Abstract
Estrogen receptor alpha (ERα, NR3A1) contributes through its expression in different tissues to a spectrum of physiological processes, including reproductive system development and physiology, bone mass maintenance, as well as cardiovascular and central nervous system functions. It is also one of the main drivers of tumorigenesis in breast and uterine cancer and can be targeted by several types of hormonal therapies. ERα is expressed in a subset of luminal cells corresponding to less than 10% of normal mammary epithelial cells and in over 70% of breast tumors (ER+ tumors), but the basis for its selective expression in normal or cancer tissues remains incompletely understood. The mapping of alternative promoters and regulatory elements has delineated the complex genomic structure of the ESR1 gene and shed light on the mechanistic basis for the tissue-specific regulation of ESR1 expression. However, much remains to be uncovered to better understand how ESR1 expression is regulated in breast cancer. This review recapitulates the current body of knowledge on the structure of the ESR1 gene and the complex mechanisms controlling its expression in breast tumors. In particular, we discuss the impact of genetic alterations, chromatin modifications, and enhanced expression of other luminal transcription regulators on ESR1 expression in tumor cells.
Keywords: breast cancer, estrogen receptor alpha, luminal breast cancer, mammary gland, ESR1, FOXA1, GATA3
1. Introduction
Breast cancer is the most frequent malignancy in women worldwide, with outcomes strongly affected by the stage of the disease [1]. It is characterized by the unregulated proliferation of epithelial cells normally located in the mammary ducts or alveoli. However, immunohistochemical (IHC) analysis reveals that breast cancer is a heterogeneous disease as evidenced by the differential detection of two main targetable tumorigenic drivers: estrogen receptor alpha (ERα), a nuclear receptor, and ERBB2/HER2, a membrane receptor belonging to the ERBB1-4 growth factor receptor family whose gene is frequently amplified in breast cancer. Use of positivity thresholds for detection of each marker by IHC (and for detection of ERBB2/HER2 amplification by fluorescence in situ hybridization) identifies four tumor subtypes, ER+HER2−, ER+HER2+, ER−HER2+ and ER−HER2− [2]. The expression of a third marker, the progesterone receptor (PR, NR3C3), an ERα target gene, reflects active estrogenic signaling and is a marker of improved prognosis compared to PR-negative tumors [3]. ER+ and/or PR+ tumors represent more than two-thirds of breast cancer cases and are currently treated with hormonal therapies that most commonly include aromatase inhibitors, which suppress endogenous estrogen production, or antiestrogens, which compete with estrogens for binding to estrogen receptors and inactivate them by inducing alternative conformations of their ligand-binding domains [4,5]. On the other hand, primary tumors with undetectable ER expression levels and activity are intrinsically resistant to hormonal therapies. These include ER-HER2+ tumors, and also tumors negative for ER, PR and HER2, called Triple Negative Breast Cancer (TNBC). It is estimated that approximately 30–50% of patients relapse after one or several lines of endocrine treatment. Expression of ERα is maintained in most relapsed tumors, which can remain sensitive to different hormonal therapy agents. However, about 15–40% of relapsed tumors lose expression of ERα and are therefore insensitive to additional hormonal treatment [6,7,8]. This review will discuss specifically the mechanism controlling ERα expression levels in normal and breast cancer cells. Other mechanisms of resistance to hormonal therapies, which include activation of other proliferative pathways and modulation of ERα or coregulator activity via genetic or epigenetic alterations, have been reviewed elsewhere (e.g., [7,9,10,11]).
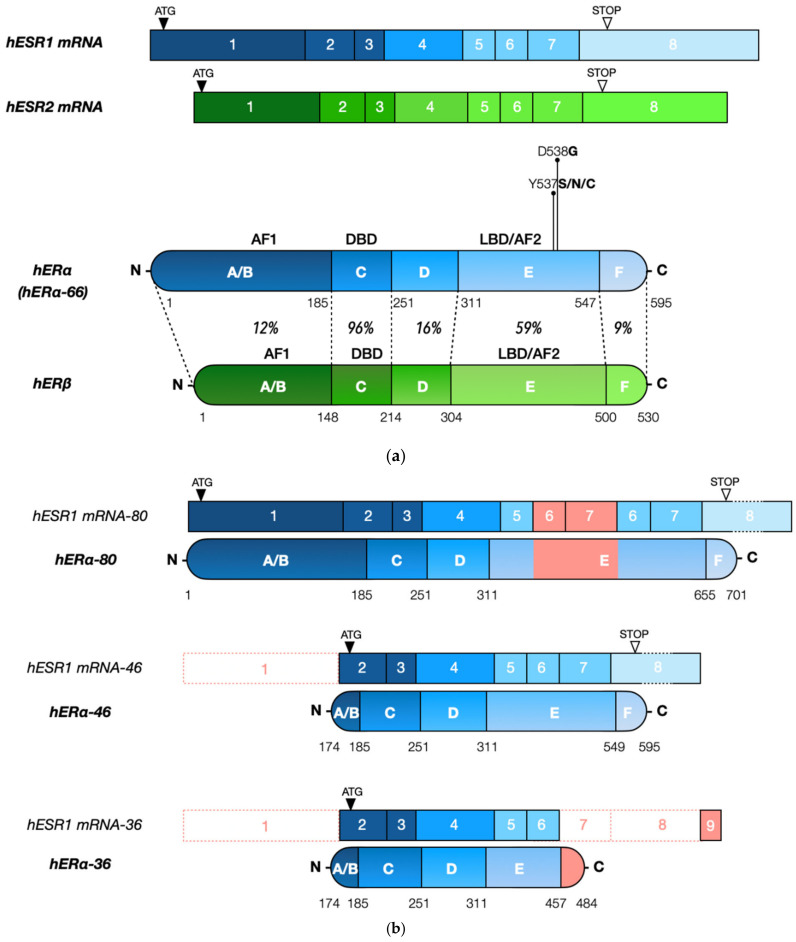
ERα and its paralog estrogen receptor beta (ERβ), encoded by the estrogen receptor 1 (ESR1) and the estrogen receptor 2 (ESR2) genes, respectively [12] (Figure 1a), mediate the systemic physiological effects of circulating estrogens [13]. ERα can be found in three major protein isoforms differing in molecular weight, ERα-66, ERα-46 and ERα-36 (Figure 1b), while ERβ has five isoforms, ERβ1-5. These receptors are part of the broader nuclear receptor family, composed of transcription factors whose activity can be regulated by small ligands and/or post-translational modifications [14]. Human ERs have only 44% overall identity in their primary structures but contain a well conserved DNA binding domain (96% identity between human ERα/β, Figure 1a) [15] composed of two zinc fingers folding into a single structural entity that directs binding of ER dimers to palindromic estrogen response elements (EREs) [16,17,18]. ER binding sites can be found at promoters of estrogen target genes but also at enhancers situated in intergenic or intronic sequences and having long-range impacts on gene transcription [19,20,21,22,23]. However, only a minority of predicted binding sites are used under any given experimental conditions [24], occupancy being controlled by chromatin accessibility [25] and/or cooperativity with other transcription factors, some of them acting as pioneer factors to enable access of ERs to cognate binding sites [26]. In addition to direct binding to EREs, ERs can also be recruited onto DNA by tethering via interactions with other transcription factors. Ligand addition leads to increased association of ERα with DNA and assembly of clusters of ERα-bound enhancers, or super-enhancers, organized around “mother enhancers” that are enriched in strong EREs and are detected in estrogen-deprived cells. By contrast, “daughter enhancers” appearing near mother enhancers upon ligand stimulation are enriched in sites for cooperating factors, suggestive of protein–protein interactions driven by the increased local concentrations of ERs on DNA [27]. Some enhancers are “hotspots” of colocalization of many transcription factors, which may be assembled in a “MegaTrans” complex marking the most active enhancers [28,29,30]. In particular, interaction with CTCF and components of the cohesin complex, which play roles in chromatin loop formation [31], has been described as a hall-mark of “hub” enhancers driving local chromatin conformation within topologically associated domains (TADs) [32].
Figure 1.
(a). ERα and ERβ have a conserved gene and protein organization. Amino acid identity between human ERα and ERβ is indicated for each domain [12]. The most frequent ERα mutations found in relapsed ER-positive tumors are indicated. (b). The main ERα protein isoforms and their corresponding mRNA structures are shown.
The ligand binding domains of ERs are also conserved, although to a lower degree (59% identity between human ERα/β) [15], which has made it possible to design receptor-specific synthetic ligands [33]. Both receptors are nevertheless activated by circulating estrogens, which trigger conformational changes in the ligand binding domain inducing receptor binding to DNA and recruitment of a variety of transcriptional coactivators or co-repressors [12,15,34,35,36,37,38]. The two receptors diverge more in other domains (Figure 1a), and as a result differ in their functional properties [39] and in their regulation by post-translational modifications (please refer to Phosphosite.org for a summary of ERα/β post-translational modifications and to [11,40,41] for reviews). Modifications can affect diverse ER functional properties, including subcellular localization, DNA binding, interaction with other transcriptional regulators or chromatin components, and protein stability. Post-translational modifications include phosphorylation by diverse kinases at multiple sites in a manner regulated by ligand binding. In addition, ERs can be acetylated, methylated and SUMOylated in a ligand-modulated manner. In addition, ubiquitination can be induced by agonist binding but also by some antiestrogens, such as fulvestrant, the prototype of Selective Estrogen Receptor Downregulators (SERDs), resulting in enhanced degradation of ERα by the proteasome machinery [42]. Finally, palmitoylation regulates localization of a pool of ERα at the plasma membrane, where it initiates non-genomic estrogenic signaling that can cross-talk with the nuclear pool of ERα [43]; mouse models with a mutation abolishing palmitoylation support a tissue-specific contribution of membrane signaling in estrogen physiology, while lack of nuclear ERα abrogates most responses [34,39].
ERα is the main tumorigenesis driver in breast cancer, the roles of the ERβ isoforms being less clear, although it was shown that they can repress the activity of ERα [44]. The human ESR1 gene, located at the q25.1-q25.2 locus of chromosome 6, has a complex structure. A cDNA was first cloned from MCF-7 cells in 1985 and was found to result from the transcription of eight exons, which together with introns span 296 kb [45,46,47,48]. However, characterization of transcripts in different estrogen-responsive tissues has revealed that the overall gene unit spreads over ~447 kb, comprising several alternative promoters and non-coding exons. Various transcriptional regulators bound to its distinct promoters or to enhancers acting at a large distance via the formation of chromatin loops control ESR1 gene expression during the development, normal physiology and tumorigenesis of the mammary tissue and in other estrogen-responsive tissues [49]. In this review, we will focus on the mechanisms known to control ESR1 expression, with a focus on positive regulation by transcription factors expressed in ER+ breast cancer, also called luminal breast cancer.
2. Expression of ESR1 Is Transcriptionally Regulated in Normal Mammary Tissue and in Breast Tumorigenesis
2.1. Expression in Normal Tissue
The mammary gland, an organ uniquely found in mammals, is composed of an epithelial and a stromal compartment [50]. The first stage of mammary tissue development occurs during mammalian embryogenesis and leads to the formation of a rudimentary ductal tree connected to the nipple [50,51]. During puberty, the mammary tissue will further develop to form an expanded tree of epithelial ducts ending in terminal end buds, embedded in the mammary fat pad. Throughout pregnancy, the ductal tree further expands and lactogenic differentiation of luminal cells in alveoli enables milk production for lactation [51].
In normal human breast tissue, ESR1 expression is restricted to the epithelial compartment, where ERα is detected in only about 7–10% of cells [52,53]. ERα is absent in differentiated myoepithelial cells and present only in a fraction of luminal cells in the human mammary gland where its expression levels are variable throughout life, particularly following puberty, during menstrual cycles and throughout pregnancy and lactation [54,55]. During mouse mammary gland development, ductal growth is initiated at terminal end buds (TEBs); ERα is not expressed in cap cells, located around the leading tip of the TEB, but is detected in a fraction of the underlying body cells, which also express luminal markers keratin 8/18 [51,56]. In mice, expression of the Esr1 gene is necessary for the development and differentiation of the mammary gland, which is under control by estrogens and progesterone [57,58,59]. Indeed, suppression of Esr1 expression disrupts the morphogenesis of the mammary gland and the function of the entire reproductive system [59,60].
The synthesis of sex hormones in the ovaries is regulated throughout the hormonal cycle after puberty and before menopause. While estrogens are produced during the entire hormonal cycle with a peak between the follicular phase and ovulation, progesterone is produced mostly during the second half (luteal phase). Increased expression of the ESR1 gene in epithelial cells during the follicular phase correlates with subsequent increased mitotic activity of epithelial cells in breast lobules [61,62,63]. However, ERα expression in individual normal epithelial cells does not correlate with proliferation (KI67 positivity, tritiated thymidine incorporation) [64], and it has been shown that paracrine signaling via ERα stimulates mammary epithelial cell proliferation during mammary morphogenesis [65]. However, studies in rodents and rhesus monkey have indicated that ERα protein expression is down-regulated by estrogens, which may explain the apparent lack of association of ERα-expressing cells with proliferative markers [66,67]. In breast cancer cell lines, ERα drives cell proliferation by activating expression of proliferative genes, including CCND1, MYC, E2Fs and FOXM1 [68,69,70,71]. In ER+ MCF-7 breast cancer cells, ERα protein levels are highest in the S and G2/M phases of the cell cycle [72]. The proliferative action of estrogens in tumors as well as cell lines is confirmed by the observation that hormonal therapies suppress proliferation of breast tumor cells [73].
2.2. Expression in Tumoral Tissue
In the clinic, breast tumors have been deemed ERα-positive (ER+ tumors) if more than 1% of epithelial cells demonstrate nuclear staining in immunohistochemistry with anti-hERα antibodies, qualifying patients for hormonal therapies, although rates of response are much lower for tumors with low expression of ERα [74]. While there is a range of ERα expression patterns between tumors, expression levels of the ERα protein are often higher and more homogeneous than in normal tissue, especially in the LumA subgroup. Nevertheless, some tumors exhibit heterogeneous expression, with variable proportions of cells expressing ERα while staining is faint or absent in others.
Elevated ESR1 expression has been observed in atypical ductal hyperplasia and in situ carcinoma, suggesting that deregulation of the ESR1 expression level may be implicated in early pathogenic changes during breast tumorigenesis [62,75]. Whether ESR1 overexpression is sufficient to drive tumorigenesis in the human mammary gland is unclear. Interestingly, transgenic expression of wild type (wt) murine ERα in mice using an MMTV-tTA controlled expression system led to ductal hyperplasia and ductal carcinoma in situ by four months of age and to a low incidence of invasive carcinoma by 12 months [76,77]. However, it remains unclear whether deregulated ESR1 expression is sufficient to drive tumorigenesis in the human mammary gland.
