Abstract
Mitochondria have been recognized as key organelles in cardiac physiology and are potential targets for clinical interventions to improve cardiac function. Mitochondrial dysfunction has been accepted as a major contributor to the development of heart failure. The main function of mitochondria is to meet the high energy demands of the heart by oxidative metabolism. Ionic homeostasis in mitochondria directly regulates oxidative metabolism, and any disruption in ionic homeostasis causes mitochondrial dysfunction and eventually contractile failure. The mitochondrial ionic homeostasis is closely coupled with inner mitochondrial membrane potential. To regulate and maintain ionic homeostasis, mitochondrial membranes are equipped with ion transporting proteins. Ion transport mechanisms involving several different ion channels and transporters are highly efficient and dynamic, thus helping to maintain the ionic homeostasis of ions as well as their salts present in the mitochondrial matrix. In recent years, several novel proteins have been identified on the mitochondrial membranes and these proteins are actively being pursued in research for roles in the organ as well as organelle physiology. In this article, the role of mitochondrial ion channels in cardiac function is reviewed. In recent times, the major focus of the mitochondrial ion channel field is to establish molecular identities as well as assigning specific functions to them. Given the diversity of mitochondrial ion channels and their unique roles in cardiac function, they present novel and viable therapeutic targets for cardiac diseases.
Keywords: bioenergetics, cardiac function, cardioprotection, ion channels, mitochondria
INTRODUCTION
Heart diseases are among the top causes of morbidity and mortality (1), but the underlying mechanisms that lead to heart failure are not fully understood. There is an urgency to understand and decipher the precise molecular mechanism(s) involved in cardiac diseases. Ion channels and transporters located in different cardiac cells are often targeted in therapeutic interventions for over 60 years (2–7). Amiodarone and lidocaine were the first groups of compounds targeted for sarcolemmal ion fluxes to treat arrhythmias (8). An intricate analysis of the molecular basis of cardiac arrhythmias has been the driving force behind establishing the molecular identity of the ion channels that generate the cardiac action potential (9). As of today, the genes encoding all the major cardiac membrane ion channels have been sequenced and identified (9, 10). As expected, several studies using pharmacological and genetic approaches have revealed a greater complexity than originally predicted (9, 11). Several of these ion channels function as macromolecular complexes. They either physically interact with each other or are functionally coupled at specific sites within the plasma membrane (9). Molecular and technical advancements that regulate the action potential hold the promise that these genes could be manipulated to treat arrhythmias (10, 11).
Even though technical advances have facilitated the establishment of proof of principle to treat cardiac diseases, the precise mechanism for cardiac diseases such as arrhythmias is still not completely deciphered. There are several gaps in our understanding of the molecular basis of cardiac function and dysfunction. It is becoming clear that there are subcellular pathways responsible for aberrant cardiac dysfunction. One of the key organelles responsible for meeting the crucial energy demand of the heart is the mitochondria. Heart dysfunction is often accompanied by mitochondrial dysfunction, providing a crucial link between bioenergetics and cardiac mechanics (12–14). In a typical mammalian heart, mitochondria account for 30% of the myocardium by volume and produce over 90% of cardiac adenosine triphosphate (ATP) (15, 16). Mitochondria oxidize dietary hydrogen (from food fed through the pyruvate cycle) with O2 to generate heat and ATP. Ideally, the inner membrane of mitochondria is maintained at around −180 to −240 mV. The depolarization of the inner mitochondrial membrane is often linked to disruption of ATP production, generation of reactive oxygen species (ROS), and triggering apoptosis. Mitochondria are one of the most negatively charged organelles in the cell and also maintain ionic homeostasis inside the matrix, which is different from cytosol and extra cellular environment (Table 1). Ionic homeostasis in mitochondria is regulated by ion channels and transporters present in mitochondrial membranes.
Table 1.
Ion concentrations in a different compartment of the cell
| Ion | Cytosol | Mitochondrial Matrix | Extracellular (Serum) | References |
|---|---|---|---|---|
| H+ | 62 nM | 10–20 mM | 0.1 mM | (18–20) |
| K+ | 150 mM | 215 mM | 5 mM | (6, 21–23) |
| Na+ | 10 mM | 5 mM | 150 mM | (24–28) |
| Ca2+ | 40–100 nM | 0.1–2 µM | 2.5 mM | (18, 29–34) |
| Mg2+ | 17–20 mM | 0.35–2.4 mM | 0.85–1.10 mM | (28, 31, 35, 36) |
| Cl− | 5–60 mM | 0.9–22.2 mM | 96–105 mM | (37–39) |
| PO4− | ∼6 mM | ∼15 mM | 0.83–1.5 mM | (40, 41) |
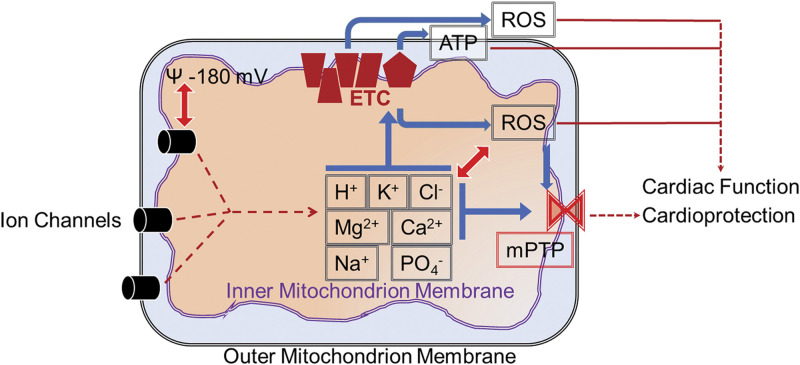
Mitochondria have two membranes, outer and inner that create two isolated compartments (Fig. 1): the intermembrane space and the mitochondrial matrix (17). Though the outer membrane of mitochondria possesses ion channels (42), it is less selective and acts as a molecular sieve. In contrast, the inner membrane is highly ion-selective (43–47). The key function of the inner mitochondrial membrane is the generation of proton motive force, utilizing the energy produced by burning fats and sugars (48–50). The proton motive force is then used by ATP synthase in mitochondria to synthesize ATP from ADP and inorganic phosphate (Pi). Membrane potential across the inner membrane is very high, which causes the mitochondrial matrix to accumulate Ca2+ ions and form complexes with phosphates and carbonates (51). The majority of the ion channels characterized so far are present in the inner mitochondrial membranes (14, 51). Surprisingly, there are continuous dynamic processes involving the transportation of ions across the inner membrane resulting in the accumulation of salt precipitates in the mitochondrial matrix (24). These salt precipitates (24) are vital during pathological stressors such as ischemic or hypoxic insults where they act as pH buffers and contribute to the continuous production of ATP. Mitochondrial ion channels are extensively studied in terms of protecting the heart or cardiac cells from ischemia-reperfusion injury. The molecular identity of several of these cardioprotective mitochondrial ion channels has been established, facilitating the more focused approach to target them for therapeutics. However, whether mitochondrial ion channels play any role in the physiology of the heart is still being elucidated (Fig. 1).
Figure 1.
Ion channels present in outer, inner, or both membranes regulate ion flux across mitochondrial membranes. Mitochondrial ionic homeostasis regulates the membrane potential of the mitochondrion. Electron transport chain (ETC) coupling present in the inner membrane is controlled by mitochondrial membrane potential which in turn modulates ATP and reactive oxygen species (ROS) production. Mitochondrial ions also directly regulate ROS production by mitochondrial ETC complexes. Mitochondrial ions and ROS in turn regulate mitochondrial permeability transition pore (mPTP). Ionic homeostasis is coupled with mitochondrial ROS either directly or indirectly via regulating ion channels in mitochondria or other organelles. All the factors, mitochondrial ions, ROS, and ATP, modulate the cardiac function and also participate in cardioprotection against ischemia-reperfusion injury.
This review aims to highlight the role of mitochondrial ion channels in cardiac function, emphasizing that mitochondrial ion channels, similar to the cardiac plasma membrane channels, also exist as macromolecules and are functionally coupled with each other either in specific sites or by physical interactions. The majority of the focus for this review will concentrate on ion channels present in cardiac mitochondria.
MITOCHONDRIA AS THERAPEUTIC TARGETS
Due to the extensive role of mitochondria in different physiological and pathophysiological conditions, a new term, “mitochondrial medicine,” has been coined to focus on mitochondrial diseases as well as mitochondria as a therapeutic target (52, 53). During oxidative phosphorylation (OXPHOS), electrons are collected from the oxidation process to allow the generation of reducing equivalents nicotinamide adenine dinucleotide (NAD) + hydrogen (H) and flavin adenine dinucleotide (FADH2). Electrons flow through the complexes of the electron transport chain (ETC) (Fig. 1), H+ are pumped out into the intermembrane space from the matrix. This creates a strong negative mitochondrial membrane potential designated as Δψm, which can be utilized to target drugs to the mitochondria. The movement of H+ down the proton gradient across the inner membrane drives the final complex of the ETC, ATP synthase, which converts ADP to ATP. The proton gradient is coupled with other mitochondrial ions to maintain appropriate ionic homeostasis. Any disruption in mitochondrial ion homeostasis due to activation or deactivation or abnormal behavior of mitochondrial channels could lead to mitochondrial dysfunction. The major readouts from mitochondria such as ATP, ROS, and Ca2+ that are regulated by mitochondrial ion channels and transporters play a significant role in cardiac function and cardioprotection (Fig. 1). Therefore, it is imperative to establish the molecular identity of mitochondrial ion channels and transporters. Once the molecular identity is established, it will facilitate elucidation of the specific role and function of cardiac mitochondrial ion channels. The next key question is how these mitochondrial ion channels target specifically to outer/inner or both membranes of mitochondria. Some of the channels possess a classical mitochondrial targeting sequence (54, 55) but several of these channels lack the mitochondrial targeting sequence. Another important mitochondrial targeting factor is splice variation (56, 57), which facilitates the interaction of ion channels with chaperones (58) to target them to mitochondria (59–62).
MITOCHONDRIAL CALCIUM CHANNELS
Mitochondrial Ca2+ plays an important role in the regulation of mitochondrial and cellular function. Mitochondria take up Ca2+ from the cytosol in response to cellular stimuli which in turn modulate the TCA cycle, energy production, and cellular metabolism. To uptake Ca2+, mitochondria are often located in close proximity to a Ca2+ source [e.g., near the plasma membrane, endoplasmic reticulum (ER), or sarcoplasmic reticulum (SR)]. Ca2+ transport into mitochondria is tightly regulated as excessive Ca2+ influx will activate the formation and opening of mitochondrial permeability transition pore (mPTP). When the oxidative phosphorylation chain is active, the membrane potential on the inner mitochondrial membrane is highly negative (∼−180 mV), which causes the influx of calcium to the matrix through a high capacity Ca2+-selective ion channel called mitochondrial calcium uniporter (MCU) channel (63–65). The channel consists of four MCU subunits. The MCU super complex consists of EMRE, MICU1/2, MCUb, MCUR1, and SLC25A23 (66). Ca2+ flux through the MCU channel (67) super complexes regulates Ca2+-dependent cell functions such as apoptosis, autophagy, transcription, and cell survival (68).
In cardiac muscle, Ca2+ plays a significant role in contraction and relaxation. Mitochondrial Ca2+ in cardiac tissue plays a critical role in meeting the energy demands. Though pharmacological and mitochondrial Ca2+ studies implicated MCU in heart function, the major breakthrough came after the discovery of MCU and its components as well as genetic mice models. Global and cell-specific null-mutant mice as well as gain-of-function indicated that MCU is involved in the onset and transmission of Ca2+ transients during cardiac contractions. At the resting stage, null-mutant mice for MCU present normal cardiac function (69) but during stress such as fight-or-flight responses MCU null-mutant mice showed an impaired heart rate acceleration (70). During ischemia and reperfusion, Ca2+ flux increases in mitochondria, resulting in the formation and opening of mitochondrial permeability transition pore (mPTP). The absence of MCU prevents Ca2+ overload of mitochondria, hence offers cardioprotection against ischemia-reperfusion injury (71, 72). Given the importance of Ca2+ in mitochondria function, it is expected to possess several other Ca2+ channels and transporters such as mitochondrial Na+/Li/Ca2+ exchanger (NCLX) (73). In agreement, the lack of MCU significantly reduced mitochondrial matrix Ca2+ (to around 25% of wild type) but does not completely eliminate it (74), indicating the presence of an independent Ca2+ uptake mechanism. Therefore, to decipher the role of mitochondrial Ca2+ channels in cardiac function, other possible Ca2+ channels are to be identified. On these lines, mitochondrial NCLX encoded by Slc8b1 was shown to be a vital mechanism involved in mitochondrial Ca2+ flux. Overexpression of NCLX offers cardioprotection to mice subjected to the permanent occlusion of the left coronary artery or pressure overload (73). In contrast, ablation of NCLX caused sudden death, and surviving mice presented left ventricular dilation and compromised left ventricular function (73). Mice lacking NCLX presented cardiac fibrosis and hypertrophy (73) indicating that Ca2+ is important for the maintenance of cardiac structure and function.
