Keywords: acid-base, ammonia, androgen receptor, sex differences, testosterone
Abstract
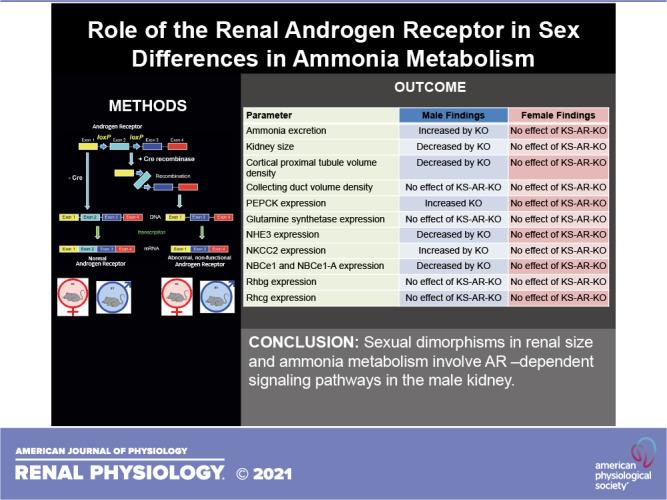
There are sex differences in renal ammonia metabolism and structure, many of which are mediated by testosterone. The goal of the present study was to determine the role of renal expression of testosterone’s canonical receptor, androgen receptor (AR), in these sexual dimorphisms. We studied mice with kidney-specific AR deletion [KS-AR-knockout (KO)] generated using Cre/loxP techniques; control mice were Cre-negative littermates (wild type). In male but not female mice, KS-AR-KO increased ammonia excretion, which eliminated sex differences. Although renal structural size typically parallel ammonia excretion, KS-AR-KO decreased kidney size, cortical proximal tubule volume density, and cortical proximal tubule cell height in males—neither were altered in females and collecting duct volume density was unaltered in both sexes. Analysis of key protein involved in ammonia handling showed in male mice that KS-AR-KO increased both phosphoenolpyruvate carboxykinase (PEPCK) and Na+-K+-2Cl− cotransporter (NKCC2) expression and decreased Na+/H+ exchanger isoform 3 (NHE3) and electrogenic Na+-bicarbonate cotransporter 1 (NBCe1)-A expression. In female mice, KS-AR-KO did not alter these parameters. These effects occurred even though KS-AR-KO did not alter plasma testosterone, food intake, or serum Na+, K+, or significantly in either sex. In conclusion, AR-dependent signaling pathways in male, but not female, kidneys regulate PEPCK and NKCC2 expression and lead to the sexual differences in ammonia excretion. Opposing effects on NHE3 and NBCe1-A expression likely limit the magnitude of ammonia excretion changes. As AR is not present in the thick ascending limb, the effect of KS-AR-KO on NKCC2 expression is indirect. Finally, AR mediates the greater kidney size and proximal tubule volume density in male compared with female mice.
NEW & NOTEWORTHY Sexual dimorphisms in ammonia metabolism involve androgen receptor (AR)-dependent signaling pathways in male, but not female, kidneys that lead to altered proximal tubule (PT), phosphoenolpyruvate carboxykinase, and thick ascending limb Na+-K+-2Cl− cotransporter expression. Adaptive responses in Na+/H+ exchanger 3 and electrogenic Na+-bicarbonate cotransporter 1-A expression limit the magnitude of the effect on ammonia excretion. Finally, the greater kidney size and PT volume density in male mice is the result of PT androgen signaling through AR.
INTRODUCTION
The presence of sex differences in many aspects of mammalian biology has been well established with sexual dimorphisms in structure and/or function have been identified in nearly every organ, including the kidney (1–3). Sex affects many aspects of kidney function and disease. Sex differences in blood pressure are widely recognized and thought to be related to sex-specific variations in nitric oxide, the renin-angiotensin-aldosterone system, and inflammation (4, 5). There are also differences in the expression of many renal epithelial cell Na+ transporters (6, 7). Understanding these differences can identify new disease mechanisms and/or new and improved therapeutic opportunities.
The kidney regulates acid-base homeostasis, which is critical for normal health (8, 9). Renal ammonia1 metabolism has a major role in acid-base homeostasis; ammonia excretion is the predominant component of basal net acid excretion, and changes in ammonia metabolism account for >80% of the response to exogenous stimuli (10–16). Dysregulation of acid-base homeostasis directly effects essentially every major organ system, accelerates the progression of chronic kidney disease, and correlates with increased mortality (17–19).
Our studies have shown that there are important sex effects on both ammonia metabolism and renal structure. The male kidney excretes less ammonia than the female kidney and does so despite the male kidney being larger and having greater cortical volume than the female kidney (20). This difference in ammonia excretion correlates with variations in multiple renal aspects, including several enzymes involved in ammonia generation and recycling, in proximal tubule size, in thick ascending limb of the loop of Henle (TAL) Na+-K+-2Cl− cotransporter (NKCC2) expression, and in collecting duct intercalated cell size and Rhesus (Rh) glycoprotein expression (20). Finally, there are sex differences in the response to acid loading (21). Many of these sex differences are reversed by orchiectomy through a mechanism abrogated by physiological testosterone replacement, suggesting that they involve testosterone-dependent signaling pathways (22).
