Abstract
Simple Summary
The management of unresectable and metastatic cutaneous melanoma has substantially improved with the introduction of molecular targeted therapy (BRAF and MEK inhibitors) and immunotherapy (Immune checkpoint inhibitors). The avenue of precision oncology holds promise in melanomas due to the high rate of somatic mutations that contribute to tumor progression. In this review article, we discuss common mutations and altered pathways that are implicated in melanomagenesis including oncogenic driver mutations, tumor suppressor gene alterations, fusion oncogenes, epigenetic regulators and alterations in the DNA-damage response pathway. We also provide a comprehensive review of promising individualized novel treatment approaches in non-BRAF mutant melanoma.
Abstract
Melanomas exhibit the highest rate of somatic mutations among all different types of cancers (with the exception of BCC and SCC). The accumulation of a multimode of mutations in the driver oncogenes are responsible for the proliferative, invasive, and aggressive nature of melanomas. High-resolution and high-throughput technology has led to the identification of distinct mutational signatures and their downstream alterations in several key pathways that contribute to melanomagenesis. This has enabled the development of individualized treatments by targeting specific molecular alterations that are vital for cancer cell survival, which has resulted in improved outcomes in several cancers, including melanomas. To date, BRAF and MEK inhibitors remain the only approved targeted therapy with a high level of evidence in BRAFV600E/K mutant melanomas. The lack of approved precision drugs in melanomas, relative to other cancers, despite harboring one of the highest rates of somatic mutations, advocates for further research to unveil effective therapeutics. In this review, we will discuss potential druggable mutations and the ongoing research of novel individualized treatment approaches targeting non-BRAF mutations in melanomas.
Keywords: melanoma, targeted therapy, precision oncology, BRAF, MEK, NF1, NRAS, epigenetic, homologous recombination deficiency, DNA damage repair, tumor suppressor gene, molecular alteration
1. Introduction
Melanoma remains the deadliest skin cancer despite substantial advances achieved in its management. According to the International Agency for Research on Cancer, the estimated global new cases of melanoma in 2020 was 324,635, and the number of new deaths was 57,043 [1]. The age-standardized death rate due to melanoma is one death per 100,000 persons globally [2]. Incidence rates vary according to the geographical region and the Fitzpatrick skin phenotype, with the highest incidence rates reported in Australia, New Zealand, Northern Europe, and North America (Figure 1A) [3]. The incidence in the African-American population is one case per 100,000, compared to 22.1 cases per 100,000 in white patients (Figure 1B) [4]. The projected increase in the incidence of melanomas in the United States is estimated to reach 56.1 in males and 36.2 in females by 2036, which represents a three- to four-fold increase in melanoma incidence [5]. This trend of an increase in incidence and mortality in melanoma patients in the last three decades in the United States represents a major challenge in healthcare due to the burden of the disease (Figure 1C,D) [6].
Figure 1.
Epidemiology and trends of melanomas. (A) Age-standardized rate of melanoma cases per 100,000 in 2018 in selected countries per GLOBOCAN statistics [3]; (B) the 2011 incidence distribution among different ethnicities in the US [4]; (C) estimated incidence of melanoma cases in the US between 1990–2021 according to the American Cancer Society Statistics [6]; (D) estimated melanoma annual deaths in the US between 1990–2021 according to the American Cancer Society Statistics [6]. GLOBOCAN: Global Cancer Observatory.
Locally advanced and metastatic melanomas constitute 13% of newly diagnosed cases (SEER database statistics 2021). Despite the small proportion of the metastatic version of the disease, the mortality rate remains high and lags behind the number of newly diagnosed cases. The treatment of metastatic melanomas has substantially improved in the last decade, owing to the collaborative work that unveiled the role of oncogenic driver mutations and the immune system dysfunction involved in melanoma progression. The identification of the BRAFV600E/K as an oncogenic driver of somatic mutation in melanomas resulted in the development of the targeted treatment of the BRAF gain-of-function mutation (GOF) [7]. Similarly, the discovery of essential checkpoint receptors (including CTLA-4 and PD-1/PD-L1) and their role in immune evasion in cancers, including melanoma, reinvigorated the effort to develop immunotherapies that have proved crucial in prolonging survival [8,9,10,11,12].
The first BRAF inhibitor in melanomas was vemurafenib, which demonstrated efficacy in BRAFV600E/K mutant melanomas in the BRIM-3 phase 3 randomized clinical trial that demonstrated the overall survival (OS) advantage compared to dacarbazine, with an objective response rate (ORR) of 48% [13]. Following the BRIM trial, several BRAF inhibitors emerged as effective treatment options in BRAFV600E/K mutant melanomas. BRAF inhibitors were combined with MEK inhibitors in clinical trials due to their synergism, which can delay tumor progression and increase acquired resistance [14]. Three clinical trials (COMBI-d, coBRIM, and COLUMBUS) demonstrated superior outcomes in terms of the ORR and progression-free survival (PFS) for the combinations dabrafenib + trametinib, vemurafenib + cobmitinib, and encorafenib + binmetinib, compared to vemurafenib monotherapy in unresectable and metastatic melanomas harboring BRAFV600E or BRAFV600K mutations [15,16,17].
Unlike targeted therapy in BRAFV600E/K mutant melanomas, immune checkpoint inhibitors (ICI) demonstrated improved clinical outcomes in melanoma patients regardless of the presence of an oncogenic mutational signature or biomarker. Several phase 3 randomized clinical trials have demonstrated a high ORR and prolonged PFS and OS in front-line and recurrent metastatic melanomas, regardless of their BRAF mutation status [18,19,20,21].
The trials of the BRAF/MEK inhibitors were limited to patients with BRAFV600E and BRAFV600K mutations and who showed an ORR between 60 and70%, which indicated that 30–40% of BRAF mutant melanoma patients did not respond to the targeted therapy. Similarly, the ICIs showed an ORR in melanomas of 35–40% with single checkpoint inhibitors, and 58% with combined nivolumab and ipilimumab [18,19,20,21]. Accordingly, 40–60% of melanoma patients did not respond to ICI. In addition, the PFS rate at 5 years was 15–20% with BRAF/MEK inhibitors, and 21–36% with ICIs [22,23,24,25]. Taken together, these results demonstrate the unmet need for further effective therapeutics in melanoma patients, due to the substantial rate of non-responders to either targeted therapy or ICI, as well as the high rate of progression after the initial response.
The distinctive genetic alterations in melanomas provide an advantage for cancer cells to operate in an aggressive and unpredicted manner. For example, the frequency of somatic mutations is the highest in melanomas among solid and hematological malignancies (>100 somatic mutations per megabase) with the exception of non-melanoma skin cancers [26]. The characterization of the mutational landscape in melanomas from The Cancer Genome Atlas (TCGA) revealed specific molecular and genetic alterations including GOF mutations, loss-of-function (LOF) mutations (nonsense, splice, frameshift) in tumor suppressor genes, recurrent hotspot mutations, and copy number variations (amplifications and deletions) as well as different epigenetic, transcriptomic, and proteomic changes [27]. This has led to a new genomic classification of melanomas, which identifies subsets with distinct genetic signatures [28].
The diverse mutational landscape in melanomas represents an insufficiently explored territory of multiple potential actionable genetic alterations. Of importance, despite the high rate of mutations in melanomas, many of these are considered passenger mutations (i.e., they are not vital for melanoma progression). Research efforts are ongoing to elucidate the pathogenic implications of the vital molecular alterations to develop novel treatments. In this review, we will discuss common and infrequent/rare mutations in melanomas. We will also discuss current novel therapeutic approaches that are underway to target non-BRAF mutant melanomas. This review is tailored to cutaneous melanomas and, where relevant, we will mention acral and mucosal melanomas. Emphasis is drawn on the importance of including patients with refractory melanomas in clinical trials whenever possible.
2. The Mutational Landscape in Metastatic Melanomas
The two most commonly altered cellular pathways in melanomas are: (1) the mitogen-activated protein kinase (MAPK) pathway, and (2) the phosphatidylinositol 3-kinase (PI3K) pathway. Together, the MAPK and PI3K pathways form important signaling cascade networks that transmit extracellular signals intracellularly to regulate cell division and differentiation. Putative oncogenic activating mutations in BRAF (with a frequency of 40–60% in melanomas), NRAS, and KIT, as well as gene fusions, can signal through the MAPK and PI3K pathways, leading to uncontrolled cellular growth, proliferation, and survival (Figure 2). These mutations appear to be mutually exclusive with rare exceptions [29]. Further recurrent mutations have been identified through major collaborative work using high-resolution whole genome/exome sequencing by the Pan-Cancer Analysis of Whole Genomes Consortium, TCGA, and the cBioPortal, among other platforms (Figure 3) [28,30,31,32]. Recurrent mutations can be GOF mutations such as: MAP2K1/MAP2K2, mTOR, KRAS/HRAS, RAC1, and TERT, while LOF mutations occur in tumor suppressor genes such as: NF1, PTEN, CDKN2A, and TP53. Likewise, copy number variations (CNV) have been identified in certain genes in association with melanomas, such as in PTEN, CDKN2A, and KIT. In addition, alterations in epigenetic regulators like EZH2, ARID2, and IDH1/2, as well as genomic instability caused by mutations in the DNA damage response (DDR) genes, can lead to the altered transcription of key gene regulators in melanomas. In addition, mutations in melanomas can be either driver or passenger mutations. Driver mutations are able to drive malignant transformations in melanoma cells by virtue of the constitutive activation of growth signaling pathways. Conversely, passenger mutations can occur by chance, and do not confer the survival advantage to tumor cells. The distinction between these two types of mutations is challenging, but is important for the development of effective targeted therapy.
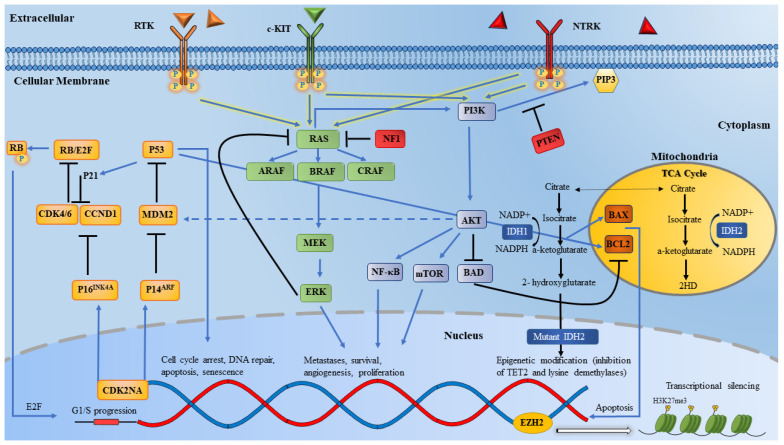
Figure 2.
Major signaling pathways in melanomas. The most essential pathways in melanomagenesis are the MAPK pathway (in green) and the PI3K pathway (violet). The MAPK pathway is activated by receptor tyrosine kinase and G-protein-coupled receptors. This activates RAS proteins, which in turn activates MEK, and then ERK. ERK can then translocate to the nucleus and phosphorylate transcriptional factor substrates involved in cell survival. NF1 negatively regulates RAS proteins which inhibits downstream RAS signaling. The PI3K pathway can be activated by RAS or through the inactivation of PTEN. PI3K can activate several pathways (BAD, NF-kB) and AKT pathways, which in turn leads to phosphorylation of mTOR, which leads to increased cellular proliferation. The CDKN2A pathway is another essential network in melanomas (yellow). CDKN2A encodes two inhibitory variants of the G1–S phase. These inhibitors (tumor suppressors) are P16INK4A, which can bind to CDK4/6, preventing them from interacting with CCND1 and RB phosphorylation. When phosphorylated, RB can release an E2F factor which leads to G1–S cell cycle progression. P14ARF can bind to MDM2 which is responsible for p53 degradation. Epigenetic regulators can be altered in melanomas. IDH1/2 are enzymes that convert isocitrate to a-ketoglutarate. IDH1/2 mutations can lead to the formation of the oncometabolite D2H that can alter and silence several key regulator genes (although the exact role of IDH mutations in melanomas has not been elucidated). Hotspot mutations and amplifications in EZH2 can lead to aberrant methylation of H3K27, leading to dysregulation of transcriptional factors. Alterations in c-KIT and NTRK fusions (although rare in melanomas) can contribute to melanomagenesis through downstream signaling of several pathways, including MAPK and PI3K, to drive cellular proliferation. Abbreviation: RTK: receptor tyrosine-kinase; NTRK: neurotrophic tyrosine receptor kinase; PIP3: phosphatidylinositol 3; PI3K: phosphoinositide 3-kinase; PTEN: phosphatase and tensin homolog; NF1: neurofibromatosis type 1; RAS: rat sarcoma; RB: retinoblastoma; E2F: E2 factor; CDK4/6: cyclin-dependent kinase 4/6; CCND1: cyclin D1; MDM2: mouse double minute 2 homolog; MEK: mitogen-activated protein kinase; CDK2NA: cyclin-dependent kinase inhibitor 2A; ERK: extracellular signal-regulated kinase; AKT: ak strain transforming; NADP+/NADPH: nicotinamide adenine dinucleotide phosphate; IDH1: isocitrate dehydrogenase 1; NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells; mTOR: mammalian target of rapamycin; BAD: BCL2 associated agonist of cell death; BAX: BCL2-associated X protein; BCL2: B-cell lymphoma 2; IDH2: isocitrate dehydrogenase 2; EZH2: enhancer of zeste homolog 2.
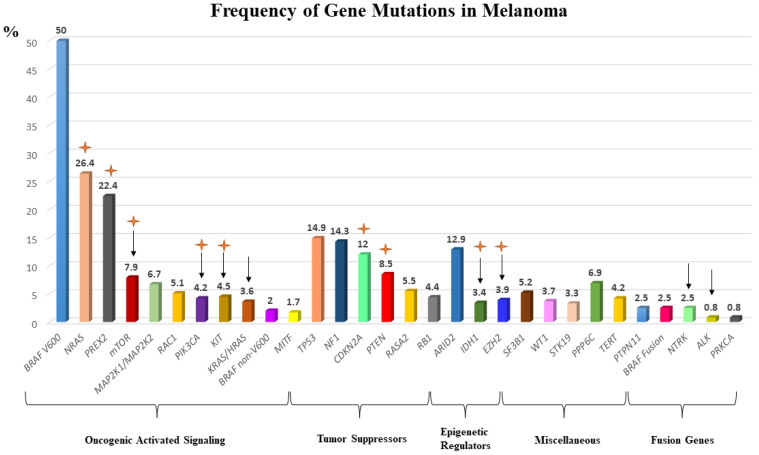
Figure 3.
Frequency of common somatic mutations in melanomas are estimated based on Vanni et al.’s analysis of the frequency of somatic mutations from different studies [33]. Fusion gene frequencies were obtained from non-TCGA database, and the frequency varies among different studies. Arrows indicate the presence of FDA-approved inhibitors in cancers harboring mutations for the specific gene in non-melanoma cancers. Orange asterisks indicate the presence of ongoing trials in melanoma patients with mutation-specific alterations or melanoma patients receiving targeted therapy for the specific mutant gene. TCGA: the cancer genome atlas; FDA: food and drug administration.
Tumorigenesis in melanomas is not driven by one single genetic alteration, but rather it requires collaboration between several altered pathways. However, BRAF, NRAS, and NF1 mutations were found to be prevalent and highly oncogenic [34,35]. As a result, melanomas have been classified into four distinct genetic subsets based on the molecular signature (BRAF mutant, NRAS mutant, NF1 mutant, and triple negative) [28]. The improved sequencing techniques helped identify novel mutations and alterations (overexpression/amplification) that can drive melanomagenesis, such as mutations in the microphthalmia-associated transcription factor (MITF), Rac family small GTPase1 (RAC1), serine/threonine kinase-19 (STK19), and RAB7 [27,28]. It is noteworthy that the implementation of BRAF and MEK inhibitors in clinical practice is a testimony of the impressive improvement in outcomes by targeting a subset of oncogenic driver mutations in melanomas. Nevertheless, the lack of targeted therapy in non-BRAF mutant melanomas, the eventual progression during treatment with BRAF/MEK inhibitors, and the high rate of somatic mutations in melanomas relative to other malignancies advocates for further research into their biological effects on tumor progression [33,36].
