Abstract
Simple Summary
Breast cancer (BrCa) is a heterogeneous disease and has important variability according to ethnicity and race with respect to incidence, clinical characteristics, and prognosis. Numerous epidemiological studies indicate that BrCa and it’s also related to environmental factors. We, therefore, undertook a systematic review of the literature regarding BrCa risk in women who used OCs based on case-control studies carried out in the years 2009–March 2020 and then performed a meta-analysis of relevant data. Increased BrCa risk was associated with early menarche, nulliparous, non-breastfeeding, older age at first parity, postmenopause, obesity, smoking, and family history of BrCa.
Abstract
To perform a meta-analysis of case-control studies that addressed the association between oral contraceptive pills (OC) use and breast cancer (BrCa), PubMED (MEDLINE), Embase, and the Cochrane Library were searched to identify case-control studies of OC and BrCa published between 2009 and 2020. We used the DerSimonian–Laird method to compute pooled odds ratios (ORs) and confidence intervals (CIs), and the Mantel–Haenszel test to assess the association between OC use and cancer. Forty-two studies were identified that met the inclusion criteria and we included a total of 110,580 women (30,778 into the BrCa group and 79,802 into the control group, of which 15,722 and 38,334 were using OC, respectively). The conducted meta-analysis showed that the use of OC was associated with a significantly increased risk of BrCa in general, OR = 1.15, 95% CI: 1.01 to 1.31, p = 0.0358. Regarding other risk factors for BrCa, we found that increased risk was associated significantly with early menarche, nulliparous, non-breastfeeding, older age at first parity, postmenopause, obesity, smoking, and family history of BrCa. Despite our conclusion that birth control pills increase the cancer risk being supported by extensive previous studies and meta-analyzes, further confirmation is required.
Keywords: oral contraceptives, breast cancer, risk, risk factors, breast malignancies
1. Introduction
Among malignant tumors, BrCa is the leading type of cancer that affects women in most countries. According to the predictions of the WHO’s International Agency for Research on Cancer, the specific age-standardized incidence rate for cancer of the female breast worldwide is 46 cases per 100,000 women. Herein, incidence rates are elevated in Australia/New Zealand (94), Western Europe (93), Northern Europe (90), and North America (85). In contrast, incidence rates in sub-Saharan African regions, particularly in Eastern (30) and Middle Africa (28), as well as South-Central Asia (26), are considerably lower [1].
BrCa is a heterogeneous disease and has important variability according to ethnicity and race with respect to incidence, clinical characteristics, and prognosis. The majority of BrCa cases are sporadic; however, it is estimated that approximately 5–10% have a genetic predisposition related to, among others, family cancer histories for first-degree relatives or genetic mutation carrier status [2,3]. Of these, 40% are due to mutations in either of the two tumor suppressor genes BRCA (BReast CAncer gene) 1 and 2, which are localized on chromosomes 17g21 and 13q12.3, respectively [4,5].
Furthermore, variations in the risk of developing BrCa have been reported in relation to molecular subtypes, defined by estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression [6,7,8].
The variations in the incidence of BrCa between countries may depend on differences in the incidence and distribution of risk factors, as well as the level of early detection and screening carried out in these countries. Numerous epidemiological studies indicate that BrCa is related to reproductive factors, such as early menarche below 12 years of age, late menopause above 55 years of age, nulliparity, miscarriages before the first full-term pregnancy, late age at first birth, infertility, and hormone usage. BrCa is also related to environmental factors that include high socio-cultural level, obesity, selected dietary habits, alcohol consumption, low physical activity, and exposure to ionizing radiation (used for therapeutic purposes) [9,10,11,12,13,14,15].
Our first meta-analysis on the effects of OC on BrCa risk covered the period 1960–2010 [16]. The meta-analysis included studies that examined the mixture of pills from four generations: the first-generation progestin included norethindrone, lynestrenol, ethynodiol diacetate, and norethisterone; the second-generation included levonorgestrel, and norgestrel, introduced in the 1990s; the third-generation included desogestrel, gestodene, and norgestodene introduced in the 1990s; and the last approved fourth-generation ethinyloestradiol/drospirone and estradiol valerate/dienogest. The continuation of the previous publication is a meta-analysis covering the period from 2009 to 2020. Third- and fourth-generation OC dominated this meta-analysis. Both publications constitute a research cycle for assessing the impact of contraception on BrCa. Although the relationship between OC use and BrCa risk has been extensively studied, this topic remains an important research area. Indeed, this issue is still pending final explanation [17,18]. We, therefore, undertook a systematic review of the literature regarding BrCa risk in women who used OCs based on case-control studies carried out in the years 2009–March 2020 and then performed a meta-analysis of relevant data.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
A systematic review and meta-analysis of published case-control studies assessing the impact of OC on the risk of female BrCa development was performed based on the guidance “Preferred Reporting Items for Systematic Reviews and Meta-analysis for 2015 protocols (PRISMA-P 2015)” [19]. Our research was limited to articles published from 2009 to March 2020.
The contents of the medical databases of PubMed (MEDLINE), Embase, and the Cochrane Library were reviewed to identify studies related to the assumptions of our work. To provide a complete overview of the available relevant studies, we additionally scrutinized references to previously published review articles, meta-analyses, and other publications.
The following inclusion criteria were established in the selection of studies: (i) full-text articles published between 2009 and 2020; (ii) case-control design (design (population- and hospital-based)); (iii) data on the correlation between OC use and BrCa; (iv) articles written in English; (v) data included in the articles were sufficient to calculate the odds ratio (OR) and 95% confidence interval (CI); (vi) at least 20 subjects use of OC included in the case group; and (vii) if there was an overlap in the cases included, only the latest and most comprehensive data were selected. The exclusion criteria were as follows: insufficient quantitative data; the results were reported as graphics; duplicate reports; and articles published in languages other than English [20].
Two reviewers independently checked the titles and abstracts of all papers retrieved from databases. They next extracted relevant study data from the full-text papers selected for inclusion. Any disagreements were resolved by consensus. Articles were initially evaluated according to title and/or abstract. Subsequently, the decision was made to include or exclude after independent and double analysis and full tests of selected research. After approval, the studies were qualified for meta-analysis and collection of data on their clinical and methodological characteristics.
2.2. Data Extraction
The following data were extracted for each study: (i) clinical and methodological study characteristics such as last name of the first author, publication year, the country in which the study was performed, name of the study, years of data collection, number of cases and control subjects, and source of cases; (ii) information on the usage of OC in both groups (ever/never); and (iii) BrCa incidence depending on menarche, parity, breastfeeding, menopausal status, family history of BrCa, nutritional status, diabetes, and tobacco smoking.
2.3. Assessment of Study Quality
Methodological quality was evaluated by means of the Newcastle–Ottawa scale (NOS) for quality assessment of the included studies. In this evaluation, scores from 0 to 3, from 4 to 6, and from 7 to 9 were given for low, medium, and high quality, respectively [21]. With this tool, each study in the meta-analysis was assessed in three separate categories: selection of cases and controls, comparability of cases and controls on the basis of the design or analysis, and ascertainment of exposure. The NOS quality stars ranged between 4–8, and the average score was 5.76 for included studies. Fifteen (35.71%) studies were regarded as high quality (NOS ≥ 7 points), and 27 (64.29%) studies were regarded as medium quality (NOS ≥ 4 points).