Studies carried out on mitochondrial ion fluxes and mechanisms involved demonstrate that mitochondrial Ca2+ homeostasis is regulated by MCU, and NCLX is indispensable for cardiac function at the basal as well as during stress.
MITOCHONDRIAL SODIUM CHANNELS
Analysis of Na+ ions in mitochondria (mitoNa+) versus cytosol (cytoNa+) indicate that mitoNa+ concentrations are either equal or lower than cytoNa+ concentrations (27, 28, 75). Using different types of assays, mitoNa concentration is estimated to be around 5 mM in cardiac cells (28, 75), and 50–113 mM in Madin-Darby Canine Kidney (MDCK) cells (26). When compared with other ions, mitoNa+ was nearly equivalent to the H+ gradient. However, the uptake of Pi by respiring mitochondria decreases matrix pH and increases matrix [Na+]. The concentration of mitoNa+ is maintained as a result of its coupling with an endogenous Na+-H+ antiporter. In ventricular myocytes, inhibition of both aerobic metabolism and glycolysis produces a gradual increase in mitoNa. An increase in mitochondrial Na+ concentration is coupled with a decrease in systolic contraction and ATP levels (28, 75). The existence of Na+/H+ exchanger (NHE) was proposed by Mitchell and Moyle in 1969 (76), and later on, NHE was proposed by Garlid in 1991 (77). NHE was shown to be the major cation transporter in mitochondria.
In myocardial infarction (MI), mitoNa+ levels show an early increase which could be due to decreased activity of NHE and hence restricted efflux of Na+. During MI, NCX could still be active causing accumulation of cytosolic Na+. In due course, mitochondrial NCX (NCLX) is also reversed which will cause the second phase decrease in mitoNa+ levels. The second phase decrease was completely abolished in the presence of CGP-37157, a specific inhibitor of the mitochondrial NCLX (36). These results indicate that levels of mitoCa2+, mitoNa+, and mitoH+ and the mitochondrial membrane potential influence mitochondrial Na+ handling.
One of the key roles attributed to Na+ ions in mitochondria is to regulate Ca2+ homeostasis. Earlier experiments using nigericin (acts as K+, Na+, H+, and Pb2+ ionophore, and usually used as antiporter for K+ and H+ across biological membranes) showed that high rates of Ca2+ efflux can be maintained by mitoNa+ levels. Na+ gradient is usually maintained by the Na+-H+ antiporter and provides a strong electromotive force to drive Ca2+ into mitochondria. Though there is clear evidence for the existence of an efficient Na+ exchange mechanism, there are no specific ion channels present to transport Na2+. Inhibition of Na+ uniporter usually by Mg2+ directly impacts mitochondrial function (28). In contrast, mitochondrial ROS and NAD+ levels are known to regulate cardiac voltage-gated sodium channel (SCN5A) currents (INa). Using SCN5 null-mutant mice (28) it was shown that an increased cytosolic NADH results in decreased INa, which can be reversed by NAD+, suggesting a link between metabolism and INa.
MITOCHONDRIAL POTASSIUM CHANNELS
Mitochondrial matrix volume is regulated by the net balance of K+ uptake and release (78). As the concentration of K+ is higher in the cytosol compared with mitochondria, they can be assumed as “potassium sinks” within the cell. Mitochondrial membrane potential across the inner membrane drives the electrophoretic influx of K+ and is balanced by the electroneutral K+/H+ antiporter (79, 80). Dysregulation of the K+ influx could result in mitochondrial depolarization and matrix swelling. Therefore, K+ flux in and out of mitochondria is required to be tightly regulated by modulating and fine-tuning ion channels and transporters. Opening of ion channels permeable to K+ is associated with a mild depolarization of mitochondrial membrane potential which could result in cytoprotective effects (80, 81). Complete depolarization of mitochondrial membrane potential could result in mitophagy, which is a highly energy-consuming process and cells will need to invest their resources in producing new mitochondria to replace degraded mitochondria.
Over the last two decades, several K+ channels have been described in the inner membrane of mitochondria (Table 2; 56, 82, 83, 87, 89, 92–94, 98–100, 102, 103). The majority of the focus is on ATP-sensitive K+ channel (mitoKATP, inhibited by physiological levels of ATP) (4, 86, 104, 105) and large-conductance Ca2+ and voltage-gated K+ channel (mitoBKCa) (2, 6, 56, 90, 106–109). Recently, additional K+ channels were identified in the inner membrane of mitochondria (86, 110). There are several outstanding questions on the existence of multiple potassium channels in mitochondria. It is possible that the presence of mitochondrial potassium channels is cellular or organ-specific. One or more potassium channels co-exist in the inner membrane of mitochondria which are functionally coupled with each other as well as with K+/H+ antiporter and other transporters (81). Mitochondrial potassium channels form a dedicated potassium ion transport mechanism that can either function in tandem or compensate for the loss of one or the other channel. At the organelle level, these potassium channels are implicated in regulating mitochondrial function, such as energy-production, ROS generation, ionic homeostasis, Ca2+ retention capacity (CRC) handling, and membrane potential (2, 111). At cellular and organ levels, the opening of mitochondrial K+ channels is associated with protecting cells and organs from hypoxia/ischemia-reperfusion injury (2, 80, 112). There is a well-established cross-coupling between sarcolemmal K+ channels and fluctuation in Ψmito. Specifically, the opening of sarcolemmal KATP channels causes hyperpolarization of the membrane, which creates a conduction block and reduces mitochondrial energy production which is termed as a “metabolic sink” (113).
Table 2.
Potassium channels in mitochondrial membranes
| Potassium Channels | Gene | References |
|---|---|---|
| mitoKATP channel | KCNJ1, CCDC51 | (82–86) |
| mitoSKCa channel | KCNN1, KCNN2, and KCNN3 | (87, 88) |
| mitoIKCa channel | KCNN4 | (89) |
| mitoBKCa channel | KCNMA1 | (56, 90, 91) |
| mitoKv1.3 channel | KCNA3 | (92) |
| mitoKv1.5 channel | KCNA5 | (93) |
| mitoKv7.4 channel | KCNQ4 | (94) |
| mitoTASK3 channel | KCNK9 | (95–97) |
| mitoSLO2 | KCNT2 | (98, 99) |
| mitoHCN | HCN1, HCN2, HCN3, and HCN4 | (100, 101) |
BKCa, large conductance calcium-activated potassium channels; HCN, hyperpolarization-activated cyclic nucleotide-gated; IKCa, intermediate-conductance Ca2+-gated K+ channel; KATP, ATP-gated potassium channel; Kv, voltage-gated potassium channel; SKCa, small-conductance Ca2+-gated K+ channel.
Though the role of mitochondrial potassium channels in cardioprotection is well established and reviewed by several independent groups, here, I will focus on the role of mitochondrial potassium channels in cardiac function. The major challenge in defining roles for mitochondrial potassium channels is their presence in other membranes such as plasma membrane, and nuclear or endoplasmic reticulum membranes. Unless otherwise specified, these functions could also originate from nonmitochondrial structures. A significant increase in ventricular funny currents (If) in a variety of cardiac diseases due to hypertension, and ischemic and dilated cardiomyopathy has been reported (114). If rises from hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, and their expression also varies in the myocardium and is highly dependent on the development and the presence of pathological state of the heart. Na+-activated potassium channels (Slick, KNa, SLO2) are activated by Ca2+ and Cl− and present high conductance and prominent subconductance states (115). These channels were shown to be present in cardiomyocyte plasma and mitochondrial membranes. Activating KNa results in cardioprotection from ischemia-reperfusion injury. Rate pressure product (RPP), a measurement of the internal workload of the heart, indicates that the expression of Slo2 is vital for maintaining heart rate and systolic blood pressure (99). A member of the KCNK gene family, TASK3 channels are leaking conductances that result in background currents and are usually present in most cell membranes. They are also localized to the mitochondria where they are predicted to regulate cristae morphology, membrane potential, apoptosis, and aldosterone biosynthesis (95–97). Aldosterone receptors are present in the heart and are known to modulate heart rate (116).
Among voltage-gated potassium channels, Kv1.3, Kv1.5, and Kv7.4 have been reported in inner mitochondrial membranes (92–94). Specifically, retigabine, a Kv7.4 opener in the rat heart, improved cardiac function such as RPP and change in pressure over time (dP/dt) in a Langendorff model albeit at a higher concentration (94). Though Kv1.5 is known to play a vital role in repolarizing K+ currents, the role of mitoKv1.5 is not yet elucidated. Kv1.3 is a key player in metabolism as the null mutant mice present lower body weight and decreased adiposity, increased total energy expenditure, increased locomotor activity, and resistance to either diet- or genetic-induced obesity. Both Kv1.3 and Kv1.5 are shown to play a crucial role in coupling myocardial blood flow to cardiac metabolism.
mitoKATP is one of the most extensively studied mitochondrial K+ channels. Opening of KATP protects the heart from IR injury but under physiological conditions, the channel is expected to be dormant. The molecular identity and precise pharmacological tools for mitoKATP are still being characterized. Recently, CCDC51 together with a member of the ABC transporter family (ABCB8) was shown to form a mitoKATP-like channel. The renal-outer-medullary-potassium (ROMK, KCNJ1) channel and MITOK (CCDC51) are the forefront candidates for the mitoKATP channel (85, 86). Pharmacological opening of KATP by diazoxide improves cardiac function including left ventricular ejection fraction (LVEF) and fractional shortening (LVFS). ROMK null-mutant mice have reduced blood pressure at baseline (117), a decreased afterload, and altered arterial elastance, but increased preload-independent indexes indicating a direct effect on cardiac contractility (85). ROMK null-mutant mice do not develop bradycardia or ECG abnormalities at the neonatal stage but adult mice present significant abnormalities in ECG, which are predicted to be caused by electrolyte imbalances associated with hypo-hyperkalemia (85). The decrease in left ventricle dimension presented in ROMK null-mutant mice (KCNJ1−/−) is also caused by systemic defects. However, the ROMK cardiomyocyte-specific knockout mouse (KCNJ1fl/fl × α-MHC-Cre mouse) presented no changes in the cardiac structure (85). The majority of the pharmacological studies focused on KATP involve diazoxide or the substituted fatty acid 5-hydroxydecanoate (5-HD) (118–120). In the literature, there is evidence of nonspecific actions of diazoxide and 5-HD as well as the existence of ATP-sensitive K+ currents that are not sensitive to known “mitoKATP” activators or blockers (68, 118–121). However, pharmacological blocking of mitoKATP does prevent the beneficial antiarrhythmic effects seen with ischemic preconditioning. However, activation of mitoKATP channels presents contrasting results in preventing arrhythmia before the onset of ischemia but not after ischemic insult (122, 123). Genetic models do provide an insight into the possible role of ATP-sensitive channels in cardiac function or at least with respect to cardioprotection against ischemia-reperfusion injury (86).
Our group and others have established that BKCa is present in cardiac mitochondria (2, 56, 79, 90, 91, 106, 124–130), and is a C-terminus splice variant encoded by the Kcnma1 gene (56). In adult cardiomyocytes, BKCa is exclusively present in the inner membrane of mitochondria and not in the plasma membrane (56, 131). BKCa has been shown to play a role in cardiac function. Inhibition of BKCa channels mediated bradycardia is the direct result of sinoatrial (SA) nodal cell inhibition and reduction of SA nodal action potential generation (132, 133). Using pharmacological tools, it was shown that BKCa does play a role in cardiac function (88, 134). Paxilline (cell-permeable blocker, possibly targeting mitoBKCa) altered the heart rate, LVEF, and global longitudinal strain within a range of 1–15 min, but iberiotoxin [IBTX, cell impermeable blocker (135)] did not present any change up to 40 µg/mL. IBTX did reduce the coronary artery mean velocity indicating the involvement of plasma membrane BKCa in coronary arteries (134). In the guinea pig, blocking BK with Paxilline or SK with NS8593 or both antagonists at the same time increased myocardial infarction and worsened the left ventricular pressure (88). In agreement with pharmacological data (134), implicating mitoBKCa in cardiac function, a cardiomyocyte-specific BKCa knockout mouse also showed a reduction in global longitudinal strain, LVEF, and an increase in fibrosis (90). These studies exclusively performed in adult animal models show that mitoBKCa participate in cardiac function as well as cardioprotection. The expression of BK changes with age and specifically in coronary myocytes that affect its contractility (136). In the future, it will be of interest to study how changes in the expression of cardiac BK with age (neonates, young, and old) affect cardioprotection from IR injury and cardiac function.
Surprisingly, most of the mitoK channels are opened by cAMP (137–139) or cGMP (90, 140–143) or both (144), which provides a common theme for their physiological activation in mitochondria in addition of voltage and Ca2+ and other factors. Initial data, from studies on ROMK and BKCa suggest that regardless of the mechanism involved, mitochondrial K+ channels can be tested as possible targets on a short-term or acute basis to modulate cardiac inotropy. The key finding is that these channel-mediated impacts are completely reversible which should be taken into account for use during short-term treatment and emergencies.
