Keywords: colonoids, enteroids, extracellular matrix, intestinal organoids, transcriptome
Abstract
Human intestinal epithelial organoids (enteroids and colonoids) are tissue cultures used for understanding the physiology of the human intestinal epithelium. Here, we explored the effect on the transcriptome of common variations in culture methods, including extracellular matrix substrate, format, tissue segment, differentiation status, and patient heterogeneity. RNA-sequencing datasets from 276 experiments performed on 37 human enteroid and colonoid lines from 29 patients were aggregated from several groups in the Texas Medical Center. DESeq2 and gene set enrichment analysis (GSEA) were used to identify differentially expressed genes and enriched pathways. PERMANOVA, Pearson’s correlation, and dendrogram analysis of the data originally indicated three tiers of influence of culture methods on transcriptomic variation: substrate (collagen vs. Matrigel) and format (3-D, transwell, and monolayer) had the largest effect; segment of origin (duodenum, jejunum, ileum, colon) and differentiation status had a moderate effect; and patient heterogeneity and specific experimental manipulations (e.g., pathogen infection) had the smallest effect. GSEA identified hundreds of pathways that varied between culture methods, such as IL1 cytokine signaling enriched in transwell versus monolayer cultures and E2F target genes enriched in collagen versus Matrigel cultures. The transcriptional influence of the format was furthermore validated in a synchronized experiment performed with various format-substrate combinations. Surprisingly, large differences in organoid transcriptome were driven by variations in culture methods such as format, whereas experimental manipulations such as infection had modest effects. These results show that common variations in culture conditions can have large effects on intestinal organoids and should be accounted for when designing experiments and comparing results between laboratories. Our data constitute the largest RNA-seq dataset interrogating human intestinal epithelial organoids.
INTRODUCTION
The human intestine is a highly dynamic organ that is essential for life and subject to common diseases such as cancer, infection, and chronic inflammation. The epithelial lining of the luminal surface of the intestines confers segment-specific functions to these organs: digestion and absorption of nutrients primarily occurs in the proximal small intestine (duodenum and jejunum), while the distal small intestine (ileum) absorbs remaining nutrients, bile acids, and B12; the colon/large intestine is primarily responsible for absorbing water and electrolytes and hosting the gut microbiome. The small intestinal mucosa consists of the proliferative crypt and differentiated villus compartments, whereas the epithelium of the large intestine is made up of crypts with a lower proliferative compartment and an upper differentiated compartment. Intestinal stem cells (ISCs) are located in the crypt base, where they rapidly renew and give rise to transit amplifying cells (TACs) which further differentiate into the various specialized cells of the epithelium (1–4). Paneth cells, primarily present in the small intestine, secrete antimicrobial peptides and provide signals for stem cell renewal and maintenance; goblet cells produce mucus; enteroendocrine cells release hormones; tuft cells are chemosensory in nature and also regulate immune responses; and enterocytes/colonocytes are responsible for the uptake of nutrients, water, and electrolytes (5–8). Apart from Paneth and stem cells, which remain at the crypt base, cells migrate up the crypt and differentiate before migrating onto the villus/colonic surface where they reside for 3–5 days before undergoing anoikis and being extruded into the lumen (9, 10).
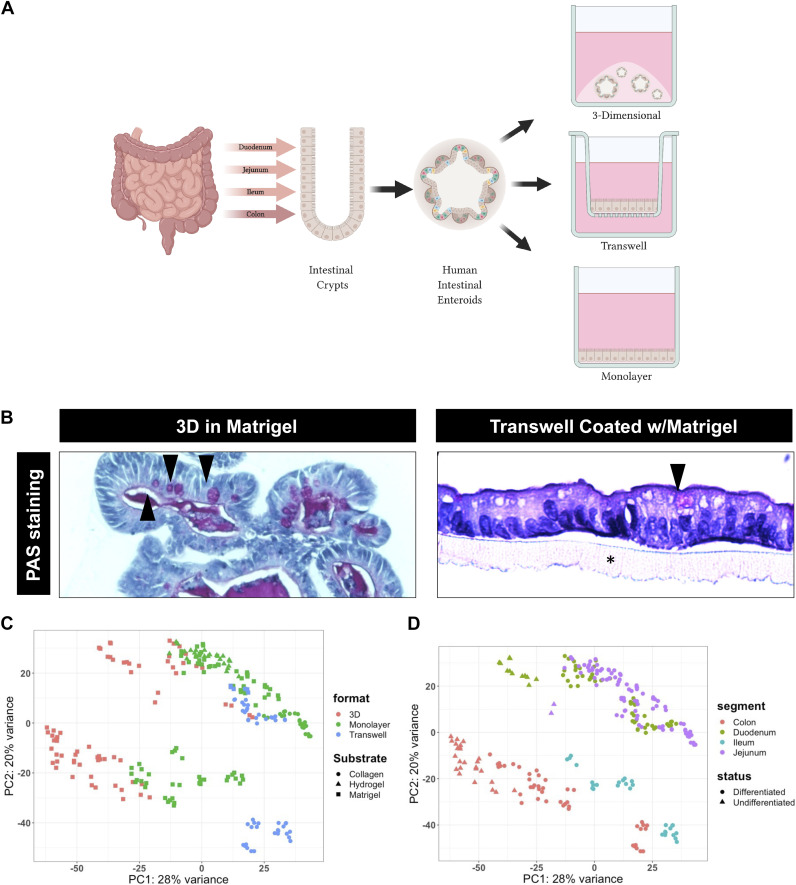
Experimental systems that recapitulate intestinal organs and can be cultured indefinitely are being validated and becoming vital for the progression of biomedical research. Small and large intestinal epithelial organoids (enteroids and colonoids respectively) provide researchers with an experimental platform composed of the epithelial cells and a simulacrum of the intestinal microenvironment similar to that seen in vivo, and thus provide excellent models for both basic and translational inquiries. Self-renewing mouse and human enteroids reported by Clevers’ laboratory in 2009 and 2011 replicate the polarized monolayer of the intestinal epithelium in a three-dimensional (3-D) culture (11, 12). Human enteroids/colonoids are derived by isolating crypts from endoscopic biopsy or surgically resected tissue. Purified crypts are collected and embedded in a 3-D matrix and cultured in a media containing key growth factors [epidermal growth factor (EGF), WNT3A, R-SPONDIN, and NOGGIN] for intestinal stem cell renewal and maintenance (1, 12–15) (Fig. 1A).
Figure 1.
Generation of enteroid models and RNA-seq data. A: schematic overview showing the derivation of three-dimensional (3-D) enteroids and colonoids from the crypts of various segments of the intestine. Enteroids and colonoids are then able to be maintained in their 3-D conformation or plated and grown in two-dimensional (2-D) as transwells or monolayers. B: periodic acid-Schiff (PAS) staining showing goblet cells (arrows) and the architecture of differentiated jejunal enteroids grown in 3-D in Matrigel (left) and in 2-D (Transwell) on Matrigel(*) (right). C: principal component analysis (PCA) of all RNA-seq samples included in the analyzed dataset [sample size (n) = 251] labeled by the format of enteroids/colonoids (color) and the substrate used (shape): 3-D-Matrigel (n = 69), monolayer-collagen (n = 24), monolayer-hydrogel (n = 26), monolayer-Matrigel (n = 80), transwell-collagen (n = 46), and transwell-Matrigel (n = 6). D: PCA of all RNA-sequencing samples included in the analyzed dataset (n = 251) labeled by segment of derivation (color) and differentiation status (shape): duodenum-differentiated (n = 49), duodenum-undifferentiated (n = 12), jejunum-differentiated (n = 90), jejunum-undifferentiated (n = 2), ileum-differentiated (n = 29), colon-differentiated (n = 44), and colon-undifferentiated (n = 25); format: growth formats (3-D, monolayers and transwells), substrate (Matrigel, collagen IV, and Matrigel-coated hydrogels), segment: segment organoids originated from (duodenum, jejunum, ileum, and colon), status: differentiation status (differentiated and undifferentiated) (n = 251). [Created with BioRender.com.]
This organoid model closely resembles the morphology, functionality, and cellular composition of the intestine in vivo, and recapitulates the expression of segment-specific markers of the tissue from which they are derived (12, 14, 16, 17). These properties contribute another unique aspect of this model allowing for the exploration of segment-specific functions or responses ex vivo. Enteroids/colonoids can also be grown in different “formats,” either three-dimensionally (3-D) or two-dimensionally (2-D), dependent on the requirements for experimentation and need for access to the apical or basal surfaces (Fig. 1A). Three-dimensional enteroid/colonoid cultures are generally grown embedded in an extracellular matrix (ECM) substitute, most commonly Matrigel. These cultures are generally oriented with the apical surface of the cells facing inward and are anchored by basolateral attachments to the ECM. Although 3-D cultures are widely used, limited access to the luminal surface present challenges for studies such as nutrient absorption and pathogen interactions. To overcome this challenge, enteroids have been grown without ECM, resulting in apical-out enteroids (18–26). In additon, 2-D cultures can be grown as monolayers on ECM or transwells on ECM-coated inserts (12, 21, 24–28). The epithelial layer is polarized with the basolateral surface attaching to the underlying ECM (Matrigel, collagen, or hydrogels) and the apical surface directed up. This allows the apical surface to be easily accessed for experimental manipulations. The ability to change the format of enteroids/colonoids allows the study of stimuli applied specifically to the apical or basolateral surfaces.
The maintenance and expansion of enteroids is achieved using proliferation media conditions that promote the growth of ISCs and suppress the differentiation of the stem cells, recapitulating the intestinal crypt compartment. In addition, enteroids can be differentiated by the withdrawal of WNT3A and R-SPONDIN, and reduced NOGGIN (key factors in maintaining the stem cell state) resulting in the terminal differentiation of ISCs into absorptive and secretory cell types recapitulating the upper-crypt or villus compartment. Typically, samples grown as monolayers or on transwells are differentiated before experimental manipulation to mimic the in vivo interaction between the upper-differentiated compartment of the intestinal epithelium and microbes, pathogens, or metabolites. Conversely, the undifferentiated crypt compartment is recapitulated through the continued growth of enteroids in proliferation media conditions. These two conditions also provide the opportunity to not only study the baseline differences between the differentiated and undifferentiated compartments but also to understand the differences in their response to particular stimuli and provide insight into compartmental-specific responses and pathway enrichment.
The enteroid/colonoid model is a reproducible, scalable, and physiologically relevant system applicable for a variety of high throughput screens and novel applications similar to the previous conventional culture of immortalized or cancer cell lines which lack physiological relevance (15, 29–31). Though the enteroid/colonoid model is a significant step forward compared with the conventional cancer or immortalized cell lines, there exist differences in methods of culture that have yet to be fully compared and characterized. Here, we aggregated 276 RNA-sequencing datasets and investigated the effect of format, substrate, segment of origin, differentiation status, project and patient line on driving transcriptional variance in human enteroids/colonoids.
METHODS
Experimentation
Human intestinal organoid (enteroids and colonoids) data from RNA-seq data samples were acquired from multiple laboratories associated with the Texas Medical Center’s Digestive Disease Core, who obtained organoids from the Digestive Disease Consortium Tissue Bank. For all studies, enteroids and colonoids were established from fresh endoscopic biopsies or resected surgical tissue under an institutional review board (IRB) approved protocol from healthy patients unless indicated otherwise (samples ending with “_CD” from project 2 were derived from patients with active Crohn’s disease), and maintained as 3-D organoid cultures in Matrigel. Some enteroid and colonoid lines were frozen and stored in liquid nitrogen, and subsequently thawed and expanded again as 3-D organoid cultures before use. Organoids were passaged every 7–14 days and were replated in enteroid/colonoid medium before experimentation. Several types of medium were used in the culturing of the enteroids and colonoids used in this study as described previously (11, 32). CMGF(−) (complete medium without growth factors), consists of advanced DMEM/F-12 medium (Gibco) supplemented with 1× GlutaMAX (Invitrogen), 10 mM HEPES buffer (Gibco), and 100 U/mL penicillin-streptomycin (Gibco). CMGF(+) (complete medium with growth factors) is CMGF(−) supplemented with 10% NOGGIN-conditioned medium, 20% R-SPONDIN-conditioned medium, 50% Wnt3A-conditioned medium, 1× B-27 supplement (Gibco), 1× N-2 supplement (Gibco), 10 mM nicotinamide (Sigma-Aldrich), 1 mM N-acetylcysteine (Sigma-Aldrich), 10 nM human gastrin I (Sigma-Aldrich), 500 nM A 83-01 (Sigma-Aldrich), 10 μM SB202190 (Sigma-Aldrich), and 50 ng/mL epidermal growth factor (EGF) (R&D Systems). Differentiation medium is CMGF(+) without WNT3A conditioned-medium, nicotinamide, and SB202190, and reduced NOGGIN (5%) and R-SPONDIN (10%) conditioned mediums. These mediums were prepared by the Texas Medical Center’s Digestive Disease Core.
Transcriptional response of human duodenal enteroids and colonoids to calcitriol.
In projects 1 and 4, 3-D enteroids were generated, maintained and experimentally manipulated in CMGF(+) as previously described (33). Briefly, undifferentiated and differentiated enteroids/colonoids were grown in CMGF(+) and differentiation medium, respectively, for 3 days, then treated with equal volumes of calcitriol (100 nM) or control [0 nM (ethanol)]. Twenty-four hours following treatment, enteroids/colonoids were collected and RNA isolated with the E.Z.N.A. Total RNA Kit I (omega BIO-TEK). Paired-end Illumina sequencing libraries were prepared by Novogene (Sacramento, CA). The mRNA sequencing was performed using Illumina platforms for 150 bp paired-end reads. The RNA-seq data (GSE159811) were deposited in the Gene Expression omnibus of the National Center for Biotechnology.
Transcriptional differences between colonoids derived from healthy patients and patients with Crohn’s disease.
In project 2, colonoids derived from healthy patients and patients with Crohn’s disease (indicated with “_CD” ending) were grown and maintained in CMGF(+). After 5–7 days of culture in Matrigel (BD Biosciences), the organoids were dissociated and RNA was isolated with the Qiagen RNeasy kit (Qiagen, Germantown, MD). Paired-end Illumina sequencing libraries were prepared and total RNA-seq was performed with the Hi-seq 2500 (Illumina Inc.). The RNA-seq data (GSE182424) were deposited in the Gene Expression omnibus of the National Center for Biotechnology.
Transcriptional response of human enteroids/colonoids to Escherichia coli infections.
In project 3, enteroids/colonoids on 96-well plate were generated as previously published (34). The undifferentiated enteroids/colonoids monolayers were grown in CMGF(+) for 3 days and in differentiation media from days 3–5. The differentiated monolayers were then infected with bacterial cultures at a multiplicity of infection of 10 for 3 h. At the end of infection, the cells were lysed in TRIzol (Invitrogen, Waltham, MA) and stored at −80°C. RNA isolated and prepared for paired-end Illumina sequencing by Novogene (Sacramento, CA). The RNA-seq data (GSE182426) were deposited in the Gene Expression omnibus of the National Center for Biotechnology.
Transcriptional response of human enteroids/colonoids on transwells to infection.
