Abstract
As the number of confirmed cases and deaths occurring from Coronavirus disease 2019 (COVID-19) surges worldwide, health experts are striving hard to fully comprehend the extent of damage caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although COVID-19 primarily manifests itself in the form of severe respiratory distress, it is also known to cause systemic damage to almost all major organs and organ systems within the body. In this review, we discuss the molecular mechanisms leading to multi-organ failure seen in COVID-19 patients. We also examine the potential of stem cell therapy in treating COVID-19 multi-organ failure cases.
Keywords: SARS-CoV-2, COVID-19, stem cells, mesenchymal, therapeutics, regenerative, blood-brain-barrier, oxidative stress
1. Introduction
The first outbreak of COVID-19 was reported in Wuhan, China, in December 2019. Subsequently, the outbreak spread globally and was declared a pandemic by the World Health Organization in March 2020 [1]. As of October 2021, over 236 million cases have been reported, with 4.8 million deaths worldwide [2]. The mortality rate of COVID-19 is around 2%, and the disease’s spread is exceedingly high [3,4]. A significant proportion of patients display mild symptoms or are asymptomatic [5]. However, the remainder of the cases are severe, especially in the aged and immune-compromised population [6]. Patients with existing comorbidities such as cardiovascular disease, diabetes mellitus, hypertension, renal dysfunction, liver damage, and cancers exhibit poor prognoses [7]. Death is ultimately caused due to acute respiratory distress syndrome (ARDS), septic shock, cardiac damage, renal dysfunction, and multi-organ failure [8,9].
The host immune response is thought to play a vital role in the pathogenesis and clinical expression of COVID-19. While immune suppression is a risk factor for infection, the immune system’s hyperactivation in response to infection can cause severe complications and organ damage [10]. Though vaccines are in various development stages, testing, and distribution, there are no robust therapeutics for either treatment or symptomatic management of the disease yet. Another alarming fact is that many recovered patients will suffer from lasting effects and disability due to COVID-19 [11]. Therefore, alternative therapeutic modalities need to be studied and developed for managing severe outcomes of the disease. The US Food and Drug Administration (FDA) is currently exploring various single-agent and combination treatments for the disease. These include antivirals, cell and gene therapies, immunomodulators, and neutralizing antibodies [12].
Stem cell-based regenerative medicine is one such field that might hold the key to reviving tissue damage caused by COVID-19. Stem cells play a significant role in developmental biology because they can differentiate into many cell types. Another advantage is that mesenchymal stem cells (MSCs) exhibit immunomodulatory qualities, regulating the immune responses in patients [13]. There are currently several stem cell-based clinical trials in various stages of development, of which quite a few have shown promise in treating patients [14]. Therefore, it is crucial to understand the molecular mechanisms associated with COVID-19 mediated multi-organ damage to efficiently apply stem cell therapy for its treatment [15].
2. Current Knowledge
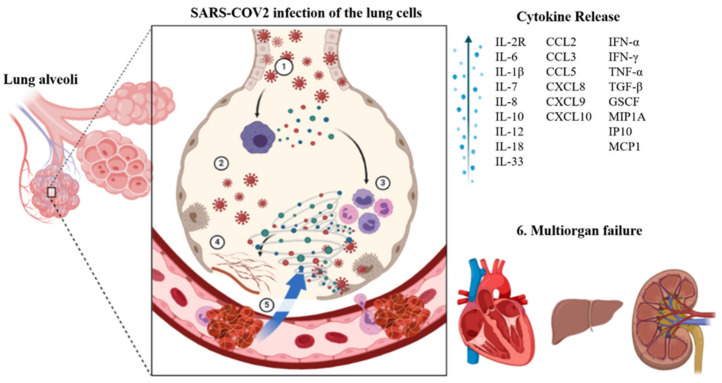
The Angiotensin-converting enzyme 2 (ACE2) receptor plays a crucial role in viral entry into the cells [16,17]. This receptor is found on many cell types present throughout the body, primarily the epithelial cells in the lungs [18] and small intestines [19]. The virus uses its spike proteins to bind to the ACE2 receptor and enter the cell [20]. Once inside a cell, the virus uses the cell’s replication machinery to create several viral copies [21]. These infect neighboring cells leading to loss of physiological function. Once the immune system recognizes an infection, it mounts an immune response to fight that infection. This can lead to inflammation in the infected areas. The immune response usually is well regulated and confined to infected regions [22]. However, in the case of COVID-19, we see a hyperactivation of the immune system, whereby it loses the ability to distinguish between self and foreign, therefore causing damage to healthy uninfected tissue. As damage to the tissue builds up, irreparable damage to the organ can occur, causing the organ to fail. This cascade can occur sequentially or in parallel within other organ systems leading to multi-organ failure and death (Figure 1) [23,24,25,26].
Figure 1.
SARS-CoV-2 infection of lungs leading to cytokine storm resulting in multi-organ failure. ① Lung cells infected by the coronavirus, ② Macrophages recognize the virus and release cytokines, ③ Cytokines attract additional immune cells, such as lymphocytes and monocytes, and they generate more cytokines, causing a storm-like cycle of inflammation that damages lung cells. ④ Damage can also occur because of fibrin production (clot formation), ⑤ Blood vessels surrounding the lungs are weakened, allowing fluid to leak into the lung cavities, resulting in respiratory failure, 6. Due to the heightened coagulation state, blood that is meant to flow to other organs such as the heart, liver, and kidneys will be obstructed, causing these organs to fail and lead to multiorgan failure.
2.1. Immunological Complications
Cytokines are proteins that recruit immune cells to the site of infection. A moderate immune response leads to a rise in proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and interleukin 1 (IL-1), and a host of lymphocytes and T cells. However, in a severe immune reaction, we see a sudden extreme induction of these proinflammatory cytokines, also known as the cytokine storm. Cytokine storms lead to widespread inflammation in the body [27]. As a result, vascular membranes become highly permeable, giving rise to fluid movement from blood vessels into organ tissue [28]. The resulting effect is an organ/tissue reaching the brink of failure due to the lack of blood and oxygen. Anti-inflammatory therapies targeting the cytokine storm are suggested to decrease the mortality of COVID-19 patients. Downstream signaling pathways such as JAK/STAT, NF-κB, and NLRP3 as well as cytokine targets such as IL-6, IL-1 β, IFN-γ, TNF-α, IL-12/23, IL-17A, GM-CSF (granulocyte-macrophage colony-stimulating factor) are being explored for treating COVID-19 induced cytokine storm [29].
2.2. Hematological Complications
Lymphopenia, or reduction of white blood cells in the blood, is a characteristic clinical hallmark of COVID-19 infection. Typically, CD4, CD8, T, and NK cell counts are significantly decreased. Other observed effects are abnormalities of granulocytes and monocytes along with hypercoagulability leading to thrombocytopenia. An enhanced threat of disseminated intravascular coagulation, specifically, the propensity of clot formation throughout the body, is reported in patients [30,31]. Increased D-dimer concentrations in the non-survivors COVID-19 patients were typical [32,33]. Treatment approaches targeting thrombin, coagulation factor Xa, and thrombin receptor [34] should be considered to reduce SARS-CoV-2 micro thrombosis.
2.3. Respiratory Complications
Injury to the lungs in COVID-19 may occur through direct or indirect mechanisms. Various cell types in the airways and lungs exhibit the ACE2 receptor. These include type II alveolar cells, ciliated epithelial cells, and pulmonary vascular endothelium. SARS-CoV-2 can infect these cell types and cause cell death directly. Another mechanism involves activating angiotensin-II, resulting in increased vascular leakiness and pulmonary edema leading to pneumonia [35]. The immune system also plays a critical role in the clinical manifestation of COVID-19 fibroproliferative lung disease [36]. The release of proinflammatory cytokines and activation of macrophages and dendritic cells triggers cell death of infected cells [37,38]. Another factor responsible for pulmonary failure is microthrombi formation in the vasculature of the lungs [39]. Lung biopsies of COVID-19 patients showed an activated complement system in the alveolar epithelial cells with acute and chronic inflammation [38].