MITOCHONDRIAL ANION CHANNELS
Chloride currents are implicated in the diastolic potential, action potential duration, and membrane conductance of cardiac tissues. In fact, voltage-clamp experiments indicated that a transient outward current is carried by Cl− ions (145, 146). Though there was some initial evidence of the physiological roles of Cl− in cardiac function, they were largely ignored once potassium was shown to be a major charge carrier for the transient outward current in sheep Purkinje fibers (147). However, persistent efforts by several groups indicated that a distinct Cl− current exists in cardiac cells (148) and in mitochondria (149).
Anion channels, originally termed as leak channels, are being increasingly recognized as key players in regulating mitochondrial ionic homeostasis. Prominent anion channels shown by experiments are presented in Table 3. Though anion channels are often ignored, the very first ion channel to be recognized in mitochondrial was the voltage-dependent anion channel (VDAC). VDAC is predominantly present in the outer mitochondrial membrane and also allows Ca2+ to pass through. There are three VDAC isoforms identified of which VDAC1 is the most abundant protein. One of the most prominent roles associated with VDAC apart from the transport of anions, cations, and metabolites is apoptosis. The role of VDAC in cardiac IR injury or function is not yet deciphered but inhibiting VDAC opening significantly attenuates myocardial IR injury (177, 178). Recent studies (179) show that the expression of VDAC1 increases in the human heart after MI, as well as in the left ventricle of patients with chronic cardiac dilation, hypertrophy, and dysfunction. Similar overexpression of VDAC1 was observed in the left atrium and left ventricle of rodents after MI or excessive aldosterone levels. These studies implicate VDAC1 in the myocardium under different pathological conditions, and blocking VDAC1 could provide beneficial effects. However, it is not clear whether the flux of anions or Ca2+ is responsible for VDAC1-related myocardial dysfunction. A recent study implicated VDAC2 in influencing excitation-contraction coupling (ECC) and cardiac disease progress progression (180). So far, both VDAC1 and VDAC2 are projected as therapeutic targets for heart failure.
Table 3.
Anion channels in mitochondrial membranes
| Anion Channels | Gene | References |
|---|---|---|
| VDAC1 | Vdac1 | (150–153) |
| VDAC2 | Vdac2 | (154–158) |
| VDAC3 | Vdac3 | (54, 153, 159–162) |
| CLIC4 | Clic4 | (163–168) |
| CLIC5 | Clic5 | (149, 169) |
| IMAC | Not identified | (170–172) |
| Ca2+-activated chloride channel, anoctamin-1 | TMEM16A | (173,174) |
| Ceramide channels | Lipid molecules | (175,176) |
CLIC, chloride intracellular channel proteins; IMAC, inner membrane anion channel; VDAC, voltage-dependent anion channel.
Although VDAC was the first mitochondrial ion channel characterized, it was the only anion channel localized to the outer mitochondrial membranes. In the inner mitochondrial membranes, the existence of an Mg2+ and pH-dependent anion transport mechanism was described in the early 1960s (181), which was reiterated by a study that indicated anion transportation decreases in low pH (182). Inner mitochondrial membrane presents a varying degree of permeability to anions like Cl−, Br−, I−, SCN−, NO3−, PO4−, HCO3−, and SO42− indicating a presence of inner membrane anion channel (IMAC) (183). The single-channel conductance of IMAC is around ∼107 pS (184). Though the molecular identity of IMAC is still not deciphered, it is involved in volume homeostasis. In the heart, IMAC has been implicated in electrical and contractile dysfunction in the heart after ischemic injury (51, 185, 186). In cardiac cells, IMAC inhibition prevents reverse oscillatory mitochondrial depolarizations induced by substrate deprivation and oxidative stress (51, 113, 186, 187). Along with porins, channels made by lipid molecules, Ceramide [C2, C16, C18,-ceramide (N-palmitoyl-d-erythro-sphingosine)] have been proposed to exist in the outer mitochondrial membrane forming a large channel with a diameter of 10 nm (175). These channels are implicated in regulation of apoptosis and could play a role in cardiac protection, but the area remains largely unexplored (175, 176).
Another anion channel, anoctamin 1 (ANO1), a Ca2+-activated Cl− channel (CaCC) encoded by TMEM16A, usually found in gastrointestinal epithelia, skeletal, and smooth muscle cells were recently identified in the inner membrane of mitochondria of rat lung microvascular endothelial cells (173). In mitochondria, ANO1 interacts with OPA1 and is implicated in the regulation of mitochondrial bioenergetics and cristae formation. The role of ANO1 in cardiac function is not known but after ischemic injury the expression of ANO1 increases in the cardiac tissue. The channel is associated with accelerated early phase 1 repolarization of action potentials (APs) and a deeper “spike and dome” after ischemia. The role of ANO1 in ischemia-induced arrhythmias is attributed to the plasma membrane variant as information on the mitochondrial variant is not yet complete in cardiac cells.
A novel class of anion channel (37, 188, 189), chloride intracellular channel proteins 4 (CLIC4) and 5 (CLIC5) were recently characterized in mitochondrial membranes (3, 149, 169). CLIC4, originally known as p64H1 was localized to mitochondrial membranes and is also called mtCLIC (163,164, 190,191). Our recent study revealed that CLIC4 is localized to the outer membranes of cardiac mitochondria. On the other hand, CLIC5 was localized to the inner mitochondrial membranes. There are no specific roles assigned to CLIC4 or CLIC5 in cardiac function. The single-channel conductance of CLIC4 is ∼15 pS but CLIC5 can present up to ∼110 pS conductance. The channel activity of CLIC4 and CLIC5 is blocked by IAA-94. IAA-94 was originally used to purify p64 protein (192) and which was later named CLIC. IAA-94 increases myocardial infarction after ischemia-reperfusion injury and cardiac cell death implicating CLIC proteins in cardioprotection (193–196). IAA-94 also reverses and even stopped high ventricular fibrillation frequencies caused by hypo-osmotic stress, indicating that IAA-94-sensitive Cl− channels in the maintenance of ventricular fibrillation (197). All the studies carried out so far implicate IAA-94-sensitive Cl channels, such as CLICs in cardioprotection and arrhythmias. In the future, null-mutant mice could provide information on mitochondrial anion channels in cardiac function.
Mitochondrial anion channels are increasingly being implicated in cell death, pH and volume regulation, cardioprotection against ischemic injury as well as arrhythmias. A combination of genetic and specific pharmacological tools is required to dissect the specific roles of these channels in cardiac function.
MITOCHONDRIAL GAP JUNCTIONS
The key mechanism of communications between cardiac cells is the gap junction. Gap junctions, also termed connexins, are also responsible for the transport of regulatory molecules between cells (198). There are four different members of the connexin (Cx) family of gap junctions in the heart: Cx43, Cx45, Cx40, and Cx37. So far, Cx43 is localized to the cardiac mitochondria with its carboxy-terminus directed toward the intermembrane space (199, 200). Cx43 forms a channel with six subunits allowing a low-resistant current propagation and passage of small molecules. In cardiac mitochondria, it regulates oxygen consumption and potassium fluxes. Inhibition of Cx43 by pharmacological and genetic approaches reduces complex I-mediated oxygen consumption and ATP production (200). Cx43 also regulates mitochondrial ROS and calcium levels (201, 202) in heart cells, implicating them in cardioprotection as well as cardiac function. The expression of mitochondrial Cx43 declines with age (203), hypertension, diabetes, and hypercholesterolemia, and heart failure (199). In the myocardial infarction model, an increase in ROS generation has been linked to the downregulation of Cx43 (204). Decreasing mitochondrial superoxide production rescues mitochondrial function and restores Cx43 expression, which increases conduction velocity and reduces arrhythmias inducibility (122, 205, 206). Though the role of Cx43 in heart function is well established, it is vital to dissect the role of mitochondrialCx43 from the plasma membrane from Cx43.
OTHER ION TRANSPORT MECHANISMS
Another important class of ion transport mechanism in the mitochondrion is SLC25 family proteins. The SLC25 is also known as mitochondrial carrier family (MCF) and has a large diversity in terms of solutes being transported through them. The transport facilitated by these MCFs could be electroneutral or electrogenic. They are divided into citrate (CIC), phosphate (PIC), ADP and ATP (AAC), dicarboxylates (DIC), aspartate, glutamate (AGC), and sulfate (UCPs). MCFs transport ions, amino acids, nucleotides, dinucleotides, carboxylates, keto acids, and substrates transported through them and play a significant role in regulating mitochondrial ionic homeostasis and function. To keep the review focused on ion homeostasis, I have not included nonionic transports through MCFs or some ion channels which are capable of transporting small molecules (150, 151, 157, 207–212).
The ion transport mechanism responsible for regulating mitochondrial proton concentrations is uncoupling proteins (UCPs). There are five UCP homologs; UCP1 (SLC25A7), UCP2 (SLC25A8), UCP3 (SLC25A9), UCP4 (SLC25A27), and UCP5 (SLC25A14) in mammals, and they are located in the inner membrane of the mitochondrion. UCP1 is chiefly localized to the brown adipose tissue, UCP2 is present in the heart, and UCP-3 is present in skeletal muscles. In the heart, loss of UCP2 from fibroblasts results in loss of right ventricular function upon pressure overload (213), however, in heart failure, UCP-2 inhibition in cardiomyocytes is beneficial as it improves the efficiency of glucose oxidation (214). In failing rat hearts, levels of UCP2 increase in pressure overload (215) and after myocardial infarction (216). These studies emphasize the role of UCPs in the transition from cardiac hypertrophy to heart failure. In terms of mechanism, there are two possible mechanisms proposed: 1) regulation of ROS by UCPs and 2) regulation of metabolic pathways. As proton is a major player in regulating ionic homeostasis, the key mechanism involved could be directly associated with proton concentration inside the mitochondria modulated by UCPs, and disruption will result in loss of mitochondrial membrane potential. The loss of mitochondrial membrane potential will increase ROS generation and decoupling of ETC, hence causing disruption of metabolic pathways.
Phosphates are the major anion present in the mitochondrial matrix. Mitochondrial phosphate carrier, SLC25A3, is the major mechanism in PO4− transport, and energy production. Two MCFs, PiCA and PiCB are responsible for PO4− transport for ADP phosphorylation but no specific ion channel has been identified for PO4− transport. Abnormal activity or expression of MCFs is known to cause several diseases and disorders including cardiomyopathy, ventriculomegaly, and congenital heart defects. Mice lacking PiC develop cardiac phenotype reminiscent of SLC25A3 deficiency in the long-term, such as hypertrophy with ventricular dilation and compromised cardiac function. However, the deletion of PiC attenuated ischemia-reperfusion injury and partially protected cells from apoptosis (217). In patients with coronary artery disease, changes in PO4− transport result in mitochondrial DNA deletions and damage to ETC complexes (218). Taken together these studies indicate that PO4− transportation is tightly coupled with ETC function and metabolism.
Another key ion involved in several important enzymes as a cofactor is Mg2+. Mg2+ is the fourth most abundant mineral in the mammalian body, and the second most abundant cation. It is also essential for the energy production and function of several kinases, ATPases, and nucleic acid metabolism. The mitochondrion is capable of take-up and extrudes Mg2+ by respiration-dependent reactions. Mg2+ is directly involved in the regulation of Ca2+ transport in mitochondria. Mitochondrial Mg2+ transport was first described around 60 years ago (219, 220). The transporters of Mg2+ recently identified in mitochondria are Ymr166c/Mme1 (221) and SLC41A3 (222). Mg2+ levels are directly involved in cardiovascular diseases, type 2 diabetes mellitus, heart failure, arrhythmias, and its serum levels also influence the outcome after heart failure in patients (223–230). Mg2+ supplementation restores mitochondrial membrane potential, increased ATP production, decreased ROS and Ca2+ overload, and cardiac diastolic function in diabetes mellitus mice (230). In patients, Mg2+ supplementation is used to acutely treat arrhythmias, decrease mortality after MI, treat hypokalemia, reduce stroke, and mortality associated with diabetes mellitus (231). Although promising in some cardiovascular diseases and associated mortality, Mg2+ supplementation does not have the same outcome for re-entrant rhythms, atrial fibrillation, and ischemic heart diseases (231). Similar to other ions, it is vital to dissect the role of mitochondrial versus extramitochondrial Mg2+ impact on cardiac function.
PERSPECTIVE VIEW
The field of mitochondrial ion channels has been increasingly recognized for their roles during stress or in apoptosis. A growing number of ion channels have been recognized and reported in mitochondrial membranes. The lack of information on the identity of mitochondrial ion channels is the biggest challenge in the field. So far a handful of cation and anion channels have been identified at the molecular level and in the future, we anticipate the unmasking of additional mitochondrial ion channel proteins. Another challenge in the field is to specifically target mitochondrial ion channels and associated post-translational modifications. All of the ion channels or their complex components are encoded by nuclear DNA (with an exception of ceramides which are made by lipid molecules). Some of the mitochondrial ion channels share biophysical and pharmacological properties with plasma membrane ion channel counterparts (232) making it challenging to specifically target mitochondrial ion channels (233).
