Abstract
To investigate how bacterial pathogens spread from child to child in a day care center, we monitored six children, two boys and four girls, born between August 1995 and November 1997, attending a day care center and analyzed nasopharyngeal samples from them using pulsed-field gel electrophoresis (PFGE). We obtained nasopharyngeal cultures from all of the affected children and almost all of the unaffected children between September 1998 and March 1999 after some children presented simultaneously with purulent rhinorrhea. Moreover, when a child was found to have acute otitis media, nasopharyngeal secretions from the child were independently cultured during treatment. During this period, 28 isolates of Moraxella catarrhalis, 13 of Streptococcus pneumoniae, and 4 of Haemophilus influenzae were recovered. PFGE gave 8 patterns for M. catarrhalis, 10 for S. pneumoniae, and 1 for H. influenzae. PFGE patterns demonstrated spread of M. catarrhalis between children. However, each occurrence of clusters of infection with M. catarrhalis lasted 2 to 6 weeks, with a change in PFGE pattern between occurrences of clusters. The M. catarrhalis strain infecting each child also changed. Similarly, the S. pneumoniae strain in each child also changed. In contrast, infection with H. influenzae persisted for about 3 months in an affected child.
Acute otitis media (AOM) is the most common disease of the upper respiratory airway in childhood and occurs at least once in about two-thirds of children under 3 years of age (17). Recurrent AOM (rAOM) tends to occur in children under 2 years of age, particularly with episodes of AOM in the first year of life (9).
AOM has a multifactorial etiology, and risk factors for rAOM are classified by host, bacterial, and environmental characteristics. Host factors include immature immunity (15), lack of breast feeding, and tubal dysfunction (4). With respect to bacterial factors, penicillin-resistant Streptococcus pneumoniae has become a major concern in respiratory tract infections in children. Recent studies have shown a high incidence of penicillin-resistant S. pneumoniae in middle ear secretions (6). As for environmental factors, the relationship between day care center attendance and occurrence of otitis media was reported by Hesselvic (8) as early as 50 years ago. Since then, many other studies have shown that day care center attendance is a strong risk factor for rAOM compared with care at home (7, 14, 16).
Although attendance at a day care center is a risk factor for rAOM, no epidemiologic study has demonstrated that children acquire infecting organisms from other children. Moreover, the incidence of rAOM has increased recently, with some children requiring hospitalization and treatment with injectable antibiotics because of persistent purulent otorrhea, high fever, and complications, e.g., bacterial meningitis. Therefore, it is important to analyze clinical nasopharyngeal samples from children attending day care centers. In this study, we analyzed nasopharyngeal cultures from children attending a day care center and investigated how bacterial pathogens spread from child to child in this setting, using pulsed-field gel electrophoresis (PFGE).
MATERIALS AND METHODS
Monitoring.
We prospectively monitored six children attending a day care center attached to Tohoku Rosai Hospital in Sendai City from December 1997 to March 1999. The six children, two boys and four girls, were born between August 1995 and November 1997. Five children were between the ages of 7 months and 2 years 4 months when our study started. The other one was 6 months old when the child entered the day care center in May 1998. These six children were the only ones in the day care center and were cared for in one room measuring about 6 by 8 m.
Nasopharyngeal cultures.
Between September 1998 and March 1999, when some children presented simultaneously with purulent rhinorrhea, nasopharyngeal secretions from all of the affected children and almost all of the unaffected children were cultured by an otolaryngologist (M.S.). In addition, when a child was found to have AOM, nasopharyngeal secretions from the child were independently cultured during treatment. The diagnosis of AOM was made by the same otolaryngologist. This study protocol was approved by Tohoku Rosai Hospital's ethics committee.