In project 5, enteroids/colonoids were plated on collagen-coated transwell membranes (Corning) and differentiated for 5 days following established protocols (35). Monolayers on transwells were mock-inoculated with TNC (10 mm Tris-HCl, 140 mM NaCl, 10 mM CaCl2, pH 7.4) or inoculated with purified triple-layered human rotavirus (Ito strain) in TNC at a high multiplicity of infection (36, PNAS 2017). After 2 h of virus adsorption in the presence of 0.2 mg/mL of porcine pancreatin (Sigma-Aldrich) prepared in CMGF(−), enteroids/colonoids were washed twice with CMGF(−) medium, and then differentiation media containing 0.2 mg/mL of porcine pancreatin was added to the transwell. Two (mock) and four (Ito-infected) transwell membranes per experimental treatment and time point were circumferentially excised and total RNA was extracted immediately using the Qiagen RNeasy Mini Kit. Undifferentiated jejunal 3-D organoids from two lines grown in CMGF(+) were similarly harvested. cDNA libraries were prepared using Illumina Epidemiology RiboZero rRNA removal with TruSeq Stranded RNA library prep following the provided protocol (Illumina). Sequencing of the cDNA libraries was performed on a high output v4 flow cell (Illumina) using a paired-end 100 cycle run on a HiSeq 2500 Sequencing System (Illumina). The RNA-seq data (GSE168005 and GSE183222) were deposited in the Gene Expression omnibus of the National Center for Biotechnology.
Transcriptional response of human duodenal enteroid monolayers to norovirus infection.
In project 6, monolayer cultures were prepared as previously described (37). Briefly, cell pellets resulting from dispersion of 3-D HIEs were suspended in IntestiCult (INT) human organoid growth medium (Stem Cell Technologies) proliferation medium, prepared by mixing equal volumes of components A and B of INT human organoid growth medium, and supplemented with 10 μM ROCK inhibitor Y-27632. After 1 day of cell growth as a monolayer, the proliferation medium was changed with differentiation medium, consisting of an equal volume of component A of INT human organoid growth medium and CMGF(−). The cell monolayers were differentiated for 5 days as previously described. Five-day-differentiated HIE cell monolayers were washed once with CMGF(−) and either mock-infected or inoculated with 5 μL human norovirus diluted in 100 μL CMGF(−) medium supplemented with 500 μM glycochenodeoxycholic acid (GCDCA), for 1–2 h at 37°C. The inoculum was removed, and monolayers were washed three times with CMGF(−) to remove the unbound virus. Differentiation medium containing 500 μM GCDCA was then added, and the cultures were incubated at 37°C for 24 h. Total RNA was extracted using the Qiagen RNeasy kit and paired-end Illumina sequencing was performed by Novogene (South Plainsfield, NJ). The RNA-seq data (GSE183223) were deposited in the Gene Expression omnibus of the National Center for Biotechnology.
Transcriptional responses of human intestinal enteroids to enteric virus infection.
In project 7, 3-D jejunal enteroids were maintained in GMCF(+) as previously described (32). Three-dimensional cultures were differentiated for 3–4 days with differentiation medium before inoculation, and were mock-inoculated with TNC or inoculated with purified triple-layered human rotavirus (Ito strain) in TNC at a high multiplicity of infection (36). The HIEs were vigorously pipetted 10–20 times with a P200 pipette to disperse and open the HIEs for apical exposure to the virus. After 2 h of virus adsorption in the presence of 0.1–0.2 mg/mL of porcine pancreatin (Sigma-Aldrich) prepared in CMGF(−) medium, HIEs were washed twice with CMGF(−) medium and centrifuged at 50 g to 70 g to remove the inoculum and pancreatin that were present during virus adsorption. RNA was extracted using the Qiagen RNeasy Mini kit. Total RNA was prepared by using the Illumina TruSeq Stranded RNA Sample preparation protocol. Paired-end sequencing was performed by using the Illumina HiSeq 2500 machine. The RNA-seq data (GSE90796) were deposited in the Gene Expression omnibus of the National Center for Biotechnology.
Transcriptional responses of human intestinal enteroids to norovirus infection.
In project 8, 3-D enteroids initially maintained as 3-D cultures in CMGF(+) were disassociated in trypsin/EDTA and seeded onto collagen IV-coated 96-well plates as monolayers. Following culture in CMGF(+) for 1 day, monolayers were cultured in differentiation medium for 5 days and then mock-inoculated or inoculated with a pandemic GII.4 Syndey strain of human norovirus as a 10% stool filtrate containing a high multiplicity of infection (MOI) of virus (1.8 × 106 genome equivalents/well) or a similar amount of γ-irradiated stool. After a 1-h adsorption period, the HIEs were washed twice with CMGF(−) medium to remove unbound viruses. The cultures were harvested at 6-, 10-, and 24-h postinfection (hpi) for RNA-seq analyses. Total RNA was extracted from 5-day differentiated, mock-inoculated monolayers at 3 hpi (treated +/− GCDCA for 3 h), and GII.4- or gGII.4-inoculated monolayers at 6, 10, and 24 hpi using the Qiagen RNeasy mini Kit. Libraries were subsequently created and sequencing was performed on an IlluminaHiSeq 2500. The RNA-seq data (GSE150918) were deposited in the Gene Expression omnibus of the National Center for Biotechnology.
Effect of substrate stiffness on human enteroids’ transcriptome.
In project 9, 3-D enteroids were established from human jejunal and duodenal epithelium and cultured as previously described (32). Monolayer cultures of enteroids were cultured atop crosslinked poly(ethylene glycol)-based hydrogels that varied in stiffness as described by Swaminathan et al. (38). Briefly, 3-D enteroids maintained in CMGF(+) were dissociated into single cells using trypsin, and seeded atop Matrigel-coated hydrogel surfaces at a density of 3 × 105 cells/cm2 for 1 day to form monolayers. After the cells were adhered to the hydrogels, they were grown in differentiation medium for 5 days with medium changed every 48 h. Subsequently, enteroids monolayers were harvested in TRIzol, homogenized, and stored at −80°C before being shipped for purification and mRNA-sequencing was performed by Novogene (Sacramento, CA). The RNA-seq data (GSE182428) were deposited in the Gene Expression omnibus of the National Center for Biotechnology.
Baseline transcription profile of human enteroids with inducible neurogenin-3 expression.
In project 10, enteroids on transwell were generated and experimentally manipulated as previously published (39). Briefly, J2 enteroids, which underwent lentivirus transduction to integrate into the genome, a doxycycline-inducible NGN3 cassette, were seeded onto 24-well Transwells coated with Matrigel and differentiated without doxycycline. After 5 days, differentiation medium from the apical side was removed and enteroids were treated with 100 µL of lyophilized LDM4 that had been resuspended in enteroid differentiation medium (40). After a 3-h incubation at 37°C, the Transwells were placed into TRIzol and RNA was extracted and purified with a Qiagen RNeasy kit. The RNA-seq data (GSE138350) were deposited in the Gene Expression omnibus of the National Center for Biotechnology.
Evaluating substrate and format as drivers of transcriptional variance in human jejunal enteroids.
In project 11, jejunal enteroids initially were maintained as 3-D cultures in CMGF(+). Three-dimensional enteroid samples were then cultured in differentiation medium for 5 days and harvested. Simultaneously, 3-D enteroids were disassociated in trypsin/EDTA and were seeded onto collagen IV or Matrigel-coated 96-well plates as monolayers or transwells. Following culture in CMGF(+) for 1 day, 2-D cultures were cultured in differentiation medium for 5 days, washed with CMGF(−) medium, and harvested. Total RNA was extracted using the Qiagen RNeasy kit and RNA-seq data were generated on a Illumina NovaSeq instrument with read lengths of 2 × 150 bp, performed by the Texas Medical Center Genomic Center for Infectious Diseases Sequencing and Technology Core (Houston, TX). The RNA-seq data (GSE182437) were deposited in the Gene Expression omnibus of the National Center for Biotechnology.
RNA-Seq and Statistical Analysis
For global assessment of RNA levels from human enteroids samples, Kallsito (version 46.1) was utilized to align RNA-seq libraries to the human reference genome (hg38) and to quantify the abundance of those transcripts (41). The tximport (version 1.18.0) package was run in R (version 4.0) to create gene-level count matrices for use with DESeq2 (version 1.30) by importing quantification data obtained from Kallisto (42, 43). DESeq2 was then used to generate transcript levels in each tissue sample. False discovery rate (FDR)/adjusted P values were generated with DESeq2 in R using a Benjamini–Hochberg procedure that corrects for the number of tests and the rank of each test. Genes were considered differentially expressed with a FDR ≤ 0.01 and a fold change ≥ 2 or ≤ 0.5. Permutational multivariate analysis of variance (PERMANOVA) was performed in R using the adonis function in the vegan package with 999 permutations and pairwise distances were calculated using the Euclidean method. The model used included Line, Status, Segment, Format, and Substrate.
Gene set enrichment analysis (GSEA) was used for pathway analysis of gene expression data to find enriched canonical pathways, hallmark pathways, and gene ontology terms as defined by MiSigDB, https://www.broadinstitute.org/gsea/msigdb/index.jsp (44, 45). Preranked lists of gene fold changes were generated in DESeq2 between duodenal and colonic biopsies. Principal component analysis (plotPCA) and volcano plots (EnhancedVolcano) were all generated in R. Dendrograms were generated using hierarchical clustering in the web tool version of iDEP (version 0.91) (46). Venn diagrams were generated using web-based tool Venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/index.html).
RESULTS
Format and Substrate Contribute the Most Variation to Transcriptomic Data from 251 Human Intestinal Epithelial Organoids Samples
To analyze the drivers of transcriptomic variation in enteroids/organoids, we aggregated RNA-seq data from human enteroid/colonoid samples. These enteroids/colonoids were propagated in 3-D growth conditions and replated into 3-D, transwell or monolayer formats (Fig. 1A). Subsequently, these were differentiated (where indicated) and subjected to a variety of experimental manipulations including but not limited to exposure to calcitriol (the active form of Vitamin D), human rotavirus, Escherichia coli, and human norovirus. Organoids were then collected, RNA extracted, and RNA-sequencing was performed resulting in a total of 251 enteroid/colonoid samples that were included in this study (Table 1). Several of these datasets have previously been published or are in preparation (23, 33, 36, 39, 47). The organoids used were derived from various segments (duodenum, jejunum, ileum, or colon), from various patients (28 patients), grown in various formats [3-D, monolayers on 96-well plates (monolayers) or monolayers on transwells (transwells)] and substrates (collagen IV, Matrigel, or Matrigel-coated hydrogels), grown under different differentiation statuses (differentiated and undifferentiated) and given different experimental manipulations (20), which were performed by different experimenters/projects (10) (Supplemental Tables S1 and S2; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.16877230.v1).
Table 1.
251 RNA-sequencing samples used in this analysis
| Duodenum | Jejunum | Ileum | Colon | |
|---|---|---|---|---|
| Formats | 3 | 3 | 2 | 3 |
| Substrates | 2 | 3 | 2 | 2 |
| Statuses | 2 | 2 | 1 | 2 |
| Lines | 8 | 4 | 3 | 20 |
| Treatments | 13 | 15 | 10 | 12 |
| Projects | 4 | 6 | 2 | 5 |
| No. of samples | 61 | 92 | 29 | 69 |
Shown are the abbreviated demographics with the number of formats (3-D, monolayers, and transwells), substrates (matrigel, collagen IV, and Matrigel-coated hydrogels), statuses (differentiated and undifferentiated), lines, treatments, and projects (n = 251).
To begin investigating the drivers of variance among samples, we performed permutational multivariate analysis of variance (PERMANOVA) on our nonparametric dataset (Table 2). These revealed that the top two drivers of variance in this large dataset were format and substrate (F = 96.1 and 85.3, respectively), which accounted for 21.3% and 18.9%, respectively, of the variation in our model. Differentiation status, project, and segment were moderate drivers of variance (F = 26.6, 18.1, and 16.1, respectively) and explained 2.9%, 10%, and 5.3%, respectively, of the model, and finally line (representing patient-to-patient variation), and experimental treatment (including various experimental treatments such as infection and calcitriol treatment; see methods for details) had the smallest contribution to the variance (F = 5.9 and 1.1, respectively) and explained 17.7% and 1.9% of the variance in the model respectively).
Table 2.
PERMANOVA results
| Source | Df | Sum Sqs. | Mean Sq. | F Model | Pr(>F) |
|---|---|---|---|---|---|
| Format | 2 | 6.83E+10 | 0.21338 | 96.1452 | 0.001*** |
| Substrate | 2 | 6.06E+10 | 0.18934 | 85.3136 | 0.001*** |
| Status | 1 | 9.46E+09 | 0.02955 | 26.6331 | 0.001*** |
| Project | 5 | 3.23E+10 | 0.10089 | 18.1835 | 0.001*** |
| Segment | 3 | 1.73E+10 | 0.05391 | 16.1951 | 0.001*** |
| Line | 27 | 5.69E+10 | 0.17785 | 5.936 | 0.001*** |
| Treatment | 16 | 6.34E+09 | 0.01979 | 1.1147 | 0.239 |
| Residual | 194 | 6.89E+10 | 0.21528 | ||
| Total | 250 | 3.2012E+11 | 1 |
PERMANOVA output showing the effects of format, substrate, segment (segmental origin of enteroid/colonoid), status (differentiation status), project, line (patient line), and treatment based on Euclidian distances and 999 permutations. Df, degrees of freedom; F model, F statistics; mean sq., mean sum of squares; PERMANOVA, permutational multivariate analysis of variance; Pr (>F), P values; R2, partial R-squared; sums sqs., sum of squares.
***P = 0.001.
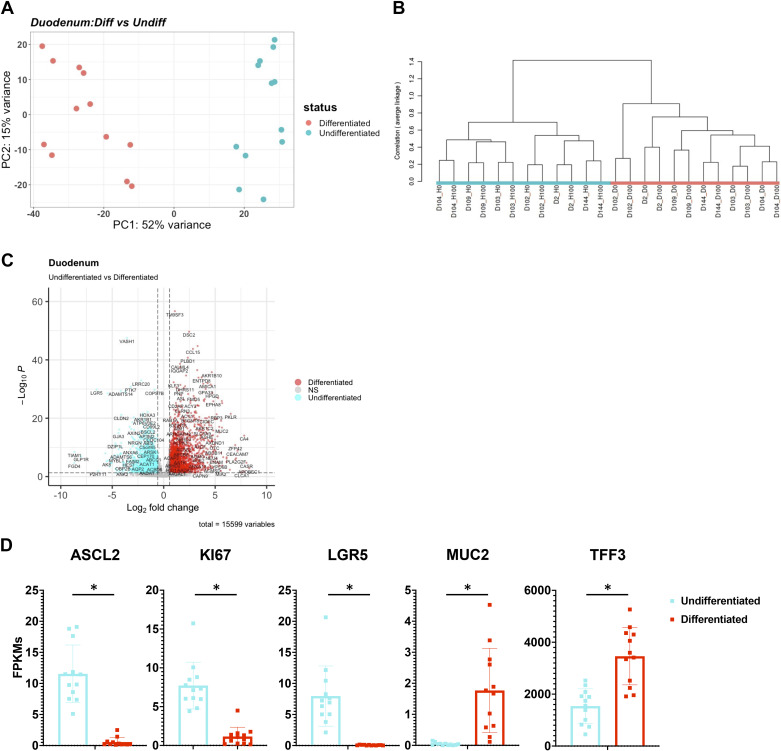
Morphologically, we observed no significant differences between enteroids grown in 3-D or 2-D with an organized cellular polarity with the presence of goblet cells (Fig. 1B). Thus, next we visualized the effect of potential sources of variation of the data on the transcriptome using principal component analysis (PCA) (Fig. 1, C and D). The PCA of the entire dataset was used to visualize clustering of the samples, with the first two principal components accounting for 29% and 19% variance of the samples. We observed that enteroids/colonoids grown in 3-D (red) were left-shifted on the x-axis compared with samples from the same segment grown in 2-D [transwells (blue) and monolayers (green)] (Fig. 1C). The PCA plot exhibited that duodenal (green) and jejunal (purple) samples clustered together in the first and second quadrant whereas the colon (red) and ileal (blue) samples existed in the third and fourth quadrant, respectively, with undifferentiated enteroids/colonoids being left-shifted on the x-axis compared with differentiated samples (Fig. 1D).