The ESR1 gene is affected by copy number variations, with amplification events initially reported at a high frequency (20%), correlating with higher ERα levels [78]. On the other hand, Brown et al. found amplification in only 1% of tumors [79]. Amplification is predicted by GISTIC in less than 4% of tumors in the TCGA Firehose Legacy dataset (Figure 2) and at similar low frequencies in other large breast tumor collections such as METABRIC. However, amplification does not correlate strongly with increased mRNA (Figure 3a) or protein expression. ERα is not frequently mutated in primary cancer, while recurrent mutations occur in a fraction of tumors progressing to resistance after hormonal treatment [80]. Several of these mutations (e.g., hot spot mutations Y537S/N/C and D538G, Figure 1a), lead to constitutive ERα activity, which generates resistance to aromatase inhibitors. ERα mutations can also differentially impact the potency and/or efficacy of clinically-relevant antiestrogens [80,81,82,83,84].
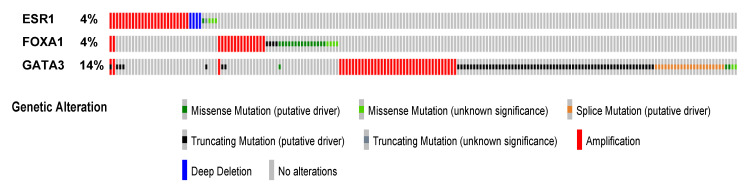
Figure 2.
Genetic alterations in ESR1, GATA3 and FOXA1 in the TCGA Firehose Legacy breast invasive carcinoma dataset. Amplification, deep deletion or mutations in genes for luminal transcription factors ERα, FOXA1 and GATA3 are shown. The figure was downloaded from cbioportal.org by selecting the TCGA Firehose Legacy dataset, querying the ESR1, FOXA1 and GATA3 genes and using the OncoPrint tool.
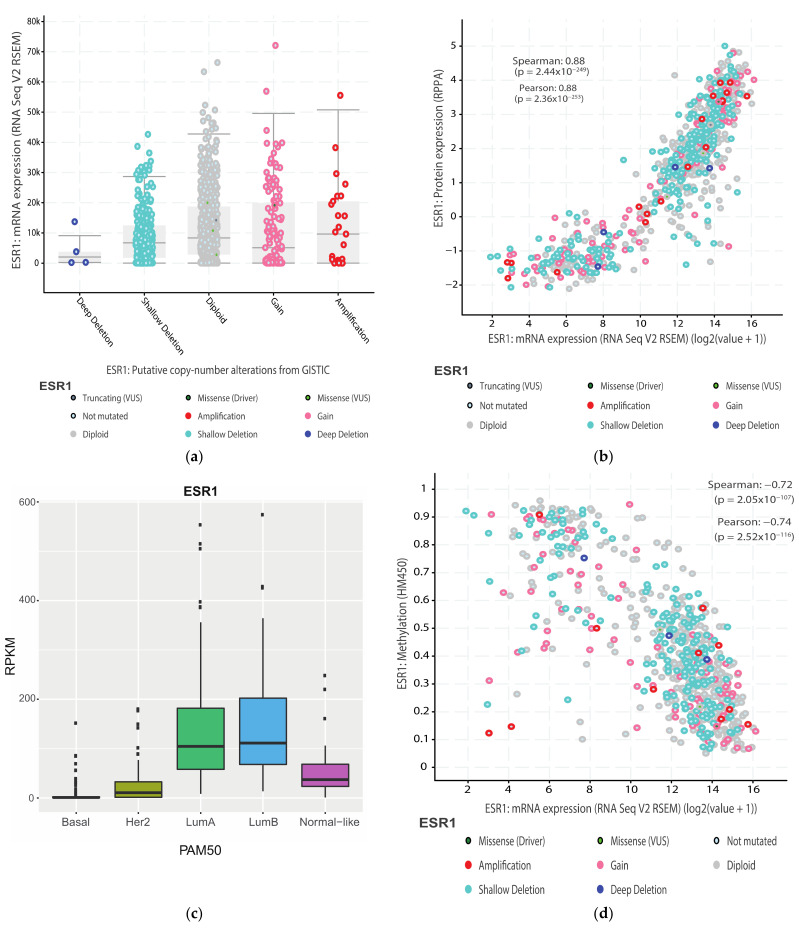
Figure 3.
Expression of the ESR1 gene is epigenetically controlled (a). Impact of CNVs on ESR1 expression in the TCGA Firehose Legacy breast invasive carcinoma dataset. (b). Correlation between ESR1 mRNA and protein expression in the same dataset. (c). Differential expression of ESR1 within PAM50 breast tumor types (d). Correlation between ESR1 gene expression and DNA methylation in the first intron in the TCGA Firehose Legacy breast invasive carcinoma dataset. Panels (a,b,d) were downloaded from cbioportal.org by querying the ESR1 gene and using the Plots tool. Panel c was produced using the PAM50 classifier on the TCGA breast invasive carcinoma dataset in [99].
In primary tumors, ESR1 mRNA expression correlates very well with protein expression, suggesting that regulation of ESR1 expression takes place mainly at the RNA level (transcription and/or mRNA stability) or that a balance between protein and RNA levels is maintained via tight feedback regulation (Figure 3b). The ESR1 mRNA is differentially expressed in breast cancer subtypes (Figure 3c). In the transcriptome-based PAM50 classification, ESR1 expression is highest in the luminal A and luminal B tumors, the latter being associated with higher grade and worse prognosis [85]. On the other hand, the HER2+ tumors express the ESR1 mRNA at variable and overall weaker levels while the basal-like subtype has very weak to undetectable levels of ESR1 mRNA and of the ERα protein (Figure 3c). The existence of discrete “intrinsic” subtypes with differential expression of the ESR1 gene likely reflects the phenotype of the cell of origin giving rise to these tumors (stem cell, bi-valent or univalent progenitor) and the inhibitory impact of genetic/epigenetic aberrations in breast cancer cells on normal epithelial cell differentiation.
ESR1 expression is rapidly lost in primary cultures of normal epithelial cells, either through epigenetic silencing or lack of proliferation/death of ER-positive cells. In addition, only a handful of ERα-expressing cell lines have been derived from ER+ tumors. ER+ tumors are also more difficult to engraft in Pdx models. Thus, our knowledge of ER signaling in tumors obtained from existing models may be biased toward more aggressive tumors. Nevertheless, ER+ cell lines such as MCF-7, T-47D and ZR-75-1 cells have proven very useful to dissect pathways regulating ERα expression as well as its role in the control of BC cell proliferation [86].
Methylation studies of the ESR1 gene have demonstrated that a CpG region located from the acceptor splice site in the first coding exon to the end of this exon (exon 1, Figure 4) is unmethylated in ER+/PR+ tumors and in ER+ cell lines MCF-7, T-47D and ZR-75-1, while it is partially methylated in ER− MDA-MB-468 cells and completely methylated in mesenchymal MDA-MB-231 cells [87]. Surprisingly, ER+/PR- tumors also presented some methylated CpG sites [87]. These results may be explained by the inclusion in the ER+ group of tumors with low ERα levels, resulting in undetectable expression of PR, whose gene is under estrogenic regulation. In addition, ESR1 expression is strongly negatively correlated with DNA methylation downstream of the major transcription start site in the TCGA breast tumor dataset (Figure 3d). Treatment of triple negative cell lines with DNA methyltransferase inhibitors such as 5′aza-deoxycytidine and/or histone deacetylase (HDAC) inhibitors was reported to induce expression of the ESR1 gene, leading to sensitivity to tamoxifen [88,89,90,91]. However, other studies have failed to reproduce these observations [92]. On the other hand, treatment of ERα-expressing breast and uterine cancer cells with HDAC inhibitors leads to suppression of ESR1 expression [93,94,95,96]. Recently, treatment of triple negative breast tumor cells MDA-MB-231 LM2 with EZH2 inhibitors has been shown to induce GATA3 expression and, more modestly, ESR1 expression, and to sensitize these cells as well as another TNBC cell line (MDA-MB-468) to treatment with the antiestrogen fulvestrant [97].
These observations suggest that epigenetic control through DNA methylation and histone acetylation/methylation plays a role in ESR1 expression in breast cancer cells. In addition to this, mutations activating or inactivating one of the multiple regulatory elements in the ESR1 gene [98], from changes in expression/activity of upstream regulators (see below), or a combination of both, may also contribute to the regulation of ESR1 expression. Altogether, the existence of different mechanisms of ESR1 gene expression activation likely contributes to the heterogeneity of ESR1 expression levels and patterns between and within tumors.
3. ESR1 Gene Organization and Regulatory Sequences
3.1. ESR1 Gene Structure and Alternative Transcripts
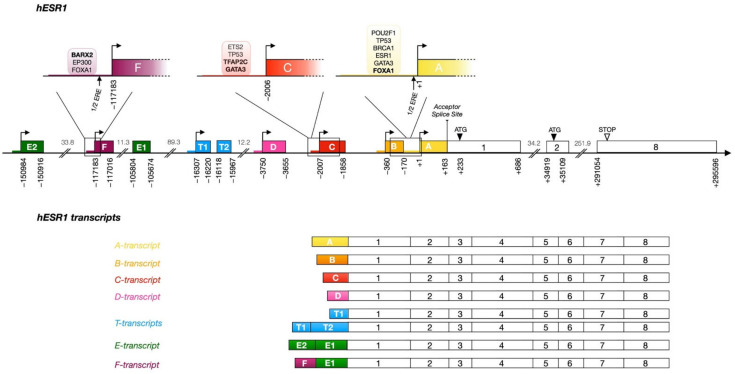
The analysis of different cDNA clones for the ESR1 gene has led to the characterization of seven promoters controlling nine upstream exons (A, B, C, D, T1/T2, F, E2/E1, Figure 4) [49,100,101]. The splicing of exons E1, T1, T2, D, C, and B to a common acceptor splice site located at position +163 in the first exon transcribed from promoter A yields 7 ESR1 transcript isoforms that can all be translated into the same 66 kDa protein [49,100,101]. The seven alternative promoters are regulated in a tissue-specific manner and generate transcripts with unique 5′ untranslated regions (5′ UTRs).
Nevertheless, several isoforms of the ERα protein can result from alternative splicing. A truncated form of ERα with an alternative, shorter N-terminal end, ERα-46 (Figure 1b), has been identified in breast cancer cells. It results from transcription initiated at the E2 or F exons, spliced to exon E1 and then directly to exon 2, deleting exon 1 [102,103]. Expression of this isoform is regulated by specific transcription factors [104,105]. The homeobox transcription factor BARX2 has been shown to bind to the ESR1 gene at the level of the E1 and F promoters and to upregulate transcription of this isoform [104]. The high mobility group A protein 1a (HMGA1a) is involved in the alternative splicing mechanism of this transcript by the recruitment of the RNA-protein complex U1 snRNP at the acceptor splice site [105]. ERα-46 translation is intiated at an ATG codon in a favorable Kozak consensus sequence in exon 2 and lacks most of the AF1 transcriptional activation domain, acting in a dominant negative manner to suppress genomic estrogen signaling and estrogen-dependent proliferation [106]. Furthermore, a shorter 36 kDa isoform has been characterized [107]. This protein, encoded by exons 2 to 6 and an alternative exon 9, located 64 kbp downstream of the ESR1 gene, is truncated both in the AF1 (lack of exon 1) and AF2 (lack of exons 7–8) transcriptional activation domains but preserves the DNA binding domain, as well as a truncated ligand binding domain with a different 27 aa C-terminus. The function of these truncated isoforms is not yet fully elucidated, although different lines of research suggest both short isoforms are localized preferentially at the plasma membrane [108]. This may result from the absence of exon 1, placing three potential myristoylation sites at residues 25–30 (GVWSCE), 76–81 (GMMKGG), and 171–176 (ELLTNL) in both shorter isoforms, and therefore much closer to the N-terminus of the protein than in the full length ER [107]. Moreover, the expression level of ERα-36 has been shown to correlate with a worse prognosis, the proposed mechanisms being, in spite of the LBD truncation, estrogen-dependent stimulation of mitogen signaling activity, activation by antiestrogens such as tamoxifen, and increased stemness and metastasis of breast cancer through upregulation of the aldehyde dehydrogenase 1A1 (ALDH1A1) gene [108,109].
Rearranged forms of the ESR1 gene have also been described. A duplication of exons 6 and 7, encoding part of the E domain, has been reported, leading to an ERα isoform of 80 kDa [110]. This longer isoform was found in a subclone of the MCF-7 cell line, MCF-7:2A, which contains four to five copies of the ESR1 gene and is estrogen independent. Moreover, 88 ESR1 gene fusions with a break point in or near intron 6, resulting in truncation of the LBD, have been characterized in metastatic ER-positive disease with a frequency estimated at more than 1% [111]. Contrary to hERα-36, which lacks exons 7 and 8 but was found to be activated by estrogens [108], these fusions are reportedly devoid of estrogen-dependent activity but have different levels of constitutive activity dependent on their fusion partners.
3.2. ESR1 Alternative Promoters Have Tissue-Specific Activity
Alternative transcripts of the ESR1 gene have been detected in both normal and cancerous tissues. In human cell models of ER+ breast tumors, ESR1 expression is driven predominantly by the proximal A promoter and the C promoter located 1.9 kb upstream [112]. Experiments from Grandien et al. demonstrated that both the A and C mRNA isoforms were also expressed in endometrial tissue and uterine adenocarcinoma cell lines. Interestingly, the mRNA levels of the A and C transcripts are widely different in breast cancer cells, with a ratio of approximately 20:1 [112]. The A transcript is more highly expressed in breast tumors than in the normal mammary gland, but the opposite is observed for the C mRNA isoform [112]. All transcripts except the T-transcript have been cloned from the MCF-7 cell line and have been shown to be expressed in the mammary gland, in endometrium and/or in liver [112].
Promoter A: Despite a degenerate TATA box (TACTTAAAG), the A transcript is the most abundant, whether in healthy mammary tissue or in luminal tumors [113,114]. DNA methylation analyses performed on a large panel of breast cancer cell lines revealed that none of the 22 CpG sites identified in promoter A were methylated [115]. However, data from ENCODE indicates that these sites are highly methylated in the HeLa cell line, consistent with the lack of expression of ESR1 in these cells.
ENCODE ChIP-Seq data reveals binding of several transcription factors (TFs) within 500 bp of the transcriptional start site (TSS) of promoter A. FOXA1, GATA3 and ERα interact with the promoter in the T-47D BC cell line and CTCF, MYC and POLR2A interact in the MCF-7 cell line. A predicted binding site for FOXA1 coincides with a FOXA1 ChIP peak 400 bp upstream of the promoter A TSS. The existence of a half ERE in promoter A (arrow, Figure 4) could possibly contribute to the recruitment of ERα [114,116]. ChIP-Seq assays targeting the breast cancer type 1 susceptibility protein (BRCA1) have shown that this protein binds the A promoter indirectly via Oct-1, encoded by the POU2F1 gene [117]. Oct-1 binds a region of promoter A between -385 and -169 bp and recruits BRCA1 as a coactivator, resulting in positive transcriptional regulation of ESR1 [117]. In addition, functional genomic studies have shown the presence of the p53 protein in the region between −128 to −40 bp of promoter A, accompanied by other factors such as Sp-1, c-Jun and the coactivator CBP, suggesting regulation of ESR1 by p53 via this promoter [118]. This is consistent with the higher rate of p53 mutations in ER-negative vs. ER-positive and in luminal B vs. luminal A tumors, although the extent to which p53 controls ESR1 expression remains unclear. The recruitment of Sp-1 within the −245 to −192 bp region was found to be essential for ESR1 expression and to mediate positive regulation of promoter A by exogenous Sp-1 expression in Drosophila Schneider SL2 cells [119].
