER1 protein [18]. In agreement, inhibition of 5-oxo-ETE production by blocking 12-LOX and 5-LOX results in reduced proliferation and induced cytotoxicity and apoptosis, further supporting OXER1 role in breast cancer [36,37]. Data mining analysis also reveals that high OXER1 expression in tumor breast invasive carcinoma (TCGA-1097) correlates with a worse overall survival probability [38]. In addition, a recent study based on RNA-sequencing data proposed OXER1 as a key gene and possible molecular marker of tumorigenesis of Hormone-Receptor-positive/Human Epidermal Growth Factor Receptor 2 (also known as Receptor Tyrosine-Protein Kinase erbB-2, cluster of differentiation 340 (CD340), or proto-oncogene Neu)-negative (HR(+)/HER2(−)) breast cancer in adolescents and young adults [39].
2.1.3. GPRC6A
GPRC6A is a Ca2+ and amino acid sensing GPCR (widely expressed both in humans and in rodents) whose activity has been linked to rapid, non-genomic signaling responses exerted by androgens [40]. This receptor is involved in different physiological and pathological contexts, including male fertility, insulin secretion, bone and energy metabolism, inflammatory responses, and androgen production [9]. GPRC6A is coupled to a Gαi protein and has been proposed to be a multi-sensing receptor, since its activation can be induced by Ca2+, Mg2+, osteocalcin, steroid hormones, and a variety of amino acids [40]. In this regard, GPRC6A has been demonstrated to mediate rapid and non-genomic signaling in response to androgen binding in AR-lacking cells, when overexpressed in these cellular models, while its silencing impaired exogenous androgen response [40]. As for the ability of this receptor to bind androgens, both computational analyses and in vitro assays demonstrated that testosterone can bind GPRC6A, leading to ERK1/2 phosphorylation and activation and to a decrease in tumor-suppressor Early Growth Response Protein 1 (Egr-1, also known as Zinc Finger protein 268, ZNF268, or Nerve Growth Factor-Induced protein A, NGFI-A) [40]. In addition, GPRC6A activation in different tissues led to the production of different cytokines (including IL-6 and adiponectin) and hormones (insulin, Glucagon-Like Peptide 1 (GLP-1) and testosterone) [40]. Noteworthy, compared to normal prostate cells, GPRC6A upregulation was observed in different prostate cancer cell lines, where its activation led to an increase in chemotaxis and cell proliferation in vitro [40]. Conversely, ablation of GPRC6A in prostate cancer mice xenografts resulted in decreased tumor progression and enhanced survival [41]. Indeed, testosterone binding to GPRC6A has been demonstrated to activate ERK, Akt and mammalian Target of Rapamycin (mTOR) signaling pathways in a time and dose-dependent manner, resulting in an increased cell proliferation and inhibition of autophagy in prostate cancer cells [42] (Figure 1). Noteworthy, the association between GPRC6A gene and prostate cancer risk has been widely reported in Eastern Asian populations [43,44,45], and the rs2274911 polymorphism in GPRC6A has been associated with increased risk of prostate cancer, since this mutation promotes prostate cancer cell proliferation and is associated with increased prostate-specific antigen (PSA) serum levels [46]. In addition, clinical and in vitro data revealed that GPRC6A is associated with aggressive prostate cancer, with a pivotal role in cell proliferation, migration, invasion, and Epithelial-Mesenchymal Transition (EMT) [47].
2.1.4. TRPM8
TRPM8 (also known as the Cold and Menthol Receptor 1, CMR1) is an androgen-regulated, Ca2+-selective cation channel sensitive to cold physical stimulus, menthol and icilin (AG-3-5), located at the endoplasmic reticulum and plasma membranes of androgen-responsive cells [9]. Indeed, TRPM8 has been recognized as an androgen-related receptor due to its androgen binding affinity and steroid specificity [48], and this strong binding of androgens, such as testosterone and DHT, affects its Ca2+ channeling activity [49]. TRPM8 is widely expressed in different tissues, including hepatic and intestinal tissues, peripheral nervous system, male urogenital tract, and cancer tissues [50]. Indeed, TRPM8 is involved in the regulation of several key processes, including inflammatory and immunomodulatory responses, cell proliferation, migration, and apoptosis [51]. In this regard, TRPM8 is a possible prostate cancer biomarker [52,53] reported to be significantly increased in early-stage prostate cancer, while its expression is significantly decreased in advanced stages androgen-independent prostate tumors [54]. Moreover, TRPM8 is highly expressed in the prostate epithelium, its levels rise in primary and hormone naïve prostate cancer metastasis [55], and several clinical data report TRPM8 among other candidate gene markers for metastatic prostate [56,57] and TRPM8 mRNA levels were found significantly upregulated in prostate cancer samples [58] and correlated with those of kallikrein-3 (KLK3, also known as PSA, gamma-seminoprotein or P-30 antigen) [59]. Although restricted to a limited number of studies, TRPM8 involvement in tumor progression has also been investigated in breast cancer since it was found overexpressed in human Breast Ductal Adenocarcinoma (hBDA) samples [60]. In this regard, TRPM8 has been reported to activate Akt and Glycogen Synthase Kinase 3 beta (GSK-3β) pathway to promote EMT and breast cancer aggressiveness [61] and to regulate proliferation, migration, and autophagy by activating AMP-Activated Protein Kinase (AMPK) and Unc-51 Like Autophagy Activating Kinase (ULK1) pathway [62] (Figure 1). Furthermore, in vitro and clinical data support the role of TRPM8 as a biomarker for poor clinical outcome prediction in estrogen receptor (ER)-negative breast cancer patients [63,64].
2.1.5. CaV1.2
CaV1.2 is a member of the L-type Voltage-Gated Ca2+ Channel (VGCC) family (CaV1.1–CaV1.4), known to play pivotal roles in the regulation of Ca2+ homeostasis, secretion, and tissue development. L-type VGCC are multi-subunit channels formed by three distinct proteins, namely, CaVα1, CaVα2δ and CaVβ [65]. The ion-conducting channel is formed by the four CaVα1 subunits, which have multiple splice variants that confer their pleiotropic effects and their association with a wide range of pathologies, including cancer [65]. Noteworthy, an increased intracellular Ca2+ influx induced by CaV1.2 can lead to the activation of Nuclear Factor of Activated T cells (NFAT) via calcineurin-mediated dephosphorylation and nuclear translocation [66,67]. In this regard, increased NFAT transcriptional activity has been implicated with increased cancer progression events, including proliferation, migration, invasion, autophagy, and tumor neo-angiogenesis [67,68]. Cav1.2-mediated Ca2+ influx has been associated with the activation of transcription factors Fos; c-Jun; cAMP Response Element-Binding protein (CREB); NFAT; and the expression of D-cyclins to control G1/S transition and, thus, cell proliferation [69,70] (Figure 1). Interestingly, not only testosterone has been demonstrated to bind and inhibit CaV1.2-induced Ca2+ influx [71] but also 5α-dihydrotestosterone decreased CaV1.2 expression in the luminal breast cancer MCF-7 cells, affecting their viability and proliferation [70].
2.2. Membrane Estrogen Receptors
nSRs, known as estrogen receptors alpha and beta (ERα and ERβ), are the most characterized and well-studied receptors known to exert the effects of endogenous and exogenous estrogens, estrogen-like substances and xenoestrogens. However, other molecular mediators of estrogenic action are located at the cellular plasma membrane and include G-Protein Estrogen Receptor (GPER), ERx, ER-X and Gq-coupled membrane Estrogen Receptor (Gq-mER) [72]. In addition, Voltage-Gated Sodium Channel Nav1.2 is also reported to bind estrogens and to activate molecular cascades upon their binding [10].
2.2.1. GPER
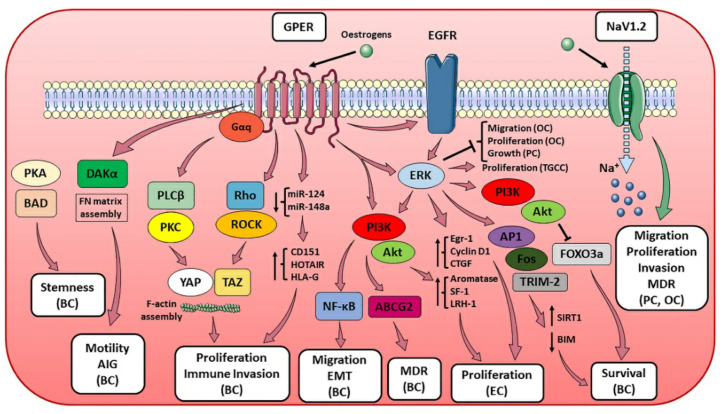
While for ER-X and Gq-mER there are almost no literature data on their possible role in cancer progression [73], and for ERx available data are limited to in vitro observations regarding breast cancer [74], GPER (previously known as G Protein-coupled Receptor 30, GPR30) has been associated to the modulation of signaling pathways involved in tumor growth both in vitro and in vivo [75,76]. GPER is a 7TM receptor coupled to G protein (encoded by GPER gene in humans) capable of binding endogenous estradiol (E2) with high affinity and responsible for the activation of rapid, non-genomic estrogenic effects [77]. GPER is a member of the Rhodopsin-like GPCR family mainly localized at the cellular plasma membrane, where it has been shown to signal through the activation of both Gαs and Gαi [78,79]. Its activation results in the induction of Ca2+ mobilization and synthesis of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), and several studies provided evidence of GPER localization also in intracellular compartments, including the endoplasmic reticulum, Golgi apparatus and nucleus [80]. However, its distribution appears to vary depending on species, tissue, and cell type and to dynamically change in response to specific environmental signals. GPER is expressed in different tissues, including breast, prostate, and ovary, as well as in immune cells [81,82], and its expression is suggested to be species-, gender-, tissue- and age-dependent [83]. In addition, GPER abundance has been reported to be developmentally regulated, since its expression in elongating ducts of the mammary gland not only is lower during puberty and increases during sexual maturity but also appears to be dependent on estrous cycle [78]. These findings are of pivotal interest not only in the investigation of GPER physiological function but also for its pathologic role in different contexts, including metabolic functions, reproduction, immune regulation, and cancer development and progression [82,84]. GPER expression has been associated with metastasis formation, tumor size and recurrence in both breast and ovarian cancer [85], and with the expression of a gene signature involved in the metastasis of ER-negative breast tumors [86]. In addition, GPER activation has been observed to play a critical role in breast cancer growth, proliferation, migration, and metastasis [76] via the modulation of different molecular pathways. These include the stabilization of the F-actin cytoskeleton and the upregulation of Yes-Associated Protein 1 (YAP) and Transcriptional coactivator with a PDZ-binding domain (TAZ) via the activation of Gαq-11, Phospholipase C beta (PLCβ)/PKC and Rho/Rho-associated protein kinase (ROCK) signaling [87,88]; the downregulation of microRNA-124 (miR124) and miR-148a to promote proliferation and support immune invasion via the upregulation of CD151, HOX Transcript Antisense RNA (HOTAIR) and Histocompatibility Antigen, class I, G (HLA-G) [89,90,91]; the enhancement of Fibronectin (FN) matrix assembly and anchorage-independent growth [92]; and cell survival through the activation of epidermal growth factor receptor (EGFR)/ERK/c-Fos/Activator Protein 1 (AP-1) for Sirtuin 1 (SIRT1, also known as NAD-dependent deacetylase sirtuin-1) upregulation [93], MAPK/ERK/Tripartite Motif Containing 2 (TRIM-2) for Bcl-2-like protein 11 (BIM) downregulation [94], and EGFR/PI3K for Forkhead Box O3s (FOXO3a) inhibition [95]. Moreover, GPER appears to be required for stemness maintenance in cancer stem cells via PKA/Bcl-2 associated agonist of cell death (BAD) pathway [96] and the development of chemoresistance via EGFR/ERK/Akt-mediated ATP Binding Cassette, Subfamily G, Member 2 (ABCG2) expression [97]. This is in line with the increasing evidence that GPER expression and activation is correlated with a poor response to chemotherapy with selective estrogen receptor modulators [98,99]. However, GPER role in breast cancer still needs to be fully clarified due to contrasting literature data on its significance as prognosis predictor [100,101,102]. This could be attributed not only to the variation of GPER expression during carcinogenesis due to the progressive hypermethylation of its promoter [103] but also to GPER different role depending on the specific breast cancer context.
Clinical data reported a correlation between GPER expression and poor prognosis also in other female reproductive cancers [104]. GPER has been proposed to act as tumor suppressor in ovarian cancer [105] and to have, together with Wnt pathway modulator Dickkopf 2 (Dkk2) expression, a positive prognostic impact in ovarian cancer patients [106,107]. GPER not only suppresses the proliferation of ovarian cancer cells by blocking tubulin polymerization [108,109], but its activation also led to a transcriptome response associated with growth inhibition in ovarian cancer cells [110] and triggered a ERK1/2-mediated trimethylation of Histone H3 at Lysine 4 (H3K4me3) to repress migration and proliferation in vitro [111]. However, contrasting evidence supports the role of GPER in mediating proliferation, migration, and invasion in vitro—through the expression of c-Fos, cyclin D1, Matrix Metallopeptidase 2 (MMP2, also known as gelatinase A, GELA or 72 kDa type IV collagenase) and MMP9 9 (also known as gelatinase B, GELB)—in both an E2-mediated [112,113] and a ligand-independent manner [114]. In endometrial cancer, GPER has been observed to promote cell proliferation by enhancing the expression of aromatase, nuclear hormone receptors Steroidogenic Factor 1 (SF-1) and Liver Receptor Homolog-1 (LRH-1) via a PI3K/Akt- and MAPK-mediated mechanism [115], and through a GPER/EGFR/ERK/Egr-1 transduction pathway resulting in the expression of cyclin D1 and Connective Tissue Growth Factor (CTGF, also known as CCN2) [116]. Moreover, upon E2 binding, GPER also regulates in vitro endometrial cancer cells motility and anchorage-independent growth through Diacylglycerol Kinase alpha (DGKα) [117]. GPER pro-tumorigenic role in endometrial cancer is also supported by evidence reporting anti-EMT effects by GPER targeting via miR-195 [118] and cell proliferation via miR-424 [119].