At the pharmaceutical and therapeutical end, it is important to unravel methods to specifically target these channels. The high throughput screening is one of the most promising pathways to identify channel-specific modulators. The next significant challenge will be to specifically deliver these compounds to mitochondria and explicitly to channels present in the inner membrane. Recent developments in mitochondrial medicine by using lipophilic cation moieties [e.g., triphenylphosphonium (TPP) and Dequalinium (DQA)], cardiolipin targeting peptides (also known as Szeto-Schiller peptides), and mitochondrial targeting signal peptides (20–40 amino acids N-terminal targeting sequences) have shown promise to target mitochondrial ion channels. In clinical studies, TPP-based compounds MitoQ and SkQ1 are used for trials for diastolic dysfunction (NCT03586414), peripheral arterial disease (NCT03506633), heart failure (NCT02966665), and hypogonadism (NCT02758431) (234). In all the clinical trials, patients are being recruited and it will be interesting to assess the outcomes of these studies in the context of mitochondrial medicine.
In summary, mitochondrial ion channels are potential therapeutic targets for transplant medicine and cardiovascular and neurogenerative diseases. These channels, once identified and characterized, can be targeted by incorporating novel and specific mechanisms to treat diseases and disorders.
GRANTS
The research in the laboratory is supported by NIH Grant HL133050, American Heart Association (AHA) Grants 16GRNT29430000 and 11SDG230059, and W. W. Smith Charitable Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
H.S. analyzed data; prepared figures; drafted manuscript; edited and revised manuscript; approved final version of manuscript.
ACKNOWLEDGMENTS
I thank Shridhar Sanghvi (OSU), Drs. Devasena Ponnalagu (OSU), Kalina Szteyn (OSU), and Shubha Gururaja Rao (ONU) for stimulating discussions and suggestions.
REFERENCES
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141: e139–e596, 2020. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Szteyn K, Singh H. BKCa channels as targets for cardioprotection. Antioxidants (Basel) 9: 760, 2020. doi: 10.3390/antiox9080760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponnalagu D, Singh H. Insights into the role of mitochondrial ion channels in inflammatory response. Front Physiol 11: 258, 2020. doi: 10.3389/fphys.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichols CG, Singh GK, Grange DK. KATP channels and cardiovascular disease: suddenly a syndrome. Circ Res 112: 1059–1072, 2013. doi: 10.1161/CIRCRESAHA.112.300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan DD. Phenomics of cardiac chloride channels. Compr Physiol 3: 667–692, 2013. doi: 10.1002/cphy.c110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh H, Stefani E, Toro L. Intracellular BK(Ca) (iBK(Ca)) channels. J Physiol 590: 5937–5947, 2012. doi: 10.1113/jphysiol.2011.215533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terracciano CM, Yacoub MH. Heart failure: a SHIFT from ion channels to clinical practice. Nat Rev Cardiol 7: 669–670, 2010. doi: 10.1038/nrcardio.2010.179. [DOI] [PubMed] [Google Scholar]
- 8.Kudenchuk PJ, Brown SP, Daya M, Rea T, Nichol G, Morrison LJ, Leroux B, Vaillancourt C, Wittwer L, Callaway CW, Christenson J, Egan D, Ornato JP, Weisfeldt ML, Stiell IG, Idris AH, Aufderheide TP, Dunford JV, Colella MR, Vilke GM, Brienza AM, Desvigne-Nickens P, Gray PC, Gray R, Seals N, Straight R, Dorian P; Resuscitation Outcomes Consortium Investigators. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med 374: 1711–1722, 2016. doi: 10.1056/NEJMoa1514204. [DOI] [PubMed] [Google Scholar]
- 9.Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol 2: 185–194, 2009. doi: 10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 10.Wilde AA, Bezzina CR. Genetics of cardiac arrhythmias. Heart 91: 1352–1358, 2005. doi: 10.1136/hrt.2004.046334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klabunde RE. Cardiac electrophysiology: normal and ischemic ionic currents and the ECG. Adv Physiol Educ 41: 29–37, 2017. doi: 10.1152/advan.00105.2016. [DOI] [PubMed] [Google Scholar]
- 12.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res 77: 334–343, 2008. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 13.O'Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 20: 303–315, 2005. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Rourke B, Cortassa S, Akar F, Aon M. Mitochondrial ion channels in cardiac function and dysfunction. Novartis Found Symp 287: 140–151, 2007. doi: 10.1002/9780470725207.ch10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu Rev Physiol 69: 51–67, 2007. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 16.Murphy E, Ardehali H, Balaban RS, DiLisa F, Dorn GW 2nd, Kitsis RN, Otsu K, Ping P, Rizzuto R, Sack MN, Wallace D, Youle RJ; American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology. Mitochondrial function, biology, and role in disease: a scientific statement from the american heart association. Circ Res 118: 1960–1991, 2016. doi: 10.1161/RES.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palade GE. The fine structure of mitochondria. Anat Rec 114: 427–451, 1952. doi: 10.1002/ar.1091140304. [DOI] [PubMed] [Google Scholar]
- 18.Astarie C, Levenson J, Simon A, Meyer P, Devynck MA. Platelet cytosolic proton and free calcium concentrations in essential hypertension. J Hypertens 7: 485–491, 1989. doi: 10.1097/00004872-198906000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Poburko D, Santo-Domingo J, Demaurex N. Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J Biol Chem 286: 11672–11684, 2011. doi: 10.1074/jbc.M110.159962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bangsbo J, Juel C, Hellsten Y, Saltin B. Dissociation between lactate and proton exchange in muscle during intense exercise in man. J Physiol 504: 489–499, 1997. doi: 10.1111/j.1469-7793.1997.489be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoeteweij JP, van de Water B, de Bont HJ, Nagelkerke JF. Mitochondrial K+ as modulator of Ca(2+)-dependent cytotoxicity in hepatocytes. Novel application of the K(+)-sensitive dye PBFI (K(+)-binding benzofuran isophthalate) to assess free mitochondrial K+ concentrations. Biochem J 299: 539–543, 1994. doi: 10.1042/bj2990539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dordick RS, Brierley GP, Garlid KD. On the mechanism of A23187-induced potassium efflux in rat liver mitochondria. J Biol Chem 255: 10299–10305, 1980. doi: 10.1016/S0021-9258(19)70464-5. [DOI] [PubMed] [Google Scholar]
- 23.Costa ADT, Quinlan CL, Andrukhiv A, West IC, Jabůrek M, Garlid KD. The direct physiological effects of mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ Physiol 290: H406–H415, 2006. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- 24.Jasielec JJ, Filipek R, Dołowy K, Lewenstam A. Precipitation of inorganic salts in mitochondrial matrix. Membranes (Basel) 10: 81, 2020. doi: 10.3390/membranes10050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clausen T, Van Hardeveld C, Everts ME. Significance of cation transport in control of energy metabolism and thermogenesis. Physiol Rev 71: 733–774, 1991. doi: 10.1152/physrev.1991.71.3.733. [DOI] [PubMed] [Google Scholar]
- 26.Baron S, Caplanusi A, van de Ven M, Radu M, Despa S, Lambrichts I, Ameloot M, Steels P, Smets I. Role of mitochondrial Na+ concentration, measured by CoroNa red, in the protection of metabolically inhibited MDCK cells. J Am Soc Nephrol 16: 3490–3497, 2005. doi: 10.1681/ASN.2005010075. [DOI] [PubMed] [Google Scholar]
- 27.Somlyo AP, Somlyo AV, Shuman H. Electron probe analysis of vascular smooth muscle. Composition of mitochondria, nuclei, and cytoplasm. J Cell Biol 81: 316–335, 1979. doi: 10.1083/jcb.81.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung DW, Apel LM, Brierley GP. Transmembrane gradients of free Na+ in isolated heart mitochondria estimated using a fluorescent probe. Am J Physiol Cell Physiol 262: C1047–C1055, 1992. doi: 10.1152/ajpcell.1992.262.4.C1047. [DOI] [PubMed] [Google Scholar]
- 29.Schreur JH, Figueredo VM, Miyamae M, Shames DM, Baker AJ, Camacho SA. Cytosolic and mitochondrial [Ca2+] in whole hearts using indo-1 acetoxymethyl ester: effects of high extracellular Ca2+. Biophys J 70: 2571–2580, 1996. doi: 10.1016/S0006-3495(96)79828-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno-Sanchez R, Hansford RG. Dependence of cardiac mitochondrial pyruvate dehydrogenase activity on intramitochondrial free Ca2+ concentration. Biochem J 256: 403–412, 1988. doi: 10.1042/bj2560403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol Heart Circ Physiol 261: H1123–H1134, 1991. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 32.Lukacs GL, Kapus A. Measurement of the matrix free Ca2+ concentration in heart mitochondria by entrapped fura-2 and quin2. Biochem J 248: 609–613, 1987. doi: 10.1042/bj2480609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis MH, Altschuld RA, Jung DW, Brierley GP. Estimation of intramitochondrial pCa and pH by fura-2 and 2,7 biscarboxyethyl-5(6)-carboxyfluorescein (BCECF) fluorescence. Biochem Biophys Res Commun 149: 40–45, 1987. doi: 10.1016/0006-291X(87)91602-0. [DOI] [PubMed] [Google Scholar]
- 34.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem 278: 19062–19070, 2003. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 35.Rutter GA, Osbaldeston NJ, McCormack JG, Denton RM. Measurement of matrix free Mg2+ concentration in rat heart mitochondria by using entrapped fluorescent probes. Biochem J 271: 627–634, 1990. doi: 10.1042/bj2710627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gout E, Rebeille F, Douce R, Bligny R. Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: unravelling the role of Mg2+ in cell respiration. Proc Natl Acad Sci USA 111: E4560–E4567, 2014. doi: 10.1073/pnas.1406251111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gururaja Rao S, Patel NJ, Singh H. Intracellular chloride channels: novel biomarkers in diseases. Front Physiol 11: 96, 2020. doi: 10.3389/fphys.2020.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jahn SC, Rowland-Faux L, Stacpoole PW, James MO. Chloride concentrations in human hepatic cytosol and mitochondria are a function of age. Biochem Biophys Res Commun 459: 463–468, 2015. doi: 10.1016/j.bbrc.2015.02.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shcheynikov N, Son A, Hong JH, Yamazaki O, Ohana E, Kurtz I, Shin DM, Muallem S. Intracellular Cl− as a signaling ion that potently regulates Na+/HCO3− transporters. Proc Natl Acad Sci USA 112: E329–E337, 2015. doi: 10.1073/pnas.1415673112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suen HE, Pasvol G, Cunnington AJ. Clinical and laboratory features associated with serum phosphate concentrations in malaria and other febrile illnesses. Malar J 19: 85, 2020. doi: 10.1186/s12936-020-03166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei AC, Liu T, O'Rourke B. Dual effect of phosphate transport on mitochondrial Ca2+ dynamics. J Biol Chem 290: 16088–16098, 2015. doi: 10.1074/jbc.M114.628446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colombini M. Mitochondrial outer membrane channels. Chem Rev 112: 6373–6387, 2012. doi: 10.1021/cr3002033. [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen H. Mitochondrial ion transport: mechanism and physiological significance. Fed Proc 25: 903–911, 1966. [PubMed] [Google Scholar]
- 44.De Pinto V, Ludwig O, Krause J, Benz R, Palmieri F. Porin pores of mitochondrial outer membranes from high and low eukaryotic cells: biochemical and biophysical characterization. Biochim Biophys Acta 894: 109–119, 1987. doi: 10.1016/0005-2728(87)90180-0. [DOI] [PubMed] [Google Scholar]
- 45.Antonenko YN, Kinnally KW, Tedeschi H. Identification of anion and cation pathways in the inner mitochondrial membrane by patch clamping of mouse liver mitoplasts. J Membr Biol 124: 151–158, 1991. doi: 10.1007/BF01870459. [DOI] [PubMed] [Google Scholar]