Figure 4.
Schematic structural organization of human ESR1 promoters and alternative transcripts. The structure of the human ESR1 gene is presented along the main axis, boxes corresponding to exons. Alternative upstream exons are colored and labeled with letters according to the nomenclature proposed by Kos et al. [49]. White boxes correspond to exons downstream of the common acceptor splice site and are numbered according to the same nomenclature. Alternative promoters are represented by a colored thick line matching the color of the corresponding 5′ exon. The arrows define transcriptional initiation sites. The numbers under the main axis are exon start/end distances from the transcription start site originally defined as +1 (transcript A), calculated based on mapped transcripts in the hg19 genome version. The numbers between the exons represent the size of the introns in kilobase pairs. The common acceptor splice site is represented by a vertical bar before exon 1 and the ATG start codon is indicated by a black arrow. The upper part of the diagram details the promoters of exons A, C and F. Reported half-estrogen response elements (1/2 EREs) in these promoters are indicated by arrows. Transcription factors bound in these regions based on published ChIP-Seq data are shown in bubbles and factors directly binding DNA (motif predicted by HOMER) are in bold. The different transcripts produced after transcription and splicing from each of the seven regulated upstream exons to exon 1 are also presented in the lower part of the diagram.
The presence of the repressive histone methylation mark H3K9me3 has been reported at promoter A in basal breast cancer cells. ChIP-qPCR on FOXC1 wt and knock-out BT549 basal-like breast cancer cells showed a correlation between trimethylation of H3K9, FOXC1 expression and loss of RNA PolII recruitment at the ESR1 regulatory sequence. This negative regulation of ESR1 gene expression reinforces the hypothesis that ESR1 heterogeneity between different breast cancer types is due to the establishment of distinct epigenetic marks [120].
Promoter B: As there are only about 360 bp between the B and A transcript start sites, several of the TF binding sites upstream of promoter A overlap with promoter/exon B. The B transcript has been detected in breast tissue at very low mRNA levels compared to the A transcript. The B promoter and exon B include 18 CpG sites that are unmethylated both in normal MCF-7 cells or in antiestrogen and anti-aromatase inhibitors resistant MCF-7 cells, which express lower levels of ERα [115].
Promoter C: Exon C presents two main transcription start sites located at −1974 and −2007 bp, generating C-transcripts with different 5′-ends [113]. C transcripts are ten times less abundant than the A-transcript in MCF-7 cells and absent from ZR-75 cells. Promoter C is highly methylated in ZR-75-1 cells, correlating with the absence of the C-transcript in that cell line, while it is unmethylated to moderately methylated in ER-negative SK-BR-3 and MDA-MB-231 cells [115].
ENCODE ChIP-Seq data and literature reports suggest binding of multiple TFs to this promoter in MCF-7 cells. The activity of the promoter C in luminal cell lines was observed to depend on the binding of estrogen receptor associated factor 1 (ERF-1), corresponding to the transcription factor AP-2 gamma (TFAP2C) [121,122]. This factor binds promoter C at −1877 bp in a complex with Jun/Fos and plays an important role in the expression of the ESR1 gene [123,124,125]. Sodium bisulfite genomic sequencing revealed that promoter C comprises nine CpG sites [115,121]. The ninth methylation site is located in a TFAP2C binding motif [115,121]. TFAP2C is expressed in both the luminal and myoepithelial cells in the adult mammary gland and is expressed in all BC subtypes [126]. MMTV-Cre induced knockout in mouse models leads to delayed migration through the mammary fat pad without deleterious impact on mammary gland function, to an increase in the CD24midCD49fhi population, an increase in basal CK5 staining and a decrease in the normalized luminal:basal ratio. Down-regulation of TFAP2C in ER+ BC cells leads to epithelial to mesenchymal transition (EMT) characterized by increased VIM and CD44 expression, with decreased CDH1 and CD24 expression and decreased luminal gene (ESR1, FOXA1, GATA3) expression [127]. TFAP2C also regulates FOXM1 expression and its down-regulation suppresses estrogen-dependent proliferation [128]. The related TFAP2A on the other hand specifically induces CDKN1A and is inactive on luminal genes [129].
Specific methylation of the fourth CpG site in promoter C was responsible for a decreased expression of ESR1 and resistance to endocrine therapy; the absence of methylation at this specific CpG site allows the recruitment of the methylation-sensitive transcription factor Ets-2, which leads to ESR1 expression in normal MCF-7 cells [115,121]. In the MCF-7 cell line, GATA3 and the negative regulator FOXC1 were reported to compete for binding to DNA at overlapping recognition motifs [120]. Induced expression of FOXC1 in MCF-7 cells leads to a loss of GATA3 recruitment to promoter C, indicating that FOXC1 can abolish the positive regulation of that promoter [120]. More distally, CTCF also binds promoter C in the MCF-7 cell line. Finally, p53 is recruited at the C promoter in ChIP-qPCR assays in the DNA region between −2094 to −1941 bp from the main TSS, which contains seven of the nine methylation sites described previously [118,124,130].
Promoter D: Promoter D was shown to be fully methylated in the MCF-7 cell line. DNA methylation studies identified seven CpG sites methylated in that promoter, but the expression level of the D-transcript did not correlate with the methylation status [115]. Moreover, an AP-1 binding site has been described as an enhancer active in MCF-7 cells and inactive in ER-negative MDA-231 cells [125].
Promoter F: The F-transcript, produced from the splicing of exons F and E1, is the predominant variant expressed in primary osteoblasts but is also highly expressed in breast cancer tissue [114,131]. As mentioned above, promoter F was found to be regulated by BARX2 in breast normal and tumoral tissue [104]. FOXA1, GATA3 and p300 also interact with this promoter in the MCF-7 and T-47D cell lines, correlating with the levels of F-transcripts in ER-positive breast cancer cells [132]. The existence of a half ERE in promoter F (arrow, Figure 4) was suggested to contribute to the recruitment of ERα [114,116]. E2F1 is also associated with promoter F in ENCODE MCF-7 ChIP-Seq data. DNA methylation analysis in this promoter has shown the presence of 10 CpGs, unmethylated in MCF-7 but all methylated in the MDA-MB-231 cell line [132].
Promoter E: The E-transcript is produced by the transcription of exons E2 (upstream of promoter F) and E1 (downstream of promoter F) and is expressed mainly in the liver and at very low levels in breast cancer cells [114].
Promoter T: This promoter is the only one not to be active in mammary glands or in breast tumors. The T-transcript is predominantly expressed in testis and epididymal tissues. The T1 and T2 exons are separated by a 101 bp intron and are located about 16 kb upstream of the first exon [49,133]. Transcription from the T promoter can form either a longer variant from the splicing of exon T1 to exon T2 and finally to exon 1, or a shorter variant from the splicing of the T1 exon directly to exon 1.
3.3. Upstream Enhancers
Distal enhancers can regulate transcription at up to several hundred kilobases away from target genes via chromatin looping [134]. ChIP experiments have revealed binding of luminal TFs FOXA1, GATA3, and ERα itself to far upstream regulatory sequences in the ESR1 gene [19,135,136,137]. All regions interacting with these luminal TFs are associated with enhancer chromatin marks H3K4Me1 and H3K27Ac in ENCODE datasets generated in MCF-7 cells, supporting the existence of at least five “luminal” enhancers located at distances greater than 120 kb upstream of exon A (Figure 5; enhancers 1–3 are upstream of exon E2, enhancers 4 and 5 are found between exons E2 and F). FOXA1 and GATA3 bind to enhancers 1–5 and ERα to enhancers 1–4. Thus, ERα, GATA-3 and FOXA1 appear to share cis regulatory sites in the ESR1 gene as well as upstream of many estrogen target genes [19,135,136,137]. Enhancers 1 and 4 contain motifs related to the consensus ERE sequence 5′-(A/G)GGTCANNNTGACC(T/C)-3′ [138,139,140]. Predicted FOXA1 motifs related to the consensus 5′-A(A/T)TRTT(G/T)RYTY-3′ [141] are found in enhancers 2, 3 and 4. Sequences matching the consensus GATA binding motif 5′-(A/T)GATA(A/G)-3′ [142,143,144] are predicted by HOMER in enhancers 4 and 5. A reported GATA3 site [98] not predicted by HOMER is located in enhancer 2. Thus, one or more predicted binding motifs for ERα, FOXA1 and GATA3 are found in each of the five enhancers. While ERα, FOXA1 and GATA3 are detected at regions that do not contain mapped binding motifs, lower affinity sequences may be accessed through DNA binding cooperativity and/or pioneer activity. Alternatively, these TFs may be tethered to other TFs directly recruited to DNA. Other transcription factors are also present at these sites as annotated in ENCODE ChIP-Seq datasets in MCF-7 cells, suggesting that they correspond to active enhancers [30]. The coactivator p300 (EP300), which acts as a general transcriptional coregulator with histone acetyltransferase activity [145], and is present at 51% of the ERα binding sites after E2 treatment [146], is strongly recruited to enhancers 1–4, as well as RNApolII. While these enhancers are not clustered in super-enhancers, we note that enhancers 3 and 4 are associated with CTCF and components of the cohesin complex, corresponding to the profile of “hub” enhancers [32], which play major roles in local chromatin organization. Finally, chromatin loops were observed to form between enhancers 1 and 2/3, 1 and 4, 2–3 and 4, and 1 and promoter F by ChIA-PET with ERα in MCF-7 cells [147]. Looping with other regions not strongly associated with luminal factors in MCF-7 cells was also observed, suggesting that other regulatory sequences play roles in ESR1 transcription, in keeping also with the existence of several other regions associated with H3K4Me1/H3K27Ac and DNAse hypersensitivity.
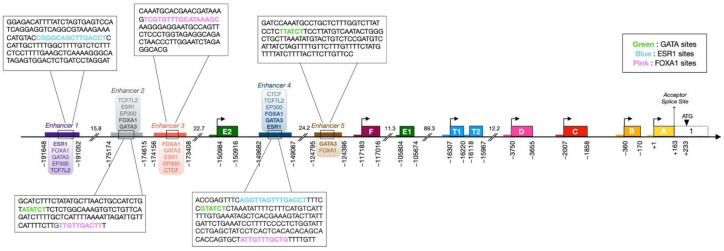
Figure 5.
Enhancer regions of the human ESR1 gene bound by luminal TFs. Colored rectangles highlight the luminal enhancers defined by ChIP-Seq assays with luminal factors ERα, FOXA1 and GATA3 and coactivator p300 [19,135,136,137]. Alternative exons spliced with coding exon 1 are shown as in Figure 4. The promoters are represented by a colored thick line upstream of the corresponding alternative first exons. Broken arrows define the different transcription initiation sites. Distances between the beginning or end of each defined feature and the transcription start site in promoter A are indicated. The distance in kilobase pairs between two highlighted elements is shown under the DNA axis. The common acceptor splice site is represented by a black line and the ATG start codon is indicated by a black triangle. Transcription factors bound to each enhancer based on ChIP-Seq data are shown in bubbles above or below each element, and factors for which a recognition motif is predicted by HOMER or mapped in a referenced study are in bold. DNA sequences from the enhancers containing one or more binding motifs for GATA3 (Green), FOXA1 (Pink) and/or ERα (Blue) are shown in magnification boxes (motifs for each TF are in bold).
Breast cancer risk-associated single nucleotide variants (SNVs) and somatic mutations have been mapped to luminal enhancers and other DNAse hypersensitive sites within 1 Mb of the ESR1 start site [98]. The rs9383590 SNV affects the reported GATA3 binding site in enhancer 2 and is predicted to increase activity of the enhancer. This suggests that this GATA3 element acts as a negative rather than positive regulator of ESR1 expression. Of interest, this motif is located within a TCF7L2 ChIP binding region (ENCODE). The transcription factor TCF7L2 has binding patterns in MCF-7 cells that coincide with those of GATA3, resulting in repression of their common target genes [148] (see also below). In addition, mutation chr6:151979547:A>G in enhancer 4 affects a nucleotide within a predicted FOXA1 motif (5′ ATTGTTTGCTG 3′) [98]. While it is unclear whether this mutation affects FOXA1 binding, residues flanking the core FOX motif (5′-RTAAAYA-3′) can alter FOX factor binding specificity (see below). Point mutations that led to increased activity in reporter gene assays were also found in tumors between enhancers 2 and 3 (chr6:151954506 C>A), after enhancer 3 (chr6: 151955192A>G; chr6:151955219:G>T), downstream of the E2 promoter (chr6:152024472C>G) or upstream of promoter C (chr6: 152125116G>C); these mutations may suppress binding by transcriptional repressors or create new binding sites for transcriptional activators, suggesting that a variety of transcription factors bound to different regulatory regions regulated ESR1 expression. Based on their data, Bailey et al. predicted that mutations leading to increased enhancer activity may explain the sustained expression of ESR1 in some (about 7%) luminal breast tumors [98].
4. Luminal ESR1 Transcriptional Regulators
4.1. GATA3
The GATA3 gene, located at 10p14 in the human genome, codes for a 48 kDa protein composed of two N-terminal transactivation domains, two zinc-finger DNA-binding motifs followed by two highly conserved and distinct basic regions (basic region 1 and 2) and a C-terminal region required for GATA3 transactivation, which contains a conserved YXKXHXXXRP motif [144,149,150]. The GATA proteins form a family of six zinc finger DNA binding proteins that recognize motifs related to the consensus sequence 5′-A/TGATAA/G-3′ [142,143,144]. GATA3 was shown to bind DNA with its C-terminal zinc-finger region and form homodimers, or heterodimers with other GATA members via its N-terminal zinc-finger region [144,151]. On certain specific DNA sequences, two close GATA sites can be bound simultaneously by the two zinc fingers of one GATA3 molecule with higher affinity [144]. Like ERα, GATA3 interacts with the acetyltransferase CREBBP/CBP, which acetylates it at K119, contributing to its capacity to downregulate mesenchymal transcription factors ZEB1/2 and Slug [152].
In breast tumors and cell lines, expression of GATA3 is strongly associated with those of ESR1 and FOXA1 [153]. On the other hand, GATA1, 2, 4 and 5 are only mildly or not correlated, while GATA6, the only GATA gene whose expression is biased toward basal-like tumors, is mildly anti-correlated with ESR1 in the TCGA dataset [99]. GATA3 was identified as a regulator of mammary branching morphogenesis by genome-wide transcript analysis [154] and shown to maintain the differentiation of luminal cells in mouse models [155,156,157]. High levels of expression of GATA3 in ER-positive cells are consistent with the reported positive cross-regulation feedback loop between the GATA3 and ESR1 genes [158], which also likely involves the FOXA1 luminal transcription factor. As mentioned above, GATA3 interacts with the ESR1 promoters A and C but also with the five enhancers associated with FOXA1 in ChIP-Seq datasets [120,136] (Figure 5). Conversely, an enhancer recruiting ERα, FOXA1 and GATA3 itself, together with the coactivator p300, was described approximately 10 kb downstream of the GATA3 gene (about 30 kb downstream of the TSS) [158]. Another region located 53.4 kb downstream of the gene (73.4 kb downstream of the TSS) is even more strongly associated with all factors in relevant ChIP-Seq/Exo datasets [136,137] and contains a predicted ERE and several FOX and GATA motifs, supporting the existence of regulatory loops between these factors.