3. Targeting Activating Mutations in the KIT Oncogene
KIT was first described in 1987 as a receptor tyrosine kinase (RTK) encoded by the proto-oncogene c-KIT localized on chromosome 4 [37,38]. The receptor has the characteristic structure of other RTKs, which includes an N-terminal signal peptide, an extracellular segment, a transmembrane domain, and a C-terminal intracellular segment. KIT is an RTK type III, which is characterized by five immunoglobulin-like repeats in the extracellular domain. The C-terminal intracellular segment contains the kinase domain, which is subdivided into an N-terminal segment that corresponds to an ATP-binding site, and a C-terminal segment that corresponds to the kinase autophosphorylation site [39,40].
KIT activation occurs by the binding of the stem cell factor ligand (SCF, also known as the KIT ligand), a growth factor that stimulates the survival, differentiation, and proliferation of several cell types, including melanocytes [41,42]. The ligand binds to the three immunoglobulin-like domains in the N-terminal portion of the extracellular segment and causes the dimerization of the receptor by enabling a homotypic interaction between the two domains closer to the cellular membrane. This causes the phosphorylation of the tyrosine domains intracellularly, as well as subsequent transmembrane signal transductions [40,43,44]. Once activated, KIT promotes the survival and development of hematopoietic stem cells, germ cells, and melanocytes. The most important signaling pathways in c-KIT-derived melanomas are the PI3K/AKT and the MAPK pathways [45,46,47].
Mutations in c-KIT are relatively infrequent in melanomas and were first reported by Went et al. who sequenced 36 tumors that were strongly positive for KIT by immunohistochemistry (IHC). These included two melanomas, of which one had a transition mutation of leucine to proline in codon 576 (L576P) of exon 11. It was later found that L576P is the most common mutation in c-KIT in melanomas and that most mutations occur in exons 11 and 13 [47,48,49]. In addition, mutations of c-KIT seem to be more common than copy amplifications in melanomas and most of them occur in the kinase domain or in the juxta-membrane domain. These alterations seem to vary in frequency depending on the melanoma subtype and are mutually exclusive to BRAF and NRAS mutations, with rare exceptions. Both the mutations and amplifications of c-KIT are uncommon in cutaneous melanomas (3–4%) but are enriched in acral (8.6–36%) and mucosal melanomas (9.6–39%) (KIT mutations are enriched in sinonasal mucosal melanomas, as opposed to anogenital) [50,51,52,53].
The standard of care for metastatic melanomas with c-KIT mutations is immunotherapy [54]. There are no prospective studies evaluating the response of KIT mutant melanomas to immunotherapy, with one retrospective study showing an ORR of 20% in patients who received anti-CTLA-4 agents and had KIT mutations in exons 2, 11, 13, and 17. The same study reported an ORR of 35% with anti-PD1 immunotherapy in patients with KIT mutations in exons 2, 10, 11, 13, and 17 [55]. The authors described that the patients had similar response rates to ICI, regardless of the exon affected [55].
Treatment approaches with tyrosine kinase inhibitors (TKIs) targeting KIT mutations have been conducted in several clinical trials in relapsed and refractory melanomas. Imatinib, which has multiple therapeutic targets including BCR-ABL, KIT, and the platelet-derived growth factor receptor (PDGFR), was approved for the treatment of gastrointestinal stromal tumors (GIST) (the majority of these tumors have the KIT mutation) [56]. The first trials evaluating imatinib in melanomas were negative and noted significant treatment toxicity. However, these trials did not select patients with KIT mutations for enrollment [57,58]. Following that, one phase 2 trial enrolled 21 patients with metastatic melanomas expressing at least one protein tyrosine-kinase (c-KIT, PDGFR, c-abl, or abl-related genes) to receive imatinib. One patient with the highest c-KIT expression had a dramatic response, four patients (three expressing c-KIT) had a stable disease, and the remaining patients had disease progression [59]. This trial has paved the way for further investigation into the role of TKIs in KIT mutant melanomas.
The first trial investigating the targeted therapy of KIT mutations was reported in 2011 and included a total of 51 melanoma patients who received imatinib. This trial demonstrated two complete responses (CR) lasting 53 and 89 weeks, and two partial responses (PR) lasting 12 and 18 weeks, among 25 evaluable patients. The reported ORR was 16% with a median progression-free survival (PFS) of 12 weeks and median overall survival (OS) of 46.3 weeks. Patients who achieved a CR or a PR had KIT mutations in exons 11 and 13 in acral or mucosal melanomas [60]. Two other trials and one retrospective study evaluated imatinib in this setting and the results were similar, as shown in Table 1. However, no patients achieved a CR as reported in the first trial. In trial 2, 10 patients out of 43 had a PR, of which nine had a KIT mutation in exon 11 or 13, and one patient had a KIT amplification. In trial 3, seven patients out of 25 achieved a PR, six of which had exon 11 or 13 mutations and one who had an exon 17 mutation [61,62]. The largest study evaluating imatinib was a retrospective study with 78 patients that did not report a CR, but there were 17 PRs (11 in patients with exon 11 or 13 mutations, two with multiple mutations, one with an amplification, and one in exons 9, 17, and 18) [63].
Table 1.
Clinical trials and retrospective studies of targeted therapy in c-KIT mutant melanoma.
| Study Design | Study Drug | Dose | Number of Patients | Type of Mutations | Special Characteristics | Overall Response Rate | Duration of Response | Median Progression Free Survival | Median Overall Survival | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Single group, open label, multicenter, phase 2 trial | Imatinib | 400 mg twice daily | 28 | Mutation and/or amplification | 328 patients screened, 51 had KIT alterations, 28 were treated, 25 analyzed (3 excluded due to toxicity) | 16% | 2 CR (94 and 95 weeks), 2 PR (53 and 89 weeks) and 2 transient PR (12 and 18 weeks) | 12 weeks | 46.3 weeks | [60] |
| Single arm, open label, single center, phase 2 trial | Imatinib | 400 mg daily | 43 | Mutation and/or amplification | Dose allowed to be escalated to 600 mg or 800 mg daily if POD | 22% | Not available | 3.5 months | 14 months | [61] |
| Single arm, open label, multicenter, phase 2 trial | Imatinib | 400 mg daily | 25 | Mutation and/or amplification | Dose escalated to 400 mg twice daily if no response | 29% (21% excluding non-confirmed responses) | Not available | 3.7 months | 12.5 months | [62] |
| Retrospective chart review, single center | Imatinib | 400 mg daily | 78 | Mutation and/or amplification | Largest study, but retrospective | 21.8% | Not available | 4.2 months | 13.1 months | [63] |
| Single arm, open label, single center, phase 2 trial | Nilotinib | 400 mg twice daily | 11 | Mutation and/or amplification | Of the 11 patients, 9 were evaluated for a response. Most patients had M1c disease | 22.2% | 2 w/KIT mutations responded for 8.4 and 10.0 months. 1 w/amplification had SD for 6 months | 2.5 months | 7.7 months | [64] |
| Phase 2 trial with 2 study groups, open label | Nilotinib | 400 mg twice daily | 19 | Mutation and/or amplification | 20 enrolled, 19 treated. 4 were not evaluated for radiographic responses to therapy. 2 cohorts: (A) those refractory or intolerant to a prior KIT inhibitor; and B) those with brain metastases | 10.5% overall (18.1% in cohort A, 2 PR; 0 in cohort B) | One patient in cohort A had ongoing response for 34.5 months. One in cohort B had a 3.9-month response in CNS disease | 3.3 months | 9.1 months | [65] |
| Open label, single arm, multicenter, phase 2 | Nilotinib | 400 mg twice daily | 42 | Mutation and/or amplification | 176 patients screened, 42 enrolled. PFS 8.5 months in CR/PR/SD vs. 7 weeks in PD | 16.7% | 34 weeks | 3.3 months | 11.9 months | [66] |
| Open label, single arm, multicenter, phase 2 | Nilotinib | 400 mg twice daily | 42 | Mutation only | First to evaluate nilotinib without prior KIT inhibitor therapy. Originally designed for 2 groups (prior dacarbazine vs. nilotinib), but not enough patients | 26.2% | 7.1 months | 4.2 months | 18.0 months | [67] |
| Open label, single arm, multicenter, phase 2 | Nilotinib | 400 mg twice daily | 25 | Mutation and/or amplification | 4 patients exhibited durable response, 3 persisting (3.6 and 2.8 years for 2 patients with stage IIIC and 2.5 years for 1 with IVM1b) | 20% | In patients with CR and PR, 46.8 months | 6.0 months | 13.2 months | [68] |
| Two-stage, open label, single arm, single center, phase 2 | Dasatinib | 70 mg twice daily | 30 in stage 2 | Mutation only | 2 stages: (1) both KIT+ and KIT-wt w/ mucosal, acral and CSD melanoma; 57 patients, 51 analyzed; (2) trial amended for KIT+ only and added vulvo-vaginal and excluded CSD melanoma; 30 patients, 22 analyzed | 18.2% in stage 2 5.9% in stage 1 | 4.2 months in stage 2 | 2.1 months (stage 1 and 2 combined) 2.7 months in KIT+ patients (both stages) | 7.5 months (stage 1 and 2 combined) 11.8 months in KIT+ patients (both stages) | [69] |
| Open label, single arm, single center, phase 2 | Sunitinib | 50 mg daily | 12 (10 analyzed) | Mutation, amplification or over-expression | 90 enrolled, 12 treated with sunitib (KIT alterations). Sunitib given 4 weeks on, 2 off. Dose reduced to 37.5 or 25 mg if AEs | 40% | 1 CR for 15 months, 2 PR (1 month and 7 months) in patients with mutations. 1 PR in amplification or over-expression. | Not available | Not available | [70] |
Abbreviation: POD: progression of disease; KIT: c-KIT receptor; CR: complete response; PR: partial response; CNS: central nervous system; CSD: chronic sun damage; PFS: progression-free survival; PD: progressive disease; SD: stable disease; w/: with; c-KIT: c-KIT receptor; KIT+: positive for c-KIT receptor.
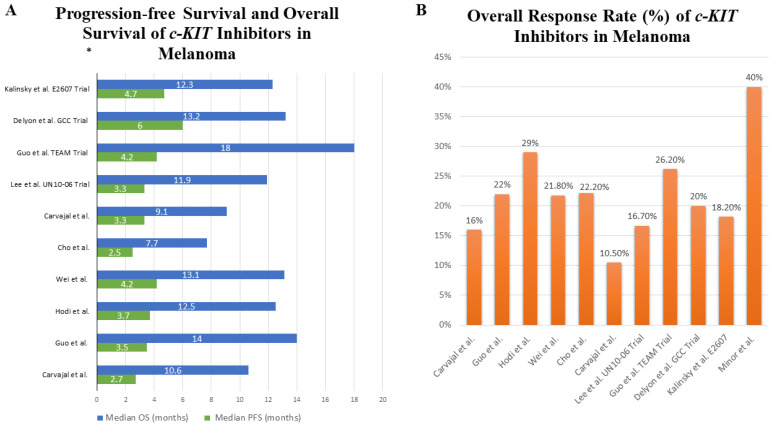
Trials with a similar design were conducted using newer TKIs, including nilotinib, sunitinib, and dasatinib, and are summarized in Table 1. These trials demonstrated similar results in terms of response rates, PFS, and OS (Figure 4A,B). Most responses in the studies were noted in patients with KIT mutations in exons 11 and 13. Other KIT alterations, such as mutations in other exons, amplifications, and overexpressions, may have lower response rates [60,61,62,63,64,65,66,67,68,69,70]. One phase 2 trial using dasatinib did not include exclusively patients with KIT mutations. The ORR was 5% (a total of two PRs, one in a patient with a c-KIT exon 13 mutation, and one in a patient with a wild-type c-KIT), which emphasizes the concept of using these TKIs in c-KIT mutant melanomas only [71].
Figure 4.
Clinical trials and retrospective study results of c-KIT inhibitors in melanomas [60,61,62,63,64,65,66,67,68,69,70]. (A) Median progression-free survival and overall survival in melanoma patient cohorts treated with different c-KIT inhibitors. These trials and retrospective studies must not be compared as patient inclusion criteria, trial designs, and KIT mutation subtypes are different. (B) Overall response rates in melanoma cohorts treated with c-KIT inhibitors. * Kalinsky et al. study analyzed PFS and OS in melanoma patients with KIT mutations involving exons 11 and 13 only [69]. PFS: progression-free survival; OS: overall survival.
Some case reports suggested the efficacy of other TKIs in melanomas with KIT alterations. For example, a patient with anal mucosal melanomas and a KIT mutation (Val560Asp) in exon 11 had a complete response to sorafenib with temozolomide that lasted 5 months [72]. Another case report demonstrated PR (including an intracranial disease) that lasted for 3 months in a patient with a primary esophageal mucosal melanoma and a KIT mutation in exon 11 treated with masitinib [73].
In general, the responses reported in all previous studies were short-lived, suggesting that other pathways may contribute to resistance. One possible mechanism of resistance explored is the activation of the MET receptor. One study reported the case of a patient with a metastatic melanoma of unknown origin that harbored a KIT mutation, N822Y, in exon 17. The patient received dasatinib for KIT inhibition and crizotinib for MET inhibition and had a response sustained for 34 months. The authors demonstrated in vitro that the addition of the hepatocyte growth factor (MET ligand) to the melanoma cell lines with the KIT mutation was able to increase cell viability despite the presence of dasatinib, which was reversed by the addition of crizotinib [74].
There is also preclinical evidence that ponatinib may have a role in the treatment of KIT mutant melanomas. One study obtained patient-derived tumor xenograft cells from KIT mutant melanomas and tested the efficacy of KIT inhibitors in vitro and in vivo. Ponatinib had a stronger affinity for KIT and was a more potent inhibitor when compared to imatinib, suggesting the need for future studies with newer TKIs in KIT mutant melanomas [75].
Some studies have also looked into combining a TKI with other systemic therapies. One phase 1/2 trial tested the combination of 800 mg of imatinib daily and 10 mg/kg of bevacizumab every 2 weeks in 23 patients with metastatic melanomas. One patient (4%) had PR and there was a stable disease in seven patients (30%). The median PFS was 7.7 weeks, and five patients remained on the study for more than 4 months [76]. Another study combined imatinib and ipilimumab in 35 patients with different advanced malignancies and demonstrated one PR in a patient with melanoma and a KITL576P mutation in exon 11. The duration of the response observed was 10 months [77]. The combination of pembrolizumab and imatinib was reported in the case of a patient with a metastatic melanoma and double KIT mutations (V559 and N822I). The patient had oligometastatic disease in the lung and achieved a CR after 6 months of therapy, which lasted for 12 months [78].
Several clinical trials are testing novel TKIs and combinations of TKIs with other systemic therapies for melanomas, such as NCT02071940, which involves evaluating PLX3397 (plexidartinib, a multi-target TKI) in advanced KIT mutant acral and mucosal melanomas. Another trial (NCT01738139) is investigating the combination of ipilimumab and imatinib in advanced solid tumors including metastatic melanomas. NCT04598009 will evaluate binimetinib and imatinib in patients with stage III and IV KIT mutant melanomas. In addition, NCT02571036 is a phase 1 trial that will evaluate DCC-2618 (ripretinib, a TKI designed to inhibit specifically KIT and PDGFR-A kinases) in patients with advanced cancers, and the trial NCT04771520 is currently recruiting patients with advanced or metastatic solid tumors with c-KIT or PDGFR-A mutations to receive avapritinib (BLU-285).
In summary, KIT is evolving as an important therapeutic target in advanced melanomas. Currently, the use of TKI is reserved for the second or later line treatment in KIT mutant melanomas. The role of KIT inhibition in patients with advanced melanomas and KIT alterations is rapidly expanding. So far, the response rates have been low and most of the responders have sensitized mutations in exons 11 and 13. Clinical trials are currently evaluating more potent inhibitors and the combination of current inhibitors with immunotherapy. More studies are needed to further evaluate the characteristics of patients that achieve a response, the mechanism of resistance development, and the role of combining immunotherapy and MET inhibitors with KIT inhibitors.