- 46.Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature 352: 244–247, 1991. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- 47.Kinnally KW, Antonenko YN, Zorov DB. Modulation of inner mitochondrial membrane channel activity. J Bioenerg Biomembr 24: 99–110, 1992. doi: 10.1007/BF00769536. [DOI] [PubMed] [Google Scholar]
- 48.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Balaban RS. Modeling mitochondrial function. Am J Physiol Cell Physiol 291: C1107–C1113, 2006. doi: 10.1152/ajpcell.00223.2006. [DOI] [PubMed] [Google Scholar]
- 50.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell 148: 1145–1159, 2012. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Rourke B. Mitochondrial ion channels. Annu Rev Physiol 69: 19–49, 2007. doi: 10.1146/annurev.physiol.69.031905.163804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39: 359–407, 2005. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michelakis ED. Mitochondrial medicine: a new era in medicine opens new windows and brings new challenges. Circulation 117: 2431–2434, 2008. doi: 10.1161/CIRCULATIONAHA.108.775163. [DOI] [PubMed] [Google Scholar]
- 54.Michaud M, Ubrig E, Filleur S, Erhardt M, Ephritikhine G, Marechal-Drouard L, Duchene AM. Differential targeting of VDAC3 mRNA isoforms influences mitochondria morphology. Proc Natl Acad Sci USA 111: 8991–8996, 2014. doi: 10.1073/pnas.1402588111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukasawa Y, Tsuji J, Fu SC, Tomii K, Horton P, Imai K. MitoFates: improved prediction of mitochondrial targeting sequences and their cleavage sites. Mol Cell Proteomics 14: 1113–1126, 2015. doi: 10.1074/mcp.M114.043083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh H, Lu R, Bopassa JC, Meredith AL, Stefani E, Toro L. mitoBKCa is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc Natl Acad Sci USA 110: 10836–10841, 2013. [Erratum in Proc Natl Acad Sci USA 110: 18024, 2013]. doi: 10.1073/pnas.1302028110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gang H, Hai Y, Dhingra R, Gordon JW, Yurkova N, Aviv Y, Li H, Aguilar F, Marshall A, Leygue E, Kirshenbaum LA. A novel hypoxia-inducible spliced variant of mitochondrial death gene Bnip3 promotes survival of ventricular myocytes. Circ Res 108: 1084–1092, 2011. doi: 10.1161/CIRCRESAHA.110.238709. [DOI] [PubMed] [Google Scholar]
- 58.Singh H, Li M, Hall L, Chen S, Sukur S, Lu R, Caputo A, Meredith AL, Stefani E, Toro L. MaxiK channel interactome reveals its interaction with GABA transporter 3 and heat shock protein 60 in the mammalian brain. Neuroscience 317: 76–107, 2016. doi: 10.1016/j.neuroscience.2015.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neupert W. Protein import into mitochondria. Annu Rev Biochem 66: 863–917, 1997. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 60.Kutik S, Guiard B, Meyer HE, Wiedemann N, Pfanner N. Cooperation of translocase complexes in mitochondrial protein import. J Cell Biol 179: 585–591, 2007. doi: 10.1083/jcb.200708199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng MY, Hartl FU, Horwich AL. The mitochondrial chaperonin hsp60 is required for its own assembly. Nature 348: 455–458, 1990. doi: 10.1038/348455a0. [DOI] [PubMed] [Google Scholar]
- 62.Geissler A, Chacinska A, Truscott KN, Wiedemann N, Brandner K, Sickmann A, Meyer HE, Meisinger C, Pfanner N, Rehling P. The mitochondrial presequence translocase: an essential role of Tim50 in directing preproteins to the import channel. Cell 111: 507–518, 2002. doi: 10.1016/s0092-8674(02)01073-5. [DOI] [PubMed] [Google Scholar]
- 63.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340, 2011. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saris NE, Sirota TV, Virtanen I, Niva K, Penttila T, Dolgachova LP, Mironova GD. Inhibition of the mitochondrial calcium uniporter by antibodies against a 40-kDa glycoproteinT. J Bioenerg Biomembr 25: 307–312, 1993. doi: 10.1007/BF00762591. [DOI] [PubMed] [Google Scholar]
- 65.Broekemeier KM, Krebsbach RJ, Pfeiffer DR. Inhibition of the mitochondrial Ca2+ uniporter by pure and impure ruthenium red. Mol Cell Biochem 139: 33–40, 1994. doi: 10.1007/BF00944201. [DOI] [PubMed] [Google Scholar]
- 66.Alevriadou BR, Patel A, Noble M, Ghosh S, Gohil VM, Stathopulos PB, Madesh M. Molecular nature and physiological role of the mitochondrial calcium uniporter channel. Am J Physiol Cell Physiol 320: C465–C482, 2021. doi: 10.1152/ajpcell.00502.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427: 360–364, 2004. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 68.De Stefani D, Rizzuto R, Pozzan T. Enjoy the trip: calcium in mitochondria back and forth. Annu Rev Biochem 85: 161–192, 2016. doi: 10.1146/annurev-biochem-060614-034216. [DOI] [PubMed] [Google Scholar]
- 69.Holmstrom KM, Pan X, Liu JC, Menazza S, Liu J, Nguyen TT, Pan H, Parks RJ, Anderson S, Noguchi A, Springer D, Murphy E, Finkel T. Assessment of cardiac function in mice lacking the mitochondrial calcium uniporter. J Mol Cell Cardiol 85: 178–182, 2015. doi: 10.1016/j.yjmcc.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu Y, Rasmussen TP, Koval OM, Joiner ML, Hall DD, Chen B, Luczak ED, Wang Q, Rokita AG, Wehrens XH, Song LS, Anderson ME. The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun 6: 6081, 2015. [Erratum in Nat Commun 6: 7241, 2015]. doi: 10.1038/ncomms7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luongo TS, Lambert JP, Yuan A, Zhang X, Gross P, Song J, Shanmughapriya S, Gao E, Jain M, Houser SR, Koch WJ, Cheung JY, Madesh M, Elrod JW. The mitochondrial calcium uniporter matches energetic supply with cardiac workload during stress and modulates permeability transition. Cell Rep 12: 23–34, 2015. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, York AJ, Zhang J, Bers DM, Molkentin JD. The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep 12: 15–22, 2015. doi: 10.1016/j.celrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, Carpenter AC, Kolmetzky D, Gao E, van Berlo JH, Tsai EJ, Molkentin JD, Chen X, Madesh M, Houser SR, Elrod JW. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature 545: 93–97, 2017. doi: 10.1038/nature22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, Gucek M, Balaban RS, Murphy E, Finkel T. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol 15: 1464–1472, 2013. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Donoso P, Mill JG, O'Neill SC, Eisner DA. Fluorescence measurements of cytoplasmic and mitochondrial sodium concentration in rat ventricular myocytes. J Physiol 448: 493–509, 1992. doi: 10.1113/jphysiol.1992.sp019053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitchell P, Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem 9: 149–155, 1969. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 77.Garlid KD, Shariat-Madar Z, Nath S, Jezek P. Reconstitution and partial purification of the Na(+)-selective Na+/H+ antiporter of beef heart mitochondria. J Biol Chem 266: 6518–6523, 1991. doi: 10.1016/S0021-9258(18)38148-1. [DOI] [PubMed] [Google Scholar]
- 78.Szabo I, Zoratti M. Mitochondrial channels: ion fluxes and more. Physiol Rev 94: 519–608, 2014. doi: 10.1152/physrev.00021.2013. [DOI] [PubMed] [Google Scholar]
- 79.Aldakkak M, Stowe DF, Cheng Q, Kwok WM, Camara AK. Mitochondrial matrix K+ flux independent of large-conductance Ca2+-activated K+ channel opening. Am J Physiol Cell Physiol 298: C530–C541, 2010. doi: 10.1152/ajpcell.00468.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Szewczyk A, Jarmuszkiewicz W, Kunz WS. Mitochondrial potassium channels. IUBMB Life 61: 134–143, 2009. doi: 10.1002/iub.155. [DOI] [PubMed] [Google Scholar]
- 81.Garlid KD, Paucek P. Mitochondrial potassium transport: the K(+) cycle. BiochimBiophysActa 1606: 23–41, 2003. doi: 10.1016/s0005-2728(03)00108-7. [DOI] [PubMed] [Google Scholar]
- 82.Bednarczyk P, Kicinska A, Laskowski M, Kulawiak B, Kampa R, Walewska A, Krajewska M, Jarmuszkiewicz W, Szewczyk A. Evidence for a mitochondrial ATP-regulated potassium channel in human dermal fibroblasts. Biochim Biophys Acta Bioenerg 1859: 309–318, 2018. doi: 10.1016/j.bbabio.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 83.Foster DB, Ho AS, Rucker J, Garlid AO, Chen L, Sidor A, Garlid KD, O'Rourke B. Mitochondrial ROMK channel is a molecular component of mitoK(ATP). Circ Res 111: 446–454, 2012. doi: 10.1161/CIRCRESAHA.112.266445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laskowski M, Augustynek B, Bednarczyk P, Żochowska M, Kalisz J, O’Rourke B, Szewczyk A, Kulawiak B. Single-channel properties of the ROMK-pore-forming subunit of the mitochondrial ATP-sensitive potassium channel. Int J Mol Sci 20: 5323, 2019. doi: 10.3390/ijms20215323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Papanicolaou KN, Ashok D, Liu T, Bauer TM, Sun J, Li Z, da Costa E, D'Orleans CC, Nathan S, Lefer DJ, Murphy E, Paolocci N, Foster DB, O'Rourke B. Global knockout of ROMK potassium channel worsens cardiac ischemia-reperfusion injury but cardiomyocyte-specific knockout does not: Implications for the identity of mitoKATP. J Mol Cell Cardiol 139: 176–189, 2020. doi: 10.1016/j.yjmcc.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paggio A, Checchetto V, Campo A, Menabo R, Di Marco G, Di Lisa F, Szabo I, Rizzuto R, De Stefani D. Identification of an ATP-sensitive potassium channel in mitochondria. Nature 572: 609–613, 2019. doi: 10.1038/s41586-019-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dolga AM, Netter MF, Perocchi F, Doti N, Meissner L, Tobaben S, Grohm J, Zischka H, Plesnila N, Decher N, Culmsee C. Mitochondrial small conductance SK2 channels prevent glutamate-induced oxytosis and mitochondrial dysfunction. J Biol Chem 288: 10792–10804, 2013. doi: 10.1074/jbc.M113.453522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stowe DF, Yang M, Heisner JS, Camara AKS. Endogenous and agonist-induced opening of mitochondrial big versus small Ca2+-sensitive K+ channels on cardiac cell and mitochondrial protection. J Cardiovasc Pharmacol 70: 314–328, 2017. doi: 10.1097/FJC.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Marchi U, Sassi N, Fioretti B, Catacuzzeno L, Cereghetti GM, Szabo I, Zoratti M. Intermediate conductance Ca2+-activated potassium channel (KCa3.1) in the inner mitochondrial membrane of human colon cancer cells. Cell calcium 45: 509–516, 2009. doi: 10.1016/j.ceca.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 90.Frankenreiter S, Bednarczyk P, Kniess A, Bork NI, Straubinger J, Koprowski P, Wrzosek A, Mohr E, Logan A, Murphy MP, Gawaz M, Krieg T, Szewczyk A, Nikolaev VO, Ruth P, Lukowski R. cGMP-elevating compounds and ischemic conditioning provide cardioprotection against ischemia and reperfusion injury via cardiomyocyte-specific BK channels. Circulation 136: 2337–2355, 2017. doi: 10.1161/CIRCULATIONAHA.117.028723. [DOI] [PubMed] [Google Scholar]
- 91.Soltysinska E, Bentzen BH, Barthmes M, Hattel H, Thrush AB, Harper ME, Qvortrup K, Larsen FJ, Schiffer TA, Losa-Reyna J, Straubinger J, Kniess A, Thomsen MB, Bruggemann A, Fenske S, Biel M, Ruth P, Wahl-Schott C, Boushel RC, Olesen SP, Lukowski R. KCNMA1 encoded cardiac BK channels afford protection against ischemia-reperfusion injury. PLoS One 9: e103402, 2014. doi: 10.1371/journal.pone.0103402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szabo I, Bock J, Jekle A, Soddemann M, Adams C, Lang F, Zoratti M, Gulbins E. A novel potassium channel in lymphocyte mitochondria. J Biol Chem 280: 12790–12798, 2005. doi: 10.1074/jbc.M413548200. [DOI] [PubMed] [Google Scholar]
- 93.Leanza L, Zoratti M, Gulbins E, Szabo I. Induction of apoptosis in macrophages via Kv1.3 and Kv1.5 potassium channels. Curr Med Chem 19: 5394–5404, 2012. doi: 10.2174/092986712803833281. [DOI] [PubMed] [Google Scholar]