Consistent with the role of GATA3 in the regulation ESR1 expression, GATA3 silencing by two different siRNAs was observed to reduce ESR1 expression in T-47D and MCF-7 cells, to blunt induction of transcription of estrogen target genes, and to inhibit estrogen-induced proliferation [158]. On the other hand, expression of GATA3 in MDA-MB-231 cells results in reprogramming of these cells from a mesenchymal to a luminal subtype, associated with a reduction of tumorigenesis and metastasis in implanted xenograft assays; GATA3 also induces a growth inhibitory response to TGFβ [159,160].
Moreover, GATA3 may act as a pioneer factor by binding target sites within nucleosomes and making neighboring cis-regulatory elements accessible for the recruitment of additional transcription factors [161,162,163]. During the mesenchymal-to-epithelial transition induced by GATA3, cellular reprogramming involves in part binding to closed chromatin regions, nucleosome eviction and chromatin remodeling in a transcriptional activation domain-dependent manner [162]. In ER-positive breast cancer cell lines, enrichment in GATA motifs was observed in ERα and FOXA1 ChIP-Seq/Exo peaks [136,137] and GATA3 silencing decreased or even abolished the recruitment of ERα at distal regulatory regions normally co-occupied with GATA3 [161]. Recruitment of common cofactors may contribute to complex formation between GATA3 and other luminal TFs. GATA3 may also recruit other transcription factors by tethering. GATA3 expression was shown to be required for the high mobility group box-containing factor TCF7L2 to bind to about 50% of TCF7L2 sites in the ER-positive breast cancer cell line MCF-7, and these sites were enriched in GATA3 but not TCF7L2 motifs [148]. GATA3 and TCF7L2 bound to shared sites simultaneously and tagged TCF7L2 could co-immunoprecipitate with endogenous GATA3 in transfected MCF-7 cells. TCF7L2 functioned mainly as a transcriptional repressor at shared sites [148]. TCF7L2 is recruited at the level of enhancers 1, 2 and 4 of the ESR1 gene; however, whether TCF7L2 represses ESR1 expression needs to be confirmed.
GATA3 is the third most mutated gene in luminal breast cancers with a prevalence of approximately 14% and 15% in luminal A and B tumors, respectively (cbioportal.org, TCGA Firehose Legacy dataset). GATA3 is also amplified in breast tumors (Figure 2), mostly in ER-negative tumors where amplification correlates with decreased rather than increased GATA3 expression, suggesting the existence of different driver genes in this 10p14 amplicon [164]. On the other hand, GATA3 is frequently affected by mutations in ER+ BC, with a bias for invasive ductal carcinoma, including splicing mutations (mostly X308_splice) and truncating frameshift mutations (in or after the second zinc finger) [165,166]. GATA3 mutations can lead to active forms of the GATA3 protein that contribute to tumor growth in BC cell xenografts and promote precocious lobuloalveolar development in transgenic mice [167]. In addition, ER-positive breast tumors with GATA3 mutations were reported to have a worse prognosis compared to wt tumors [168]. In the MCF7 cell line, GATA3 is affected by a heterozygous D336fs mutation in the second zinc finger that leads to a truncated protein with reduced affinity for DNA. Both wt and mt GATA3 have increased stability in MCF-7 vs. wt GATA3 in T-47D cells, leading to increased genomic occupancy by GATA3; genomic distribution was similar as in wt T47D but with gene-specific loss of binding, such as at the progesterone receptor gene [169]. Mutation R330fs was on the other hand reported to alter genomic distribution of ERα and FOXA1, leading to changes in gene regulation affecting genes involved in mammary gland development and epithelial cell biology [170]. In contrast, the X308_splice mutation, which generates a GATA3 protein lacking the second zinc finger but containing a novel 44 aa C-terminal end, downregulates ER genomic signaling and is associated with improved overall survival in ER+ patients [171].
4.2. FOXA1
The forkhead box (FOX) family of transcription factors comprises 50 human members that share a characteristic ‘winged-helix’ DBD composed of a helix-turn-helix motif with two C-terminal loops or “wings” [172] and regulate gene expression spatially and temporally during development [173]. These transcription factors can be divided into 19 subgroups (FOXA to FOXS) based on the sequence of their DBDs. Although all FOX proteins recognize core motifs related to the consensus 5′-RTAAAYA-3′, flanking sequences contribute to DNA binding specificity within the family [172,174]. Further, divergence in FOX factor primary sequence outside of the DBD results in a wide range of biological functions. In particular, FOXA1 plays an essential role in mammary ductal morphogenesis [175], and its expression is strongly correlated with that of ERα in breast luminal tumors [153]. FOXA1 has been identified as an upstream regulator of ESR1 expression in mouse and human breast cancer cells [175,176] and as a repressor of basal-like genes [177]. Notably, FOXA1 is co-associated with GATA3 at several enhancers in the ESR1 gene (Figure 5) and may thus cooperate with it for regulation of ESR1 expression. In addition, FOXA1 can act as a pioneer transcription factor, directly binding and opening condensed chromatin [178,179]. FOXA1 can enable recruitment of ERα and the androgen receptor (AR) to their respective response elements in breast and prostate cancer, respectively [19,26,180,181,182]. Beyond facilitation of hormone receptor signaling, FOXA1 overexpression has been shown to contribute to tamoxifen insensitivity of breast cancer cells in a mechanism that involves induction of interleukin-8 [183].
The FOXA1 gene is infrequently amplified (Figure 2), and copy number gain does not associate with markedly higher expression levels, while shallow deletion and DNA methylation may account for low FOXA1 expression (cbioportal.org). Mutations in FOXA1 are less frequent than those in GATA3 and are mostly missense mutations associated with high FOXA1 mRNA levels. Contrary to GATA3 mutations, they are frequent in invasive lobular carcinoma [166]. These mutations affect mostly the forkhead domain, the most common in primary tumors being I176M/V, D226G/N and S250F (cbioportal.org, Breast invasive carcinoma, TCGA Firehose Legacy dataset). Mutations are enriched in metastatic tumors and correlate with resistance to aromatase inhibitors. Mutations in the Wing 2 region increase cooperativity with ER while mutation SY242CS, located in the third beta strand, leads to altered DNA binding patterns [184]. In addition, a G>A mutational hotspot was discovered in ER+ breast tumors at position -81 relative to the FOXA1 TSS, resulting in increased binding of E2F1 and FOXA1 overexpression [185]. Thus, enhanced expression or activity of FOXA1 could in turn result in increased ERα levels via direct transcriptional regulation of ESR1 expression, in addition to the role of FOXA1 in cooperating with ERα for transcriptional regulation.
FOXA2 and FOXA3 are the closest homologs of FOXA1 in the FOX family. RNA levels of both factors are much lower than those of FOXA1 in breast cancer, and neither are positively correlated with ESR1 expression. FOXO family members are also expressed in breast tumors, without a strong bias for luminal tumors. Activity of these factors is under post-translational regulation. For instance, FOXO protein expression is downregulated by the Akt pathway [186]. FOXO3 was reported to positively regulate ESR1 expression, the expression of a dominant negative form repressing expression of ESR1 in MCF-7 cells [187], and binding sites were identified in promoters A and B. However, FOXO3 was also reported to suppress the transcriptional activation properties of ERα via protein–protein interactions and to suppress growth of ER+ breast tumor cells [188]. In addition, FOXO3 mediates the cytostatic and cytotoxic properties of breast cancer therapeutics, such as anthracyclins and taxanes, and is down-regulated in MCF-7 cells resistant to epirubicin and paclitaxel [189].
A number of other FOX family members have been reported to regulate ERα function and/or expression. For instance, FOXM1 drives breast cancer cell proliferation under transcriptional control by ERα. It also activates ESR1 transcription via binding to several sites upstream of promoters A, B and C, possibly in interaction with FOXO3, which bound the same sites and interacted with FOXM1 in co-immunoprecipitation experiments [190]. One site identified in promoter A overlaps with a ChIP peak for FOXA1 [137]. Despite these observations, FOXM1 is highly expressed in triple negative tumors [191], and in mice inhibits luminal differentiation via inhibition of GATA3 expression [192].
FOXC1 and FOXC2 expression patterns are likewise elevated mostly in basal-like tumors [193,194]. FOXC1 repressed ESR1 expression when overexpressed in three ER+ BC cell lines. ChIP qPCR and pulldown with biotinylated antibodies indicated binding of GATA3 and FOXC1 to overlapping motifs in promoter C and enhancer 4. FOXC1-mediated suppression of ESR1 was accompanied by exclusion of GATA3 binding, increased H3K9me3, and reduced KDM4B and RNApolII interaction at these sites and upstream of promoter A [120].
Together, these studies suggest a positive role for FOXA1 in ESR1 up-regulation in ER+ breast cancer cells, with possible cross-talk with several FOX factors.
4.3. Estrogen Receptor Alpha
Published ChIP-Seq/ChIP-Exo datasets [19,135,136,137,195] show that ERα binds ESR1 enhancers 1 to 4; in addition, EREs were detected in the center of the ChIP peaks corresponding to enhancers 1 and 4 (Figure 5). Previous studies reported half-ERE motifs in promoters A [116] and F [114] (Figure 4), although these motifs do not correlate with strong recruitment of ERα at those sites in the ChIP-Exo study. The presence of ERα at distal enhancers is in agreement with reports that ERα is primarily recruited at enhancers rather than promoters of estrogen-modulated genes in breast cancer cell lines [19,135].
Consistent with its recruitment on ESR1 regulatory regions, ERα exerts feedback regulation on its own expression in a cell context-dependent manner [116,158]. Indeed, ESR1 mRNA levels are decreased by 17-β-estradiol in MCF-7 and induced in T-47D [196]. The basis for this differential regulation, as well as the reasons dictating the wide variations in ESR1 expression levels in BC cell lines and tumors, remain unclear. Of note, exogenous expression of ERβ in MCF-7 cells identified genomic targets that for the vast majority overlapped with ERα binding regions [197]. This likely results from the capacity of both receptors to recognize the same binding sites and to heterodimerize; however, divergence in their transactivation regions often result in different effects on transcription, heterodimers having intermediate profiles [198,199]. Whether ERβ can bind ESR1 regulatory regions and cross-regulate its expression in normal tissue, in which it is expressed to higher levels than in breast tumors, remains to be clarified.
Estradiol treatment also induces ERα turnover in tumor cells, leading to an attenuation of ER signaling [196,200]. The existence of negative feedback mechanisms on ERα levels in the presence of agonists in normal tissue [201] may contribute to the transient nature of the proliferative response to estrogens during the hormonal cycle and may explain in part the lack of correlation between ERα and proliferation markers such as KI67 in the normal human mammary gland (see above). In tumors or in normal tissue of high-risk women [201], bypass of these control mechanisms and ERα overexpression may drive mammary cell proliferation.
5. Conclusions
The identification of long-range regulatory elements in ESR1 by ChIP-Seq analysis together with the mapping of alternative promoters outlines a complex mode of ESR1 regulation. Transcription factors bind these regulatory elements directly via the recognition of cognate DNA target motifs but may also interact indirectly with other regions via long-range chromatin loops, through direct or co-factor-mediated interactions with other transcription factors. Luminal factors FOXA1 and GATA3 contribute to the positive regulation of the ESR1 gene, with ERα and possibly ERβ exerting context-dependent feedback regulation. CpG sites methylated in a cell-specific manner are found across the entire ESR1 gene and likely modulate expression of alternative promoters. Epigenetic modulation via molecules inhibiting histone/DNA methyltransferases or histone deacetylases suggests that ESR1 expression is controlled in a cell-specific manner by chromatin-modifying/remodeling enzymes potentially recruited to the ESR1 gene by specific transcription factors [202]. Luminal transcription factors described above likely play an important role in recruiting coactivators such as histone acetyl transferases, and chromatin remodelers such as the SWI-SNF complex. Repressive complexes may be recruited by negative regulators such as FOXC1 or other repressors, including the transcription factor ELF5, shown to repress ESR1 and a set of ERα-associated genes with promotion of basal-like breast cancer characteristics [203]. Mesenchymal transcription factors have also been observed to repress ESR1 expression [204,205]. Other regulators not discussed in this review include miRNAs; several are capable of targeting the ESR1 3′UTR and some are negatively correlated with ESR1 in breast cancer [206].
In spite of the accumulated knowledge about regulators of ESR1 transcription, our understanding of the mechanisms that control ERα expression in normal tissue and how they are altered during breast tumorigenesis requires further investigation. A better dissection of the hierarchical nature of enhancers controlling the ESR1 gene is required to identify which factors are key to enhancing or silencing ESR1 expression. Several epigenetic regulators have been proposed to induce ESR1 expression in triple negative tumors, but whether this will result in therapeutic approaches based on subtype conversion remains unclear. Finally, it is crucially important to better understand reasons for the variable expression patterns of ERα in tumors, the nature of ER-negative cells in heterogeneous ER-positive tumors, the impact of hormonal therapies on these tumors and the mechanisms of the apparent conversion of some ER-positive to ER-negative tumors during cancer progression. The recent availability of single cell and spatially resolved genomic analyses should greatly facilitate the study of intra-tumoral heterogeneity in ERα expression in the near future.
Acknowledgments
The authors apologize for not being able to cite all relevant articles due to space limitations and are grateful to John White for manuscript reading and comments.
