Abstract
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (Apo2 ligand [Apo2L]) is a member of the TNF superfamily and has been shown to have selective antitumor activity. Although it is known that TRAIL (Apo2L) induces apoptosis and activates NF-κB and Jun N-terminal kinase (JNK) through receptors such as TRAIL-R1 (DR4) and TRAIL-R2 (DR5), the components of its signaling cascade have not been well defined. In this report, we demonstrated that the death domain kinase RIP is essential for TRAIL-induced IκB kinase (IKK) and JNK activation. We found that ectopic expression of the dominant negative mutant RIP, RIP(559–671), blocks TRAIL-induced IKK and JNK activation. In the RIP null fibroblasts, TRAIL failed to activate IKK and only partially activated JNK. The endogenous RIP protein was detected by immunoprecipitation in the TRAIL-R1 complex after TRAIL treatment. More importantly, we found that RIP is not involved in TRAIL-induced apoptosis. In addition, we also demonstrated that the TNF receptor-associated factor 2 (TRAF2) plays little role in TRAIL-induced IKK activation although it is required for TRAIL-mediated JNK activation. These results indicated that the death domain kinase RIP, a key factor in TNF signaling, also plays a pivotal role in TRAIL-induced IKK and JNK activation.
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (Apo2 ligand [Apo2L]) is a member of the TNF superfamily, which includes TNF, FasL, lymphotoxin, CD27L, OX40, CD30L, and CD40L (1, 30, 40). All members in this superfamily are type II membrane proteins, and many of them are involved in a variety of cellular processes, including cell proliferation, differentiation, and apoptosis (42, 44). Unlike other members, whose expression is transitorily regulated and detected only in certain tissues, TRAIL (Apo2L) is constitutively expressed in most types of tissues and cells (25, 34, 49). It is believed that, like the active forms of TNF and FasL, the active form of TRAIL is a trimer (15, 28). Five proteins, TRAIL-R1 (DR4), TRAIL-R2 (DR5), TRAIL-R3 (DcR1), TRAIL-R4 (DcR2), and osteoprotegerin, have been identified as TRAIL receptors (9, 32, 33). Among these receptors, TRAIL-R1, TRAIL-R2, and TRAIL-R4 are type I membrane proteins and belong to the TNF receptor (TNF-R) superfamily (38, 47). Like TNF-R1 and Fas, which are known as death receptors, TRAIL-R1 and TRAIL-R2 also contain a death domain in their cytoplasmic region and are able to transduce a TRAIL-induced death signal (5, 6, 33, 37). TRAIL-R4 only has a truncated death domain and functions as a decoy receptor to block TRAIL-induced apoptosis (32). TRAIL-R3 is also a decoy receptor because it lacks a cytoplasmic region (38). Recently, it has been shown that osteoprotegerin, which was originally identified as a regulator of bone density, is able to bind to TRAIL (9).
Upon binding to TRAIL-R1, TRAIL-R2, or TRAIL-R4, TRAIL can also activate the transcriptional factor NF-κB and c-Jun N-terminal kinase (JNK) (5, 6, 29, 37). In response to many stimuli such as TNF and interleukin-1 (IL-1), the activation of NF-κB is mediated through IκB kinase (IKK) and JNK is activated through the mitogen-activated protein kinase cascade, namely, JNKK1 (MKK4) and MEKK1 (8, 18, 24, 26, 27, 35). The activation of JNK is a major regulatory step to activate the transcription factor AP-1 (18). Inactive NF-κB is located in the cytoplasm because its interaction with the inhibitory proteins, IκBs, masks its nuclear translocation signal (3, 39). When IKK is activated, it phosphorylates IκBs. Then the phosphorylated IκBs will be polyubiquitinated and rapidly degraded by the proteasome (3). The degradation of IκBs leads to the release of NF-κB and allows NF-κB to translocate into the nucleus and to activate its target genes, some of which are the crucial mediators of the NF-κB antiapoptotic function (4, 23, 43, 48). It has been found that NF-κB activation also protects cells against TRAIL-induced apoptosis (16, 17).
Although some effort has been made to elucidate the molecular mechanism of TRAIL signaling, the components of different TRAIL signaling pathways are still largely undefined, despite the fact that the possible role of TRADD (TNF-R1-associated death domain protein), FADD (Fas-associated death domain factor), TRAF2 (TNFR-associated factor 2), or RIP (receptor-interacting protein) in TRAIL signaling has been suggested (1). All of these four proteins are known to be essential for TNF-R1 signaling: (i) TRADD serves as an adapter molecule that recruits other proteins into the TNF-R1 complex (11–13); (ii) FADD is required for TNF-induced apoptosis (50, 53); (iii) RIP is essential for TNF-mediated NF-κB activation (20, 41); and (iv) TRAF2 mediates TNF-induced JNK activation (21, 23, 31, 36, 51). In this study, we investigated the role of RIP and TRAF2 in TRAIL signaling, especially TRAIL-mediated IKK and JNK activation. Using RIP−/− and TRAF2−/− fibroblasts, we demonstrated that RIP is essential for TRAIL-induced IKK activation, and both RIP and TRAF2 are involved in TRAIL-mediated JNK activation. However, neither RIP nor TRAF2 is required for TRAIL-induced apoptosis.
MATERIALS AND METHODS
Reagents and plasmids.
Soluble recombinant human TRAIL was purchased from Biomol. Glutathione S-transferase (GST)–TRAIL was expressed and purified from Escherichia coli as described elsewhere (19). Antibodies specific to RIP, DR4, c-Myc, and JNK1 were purchased from Pharmingen. Anti-FADD antibody was from Transduction Laboratories. Antibodies directed against TRADD, IKKα, IKKβ, IκBα, and hemagglutinin epitope (HA) were from Santa Cruz Biotechnology. Anti-phospho-IκBα antibody was from New England Biolabs. Anti-Flag antibody (M2) was from Sigma. The mammalian expression plasmids for RIP, RIP(559–671), TRAF2, TRAF2(87–501), CrmA, FADD, TRADD, DR4, HA-JNK1, and HA-IKKβ have been previously described (12, 13, 23, 32, 52).