2.4. Cardiac Complications
Patients with cardiac complications caused due to COVID-19 are termed as suffering from Acute COVID-19 Cardiovascular Syndrome (ACovCS) [40]. There are two different mechanisms for an individual to develop ACovCS. A hypoxia-induced myocardial injury can occur due to a cytokine storm’s precipitation, as discussed before. This is accompanied by intracellular acidosis and increased oxidative stress. On the other hand, myocarditis can occur through ACE2 facilitated direct infection of cardiac myocytes resulting in arrhythmias or cardiac arrest [41,42]. However, it is important to note that in a majority of individuals COVID-19 related myocarditis did not accompany immune cell infiltration pointing to cell death from obstructed blood flow likely due to constricted pericytes or clumping of red blood cells [43]. The histopathological analysis reported fibrosis and myocyte hypertrophy in most COVID-19 patients [44]. Thus, patients with existing cardiovascular disease and hypertension are at heightened risk for mortality due to ischemia and myocardial necrosis factors. Comorbidities such as obesity and diabetes have also been found to indirectly cause adverse cardiac complications. For example, obesity arising out of COVID-19 quarantining is linked with stress, causing a persistent inflammatory state that leads to the deposition of atherosclerotic plaques, rendering obese individuals more susceptible to cardiovascular events [45,46]. A study reported a greater incidence of diabetes in patients with COVID-19 associated cardiac injury [47].
2.5. Renal Complications
Patients with renal complications often suffer from acute kidney injury (AKI) and proteinuria. Lymphopenia, macrophage activation syndrome, hypercoagulability, and cytokine storm, among other factors, can promote AKI. Other potential mechanisms can include sepsis, endothelial impairment, and rhabdomyolysis. Furthermore, the microthrombi formation may lead to acute ischemic injury and hypoxia, resulting in renal tubular necrosis [48,49,50]. Various reports confirmed that the development of AKI significantly increased the mortality rate in COVID-19 patients. SARS-CoV-2 infection increased blood urea nitrogen (BUN) and serum creatinine levels, leading to renal failure in many patients [48]. Autopsies of COVID-19 patients detected fibrin thrombi, indicating severe endothelial damage in the glomerular region [51].
2.6. Hepatic Complications
Studies have observed that the severity of COVID-19 infection corresponds to the severity of hepatic complications in patients. Very high levels of liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were found in the blood of patients with severe COVID-19 infections [52]. Cytotoxic T cell activation and dysregulated immune responses increased liver biomarkers and COVID-19 severity [53]. One mechanism for viral entry to the liver might be through the ACE2 receptors found on cells lining the bile duct. Other liver injury mechanisms are thought to be linked to oxygen deprivation, the toxicity of antiviral/antimalarial treatments, and inflammatory cells’ passage to the liver [54,55].
2.7. Neurological Complications
ACE 2 receptors responsible for attachment and subsequent internalization of SARS-CoV-2 are also found in glial cells in the brain and spinal neurons. Hence, the virus can damage the neuronal tissue and result in hypoxic brain injury and immune-mediated damage to the Central Nervous system (CNS) [56].
A study conducted by the National Hospital, Queen Square, London, and University College London Hospital detailed clinical and paraclinical data on neurological disorders observed during and after the COVID-19 infection. Patients displayed a wide range of CNS and Peripheral Nervous System (PNS) complications together with neuroinflammation. These pathological manifestations can be a direct effect of the virus on the nervous system, para or post-infectious immune-mediated disease, and neurological complications of the systemic impacts of COVID-19 infection. Out of 43 COVID-19 patients, ten patients above the age of 50 suffered septic encephalopathy and presented symptoms such as confusion, psychosis, seizures, etc. Complications such as difficulty speaking, deteriorating vision, cognitive abilities, and Acute Disseminated Encephalomyelitis (ADEM) with hemorrhagic transformations were reported in critically ill patients [57]. Alteration in mental status was frequent in patients with severe infection, especially in those requiring intensive care management. However, this was documented more in older groups, which might be suffering from latent neurocognitive degenerative disease or multiple medical comorbidities, often associated with sepsis and hypoxia. MRI diagnosis in severe cases showed abnormalities in the brain’s temporal lobe with hemorrhagic lesions. Furthermore, features like vascular and ADEM-like pathology, with macrophages and axonal injury, were reported.
Further, seizures were reported with viral encephalitis and subsequent activation of neuro-inflammatory pathways in critically ill patients. Lymphocytic panencephalitis, meningitis, and brainstem inflammatory change with neuronal loss were observed in post-mortem reports [58]. A recent report on neurological complications of children of age < 18 years described a distinct neurological syndrome associated with lesions in the corpus callosum’s splenium. These patients were previously healthy but had the onset of neurological symptoms after the COVID-19 infection. Symptoms observed were encephalopathy, headaches, brainstem-cerebellar signs, muscle weakness, and reduced reflexes [59].
To summarize, the neurological manifestations of COVID-19 can be divided into direct and indirect effects. Symptoms like anosmia, hypogeusia meningitis, encephalitis, cerebral vasculitis, and myalgia result from a direct viral invasion of the CNS and PNS. Encephalopathy arising out of hypoxia, cytokine storm, and hypercoagulable state leading to stroke are some of the indirect manifestations seen in the CNS [60]. Another critical factor that needs to be considered is the impairment of the blood–brain barrier (BBB) due to endothelial dysfunction. This may give rise to the virus’s invasion and inflammatory cells into the CNS, leading to further neurological manifestations [54,61].
3. Stem Cell Therapy
Stem cells are undifferentiated or partially differentiated cells in the body that can differentiate into various cell types and proliferate indefinitely (self-renewal). Their primary function is to serve as a reserve for the body [62]. Stem cells can be of embryonic or adult origin. Based on their functionality, stem cells can be grouped into three categories, pluripotent, hematopoietic, and mesenchymal types [63,64,65]. Pluripotent stem cells can differentiate and mature into any of the three fundamental groups of cells important in human developmental biology. Embryonic stem cells are used for in vitro fertilization purposes [66]. Hematopoietic stem cells can differentiate into various types of blood cells. They are obtained from bone marrow or umbilical cord blood and are used in bone marrow transplants. However, both these types of stem cells are currently not used to treat COVID-19. Lastly, MSCs are non-hematopoietic cells that can be differentiated into skeletal tissue such as muscle, bone, cartilage, fat, etc. These cells have immunomodulatory capabilities and have been approved as treatments for a host of autoimmune diseases [67,68]. Additionally, the therapeutic effects of stem cells were recently ascribed to their ability to replace damaged cells. However, we now know that stem cells’ pro-regenerative quality is also due to paracrine functions and the ability to release microvesicles. Microvesicles are known to contain growth factors, bioactive lipids, anti-apoptotic factors which enhance cell function and stimulate angiogenesis in damaged tissues. They are also known to transfer proteins, mRNA, and microRNA between cells [69].
MSCs can be isolated from bone marrow, placenta, umbilical cord blood, and adipose tissue of the same individual (autologous) or another individual (allogeneic) [70]. These are then cultured in vitro and can be injected back into the diseased body. Once inside, they secrete a host of anti-inflammatory mediators that can accelerate tissue repair and revival [71]. MSCs are most likely to have favorable effects when treating acute inflammatory conditions [72]. Conditions frequently encountered in COVID-19 patients, such as cytokine storm, ARDS, and sepsis, are likely to be prime candidates for MSC-based therapy.