Author Contributions
Writing—original draft preparation, L.P. and H.I.; diagrams—creation and design, L.P.; writing—review and editing, supervision, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Canadian Institutes of Health Research, grant number PJT153178.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P., Ruddy K., Tsang J., Cardoso F. Breast cancer. Nat. Rev. Dis. Prim. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 2.Onitilo A.A., Engel J.M., Greenlee R.T., Mukesh B.N. Breast Cancer Subtypes Based on ER/PR and Her2 Expression: Comparison of Clinicopathologic Features and Survival. Clin. Med. Res. 2009;7:4–13. doi: 10.3121/cmr.2008.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stierer M., Rosen H., Weber R., Hanak H., Spona J., Tüchler H. Immunohistochemical and Biochemical Measurement of Estrogen and Progesterone Receptors in Primary Breast Cancer Correlation of Histopathology and Prognostic Factors. Ann. Surg. 1993;218:13–21. doi: 10.1097/00000658-199307000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traboulsi T., El Ezzy M., Gleason J., Mader S. Antiestrogens: Structure-activity relationships and use in breast cancer treatment. J. Mol. Endocrinol. 2017;58:R15–R31. doi: 10.1530/JME-16-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caciolla J., Bisi A., Belluti F., Rampa A., Gobbi S. Reconsidering Aromatase for Breast Cancer Treatment: New Roles for an Old Target. Molecules. 2020;25:5351. doi: 10.3390/molecules25225351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston S.R., Saccani-Jotti G., Smith I.E., Salter J., Newby J., Coppen M., Ebbs S.R., Dowsett M. Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Cancer Res. 1995;55:3331–3338. [PubMed] [Google Scholar]
- 7.Lykkesfeldt A.E. Mechanisms of Tamoxifen Resistance in the Treatment of Advanced Breast Cancer. Acta Oncol. 1996;35((Suppl. 5)):9–14. doi: 10.3109/02841869609083961. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez M.C., Detre S., Johnston S., Mohsin S.K., Shou J., Allred D.C., Schiff R., Osborne C.K., Dowsett M. Molecular Changes in Tamoxifen-Resistant Breast Cancer: Relationship Between Estrogen Receptor, HER-2, and p38 Mitogen-Activated Protein Kinase. J. Clin. Oncol. 2005;23:2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 9.Ali S., Coombes R.C. Estrogen Receptor Alpha in Human Breast Cancer: Occurrence and Significance. J. Mammary Gland. Biol. Neoplasia. 2000;5:271–281. doi: 10.1023/A:1009594727358. [DOI] [PubMed] [Google Scholar]
- 10.Sukocheva O.A., Lukina E., Friedemann M., Menschikowski M., Hagelgans A., Aliev G. The crucial role of epigenetic regulation in breast cancer anti-estrogen resistance: Current findings and future perspectives. Semin. Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Jeffreys S.A., Powter B., Balakrishnar B., Mok K., Soon P., Franken A., Neubauer H., de Souza P., Becker T.M. Endocrine Resistance in Breast Cancer: The Role of Estrogen Receptor Stability. Cells. 2020;9:2077. doi: 10.3390/cells9092077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ascenzi P., Bocedi A., Marino M. Structure–function relationship of estrogen receptor α and β: Impact on human health. Mol. Asp. Med. 2006;27:299–402. doi: 10.1016/j.mam.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton K.J., Hewitt S.C., Arao Y., Korach K.S. Estrogen Hormone Biology. Curr. Top. Dev. Biol. 2017;125:109–146. doi: 10.1016/bs.ctdb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans R.M., Mangelsdorf D.J. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014;157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosselman S., Polman J., Dijkema R. ERβ: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-X. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez R., Nguyen D., Rocha W., White J.H., Mader S. Diversity in the mechanisms of gene regulation by estrogen receptors. BioEssays. 2002;24:244–254. doi: 10.1002/bies.10066. [DOI] [PubMed] [Google Scholar]
- 17.Klinge C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotnoir-White D., Laperrière D., Mader S. Evolution of the repertoire of nuclear receptor binding sites in genomes. Mol. Cell. Endocrinol. 2011;334:76–82. doi: 10.1016/j.mce.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Carroll J.S., Liu X.S., Brodsky A.S., Li W., Meyer C.A., Szary A.J., Eeckhoute J., Shao W., Hestermann E.V., Geistlinger T.R., et al. Chromosome-Wide Mapping of Estrogen Receptor Binding Reveals Long-Range Regulation Requiring the Forkhead Protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Deschênes J., Bourdeau V., White J.H., Mader S. Regulation of GREB1 Transcription by Estrogen Receptor α through a Multipartite Enhancer Spread Over 20 kb of Upstream Flanking Sequences. J. Biol. Chem. 2007;282:17335–17339. doi: 10.1074/jbc.C700030200. [DOI] [PubMed] [Google Scholar]
- 21.Barnett D.H., Sheng S., Charn T.H., Waheed A., Sly W.S., Lin C.-Y., Liu E.T., Katzenellenbogen B.S. Estrogen Receptor Regulation of Carbonic Anhydrase XII through a Distal Enhancer in Breast Cancer. Cancer Res. 2008;68:3505–3515. doi: 10.1158/0008-5472.CAN-07-6151. [DOI] [PubMed] [Google Scholar]
- 22.Pan Y.F., Wansa K.D.S.A., Liu M.H., Zhao B., Hong S.Z., Tan P.Y., Lim K.S., Bourque G., Liu E.T., Cheung E. Regulation of Estrogen Receptor-mediated Long Range Transcription via Evolutionarily Conserved Distal Response Elements. J. Biol. Chem. 2008;283:32977–32988. doi: 10.1074/jbc.M802024200. [DOI] [PubMed] [Google Scholar]
- 23.Charn T.H., Liu E.T.-B., Chang E.C., Lee Y.K., Katzenellenbogen J.A., Katzenellenbogen B.S. Genome-Wide Dynamics of Chromatin Binding of Estrogen Receptors α and β: Mutual Restriction and Competitive Site Selection. Mol. Endocrinol. 2010;24:47–59. doi: 10.1210/me.2009-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vega V.B., Lin C.-Y., Lai K.S., Kong S.L., Xie M., Su X., Teh H.F., Thomsen J.S., Yeo A.L., Sung W.K., et al. Multiplatform genome-wide identification and modeling of functional human estrogen receptor binding sites. Genome Biol. 2006;7:R82. doi: 10.1186/gb-2006-7-9-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph R., Orlov Y., Huss M., Sun W., Kong S.L., Ukil L., Pan Y.F., Li G., Lim M., Thomsen J.S., et al. Integrative model of genomic factors for determining binding site selection by estrogen receptor-α. Mol. Syst. Biol. 2010;6:456. doi: 10.1038/msb.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lupien M., Brown M. Cistromics of hormone-dependent cancer. Endocr.-Relat. Cancer. 2009;16:381–389. doi: 10.1677/ERC-09-0038. [DOI] [PubMed] [Google Scholar]
- 27.Bojcsuk D., Nagy G., Balint B.L. Inducible super-enhancers are organized based on canonical signal-specific transcription factor binding elements. Nucleic Acids Res. 2016;45:3693–3706. doi: 10.1093/nar/gkw1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siersbaek R., Nielsen R., John S., Sung M.-H., Baek S., Loft A., Hager G.L., Mandrup S. Extensive chromatin remodelling and establishment of transcription factor ‘hotspots’ during early adipogenesis. EMBO J. 2011;30:1459–1472. doi: 10.1038/emboj.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siersbæk R.D., Baek S., Rabiee A., Nielsen R., Traynor S., Clark N., Sandelin A., Jensen O.N., Sung M.-H., Hager G.L., et al. Molecular Architecture of Transcription Factor Hotspots in Early Adipogenesis. Cell Rep. 2014;7:1434–1442. doi: 10.1016/j.celrep.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z., Merkurjev D., Yang F., Li W., Oh S., Friedman M.J., Song X., Zhang F., Ma Q., Ohgi K.A., et al. Enhancer Activation Requires trans-Recruitment of a Mega Transcription Factor Complex. Cell. 2014;159:358–373. doi: 10.1016/j.cell.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao S.S., Huang S.-C., Hilaire B.G.S., Engreitz J.M., Perez E., Kieffer-Kwon K.-R., Sanborn A.L., Johnstone S.E., Bascom G.D., Bochkov I., et al. Cohesin Loss Eliminates All Loop Domains. Cell. 2017;171:305–320. doi: 10.1016/j.cell.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang J., Li K., Cai W., Liu X., Zhang Y., Orkin S.H., Xu J., Yuan G.-C. Dissecting super-enhancer hierarchy based on chromatin interactions. Nat. Commun. 2018;9:943. doi: 10.1038/s41467-018-03279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sepehr E., Lebl-Rinnova M., Mann M.K., Pisani S.L., Churchwell M.I., Korol D.L., Katzenellenbogen J.A., Doerge D.R. Pharmacokinetics of the estrogen receptor subtype-selective ligands, PPT and DPN: Quantification using UPLC-ES/MS/MS. J. Pharm. Biomed. Anal. 2012;71:119–126. doi: 10.1016/j.jpba.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnal J.F., Lenfant F., Metivier R., Flouriot G., Henrion D., Adlanmerini M., Fontaine C., Gourdy P., Chambon P., Katzenellenbogen B., et al. Membrane and Nuclear Estrogen Receptor Alpha Actions: From Tissue Specificity to Medical Implications. Physiol. Rev. 2017;97:1045–1087. doi: 10.1152/physrev.00024.2016. [DOI] [PubMed] [Google Scholar]
- 35.White J.H., Fernandes I., Mader S., Yang X.-J. Corepressor Recruitment by Agonist-Bound Nuclear Receptors. Vitam. Horm. 2004;68:123–143. doi: 10.1016/s0083-6729(04)68004-6. [DOI] [PubMed] [Google Scholar]
- 36.Hall J.M., McDonnell D.P. Coregulators in Nuclear Estrogen Receptor Action: From Concept to Therapeutic Targeting. Mol. Interv. 2005;5:343–357. doi: 10.1124/mi.5.6.7. [DOI] [PubMed] [Google Scholar]
- 37.Manavathi B., Samanthapudi V.S.K., Gajulapalli V.N.R. Estrogen receptor coregulators and pioneer factors: The orchestrators of mammary gland cell fate and development. Front. Cell Dev. Biol. 2014;2:34. doi: 10.3389/fcell.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi P., Wang Z., Feng Q., Pintilie G.D., Foulds C., Lanz R.B., Ludtke S., Schmid M.F., Chiu W., O’Malley B.W. Structure of a Biologically Active Estrogen Receptor-Coactivator Complex on DNA. Mol. Cell. 2015;57:1047–1058. doi: 10.1016/j.molcel.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hewitt S., Korach K.S. Estrogen Receptors: New Directions in the New Millennium. Endocr. Rev. 2018;39:664–675. doi: 10.1210/er.2018-00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lannigan D.A. Estrogen receptor phosphorylation. Steroids. 2003;68:1–9. doi: 10.1016/S0039-128X(02)00110-1. [DOI] [PubMed] [Google Scholar]
- 41.Le Romancer M., Poulard C., Cohen P., Sentis S., Renoir J.-M., Corbo L. Cracking the Estrogen Receptor’s Posttranslational Code in Breast Tumors. Endocr. Rev. 2011;32:597–622. doi: 10.1210/er.2010-0016. [DOI] [PubMed] [Google Scholar]
- 42.Wijayaratne A.L., McDonnell D.P. The Human Estrogen Receptor-α Is a Ubiquitinated Protein Whose Stability Is Affected Differentially by Agonists, Antagonists, and Selective Estrogen Receptor Modulators. J. Biol. Chem. 2001;276:35684–35692. doi: 10.1074/jbc.M101097200. [DOI] [PubMed] [Google Scholar]
- 43.Levin E.R. Integration of the Extranuclear and Nuclear Actions of Estrogen. Mol. Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng B., Lu B., Leygue E., Murphy L.C. Putative functional characteristics of human estrogen receptor-beta isoforms. J. Mol. Endocrinol. 2003;30:13–29. doi: 10.1677/jme.0.0300013. [DOI] [PubMed] [Google Scholar]
- 45.Walter P., Green S., Greene G., Krust A., Bornert J.M., Jeltsch J.M., Staub A., Jensen E., Scrace G., Waterfield M. Cloning of the human estrogen receptor cDNA. Proc. Natl. Acad. Sci. USA. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green S., Walter P., Greene G., Krust A., Goffin C., Jensen E., Scrace G., Waterfield M., Chambon P. Cloning of the human oestrogen receptor cDNA. J. Steroid Biochem. 1986;24:77–83. doi: 10.1016/0022-4731(86)90035-X. [DOI] [PubMed] [Google Scholar]
- 47.Greene G.L., Gilna P., Waterfield M., Baker A., Hort Y., Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- 48.Ponglikitmongkol M., Green S., Chambon P. Genomic organization of the human oestrogen receptor gene. EMBO J. 1988;7:3385–3388. doi: 10.1002/j.1460-2075.1988.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koš M., Reid G., Denger S., Gannon F. Minireview: Genomic Organization of the Human ERα Gene Promoter Region. Mol. Endocrinol. 2001;15:2057–2063. doi: 10.1210/mend.15.12.0731. [DOI] [PubMed] [Google Scholar]
- 50.Hennighausen L., Robinson G.W. Information networks in the mammary gland. Nat. Rev. Mol. Cell Biol. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- 51.Paine I.S., Lewis M.T. The Terminal End Bud: The Little Engine that Could. J. Mammary Gland. Biol. Neoplasia. 2017;22:93–108. doi: 10.1007/s10911-017-9372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson E., Clarke R.B., Howell A. Estrogen responsiveness and control of normal human breast proliferation. J. Mammary Gland. Biol. Neoplasia. 1998;3:23–35. doi: 10.1023/A:1018718117113. [DOI] [PubMed] [Google Scholar]
- 53.Petersen O.W., E Høyer P., van Deurs B. Frequency and distribution of estrogen receptor-positive cells in normal, nonlactating human breast tissue. Cancer Res. 1987;47:5748–5751. [PubMed] [Google Scholar]
- 54.Macias H., Hinck L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012;1:533–557. doi: 10.1002/wdev.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bondesson M., Hao R., Lin C.-Y., Williams C., Gustafsson J.- Åke Estrogen receptor signaling during vertebrate development. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015;1849:142–151. doi: 10.1016/j.bbagrm.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petersen O.W., Polyak K. Stem Cells in the Human Breast. Cold Spring Harb. Perspect. Biol. 2010;2:a003160. doi: 10.1101/cshperspect.a003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silberstein G.B., van Horn K., Shyamala G., Daniel C.W. Essential role of endogenous estrogen in directly stimulating mammary growth demonstrated by implants containing pure antiestrogens. Endocrinology. 1994;134:84–90. doi: 10.1210/endo.134.1.8275973. [DOI] [PubMed] [Google Scholar]