2.4. Statistical Analysis
The meta-analysis of summary statistics from individual studies was performed through STATISTICA 13.3 software (StatSoft Poland, Krakow, Poland), using the Medical Package program. For each study, we constructed separate two-by-two (2 × 2) contingency tables to calculate the odds ratios (OR) and 95% confidence intervals (CIs), cross-classifying OC users and occurrence of BrCa. The Mantel-Haenszel test was used to assess the relationship between OC use and BrCa. The meta-analysis combining the ORs across studies was conducted using the DerSimonian–Laird random-effects model [22]. The random-effects meta-analysis model was used due to the diversity of research in terms of, for example, design and population. In the random-effects model, it is assumed that there is no common effect size for independent studies. Instead, each study is assumed to have a different population effect size, which is a random variable and has a normal distribution. Therefore, there is a difference between the size of the effects of individual studies. Thus, the variance of the effect size in the random-effects model is the sum of the variance within and between studies. The weighting of the studies in the meta-analysis was calculated on the basis of the inverse of the sum of ‘within study’ and between studies variances.
Heterogeneity was assessed graphically by employing a forest plot and statistically by applying the Q test and I2 index. I2 values of 25%, 50%, and 75% have been regarded as respectively representing low, moderate, and high heterogeneity between studies [23].
Publication bias was explored via funnel plots and estimated using Begg’s and Egger’s tests [24,25]. For all the analyses, a forest plot was generated to display results, whereby diamonds represent study-specific odds ratios; 95% CIs for individual studies are represented by horizontal lines.
2.5. Subgroup Analysis
Prespecified subgroup analyses were carried out to identify the sources of heterogeneity between studies in accordance with reproductive factors: age of menarche (≤12 years/>12 years), parity (nulliparous/parous), age at first pregnancy (≥25 years/>25 years; ≥30 years/<30 years), breastfeeding (no/yes) and menopausal status (pre-/post-menopausal); as well as personal risk factors: the period of OC use (≤5 years; >5 years), body mass index (≥30 kg/m2/<30 kg/m2), diabetes (yes/no), tobacco smoking (ever/never), and family history of BrCa (yes/no).
3. Results
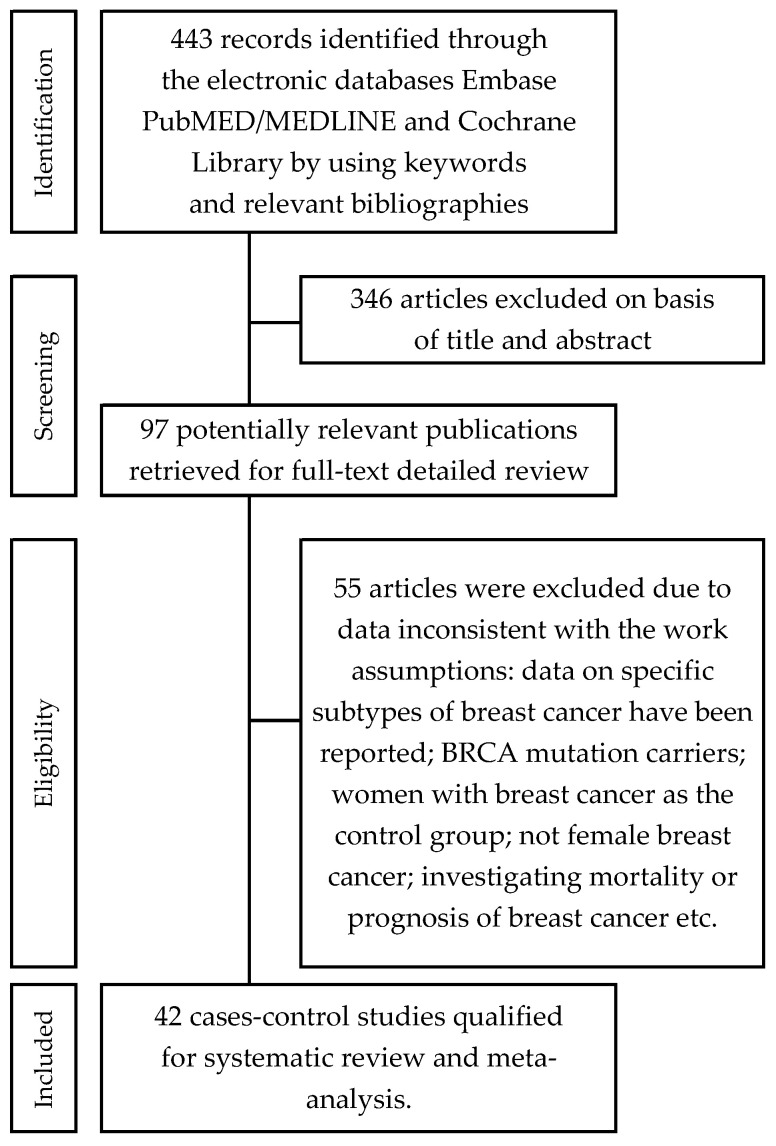
We identified 443 references through the medical electronic databases PubMed (MEDLINE), Embase, and the Cochrane Library up to March 2020. We excluded 346 citations on the basis of titles and abstracts, and 99 citations after reviewing the full texts. These studies were excluded because the full text was not available in the English language, they were letters to the editor, commentaries, review articles, of inappropriate designs (studies of cross-sectional, randomized controlled trials, prospective/retrospective cohort studies), or BrCa was only reported. Moreover, exclusion occurred because the subjects were BRCA mutation carriers, women with BrCa were the control group, the papers were not about female BrCa, the researchers were investigating mortality or prognosis of BrCa, etc. Eventually, 42 case-control studies were qualified in the review and meta-analysis (Figure 1).
Figure 1.
Flowchart of the selection procedure for studies included in the current review and meta-analysis.
In total, 110,580 women (30,778 in the group with BrCa and 79,802 in the control group, of which 15,722 and 38,334 used of OC, respectively) were included in the meta-analysis. Of the studies included:
seven (7) case-control studies were conducted in the Americas,
six (6) in Africa,
sixteen (16) in the Middle East,
eleven (11) in Asia,
one (1) in Europe,
one (1) was an international, multicenter research study.
Characteristics of selected works are shown in Table 1.
Table 1.
Characteristics of the case-control studies included in the meta-analysis on the association between OC use and BrCa risk.
