Abstract
Background
SARS-CoV-2 variants of concern (VOCs) have threatened COVID-19 vaccine effectiveness. We aimed to assess the effectiveness of the ChAdOx1 nCoV-19 vaccine, predominantly against the delta (B.1.617.2) variant, in addition to the cellular immune response to vaccination.
Methods
We did a test-negative, case-control study at two medical research centres in Faridabad, India. All individuals who had a positive RT-PCR test for SARS-CoV-2 infection between April 1, 2021, and May 31, 2021, were included as cases and individuals who had a negative RT-PCR test were included as controls after matching with cases on calendar week of RT-PCR test. The primary outcome was effectiveness of complete vaccination with the ChAdOx1 nCoV-19 vaccine against laboratory-confirmed SARS-CoV-2 infection. The secondary outcomes were effectiveness of a single dose against SARS-CoV-2 infection and effectiveness of a single dose and complete vaccination against moderate-to-severe disease among infected individuals. Additionally, we tested in-vitro live-virus neutralisation and T-cell immune responses to the spike protein of the wild-type SARS-CoV-2 and VOCs among healthy (anti-nucleocapsid antibody negative) recipients of the ChAdOx1 nCoV-19 vaccine.
Findings
Of 2379 cases of confirmed SARS-CoV-2 infection, 85 (3·6%) were fully vaccinated compared with 168 (8·5%) of 1981 controls (adjusted OR [aOR] 0·37 [95% CI 0·28–0·48]), giving a vaccine effectiveness against SARS-CoV-2 infection of 63·1% (95% CI 51·5–72·1). 157 (6·4%) of 2451 of cases and 181 (9·1%) of 1994) controls had received a single dose of the ChAdOx1 nCoV-19 vaccine (aOR 0·54 [95% CI 0·42–0·68]), thus vaccine effectiveness of a single dose against SARS-CoV-2 infection was 46·2% (95% CI 31·6–57·7). One of 84 cases with moderate-to-severe COVID-19 was fully vaccinated compared with 84 of 2295 cases with mild COVID-19 (aOR 0·19 [95% CI 0·01–0·90]), giving a vaccine effectiveness of complete vaccination against moderate-to-severe disease of 81·5% (95% CI 9·9–99·0). The effectiveness of a single dose against moderate-to-severe disease was 79·2% (95% CI 46·1–94·0); four of 87 individuals with moderate-to-severe COVID-19 had received a single dose compared with 153 of 2364 participants with mild disease (aOR 0·20 [95% CI 0·06–0·54]). Among 49 healthy, fully vaccinated individuals, neutralising antibody responses were lower against the alpha (B.1.1.7; geometric mean titre 244·7 [95% CI 151·8–394·4]), beta (B.1.351; 97·6 [61·2–155·8]), kappa (B.1.617.1; 112·8 [72·7–175·0]), and delta (88·4 [61·2–127·8]) variants than against wild-type SARS-CoV-2 (599·4 [376·9–953·2]). However, the antigen-specific CD4 and CD8 T-cell responses were conserved against both the delta variant and wild-type SARS-CoV-2.
Interpretation
The ChAdOx1 nCoV-19 vaccine remained effective against moderate-to-severe COVID-19, even during a surge that was dominated by the highly transmissible delta variant of SARS-CoV-2. Spike-specific T-cell responses were maintained against the delta variant. Such cellular immune protection might compensate for waning humoral immunity.
Funding
Department of Biotechnology India, Council of Scientific and Industrial Research India, and Fondation Botnar.
Introduction
As of Oct 12, 2021, SARS-CoV-2 has affected more than 219 million people and caused more than 4 million deaths worldwide. The emergence of mutations in the virus has led to concerns regarding vaccine effectiveness. Some of the SARS-CoV-2 variants include alpha (B.1.1.7), beta (B.1.351), kappa (B.1.617.1), and delta (B.1.617.2).1, 2 At the time of writing, the delta variant of concern (VOC) was the predominant strain of SARS-CoV-2 worldwide. The virus enters the host respiratory epithelial cells via an interaction between the receptor-binding domain (RBD) of its spike protein and the human angiotensin-converting enzyme 2 receptors. Vaccines are designed to generate neutralising antibodies against the spike protein to block viral entry. Mutations in the RBD might lead to decreased neutralising ability of vaccine-derived antibodies, resulting in reduced effectiveness.3, 4, 5, 6 However, cellular immune responses might remain protective against COVID-19 even if the neutralising activity is suboptimal and the virus gains entry to host cells.7 In India, a substantial surge in SARS-CoV-2 infections was observed between April and May, 2021, largely due to the delta variant.8 According to the CoWIN dashboard (Ministry of Health and Family Welfare, New Delhi, India) until September, 2021, of 888 064 065 individuals who had been vaccinated in India, 649 886 100 (73·2%) had received a single dose and 238 177 965 (26.8%) had received two doses. The vaccination programme in India is driven largely by the Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine (Covishield; Serum Institute of India, Pune, India). In this study, we aimed to evaluate the real-world vaccine effectiveness of ChAdOx1 nCoV-19, a chimpanzee adenovirus-vectored vaccine expressing the SARS-CoV-2 spike protein,9 during the surge in SARS-CoV-2 infections between April and May, 2021, in India. Additionally, we aimed to assess live-virus neutralising activity and cellular immune responses against VOCs in healthy recipients of the ChAdOx1 nCoV-19 vaccine to understand the mechanisms of protection.
Research in context.