Human Enteroid/Colonoid Substrate Potentially as a Major Driver of Variation
To further examine the drivers of transcriptomic variance, we investigated the variance across the dataset subset by segment of origin (duodenum, jejunum, ileum, and colon). Using PCA of datasets derived from individual segments, we observed that the samples clustered by format and substrate (Fig. 2A and Supplemental Figs. S1–S3). For example, in duodenum samples there are four observable clusters: monolayers grown on Matrigel, transwells and monolayers grown on collagen, and two 3-D clusters grown in Matrigel (Fig. 2A). PC1 in this analysis, accounting for 38% variance, appears to be driven by the dimensionality of the format, with the 2-D enteroids clustered in the first and fourth quadrants of the plot whereas the 3-D enteroids clustered in the second and third quadrant of the PCA plot (Fig. 2A). PC2, accounting for 20% variance, appears to be driven primarily by substrate with monolayers grown on Matrigel clustering in the first quadrant of the plot and transwells and monolayers grown on collagen clustering in the fourth quadrant of the PCA plot. Furthermore, subclusters within the fourth quadrant separate monolayer and transwell samples grown on collagen. The separation of 3-D samples into two clusters is driven by their differentiation status, which will be examined in greater detail subsequently. Additionally, a dendrogram of the data shows a clear bifurcation of the duodenal monolayers based on the substrate regardless of the line or other experimental manipulations (Fig. 2B). This same effect was observed in a Pearson correlation matrix of duodenal enteroids (Supplemental Fig. S4). Patient-to-patient variation and the experimental treatments (e.g., infection) had small effects on the variation within the dataset, which could be observed in the dendrograms as the final branch points in the tree, all reinforcing the results of the PERMANOVA analysis of our data (Fig. 2B and Table 2). Similar observations were made by analyzing clustering of jejunal, ileal, and colonic datasets using PCA, dendrogram, and Pearson correlation analysis which demonstrated clustering of samples by format and substrate (Supplemental Fig. S1, A and B; Fig. S2, A and B; Fig. S3, A and B; and Figs. S4–S7). In addition, in the jejunum, monolayer samples were also grown on synthetic hydrogel bases that were coated with Matrigel (thus providing different stiffness to the Matrigel substrate). Data from these monolayers that were grown on hydrogels clustered together with other monolayer samples grown on Matrigel-coated plastic, with distinct subclusters associated with differing hydrogel properties (Supplemental Fig. S1, A and B). An analysis of the influence of substrate stiffness on enteroid physiology was be published by Swaminathan et al. (38); however, these results underscore that the composition of the substrate matrix is a major driver of variance.
Figure 2.
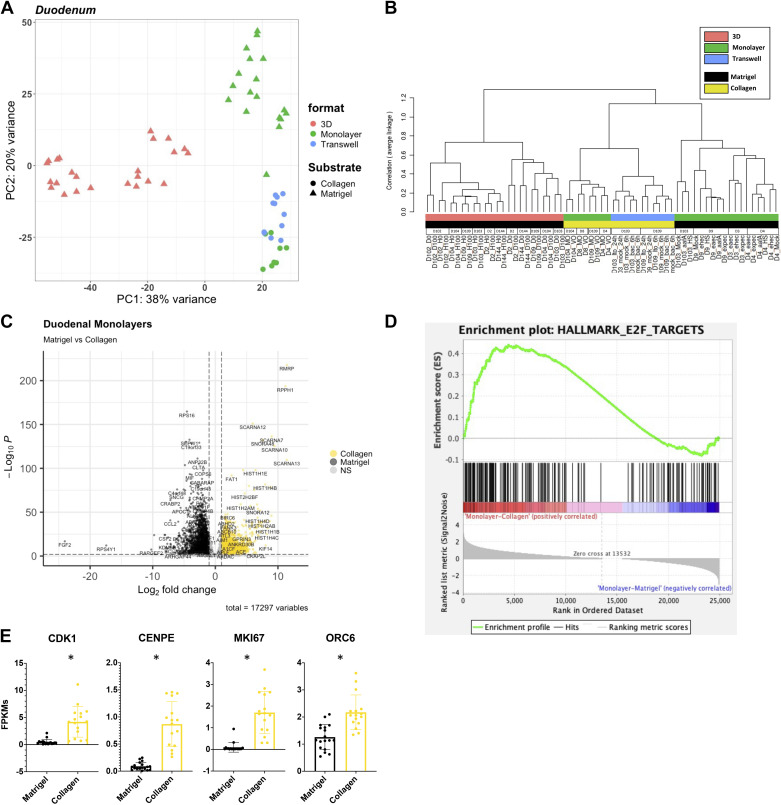
E2F target genes are upregulated in duodenal monolayers grown on Matrigel. A: principal component analysis (PCA) of the RNA-sequencing datasets for duodenal enteroids: three-dimensional (3-D)-Matrigel (n = 24), monolayer-Matrigel (n = 18), monolayer-collagen (n = 8), and transwell-collagen (n = 11). B: a dendrogram with agglomerative hierarchal clustering of the duodenal gene set from RNA-sequencing. Branch length indicates degree of difference between samples. C: volcano plot of differentially expressed genes when comparing duodenal monolayers on collagen (gold) and Matrigel (black). Gold/black dots indicate differentially expressed genes (false discovery rate, FDR ≤ 0.01 and a fold change ≥2 or ≤0.5) that are upregulated or downregulated (respectively) in duodenal monolayers on collagen (3,136 genes) vs. duodenal monolayers on Matrigel (2,868 genes). D: gene set enrichment analysis (GSEA) showing an enrichment of the hallmark E2F targets gene set signature in duodenal monolayers grown on collagen when compared with duodenal monolayers grown on Matrigel. E: normalized fragments per kilobase of transcript per million mapped reads (FPKMs) of E2F target genes from RNA-sequencing of duodenal monolayers grown on Matrigel (black) and collagen IV (gold)s. *False discovery rate ≤1% (0.01) according to DESeq2.
We used DESeq2 to identify differentially expressed genes (DEGs) when comparing datasets from organoids in which only one of the four major variables (segment, substrate, format, differentiation status) was different (42). Thus, a total of 39 comparisons were made (Supplemental Table S3). Genes were considered differentially expressed with a false discovery rate (FDR) ≤ 0.01 and a fold change ≥2 or ≤0.5. We identified genes differentially regulated in response to substrate. Specifically, as visualized in a volcano plot 5,244 genes were differentially expressed in duodenal enteroids grown as monolayers on collagen (3,136 genes upregulated) versus Matrigel (2,868 genes upregulated) (Fig. 2C and Supplemental Table S3). We next assessed pathways that were differentially regulated by substrate using gene set enrichment analysis (GSEA) with FDR ≤ 0.05 (44, 45). GSEA revealed a total of 36 of the Hallmark collection gene sets that were enriched in duodenal monolayers grown on collagen (6 gene sets) and duodenal monolayers grown on Matrigel (30 gene sets). There were also 238 GSEA canonical pathway gene sets that were enriched in duodenal monolayers grown on collagen (4 gene sets) and duodenal monolayers grown on Matrigel (234 gene sets). And 326 Gene Ontology (GO) gene sets that were enriched in duodenal monolayers grown on collagen (10 gene sets) and duodenal monolayers grown on Matrigel (326 gene sets). Similar analyses comparing jejunal monolayers grown on collagen and Matrigel identified 24 Hallmark pathway gene sets, 193 GSEA canonical pathway gene sets, and 278 GO term gene sets that were enriched (FDR ≤ 0.05). Datasets from ileum and colon did not include organoids grown in the same format with different substrates, therefore a focused analysis of pathways that were driven by substrate alone is confounded by differences in format (Supplemental Figs. S2 and S3 and Supplemental Table S3).
Of interest, the E2F targets and Hallmark gene set from MSigDB were enriched in duodenal monolayers grown on collagen compared with duodenal monolayers grown on Matrigel (normalized enrichment score, NES = 1.84 and FDR = 9.50E−04) (Fig. 2D and Supplemental Fig. S1). Several E2F targets (CDK1, CENPE, ORC6, and MKI67) were upregulated in monolayers on collagen compared with Matrigel (Fig. 2E). Also, the cholesterol homeostasis Hallmark gene set was enriched in jejunal monolayers grown on collagen compared with duodenal monolayers grown on Matrigel. SREBF1 (Sterol Regulatory Element Binding Transcription Factor 1), a regulator of cholesterol biosynthesis, was also significantly upregulated in duodenal monolayers that were grown on Matrigel corresponding with the increase in enzymes that are involved in cholesterol biosynthesis (Supplemental Fig. S1C).
Growth Format is a Major Driver of Variation in Human Enteroids and Colonoids
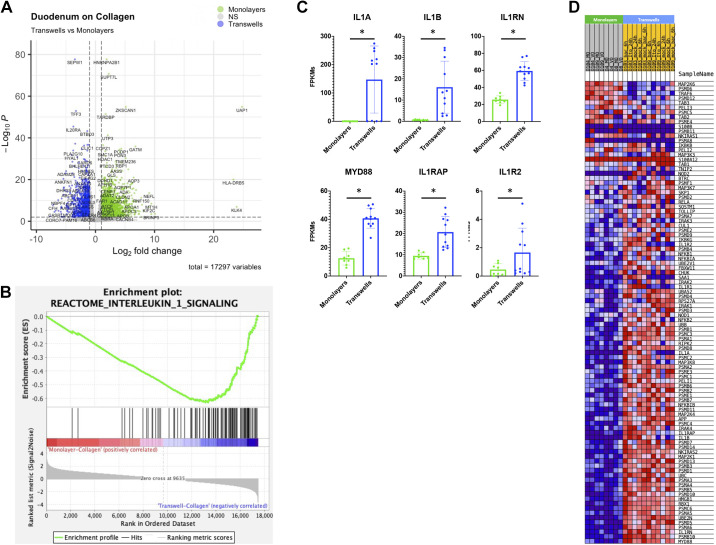
In addition to observing the effects of the substrates on enteroid monolayers in PCA plots and dendrograms, we also observed differences between enteroids grown in different growth formats with the same substrate (Fig. 2, A and B and Supplemental Fig. S1). DESeq2 analysis identified 4,695 genes that were differentially expressed between duodenal enteroids grown on collagen in the monolayer (3,378 genes upregulated) or transwell (1,317 genes upregulated) formats (Fig. 3A and Supplemental Table S3). Thus, we further investigated monolayers and transwells that were both grown on collagen. GSEA (FDR ≤ 0.05) revealed a total of 31 of Hallmark pathways gene sets that were enriched in duodenal monolayers grown on collagen (2 gene sets) or in duodenal transwells grown on collagen (29 gene sets). There were also 505 GSEA canonical pathway gene sets that were enriched in duodenal monolayers grown on collagen (2 gene sets) or in duodenal transwells grown on collagen (503 gene sets). Four hundred GO gene sets were only enriched in duodenal transwells grown on collagen.
Figure 3.
Interleukin-1 signaling pathway is enriched in duodenal transwells grown on collagen. A: volcano plot of differentially expressed genes when comparing duodenal monolayers (green) and transwells (blue) grown on collagen. Green/blue dots indicate differentially expressed genes (false discovery rate, FDR ≤ 0.01 and a fold change ≥2 or ≤0.5) that are upregulated or downregulated (respectively) in duodenal monolayers on collagen (3,378 genes) vs. duodenal transwells on collagen (1,317 genes). B: gene set enrichment analysis (GSEA) showing an enrichment of the Reactome interleukin-1 signaling gene set signature in duodenal transwells grown on collagen when compared with duodenal monolayers grown on collagen. C: normalized fragments per kilobase of transcript per million mapped reads (FPKMs) of Interleukin-1 signaling pathway genes that encode for secreted cytokines (IL1A and IL1B) and inhibitors (IL1RN) (top), and interleukin-1 receptor machinery (bottom) on duodenal monolayers (green) and transwells (blue) grown on collagen. D: GSEA showing an enrichment of the reactome interleukin-1 signaling gene set signature in duodenal transwells grown on collagen when compared with duodenal monolayers grown on collagen. *False discovery rate ≤1% (0.01) according to DESeq2.
Of note, the Reactome interleukin 1 signaling gene set was enriched in duodenal transwells grown on collagen when compared with monolayers grown on collagen (NES = −3.05 and FDR = 0) (Fig. 3B). Similarly, the Reactome interleukin 1 signaling gene set was also enriched in jejunal transwells grown on collagen when compared with monolayers grown on collagen (NES = −2.56 and FDR = 2.32E−04) (Supplemental Fig. S1D). Several of the enriched genes were associated with the receptor machinery for IL-1 signaling (ILRAP, IL1R2, and MYD88). Moreover, secreted protein transcripts were also enriched in transwells, those being proinflammatory IL-1 cytokines (IL1A and IL1B) as well as an IL-1 receptor antagonist (IL1RN) (Fig. 3C). We also observed an enrichment in the hypoxia hallmark gene set in duodenal (NES = −1.72 and FDR = 0.001) and jejunal (NES = 1.78 and FDR = 0.003) transwells grown on collagen compared with monolayers grown on collagen (Supplemental Fig. S8).
In addition, a comparison of 3-D and monolayers grown on/in Matrigel revealed several genes and gene sets that were differentially expressed. These comparisons were made in duodenal, jejunal, and colonic organoids (Fig. 2A and Supplemental Fig. S9). DESeq2 analysis identified genes that were differentially expressed between duodenal (5,139 genes), jejunal (5,195 genes), and colonic (6,049 genes) organoids grown in 3-D (duodenum-2,615, jejunum-1,569, and colon-3,481 upregulated genes) or on monolayers (duodenum-2,524, jejunum-3,635, and colon-2,568 upregulated genes) with Matrigel (Supplemental Table S3). In addition, the Hallmark MYC targets gene set was enriched for 3-D organoids in Matrigel compared with monolayers on Matrigel (Supplemental Fig. S9, A and B). We also observed an enrichment for the Reactome antigen processing and cross presentation gene set in colonoid monolayers grown on Matrigel compared with 3-D colonoids in Matrigel (Supplemental Fig. S3, C and D). Colonoid monolayers on Matrigel had an enrichment specifically in the HLA-E transcripts, a nonclassical major histocompatibility complex (MHC) I molecule (48, 49). In another case, we observed an enrichment of several ATP-binding cassette (ABC) transporters, known for their role in the transport of molecules and drug resistance, in ileal transwells on collagen compared with ileal monolayers on Matrigel (Supplemental Fig. S2, C and D) (50, 51).