| First Autor Pub Year (References) | Region | Recruitment (Year) | Number of Case Subjects (%) | Age (Mean ± SD or Range) | Number of Controls Subjects (%) | Source of Subjects | NOS Score |
|---|---|---|---|---|---|---|---|
| Engbang 2020 [26] | Duala; Cameroon | 2012–2018 | 297 (50.5) | 53.3 ± 12.7 | 1158 (36.7) | Hospital | 8 |
| Hamdi-Cherif 2020 [27] | Setif; Algeria | 2012–2017 | 547 (63.1) | 28–77 | 543 (59.1) | Hospital | 4 |
| Alipour 2019 [28] | Golestan; Iran | 2004–2008 | 99 (34.3) | 40–75 | 400 (33.2) | Population | 8 |
| Alsolami 2019 [29] | Mahhah; Saudi Arabia | 2014–2016 | 214 (43.9) | 57.0 ± 7.3 | 218 (25.2) | Population | 5 |
| Andarieh 2019 [30] | Babolsar; Iran | 2014–2016 | 1177 (58.5) | 48.8 ± 8.5 | 1204 (27.3) | Hospital | 7 |
| Yuan 2019 [31] | Chengdu; China | 2014–2015 | 448 (19.2) | 43.7 ± 6.1 | 463 (10.6) | Clinic | 5 |
| Bashamakha 2019 [32] | Seiyun; Yemen | 2011–2015 | 105 (49.5) | N/A | 210 (55.2) | Population | 6 |
| Wahidin 2018 [33] | Jakarta; Indonesia | 2018 | 381 (35.4) | 40–49 | 381 (20.2) | Hospital | 4 |
| Khalis 2018 [34] | Fez; Morocco | 2014–2015 | 237 (62.2) | 45–54 | 237 (61.2) | Hospital | 6 |
| Sofi 2018 [35] | New Delhi; India | 2015–2017 | 195 (12.3) | 45.0 ± 10.0 | 191 (25.1) | Hospital | 7 |
| Tan 2018 [36] | Malaysia | 2002–2016 | 3387 (27.6) | 58.0 | 3951 (29.0) | Hospital | 4 |
| Ellingjord-Dale 2017 [37] | Norway | 2006–2014 | 5050 (51.6) | 50–69 | 24,343 (50.7) | Population | 8 |
| Balekouzou 2017 [38] | Central African Republic | 2003–2015 | 174 (28.9) | 45.8 ± 13.6 | 348 (41.1) | Population | 6 |
| Kariri 2017 [39] | Gaza Strip; Palestine | 2014–2015 | 96 (28.1) | 18–60 | 197 (28.9) | Hospital | 7 |
| Dianatinasab 2017 [40] | Shiraz; Iran | 2014–2016 | 526 (46.8) | <40–60> | 562 (40.7) | Hospital | 7 |
| Chollet-Hinton 2017 [41] | USA | 2005 | 1589 (80.7) | 22–75 | 5137 (86.1) | Community | 7 |
| Nguyen 2016 [42] | Hanoi; Wietnam | 2007–2013 | 291 (8.6) | 24–65 | 291 (4.5) | Hospital | 7 |
| Wang 2016 [43] | Hong Kong SAR | 2011–2015 | 923 (32.6) | 56.0 ± 11.8 | 918 (36.3) | Hospital | 6 |
| Al-Amri 2015 [44] | Riyadh; Saudi Arabia | 2013–2014 | 58 (62.1) | 30–69 | 290 (73.8) | Hospital | 6 |
| Ichida 2015 [45] | Tokyo; Japan | 2007–2013 | 155 (23.2) | 20–69 | 12,223 (26.8 | Clinic | 6 |
| Karim 2015 [46] | Jeddah; Saudi Arabia | 2001–2013 | 92 (58.7) | 30–65 | 100 (67.0) | Clinic | 4 |
| Mohite 2015 [47] | Satara district, India | 2009–2011 | 217 (31.8) | 40–49 | 217 (22.1) | Hospital | 4 |
| Laamiri 2015 [48] | Rabat; Morocco | 2008–2010 | 400 (74.5) | 45.8 ± 11.1 | 400 (76.0) | Hospital | 5 |
| Kawai 2014 [49] | USA | 2004–2010 | 960 (88.9) | 20–44 | 938 (89.1) | Population | 6 |
| Work 2014 [50] | USA, Canada, Australia | 1995–2004 | 4011 (72.9) | 18–69 | 2997 (77.8) | Community | 6 |
| Beaber 2014 [51] | USA | 2004–2010 | 985 (87.9) | 20–44 | 882 (88.3) | Population | 7 |
| Hosseinzadeh 2014 [52] | Tabriz; Iran | 2012–2013 | 140 (69.3) | 47.6 ± 10.7 | 280 (38.6) | Clinic | 4 |
| Vaisy 2014 [53] | Urmia; Iran | 2013–2014 | 228 (72.4) | 47.63 | 216 (57.4) | Clinic | 5 |
| Pimhanam 2014 [54] | Bangkok; Thailand | 2007–2011 | 444 (45.9) | 45.8 ± 10.1 | 444 (38.7) | Hospital | 4 |
| Sepandi 2014 [55] | Shiraz; Iran | 2001–2012 | 197 (57.9) | 26–68 | 11,653 (55.8) | Hospital | 4 |
| Tazhibi 2014 [56] | Isfahan; Iran | 1999–2010 | 216 (63.9) | 20–75 | 41 (75.6) | Hospital | 5 |
| Amadou 2014 [57] | Mexico | 2004–2007 | 263 (17.9) | 35–64 | 314 (19.4) | Hospital | 6 |
| Morales 2013 [58] | Puerto Rico | 2005–2009 | 462 (48.5) | 56.4 ± 12.6 | 649 (55.0) | Hospital | 6 |
| Ehsanpour 2013 [59] | Isfahan; Iran | 2011 | 175 (43.4) | <41–60+ | 350 (25.4) | Clinic | 5 |
| Urban 2012 [60] | South Africa | 1995–2006 | 1112 (23.0) | 49.0 | 1102 (14.2) | Hospital | 4 |
| Ronco 2012 [61] | Montevideo; Uruguay | 2004–2010 | 251 (69.7) | <30–50≥ | 497 (65.2) | Hospital | 8 |
| Matalqah 2011 [62] | Penang, Malaysia | 2009–2010 | 150 (26.7) | 52.8 ± 1.1 | 150 (18.0) | Population | 7 |
| Ghiasvand 2011 [63] | Shiraz; Iran | 2005–2008 | 442 (66.3) | 41.2 ± 5.7 | 463 (62.9) | Hospital | 5 |
| Ekpanyaskul 2010 [64] | Khon Kaen, Thailand | 2002–2004 | 516 (42.0) | 46.9 ± 10.6 | 516 (34.9) | Hospital | 6 |
| Ma 2010 [65] | USA | 1994–1998 | 1197 (78.8) | 36–64 | 2015 (79.6) | Community | 8 |
| Hahjisavvas 2010 [66] | Cyprus | 1999–2005 | 1103 (25.4) | 50–59 | 1173 (25.1) | Hospital | 7 |
| Ozmen 2009 [67] | Istanbul, Turkey | 2000–2006 | 1492 (18.4) | 18–70 | 2167 (27.8) | Hospital | 7 |
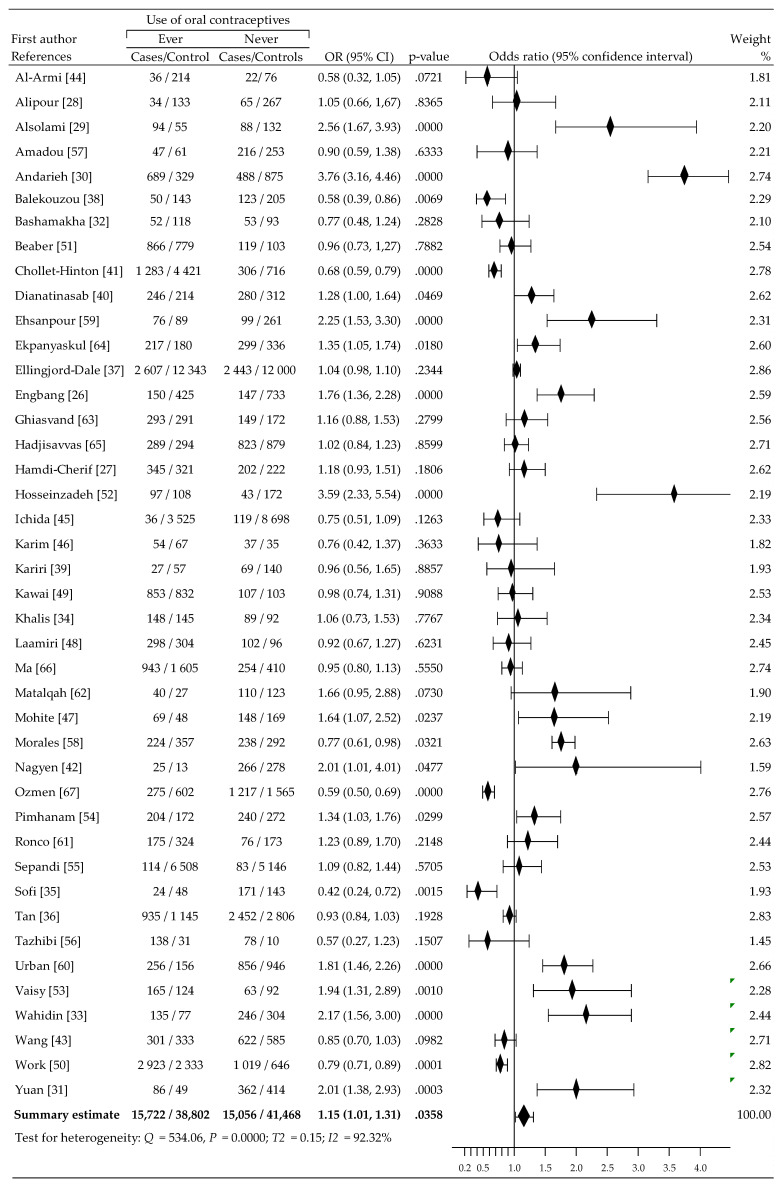
Figure 2 shows the change incidence of BrCa risk related to OC use in each study and overall. An increase in the risk of BrCa among women using OC was reported in 23 of 42 trials, including the increase being statistically significant at 14 trials. In comparison, a decrease in the risk of BrCa was observed in participants not using OC in 19 of 42 studies, with the reduction being statistically significant in 6 trials. Meta-analysis using a random-effects model show moderate, statistically significant increase of BrCa risk: OR = 1.15 (95% CI, 1.01 to 1.31), p = 0.0358. This was accompanied by high heterogeneity: I2 = 92.32%.