Cell culture and transfection.
HeLa, HEK293, and mouse fibroblast cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% calf serum, 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells were transfected with Lipofectamine (Gibco) as described previously (22).
Western blot analysis and coimmunoprecipitation.
After treatment with different reagents as described in the figure legends, cells were collected and lysed in M2 buffer (20 mM Tris [pH 7], 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 20 mM β-glycerol phosphate, 1 mM sodium vanadate, 1 μg of leupeptin/ml). Fifty micrograms of the cell lysate from each sample was fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotted. The proteins were visualized by enhanced chemiluminescence as instructed by the manufacturer (Amersham) (22). For immunoprecipitation assays of transfected proteins, HEK293 cells were transiently cotransfected with different plasmids and then lysed in lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 30 mM NaF, 2 mM sodium pyrophosphate, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml). The expression of each transfected protein was verified by Western blotting. The immunoprecipitation experiments were performed with anti-Flag antibody (M2) and protein A-Sepharose beads by incubation at 4°C overnight. The beads were washed three times with lysis buffer, and the bound proteins were resolved by SDS-PAGE on a 10% gel. Detection was accomplished by Western blot analysis (22). For immunoprecipitation assays of endogenous proteins, 5 × 107 HeLa cells were treated with TRAIL (1 μg/ml) for 30 min as indicated in the legend to Fig. 6. The cells were then lysed in lysis buffer and precipitated with 2 μg of anti-DR4 antibody as described above.
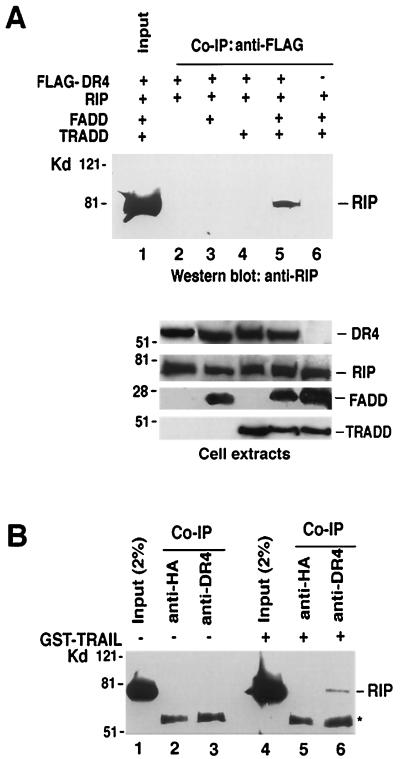
FIG. 6.
RIP is present in the TRAIL-R1 complex. (A) HEK293 cells were cotransfected with RIP (2.5 μg) and CrmA (1 μg) along with 2.5 μg of Flag-DR4, FADD, or TRADD as indicated. Cells were collected 24 hours after transfection. Expression of Flag-DR4, RIP, TRADD, and FADD was determined by Western blotting (bottom). Coimmunoprecipitation (Co-IP) experiments were performed with anti-Flag antibody (M2), and coprecipitated RIP proteins were detected by Western blotting (top). (B) HeLa cells (2 × 107) were treated with TRAIL (1 μg/ml) for 20 min or not treated. Immunoprecipitation experiments were performed with anti-DR4 (lanes 3 and 6) or anti-HA (lanes 2 and 5) antibody, and the coprecipitated RIP protein was detected by Western blotting.
Kinase assays.
HeLa cells or mouse fibroblasts (5 × 105) were treated with TRAIL or GST-TRAIL, respectively, as described in the figure legends. Cells were collected in 300 μl of M2 lysis buffer. IKK complex and JNK1 were immunoprecipitated with anti-IKKα and anti-JNK1 antibodies, respectively. IKK and JNK kinase activities were determined by using 2 μg of GST-IκBα(1–54) and GST–c-Jun(1–79), respectively, as substrates.
Transfected cells were collected in 300 μl of M2 lysis buffer 24 h after transfection as described elsewhere (23). HA-JNK1 and HA-IKKβ were immunoprecipitated with HA antibody, and their kinase activities were determined as described above.
RESULTS
TRAIL induces IKK and JNK activation in HeLa cells.
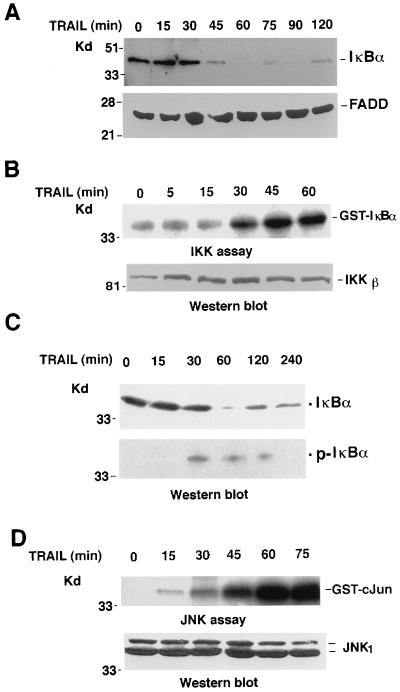
Upon NF-κB-inducing stimulation, IκBα is phosphorylated by IKK and degraded in the proteasome. To investigate whether TRAIL induces IKK activation, a time course of TRAIL treatment in HeLa cells was conducted, and the levels of IκBα protein were detected at different time points after treatment by Western blotting. We found that the IκBα protein level began to decline after 45 min of TRAIL treatment and started to recover by 120 min of treatment; the FADD protein level, measured as a control, showed no significant change (Fig. 1A). Then we tested whether TRAIL-induced IκBα degradation is induced by IKK. To do this, we treated HeLa cells with TRAIL for different times and measured the IKK activity of each sample by in vitro kinase assay with GST-IκBα(1–54) as the substrate (52). As shown in Fig. 1B, IKK activity started to increase after 30 min of treatment and peaked at 45 min posttreatment. Although the kinetics of TRAIL-induced IKK is slower than the kinetics of TNF treatment (8), TRAIL potently activates IKK, suggesting that TRAIL induces IκB degradation through the IKK pathway. This conclusion is further supported by the observation that IκBα is phosphorylated at Ser32 following TRAIL treatment (Fig. 1C). The ability of TRAIL to induce JNK activation was also measured in HeLa cells by in vitro kinase assay with GST–c-Jun(1–79) as the substrate (23). Again, TRAIL induced a slower but similar extent of JNK activation as TNF does in HeLa cells (Fig. 1D). These results indicated that TRAIL, like TNF, activated IKK and JNK in HeLa cells.