GPER tumorigenic properties have also been investigated in male cancers, namely, prostate and testicular cancers. Indeed, GPER activation led to the ERK1/2-mediated inhibition of prostate cancer cell growth [120], although its role and significance still need to be completely elucidated. Finally, GPER is also involved in regulating the proliferation of testicular germ cell cancer [121,122] and has been reported to be overexpressed in human seminoma (a testicular germ cell tumor subtype) [122]. Indeed, clinical and experimental studies support the hypothesis that estrogens, through GPER activity, contribute to the regulation of tumor testicular germ cells proliferation via ERK1/2 [121,122]. Noteworthy, two polymorphisms in the promoter region of GPER (rs3808350 and rs3808351) in seminomas are correlated to its overexpression and confer genetic susceptibility for testicular carcinogenesis [122]. Conversely, in Leydig cell tumor (a different testicular germ cell tumor subtype compared to seminoma), GPER activation is correlated with decreased proliferation, increased apoptosis [121] and perturbance of lipid metabolism and steroidogenesis via PI3K/Akt/mTOR pathway impairment [123], suggesting that, for testicular germ cell tumors, GPER-mediated effects on cell survival and proliferation depend on specific cell type (Figure 2).
Figure 2.
mER-associated pathways and their effect on hormone-sensitive cancer progression. The figure illustrates the molecular pathways correlated to the different mERs and their biological effects on multiple hormone-sensitive cancer types. When one or more tumor-related processes were reported only for a specific cancer type, the latter was made explicit in the figure and put in parentheses (e.g., breast cancer as BC, prostate cancer as PC, endometrial cancer as EC, ovarian cancer as OC and testicular germ cell cancer as TGCC). Other abbreviations: multi-drug resistance (MDR), anchorage-independent growth (AIG) (see text for details).
2.2.2. NaV1.2
Voltage-Gated Sodium Channels (VGSCs) are integral membrane glycoprotein complexes that, in response to membrane potential depolarization, undergo a conformational change, open a transmembrane pore and mediate a Na+ influx. VGSCs are composed of principal subunits alpha (VGSCα) and auxiliary subunits beta (VGSCβ), and, since they are functionally expressed in different tissues and cell types [124], VGSCs are involved in a variety of molecular events, including neurotransmission, nerve skeletal and cardiac muscle contraction, and secretion. In this regard, VGSCs play an important role in the development and progression of different cancers, since they have been correlated with different events of the carcinogenic process, including cell proliferation, migration, invasion, and Multi-Drug Resistance (MDR) [125,126]. NaV1.2 channel increased expression has been observed in different cancer types, including prostate [127] and ovarian cancer, where it is involved in regulating migration and invasion of highly metastatic cancer cells [128]. Interestingly, rapid estrogen actions on different ion channels’ functionality have been reported, and both endogenous and exogenous estrogens can bind NaV1.2, other VGSCs and other ion channels, to regulate their activity [129,130] (Figure 2).
2.3. Membrane Progesterone Receptors
In the same way for androgen and estrogen signaling, the progesterone receptor (PR), with its two isoforms PR-A and PR-B, is the most prominent and studied mediator of progestogenic effects, although other receptors localized at the plasma membrane level can participate in mediating progesterone (P4) and progesterone-like substances effects. These membrane progesterone receptors that mediate non-traditional progesterone actions are classified into two groups: the class II progestin and adipoQ receptor (PAQR) family (also called membrane Progesterone Receptors, mPRs) includes mPRα, mPRβ, mPRγ, mPRδ, mPRε encoded by PAQR7, PAQR8, PAQR5, PAQR6 and PAQR9 genes, respectively [131], while the b5-like haem/steroid-binding protein family (also called Membrane Associated Progesterone Receptors, MAPRs) comprises Progesterone Receptor Membrane Component 1 (PGRMC1), PGRMC2, GIG47 (previously known as Neuron-Derived Neurotrophic Factor, NENF, and neudesin) and neuferricin (alternatively termed Cytochrome B5 Domain Containing 2, CYB5D2) [132].
2.3.1. mPRs
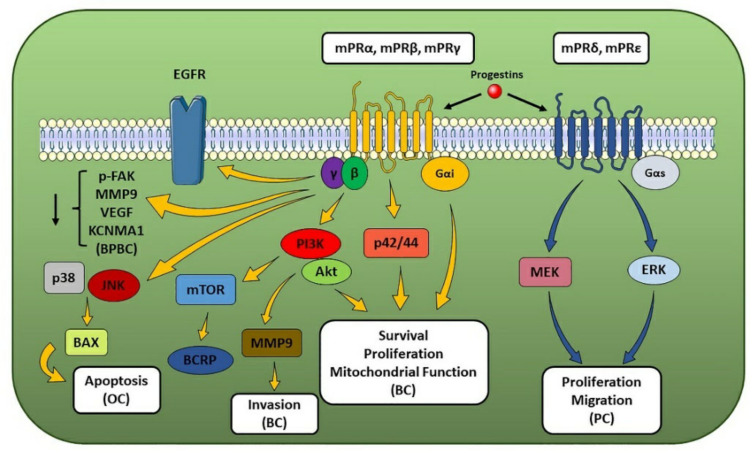
The mPRs are 7-transmembrane protein receptors located in the cell plasma membrane that transduce via G-proteins, even though they do not share structural nor sequence homology to GPCRs and nuclear steroid receptors [131]. In mammals, these receptors show a differential expression in both reproductive (e.g., ovary, uterus, placenta, and testis) and non-reproductive (e.g., brain, kidney and intestinal) tissues [133] and display a high binding affinity for progestins. Although several members of the class II PAQR family are known, most literature data reported and focused on the functional characterization of mPRα, which is the most abundant mPR expressed in different human tissues [134], while information is still lacking for the other members. Several studies reported that mPRα, mPRβ and mPRγ are coupled and activate a Gαi, leading to a progestin-triggered down-regulation of adenylyl cyclase activity [131,134], while mPRδ and mPRε have been shown to signal through a Gαs [135]. In addition, after progestin activation, the βγ subunit has been reported to participate in signal transduction by upregulating PI3K/Akt, ERK1/2 and p38 MAPK molecular pathways [136]. Progestin functions correlated to the presence of mPRs and mPR-dependent signaling in mammalian cells have been suggested to mediate the development of breast and ovarian hormone-sensitive cancers [137,138] since P4 has been observed to inhibit the EMT process in breast cancer cells through mPRs-mediated PI3K and EGFR activation [139] and to transduce through mPRs in ovarian cancer cells [140]. Regarding breast cancer, mPRα and MMP9 expression and Akt phosphorylation have been reported to be higher in breast cancer tissue compared to non-cancerous one. A positive correlation between mPRα, HER2 and MMP9 expression and tumor size has been reported, while a negative correlation with ER and PR status has also been observed [141]. In addition, mPRα expression also showed a positive correlation with EGFR, HER2 and Ki67 expression pattern [142]. Indeed, P4-mediated mPRα activation has been found to modulate cell proliferation via PI3K molecular pathway [142]; to decrease apoptosis; to increase mitochondrial potential through Gαi, p42/44 MAPK and Akt signaling cascades [143]; and to upregulate the breast cancer resistance protein (BCRP)—an independent risk factor for breast cancer and possible marker for poor prognosis—through PI3K/Akt/mTOR pathway [144], supporting a role for mPRα in the development and progression of breast cancer through cell death inhibition and its role as a major prognostic marker of poor prognosis (Figure 3). Conversely, upon its P4-mediated activation, mPRα exerts inhibitory effects on cell proliferation and migration in triple-negative breast cancer [145] and in basal phenotype breast cancer via FAK dephosphorylation, MMP9, Vascular-Endothelial Growth Factor (VEGF), and Calcium-Activated Potassium Channel Subunit alpha-1 (KCNMA1, also known as BK channel alpha subunit or large conductance Ca2+-activated potassium channel, subfamily M, alpha member 1, KCa1.1) downregulation mechanisms [146]. Regarding ovarian cancer, expression profiles of mPRα and mPRβ suggested a potential role in the pathogenesis and development of ovarian tumors [147]. However, unlike in breast cancer cells, P4-triggered mPR activity has been shown to indirectly induce cAMP levels by enhancing β1,2-adrenergic receptor activation and to up-regulate apoptosis regulator BAX (also known as Bcl-2-like protein 4) through JNK1/2 and p38 MAPKs activation [148], indicating the existence of mPR-induced molecular pathway that, through pro-apoptotic MAPK signaling modules, can lead to ovarian cancer cell death and suggesting that P4-based hormone therapy could provide a suitable and effective strategy for the treatment of ovarian cancer [148] (Figure 3). In addition to breast and ovarian cancers, mPR have been associated also with other hormone-sensitive tumors like endometrial and prostate cancers. Indeed, analyses of samples of endometrial cancer patients not only revealed that mPRβ and mPRγ displayed a different subcellular localization in cancerous tissue compared to non-cancerous one but also suggest, based on expression data, these receptors as potential prognostic biomarker for endometrial cancer [149]. Regarding prostate cancer, mRNA expression analyses of samples from patients revealed that PAQR6 levels were upregulated compared to non-cancerous tissue and positively correlated with a lower survival rate. In addition, gene silencing data correlated mPRδ activity with MEK and ERK1/2 signaling pathway involved in proliferation and migration of tumor cells, suggesting its potential role in prostate cancer development and serving as potential prognosis biomarker [150] (Figure 3).
Figure 3.
mPR-associated pathways and their effect on hormone-sensitive cancer progression. The figure illustrates the molecular pathways correlated to the different mPRs and their biological effects on multiple hormone-sensitive cancer types. When one or more tumor-related processes were reported only for a specific cancer type, the latter has been made explicit in the figure and put in parentheses (e.g., breast cancer as BC, prostate cancer as PC, ovarian cancer as OC, and basal phenotype breast cancer as BPBC) (see text for details).
2.3.2. MAPRs
MAPRs proteins, like mPRs, are membrane-bound receptors—evolutionarily conserved and distant homologues of the small haemoprotein Cytochrome B5 (CYB5)—able to bind P4 and activate rapid, non-genomic and PR-independent effects. MAPRs can interact with the superfamily of microsomal and mitochondrial haemoproteins Cytochrome P450 enzymes (CYP) and other different proteins to regulate a variety of molecular processes, including cell proliferation and migration, steroid homeostasis, and autophagy [132].
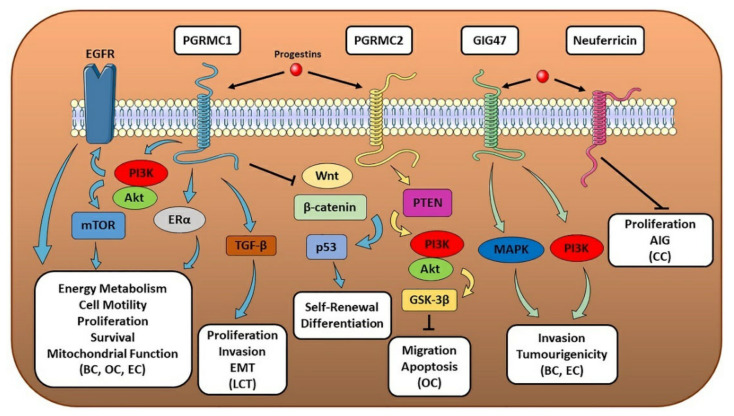
PGRMC1 features a single transmembrane domain and, besides progestins, binds several other ligands, including cholesterol, glucocorticoids, and other steroids [134]. It is localized, together with CYPs, in different subcellular compartments, including the plasma membrane, nucleus, endoplasmic reticulum and mitochondria [151]. Its expression is upregulated in different tumor types, and it is involved in promoting cancer progression and EMT [152,153]. This is in line with the reported role of PGRMC1 in promoting cell self-renewal and inhibiting differentiation by downregulating Wnt/β-catenin and p53 pathways [154], as well as upregulating energy metabolism, mitochondrial function, glycolysis, cell motility and tumor growth, through PI3K/Akt pathway [155]. Regarding hormone-sensitive cancers, PGRMC1 has been shown to regulate some breast cancer hallmarks [156]. Indeed, PGRMC1 can promote tumorigenesis [157], and its expression is associated with a malignant breast cancer phenotype [158]. Recently, independent studies showed a positive correlation between PGRMC1 expression levels and breast features associated with poor prognosis [159,160], further suggesting its role as tumor marker and strengthening PGRMC1 prognostic value for this malignancy. PGRMC1 can promote breast cancer development by increasing survival and growth of tumor cells [161] and inducing neovascularization in tumor tissue through VEGF activation [162]. These observations on its tumor-promoting role were confirmed by the reduced migration and metastasis of breast cancer cells after PGRMC1 knock-out [163] and by the suppression effect on PGRMC1 tumorigenicity exerted by miR-181a [164]. At molecular level, although conflicting data are reported in literature regarding PGRMC1-initiated effects in breast cancer cells [165], PGRMC1 activation enhances PI3K/Akt/mTOR and EGFR-mediated signaling pathways, resulting in the increased proliferation [166] and alteration of lipid metabolism [167] in ER+ and triple-negative breast tumors. This is in line with the reported association between PGRMC1 and ER levels [160], the observed PGRMC1-mediated ERα activation [167] and the increased breast cancer cell proliferation correlated with PGRMC1-ERα crosstalk recently reported [168]. However, PGRMC1’s role in tumor development and progression is not limited to breast cancer. Indeed, several studies reported its involvement also in other hormone-sensitive cancers, including testicular Leydig cell tumor proliferation and invasion through Transforming Growth Factor beta (TGF-β) alternative pathway [169], endometrial cancer [170] and ovarian cancer, in which PGRMC1 activation promotes cell viability, growth, proliferation, migration and survival through PI3K/Akt-mediated inhibition of apoptosis [171,172] (Figure 4).
Figure 4.
MAPR-associated pathways and their effect on hormone-sensitive cancer progression. The figure illustrates the molecular pathways correlated to the different MAPRs and their biological effects on multiple hormone-sensitive cancer types. When one or more tumor-related processes were reported only for a specific cancer type, the latter has been made explicit in the figure and put in parentheses (e.g., breast cancer as BC, endometrial cancer as EC, ovarian cancer as OC, Leydig cell tumor as LCT and cervical cancer as CC). Other abbreviations: anchorage-independent growth (AIG) (see text for details).
PGRMC2 is a membrane-bound receptor structurally similar to PGRMC1, although it displays a different N-terminal transmembrane domain and is less functionally characterized [173]. Like PGRMC1, PGRMC2 is expressed in different tissues and with a similar subcellular localization. Although limited studies on its role in cancer development and progression are available, PGRMC2 expression, which has been shown to be post-transcriptionally regulated by miR-142-3p [174], is higher in breast cancer tissue compared to non-cancerous one, suggesting its potential contribution to the tumorigenesis of the breast [175] and serving as possible biomarker for breast cancer staging [176]. Moreover, PGRMC2 is highly expressed in ovarian cancer cells, and in vitro studies showed that its P4-mediated activation resulted in the inhibition of cell migration [177] and apoptosis through PI3K/Phosphatase and Tensin Homolog (PTEN)/Akt/GSK-3β axis [172] (Figure 4).