Evidence before this study
The delta (B.1.617.2) variant of SARS-CoV-2 has emerged as the dominant circulating strain contributing to the majority of infections worldwide. Evidence regarding immunological response and clinical effectiveness in individuals who have received the ChAdOx1 nCoV-19 vaccine is still emerging. We searched PubMed for articles published between Jan 1, 2021, and June 30, 2021, using the search terms “SARS-CoV-2”, “COVID-19”, “vaccine”, “ChAdOx1 nCoV-19”, and “immune response” alone or in combination. Our search yielded 147 articles about vaccine effectiveness and 56 about immune response. We identified five vaccine efficacy or effectiveness studies and ten articles on immune response to vaccines that were relevant to our study. Preliminary reports suggest that the delta variant is less susceptible than wild-type SARS-CoV-2 to in-vitro neutralisation by antibodies from recipients of two doses of the ChAdOx1 nCoV-19 vaccine. Surveillance data from the UK suggested that two doses of ChAdOx1 nCoV-19 vaccine might have a reduced effectiveness against infection, but comparable effectiveness against hospitalisation with the delta variant compared with wild-type SARS-CoV-2.
Added value of this study
This test-negative, case-control study showed that two doses of ChAdOx1 nCoV-19 vaccine provided 63·1% (95% CI 51·5–72·1) protection against infection attributed predominantly to the SARS-CoV-2 delta variant during the surge in infections observed in India between April and May, 2021. Protection against moderate-to-severe disease was higher than protection against infection. The effectiveness of single-dose vaccine was 46·2% (31·6–57·7) against infection. In the mechanistic study among 49 healthy, fully vaccinated individuals, we found that protective humoral immune response against the delta variant was significantly reduced when compared with wild-type SARS-CoV-2, but T-cell-mediated immunity was maintained.
Implications of all the available evidence
Our data suggest that two doses of the ChAdOx1 nCoV-19 vaccine provide modest protection against infection with the SARS-CoV-2 delta variant and high levels of protection against moderate-to-severe COVID-19. Although infection with a variant of concern remains possible due to reduced neutralisation by the vaccine-elicited antibodies that primarily prevent virus entry into host cells, the protection against severe disease might be attributed to the maintenance of T-cell-mediated immune responses conferred by the vaccine. The COVID-19 vaccination policy should encourage two doses of ChAdOx1 nCoV-19 in addition to other epidemiological public health measures to overcome the threat posed by the delta variant and other emerging SARS-CoV-2 variants.
Methods
Study design and participants
We did a test-negative, case-control study to assess vaccine effectiveness among individuals who attended Employee State Insurance Corporation Medical College (ESICMC) Hospital (Faridabad, India) or Translational Health Science and Technology Institute (Faridabad, India) for RT-PCR testing for suspected SARS-CoV-2 infection between April 1 and May 31, 2021.10 These two centres are the largest COVID-19 testing centres in Faridabad, contributing to 398 828 (91%) of 439 571 tests of all tests done in Faridabad.
All individuals who had a positive RT-PCR test for SARS-CoV-2 infection were included as cases. Controls were selected randomly using a computer program from individuals who tested negative on RT-PCR and were matched on the basis of calendar week of test receipt during the study period.
For the mechanistic study, we enrolled 59 healthy individuals vaccinated at ESICMC Hospital who had received full ChAdOx1 nCoV-19 vaccination (appendix p 11). These participants were derived from the same population as those enrolled for the vaccine effectiveness study. We excluded individuals who reported SARS-CoV-2 positivity either by RT-PCR any time before blood collection or who tested positive for anti-nucleocapsid antibody on the collected blood sample by ELISA.
The study was approved by the institutional ethics committees of ESICMC Hospital and the Translational Health Science and Technology Institute. Written informed consent was obtained from participants who contributed biospecimens, and verbal consent was obtained for the information collected for the vaccine effectiveness objective.
Data collection
Vaccination data, including the name of the vaccine, number of doses received, dates, and place of administration, and clinical manifestations of COVID-19 were obtained from cases and controls through a telephone questionnaire done by trained research staff. Quality control measures, methodological considerations for assessment of COVID-19 vaccination status, bias control, and confounding adjustments are described in the appendix (pp 2–5). Complete vaccination was defined as having had the second dose of the vaccine at least 14 days previously. Single-dose vaccination was defined as having had the first dose of the vaccine at least 21 days previously but not having had the second dose. Unvaccinated individuals were those who had not received a single dose of vaccine and were included as the control group to estimate vaccine effectiveness for all analyses. Moderate-to-severe COVID-19 was defined if a patient reported at least one of the following: the need for oxygen supplementation, admission to intensive care, mechanical ventilation, or death.
Mechanistic study procedures
For the mechanistic study, blood samples were collected and processed for plasma and peripheral blood mononuclear cells (PBMCs; appendix p 7).
To measure IgG antibodies, a recombinant spike protein RBD ELISA was done as described previously but with minor modifications,11 and antibody levels were calibrated against the WHO reference reagent (appendix p 6). Virus neutralisation assay titres were estimated as described previously.12 Briefly, plasma samples were serially diluted from 1/20 to 1/640 and incubated with one of four SARS-CoV-2 isolates (appendix p 5). 50% neutralisation values were calculated with four-parameter logistic regression using GraphPad Prism software (version 7.0e).