Nasopharyngeal secretions were obtained with a sterile cotton swab (Seed Swab No. 2; Eiken Chemical Co., Ltd., Tokyo, Japan). The swab with the sample was shaken in 1.0 ml of buffered saline with gelatin (BSG), which consisted of 8.5 g of NaCl, 0.3 g of KH2PO4, 0.6 g of Na2HPO4, 0.1 g of gelatin, and 1,000 ml of distilled water (11), to suspend microorganisms, and 20 μl of the suspension was plated onto chocolate and sheep blood agar plates, which were incubated at 35°C for 18 to 24 h in a candle jar. Alpha-hemolytic colonies were selected and transferred to sheep blood agar, and S. pneumoniae was identified by its sensitivity to optochin. Colonies with morphology typical of Haemophilus influenzae were purified by passage on chocolate agar and identified using paper disks impregnated with an NAD solution (Factor X; Eiken Chemical Co.) and hemin (Factor V; Eiken Chemical Co.). Moraxella catarrhalis was identified by failure of the organism to utilize carbohydrates, reduction of nitrate, and production of DNase (13). Gram stains were performed for each isolate.
Antimicrobial agents.
Reference powders of different drugs with known potency were as follows: benzylpenicillin (Banyu Pharmaceutical Co., Ltd., Tokyo, Japan), ampicillin (Meiji Seika Kaisha., Ltd., Tokyo, Japan), clavulanic acid (SmithKline Beecham Pharmaceuticals, Surrey, United Kingdom), cefaclor (Eizai Co., Ltd., Tokyo, Japan), cefpodoxime (Sankyo Co., Ltd., Tokyo, Japan), cefditoren (Meiji Seika Kaisha), cefotiam (Takeda Chemical Industries, Ltd., Osaka, Japan), cefmetazole (Sankyo Co.), cefotaxime (Nippon Hoechst Marion Roussel, Tokyo, Japan), and imipenem (Banyu Pharmaceutical Co.). All of these were the kind gifts of the respective manufactures.
MIC determinations.
MICs were determined in Sensitivity Test Agar (Mueller-Hinton agar medium; Eiken Chemical Co.) with Strepto Haemo supplement (Eiken Chemical Co.) by agar dilution with an inoculum of 5 × 104 CFU/spot delivered by a Microplanter inoculator (Sakuma Seisaku, Tokyo, Japan). The MIC of each drug was scored after 18 h of incubation at 35°C (10).
Genotyping.
M. catarrhalis, S. pneumoniae, and H. influenzae were grown in Mueller-Hinton broth (Eiken Chemical Co.) with Strepto Haemo supplement (Eiken Chemical Co.) at 35°C for 16 h. The cells were harvested by centrifugation at 4,000 × g and 4°C for 5 min, washed with a saline-EDTA solution (0.15 M NaCl, 10 mM EDTA [pH 8.0]), and resuspended in a Pett IV solution (1 M NaCl, 10 mM EDTA [pH 8.0]). An equal volume of melted 2.0% low-melting-point agarose (InCert agarose; FMC BioProducts, Rockland, Maine) was added to this suspension. The mixture was poured into an insert former and chilled at 4°C for 20 min. The plugs removed from the former were treated at 37°C with 1 to 5 mg of lysozyme (Seikagaku Co., Tokyo, Japan) per ml of lysis solution (1 M NaCl, 0.1 M EDTA [pH 8.0], 10 mM Tris-HCl, 0.5% Brij 58, 0.2% sodium deoxycholate, 0.5% Sarkosyl). After 12 h, the lysis solution was decanted and replaced with 0.1 to 1.0 mg of proteinase K (Wako Pure Chemical Industries, Ltd., Osaka, Japan) per ml of ES solution (0.25 M EDTA [pH 8.0], 1% Sarkosyl) at 50°C for 24 h. The ES solution was decanted, and the plugs were placed in TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA [pH 8.0]) containing 1 mM phenylmethylsulfonyl fluoride at room temperature for 4 h. Next, the plugs were washed in TE buffer for 20 min at room temperature. For restriction endonuclease digestion, the plugs were incubated in enzyme restriction buffer for 30 min at room temperature to remove the EDTA. The plugs were incubated in restriction enzyme buffer with 20 U of SpeI (TaKaRa Shuzo Co., Ltd., Kyoto, Japan), NotI (TaKaRa Shuzo Co.), and NheI (TaKaRa Shuzo Co.) for M. catarrhalis isolates; SmaI (TaKaRa Shuzo Co.) and ApaI (TaKaRa Shuzo Co.) for S. pneumoniae isolates; and SmaI (TaKaRa Shuzo Co.) and SpeI (TaKaRa Shuzo Co.) for H. influenzae isolates, respectively. The digestions with SpeI, NotI, NheI, and ApaI were performed at 37°C, and those with SmaI were done at 30°C for 16 h, respectively.