Figure 2.
A forest plot and the summary odds ratios for the relationship between BrCa risk and ever OC use: case-control studies conducted 2009–2020 in alphabetical order.
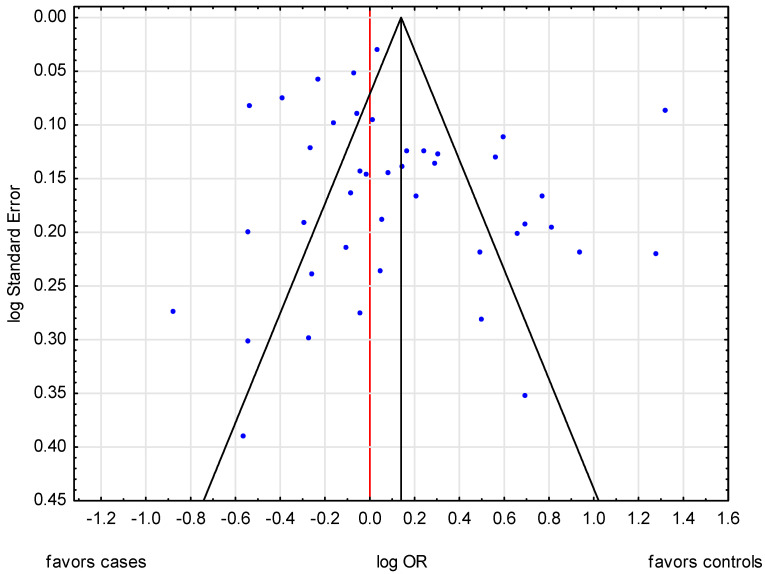
No obvious evidence of publication bias was detected by inspection of the funnel plot (Figure 3). Moreover, the Begg and Mazumdar’s test for rank correlation did not indicate evidence of publication bias. Accordingly, Kendall’s tau = 0.0580; Z = 0.5202; p = 0.6029; similarly, Begg and Egger’s test: b0 = 1.2587 (95% CI: −0.6964 to 3.2138); t = 1.3012, p = 0.2006.
Figure 3.
A funnel plot of publication bias in the meta-analysis of the relationship between BrCa risk and ever use of OC.
A summary of the results of the studies providing data for the assessment of BrCa risk is shown in Table 2. For the period of contraceptive usage, as indicated through meta-analysis, 13 studies [28,33,37,38,41,48,49,50,51,53,59,62,66] showed a slight statistical insignificant increase of BrCa risk in women taking OC longer than 5 years (OR = 1.05, 95% CI: 0.88 to 1.25, p = 0.5787) as well as a slight statistically insignificant decrease in the risk of BrCa in women taking OC for less than or equal to 5 years (OR = 0.92, 95% CI: 0.77 to 1.10, p = 0.3674).
Table 2.
Analysis of the potential modifying effects of other risk factors on the relationship of OC use and BrCa risk.
| Variables | Studies, N (Ref.) | OR (95% CI) | p | I2 (%) |
|---|---|---|---|---|
| Taking OC | ||||
| ever vs. never | 42 [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] | 1.15 (1.01, 1.31) | 0.0358 | 92.32 |
| The period of OC using | ||||
| ≤5 years | 13 [28,33,37,38,41,48,49,50,51,53,59,62,66] | 0.92 (0.77, 1.10) | 0.3674 | 85.86 |
| >5 years | 1.05 (0.88, 1.25) | 0.5787 | 82.95 | |
| Age of menarche | ||||
| ≤12 years vs. >12 years | 29 [26,27,30,32,33,34,35,36,37,38,39,41,43,44,47,48,49,50,51,52,54,55,56,57,58,61,62,64,66] | 1.18 (1.07, 1.31) | 0.0016 | 80.71 |
| Parity | ||||
| Nulliparous vs. Parous | 30 [26,27,30,34,35,36,37,38,39,41,42,43,45,47,48,49,50,51,52,54,55,56,57,58,61,62,63,65,66,67] | 1.22 (1.04, 1.43) | 0.0146 | 89.88 |
| Age at first pregnancy | ||||
| ≥25 years vs. <25 years | 14 [27,35,36,37,39,41,43,49,51,55,62,63,66,67] | 1.09 (0.94, 1.25) | 0.2599 | 86.64 |
| ≥30 years vs. <30 years | 6 [26,48,52,58,64,65] | 3.08 (1.10, 8.60) | 0.0322 | 97.49 |
| Breastfeeding | ||||
| No vs. Yes | 27 [26,29,30,32,33,36,37,38,39,41,43,44,45,46,47,48,50,51,52,54,58,61,62,64,65,66,67] | 1.36 (1.13, 1.63) | 0.0010 | 91.81 |
| Menopausal status | ||||
| Post- vs. Pre- | 22 [26,32,34,35,36,37,39,42,43,44,45,46,47,48,50,52,54,55,58,62,63,64] | 1.36 (1.14, 1.63) | 0.0007 | 92.86 |
| Family history of BrCa | ||||
| Yes vs. No | 29 [27,28,29,30,32,33,34,35,37,39,41,43,44,45,46,48,49,50,51,52,54,55,57,58,61,63,64,66,67] | 1.72 (1.32, 2.24) | 0.0001 | 93.07 |
| Body mass index (kg/m2) | ||||
| ≥30 (obesitas) vs. <30 | 16 [26,27,28,29,32,34,39,41,44,49,51,54,55,57,63,66] | 1.19 (0.95, 1.5) | 0.1289 | 90.46 |
| Tobacco smoking | ||||
| Yes vs. No | 14 [26,28,29,30,36,42,49,52,54,58,62,64,66,67] | 1.52 (1.26, 1.83) | 0.0000 | 72.32 |
| Diabetes | ||||
| Yes vs. No | 4 [26,29,32,39] | 1.99 (0.60, 6.62) | 0.2605 | 93.49 |
Reproductive factors included age at menarche, parity, age at first birth, breastfeeding and menopausal status. The earlier age of menarche (≤12 years) was associated with a statistically significant higher BrCa risk (OR = 1.18, 95% CI: 1.07 to 1.31, p = 0.0016), as shown in the meta-analysis of 29 studies [26,27,30,32,33,34,35,36,37,38,39,41,43,44,47,48,49,50,51,52,54,55,56,57,58,61,62,64,66]. In addition, analysis of 30 studies of parity [26,27,30,34,35,36,37,38,39,41,42,43,45,47,48,49,50,51,52,54,55,56,57,58,61,62,63,65,66,67] indicated that nulliparous women had a significantly increased risk for BrCa compared to women who gave birth (OR = 1.22, 95% CI: 1.04 to 1.43, p = 0.0146). A total of 14 studies [27,35,36,37,39,41,43,49,51,55,62,63,65,67] evaluated the association between first pregnancy above 25 years and risk of developing BrCa. Compared to the first births under the age of 25 years, increased risk was noted (OR = 1.09, 95% CI: 0.94 to 1.25, p = 0.2599). What is more, the analysis of six studies [26,48,52,58,64,65] showed a statistically significant higher risk of BrCa in women giving birth for the first time after 30 than before the age of 30 (OR = 3.08, 95% CI: 1.10 to 8.60, p = 0.0322). Beyond the aforementioned, in the meta-analysis of 27 studies [26,29,30,32,33,36,37,38,39,41,43,44,45,46,47,48,50,51,52,54,58,61,62,64,65,66,67], non-breastfeeding was associated with a significant increase in the risk for BrCa, in comparison to breastfeeding (OR = 1.36, 95% CI: 1.13 to 1.63, p = 0.0010). Finally, the data from 22 studies [26,32,34,35,36,37,39,42,43,44,45,46,47,48,50,52,54,55,58,62,63,64] demonstrated a statistically significant increase in BrCa risk among postmenopausal women (OR = 1.36, 95% CI: 1.14 to 1.63, p = 0.0007) when compared to premenopausal women.