3.1. Immunomodulatory and Regenerative Effects of MSCs
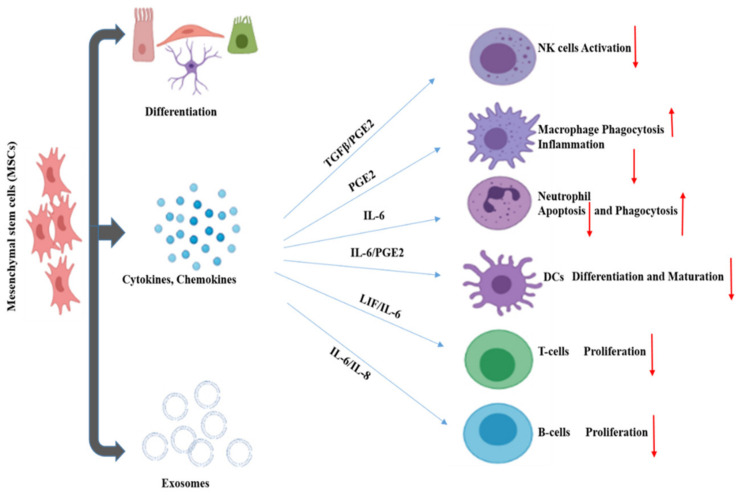
MSCs are referred to as “guardians of inflammation” because of their immunomodulatory effect through the secretion of cytokines, chemokines, growth factors, exosomes, etc. MSCs regulate the inflammatory microenvironment through cell-to-cell contact and the secretion of regulatory molecules. These affect the activation, maturation, proliferation, differentiation, and effector functions of various immune cells involved in innate and adaptive immunity. Innate immunity is mediated through NK cells, macrophages, neutrophils. On the other hand, adaptive immunity is facilitated by T cells and B cells (Figure 2). Dendritic cells (DC) act as the connecting link between innate and adaptive immunity.
Figure 2.
Immunomodulatory effects of mesenchymal stem cells.
NK cells secrete cytokines like IFN-γ and exhibit cytotoxic functions in response to viral infection. MSCs can reduce the proliferation of NK cells, inhibiting their cytotoxic functions through key mediators such prostaglandin E2 (PGE2), indolamine 2,3-dioxygenase (IDO), and human leukocyte antigen G5 (HLA-G5) [73]. Macrophages play a vital role in innate immunity by engulfing foreign agents or aberrant cells. There are mainly two forms of activated macrophages grouped into M1 and M2. M1 exhibits a proinflammatory response in contrast to M2, which displays an anti-inflammatory response. PGE2 secreted by MSC influences the macrophage transition from proinflammatory M1 into an anti-inflammatory M2. M2 macrophage expresses high levels of anti-inflammatory cytokines, reduces levels of TNF-α, and IL-12 with higher phagocytic activity [74]. In the inflammatory process, neutrophils generate more reactive oxygen species (ROS) and reduce antioxidant levels. MSCs secrete IL-6, which reduces ROS levels without affecting the phagocytic activity of neutrophils [75]. T-cells, once activated, proliferate and secrete inflammatory cytokines and chemokines. MSCs facilitate their immunomodulatory activity by recruiting local helper (Th) and effector T cells in the inflammatory environment via Chemokine (C-X-C motif) ligands-CXCL9 and CXCL10. B-cells are vital for humoral immunity and secrete antibodies when stimulated. MSCs inhibit B cell activation, proliferation, and differentiation during inflammation through contact inhibition [76,77,78].
MSCs not only play a role in immune regulation, but also the regeneration and reconstruction of tissue. MSCs have differentiation properties conducive to tissue regeneration and can secrete hepatocyte growth factor, vascular endothelial growth factor, and keratinocyte growth factor. These functions can promote the regeneration of type II alveolar epithelial cells [79]. This shows a potential use for MSCs in recovery for severe COVID-19 cases wherein alveolar injury has occurred. It is suggested that MSCs suppress the over-activated inflammatory response, promote recovery of lung function, and potentially influence the progress of pulmonary fibrosis. MSCs have already been shown to significantly contribute to the recovery of patients from severe COVID-19 in Phase 1 clinical trial [14]. A larger phase 2/3 trial is in progress, with 100 participants recruited to evaluate the safety and efficacy of human umbilical cord-derived MSCs as a treatment for severe COVID-19 cases [80]. Additionally, MSCs and other stem cell types and derivatives have been indicated to be capable of promoting regeneration in other tissues, such as vascular [81], renal [82], hepatic [83], and neurological [84]. Through their immune regulatory functions, and role in contributing to tissue repair MSCs represent a promising area for the treatment of COVID-19 [81].
3.2. Clinical Trials
The treatment potential of stem cell therapy has been widely explored in immunological, cardiovascular, renal, pulmonary, and hepatic diseases at the preclinical level. However, the current bulk of clinical data is insufficient to demonstrate the unequivocal efficacy of stem cell therapy in patients with complex diseases like organ failure. Patients with dysfunctional renal and hepatic organs are likely to need organ transplants toward their disease’s end-stage. Kidney failure in chronic kidney disease (CKD) or AKI can be attributed to various complicating factors, including diabetes and heart disease. Similarly, liver failure may be caused by multiple factors such as cirrhosis, Hepatitis B or C, and hemochromatosis, etc. However, there are not enough kidney and liver donors available for the number of needing patients. In this backdrop, stem cell therapy has emerged as a promising option for these patients. Studies have shown minimal adverse events and, in some cases, even positive outcomes for patients undergoing stem cell treatments for liver conditions [85,86].
MSC therapies have been approved in several countries for treating several diseases due to their immuno-modulatory effects. They are potent candidates to treat severe cases of COVID-19 and have been used in clinical trials for therapeutic purposes [87]. A list of completed and active clinical trials using MSC therapy to treat COVID-19 associated conditions is provided in Table 1 and Table 2, respectively. Similarly, we have outlined the available results of specific studies in Table 3.
Table 1.
List of completed clinical trials studying MSC therapy’s safety and efficacy to treat COVID-19 conditions.
| Title | Interventions | Location | URL (accessed on 15 October 2021) | |
|---|---|---|---|---|
| 1 | Mesenchymal Stem Cells Therapy in Patients With COVID-19 Pneumonia [88] | Other: Mesenchymal stem cells | Turkey | https://ClinicalTrials.gov/show/NCT04713878 |
| 2 | A Proof of Concept Study for the DNA Repair Driven by the Mesenchymal Stem Cells in Critical COVID-19 Patients [89] | Biological: Mesenchymal Stem Cells Transplantation | Turkey | https://ClinicalTrials.gov/show/NCT04898088 |
| 3 | Treatment With Human Umbilical Cord-derived Mesenchymal Stem Cells for Severe Corona Virus Disease 2019 (COVID-19) [80] | Biological: UC-MSCs|Biological: Saline containing 1% Human serum albuminï¼ solution without UC-MSCsï | China | https://ClinicalTrials.gov/show/NCT04288102 |
| 4 | A Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Autologous Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection Against COVID-19 [90] | Biological: HB-adMSCs | United States | https://ClinicalTrials.gov/show/NCT04349631 |
| 5 | A Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Allogeneic Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection Against COVID-19 [91] | Biological: HB-adMSCs|Other: Placebos | United States | https://ClinicalTrials.gov/show/NCT04348435 |
| 6 | Mesenchymal Stem Cells for the Treatment of COVID-19 [92] | Biological: PrimePro|Other: Placebo | United States | https://ClinicalTrials.gov/show/NCT04573270 |
| 7 | Treatment of COVID-19 Associated Pneumonia With Allogenic Pooled Olfactory Mucosa-derived Mesenchymal Stem Cells [93] | Biological: Allogenic pooled olfactory mucosa-derived mesenchymal stem cells|Other: Standard treatment according to the Clinical protocols | Belarus | https://ClinicalTrials.gov/show/NCT04382547 |
| 8 | Use of UC-MSCs for COVID-19 Patients [94] | Biological: Umbilical Cord Mesenchymal Stem Cells + Heparin along with best supportive care.|Other: Vehicle + Heparin along with best supportive care | United States | https://ClinicalTrials.gov/show/NCT04355728 |
| 9 | An Exploratory Study of ADR-001 in Patients With Severe Pneumonia Caused by SARS-CoV-2 Infection [95] | Biological: Mesenchymal stem cell | Japan | https://ClinicalTrials.gov/show/NCT04522986 |
| 10 | Therapeutic Study to Evaluate the Safety and Efficacy of DW-MSC in COVID-19 Patients [96] | Drug: allogeneic mesenchymal stem cell|Other: Placebo | Indonesia | https://ClinicalTrials.gov/show/NCT04535856 |
| 11 | Extracellular Vesicle Infusion Treatment for COVID-19 Associated ARDS [97] | Biological: DB-001|Other: Intravenous normal saline | United States | https://ClinicalTrials.gov/show/NCT04493242 |
| 12 | A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia [98] | Biological: MSCs-derived exosomes | China | https://ClinicalTrials.gov/show/NCT04276987 |
| 13 | Investigational Treatments for COVID-19 in Tertiary Care Hospital of Pakistan [99] | Procedure: Therapeutic Plasma exchange|Biological: Convalescent Plasma|Drug: Tocilizumab|Drug: Remdesivir|Biological: Mesenchymal stem cell therapy | Pakistan | https://ClinicalTrials.gov/show/NCT04492501 |
| 14 | Clinical Use of Stem Cells for the Treatment of COVID-19 [100] | Biological: MSC Treatment|Biological: Saline Control | Turkey | https://ClinicalTrials.gov/show/NCT04392778 |
| 15 | Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia [101] | Drug: EXO 1 inhalation|Drug: EXO 2 inhalation|Drug: Placebo inhalation | Russian Federation | https://ClinicalTrials.gov/show/NCT04491240 |
| 16 | Menstrual Blood Stem Cells in Severe COVID-19 [102] | Biological: Allogeneic human menstrual blood stem cells secretome|Other: Intravenous saline injection | Islamic Republic of Iran | https://ClinicalTrials.gov/show/NCT05019287 |
| 17 | Cellular Immuno-Therapy for COVID-19 Acute Respiratory Distress Syndrome [103] | Biological: Mesenchymal Stromal Cells | Canada | https://ClinicalTrials.gov/show/NCT04400032 |
Table 2.