Electrophoresis was performed using a CHEF Mapper (Bio-Rad Laboratories, Hercules, Calif.). Agarose gels were prepared at a 1% concentration in 0.5× TBE buffer (45 mM Tris base, 45 mM boric acid, 1 mM EDTA [pH 8.0]). Separation of fragments was done at 6 V/cm at 14°C for 20 h 18 min. The pulse time, which changed linearly, was 0.47 to 63.80 s. Lambda Ladder (Bio-Rad Laboratories) was used as the size standard. The gel was stained for 30 min in ethidium bromide at 1 μg/ml and decolorized in distilled water for 15 min. The gel was photographed by UV transillumination.
RESULTS
Occurrence of upper respiratory infection and AOM.
All of the children had upper respiratory infections during the monitoring period. Five children (83%) had at least one episode of AOM, and four had more than four episodes (Table 1). Although child 3 did not have AOM before entrance into the day care center, he had rAOM episodes after entrance. On the other hand, children 2 and 5 rarely had episodes of AOM during the monitoring period, although they attended the same day care center.
TABLE 1.
Clinical courses of the six children studied
| Child | Dec. 1997 | 1998
|
1999
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jan. | Feb. | March | April | May | June | July | Aug. | Sept. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | ||
| 1 | AOM | AOM | URIa | AOM | AOM | URI | URI | URI | URI | |||||||
| 2 | URI | URI | URI | URI | ||||||||||||
| 3 | Eb | AOM | AOM | AOM | AOM | URI | ||||||||||
| 4 | AOM | AOM | AOM | AOM | URI | AOM | AOM | URI | ||||||||
| 5 | AOM | URI | ||||||||||||||
| 6 | AOM | AOM | URI | AOM | URI | AOM | AOM | URI | ||||||||
URI, upper respiratory infection.
E, entrance into the day care center.
Nasopharyngeal culture.
Between September 1998 and March 1999, 28 isolates of M. catarrhalis, 13 of S. pneumoniae, and 4 of H. influenzae were recovered from the six children, mainly from the nasopharynx.
PFGE.
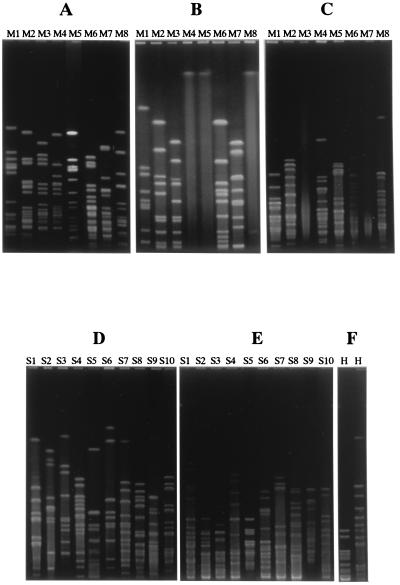
We examined whether isolates from the nasopharynx belonged to the same strain by PFGE after cutting chromosomal DNA with two or three restriction enzymes. As shown in Fig. 1, we obtained 8 PFGE patterns from M. catarrhalis isolates, 10 from S. pneumoniae isolates, and 1 from H. influenzae isolates. The isolates which showed the same PFGE pattern with one restriction enzyme were identified as the same strains after digestion with the other enzymes for each organism. We designated these strains M1 to M8, S1 to S10, and H, respectively.
FIG. 1.
Analysis of PFGE patterns of M. catarrhalis, S. pneumoniae, and H. influenzae. M. catarrhalis isolates were classified as M1, M2, M3, M4, M5, M6, M7, or M8 based on their PFGE patterns with SpeI (A), NotI (B), and NheI (C). S. pneumoniae isolates were classified as S1, S2, S3, S4, S5, S6, S7, S8, S9, or S10 based on their PFGE patterns with SmaI (D) and ApaI (E). All H. influenzae isolates had the same PFGE pattern with SpeI (F, left lane) and SmaI (F, right lane).