Known Transcriptional Regional Identity Markers Are Observed in Enteroids and Colonoids
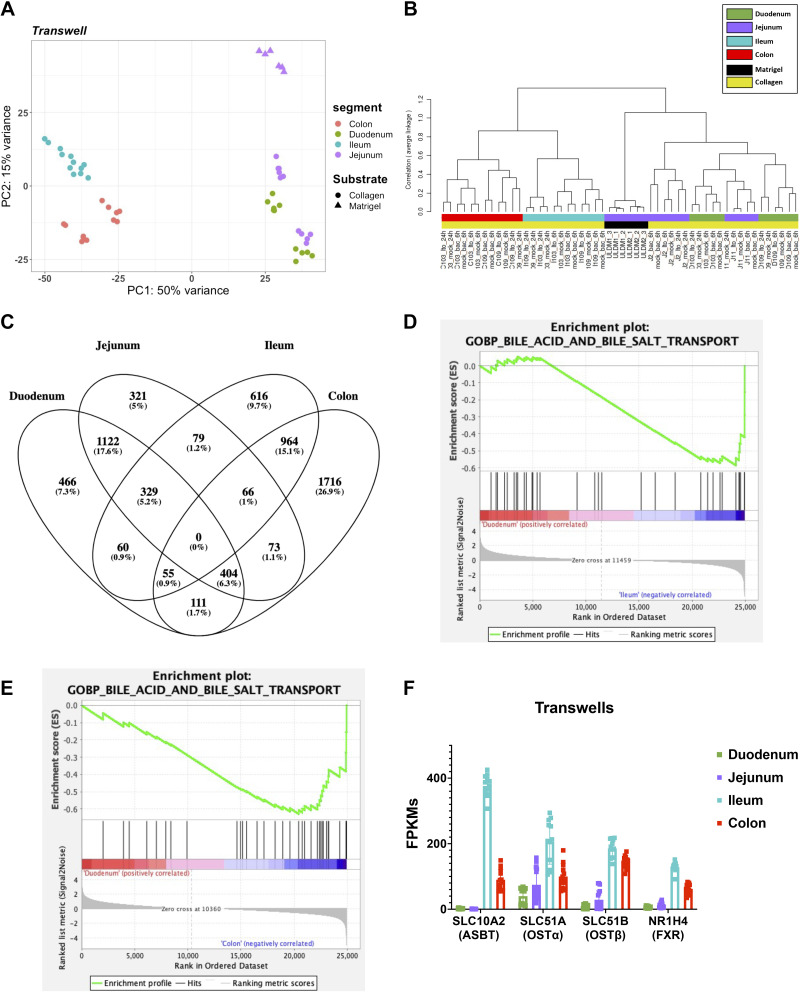
The human intestine is composed of several specialized segments with dedicated functional capabilities. Gene expression profiles of each of the segments have been characterized in previous reports (52–54). To highlight the variation in gene expression between human enteroids derived from different segments of the intestine (duodenum, jejunum, ileum, and colon), we performed PCA of enteroids/colonoids that were grown using the same format (3-D, monolayer, or transwell). PCA of organoids cultured on transwells only showed that colonic and ileal samples were separated from the jejunal and duodenal samples by PC1 on the x-axis, accounting for 50% variance in this dataset. Specifically, the ileal and colonic samples clustered in the second and third quadrant, respectively, on the PCA plot. The duodenal and jejunal samples clustered in the first and fourth quadrant of the PCA plot, suggesting a higher level of similarity between duodenal and jejunal samples than ileal and colon samples (Fig. 4A). This observation was replicated when using a dendrogram which showed clear separations between the colonic and ileal samples being broadly separated from the duodenal and jejunal samples that were intermingled (Fig. 4B). Similar observations could also be made across formats, where samples derived from duodenum and jejunum clustered together and samples from ileum and colon also clustered together (Fig. 4, A and B and Supplemental Fig. S10, A–D). As previously mentioned, it was observed that the substrate that the enteroids were grown on is a large driver of variance, in this case appears to be the driver of PC2 and a delineator of jejunal and duodenal samples in the dendrogram (Fig. 4, A and B). In addition, the jejunal enteroids that were grown on Matrigel (ULDM1 and ULDM2) were also transduced with a doxycycline-inducible neurogenin-3 (NGN3) (uninduced) construct, an additional factor that may have driven the differences between other jejunal samples.
Figure 4.
Bile acid transport machinery is enriched in ileal enteroids and colonoids. A: principal component analysis (PCA) of the RNA-sequencing datasets for enteroids on transwells: duodenum-collagen (n = 11), jejunum-collagen (n = 11), jejunum-Matrigel (n = 6), ileum-collagen (n = 12), and colon-collagen (n = 12). B: a dendrogram with agglomerative hierarchal clustering of the transwell gene set from RNA-sequencing. Branch length indicates degree of difference between samples. C: Venn diagram displaying the overlap of differentially expressed genes (DEGs) between duodenal, jejunal, ileal, and colonic intestinal organoids grown on transwells. DEGs were defined as any gene that was differentially enriched in the segment of interest compared with any other segment with DESeq2 (false discovery rate, FDR ≤ 0.01 and fold change ≥2 and ≤0.5). Gene set enrichment analysis (GSEA) showing an enrichment of the Gene Ontology (GO) bile acid and bile salt transport get set signature in ileal enteroids (D) and colonoids (E) on transwells when compared with duodenal enteroids grown on transwells. F: normalized fragments per kilobase of transcript per million mapped reads (FPKMs) of bile acid transport genes in duodenal (green), jejunal (purple), ileal (blue), and colonic (red) intestinal organoids grown on transwells. SLC10A2, SLC51A, SLC51B, and NR1H4 were upregulated in the ileum and colon compared with the duodenum and jejunum with a false discovery rate ≤ 1% (0.01) according to DESeq2.
We utilized DESeq2 analysis to generate differentially expressed gene lists between segments within the same format. Samples were considered differentially expressed with an FDR ≤ 0.01 and fold changes ≥2 or ≤0.5 (Supplemental Table S3). To show the similarities and differences of intestinal organoids derived from various segments, we generated Venn diagrams using the DEGs for each segment (Fig. 4C and Supplemental Fig. S10E). The DEG list for each segment was composed of all enriched genes in the segment of interest compared with any other segment (e.g., union list of all duodenal enriched genes in Duodenum vs. Jejunum, Duodenum vs. Ileum, and Duodenum vs. Colon). As indicated by the PCA plot, the duodenum and jejunum (1,122 shared DE genes) shared the most similarity and the ileum and colon (964 shared DE genes) were also similar to each other (Fig. 4C).
To understand the potential phenotypic impact due to the differences in gene expression between segments, GSEA was performed (FDR ≤ 0.05) (Fig. 4, D–F, and Supplemental Information 3; see https://doi.org/10.6084/m9.figshare.14575647). GSEA revealed a total of 92 of GO gene sets that were enriched in duodenal transwells grown on collagen (14 gene sets) and ileum transwells grown on collagen (78 gene sets). In addition, a total of 141 GO gene sets were enriched in duodenal (133 gene sets) and colon transwells grown on collagen (8 gene sets). There was an enrichment in the bile acid and bile salt transport gene ontology term in ileal (NES = −1.61 and FDR = 0.11) and colonic (NES = −1.88 and FDR = 0.04) transwells relative to the duodenal transwells (Fig. 4, D and E). As anticipated, these transcripts for bile acid transport genes were upregulated in the ileum and colon compared with the duodenum and jejunum (Fig. 4F).
Enteroids/colonoids have been used as in vitro surrogates of the human intestine. Thus, we also explored which enteroid/colonoid format held the highest level of transcriptional similarity to human biopsy samples. RNA-seq data from healthy human duodenal and colonic mucosal biopsies (PRJNA528755 and GSE88710) collected from two separate studies were compiled and compared with our duodenal enteroid and colonoid datasets (55, 56). A Pearson correlation matrix of the previously mentioned datasets exhibited a separation between mucosal biopsies and in vitro cultured enteroids/colonoids (Supplemental Fig. S11A). Similar expression profiles of regional markers such GATA4 and SATB2, which mark the proximal and distal intestine respectively, were matched in enteroids/colonoids and biopsies from the same segment (Supplemental Fig. S11B). However, enteroids/colonoids showed a reduced or complete lack of stromal (ACTA2 and VIM) and endothelial (PECAM1 and VCAM1) markers, which were present in mucosal biopsy samples (Supplemental Fig. S11B). Although all three formats matched the biopsy data very well with Pearson coefficients ≥0.77, GSEA analysis comparing enteroid expression patterns to genes differentially expressed between duodenal and colonic biopsies suggested that 3-D formatted enteroids best reflect expression patterns in biopsies (Supplemental Fig. S11, C and D). Three-dimensional undifferentiated and differentiated enteroid/colonoid gene sets had the highest normalized enrichment scores (NESs) of 2.42(duodenal)/−4.08 (colonic) and 2.37(duodenal)/−3.98 (colonic) respectively. Differentiated monolayer and transwell enteroid/colonoid gene sets had NESs of 2.18 (duodenal)/−3.76 (colonic) and 1.66 (duodenal)/1.15 (colonic) respectively (Supplemental Fig. S11E).
Differentiation Status Drives Known Changes in Transcriptional Markers Proliferating and Differentiated Enteroids and Colonoids
The transcriptional and functional differences between the undifferentiated crypt compartment and the upper villus/differentiated compartment of the intestine have been well characterized in vivo and in vitro. Here, we confirmed and expanded on the importance of differentiation on the enteroid/colonoid model.
As shown earlier, the format is a major driver of variation in enteroids, however further subclusters can be observed that are distinguished by differentiation status (Fig. 1D). By analyzing duodenal and colonic organoids grown in 3-D, differences between the differentiated (red) and undifferentiated (cyan) cultures can be observed in PCA plots and dendrograms (Fig. 5, A and B, and Supplemental Fig. S12, A and B). The dendrogram and the first principal component of the PCA revealed a clear separation between differentiated and undifferentiated enteroids (Fig. 5, A and B, and Supplemental Fig. S12, A and B). DESeq2 analysis identified 2,902 genes that were differentially expressed between duodenal enteroids that were differentiated (1,474 genes enriched) and undifferentiated (1,428 genes enriched). In this analysis, only control samples were used due to the presence of calcitriol responsive genes that were compartment specific, which will be explored in a manuscript in preparation (Fig. 5C and Supplemental Table S3). Similarly, there were 2,756 genes that were differentially expressed between colonoids that were differentiated (1,338 genes enriched) and undifferentiated (1,418 genes enriched) (FDR ≤ 0.01 and fold changes ≥2 or ≤0.5) (Supplemental Fig. S12C and Supplemental Table S3). Consistent with their proliferative and self-renewing nature, markers of proliferation and intestinal stem cells were upregulated in undifferentiated enteroids and colonoids (ASCL2, KI67, and LGR5) (Fig. 5, C and D, and Supplemental Fig. S12, C and D). Conversely, differentiated enteroids and colonoids expressed genes that are associated with differentiated cells in the duodenum (MUC2 and TFF3) and colon (CA2 and TFF3) (Fig. 5D and Supplemental Fig. S12D). Here, we explored the well-known effect of changing media conditions resulting in the shift from crypt-like stem cells to villus-like differentiated cell types.
Figure 5.
Differentiation media conditions drive changes in proliferation and differentiation markers in three-dimensional (3-D) duodenal enteroids. A: principal component analysis (PCA) of the RNA-sequencing datasets for 3-D duodenal enteroids: differentiated (n = 12) and undifferentiated (n = 12). B: a dendrogram with agglomerative hierarchal clustering of the 3-D duodenal gene set from RNA-sequencing. Branch length indicates degree of difference between samples. C: volcano plot of differentially expressed genes when comparing differentiated (red) and undifferentiated (cyan) 3-D duodenal enteroids. Red/cyan dots indicate differentially expressed genes (false discovery rate, FDR ≤ 0.01 and a fold change ≥2 or ≤0.5) that are upregulated or downregulated (respectively) in differentiated (1,474 genes) vs. undifferentiated (1,428 genes) 3-D duodenal enteroids. D: normalized fragments per kilobase of transcript per million mapped reads (FPKMs) of undifferentiated (cyan) and differentiated (red) 3-D duodenal enteroids for stem cell (ASCL2, KI67, and LGR5) and differentiation (MUC2 and TFF3) markers. *False discovery rate ≤ 1% (0.01) according to DESeq2.
Patient-to-Patient Variability is a Driver of Variation between Samples
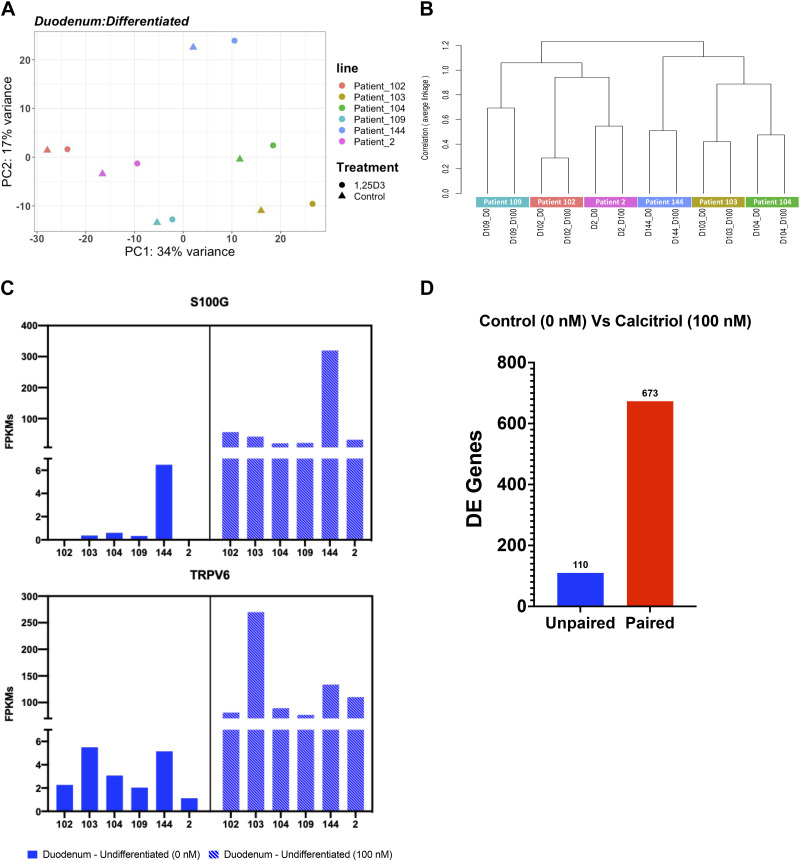
Another variable of interest is the difference between patients. We previously described patient-to-patient variability in jejunal enteroid lines suggesting significant baseline variability between enteroids derived from different individuals, but with little variation between technical replicates (36, 47). Here, we examined patient-to-patient variation on a larger scale with a study encompassing 28 patient lines from the duodenum, jejunum, ileum, and colon (Supplemental Fig. S1A). Analysis of differentiated 3-D duodenal enteroids shows that samples from the same patient pair together (control and treatment) on a PCA plot, and the experimental treatment (in this case, exposure to calcitriol for 24 h) results in small shifts on the x-axis on the PCA plot compared with the controls (Fig. 6A). In addition, the dendrogram shows clustering of patient lines regardless of the experimental manipulation (Fig. 6B). A parallel analysis of undifferentiated 3-D duodenal enteroids and colonoids shows that patient samples clustered together despite experimental manipulations (Fig. 5B and Supplemental Fig. S12B). Clustering by patient is also observed in samples differentiated on transwells, where samples from the same patient line cluster together as well despite undergoing several distinct experimental manipulations such as bacteria, rotavirus, calcitriol, or vehicle exposures (Fig. 4, A and B).