- 94.Testai L, Barrese V, Soldovieri MV, Ambrosino P, Martelli A, Vinciguerra I, Miceli F, Greenwood IA, Curtis MJ, Breschi MC, Sisalli MJ, Scorziello A, Canduela MJ, Grandes P, Calderone V, Taglialatela M. Expression and function of Kv7.4 channels in rat cardiac mitochondria: possible targets for cardioprotection. Cardiovasc Res 110: 40–50, 2016. doi: 10.1093/cvr/cvv281. [DOI] [PubMed] [Google Scholar]
- 95.Yao J, McHedlishvili D, McIntire WE, Guagliardo NA, Erisir A, Coburn CA, Santarelli VP, Bayliss DA, Barrett PQ. Functional TASK-3-like channels in mitochondria of aldosterone-producing zona glomerulosa cells. Hypertension 70: 347–356, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rusznak Z, Bakondi G, Kosztka L, Pocsai K, Dienes B, Fodor J, Telek A, Gonczi M, Szucs G, Csernoch L. Mitochondrial expression of the two-pore domain TASK-3 channels in malignantly transformed and non-malignant human cells. Virchows Arch 452: 415–426, 2008. doi: 10.1007/s00428-007-0545-x. [DOI] [PubMed] [Google Scholar]
- 97.Toczyłowska-Mamińska R, Olszewska A, Laskowski M, Bednarczyk P, Skowronek K, Szewczyk A. Potassium channel in the mitochondria of human keratinocytes. J Invest Dermatol 134: 764–772, 2014. doi: 10.1038/jid.2013.422. [DOI] [PubMed] [Google Scholar]
- 98.Wojtovich AP, Sherman TA, Nadtochiy SM, Urciuoli WR, Brookes PS, Nehrke K. SLO-2 is cytoprotective and contributes to mitochondrial potassium transport. PloS One 6: e28287, 2011. doi: 10.1371/journal.pone.0028287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wojtovich AP, Smith CO, Urciuoli WR, Wang YT, Xia XM, Brookes PS, Nehrke K. Cardiac Slo2.1 is required for volatile anesthetic stimulation of K+ transport and anesthetic preconditioning. Anesthesiology 124: 1065–1076, 2016. doi: 10.1097/ALN.0000000000001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leon-Aparicio AD, Salvador C, Aparicio-Trejo TO, Briones-Herrera HA, Pedraza- Chaverri CJ, Vaca L, Sampieri A, Padilla-Flores FT, Lopez-González GZ, Leon-Contreras CJC, Hernandez-Pando PR, Escobar LI. Novel potassium channels in kidney mitochondria: the hyperpolarization-activated and cyclic nucleotide-gated HCN channels. Int J Mol Sci 20: 4995, 2019. doi: 10.3390/ijms20204995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Padilla-Flores T, Lopez-Gonzalez Z, Vaca L, Aparicio-Trejo OE, Briones-Herrera A, Riveros-Rosas H, Pedraza-Chaverri J, Leon-Aparicio D, Salvador C, Sampieri A, Escobar LI. “Funny” channels in cardiac mitochondria modulate membrane potential and oxygen consumption. Biochem Biophys Res Commun 524: 1030–1036, 2020. doi: 10.1016/j.bbrc.2020.02.033. [DOI] [PubMed] [Google Scholar]
- 102.Bednarczyk P, Kowalczyk JE, Beresewicz M, Dołowy K, Szewczyk A, Zabłocka B. Identification of a voltage-gated potassium channel in gerbil hippocampal mitochondria. Biochem Biophys Res Commun 397: 614–620, 2010. doi: 10.1016/j.bbrc.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 103.Pocsai K, Kosztka L, Bakondi G, Gonczi M, Fodor J, Dienes B, Szentesi P, Kovacs I, Feniger-Barish R, Kopf E, Zharhary D, Szucs G, Csernoch L, Rusznak Z. Melanoma cells exhibit strong intracellular TASK-3-specific immunopositivity in both tissue sections and cell culture. Cell Mol Life Sci 63: 2364–2376, 2006. doi: 10.1007/s00018-006-6166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu Z, Ji X, Boysen PG. Exogenous nitric oxide generates ROS and induces cardioprotection: involvement of PKG, mitochondrial KATP channels, and ERK. Am J Physiol Heart Circ Physiol 286: H1433–H1440, 2004. doi: 10.1152/ajpheart.00882.2003. [DOI] [PubMed] [Google Scholar]
- 105.Anastacio MM, Kanter EM, Makepeace CM, Keith AD, Zhang H, Schuessler RB, Nichols CG, Lawton JS. Relationship between mitochondrial matrix volume and cellular volume in response to stress and the role of ATP-sensitive potassium channel. Circulation 128: S130–S135, 2013. doi: 10.1161/CIRCULATIONAHA.112.000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O'Rourke B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science 298: 1029–1033, 2002. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 107.Wang X, Yin C, Xi L, Kukreja RC. Opening of Ca2+-activated K+ channels triggers early and delayed preconditioning against I/R injury independent of NOS in mice. Am J Physiol Heart Circ Physiol 287: H2070–H2077, 2004. doi: 10.1152/ajpheart.00431.2004. [DOI] [PubMed] [Google Scholar]
- 108.Bentzen BH, Osadchii O, Jespersen T, Hansen RS, Olesen SP, Grunnet M. Activation of big conductance Ca(2+)-activated K (+) channels (BK) protects the heart against ischemia-reperfusion injury. Pflugers Arch 457: 979–988, 2009. doi: 10.1007/s00424-008-0583-5. [DOI] [PubMed] [Google Scholar]
- 109.Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation 111: 198–203, 2005. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- 110.Szabo I, Bock J, Grassme H, Soddemann M, Wilker B, Lang F, Zoratti M, Gulbins E. Mitochondrial potassium channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc Natl Acad Sci USA 105: 14861–14866, 2008. [Erratum in Proc Natl Acad Sci USA 105: 17587, 2008] doi: 10.1073/pnas.0804236105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baines CP. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol 72: 61–80, 2010. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- 112.Tano JY, Gollasch M. Calcium-activated potassium channels in ischemia reperfusion: a brief update. Front Physiol 5: 381, 2014. doi: 10.3389/fphys.2014.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brown DA, O'Rourke B. Cardiac mitochondria and arrhythmias. Cardiovasc Res 88: 241–249, 2010. doi: 10.1093/cvr/cvq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stillitano F, Lonardo G, Zicha S, Varro A, Cerbai E, Mugelli A, Nattel S. Molecular basis of funny current (If) in normal and failing human heart. J Mol Cell Cardiol 45: 289–299, 2008. doi: 10.1016/j.yjmcc.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 115.Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, Kaczmarek L, Crowder CM, Salkoff L. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron 37: 765–773, 2003. doi: 10.1016/s0896-6273(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 116.Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, Swynghedauw B, Delcayre C. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem 273: 4883–4891, 1998. doi: 10.1074/jbc.273.9.4883. [DOI] [PubMed] [Google Scholar]
- 117.Lorenz JN, Baird NR, Judd LM, Noonan WT, Andringa A, Doetschman T, Manning PA, Liu LH, Miller ML, Shull GE. Impaired renal NaCl absorption in mice lacking the ROMK potassium channel, a model for type II Bartter's syndrome. J Biol Chem 277: 37871–37880, 2002. doi: 10.1074/jbc.M205627200. [DOI] [PubMed] [Google Scholar]
- 118.Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol 542: 735–741, 2002. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hanley PJ, Daut J. K(ATP) channels and preconditioning: a re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms. J Mol Cell Cardiol 39: 17–50, 2005. doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 120.Drose S, Brandt U, Hanley PJ. K+-independent actions of diazoxide question the role of inner membrane KATP channels in mitochondrial cytoprotective signaling. J Biol Chem 281: 23733–23739, 2006. doi: 10.1074/jbc.M602570200. [DOI] [PubMed] [Google Scholar]
- 121.Garlid KD, Halestrap AP. The mitochondrial K(ATP) channel—fact or fiction? J Mol Cell Cardiol 52: 578–583, 2012. doi: 10.1016/j.yjmcc.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rutledge C, Dudley S. Mitochondria and arrhythmias. Expert Rev Cardiovasc Ther 11: 799–801, 2013. doi: 10.1586/14779072.2013.811969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Das B, Sarkar C. Is the sarcolemmal or mitochondrial K(ATP) channel activation important in the antiarrhythmic and cardioprotective effects during acute ischemia/reperfusion in the intact anesthetized rabbit model? Life Sci 77: 1226–1248, 2005. doi: 10.1016/j.lfs.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 124.Gururaja Rao S, Bednarczyk P, Towheed A, Shah K, Karekar P, Ponnalagu D, Jensen HN, Addya S, Reyes BAS, Van Bockstaele EJ, Szewczyk A, Wallace DC, Singh H. BKCa (Slo) channel regulates mitochondrial function and lifespan in Drosophila melanogaster. Cells 8: 945, 2019. doi: 10.3390/cells8090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bednarczyk P, Wieckowski MR, Broszkiewicz M, Skowronek K, Siemen D, Szewczyk A. Putative structural and functional coupling of the mitochondrial BK channel to the respiratory chain. PLoS One 8: e68125, 2013. doi: 10.1371/journal.pone.0068125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Skalska J, Bednarczyk P, Piwońska M, Kulawiak B, Wilczynski G, Dołowy K, Kudin AP, Kunz WS, Szewczyk A. Calcium ions regulate K(+) uptake into brain mitochondria: the evidence for a novel potassium channel. Int J Mol Sci 10: 1104–1120, 2009. doi: 10.3390/ijms10031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bednarczyk P, Koziel A, Jarmuszkiewicz W, Szewczyk A. (2)(+)-Activated potassium channel in mitochondria of endothelial EA.hy926 cells. Am J Physiol Heart Circ Physiol 304: H1415–H1427, 2013. doi: 10.1152/ajpheart.00976.2012. [DOI] [PubMed] [Google Scholar]
- 128.Stowe DF, Aldakkak M, Camara AK, Riess ML, Heinen A, Varadarajan SG, Jiang MT. Cardiac mitochondrial preconditioning by Big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am J Physiol Heart Circ Physiol 290: H434–H440, 2006. doi: 10.1152/ajpheart.00763.2005. [DOI] [PubMed] [Google Scholar]
- 129.Heinen A, Camara AK, Aldakkak M, Rhodes SS, Riess ML, Stowe DF. Mitochondrial Ca2+-induced K+ influx increases respiration and enhances ROS production while maintaining membrane potential. Am J Physiol Cell Physiol 292: C148–C156, 2007. doi: 10.1152/ajpcell.00215.2006. [DOI] [PubMed] [Google Scholar]
- 130.Frankenreiter S, Groneberg D, Kuret A, Krieg T, Ruth P, Friebe A, Lukowski R. Cardioprotection by ischemic postconditioning and cyclic guanosine monophosphate-elevating agents involves cardiomyocyte nitric oxide-sensitive guanylyl cyclase. Cardiovasc Res 114: 822–829, 2018. doi: 10.1093/cvr/cvy039. [DOI] [PubMed] [Google Scholar]
- 131.Goswami SK, Ponnalagu D, Hussain AT, Shah K, Karekar P, Rao Meredith GS, Khan AL, Singh MH. . Expression and activation of BKCa channels in mice protects against ischemia-reperfusion injury of isolated hearts by modulating mitochondrial function. Front Cardiovasc Med 5: 194, 2018. doi: 10.3389/fcvm.2018.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Imlach WL, Finch SC, Miller JH, Meredith AL, Dalziel JE. A role for BK channels in heart rate regulation in rodents. PloS one 5: e8698, 2010. doi: 10.1371/journal.pone.0008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lai MH, Wu Y, Gao Z, Anderson ME, Dalziel JE, Meredith AL. BK channels regulate sinoatrial node firing rate and cardiac pacing in vivo. Am J Physiol Heart Circ Physiol 307: H1327–H1338, 2014. doi: 10.1152/ajpheart.00354.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Patel NH, Johannesen J, Shah K, Goswami SK, Patel NJ, Ponnalagu D, Kohut AR, Singh H. Inhibition of BKCa negatively alters cardiovascular function. Physiol Rep 6: e13748, 2018. doi: 10.14814/phy2.13748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bingham JP, Bian S, Tan ZY, Takacs Z, Moczydlowski E. Synthesis of a biotin derivative of iberiotoxin: binding interactions with streptavidin and the BK Ca2+-activated K+ channel expressed in a human cell line. Bioconjug Chem 17: 689–699, 2006. doi: 10.1021/bc060002u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L. Decreased expression of voltage- and Ca(2+)-activated K(+) channels in coronary smooth muscle during aging. Circ Res 88: 210–216, 2001. doi: 10.1161/01.res.88.2.210. [DOI] [PubMed] [Google Scholar]