List of active clinical trials studying the safety and efficacy of MSC therapy to treat COVID-19 induced conditions that have completed recruitment as of October 2021.
| Title | Interventions | Locations | URL (accessed on 15 October 2021) | |
|---|---|---|---|---|
| 1 | Safety and Efficacy of Mesenchymal Stem Cells in the Management of Severe COVID-19 Pneumonia [105] | Biological: Umbilical cord-derived mesenchymal stem cells|Biological: Placebo | https://ClinicalTrials.gov/show/NCT04429763 | |
| 2 | Novel Coronavirus Induced Severe Pneumonia Treated by Dental Pulp Mesenchymal Stem Cells [106] | Biological: Dental pulp mesenchymal stem cells | https://ClinicalTrials.gov/show/NCT04302519 | |
| 3 | NestaCell® Mesenchymal Stem Cell to Treat Patients with Severe COVID-19 Pneumonia [107] | Biological: NestaCell®|Biological: Placebo | Brazil | https://ClinicalTrials.gov/show/NCT04315987 |
| 4 | Safety and Effectiveness of Mesenchymal Stem Cells in the Treatment of Pneumonia of Coronavirus Disease 2019 [108] | Drug: Oseltamivir|Drug: hormones|Device: oxygen therapy|Procedure: mesenchymal stem cells | China | https://ClinicalTrials.gov/show/NCT04371601 |
| 5 | Bone Marrow-Derived Mesenchymal Stem Cell Treatment for Severe Patients with Coronavirus Disease 2019 (COVID-19) [109] | Biological: BM-MSCs|Biological: Placebo | China | https://ClinicalTrials.gov/show/NCT04346368 |
| 6 | Use of Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome Caused by COVID-19 [110] | Biological: Mesenchymal Stem Cells derived from Wharton Jelly of Umbilical cords | Mexico | https://ClinicalTrials.gov/show/NCT04456361 |
| 7 | Study of Allogeneic Adipose-Derived Mesenchymal Stem Cells for Treatment of COVID-19 Acute Respiratory Distress [111] | Biological: COVI-MSC|Drug: Placebo | https://ClinicalTrials.gov/show/NCT04905836 | |
| 8 | Efficacy of Infusions of MSC From Wharton Jelly in the SARS-CoV-2 (COVID-19) Related Acute Respiratory Distress Syndrome [112] | Biological: Ex vivo expanded Wharton’s Jelly Mesenchymal Stem Cells|Biological: Placebo | https://ClinicalTrials.gov/show/NCT04625738 | |
| 9 | Study of Allogeneic Adipose-Derived Mesenchymal Stem Cells to Treat Post COVID-19 “Long Haul” Pulmonary Compromise [113] | Biological: COVI-MSC|Biological: Placebo | https://ClinicalTrials.gov/show/NCT04992247 | |
| 10 | Study of Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Severe COVID-19 [114] | Biological: UC-MSCs|Drug: Placebo | China | https://ClinicalTrials.gov/show/NCT04273646 |
| 11 | Clinical Study for Subjects With COVID-19 Using Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells [115] | Biological: Autologous adipose-derived stem cells | https://ClinicalTrials.gov/show/NCT05017298 | |
| 12 | Expanded Access Protocol on Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicle Infusion Treatment for Patients With COVID-19 Associated ARDS [116] | Biological: Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicles Infusion Treatment | https://ClinicalTrials.gov/show/NCT04657458 | |
| 13 | Clinical Trial to Assess the Safety and Efficacy of Intravenous Administration of Allogeneic Adult Mesenchymal Stem Cells of Expanded Adipose Tissue in Patients with Severe Pneumonia Due to COVID-19 [117] | Drug: Allogeneic and Expanded Adipose Tissue-Derived Mesenchymal Stem Cells | Spain | https://ClinicalTrials.gov/show/NCT04366323 |
| 14 | Study to Evaluate the Efficacy and Safety of AstroStem-V in Treatment of COVID-19 Pneumonia [118] | Drug: AstroStem-V | https://ClinicalTrials.gov/show/NCT04527224 | |
| 15 | Autologous Adipose-derived Stem Cells (AdMSCs) for COVID-19 [119] | Biological: autologous adipose-derived stem cells | https://ClinicalTrials.gov/show/NCT04428801 | |
| 16 | Adipose Mesenchymal Cells for Abatement of SARS-CoV-2 Respiratory Compromise in COVID-19 Disease [120] | Biological: Autologous Adipose MSC’s | https://ClinicalTrials.gov/show/NCT04352803 | |
| 17 | Umbilical Cord Tissue (UC) Derived Mesenchymal Stem Cells (MSCs) Versus Placebo to Treat Acute Pulmonary Inflammation Due to COVID-19 [121] | Biological: UCMSCs|Other: Placebo | United States | https://ClinicalTrials.gov/show/NCT04490486 |
| 18 | Efficacy and Safety Study of Allogeneic HB-adMSCs for the Treatment of COVID-19 [122] | Biological: HB-adMSC|Other: Placebo | United States | https://ClinicalTrials.gov/show/NCT04362189 |
| 19 | Study of Intravenous COVI-MSC for Treatment of COVID-19-Induced Acute Respiratory Distress [123] | Biological: COVI-MSC|Drug: Placebo | https://ClinicalTrials.gov/show/NCT04903327 | |
| 20 | Mesenchymal Stem Cells (MSCs) in Inflammation-Resolution Programs of Coronavirus Disease 2019 (COVID-19) Induced Acute Respiratory Distress Syndrome (ARDS) [124] | Biological: MSC | Germany | https://ClinicalTrials.gov/show/NCT04377334 |
| 21 | Study of the Safety of Therapeutic Tx with Immunomodulatory MSC in Adults With COVID-19 Infection Requiring Mechanical Ventilation [125] | Biological: BM-Allo. MSC|Biological: Placebo | United States | https://ClinicalTrials.gov/show/NCT04397796 |
| 22 | Use of hUC-MSC Product (BX-U001) for the Treatment of COVID-19 With ARDS [126] | Biological: Human umbilical cord mesenchymal stem cells + best supportive care|Other: Placebo control + best supportive care | https://ClinicalTrials.gov/show/NCT04452097 | |
| 23 | The Use of Exosomes for the Treatment of Acute Respiratory Distress Syndrome or Novel Coronavirus Pneumonia Caused by COVID-19 [127] | Drug: MSC-exosomes delivered intravenously every other day on an escalating dose: (2:4:8)|Drug: MSC-exosomes delivered intravenously every other day on an escalating dose (8:4:8)|Drug: MSC-exosomes delivered intravenously every other day (8:8:8) | United States | https://ClinicalTrials.gov/show/NCT04798716 |
| 24 | Safety and Feasibility of Allogenic MSC in the Treatment of COVID-19 [128] | Biological: Mesenchymal Stromal Cells infusion | https://ClinicalTrials.gov/show/NCT04467047 | |
| 25 | ACT-20 in Patients with Severe COVID-19 Pneumonia [129] | Biological: ACT-20-MSC|Biological: ACT-20-CM|Biological: Placebo | https://ClinicalTrials.gov/show/NCT04398303 | |
| 26 | Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration (REALIST) (COVID-19) [130] | Biological: Human umbilical cord derived CD362 enriched MSCs|Biological: Placebo (Plasma-Lyte 148) | United Kingdom | https://ClinicalTrials.gov/show/NCT03042143 |
| 27 | Safety and Efficiency of Method of Exosome Inhalation in COVID-19 Associated Pneumonia [131] | Drug: EXO 1 inhalation|Drug: EXO 2 inhalation|Drug: Placebo inhalation | Russian Federation | https://ClinicalTrials.gov/show/NCT04602442 |