Although originally identified as a secreted protein with neurotrophic actions mediated via MAPK and PI3K pathways, Nuclear Magnetic Resonance (NMR) studies revealed that GIG47 shares a similar structure with PGRMC1 and 2 [178]. GIG47 increased expression has been reported in several cancer types, including uterine and breast cancer, in which it promotes cancer cell invasion and increased tumorigenicity through MAPK and PI3K pathways [178] (Figure 4). In addition to its receptor-correlated activity, an extracellularly secreted GIG47 has been shown to be involved in cancer cell immortalization and resistance to carcinogens [179].
A homology-based search of the same CYB5-like haem/steroid-binding domain of PGRMC1, PGRMC2 and GIG47 led to the discovery of Neuferricin that, like the other MAPRs, is expressed in a variety of different tissues [132]. Like GIG47, Neuferricin can be detected as both secreted haemoprotein and membrane-bound receptor, especially localized in the endoplasmic reticulum, where it can interact with CYP Reductase (POR) [180]. Its expression has been correlated with the inhibition of cell proliferation and anchorage-independent colony growth in vitro in cervical cancer cells [180] and has been suggested as a potential candidate tumor suppressor of cervical tumorigenesis [181]. However, correlations between Neuferricin expression or its activity and the development and progression of hormone-sensitive cancers have not been found yet.
3. Beyond mSRs Physiologic Role
Since the mSRs reported and dissected here show widespread expression in human tissues and can regulate the activation of well-known and pivotal signaling pathways, the exposure to exogenous hormone-active substances, e.g., EDCs, can cause their aberrant and non-physiologic activation. This can trigger different molecular mechanisms and initiate a variety of pleiotropic effects that, due to the uncertainty about time, duration, and concentration of exposure, may play a role in the development and progression of different diseases that rely on the activity of hormones and hormone-like compounds. Therefore, in a hormone-sensitive cancer context, mSR abnormal activation in different but adjacent tissues may shape the characteristics of the TME to drive and sustain the formation of the cancer mass [3]. On the other hand, this ability to affect not only cancer cells but also other cellular subpopulations within the TME, including immune cells, makes mSRs possible and interesting drug targets able to interfere not only with the biology of cancer cells but also with cancer-promoting and cancer-sustaining mechanisms activated by other adjacent cell types. In the following section, the role of different mSRs will be firstly dissected as possible TME shapers due to their role as EDCs receptors and, secondly, as potential drug targets for different hormone-sensitive cancers.
3.1. mSRs as EDCs Targets
In the classic definition of endocrine disruption, hormonally active agents are considered for their ability to interfere with the action of hormones and endocrine signaling primarily through nSRs. Noteworthy, EDCs have been shown to affect hormone signaling within the TME to promote a crosstalk between cancers cells, stromal cells, and immune cells and to influence the extracellular matrix remodeling in a pro-tumorigenic manner, finally contributing to cancer cell progression, invasion, and metastasis formation (reviewed in [3]). However, recent and increasing evidence suggests that also mSRs should be considered in the mechanism of endocrine disruption since EDCs have been shown to bind and modulate mSR-initiated downstream molecular processes.
Regarding mARs, in prostate cancer cells, a variety of pesticides, including prochloraz, vinclozolin and its metabolite M2 (3’,5’-dichloro-2-hydroxy-2-methylbut-3-enanilide), have been shown to exert antiandrogen actions through ZIP9, competing with testosterone and antagonising ERK-mediated pro-apoptotic gene BAX expression and reducing ZIP-mediated intracellular free Zn2+ influx [182]. However, potential adverse effects on human health as a consequence of the inhibition of ZIP9-dependent functions could include the attenuation of testosterone-activated pro-apoptotic and antitumorigenic effects in prostate cancer cells [9,12]. Moreover, in addition to environmental chemicals and pollutants, also plant-derived compounds have been shown to exert anti-androgenic and mAR-directed mechanism of endocrine disruption [32], although the roles of ZIP9, OXER1, and GPRC6A in mediating mechanisms of cancer progression activated by phyto-antiandrogens have still to be elucidated.
Among the different mERs, GPER is the most studied for its correlations with mechanisms of endocrine disruption [183,184]. In this regard, upon the binding of a variety of estrogen-active compounds—namely, diethylstilbestrol (DES), zearalenone (ZEA), bisphenols A, AF, B and S (BPA, BPAF, BPB and BPS), Tetrachlorobisphenol A (TCBPA) and Tetrabromobisphenol A (TBBPA) – GPER has been shown to enhance migration and proliferation of multiple breast cancer cell lines [185,186,187,188,189,190] by activating FAK, Src and ERK2 (via EGFR) to promote focal adhesion (FA) assembly [191]; to enhance cancer cell proliferation in testicular seminoma [192]; and, via PI3K/Akt/mTOR pathway, in Leydig cell tumor [193]; to induce, through a Receptor for Activated C Kinase 1 (RACK1)/PKC-dependent mechanism, an increased production of pro-inflammatory cytokines TNF-α and IL-8 in THP-1 cells, thus predisposing the cell to an increased response to pro-inflammatory stimuli [194,195,196]. These effects could be particularly relevant in shaping pivotal TME characteristics, since the misregulated and unhealthy Pro-tumor Inflammation (PTI), directly correlated with TNF-α and monocytes/macrophages action, has been shown to facilitate the oncogenic process by promoting tumor growth, survival and metastasis and to suppress the antitumoral immune response, leading to TME alterations [197]. In addition, 3-methylcholanthrene has been reported to activate EGFR/ERK/c-Fos transduction signaling through an Aryl hydrocarbon Receptor (AhR) and GPER-mediated mechanism in both breast cancer cells and Cancer-Associated Fibroblasts (CAFs). This activation led to the upregulation of cyclin D1 and CYP1B1 (an enzyme required for estrogen metabolism and oncogenic activation of environmental pollutants), which resulted in an increased tumor growth [198], further outlining GPER potential role in shaping the TME in a pro-tumorigenic manner.
On the other hand, literature data on mPR- and MAPR-related endocrine disruption mechanisms are still limited and uncertain. In this regard, in vitro analyses in MDA-MB-231 breast cancer cells showed that DES and its analogues can bind and activate mPRα, thus mimicking non-genomic progestins action [199]. In addition, the activation of PGRMC1 triggered by ethynylestradiol (EE) and other estrogen-like compounds has been shown to induce and enhance breast cancer cell proliferation both in vitro and in xenograft [200,201], in line with the previous observation that PGRMC1 can promote breast cancer cell proliferation through an E2-induced mechanism [202].
3.2. mSRs as Drug Targets
The ability to bind hormones and hormone-like substances and to activate non-genomic pathways with a pivotal role in cancer progression makes mSRs interesting and potential new drug targets for the development of structure-based compounds tailored to activate or antagonize one or more mSR, depending on the receptor, the activated pathways and the hormone-sensitive cancer considered. In addition, the expression of the same mSR in different but strictly connected tissues in the TME also offers the possibility to intervene not only on the tumorigenic process itself but also in cancer-sustaining mechanisms. Synthetic and natural compounds able to bind mSRs here presented—apart from TRPM8 and GPER, whose molecular modulators were recently and excellently reviewed [203,204]—are listed in Table 1.
Table 1.
mSR-targeting compounds in pre-clinical studies and their correlated effects.
| Compound | Receptor | Profile | Cell Line/Model | Pathway | Effect | Ref. |
|---|---|---|---|---|---|---|
| (−)-Epicatechin | ZIP9 | agonist | PC3 (PC) MDA-MB-468 (BC) |
ERK1/2, JNK and Bax | Proapoptotic action (increased Caspase-3 levels), increased cAMP and intracellular Zn2+ levels | [205] |
| (+)-Catechin | ZIP9 | antagonist | PC3 MDA-MB-468 |
ERK 1/2, JNK and Bax | [205] | |
| Bicalutamide | ZIP9 | antagonist | 93RS2 (non -cancerous testicular cell line) | ERK1/2, CREB and ATF-1 | Reduced claudin-5 and zonula occludens-1 (ZO-1) expression | [206] |
| Nandrolone | OXER1 | antagonist | MCF-7, MDA-MB-231 (BC) | PI3K/Akt/NF-κB and RACK1 | Reduced proliferation and migration | [35] |
| 5-oxo-EPE | OXER1 | agonist | In vitro assay | Increased β-Arrestin recruitment | [207] | |
| S-230 | OXER1 | antagonist | In vivo (monkeys), human neutrophils | Gβγ-mediated signaling | Reduced Gβγ-mediated Ca2+ mobilization | [208,209] |
| S-Y048 | OXER1 | antagonist | In vivo (monkeys), human neutrophils and human eosinophils | Gβγ-mediated signaling | Reduced Gβγ-mediated Ca2+ mobilization, actin polymerization and eosinophil infiltration | [210] |
| S-C025 | OXER1 | antagonist | In vivo (monkeys), human neutrophils | Gβγ-mediated signaling | Reduced Gβγ-mediated Ca2+ mobilization and eosinophil activation | [211] |
| 264 | OXER1 | antagonist | In vivo (monkeys, rats), monkey eosinophils and monkey neutrophils | Gβγ-mediated signaling | Reduced Gβγ-mediated Ca2+ mobilization, actin polymerization and chemotaxis in granulocytes | [212] |
| DJ-V-159 | GPRC6A | agonist | HEK-293 (human embryonic kidney), MIN-6 (mouse pancreatic β-cell) and in vivo (mice) | Gαs-dependent signaling, ERK1/2 | Increased cAMP levels, insulin secretion and decreased serum glucose (in vivo, mouse) | [213] |
| Diltiazem | CaV1.2 | antagonist | In vivo (mice) | CaV1.2-PKC | Inhibition of Ca2+ influx, PLCδ1 | [214] |
| Lercanidipine | CaV1.2 | antagonist | Healthy and pediatric acute myeloid leukemia (AML) mesenchymal stromal cells (MSCs) | Inhibition of Ca2+ influx | [215] | |
| Ketamine | CaV1.2 | antagonist | In vivo (mice), Xenopus laevis oocytes (ex vivo) | Inhibition of CaV1.2 expression; Ca2+ influx; and vascular smooth muscle contraction | [216] | |
| Ritanserin | CaV1.2 | antagonist | Rat vascular myocytes (ex vivo) | Inhibition of Ca2+ influx; in vitro vasodilation; and vascular smooth muscle relaxation | [217] | |
| (R)-Roscovitine | CaV1.2 | antagonist | HEK-293 | Slows activation and enhances inactivation | [218] | |
| Metergoline | NaV1.2 | antagonist | Xenopus laevis oocytes (ex vivo) | Inhibition of Na+ influx | [219] | |
| Ranolazine | NaV1.2 | antagonist | CHO | Inhibition of Na+ influx | [220] | |
| 2,4(5)-diarylimidazoles | NaV1.2 | antagonist | In vitro assay | Inhibition of Na+ influx | [221] | |
| Org OD 02-0 | mPRα | agonist | A549, PC-9 (human lung adenocarcinoma), HBE (human bronchial epithelial) and MCF-7 | PKA/CREB and PKA/β-catenin | Inhibition of cell growth and tumor growth (in vivo) | [222] |
| Ganaxolone | mPRδ | agonist | GT1-7 (rat hypothalamic cells), H19-7 (rat hippocampal neuronal cells) | Gαs-dependent signaling | Reduction of apoptosis and cell death | [223] |
| AG-205 | PGRMC1 | antagonist | CHO-K1, HeLa, COS-7, and H4 glioma Cells | Increased endosome formation | [224] | |
| PaCa-2 cells (pancreatic cancer) | RACK1, alpha-Actinin-1 | Reduced PGRMC1 interactions with the actin cytoskeleton | [225] | |||
| Human granulosa/luteal cell | B-cell lymphoma 2 (BCL2) pathway | Increased PGRMC1 monomeric form, increased proapoptotic Harakiri (Hrk) expression | [226] |
Due to the relative novelty of mSRs as mediators of steroid signaling, studies on mSR-active compounds here presented were limited to the pre-clinical phase. Regarding ZIP9, both an antagonist and agonist compounds were analyzed for their ability to modulate ERK1/2, JNK and Bax pathways in both prostate and breast cancer cell lines with a final effect on apoptosis regulation. For OXER1, different antagonists were synthetized and screened both in vitro and in vivo, mostly for their ability to reduce inflammation-related pathways. In addition, several natural compounds were examined for their binding affinity for ZIP9, OXER1 and GPRC6A, although this analysis was limited to in silico data [227]. For mPRα and PGRMC1, an agonist and antagonist compounds have been reported, respectively. On the other hand, for CaV1.2, only antagonist compounds were described, even though cardiotoxicity or neurotoxocity were reported upon its blockade [228,229]. Finally, for GPRC6A, only an agonist has been reported, although an antagonist profile for its targeting could be more useful considering its inflammatory role regarding cytokine release [230]. Considering different and opposite tumor-dependent effects of the different mSRs, it will be important to develop targeted delivery systems to limit unwanted off-target effects.
4. Conclusions
Hormone-mediated rapid non-genomic effects, which cannot be attributed to the genomic action of nSRs, have raised attention on the potential involvement of mSRs in modulating specific molecular pathways pivotal for the physiologic hormone action. More recently, a possible contribution of mSRs to the development of hormone-linked diseases has been hypothesized, including hormone-related cancers. Because of their widespread expression in different human tissues, their role in promoting and driving not only the cancerous process itself but also other cancer-sustaining mechanisms in the surrounding TME Therefore, their ability to bind and exert the action of hormones and hormone-like substances (e.g., EDCs), also in a pathologic context, indicated, on one hand, their potential involvement in shaping the TME characteristic to support the tumor growth and, on the other, offered new insights into their potential as therapeutic targets. However, the limited knowledge on mSRs also as EDCs targets warrants further studies to dissect and elucidate their role in mediating non-physiologic mechanisms of endocrine disruption that can have an important impact on the development and progression of different hormone-linked diseases with a strong or hypothesized environmental component, including hormone-sensitive cancers. Moreover, the early-stage translational research on mSRs as novel drug targets requires additional studies for the development of new small molecules capable to specifically target these receptors and hinder or amplify their correlated action to counteract cancer development.
Acknowledgments
The authors would like to thank SMART—Servier Medical ART (https://smart.servier.com/, accessed on 14 September 2021) used for performing Figure 1, Figure 2, Figure 3 and Figure 4.
Author Contributions
Conceptualization, M.M., E.C. and E.B. writing—original draft, M.M. and E.B. writing—review and editing, M.M., M.R., C.T., E.C. and E.B. visualization, M.M., M.R., C.T., E.C. and E.B. critical discussion, M.M., M.R., C.T., E.C. and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
Research has been supported by Ministero dell’Istruzione, dell’Università e della Ricerca to Emanuela Corsini (PRIN2017, Project number 2017MLC3NF) and to Marco Racchi (PRIN2017, Project number 2017B9NCSX).