Spread of bacteria in the day care center.
Clusters of infections with M. catarrhalis strains M1, M2, M4, and M7 occurred in children attending the day care center from September 1998 to March 1999. However, the duration of each occurrence of clusters of infection lasted 2 to 6 weeks. The PFGE pattern of the M. catarrhalis strains changed between periods of clusters. Similarly, the strain of M. catarrhalis changed in each child in the day care center between periods of infection (Table 2).
TABLE 2.
M. catarrhalis, S. pneumoniae, and H. influenzae isolates recovered from the nasopharynges of the six children studied and occurrence of clusters of infection with M. catarrhalisa
| Child | 1998
|
1999
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9/24 | 10/29 | 11/9 | 11/17 | 11/24 | 12/4 | 12/15 | 1/21 | 1/25 | 1/28 | 3/1 | 3/2 | 3/9 | 3/15 | 3/23 | |
| 1 | (−) | M1 | — | M1 | M4 | M1, S5, S6 | — | M6 | M7 | — | M7 | — | S9 | — | (−) |
| 2 | (−) | M1, S1 | — | M1 | — | M1 | — | — | (−) | — | (−) | — | — | H | S10 |
| 3 | (−) | (−) | — | M2, S4 | — | M4, H | M4, S7 | — | M7, S8 | M7, H, S8 | — | M8, H | — | — | (−) |
| 4 | (−) | (−) | M2 | M3 | — | M5 | — | — | M7 | — | — | — | M7 | — | (−) |
| 5 | (−) | S2, S3 | — | (−) | — | M4 | — | — | M5 | — | — | — | M7 | — | (−) |
| 6 | (−) | (−) | M2 | M2 | — | M4 | — | M7 | — | — | — | — | S8 | — | S8 |
Clusters of infection spanned the following dates: M1, 10/29/98 to 12/4/98; M2, 11/9/98 to 11/17/98; M4, 11/24/98 to 12/15/98; M7, 1/21/99 to 3/9/99. M1 to M8, M. catarrhalis; S1, S2, and S10, penicillin-resistant S. pneumoniae; S3, S4, S5, and S9, intermediately penicillin-resistant S. pneumoniae; S6, S8, and S9, penicillin-susceptible S. pneumoniae; H, H. influenzae; (−), only nasopharyngeal flora isolated or no bacterial species isolated from the nasopharynx; —, not screened.
In this study, the clusters of infection with S. pneumoniae and H. influenzae could not been measured. As with M. catarrhalis, the strain of S. pneumoniae also changed with time in each child. However, child 3 was infected with the same H. influenzae strain for 3 months (Table 2).
MIC determinations.
The MICs of 10 antibiotics against 28 isolates of M. catarrhalis, 13 of S. pneumoniae, and 4 of H. influenzae were determined. When we compared susceptibility patterns with PFGE profiles, the MICs were almost equal for the strains which showed the same PFGE profile. Therefore, the MICs for the representative strains which showed different PFGE profiles are shown in Table 3.
TABLE 3.