4. Targeting Activating Mutations in RAS Oncogenes
4.1. NRAS
4.1.1. NRAS Biology
The NRAS oncogene was first identified in a melanoma cell line in 1984 [79]. Since then, several efforts have been conducted to develop targeted therapy strategies for NRAS mutant melanomas. NRAS is part of the RAS proteins (NRAS, KRAS and HRAS) and is a small plasma membrane-associated guanosine 5′-triphosphate (GTP)-binding protein. The most frequent oncogenic mutation (>80%) is a point mutation at position 61, leading to the substitution of leucine with glutamine. This point mutation results in impaired GTPase activity and the locking of the RAS protein into its activated (GTP-associated) conformation. The activated RAS subsequently transmits the signal from RTK to several downstream transduction pathways involved in growth, motility, cell-to-cell signaling, differentiation, and survival.
In normal melanocytes, MAPK signaling occurs selectively through BRAF rather than CRAF, as the cyclic AMP pathway activation promotes protein kinase A (PKA) mediated inhibition of CRAF. In NRAS mutant melanomas, the inhibition of PKA signaling (that prevents CRAF inactivation) leads to the negative feedback inhibition of BRAF and promotes CRAF-mediated MAPK signaling instead [80].
Upstream effectors controlling NRAS, such as RTK, have been identified. More recently, Yin et al. identified a critical kinase (STK19) upstream that phosphorylates NRAS on the S89, promoting oncogenic NRAS signaling. This led to the development of a selective STK19 inhibitor with preclinical results in vitro and in vivo exhibiting inhibitory effects on NRAS mutant melanomas [81].
4.1.2. The Frequency in Melanomas and Characteristics
NRAS mutations account for 26% of all mutations present in melanomas registered in TCGA [28]. Comparing the genetic alterations in 126 melanomas, Curtin et al. identified that 81% of melanomas on skin without chronic sun damage had mutations in BRAF or NRAS [82]. NRAS mutations occur at a higher rate in the older population, where the median age is 55.7 years compared to 49.8 years in patients with the BRAF mutation [83]. In addition, NRAS mutations are present predominantly in upper extremities and in one third of nodular melanomas [84,85].
In a retrospective study of 677 patients, 82.4% of NRAS mutations accounted for substitutions in positions 60–61 and, most frequently, a glutamine to arginine/lysine/leucine/histidine substitution at position 61 (Q61R/K/L/H) [83,86]. Uncommonly, 20% of all NRAS mutations are due to a glycine to aspartic acid/arginine substitution in positions 12–13 G12 (G12D), and G13 (G13R, G13D). Burd et al. demonstrated in vitro and in “knock-in” mouse models that the expression of the NRAS mutation at codon 61 drives melanoma formation with increased melanomagenecity compared to the NRAS mutation at codon 12. Therefore, this explains the predominance of NRAS mutations in Q61R [87].
4.1.3. The Prognostic Impact of NRAS in Melanomas
The prognostic impact of NRAS mutations in melanomas is controversial. A large retrospective review of patients with melanomas reported that BRAF and NRAS mutations were more likely to have CNS involvement at the time of diagnosis, and NRAS mutant melanomas were an independent predictor of shorter survival times in metastatic melanomas compared to BRAF mutant and wild-types [83]. In addition, NRAS mutation is associated with the deepest Breslow and Clark levels of invasion and patients tend to present with regional metastases compared with wild-type tumors. In the setting of stage III disease, Ellerhorst et al. reported no difference in survival between mutant (BRAF and NRAS) and wild-type melanomas; however, in this study the mutation pattern was only obtained from the primary site and metastases were not sequenced [85].
A European retrospective multicenter analysis (n = 364) comparing ICI therapy between NRAS mutant and wild-type melanomas showed a shorter median OS in patients with NRAS mutations (21 months compared to 33 months) despite similar response rates. In this study, loco-regional or distant metastases were used for mutation patterns [88].
In contrast, a large retrospective analysis (n = 656) of a Clinico-Genomic Database evaluating outcomes in a real-world setting demonstrated that after the first line of immunotherapy, the median OS of NRAS mutant melanomas was 44.9 months compared to 38.6 months in BRAF mutants, 27.1 months in NF1 mutants, and 19.8 months in triple wild-type melanomas [89].
Similar findings were reported by Mangana et al. in a smaller study (n = 101) evaluating anti-CTLA-4 in NRAS and BRAF mutant melanomas, however, results did not reach statistical significance [90].
4.1.4. Targeting Upstream Effectors of NRAS
Targeting RTK remains a challenge in melanomas. Early studies have been conducted in combination with downstream inhibitors with mixed results. A phase 1 study with an antibody targeting ERBB3 in combination with trametinib in NRAS mutant or wild-type melanomas was terminated in 2020 (NCT03580382). The MET receptor tyrosine kinase, activated in NRAS mutant cancers, has been targeted with the oral inhibitor tivantinib. A phase 1 study in combination with sorafenib was extended to a cohort of patients with melanoma. Among the eight patients with NRAS mutations, there was one CR, one PR, and two stable diseases (SD) [91].
4.1.5. Targeting NRAS
The direct targeting of the ligand binding sites on all RAS proteins remains challenging due to their high affinity for GDP, GTP, and suspected high toxicity. Given RAS is essential in cell signaling, the deletion of all three RAS proteins results in embryonic lethality in mouse models and no cellular proliferation in vitro. Other strategies have been studied, such as small binding compounds inhibiting the SOS1-mediated nucleotide exchange on RAS and the reduced phosphorylation of the downstream kinases ERK and AKT. However, the compounds are not mutant-selective inhibitors and no specific strategy targeting NRAS has been developed [86].
4.1.6. Post-Translational Targets in RAS Proteins
RAS proteins undergo a lipid post-translational modification in order to get access to the effectors in the membrane compartments. Farnesylation is one of the key post-translational modifications which is catalyzed by the enzyme Farnesyltransferase (FT). Therefore, FT inhibitors have been developed as post-translation targets to reduce RAS- mediated downstream activation. In melanomas, the FT inhibitor lonafarnib, in combination with sorafenib, showed preclinical activity against tumor cell growth in vitro and in vivo. In a phase 2 clinical trial, R115777 (an oral selective FT inhibitor) did not show evidence of clinical activity in a cohort of 14 patients with melanoma [92,93].
4.1.7. Targeting the Downstream Effectors of NRAS
The activation of the RAS/RAF/MEK/ERK signaling pathway through NRAS mutations occurs in 15–20% of melanoma cases. In vertebrates, there are three RAF proteins called ARAF, BRAF, and CRAF, and they are the initial effectors in the kinase cascade. Dorard et al. demonstrated that the concomitant ablation of BRAF and CRAF in NRAS Q61K mutant mouse melanoma models resulted in a blockage of tumor growth, providing evidence that the MAPK pathway is an important downstream effector of oncogenicity in NRAS mutant melanomas [94].
The downstream oncogenic effect of NRAS mutations is mainly driven by CRAF, which subsequently signals to MEK, and is associated with cAMP signaling dysregulation [95]. Therefore, the selective inhibition of BRAF in NRAS mutant melanomas is not an adequate approach, as it can induce the paradoxical activation of RAF proteins and the concomitant ablation of BRAF and CRAF, leading to the emergence of resistant cells showing the ARAF-dependent reactivation of ERK [94].
Solit et al. emphasized the concept that RAF/MAPK signaling is dispensable for the oncogenic activity of NRAS mutant melanomas, and they suggested that a single-agent therapeutic strategy may be insufficient in RAS mutant tumors [96].
Pan-RAF inhibitors, such as belvarafenib, which can target BRAFV600E, the BRAF wild-type, and CRAF have shown anti-tumor efficacy in NRAS mutant melanomas in a phase 1 dose escalation study with an ORR of 44% [97]. Subsequently, in a phase 1b study in combination with cobimetinib (a MEK inhibitor), it showed an ORR of 38.5% with median PFS of 7.3 months. Another pan-RAF inhibitor (PRi, Amgen Compd), in combination with trametinib, demonstrated anti-proliferation properties in vitro in NRAS mutant melanomas [98].
Targeting MEK is currently the most developed strategy in NRAS mutant melanomas. First-generation MEK inhibitors (selumetinib) failed to show positive outcomes in patients with unselected BRAF/NRAS mutations, as well as patients with NRAS mutations [99]. Similarly, a phase 2 trial double-blind study that analyzed the NRAS mutation status retrospectively did not show an improvement between selumetinib with docetaxel vs. a placebo [100].
Second- and third-generation MEK inhibitors are the most advanced in this setting, especially binimetinib, which was evaluated in the NEMO study. This study was a randomized phase 3 multicenter trial comparing binimetinib vs. dacarbazine in NRAS mutant melanoma patients that showed an improvement in their median PFS at 2.8 months in binimetinib groups, vs. 1.5 months in the dacarbazine group. However, no difference was observed in OS [101]. Pimasertib, a second-generation MEK1/2 inhibitor, also showed an improvement in the PFS over dacarbazine (13.0 vs. 6.9 weeks) in a phase 2 study in NRAS mutant melanomas; however, the OS was similar [102].
Given that NRAS activates both the MAPK and PI3K pathways, the combination of the MEK inhibitor (trametinib) with the PI3K/mTOR inhibitor has shown to enhance cell growth inhibition in vitro [103]. This combination was found to be synergistic, and was effective in inducing tumor reduction in a nude mouse NRAS mutant xenograft tumor model [104]. Nevertheless, a non-randomized multicenter phase 2 study using trametinib in combination with GSK2141795 (a pan AKT inhibitor) did not yield significant clinical activity in NRAS mutant melanomas [105].
The CDKN2A/CDK4/6 pathway has a role in the G1–S transition in the cell cycle, which is dysregulated in BRAF and NRAS mutant melanomas. In addition, NRAS activation causes increased cyclinD1 (CCND1) expression and the upregulation of CDK4/6. Alterations in CDKN2A and in CCND1 are present in NRAS mutant melanomas in 70% and 10% of cases, respectively [28,106]. A combination of ribociclib (a CDK4/6 inhibitor) and a MEK inhibitor (binimetinib) was evaluated in a phase 1b/2b trial in 63 patients with NRAS mutant melanomas, obtaining an ORR of 19.5% (n = 41) and a PFS of 3.7 months in the phase 2 expansion [107].
Finally, MEK inhibitors have also been studied in combination with ROCK inhibitors (GSK269962A) in vitro and in vivo, showing the suppression of the growth of NRAS mutant melanomas. In this case, ROCK 1 and 2 are Rho GTPase-activated serine/threonine kinases implicated in tumor cell proliferation [108]. To date, there have been no clinical trials with this combination.
Despite the lack of clinically effective targeted therapy in NRAS mutant melanomas, studies are ongoing to address novel treatment approaches and are summarized in Table 2.
Table 2.
Summary of ongoing targeted therapy trials in NRAS mutant melanomas.
| Drug(s) | Study Phase | Target Population | Sample Size | ClinicalTrials.gov (Accessed on 1 September 2021) Registration |
|---|---|---|---|---|
|
MEK + Autophagy inhibitor Trametinib + Hydroxychloroquine |
Phase 1b/2 | Metastatic or locally advanced unresectable NRAS melanoma | 29 | NCT03979651 |
| MEK inhibitor Binimetinib | Phase 2 | BRAF or NRAS mutant locally advanced or metastatic melanoma | 183 | NCT01320085 |
| MEK inhibitor HL-085 | Phase 1/2 | Stage III or Stage IV NRAS mutated melanoma | 54 | NCT03973151 |
| MEK inhibitor FCN-159 | Phase 1a/1b | NRAS aberrant and NRAS mutated melanoma | 37 | NCT03932253 |
|
RAF inhibitor + ERK inhibitor +/− other agents Encorafenib + LY3214996 |
Phase 1 | Part B: BRAF mutant metastatic melanoma refractory or relapse, NRAS metastatic melanoma, BRAF mutant NSCLC | 245 | NCT02857270 |
| ERK inhibitor ASN007 | Phase 1 | NRAS/BRAF mutant melanoma | 49 | NCT03415126 |
|
ARF-sparing inhibitor of BRAF and CRAF + ERK inhibitor or MEK inhibitor or CD4/6 inhibitor LXH254 + LTT462 or Trametinib or Ribociclib |
Phase 1b | KRAS/BRAF NSCLC | 331 | NCT02974725 |
| Phase 2 | NRAS mutant melanoma | 320 | NCT04417621 | |
| ERBB3 antibody + MEK inhibitor CDX-3370 + Trametinib |
Phase 1b/2 Terminated |
NRAS melanoma | 3 | NCT03580382 |
| RAF Inhibitor + MEK inhibitor + immune check point inhibitor | Phase 1b | Stage IV or Stage III NRAS melanoma relapse after check point inhibitor | 83 | NCT04835805 |
NRAS: neuroblastoma RAS viral oncogene; BRAF: BRAF gene; NSCLC: non-small cell lung cancer; MEK: mitogen-activated protein kinase; RAF: RAF gene; ERK: extracellular signal-regulated kinase; CRAF: CRAF gene.
4.2. Targeting KRAS/HRAS
Mutations in KRAS and HRAS occur at a low frequency in melanomas, occurring at 1.7% and 1.9%, respectively [33]. Targeting KRAS and HRAS mutations in melanomas is not currently being pursued due to the lack of insight on the biological impact in melanomagenesis. Nevertheless, the projected number of new melanoma cases with KRAS/HRAS mutations is substantial and is around 2600 persons per year [109]. Therefore, further research is essential to investigate the role of these alterations in melanomas. Mutations in KRAS have been regarded as undruggable until a recent novel molecule (sotorosib), which targets the KRASG12C mutation, demonstrated efficacy in phase 1 and 2 trials in non-small cell lung cancer [110]. Of interest, in the phase 1 trial which included multiple solid tumors with KRASG12C mutations, there was one melanoma patient with KRASG12C [111]. The patient received 960 mg of sotorasib daily and achieved PR within 2 months of starting treatment. The duration of response was 5.6 months which was followed by disease progression.
5. Targeting Tumor Suppressor Gene Alterations
Tumor suppressor genes are responsible for maintaining normal cellular division. Tumor suppressor genes can halt cellular growth, induce apoptosis, and provide a checkpoint state to allow for the repair of damaged DNA, or can induce apoptosis if genomic integrity is not achieved. The most commonly mutated tumor suppressor genes identified in melanomas are: NF1, TP53, CKDN2A, and PTEN [28]. The targeted treatment of tumor suppressor gene alterations in cancer has proved challenging despite extensive research efforts [112]. Nonetheless, several promising approaches are being investigated in cancers with tumor suppressor gene alterations.
5.1. Targeting NF1 in Melanomas
The neurofibromatosis-1 protein NF1, which is encoded by its parent tumor suppressor gene (NF1) on chromosome 17, operates by the negative regulation of the MAPK and PI3K pathways. This function is mediated by the GTPase-activating protein-related domain which converts active RAS-guanosine triphosphate (RAS-GTP) to the inactive guanosine diphosphate form, leading to the downregulation of the RAS cascade, which prevents uncontrolled cellular growth [113]. The majority of NF1 mutations are LOF nonsense mutations which deprive the cell of the negative regulation of growth pathways. The frequency of NF1 mutations in melanomas is approximately 15%, with a higher frequency observed in older patients, chronic sun damage lesions, desmoplastic melanomas, and BRAF/NRAS wild-type melanomas (a frequency of 70%) [114,115]. In addition, NF1 mutations can co-occur with BRAF/NRAS mutations and can mediate the resistance to BRAF inhibitors. To date, no studies have identified an effective targeted therapy in NF1 mutant melanomas. However, preclinical evidence suggests that NF1 mutant melanomas are dependent on MEK signaling and may be sensitive to MEK inhibitors [116,117]. Recently, Py et al. reported the efficacy of trametinib (a MEK inhibitor) in a patient with an immunotherapy-refractory melanoma with BRAF/NRAS wild-types and mutations in NF1 and PTPN11 [118]. The patient had a PR, including the regression of brain metastases, with a duration of response lasting 5 months prior to disease progression. In a phase 1 study using trametinib in advanced melanomas, trametinib did not demonstrate efficacy in the majority of patients with BRAF/NRAS wild-types, but one patient with an NF1 mutation had a stable disease [119]. No specific clinical trials are currently ongoing in patients with NF1 mutant melanomas, but several trials are evaluating targeted therapy in NF1 mutant solid tumors, including melanomas: (1) The MATCH screening trial evaluating trametinib for the treatment of NF1 mutant refractory solid cancers (NCT02465060); (2) the MatchMel trial which includes a cohort with NF1 mutant refractory tumors receiving trametinib, sorafenib, or everolimus (NCT02645149); and (3) the use of RMC4630 (a potent PTPN11 inhibitor) and cobmitinib in solid tumors with NF1 mutations (NCT03634982).