We expressed and purified recombinant RBD protein representing wild-type SARS-CoV-2 sequences, which were cloned in pCDN3.1 expression vector with CD5 secretory sequences. The RBD construct representing the VOCs was generated by introducing desired mutations one by one by site-directed mutagenesis, including Glu484Gln, Leu452Arg, Asn501Tyr, Lys417Asn, Glu484Lys, and Asn501Tyr, which are characteristic mutations in the alpha, beta, kappa, and delta VOCs (appendix pp 6, 7); the mutant RBD protein was then expressed in Expi293 cells (Thermo Fisher Scientific, Waltham, MA, USA).13
CD4 and CD8 T-cell responses to stimulation with whole-spike peptide pools of wild-type and delta SARS-CoV-2 were tested with a cytokine bead array in culture supernatant of activated PBMCs, an activation induced marker assay, and an intracellular cytokine assay. To stimulate T cells, PBMCs were incubated with pools of 157 and 158 synthetic peptides (15-mers peptide pools overlapping by 11 amino acids) spanning the entire spike protein (PepMix; catalogue numbers PM-WCPV-S-1 and PM-SARS2-SMUT06-1; JPT Peptide Technologies, Berlin, Germany).14 To study the T-cell responses to mutant regions of the delta variant, we stimulated PBMCs with the commercially available PepTivator Prot_S B.6.1.7.2 mutant pool (Miltenyi Biotec, North Rhine-Westphalia, Germany). The mutant pool selectively covers the ten mutated regions in the spike protein of the delta variant with 32 15-mer peptides that include Thr19Arg, Gly142Asp, Glu156Gly, Phe157del, Arg158del, Leu452Arg, Thr478Lys, Asp614Gly, Pro681Arg, and Asp950Asn. A respective wild-type reference pool (Miltenyi Biotec) consisting of the 32 homologous peptides of wild-type SARS-CoV-2 was used as a control.14
Antigen-specific CD4 and CD8 T-cell responses were determined by the production of the cytokines interferon-γ (IFNγ), interleukin-2 (IL-2), and tumour necrosis factor α (TNFα), and the effector molecules granzyme B and perforin expression. For cytokine production, 1 × 106 cells per well were stimulated with 2 μg/mL peptide pool (PepMix) for 48 h and the culture supernatant was used to analyse the secretion of IFNγ, IL-2, and TNFα (appendix p 7).15 A similar number of PBMCs were seeded with dimethyl sulfoxide alone (unstimulated) as a negative control and the T-cell immune response was calculated by subtracting the readings of unstimulated PBMCs from the stimulated PBMCs. In all T-cell assays, phytohemagglutinin 5 μg/mL was used as a positive control. Any sample with a low phytohemagglutinin signal was removed for quality control.
To perform intracellular staining, 1 million cells per well were stimulated with 2 μg/mL peptide pool (PepMix) for 18–22 h and with monensin (Golgi Stop; BD Biosciences, Franklin Lakes, NJ, USA) for the final 6 h. Intracellular expression of IFNγ, IL-2, and TNFα in CD4 T cells and IFNγ, granzyme B, and perforin in CD8 T cells was tested in response to spike peptides pools of wild-type and delta variant SARS-CoV-2. The cells were stained for extracellular and intracellular markers and analysed using flow cytometry (appendix pp 7, 8).15 IFNγ-linked enzyme linked immunospot (ELISpot) was done to determine the frequency of IFNγ-secreting cells in response to wild-type and mutant peptide pools specific to delta variants (appendix p 9).
We did an activation-induced marker assay to gain a broader understanding of the total antigen-specific T-cell response. For the activation-induced marker assay, PBMCs were assessed as described previously (appendix pp 7, 8).7 Activation of both CD4 and CD8 T cells was measured by determining the surface expression of OX40 (TNFSF4) and CD137 (TNFRSF9) on CD4 T cells and CD69 and CD137 on CD8 T cells in response to stimulation with spike peptides pools of wild-type or delta SARS-CoV-2. To understand the RBD specific T-cell response, PBMCs were stimulated with wild-type and mutant RBD proteins as described previously.16 Response after 72 h of incubation was evaluated by IFNγ secretion in the culture supernatant (appendix p 8).
Sequencing libraries were prepared from the archived SARS-CoV-2 RNA samples using the amplicon-based COVIDSeq test kit (Illumina, San Diego, CA, USA).17 All synthesised libraries were sequenced on the NovaSeq 6000 platform with a read length of 2 × 100 bp (appendix p 9). Lineages were assigned using pangolin (version 3.0.5, pangoLEARN 2021-06-05).18
Outcomes
Our primary outcome was real-world vaccine effectiveness of two doses of the ChAdOx1 nCoV-19 vaccine against laboratory confirmed SARS-CoV-2 infection. The secondary outcomes were vaccine effectiveness of a single dose of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection and vaccine effectiveness of a single dose and complete vaccination against moderate-to-severe disease among individuals infected with SARS-CoV-2. Additional outcomes for the mechanistic study were live-virus neutralisation and T-cell immune responses against VOCs among ChAdOx1 nCoV-19 vaccine recipients.
Statistical analysis
To detect a vaccine effectiveness of 60% for complete vaccination with ChAdOx1 against infection with 10% precision, and assuming that vaccine coverage among the controls was 10% (based on vaccination rates at the time of writing),19 we estimated that we needed a minimum of 4474 participants, with 2237 participants in each group, using the WHO vaccine effectiveness sample size tool (appendix p 2).
Data are presented as mean (SD) or median (IQR), as appropriate. The vaccine effectiveness of complete vaccination was estimated by comparison with unvaccinated individuals after exclusion of those who received a single dose and similarly, for the estimation of vaccine effectiveness for single dose vaccination, we excluded individuals with complete vaccination. Adjusted odds ratios (aORs) with 95% CIs were estimated using a multivariable logistic regression model, which included the following confounders decided a priori: age, sex, and risk of exposure (appendix p 4) as independent variables in addition to vaccination status. The final vaccine effectiveness estimate was calculated as vaccine effectiveness = (1–aOR) × 100%. Among individuals infected with SARS-CoV-2, the vaccine effectiveness of a single dose and complete vaccination for the prevention of moderate-to-severe COVID-19 was ascertained using individuals with mild COVID-19 as controls. To compare the live-virus neutralisation between different VOCs, the geometric mean titre for each variant was calculated and analysis of variance was done with the log-transformed titre values, using wild-type titres as the reference. We used Pearson's correlation coefficient to assess the correlations between geometric mean titres of neutralising antibodies or quantitative IgG antibody levels and T-cell IFNγ and IL-2 production (measured in culture supernatant). The pair-wise fold change in IFNγ response against each variant versus wild-type was calculated and compared using the Wilcoxon matched-pairs signed-rank test. All statistical analyses were done using R (version 4.0.4).