| 28 | Mesenchymal Stromal Cell Therapy for The Treatment of Acute Respiratory Distress Syndrome [132] | Drug: Mesenchymal Stromal Stem Cells-KI-MSC-PL-205 | Sweden | https://ClinicalTrials.gov/show/NCT04447833 |
| 29 | MSCs in COVID-19 ARDS [133] | Biological: Remestemcel-L|Drug: Placebo | United States | https://ClinicalTrials.gov/show/NCT04371393 |
| 30 | Cell Therapy Using Umbilical Cord-derived Mesenchymal Stromal Cells in SARS-CoV-2-related ARDS [134] | Biological: Umbilical cord Wharton’s jelly derived human|Other: NaCl 0.9% | France | https://ClinicalTrials.gov/show/NCT04333368 |
| 31 | Stem Cell Educator Therapy Treat the Viral Inflammation in COVID-19 [135] | Combination Product: Stem Cell Educator-Treated Mononuclear Cells Apheresis | https://ClinicalTrials.gov/show/NCT04299152 | |
| 32 | Multiple Dosing of Mesenchymal Stromal Cells in Patients with ARDS (COVID-19) [136] | Biological: Mesenchymal stromal cells|Other: Placebo | United States | https://ClinicalTrials.gov/show/NCT04466098 |
Table 3.
Results of completed clinical trials studying MSC therapy to treat COVID-19 conditions.
| Therapy | Side effects of therapy | Results | No. of Patients |
|---|---|---|---|
| Exosomes (ExoFlo)-allogeneic-bone marrow-derived-mesenchymal stem cells [138] |
Hypoxic respiratory failure, pulmonary embolism, acute renal failure, and expiration. | Improved PaO2/FiO2 ratio. Substantial reduction in levels of the CRP, ferritin, acute phase reactants, and D-dimer. Reductions in neutrophil count. Increase in lymphocyte count, including subsets staining positive for CD3+, CD4+, CD8+. |
24 |
Preclinical and early clinical trials have demonstrated systemic infusions and bronchial instillations as a route of MSC administration for treating ARDS and other respiratory complications. MSC therapy can suppress cytokine storms and lung inflammation by promoting endogenous repair. Adipose-derived MSCs (AD-MSCs), Umbilical cord MSCs (UC-MSCs), bone marrow-derived MSCs (BM-MSCs), and other MSCs, as well as exosomes from MSCs, are being tested in clinical trials. UC-MSCs are the most effective for treating COVID-19 patients due to their proliferative capability and immunomodulatory effects. In a recent clinical study, laboratory tests of C-reactive protein (CRP), alanine aminotransferase (ALT), creatinine, serum ferritin (SF), and platelets before and after the UC-MSCs treatment at days 0, 3, and 7 were recorded for both experimental and control group. In the UC-MSCs treatment group, there was a decline of IL-6 within three days after UC-MSCs infusion, which remained stable for the following four days. The partial pressure of arterial oxygen: percentage of inspired oxygen (PaO2/FiO2) ratio improved in most severe cases. Representative chest CT scan images showed controlled lung lesions within six days, which completely disappeared within two weeks of treatment. A reduced trend in the levels of proinflammatory cytokines was noted within 14 days [104].
In another study, a double-blind, phase 1/2a, randomized, controlled trial was performed in subjects with ARDS secondary to COVID-19. Twenty-four subjects (12 per group) were recruited for this study. At 28 days post the last infusion, patient survival was 91% and 42% in the UC-MSC and control groups, respectively (p = 0.015). No serious adverse events were observed related to UC-MSC infusions [137]. Although several studies have been completed, their results have not been declared. As such, several concerns regarding the safety and efficacy of MSCs treatment for COVID-19 associated lung disease are unanswered. For example, we do not know the best administration route, whether intravenous, intramuscular, or through the nasopharyngeal route. Apart from this, there is no consensus on critical factors such as MSC tissue of origin, type of culture environment, and dosing.
4. Future Directions
The field of stem cell therapy for treating COVID-19 is gaining a lot of traction. As of October 2021, 100 studies in various countries were displayed on the clinicaltrials.gov website. During a pandemic, as in the case of COVID-19, treatment options are often accelerated and provided emergency authorization to save as many lives as possible. However, it is difficult to ascertain the cause and effect of therapy and outcome based on observational studies alone. Therefore, it is crucial to conduct randomized, double-blinded, placebo-control trials to fully ascertain the efficacy of stem cell therapy as a viable option for COVID-19. Furthermore, it is essential to know the underlying molecular mechanisms through which stem cells fight disease. As such, future studies, both preclinical and clinical with mechanistic approaches, would propel the field further in the right direction.
SARS-CoV-2 attracts leukocytes to the site of infection, thereby initiating the immune response with cytokines’ help. It is known that an increase in ROS accompanies an increase in the immune response. This causes oxidative stress, cell apoptosis, lipid peroxidation, and protein oxidation, further worsening the immune response [139,140]. We hypothesize that facilitating ROS elimination through a better understanding of antioxidant pathways may prevent the oxidative injury caused by SARS-CoV-2 infection. Many studies support the antioxidant and regenerative properties of MSCs [141,142]. A key transcriptional factor, nuclear factor erythroid 2-related factor 2 (NRF2), has been studied for its involvement in regulating antioxidant signals [143]. A recent study published by Olagnier et al. showed suppression of NRF2 in COVID-19 patients, thereby strengthening our hypothesis [144]. Therefore, further studies focusing on NRF2 as a molecular target for treating COVID-19 related complications can provide additional insights into the disease.
5. Conclusions
Patients with severe COVID-19 infection often develop multi-organ failure. The damage to organs and organ systems is either through direct infection or hampered physiological processes in response to the infection. It is crucial to consider the immune system as the focal point to understand better and integrate the other organs’ complications. Given the immunomodulatory properties of stem cells, it is essential to conduct further research to study stem cell therapy’s potential in alleviating COVID-19 multi-organ failure.
Acknowledgments
Figures created using www.biorender.com.