Finally, an approach involving the stabilization of the NF1 protein by reducing its degradation has been suggested to enhance sensitivity to trametinib in melanoma cell lines. In this study, Alon et al. demonstrated that NF1 could be rescued through the inhibition of calpain1 (CAPN1), which is a calcium-dependent neutral cysteine protease that plays a role in NF1 degradation, therefore providing a blockage of RAS activation in melanoma cells [120]. However, prospective clinical trials are still needed to study the safety and efficacy of this approach. To this end, the current standard of care in melanoma patients with NF1 mutations remains the use of ICI.
5.2. Targeting TP53
TP53 is an essential tumor suppressor gene which maintains the integrity of the cellular genome. Inactivating mutations of TP53 are the most prevalent mutations in solid tumors [121]. In melanomas, mutations in TP53 occur at a frequency of 15% with the majority being LOF due to the inactivating missense mutations, as well as a simultaneous loss of heterozygosity (LOH) deletion in chromosome 17 [28,122]. TP53 mutations can co-exist with other driver mutations, such as with BRAF and NRAS [123]. The loss of the regulatory function of p53 (the transcribed protein of TP53) is a late event in melanomas and is caused by several mechanisms, including structural mutations in TP53; the overexpression of the mouse double minute 2 (MDM2) oncoprotein, which is responsible for the ubiquitination and degradation of p53; mutations in P14ARF, which lead to an overexpression of MDM2; and the overexpression of the MDM2 homolog murine double minute x (MDMX) which inhibits p53 [124,125]. The research efforts to overcome the LOF of p53 is based on two different approaches: (1) the restoration of mutant p53 function, and (2) the restoration of wild-type p53 function through the inhibition of negative regulators such as MDM2 and MDMX. Several preclinical studies have demonstrated anti-tumor effects in wild-type TP53 tumors using novel inhibitors of negative regulators, such as inhibitors of MDM2/MDMX, in melanoma cell lines and animal xenografts. However, there are no current trials in humans evaluating the safety and efficacy of this approach [126,127,128,129]. A phase 1 study of AMG-232 (an MDM2 inhibitor) and trametinib evaluated the safety and maximum tolerated dose in patients with metastatic cutaneous melanomas with wild-type TP53 [130]. Of the 15 evaluable patients, 13% (2/15) patients had a PR, 73% (11/15) had a SD, and 13% (2/15) had a progressive disease.
The ability to target p53 mutant cancers by restoring p53 function was recently supported by two studies in hematological malignancies using eprenetapopt (APR-246), which is a small molecule prodrug administered intravenously. When activated, eprenetapopt can bind to cysteine residues in mutant p53, leading to thermodynamic stabilization as well as shifting the equilibrium of p53 into its active conformation. However, its efficacy was only reported in myeloid dysplastic syndrome (MDS) and acute myeloid leukemia (AML) when used in combination with azacitidine [131,132].
5.3. Targeting CDKN2A and CDK4/6 Networks
The cyclin-dependent kinase inhibitor 2A (CDKN2A) plays an important role in cell cycle regulation through its complex networks, which converges with other important genes such as TP53 and RB. Germline mutations in CDKN2A are an established risk factor for the development of hereditary melanoma syndromes, and increase the risk of hereditary melanomas by at least 10-fold [133]. The CDKN2A is located on chromosome 9p21 and encodes two distinct proteins, p16INK4A and p14ARF. The main function of p16INK4A is to regulate the G1–S phase and cellular senescence by binding to CDK4/6, leading to the interruption of the interaction with CCND1 and the phosphorylation of RB. This, in turn, locks the RB/E2F in its inactive form, preventing the release of the transcription factor E2F and G1–S phase progression. P14ARF exerts its tumor suppressor effects through binding to MDM2, which leads to the inhibition of p53 degradation [134].
Alterations in the CDKN2A pathway in melanomas can occur due to either copy number changes (deletions of CDKN2A, the amplification of CCND1, and the amplification of CDK4/6), mutations in CDKN2A or CDK4/6, and promoter hypermethylation of CDKN2A [27]. The frequency of mutations of CDKN2A is approximately 12% and deletions can be present in up to 28% of cutaneous melanomas [33].
Several trials are ongoing to assess the safety and efficacy of drugs targeting the dysregulation of the CDKN2A/CDK4/6 pathways using CDK4/6 inhibitors such as palbociclib, ribociclib, and abemaciclib. Preclinical evidence suggests the antitumor synergistic efficacy of CDK4/6 inhibitors, with BRAF/MEK inhibitors and MEK inhibitors, in BRAF and NRAS mutant melanomas, respectively [135,136,137]. The anti-tumor efficacy of single agent CDK4/6 inhibitors is unknown in melanomas bearing specific dysregulations in the CDKN2A pathway. However, some evidence exists regarding their potential anti-tumor effects. For example, in a phase 1 dose escalation trial of abemaciclib in solid tumors including melanoma, there was one PR and six patients with stable diseases among 26 patients who had melanoma [138]. Of interest, the patient who achieved a partial response was found to have a copy number loss of the INK4 locus. Unfortunately, a phase 2 study of abemaciclib in 23 melanoma patients with intracranial metastases showed 0% objective responses, but patients were selected irrespective of the presence of the CDKN2A pathway alteration [139]. Ribociclib was studied in a phase 1 trial in patients with advanced solid tumors. Among 132 patients, there were three patients with melanomas, with one achieving a PR and one achieving a stable disease [140]. Of interest, the patient with the partial response had CCND1 amplification. In regards to Palbociclib, a phase 1 trial in China evaluated its efficacy in patients with acral lentiginous melanomas with CDKN2A pathway alterations [141]. Among the 15 patients enrolled in this trial, there were three documented responses (an ORR of 20%) with one achieving a PR with palbociclib monotherapy. Given the lack of insight into the efficacy of the CDK4/6 inhibitor monotherapy in melanomas, and the possible synergy and efficacy when combined with other inhibitors in BRAF and NRAS mutant melanomas, most of the current ongoing trials are evaluating combination CDK4/6 inhibitors with other targeted therapies [142].
6. Targeting Fusion Gene Alterations
Fusion genes refer to chromosome rearrangements during cell mitosis by joining separate segments of different genes [143]. The rearrangement process can happen due to chromosome translocation, inversion, deletion, or duplication. The newly fused chimeric proteins can lead to the constitutive activation of the kinase domains of several pathways, including MAPK, PI3K, and phospholipase C (PLC), that promote cell survival in both hematological and solid malignancies [144]. The most common characterized gene fusions in cancer include ALK, ROS1, MET, BRAF, RET, and NTRK. Kinase fusions have been detected at a high frequency in Spitz tumors and are frequently linked to Spitzoid melanomas [145]. TCGA analysis detected fusions in BRAF and RAF1 kinases in melanomas, but did not report fusions in ALK, ROS1, MET, RET, or NTRK, which are clinically targetable [28,146]. However, other studies identified a subset of melanoma patients with targetable gene fusions, albeit with a rare frequency. For example, a Chinese study identified ALK break points in four patients among 30 acral melanomas (13.3%), and no ALK fusions were identified in 28 mucosal melanoma patients [147]. Busam et al. did not report ALK fusions in a cohort of 600 melanoma patients, but reported ALK expression by IHC that was detected in 16 samples [148]. Turner et al. reported chromosomal rearrangement in RET (a patient with acral melanoma), BRAF, and ROS1 (a superficial spreading melanoma subtype and an unknown primary) in four patients (n = 59) [149]. Another recent study using next-generation sequencing (NGS) identified gene fusions in a cohort of 122 patients with a frequency of 2.5% BRAF (three cases), 2.5% NTRK3 (three cases), 0.8% ALK (one case), and 0.8% PRKCA (one case) [32]. Taken together, these results suggest that gene fusions in melanomas might be subtype-specific and enriched (i.e., most detected cases have been found in acral melanomas) although some fusions like NTRK were identified at a very low rate in cutaneous melanomas [150,151]. In addition, the majority of gene fusions were detected in cases where no driver mutations were present, suggesting the oncogenic potential of these chimeric fusions.
The targeted treatment of oncogenic gene fusions is already a standard of care in tumors such as lung cancer. Evidence on the efficacy of these targeted treatments in melanomas harboring structural chromosomal abnormalities is lacking due to the rarity of these alterations, but some evidence suggests a possible role in melanoma. The NTRK inhibitor larotrectinib demonstrated clinical efficacy in refractory solid tumors with NTRK fusions in a phase 1/2 trial. In this study, a total of 55 patients (including four melanoma patients) were enrolled, and the ORR was 75% with a median duration of responses and a PFS that has not been reached at a median follow up of 9.4 months [152]. A pooled analysis of three phase 1/2 trials of larotrectinib included seven melanoma patients, and they reported three confirmed responses and an ORR of 43%, but information on the duration of the response was not reported on in melanoma patients [153]. Another NTRK/ROS1/ALK inhibitor (entrectinib) demonstrated clinical safety and efficacy in phase 1/2 trials, but no melanoma patients were included in these studies [154]. Of interest, one case report demonstrated the efficacy of entrectinib in a patient with an acral melanoma harboring ROS1 gene fusion [155]. The patient received 600 mg of entrectinib orally daily and achieved a PR within 2 weeks of treatment initiation, and the response was ongoing at 11 months, with side effects that included dyspnea and weight gain. In contrast, entrectinib did not yield a response in another patient with a mucosal vulvar melanoma who had an ALK isoform with an alternative transcription initiation (ALKATI) who experienced rapid progression and dyspnea with pain after receiving the targeted treatment [156]. Of note, ALKATI is not associated with genetic aberrations on the ALK locus.
Despite the lack of strong evidence for targeting gene fusions in melanomas, the FDA approval of larotrectinib in solid tumors (including melanomas) with NTRK fusions provides a base for further research on the efficacy of other fusion protein inhibitors in a small subset of melanoma patients.
7. Targeting Epigenetic Regulators
Epigenetics involves the role of gene expression alterations associated with diseases that are independent of structural DNA changes (i.e., enzymes and proteins that regulate histone function and gene expression through methylation and acetylation) [157]. There is compelling evidence of the oncogenic role of several somatic mutations in epigenetic modifiers in multiple human cancers, including melanomas. Most of these mutations are in genes that code for chromatin modifier proteins [158].
One of the most frequently mutated chromatin regulators occurs in the enhancer of the zeste homolog 2 (EZH2) gene, a trimethylator of lysine-27 in histone (H3K27me3), which controls the expression of genes essential to cell cycle regulation, senescence, and apoptosis [159]. Of interest, EZH2 can function as either an oncogene or a tumor suppressor gene in different cancers for unknown reasons. In melanomas, the most frequent hotspot mutations in EZH2 are in the enzymatic SET-domain Y641, and the frequency of different EZH2 mutations in melanomas is approximately 5% [160]. Focal amplifications of EZH2 were also identified in 5.7% of melanoma cases from TCGA database. Of interest, EZH2 is suggested to mediate resistance in BRAF mutant melanomas, and to cooperate with BRAF to maintain tumor progression [161]. In addition, EZH2 can increase melanoma growth and is associated with a high proliferation rate through the silencing of tumor suppressors [162,163]. The use of EZH2 inhibitors in melanoma cell lines demonstrated anti-tumor efficacy in both EZH2 wild-types and mutant forms [164]. Tazemetostat is a selective, reversible, small molecule inhibitor of the histone methyl transferase of EZH2 that can inhibit both wild-type and mutant forms. The medication is FDA approved for the treatment of relapsed follicular lymphomas and advanced epitheloid sarcomas. Currently, an ongoing trial is underway to evaluate the safety and efficacy of adding tazemetostat to dabrafenib and trametenib in progressive metastatic melanoma patients with EZH2 alterations (NCT04557956).
Isocitrate hydrogynase 1/2 (IDH1/2) are a set of enzymes that function within the mitochondria and cytoplasm, respectively, in the TCA cycle to convert isocitrate into a-ketoglutarate to generate NADPH from NADP+, which is important in cellular metabolism. Somatic point mutations in arginine residues can lead to the altered function of the enzyme, leading to the formation of the oncometabolite D-2-hydroxyglutarate (D2HG) which, in turn, can lead to the CpG island promotor and hypermethylation, as well as suppressing the transcription of key gene regulators [165,166]. IDH mutations have been identified in several malignancies (especially IDH1 R132), and were found to be targetable in cancers such as AML and gliomas [165]. Two IDH inhibitors, ivosedinib (an IDH1 inhibitor) and enasedinib (an IDH2 inhibitor) demonstrated efficacy against gliomas, cholangiocarcinomas, and AML in clinical trials [167,168,169,170]. In melanomas, IDH mutations were first described in 2010, and a follow-up analysis of TCGA data identified a frequency of 4.9% [28,171]. However, the presence of IDH mutations in melanomas does not appear to have a prognostic impact on survival compared to IDH wild-types [165]. Despite the evidence that IDH mutations can confer the in vivo growth ability in melanoma cell lines with BRAF mutations, the biological implications of IDH mutations in melanomas and their impacts on tumorigenesis and progression remain to be elucidated [172]. Currently, an ongoing phase 1 trial is underway to evaluate the safety and efficacy of a novel IDH1 inhibitor (LY3410738) in advanced solid tumors with IDH1 mutations, including melanomas (NCT04521686).
The biological impact of alterations in other epigenetic regulators, such as ARID2 which occurs at a high frequency in melanomas, are lacking, and further research is essential to illustrate their role in melanomagenesis. The main challenge will be integrating epigenetic regulators in the melanoma treatment paradigm, which is dependent on better understanding of the role of epigenetic alterations in tumorigenesis.