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
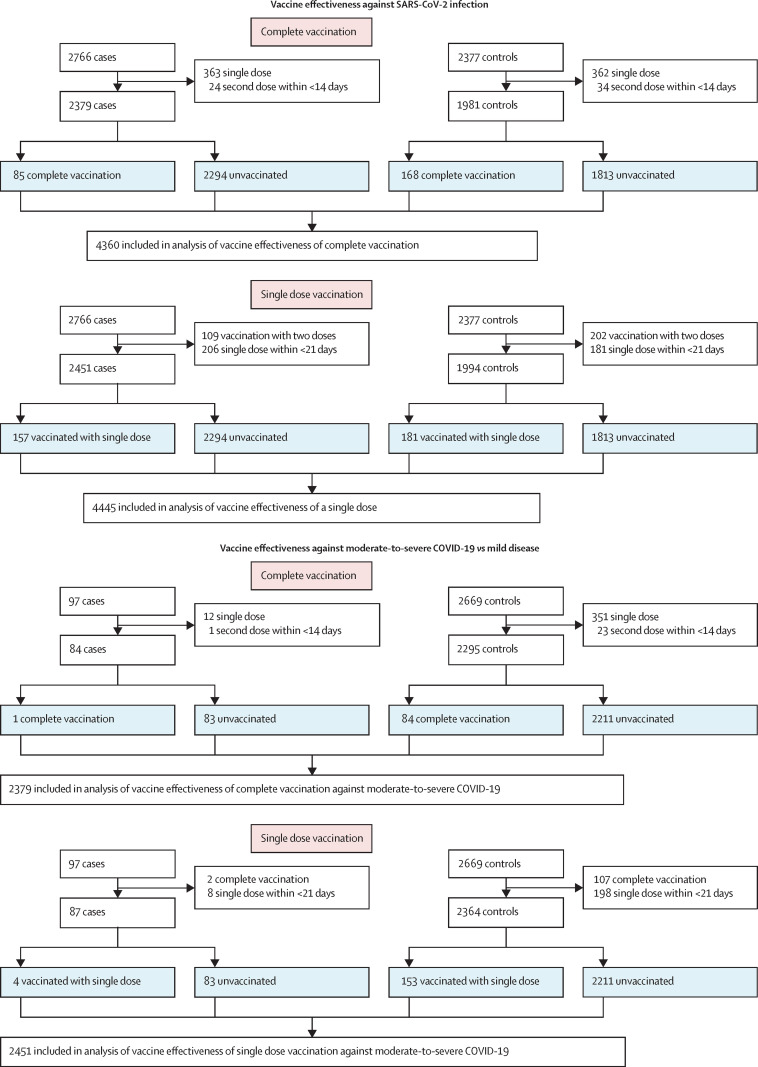
Between April 1, 2021, and May 31, 2021, 8850 individuals had an RT-PCR test at the two centres, of whom 7132 (80·6%) responded when invited to participate in the study (4036 [78·2%] of 5163 RT-PCR test-negative individuals [controls]; 3086 [83·7%] of 3687 RT-PCR test-positive individuals [cases]). 169 individuals did not consent to the interview, 194 reported testing positive for SARS-CoV-2 during the previous wave of infections in 2020, and 191 were sampled before April 1, 2021, thus 3695 controls and 2883 cases were eligible for inclusion. After matching cases and controls and excluding individuals who had a negative test recorded in the database but reported a positive test later (n=476) and those who had received COVID-19 vaccines other than ChAdOx1 nCoV-19 (n=276), 2766 cases and 2377 controls were included in the vaccine effectiveness analysis (appendix p 3). The demographic, vaccination, and COVID-19 clinical characteristics of included participants are shown in table 1 . The median interval between vaccine doses was 33 days (IQR 29–50) among cases and 31 days (28–45) among controls.
Table 1.
Baseline characteristics of the participants included in vaccine effectiveness analyses
| Cases (n=2766) | Controls (n=2377) | ||
|---|---|---|---|
| Age, years | 35 (28–45) | 32 (26–42) | |
| Sex | |||
| Male | 1804 (65·2%) | 1626 (68·4%) | |
| Female | 962 (34·8%) | 751 (31·6%) | |
| High risk of COVID-19 exposure at workplace | 56 (2·0%) | 60 (2·5%) | |
| Duration between the two vaccine doses among vaccinated participants, days | 33 (29–50; n=109) | 31 (28–45; n=202) | |
| Mild COVID-19 symptoms | 2679 (96·5%) | NA | |
| Oxygen supplementation | 89 (3·2%) | NA | |
| Admission to the intensive care unit | 26 (0·9%) | NA | |
| Ventilatory support | 7 (0·3%) | NA | |
| Death | 16 (0·6%) | NA | |
Data are median (IQR) or n (%). NA=not applicable.
To estimate the effectiveness of two doses of ChAdOx1 nCoV-19 against SARS-CoV-2 infection, 2379 cases and 1981 controls were included in the analysis after exclusions (figure 1 ). 85 (3·6%) cases were fully vaccinated compared with 168 (8·5%) controls (aOR 0·37 [95% CI 0·28–0·48]), giving a vaccine effectiveness against SARS-CoV-2 infection of 63·1% (95% CI 51·5–72·1). 157 (6·4%) of 2451 cases and 181 (9·1%) of 1994 controls had received only one dose of the vaccine (aOR 0·54 [95% CI 0·42–0·68]; figure 1), giving a vaccine effectiveness of a single dose against SARS-CoV-2 infection of 46·2% (95% CI 31·6–57·7; table 2 ). Among the 84 cases of moderate-to-severe COVID-19, only one (1·2%) was completely vaccinated compared with 84 (3·7%) of 2295 cases with mild COVID-19 (aOR 0·19 [95% CI 0·01–0·90]), thus effectiveness of complete vaccination against moderate-to-severe COVID-19 was 81·5% (95% CI 9·9–99·0; table 2; figure 1). 16 deaths were reported in the unvaccinated or incomplete vaccination group, but none in the completely vaccinated group. Among 87 participants with moderate-to-severe COVID-19, four (4·6%) were vaccinated with a single dose compared with 153 (6·5%) of 2364 with mild disease. Thus, a single dose of ChAdOx1 nCoV-19 prevented moderate-to-severe disease with an effectiveness of 79·2% (95% CI 46·1–94·0).