Abbreviations
| ACE2 | Angiotensin-Converting Enzyme 2 |
| ACovCS | Acute COVID-19 Cardiovascular Syndrome |
| AKI | Acute Kidney Injury |
| ALT | Alanine Aminotransferase |
| ARDS | Acute Respiratory Distress Syndrome |
| AST | Aspartate Aminotransferase |
| BBB | Blood–brain barrier |
| BUN | Blood Urea Nitrogen |
| CD4 | Cluster of Differentiation 4 |
| CD8 | Cluster of Differentiation 8 |
| CKD | Chronic Kidney Disease |
| CNS | Central Nervous System |
| COVID-19 | Coronavirus Disease 2019 |
| DIC | Disseminated Intravascular Coagulation |
| FDA | Food and Drug Administration |
| FiO2 | Percentage of inspired oxygen |
| IFN-γ | Interferon Gamma |
| IL-1 | Interleukin 1 |
| IL-1RA | Interleukin Receptor type 1 |
| IL-6 | Interleukin 6 |
| IP-10 | Interferon Inducible Cytokine IP10 |
| MCP-1 | Monocyte Chemoattractant Protein 1 |
| MIP-1 | Macrophage Inflammatory Protein 1-α |
| MSCs | Mesenchymal Stem Cells |
| NK | Natural Killer |
| NRF2 | Nuclear factor erythroid 2-Related Factor 2 |
| PaO2 | Partial Pressure of arterial oxygen |
| PNS | Peripheral Nervous System |
| ROS | Reactive oxygen species |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome CoronaVirus 2 |
| TNF-α | Tumor Necrosis Factor-alpha |
Author Contributions
A.B., conceived the study and prepared the drafting of the manuscript. S.R., B.N. and S.M. edited and revised the manuscript. L.C., assisted with the drafting and editing of the manuscript, oversaw the entire project, and provided funding support. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institutes of Health/National Institute on Drug Abuse 2R01DA029121-01A1, 1R01DA049737-01, and National Institute of Neurological Disorders and Stroke 1R01NS117906-01 to Dr. Luca Cucullo.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio Med. Atenei Parm. 2020;91:157. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roser M., Ritchie H., Ortiz-Ospina E., Hasell J. Coronavirus Disease (COVID-19)–Statistics and Research. Our World Data. 2020. [(accessed on 24 October 2021)]. Available online: https://ourworldindata.org/coronavirus.
- 3.Mahase E. Coronavirus: COVID-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 4.Tindale L.C., Stockdale J.E., Coombe M., Garlock E.S., Lau W.Y.V., Saraswat M., Zhang L., Chen D., Wallinga J., Colijn C. Evidence for transmission of COVID-19 prior to symptom onset. eLife. 2020;9:e57149. doi: 10.7554/eLife.57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G., Li W., He X., Cao Y. Asymptomatic and Presymptomatic Infectors: Hidden Sources of Coronavirus Disease 2019 (COVID-19) Clin. Infect. Dis. 2020;71:2018. doi: 10.1093/cid/ciaa418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395:1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020;5:1–8. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585:339–341. doi: 10.1038/d41586-020-02598-6. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration Coronavirus Treatment Acceleration Program (CTAP) [(accessed on 15 October 2021)];2020 Available online: https://www.fda.gov/drugs/coronavirus.
- 13.Golchin A., Seyedjafari E., Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: Present or future. Stem Cell Rev. Rep. 2020;16:427–433. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng F., Xu R., Wang S., Xu Z., Zhang C., Li Y., Yang T., Shi L., Fu J., Jiang T., et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: A phase 1 clinical trial. Signal Transduct. Target. Ther. 2020;5:172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multi-organ response. Curr. Probl. Cardiol. 2020 doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C., et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, thereceptor of SARS-CoV-2. Biorxiv. 2020 doi: 10.1101/2020.01.26.919985e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G.V., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz J.H. Hypothesis: Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J. Travel Med. 2020:27. doi: 10.1093/jtm/taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perlman S., Netland J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein K.E., Khan Z., Giani J.F., Cao D.Y., Bernstein E.A., Shen X.Z. Angiotensin-converting enzyme in innate and adaptive immunity. Nat. Rev. Nephrol. 2018;14:325. doi: 10.1038/nrneph.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J., Zhong S., Liu J., Li L., Li Y., Wu X., Li Z., Deng P., Zhang J., Zhong N., et al. Detection of severe acute respiratory syndrome coronavirus in the brain: Potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y.-T., Shao S.-C., Hsu C.-K., Wu I.-W., Hung M.-J., Chen Y.-C. Incidence of acute kidney injury in COVID-19 infection: A systematic review and meta-analysis. Crit. Care. 2020;24:1–4. doi: 10.1186/s13054-020-03009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chua R.L., Lukassen S., Trump S., Hennig B.P., Wendisch D., Pott F., Debnath O., Thürmann L., Kurth F., Völker M.T., et al. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020;38:970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 26.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet (Lond. Engl.) 2020;395:1033. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meduri G.U., Kohler G., Headley S., Tolley E., Stentz F., Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS: Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 29.Yang L., Xie X., Tu Z., Fu J., Xu D., Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021;6:255. doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.José R.J., Williams A.E., Chambers R.C. Proteinase-activated receptors in fibroproliferative lung disease. Thorax. 2014;69:190–192. doi: 10.1136/thoraxjnl-2013-204367. [DOI] [PubMed] [Google Scholar]
- 31.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: A pooled analysis. Thromb. Haemost. 2020;120:876. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J. Thromb. Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antoniak S., Owens A.P., Baunacke M., Williams J.C., Lee R.D., Weithäuser A., Sheridan P.A., Malz R., Luyendyk J.P., Esserman D.A., et al. PAR-1 contributes to the innate immune response during viral infection. J. Clin. Investig. 2013;123:1310–1322. doi: 10.1172/JCI66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Maat S., De Mast Q., Jan Danser A.H., Van De Veerdonk F.L., Maas C. Seminars in Thrombosis and Hemostasis. Thieme Medical Publishers; New York, NY, USA: 2020. Impaired Breakdown of Bradykinin and Its Metabolites as a Possible Cause for Pulmonary Edema in COVID-19 Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercer P.F., Williams A.E., Scotton C.J., José R.J., Sulikowski M., Moffatt J.D., Murray L.A., Chambers R.C. Proteinase-activated receptor-1, CCL2, and CCL7 regulate acute neutrophilic lung inflammation. Am. J. Respir. Cell Mol. Biol. 2014;50:144–157. doi: 10.1165/rcmb.2013-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatia M., Zemans R.L., Jeyaseelan S. Role of chemokines in the pathogenesis of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2012;46:566–572. doi: 10.1165/rcmb.2011-0392TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao T., Hu M., Zhang X., Li H., Zhu L., Liu H., Dong Q., Zhang Z., Wang Z., Hu Y., et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. MedRxiv. 2020 doi: 10.1101/2020.03.29.20041962. [DOI] [Google Scholar]
- 39.Chen J., Wang X., Zhang S., Liu B., Wu X., Wang Y., Wang X., Yang M., Sun J., Xie Y. Characteristics of Acute Pulmonary Embolism in Patients with COVID-19 Associated Pneumonia from the City of Wuhan. Clin. Appl. Thromb./Hemost. 2020 doi: 10.1177/1076029620936772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T., Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: From vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fremont-Smith M., Gherlone N., Smith N., Tisdall P., Ricke D.O. Models for COVID-19 Early Cardiac Pathology Following SARS-CoV-2 Infection. Int. J. Infect. Dis. 2021 doi: 10.1016/j.ijid.2021.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradley B.T., Maioli H., Johnston R., Chaudhry I., Fink S.L., Xu H., Najafian B., Deutsch G., Lacy J.M., Williams T., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abbas A.M., Fathy S.K., Fawzy A.T., Salem A.S., Shawky M.S. The mutual effects of COVID-19 and obesity. Obes. Med. 2020;19:100250. doi: 10.1016/j.obmed.2020.100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nasi M., Patrizi G., Pizzi C., Landolfo M., Boriani G., Dei Cas A., Cicero A.F., Fogacci F., Rapezzi C., Sisca G., et al. The role of physical activity in individuals with cardiovascular risk factors: An opinion paper from Italian Society of Cardiology-Emilia Romagna-Marche and SIC-Sport. J. Cardiovasc. Med. (Hagerstown) 2019;20:631–639. doi: 10.2459/JCM.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 47.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z., Wu M., Yao J., Guo J., Liao X., Song S., Li J., Duan G., Zhou Y., Wu X., et al. Caution on kidney dysfunctions of 2019-nCoV patients. MedRxiv. 2020 doi: 10.1101/2020.02.08.20021212. [DOI] [Google Scholar]