Among personal risk factors, the meta-analysis for BMI based on 16 studies [26,27,28,29,32,34,39,41,44,49,51,54,55,57,63,66] indicated a non-significant increase in risk for obese women (OR = 1.19, 95% CI: 0.95 to 1.50, p = 0.1289) compared to women who are underweight/normal/overweight. Tobacco use also had significant associations with BrCa risks (OR = 1.52, 95% CI: 1.26 to 1.83, p = 0.0000) based on the meta-analysis of 14 studies [26,28,29,30,36,42,49,52,54,58,62,64,66,67]. Likewise, 29 studies [27,28,29,30,32,33,34,35,37,39,41,43,44,45,46,48,49,50,51,52,54,55,57,58,61,63,64,66,67] showed that family history of BrCa in the first or second relatives was significantly associated with higher risk (OR = 1.72, 95% CI: 1.32 to 2.24, p = 0.0001). Finally, an analysis of four studies [26,29,32,39] found a slightly increased, but not statistically significant, risk of BrCa in women with diabetes (OR = 1.99, 95% CI: 0.60 to 6.62, p = 0.2605).
In most analyses, no obvious evidence of publication bias was detected by inspection of the funnel plot or through statistical tests. There was evidence, however, suggesting publication bias among studies that investigated BrCa, menopausal status (post-/pre-) (Begg–Mazudar’s test: p = 0.6769; Egger’s test: p = 0.0031), and smoking (yes/no) (Begg–Mazudar’s test: p = 0.0381; Egger’s test: p = 0.2327).
4. Discussion
The presented meta-analysis of the magnitude of effects of OC on BrCa risk revealed a statistically significant slight increase of cancer risk, OR = 1.15 (95% CI; 1.01 to 1.31). The results from our study were, to a large extent, consistent with the results described in previous studies. For example, in 2018, 1245 BrCa cases were found in the analysis of data derived from a prospective national population-based cohort study carried out from 1991 to 2007 of 74,862 Norwegian premenopausal women. These authors saw a statistically insignificant increased risk of BrCa in participants using hormonal contraception. Here, HR (hazard risk) = 1.12, 95%: CI 0.99 to 1.26 [68]. Moreover, over 11,000 BrCa cases were diagnosed from 1995 to 2012 in a large prospective cohort study published in 2017 involving 1.8 million Danish women. The authors of this work noted a moderate significant increase in the relative risk of BrCa (RR = 1.20, 95% CI: 1.14 to 1.26) among users of hormonal contraceptives [69]. Furthermore, the Royal College of General Practitioners Oral Contraception Study conducted in the years 1968–2012 involved a total of 28,993 OC taking women (cancer cases = 4661) and a total of 17,039 women who never took OC (cancer cases = 2341) and found a slight, statistically non-significant increase in the risk of BrCa was evident. Here, IRR (incidence rate ratio) = 1.04, 95% CI: 0.91 to 1.17 [70]. Our previous meta-analysis covering the period 1960–2010 [16] estimated 79 case-control studies conducted between 1960–2010, including a total of 72,030 incidents, histologically confirmed cases of BrCa and 123,650 population/hospital controls. A decrease was observed in cancer risk in OC users before age 25 years (0.91, 0.83–1.00). However, the use of OCs before the first full-term pregnancy significantly increased the risk of BrCa (OR, 1.14, 1.01–1.28, p = 0.04), as did OC use longer than 5 years (1.09, 1.01–1.18, p = 0.02). Pooled crude odds ratios of BrCa in ever-users of OC was 1.01 [95% confidence interval (CI), 0.95–1.07], compared with never-users. There was no significant increase in risk among premenopausal women (1.06, 0.92–1.22), postmenopausal women (0.99, 0.89–1.10), or nulliparous women (1.02, 0.82–1.26) [16].
Gierisch et al. [71] also conducted a meta-analysis. This work covered fifteen case-control studies (38,682 women) and 8 cohort studies (317,341 women) published between 2000 and 2012. It assessed the association between OC use and BrCa incidence. Overall, in women taking OC, BrCa risk increased slightly, albeit statistically significantly. Here, OR = 1.08 (95% CI, 1.00–1.17). These results were similar to those derived from the meta-analysis by Zhu et al. [72], which included a total of 13 prospective cohort studies on OC use and BrCa risk (11,722 BrCa cases and 859,894 participants) that were identified by searching databases from 1960–2012. In the meta-analysis, the RR (relative risk) of BrCa in women using OC was 1.08 (95% CI: 0.99.17) when compared with never-users. What is more, in a recent publication by Del Puo et al. [73], which analyzed the results of two meta-analyses on the incidence of BrCa among users of hormonal OC, a statistically significant overall increase in the risk of BrCa (OR = 1.08 (95% CI 1.00 to 1.17)) was indicated.
The results of our meta-analyses of potentially modifying risk factors for BrCa indicate that menarche before the age of 12 years, nulliparous, non-breastfeeding, first pregnancy in old age, postmenopause, obesity, smoking, and family history of BrCa significantly increase the risk of development of BrCa. The majority of the above risk factors are associated with a longer duration of estrogen exposure [74,75,76,77,78,79].
We are aware that drawing final conclusions from the results of our meta-analysis requires caution given the number of limitations encountered in its construction. First, we limited the search to studies published in English, and these were identified through electronic databases. The possibility of not reaching out to all publications on this topic may have an effect on the value of the results [80]. Secondly, the studies, including summary estimates, are vulnerable to various types of bias. Retrospective self-reporting of using OC, for example, may be associated with overestimation or underestimation of actuality. Moreover, mistakes may be made in control group recruitment under hospital scenarios. Additionally, mistakes can be made when cases are recruited for study based on the admission of OC use. Thirdly, a lack of a uniform definition of “ever” use of OC exists (this affects the various periods of exposure to OCs), as defined by the times that pill self-administration was begun and ended. Such fuzziness may lead to misclassification, and this, in turn, may weaken the true association between OC use and BrCa affliction (as may occur equally among cases and controls). The limitations should also take into account other coexisting factors, such as the use of different OC and other genetic factors that were not explored in the studies analyzed, such as BRCA1 and BRCA2 mutations (participants were not tested for the presence of mutations in the BRCA1 and BRCA2 genes). Furthermore, a prospective study needs to be conducted to confirm our findings.
5. Conclusions
In conclusion, the results from this meta-analysis suggest that OC using is associated with a modest, statistically significant increase in the risk of BrCa. Despite our conclusion that birth control pills increase cancer risk, and that our conclusion is supported by extensive previous studies and meta-analyzes, further confirmation is required.