8. Targeting Homologous Recombination Deficiency and the DNA Damage Response Pathway
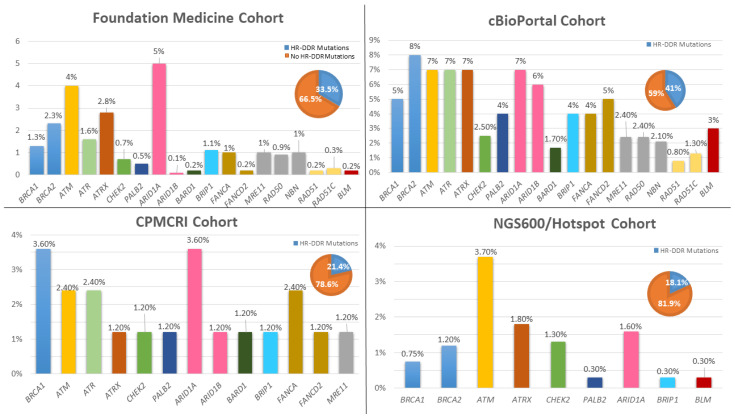
Genomic integrity is contingent on the presence of effective cellular machinery that can recognize DNA damage caused by reactive oxygen species and spontaneous mutagenesis. Subsequentially, this system operates by recruiting and providing cellular instruments to repair damaged DNA. This is achieved by DNA damage response (DDR) genes that function through two main mechanisms: (1) homologous recombination (HR), and (2) non-homologous end-joining (NHEJ). The advantage of the HR pathway is its high fidelity in DNA repair, as it uses the non-damaged DNA template to repair damaged DNA, but it can only function during the S and G2 phases. In contrast, NHEJ provides an immediate repair mechanism, but is more error prone. The role of genomic instability due to alterations in DDR genes has been well established as an essential process in tumorigenesis and cancer progression in multiple malignancies [173]. More than 400 genes have been identified to play a role in the DDR pathway which can repair double-stranded and single-stranded DNA breaks [173]. Major DDR pathways are functionally grouped into multiple categories that are responsible for base/nucleotide excision repair, direct damage reversal/repair, mismatch repair, and the Fanconi anemia pathway, among others [174]. Of interest, while alterations in DDR genes provide an advantage to cancer cells, they generate vulnerability which, when exploited, can lead to anti-tumor effects. This concept was emphasized through the development of poly (ADP-ribose) polymerase (PARP) inhibitors, which showed safety and efficacy in ovarian, breast, prostate, and pancreatic cancers that harbor defects in HR genes (mainly BRCA1/2 mutations), which led to their FDA approvals [175,176,177,178,179,180]. Currently approved inhibitors are olaparib, rucaparib, talazoparib, and niraparib, which are orally administered, and exert their effect through the trapping of PARP enzymes, which are essential for single-strand break repair. The efficacy is highly evident in cancer cells with HR disruptions due to BRCA 1/2 mutations leading to synthetic lethality. The term homologous recombination deficiency (HRD) represents the breach in HR repair mechanisms due to mutations in DDR genes such as BRCA1/2 and ATM, among many others [181]. Several assays have been developed in an attempt to identify the HRD status in cells through the analysis of promiscuous mutations in DDR genes, a LOH, large-scale state transitions, the number of telomeric allelic imbalances, and epigenetic alterations that lead to the silencing of DDR genes [182]. These methods are still under investigation as a biomarker surrogate for the response to PARP inhibitors, as the majority of the approvals of PARP inhibitors are in specific cancers harboring mutations in BRCA1/2 [183]. The presence of DDR gene mutations has been described in melanomas from TCGA [182]. Heeke et al. identified an HR mutation frequency of 18.1% in 670 melanoma samples [184]. Moreover, studies investigating samples from FoundationOne medicine, cBioPortal, and CPMCRI reported different DDR gene mutation frequencies in melanomas (Figure 5) [185]. The frequency in DDR mutations varies in melanomas in comparison with other cancers. For example, the rate of BRCA1/2 mutations is lower in melanomas compared to breast, ovarian, and prostate cancers [184]. Conversely, higher rates of ATM mutations are observed in melanomas compared to breast and ovarian cancers [184]. Preclinical evidence exists regarding the possible anti-tumor efficacy of targeting DDR genes in melanoma cells, such as the inhibition of RAD51 [186]. Recently, the anti-tumor activity of niraparib was demonstrated in melanoma cell lines with mutations in BRCA1, ARID1,B and CHD2 [187]. Of interest, there is mounting evidence that PARP inhibition can reprogram the tumor microenvironment, leading to enhanced tumor– cell intrinsic immunity. This suggests a possible synergistic role of PARP inhibitors with immunotherapy, such as ICI, to enhance anti-tumor immunity [188,189].
Figure 5.
Frequency of commonly mutated genes of the HR-DDR pathway in melanomas from different cohorts including: FoundationOne medicine (n = 1986), cBioPortal (1088), CPMCRI (84), and NGS600/Hotspot (n = 670) cohorts. Bar charts represent the percentage of specific gene mutations in each cohort. Pie charts represent the percentage of melanoma samples with at least one mutation in the HR-DDR pathway. HR-DDR: Homologous recombination-DNA damage response. Pie charts: blue color represents the percentage of melanoma specimens harboring at least 1 mutant gene from homologous recombination pathway. The orange color represents percentage of melanoma specimens without homologous recombination gene mutations. CPMCRI: California Pacific Medical Center Research Institute. NGS: Next-generation Sequencing.
To date, there is no evidence available from prospective trials on the role of PARP inhibition in melanomas or the combination of PARP inhibition with immunotherapy. However, several case reports provide a proof-of-concept for the clinical efficacy of PARP inhibitors. For example, Lau et al. reported a PR in a melanoma patient with a PALB2 mutation who progressed during treatment with ICI and was later treated with a PARP inhibitor [190]. In contrast, two case reports suggested possible synergism between olaparib and nivolumab in two melanoma patients who were immunotherapy-refractory patients and who had evidence of HRD [191,192]. Of importance, one patient developed hepatitis as a side effect during combination treatment, which raises a safety concern. Currently, several prospective trials are ongoing to assess the safety and efficacy of combination treatment (PARP inhibitors with ICI or PARP inhibitor monotherapy) in patients with HRD melanomas (NCT03925350; NCT04187833; NCT04633902). Finally, novel inhibitors of other DDR gene alterations are currently undergoing early phase trials to assess their safety and efficacy in melanomas with HRD (i.e., inhibitors of ATR, ATM, CHK1/2, and WEE1, among others) [183].
9. Conclusions
The considerable advances in understanding molecular alterations in melanomas have opened the door for extensive research on novel individualized therapeutic approaches. Distinguishing driver and passenger mutations in melanomas and their implications in melanomagenesis is important for the development of effective targeted therapies. Immune checkpoint inhibitors remain the mainstay of treatment for unresectable, locally advanced, and metastatic melanomas, irrespective of a presence of a biomarker. Targeted therapy including BRAF and MEK inhibitors and immune checkpoint inhibitors are the standard of care for unresectable locally advanced and metastatic melanomas with BRAFV600E/K mutations. The innovative high-resolution technology and integration of next-generation sequencing in cancer characterization have allowed for the identification of melanoma driver mutations and have led to the integration of alternative targeted therapies such as tyrosine kinase inhibitors in c-KIT relapsed and refractory melanomas. In contrast, common mutations in melanomas such as NRAS and NF1 remain unable to be targeted to date. Current research efforts are focusing on understanding the interplay between specific somatic mutations, their downstream signaling pathways, and other signaling networks. Part of the investigational effort focuses on targeting downstream effectors or parallel pathways that are essential for NRAS and NF1 mutant melanomas, and another investigation is focused on the combined targeted therapy approach. The surge of multiple effective targeted therapies in non-melanoma cancers warrants the rational and cautious investigation of their safety and efficacy in a subset of melanoma patients harboring the specific desired molecular alterations, as they could prove useful. Moreover, there is a need for further investigation into the role of epigenetic changes and DNA damage response alterations/homologous recombination deficiencies in tumorigenesis, aggressiveness, and the resistance to treatment in melanomas. Finally, it is important to note that inclusion of melanoma patients in well-designed clinical trials testing targeted therapies should be emphasized whenever possible to provide a level of evidence on their safety and efficacy.
Abbreviations
ALK: Anaplastic lymphoma kinase; AML: Acute myeloid leukemia; ARID2: AT-Rich Interaction Domain 2; BRAF: B-rapidly accelerated fibrosarcoma; cAMP: Cyclic adenosine 3′,5′-cyclic monophosphate; CAPN1: Calpain1; CCND1: cyclinD1; CDKN2A: cyclin dependent kinase inhibitor 2A; CNS: Central nervous system; CNV: Copy number variation; CR: Complete response; CRAF: Proto-oncogene c-RAF; CTLA-4: Cytotoxic T-lymphocyte associated antigen-4; DDR: DNA-damage response; D2HG: D-2-hydroxyglutarate; E2F: E2 Transcription Factor; ERBB3: Erb-B2 Receptor Tyrosine Kinase 3; EZH2: Enhancer of zeste homolog 2; FDA: Food and Drug Administration; FT: Farnesyltransferase; GIST: Gastrointestinal stromal tumor; GOF: Gain of Function; HR: Homologous recombination; HRAS: Harvey rat sarcoma viral oncogene homolog; HRD: Homologous recombination deficiency; ICI: Immune checkpoint inhibitor; IDH1/2: Isocitrate dehydrogenase 1/2; IHC: Immunohistochemistry; JAK: Janus kinase; KIT: Mast/Stem cell growth factor receptor kit; KRAS: Kirsten rat sarcoma viral oncogene homolog; LOF: Loss of function; LOH: Loss of heterozygosity; MAPK: Mitogen-activated protein kinase; MAP2K1: mitogen-activated protein kinase kinase 1; MAP2K2: mitogen-activated protein kinase kinase 2; MDM2: Mouse double minute-2; MDMX: Murine double minute X; MDS: Myelodysplastic syndrome; MEK: Mitogen-activated protein kinase kinase; MET: Mesenchymal–epithelial transition factor; mTOR: mammalian target of rapamycin; NF1: Neurofibromatosis type 1; NGS: Next-generation sequencing; NHEJ: Non-homologous end-joining; NRAS: Neuroblastoma RAS viral oncogene homolog; NTRK: Neurotrophic tyrosine receptor kinase; ORR: Objective response rate; OS: Overall survival; PARP: Poly (ADP-ribose) polymerase; PD1: Programmed death-1; PDGFR: Platelet-derived growth factor receptor; PDL1: Programmed death ligand-1; PFS: Progression-free survival; PI3K: Phosphatidylinositol 3-kinase; PKA: Promotes protein kinase A; PLC: Phospholipase C; PR: Partial response; PRKCA: Protein kinase C α; PTEN: Phosphatase and TENsin homolog; PTPN11: Tyrosine-protein phosphatase non-receptor type 11; RAC1: Ras-related C3 botulinum toxin substrate 1; RB: Retinoblastoma; RTK: Receptor tyrosine kinase; SCF: Stem cell factor ligand; SD: Stable disease; SEER: Surveillance, Epidemiology, and End Result Program.; SOS1: Son of sevenless 1; STAT: Signal transducers and activators of transcription; STK19: Serine/threonine-protein kinase 19; TCA: Tricarboxylic acid; TCGA: The Cancer Genome Atlas; TERT: Telomerase Reverse Transcriptase; TKI: Tyrosine kinase inhibitor; TP53: Tumor protein 53.
Author Contributions
Conceptualization, K.K.; methodology, K.K.; validation, K.K., L.M., A.M.A.-R. and G.A.; data curation, K.K.; writing—original draft preparation, K.K.; writing K.K., L.M. (KIT section), A.M.A.-R. (NRAS section); figure creation, K.K.; review and editing, K.K., L.M., A.M.A.-R., Y.M., S.S., G.A., supervision, K.K., G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Karimkhani C., Green A., Nijsten T., Weinstock M., Dellavalle R., Naghavi M., Fitzmaurice C. The global burden of melanoma: Results from the Global Burden of Disease Study. Br. J. Dermatol. 2017;177:134–140. doi: 10.1111/bjd.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Guy G.P., Jr., Thomas C.C., Thompson T., Watson M., Massetti G.M., Richardson L.C. Centers for Disease Control and Prevention (CDC). Vital signs: Melanoma incidence and mortality trends and projections—United States, 1982–2030. MMWR Morb Mortal Wkly Rep. 2015;64:591. [PMC free article] [PubMed] [Google Scholar]
- 5.Garbe C., Keim U., Gandini S., Amaral T., Katalinic A., Hollezcek B., Martus P., Flatz L., Leiter U., Whiteman D. Epidemiology of cutaneous melanoma and keratinocyte cancer in white populations 1943–2036. Eur. J. Cancer. 2021;152:18–25. doi: 10.1016/j.ejca.2021.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 7.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 8.Krummel M.F., Allison J. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leach D.R., Krummel M.F., Allison J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 10.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., Walsh L.A., Postow M.A., Wong P., Ho T.S., et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene super-family, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blank C., Brown I., Peterson A.C., Spiotto M., Iwai Y., Honjo T., Gajewski T.F. PD-L1/B7H-1 Inhibits the Effector Phase of Tumor Rejection by T Cell Receptor (TCR) Transgenic CD8+ T Cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.CAN-03-3259. [DOI] [PubMed] [Google Scholar]
- 13.Chapman P.B., Hauschild A., Robert C., Haanen J.B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N. Engl. J. Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paraiso K.H.T., Fedorenko I.V., Cantini L.P., Munko A.C., Hall M., Sondak V.K., Messina J.L., Flaherty K.T., Smalley K.S.M. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br. J. Cancer. 2010;102:1724–1730. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert C., Karaszewska B., Schachter J., Rutkowski P., Mackiewicz A., Stroiakovski D., Lichinitser M., Dummer R., Grange F., Mortier L., et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Engl. J. Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 16.Larkin J., Ascierto P.A., Dréno B., Atkinson V., Liszkay G., Maio M., Mandalà M., Demidov L., Stroyakovskiy D., Thomas L., et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N. Engl. J. Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 17.Dummer R., Ascierto P.A., Gogas H.J., Arance A., Mandala M., Liszkay G., Garbe C., Schadendorf D., Krajsova I., Gutzmer R., et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF -mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–615. doi: 10.1016/S1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 18.Schachter J., Ribas A., Long G.V., Arance A., Grob J.-J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M., et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390:1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 19.Robert C., Long G.V., Brady B., Dutriaux C., Maio M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., Kalinka-Warzocha E., et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 20.Weber J.S., D’Angelo S.P., Minor D., Hodi F.S., Gutzmer R., Neyns B., Hoeller C., Khushalani N.I., Miller W.H., Lao C.D., et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 21.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Rutkowski P., Grob J.-J., Cowey C.L., Lao C.D., Wagstaff J., Schadendorf D., Ferrucci P.F., et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert C., Grob J.J., Stroyakovskiy D., Karaszewska B., Hauschild A., Levchenko E., Sileni V.C., Schachter J., Garbe C., Bondarenko I., et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019;381:626–636. doi: 10.1056/NEJMoa1904059. [DOI] [PubMed] [Google Scholar]