- 137.van der Horst J, Greenwood IA, Jepps TA. Cyclic AMP-dependent regulation of Kv7 voltage-gated potassium channels. Front Physiol 11: 727, 2020. doi: 10.3389/fphys.2020.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aoki I, Tateyama M, Shimomura T, Ihara K, Kubo Y, Nakano S, Mori I. SLO potassium channels antagonize premature decision making in C. elegans. Commun Biol 1: 123, 2018. doi: 10.1038/s42003-018-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ren Y, Barnwell LF, Alexander JC, Lubin FD, Adelman JP, Pfaffinger PJ, Schrader LA, Anderson AE. Regulation of surface localization of the small conductance Ca2+-activated potassium channel, Sk2, through direct phosphorylation by cAMP-dependent protein kinase. J Biol Chem 281: 11769–11779, 2006. doi: 10.1074/jbc.M513125200. [DOI] [PubMed] [Google Scholar]
- 140.Han J, Kim N, Kim E, Ho WK, Earm YE. Modulation of ATP-sensitive potassium channels by cGMP-dependent protein kinase in rabbit ventricular myocytes. J Biol Chem 276: 22140–22147, 2001. doi: 10.1074/jbc.M010103200. [DOI] [PubMed] [Google Scholar]
- 141.Ferreira R, Wong R, Schlichter LC. KCa3.1/IK1 channel regulation by cGMP-dependent protein kinase (PKG) via reactive oxygen species and CaMKII in microglia: an immune modulating feedback system? Front Immunol 6: 153, 2015. doi: 10.3389/fimmu.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nunez L, Vaquero M, Gomez R, Caballero R, Mateos-Caceres P, Macaya C, Iriepa I, Galvez E, Lopez-Farre A, Tamargo J, Delpon E. Nitric oxide blocks hKv1.5 channels by S-nitrosylation and by a cyclic GMP-dependent mechanism. Cardiovasc Res 72: 80–89, 2006. doi: 10.1016/j.cardiores.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 143.Stott JB, Greenwood IA. Complex role of Kv7 channels in cGMP and cAMP-mediated relaxations. Channels (Austin) 9: 117–118, 2015. doi: 10.1080/19336950.2015.1046732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Surges R, Brewster AL, Bender RA, Beck H, Feuerstein TJ, Baram TZ. Regulated expression of HCN channels and cAMP levels shape the properties of the h current in developing rat hippocampus. Eur J Neurosci 24: 94–104, 2006. doi: 10.1111/j.1460-9568.2006.04880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hutter OF, Noble D. Anion conductance of cardiac muscle. J Physiol 157: 335–350, 1961. doi: 10.1113/jphysiol.1961.sp006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carmeliet EE. Chloride ions and the membrane potential of Purkinje fibres. J Physiol 156: 375–388, 1961. doi: 10.1113/jphysiol.1961.sp006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kenyon JL, Gibbons WR. Influence of chloride, potassium, and tetraethylammonium on the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol 73: 117–138, 1979. doi: 10.1085/jgp.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Varro A, Tomek J, Nagy N, Virag L, Passini E, Rodriguez B, Baczko I. Cardiac transmembrane ion channels and action potentials: cellular physiology and arrhythmogenic behavior. Physiol Rev 101: 1083–1176, 2021. doi: 10.1152/physrev.00024.2019. [DOI] [PubMed] [Google Scholar]
- 149.Ponnalagu D, Gururaja Rao S, Farber J, Xin W, Hussain AT, Shah K, Tanda S, Berryman M, Edwards JC, Singh H. Molecular identity of cardiac mitochondrial chloride intracellular channel proteins. Mitochondrion 27: 6–14, 2016. doi: 10.1016/j.mito.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 150.Camara AKS, Zhou Y, Wen PC, Tajkhorshid E, Kwok WM. Mitochondrial VDAC1: a key gatekeeper as potential therapeutic target. Front Physiol 8: 460, 2017. doi: 10.3389/fphys.2017.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399: 483–487, 1999. [Erratum in Nature 407: 767, 2000] doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 152.Xu X, Decker W, Sampson MJ, Craigen WJ, Colombini M. Mouse VDAC isoforms expressed in yeast: channel properties and their roles in mitochondrial outer membrane permeability. J Membr Biol 170: 89–102, 1999. doi: 10.1007/s002329900540. [DOI] [PubMed] [Google Scholar]
- 153.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9: 550–555, 2007. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Naghdi S, Hajnoczky G. VDAC2-specific cellular functions and the underlying structure. Biochim Biophys Acta 1863: 2503–2514, 2016. doi: 10.1016/j.bbamcr.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maurya SR, Mahalakshmi R. Mitochondrial VDAC2 and cell homeostasis: highlighting hidden structural features and unique functionalities. Biol Rev Camb Philos Soc 92: 1843–1858, 2017. doi: 10.1111/brv.12311. [DOI] [PubMed] [Google Scholar]
- 156.Lauterwasser J, Todt F, Zerbes RM, Nguyen TN, Craigen W, Lazarou M, van der Laan M, Edlich F. The porin VDAC2 is the mitochondrial platform for Bax retrotranslocation. Sci Rep 6: 32994, 2016. doi: 10.1038/srep32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dadsena S, Bockelmann S, Mina JGM, Hassan DG, Korneev S, Razzera G, Jahn H, Niekamp P, Muller D, Schneider M, Tafesse FG, Marrink SJ, Melo MN, Holthuis JCM. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat Commun 10: 1832, 2019. doi: 10.1038/s41467-019-09654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301: 513–517, 2003. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 159.Sabirov RZ, Sheiko T, Liu H, Deng D, Okada Y, Craigen WJ. Genetic demonstration that the plasma membrane maxianion channel and voltage-dependent anion channels are unrelated proteins. J Biol Chem 281: 1897–1904, 2006. doi: 10.1074/jbc.M509482200. [DOI] [PubMed] [Google Scholar]
- 160.Checchetto V, Reina S, Magri A, Szabo I, De Pinto V. Recombinant human voltage dependent anion selective channel isoform 3 (hVDAC3) forms pores with a very small conductance. Cell Physiol Biochem 34: 842–853, 2014. doi: 10.1159/000363047. [DOI] [PubMed] [Google Scholar]
- 161.Reina S, Guarino F, Magri A, De Pinto V. VDAC3 as a potential marker of mitochondrial status is involved in cancer and pathology. Front Oncol 6: 264, 2016. doi: 10.3389/fonc.2016.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Anflous-Pharayra K, Lee N, Armstrong DL, Craigen WJ. VDAC3 has differing mitochondrial functions in two types of striated muscles. Biochim Biophys Acta 1807: 150–156, 2011. doi: 10.1016/j.bbabio.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Duncan RR, Westwood PK, Boyd A, Ashley RH. Rat brain p64H1, expression of a new member of the p64 chloride channel protein family in endoplasmic reticulum. J Biol Chem 272: 23880–23886, 1997. doi: 10.1074/jbc.272.38.23880. [DOI] [PubMed] [Google Scholar]
- 164.Arnould T, Mercy L, Houbion A, Vankoningsloo S, Renard P, Pascal T, Ninane N, Demazy C, Raes M. mtCLIC is up-regulated and maintains a mitochondrial membrane potential in mtDNA-depleted L929 cells. FASEB J 17: 2145–2147, 2003. doi: 10.1096/fj.03-0075fje. [DOI] [PubMed] [Google Scholar]
- 165.Fernandez-Salas E, Suh KS, Speransky VV, Bowers WL, Levy JM, Adams T, Pathak KR, Edwards LE, Hayes DD, Cheng C, Steven AC, Weinberg WC, Yuspa SH. mtCLIC/CLIC4, an organellular chloride channel protein, is increased by DNA damage and participates in the apoptotic response to p53. Mol Cell Biol 22: 3610–3620, 2002. doi: 10.1128/MCB.22.11.3610-3620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Suh KS, Mutoh M, Nagashima K, Fernandez-Salas E, Edwards LE, Hayes DD, Crutchley JM, Marin KG, Dumont RA, Levy JM, Cheng C, Garfield S, Yuspa SH. The organellular chloride channel protein CLIC4/mtCLIC translocates to the nucleus in response to cellular stress and accelerates apoptosis. J Biol Chem 279: 4632–4641, 2004. doi: 10.1074/jbc.M311632200. [DOI] [PubMed] [Google Scholar]
- 167.Shiio Y, Suh KS, Lee H, Yuspa SH, Eisenman RN, Aebersold R. Quantitative proteomic analysis of myc-induced apoptosis: a direct role for Myc induction of the mitochondrial chloride ion channel, mtCLIC/CLIC4. J Biol Chem 281: 2750–2756, 2006. doi: 10.1074/jbc.M509349200. [DOI] [PubMed] [Google Scholar]
- 168.Xu Y, Kang J, Yuan Z, Li H, Su J, Li Y, Kong X, Zhang H, Wang W, Sun L. Suppression of CLIC4/mtCLIC enhances hydrogen peroxide-induced apoptosis in C6 glioma cells. Oncol Rep 29: 1483–1491, 2013. doi: 10.3892/or.2013.2265. [DOI] [PubMed] [Google Scholar]
- 169.Ponnalagu D, Singh H. Anion channels of mitochondria. Handb Exp Pharmacol 240: 71–101, 2017. doi: 10.1007/164_2016_39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Beavis AD, Davatol-Hag H. The mitochondrial inner membrane anion channel is inhibited by DIDS. J Bioenerg Biomembr 28: 207–214, 1996. doi: 10.1007/BF02110652. [DOI] [PubMed] [Google Scholar]
- 171.Misak A, Grman M, Malekova L, Novotova M, Markova J, Krizanova O, Ondrias K, Tomaskova Z. Mitochondrial chloride channels: electrophysiological characterization and pH induction of channel pore dilation. Eur Biophys J 42: 709–720, 2013. doi: 10.1007/s00249-013-0920-2. [DOI] [PubMed] [Google Scholar]
- 172.Schonfeld P, Sayeed I, Bohnensack R, Siemen D. Fatty acids induce chloride permeation in rat liver mitochondria by activation of the inner membrane anion channel (IMAC). J Bioenerg Biomembr 36: 241–248, 2004. doi: 10.1023/B:JOBB.0000031975.72350.c6. [DOI] [PubMed] [Google Scholar]
- 173.Allawzi AM, Vang A, Clements RT, Jhun BS, Kue NR, Mancini TJ, Landi AK, Terentyev D, O-Uchi J, Comhair SA, Erzurum SC, Choudhary G. Activation of anoctamin-1 limits pulmonary endothelial cell proliferation via p38-mitogen-activated protein kinase-dependent apoptosis. Am J Respir Cell Mol Biol 58: 658–667, 2018. doi: 10.1165/rcmb.2016-0344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Colombini M. Ceramide channels and mitochondrial outer membrane permeability. J Bioenerg Biomembr 49: 57–64, 2017. doi: 10.1007/s10863-016-9646-z. [DOI] [PubMed] [Google Scholar]
- 176.Colombini M. Ceramide channels. Adv Exp Med Biol 1159: 33–48, 2019. doi: 10.1007/978-3-030-21162-2_3. [DOI] [PubMed] [Google Scholar]
- 177.Das S, Steenbergen C, Murphy E. Does the voltage dependent anion channel modulate cardiac ischemia-reperfusion injury? Biochim Biophys Acta 1818: 1451–1456, 2012. doi: 10.1016/j.bbamem.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res 103: 983–991, 2008. doi: 10.1161/CIRCRESAHA.108.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Klapper-Goldstein H, Verma A, Elyagon S, Gillis R, Murninkas M, Pittala S, Paul A, Shoshan-Barmatz V, Etzion Y. VDAC1 in the diseased myocardium and the effect of VDAC1-interacting compound on atrial fibrosis induced by hyperaldosteronism. Sci Rep 10: 22101, 2020. doi: 10.1038/s41598-020-79056-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shankar TS, Ramadurai DKA, Steinhorst K, Sommakia S, Badolia R, Thodou Krokidi A, Calder D, Navankasattusas S, Sander P, Kwon OS, Aravamudhan A, Ling J, Dendorfer A, Xie C, Kwon O, Cheng EHY, Whitehead KJ, Gudermann T, Richardson RS, Sachse FB, Schredelseker J, Spitzer KW, Chaudhuri D, Drakos SG. Cardiac-specific deletion of voltage dependent anion channel 2 leads to dilated cardiomyopathy by altering calcium homeostasis. Nat Commun 12: 4583, 2021. doi: 10.1038/s41467-021-24869-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Azzi A, Azzone GF. Swelling and shrinkage phenomena in liver mitochondria. II. Low amplitude swelling-shrinkage cycles. Biochim Biophys Acta 105: 265–278, 1965. doi: 10.1016/S0926-6593(65)80151-5. [DOI] [PubMed] [Google Scholar]
- 182.Brierley GP. Energy-linked alteration of mitochondrial permeability to anions. Biochem Biophys Res Commun 35: 396–402, 1969. doi: 10.1016/0006-291X(69)90512-9. [DOI] [PubMed] [Google Scholar]
- 183.Garlid KD, Beavis AD. Evidence for the existence of an inner membrane anion channel in mitochondria. Biochim Biophys Acta 853: 187–204, 1986. doi: 10.1016/0304-4173(87)90001-2. [DOI] [PubMed] [Google Scholar]
- 184.Sorgato MC, Keller BU, Stuhmer W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature 330: 498–500, 1987. doi: 10.1038/330498a0. [DOI] [PubMed] [Google Scholar]