Conflicts of Interest
The authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., Bray F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 2.Henderson B.E., Feigelson H.S. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 3.Buoso E., Masi M., Racchi M., Corsini E. Endocrine-Disrupting Chemicals’ (EDCs) Effects on Tumour Microenvironment and Cancer Progression: Emerging Contribution of RACK1. Int. J. Mol. Sci. 2020;21:9229. doi: 10.3390/ijms21239229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kravchenko J., Corsini E., Williams M.A., Decker W., Manjili M.H., Otsuki T., Singh N., Al-Mulla F., Altemaimi R., Amedei A., et al. Chemical compounds from anthropogenic environment and immune evasion mechanisms: Potential interactions. Carcinogenesis. 2015;36:S111–S127. doi: 10.1093/carcin/bgv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whirledge S., Cidlowski J.A. Chapter 5-Steroid Hormone Action. In: Strauss J., Barbieri R., editors. Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 8th ed. Elsevier; Amsterdam, The Netherlands: 2018. pp. 115–131. [Google Scholar]
- 6.Levin E.R. Translating extranuclear steroid receptor signaling to clinical medicine. Horm. Cancer. 2014;5:140–145. doi: 10.1007/s12672-014-0179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammes S.R., Levin E.R. Minireview: Recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152:4489–4495. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedram A., Razandi M., Sainson R.C., Kim J.K., Hughes C.C., Levin E.R. A Conserved Mechanism for Steroid Receptor Translocation to the Plasma Membrane. J. Biol. Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 9.Thomas P. Membrane Androgen Receptors Unrelated to Nuclear Steroid Receptors. Endocrinology. 2019;160:772–781. doi: 10.1210/en.2018-00987. [DOI] [PubMed] [Google Scholar]
- 10.Treviño L.S., Gorelick D.A. The Interface of Nuclear and Membrane Steroid Signaling. Endocrinology. 2021;162:bqab107. doi: 10.1210/endocr/bqab107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichten L.A., Cousins R.J. Mammalian Zinc Transporters: Nutritional and Physiologic Regulation. Annu. Rev. Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 12.Thomas P., Converse A., Berg H. ZIP9, a novel membrane androgen receptor and zinc transporter protein. Gen. Comp. Endocrinol. 2018;257:130–136. doi: 10.1016/j.ygcen.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Thomas P., Pang Y., Dong J. Membrane androgen receptor characteristics of human ZIP9 (SLC39A) zinc transporter in prostate cancer cells: Androgen-specific activation and involvement of an inhibitory G protein in zinc and MAP kinase signaling. Mol. Cell. Endocrinol. 2017;447:23–34. doi: 10.1016/j.mce.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi M., Fukunaka A., Hagihara M., Watanabe K., Kamino S., Kambe T., Enomoto S., Hiromura M. Essential Role of the Zinc Transporter ZIP9/SLC39A9 in Regulating the Activations of Akt and Erk in B-Cell Receptor Signaling Pathway in DT40 Cells. PLoS ONE. 2013;8:e58022. doi: 10.1371/journal.pone.0058022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas P., Pang Y., Dong J., Berg H. Identification and Characterization of Membrane Androgen Receptors in the ZIP9 Zinc Transporter Subfamily: II. Role of Human ZIP9 in Testosterone-Induced Prostate and Breast Cancer Cell Apoptosis. Endocrinology. 2014;155:4250–4265. doi: 10.1210/en.2014-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pascal L., Wang Z. Unzipping Androgen Action Through ZIP9: A Novel Membrane Androgen Receptor. Endocrinology. 2014;155:4120–4123. doi: 10.1210/en.2014-1749. [DOI] [PubMed] [Google Scholar]
- 17.Bulldan A., Bartsch J.W., Konrad L., Scheiner-Bobis G. ZIP9 but not the androgen receptor mediates testosterone-induced migratory activity of metastatic prostate cancer cells. Biochim. Biophys. Acta Bioenerg. 2018;1865:1857–1868. doi: 10.1016/j.bbamcr.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Kalyvianaki K., Panagiotopoulos A.A., Malamos P., Moustou E., Tzardi M., Stathopoulos E.N., Ioannidis G.S., Marias K., Notas G., Theodoropoulos P.A., et al. Membrane androgen receptors (OXER1, GPRC6A AND ZIP9) in prostate and breast cancer: A comparative study of their expression. Steroids. 2019;142:100–108. doi: 10.1016/j.steroids.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Liu L., Yang J., Wang C. Analysis of the prognostic significance of solute carrier (SLC) family 39 genes in breast cancer. Biosci. Rep. 2020;40:BSR20200764. doi: 10.1042/BSR20200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J., Kim S., Ko S., In Y.-H., Moon H.-G., Ahn S.K., Kim M.K., Lee M., Hwang J.-H., Ju Y.S., et al. Recurrent fusion transcripts detected by whole-transcriptome sequencing of 120 primary breast cancer samples. Genes Chromosom. Cancer. 2015;54:681–691. doi: 10.1002/gcc.22279. [DOI] [PubMed] [Google Scholar]
- 21.Hosoi T., Koguchi Y., Sugikawa E., Chikada A., Ogawa K., Tsuda N., Suto N., Tsunoda S., Taniguchi T., Ohnuki T. Identification of a Novel Human Eicosanoid Receptor Coupled to Gi/o. J. Biol. Chem. 2002;277:31459–31465. doi: 10.1074/jbc.M203194200. [DOI] [PubMed] [Google Scholar]
- 22.Grant G.E., Rokach J., Powell W.S. 5-Oxo-ETE and the OXE receptor. Prostaglandins Lipid Mediat. 2009;89:98–104. doi: 10.1016/j.prostaglandins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarveswaran S., Ghosh J. OXER1, a G protein-coupled oxoeicosatetraenoid receptor, mediates the survival-promoting effects of arachidonate 5-lipoxygenase in prostate cancer cells. Cancer Lett. 2013;336:185–195. doi: 10.1016/j.canlet.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konya V., Blättermann S., Jandl K., Platzer W., Ottersbach P.A., Marsche G., Gütschow M., Kostenis E., Heinemann A. A Biased Non-Gαi OXE-R Antagonist Demonstrates That Gαi Protein Subunit Is Not Directly Involved in Neutrophil, Eosinophil, and Monocyte Activation by 5-Oxo-ETE. J. Immunol. 2014;192:4774–4782. doi: 10.4049/jimmunol.1302013. [DOI] [PubMed] [Google Scholar]
- 25.Hosoi T., Sugikawa E., Chikada A., Koguchi Y., Ohnuki T. TG1019/OXE, a Gαi/o-protein-coupled receptor, mediates 5-oxo-eicosatetraenoic acid-induced chemotaxis. Biochem. Biophys. Res. Commun. 2005;334:987–995. doi: 10.1016/j.bbrc.2005.06.191. [DOI] [PubMed] [Google Scholar]
- 26.Sarveswaran S., Thamilselvan V., Brodie C., Ghosh J. Inhibition of 5-lipoxygenase triggers apoptosis in prostate cancer cells via down-regulation of protein kinase C-epsilon. Biochim. Biophys. Acta Bioenerg. 2011;1813:2108–2117. doi: 10.1016/j.bbamcr.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langlois A., Chouinard F., Flamand N., Ferland C., Rola-Pleszczynski M., Laviolette M. Crucial implication of protein kinase C (PKC)-, PKC-, ERK-1/2, and p38 MAPK in migration of human asthmatic eosinophils. J. Leukoc. Biol. 2009;85:656–663. doi: 10.1189/jlb.0808492. [DOI] [PubMed] [Google Scholar]
- 28.O’Flaherty J.T., Rogers L.C., Paumi C.M., Hantgan R.R., Thomas L.R., Clay C.E., High K., Chen Y.Q., Willingham M.C., Smitherman P.K., et al. 5-Oxo-ETE analogs and the proliferation of cancer cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2005;1736:228–236. doi: 10.1016/j.bbalip.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Sundaram S., Ghosh J. Expression of 5-oxoETE receptor in prostate cancer cells: Critical role in survival. Biochem. Biophys. Res. Commun. 2006;339:93–98. doi: 10.1016/j.bbrc.2005.10.189. [DOI] [PubMed] [Google Scholar]
- 30.Grant G.E., Rubino S., Gravel S., Wang X., Patel P., Rokach J., Powell W.S. Enhanced formation of 5-oxo-6,8,11,14-eicosatetraenoic acid by cancer cells in response to oxidative stress, docosahexaenoic acid and neutrophil-derived 5-hydroxy-6,8,11,14-eicosatetraenoic acid. Carcinogenesis. 2011;32:822–828. doi: 10.1093/carcin/bgr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalyvianaki K., Gebhart V., Peroulis N., Panagiotopoulou C., Kiagiadaki F., Pediaditakis I., Aivaliotis M., Moustou E., Tzardi M., Notas G., et al. Antagonizing effects of membrane-acting androgens on the eicosanoid receptor OXER1 in prostate cancer. Sci. Rep. 2017;7:srep44418. doi: 10.1038/srep44418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kampa M., Notas G., Castanas E. Natural extranuclear androgen receptor ligands as endocrine disruptors of cancer cell growth. Mol. Cell. Endocrinol. 2017;457:43–48. doi: 10.1016/j.mce.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Notas G., Pelekanou V., Castanas E., Kampa M. Conjugated and non-conjugated androgens differentially modulate specific early gene transcription in breast cancer in a cell-specific manner. Steroids. 2010;75:611–618. doi: 10.1016/j.steroids.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Kampa M., Nifli A.-P., Charalampopoulos I., Alexaki V.-I., Theodoropoulos P.A., Stathopoulos E.N., Gravanis A., Castanas E. Opposing effects of estradiol- and testosterone-membrane binding sites on T47D breast cancer cell apoptosis. Exp. Cell Res. 2005;307:41–51. doi: 10.1016/j.yexcr.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 35.Masi M., Garattini E., Bolis M., Di Marino D., Maraccani L., Morelli E., Grolla A.A., Fagiani F., Corsini E., Travelli C., et al. OXER1 and RACK1-associated pathway: A promising drug target for breast cancer progression. Onco-Genesis. 2020;9:1–15. doi: 10.1038/s41389-020-00291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh A.K., Singh R., Naz F., Chauhan S.S., Dinda A., Shukla A.A., Gill K., Kapoor V., Dey S. Structure Based Design and Synthesis of Peptide Inhibitor of Human LOX-12: In Vitro and In Vivo Analysis of a Novel Therapeutic Agent for Breast Cancer. PLoS ONE. 2012;7:e32521. doi: 10.1371/journal.pone.0032521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar R., Singh A.K., Kumar M., Shekhar S., Rai N., Kaur P., Parshad R., Dey S. Serum 5-LOX: A progressive protein marker for breast cancer and new approach for therapeutic target. Carcinogenesis. 2016;37:912–917. doi: 10.1093/carcin/bgw075. [DOI] [PubMed] [Google Scholar]
- 38.R2: Genomics Analysis and Visualization Platform. [(accessed on 12 July 2021)]. Available online: http://r2.amc.nl.