- 23.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.-J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 24.Hamid O., Robert C., Daud A., Hodi F.S., Hwu W.J., Kefford R., Wolchok J.D., Hersey P., Joseph R., Weber J.S., et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE. Ann. Oncol. 2019;30:582–588. doi: 10.1093/annonc/mdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert C., Long G.V., Brady B., Dutriaux C., di Giacomo A.M., Mortier L., Rutkowski P., Hassel J.C., McNeil C.M., Kalinka E.A., et al. Five-Year Outcomes With Nivolumab in Patients With Wild-Type BRAF Advanced Melanoma. J. Clin. Oncol. 2020;38:3937–3946. doi: 10.1200/JCO.20.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence M.S., Stojanov P., Polak P., Kryukov G.V., Cibulskis K., Sivachenko A., Carter S.L., Stewart C., Mermel C.H., Roberts S.A., et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nat. Cell Biol. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodis E., Watson I.R., Kryukov G.V., Arold S.T., Imielinski M., Theurillat J.-P., Nickerson E., Auclair D., Li L., Place C., et al. A Landscape of Driver Mutations in Melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akbani R., Akdemir K.C., Aksoy B.A., Albert M., Ally A., Amin S., Arachchi H., Arora A., Auman J.T., Ayala B., et al. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raaijmakers M.I.G., Widmer D.S., Narechania A., Eichhoff O., Freiberger S.N., Wenzina J., Cheng P., Mihic-Probst D., DeSalle R., Dummer R., et al. Co-existence of BRAF and NRAS driver mutations in the same melanoma cells results in heterogeneity of targeted therapy resistance. Oncotarget. 2016;7:77163–77174. doi: 10.18632/oncotarget.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium Pan-cancer analysis of whole genomes. Nature. 2020;578:82–93. doi: 10.1038/s41586-020-1969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zehir A., Benayed R., Shah R.H., Syed A., Middha S., Kim H.R., Srinivasan P., Gao J., Chakravarty D., Devlin S.M., et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh I., Jorgenson E., Shen L., Xu M., North J.P., Shain A.H., Reuss D., Wu H., Robinson W., Olshen A., et al. Targeted Genomic Profiling of Acral Melanoma. J. Natl. Cancer Inst. 2019;111:1068–1077. doi: 10.1093/jnci/djz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanni I., Tanda E.T., Dalmasso B., Pastorino L., Andreotti V., Bruno W., Boutros A., Spagnolo F., Ghiorzo P. Non-BRAF Mutant Melanoma: Molecular Features and Therapeutical Implications. Front. Mol. Biosci. 2020;7:172. doi: 10.3389/fmolb.2020.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uribe P., Wistuba I.I., González S. BRAF Mutation. Am. J. Dermatopathol. 2003;25:365–370. doi: 10.1097/00000372-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Bauer J., Curtin J., Pinkel D., Bastian B.C. Congenital Melanocytic Nevi Frequently Harbor NRAS Mutations but no BRAF Mutations. J. Investig. Dermatol. 2007;127:179–182. doi: 10.1038/sj.jid.5700490. [DOI] [PubMed] [Google Scholar]
- 36.Zhang T., Dutton-Regester K., Brown K.M., Hayward N.K. The genomic landscape of cutaneous melanoma. Pigment. Cell Melanoma Res. 2016;29:266–283. doi: 10.1111/pcmr.12459. [DOI] [PubMed] [Google Scholar]
- 37.Besmer P., Murphy J.E., George P.C., Qiu F., Bergold P., Lederman L., Snyder H.W., Jr., Brodeur D., Zuckerman E.E., Hardy W.D. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nat. Cell Biol. 1986;320:415–421. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- 38.Yarden Y., Kuang W.J., Yang-Feng T., Coussens L., Munemitsu S., Dull T.J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: A new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu F.H., Ray P., Brown K., Barker P.E., Jhanwar S., Ruddle F.H., Besmer P. Primary structure of c-kit: Relationship with the CSF-1/PDGF receptor kinase family—oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988;7:1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-K. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z., Zhang R., Joachimiak A., Schlessinger J., Kong X.-P. Crystal structure of human stem cell factor: Implication for stem cell factor receptor dimerization and activation. Proc. Natl. Acad. Sci. USA. 2000;97:7732–7737. doi: 10.1073/pnas.97.14.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang E., Nocka K., Beier D.R., Chu T.-Y., Buck J., Lahm H.-W., Wellner D., Leder P., Besmer P. The hematopoietic growth factor KL is encoded by the SI locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-V. [DOI] [PubMed] [Google Scholar]
- 43.Lemmon M., Pinchasi D., Zhou M., Lax I., Schlessinger J. Kit Receptor Dimerization Is Driven by Bivalent Binding of Stem Cell Factor. J. Biol. Chem. 1997;272:6311–6317. doi: 10.1074/jbc.272.10.6311. [DOI] [PubMed] [Google Scholar]
- 44.Verstraete K., Savvides S.N. Extracellular assembly and activation principles of oncogenic class III receptor tyrosine kinases. Nat. Rev. Cancer. 2012;12:753–766. doi: 10.1038/nrc3371. [DOI] [PubMed] [Google Scholar]
- 45.Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int. J. Biochem. Cell Biol. 1999;31:1053–1074. doi: 10.1016/S1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 46.Kitamura Y., Hirotab S. Kit as a Human Oncogenic tyrosine kinase. Cell. Mol. Life Sci. 2004;61:2924–2931. doi: 10.1007/s00018-004-4273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pham D.M., Guhan S., Tsao H. KIT and Melanoma: Biological Insights and Clinical Implications. Yonsei Med. J. 2020;61:562–571. doi: 10.3349/ymj.2020.61.7.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willmore-Payne C., Holden J.A., Tripp S., Layfield L.J. Human malignant melanoma: Detection of BRAF- and c-kit–activating mutations by high-resolution amplicon melting analysis. Hum. Pathol. 2005;36:486–493. doi: 10.1016/j.humpath.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 49.Went P.T., Dirnhofer S., Bundi M., Mirlacher M., Schraml P., Mangialaio S., Dimitrijevic S., Kononen J., Lugli A., Simon R., et al. Prevalence of KIT Expression in Human Tumors. J. Clin. Oncol. 2004;22:4514–4522. doi: 10.1200/JCO.2004.10.125. [DOI] [PubMed] [Google Scholar]
- 50.Beadling C., Jacobson-Dunlop E., Hodi F.S., Le C., Warrick A., Patterson J., Town A., Harlow A., Cruz F., Azar S., et al. KIT Gene Mutations and Copy Number in Melanoma Subtypes. Clin. Cancer Res. 2008;14:6821–6828. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- 51.Curtin J.A., Busam K., Pinkel D., Bastian B.C. Somatic Activation of KIT in Distinct Subtypes of Melanoma. J. Clin. Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 52.Kong Y., Si L., Zhu Y., Xu X., Corless C.L., Flaherty K.T., Li L., Li H., Sheng X., Cui C., et al. Large-Scale Analysis of KIT Aberrations in Chinese Patients with Melanoma. Clin. Cancer Res. 2011;17:1684–1691. doi: 10.1158/1078-0432.CCR-10-2346. [DOI] [PubMed] [Google Scholar]
- 53.Hayward N.K., Wilmott J.S., Waddell N., Johansson P.A., Field M.A., Nones K., Patch A.-M., Kakavand H., Alexandrov L.B., Burke H., et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–180. doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- 54.Jenkins R.W., Fisher D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2021;141:23–31. doi: 10.1016/j.jid.2020.03.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKean M., Oba J., Ma J., Roth K.G., Wang W.-L., Macedo M.P., Carapeto F.C.L., Haydu L.E., Siroy A.E., Vo P., et al. Tyrosine Kinase Inhibitor and Immune Checkpoint Inhibitor Responses in KIT-Mutant Metastatic Melanoma. J. Investig. Dermatol. 2019;139:728–731. doi: 10.1016/j.jid.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 56.Huang L., Jiang S., Shi Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020) J. Hematol. Oncol. 2020;13:143. doi: 10.1186/s13045-020-00977-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wyman K., Atkins M.B., Prieto V., Eton O., McDermott D.F., Hubbard F., Byrnes C., Sanders K., Sosman J.A. Multicenter Phase II trial of high-dose imatinib mesylate in metastatic melanoma: Significant Toxicity with No Clinical Efficacy. Cancer. 2006;106:2005–2011. doi: 10.1002/cncr.21834. [DOI] [PubMed] [Google Scholar]
- 58.Ugurel S., Hildenbrand R., Zimpfer A., La Rosée P., Paschka P., Sucker A., Keikavoussi P., Becker J.C., Rittgen W., Hochhaus A., et al. Lack of clinical efficacy of imatinib in metastatic melanoma. Br. J. Cancer. 2005;92:1398–1405. doi: 10.1038/sj.bjc.6602529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim K.B., Eton O., Davis D.W., Frazier M.L., McConkey D.J., Diwan A.H., Papadopoulos N.E., Bedikian A.Y., Camacho L.H., Ross M.I., et al. Phase II trial of imatinib mesylate in patients with metastatic melanoma. Br. J. Cancer. 2008;99:734–740. doi: 10.1038/sj.bjc.6604482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carvajal R.D., Antonescu C.R., Wolchok J.D., Chapman P.B., Roman R.-A., Teitcher J., Panageas K.S., Busam K.J., Chmielowski B., Lutzky J., et al. KIT as a Therapeutic Target in Metastatic Melanoma. JAMA. 2011;305:2327–2334. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo J., Si L., Kong Y., Flaherty K.T., Xu X., Zhu Y., Corless C.L., Li L., Li H., Sheng X., et al. Phase II, Open-Label, Single-Arm Trial of Imatinib Mesylate in Patients with Metastatic Melanoma Harboring c-Kit Mutation or Amplification. J. Clin. Oncol. 2011;29:2904–2909. doi: 10.1200/JCO.2010.33.9275. [DOI] [PubMed] [Google Scholar]
- 62.Hodi F.S., Corless C.L., Giobbie-Hurder A., Fletcher J.A., Zhu M., Marino-Enriquez A., Friedlander P., Gonzalez R., Weber J.S., Gajewski T.F., et al. Imatinib for Melanomas Harboring Mutationally Activated or Amplified KIT Arising on Mucosal, Acral, and Chronically Sun-Damaged Skin. J. Clin. Oncol. 2013;31:3182–3190. doi: 10.1200/JCO.2012.47.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei X., Mao L., Chi Z., Sheng X., Cui C., Kong Y., Dai J., Wang X., Li S., Tang B., et al. Efficacy Evaluation of Imatinib for the Treatment of Melanoma: Evidence from a Retrospective Study. Oncol. Res. 2019;27:495–501. doi: 10.3727/096504018X15331163433914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho J.H., Kim K.M., Kwon M., Kim J.H., Lee J. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Investig. New Drugs. 2012;30:2008–2014. doi: 10.1007/s10637-011-9763-9. [DOI] [PubMed] [Google Scholar]
- 65.Carvajal R.D., Lawrence D.P., Weber J.S., Gajewski T.F., Gonzalez R., Lutzky J., O’Day S.J., Hamid O., Wolchok J.D., Chapman P.B., et al. Phase II Study of Nilotinib in Melanoma Harboring KIT Alterations Following Progression to Prior KIT Inhibition. Clin. Cancer Res. 2015;21:2289–2296. doi: 10.1158/1078-0432.CCR-14-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee S.J., Kim T.M., Kim Y.J., Jang K.-T., Lee H.J., Lee S.N., Ahn M.S., Hwang I.G., Lee S., Lee M.-H., et al. Phase II Trial of Nilotinib in Patients with Metastatic Malignant Melanoma Harboring KIT Gene Aberration: A Multicenter Trial of Korean Cancer Study Group (UN10-06) Oncologist. 2015;20:1312–1319. doi: 10.1634/theoncologist.2015-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo J., Carvajal R.D., Dummer R., Hauschild A., Daud A., Bastian B.C., Markovic S.N., Queirolo P., Arance A., Berking C., et al. Efficacy and safety of nilotinib in patients with KIT-mutated metastatic or inoperable melanoma: Final results from the global, single-arm, phase II TEAM trial. Ann. Oncol. 2017;28:1380–1387. doi: 10.1093/annonc/mdx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delyon J., Chevret S., Jouary T., Dalac S., Dalle S., Guillot B., Arnault J.-P., Avril M.-F., Bedane C., Bens G., et al. STAT3 Mediates Nilotinib Response in KIT-Altered Melanoma: A Phase II Multicenter Trial of the French Skin Cancer Network. J. Investig. Dermatol. 2018;138:58–67. doi: 10.1016/j.jid.2017.07.839. [DOI] [PubMed] [Google Scholar]
- 69.Kalinsky K., Lee S., Rubin K., Lawrence D.P., Iafrarte A.J., Borger D.R., Margolin K.A., Leitao M.M., Tarhini A.A., Koon H.B., et al. A Phase II Trial of Dasatinib in Patients with Locally Advanced or Stage IV Mucosal, Acral and Vulvovaginal Melanoma: A Trial of the ECOG-ACRIN Cancer Research Group (E2607) Cancer. 2017;123:2688–2697. doi: 10.1002/cncr.30663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Minor D.R., Kashani-Sabet M., Garrido M., O’Day S.J., Hamid O., Bastian B.C. Sunitinib Therapy for Melanoma Patients with KIT Mutations. Clin. Cancer Res. 2012;18:1457–1463. doi: 10.1158/1078-0432.CCR-11-1987. [DOI] [PubMed] [Google Scholar]
- 71.Kluger H.M., Dudek A.Z., McCann C., Ritacco J., Southard N., Jilaveanu L.B., Molinaro A., Sznol M. A phase II trial of dasatinib in advanced melanoma. Cancer. 2011;117:2202–2208. doi: 10.1002/cncr.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quintás-Cardama A., Lazar A.J., Woodman S.E., Kim K., Ross M., Hwu P., Quint A. Complete response of stage IV anal mucosal melanoma expressing KIT Val560Asp to the multikinase inhibitor sorafenib. Nat. Clin. Pract. Oncol. 2008;5:737–740. doi: 10.1038/ncponc1251. [DOI] [PubMed] [Google Scholar]
- 73.Prosvicova J., Lukesova S., Kopecky J., Grim J., Papik Z., Kolarova R., Navratilova B., Dubreuil P., Agopian J., Mansfield C.D., et al. Rapid and clinically significant response to masitinib in the treatment of mucosal primary esophageal melanoma with somatic KIT exon 11 mutation involving brain metastases: A case report. Biomed. Pap. 2015;159:695–697. doi: 10.5507/bp.2015.061. [DOI] [PubMed] [Google Scholar]
- 74.Oba J., Kim S.-H., Wang W.-L., Macedo M.P., Carapeto F., Mckean M.A., van Arnam J., Eterovic A.K., Sen S., Kale C.R., et al. Targeting the HGF/MET Axis Counters Primary Resistance to KIT Inhibition in KIT-Mutant Melanoma. JCO Precis. Oncol. 2018;2:1–8. doi: 10.1200/PO.18.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han Y., Gu Z., Wu J., Huang X., Zhou R., Shi C., Tao W., Wang L., Wang Y., Zhou G., et al. Repurposing Ponatinib as a Potent Agent against KIT Mutant Melanomas. Theranostics. 2019;9:1952–1964. doi: 10.7150/thno.30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flaherty K.T., Hamilton B.K., Rosen M.A., Amaravadi R.K., Schuchter L.M., Gallagher M., Chen H., Sehgal C., O’Dwyer P.J. Phase I/II Trial of Imatinib and Bevacizumab in Patients with Advanced Melanoma and Other Advanced Cancers. Oncologist. 2015;20:952–959. doi: 10.1634/theoncologist.2015-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reilley M.J., Bailey A., Subbiah V., Janku F., Naing A., Falchook G., Karp D., Piha-Paul S., Tsimberidou A., Fu S., et al. Phase I clinical trial of combination imatinib and ipilimumab in patients with advanced malignancies. J. Immunother. Cancer. 2017;5:35. doi: 10.1186/s40425-017-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abdou Y., Kapoor A., Hamad L., Ernstoff M.S. Combination of pembrolizumab and imatinib in a patient with double KIT mutant melanoma. Medicine. 2019;98:e17769. doi: 10.1097/MD.0000000000017769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Padua R.A., Barrass N., Currie G.A. A novel transforming gene in a human malignant melanoma cell line. Nat. Cell Biol. 1984;311:671–673. doi: 10.1038/311671a0. [DOI] [PubMed] [Google Scholar]