Figure 1.
Study flow diagram
Table 2.
Effectiveness of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection and moderate-to-severe COVID-19
| Cases, n/N (%) | Controls, n/N (%) | Adjusted OR*(95% CI) | Effectiveness against infection (95% CI) | Moderate-to-severe COVID-19, n/N (%) | Mild COVID-19, n/N (%) | Adjusted OR*(95% CI) | Effectiveness against moderate-to-severe COVID-19 (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Effectiveness of two doses | ||||||||
| Unvaccinated | 2294/2379 (96·4%) | 1813/1981 (91·5%) | .. | .. | 83/84 (98·8%) | 2211/2295 (96·3%) | .. | .. |
| Complete vaccination | 85/2379 (3·6%) | 168/1981 (8·5%) | 0·37 (0·28–0·48) | 63·1% (51·5–72·1) | 1/84 (1·2%) | 84/2295 (3·7%) | 0·19 (0·01–0·90) | 81·5% (9·9–99·0) |
| Effectiveness of a single dose | ||||||||
| Unvaccinated | 2294/2451 (93·6%) | 1813/1994 (90·9%) | .. | .. | 83/87 (95·4%) | 2211/2364 (93·5%) | .. | .. |
| Single-dose vaccination | 157/2451 (6·4%) | 181/1994 (9·1%) | 0·54 (0·42–0·68) | 46·2% (31·6, 57·7) | 4/87 (4·6%) | 153/2364 (6·5%) | 0·20 (0·06–0·54) | 79·2% (46·1–94·0) |
OR=odds ratio.
OR adjusted for differences in age, sex, and risk of exposure to COVID-19-positive individuals.
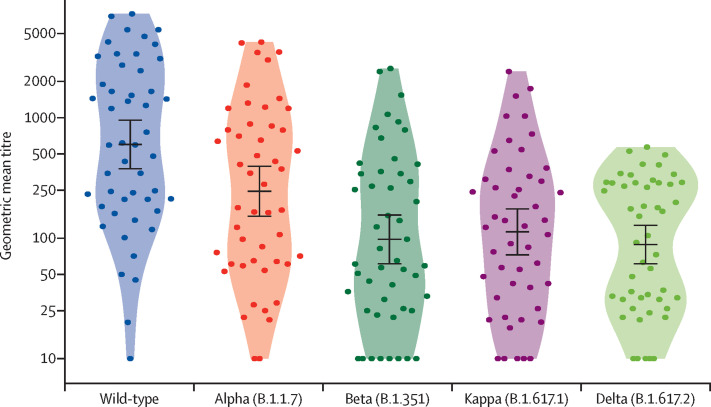
The neutralisation ability was tested in plasma from 49 of 59 fully vaccinated healthy participants. Their clinical characteristics are provided in the appendix (p 11). Blood samples were collected a median of 61 days (IQR 52–79) after the second vaccine dose. The median plasma IgG RBD antibody level was 234·6 ELISA laboratory unit/mL (IQR 88·0–452·4). The geometric mean titre of neutralising antibodies was 599·4 (95% CI 376·9–953·2) against the wild-type virus. A strong correlation was identified between the geometric mean titre against the wild-type strain and the corresponding IgG antibody levels (r=0·96 [95% CI 0·92–0·97]; appendix p 10). Geometric mean titres of neutralising antibodies were significantly reduced against SARS-CoV-2 variants compared with the wild-type strain: geometric mean titres were 244·7 (95% CI 151·8–394·4; p=0·036) for the alpha variant, 112·8 (72·7–175·0; p<0·0001) for the kappa variant, 97·6 (61·2–155·8; p<0·0001) for the beta variant, and 88·4 (61·2–127·8; p<0·0001) for the delta variant (figure 2 ; appendix p 12).
Figure 2.
Titres of neutralising antibodies targeting wild-type SARS-CoV-2 and variants in plasma from ChAdOx1 nCoV-19 vaccine recipients
Neutralisation titres estimated by focus reduction neutralisation test are plotted in log-scale on the y-axis. The dots represent neutralisation titres of individual participants (n=49). The solid black line with error bars indicate geometric mean titres and 95% CIs. The sample numbers, geometric mean titre values, and fold change in titres between the variants of concern are shown in the appendix (p 12).
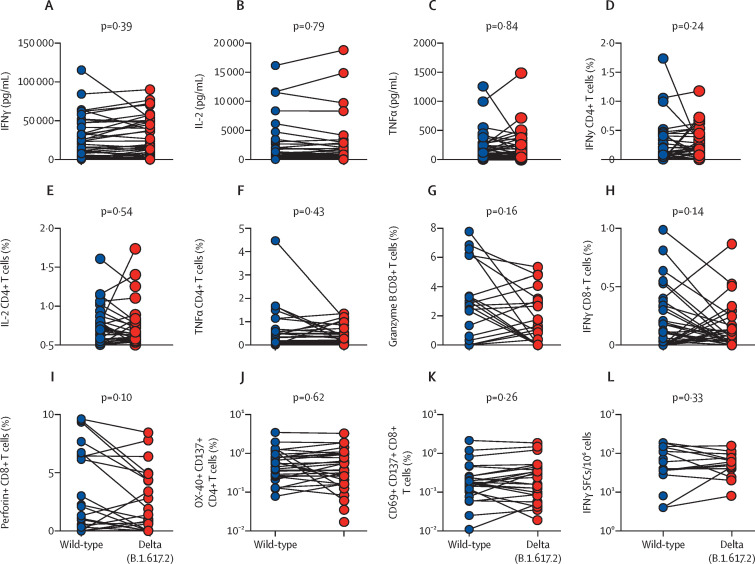
The cytokine bead array was done in 48 available samples from vaccinated individuals, of whom 47 responded. No significant differences were observed in IFNγ, IL-2, and TNFα production between samples stimulated with wild-type and delta spike-specific peptide pools (figure 3A–C , table 3 ). The intracellular cytokine assay showed that the spike peptide pools of both wild-type and delta SARS-CoV-2 similarly induced the expression of IFNγ, IL-2, and TNFα in CD4 T cells and IFNγ, granzyme B, and perforin in CD8 T cells (figure 3D–I, table 3). Total antigen-specific T-cell responses also showed that both wild-type and delta SAR-CoV-2 spike peptides pools effectively and comparably induced surface expression of activation induced markers on CD4 and CD8 T-cells (figure 3J, K, table 3).