Author Contributions
Conceptualization, W.K., A.B. (Agnieszka Barańska); Data curation, A.B. (Agata Błaszczuk), W.K.; Formal analysis, M.M.; Funding acquisition, K.D., A.B. (Agnieszka Barańska); Investigation, A.B. (Agata Błaszczuk); Methodology, W.K., A.B. (Agnieszka Barańska) and M.M.; Project administration, M.P.-D.; Resources, A.B. (Agnieszka Barańska); Software, W.K., K.D.; Supervision, A.B. (Agata Błaszczuk); Validation, W.K.; Visualization, A.B. (Agata Błaszczuk), A.B. (Agnieszka Barańska); Writing—original draft, W.K., A.B. (Agnieszka Barańska); Writing—review & editing, A.B. (Agnieszka Barańska), A.B (Agata Błaszczuk). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The Cancer Atlas: Breast Cancer Accounts for Almost a Quarter of New Cancer Cases among Women. [(accessed on 12 March 2021)]. Available online: https://canceratlas.cancer.org/wp-content/uploads/2019/09/CA3_BreastCancer.pdf.
- 2.Harvey J., Murff H.J., David R., Spigel D.R., Syngal S. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. JAMA. 2004;292:1480–1489. doi: 10.1001/jama.292.12.1480. [DOI] [PubMed] [Google Scholar]
- 3.Palma M., Ristori E., Ricevuto E., Giannini G., Gulino A. BRCA1 and BRCA2: The genetic testing and the current management options for mutation carriers. Crit. Rev. Oncol. Hematol. 2006;57:1–23. doi: 10.1016/j.critrevonc.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Hall J.M., Friedman L., Guenther C., Lee M.K., Weber J., Black D.M., King M.C. Closing in on a breast cancer gene on chromosome 17q. Am. J. Hum. Genet. 1992;50:1235–1242. [PMC free article] [PubMed] [Google Scholar]
- 5.Wooster R., Neuhausen S.L., Mangion J., Quirk Y., Ford D., Collins N., Nguyen K., Seal S., Tran T., Averill D. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12–13. Science. 1994;265:2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]
- 6.Blows F.M., Driver K.E., Schmidt M.K., Broeks A., van Leeuwen F.E., Wesseling J., Cheang M.C., Gelmon K., Nielsen T.O., Blomqvist C., et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: A collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;75:e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurebayashi J., Moriya T., Ishida T., Hirakawa H., Kurosumi M., Akiyama F., Kinoshita T., Takei H., Takahashi K., Ikeda M., et al. The prevalence of intrinsic subtypes and prognosis in breast cancer patients of different races. Breast. 2007;16((Suppl. S2)):72–77. doi: 10.1016/j.breast.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Yang X.R., Chang-Claude J., Goode E.L., Yang X.R., Chang-Claude J., Goode E.L., Couch F.J., Nevanlinna H., Milne R.L., Gaudet M., et al. Associations of breast cancer risk factors with tumor subtypes: A pooled analysis from the Breast Cancer Association Consortium studies. J. Natl. Cancer Inst. 2011;103:250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collaborative Group on Hormonal Factors in Breast Cancer Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118,964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikkelsen A.P., Egerup P., Ebert J.F.M., Kolte A.M., Nielsen H.S., Lidegaard Ø. Pregnancy loss and cancer risk: A nationwide observational study. Clin. Med. 2019;15:80–88. doi: 10.1016/j.eclinm.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuzhan A., Adli M. The effect of socio-economic-cultural factors on breast cancer. J. Breast Health. 2015;11:17–21. doi: 10.5152/tjbh.2014.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K., Kruper L., Dieli-Conwright C.M., Mortimer J.E. The impact of obesity on breast cancer diagnosis and treatment. Curr. Oncol. Rep. 2019;1:41. doi: 10.1007/s11912-019-0787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dydjow-Bendek D., Zagozdzon P. Selected dietary factors and breast cancer risk. Przeg Epidemiol. 2019;73:361–368. doi: 10.32394/pe.73.29. [DOI] [PubMed] [Google Scholar]
- 14.McDonald J.A., Abhishek Goyal A., Terry M.B. Alcohol intake and breast cancer risk: Weighing the overall evidence. Curr. Breast Cancer Rep. 2013;5:10. doi: 10.1007/s12609-013-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preston D.L., Kitahara C.M., Freedman D.M., Sigurdson A.J., Simon S.L., Little M.P., Cahoon E.K., Rajaraman P., Miller J.S., Alexander B.H., et al. Breast cancer risk and protracted low-to-moderate dose occupational radiation exposure in the US Radiologic Technologists Cohort, 1983–2008. Br. J. Cancer. 2016;115:1105–1112. doi: 10.1038/bjc.2016.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanadys W., Barańska A., Malm M., Błaszczuk A., Polz-Dacewicz M., Janiszewska J., Jędrych M. Use of Oral Contraceptives as a Potential Risk Factor for Breast Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies Up to 2010. Int. J. Environ. Res. Public Health. 2021;18:4638. doi: 10.3390/ijerph18094638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlton B.M., Rich-Edwards J.W., Colditz G.A., Missmer S.A., Rosner B.A., Hankinson S.E., Speizer F.E., Michels K.B. Oral contraceptive use and mortality after 36 years of follow-up in the Nurse’ Health Study: Prospective cohort study. BMJ. 2014;349:g6356. doi: 10.1136/bmj.g6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vessey M., Yeates D. Oral contraceptive use and cancer: Final report from the Oxford-Family Planning Association contraceptive study. Contraception. 2013;88:678–683. doi: 10.1016/j.contraception.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Hoboken, NJ, USA: 2019. (Version 6.0). [Google Scholar]
- 21.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non Randomised Studies in Meta-Analyses. [(accessed on 12 March 2021)]. Available online: www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 22.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 25.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engbang N.J.P., Essome H., Mve K.V., Sukam N.D.S., Mboudou T.E. Risk factors for breast cancer in the city of Douala: A case control study. Adv. Breast Cancer Res. 2020;9:66–77. doi: 10.4236/abcr.2020.93006. [DOI] [Google Scholar]
- 27.Hamdi-Cherif M., Serraino D., Bouad S., Dib A., Boudaoud K., Atoui S., Mergherm I., Toffolutti F., Bidoli E., Kara L., et al. Sociodemographic and reproductive risk factors for breast cancer: A case-control study in the Setif Province, Northern Algeria. Asian Pac. J. Cancer Prev. 2020;21:457–464. doi: 10.31557/APJCP.2020.21.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alipour S., Omranipour R., Malekzadeh R., Poustchi H., Pourshams A., Khoshnia M., Gharavi A., Roshandel G., Eslami B. A Case-control study of breast cancer in Northeast of Iran: The Golestan Cohort Study. Arch. Iran. Med. 2019;22:355–360. [PubMed] [Google Scholar]
- 29.Alsolami F.J., Azzeh F.S., Ghafouri K.J., Ghaith M.M., Almaimani R.A., Almasmoum H.A., Abdulal R.H., Abdulaal W.H., Jazar A.S., Tashtoush S.H. Determinants of breast cancer in Saudi women from Makkah region: A case-control study (breast cancer risk factors among Saudi women) BMC Public Health. 2019;19:1554. doi: 10.1186/s12889-019-7942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andarieh M.G., Delavar M.A., Moslemi D., Ahmadi M.H., Zabihi E., Esmaeilzadeh S. Infertility as a risk factor for breast cancer: Results from a hospital-based case-control study. J. Cancer Res. Ther. 2019;15:976–980. doi: 10.4103/jcrt.JCRT_905_16. [DOI] [PubMed] [Google Scholar]