- 58.Daniel C.W., Silberstein G.B., Strickland P. Direct action of 17 beta-estradiol on mouse mammary ducts analyzed by sustained release implants and steroid autoradiography. Cancer Res. 1987;47:6052–6057. [PubMed] [Google Scholar]
- 59.Korach K. Insights from the study of animals lacking functional estrogen receptor. Science. 1994;266:1524–1527. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- 60.Hewitt S., Winuthayanon W., Korach K.S. What’s new in estrogen receptor action in the female reproductive tract. J. Mol. Endocrinol. 2016;56:R55–R71. doi: 10.1530/JME-15-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atalay C., Kanliöz M., Altinok M. Menstrual cycle and hormone receptor status in breast cancer patients. Neoplasma. 2002;49:278–284. [PubMed] [Google Scholar]
- 62.Bartow S.A. Use of the autopsy to study ontogeny and expression of the estrogen receptor gene in human breast. J. Mammary Gland. Biol. Neoplasia. 1998;3:37–48. doi: 10.1023/A:1026641401184. [DOI] [PubMed] [Google Scholar]
- 63.Söderqvist G., von Schoultz B., Tani E., Skoog L. Estrogen and progesterone receptor content in breast epithelial cells from healthy women during the menstrual cycle. Am. J. Obstet. Gynecol. 1993;168:874–879. doi: 10.1016/S0002-9378(12)90837-6. [DOI] [PubMed] [Google Scholar]
- 64.Clarke R., Howell A., Potten C.S., Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- 65.Mallepell S., Krust A., Chambon P., Brisken C. Paracrine signaling through the epithelial estrogen receptor α is required for proliferation and morphogenesis in the mammary gland. Proc. Natl. Acad. Sci. USA. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng G., Weihua Z., Warner M., Gustafsson J.-A. Estrogen receptors ER and ER in proliferation in the rodent mammary gland. Proc. Natl. Acad. Sci. USA. 2004;101:3739–3746. doi: 10.1073/pnas.0307864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng G., Li Y., Omoto Y., Wang Y., Berg T., Nord M., Vihko P., Warner M., Piao Y.-S., Gustafsson J.-Å. Differential Regulation of Estrogen Receptor (ER)α and ERβ in Primate Mammary Gland. J. Clin. Endocrinol. Metab. 2005;90:435–444. doi: 10.1210/jc.2004-0861. [DOI] [PubMed] [Google Scholar]
- 68.Tan H., Zhong Y., Pan Z. Autocrine regulation of cell proliferation by estrogen receptor-alpha in estrogen receptor-alpha-positive breast cancer cell lines. BMC Cancer. 2009;9:31. doi: 10.1186/1471-2407-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stender J.D., Frasor J., Komm B., Chang K.C.N., Kraus W.L., Katzenellenbogen B.S. Estrogen-Regulated Gene Networks in Human Breast Cancer Cells: Involvement of E2F1 in the Regulation of Cell Proliferation. Mol. Endocrinol. 2007;21:2112–2123. doi: 10.1210/me.2006-0474. [DOI] [PubMed] [Google Scholar]
- 70.Bourdeau V., Deschênes J., Laperrière D., Aid M., White J., Mader S. Mechanisms of primary and secondary estrogen target gene regulation in breast cancer cells. Nucleic Acids Res. 2007;36:76–93. doi: 10.1093/nar/gkm945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Millour J., Constantinidou D., Stavropoulou A.V., Wilson M., Myatt S.S., Kwok J.M.-M., Sivanandan K., Coombes R.C., Medema R., Hartman J., et al. FOXM1 is a transcriptional target of ERα and has a critical role in breast cancer endocrine sensitivity and resistance. Oncogene. 2010;29:2983–2995. doi: 10.1038/onc.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.JavanMoghadam S., Weihua Z., Hunt K.K., Keyomarsi K. Estrogen receptor alpha is cell cycle-regulated and regulates the cell cycle in a ligand-dependent fashion. Cell Cycle. 2016;15:1579–1590. doi: 10.1080/15384101.2016.1166327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fabian C.J., Kimler B.F., Zalles C.M., Khan Q.J., Mayo M.S., Phillips T.A., Simonsen M., Metheny T., Petroff B.K. Reduction in proliferation with six months of letrozole in women on hormone replacement therapy. Breast Cancer Res. Treat. 2007;106:75–84. doi: 10.1007/s10549-006-9476-5. [DOI] [PubMed] [Google Scholar]
- 74.Allison K.H., Hammond M.E.H., Dowsett M., McKernin S.E., Carey L.A., Fitzgibbons P.L., Hayes D.F., Lakhani S.R., Chavez-MacGregor M., Perlmutter J., et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 75.Giri D.D., Dundas S.A.C., Nottingham J.F., Underwood J.C.E. Oestrogen receptors in benign epithelial lesions and intraduct carcinomas of the breast: An immunohistological study. Histopathology. 1989;15:575–584. doi: 10.1111/j.1365-2559.1989.tb01623.x. [DOI] [PubMed] [Google Scholar]
- 76.Frech M.S., Halama E.D., Tilli M.T., Singh B., Gunther E.J., Chodosh L.A., Flaws J.A., Furth P.A. Deregulated estrogen receptor alpha expression in mammary epithelial cells of transgenic mice results in the development of ductal carcinoma in situ. Cancer Res. 2005;65:681–685. [PMC free article] [PubMed] [Google Scholar]
- 77.Dabydeen S.A., Furth P.A. Genetically engineered ERα-positive breast cancer mouse models. Endocr.-Relat. Cancer. 2014;21:R195–R208. doi: 10.1530/ERC-13-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holst F., Stahl P.R., Ruiz C., Hellwinkel O., Jehan Z., Wendland M., Lebeau A., Terracciano L., Al-Kuraya K., Jänicke F., et al. Estrogen receptor alpha (ESR1) gene amplification is frequent in breast cancer. Nat. Genet. 2007;39:655–660. doi: 10.1038/ng2006. [DOI] [PubMed] [Google Scholar]
- 79.Brown L., Hoog J., Chin S.-F., Tao Y., Zayed A.A., Chin K., E Teschendorff A., Quackenbush J.F., Marioni J.C., Leung S., et al. ESR1 gene amplification in breast cancer: A common phenomenon? Nat. Genet. 2008;40:806–807. doi: 10.1038/ng0708-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeselsohn R., Buchwalter G., de Angelis C., Brown M., Schiff R. ESR1 mutations—A mechanism for acquired endocrine resistance in breast cancer. Nat. Rev. Clin. Oncol. 2015;12:573–583. doi: 10.1038/nrclinonc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toy W., Weir H., Razavi P., Lawson M., Goeppert A.U., Mazzola A.M., Smith A., Wilson J., Morrow C., Wong W.L., et al. Activating ESR1 Mutations Differentially Affect the Efficacy of ER Antagonists. Cancer Discov. 2017;7:277–287. doi: 10.1158/2159-8290.CD-15-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fanning S.W., Jeselsohn R., Dharmarajan V., Mayne C.G., Karimi M., Buchwalter G., Houtman R., Toy W., E Fowler C., Han R., et al. The SERM/SERD bazedoxifene disrupts ESR1 helix 12 to overcome acquired hormone resistance in breast cancer cells. eLife. 2018;7 doi: 10.7554/eLife.37161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guan J., Zhou W., Hafner M., Blake R.A., Chalouni C., Chen I., de Bruyn T., Giltnane J.M., Hartman S., Heidersbach A., et al. Therapeutic Ligands Antagonize Estrogen Receptor Function by Impairing Its Mobility. Cell. 2019;178:949–963. doi: 10.1016/j.cell.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 84.Lei J.T., Gou X., Seker S., Ellis M.J. ESR1 alterations and metastasis in estrogen receptor positive breast cancer. J. Cancer Metastasis Treat. 2019;5:38. doi: 10.20517/2394-4722.2019.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sørlie T., Tibshirani R., Parker J., Hastie T., Marron J.S., Nobel A., Deng S., Johnsen H., Pesich R., Geisler S., et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu Y., Wang A., Liu M.C., Zwart A., Lee R.Y., Gallagher A., Wang Y., Miller W.R., Dixon J.M., Clarke R. Estrogen receptor alpha positive breast tumors and breast cancer cell lines share similarities in their transcriptome data structures. Int. J. Oncol. 2006;29:1581–1589. doi: 10.3892/ijo.29.6.1581. [DOI] [PubMed] [Google Scholar]
- 87.Lapidus R.G., Nass S.J., A Butash K., Parl F.F., A Weitzman S., Graff J.G., Herman J.G., Davidson N.E. Mapping of ER gene CpG island methylation-specific polymerase chain reaction. Cancer Res. 1998;58:2515–2519. [PubMed] [Google Scholar]
- 88.Ferguson A.T., Lapidus R.G., Baylin S.B., E Davidson N. Demethylation of the estrogen receptor gene in estrogen receptor-negative breast cancer cells can reactivate estrogen receptor gene expression. Cancer Res. 1995;55:2279–2283. [PubMed] [Google Scholar]
- 89.Yang X., Phillips D.L., Ferguson A.T., Nelson W.G., Herman J.G., Davidson N.E. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001;61:7025–7029. [PubMed] [Google Scholar]
- 90.Fan J., Yin W.-J., Lu J.-S., Wang L., Wu J., Wu F.-Y., Di G.-H., Shen Z.-Z., Shao Z.-M. ERα negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J. Cancer Res. Clin. Oncol. 2008;134:883–890. doi: 10.1007/s00432-008-0354-x. [DOI] [PubMed] [Google Scholar]
- 91.Billam M., Witt A.E., Davidson N.E. The silent estrogen receptor—Can we make it speak? Cancer Biol. Ther. 2009;8:485–496. doi: 10.4161/cbt.8.6.7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Cremoux P., Dalvai M., N’Doye O., Moutahir F., Rolland G., Chouchane-Mlik O., Assayag F., Lehmann-Che J., Kraus-Berthie L., Nicolas A., et al. HDAC inhibition does not induce estrogen receptor in human triple-negative breast cancer cell lines and patient-derived xenografts. Breast Cancer Res. Treat. 2015;149:81–89. doi: 10.1007/s10549-014-3233-y. [DOI] [PubMed] [Google Scholar]
- 93.Alao J.P., Lam E., Ali S., Buluwela L., Bordogna W., Lockey P., Varshochi R., Stavropoulou A.V., Coombes R.C., Vigushin D.M. Histone Deacetylase Inhibitor Trichostatin A Represses Estrogen Receptor α-Dependent Transcription and Promotes Proteasomal Degradation of Cyclin D1 in Human Breast Carcinoma Cell Lines. Clin. Cancer Res. 2004;10:8094–8104. doi: 10.1158/1078-0432.CCR-04-1023. [DOI] [PubMed] [Google Scholar]
- 94.Reid G., Métivier R., Lin C.-Y., Denger S., Ibberson D., Ivacevic T., Brand H., Benes V., Liu E.T., Gannon F. Multiple mechanisms induce transcriptional silencing of a subset of genes, including oestrogen receptor α, in response to deacetylase inhibition by valproic acid and trichostatin A. Oncogene. 2005;24:4894–4907. doi: 10.1038/sj.onc.1208662. [DOI] [PubMed] [Google Scholar]
- 95.Margueron R., Duong V., Bonnet S., Escande A., Vignon F., Balaguer P., Cavailles V. Histone deacetylase inhibition and estrogen receptor alpha levels modulate the transcriptional activity of partial antiestrogens. J. Mol. Endocrinol. 2004;32:583–594. doi: 10.1677/jme.0.0320583. [DOI] [PubMed] [Google Scholar]
- 96.Rocha W., Sanchez R., Deschênes J., Auger A., Hébert E., White J.H., Mader S. Opposite Effects of Histone Deacetylase Inhibitors on Glucocorticoid and Estrogen Signaling in Human Endometrial Ishikawa Cells. Mol. Pharmacol. 2005;68:1852–1862. doi: 10.1124/mol.105.014514. [DOI] [PubMed] [Google Scholar]
- 97.Yomtoubian S., Lee S.B., Verma A., Izzo F., Markowitz G., Choi H., Cerchietti L., Vahdat L., Brown K.A., Andreopoulou E., et al. Inhibition of EZH2 Catalytic Activity Selectively Targets a Metastatic Subpopulation in Triple-Negative Breast Cancer. Cell Rep. 2020;30:755–770. doi: 10.1016/j.celrep.2019.12.056. [DOI] [PubMed] [Google Scholar]
- 98.Bailey S.D., Desai K., Kron K.J., Mazrooei P., Sinnott-Armstrong N.A., Treloar A.E., Dowar M., Thu K.L., Cescon D.W., Silvester J., et al. Noncoding somatic and inherited single-nucleotide variants converge to promote ESR1 expression in breast cancer. Nat. Genet. 2016;48:1260–1266. doi: 10.1038/ng.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lemieux S., Sargeant T., Laperrière D., Ismail H., Boucher G., Rozendaal M., Lavallée V.-P., Ashton-Beaucage D., Wilhelm B., Hébert J., et al. MiSTIC, an integrated platform for the analysis of heterogeneity in large tumour transcriptome datasets. Nucleic Acids Res. 2017;45:e122. doi: 10.1093/nar/gkx338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keaveney M., Klug J., Dawson M., Nestor P., Neilan J., Forde R., Gannon F. EVIDENCE FOR A PREVIOUSLY UNIDENTIFIED UPSTREAM EXON IN THE HUMAN OESTROGEN RECEPTOR GENE. J. Mol. Endocrinol. 1991;6:111–115. doi: 10.1677/jme.0.0060111. [DOI] [PubMed] [Google Scholar]
- 101.Green S., Walter P., Kumar V., Krust A., Bornert J.-M., Argos P., Chambon P. Human oestrogen receptor cDNA: Sequence, expression and homology to v-erb-A. Nat. Cell Biol. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 102.Flouriot G., Brand H., Denger S., Métivier R., Koš M., Reid G., Sonntag-Buck V., Gannon F. Identification of a new isoform of the human estrogen receptor-alpha (hER-α) that is encoded by distinct transcripts and that is able to repress hER-α activation function 1. EMBO J. 2000;19:4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hattori Y., Ishii H., Morita A., Sakuma Y., Ozawa H. Characterization of the fundamental properties of the N-terminal truncation (Δ exon 1) variant of estrogen receptor α in the rat. Gene. 2015;571:117–125. doi: 10.1016/j.gene.2015.06.086. [DOI] [PubMed] [Google Scholar]
- 104.A Stevens T., Meech R. BARX2 and estrogen receptor-α (ESR1) coordinately regulate the production of alternatively spliced ESR1 isoforms and control breast cancer cell growth and invasion. Oncogene. 2006;25:5426–5435. doi: 10.1038/sj.onc.1209529. [DOI] [PubMed] [Google Scholar]
- 105.Ohe K., Miyajima S., Abe I., Tanaka T., Hamaguchi Y., Harada Y., Horita Y., Beppu Y., Ito F., Yamasaki T., et al. HMGA1a induces alternative splicing of estrogen receptor alpha in MCF-7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2018;182:21–26. doi: 10.1016/j.jsbmb.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 106.Penot G., Le Péron C., Mérot Y., Grimaud-Fanouillére E., Ferriere F., Boujrad N., Kah O., Saligaut C., Ducouret B., Métivier R., et al. The Human Estrogen Receptor-α Isoform hERα46 Antagonizes the Proliferative Influence of hERα66 in MCF7 Breast Cancer Cells. Endocrinology. 2005;146:5474–5484. doi: 10.1210/en.2005-0866. [DOI] [PubMed] [Google Scholar]