FIG. 1.
Activation of IKK and JNK in HeLa cells by TRAIL. (A) Time course of IκBα degradation in TRAIL-treated HeLa cells. Cells were treated with TRAIL (1 μg/ml) and incubated for the indicated time periods. IκBα and FADD were detected by Western blot analysis. (B) Time course of IKK activation in TRAIL-treated HeLa cells. Cells were treated with TRAIL and collected in lysis buffer. Then IKKβ expression was detected by Western blotting (bottom), and its activity was measured by an IKK assay (top). (C) HeLa cells were treated with TRAIL (1 μg/ml) and incubated for the indicated time periods. IκBα and phospho-IκBα were detected by Western blot analysis. (D) Time course of JNK activation in TRAIL-treated HeLa cells. Cells were treated as indicated; then JNK1 expression or activity from each sample was measured by Western blotting (bottom) or kinase assay (top), respectively.
The dominant negative mutant of RIP, RIP(559–671), blocked TRAIL-induced IKK and JNK activation.
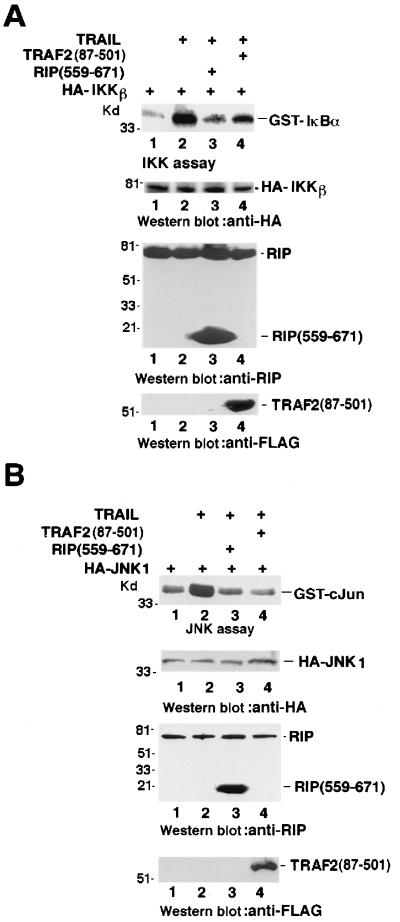
RIP and TRAF2 are essential for TNF-induced activation of NF-κB and JNK (20–23, 31, 36, 41, 51). It has been suggested that TRAF2 plays a similar role in TRAIL-R1- and TRAIL-R2-mediated NF-κB and JNK activation (14). To investigate whether TRAIL-induced IKK activation is mediated by RIP, we tested the effect of the dominant negative mutant of RIP, RIP(559–671), on TRAIL-induced IKK activation in HeLa cells. The role of TRAF2 on TRAIL-induced IKK activation in HeLa cells was also evaluated by overexpression of the dominant negative mutant of TRAF2, TRAF2(87–501). HA-tagged IKKβ was cotransfected with the RIP(559–671) or TRAF2(87–501) expression vector, and its kinase activity was measured by in vitro kinase assay after immunoprecipitation with the anti-HA antibody. In the case of RIP(559–671), the expression vector for the cowpox virus protein CrmA, a potent apoptosis inhibitor, was also included in order to inhibit RIP(559–671)-induced apoptosis. As shown in Fig. 2A, the ectopic expression of RIP(559–671) almost completely abolished TRAIL-induced IKK activation (top panel, lane 3), while overexpression of TRAF2(87–501) only partially inhibited IKK activation after TRAIL treatment (top panel, lane 4). As controls, the CrmA, wild-type (wt) RIP, or wt TRAF2 expression vector was also used to cotransfect cells with HA-IKKβ. The expression of CrmA, wt RIP, or TRAF2 has no effect on TRAIL-induced IKK activation (data not shown). The expression level of HA-IKKβ, RIP(559–671), or Flag-TRAF2(87–501) was detected with anti-HA, anti-RIP, or anti-Flag antibody, respectively. To understand the roles of RIP and TRAF2 in TRAIL-mediated JNK activation, cotransfection experiments were performed with HA-JNK1 and RIP(559–671) or TRAF2(87–501) as described above. HA-JNK1 was immunoprecipitated with the anti-HA antibody, and its kinase activity was determined by in vitro kinase assay. In the presence of either RIP(559–671) or TRAF2(87–501), as detected by Western blotting with anti-RIP or anti-Flag antibody (Fig. 2B), TRAIL-induced JNK activation was completely blocked (Fig. 2B, top). The expression level of HA-JNK was measured by Western blotting with the anti-HA antibody. The CrmA, wt RIP, and wt TRAF2 expression vectors were used as controls. The presence of CrmA, wt RIP, or wt TRAF2 has no effect on TRAIL-induced JNK activation (data not shown). These results indicated that ectopic expression of the dominant negative mutant RIP potently disrupted TRAIL-induced activation of both IKK and JNK, implying that RIP may play an essential role in both IKK and JNK activation by TRAIL treatment. However, because overexpression of dominant negative mutant TRAF2 had more profound, disruptive effect on TRAIL-induced JNK activation than on IKK activation, the function of TRAF2 may be more critical for JNK activation than for IKK activation by TRAIL.