Figure 6.
Patient-to-patient variability results in variable basal gene expression and response to stimuli. A: principal component analysis (PCA) of the RNA-sequencing datasets for three-dimensional (3-D) differentiated duodenal enteroids (n = 12) with six patient samples indicated by six different colors. B: a dendrogram with agglomerative hierarchal clustering of the 3-D differentiated duodenal gene set from RNA-sequencing. Branch length indicates degree of difference between samples. C: normalized fragments per kilobase of transcript per million mapped reads (FPKMs) of S100G (top) and TRPV6 (bottom) expression in individual differentiated 3-D duodenal enteroids lines comparing the gene expression level between the control group (0 nM, solid) and the calcitriol treatment group (100 nM, dashed). Gene expression was upregulated upon the calcitriol treatment with a false discovery rate ≤ 1% (0.01). D: the number of differentially expressed (DE) genes that were output from DESeq2 as a result of using an unpaired (blue) or paired (red) experimental design.
Upon further investigation of differentiated 3-D duodenal enteroids, we observed that basal gene expression as well as transcriptional response to calcitriol treatment was highly variable among six distinct patient lines (D102, D103, D104, D109, D144, and D2). Basal gene expression of S100G, which encodes the calcium binding protein calbindin-D9k, was highly variable with FPKM values of 0 (D102), 0.37 (D103), 0.59 (D104), 0.33 (D109), 6.47 (D144), and 0 (D2). Upon calcitriol treatment, S100G expression was increased proportionally with FPKM values of 56.44 (D102), 41.52 (D103), 20.01 (D104), 21.47 (D109), 319.58 (D144), and 32.02 (D2) (Fig. 6C). Likewise, TRPV6, encoding the transporter which mediates uptake of the Ca2+ from the intestinal lumen to the cytosol, also showed variable expression that increased proportionally upon calcitriol treatment: FPKM values in differentiated enteroids were 2.27 (D102), 5.49 (D103), 3.06 (D104), 2.03 (D109), 5.14 (D144), and 1.12 (D2); and increased upon calcitriol treatment to 81.15 (D102), 269.82 (D103), 89.22 (D104), 76.78 (D109), 133.54 (D144), and 110.14 (D2). This same pattern of patient-specific response to treatment was also observed in undifferentiated duodenal enteroids as well as in differentiated and undifferentiated colonoids (Supplemental Fig. S13, A–C). In-depth analysis of the influence of calcitriol on human enteroids and colonoids is in preparation and has been previewed previously; however, these results briefly underscore one of the points of patient-to-patient variability that is observed in different enteroid/colonoid lines (33).
These data demonstrate what was observed with PERMANOVA that although patient-to-patient variation in enteroids and colonoids is low compared with the influence of other variables—such as substrate, format, segment, and differentiation status—it is nevertheless significant and often greater than the experimental manipulation such as infection or hormone treatment. Because the experimental treatment of interest frequently does not drive transcriptional differences as strongly as patient-to-patient variation, we sought to compare analytical results using DESeq2 to observe the differences between the comparison of group means (unpaired) versus a paired statistical analysis. Thus, when examining differentially expressed genes in response to calcitriol treatment in differentiated duodenal enteroids, the number of DEGs was markedly higher using a paired analysis (673 genes) compared with an unpaired analysis (110 genes) (Fig. 6D). Similarly, the number of genes differentially expressed in differentiated colonoids treated with calcitriol was markedly higher using a paired analysis (447 genes) versus an unpaired analysis (64 genes). This observation was consistent in undifferentiated duodenal enteroids (unpaired: 53 genes and paired: 210) and colonoids (unpaired: 16 genes and paired: 106) (Supplemental Fig. S13D). Likewise, the number of differentially expressed genes in response to differentiation is also increased using a paired analysis, although the magnitude of the affect is proportionally smaller because of the large effect size of differentiation compared with patient-to-patient variation (Supplemental Fig. S12E).
Simultaneously Grown Jejunal Enteroids Validate Growth Format as a Significant Driver of Transcriptional Variance in Human Enteroids
To validate the observations previously presented, five differentiated jejunal enteroid patient lines were grown as 3-D, transwell, or monolayer jejunal enteroids on/in Matrigel (3-D, transwells, monolayers) and collagen (monolayers and transwells), resulting in a total of 25 samples in addition to the original 251 samples (276 samples total) (Supplemental Table S1). A Pearson correlation matrix and PCA plot of the data demonstrated a clustering of samples by format with 3-D and transwell enteroids forming a cluster separate from monolayer enteroids (Fig. 7A and Supplemental Fig. S14A). Of note, DESeq2 analysis identified several gene sets that were differentially expressed between various formats and substrates in jejunal enteroids. 4,300 genes were differentially expressed between jejunal enteroids grown with Matrigel in the monolayer (2,736 genes upregulated) or 3-D (1,564 genes upregulated) formats. 2,828 genes were differentially expressed between jejunal enteroids grown on collagen in the monolayer (1,629 genes upregulated) or transwell (1,199 genes upregulated) formats. And 16 genes were differentially expressed between jejunal enteroids grown as monolayers on Matrigel (7 genes upregulated) or Collagen (9 genes upregulated) (Supplemental Fig. S14D and Supplemental Table S3). The differentially expressed genes from the validation experiments shared 1,725 (22%), 1,445 (28%), and 6 (0.1%) genes with the previously procured jejunal datasets (Supplemental Fig. S14D).
Figure 7.
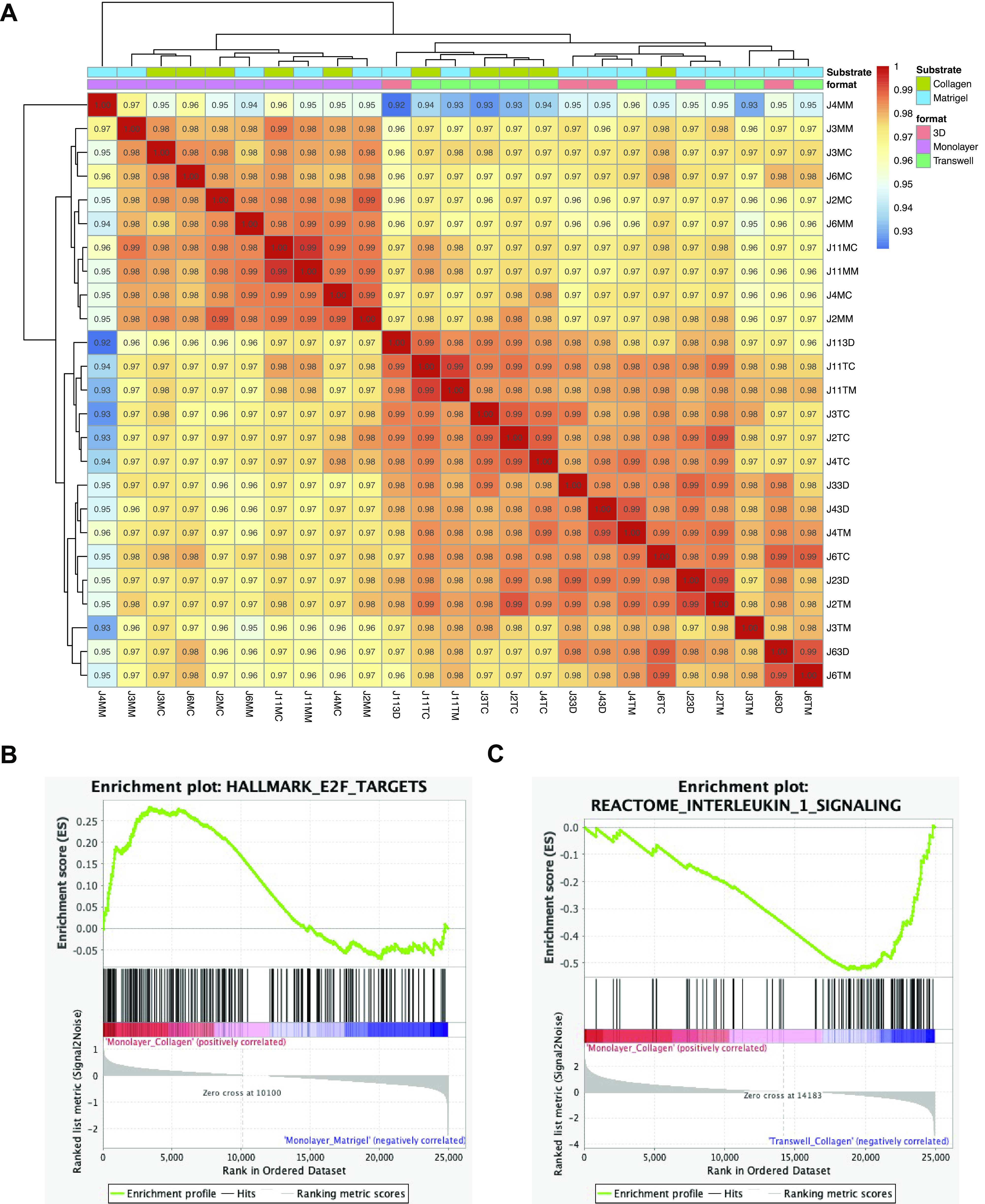
Format validated as a strong driver of variance in a synchronized experiment with control jejunal enteroids. A: validation analysis of three-dimensional (3-D), monolayer (on collagen and Matrigel) and transwell (on collagen and Matrigel) differentiated jejunal enteroids grown simultaneously with five jejunal lines (J2, J3, J4, J6, and J11) for each substrate-format combination (n = 25). B: gene set enrichment analysis (GSEA) showing an enrichment of the hallmark E2F targets gene set signature in jejunal (validation experiment) transwells grown on collagen (normalized enrichment score, NES = 1.36 and false discovery rate, FDR = 0.08) when compared with jejunal (validation experiment) transwells grown on Matrigel. C: GSEA showing an enrichment of the reactome interleukin-1 signaling gene set signature in jejunal (validation experiment) transwells grown on collagen (NES = −2.03 and FDR = 0.003) when compared with jejunal (validation experiment) monolayers grown on collagen. Correlation coefficients of the matrix were generated using the entire transcriptome.
GSEA revealed an enrichment in the E2F targets hallmark gene set in the jejunal monolayers (NES = 1.36 and FDR = 0.08) and transwells (NES = 1.75 and FDR = 0.004) grown on collagen compared with Matrigel (Fig. 7B and Supplemental Fig. S14B). GSEA also revealed an enrichment in the Reactome interleukin 1 signaling gene set in the jejunal monolayers on collagen (NES = −2.03 and FDR = 0.003) and monolayers on Matrigel (NES = −1.94 and FDR = 0.018) compared with transwells (and Supplemental Figs. 7C and S14C). These results similarly reflected enrichments in the E2F targets and Reactome interleukin 1 signaling gene sets in corresponding comparisons as observed previously (Figs. 2D and 3B). In addition, although there was a similar enrichment of E2F target genes in enteroids grown on collagen in comparison with those grown on Matrigel, the previous observation of samples clustering by substrate was not observed (Figs. 2A and 7A). Moreover, the enrichment of the GSEA MYC targets (V2) in 3-D enteroids compared with monolayers was validated in our jejunal validation experiment (Supplemental Fig. S9B). In addition, the jejunal validation experiment that confirmed the enrichment of GSEA hypoxia gene sets was also enriched in Transwells (on Matrigel and collagen) in comparisons to monolayers (Supplemental Fig. S8C).
DISCUSSION
Human enteroids and colonoids have been used increasingly over the past decade as in vitro organoid models to recapitulate human intestinal physiology and response to stimuli. However, a comprehensive examination of experimental parameters that drive transcriptomic changes in these organoids had not yet been performed. Here, we compiled 276 RNA-seq samples from several studies across a consortium of laboratories to explore the effects of common variables on the transcriptome of this intestinal organoid platform. Initially with PERMANOVA we showed that experimental variables could be separated into three tiers based on their magnitude of effect: 1) growth format and substrate, 2) project/experiment, segment, and differentiation status, and 3) patient line and experimental treatment. Upon subsequent investigation with an experiment utilizing five jejunal lines on various format and substrate combinations, we validated format as a strong driver of transcriptional variation. The variation due to growth substrate was not as strong as initially anticipated. Initially, the number of differentially expressed genes between monolayers on collagen and monolayers on Matrigel in duodenal and jejunal enteroids were 6,004 and 5,148, respectively. However, in the jejunal validation dataset the number of differentially expressed genes between Matrigel and collagen in monolayers and transwells were much lower at 16 and 30 genes, respectively, indicating a potential unidentified variable that contributed to the initial variation that was attributed to the substrate. We also observed that the project/experiment was a significant driver of variation in our dataset. This observation highlights a source of transcriptional variation which could be a result of multiple factors such as batch-to-batch variations in conditioned media, differences in incubator conditions, passage rate, intervals of media changes, or differences in sequencing platforms. This was made evident in Supplemental Fig. S14 comparing projects 5 and 8 to project 11 (validation experiment) that were grown in the same formats and on the same substrates but were separated on the x-axis of the PCA plot. Here, we also explored specific examples of biological pathways that were impacted by differences in the growth format, substrate, and segment of origin using GSEA. We were able to observe the well-known effect of manipulating differentiation status by growth factor withdrawal. In addition, the importance of accounting for patient-to-patient variability was addressed when using lines derived from several patients. Moreover, lines from the same patient were found to be transcriptionally like each other even after experimental treatments, which were shown to have the smallest impact on the transcriptome.