- 31.Yuan X., Yi F., Hou C., Lee H., Zhong X., Tao P., Li H., Xu Z., Li J. Induced abortion, birth control methods, and breast cancer risk: A case-control study in China. J. Epidemiol. 2019;29:173–179. doi: 10.2188/jea.JE20170318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bashamakha G., Sumait H.B., Bashamakha M., Al Serouri A., Khader Y. Risk factors of breast cancer in Hadramout Valley and Desert, Yemen. Int. J. Prev. Med. 2019;10:161. doi: 10.4103/ijpvm.IJPVM_251_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wahidin M., Djuwita R., Adisasmita A. Oral contraceptive and breast cancer risks: A case control study in six referral hospitals in Indonesia. Asian Pac. J. Cancer Prev. 2018;19:2199–2203. doi: 10.22034/APJCP.2018.19.8.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khalis M., Charbotel B., Chajès V., Rinaldi S., Moskal A., Biessy C., Dossus L., Huybrechts I., Fort E., Mellas N., et al. Menstrual and reproductive factors and risk of breast cancer: A case-control study in the Fez Region, Morocco. PLoS ONE. 2018;13:e0191333. doi: 10.1371/journal.pone.0191333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sofi N.Y., Jain M., Kapil U., Seenu V.R.L., Yadav C.P., Pandey R.M., Sareen N. Reproductive factors, nutritional status and serum 25(OH)D levels in women with breast cancer: A case control study. J. Steroid Biochem. Mol. Biol. 2018;175:200–204. doi: 10.1016/j.jsbmb.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Tan M.M., Ho W.K., Yoon S.Y., Mariapun S., Hasan S.N., Lee D.S.C., Hassan T., Lee S.Y., Phuah S.Y., Sivanandan K., et al. A case-control study of breast cancer risk factors in 7663 women in Malaysia. PLoS ONE. 2018;13:e0203469. doi: 10.1371/journal.pone.0203469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellingjord-Dale M., Vos L., Tretli S., Hofvind S., Dos-Santos-Silva I., Ursin G. Parity, hormones and breast cancer subtypes—Results from a large nested case-control study in a national screening program. Breast Cancer Res. 2017;19:10. doi: 10.1186/s13058-016-0798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balekouzou A., Yin P., Pamatika C.M., Bekolo C.E., Nambei S.W., Djeintote M., Kota K., Mossoro-Kpinde C.D., Shu C., Yin M., et al. Reproductive risk factors associated with breast cancer in women in Bangui: A case-control study. BMC Womens Health. 2017;17:14. doi: 10.1186/s12905-017-0368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kariri M., Jalambo M.O., Kanou B., Deqes S., Younis S., Zabut B., Balawi U. Risk factors for breast cancer in Gaza Strip, Palestine: A case-control study. Clin. Nutr. Res. 2017;6:161–171. doi: 10.7762/cnr.2017.6.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dianatinasab M., Fararouei M., Mohammadianpanah M., Zare-Bandamiri M., Rezaianzadeh A. Hair Coloring, Stress, and Smoking Increase the Risk of Breast Cancer: A Case-Control Study. Clin. Breast Cancer. 2017;17:650–659. doi: 10.1016/j.clbc.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Chollet-Hinton L., Olshan A.F., Nichols H.B., Anders C.K., Lund J.L., Allott E.H., Bethea T.N., Hong C.C., Cohen S.M., Khoury T., et al. Biology and Etiology of Young-Onset Breast Cancers among Premenopausal African American Women: Results from the AMBER Consortium. Cancer Epidemiol. Biomark. Prev. 2017;26:1722–1729. doi: 10.1158/1055-9965.EPI-17-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen J., Le Q.H., Duong B.H., Sun P., Pham H.T., Ta V.T., Kotsopoulos J., Narod S.A., Ginsburg O. A matched case-control study of risk factors for breast cancer risk in Vietnam. Int. J. Breast Cancer. 2016;2016:1–7. doi: 10.1155/2016/7164623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F., Dai J., Li M., Chan W., Kwok C.C., Leung S., Wu C., Li W., Yu W., Tsang K.-H., et al. Risk assessment model for invasive breast cancer in Hong Kong women. Medicine. 2016;95:e4515. doi: 10.1097/MD.0000000000004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Amri F.A., Saeedi M.Y., Al-Tahan F.M., Ali A.M., Alomary S.A., Arafa M., Ibrahim A.K., Kassim K.A. Breast cancer correlates in a cohort of breast screening program participants in Riyadh, KSA. J. Egypt Natl. Cancer Inst. 2015;27:77–82. doi: 10.1016/j.jnci.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Ichida M., Kataoka A., Tsushima R., Taguchi T. No increase in breast cancer risk in Japanese women taking oral contraceptives: A case-control study investigating reproductive, menstrual and familial risk factors for breast cancer. Asian Pac. J. Cancer Prev. 2015;16:3685–3690. doi: 10.7314/APJCP.2015.16.9.3685. [DOI] [PubMed] [Google Scholar]
- 46.Karim S.M., Baeshen W., Neamatullah S.N., Bin B. Oral contraceptives, abortion and breast cancer risk: A case control study in Saudi Arabia. Asian Pac. J. Cancer Prev. 2015;16:3957–3960. doi: 10.7314/APJCP.2015.16.9.3957. [DOI] [PubMed] [Google Scholar]
- 47.Mohite V.R., Pratinidhi A.K., Mohite R.V. Reproductive risk factors and breast cancer: A case control study from rural India. Bangladesh J. Med. Sci. 2015;14:258–264. doi: 10.3329/bjms.v14i3.21865. [DOI] [Google Scholar]
- 48.Laamiri F.Z., Bouayad A., Hasswane N., Ahid S., Mrabet M., Amina B. Risk factors for breast cancer of different age groups: Moroccan Data? Open J. Obst. Gynecol. 2015;5:79–87. doi: 10.4236/ojog.2015.52011. [DOI] [Google Scholar]
- 49.Kawai M., Malone K.E., Tang M.T.C., Li C.I. Active smoking and the risk of estrogen receptor-positive and triple-negative breast cancer among women ages 20 to 44 years. Cancer. 2014;120:1026–1034. doi: 10.1002/cncr.28402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Work M.E., John E.M., Andrulis I.L., Knight J.A., Liao Y., Mulligan A.M., Southey M.C., Giles G.G., Dite G.S., Apicella C., et al. Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the Breast Cancer Family Registry. Brit. J. Cancer. 2014;110:1367–1377. doi: 10.1038/bjc.2013.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beaber E.F., Malone K.E., Tang M.T., Barlow W.E., Porter P.L., Daling J.R., Li C.I. Oral contraceptives and breast cancer risk overall and by molecular subtype among young women. Cancer Epidemiol. Biomark. Prev. 2014;23:755–764. doi: 10.1158/1055-9965.EPI-13-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hosseinzadeh M., Eivazi Ziaei J., Mahdavi N., Aghajari P., Vahidi M., Fateh A., Asghari E. Risk factors for breast cancer in Iranian women: A hospital-based case-control study in Tabriz. Iran. J. Breast Cancer. 2014;17:236–243. doi: 10.4048/jbc.2014.17.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaisy A., Lotfinejad S., Zhian F. Risk of cancer with combined oral contraceptive use among Iranian women. Asian Pac. J. Cancer Prev. 2014;15:5517–5522. doi: 10.7314/APJCP.2014.15.14.5517. [DOI] [PubMed] [Google Scholar]
- 54.Pimhanam C., Sangrajrang S., Ekpanyaskul C. Tobacco smoke exposure and breast cancer risk in Thai urban females. Asian Pac. J. Cancer Prev. 2014;15:7407–7411. doi: 10.7314/APJCP.2014.15.17.7407. [DOI] [PubMed] [Google Scholar]