- 39.Yi S., Zhou W. Tumorigenesis-related key genes in adolescents and young adults with HR(+)/HER2(−) breast cancer. Int. J. Clin. Exp. Pathol. 2020;13:2701–2709. [PMC free article] [PubMed] [Google Scholar]
- 40.Pi M., Nishimoto S.K., Quarles L.D. GPRC6A: Jack of all metabolism (or master of none) Mol. Metab. 2017;6:185–193. doi: 10.1016/j.molmet.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye R., Pi M., Cox J.V., Nishimoto S.K., Quarles L.D. CRISPR/Cas9 targeting of GPRC6A suppresses prostate cancer tumorigenesis in a human xenograft model. J. Exp. Clin. Cancer Res. 2017;36:1–13. doi: 10.1186/s13046-017-0561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye R., Pi M., Nooh M.M., Bahout S.W., Quarles L.D. Human GPRC6A Mediates Testosterone-Induced Mitogen-Activated Protein Kinases and mTORC1 Signaling in Prostate Cancer Cells. Mol. Pharmacol. 2019;95:563–572. doi: 10.1124/mol.118.115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takata R., Akamatsu S., Kubo M., Takahashi A., Hosono N., Kawaguchi T., Tsunoda T., Inazawa J., Kamatani N., Ogawa O., et al. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat. Genet. 2010;42:751–754. doi: 10.1038/ng.635. [DOI] [PubMed] [Google Scholar]
- 44.Long Q.-Z., Du Y.-F., Ding X.-Y., Li X., Song W.-B., Yang Y., Zhang P., Zhou J.-P., Liu X.-G. Replication and Fine Mapping for Association of the C2orf43, FOXP4, GPRC6A and RFX6 Genes with Prostate Cancer in the Chinese Population. PLoS ONE. 2012;7:e37866. doi: 10.1371/journal.pone.0037866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X.-H., Xu Y., Yang K., Shi J.-J., Zhang X., Yang F., Yuan H., Zhu X., Zhang Y.-H., Wang J.-Y., et al. Association of THADA, FOXP4, GPRC6A/RFX6 genes and 8q24 risk alleles with prostate cancer in Northern Chinese men. Off. J. Balk. Union Oncol. 2015;20:1223–1228. [PubMed] [Google Scholar]
- 46.Qi N., Chen Y., Zeng Y., Bao M., Long X., Guo Y., Tan A., Gao Y., Zhang H., Yang X., et al. rs2274911 polymorphism in GPRC6A associated with serum E2 and PSA in a Southern Chinese male population. Gene. 2020;763:145067. doi: 10.1016/j.gene.2020.145067. [DOI] [PubMed] [Google Scholar]
- 47.Liu M., Zhao Y.-Y., Yang F., Wang J.-Y., Shi X.-H., Zhu X.-Q., Xu Y., Wei D., Sun L., Zhang Y.-G., et al. Evidence for a role of GPRC6A in prostate cancer metastasis based on case-control and in vitro analyses. Eur. Rev. Med. Pharmacol. Sci. 2016;20:2235–2248. [PubMed] [Google Scholar]
- 48.Asuthkar S., Elustondo P.A., Demirkhanyan L., Sun X., Baskaran P., Velpula K.K., Thyagarajan B., Pavlov E.V., Zakharian E. The TRPM8 Protein Is a Testosterone Receptor. J. Biol. Chem. 2015;290:2659–2669. doi: 10.1074/jbc.M114.610824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asuthkar S., Demirkhanyan L., Sun X., Elustondo P.A., Krishnan V., Baskaran P., Velpula K.K., Thyagarajan B., Pavlov E.V., Zakharian E. The TRPM8 Protein Is a Testosterone Receptor. J. Biol. Chem. 2015;290:2670–2688. doi: 10.1074/jbc.M114.610873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Wang X., Yang Z., Zhu G., Chen D., Meng Z. Menthol Inhibits the Proliferation and Motility of Prostate Cancer DU145 Cells. Pathol. Oncol. Res. 2012;18:903–910. doi: 10.1007/s12253-012-9520-1. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y., Mikrani R., He Y., Baig M.M.F.A., Abbas M., Naveed M., Tang M., Zhang Q., Li C., Zhou X. TRPM8 channels: A review of distribution and clinical role. Eur. J. Pharmacol. 2020;882:173312. doi: 10.1016/j.ejphar.2020.173312. [DOI] [PubMed] [Google Scholar]
- 52.Cunha A., Weigle B., Kiessling A., Bachmann M., Rieber E.P. Tissue-specificity of prostate specific antigens: Comparative analysis of transcript levels in prostate and non-prostatic tissues. Cancer Lett. 2006;236:229–238. doi: 10.1016/j.canlet.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 53.Lunardi A., Barbareschi M., Carbone F.G., Morelli L., Brunelli M., Fortuna N., Genovesi S., Alaimo A. TRPM8 protein expression in hormone naïve local and lymph node metastatic prostate cancer. Pathologica. 2021;113:95–101. doi: 10.32074/1591-951X-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henshall S.M., Afar D.E., Hiller J., Horvath L.G., Quinn D.I., Rasiah K.K., Gish K., Willhite D., Kench J.G., Gardiner-Garden M., et al. Survival analysis of genome-wide gene expression pro-files of prostate cancers identifies new prognostic targets of disease relapse. Cancer Res. 2003;63:4196–4203. [PubMed] [Google Scholar]
- 55.Alaimo A., De Felice D., Genovesi S., Lorenzoni M., Lunardi A. Tune the channel: TRPM8 targeting in prostate cancer. Oncoscience. 2021;8:97–100. doi: 10.18632/oncoscience.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X.S., Zhang Y., Wu P.X., Liu S.J., Zhou J.Y., Liu S.X. Early diagnosis of prostate cancer by combined use of Trp-p8 expres-sion and PSA density of the transition zone. Zhonghua Nan Ke Xue. 2015;21:724–728. [PubMed] [Google Scholar]
- 57.Lunger L., Retz M., Bandur M., Souchay M., Vitzthum E., Jäger M., Weirich G., Schuster T., Autenrieth M., Kübler H., et al. KLK3 and TMPRSS2 for molecular lymph-node staging in prostate cancer patients undergoing radical prostatectomy. Prostate Cancer Prostatic Dis. 2021;24:362–369. doi: 10.1038/s41391-020-00283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bai V.U., Murthy S., Chinnakannu K., Muhletaler F., Tejwani S., Barrack E.R., Kim S.H., Menon M., Veer Reddy G.P. Androgen regulated TRPM8 expression: A potential mRNA marker for metastatic prostate cancer detection in body fluids. Int. J. Oncol. 2010;36:443–450. [PubMed] [Google Scholar]
- 59.Huskova Z., Knillova J., Kolar Z., Vrbkova J., Kral M., Bouchal J. The Percentage of Free PSA and Urinary Markers Distinguish Prostate Cancer from Benign Hyperplasia and Contribute to a More Accurate Indication for Prostate Biopsy. Biomedicines. 2020;8:173. doi: 10.3390/biomedicines8060173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhennin-Duthille I., Gautier M., Faouzi M., Guilbert A., Brevet M., Vaudry D., Ahidouch A., Sevestre H., Ouadid-Ahidouch H. High Expression of Transient Receptor Potential Channels in Human Breast Cancer Epithelial Cells and Tissues: Correlation with Pathological Parameters. Cell. Physiol. Biochem. 2011;28:813–822. doi: 10.1159/000335795. [DOI] [PubMed] [Google Scholar]
- 61.Liu J., Chen Y., Shuai S., Ding D., Li R., Luo R. TRPM8 promotes aggressiveness of breast cancer cells by regulating EMT via activating AKT/GSK-3β pathway. Tumor Biol. 2014;35:8969–8977. doi: 10.1007/s13277-014-2077-8. [DOI] [PubMed] [Google Scholar]
- 62.Huang Y., Li S., Jia Z., Zhao W., Zhou C., Zhang R., Ali D.W., Michalak M., Chen X.-Z., Tang J. Transient Receptor Potential Melastatin 8 (TRPM8) Channel Regulates Proliferation and Migration of Breast Cancer Cells by Activating the AMPK-ULK1 Pathway to Enhance Basal Autophagy. Front. Oncol. 2020;10:2645. doi: 10.3389/fonc.2020.573127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ouadid-Ahidouch H., Dhennin-Duthille I., Gautier M., Sevestre H., Ahidouch A. Canaux cationiques TRP dans le cancer du sein: Expression, rôle et corrélation avec des paramètres cliniques. Bull. Cancer. 2012;99:655–664. doi: 10.1684/bdc.2012.1595. [DOI] [PubMed] [Google Scholar]
- 64.Pratt S.J.P., Lee R.M., Chang K.T., Hernández-Ochoa E.O., Annis D.A., Ory E.C., Thompson K.N., Bailey P.C., Mathias T.J., Ju J.A., et al. Mechanoactivation of NOX2-generated ROS elicits persistent TRPM8 Ca2+ signals that are inhibited by oncogenic KRas. Proc. Natl. Acad. Sci. USA. 2020;117:26008–26019. doi: 10.1073/pnas.2009495117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zamponi G., Striessnig J., Koschak A., Dolphin A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy J.G., Sanderson J.L., Gorski J.A., Scott J.D., Catterall W.A., Sather W.A., Dell’Acqua M.L. AKAP-Anchored PKA Maintains Neuronal L-type Calcium Channel Activity and NFAT Transcriptional Signaling. Cell Rep. 2014;7:1577–1588. doi: 10.1016/j.celrep.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qin J., Nag S., Wang W., Zhou J., Zhang W.-D., Wang H., Zhang R. NFAT as cancer target: Mission possible? Biochim. Biophys. Acta Bioenerg. 2014;1846:297–311. doi: 10.1016/j.bbcan.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mancini M., Toker A. NFAT proteins: Emerging roles in cancer progression. Nat. Rev. Cancer. 2009;9:810–820. doi: 10.1038/nrc2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Déliot N., Constantin B. Plasma membrane calcium channels in cancer: Alterations and consequences for cell proliferation and migration. Biochim. Biophys. Acta Biomembr. 2015;1848:2512–2522. doi: 10.1016/j.bbamem.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 70.Marques R., Peres C.G., Vaz C.V., Gomes I.M., Figueira M.I., Cairrão E., Verde I., Maia C.J., Socorro S. 5α-Dihydrotestosterone regulates the expression of L-type calcium channels and calcium-binding protein regucalcin in human breast cancer cells with suppression of cell growth. Med. Oncol. 2015;32:228. doi: 10.1007/s12032-015-0676-x. [DOI] [PubMed] [Google Scholar]
- 71.Scragg J.L., Dallas M.L., Peers C. Molecular requirements for L-type Ca2+ channel blockade by testosterone. Cell Calcium. 2007;42:11–15. doi: 10.1016/j.ceca.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 72.Soltysik K., Czekaj P. Membrane estrogen receptors—Is it an alternative way of estrogen action? J. Physiol. Pharmacol. 2013;64:129–142. [PubMed] [Google Scholar]
- 73.Taheri M., Shoorei H., Dinger M.E., Ghafouri-Fard S. Perspectives on the Role of Non-Coding RNAs in the Regulation of Expression and Function of the Estrogen Receptor. Cancers. 2020;12:2162. doi: 10.3390/cancers12082162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kampa M., Notas G., Pelekanou V., Troullinaki M., Andrianaki M., Azariadis K., Kampouri E., Lavrentaki K., Castanas E. Early membrane initiated transcriptional effects of estrogens in breast cancer cells: First pharmacological evidence for a novel membrane estrogen receptor element (ERx) Steroids. 2012;77:959–967. doi: 10.1016/j.steroids.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 75.Elappano R., Episano A., Maggiolini M. GPER Function in Breast Cancer: An Overview. Front. Endocrinol. 2014;5:66. doi: 10.3389/fendo.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marjon N.A., Hu C., Hathaway H.J., Prossnitz E.R. G Protein–Coupled Estrogen Receptor Regulates Mammary Tumorigenesis and Metastasis. Mol. Cancer Res. 2014;12:1644–1654. doi: 10.1158/1541-7786.MCR-14-0128-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prossnitz E.R., Arterburn J.B. International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators. Pharmacol. Rev. 2015;67:505–540. doi: 10.1124/pr.114.009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prossnitz E.R., Arterburn J.B., Smith H.O., Oprea T.I., Sklar L.A., Hathaway H.J. Estrogen Signaling through the Transmembrane G Protein-Coupled Receptor GPR30. Annu. Rev. Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 79.Thomas P., Pang Y., Filardo E., Dong J. Identity of an Estrogen Membrane Receptor Coupled to a G Protein in Human Breast Cancer Cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 80.Revankar C.M., Cimino D.F., Sklar L.A., Arterburn J.B., Prossnitz E.R. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 81.Hugo E.R., Brandebourg T.D., Woo J.G., Loftus J., Alexander J.W., Ben-Jonathan N. Bisphenol A at Environmentally Relevant Doses Inhibits Adiponectin Release from Human Adipose Tissue Explants and Adipocytes. Environ. Health Perspect. 2008;116:1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi H., Kumar S.P.D.S., Liu X. G Protein-Coupled Estrogen Receptor in Energy Homeostasis and Obesity Pathogenesis. Prog. Mol. Biol. Transl. Sci. 2013;114:193–250. doi: 10.1016/b978-0-12-386933-3.00006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo J., Liu D. Does GPER Really Function as a G Protein-Coupled Estrogen Receptor in vivo? Front. Endocrinol. 2020;11:148. doi: 10.3389/fendo.2020.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prossnitz E.R., Hathaway H.J. What have we learned about GPER function in physiology and disease from knockout mice? J. Steroid Biochem. Mol. Biol. 2015;153:114–126. doi: 10.1016/j.jsbmb.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jung J. Role of G Protein-Coupled Estrogen Receptor in Cancer Progression. Toxicol. Res. 2019;35:209–214. doi: 10.5487/TR.2019.35.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Talia M., De Francesco E.M., Rigiracciolo D.C., Muoio M.G., Muglia L., Belfiore A., Maggiolini M., Sims A.H., Lappano R. The G Protein-Coupled Estrogen Receptor (GPER) Expression Correlates with Pro-Metastatic Pathways in ER-Negative Breast Cancer: A Bioinformatics Analysis. Cells. 2020;9:622. doi: 10.3390/cells9030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou X., Wang S., Wang Z., Feng X., Liu P., Lv X.-B., Kun-Liang G., Yu F.-X., Sun Y., Yuan H., et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J. Clin. Investig. 2015;125:2123–2135. doi: 10.1172/JCI79573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Z., Sun L., Liang S., Liu Z.-C., Zhao Z.-Y., Yang J., Wang D., Yang D.-Q. GPER stabilizes F-actin cytoskeleton and activates TAZ via PLCβ-PKC and Rho/ROCK-LIMK-Cofilin pathway. Biochem. Biophys. Res. Commun. 2019;516:976–982. doi: 10.1016/j.bbrc.2019.06.132. [DOI] [PubMed] [Google Scholar]
- 89.Yang H., Wang C., Liao H., Wang Q. Activation of GPER by E2 promotes proliferation, invasion and migration of breast cancer cells by regulating the miR-124/CD151 pathway. Oncol. Lett. 2021;21:1–9. doi: 10.3892/ol.2021.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tao S., He H., Chen Q., Yue W. GPER mediated estradiol reduces miR-148a to promote HLA-G expression in breast cancer. Biochem. Biophys. Res. Commun. 2014;451:74–78. doi: 10.1016/j.bbrc.2014.07.073. [DOI] [PubMed] [Google Scholar]