From this study, a strong case is made that the format the enteroids and colonoids are grown in should be taken into consideration when designing experiments. Here, we observed that with the same segment and substrate, the format that enteroids are grown on is a large driver of variance particularly between monolayers and 3-D and monolayers and transwells (Table 2, Figs. 2 and 7, Supplemental Figs. S1D, S9B, S8C, and S14). We also observed variations in the transcriptome of organoids grown in transwells compared with monolayers. Although both formats are 2-D, significant differences between transwells and monolayers were observed that may be due to transwells allowing exposure of media to both the apical and basolateral surfaces, while in the monolayer cultures the exposure to medium is limited to the apical surface of the cells. For instance, we observed an enrichment in hypoxia signaling in duodenal and jejunal enteroids grown on transwells compared with monolayers, with GSEA. Oxygen levels have been reported to have an effect on the differentiation of pluripotent stem cell-derived pancreatic cells with a hypoxic state resulting in a lower amount of differentiated beta cells (57). Changes in metabolism have also been linked with changes in the availability of oxygen. Kondo et al. (58) reported increased oxidative phosphorylation with an increase in oxygen levels (decreased thickness in the culture medium layer). It was reported that under standard cell culture conditions Caco-2 monolayers had an oxygen concentration at ∼18% and transwell inserts were reported to have oxygen levels at ∼16% (59, 60). Another study examined the differences between transwells, and air-liquid interface (ALI) cultures based on the diffusion of oxygen into the growth media. They found that ALI cultures, which had a smaller distance between the cell layer and the top of the medium layer, had lower levels of hypoxia-inducible factor (HIF)1α because of the increased level of oxygen to the cultures. Furthermore, they reported that ALI cultures had lower HIF1α target genes expressed, and a metabolic shift from producing lactate and performing glycolysis to oxidative phosphorylation (61). The availability of oxygen has been shown to cause changes in cells’ differentiation capacity as well as their capacity to shift metabolic pathways. Another reported response to hypoxia is the increase in the production of proinflammatory cytokines at baseline and in response to stimuli (62–65). Likewise, we observed an enrichment in interlukin-1 signaling pathways using GSEA of duodenal and jejunal enteroids grown on transwells compared with monolayers. Thus, we believe that the enrichment in the interleukin-1 proinflammatory pathway in transwells may be caused by the hypoxic environment compared with that of monolayers. The enrichment of interlukin-1 signaling pathways on transwells were initially observed in our samples that were aggregated across several studies and further validated in jejunal enteroids (Fig. 7 and Supplemental Fig. S14). In total, our aggregate analyses can serve as a resource to identify pathways or genes of interest that are enriched in particular culture formats (Supplemental Information S3).
Although the exact origin of the variables that makes format a large driver of variance remains unclear, few investigators have compared organoids grown as 3-D, 2-D monolayers and/or transwells (66–71). The differences that we observed between 3-D and 2-D (monolayers and transwells) formats could be potentially due to the different cell shapes in sheet and spherical conformations, which may alter attachment to the Matrigel substrate. In addition, the 3-D format creates an oxygen gradient with lower oxygen tension in the lumen/apical surface of the organoids, and furthermore requires diffusion of growth factors, nutrients, and cellular metabolites through the matrix; thus, 3-D culture creates a niche distinct from 2-D culture even when the media and substrate are identical (72).
In this study, we also explored the effect of the substrate that enteroids and colonoids are grown on. A recent study performed in 3-D enteroids showed that the loss of contact with ECM results in re-oriented, apical-out enteroids, underscoring the importance of the substrate in establishing orientation and cell polarity (18). We also found that jejunal monolayers grown on Matrigel had increased expression of the cholesterol biosynthesis pathway compared with those grown on collagen. We speculate that the effect of the substrate is either due to the transduction of mechanical forces to the cells or the presence of a ligand that induces transcriptional changes. In vivo it is known that the basal surface of the intestinal epithelium binds to and interacts with the underlying basement membrane via integrins that are differentially expressed along the crypt and villus axis (73). This binding of integrins to the ECM components of the basement membrane is known to result in a mechanical stress on the epithelium (74). Mechanical stress on the intestine has been shown to result in transcriptional changes, for instance an in vivo model of cancer exhibited that a prolonged mechanical pressure resulted in the nuclear translocation of β-catenin in the intestine (75). In vitro substrate stiffness has also been shown to affect the proliferation and migration of Caco-2 cells, a colorectal adenocarcinoma cell line, as well in other cell types (38, 76–79). Specifically in the context of human enteroids it was observed that changes in a substrates mechanical properties result in differences in growth and viability, in one case even the ability for pathogens to adhere to enteroid (38, 77, 80, 81). Substrate stiffness has been reported to affect cell migration and proliferation through the integrin-mediated focal adhesion kinase (FAK) pathway, with collagen IV being shown to promote FAK dependent pathways in the Caco-2 cell line (76, 78, 79). Collagen IV substrates were (compared to a poly-l-lysine substrate) also found to influence migration and proliferation of cells through FAK-independent pathways (78, 79).
Matrigel, an extract derived from a mouse sarcoma line, contains several ECM components including fibronectin, laminin, perlecan, and nidogen-1 (82–84). Matrigel is commonly used in 3-D culture of enteroids due to the ease of polymerizing the matrix at room temperature and depolymerization of the matrix by simply lowering the temperature. However, there have also been reports of successfully using collagen I as the matrix to grow 3-D cultures (27, 85, 86). In addition, 2-D cultures have more often been grown on a broader number of substrates such as Matrigel, collagen I, collagen IV, and hydrogels (11, 22–24, 38, 47, 80, 85, 87, 88). The components of Matrigel and collagen IV used in the studies explored for this analysis differ in their composition. Thus, the protein attachments to the matrix are likely to be significantly different, and the differential interactions of media components with the matrix are likely to result in altered signaling to the enteroids/colonoids. Furthermore, mechanical stress is likely to be transduced differently by these distinct matrices to the overlying 2-D organoid cultures. For example, collagens I and IV vary in their ability to transduce mechanical stress to cells due to differences in how integrins of the cells bind to the substrates (74). In addition to chemical and mechanical forces potentially driving the transcriptional differences between 2-D organoids grown on different substrates, there may also be other unknown mechanisms at work, such as the presence of residual growth factors or a yet to be described physical signal. In addition, it would also be interesting to investigate if the differences that we observed between collagen IV and Matrigel in 2-D organoids also exist in 3-D organoids.
Here, we highlighted the enrichment of E2F targets in monolayers on collagen in comparison with monolayers on Matrigel. E2Fs are regulators of cell cycle-dependent gene expression, thus suggesting the role of substrates on influencing the cell cycle, proliferation, and potentially the differentiation capacity of the enteroids on various substrates (89–92). In addition, cholesterol homeostasis was shown to be regulated by substrate as well. Absorption of cholesterol by the intestine is a major determinant of cholesterol homeostasis and the target of an important cholesterol-lowering drug, ezetimibe (93, 94). Furthermore, the cholesterol biosynthesis pathway is essential for homeostasis of the intestinal epithelium (95, 96). Thus, a better understanding of cholesterol homeostasis in the intestinal epithelium is important for understanding both cholesterol absorption and epithelial homeostasis.
Although in our initial analysis we found that substrate was a significant driver of variation in enteroids/colonoids, in our validation experiment similar results were not reproduced. Our results suggest that substrates may have an influence on the expression of genes and consequently several pathways but will require further validation. Although our study did not validate the effect of substrate on enteroids, a subset of the data from Swaminathan et al. (38) (project 9) did exhibit the effect of substrate stiffness on enteroids monolayers and the ability for bacteria to bind to the epithelium in vitro. It is also important to mention that collagen IV is a basement membrane collagen which is more similar to what the epithelium would be exposed to in vivo than collagen I, a highly abundant collagen that is most often found in the bone, skin, or tendons, which has been used in intestinal organoid 2-D and 3-D cultures. Furthermore, we have provided a resource that can help identify pathways or genes of interest that are enriched in cells grown on these common substrates—we recommend using our current dataset and when designing 2-D organoids experiments [Supplemental Information S1 (see https://doi.org/10.6084/m9.figshare.14575650), Supplemental Information S2 (see https://doi.org/10.6084/m9.figshare.14575653), and Supplemental Information S3].
To understand the impact of tissue origin on enteroids in specific culture format, we performed unsupervised clustering analyses of the gene expression data. PCA and dendrograms reflected the physical proximity of the segments, where colonoids and ileal enteroids clustered together and were separated from duodenal and jejunal enteroids, which also clustered together (Supplemental Figs. S1, S9, A and B, and S10). An exception to this was observed in jejunal enteroids transduced with NGN3-expressing lentivirus, which formed a separate cluster even when uninduced, either due to the effect of transduction, leaky expression of NGN3, or the Matrigel substrate that the enteroids were grown on (Fig. 4, A and B). Without taking the Matrigel substrate into account, this highlights the importance of comparing transduced samples to a control that should also be a transduced enteroid line to account for these confounding factors such as leaky expression and the influence of transduction on the transcriptome. Ileal enteroids were acquired from the terminal ileum, and the majority of colonic enteroids were derived from ascending colon, therefore it is possible that the physical proximity of the donor tissues may explain the similarity in gene expression as shown by their clustering on PCAs and dendrograms (97–99). A transcriptomic analysis of mouse epithelium and enteroids found a similar segment-specific gene expression pattern but did not include colonoids in their analysis (14). Additional analyses of human duodenal and ileal enteroids showed that segment-specific markers such as ASBT (SLC10A2) and OSTα/β (SLC51A/B) were differentially expressed only in differentiated enteroids (14). We also observed the differential expression of these genes when comparing our duodenal and ileal enteroids [Supplemental Information S2 (see https://doi.org/10.6084/m9.figshare.14575653)].
To further understand these gene expression patterns, we used GSEA analysis to identify pathways enriched in ileal enteroids and colonoids compared with duodenal enteroids. The results for intersegmental comparisons within specific culture formats revealed novel enriched terms as well as those that confirm known functional differences between segments (66, 100–102). It is well known that bile acids are released into the duodenum and are reabsorbed by the enterocytes in the ileum and recirculated back to the liver via enterohepatic circulation. The bile acids that escape into the colon are either reabsorbed or interact with the colonic micro-environment and undergo chemical modification by the colonic microbiome (67, 103, 104). ASBT (SLC10A2) is responsible for the transport of bile acids from the lumen into enterocytes. Subsequently, bile acids are exported out of the basolateral side of enterocytes via OSTα (SLC51A) and OSTβ (SLC51B) (67, 103, 104). In addition, Farnesoid X receptor (FXR; NR1H4), a nuclear receptor, is activated by bile acids and promotes the reuptake of bile acids from the intestine to the liver (105). As expected, this analysis showed an enrichment in the bile acid and salt transport gene set in the ileal enteroids and colonoids relative to the duodenal enteroids (45). As expected, the segment that organoids are derived from has been shown to maintain their regional transcriptional patterning in vitro. This is advantageous because it allows one to study regional-specific responses to specific stimuli over other immortalized or cancer cell lines. For instance, if one is studying the effect of a drug on iron absorption using colonoids or a colon cancer cell line would not accurately recapitulate what would occur in the duodenum where iron is absorbed. Therefore, it is important to consider the specific region being studied when using a specific intestinal organoid line. We also further investigated the effect of differentiation status on 3-D duodenal and colonic organoids. PCA plots, dendrograms, and DESeq2 differential gene expression analyses showed the strong effect differentiation media conditions had on 3-D enteroids. We found that in dendrograms and PCA plots, there was a bifurcation of the samples into the differentiated and undifferentiated groups. We also employed differential gene expression analyses with DESeq2 and found as expected the genes associated with proliferation and stem cells were upregulated in undifferentiated enteroids and genes associated with secretory and absorptive cell types were upregulated in the differentiated organoids (Fig. 5 and Supplemental Fig. S12). These results were expected due to the previously mentioned well-studied functional and transcriptional differences between the differentiated and undifferentiated compartments of the intestinal epithelium (1, 14). This also highlights an advantage of human enteroid/colonoid cultures with the ability to separately mimic the crypt or villus compartment of the human intestine. Prior studies using primary cultures, cancer cell lines such as Caco-2 or conditionally immortalized cells were limited in their ability to continuously expand and easily differentiate multiple intestinal epithelial cells as shown in our study (106–109). Thus, we find that enteroids/colonoids are a facile system for comparing stem/progenitor versus differentiated cells and can be easily incorporated into the experimental design. For example, it may be preferable to apply luminal stimuli (e.g., food products or infectious agents) to differentiated monolayers or transwell cultures that more closely reflect the apical cell surfaces that first contact these substances. Conversely, to mimic exposure to compounds that will circulate systemically (e.g., chemotherapeutic drugs) and expose the compound to the basal lateral side of the epithelium through the capillaries, it may be important to apply the stimuli to both the differentiated and undifferentiated cultures in either 3-D or transwell format. Although our analysis of differentiated versus undifferentiated organoids was focused primarily on 3-D cultures, we anticipate that other formats will show similar patterns of gene expression. Therefore, we conclude that the compartment (crypt or villus/upper-differentiated) can be readily incorporated into the experimental design.
An advantage of using enteroids and colonoids over immortalized or cancer cell lines is that organoids more accurately recapitulate normal human intestinal epithelium at homeostasis. Human enteroids and colonoids are also advantageous over inbred mouse strains that lack genetic diversity because with the use of multiple patient-derived lines one can more accurately understand the broad response of the human population to a specific stimulus. Therefore, we sought to understand the effect of using enteroid/colonoid lines from various patients on a study. Initially through observing PCA plots and dendrograms, we noted that patient samples were paired together despite the experimental conditions that the enteroids/colonoids were exposed to (Fig. 6 and Supplemental Fig. S13). This phenomenon of patient clustering regardless of experimental manipulation was observed across various segments and formats, indicating that a greater amount of transcriptomic variability exists between different patients than that of experimental conditions such as calcitriol, bacteria, and rotavirus infections. It can be observed in the dendrogram for transwells that the batch-to-batch variation in transduced jejunal samples (J2: ULDM1 versusULDM2) is larger than the variation between technical replicates (J2: ULDM1_1/2/3). And these technical replicates were more similar to each other than two different jejunal patient lines from the same segment (Fig. 4, A and B). To account for this patient-to-patient variability, a paired statistical analysis can be performed; using this approach, we identified six- to sevenfold more differentially expressed genes in 3-D duodenum and colon organoids than using a standard test of means (Fig. 6 and Supplemental Fig. S13). This observation along with the observed patient-to-patient variability that exists in the clinical setting reinforces the need for multiple biological replicates rather than using technical replicates from the same line multiple times. As explored in this manuscript, sources of the variation between patients are the variations in their basal gene expression as well as differences in their response to stimuli. Therefore, we recommend using multiple patient lines in human organoid experiments.
A primary goal of enteroids is to recapitulate the human intestine in vitro. Here, we also set out to identify which format most closely recapitulates the human intestine in vivo. In an attempt to answer this question, we compared data from fresh duodenal and colonic biopsies to our enteroid/colonoid datasets. The Pearson correlation matrix revealed that while overall correlations between enteroids and biopsy data were strong (Pearson correlation > 0.77), the biopsy samples clustered separately from the enteroid/colonoid samples. We speculate that the transcriptomic differences are primarily driven by the multiple cell types (stromal, endothelial, etc.) present in biopsies that are absent in the enteroids/colonoids. Despite these differences, there existed a high level of similarity between enteroids/colonoids and their segment matching biopsies, seen via correlation coefficients being greater than 0.77. In addition, we created a pre-ranked list of 6,945 differentially expressed genes from duodenal and colonic biopsies as a unique expression gene set, and then performed a GSEA with enteroid/colonoid datasets and found that 3-D enteroids had the highest enrichment scores (with FDRs of 0.0) and therefore were the most like duodenal and colonic biopsies. Monolayers had the next highest level of similarity to the biopsies (with FDRs of 0.0) and transwells were the least similar transcriptionally to their biopsy counterparts with duodenal transwells DEGs showing enrichment with an FDR of 0.0, but colonoid transwells DEGs were not enriched in the colonic biopsy gene set. Therefore, all formats give enteroids the ability to recapitulate functional regional markers, however, 3-D enteroids seem to have the highest level of similarity to biopsies and may serve as the closest surrogate to the human intestine in vitro.