FIG. 2.
Effects of dominant negative mutants of RIP and TRAF2 on TRAIL-induced IKK and JNK activation in HeLa cells. (A) HeLa cells were transfected with 0.5 μg of HA-IKKβ (lanes 1 and 2) or cotransfected with 0.5 μg of HA-IKKβ, 0.2 μg of CrmA, and 1 μg of RIP(559–671) (lane 3) or TRAF2(87–501) (lane 4). Twenty-four hours posttransfection, the cells were treated with TRAIL (1 μg/ml) for 30 min (lanes 2 to 4). IKK activity was detected by kinase assay. The expression of each introduced factor is shown. (B) HeLa cells were transfected with 0.5 μg of HA-JNK1 (lanes 1 and 2) or cotransfected with 0.5 μg of HA-JNK1, 0.2 μg of CrmA, and 1 μg of RIP(559–671) (lane 3) or TRAF2(87–501) (lane 4). Twenty-four hours posttransfection, the cells were treated with TRAIL (1 μg/ml) for 30 min (lanes 2 to 4). Cells were collected, and the expression of HA-JNK1, RIP(559–671) and TRAF2(87–501) was detected by Western blotting. The JNK assay was performed as described in Materials and Methods.
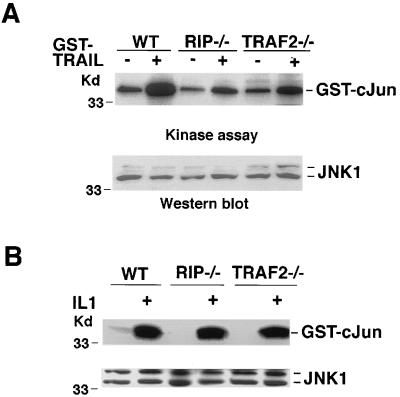
TRAIL failed to induce IKK activation in RIP−/− fibroblasts, while TRAIL-induced JNK activation was impaired in both RIP−/− and TRAF2−/− cells.
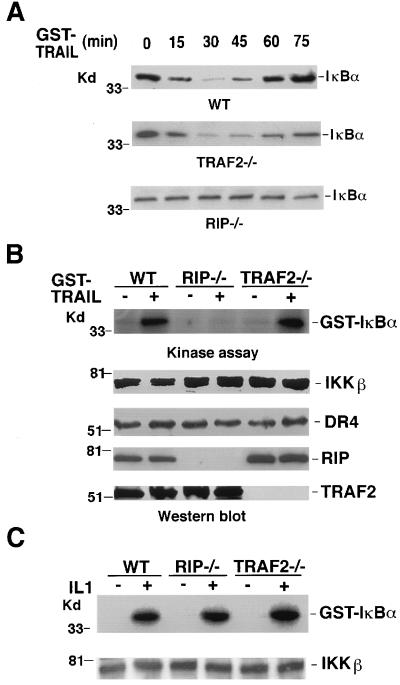
To further evaluate the roles of RIP and TRAF2 in TRAIL-induced NF-κB activation, we investigated IκB degradation by Western blotting and IKK activation by in vitro kinase assay in response to TRAIL treatment in RIP−/− and TRAF2−/− mouse fibroblasts. The wt fibroblasts were used as controls. In these experiments, GST-TRAIL, instead of TRAIL, was used to treat cells, as it exerted better activity in mouse fibroblasts (data not shown). In the wt cells, IκBα degradation was detectable by 15 min after treatment, and most degradation was observed by 30 min after TRAIL treatment (Fig. 3A, top). The IκBα level returned to its normal level by 75 min after TRAIL treatment. In TRAF2−/− cells, IκBα degradation showed similar kinetics (Fig. 3A, middle). However, there was no detectable degradation of the IκBα protein in the RIP−/− cells after TRAIL treatment (Fig. 3A, bottom). The activation of IKK in these cells was also determined. TRAIL treatment efficiently activated IKK in both wt and TRAF2−/− cells (Fig. 3B, top). In contrast, TRAIL-induced IKK activation was barely detected in RIP−/− cells. Since comparable expression levels of IKKα, IKKβ, and one of the major TRAIL receptors, TRAIL-R1, were detected in these three types of cells (Fig. 3B [middle panels] and data not shown), it is unlikely that the decrease of IKK activation in RIP−/− cells resulted from the altered expression of IKK or TRAIL receptor. The protein levels of RIP and TRAF2 were also measured by Western blotting (Fig. 3B, bottom). Furthermore, the absence of the IKK activation in RIP−/− cells is TRAIL specific, because these RIP−/− cells displayed normal IL-1-induced IKK activation as the wt and TRAF2−/− cells did (Fig. 3C). Taken together, these results further supported the observation from the transient transfection experiments: RIP, not TRAF2, plays an essential role in TRAIL-induced IKK activation.
FIG. 3.
RIP−/− cells are insensitive to TRAIL-induced IκBα degradation and IKK activation. (A) Wild-type, RIP−/−, and TRAF2−/− mouse fibroblasts were treated with GST-TRAIL (10 μg/ml) for the indicated time periods. IκBα was detected by Western blot analysis. (B) Wild-type, RIP−/−, and TRAF2−/− mouse fibroblasts were treated with GST-TRAIL (10 μg/ml) for 30 min. IKKβ, DR4, RIP, and TRAF2 were detected by Western blot analysis. The IKK complex was precipitated, and a kinase assay was performed as described in Materials and Methods. Nontreated cells served as controls. (C) Wild-type, RIP−/−, and TRAF2−/− mouse fibroblasts were treated with IL1 (4 ng/ml) for 10 min and subjected to an IKK assay.