- 91.Tao S., He H., Chen Q. Estradiol induces HOTAIR levels via GPER-mediated miR-148a inhibition in breast cancer. J. Transl. Med. 2015;13:1–8. doi: 10.1186/s12967-015-0489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Magruder H.T., Quinn J.A., Schwartzbauer J.E., Reichner J., Huang A., Filardo E.J. The G Protein-Coupled Estrogen Receptor-1, GPER-1, Promotes Fibrillogenesis via a Shc-Dependent Pathway Resulting in Anchorage-Independent Growth. Horm. Cancer. 2014;5:390–404. doi: 10.1007/s12672-014-0195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Santolla M.F., Avino S., Pellegrino M.A., De Francesco E.M., De Marco P., Lappano R., Vivacqua A., Cirillo F., Rigiracciolo D.C., Scarpelli A., et al. SIRT1 is involved in oncogenic signaling mediated by GPER in breast cancer. Cell Death Dis. 2015;6:e1834. doi: 10.1038/cddis.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yin H., Zhu Q., Liu M., Tu G., Li Q., Yuan J., Wen S., Yang G. GPER promotes tamoxifen-resistance in ER+ breast cancer cells by reduced Bim proteins through MAPK/Erk-TRIM2 signaling axis. Int. J. Oncol. 2017;51:1191–1198. doi: 10.3892/ijo.2017.4117. [DOI] [PubMed] [Google Scholar]
- 95.Zekas E., Prossnitz E.R. Estrogen-mediated inactivation of FOXO3a by the G protein-coupled estrogen receptor GPER. BMC Cancer. 2015;15:1–12. doi: 10.1186/s12885-015-1699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chan Y., Lai A.C., Lin R., Wang Y., Wang Y., Chang W.-W., Wu H., Lin Y., Wu J., Yu J., et al. GPER-induced signaling is essential for the survival of breast cancer stem cells. Int. J. Cancer. 2020;146:1674–1685. doi: 10.1002/ijc.32588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu T., Cheng H., Ding Z., Wang Z., Zhou L., Zhao P., Tan S., Xu X., Huang X., Liu M., et al. GPER mediates decreased chemosensitivity via regulation of ABCG2 expression and localization in tamoxifen-resistant breast cancer cells. Mol. Cell. Endocrinol. 2020;506:110762. doi: 10.1016/j.mce.2020.110762. [DOI] [PubMed] [Google Scholar]
- 98.Ignatov T., Treeck O., Kalinski T., Ortmann O., Ignatov A. GPER-1 expression is associated with a decreased response rate to primary tamoxifen therapy of breast cancer patients. Arch. Gynecol. Obstet. 2020;301:565–571. doi: 10.1007/s00404-019-05384-6. [DOI] [PubMed] [Google Scholar]
- 99.Molina L., Bustamante F., Ortloff A., Ramos I., Ehrenfeld P., Figueroa C.D. Continuous Exposure of Breast Cancer Cells to Tamoxifen Upregulates GPER-1 and Increases Cell Proliferation. Front. Endocrinol. 2020;11:563165. doi: 10.3389/fendo.2020.563165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weißenborn C., Ignatov T., Poehlmann A., Wege A.K., Costa S.D., Zenclussen A., Ignatov A. GPER functions as a tumor suppressor in MCF-7 and SK-BR-3 breast cancer cells. J. Cancer Res. Clin. Oncol. 2014;140:663–671. doi: 10.1007/s00432-014-1598-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martin S., Lebot M.N., Sukkarn B., Ball G., Green A., Rakha E.A., Ellis I., Storr S.J. Low expression of G protein-coupled oestrogen receptor 1 (GPER) is associated with adverse survival of breast cancer patients. Oncotarget. 2018;9:25946–25956. doi: 10.18632/oncotarget.25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tutzauer J., Sjöström M., Bendahl P.-O., Rydén L., Fernö M., Leeb-Lundberg L.M.F., Alkner S. Plasma membrane expression of G protein-coupled estrogen receptor (GPER)/G protein-coupled receptor 30 (GPR30) is associated with worse outcome in metachronous contralateral breast cancer. PLoS ONE. 2020;15:e0231786. doi: 10.1371/journal.pone.0231786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weissenborn C., Ignatov T., Nass N., Kalinski T., Costa S.D., Zenclussen A.C., Ignatov A. GPER Promoter Methylation Controls GPER Expression in Breast Cancer Patients. Cancer Investig. 2017;35:1–8. doi: 10.1080/07357907.2016.1271886. [DOI] [PubMed] [Google Scholar]
- 104.Hernández-Silva C.D., Villegas-Pineda J.C., Pereira-Suárez A.L. Expression and Role of the G Protein-Coupled Estrogen Receptor (GPR30/GPER) in the Development and Immune Response in Female Reproductive Cancers. Front. Endocrinol. 2020;11:544. doi: 10.3389/fendo.2020.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ignatov T., Modl S., Thulig M., Weißenborn C., Treeck O., Ortmann O., Zenclussen A., Costa S.D., Kalinski T., Ignatov A. GPER-1 acts as a tumor suppressor in ovarian cancer. J. Ovarian Res. 2013;6:51. doi: 10.1186/1757-2215-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fraungruber P., Kaltofen T., Heublein S., Kuhn C., Mayr D., Burges A., Mahner S., Rathert P., Jeschke U., Trillsch F. G Protein-Coupled Estrogen Receptor Correlates with Dkk2 Expression and Has Prognostic Impact in Ovarian Cancer Patients. Front. Endocrinol. 2021;12:18. doi: 10.3389/fendo.2021.564002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heublein S., Mayr D., Vrekoussis T., Friese K., Hofmann S.S., Jeschke U., Lenhard M. The G-Protein Coupled Estrogen Receptor (GPER/GPR30) is a Gonadotropin Receptor Dependent Positive Prognosticator in Ovarian Carcinoma Patients. PLoS ONE. 2013;8:e71791. doi: 10.1371/journal.pone.0071791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang C., Lv X., Jiang C., Davis J.S. The putative G-protein coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian and breast cancer cells in a GPER-independent manner. Am. J. Transl. Res. 2012;4:390–402. [PMC free article] [PubMed] [Google Scholar]
- 109.Wang C., Lv X., He C., Hua G., Tsai M.-Y., Davis J.S. The G-protein-coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian cancer cells by blocking tubulin polymerization. Cell Death Dis. 2013;4:e869. doi: 10.1038/cddis.2013.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schüler-Toprak S., Skrzypczak M., Ignatov T., Ignatov A., Ortmann O., Treeck O. G protein-coupled estrogen receptor 1 (GPER-1) and agonist G-1 inhibit growth of ovarian cancer cells by activation of anti-tumoral transcriptome responses: Impact of GPER-1 mRNA on survival. J. Cancer Res. Clin. Oncol. 2020;146:3175–3188. doi: 10.1007/s00432-020-03333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Han N., Heublein S., Jeschke U., Kuhn C., Hester A., Czogalla B., Mahner S., Rottmann M., Mayr D., Schmoeckel E., et al. The G-Protein-Coupled Estrogen Receptor (GPER) Regulates Trimethylation of Histone H3 at Lysine 4 and Represses Migration and Proliferation of Ovarian Cancer Cells In Vitro. Cells. 2021;10:619. doi: 10.3390/cells10030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yan Y., Liu H., Wen H., Jiang X., Cao X., Zhang G., Liu G. The novel estrogen receptor GPER regulates the migration and invasion of ovarian cancer cells. Mol. Cell. Biochem. 2013;378:1–7. doi: 10.1007/s11010-013-1579-9. [DOI] [PubMed] [Google Scholar]
- 113.Liu H., Yan Y., Wen H., Jiang X., Cao X., Zhang G., Liu G. A novel estrogen receptor GPER mediates proliferation induced by 17β-estradiol and selective GPER agonist G-1 in estrogen receptor α (ERα)-negative ovarian cancer cells. Cell Biol. Int. 2014;38:631–638. doi: 10.1002/cbin.10243. [DOI] [PubMed] [Google Scholar]
- 114.Yan Y., Jiang X., Zhao Y., Wen H., Liu G. Role of GPER on proliferation, migration and invasion in ligand-independent manner in human ovarian cancer cell line SKOV3. Cell Biochem. Funct. 2015;33:552–559. doi: 10.1002/cbf.3154. [DOI] [PubMed] [Google Scholar]
- 115.Lin B.C., Suzawa M., Blind R., Tobias S.C., Bulun S.E., Scanlan T.S., Ingraham H.A. Stimulating the GPR30 Estrogen Receptor with a Novel Tamoxifen Analogue Activates SF-1 and Promotes Endometrial Cell Proliferation. Cancer Res. 2009;69:5415–5423. doi: 10.1158/0008-5472.CAN-08-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vivacqua A., Romeo E., De Marco P., De Francesco E.M., Abonante S., Maggiolini M. GPER mediates the Egr-1 expression induced by 17β-estradiol and 4-hydroxitamoxifen in breast and endometrial cancer cells. Breast Cancer Res. Treat. 2011;133:1025–1035. doi: 10.1007/s10549-011-1901-8. [DOI] [PubMed] [Google Scholar]
- 117.Filigheddu N., Sampietro S., Chianale F., Porporato P.E., Gaggianesi M., Gregnanin I., Rainero E., Ferrara M., Perego B., Riboni F., et al. Diacylglycerol kinase α mediates 17-β-estradiol-induced proliferation, motility, and anchorage-independent growth of Hec-1A endometrial cancer cell line through the G protein-coupled estrogen receptor GPR30. Cell. Signal. 2011;23:1988–1996. doi: 10.1016/j.cellsig.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 118.Deng J., Wang W., Yu G., Ma X. MicroRNA-195 inhibits epithelial-mesenchymal transition by targeting G protein-coupled estrogen receptor 1 in endometrial carcinoma. Mol. Med. Rep. 2019;20:4023–4032. doi: 10.3892/mmr.2019.10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang H., Wang X., Chen Z., Wang W. MicroRNA-424 suppresses estradiol-induced cell proliferation via targeting GPER in endometrial cancer cells. Cell. Mol. Boil. 2015;61:96–101. [PubMed] [Google Scholar]
- 120.Lau K.-M., Ma F.M.-T., Xia J.T., Chan Q.K.Y., Ng C.-F., To K.-F. Activation of GPR30 stimulates GTP-binding of Gαi1 protein to sustain activation of Erk1/2 in inhibition of prostate cancer cell growth and modulates metastatic properties. Exp. Cell Res. 2017;350:199–209. doi: 10.1016/j.yexcr.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 121.Chimento A., De Luca A., Nocito M.C., Avena P., La Padula D., Zavaglia L., Pezzi V. Role of GPER-Mediated Signaling in Testicular Functions and Tumorigenesis. Cells. 2020;9:2115. doi: 10.3390/cells9092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chevalier N., Hinault C., Clavel S., Paul-Bellon R., Fenichel P. GPER and Testicular Germ Cell Cancer. Front. Endocrinol. 2021;11:1084. doi: 10.3389/fendo.2020.600404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kotula-Balak M., Gorowska-Wojtowicz E., Milon A., Pawlicki P., Tworzydlo W., Płachno B.J., Krakowska I., Hejmej A., Wolski J.K., Bilinska B. Towards understanding leydigioma: Do G protein-coupled estrogen receptor and peroxisome proliferator–activated receptor regulate lipid metabolism and steroidogenesis in Leydig cell tumors? Protoplasma. 2020;257:1149–1163. doi: 10.1007/s00709-020-01488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Börjesson S.I., Elinder F. Structure, Function, and Modification of the Voltage Sensor in Voltage-Gated Ion Channels. Cell Biophys. 2008;52:149–174. doi: 10.1007/s12013-008-9032-5. [DOI] [PubMed] [Google Scholar]
- 125.Fiske J.L., Fomin V.P., Brown M.L., Duncan R.L., Sikes R.A. Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev. 2006;25:493–500. doi: 10.1007/s10555-006-9017-z. [DOI] [PubMed] [Google Scholar]
- 126.Jean-Yves L., Halima O.-A., Olivier S., Pierre B., Ahmed A., Christophe V. Voltage-Gated Ion Channels, New Targets in Anti-Cancer Research. Recent Pat. Anti-Cancer Drug Discov. 2007;2:189–202. doi: 10.2174/157489207782497244. [DOI] [PubMed] [Google Scholar]
- 127.Shan B., Dong M., Tang H., Wang N., Zhang J., Yan C., Jiao X., Zhang H., Wang C. Voltage-gated sodium channels were differentially expressed in human normal prostate, benign prostatic hyperplasia and prostate cancer cells. Oncol. Lett. 2014;8:345–350. doi: 10.3892/ol.2014.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Z., Gao R., Shen Y., Cai J., Lei M., Wang L.-Y. Expression of voltage-gated sodium channel α subunit in human ovarian cancer. Oncol. Rep. 2010;23:1293–1299. doi: 10.3892/or_00000763. [DOI] [PubMed] [Google Scholar]
- 129.Kow L.-M., Pfaff D.W. Rapid estrogen actions on ion channels: A survey in search for mechanisms. Steroids. 2016;111:46–53. doi: 10.1016/j.steroids.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sula A., Hollingworth D., Ng L.C., Larmore M., DeCaen P.G., Wallace B. A tamoxifen receptor within a voltage-gated sodium channel. Mol. Cell. 2021;81:1160–1169. doi: 10.1016/j.molcel.2020.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thomas P., Pang Y., Dong J., Groenen P., Kelder J., De Vlieg J., Zhu Y., Tubbs C. Steroid and G Protein Binding Characteristics of the Seatrout and Human Progestin Membrane Receptor α Subtypes and Their Evolutionary Origins. Endocrinology. 2007;148:705–718. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- 132.Ryu C.S., Klein K., Zanger U.M. Membrane Associated Progesterone Receptors: Promiscuous Proteins with Pleiotropic Functions—Focus on Interactions with Cytochromes P450. Front. Pharmacol. 2017;8:159. doi: 10.3389/fphar.2017.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhu Y., Bond J., Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. USA. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thomas P. characteristics of membrane progesterone alpha (mPR?) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front. Neuroendocrinol. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pang Y., Dong J., Thomas P. Characterization, Neurosteroid Binding and Brain Distribution of Human Membrane Progesterone Receptors δ and ϵ (mPRδ and mPRϵ) and mPRδ Involvement in Neurosteroid Inhibition of Apoptosis. Endocrinology. 2013;154:283–295. doi: 10.1210/en.2012-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dosiou C., Hamilton A.E., Pang Y., Overgaard M.T., Tulac S., Dong J., Thomas P., Guidice L.C. Expression of membrane proges-terone receptors (mPRs) on human T lymphocytes and Jurkat cells and activation of G proteins by progesterone. J. Endocrinol. 2008;196:67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- 137.Dressing G.E., Thomas P. Identification of membrane progestin receptors in human breast cancer cell lines and biopsies and their potential involvement in breast cancer. Steroids. 2007;72:111–116. doi: 10.1016/j.steroids.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 138.Pang Y.F., Thomas P. Progesterone signals through membrane progesterone receptors (mPRs) in MDA-MB-468 and mPR-transfected MDA-MB-231 breast cancer cells which lack full-length and C-terminal truncated isoforms of the nuclear progesterone receptor. Steroids. 2011;76:921–928. doi: 10.1016/j.steroids.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zuo L., Li W., You S. Progesterone reverses the mesenchymal phenotypes of basal phenotype breast cancer cells by a mem-brane progesterone receptor alpha mediated pathway. Breast Cancer Res. 2010;12:R34. doi: 10.1186/bcr2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Charles N.A., Thomas P., Lange C.A. Signaling events mediated by membrane progesterone receptors (mPRs) in ovarian cancer cells. Horm. Cancer. 2010;1:167–176. doi: 10.1007/s12672-010-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu X., Sun L., Wang X., Su P., Li Z., Zhang C., Wang Y., Gao P., Ma R. Breast Cancer Invasion and Metastasis by mPRα Through the PI3K/Akt Signaling Pathway. Pathol. Oncol. Res. 2015;22:471–476. doi: 10.1007/s12253-015-0023-8. [DOI] [PubMed] [Google Scholar]