- 50.Naicker S., Yang C.-W., Hwang S.-J., Liu B.-C., Chen J.-H., Jha V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y., Yi F., Yang H.C., Fogo A.B., Nie X., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Zheng L., Liu L., Zhao M., Xiao J., Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 53.Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol. Hepatol. 2020;5:529. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dorche M.S., Huot P., Osherov M., Wen D., Saveriano A., Giacomini P., Antel J.P., Mowla A. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J. Neurol. Sci. 2020;417:117085. doi: 10.1016/j.jns.2020.117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L., et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varatharaj A., Thomas N., Ellul M., Davies N.W., Pollak T., Tenorio E.L., Sultan M., Easton A., Breen G., Zandi M., et al. UK-wide surveillance of neurological and neuropsychiatric complications of COVID-19: The first 153 patients. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdel-Mannan O., Eyre M., Löbel U., Bamford A., Eltze C., Hameed B., Hemingway C., Hacohen Y. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77:1440–1445. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Virhammar J., Kumlien E., Fällmar D., Frithiof R., Jackmann S., Sköld M.K., Kadir M., Frick J., Lindeberg J., Olivero-Reinius H., et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020;95:445–449. doi: 10.1212/WNL.0000000000010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berger J.R. COVID-19 and the nervous system. J. Neurovirology. 2020;26:143–148. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blau H., Brazelton T., Weimann J. The evolving concept of a stem cell: Entity or function? Cell. 2001;105:829–841. doi: 10.1016/S0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- 63.Lagasse E., Connors H., Al-Dhalimy M., Reitsma M., Dohse M., Osborne L., Wang X., Finegold M., Weissman I.L., Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 64.Tuan R.S., Boland G., Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 2002;5:1–14. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okita K., Ichisaka T., Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 66.Lin G., OuYang Q., Zhou X., Gu Y., Yuan D., Li W., Liu G., Liu T., Lu G. A highly homozygous and parthenogenetic human embryonic stem cell line derived from a one-pronuclear oocyte following in vitro fertilization procedure. Cell Res. 2007;17:999–1007. doi: 10.1038/cr.2007.97. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Q., Ren H., Han Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J. Cell. Immunother. 2016;2:3–20. doi: 10.1016/j.jocit.2014.12.001. [DOI] [Google Scholar]
- 68.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ratajczak M.Z., Kucia M., Jadczyk T., Greco N.J., Wojakowski W., Tendera M., Ratajczak J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26:1166–1173. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- 70.Wagner W., Wein F., Seckinger A., Frankhauser M., Wirkner U., Krause U., Blake J., Schwager C., Eckstein V., Ansorge W., et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Schmuck E.G., Koch J.M., Centanni J.M., Hacker T.A., Braun R.K., Eldridge M., Hei D.J., Hematti P., Raval A.N. Biodistribution and clearance of human mesenchymal stem cells by quantitative three-dimensional cryo-imaging after intravenous infusion in a rat lung injury model. Stem Cells Transl. Med. 2016;5:1668–1675. doi: 10.5966/sctm.2015-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prockop D.J., Oh J.Y. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol. Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uccelli A., Pistoia V., Moretta L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 74.Cho D.I., Kim M.R., Jeong H.Y., Jeong H.C., Jeong M.H., Yoon S.H., Kim Y.S., Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 2014;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salami F., Tavassoli A., Mehrzad J., Parham A. Immunomodulatory effects of mesenchymal stem cells on leukocytes with emphasis on neutrophils. Immunobiology. 2018;223:786–791. doi: 10.1016/j.imbio.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp. Cell Res. 2006;312:2169–2179. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 77.Yi T., Song S.U. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch. Pharmacal Res. 2012;35:213–221. doi: 10.1007/s12272-012-0202-z. [DOI] [PubMed] [Google Scholar]
- 78.Blazquez R., Sanchez-Margallo F.M., de la Rosa O., Dalemans W., Álvarez V., Tarazona R., Casado J.G. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front. Immunol. 2014;5:556. doi: 10.3389/fimmu.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 80.Shi L., Huang H., Lu X., Yan X., Jiang X., Xu R., Wang S., Zhang C., Yuan X., Xu Z., et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6:58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang J., Jia Z. Cell-based therapy in lung regenerative medicine. Regen Med. Res. 2014;2:7. doi: 10.1186/2050-490X-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brasile L., Henry N., Orlando G., Stubenitsky B. Potentiating Renal Regeneration Using Mesenchymal Stem Cells. Transplantation. 2019;103:307–313. doi: 10.1097/TP.0000000000002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Apostolou K.G., Papanikolaou I.G., Katselis C., Feretis T., Kletsas D., Konstadoulakis M.M., Lymperi M., Saetta A.A., Tsikalakis S., Agrogiannis G., et al. Undifferentiated Adipose Tissue Stem Cell Transplantation Promotes Hepatic Regeneration, Ameliorates Histopathologic Damage of the Liver, and Upregulates the Expression of Liver Regeneration- and Liver-Specific Genes in a Rat Model of Partial Hepatectomy. Stem Cells Int. 2018;2018:1393607. doi: 10.1155/2018/1393607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu S., Xie Y.Y., Wang L.D., Tai C.X., Chen D., Mu D., Cui Y.Y., Wang B. A multi-channel collagen scaffold loaded with neural stem cells for the repair of spinal cord injury. Neural Regen Res. 2021;16:2284–2292. doi: 10.4103/1673-5374.310698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.A Phase I/II Safety and Tolerability Dose Escalation Study of Autologous Stem Cells to Patients with Liver Insufficiency. [(accessed on 1 August 2021)];2008 Available online: https://clinicaltrials.gov/ct2/show/results/NCT00655707.
- 86.Pietrosi G., Vizzini G., Gerlach J., Chinnici C., Luca A., Amico G., D’amato M., Conaldi P.G., Petri S.L., Spada M., et al. Phases I-II matched case-control study of human fetal liver cell transplantation for treatment of chronic liver disease. Cell Transplant. 2015;24:1627–1638. doi: 10.3727/096368914X682422. [DOI] [PubMed] [Google Scholar]
- 87.Lin F., Ichim T.E., Pingle S., Jones L.D., Kesari S., Ashili S. Mesenchymal stem cells as living anti-inflammatory therapy for COVID-19 related acute respiratory distress syndrome. World J. Stem Cells. 2020;12:1067. doi: 10.4252/wjsc.v12.i10.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mesenchymal Stem Cells Therapy in Patients with COVID-19 Pneumonia. NCT04713878. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04713878.
- 89.A Proof of Concept Study for the DNA Repair Driven by the Mesenchymal Stem Cells in Critical COVID-19 Patients. NCT04898088. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04898088.
- 90.A Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Autologous Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection against COVID-19. NCT04349631. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04349631.
- 91.A Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Allogeneic Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection Against COVID-19. NCT04348435. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04348435.
- 92.Mesenchymal Stem Cells for the Treatment of COVID-19. NCT04573270. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04573270.
- 93.Treatment of COVID-19 Associated Pneumonia with Allogenic Pooled Olfactory Mucosa-Derived Mesenchymal Stem Cells. NCT04382547. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04382547.
- 94.Use of UC-MSCs for COVID-19 Patients. NCT04355728. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04355728.
- 95.An Exploratory Study of ADR-001 in Patients with Severe Pneumonia Caused by SARS-CoV-2 Infection. NCT04522986. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04522986.
- 96.Therapeutic Study to Evaluate the Safety and Efficacy of DW-MSC in COVID-19 Patients. NCT04535856. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04535856.
- 97.Extracellular Vesicle Infusion Treatment for COVID-19 Associated ARDS. NCT04493242. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04493242.
- 98.Raghav A., Khan Z.A., Upadhayay V.K., Tripathi P., Gautam K.A., Mishra B.K., Ahmad J., Jeong G.B. Mesenchymal Stem Cell-Derived Exosomes Exhibit Promising Potential for Treating SARS-CoV-2-Infected Patients. Cells. 2021;10:587. doi: 10.3390/cells10030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Investigational Treatments for COVID-19 in Tertiary Care Hospital of Pakistan. NCT04492501. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04492501.