Figure 3.
T-cell immune responses against spike antigens of wild-type and delta variant SARS-CoV-2 in samples from vaccinated participants
IFNγ (A), IL-2 (B), and IFNγ (C) concentrations in culture supernatant of peripheral blood mononuclear cells stimulated with peptide pools of wild-type and delta variant spike protein for 48 h (n=48; 47 of 48 samples responded to stimulation). Intracellular cytokine staining for IFNγ (D), IL-2 (E), and TNFα (F) in CD4 cells, and granzyme B (G), IFNγ (H), and perforin (I) in CD8 cells stimulated with peptide pools of wild-type and delta variant spike protein for 18–20 h; graphs show the proportions of cells expressing these molecules (n=41). Activation-induced marker assay of CD4 T cells (J) and CD8 T cells (K) stimulated with peptide pools of wild-type and delta variant spike protein for 24 h; graphs show the proportions of cells expressing these activation markers (n=38). (L) Antigen-specific activation of T cells by peptide pools exclusively covering the mutant regions of the delta variant spike protein compared with the homologous wild-type reference peptide pool (n=19; 19 of 24 samples responded to simulation; mean 63·78 SFCs/106 PBMCs for wild-type, and 45.68 SFCs/106 PBMCs for the delta variant). Each datapoint represents the readings from the peptide pool-stimulated wells for one study participant, after subtraction of the dimethyl sulfoxide-stimulated wells. IFNγ=interferon γ. SFCs=spot forming cells. TNFα=tumour necrosis factor α.
Table 3.
T-cell immune responses among healthy ChAdOx1 nCoV-19-vaccinated participants
| Total number of samples tested | Wild-type SARS-CoV-2 spike peptide pool | Delta (B.1.617.2) SARS-CoV-2 spike peptide pool | p value | ||
|---|---|---|---|---|---|
| Cytokines secreted by stimulated PBMCs (pg/mL) | |||||
| IFNγ | 48 | 22 947 (23 159) | 28 316 (28 955) | 0·39 | |
| IL-2 | 48 | 2049 (3524) | 2053 (3731) | 0·79 | |
| TNFα | 48 | 169 (247) | 204 (330) | 0·83 | |
| Intracellular cytokine staining (%)* | |||||
| CD4 T cells | |||||
| IFNγ | 41 | 0·22% (0·35) | 0·21% (0·25) | 0·24 | |
| IL-2 | 41 | 0·24% (0·32) | 0·24% (0·35) | 0·54 | |
| TNFα | 41 | 0·42% (0·79) | 0·29% (0·38) | 0·43 | |
| CD8 T cells | |||||
| Granzyme | 41 | 1·5% (2·27) | 0·97% (1·55) | 0·16 | |
| IFNγ | 41 | 0·16% (0·24) | 0·11% (0·18) | 0·14 | |
| Perforin | 41 | 1·82% (2·91) | 1·34% (2·27) | 0·26 | |
| Activation-induced markers (%)* | |||||
| CD4+CD137+OX-40+ | 38 | 0·60% (0·74) | 0·66% (1·01) | 0·62 | |
| CD8+CD137+CD69+ | 38 | 0·25% (0·42) | 0·29% (0·43) | 0·26 | |
Data are mean (SD). PBMCs=peripheral blood mononuclear cells. IFN=interferon. IL=interleukin. TNF=tumour necrosis factor.
Data are the proportions of cells expressing the indicated molecules.
To further assess a more specific effect of mutant regions of the delta variant on the T-cell response, IFNγ-linked ELISpot was done. The antigen-specific T-cell immune response was conserved against the delta variant compared with wild-type SARS-CoV-2 (n=24; figure 3l). Compared with unstimulated controls, a robust T-cell response was observed from stimulated cells; secretion of IFNγ was higher for 19 of 24 samples, and thus these samples were considered as responders (appendix p 9).
No significant correlation was identified between geometric mean titres of neutralising antibodies and T-cell IFNγ response (n=23; r=–0·11) or between serum anti-RBD IgG antibody levels and T-cell IFNγ response (n=17; r=0·14). A significant positive correlation was identified between IL-2 expression and serum anti-RBD IgG antibody levels (n=17; r=0·58).
To understand the T-cell response against RBD proteins of wild-type and VOCs, antigen-specific T-cell responses were tested in 59 vaccinated individuals. T-cell responses were observed after stimulation of PBMCs by wild-type and mutant RBD proteins in 40 (68%) of 59 vaccinated individuals. Among these 40 responders—classified based on wild-type RBD-induced IFNγ production—the mean IFNγ secretion was comparable across all variants of SARS-CoV-2, with the exception of the beta variant, for which IFNγ secretion was significantly reduced compared with wild-type RBD (p<0·0001; appendix p 11). Among the 19 non-responders, 14 (75%) individuals did not respond to wild-type or any other VOC RBD. A difference was identified between wild-type and the delta variant SARS-CoV-2 with regards to IFNγ secretion after stimulation of PBMCs with RBD protein; however, this difference was not statistically significant (p=0·24, n=24; appendix p 11).