- 142.Xie M., Zhu X., Liu Z., Shrubsole M., Varma V., Mayer I.A., Dai Q., Chen Q., You S. Membrane Progesterone Receptor Alpha as a Potential Prognostic Biomarker for Breast Cancer Survival: A Retrospective Study. PLoS ONE. 2012;7:e35198. doi: 10.1371/journal.pone.0035198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dressing G.E., Alyea R., Pang Y., Thomas P. Membrane Progesterone Receptors (mPRs) Mediate Progestin Induced Antimorbidity in Breast Cancer Cells and Are Expressed in Human Breast Tumors. Horm. Cancer. 2012;3:101–112. doi: 10.1007/s12672-012-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang J., Hu J., Li W., Zhang C., Su P., Wang Y., Sun W., Wang X., Li L., Wu X. Rapamycin Antagonizes BCRP-Mediated Drug Resistance Through the PI3K/Akt/mTOR Signaling Pathway in mPRα-Positive Breast Cancer. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.608570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou L., Zhou W., Zhang H., Hu Y., Yu L., Zhang Y., Zhang Y., Wang S., Wang P., Xia W. Progesterone suppresses triple-negative breast cancer growth and metastasis to the brain via membrane progesterone receptor α. Int. J. Mol. Med. 2017;40:755–761. doi: 10.3892/ijmm.2017.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xie M., Zhou L., Chen X., Gainey L.O., Xiao J., Nanes M.S., Hou A., You S., Chen Q. Progesterone and Src Family Inhibitor PP1 Synergistically Inhibit Cell Migration and Invasion of Human Basal Phenotype Breast Cancer Cells. BioMed Res. Int. 2015;2015:1–14. doi: 10.1155/2015/426429. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 147.Romero-Sánchez M., Peiper S.C., Evans B., Wang Z., Catasús L., Ribe A., Prat J., Giri J.G. Expression profile of heptahelical putative membrane progesterone receptors in epithelial ovarian tumors. Hum. Pathol. 2008;39:1026–1033. doi: 10.1016/j.humpath.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 148.Charles N.J., Thomas P., Lange C.A. Expression of Membrane Progesterone Receptors (mPR/PAQR) in Ovarian Cancer Cells: Implications for Progesterone-Induced Signaling Events. Horm. Cancer. 2010;1:167–176. doi: 10.1007/s12672-010-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sinreih M., Knific T., Thomas P., Grazio S.F., Rižner T.L. Membrane progesterone receptors β and γ have potential as prognostic biomarkers of endometrial cancer. J. Steroid Biochem. Mol. Biol. 2018;178:303–311. doi: 10.1016/j.jsbmb.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 150.Li B., Lin Z., Liang Q., Hu Y., Xu W.-F. PAQR6 expression enhancement suggests a worse prognosis in prostate cancer patients. Open Life Sci. 2018;13:511–517. doi: 10.1515/biol-2018-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kimura I., Nakayama Y., Konishi M., Terasawa K., Ohta M., Itoh N., Fujimoto M. Functions of MAPR (membrane-associated progesterone receptor) family members as heme/steroid-binding proteins. Curr. Protein Pept. Sci. 2012;13:687–696. doi: 10.2174/138920312804142110. [DOI] [PubMed] [Google Scholar]
- 152.Ruan X., Zhang Y., Mueck A.O., Willibald M., Seeger H., Fehm T., Brucker S., Neubauer H. Increased expression of progesterone receptor membrane component 1 is associated with aggressive phenotype and poor prognosis in ER-positive and negative breast cancer. Menopause. 2017;24:203–209. doi: 10.1097/GME.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 153.Lin S.-T., May E.W.S., Chang J.-F., Hu R.-Y., Wang L.H.-C., Chan H.-L. PGRMC1 contributes to doxorubicin-induced chemoresistance in MES-SA uterine sarcoma. Cell. Mol. Life Sci. 2015;72:2395–2409. doi: 10.1007/s00018-014-1831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim J.Y., Kim S.Y., Choi H.S., Kim M.K., Lee H.M., Jang Y.-J., Ryu C.J. Progesterone Receptor Membrane Component 1 suppresses the p53 and Wnt/β-catenin pathways to promote human pluripotent stem cell self-renewal. Sci. Rep. 2018;8:1–16. doi: 10.1038/s41598-018-21322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thejer B.M., Adhikary P.P., Kaur A., Teakel S.L., Van Oosterum A., Seth I., Pajic M., Hannan K.M., Pavy M., Poh P., et al. PGRMC1 phosphorylation affects cell shape, motility, glycolysis, mitochondrial form and function, and tumor growth. BMC Mol. Cell Biol. 2020;21:1–24. doi: 10.1186/s12860-020-00256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Neubauer H., Ma Q., Zhou J., Yu Q., Ruan X., Seeger H., Fehm T., Mueck A.O. Possible role of PGRMC1 in breast cancer development. Climacteric. 2013;16:509–513. doi: 10.3109/13697137.2013.800038. [DOI] [PubMed] [Google Scholar]
- 157.Ahmed I.S., Rohe H.J., Twist K.E., Mattingly M.N., Craven R.J. Progesterone Receptor Membrane Component 1 (Pgrmc1): A Heme-1 Domain Protein That Promotes Tumorigenesis and Is Inhibited by a Small Molecule. J. Pharmacol. Exp. Ther. 2010;333:564–573. doi: 10.1124/jpet.109.164210. [DOI] [PubMed] [Google Scholar]
- 158.Ji S., Wu A., Yang H. Expression of progesterone receptor membrane component-1 is associated with the malignant pheno-types of breast cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:635–638. [PubMed] [Google Scholar]
- 159.Cai G., Yang X., Ruan X., Wang J., Fang Y., Wei Y., Zhang Y., Gu M., Mueck A.O. Association of circulating Progesterone Receptor Membrane Component-1 (PGRMC1) with PGRMC1 expression in breast tumour tissue and with clinical breast tumour characteristics. Maturitas. 2020;140:64–71. doi: 10.1016/j.maturitas.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 160.Ruan X., Cai G., Wei Y., Gu M., Zhang Y., Zhao Y., Mueck A.O. Association of circulating Progesterone Receptor Membrane Component-1 (PGRMC1) with breast tumor characteristics and comparison with known tumor markers. Menopause. 2020;27:183–193. doi: 10.1097/GME.0000000000001436. [DOI] [PubMed] [Google Scholar]
- 161.Clark N.C., Friel A., Pru C.A., Zhang L., Shioda T., Rueda B.R., Peluso J.J., Pru J.K. Progesterone receptor membrane component 1 promotes survival of human breast cancer cells and the growth of xenograft tumors. Cancer Biol. Ther. 2016;17:262–271. doi: 10.1080/15384047.2016.1139240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Neubauer H., Adam G., Seeger H., Mueck A.O., Solomayer E., Wallwiener D., Cahill M., Fehm T. Membrane-initiated effects of progesterone on proliferation and activation of VEGF in breast cancer cells. Climacteric. 2009;12:230–239. doi: 10.1080/13697130802635637. [DOI] [PubMed] [Google Scholar]
- 163.Lee S.R., Lee Y.H., Jo S.L., Heo J.H., Kim G., Lee G.-S., An B.-S., Baek I.-J., Hong E.-J. Absence of progesterone receptor membrane component 1 reduces migration and metastasis of breast cancer. Cell Commun. Signal. 2021;19:1–11. doi: 10.1186/s12964-021-00719-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gu M., Wang L., Yang C., Li X., Jia C., Croteau S., Ruan X., Hardy P. Micro-RNA-181a suppresses progestin-promoted breast cancer cell growth. Maturitas. 2018;114:60–66. doi: 10.1016/j.maturitas.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 165.Cantonero C., Salido G.M., Rosado J.A., Redondo P.C. PGRMC1 Inhibits Progesterone-Evoked Proliferation and Ca2+ Entry Via STIM2 in MDA-MB-231 Cells. Int. J. Mol. Sci. 2020;21:7641. doi: 10.3390/ijms21207641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pedroza D.A., Rajamanickam V., Subramani R., Bencomo A., Galvez A., Lakshmanaswamy R. Progesterone receptor membrane component 1 promotes the growth of breast cancers by altering the phosphoproteome and augmenting EGFR/PI3K/AKT signalling. Br. J. Cancer. 2020;123:1326–1335. doi: 10.1038/s41416-020-0992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Asperger H., Stamm N., Gierke B., Pawlak M., Hofmann U., Zanger U.M., Marton A., Katona R.L., Buhala A., Vizler C., et al. Progesterone receptor membrane component 1 regulates lipid homeostasis and drives oncogenic signaling resulting in breast cancer progression. Breast Cancer Res. 2020;22:1–16. doi: 10.1186/s13058-020-01312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pedroza D.A., Subramani R., Tiula K., Do A., Rashiraj N., Galvez A., Chatterjee A., Bencomo A., Rivera S., Lakshmanaswamy R. Crosstalk between progesterone receptor membrane component 1 and estrogen receptor α promotes breast cancer cell proliferation. Lab. Investig. 2021;101:733–744. doi: 10.1038/s41374-021-00594-6. [DOI] [PubMed] [Google Scholar]
- 169.Ponikwicka-Tyszko D., Chrusciel M., Pulawska K., Bernaczyk P., Sztachelska M., Guo P., Li X., Toppari J., Huhtaniemi I.T., Wolczynski S., et al. Mifepristone Treatment Promotes Testicular Leydig Cell Tumor Progression in Transgenic Mice. Cancers. 2020;12:3263. doi: 10.3390/cancers12113263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Friel A., Zhang L., Pru C.A., Clark N.C., McCallum M.L., Blok L.J., Shioda T., Peluso J.J., Rueda B.R., Pru J.K. Progesterone receptor membrane component 1 deficiency attenuates growth while promoting chemosensitivity of human endometrial xenograft tumors. Cancer Lett. 2015;356:434–442. doi: 10.1016/j.canlet.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Peluso J.J. Progesterone signaling mediated through progesterone receptor membrane component-1 in ovarian cells with special emphasis on ovarian cancer. Steroids. 2011;76:903–909. doi: 10.1016/j.steroids.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhu X., Han Y., Fang Z., Wu W., Ji M., Teng F., Zhu W., Yang X., Jia X., Zhang C. Progesterone protects ovarian cancer cells from cisplatin-induced inhibitory effects through progesterone receptor membrane component 1/2 as well as AKT signaling. Oncol. Rep. 2013;30:2488–2494. doi: 10.3892/or.2013.2680. [DOI] [PubMed] [Google Scholar]
- 173.Hasegawa S., Kasubuchi M., Terasawa K., Kimura I. Perspectives on Membrane-associated Progesterone Receptors as Prospective Therapeutic Targets. Curr. Drug Targets. 2016;17:1189–1197. doi: 10.2174/1389450116666150518102651. [DOI] [PubMed] [Google Scholar]
- 174.Schwickert A., Weghake E., Brüggemann K., Engbers A., Brinkmann B., Kemper B., Seggewiß J., Stock C., Ebnet K., Kiesel L., et al. microRNA miR-142-3p Inhibits Breast Cancer Cell Invasiveness by Synchronous Targeting of WASL, Integrin Alpha V, and Additional Cytoskeletal Elements. PLoS ONE. 2015;10:e0143993. doi: 10.1371/journal.pone.0143993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fahlén M., Zhang H., Lofgren L., Masironi B., Von Schoultz E., Von Schoultz B., Sahlin L. Expression of Progesterone and Androgen Receptors in the Breast of Premenopausal Women, Considering Menstrual Phase. Anticancer. Res. 2018;38:1499–1510. doi: 10.21873/anticanres.12377. [DOI] [PubMed] [Google Scholar]
- 176.Causey M.W., Huston L.J., Harold D.M., Charaba C.J., Ippolito D.L., Hoffer Z.S., Brown T.A., Stallings J. Transcriptional Analysis of Novel Hormone Receptors PGRMC1 and PGRMC2 as Potential Biomarkers of Breast Adenocarcinoma Staging. J. Surg. Res. 2011;171:615–622. doi: 10.1016/j.jss.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 177.Albrecht C., Huck V., Wehling M., Wendler A. In vitro inhibition of SKOV-3 cell migration as a distinctive feature of progesterone receptor membrane component type 2 versus type. Steroids. 2012;77:1543–1550. doi: 10.1016/j.steroids.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 178.Han K.-H., Lee S.-H., Ha S.-A., Kim H.K., Lee C., Kim D.-H., Gong K.H., Yoo J., Kim S., Kim J.W. The functional and structural characterization of a novel oncogene GIG47 involved in the breast tumorigenesis. BMC Cancer. 2012;12:274. doi: 10.1186/1471-2407-12-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ohta H., Kimura I., Konishi M., Itoh N. Neudesin as a unique secreted protein with multi-functional roles in neural functions, energy metabolism, and tumorigenesis. Front. Mol. Biosci. 2015;2:24. doi: 10.3389/fmolb.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bruce A., Rybak A.P. CYB5D2 Requires Heme-Binding to Regulate HeLa Cell Growth and Confer Survival from Chemotherapeutic Agents. PLoS ONE. 2014;9:e86435. doi: 10.1371/journal.pone.0086435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xie Y., Shen Y.T., Kapoor A., Ojo D., Wei F., De Melo J., Lin X., Wong N., Yan J., Tao L., et al. CYB5D2 displays tumor suppression activities towards cervical cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2016;1862:556–565. doi: 10.1016/j.bbadis.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 182.Thomas P., Dong J. Novel mechanism of endocrine disruption by fungicides through binding to the membrane androgen receptor, ZIP9 (SLC39A9), and antagonizing rapid testosterone induction of the intrinsic apoptotic pathway. Steroids. 2019;149:108415. doi: 10.1016/j.steroids.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 183.Périan S., Vanacker J.-M. GPER as a Receptor for Endocrine-Disrupting Chemicals (EDCs) Front. Endocrinol. 2020;11:545. doi: 10.3389/fendo.2020.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Qie Y., Qin W., Zhao K., Liu C., Zhao L., Guo L.-H. Environmental Estrogens and Their Biological Effects through GPER Mediated Signal Pathways. Environ. Pollut. 2021;278:116826. doi: 10.1016/j.envpol.2021.116826. [DOI] [PubMed] [Google Scholar]