- 80.Fedorenko I.V., Gibney G.T., Smalley K.S.M. NRAS mutant melanoma: Biological behavior and future strategies for therapeutic management. Oncogene. 2013;32:3009–3018. doi: 10.1038/onc.2012.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yin C., Zhu B., Zhang T., Liu T., Chen S., Liu Y., Li X., Miao X., Li S., Mi X., et al. Pharmacological Targeting of STK19 Inhibits Oncogenic NRAS-Driven Melanomagenesis. Cell. 2019;176:1113–1127.e16. doi: 10.1016/j.cell.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 82.Curtin J.A., Fridlyand J., Kageshita T., Patel H.N., Busam K.J., Kutzner H., Cho K.-H., Aiba S., Bröcker E.-B., LeBoit P.E., et al. Distinct Sets of Genetic Alterations in Melanoma. N. Engl. J. Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 83.Jakob J.A., Bassett R.L., Ng C.S., Curry J.L., Joseph R., Alvarado G.C., Apn M.L.R., Richard J., Gershenwald J.E., Kim K.B., et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012;118:4014–4023. doi: 10.1002/cncr.26724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akslen L.A., Angelini S., Straume O., Bachmann I.M., Molven A., Hemminki K., Kumar R. BRAF and NRAS Mutations Are Frequent in Nodular Melanoma but Are not Associated with Tumor Cell Proliferation or Patient Survival. J. Investig. Dermatol. 2005;125:312–317. doi: 10.1111/j.0022-202X.2005.23788.x. [DOI] [PubMed] [Google Scholar]
- 85.Ellerhorst J.A., Greene V.R., Ekmekcioglu S., Warneke C.L., Johnson M.M., Cooke C.P., Wang L.-E., Prieto V.G., Gershenwald J.E., Wei Q., et al. Clinical Correlates ofNRASandBRAFMutations in Primary Human Melanoma. Clin. Cancer Res. 2010;17:229–235. doi: 10.1158/1078-0432.CCR-10-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moore A.R., Rosenberg S.C., McCormick F., Malek S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020;19:533–552. doi: 10.1038/s41573-020-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burd C.E., Liu W., Huynh M.V., Waqas M.A., Gillahan J.E., Clark K.S., Fu K., Martin B.L., Jeck W.R., Souroullas G.P., et al. Mutation-Specific RAS Oncogenicity Explains NRAS Codon 61 Selection in Melanoma. Cancer Discov. 2014;4:1418–1429. doi: 10.1158/2159-8290.CD-14-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kirchberger M.C., Ugurel S., Mangana J., Heppt M.V., Eigentler T.K., Berking C., Schadendorf D., Schuler G., Dummer R., Heinzerling L. MEK inhibition may increase survival of NRAS-mutated melanoma patients treated with checkpoint blockade: Results of a retrospective multicentre analysis of 364 patients. Eur. J. Cancer. 2018;98:10–16. doi: 10.1016/j.ejca.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 89.Sadetsky N., Lambert P., Julian C., Chen J., Yan Y. Comprehensive genomic profiling and outcomes among metastatic melanoma patients (pts) treated with first-line cancer immunotherapy (CIT) in a real-world setting. Ann. Oncol. 2019;30:vii2. doi: 10.1093/annonc/mdz413.011. [DOI] [Google Scholar]
- 90.Mangana J., Cheng P.F., Schindler K., Weide B., Held U., Frauchiger A.L., Romano E., Kähler K.C., Rozati S., Rechsteiner M., et al. Analysis of BRAF and NRAS Mutation Status in Advanced Melanoma Patients Treated with Anti-CTLA-4 Antibodies: Association with Overall Survival? PLoS ONE. 2015;10:e0139438. doi: 10.1371/journal.pone.0139438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Means-Powell J.A., Adjei A.A., Puzanov I., Dy G.K., Goff L.W., Ma W.W., Fetterly G.J., Michael S.A., Chai F., Lamar M., et al. Safety and efficacy of MET inhibitor tivantinib (ARQ 197) combined with sorafenib in patients (pts) with NRAS wild-type or mutant melanoma from a phase I study. J. Clin. Oncol. 2012;30:8519. doi: 10.1200/jco.2012.30.15_suppl.8519. [DOI] [Google Scholar]
- 92.Niessner H., Beck D., Sinnberg T., Lasithiotakis K., Maczey E., Gogel J., Venturelli S., Berger A., Mauthe M., Toulany M., et al. The Farnesyl Transferase Inhibitor Lonafarnib Inhibits mTOR Signaling and Enforces Sorafenib-Induced Apoptosis in Melanoma Cells. J. Investig. Dermatol. 2011;131:468–479. doi: 10.1038/jid.2010.297. [DOI] [PubMed] [Google Scholar]
- 93.Gajewski T.F., Salama A.K., Niedzwiecki D., Johnson J., Linette G., Bucher C., Blaskovich M.A., Sebti S.M., Haluska F. Phase II study of the farnesyltransferase inhibitor R115777 in advanced melanoma (CALGB 500104) J. Transl. Med. 2012;10:246. doi: 10.1186/1479-5876-10-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dorard C., Estrada C., Barbotin C., Larcher M., Garancher A., Leloup J., Beermann F., Baccarini M., Pouponnot C., LaRue L., et al. RAF proteins exert both specific and compensatory functions during tumour progression of NRAS-driven melanoma. Nat. Commun. 2017;8:15262. doi: 10.1038/ncomms15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dumaz N., Hayward R., Martin J., Ogilvie L., Hedley D., Curtin J.A., Bastian B.C., Springer C., Marais R. In Melanoma, RAS Mutations are Accompanied by Switching Signaling from BRAF to CRAF and Disrupted Cyclic AMP Signaling. Cancer Res. 2006;66:9483–9491. doi: 10.1158/0008-5472.CAN-05-4227. [DOI] [PubMed] [Google Scholar]
- 96.Solit D.B., Garraway L.A., Pratilas C.A., Sawai A., Getz G., Basso A., Ye Q., Lobo J.M., She Y., Osman I., et al. BRAF mutation predicts sensitivity to MEK inhibition. Nat. Cell Biol. 2005;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim T.W., Lee J., Shin S.J., Kim J.-S., Kim Y.J., Han H.S., Lee S.J., Lim H.-S., Hong Y.-H., Noh Y.S., et al. Belvarafenib, a novel pan-RAF inhibitor, in solid tumor patients harboring BRAF, KRAS, or NRAS mutations: Phase I study. J. Clin. Oncol. 2019;37:3000. doi: 10.1200/JCO.2019.37.15_suppl.3000. [DOI] [Google Scholar]
- 98.Atefi M., Titz B., Avramis E., Ng C., Wong D.J., Lassen A., Cerniglia M., Escuin-Ordinas H., Foulad D., Comin-Anduix B., et al. Combination of pan-RAF and MEK inhibitors in NRAS mutant melanoma. Mol. Cancer. 2015;14:27. doi: 10.1186/s12943-015-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kirkwood J.M., Bastholt L., Robert C., Sosman J.A., Larkin J., Hersey P., Middleton M.R., Cantarini M.V., Zazulina V., Kemsley K., et al. Phase II, Open-Label, Randomized Trial of the MEK1/2 Inhibitor Selumetinib as Monotherapy versus Temozolomide in Patients with Advanced Melanoma. Clin. Cancer Res. 2012;18:555–567. doi: 10.1158/1078-0432.CCR-11-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gupta A., Love S., Schuh A., Shanyinde M., Larkin J.M., Plummer R., Nathan P.D., Danson S., Ottensmeier C.H., Lorigan P., et al. DOC-MEK: A double-blind randomized phase II trial of docetaxel with or without selumetinib in wild-type BRAF advanced melanoma. Ann. Oncol. 2014;25:968–974. doi: 10.1093/annonc/mdu054. [DOI] [PubMed] [Google Scholar]
- 101.Dummer R., Schadendorf D., Ascierto P.A., Arance A., Dutriaux C., di Giacomo A.M., Rutkowski P., del Vecchio M., Gutzmer R., Mandala M., et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:435–445. doi: 10.1016/S1470-2045(17)30180-8. [DOI] [PubMed] [Google Scholar]
- 102.Lebbé C., Dutriaux C., Lesimple T., Kruit W., Kerger J., Thomas L., Guillot B., de Braud F., Garbe C., Grob J.-J., et al. Pimasertib Versus Dacarbazine in Patients with Unresectable NRAS-Mutated Cutaneous Melanoma: Phase II, Randomized, Controlled Trial with Crossover. Cancers. 2020;12:1727. doi: 10.3390/cancers12071727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Greger J.G., Eastman S.D., Zhang V., Bleam M.R., Hughes A.M., Smitheman K.N., Dickerson S.H., Laquerre S.G., Liu L., Gilmer T.M. Combinations of BRAF, MEK, and PI3K/mTOR Inhibitors Overcome Acquired Resistance to the BRAF Inhibitor GSK2118436 Dabrafenib, Mediated by NRAS or MEK Mutations. Mol. Cancer Ther. 2012;11:909–920. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
- 104.Posch C., Moslehi H., Feeney L., Green G.A., Ebaee A., Feichtenschlager V., Chong K., Peng L., Dimon M.T., Phillips T., et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2013;110:4015–4020. doi: 10.1073/pnas.1216013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Algazi A.P., Esteve-Puig R., Nosrati A., Hinds B., Hobbs-Muthukumar A., Nandoskar P., Ortiz-Urda S., Chapman P.B., Daud A. Dual MEK/AKT inhibition with trametinib and GSK2141795 does not yield clinical benefit in metastatic NRAS-mutant and wild-type melanoma. Pigment. Cell Melanoma Res. 2018;31:110–114. doi: 10.1111/pcmr.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garcia-Alvarez A., Ortiz C., Muñoz-Couselo E. Current Perspectives and Novel Strategies of NRAS-Mutant Melanoma. OncoTargets Ther. 2021;14:3709–3719. doi: 10.2147/OTT.S278095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sosman J.A., Kittaneh M., Lolkema M.P.J.K., Postow M.A., Schwartz G., Franklin C., Matano A., Bhansali S., Parasuraman S., Kim K. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: Early encouraging clinical activity. J. Clin. Oncol. 2014;32:9009. doi: 10.1200/jco.2014.32.15_suppl.9009. [DOI] [Google Scholar]
- 108.Vogel C.J., Smit M.A., Maddalo G., Possik P.A., Sparidans R.W., van der Burg S.H., Verdegaal E.M., Heck A.J.R., Samatar A.A., Beijnen J.H., et al. Cooperative induction of apoptosis in NRAS mutant melanoma by inhibition of MEK and ROCK. Pigment Cell Melanoma Res. 2015;28:307–317. doi: 10.1111/pcmr.12364. [DOI] [PubMed] [Google Scholar]
- 109.Prior I.A., Hood F.E., Hartley J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020;80:2969–2974. doi: 10.1158/0008-5472.CAN-19-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Skoulidis F., Li B.T., Dy G.K., Price T.J., Falchook G.S., Wolf J., Italiano A., Schuler M., Borghaei H., Barlesi F., et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hong D.S., Fakih M.G., Strickler J.H., Desai J., Durm G.A., Shapiro G.I., Falchook G.S., Price T.J., Sacher A., Denlinger C.S., et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morris L.G.T., Chan T.A. Therapeutic targeting of tumor suppressor genes. Cancer. 2015;121:1357–1368. doi: 10.1002/cncr.29140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kiuru M., Busam K.J. The NF1 gene in tumor syndromes and melanoma. Lab. Investig. 2017;97:146–157. doi: 10.1038/labinvest.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Krauthammer M., Kong Y., Bacchiocchi A., Evans P., Pornputtapong N., Wu C., McCusker J., Ma S., Cheng E., Straub R., et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat. Genet. 2015;47:996–1002. doi: 10.1038/ng.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wiesner T., Kiuru M., Scott S.N., Arcila M., Halpern A.C., Hollmann T., Berger M.F., Busam K.J. NF1 Mutations Are Common in Desmoplastic Melanoma. Am. J. Surg. Pathol. 2015;39:1357–1362. doi: 10.1097/PAS.0000000000000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nissan M.H., Pratilas C.A., Jones A.M., Ramirez R., Won H., Liu C., Tiwari S., Kong L., Hanrahan A.J., Yao Z., et al. Loss of NF1 in Cutaneous Melanoma Is Associated with RAS Activation and MEK Dependence. Cancer Res. 2014;74:2340–2350. doi: 10.1158/0008-5472.CAN-13-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ranzani M., Alifrangis C., Perna D., Dutton-Regester K., Pritchard A., Wong K., Rashid M., Espinoza C.D.R., Hayward N., McDermott U., et al. BRAF / NRAS wild-type melanoma, NF 1 status and sensitivity to trametinib. Pigment. Cell Melanoma Res. 2015;28:117–119. doi: 10.1111/pcmr.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Py C., Christinat Y., Kreutzfeldt M., McKee T.A., Dietrich P.-Y., Tsantoulis P. Response of NF1-Mutated Melanoma to an MEK Inhibitor. JCO Precis. Oncol. 2018;2:1–11. doi: 10.1200/PO.18.00028. [DOI] [PubMed] [Google Scholar]
- 119.Falchook G.S., Lewis K.D., Infante J.R., Gordon M.S., Vogelzang N.J., DeMarini D.J., Sun P., Moy C., Szabo S.A., Roadcap L.T., et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: A phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782–789. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alon M., Arafeh R., Lee J.S., Madan S., Kalaora S., Nagler A., Abgarian T., Greenberg P., Ruppin E., Samuels Y. CAPN1 is a novel binding partner and regulator of the tumor suppressor NF1 in melanoma. Oncotarget. 2018;9:31264–31277. doi: 10.18632/oncotarget.25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vogelstein B., Lane D., Levine A.J. Surfing the p53 network. Nat. Cell Biol. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 122.Liu Y., Chen C., Xu Z., Scuoppo C., Rillahan C.D., Gao J., Spitzer B., Bosbach B., Kastenhuber E.R., Baslan T., et al. Deletions linked to TP53 loss drive cancer through p53-independent mechanisms. Nat. Cell Biol. 2016;531:471–475. doi: 10.1038/nature17157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Viros A., Sanchez-Laorden B., Pedersen M., Furney S., Rae J., Hogan K., Ejiama S., Girotti M.R., Cook M., Dhomen N., et al. Ultraviolet radiation accelerates BRAF-driven melanomagenesis by targeting TP53. Nat. Cell Biol. 2014;511:478–482. doi: 10.1038/nature13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shadfan M., Lopez-Pajares V., Yuan Z.-M. MDM2 and MDMX: Alone and together in regulation of p53. Transl. Cancer Res. 2012;1:88–89. [PMC free article] [PubMed] [Google Scholar]
- 125.Loureiro J., Abrantes M., Oliveira P., Saraiva L. P53 in skin cancer: From a master player to a privileged target for prevention and therapy. Biochim. Biophys. Acta (BBA)-Bioenerg. 2020;1874:188438. doi: 10.1016/j.bbcan.2020.188438. [DOI] [PubMed] [Google Scholar]
- 126.Gembarska A., Luciani F., Fedele C., Russell E.A., Dewaele M., Villar S., Zwolinska A., Haupt S., de Lange J., Yip D., et al. MDM4 is a key therapeutic target in cutaneous melanoma. Nat. Med. 2012;18:1239–1247. doi: 10.1038/nm.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu C.-E., Esfandiari A., Ho Y.-H., Wang N., Mahdi A., Aptullahoglu E., Lovat P., Lunec J. Targeting negative regulation of p53 by MDM2 and WIP1 as a therapeutic strategy in cutaneous melanoma. Br. J. Cancer. 2018;118:495–508. doi: 10.1038/bjc.2017.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu M., Breyssens H., Salter V., Zhong S., Hu Y., Baer C., Ratnayaka I., Sullivan A., Brown N.R., Endicott J., et al. Restoring p53 Function in Human Melanoma Cells by Inhibiting MDM2 and Cyclin B1/CDK1-Phosphorylated Nuclear iASPP. Cancer Cell. 2013;23:618–633. doi: 10.1016/j.ccr.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 129.Loureiro J., Raimundo L., Calheiros J., Carvalho C., Barcherini V., Lima N., Gomes C., Almeida M., Alves M., Costa J., et al. Targeting p53 for Melanoma Treatment: Counteracting Tumour Proliferation, Dissemination and Therapeutic Resistance. Cancers. 2021;13:1648. doi: 10.3390/cancers13071648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moschos S.J., Sandhu S.K., Lewis K.D., Sullivan R.J., Johnson D.B., Zhang Y., Rasmussen E., Henary H.A., Long G.V. Phase 1 study of the p53-MDM2 inhibitor AMG 232 combined with trametinib plus dabrafenib or trametinib in patients (Pts) with TP53 wild type (TP53WT) metastatic cutaneous melanoma (MCM) J. Clin. Oncol. 2017;35:2575. doi: 10.1200/JCO.2017.35.15_suppl.2575. [DOI] [Google Scholar]
- 131.Sallman D.A., DeZern A.E., Garcia-Manero G., Steensma D.P., Roboz G.J., Sekeres M.A., Cluzeau T., Sweet K.L., McLemore A., McGraw K.L., et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J. Clin. Oncol. 2021;39:1584–1594. doi: 10.1200/JCO.20.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cluzeau T., Sebert M., Rahmé R., Cuzzubbo S., Lehmann-Che J., Madelaine I., Peterlin P., Bève B., Attalah H., Chermat F., et al. Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone des Myélodysplasies (GFM) J. Clin. Oncol. 2021;39:1575–1583. doi: 10.1200/JCO.20.02342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goldstein A.M., Chan M., Harland M., Gillanders E.M., Hayward N.K., Avril M.-F., Azizi E., Scarrà G.B., Bishop D.T., Paillerets B.B.-D., et al. High-risk Melanoma Susceptibility Genes and Pancreatic Cancer, Neural System Tumors, and Uveal Melanoma across GenoMEL. Cancer Res. 2006;66:9818–9828. doi: 10.1158/0008-5472.CAN-06-0494. [DOI] [PubMed] [Google Scholar]