MICs of 10 antibiotics against 8 M. catarrhalis strains, 10 S. pneumoniae strains, and 1 H. influenzae strain
| Strain | MICa (μg/ml) of:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCG | AMP | CLA-AMP | CEC | CPD | CDT | CTM | CMZ | CTX | IPM | |
| M1 | 1 | 0.25 | 0.06 | 0.25 | 0.25 | 0.06 | 0.25 | 0.25 | 0.13 | 0.06 |
| M2 | 1 | 0.25 | 0.06 | 0.25 | 0.5 | 0.06 | 0.5 | 1 | 0.5 | 0.06 |
| M3 | 1 | 0.25 | 0.06 | 0.25 | 0.5 | 0.06 | 0.5 | 1 | 0.5 | 0.06 |
| M4 | 16 | 2 | 0.06 | 1 | 1 | 0.25 | 1 | 1 | 1 | 0.06 |
| M5 | 1 | 0.25 | 0.06 | 0.25 | 0.25 | 0.06 | 0.25 | 0.13 | 0.06 | 0.06 |
| M6 | 32 | 16 | 0.06 | 8 | 1 | 0.25 | 2 | 1 | 0.5 | 0.06 |
| M7 | 32 | 16 | 0.06 | 32 | 1 | 1 | 2 | 1 | 0.5 | 0.06 |
| M8 | 2 | 0.5 | 0.06 | 0.5 | 0.25 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| S1 | 4 | 4 | 4 | 128 | 32 | 4 | 16 | 64 | 4 | 1 |
| S2 | 0.25 | 0.13 | 0.06 | 1 | 0.25 | 0.06 | 1 | 2 | 0.13 | 0.06 |
| S3 | 4 | 4 | 4 | 128 | 32 | 4 | 16 | 128 | 4 | 1 |
| S4 | 1 | 2 | 2 | 32 | 2 | 0.5 | 4 | 8 | 0.5 | 0.25 |
| S5 | 0.25 | 1 | 0.5 | 1 | 0.5 | 0.25 | 0.5 | 2 | 0.25 | 0.25 |
| S6 | 0.06 | 0.06 | 0.06 | 0.5 | 0.13 | 0.06 | 0.5 | 1 | 0.13 | 0.13 |
| S7 | 0.25 | 0.25 | 0.13 | 4 | 0.25 | 0.13 | 0.25 | 0.5 | 0.13 | 0.06 |
| S8 | 0.06 | 0.06 | 0.06 | 0.5 | 0.06 | 0.06 | 0.06 | 0.5 | 0.06 | 0.06 |
| S9 | 0.06 | 0.06 | 0.06 | 0.25 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| S10 | 4 | 8 | 8 | >128 | 32 | 4 | 16 | 64 | 8 | 1 |
| H | 0.25 | 0.25 | 0.06 | 2 | 0.06 | 0.06 | 2 | 2 | 0.06 | 0.5 |
PCG, benzylpenicillin; AMP, ampicillin; CLA, clavulanic acid (5 μg/ml); CEC, cefaclor; CPD, cefpodoxime; CDT, cefditoren; CTM, cefotiam; CMZ, cefmetazole; CTX, cefotaxime; IPM, imipenem.
Because the MICs decreased slightly in the presence of clavulanic acid (5 μg/ml), which inhibits β-lactamase activity, all strains of M. catarrhalis and H. influenzae were considered to produce penicillinase. In addition, All M. catarrhalis and H. influenzae isolates were considered to produce penicillinase by the P/Case test (Showa Chemical Co., Ltd., Tokyo, Japan). Of the 10 strains of S. pneumoniae, 3 were resistant to benzylpenicillin (MIC, ≧2.0 μg/ml) and 4 showed intermediate resistance (0.1 μg/ml ≦ MIC ≦ 1.0 μg/ml).
DISCUSSION
AOM is a major health problem of childhood. In the past, it was easy for physicians to treat AOM with antibiotics. However, the incidence of rAOM has increased recently, with some children requiring hospitalization and treatment with injectable antibiotics because of persistent purulent otorrhea and high fever. Such severe AOM has the possibility of suppurative complications, e.g., bacterial meningitis or brain abscess. Generally, the parents of children who attend day care centers have jobs outside the home. Thus, rAOM represents a significant burden when outpatient treatment fails. Moreover, rAOM is recognized as a social problem because of the associated high medical expenses.
Although rAOM has been reported to be associated with attendance at day care centers (7, 14, 16), no epidemiologic study has demonstrated that children acquire infecting organisms from either other children or the same children at different times. Neither has molecular biology been used to characterize the infecting agent, thereby establishing the mode of infection. Therefore, it is important to analyze isolates from the nasopharynges of children attending day care centers to answer these questions. In this study, we investigated bacteria in serial nasopharyngeal cultures repeatedly in children attending a day care center, using PFGE.