Similarly, we used RIP−/− and TRAF2−/− fibroblasts to further determine the functions of RIP and TRAF2 in TRAIL-induced JNK activation. TRAIL activated JNK efficiently in wt fibroblasts, but only marginally in both RIP−/− and TRAF2−/− cells (Fig. 4A, top). Similar levels of JNK1 expression in these cells were detected (Fig. 4A, bottom). These results suggested that both RIP and TRAF2 are required for transducing the TRAIL signal to fully activate JNK. The defectiveness of JNK activation in RIP−/− and TRAF2−/− cells was specific to TRAIL treatment because those RIP−/− and TRAF2−/− cells responded to IL-1 and UV treatment as efficiently as the wt cells did in terms of JNK activation (Fig. 4B and data not shown).
FIG. 4.
TRAIL-induced JNK activation was impaired in both RIP−/− and TRAF2−/− cells. (A) Wild-type, RIP−/−, and TRAF2−/− mouse fibroblasts were treated with GST-TRAIL (10 μg/ml) for 30 min, and a JNK assay was performed. JNK1 was detected by Western blot analysis as described in Materials and Methods. Nontreated cells served as controls. (B) Wild-type, RIP−/−, and TRAF2−/− mouse fibroblasts were treated with IL-1 (4 ng/ml) for 10 min, and a JNK assay was performed. Nontreated cells served as controls.
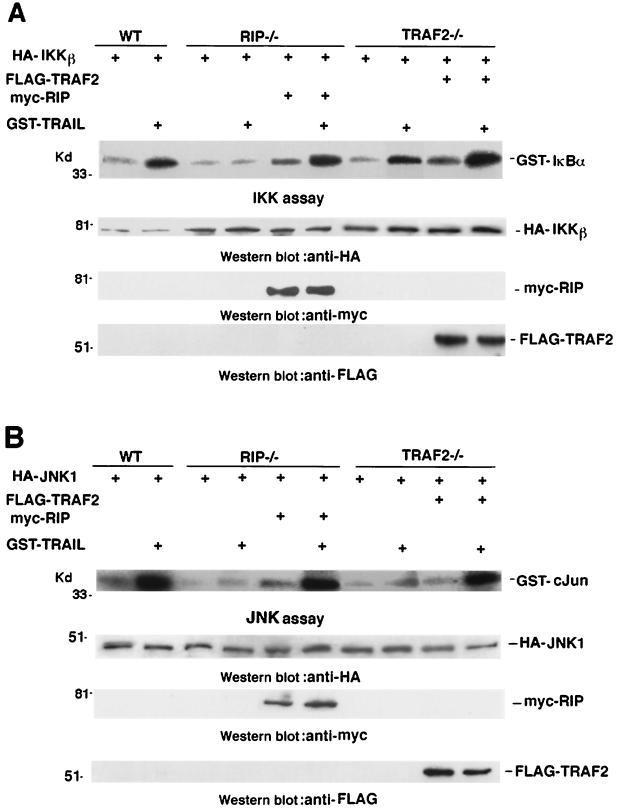
To rule out the possibility that some other defects in the signaling pathway of TRAIL-induced IKK or JNK activation are present in RIP−/− or TRAF2−/− cells, we tested whether TRAIL-induced IKK or JNK activation could be reconstituted in those cells. To examine the reconstitution of TRAIL-induced IKK activation, the expression vector for either Myc-RIP or Flag-TRAF2 was cotransfected with HA-IKKβ into RIP−/− or TRAF2−/− cells, respectively. Following treatment with GST-TRAIL, the transfected HA-IKKβ was immunoprecipitated for in vitro kinase assay. As shown in Fig. 5A, RIP expression could restore the IKK activation in response to TRAIL treatment in RIP−/− cells. Since TRAIL-induced IKK activation was normal in TRAF2−/− cells (Fig. 3B), the expression of TRAF2 had little effect on TRAIL-induced IKK activation in TRAF2−/− cells. Similarly, we also ectopically expressed Myc-RIP or Flag-TRAF2 with HA-JNK1 in RIP−/− or TRAF2−/− cells, respectively, in order to measure the reconstitution of TRAIL-induced JNK activation. In these experiments, HA-JNK1 was immunoprecipitated for in vitro kinase assay. As shown in Fig. 5B, the expression of RIP in RIP−/− cells or the expression of TRAF2 in TRAF2−/− cells restored JNK activation in response to TRAIL. These results indicated that the defects in TRAIL-induced IKK or JNK activation in RIP−/− and TRAF2−/− cells are due to the absence of RIP or TRAF2.
FIG. 5.
Reconstitution of TRAIL-induced IKK and JNK activation in RIP−/− and TRAF2−/− cells. (A) Wild-type, RIP−/−, and TRAF2−/− mouse fibroblasts cells were cotransfected with HA-IKKβ and Myc-RIP or Flag-TRAF2 expression plasmids as indicated. Twenty four hours after transfection, cells were treated with GST-TRAIL (10 μg/ml) for 30 min and subjected to an IKK assay. HA-IKKβ, Myc-RIP, and Flag-TRAF2 were detected by Western blotting. Nontreated cells served as controls. (B) Wild-type, RIP−/−, and TRAF2−/− mouse fibroblasts were cotransfected with HA-JNK1 and Myc-RIP or Flag-TRAF2 expression plasmids as indicated. Cells were treated with GST-TRAIL (10 μg/ml) for 30 min and analyzed by JNK assay. HA-JNK1, Myc-RIP, and Flag-TRAF2 were detected by Western blotting. Nontreated cells served as controls.
RIP is a component of the TRAIL-R1 signaling complex.