- 107.Wang Z., Zhang X., Shen P., Loggie B.W., Chang Y., Deuel T.F. Identification, cloning, and expression of human estrogen receptor-α36, a novel variant of human estrogen receptor-α66. Biochem. Biophys. Res. Commun. 2005;336:1023–1027. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 108.Omarjee S., Jacquemetton J., Poulard C., Rochel N., Dejaegere A., Chebaro Y., Treilleux I., Marangoni E., Corbo L., Le Romancer M. The molecular mechanisms underlying the ERα-36-mediated signaling in breast cancer. Oncogene. 2016;36:2503–2514. doi: 10.1038/onc.2016.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Q., Jiang J., Ying G., Xie X.-Q., Zhang X., Xu W., Zhang X., Song E., Bu H., Ping Y.-F., et al. Tamoxifen enhances stemness and promotes metastasis of ERα36+ breast cancer by upregulating ALDH1A1 in cancer cells. Cell Res. 2018;28:336–358. doi: 10.1038/cr.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pink J.J., Wu S.-Q., Wolf D.M., Bilimoria M.M., Jordan V.C. A Novel 80 kDa Human Estrogen Receptor Containing a Duplication of Exons 6 and 7. Nucleic Acids Res. 1996;24:962–969. doi: 10.1093/nar/24.5.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hartmaier R., Trabucco S., Priedigkeit N., Chung J., Parachoniak C., Borre P.V., Morley S., Rosenzweig M., Gay L., Goldberg M., et al. Recurrent hyperactive ESR1 fusion proteins in endocrine therapy-resistant breast cancer. Ann. Oncol. 2018;29:872–880. doi: 10.1093/annonc/mdy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grandien K., Berkenstam A., Gustafsson J.-Å. The estrogen receptor gene: Promoter organization and expression. Int. J. Biochem. Cell Biol. 1997;29:1343–1369. doi: 10.1016/S1357-2725(97)89967-0. [DOI] [PubMed] [Google Scholar]
- 113.Becherini L., Gennari L., Masi L., Mansani R., Massart F., Morelli A., Falchetti A., Gonnelli S., Fiorelli G., Tanini A., et al. Evidence of a linkage disequilibrium between polymorphisms in the human estrogen receptor alpha gene and their relationship to bone mass variation in postmenopausal Italian women. Hum. Mol. Genet. 2000;9:2043–2050. doi: 10.1093/hmg/9.13.2043. [DOI] [PubMed] [Google Scholar]
- 114.Flouriot G., Griffin C., Kenealy M., Sonntag-Buck V., Gannon F. Differentially Expressed Messenger RNA Isoforms of the Human Estrogen Receptor-α Gene Are Generated by Alternative Splicing and Promoter Usage. Mol. Endocrinol. 1998;12:1939–1954. doi: 10.1210/mend.12.12.0209. [DOI] [PubMed] [Google Scholar]
- 115.Tsuboi K., Nagatomo T., Gohno T., Higuchi T., Sasaki S., Fujiki N., Kurosumi M., Takei H., Yamaguchi Y., Niwa T., et al. Single CpG site methylation controls estrogen receptor gene transcription and correlates with hormone therapy resistance. J. Steroid Biochem. Mol. Biol. 2017;171:209–217. doi: 10.1016/j.jsbmb.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 116.Castles C., Oesterreich S., Hansen R., Fuqua S. Auto-regulation of the estrogen receptor promoter. J. Steroid Biochem. Mol. Biol. 1997;62:155–163. doi: 10.1016/S0960-0760(97)00023-X. [DOI] [PubMed] [Google Scholar]
- 117.Hosey A.M., Gorski J.J., Murray M.M., Quinn J.E., Chung W.Y., Stewart G.E., James C.R., Farragher S.M., Mulligan J.M., Scott A.N., et al. Molecular Basis for Estrogen Receptor Deficiency in BRCA1-Linked Breast Cancer. J. Natl. Cancer Inst. 2007;99:1683–1694. doi: 10.1093/jnci/djm207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shirley S.H., Rundhaug J.E., Tian J., Cullinan-Ammann N., Lambertz I., Conti C.J., Fuchs-Young R. Transcriptional Regulation of Estrogen Receptor-α by p53 in Human Breast Cancer Cells. Cancer Res. 2009;69:3405–3414. doi: 10.1158/0008-5472.CAN-08-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Degraffenried L.A., Hilsenbeck S.G., Fuqua S.A. Sp1 is essential for estrogen receptor α gene transcription. J. Steroid Biochem. Mol. Biol. 2002;82:7–18. doi: 10.1016/S0960-0760(02)00151-6. [DOI] [PubMed] [Google Scholar]
- 120.Yu-Rice Y., Jin Y., Han B., Qu Y., Johnson J., Watanabe T., Cheng L., Deng N., Tanaka H., Gao B., et al. FOXC1 is involved in ERα silencing by counteracting GATA3 binding and is implicated in endocrine resistance. Oncogene. 2016;35:5400–5411. doi: 10.1038/onc.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tanimoto K., Eguchi H., Yoshida T., Hajiro-Nakanishi K., Hayashi S.-I. Regulation of estrogen receptor gene mediated by promoter B responsible for its enhanced expression in human breast cancer. Nucleic Acids Res. 1999;27:903–909. doi: 10.1093/nar/27.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McPherson L.A., Baichwal V.R., Weigel R. Identification of ERF-1 as a member of the AP2 transcription factor family. Proc. Natl. Acad. Sci. USA. 1997;94:4342–4347. doi: 10.1073/pnas.94.9.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoshida T., Eguchi H., Nakachi K., Tanimoto K., Higashi Y., Suemasu K., Iino Y., Morishita Y., Hayashi S.-I. Distinct mechanisms of loss of estrogen receptor alpha gene expression in human breast cancer: Methylation of the gene and alteration of trans-acting factors. Carcinogenesis. 2000;21:2193–2201. doi: 10.1093/carcin/21.12.2193. [DOI] [PubMed] [Google Scholar]
- 124.Deconinck E.C., A McPherson L., Weigel R.J. Transcriptional regulation of estrogen receptor in breast carcinomas. Mol. Cell. Biol. 1995;15:2191–2196. doi: 10.1128/MCB.15.4.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tang Z., Treilleux I., Brown M. A transcriptional enhancer required for the differential expression of the human estrogen receptor in breast cancers. Mol. Cell. Biol. 1997;17:1274–1280. doi: 10.1128/MCB.17.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Friedrichs N., Steiner S., Buettner R., Knoepfle G. Immunohistochemical expression patterns of AP2α and AP2γ in the developing fetal human breast. Histopathology. 2007;51:814–823. doi: 10.1111/j.1365-2559.2007.02887.x. [DOI] [PubMed] [Google Scholar]
- 127.Cyr A.R., Kulak M.V., Park J.M., Bogachek M.V., Spanheimer P.M., Woodfield G.W., White-Baer L.S., O’Malley Y.Q., Sugg S.L., Olivier A.K., et al. TFAP2C governs the luminal epithelial phenotype in mammary development and carcinogenesis. Oncogene. 2014;34:436–444. doi: 10.1038/onc.2013.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Woodfield G.W., Horan A.D., Chen Y., Weigel R. TFAP2C Controls Hormone Response in Breast Cancer Cells through Multiple Pathways of Estrogen Signaling. Cancer Res. 2007;67:8439–8443. doi: 10.1158/0008-5472.CAN-07-2293. [DOI] [PubMed] [Google Scholar]
- 129.Bogachek M.V., Chen Y., Kulak M., Woodfield G.W., Cyr A.R., Park J.M., Spanheimer P.M., Li Y., Li T., Weigel R.J. Sumoylation Pathway Is Required to Maintain the Basal Breast Cancer Subtype. Cancer Cell. 2014;25:748–761. doi: 10.1016/j.ccr.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Angeloni S.V., Martin M.B., Garcia-Morales P., Castro-Galache M.D., A Ferragut J., Saceda M. Regulation of estrogen receptor-alpha expression by the tumor suppressor gene p53 in MCF-7 cells. J. Endocrinol. 2004;180:497–504. doi: 10.1677/joe.0.1800497. [DOI] [PubMed] [Google Scholar]
- 131.Denger S., Reid G., Brand H., Kos M., Gannon F. Tissue-specific expression of human ERα and ERβ in the male. Mol. Cell. Endocrinol. 2001;178:155–160. doi: 10.1016/S0303-7207(01)00417-8. [DOI] [PubMed] [Google Scholar]
- 132.Penolazzi L., Lambertini E., Giordano S., Sollazzo V., Traina G., del Senno L., Piva R. Methylation analysis of the promoter F of estrogen receptor α gene: Effects on the level of transcription on human osteoblastic cells. J. Steroid Biochem. Mol. Biol. 2004;91:1–9. doi: 10.1016/j.jsbmb.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 133.Brand H., Koš M., Denger S., Flouriot G., Gromoll J., Gannon F., Reid G. A Novel Promoter Is Involved in the Expression of Estrogen Receptor α in Human Testis and Epididymis. Endocrinology. 2002;143:3397–3404. doi: 10.1210/en.2001-210832. [DOI] [PubMed] [Google Scholar]
- 134.Bulger M., Groudine M. Looping versus linking: Toward a model for long-distance gene activation. Genes Dev. 1999;13:2465–2477. doi: 10.1101/gad.13.19.2465. [DOI] [PubMed] [Google Scholar]
- 135.Carroll J.S., A Meyer C., Song J., Li W., Geistlinger T.R., Eeckhoute J., Brodsky A.S., Keeton E.K., Fertuck K.C., Hall G.F., et al. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 136.Kong S.L., Li G., Loh S.L., Sung W.-K., Liu E.T. Cellular reprogramming by the conjoint action of ERα, FOXA1, and GATA3 to a ligand-inducible growth state. Mol. Syst. Biol. 2011;7:526. doi: 10.1038/msb.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.A Serandour A., Brown G.D., Cohen J.D., Carroll J.S. Development of an Illumina-based ChIP-exonuclease method provides insight into FoxA1-DNA binding properties. Genome Biol. 2013;14:R147. doi: 10.1186/gb-2013-14-12-r147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mader S., Leroy P., Chen J., Chambon P. Multiple parameters control the selectivity of nuclear receptors for their response elements. Selectivity and promiscuity in response element recognition by retinoic acid receptors and retinoid X receptors. J. Biol. Chem. 1993;268:591–600. doi: 10.1016/S0021-9258(18)54192-2. [DOI] [PubMed] [Google Scholar]
- 139.Driscoll M.D., Sathya G., Muyan M., Klinge C., Hilf R., Bambara R.A. Sequence Requirements for Estrogen Receptor Binding to Estrogen Response Elements. J. Biol. Chem. 1998;273:29321–29330. doi: 10.1074/jbc.273.45.29321. [DOI] [PubMed] [Google Scholar]
- 140.Bourdeau V., Deschênes J., Métivier R., Nagai Y., Nguyen D., Bretschneider N., Gannon F., White J.H., Mader S. Genome-Wide Identification of High-Affinity Estrogen Response Elements in Human and Mouse. Mol. Endocrinol. 2004;18:1411–1427. doi: 10.1210/me.2003-0441. [DOI] [PubMed] [Google Scholar]
- 141.Overdier D.G., Porcella A., Costa R.H. The DNA-binding specificity of the hepatocyte nuclear factor 3/forkhead domain is influenced by amino-acid residues adjacent to the recognition helix. Mol. Cell. Biol. 1994;14:2755–2766. doi: 10.1128/MCB.14.4.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lentjes M.H.F.M., Niessen H.E.C., Akiyama Y., de Bruine A.P., Melotte V., van Engeland M. The emerging role of GATA transcription factors in development and disease. Expert Rev. Mol. Med. 2016;18:e3. doi: 10.1017/erm.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Merika M., Orkin S.H. DNA-binding specificity of GATA family transcription factors. Mol. Cell. Biol. 1993;13:3999–4010. doi: 10.1128/MCB.13.7.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bates D.L., Chen Y., Kim G., Guo L., Chen L. Crystal Structures of Multiple GATA Zinc Fingers Bound to DNA Reveal New Insights into DNA Recognition and Self-Association by GATA. J. Mol. Biol. 2008;381:1292–1306. doi: 10.1016/j.jmb.2008.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hanstein B., Eckner R., DiRenzo J., Halachmi S., Liu H., Searcy B., Kurokawa R., Brown M. p300 is a component of an estrogen receptor coactivator complex. Proc. Natl. Acad. Sci. USA. 1996;93:11540–11545. doi: 10.1073/pnas.93.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zwart W., Theodorou V., Kok M., Canisius S., Linn S., Carroll J.S. Oestrogen receptor-co-factor-chromatin specificity in the transcriptional regulation of breast cancer. EMBO J. 2011;30:4764–4776. doi: 10.1038/emboj.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fullwood M., Liu M.H., Pan Y.F., Liu J., Xu H., Bin Mohamed Y., Orlov Y., Velkov S., Thoreau H., Mei P.H., et al. An oestrogen-receptor-α-bound human chromatin interactome. Nat. Cell Biol. 2009;462:58–64. doi: 10.1038/nature08497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Frietze S., Wang R., Yao L., Tak Y.G., Ye Z., Gaddis M., Witt H., Farnham P.J., Jin V.X. Cell type-specific binding patterns reveal that TCF7L2 can be tethered to the genome by association with GATA3. Genome Biol. 2012;13:1–18. doi: 10.1186/gb-2012-13-9-r52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smith V.M., Lee P.P., Szychowski S., Winoto A. GATA-3 Dominant Negative Mutant. J. Biol. Chem. 1995;270:1515–1520. doi: 10.1074/jbc.270.4.1515. [DOI] [PubMed] [Google Scholar]
- 150.Shinnakasu R., Yamashita M., Shinoda K., Endo Y., Hosokawa H., Hasegawa A., Ikemizu S., Nakayama T. Critical YxKxHxxxRP Motif in the C-Terminal Region of GATA3 for Its DNA Binding and Function. J. Immunol. 2006;177:5801–5810. doi: 10.4049/jimmunol.177.9.5801. [DOI] [PubMed] [Google Scholar]
- 151.Crossley M., Merika M., Orkin S.H. Self-association of the erythroid transcription factor GATA-1 mediated by its zinc finger domains. Mol. Cell. Biol. 1995;15:2448–2456. doi: 10.1128/MCB.15.5.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li X., Jin J., Yang S., Xu W., Meng X., Deng H., Zhan J., Gao S., Zhang H. GATA3 acetylation at K119 by CBP inhibits cell migration and invasion in lung adenocarcinoma. Biochem. Biophys. Res. Commun. 2018;497:633–638. doi: 10.1016/j.bbrc.2018.02.120. [DOI] [PubMed] [Google Scholar]
- 153.Lacroix M., Leclercq G. About GATA3, HNF3A, and XBP1, three genes co-expressed with the oestrogen receptor-α gene (ESR1) in breast cancer. Mol. Cell. Endocrinol. 2004;219:1–7. doi: 10.1016/j.mce.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 154.Kouros-Mehr H., Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev. Dyn. 2006;235:3404–3412. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kouros-Mehr H., Slorach E.M., Sternlicht M.D., Werb Z. GATA-3 Maintains the Differentiation of the Luminal Cell Fate in the Mammary Gland. Cell. 2006;127:1041–1055. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kouros-Mehr H., Kim J.-W., Bechis S.K., Werb Z. GATA-3 and the regulation of the mammary luminal cell fate. Curr. Opin. Cell Biol. 2008;20:164–170. doi: 10.1016/j.ceb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Asselin-Labat M.-L., Sutherland K.D., Barker H., Thomas R., Shackleton M., Forrest N.C., Hartley L., Robb L., Grosveld F.G., van der Wees J., et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 158.Eeckhoute J., Keeton E.K., Lupien M., Krum S.A., Carroll J., Brown M. Positive Cross-Regulatory Loop Ties GATA-3 to Estrogen Receptor α Expression in Breast Cancer. Cancer Res. 2007;67:6477–6483. doi: 10.1158/0008-5472.CAN-07-0746. [DOI] [PubMed] [Google Scholar]