Although this study is presented with limitations such as variability in passage numbers, slight variations in the handling and culturing of organoids, and unequal datasets, it serves as an excellent tool for understanding the effect of certain variables on the transcriptome of human enteroids/colonoids. We conclude that the enteroid/colonoid system is a powerful surrogate that can serve as an excellent model of the human intestinal epithelium, with the capacity to mimic various segments and compartments. We have found that the culture conditions must be taken into consideration when designing experiments, and caution must be used when comparing experiments performed under different experimental conditions and by different experimenters. Overall, we highlight the importance of understanding the nuance of in vitro model systems and the effect that common variables may have on experimental outcomes with the largest compilation and analysis of human enteroids and colonoids. Finally, our dataset can be used as the basis for rational design of experiments using enteroids/colonoids.
SUPPLEMENTAL DATA
Supplemental Tables S1–S3 and Supplemental Figs. S1–S14: https://doi.org/10.6084/m9.figshare.16877230.v1.
Supplemental Information S1: https://doi.org/10.6084/m9.figshare.14575650.
Supplemental Information S2: https://doi.org/10.6084/m9.figshare.14575653.
Supplemental Information S3: https://doi.org/10.6084/m9.figshare.14575647.
GRANTS
Research reported in this publication was supported by National Institute of Diabetes and Digestive and Kidney Disorders (NIDDK) and National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under Grant Nos. U19AI144297, P30DK056338, R01DK118904, U01DK103117, R01DK112365, and U19AI116497. Z. Criss was funded by a Howard Hughes Medical Institute Gilliam Fellowship. We also acknowledge Supplemental funding provided by Baylor Research Advocates for Student Scientists (BRASS) during the completion of this project.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.K.C., N.B., S.C.D.R., A.R., K.D.-F., G.S., N.K., D.C., J.F.P., M.A.H., R.A.D., R.A.B., A.W.M., K.J.G.-A., S.E.B., S.E.C., M.K.E., S.R., and N.F.S. conceived and designed research; Z.K.C., N.B., A.R., K.D.-F., G.S., N.K., X-L.Z., H.D., V.K.M., D.C., C.E., X.Y., K.P., A.W.M., K.J.G.-A., S.E.B., and S.E.C. performed experiments; Z.K.C., N.B., A.W.M., and N.F.S. analyzed data; Z.K.C., N.B., S.C.D.R., J.C.F., S.A., R.A.B., and N.F.S. interpreted results of experiments; Z.K.C. prepared figures; Z.K.C. drafted manuscript; Z.K.C., N.B., S.C.D.R., A.R., K.D.-F., G.S., N.K., H.D., J.C.F., M.P.V., S.C., M.A.H., S.A., R.A.B., A.W.M., K.J.G.-A., S.E.B., S.E.C., M.K.E., S.R., and N.F.S. edited and revised manuscript; Z.K.C., N.B., H.D., V.K.M., J.F.P., and N.F.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. William Lagor and Alexandria Doerfler for insights on the cholesterol biosynthesis pathway.
Preprint is available at https://doi.org/10.1101/2021.06.02.446644.
REFERENCES
- 1.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 15: 19–33, 2014. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 2.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev 22: 1856–1864, 2008. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology 134: 849–864, 2008. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 4.Van Der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71: 241–260, 2009. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 5.Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 75: 289–311, 2013. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- 6.Manley NR, Capecchi MR. The role of Hoxa-3 in mouse thymus and thyroid development. Development 121: 1989–2003, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Pras-Raves M, Sacchetti A, Hornsveld M, Oost KC, Snippert HJ, Verhoeven-Duif N, Fodde R, Burgering BMT. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 543: 424–427, 2017. doi: 10.1038/nature21673. [DOI] [PubMed] [Google Scholar]
- 8.Vermeulen L, Snippert HJ. Stem cell dynamics in homeostasis and cancer of the intestine. Nat Rev Cancer 14: 468–480, 2014. doi: 10.1038/nrc3744. [DOI] [PubMed] [Google Scholar]
- 9.Blander JM. Death in the intestinal epithelium—basic biology and implications for inflammatory bowel disease. FEBS J 283: 2720–2730, 2016. doi: 10.1111/febs.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams JM, Duckworth CA, Burkitt MD, Watson AJM, Campbell BJ, Pritchard DM. Epithelial cell shedding and barrier function: a matter of life and death at the small intestinal villus tip. Vet Pathol 52: 445–455, 2015. doi: 10.1177/0300985814559404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato T, Stange DE, Ferrante M, Vries RGJ, van Es JH, van den Brink S, van Houdt WJ, Pronk A, van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141: 1762–1772, 2011. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 13.Leushacke M, Barker N. Ex vivo culture of the intestinal epithelium: strategies and applications. Gut 63: 1345–1354, 2014. doi: 10.1136/gutjnl-2014-307204. [DOI] [PubMed] [Google Scholar]
- 14.Middendorp S, Schneeberger K, Wiegerinck CL, Mokry M, Akkerman RDL, van Wijngaarden S, Clevers H, Nieuwenhuis EES. Adult stem cells in the small intestine are intrinsically programmed with their location-specific function. Stem Cells (Dayton, Ohio) 32: 1083–1091, 2014. doi: 10.1002/stem.1655. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340: 1190–1194, 2013. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 16.Cramer JM, Thompson T, Geskin A, Laframboise W, Lagasse E. Distinct human stem cell populations in small and large intestine. PLoS One 10: e0118792, 2015. doi: 10.1371/journal.pone.0118792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phipson B, Er PX, Combes AN, Forbes TA, Howden SE, Zappia L, Yen H-J, Lawlor KT, Hale LJ, Sun J, Wolvetang E, Takasato M, Oshlack A, Little MH. Evaluation of variability in human kidney organoids. Nat Methods 16: 79–87, 2019. doi: 10.1038/s41592-018-0253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Co JY, Margalef-Català M, Li X, Mah AT, Kuo CJ, Monack DM, Amieva MR. Controlling epithelial polarity: a human enteroid model for host-pathogen interactions. Cell Rep 26: 2509–2520.e4, 2019. doi: 10.1016/j.celrep.2019.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng X-L, Qu L, Kou B, Opekun AR, Burrin D, Graham DY, Ramani S, Atmar RL, Estes MK. Replication of human noroviruses in stem cell-derived human enteroids. Science 353: 1387–1393, 2016. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozuka K, He Y, Koo-McCoy S, Kumaraswamy P, Nie B, Shaw K, Chan P, Leadbetter M, He L, Lewis JG, Zhong Z, Charmot D, Balaa M, King AJ, Caldwell JS, Siegel M. Development and characterization of a human and mouse intestinal epithelial cell monolayer platform. Stem Cell Rep 9: 1976–1990, 2017. doi: 10.1016/j.stemcr.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Qi Z, Li X, Du Y, Chen Y-G. Monolayer culture of intestinal epithelium sustains Lgr5+ intestinal stem cells. Cell Discov 4: 32, 2018. doi: 10.1038/s41421-018-0036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puzan M, Hosic S, Ghio C, Koppes A. Enteric nervous system regulation of intestinal stem cell differentiation and epithelial monolayer function. Sci Rep 8: 6313, 2018. doi: 10.1038/s41598-018-24768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajan A, Robertson MJ, Carter HE, Poole NM, Clark JR, Green SI, Criss ZK, Zhao B, Karandikar U, Xing Y, Margalef-Català M, Jain N, Wilson RL, Bai F, Hyser JM, Petrosino J, Shroyer NF, Blutt SE, Coarfa C, Song X, Prasad BV, Amieva MR, Grande-Allen J, Estes MK, Okhuysen PC, Maresso AW. Enteroaggregative E. coli adherence to human heparan sulfate proteoglycans drives segment and host specific responses to infection. PLoS Pathog 16: e1008851, 2020. doi: 10.1371/journal.ppat.1008851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott A, Rouch JD, Jabaji Z, Khalil HA, Solorzano S, Lewis M, Martín MG, Stelzner MG, Dunn JCY. Long-term renewable human intestinal epithelial stem cells as monolayers: a potential for clinical use. J Pediatr Surg 51: 995–1000, 2016. doi: 10.1016/j.jpedsurg.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorne CA, Chen IW, Sanman LE, Cobb MH, Wu LF, Altschuler SJ. Enteroid monolayers reveal an autonomous WNT and BMP circuit controlling intestinal epithelial growth and organization. Dev Cell 44: 624–633.e4, 2018. doi: 10.1016/j.devcel.2018.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong Z, Martyn K, Yang A, Yin X, Mead BE, Joshi N, Sherman NE, Langer RS, Karp JM. Towards a defined ECM and small molecule based monolayer culture system for the expansion of mouse and human intestinal stem cells. Biomaterials 154: 60–73, 2018. doi: 10.1016/j.biomaterials.2017.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jabaji Z, Brinkley GJ, Khalil HA, Sears CM, Lei NY, Lewis M, Stelzner M, Martín MG, Dunn JCY. Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS One 9: e107814, 2014. doi: 10.1371/journal.pone.0107814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim GA, Spence JR, Takayama S. Bioengineering for intestinal organoid cultures. Curr Opin Biotechnol 47: 51–58, 2017. doi: 10.1016/j.copbio.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, Janssens HM, Bronsveld I, van de Graaf EA, Nieuwenhuis EES, Houwen RHJ, Vleggaar FP, Escher JC, de Rijke YB, Majoor CJ, Heijerman HGM, de Winter–de Groot KM, Clevers H, van der Ent CK, Beekman JM. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med 8: 344ra84, 2016. doi: 10.1126/scitranslmed.aad8278. [DOI] [PubMed] [Google Scholar]
- 30.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NWM, Bijvelds MJC, Scholte BJ, Nieuwenhuis EES, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19: 939–945, 2013. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 31.Wilding JL, Bodmer WF. Cancer cell lines for drug discovery and development. Cancer Res 74: 2377–2384, 2014. doi: 10.1158/0008-5472.CAN-13-2971. [DOI] [PubMed] [Google Scholar]
- 32.Saxena K, Blutt SE, Ettayebi K, Zeng X-L, Broughman JR, Crawford SE, Karandikar UC, Sastri NP, Conner ME, Opekun AR, Graham DY, Qureshi W, Sherman V, Foulke-Abel J, In J, Kovbasnjuk O, Zachos NC, Donowitz M, Estes MK. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J Virol 90: 43–56, 2016. doi: 10.1128/jvi.01930-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, De La Cruz J, Hutchens S, Mukhopadhyay S, Criss ZK, Aita R, Pellon-Cardenas O, Hur J, Soteropoulos P, Husain S, Dhawan P, Verlinden L, Carmeliet G, Fleet JC, Shroyer NF, Verzi MP, Christakos S. Analysis of 1,25-dihydroxyvitamin D3 genomic action reveals calcium-regulating and calcium-independent effects in mouse intestine and human enteroids. Mol Cell Biol 41: e00372-20, 2020. doi: 10.1128/mcb.00372-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole NM, Rajan A, Maresso AW. Human intestinal enteroids for the study of bacterial adherence, invasion, and translocation. Curr Protoc Microbiol 50: e55, 2018. doi: 10.1002/cpmc.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou WY, Blutt SE, Crawford SE, Ettayebi K, Zeng X-L, Saxena K, Ramani S, Karandikar UC, Zachos NC, Estes MK. Human intestinal enteroids: new models to study gastrointestinal virus infections. Methods Mol Biol (Clifton, NJ) 1576: 229–247, 2019. doi: 10.1007/7651_2017_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxena K, Simon LM, Zeng X-L, Blutt SE, Crawford SE, Sastri NP, Karandikar UC, Ajami NJ, Zachos NC, Kovbasnjuk O, Donowitz M, Conner ME, Shaw CA, Estes MK. A paradox of transcriptional and functional innate interferon responses of human intestinal enteroids to enteric virus infection. Proc Natl Acad Sci USA 114: E570–E579, 2017. doi: 10.1073/pnas.1615422114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ettayebi K, Tenge VR, Cortes-Penfield NW, Crawford SE, Neill FH, Zeng X-L, Yu X, Ayyar BV, Burrin D, Ramani S, Atmar RL, Estes MK. New insights and enhanced human norovirus cultivation in human intestinal enteroids. MSphere 6: e01136-20, 2021. doi: 10.1128/msphere.01136-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swaminathan G, Kamyabi N, Carter HE, Rajan A, Karandikar U, Criss ZK, Shroyer NF, Robertson MJ, Coarfa C, Huang C, Shannon TE, Tadros M, Estes MK, Maresso AW, Grande-Allen KJ. Effect of substrate stiffness on human intestinal enteroids’ infectivity by enteroaggregative Escherichia coli. Acta Biomater 132: 245–259, 2021. doi: 10.1016/J.ACTBIO.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang-Graham AL, Danhof HA, Engevik MA, Tomaro-Duchesneau C, Karandikar UC, Estes MK, Versalovic J, Britton RA, Hyser JM. Human intestinal enteroids with inducible neurogenin-3 expression as a novel model of gut hormone secretion. Cell Mol Gastroenterol Hepatol 8: 209–229, 2019. doi: 10.1016/j.jcmgh.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engevik MA, Morra CN, Röth D, Engevik K, Spinler JK, Devaraj S, Crawford SE, Estes MK, Kalkum M, Versalovic J. Microbial metabolic capacity for intestinal folate production and modulation of host folate receptors. Front Microbiol 10: 2305, 2019. doi: 10.3389/fmicb.2019.02305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34: 525–527, 2016. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 42.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soneson C, Love MI, Robinson MD. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences [version 2; referees: 2 approved]. F1000Res 4: 1521, 2015. doi: 10.12688/F1000RESEARCH.7563.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mootha VK, Lindgren CM, Eriksson K-F, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 45.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ge SX, Son EW, Yao R. iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinformatics 19: 1–24, 2018. doi: 10.1186/s12859-018-2486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin S-C, Qu L, Ettayebi K, Crawford SE, Blutt SE, Robertson MJ, Zeng X-L, Tenge VR, Ayyar BV, Karandikar UC, Yu X, Coarfa C, Atmar RL, Ramani S, Estes MK. Human norovirus exhibits strain-specific sensitivity to host interferon pathways in human intestinal enteroids. Proc Natl Acad Sci USA 117: 23782–23793, 2020. doi: 10.1073/pnas.2010834117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perera L, Shao L, Patel A, Evans K, Meresse B, Blumberg R, Geraghty D, Groh V, Spies T, Jabri B, Mayer L. Expression of nonclassical class I molecules by intestinal epithelial cells. Inflammatory Bowel Diseases 13: 298–307, 2007. doi: 10.1002/ibd.20026. [DOI] [PubMed] [Google Scholar]
- 49.Shao L, Kamalu O, Mayer L. Non-classical MHC class I molecules on intestinal epithelial cells: mediators of mucosal crosstalk. Immunol Rev 206: 160–176, 2005. doi: 10.1111/j.0105-2896.2005.00295.x. [DOI] [PubMed] [Google Scholar]