It is believed that as for TNF, TRAIL ligation initiates its receptors' trimerization and that this aggregation of TRAIL receptors leads to the recruitment of downstream effector molecules to the receptor signaling complex (1, 15, 28). Since our data suggested that RIP is essential in TRAIL-induced activation of both IKK and JNK, it might be a component of the TRAIL receptor complex. To test this possibility, we first investigated whether RIP interacts with TRAIL receptors by ectopic expression of RIP and TRAIL-R1 in HEK293 cells. Because RIP is recruited to the TNF-R1 complex through TRADD and it has been suggested that FADD is involved in RIP–TRAIL-R1 interaction, we also studied the interaction between RIP and TRAIL-R1 in the presence of TRADD, FADD, or both. In each experiment, Flag-tagged TRAIL-R1 was immunoprecipitated with anti-Flag antibody and the immunoprecipitates were analyzed by Western blotting with an anti-RIP antibody. As shown in Fig. 6A, although RIP and TRAIL-R1 were expressed at similar levels in each transfection, RIP was not coprecipitated with Flag-TRAIL-R1 (lane 2). Even in the presence of FADD or TRADD, immunoprecipitation of Flag-TRAIL-R1 failed to pull down RIP (lanes 3 and 4). However, RIP was efficiently coprecipitated with Flag-TRAIL-R1 when both FADD and TRADD were coexpressed with TRAIL-R1 (lane 5). As a control, when TRAIL-R1 was not coexpressed with RIP, FADD, and TRADD, RIP was not precipitated (lane 6). These results suggested that RIP binds to TRAIL-R1 indirectly. To test whether endogenous RIP is recruited to the TRAIL-R1 complex, we performed coimmunoprecipitation experiments to analyze the TRAIL-R1 complex with cell extracts derived from HeLa cells with or without TRAIL treatment. In these experiments, endogenous TRAIL-R1 was immunoprecipitated with anti-DR4 antibody, and the immunoprecipitates were analyzed with different antibodies. As shown in Fig. 6B, while RIP was not coprecipitated by anti-DR4 antibody from the nontreated cell extract (lane 3), RIP was present in the TRAIL-R1 complex that was immunoprecipitated from the cell extract with TRAIL treatment (lane 6). The control antibody, anti-HA, failed to pull down RIP (lanes 2 and 5). However, no TRADD and FADD were detected in the same TRAIL-R1 complex when the same blot was probed with anti-TRADD and anti-FADD antibodies (data not shown). These results suggested that TRAIL treatment induces the recruitment of RIP to the TRAIL-R1 complex. Although overexpression of TRADD and FADD can help RIP bind to TRAIL-R1, both TRADD and FADD are not in the TRAIL-R1 complex.
Neither RIP nor TRAF2 is required for TRAIL-induced apoptosis.
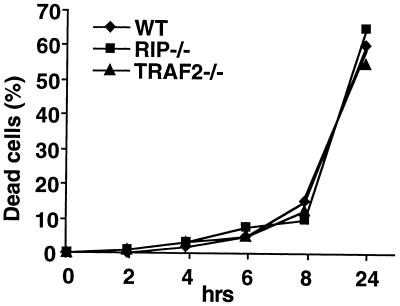
TRAIL is a potent inducer of apoptosis, but the molecular mechanism of TRAIL-induced apoptosis is still unclear (1). Since our study suggested that RIP and TRAF2 are important components of the TRAIL signaling pathway, we next investigated whether RIP and TRAF2 are involved in TRAIL-induced apoptosis. Normally, fibroblasts do not die upon TRAIL treatment, but they can be rendered sensitive to TRAIL in combination with cycloheximide (CHX) treatment. Therefore, to induce apoptosis, the wt, RIP−/−, and TRAF2−/− cells were treated with both GST-TRAIL and CHX. Cells were collected at 0, 2, 4, 6, 8, and 24 h after treatment, and the percentage of apoptosis in each type of cells was determined by trypan blue exclusion staining. As shown in Fig. 7, the absence of RIP or TRAF2 had no effect on TRAIL-induced apoptosis since both RIP−/− and TRAF2−/− cells died to the same extent as the wt cells. These results suggested that both RIP and TRAF2 are not required for TRAIL-induced apoptosis.
FIG. 7.
TRAIL-induced apoptotic cell death in wt, RIP−/−, and TRAF2−/− cells. Cells were treated with GST-TRAIL (10 μg/ml) and CHX (10 μg/ml) for the indicated periods of time. Dead cells were determined by trypan blue staining. The results shown are averages of three independent experiments.
DISCUSSION
Because TRAIL selectively induces apoptosis in tumor or transformed cells but not in normal cells, it has shown great potential to be a valuable tumor therapeutic agent (2, 10, 46). Like other members of the TNF superfamily, TRAIL has other biological functions such as activating transcription factor NF-κB and JNKs (14, 16, 17, 29, 37). Although much effort has been made to investigate the biological functions of TRAIL since it was discovered, the molecular mechanism of TRAIL signaling is still largely unknown. Recently, it was suggested that TRAF2, an important effector of TNF signaling, was involved in both NF-κB and JNK activation induced by overexpression of TRAIL receptors (14). In our study, we reported that another critical effector of TNF signaling, RIP, plays a critical role in TRAIL-induced activation of both IKK and JNK. We also found that while overexpression of the dominant negative mutant TRAF2 blocked TRAIL-induced IKK and JNK activation, the absence of TRAF2 affected TRAIL-induced JNK activation but had little effect on IKK activation. In addition, we also demonstrated that neither RIP nor TRAF2 was required for TRAIL-induced apoptosis.