- 159.Chu I.M., Lai W.-C., Aprelikova O., El Touny L.H., Kouros-Mehr H., Green J.E. Expression of GATA3 in MDA-MB-231 Triple-negative Breast Cancer Cells Induces a Growth Inhibitory Response to TGFß. PLoS ONE. 2013;8:e61125. doi: 10.1371/journal.pone.0061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yan W., Cao Q.J., Arenas R.B., Bentley B., Shao R. GATA3 Inhibits Breast Cancer Metastasis through the Reversal of Epithelial-Mesenchymal Transition. J. Biol. Chem. 2010;285:14042–14051. doi: 10.1074/jbc.M110.105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Theodorou V., Stark R., Menon S., Carroll J.S. GATA3 acts upstream of FOXA1 in mediating ESR1 binding by shaping enhancer accessibility. Genome Res. 2013;23:12–22. doi: 10.1101/gr.139469.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Takaku M., Grimm S.A., Shimbo T., Perera L., Menafra R., Stunnenberg H.G., Archer T.K., Machida S., Kurumizaka H., Wade P.A. GATA3-dependent cellular reprogramming requires activation-domain dependent recruitment of a chromatin remodeler. Genome Biol. 2016;17:36. doi: 10.1186/s13059-016-0897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tanaka H., Takizawa Y., Takaku M., Kato D., Kumagawa Y., Grimm S.A., Wade P.A., Kurumizaka H. Interaction of the pioneer transcription factor GATA3 with nucleosomes. Nat. Commun. 2020;11:4136. doi: 10.1038/s41467-020-17959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Turner N., Lambros M.B., Horlings H.M., Pearson A., Sharpe R., Natrajan R., Geyer F.C., van Kouwenhove M., Kreike B., Mackay A., et al. Integrative molecular profiling of triple negative breast cancers identifies amplicon drivers and potential therapeutic targets. Oncogene. 2010;29:2013–2023. doi: 10.1038/onc.2009.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Usary J., Llaca V., Karaca G., Presswala S., Karaca M., He X., Langerød A., Kåresen R., Oh D.S., Dressler L.G., et al. Mutation of GATA3 in human breast tumors. Oncogene. 2004;23:7669–7678. doi: 10.1038/sj.onc.1207966. [DOI] [PubMed] [Google Scholar]
- 166.Ciriello G., Gatza M., Beck A.H., Wilkerson M.D., Rhie S., Pastore A., Zhang H., McLellan M., Yau C., Kandoth C., et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell. 2015;163:506–519. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Emmanuel N., Lofgren K.A., Peterson E.A., Meier D.R., Jung E.H., Kenny P.A. Mutant GATA3 Actively Promotes the Growth of Normal and Malignant Mammary Cells. Anticancer Res. 2018;38:4435–4441. doi: 10.21873/anticanres.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Afzaljavan F., Sadr A.S., Savas S., Pasdar A. GATA3 somatic mutations are associated with clinicopathological features and expression profile in TCGA breast cancer patients. Sci. Rep. 2021;11:1679. doi: 10.1038/s41598-020-80680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Adomas A.B., A Grimm S., Malone C., Takaku M., Sims J.K., A Wade P. Breast tumor specific mutation in GATA3 affects physiological mechanisms regulating transcription factor turnover. BMC Cancer. 2014;14:278. doi: 10.1186/1471-2407-14-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Takaku M., A Grimm S., de Kumar B., Bennett B.D., A Wade P. Cancer-specific mutation of GATA3 disrupts the transcriptional regulatory network governed by Estrogen Receptor alpha, FOXA1 and GATA3. Nucleic Acids Res. 2020;48:4756–4768. doi: 10.1093/nar/gkaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hruschka N., Kalisz M., Subijana M., Graña-Castro O., Del Cano-Ochoa F., Brunet L.P., Chernukhin I., Sagrera A., de Reynies A., Kloesch B., et al. The GATA3 X308_Splice breast cancer mutation is a hormone context-dependent oncogenic driver. Oncogene. 2020;39:5455–5467. doi: 10.1038/s41388-020-1376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Clark K.L., Halay E.D., Lai E., Burley S. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nat. Cell Biol. 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 173.Lam E.W.-F., Brosens J., Gomes A.R., Koo C.Y. Forkhead box proteins: Tuning forks for transcriptional harmony. Nat. Rev. Cancer. 2013;13:482–495. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- 174.Marsden I., Jin C., Liao X. Structural changes in the region directly adjacent to the DNA-binding helix highlight a possible mechanism to explain the observed changes in the sequence-specific binding of winged helix proteins. J. Mol. Biol. 1998;278:293–299. doi: 10.1006/jmbi.1998.1703. [DOI] [PubMed] [Google Scholar]
- 175.Bernardo G.M., Lozada K.L., Miedler J.D., Harburg G., Hewitt S., Mosley J., Godwin A.K., Korach K., Visvader J.E., Kaestner K.H., et al. FOXA1 is an essential determinant of ERα expression and mammary ductal morphogenesis. Development. 2010;137:2045–2054. doi: 10.1242/dev.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Caizzi L., Ferrero G., Cutrupi S., Cordero F., Ballaré C., Miano V., Reineri S., Ricci L., Friard O., Testori A., et al. Genome-wide activity of unliganded estrogen receptor-α in breast cancer cells. Proc. Natl. Acad. Sci. USA. 2014;111:4892–4897. doi: 10.1073/pnas.1315445111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bernardo G.M., Bebek G., Ginther C.L., Sizemore S.T., Lozada K.L., Miedler J.D., A Anderson L., Godwin A.K., Abdul-Karim F.W., Slamon D.J., et al. FOXA1 represses the molecular phenotype of basal breast cancer cells. Oncogene. 2013;32:554–563. doi: 10.1038/onc.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cirillo L.A., McPherson C.E., Bossard P., Stevens K., Cherian S., Shim E.Y., Clark K.L., Burley S., Zaret K.S. Binding of the winged-helix transcription factor HNF3 to a linker histone site on the nucleosome. EMBO J. 1998;17:244–254. doi: 10.1093/emboj/17.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cirillo L.A., Lin F.R., Cuesta I., Friedman D., Jarnik M., Zaret K.S. Opening of Compacted Chromatin by Early Developmental Transcription Factors HNF3 (FoxA) and GATA-4. Mol. Cell. 2002;9:279–289. doi: 10.1016/S1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- 180.Laganiere J., Deblois G., Lefebvre C., Bataille A.R., Robert F., Giguere V. Location analysis of estrogen receptor target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc. Natl. Acad. Sci. USA. 2005;102:11651–11656. doi: 10.1073/pnas.0505575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lupien M., Eeckhoute J., Meyer C.A., Wang Q., Zhang Y., Li W., Carroll J.S., Liu X.S., Brown M. FoxA1 Translates Epigenetic Signatures into Enhancer-Driven Lineage-Specific Transcription. Cell. 2008;132:958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sahu B., Laakso M., Ovaska K., Mirtti T., Lundin J., Rannikko A., Sankila A., Turunen J.-P., Lundin M., Konsti J., et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 2011;30:3962–3976. doi: 10.1038/emboj.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fu X., Jeselsohn R., Pereira R., Hollingsworth E.F., Creighton C.J., Li F., Shea M., Nardone A., de Angelis C., Heiser L.M., et al. FOXA1 overexpression mediates endocrine resistance by altering the ER transcriptome and IL-8 expression in ER-positive breast cancer. Proc. Natl. Acad. Sci. USA. 2016;113:E6600–E6609. doi: 10.1073/pnas.1612835113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Arruabarrena-Aristorena A., Maag J.L., Kittane S., Cai Y., Karthaus W.R., Ladewig E., Park J., Kannan S., Ferrando L., Cocco E., et al. FOXA1 Mutations Reveal Distinct Chromatin Profiles and Influence Therapeutic Response in Breast Cancer. Cancer Cell. 2020;38:534–550. doi: 10.1016/j.ccell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rheinbay E., Parasuraman P., Grimsby J., Tiao G., Engreitz J.M., Kim J., Lawrence M.S., Taylor-Weiner A., Rodriguez-Cuevas S., Rosenberg M., et al. Recurrent and functional regulatory mutations in breast cancer. Nat. Cell Biol. 2017;547:55–60. doi: 10.1038/nature22992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bullock M. FOXO factors and breast cancer: Outfoxing endocrine resistance. Endocr.-Relat. Cancer. 2015;23:R113–R130. doi: 10.1530/ERC-15-0461. [DOI] [PubMed] [Google Scholar]
- 187.Guo S., Sonenshein G.E. Forkhead Box Transcription Factor FOXO3a Regulates Estrogen Receptor Alpha Expression and Is Repressed by the Her-2/neu/Phosphatidylinositol 3-Kinase/Akt Signaling Pathway. Mol. Cell. Biol. 2004;24:8681–8690. doi: 10.1128/MCB.24.19.8681-8690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zou Y., Tsai W.-B., Cheng C.-J., Hsu C., Chung Y.M., Li P.-C., Lin S.-H., Hu M.C.-T. Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis. Breast Cancer Res. 2008;10:R21. doi: 10.1186/bcr1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Alasiri G., Jiramongkol Y., Zona S., Fan L.Y.-N., Mahmud Z., Gong G., Lee H.J., Lam E.W.-F. Regulation of PERK expression by FOXO3: A vulnerability of drug-resistant cancer cells. Oncogene. 2019;38:6382–6398. doi: 10.1038/s41388-019-0890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Madureira P., Varshochi R., Constantinidou D., Francis R.E., Coombes R.C., Yao K.-M., Lam E. The Forkhead Box M1 Protein Regulates the Transcription of the Estrogen Receptor α in Breast Cancer Cells. J. Biol. Chem. 2006;281:25167–25176. doi: 10.1074/jbc.M603906200. [DOI] [PubMed] [Google Scholar]
- 191.Tan Y., Wang Q., Xie Y., Qiao X., Zhang S., Wang Y., Yang Y., Zhang B. Identification of FOXM1 as a specific marker for triple-negative breast cancer. Int. J. Oncol. 2018;54:87–97. doi: 10.3892/ijo.2018.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Carr-Ascher J., Kiefer M.M., Park H.J., Li J., Wang Z., Fontanarosa J., DeWaal D., Kopanja D., Benevolenskaya E.V., Guzman G., et al. FoxM1 Regulates Mammary Luminal Cell Fate. Cell Rep. 2012;1:715–729. doi: 10.1016/j.celrep.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang J., Ray P.S., Sim M.-S., Zhou X.Z., Lu K.P., Lee A.V., Lin X., Bagaria S.P., Giuliano A.E., Cui X. FOXC1 regulates the functions of human basal-like breast cancer cells by activating NF-κB signaling. Oncogene. 2012;31:4798–4802. doi: 10.1038/onc.2011.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Taube J., Herschkowitz J.I., Komurov K., Zhou A., Gupta S., Yang J., Hartwell K., Onder T., Gupta P.B., Evans K.W., et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc. Natl. Acad. Sci. USA. 2010;107:15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Welboren W.-J., A van Driel M., Janssen-Megens E.M., van Heeringen S., Sweep F., Span P., Stunnenberg H.G. ChIP-Seq of ERα and RNA polymerase II defines genes differentially responding to ligands. EMBO J. 2009;28:1418–1428. doi: 10.1038/emboj.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Read L.D., Greene G.L., Katzenellenbogen B.S. Regulation of Estrogen Receptor Messenger Ribonucleic Acid and Protein Levels in Human Breast Cancer Cell Lines by Sex Steroid Hormones, Their Antagonists, and Growth Factors. Mol. Endocrinol. 1989;3:295–304. doi: 10.1210/mend-3-2-295. [DOI] [PubMed] [Google Scholar]
- 197.Grober O.M.V., Mutarelli M., Giurato G., Ravo M., Cicatiello L., de Filippo M.R., Ferraro L., Nassa G., Papa M.F., Paris O., et al. Global analysis of estrogen receptor beta binding to breast cancer cell genome reveals an extensive interplay with estrogen receptor alpha for target gene regulation. BMC Genom. 2011;12:36. doi: 10.1186/1471-2164-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Matthews J., Gustafsson J.A. Estrogen signaling: A subtle balance between ER alpha and ER beta. Mol. Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 199.Cotnoir-White D., El Ezzy M., Boulay P.-L., Rozendaal M., Bouvier M., Gagnon E., Mader S. Monitoring ligand-dependent assembly of receptor ternary complexes in live cells by BRETFect. Proc. Natl. Acad. Sci. USA. 2018;115:E2653–E2662. doi: 10.1073/pnas.1716224115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Eckert R.L., Mullick A., Rorke E.A., Katzenellenbogen B.S. Estrogen Receptor Synthesis and Turnover in MCF-7 Breast Cancer Cells Measured by a Density Shift Technique. Endocrinology. 1984;114:629–637. doi: 10.1210/endo-114-2-629. [DOI] [PubMed] [Google Scholar]
- 201.A Khan S., Sachdeva A., Naim S., Meguid M.M., Marx W., Simon H., Halverson J.D., Numann P.J. The normal breast epithelium of women with breast cancer displays an aberrant response to estradiol. Cancer Epidemiol. Biomark. Prev. 1999;8:867–872. [PubMed] [Google Scholar]
- 202.Macaluso M., Cinti C., Russo G., Russo A., Giordano A. pRb2/p130-E2F4/5-HDAC1-SUV39H1-p300 and pRb2/p130-E2F4/5-HDAC1-SUV39H1-DNMT1 multimolecular complexes mediate the transcription of estrogen receptor-α in breast cancer. Oncogene. 2003;22:3511–3517. doi: 10.1038/sj.onc.1206578. [DOI] [PubMed] [Google Scholar]
- 203.Kalyuga M., Gallego-Ortega D., Lee H., Roden D.L., Cowley M., Caldon C.E., Stone A., Allerdice S.L., Valdes-Mora F., Launchbury R., et al. ELF5 Suppresses Estrogen Sensitivity and Underpins the Acquisition of Antiestrogen Resistance in Luminal Breast Cancer. PLoS Biol. 2012;10:e1001461. doi: 10.1371/journal.pbio.1001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dhasarathy A., Kajita M., Wade P.A. The Transcription Factor Snail Mediates Epithelial to Mesenchymal Transitions by Repression of Estrogen Receptor-α. Mol. Endocrinol. 2007;21:2907–2918. doi: 10.1210/me.2007-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang J., Zhou C., Jiang H., Liang L., Shi W., Zhang Q., Sun P., Xiang R., Wang Y., Yang S. ZEB1 induces ER-α promoter hypermethylation and confers antiestrogen resistance in breast cancer. Cell Death Dis. 2017;8:e2732. doi: 10.1038/cddis.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Klinge C.M. miRNAs and estrogen action. Trends Endocrinol. Metab. 2012;23:223–233. doi: 10.1016/j.tem.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.