- 185.Akar FG, Aon MA, Tomaselli GF, O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest 115: 3527–3535, 2005. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278: 44735–44744, 2003. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 187.O'Rourke B. Pathophysiological and protective roles of mitochondrial ion channels. J Physiol 529: 23–36, 2000. doi: 10.1111/j.1469-7793.2000.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Singh H. Two decades with dimorphic chloride intracellular channels (CLICs). FEBS Lett 584: 2112–2121, 2010. doi: 10.1016/j.febslet.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 189.Rao GS, Ponnalagu D, Patel NJ, Singh H. . Three decades of chloride intracellular channel proteins: from organelle to organ physiology. Curr Protoc Pharmacol 80: 11 21 11–11 21 17, 2018. doi: 10.1002/cpph.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Singh H, Ashley RH. CLIC4 (p64H1) and its putative transmembrane domain form poorly selective, redox-regulated ion channels. Mol Membr Biol 24: 41–52, 2007. doi: 10.1080/09687860600927907. [DOI] [PubMed] [Google Scholar]
- 191.Fernandez-Salas E, Sagar M, Cheng C, Yuspa SH, Weinberg WC. p53 and tumor necrosis factor alpha regulate the expression of a mitochondrial chloride channel protein. J Biol Chem 274: 36488–36497, 1999. doi: 10.1074/jbc.274.51.36488. [DOI] [PubMed] [Google Scholar]
- 192.Landry DW, Akabas MH, Redhead C, Edelman A, Cragoe EJ Jr, Al-Awqati Q. Purification and reconstitution of chloride channels from kidney and trachea. Science 244: 1469–1472, 1989. doi: 10.1126/science.2472007. [DOI] [PubMed] [Google Scholar]
- 193.Diaz RJ, Losito VA, Mao GD, Ford MK, Backx PH, Wilson GJ. Chloride channel inhibition blocks the protection of ischemic preconditioning and hypo-osmotic stress in rabbit ventricular myocardium. Circ Res 84: 763–775, 1999. doi: 10.1161/01.RES.84.7.763. [DOI] [PubMed] [Google Scholar]
- 194.Batthish M, Diaz RJ, Zeng HP, Backx PH, Wilson GJ. Pharmacological preconditioning in rabbit myocardium is blocked by chloride channel inhibition. Cardiovascular research 55: 660–671, 2002. doi: 10.1016/S0008-6363(02)00454-6. [DOI] [PubMed] [Google Scholar]
- 195.Li J, Wu X, Cui T. Functional characteristics and molecular identification of swelling-activated chloride conductance in adult rabbit heart ventricles. J Huazhong Univ Sci Technolog Med Sci 28: 37–41, 2008. doi: 10.1007/s11596-008-0109-6. [DOI] [PubMed] [Google Scholar]
- 196.Ponnalagu D, Hussain AT, Thanawala R, Meka J, Bednarczyk P, Feng Y, Szewczyk A, GururajaRao S, Bopassa JC, Khan M, Singh H. Chloride channel blocker IAA-94 increases myocardial infarction by reducing calcium retention capacity of the cardiac mitochondria. Life Sci 235: 116841, 2019. doi: 10.1016/j.lfs.2019.116841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Choi BR, Hatton WJ, Hume JR, Liu T, Salama G. Low osmolarity transforms ventricular fibrillation from complex to highly organized, with a dominant high-frequency source. Heart Rhythm 3: 1210–1220, 2006. doi: 10.1016/j.hrthm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 198.Kumar NM, Gilula NB. The gap junction communication channel. Cell 84: 381–388, 1996. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 199.Boengler K, Schulz R. Connexin 43 and mitochondria in cardiovascular health and disease. Adv Exp Med Biol 982: 227–246, 2017. doi: 10.1007/978-3-319-55330-6_12. [DOI] [PubMed] [Google Scholar]
- 200.Boengler K, Ruiz-Meana M, Gent S, Ungefug E, Soetkamp D, Miro-Casas E, Cabestrero A, Fernandez-Sanz C, Semenzato M, Di Lisa F, Rohrbach S, Garcia-Dorado D, Heusch G, Schulz R. Mitochondrial connexin 43 impacts on respiratory complex I activity and mitochondrial oxygen consumption. J Cell Mol Med 16: 1649–1655, 2012. doi: 10.1111/j.1582-4934.2011.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Srisakuldee W, Makazan Z, Nickel BE, Zhang F, Thliveris JA, Pasumarthi KB, Kardami E. The FGF-2-triggered protection of cardiac subsarcolemmal mitochondria from calcium overload is mitochondrial connexin 43-dependent. Cardiovasc Res 103: 72–80, 2014. doi: 10.1093/cvr/cvu066. [DOI] [PubMed] [Google Scholar]
- 202.Soetkamp D, Nguyen TT, Menazza S, Hirschhauser C, Hendgen-Cotta UB, Rassaf T, Schluter KD, Boengler K, Murphy E, Schulz R. S-nitrosation of mitochondrial connexin 43 regulates mitochondrial function. Basic Res Cardiol 109: 433, 2014. doi: 10.1007/s00395-014-0433-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Boengler K, Konietzka I, Buechert A, Heinen Y, Garcia-Dorado D, Heusch G, Schulz R. Loss of ischemic preconditioning's cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am J Physiol Heart Circ Physiol 292: H1764–H1769, 2007. doi: 10.1152/ajpheart.01071.2006. [DOI] [PubMed] [Google Scholar]
- 204.Sovari AA, Iravanian S, Dolmatova E, Jiao Z, Liu H, Zandieh S, Kumar V, Wang K, Bernstein KE, Bonini MG, Duffy HS, Dudley SC. Inhibition of c-Src tyrosine kinase prevents angiotensin II-mediated connexin-43 remodeling and sudden cardiac death. J Am Coll Cardiol 58: 2332–2339, 2011. doi: 10.1016/j.jacc.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, Hirst-Jensen BJ, Duffy HS, Sorgen PL. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res 104: 1103–1112, 2009. doi: 10.1161/CIRCRESAHA.108.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sovari AA, Rutledge CA, Jeong EM, Dolmatova E, Arasu D, Liu H, Vahdani N, Gu L, Zandieh S, Xiao L, Bonini MG, Duffy HS, Dudley SC Jr.. Mitochondria oxidative stress, connexin43 remodeling, and sudden arrhythmic death. Circ Arrhythm Electrophysiol 6: 623–631, 2013. doi: 10.1161/CIRCEP.112.976787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Colombini M. Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. Ann N Y Acad Sci 341: 552–563, 1980. doi: 10.1111/j.1749-6632.1980.tb47198.x. [DOI] [PubMed] [Google Scholar]
- 208.Colombini M. VDAC: the channel at the interface between mitochondria and the cytosol. Mol Cell Biochem 256–257: 107–115, 2004. doi: 10.1023/B:MCBI.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- 209.Elinder F, Akanda N, Tofighi R, Shimizu S, Tsujimoto Y, Orrenius S, Ceccatelli S. Opening of plasma membrane voltage-dependent anion channels (VDAC) precedes caspase activation in neuronal apoptosis induced by toxic stimuli. Cell death Differ 12: 1134–1140, 2005. doi: 10.1038/sj.cdd.4401646. [DOI] [PubMed] [Google Scholar]
- 210.Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol 171: 2017–2028, 2014. doi: 10.1111/bph.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275: 1129–1132, 1997. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 212.Shimizu S, Ide T, Yanagida T, Tsujimoto Y. Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J Biol Chem 275: 12321–12325, 2000. doi: 10.1074/jbc.275.16.12321. [DOI] [PubMed] [Google Scholar]
- 213.Esfandiary A, Kutsche HS, Schreckenberg R, Weber M, Pak O, Kojonazarov B, Sydykov A, Hirschhauser C, Wolf A, Haag D, Hecker M, Fink L, Seeger W, Ghofrani HA, Schermuly RT, Weissmann N, Schulz R, Rohrbach S, Li L, Sommer N, Schluter KD. Protection against pressure overload-induced right heart failure by uncoupling protein 2 silencing. Cardiovasc Res 115: 1217–1227, 2019. doi: 10.1093/cvr/cvz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kutsche HS, Schreckenberg R, Weber M, Hirschhauser C, Rohrbach S, Li L, Niemann B, Schulz R, Schluter KD. Alterations in glucose metabolism during the transition to heart failure: the contribution of UCP-2. Cells 9: 552, 2020. doi: 10.3390/cells9030552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Noma T, Nishiyama A, Mizushige K, Murakami K, Tsuji T, Kohno M, Rahman M, Fukui T, Abe Y, Kimura S. Possible role of uncoupling protein in regulation of myocardial energy metabolism in aortic regurgitation model rats. FASEB J 15: 1206–1208, 2001. doi: 10.1096/fj.000569fje. [DOI] [PubMed] [Google Scholar]
- 216.Murray AJ, Cole MA, Lygate CA, Carr CA, Stuckey DJ, Little SE, Neubauer S, Clarke K. Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol 44: 694–700, 2008. doi: 10.1016/j.yjmcc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 217.Kwong JQ, Davis J, Baines CP, Sargent MA, Karch J, Wang X, Huang T, Molkentin JD. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ 21: 1209–1217, 2014. doi: 10.1038/cdd.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ait-Aissa K, Blaszak SC, Beutner G, Tsaih SW, Morgan G, Santos JH, Flister MJ, Joyce DL, Camara AKS, Gutterman DD, Donato AJ, Porter GA Jr, Beyer AM. Mitochondrial oxidative phosphorylation defect in the heart of subjects with coronary artery disease. Sci Rep 9: 7623, 2019. doi: 10.1038/s41598-019-43761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Brierley GP, Murer E, Green DE. Participation of an intermediate of oxidative phosphorylation in ion accumulation by mitochondria. Science 140: 60–62, 1963. doi: 10.1126/science.140.3562.60. [DOI] [PubMed] [Google Scholar]
- 220.Brierley GP, Bachmann E, Green DE. Active transport of inorganic phosphate and magnesium ions by beef heart mitochondria. Proc Natl Acad Sci USA 48: 1928–1935, 1962. doi: 10.1073/pnas.48.11.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cui Y, Zhao S, Wang J, Wang X, Gao B, Fan Q, Sun F, Zhou B. A novel mitochondrial carrier protein Mme1 acts as a yeast mitochondrial magnesium exporter. Biochim Biophys Acta 1853: 724–732, 2015. doi: 10.1016/j.bbamcr.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 222.Mastrototaro L, Smorodchenko A, Aschenbach JR, Kolisek M, Sponder G. Solute carrier 41A3 encodes for a mitochondrial Mg(2+) efflux system. Sci Rep 6: 27999, 2016. doi: 10.1038/srep27999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wester PO, Dyckner T. Magnesium and hypertension. J Am Coll Nutr 6: 321–328, 1987. doi: 10.1080/07315724.1987.10720194. [DOI] [PubMed] [Google Scholar]
- 224.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med 23: 1050–1056, 2006. doi: 10.1111/j.1464-5491.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 225.Reffelmann T, Ittermann T, Dorr M, Volzke H, Reinthaler M, Petersmann A, Felix SB. Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis 219: 280–284, 2011. doi: 10.1016/j.atherosclerosis.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 226.Nielsen FH, Milne DB, Klevay LM, Gallagher S, Johnson L. Dietary magnesium deficiency induces heart rhythm changes, impairs glucose tolerance, and decreases serum cholesterol in post menopausal women. J Am Coll Nutr 26: 121–132, 2007. doi: 10.1080/07315724.2007.10719593. [DOI] [PubMed] [Google Scholar]
- 227.Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes 65: 3–13, 2016. doi: 10.2337/db15-1028. [DOI] [PubMed] [Google Scholar]
- 228.Dyckner T, Wester PO. Potassium/magnesium depletion in patients with cardiovascular disease. Am J Med 82: 11–17, 1987. doi: 10.1016/0002-9343(87)90127-6. [DOI] [PubMed] [Google Scholar]
- 229.Ceremuzynski L, Gebalska J, Wolk R, Makowska E. Hypomagnesemia in heart failure with ventricular arrhythmias. Beneficial effects of magnesium supplementation. J Intern Med 247: 78–86, 2000. doi: 10.1046/j.1365-2796.2000.00585.x. [DOI] [PubMed] [Google Scholar]
- 230.Liu M, Jeong EM, Liu H, Xie A, So EY, Shi G, Jeong GE, Zhou A, Dudley SC Jr.. Magnesium supplementation improves diabetic mitochondrial and cardiac diastolic function. JCI Insight 4: e123182, 2019. doi: 10.1172/jci.insight.123182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Liu M, Dudley SC Jr.. Magnesium, oxidative stress, inflammation, and cardiovascular disease. Antioxidants (Basel) 9: 907, 2020. doi: 10.3390/antiox9100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gałecka S, Kulawiak B, Bednarczyk P, Singh H, Szewczyk A. Single channel properties of mitochondrial large conductance potassium channel formed by BK-VEDEC splice variant. Sci Rep 11: 10925, 2021. doi: 10.1038/s41598-021-90465-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov 9: 447–464, 2010. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 234.Zinovkin RA, Zamyatnin AA. Mitochondria-targeted drugs. Curr Mol Pharmacol 12: 202–214, 2019. doi: 10.2174/1874467212666181127151059. [DOI] [PMC free article] [PubMed] [Google Scholar]