The death domain kinase RIP is an essential effector for TNF-induced NF-κB activation (20, 41). It has been suggested that RIP plays a similar role in DR3/Apo3-mediated NF-κB activation (1). Here we provided evidence that RIP is also essential in TRAIL-induced NF-κB activation. The dominant negative mutant of RIP efficiently blocked TRAIL-induced IKK activation. In RIP−/− cell lines, no IKK activity was detected following TRAIL treatment. Furthermore, we found that RIP was present in the TRAIL-R1 complex, whose formation is TRAIL dependent. However, because RIP does not directly interact with TRAIL-R1 (Fig. 6A), it seems that the recruitment of RIP requires additional adapter molecules. In previous overexpression experiments, it was shown that the presence of TRADD and FADD resulted in the interaction of TRAIL-R1 and RIP (5). Our results have confirmed this observation (Fig. 6A). But since TRADD and FADD were not found in the TRAIL-R1 complex (Fig. 6B), they may not be the molecules that mediate the endogenous TRAIL-R1–RIP interaction. Consistent with this possibility, it has been shown that FADD is not required for TRAIL-R1-mediated apoptosis (25). Therefore, it is possible that the recruitment of RIP to TRAIL receptors is mediated by other death domain-containing factors.
Previous studies involving overexpression of the dominant negative mutant RIP had shown that RIP was involved in TNF-induced JNK activation (23). However, a study using RIP knockout mice showed that RIP had little effect on TNF-induced JNK activation (20). In this study, however, we found that RIP was required for TRAIL-induced JNK activation. Overexpression of the dominant negative mutant RIP completely abolished TRAIL-induced JNK activation (Fig. 2). In addition, TRAIL-induced JNK activation was greatly decreased in RIP−/− cells (Fig. 4). These data suggested that RIP is involved in IKK and JNK activation by TRAIL treatment. Therefore, RIP is a critical effector in TRAIL signaling.
TRAF2 was initially identified as a component of the TNF-R2 complex and was also found in the TNF-R1 signaling complex (23, 31). Previous studies involving overexpression of TRAF2 and its dominant negative mutant had shown that TRAF2 played a critical role in TNF-induced NF-κB and JNK activation. However, the aforementioned study with a genetic approach reported that removal of TRAF2 caused the diminishment of TNF-induced JNK activation and had only a minor effect on TNF-induced NF-κB activation (51). In this study, we found that TRAF2 had a similar effect on TRAIL signaling: the absence of TRAF2 severely affected TRAIL-induced JNK activation but had no detectable effect on IKK activation (Fig. 3 and 4). But in the overexpression experiments, we found that TRAIL-induced activation of both IKK and JNK was blocked by the dominant negative mutant of TRAF2 (Fig. 2). This observation is consistent with a recent report (14). One possibility is that other TRAF proteins, such as TRAF5, may replace the function of TRAF2 to mediate TRAIL-induced IKK activation. Therefore, the effect of the absence of TRAF2 on TRAIL-induced IKK activation might be minimized by the presence of other TRAF proteins. However, when the dominant negative mutant of TRAF2 is overexpressed, it might also block the function of other TRAF proteins; as a result, overexpression of the dominant negative mutant of TRAF2 inhibits TRAIL-induced IKK activation. Further studies are necessary to elucidate the role of TRAF proteins in TRAIL-induced IKK activation.
It has been reported that FADD is dispensable for TRAIL-induced apoptosis although it is essential for TNF- and Fas-mediated cell death (25, 50, 53). But because overexpression of dominant negative FADD efficiently blocked TRAIL-induced apoptosis (45), it is possible that a FADD-like death factor mediates TRAIL-induced cell death. In this study, we demonstrated that neither RIP nor TRAF2 is required for TRAIL-induced apoptosis (Fig. 7). Although JNK activation is essential for cells to undergo apoptosis in some circumstances, it is unlikely that JNK activation is involved in TRAIL-induced apoptosis since TRAF2−/− cells died to the same extent as wt fibroblasts. On the other hand, because NF-κB activation provides an antiapoptotic effect (4, 23, 43, 48), RIP-mediated NF-κB activation following TRAIL treatment may protect cells against TRAIL-induced apoptosis. Unfortunately, because RIP−/− fibroblasts are insensitive to TRAIL treatment and CHX is necessary to induce death of RIP−/− cells, we failed to evaluate the antiapoptotic effect of NF-κB activation in TRAIL-induced apoptosis with those fibroblasts. However, we found in a previous study that RIP was cleaved by caspase-8 in Fas-, TNF-, and TRAIL-induced apoptosis (22). Importantly, the cleavage of RIP abolished its ability to efficiently activate NF-κB. Therefore, NF-κB activation may also be antiapoptotic in response to TRAIL treatment. This possibility is further supported by the observation that inhibition of NF-κB activation sensitized several types of tumor cells to TRAIL treatment (16, 17).
Taken together, the results of our study shed some light on the molecular mechanisms of TRAIL signaling. We demonstrated that both RIP and TRAF2 are important effectors of TRAIL signaling. In addition, neither RIP nor TRAF2 is required for TRAIL-induced apoptosis. Because TRAIL has been pursued as a potential cancer therapy, knowledge of TRAIL signaling will accelerate this process and help in developing new strategies for improving its therapeutic value.
ACKNOWLEDGMENTS
We thank W. C. Yeh and T. W. Mak for TRAF2−/− fibroblasts and C. Vincenz for DR4 plasmid. We also thank J. Lewis for assistance in manuscript preparation.