The usefulness of epidemiological typing of the strains of a bacterial species by PFGE for genomic DNA has been well established (2). At first, we analyzed PFGE patterns using SpeI for M. catarrhalis and SmaI for S. pneumoniae and H. influenzae. As a result, we obtained 8 PFGE patterns from M. catarrhalis isolates, 10 from S. pneumoniae isolates, and 1 from H. influenzae isolates. To indicate that the strains showing indistinguishable PFGE patterns are indeed the same, we further analyzed PFGE patterns using NotI and NheI for M. catarrhalis, ApaI for S. pneumoniae, and SpeI for H. influenzae. As for S. pneumoniae and H. influenzae, the isolates which showed the same PFGE pattern with the first restriction enzyme were identified as the same strains after digestion with another enzyme. As for some M. catarrhalis strains, it was very difficult to obtain PFGE bands with NotI (M4, M5, and M8) and NheI (M3 and M7) for unknown reasons. However, by using both NotI and NheI, we found that the strains showing indistinguishable PFGE patterns with SpeI were indeed the same.
In analyzing the restriction patterns, we referred to Tenover's guidelines to determine the relatedness of bacterial isolates (18). As a result of PFGE, restriction patterns were divided into two types: one is indistinguishable patterns, and the other is differences of more than four bands in each organism. Strains showing differences of more than four bands were considered unrelated because the isolates were collected within a short period (6 months) and taken from small populations (18).
Table 1 shows the clinical courses of all of the children studied with regard to AOM and upper respiratory infections between December 1997 and March 1999. There were many AOM episodes. Child 3 had no attacks prior to entrance into the day care center but had several consecutive episodes of AOM after entrance. Attendance at a day care center seemed to be a causative factor for rAOM in this case. However, children 2 and 5 rarely experienced episodes of AOM despite their attendance at the same day care center. This suggests that host factors are also very important and that AOM is a multifactorial disease.
There were five epidemic episodes of upper respiratory infection, including AOM, among the children attending the day care center between September 1998 and March 1999. M. catarrhalis was cultured repeatedly during these episodes. Twenty-eight M. catarrhalis isolates obtained from the nasopharynges of the six children were classified into eight strains by means of PFGE. Table 2 shows the spread of a particular M. catarrhalis isolate from one child to another.
For example, strain M4 was isolated from child 1 on 24 November 1998. This strain spread to children 3, 5, and 6. However, the presence of clusters of infection with M4 had ended by 15 December 1998. Strain M7 was then isolated from child 6 on 21 January 1999. After 4 days, this strain spread to children 1, 3, and 4. Finally, the strain spread to all of the children in the day care center except child 2. When the next clusters of infection with M. catarrhalis occurred, the strains had changed, as shown by their PFGE patterns. The strain of M. catarrhalis infecting each child also changed. For example, four different strains of M. catarrhalis were isolated one after another from child 3 during this period. The mechanism of the observed turnover may be very complex and probably involves many factors.
M. catarrhalis is frequently part of the nasopharyngeal microflora of small children, especially during episodes of AOM. M. catarrhalis can be transmitted in the hospital (1), as well as in the community (12). M. catarrhalis has become increasingly recognized as a cause of upper and lower respiratory tract infections. An important characteristic of M. catarrhalis is that most isolates produce β-lactamase (5); the enzyme produced by M. catarrhalis hydrolyzes not only penicillins but also cephalosporins. The phenomenon of indirect pathogenicity in mixed infections, in which a pathogen such as S. pneumoniae and H. influenzae is protected by an enzyme produced by another organism, is well recognized (3). In this study, the isolates obtained from S. pneumoniae and H. influenzae were in mixed infections with M. catarrhalis and all of the M. catarrhalis isolates produced β-lactamases; thus, it was important to inhibit these enzymes to treat S. pneumoniae and H. influenzae infections.
One episode of spread of S. pneumoniae from child 3 to child 6 was recognized (Table 2). However, the clusters of infection with S. pneumoniae and H. influenzae could not been measured in this study. As with M. catarrhalis strains, the S. pneumoniae strain changed in each child with the next upper respiratory infection, including episodes of AOM. For example, three different strains of S. pneumoniae were isolated, one after another, from child 3 during this period.
Four H. influenzae isolates were recovered from the nasopharynges of six children. PFGE showed that these isolates belonged to the same strain. Infection with H. influenzae, unlike infection with M. catarrhalis and S. pneumoniae, persisted for about 3 months in child 3. Therefore, we assume that elimination of H. influenzae from the nasopharynx is difficult, compared with that of M. catarrhalis and S. pneumoniae. Studies addressing this question are under way in our laboratory.