- 100.Clinical Use of Stem Cells for the Treatment of COVID-19. NCT04392778. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04392778.
- 101.Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia. NCT04491240. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04491240.
- 102.Menstrual Blood Stem Cells in Severe COVID-19. NCT05019287. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT05019287.
- 103.Cellular Immuno-Therapy for COVID-19 Acute Respiratory Distress Syndrome. NCT04400032. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04400032.
- 104.Shi L., Huang H., Lu X., Yan X., Jiang X., Xu R., Wang S., Zhang C., Yuan X., Xu Z., et al. Treatment with human umbilical cord-derived mesenchymal stem cells for COVID-19 patients with lung damage: A randomised, double-blind, placebo-controlled phase 2 trial. CellR4 Repair Replace Regen Reprogram. 2020;8:e2839. doi: 10.32113/cellr4_20204_2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Safety and Efficacy of Mesenchy-Mal Stem Cells in the Manage-Ment of Severe COVID-19 Pneu-Monia; NCT04429763. NCT04429763. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04429763.
- 106.Novel Coronavirus Induced Severe Pneumonia Treated by Dental Pulp Mesenchymal Stem Cells. NCT04302519. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04302519.
- 107.NestaCell® Mesenchymal Stem Cell to Treat Patients with Severe COVID-19 Pneumonia. NCT04315987. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04315987.
- 108.Safety and Effectiveness of Mes-Enchymal Stem Cells in the Treatment of Pneumonia of Coro-Navirus Disease 2019. NCT04371601. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04371601.
- 109.Bone Marrow-Derived Mesen-Chymal Stem Cell Treatment for Severe Patients with Coronavirus Disease 2019 (COVID-19) NCT04346368. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04346368.
- 110.Use of Mesenchymal Stem Cells in Acute Respiratory Distress Syn-Drome Caused by COVID-19. NCT04456361. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04456361.
- 111.Study of Allogeneic Adi-Pose-Derived Mesenchymal Stem Cells for Treatment of COVID-19 Acute Respiratory Distress. NCT04905836. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04905836.
- 112.Efficacy of Infusions of MSC From Wharton Jelly in the SARS-CoV-2 (COVID-19) Related Acute Respir-Atory Distress Syndrome. NCT04625738. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04625738.
- 113.Study of Allogeneic Adi-Pose-Derived Mesenchymal Stem Cells to Treat Post COVID-19 “Long Haul” Pulmonary Com-Promise. NCT04992247. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04992247.
- 114.Study of Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Severe COVID-19. NCT04273646. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04273646.
- 115.Clinical Study for Subjects with COVID-19 Using Allogeneic Adi-Pose Tissue-Derived Mesenchy-Mal Stem Cells. NCT05017298. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT05017298.
- 116.Expanded Access Protocol on Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicle Infusion Treatment for Patients with COVID-19 Associated ARDS. NCT04657458. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04657458.
- 117.Clinical Trial to Assess the Safety and Efficacy of Intravenous Ad-ministration of Allogeneic Adult Mesenchymal Stem Cells of Ex-Panded Adipose Tissue in Pa-Tients with Severe Pneumonia Due to COVID-19. NCT04366323. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04366323.
- 118.Study to Evaluate the Efficacy and Safety of AstroStem-V in Treat-Ment of COVID-19 Pneumonia. NCT04527224. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04527224.
- 119.Autologous Adipose-Derived Stem Cells (AdMSCs) for COVID-19. NCT04428801. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04428801.
- 120.Adipose Mesenchymal Cells for Abatement of SARS-CoV-2 Res-piratory Compromise in COVID-19 Disease. NCT04352803. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04352803.
- 121.Umbilical Cord Tissue (UC) De-Rived Mesenchymal Stem Cells (MSCs) Versus Placebo to Treat Acute Pulmonary Inflammation Due to COVID-19. NCT04490486. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04490486.
- 122.Efficacy and Safety Study of Al-logeneic HB-adMSCs for the Treatment of COVID-19. NCT04362189. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04362189.
- 123.Study of Intravenous COVI-MSC for Treatment of COVID-19-Induced Acute Respir-atory Distress. NCT04903327. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04903327.
- 124.Mesenchymal Stem Cells (MSCs) in Inflammation-Resolution Pro-Grams of Coronavirus Disease 2019 (COVID-19) Induced Acute Respiratory Distress Syndrome (ARDS) NCT04377334. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04377334.
- 125.Study of the Safety of Therapeutic Tx with Immunomodulatory MSC in Adults with COVID-19 Infection Requiring Mechanical Venti-lation. NCT04397796. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04397796.
- 126.Use of hUC-MSC Product (BX-U001) for the Treatment of COVID-19 with ARDS. NCT04452097. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04452097.
- 127.The Use of Exosomes for the Treatment of Acute Respiratory Distress Syndrome or Novel Coronavirus Pneumonia Caused by COVID-19. NCT04798716. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04798716.
- 128.Safety and Feasibility of Allogenic MSC in the Treatment of COVID-19. NCT04467047. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04467047.
- 129.ACT-20 in Patients with Severe COVID-19 Pneumonia. NCT04398303. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04398303.
- 130.Gorman E., Shankar-Hari M., Hopkins P., Tunnicliffe W.S., Perkins G.D., Silversides J., McGuigan P., Jackson C., Boyle R., McFerran J., et al. Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration in COVID-19 (REALIST-COVID-19): A structured summary of a study protocol for a randomised, controlled trial. Trials. 2020;21:462. doi: 10.1186/s13063-020-04416-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Safety and Efficiency of Method of Exosome Inhalation in COVID-19 Associated Pneumonia. NCT04602442. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04602442.
- 132.Mesenchymal Stromal Cell Ther-apy for The Treatment of Acute Respiratory Distress Syndrome. NCT04447833. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04447833.
- 133.MSCs in COVID-19 ARDS. NCT04371393. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04371393.
- 134.Cell Therapy Using Umbilical Cord-Derived Mesenchymal Stro-mal Cells in SARS-CoV-2-Related ARDS. NCT04333368. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04333368.
- 135.Stem Cell Educator Therapy Treat the Viral Inflammation in COVID-19. NCT04299152. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04299152.
- 136.Multiple Dosing of Mesenchymal Stromal Cells in Patients with ARDS (COVID-19) NCT04466098. [(accessed on 15 October 2021)]; Available online: https://ClinicalTrials.gov/show/NCT04466098.
- 137.Lanzoni G., Linetsky E., Correa D., Cayetano S.M., Marttos A.C., Alvarez R.A., Gil A.A., Poggioli R., Ruiz P., Hirani K., et al. Umbilical Cord Mesenchymal Stem Cells for COVID-19 ARDS: A Double Blind, Phase 1/2a, Randomized Controlled Trial. Stem Cells Transl. Med. 2021;10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qin M., Cao Z., Wen J., Yu Q., Liu C., Wang F., Zhang J., Yang F., Li Y., Fishbein G., et al. An Antioxidant Enzyme Therapeutic for COVID-19. Adv. Mater. 2020;32:2004901. doi: 10.1002/adma.202004901. [DOI] [PubMed] [Google Scholar]
- 141.Jiang W., Tan Y., Cai M., Zhao T., Mao F., Zhang X., Xu W., Yan Z., Qian H., Yan Y. Human umbilical cord MSC-derived exosomes suppress the development of CCl4-induced liver injury through antioxidant effect. Stem Cells Int. 2018;2018:6079642. doi: 10.1155/2018/6079642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stavely R., Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020;9:985–1006. doi: 10.1002/sctm.19-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sivandzade F., Prasad S., Bhalerao A., Cucullo L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Olagnier D., Farahani E., Thyrsted J., Blay-Cadanet J., Herengt A., Idorn M., Hait A., Hernaez B., Knudsen A., Iversen M.B., et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020;11:4938. doi: 10.1038/s41467-020-18764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]