Of the 150 cases in whom the virus was sequenced, sequences were assembled at an average genome coverage of 6830× (99·5%). 121 samples (81%) were assigned the lineage delta, including AY.1 (a sublineage of the delta variant) in one sample. Eight samples (5%) were assigned kappa lineages, two samples (1%) were assigned the B.1.604 lineage, and two (1%) the alpha lineage. The lineage could not be ascertained in 17 samples (11%; appendix p 9).
Discussion
In the absence of an effective antiviral therapy, vaccination remains the only strategy to mitigate the ongoing SARS-CoV-2 pandemic. ChAdOx1 nCoV-19 is one of the most commonly used vaccines. Multiple VOCs have the potential to jeopardise the effectiveness of vaccines. In a previous study, the ChAdOx1 nCoV-19 vaccine was found to be 10·4% (95% CI −76·8 to 54·8) effective against laboratory-confirmed symptomatic COVID-19 with the beta variant in a secondary endpoint analysis.20 In this study, we report a real-world effectiveness of 63·1% for complete vaccination with the ChAdOx1 nCoV-19 vaccine against laboratory-confirmed SARS-CoV-2 infection during the recent surge of COVID-19 cases caused by the delta variant in India.10 Studies from England and Scotland have reported effectiveness of 60·0–67·0% for the vaccine against infection by the delta variant.21, 22, 23 The delta variant has been shown to be 97% more transmissible than wild-type SAR-CoV-2 and has emerged as the dominant VOC across the world.24 Breakthrough infections due to the delta variant have been reported after vaccination with mRNA vaccines.22, 25
The strengths of our study are use of the WHO-recommended test-negative, case-control design, which balances risk profile, health-care seeking behaviour, and access to care among vaccinated and non-vaccinated people, and the comparability of the vaccine effectiveness estimates with those of randomised trials.26 We minimised the potential bias of prioritised group for vaccination, such as elderly people, by adjusting for age, sex, and risk of COVID-19 exposure. The overall vaccination coverage at the time of the study was low in the Indian population. This low coverage needs to be considered when interpreting our findings since it is known that as vaccine coverage improves, disease incidence at the population level decreases.27 In our study, in addition to the low vaccine coverage, the majority of affected patients had mild COVID-19 with few severe COVID-19 manifestations, which could potentially make the estimates of vaccine effectiveness against severe disease unstable, as evidenced by the wide 95% CIs. We considered severe COVID-19 as a secondary outcome that was intended to be hypothesis generating to aid future studies to evaluate this outcome with adequate power. Our study population included predominantly young men, with a few at high risk of exposure in the workplace. This pattern, although fairly representative of our target population, emphasises the need for vaccine effectiveness studies in different settings to improve the generalisability of our results.
Mutations in the RBD region of the spike protein might compromise binding with neutralising IgG antibodies and thus the reduced neutralising effectivity of vaccine to the delta variant observed in a previous study.4 We also found neutralisation was significantly reduced in response to VOCs, particularly against the delta variant. The mutated proteins have been shown to escape neutralisation by monoclonal antibodies that are in clinical use.5 Although neutralising antibodies are important to prevent virus entry into host cells, an important defence mechanism is the cellular immune response against the virus. We studied the CD4 and CD8 T-cell responses against the SARS-CoV-2 full spike protein using overlapping peptide pools and RBD protein. We found no significant differences with regard to T-cell immune responses against wild-type or the delta variant of SARS-CoV-2. These observations are consistent with a previously published report, which showed that the reduction in total IFNγ secretion in response to variant peptide pools from among individuals vaccinated with Pfizer-BioNTech COVID-19 vaccine or Moderna mRNA-1273 vaccine was not significant, indicating sufficient T-cell response against VOCs.28 Although CD4 and CD8 T-cell responses are key to viral clearance, it has been reported that the CD4 T-cell immune response is more important for controlling SARS-CoV-2 infection than the CD8 T-cell immune response.29 Considering the importance of CD4 T-cell responses in SARS-CoV-2 infection and that the RBD is one of the most immunogenic regions of spike proteins, we used full-length RBD protein to restimulate antigen-specific T cells, since protein stimulation primarily captures the CD4 T-cell reactivity in vaccinated individuals. Although there was a poor correlation between neutralising antibody geometric mean titres and IFNγ T-cell response, a significant positive correlation was observed between the antibody response and the IL-2 production by PBMCs. These observations are consistent with previous observations in COVID-19 convalescent individuals, in whom a poor correlation was identified between the humoral immune response and the T-cell immune response.7 A previous study showed that a conserved immunodominant S346–S365 region in the RBD protein comprising nested HLA-DR-restricted and HLA-DP-restricted epitopes is important for CD4 T-cell responses.30 This region is unaffected by the mutations in VOCs. Another study showed that almost 93% of CD4 T-cell epitopes and 97% of CD8 T-cell epitopes are conserved in SARS-CoV-2 variants. Hence, the cell-mediated immune response might be largely conserved against the point mutations generated in emerging variants of SARS-CoV-2.28
From a policy perspective, a distinction between symptomatic disease and severe disease is important. Although neutralising antibodies might prevent symptomatic disease, cellular immune responses might prevent severe disease. Our study has shown that complete vaccination protected against moderate-to-severe COVID-19 even when the delta variant was dominant. It has been argued that breakthrough infections should only be diagnosed if a vaccinated person develops lower respiratory tract involvement since the vaccines are not designed to provide nasopharyngeal mucosal immunity.31 Our findings support this viewpoint that cellular immune responses might salvage reduced neutralising ability and prevent severe disease among vaccinated people even though protection against infection is compromised.
In summary, two doses of ChAdOx1 nCoV-19 vaccine were 63·1% effective against SARS-CoV-2 infection, caused mostly by the delta variant, and prevented moderate-to-severe COVID-19 in 81·5% of cases. COVID-19 vaccination policies should encourage complete vaccination in addition to other epidemiological public health measures to overcome the threat posed by the delta variant and other emerging variants of SARS-CoV-2.