REFERENCES
- 1.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A, Pai R C, Fong S, Leung S, Lawrence D A, Marsters S A, Blackie C, Chang L, McMurtrey A E, Hebert A, DeForge L, Koumenis I L, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall R H. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Investig. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 4.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNFα-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhary P M, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-κB pathway. Immunity. 1997;7:821–830. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- 6.Degli-Esposti M A, Dougall W C, Smolak P J, Waugh J Y, Smith C A, Goodwin R G. The novel receptor TRAIL-R4 induces NF-κB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 7.DiDonato J A, Mercurio F, Karin M. Phosphorylation of IκBα precedes but is not sufficient for its dissociation from NF-κB. Mol Cell Biol. 1995;15:1302–1311. doi: 10.1128/mcb.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 9.Emery J G, McDonnell P, Burke M B, Deen K C, Lyn S, Silverman C, Dul E, Appelbaum E R, Eichman C, DiPrinzio R, Dodds R A, James I E, Rosenberg M, Lee J C, Young P R. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 10.Gazitt Y. TRAIL is a potent inducer of apoptosis in myeloma cells derived from multiple myeloma patients and is not cytotoxic to hematopoietic stem cells. Leukemia. 1999;13:1817–1824. doi: 10.1038/sj.leu.2401501. [DOI] [PubMed] [Google Scholar]
- 11.Hsu H, Shu H B, Pan M G, Goeddel D V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 12.Hsu H, Huang J, Shu H B, Baichwal V, Goeddel D V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 13.Hsu H, Xiong J, Goeddel D V. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 14.Hu W H, Johnson H, Shu H B. Tumor necrosis factor-related apoptosis-inducing ligand receptors signal NF-kappaB and JNK activation and apoptosis through distinct pathways. J Biol Chem. 1999;274:30603–30610. doi: 10.1074/jbc.274.43.30603. [DOI] [PubMed] [Google Scholar]
- 15.Hymowitz S G, Christinger H W, Fuh G, Ultsch M, O'Connell M, Kelley R F, Ashkenazi A, de Vos A M. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell. 1999;4:563–571. doi: 10.1016/s1097-2765(00)80207-5. [DOI] [PubMed] [Google Scholar]
- 16.Jeremias I, Debatin K M. TRAIL induces apoptosis and activation of NFkappaB. Eur Cytokine Netw. 1998;9:687–688. [PubMed] [Google Scholar]
- 17.Jeremias I, Kupatt C, Baumann B, Herr I, Wirth T, Debatin K M. Inhibition of nuclear factor kappaB activation attenuates apoptosis resistance in lymphoid cells. Blood. 1998;91:4624–4631. [PubMed] [Google Scholar]
- 18.Karin M, Liu Z G, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 19.Keane M M, Ettenberg S A, Nau M M, Russell E K, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999;59:734–741. [PubMed] [Google Scholar]
- 20.Kelliher M A, Grimm S, Ishida Y, Kuo F, Stanger B Z, Leder P. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 21.Lee S Y, Reichlin A, Santana A, Sokol K A, Nussenzweig M C, Choi Y. TRAF2 is essential for JNK but not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y, Devin A, Rodriguez Y, Liu Z G. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 24.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 25.Marsters S A, Pitti R M, Donahue C J, Ruppert S, Bauer K D, Ashkenazi A. Activation of apoptosis by Apo-2 ligand is independent of FADD but blocked by CrmA. Curr Biol. 1996;6:750–752. doi: 10.1016/s0960-9822(09)00456-4. [DOI] [PubMed] [Google Scholar]
- 26.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 27.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 28.Mongkolsapaya J, Grimes J M, Chen N, Xu X N, Stuart D I, Jones E Y, Screaton G. Structure of the TRAIL-DR5 complex reveals mechanisms conferring specificity in apoptotic initiation. Nat Struct Biol. 1999;6:1048–1053. doi: 10.1038/14935. [DOI] [PubMed] [Google Scholar]
- 29.Muhlenbeck F, Haas E, Schwenzer R, Schubert G, Grell M, Smith C, Scheurich P, Wajant H. TRAIL/Apo2L activates c-Jun NH2-terminal kinase (JNK) via caspase-dependent and caspase-independent pathways. J Biol Chem. 1998;273:33091–33098. doi: 10.1074/jbc.273.49.33091. [DOI] [PubMed] [Google Scholar]
- 30.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 31.Natoli G, Costanzo A, Ianni A, Templeton D J, Woodgett J R, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 32.Pan G, Ni J, Wei Y F, Yu G, Gentz R, Dixit V M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 33.Pan G, O'Rourke K, Chinnaiyan A M, Gentz R, Ebner R, Ni J, Dixit V M. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 34.Pitti R M, Marsters S A, Ruppert S, Donahue D J, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 35.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 36.Reinhard C, Shamoon B, Shyamala V, Williams L T. Tumor necrosis factor α-induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO J. 1997;16:1080–1092. doi: 10.1093/emboj/16.5.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider P, Thome M, Burns K, Bodmer J L, Hofmann K, Kataoka T, Holler N, Tschopp J. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-κB. Immunity. 1997;7:831–836. doi: 10.1016/s1074-7613(00)80401-x. [DOI] [PubMed] [Google Scholar]
- 38.Sheridan J P, Marsters S A, Pitti R M, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray C L, Baker K, Wood W I, Goddard A D, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 39.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 40.Smith C A, Farrah T, Goodwin R G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 41.Ting A T, Pimentel-Muinos F X, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-κB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 42.Tracey K J, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- 43.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-alpha-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 44.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 45.Wajant H, Johannes Haas F J, Siemienski K, Schwenzer R, Schubert G, Weiss T, Grell M, Scheurich P. Dominant-negative FADD inhibits TNFR60-, Fas/Apo1- and TRAIL-R/Apo2-mediated cell death but not gene induction. Curr Biol. 1998;8:113–116. doi: 10.1016/s0960-9822(98)70042-9. [DOI] [PubMed] [Google Scholar]
- 46.Walczak H, Miller R E, Ariail K, Gliniak B, Griffith T S, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin R G, Rauch C T, Schuh J C, Lynch D H. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 47.Walczak H, Degli-Esposti M A, Johnson R S, Smolak P J, Waugh J Y, Boiani N, Timour M S, Gerhart M J, Schooley K A, Smith C A, Goodwin R G, Rauch C T. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 49.Wiley S R, Schooley K, Smolak P J, Din W S, Huang C P, Nicholl J K, Sutherland G R, Smith T D, Rauch C, Smith C A. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 50.Yeh W C, Pompa J L, McCurrach M E, Shu H B, Elia A J, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, El-Deiry W S, Lowe S W, Goeddel D V, Mak T W. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 51.Yeh W C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa J L, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel D V, Mak T W. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 52.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Cado D, Chen A, Kabra N H, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]