- 185.Pupo M., Pisano A., Lappano R., Santolla M.F., De Francesco E.M., Abonante S., Rosano C., Maggiolini M. Bisphenol A Induces Gene Expression Changes and Proliferative Effects through GPER in Breast Cancer Cells and Cancer-Associated Fibroblasts. Environ. Health Perspect. 2012;120:1177–1182. doi: 10.1289/ehp.1104526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xu F., Wang X., Wu N., He S., Yi W., Xiang S., Zhang P., Xie X., Ying C. Bisphenol A induces proliferative effects on both breast cancer cells and vascular endothelial cells through a shared GPER-dependent pathway in hypoxia. Environ. Pollut. 2017;231:1609–1620. doi: 10.1016/j.envpol.2017.09.069. [DOI] [PubMed] [Google Scholar]
- 187.Cao L., Ren X.-M., Liang-Hong G., Zhang J., Qin W.-P., Yang Y., Wan B., Guo L.-H. Bisphenol AF and Bisphenol B Exert Higher Estrogenic Effects than Bisphenol A via G Protein-Coupled Estrogen Receptor Pathway. Environ. Sci. Technol. 2017;51:11423–11430. doi: 10.1021/acs.est.7b03336. [DOI] [PubMed] [Google Scholar]
- 188.Deng Q., Jiang G., Wu Y., Li J., Liang W., Chen L., Su Q., Li W., Du J., Wong C.K., et al. GPER/Hippo-YAP signal is involved in Bisphenol S induced migration of triple negative breast cancer (TNBC) cells. J. Hazard. Mater. 2018;355:1–9. doi: 10.1016/j.jhazmat.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 189.Lei B., Sun S., Zhang X., Feng C., Xu J., Wen Y., Huang Y., Wu M., Yu Y. Bisphenol AF exerts estrogenic activity in MCF-7 cells through activation of Erk and PI3K/Akt signals via GPER signaling pathway. Chemosphere. 2019;220:362–370. doi: 10.1016/j.chemosphere.2018.12.122. [DOI] [PubMed] [Google Scholar]
- 190.Lei B., Tang Q., Sun S., Zhang X., Huang Y., Xu L. Insight into the mechanism of tetrachlorobisphenol A (TCBPA)-induced proliferation of breast cancer cells by GPER-mediated signaling pathways. Environ. Pollut. 2021;275:116636. doi: 10.1016/j.envpol.2021.116636. [DOI] [PubMed] [Google Scholar]
- 191.Castillo-Sanchez R., Ramirez-Ricardo J., Martinez-Baeza E., Cortes-Reynosa P., Candanedo-Gonzales F., Gomez R., Salazar E.P. Bisphenol A induces focal adhesions assembly and activation of FAK, Src and ERK2 via GPER in MDA-MB-231 breast cancer cells. Toxicol. Vitr. 2020;66:104871. doi: 10.1016/j.tiv.2020.104871. [DOI] [PubMed] [Google Scholar]
- 192.Chevalier N., Bouskine A., Fenichel P. Bisphenol A promotes testicular seminoma cell proliferation through GPER/GPR. Int. J. Cancer. 2012;130:241–242. doi: 10.1002/ijc.25972. [DOI] [PubMed] [Google Scholar]
- 193.Gorowska-Wojtowicz E., Duliban M., Kudrycka M., Dutka P., Pawlicki P., Milon A., Zarzycka M., Placha W., Kotula-Balak M., Ptak A., et al. Leydig cell tumorigenesis—Implication of G-protein coupled membrane estrogen receptor, peroxisome proliferator-activated receptor and xenoestrogen exposure. In vivo and in vitro appraisal. Tissue Cell. 2019;61:51–60. doi: 10.1016/j.tice.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 194.Buoso E., Masi M., Galbiati V., Maddalon A., Iulini M., Kenda M., Dolenc M.S., Marinovich M., Racchi M., Corsini E. Effect of estrogen-active compounds on the expression of RACK1 and immunological implications. Arch. Toxicol. 2020;94:2081–2095. doi: 10.1007/s00204-020-02756-9. [DOI] [PubMed] [Google Scholar]
- 195.Buoso E., Kenda M., Masi M., Linciano P., Galbiati V., Racchi M., Dolenc M.S., Corsini E. Effects of Bisphenols on RACK1 Expression and Their Immunological Implications in THP-1 Cells. Front. Pharmacol. 2021;12:2585. doi: 10.3389/fphar.2021.743991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Racchi M., Buoso E., Ronfani M., Serafini M.M., Galasso M., Lanni C., Corsini E. Role of Hormones in the Regulation of RACK1 Expression as a Signaling Checkpoint in Immunosenescence. Int. J. Mol. Sci. 2017;18:1453. doi: 10.3390/ijms18071453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Greten F.R., Grivennikov S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cirillo F., Lappano R., Bruno L., Rizzuti B., Grande F., Guzzi R., Briguori S., Miglietta A.M., Nakajima M., Di Martino M.T., et al. AHR and GPER mediate the stimulatory effects induced by 3-methylcholanthrene in breast cancer cells and cancer-associated fibroblasts (CAFs) J. Exp. Clin. Cancer Res. 2019;38:1–18. doi: 10.1186/s13046-019-1337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tokumoto T., Tokumoto M., Thomas P. Interactions of Diethylstilbestrol (DES) and DES Analogs with Membrane Progestin Receptor-α and the Correlation with Their Nongenomic Progestin Activities. Endocrinology. 2007;148:3459–3467. doi: 10.1210/en.2006-1694. [DOI] [PubMed] [Google Scholar]
- 200.Seeger H., Ruan X., Neubauer H., Brucker S., Mueck A.O. Membrane-initiated effects of Serelys® on proliferation and apoptosis of human breast cancer cells. Gynecol. Endocrinol. 2018;34:353–356. doi: 10.1080/09513590.2017.1407751. [DOI] [PubMed] [Google Scholar]
- 201.Li X., Ruan X., Gu M., Mueck A.O. PGRMC1 can trigger estrogen-dependent proliferation of breast cancer cells: Estradiol vs. equilin vs. ethinylestradiol. Climacteric. 2019;22:483–488. doi: 10.1080/13697137.2019.1582624. [DOI] [PubMed] [Google Scholar]
- 202.Neubauer H., Yang Y., Seeger H., Fehm T., Cahill M.A., Tong X., Ruan X., Mueck A.O. The presence of a membrane-bound progesterone receptor sensitizes the estradiol-induced effect on the proliferation of human breast cancer cells. Menopause. 2011;18:845–850. doi: 10.1097/gme.0b013e31820e5ac5. [DOI] [PubMed] [Google Scholar]
- 203.Izquierdo C., Martín-Martínez M., Gómez-Monterrey I., González-Muñiz R. TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation. Int. J. Mol. Sci. 2021;22:8502. doi: 10.3390/ijms22168502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rouhimoghadam M., Lu A.S., Salem A.K., Filardo E.J. Therapeutic Perspectives on the Modulation of G-Protein Coupled Estrogen Receptor, GPER, Function. Front. Endocrinol. 2020;11:591217. doi: 10.3389/fendo.2020.591217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Thomas P., Dong J. (−)-Epicatechin acts as a potent agonist of the membrane androgen receptor, ZIP9 (SLC39A9), to promote apoptosis of breast and prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2021;211:105906. doi: 10.1016/j.jsbmb.2021.105906. [DOI] [PubMed] [Google Scholar]
- 206.Bulldan A., Malviya V.N., Upmanyu N., Konrad L., Scheiner-Bobis G. Testosterone/bicalutamide antagonism at the predicted extracellular androgen binding site of ZIP9. Biochim. Biophys. Acta Bioenerg. 2017;1864:2402–2414. doi: 10.1016/j.bbamcr.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 207.Stepniewski T.M., Torrens-Fontanals M., Rodríguez-Espigares I., Giorgino T., Primdahl K.G., Vik A., Stenstrøm Y., Selent J., Hansen T.V. Synthesis, molecular modelling studies and biological evaluation of new oxoeicosanoid receptor 1 agonists. Bioorg. Med. Chem. 2018;26:3580–3587. doi: 10.1016/j.bmc.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 208.Chourey S., Ye Q., Reddy C.N., Cossette C., Gravel S., Zeller M., Slobodchikova I., Vuckovic D., Rokach J., Powell W.S. In vivo α-hydroxylation of a 2-alkylindole antagonist of the OXE receptor for the eosinophil chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid in monkeys. Biochem. Pharmacol. 2017;138:107–118. doi: 10.1016/j.bcp.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cossette C., Chourey S., Ye Q., Reddy C.N., Gore V., Gravel S., Slobodchikova I., Vuckovic D., Rokach J., Powell W.S. Pharmacokinetics and Metabolism of Selective Oxoeicosanoid (OXE) Receptor Antagonists and Their Effects on 5-Oxo-6,8,11,14-eicosatetraenoic Acid (5-Oxo-ETE)-Induced Granulocyte Activation in Monkeys. J. Med. Chem. 2016;59:10127–10146. doi: 10.1021/acs.jmedchem.6b00895. [DOI] [PubMed] [Google Scholar]
- 210.Ye Q., Chourey S., Reddy C.N., Wang R., Cossette C., Gravel S., Slobodchikova I., Vuckovic D., Rokach J., Powell W.S. Novel highly potent OXE receptor antagonists with prolonged plasma lifetimes that are converted to active metabolites in vivo in monkeys. Br. J. Pharmacol. 2020;177:388–401. doi: 10.1111/bph.14874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chourey S., Ye Q., Reddy C.N., Wang R., Cossette C., Gravel S., Slobodchikova I., Vuckovic D., Rokach J., Powell W.S. Novel Highly Potent and Metabolically Resistant Oxoeicosanoid (OXE) Receptor Antagonists That Block the Actions of the Granulocyte Chemoattractant 5-Oxo-6,8,11,14-Eicosatetraenoic Acid (5-oxo-ETE) J. Med. Chem. 2018;61:5934–5948. doi: 10.1021/acs.jmedchem.8b00154. [DOI] [PubMed] [Google Scholar]
- 212.Reddy C.N., Alhamza H., Chourey S., Ye Q., Gore V., Cossette C., Gravel S., Slobodchikova I., Vuckovic D., Rokach J., et al. Metabolism and pharmacokinetics of a potent N-acylindole antagonist of the OXE receptor for the eosinophil chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE) in rats and monkeys. Eur. J. Pharm. Sci. 2018;115:88–99. doi: 10.1016/j.ejps.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pi M., Kapoor K., Ye R., Hwang D.-J., Miller D.D., Smith J.C., Baudry J., Quarles L.D. Computationally identified novel agonists for GPRC6A. PLoS ONE. 2018;13:e0195980. doi: 10.1371/journal.pone.0195980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ishida Y., Kitayama K., Hanada K., Shibutani S., Nishizaki K., Kinjo T., Endo T., Suzuki A., Tateyama S., Nishizaki F., et al. Diltiazem Inhibits Coronary Spasm via Inhibition of Cav1.2Phosphorylation and Protein Kinase C Activation in a Mouse Model of Coronary Spastic Angina. Int. Hearth J. 2021;62:910–918. doi: 10.1536/ihj.20-366. [DOI] [PubMed] [Google Scholar]
- 215.Borella G., Da Ros A., Borile G., Porcù E., Tregnago C., Benetton M., Marchetti A., Bisio V., Montini B., Michielotto B., et al. Targeting mesenchymal stromal cells plasticity to reroute acute myeloid leukemia course. Blood. 2021;138:7. doi: 10.1182/blood.2020009845. [DOI] [PubMed] [Google Scholar]
- 216.Chen H., Vandorpe D.H., Xie X., Alper S.L., Zeidel M.L., Yu W. Disruption of Cav1.2-mediated signaling is a pathway for ketamine-induced pathology. Nat. Commun. 2020;11:4328. doi: 10.1038/s41467-020-18167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fusi F., Trezza A., Sgaragli G., Spiga O., Saponara S., Bova S. Ritanserin blocks CaV1.2 channels in rat artery smooth muscles: Electrophysiological, functional, and computational studies. Acta Pharmacol. Sin. 2020;41:1158–1166. doi: 10.1038/s41401-020-0370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yarotskyy V., Gao G., Du L., Ganapathi S.B., Peterson B.Z., Elmslie K. Roscovitine Binds to Novel L-channel (CaV1.2) Sites That Separately Affect Activation and Inactivation. J. Biol. Chem. 2010;285:43–53. doi: 10.1074/jbc.M109.076448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lee J.-H., Liu J., Shin M., Hong M., Nah S.-Y., Bae H. Metergoline inhibits the neuronal Nav1.2 voltage-dependent Na+ channels expressed in Xenopus oocytes. Acta Pharmacol. Sin. 2014;35:862–868. doi: 10.1038/aps.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Peters C., Sokolov S., Rajamani S., Ruben P. Effects of the antianginal drug, ranolazine, on the brain sodium channel NaV1.2 and its modulation by extracellular protons. Br. J. Pharmacol. 2013;169:704–716. doi: 10.1111/bph.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rivara M., Baheti A.R., Fantini M., Cocconcelli G., Ghiron C., Kalmar C.L., Singh N., Merrick E.C., Patel M.K., Zuliani V. 2,4(5)-Diarylimidazoles: Synthesis and biological evaluation of a new class of sodium channel blockers against hNav1.2. Bioorg. Med. Chem. Lett. 2008;18:5460–5462. doi: 10.1016/j.bmcl.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 222.Xiao J., Chen X., Lu X., Xie M., He B., He S., You S., Chen Q. Progesterone/Org inhibits lung adenocarcinoma cell growth via membrane progesterone receptor alpha. Thorac. Cancer. 2020;11:2209–2223. doi: 10.1111/1759-7714.13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Thomas P., Pang Y. Anti-apoptotic Actions of Allopregnanolone and Ganaxolone Mediated Through Membrane Progesterone Receptors (PAQRs) in Neuronal Cells. Front. Endocrinol. 2020;11:417. doi: 10.3389/fendo.2020.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang-Eckhardt L., Eckhardt M. A progesterone receptor membrane component 1 antagonist induces large vesicles independent of progesterone receptor membrane component 1 expression. Biol. Chem. 2020;401:1093–1099. doi: 10.1515/hsz-2019-0417. [DOI] [PubMed] [Google Scholar]
- 225.Teakel S.L., Ludescher M., Thejer B.M., Poschmann G., Forwood J.K., Neubauer H., Cahill M.A. Protein complexes including PGRMC1 and actin-associated proteins are disrupted by AG-205. Biochem. Biophys. Res. Commun. 2020;524:64–69. doi: 10.1016/j.bbrc.2019.12.108. [DOI] [PubMed] [Google Scholar]
- 226.Will E.A., Liu X., Peluso J.J. AG 205, a progesterone receptor membrane component 1 antagonist, ablates progesterone’s ability to block oxidative stress-induced apoptosis of human granulosa/luteal cells. Biol. Reprod. 2017;96:843–854. doi: 10.1093/biolre/iox013. [DOI] [PubMed] [Google Scholar]
- 227.D’Arrigo G., Gianquinto E., Rossetti G., Cruciani G., Lorenzetti S., Spyrakis F. Binding of Androgen- and Estrogen-Like Flavonoids to Their Cognate (Non)Nuclear Receptors: A Comparison by Computational Prediction. Molecules. 2021;26:1613. doi: 10.3390/molecules26061613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kowalska M., Nowaczyk J., Nowaczyk A. KV11.1, NaV1.5, and CaV1.2 Transporter Proteins as Antitarget for Drug Cardiotoxicity. Int. J. Mol. Sci. 2020;21:8099. doi: 10.3390/ijms21218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wang X., Saegusa H., Huntula S., Tanabe T. Blockade of microglial Cav1.2 Ca2+ channel exacerbates the symptoms in a Parkinson’s disease model. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-45681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jørgensen C.V., Bräuner-Osborne H. Pharmacology and physiological function of the orphan GPRC6A receptor. Basic Clin. Pharmacol. Toxicol. 2020;126:77–87. doi: 10.1111/bcpt.13397. [DOI] [PubMed] [Google Scholar]