- 55.Sepandi M., Akrami M., Tabatabaee H., Rajaeefard A., Tahmasebi S., Angali K.A., Rezaianzadeh A., Talei A. Breast cancer risk factors in women participating in a breast screening program: A study on 11,850 Iranian females. Asian Pac. J. Cancer Prev. 2014;15:8499–8502. doi: 10.7314/APJCP.2014.15.19.8499. [DOI] [PubMed] [Google Scholar]
- 56.Tazhibi M., Dehghani M., Babazadeh S., Makkarian F., Tabatabaeian M., Sadeghi M., Rezaei P., Faghihi M. Hormonal and reproductive risk factors associated with breast cancer in Isfahan patients. J. Educ. Health Promot. 2014;3:69. doi: 10.4103/2277-9531.134818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amadou A., Fabre A., Torres-Mejía G., Ortega-Olvera C., Angeles-Llerenas A., McKenzie F., Biessy C., Hainaut P., Romieu I. Hormonal therapy and risk of breast cancer in Mexican women. PLoS ONE. 2013;8:e79695. doi: 10.1371/journal.pone.0079695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morales L., Alvarez-Garriga C., Matta J., Ortiz C., Vergne Y., Vargas W., Acosta H., Ramírez J., Perez-Mayoral J., Bayona M., et al. Factors associated with breast cancer in Puerto Rican women. J. Epidemiol. Glob. Health. 2013;3:205–215. doi: 10.1016/j.jegh.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehsanpour S., Nejad F.S., Rajabi F.M., Taleghani F. Investigation on the association between breast cancer and consumption patterns of combined oral contraceptive pills in the women of Isfahan in Iranian. Iran. J. Nurs. Midwifery Res. 2013;18:186–190. [PMC free article] [PubMed] [Google Scholar]
- 60.Urban M., Banks E., Egger S., Canfell K., O’Connell D., Beral V., Sitas F. Injectable and oral contraceptive use and cancers of the breast, cervix, ovary, and endometrium in black South African women: Case-control study. PLoS Med. 2012;9:e1001182. doi: 10.1371/journal.pmed.1001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ronco A.L., De Stefani E., Deneo-Pellegrini H. Risk factors for premenopausal breast cancer: A case-control study in Uruguay. Asian Pac. J. Cancer Prev. 2012;13:2879–2886. doi: 10.7314/APJCP.2012.13.6.2879. [DOI] [PubMed] [Google Scholar]
- 62.Matalqah L., Radaideh K., Yusoff Z.M., Awaisu A. Predictors of breast cancer among women in a northern state of Malaysia: A matched case-control study. Asian Pac. J. Cancer Prev. 2011;12:1549–1553. [PubMed] [Google Scholar]
- 63.Ghiasvand R., Maram E.S., Tahmasebi S., Tabatabaee S.H.R. Risk factor for breast cancer among young women in Southern Iran. Int. J. Cancer. 2011;129:1443–1449. doi: 10.1002/ijc.25748. [DOI] [PubMed] [Google Scholar]
- 64.Ekpanyaskul C., Khuhaprema T., Wiangnon S., Sangrajrang S. Case-control study of occupational categories and breast cancer risk in Thailand. Asian Pac. J. Cancer Prev. 2010;11:793–797. [PubMed] [Google Scholar]
- 65.Ma H., Wang Y., Sullivan-Halley J., Linda Weiss L., Marchbanks P.A., Spirtas R., Ursin G., Burkman R.T., Simon M.S., Malone K.E., et al. Use of four biomarkers to evaluate the risk of breast cancer subtypes in the Women's Contraceptive and Reproductive Experiences Study. Cancer Res. 2010;70:575–587. doi: 10.1158/0008-5472.CAN-09-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hadjisavvas A., Loizidou M.A., Middleton N., Michael T., Papachristoforou R., Kakouri E., Daniel M., Papadopoulos P., Malas S., Marcou Y., et al. An investigation of breast cancer risk factors in Cyprus: A case control study. BMC Cancer. 2010;10:447. doi: 10.1186/1471-2407-10-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ozmen V., Ozcinar B., Karanlik H., Cabioglu N., Tukenmez M., Disci R., Ozmen T., Igci A., Muslumanoglu M., Kecer M., et al. Breast cancer risk factors in Turkish women—A University Hospital based nested case control study. World J. Surg. Oncol. 2009;7:37. doi: 10.1186/1477-7819-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Busund M., Bugge N.S., Braaten T., Waaseth M., Rylander C., Lund E. Progestin-only and combined oral contraceptives and receptor-defined premenopausal breast cancer risk: The Norwegian Women and Cancer Study. Int. J. Cancer. 2018;142:2293–2302. doi: 10.1002/ijc.31266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mørch L.S., Skovlund C.W., Hannaford P.C., Iversen L., Fielding S., Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N. Engl. J. Med. 2017;377:2228–2239. doi: 10.1056/NEJMoa1700732. [DOI] [PubMed] [Google Scholar]
- 70.Iversen L., Sivasubramaniam S., Lee A.J., Fielding S., Hannafordet P.C. Lifetime cancer risk and combined oral contraceptives: The Royal College of General Practitioners’ Oral Contraception Study. Am. J. Obstet. Gynecol. 2017;216:580. doi: 10.1016/j.ajog.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 71.Gierisch J.M., Coeytaux R.R., Urrutia R.P., Havrilesky L.J., Moorman P.G., Lowery W.J., Dinan M., McBroom A.J., Hasselblad V., Sanders G.D., et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: A systematic review. Cancer Epidemiol. Biomark. Prev. 2013;22:1931–1943. doi: 10.1158/1055-9965.EPI-13-0298. [DOI] [PubMed] [Google Scholar]
- 72.Zhu H., Lei X., Feng J., Wang Y. Oral contraceptive use and risk of breast cancer: A meta-analysis of prospective cohort studies. Eur. J. Contracept. Reprod. Health Care. 2012;17:402–414. doi: 10.3109/13625187.2012.715357. [DOI] [PubMed] [Google Scholar]
- 73.Del Pup L., Codacci-Pisanelli G., Peccatori F. Breast cancer risk of hormonal contraception: Counselling considering new evidence. Crit. Rev. Oncol. Hematol. 2019;137:123–130. doi: 10.1016/j.critrevonc.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 74.Tamimi R.M., Spiegelman D., Smith-Warner S.A., Wang M., Pazaris M., Willett W.C., Eliassen A.H., Hunter D.J. Population Attributable Risk of Modifiable and Nonmodifiable Breast Cancer Risk Factors in Postmenopausal Breast Cancer. Am. J. Epidemiol. 2016;184:884–893. doi: 10.1093/aje/kww145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nguyen B., Venet D., Lambertini M., Desmedt C., Salgado R., Horlings H.M., Rothé F., Sotiriou C. Imprint of parity and age at first pregnancy on the genomic landscape of subsequent breast cancer. Breast Cancer Res. 2019;21:25. doi: 10.1186/s13058-019-1111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Unar-Munguía M., Torres-Mejía G., Arantxa Colchero M., González de Cosío T. Breastfeeding mode and risk of breast cancer: A dose-response meta-analysis. J. Hum. Lact. 2017;33:422–434. doi: 10.1177/0890334416683676. [DOI] [PubMed] [Google Scholar]
- 77.Amadou A., Hainaut P., Romieu I. Role of obesity in the risk of breast cancer: Lessons from anthropometry. J. Oncol. 2013;2013:906495. doi: 10.1155/2013/906495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duan W., Li S., Meng X., Sun Y., Jia C. Smoking and survival of breast cancer patients: A meta-analysis of cohort studies. Breast. 2017;33:117–124. doi: 10.1016/j.breast.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 79.Grabrick D.M., Hartmann L.C., Cerhan J.R., Vierkant R.A., Therneau T.M., Vachon C.M., Olson F.J., Couch F.J., Anderson K.E., Pankratz V.S., et al. Risk of breast cancer with oral contraceptive use in women with a family history of breast cancer. JAMA. 2000;284:1791–1798. doi: 10.1001/jama.284.14.1791. [DOI] [PubMed] [Google Scholar]
- 80.Petitti D. Meta-Analysis, Decision, Analysis, and Cost-Effectiveness Analysis. Oxford University Press; New York, NY, USA: 2000. [Google Scholar]