- 50.Mutch DM, Anderle P, Fiaux M, Mansourian R, Vidal K, Wahli W, Williamson G, Roberts M-A. Regional variations in ABC transporter expression along the mouse intestinal tract. Physiol Genomics 17: 11–20, 2004. doi: 10.1152/physiolgenomics.00150.2003. [DOI] [PubMed] [Google Scholar]
- 51.Tsai Y-H, Nattiv R, Dedhia PH, Nagy MS, Chin AM, Thomson M, Klein OD, Spence JR. In vitro patterning of pluripotent stem cell-derived intestine recapitulates in vivo human development. Development 144: 1045–1055, 2017. doi: 10.1242/dev.138453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camp JG, Frank CL, Lickwar CR, Guturu H, Rube T, Wenger AM, Chen J, Bejerano G, Crawford GE, Rawls JF. Microbiota modulate transcription in the intestinal epithelium without remodeling the accessible chromatin landscape. Genome Res 24: 1504–1516, 2014. doi: 10.1101/gr.165845.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu K, Mu C, Yang Y, Su Y, Zhu W. Segment-specific responses of intestinal epithelium transcriptome to in-feed antibiotics in pigs. Physiol Genomics 49: 582–591, 2017. doi: 10.1152/physiolgenomics.00020.2017. [DOI] [PubMed] [Google Scholar]
- 54.Zheng J, Wang J, Pouliot M, Authier S, Zhou D, Loose DS, Hauer-Jensen M. Gene expression profiling in non-human primate jejunum, ileum and colon after total-body irradiation: a comparative study of segment-specific molecular and cellular responses. BMC Genomics 16: 984, 2015. doi: 10.1186/s12864-015-2168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bücker R, Krug SM, Moos V, Bojarski C, Schweiger MR, Kerick M, Fromm A, Janßen S, Fromm M, Hering NA, Siegmund B, Schneider T, Barmeyer C, Schulzke JD. Campylobacter jejuni impairs sodium transport and epithelial barrier function via cytokine release in human colon. Mucosal Immunol 11: 474–485, 2018. [Erratum in Mucosal Immunol 11: 575–577, 2017]. doi: 10.1038/mi.2017.66. [DOI] [PubMed] [Google Scholar]
- 56.Leonard MM, Bai Y, Serena G, Nickerson KP, Camhi S, Sturgeon C, Yan S, Fiorentino MR, Katz A, Nath B, Richter J, Sleeman M, Gurer C, Fasano A. RNA sequencing of intestinal mucosa reveals novel pathways functionally linked to celiac disease pathogenesis. PLoS One 14: e0215132, 2019. doi: 10.1371/journal.pone.0215132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heinis M, Simon MT, Ilc K, Mazure NM, Pouysségur J, Scharfmann R, Duvillié B. Oxygen tension regulates pancreatic β-cell differentiation through hypoxia-inducible factor 1α. Diabetes 59: 662–669, 2010. doi: 10.2337/db09-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kondo M, Tamaoki J, Sakai A, Kameyama S, Kanoh S, Konno K. Increased oxidative metabolism in cow tracheal epithelial cells cultured at air-liquid interface. Am J Respir Cell Mol Biol 16: 62–68, 1997. doi: 10.1165/ajrcmb.16.1.8998080. [DOI] [PubMed] [Google Scholar]
- 59.Fofanova TY, Stewart CJ, Auchtung JM, Wilson RL, Britton RA, Grande-Allen KJ, Estes MK, Petrosino JF. A novel human enteroid-anaerobe co-culture system to study microbial-host interaction under physiological hypoxia. BioRxiv, 2019. doi: 10.1101/555755. [DOI]
- 60.von Köckritz-Blickwede M, Zeitouni N, Fandrey J, Naim HY. Measuring oxygen levels in Caco-2 cultures. Hypoxia (Auckl) 3: 53–66, 2015. doi: 10.2147/hp.s85625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klasvogt S, Zuschratter W, Schmidt A, Kröber A, Vorwerk S, Wolter R, Isermann B, Wimmers K, Rothkötter HJ, Nossol C. Air–liquid interface enhances oxidative phosphorylation in intestinal epithelial cell line IPEC-J2. Cell Death Discov 3: 17001, 2017. doi: 10.1038/cddiscovery.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi S, Chung H, Hong H, Kim SY, Kim SE, Seoh JY, Moon CM, Yang EG, Oh ES. Inflammatory hypoxia induces syndecan-2 expression through IL-1β-mediated FOXO3a activation in colonic epithelia. FASEB J 31: 1516–1530, 2017. doi: 10.1096/fj.201601098R. [DOI] [PubMed] [Google Scholar]
- 63.Fujii M, Kawashima N, Tazawa K, Hashimoto K, Nara K, Noda S, Nagai S, Okiji T. Hypoxia-inducible factor 1α promotes interleukin 1β and tumour necrosis factor α expression in lipopolysaccharide-stimulated human dental pulp cells. Int Endod J 53: 636–646, 2020. doi: 10.1111/iej.13264. [DOI] [PubMed] [Google Scholar]
- 64.Hsu Y-H, Lin R-M, Chiu Y-S, Liu W-L, Huang K-Y. Effects of IL-1β, IL-20, and BMP-2 on intervertebral disc inflammation under hypoxia. J Clin Med 9: 140, 2020. doi: 10.3390/jcm9010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tannahill GM, Curtis AM, Adamik J, Palsson-Mcdermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LA. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496: 238–242, 2013. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Song W, Wang J, Wang T, Xiong X, Qi Z, Fu W, Yang X, Chen Y-G. Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine. J Exp Med 217: e20191130, 2020. doi: 10.1084/jem.20191130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Islam KBMS, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141: 1773–1781, 2011. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 68.Guo Q, Xia B, Moshiach S, Xu C, Jiang Y, Chen Y, Sun Y, Lahti JM, Zhang XA. The microenvironmental determinants for kidney epithelial cyst morphogenesis. Eur J Cell Biol 87: 251–266, 2008. doi: 10.1016/j.ejcb.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kasendra M, Tovaglieri A, Sontheimer-Phelps A, Jalili-Firoozinezhad S, Bein A, Chalkiadaki A, Scholl W, Zhang C, Rickner H, Richmond CA, Li H, Breault DT, Ingber DE. Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci Rep 8: 2871, 2018. doi: 10.1038/s41598-018-21201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio IIC, Hwang C-I, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 214: 579–596, 2017. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Schwartz MP, Hou Z, Bai Y, Ardalani H, Swanson S, Steill J, Ruotti V, Elwell A, Nguyen BK, Bolin J, Stewart R, Thomson JA, Murphy WL. A genome-wide analysis of human pluripotent stem cell-derived endothelial cells in 2D or 3D culture. Stem Cell Reports 8: 907–918, 2017. doi: 10.1016/j.stemcr.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okkelman IA, Neto N, Papkovsky DB, Monaghan MG, Dmitriev RI. A deeper understanding of intestinal organoid metabolism revealed by combining fluorescence lifetime imaging microscopy (FLIM) and extracellular flux analyses. Redox Biol 30: 101420, 2020. doi: 10.1016/j.redox.2019.101420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lussier C, Basora N, Bouatrouss Y, Beaulieu JF. Integrins as mediators of epithelial cell-matrix interactions in the human small intestinal mucosa. Microsc Res Tech 51: 169–178, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 74.Tang VW. Collagen, stiffness, and adhesion: the evolutionary basis of vertebrate mechanobiology. Mol Biol Cell 31: 1823–1834, 2020. doi: 10.1091/mbc.E19-12-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernández-Sánchez ME, Barbier S, Whitehead J, Béalle G, Michel A, Latorre-Ossa H, Rey C, Fouassier L, Claperon A, Brullé L, Girard E, Servant N, Rio-Frio T, Marie H, Lesieur S, Housset C, Gennisson J-L, Tanter M, Ménager C, Fre S, Robine S, Farge E. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature 523: 92–95, 2015. doi: 10.1038/nature14329. [DOI] [PubMed] [Google Scholar]
- 76.Du J, Zu Y, Li J, Du S, Xu Y, Zhang L, Wang Z, Chien S, Yang C. Extracellular matrix stiffness dictates Wnt expression through integrin pathway. Sci Rep 6: 20395, 2016. doi: 10.1038/srep20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, Lutolf MP. Designer matrices for intestinal stem cell and organoid culture. Nature 539: 560–564, 2016. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 78.Sanders MA, Basson MD. Collagen IV-dependent ERK activation in human Caco-2 intestinal epithelial cells requires focal adhesion kinase. J Biol Chem 275: 38040–38047, 2000. doi: 10.1074/jbc.M003871200. [DOI] [PubMed] [Google Scholar]
- 79.Sanders MA, Basson MD. Collagen IV regulates Caco-2 cell spreading and p130Cas phosphorylation by FAK-dependent and FAK-independent pathways. Biol Chem 389: 47–55, 2008. doi: 10.1515/BC.2008.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hernandez-Gordillo V, Kassis T, Lampejo A, Choi G, Gamboa ME, Gnecco JS, Brown A, Breault DT, Carrier R, Griffith LG. Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials 254: 120125, 2020. doi: 10.1016/j.biomaterials.2020.120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ng S, Tan WJ, Pek MMX, Tan MH, Kurisawa M. Mechanically and chemically defined hydrogel matrices for patient-derived colorectal tumor organoid culture. Biomaterials 219: 119400, 2019. doi: 10.1016/j.biomaterials.2019.119400. [DOI] [PubMed] [Google Scholar]
- 82.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics, 10: 1886–1890, 2010. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 83.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 15: 378–386, 2005. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 84.Mccarty WJ, Johnson M. The hydraulic conductivity of MatrigelTM. Biorheology 44: 303–317, 2007. [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y, DiSalvo M, Gunasekara DB, Dutton J, Proctor A, Lebhar MS, Williamson IA, Speer J, Howard RL, Smiddy NM, Bultman SJ, Sims CE, Magness ST, Allbritton NL. Self-renewing monolayer of primary colonic or rectal epithelial cells. Cell Mol Gastroenterol Hepatol 4: 165–182.e7, 2017. doi: 10.1016/j.jcmgh.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18: 618–623, 2012. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 87.Jabaji Z, Sears CM, Brinkley GJ, Lei NY, Joshi VS, Wang J, Lewis M, Stelzner M, Martín MG, Dunn JCY. Use of collagen gel as an alternative extracellular matrix for the in vitro and in vivo growth of murine small intestinal epithelium. Tissue Eng Part C Methods 19: 961–969, 2013. doi: 10.1089/ten.tec.2012.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc 8: 2471–2482, 2013. doi: 10.1038/nprot.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kent LN, Leone G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer 19: 326–338, 2019. doi: 10.1038/s41568-019-0143-7. [DOI] [PubMed] [Google Scholar]
- 90.Lavia P, Jansen-Dürr P. E2F target genes and cell-cycle checkpoint control. BioEssays 21: 221–230, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 91.Ohtani K. Implication of transcription factor E2F in regulation of DNA replication. Front Biosci 4: D793–D804, 1999. doi: 10.2741/ohtani. [DOI] [PubMed] [Google Scholar]
- 92.Suzuki T, Yasui W, Yokozaki H, Naka K, Ishikawa T, Tahara E. Expression of the E2F family in human gastrointestinal carcinomas. Int J Cancer 81: 535–538, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 93.Malhotra P, Gill RK, Saksena S, Alrefai WA. Disturbances in cholesterol homeostasis and non-alcoholic fatty liver diseases. Front Med 7: 467, 2020. doi: 10.3389/fmed.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pradhan A, Bhandari M, Sethi R. Ezetimibe and improving cardiovascular outcomes: current evidence and perspectives. Cardiol Res Pract. 2020: 9815016, 2020. doi: 10.1155/2020/9815016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McFarlane MR, Cantoria MJ, Linden AG, January BA, Liang G, Engelking LJ. Scap is required for sterol synthesis and crypt growth in intestinal mucosa. J Lipid Res 56: 1560–1571, 2015. doi: 10.1194/jlr.M059709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rong S, McDonald JG, Engelking LJ. Cholesterol auxotrophy and intolerance to ezetimibe in mice with SREBP-2 deficiency in the intestine. J Lipid Res 58: 1988–1998, 2017. doi: 10.1194/jlr.M077610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Foley MH, O’Flaherty S, Barrangou R, Theriot CM. Bile salt hydrolases: gatekeepers of bile acid metabolism and host-microbiome crosstalk in the gastrointestinal tract. PLoS Pathog 15: e1007581, 2019. doi: 10.1371/journal.ppat.1007581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tian Y, Gui W, Koo I, Smith PB, Allman EL, Nichols RG, Rimal B, Cai J, Liu Q, Patterson AD. The microbiome modulating activity of bile acids. Gut Microbes 11: 979–996, 2020. doi: 10.1080/19490976.2020.1732268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vuik F, Dicksved J, Lam SY, Fuhler GM, van der Laan L, van de Winkel A, Konstantinov SR, Spaander MW, Peppelenbosch MP, Engstrand L, Kuipers EJ. Composition of the mucosa-associated microbiota along the entire gastrointestinal tract of human individuals. United European Gastroenterol J 7: 897–907, 2019. doi: 10.1177/2050640619852255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LaPointe LC, Dunne R, Brown GS, Worthley DL, Molloy PL, Wattchow D, Young GP. Map of differential transcript expression in the normal human large intestine. Physiol Genomics 33: 50–64, 2008. doi: 10.1152/physiolgenomics.00185.2006. [DOI] [PubMed] [Google Scholar]
- 101.Mach N, Berri M, Esquerré D, Chevaleyre C, Lemonnier G, Billon Y, Lepage P, Oswald IP, Doré J, Rogel-Gaillard C, Estellé J. Extensive expression differences along porcine small intestine evidenced by transcriptome sequencing. PLoS One 9: e88515, 2014. doi: 10.1371/journal.pone.0088515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, Wernerus H, Björling L, Ponten F. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol 28: 1248–1250, 2010. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 103.Molinero N, Ruiz L, Sánchez B, Margolles A, Delgado S. Intestinal bacteria interplay with bile and cholesterol metabolism: implications on host physiology. Front Physiol 10: 185, 2019. doi: 10.3389/fphys.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47: 241–259, 2006. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 105.Matsubara T, Li F, Gonzalez FJ. FXR signaling in the enterohepatic system. Mol Cell Endocrinol 368: 17–29, 2013. doi: 10.1016/j.mce.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beaulieu JF, Ménard D. Isolation, characterization, and culture of normal human intestinal crypt and villus cells. Methods Mol Biol 806: 157–173, 2012. doi: 10.1007/978-1-61779-367-7_11. [DOI] [PubMed] [Google Scholar]
- 107.Perreault N, Beaulieu JF. Primary cultures of fully differentiated and pure human intestinal epithelial cells. Exp Cell Res 245: 34–42, 1998. doi: 10.1006/excr.1998.4221. [DOI] [PubMed] [Google Scholar]
- 108.Tremblay E, Auclair J, Delvin E, Levy E, Ménard D, Pshezhetsky AV, Rivard N, Seidman EG, Sinnett D, Vachon PH, Beaulieu J-F. Gene expression profiles of normal proliferating and differentiating human intestinal epithelial cells: a comparison with the Caco-2 cell model. J Cell Biochem 99: 1175–1186, 2006. doi: 10.1002/jcb.21015. [DOI] [PubMed] [Google Scholar]
- 109.Whitehead RH, Robinson PS. Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice. Am J Physiol Gastrointest Liver Physiol 296: G455–G460 2009. doi: 10.1152/ajpgi.90381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]