Data sharing
The de-identified dataset and related codes for analysis will be made available to researchers on request after publication. Requests for data should be addressed to the corresponding author and will need to be approved by the Department of Biotechnology, Government of India (New Delhi, India).
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We gratefully acknowledge Renu Swarup (Department of Biotechnology, Government of India) for her support during this study. We acknowledge the Department of Biotechnology Consortium for COVID-19 Research for enabling this study. We sincerely thank the staff and students of the Translational Health Science and Technology Institute (THSTI; Faridabad, India) for helping to ensure the smooth conduct of the study. This study was supported by an Ind-CEPI grant (102/IFD/SAN/5477/2018-2019) from the Department of Biotechnology to the THSTI, a COVID-19 Bioresource grant (BT/PR40401/BIOBANK/03/2020) from the Department of Biotechnology to the THSTI and the Regional Centre for Biotechnology (Faridabad, India), and Council of Scientific and Industrial Research (MLP-2005) and Fondation Botnar (CLP-0031) grants to the CSIR–Institute of Genomics and Integrative Biology (New Delhi, India). The Mission COVID Suraksha grant (BT/CS0010/CS/02/20) was awarded to THSTI for cellular assays.
Contributors
PKG conceptualised and designed the study. ShB, RT, and NW designed the study for clinical objectives. AA, GM, AnA, NG, SBhatt, and SM designed the study for laboratory objectives. AA, AB, TV, AZ, and DR designed, performed, and analysed T-cell assays. RT, NW, DRM, PKM, JT, AKP, SBhatt, SM, AB, TV, AZ, DR, NAK, HS, SSi, RP, VS, AnA, CS, and SV conducted clinical and laboratory experiments. TS, SSa, PK, RK, JB, PKM, JT, AKP, and NG provided resources. BKD, RT, DRM, AA, GM, SrS, RP, and VS conducted data analyses. PKG, RT, AA, GM, SSa, and TS verified the data, compiled the results, and wrote the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Public Health England SARS-CoV-2 variants of concern and variants under investigation. Technical briefing 10. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/984274/Variants_of_Concern_VOC_Technical_Briefing_10_England.pdf
- 2.WHO COVID-19 weekly epidemiological update. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20210511_Weekly_Epi_Update_39.pdf [DOI] [PMC free article] [PubMed]
- 3.Supasa P, Zhou D, Dejnirattisai W, et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184:2201. doi: 10.1016/j.cell.2021.02.033. 11.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 6.Yadav PD, Sapkal GN, Abraham P, et al. Neutralization potential of Covishield vaccinated individuals sera against B.1.617.1. bioRxiv. 2021 doi: 10.1101/2021.05.12.443645. published online May 12. (preprint). [DOI] [PubMed] [Google Scholar]
- 7.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhar MS, Marwal R, Radhakrishnan VS, et al. Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. medRxiv. 2021 doi: 10.1101/2021.06.02.21258076. published online June 3. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . World Health Organization; Geneva: March 17, 2021. Evaluation of COVID-19 vaccine effectiveness.https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-vaccine_effectiveness-measurement-2021.1 [Google Scholar]
- 11.Mehdi F, Chattopadhyay S, Thiruvengadam R, et al. Development of a fast SARS-CoV-2 IgG ELISA, based on receptor-binding domain, and its comparative evaluation using temporally segregated samples from RT-PCR positive individuals. Front Microbiol. 2021;11 doi: 10.3389/fmicb.2020.618097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bewley KR, Coombes NS, Gagnon L, et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat Protoc. 2021;16:3114–3140. doi: 10.1038/s41596-021-00536-y. [DOI] [PubMed] [Google Scholar]
- 13.Shrivastava T, Singh B, Rizvi ZA, et al. Comparative immunomodulatory evaluation of the receptor binding domain of the SARS-CoV-2 spike protein; a potential vaccine candidate which imparts potent humoral and Th1 type immune response in a mouse model. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.641447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geers D, Shamier MC, Bogers S, et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 16.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhoyar RC, Jain A, Sehgal P, et al. High throughput detection and genetic epidemiology of SARS-CoV-2 using COVIDSeq next-generation sequencing. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rambaut A, Holmes EC, O'Toole Á, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padma TV. India's COVID-vaccine woes–by the numbers. Nature. 2021;592:500–501. doi: 10.1038/d41586-021-00996-y. [DOI] [PubMed] [Google Scholar]
- 20.Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emary KRW, Golubchik T, Aley PK, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell F, Archer B, Laurenson-Schafer H, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Public Health England Effectiveness of COVID-19 vaccines against hospital admission with the delta (B.1.617.2) variant. https://khub.net/documents/135939561/479607266/Effectiveness+of+COVID-19+vaccines+against+hospital+admission+with+the+Delta+%28B.1.617.2%29+variant.pdf/1c213463-3997-ed16-2a6f-14e5deb0b997?t=1623689315431 (preprint).
- 26.De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.37.20585. [DOI] [PubMed] [Google Scholar]
- 27.Public Health Ontario COVID-19 real-world vaccine effectiveness–what we know so far. https://www.publichealthontario.ca/-/media/documents/ncov/covid-wwksf/2021/04/wwksf-vaccine-effectiveness.pdf?la=en
- 28.Tarke A, Sidney J, Methot N, et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Low JS, Vaqueirinho D, Mele F, et al. Clonal analysis of immunodominance and cross-reactivity of the CD4 T cell response to SARS-CoV-2. Science. 2021;372:1336–1341. doi: 10.1126/science.abg8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schieffelin JS, Norton EB, Kolls JK. What should define a SARS-CoV-2 “breakthrough” infection? J Clin Invest. 2021;131 doi: 10.1172/JCI151186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The de-identified dataset and related codes for analysis will be made available to researchers on request after publication. Requests for data should be addressed to the corresponding author and will need to be approved by the Department of Biotechnology, Government of India (New Delhi, India).