- 134.Zhang S., Ramsay E.S., Mock B.A. Cdkn2a, the cyclin-dependent kinase inhibitor encoding p16INK4a and p19ARF, is a candidate for the plasmacytoma susceptibility locus, Pctr1. Proc. Natl. Acad. Sci. USA. 1998;95:2429–2434. doi: 10.1073/pnas.95.5.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martin C.A., Cullinane C., Kirby L., AbuHammad S., Lelliott E.J., Waldeck K., Young R.J., Brajanovski N., Cameron D.P., Walker R., et al. Palbociclib synergizes with BRAF and MEK inhibitors in treatment naïve melanoma but not after the development of BRAF inhibitor resistance. Int. J. Cancer. 2018;142:2139–2152. doi: 10.1002/ijc.31220. [DOI] [PubMed] [Google Scholar]
- 136.Hayes T.K., Luo F., Cohen O., Goodale A.B., Lee Y., Pantel S., Bagul M., Piccioni F., Root D.E., Garraway L.A., et al. A Functional Landscape of Resistance to MEK1/2 and CDK4/6 Inhibition in NRAS-Mutant Melanoma. Cancer Res. 2019;79:2352–2366. doi: 10.1158/0008-5472.CAN-18-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kwong L.N., Costello J., Liu H., Jiang S., Helms T.L., Langsdorf A.E., Jakubosky D., Genovese G., Muller F., Jeong J.H., et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nat. Med. 2012;18:1503–1510. doi: 10.1038/nm.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patnaik A., Rosen L.S., Tolaney S.M., Tolcher A.W., Goldman J.W., Gandhi L., Papadopoulos K.P., Beeram M., Rasco D.W., Hilton J.F., et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov. 2016;6:740–753. doi: 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 139.Sahebjam S., Le Rhun E., Queirolo P., Jerusalem G., Subramaniam D., Bear M., Yang Z., Chen Y., Conte P. A phase II study of abemaciclib in patients (pts) with brain metastases (BM) secondary to non-small cell lung cancer (NSCLC) or melanoma (MEL) Ann. Oncol. 2019;30:v117. doi: 10.1093/annonc/mdz242.026. [DOI] [Google Scholar]
- 140.Infante J.R., Cassier P.A., Gerecitano J.F., Witteveen P.O., Chugh R., Ribrag V., Chakraborty A., Matano A., Dobson J.R., Crystal A.S., et al. A Phase I Study of the Cyclin-Dependent Kinase 4/6 Inhibitor Ribociclib (LEE011) in Patients with Advanced Solid Tumors and Lymphomas. Clin. Cancer Res. 2016;22:5696–5705. doi: 10.1158/1078-0432.CCR-16-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mao L., Dai J., Cao Y., Bai X., Sheng X., Chi Z., Cui C., Kong Y., Zhang Y., Wu L., et al. Palbociclib in advanced acral melanoma with genetic aberrations in the cyclin-dependent kinase 4 pathway. Eur. J. Cancer. 2021;148:297–306. doi: 10.1016/j.ejca.2021.02.021. [DOI] [PubMed] [Google Scholar]
- 142.Garutti M., Targato G., Buriolla S., Palmero L., Minisini A., Puglisi F. CDK4/6 Inhibitors in Melanoma: A Comprehensive Review. Cells. 2021;10:1334. doi: 10.3390/cells10061334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mertens F., Johansson B., Fioretos T., Mitelman F. The emerging complexity of gene fusions in cancer. Nat. Rev. Cancer. 2015;15:371–381. doi: 10.1038/nrc3947. [DOI] [PubMed] [Google Scholar]
- 144.Mitelman F., Johansson B., Mertens F. The impact of translocations and gene fusions on cancer causation. Nat. Rev. Cancer. 2007;7:233–245. doi: 10.1038/nrc2091. [DOI] [PubMed] [Google Scholar]
- 145.Wiesner T., He J., Yelensky R., Esteve-Puig R., Botton T., Yeh I., Lipson D., Otto G., Brennan K., Murali R., et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat. Commun. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Newman S., Fan L., Pribnow A., Silkov A., Rice S.V., Lee S., Shao Y., Shaner B., Mulder H., Nakitandwe J., et al. Clinical genome sequencing uncovers potentially targetable truncations and fusions of MAP3K8 in spitzoid and other melanomas. Nat. Med. 2019;25:597–602. doi: 10.1038/s41591-019-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Niu H.-T., Zhou Q.-M., Wang F., Shao Q., Guan Y.-X., Wen X.-Z., Chen L.-Z., Feng Q.-S., Li W., Zeng Y.-X., et al. Identification of anaplastic lymphoma kinase break points and oncogenic mutation profiles in acral/mucosal melanomas. Pigment Cell Melanoma Res. 2013;26:646–653. doi: 10.1111/pcmr.12129. [DOI] [PubMed] [Google Scholar]
- 148.Busam K.J., Vilain R.E., Lum T., Busam J.A., Hollmann T.J., Saw R.P., Coit D.C., Scolyer R.A., Wiesner T. Primary and Metastatic Cutaneous Melanomas Express ALK Through Alternative Transcriptional Initiation. Am. J. Surg. Pathol. 2016;40:786–795. doi: 10.1097/PAS.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Turner J., Couts K., Sheren J., Saichaemchan S., Ariyawutyakorn W., Avolio I., Cabral E., Glogowska M., Amato C., Robinson S., et al. Kinase gene fusions in defined subsets of melanoma. Pigment Cell Melanoma Res. 2017;30:53–62. doi: 10.1111/pcmr.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stransky N., Cerami E., Schalm S., Kim J.L., Lengauer C. The landscape of kinase fusions in cancer. Nat. Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lezcano C., Shoushtari A.N., Ariyan C., Hollmann T.J., Busam K.J. Primary and Metastatic Melanoma with NTRK Fusions. Am. J. Surg. Pathol. 2018;42:1052–1058. doi: 10.1097/PAS.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Drilon A., Laetsch T.W., Kummar S., Dubois S.G., Lassen U.N., Demetri G.D., Nathenson M., Doebele R.C., Farago A.F., Pappo A.S., et al. Efficacy of Larotrectinib inTRKFusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hong D.S., DuBois S.G., Kummar S., Farago A.F., Albert C.M., Rohrberg K.S., van Tilburg C.M., Nagasubramanian R., Berlin J.D., Federman N., et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531–540. doi: 10.1016/S1470-2045(19)30856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Doebele R.C., Drilon A., Paz-Ares L., Siena S., Shaw A.T., Farago A.F., Blakely C.M., Seto T., Cho B.C., Tosi D., et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Couts K.L., McCoach C.E., Murphy D., Christiansen J., Turner J., Lewis K.D., Robinson W.A., Doebele R.C. Acral Lentiginous Melanoma Harboring a ROS1 Gene Fusion With Clinical Response to Entrectinib. JCO Precis. Oncol. 2017;1:1–7. doi: 10.1200/PO.16.00013. [DOI] [PubMed] [Google Scholar]
- 156.Couts K.L., Bemis J., Turner J.A., Bagby S.M., Murphy D., Christiansen J., Hintzsche J.D., Danielle M., Pitts T.M., Wells K., et al. ALK Inhibitor Response in Melanomas Expressing EML4-ALK Fusions and Alternate ALK Isoforms. Mol. Cancer Ther. 2018;17:222–231. doi: 10.1158/1535-7163.MCT-17-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sharma S., Kelly T.K., Jones P.A. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bates S.E. Epigenetic Therapies for Cancer. N. Engl. J. Med. 2020;383:650–663. doi: 10.1056/NEJMra1805035. [DOI] [PubMed] [Google Scholar]
- 159.Duan R., Du W., Guo W. EZH2: A novel target for cancer treatment. J. Hematol. Oncol. 2020;13:104. doi: 10.1186/s13045-020-00937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tiffen J.C., Gallagher S.J., Tseng H.-Y., Filipp F.V., Groth B.F.D.S., Hersey P. EZH2 as a mediator of treatment resistance in melanoma. Pigment Cell Melanoma Res. 2016;29:500–507. doi: 10.1111/pcmr.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zingg D., Debbache J., Schaefer S.M., Tuncer E., Frommel S.C., Cheng P., Arenas-Ramirez N., Haeusel J., Zhang Y., Bonalli M., et al. The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors. Nat. Commun. 2015;6:6051. doi: 10.1038/ncomms7051. [DOI] [PubMed] [Google Scholar]
- 163.Bachmann I.M., Halvorsen O.J., Collett K., Stefansson I.M., Straume O., Haukaas S.A., Salvesen H.B., Otte A.P., Akslen L.A. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J. Clin. Oncol. 2006;24:268–273. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- 164.Tiffen J.C., Gunatilake D., Gallagher S., Gowrishankar K., Heinemann A., Cullinane C., Dutton-Regester K., Pupo G.M., Strbenac D., Yang J., et al. Targeting activating mutations of EZH2 leads to potent cell growth inhibition in human melanoma by derepression of tumor suppressor genes. Oncotarget. 2015;6:27023–27036. doi: 10.18632/oncotarget.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Unruh D., Zewde M., Buss A., Drumm M.R., Tran A.N., Scholtens D.M., Horbinski C. Methylation and transcription patterns are distinct in IDH mutant gliomas compared to other IDH mutant cancers. Sci. Rep. 2019;9:8946. doi: 10.1038/s41598-019-45346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dang L., White D.W., Gross S., Bennett B.D., Bittinger M.A., Driggers E.M., Fantin V.R., Jang H.G., Jin S., Keenan M.C., et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mellinghoff I.K., Ellingson B.M., Touat M., Maher E., De La Fuente M.I., Holdhoff M., Cote G.M., Burris H., Janku F., Young R.J., et al. Ivosidenib in Isocitrate Dehydrogenase 1–Mutated Advanced Glioma. J. Clin. Oncol. 2020;38:3398–3406. doi: 10.1200/JCO.19.03327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Abou-Alfa G.K., Macarulla T., Javle M.M., Kelley R.K., Lubner S.J., Adeva J., Cleary J.M., Catenacci D.V., Borad M.J., Bridgewater J., et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Roboz G.J., Dinardo C.D., Stein E.M., de Botton S., Mims A.S., Prince G.T., Altman J.K., Arellano M.L., Donnellan W., Erba H.P., et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135:463–471. doi: 10.1182/blood.2019002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stein E.M., DiNardo C.D., Pollyea D.A., Fathi A.T., Roboz G.J., Altman J.K., Stone R.M., DeAngelo D.J., Levine R.L., Flinn I.W., et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lopez G.Y., Reitman Z.J., Solomon D., Waldman T., Bigner D.D., McLendon R.E., Rosenberg S.A., Samuels Y., Yan H. IDH1R132 mutation identified in one human melanoma metastasis, but not correlated with metastases to the brain. Biochem. Biophys. Res. Commun. 2010;398:585–587. doi: 10.1016/j.bbrc.2010.06.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shibata T., Kokubu A., Miyamoto M., Sasajima Y., Yamazaki N. Mutant IDH1 Confers an in Vivo Growth in a Melanoma Cell Line with BRAF Mutation. Am. J. Pathol. 2011;178:1395–1402. doi: 10.1016/j.ajpath.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pearl L.H., Schierz A.C., Ward S.E., Al-Lazikani B., Pearl F.M. Therapeutic opportunities within the DNA damage response. Nat. Rev. Cancer. 2015;15:166–180. doi: 10.1038/nrc3891. [DOI] [PubMed] [Google Scholar]
- 174.Chatterjee N., Walker G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017;58:235–263. doi: 10.1002/em.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kaufman B., Shapira-Frommer R., Schmutzler R.K., Audeh M.W., Friedlander M., Balmaña J., Mitchell G., Fried G., Stemmer S.M., Hubert A., et al. Olaparib Monotherapy in Patients with Advanced Cancer and a Germline BRCA1/2 Mutation. J. Clin. Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Moore K., Colombo N., Scambia G., Kim B.-G., Oaknin A., Friedlander M., Lisyanskaya A., Floquet A., Leary A., Sonke G.S., et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 177.Mirza M.R., Monk B.J., Herrstedt J., Oza A.M., Mahner S., Redondo A., Fabbro M., Ledermann J.A., Lorusso D., Vergote I., et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 178.Litton J.K., Rugo H.S., Ettl J., Hurvitz S.A., Gonçalves A., Lee K.-H., Fehrenbacher L., Yerushalmi R., Mina L.A., Martin M., et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.de Bono J.S., Mateo J., Fizazi K., Saad F., Shore N., Sandhu S., Chi K.N., Sartor O., Agarwal N., Olmos D., et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020;382:2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 180.Golan T., Hammel P., Reni M., van Cutsem E., Macarulla T., Hall M.J., Park J.-O., Hochhauser D., Arnold D., Oh D.-Y., et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.O’Connor M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 182.Knijnenburg T.A., Wang L., Zimmermann M.T., Chambwe N., Gao G.F., Cherniack A.D., Fan H., Shen H., Way G.P., Greene C.S., et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018;23:239–254. doi: 10.1016/j.celrep.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pilié P.G., Tang C., Mills G.B., Yap T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019;16:81–104. doi: 10.1038/s41571-018-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Heeke A.L., Pishvaian M.J., Lynce F., Xiu J., Brody J.R., Chen W.-J., Baker T.M., Marshall J.L., Isaacs C. Prevalence of Homologous Recombination–Related Gene Mutations Across Multiple Cancer Types. JCO Precis. Oncol. 2018;2:1–13. doi: 10.1200/PO.17.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chan W.Y., Brown L.J., Reid L., Joshua A.M. PARP Inhibitors in Melanoma—An Expanding Therapeutic Option? Cancers. 2021;13:4520. doi: 10.3390/cancers13184520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Makino E., Fröhlich L.M., Sinnberg T., Kosnopfel C., Sauer B., Garbe C., Schittek B. Targeting Rad51 as a strategy for the treatment of melanoma cells resistant to MAPK pathway inhibition. Cell Death Dis. 2020;11:581. doi: 10.1038/s41419-020-2702-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kim K.B., Soroceanu L., de Semir D., Millis S.Z., Ross J., Vosoughi E., Dar A.A., Nosrati M., Desprez P.-Y., Ice R., et al. Prevalence of Homologous Recombination Pathway Gene Mutations in Melanoma: Rationale for a New Targeted Therapeutic Approach. J. Investig. Dermatol. 2021;141:2028–2036. doi: 10.1016/j.jid.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 188.Chabanon R.M., Muirhead G., Krastev D.B., Adam J., Morel D., Garrido M., Lamb A., Hénon C., Dorvault N., Rouanne M., et al. PARP inhibition enhances tumor cell–intrinsic immunity in ERCC1-deficient non–small cell lung cancer. J. Clin. Investig. 2019;129:1211–1228. doi: 10.1172/JCI123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Peyraud F., Italiano A. Combined PARP Inhibition and Immune Checkpoint Therapy in Solid Tumors. Cancers. 2020;12:1502. doi: 10.3390/cancers12061502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lau B., Menzies A., Joshua A. Ongoing partial response at 6 months to olaparib for metastatic melanoma with somatic PALB2 mutation after failure of immunotherapy: A case report. Ann. Oncol. 2021;32:280–282. doi: 10.1016/j.annonc.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 191.Khaddour K., Ansstas M., Visconti J. Mutation clearance and complete radiologic resolution of immunotherapy relapsed metastatic melanoma after treatment with nivolumab and olaparib in a patient with homologous recombinant deficiency: Any role for PARP inhibitors and checkpoint blockade? Ann. Oncol. 2021;32:279–280. doi: 10.1016/j.annonc.2020.10.602. [DOI] [PubMed] [Google Scholar]
- 192.Khaddour K., Ansstas M., Ansstas G. Clinical outcomes and longitudinal circulating tumor DNA changes after treatment with nivolumab and olaparib in immunotherapy relapsed melanoma with detected homologous recombination deficiency. Cold Spring Harb Mol. Case Stud. 2021;7:a006129. doi: 10.1101/mcs.a006129. [DOI] [PMC free article] [PubMed] [Google Scholar]