ACKNOWLEDGMENT
This work was supported in part by grant-in-aid 09670296 from the Ministry of Science, Education and Culture of Japan, awarded to M. Inoue.
REFERENCES
- 1.Ahmad F, McLeod D T, Power J T, Calder M A. Branhamella catarrhalis prevalence in a hospital population. J Hosp Infect. 1985;6:71–74. doi: 10.1016/s0195-6701(85)80020-7. [DOI] [PubMed] [Google Scholar]
- 2.Arbeit R D. Laboratory procedures for the epidemiologic analysis of microorganisms. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 190–208. [Google Scholar]
- 3.Brook I. The concept of indirect pathogenicity by beta-lactamase production, especially in ear, nose and throat infection. J Antimicrob Chemother. 1989;24(Suppl. B):63–72. doi: 10.1093/jac/24.suppl_b.63. [DOI] [PubMed] [Google Scholar]
- 4.Bylander A. Pathophysiological aspects on eustachian tube function and SOM. Scand Audiol Suppl. 1986;26:59–63. [PubMed] [Google Scholar]
- 5.Doern G V, Jones R N, Pfaller M A, Kugler K The Sentry Participants Group. Haemophilus influenzae and Moraxella catarrhalis from patients with community-acquired respiratory tract infections: antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997) Antimicrob Agents Chemother. 1999;43:385–389. doi: 10.1128/aac.43.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gehanno P, N'Guyen L, Derriennic M, Pichon F, Goehrs J M, Berche P. Pathogens isolated during treatment failures in otitis. Pediatr Infect Dis J. 1998;7:885–890. doi: 10.1097/00006454-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hardy A M, Fowler M G. Child care arrangements and repeated ear infections in young children. Am J Public Health. 1993;83:1321–1325. doi: 10.2105/ajph.83.9.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesselvik L. Respiratory infections among children in day nurseries. Acta Paediatr Scand Suppl. 1949;74:1–103. [Google Scholar]
- 9.Howie V M, Ploussard J H, Sloyer J. The “otitis-prone” condition. Am J Dis Child. 1975;129:676–678. doi: 10.1001/archpedi.1975.02120430016006. [DOI] [PubMed] [Google Scholar]
- 10.Inoue M, Okamoto R, Okubo T, Inoue K, Mitsuhashi S. Comparative in vitro activity of RP59500 against clinical bacterial isolates. J Antimicrob Chemother. 1992;30(Suppl. A):45–51. doi: 10.1093/jac/30.suppl_a.45. [DOI] [PubMed] [Google Scholar]
- 11.Katsu K, Inoue M, Mitsuhashi S. Transposition of the carbenicillin-hydrolyzing beta-lactamase gene. J Bacteriol. 1982;150:483–489. doi: 10.1128/jb.150.2.483-489.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLeod D T, Ahmad F, Power J T, Calder M A, Seaton A. Bronchopulmonary infection due to Branhamella catarrhalis. Br Med J (Clin Res Ed) 1983;12:1446–1447. doi: 10.1136/bmj.287.6403.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morello J A, Janda W M, Bohnhoff M. Neisseria and Branhamella. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 324–340. [Google Scholar]
- 14.Niemela M, Uhari M, Mottonen M. A pacifier increases the risk of recurrent acute otitis media in children in day care centers. Pediatrics. 1995;96:884–888. [PubMed] [Google Scholar]
- 15.Prellner K, Kalm O, Pedersen F K. Pneumococcal antibodies and complement during and after periods of recurrent otitis. Int J Pediatr Otorhinolaryngol. 1984;7:39–49. doi: 10.1016/s0165-5876(84)80052-x. [DOI] [PubMed] [Google Scholar]
- 16.Pukander J, Luotonen J, Timonen M, Karma P. Risk factors affecting the occurrence of acute otitis media among 2–3-year-old urban children. Acta Oto-laryngol. 1985;100:260–265. doi: 10.3109/00016488509104788. [DOI] [PubMed] [Google Scholar]
- 17.Teele D W, Klein J O, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 18.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]