The canonical pathway through which testosterone mediates biological effects involves binding to the androgen receptor (AR) (23, 24). AR protein and mRNA expression has been observed in both male and female kidneys for decades (25–28). We recently showed that AR protein is expressed specifically in the proximal tubule and that expression was not detectable in either the TAL or collecting duct (22), where there are known sex differences in proteins involved in ammonia handling. Our studies further showed that proximal tubule AR expression was present in both male and female kidneys, with expression being approximately twice as much in the male kidney than in the female kidney (22). These observations suggest that AR may mediate some, but not all, of the effects of sex on renal structure and on ammonia generation and transport. They also raise the question as to the role of AR in the female kidney.
Thus, the purpose of the present study was to determine the role of renal AR in mediating these identified sexual dimorphisms in renal ammonia metabolism and renal structure. Because AR activation has numerous biological effects, which may indirectly alter acid-base homeostasis and thereby ammonia handling, interpreting studies using pharmacological AR inhibition and/or global AR deletion is complicated. Therefore, the present study used mice with kidney-specific AR deletion [KS-AR-knockout (KO)] generated using Cre-loxP techniques. We compared Cre-positive mice with their Cre-negative littermates to determine the effect of KS-AR-KO. Both male and female mice were evaluated to allow understanding of the role of AR in both sexes.
METHODS
Animals
We used mice with loxP sites flanking exon 2 of the AR gene (ARfl/fl), which have been previously described (29–31). We induced whole kidney AR deletion by breeding mice expressing Cre-recombinase under control of the Pax-8 promotor (Pax8Cre; 32). KS-AR-KO mice were ARfl/fl, Pax8-Cre+; control mice were ARfl/fl, Pax8-Cre − . All mice were on the C57/Bl6 background strain. We genotyped all mice using DNA extracted from tail clip samples. Mice used in these experiments were adult male and female mice of ∼4 mo of age. Mice were maintained on standard rodent diet (18% protein, Harlan Teklad, Madison, WI) with free access to water. The University of Florida and the North Florida/South Georgia Veterans Health System Institutional Animal Care and Use Committees approved all animal protocols. Animal breeding was performed in the University of Florida College of Medicine Cancer and Genetics Transgenic Animal Core Facility by trained personnel.
Metabolic Cage Experiments
Mice were placed in metabolic cages for 3 days and fed a powdered normal diet (18% protein, Harlan Teklad) mixed 1:1 with water. Daily food consumption was measured, and daily 24-h urine samples were collected. Urine samples were collected in tubes containing water-equilibrated mineral oil to prevent evaporation and gas molecule loss. Daily urine volume and pH were recorded. Urine samples were stored at −20°C until analyzed further.
Blood Analysis
Blood was obtained by cannulation of the abdominal aorta in mice anesthetized with isoflurane, drawn into a heparinized syringe, and immediately analyzed for Na+, K+, and bicarbonate concentrations using a Siemens Microanalytic Blood Gas Analyzer (RAPIDLab 348 Analyzer, Siemens, Munich, Germany). Serum testosterone was measured in The University of Virginia School of Medicine Center for Research in Reproduction Ligand Assay and Analysis Core (IBL America mouse testosterone ELISA, Minneapolis, MN)
Urinary Analysis
Urine ammonia was measured using a modification of a commercially available kit (A7553, Pointe Scientific, Canton, MI), as previously described (20, 33). Urine pH was measured using a micro-pH electrode (ROSS semi-micro pH, Orion 8103BN, Thermo Fisher Scientific, Waltham, MA). Titratable acid excretion was determined using previously described standard techniques (20, 33).
Antibodies
Table 1 shows the antibodies used in this study, their suppliers, and their catalog/identification numbers.
Table 1.
Antibodies
| Target | Supplier | Location | Number | Dilution |
|---|---|---|---|---|
| AR | Dr. Gail Prins | University of Illinois at Chicago, Chicago, IL | AR21 | I: 1:500 |
| GS | Abcam | Cambridge, MA | Ab73593 | W: 1:1,500I: 1:60,000 |
| NBCe1 | ProteinTech | Rosemont, IL | 885-1-AP | W: 1:2,000I: 1:20,000 |
| NBCe1-A | Dr. Michael Romero | Mayo Clinic, Rochester, MN | Α-333 | W: 1:2,000 |
| NHE3 | EMD Millipore | Temecula, CA | 2736700 | W: 1:500I: 1:5,000 |
| NKCC2 | Dr. H. Moo Kwon | Ulsan National Institute of Science and Technology, Ulsan, South Korea | NA | W: 1:4,000I: 1:20,000 |
| PEPCK | Cayman Chemical | Ann Arbor, MI | 10004943 | W: 1:3,000I: 1:5,000 |
| Rhbg | Weiner laboratory | Gainesville, FL | 359 | W: 1:400I: 1:50,000 |
| Rhcg | Weiner laboratory | Gainesville, FL | 361 | I: 1:30,000 |
AR, androgen receptor; GS, glutamine synthetase; I, immunohistochemistry; NA, not applicable; NBCe1, electrogenic Na+-bicarbonate cotransporter; NHE3, Na+/H+ exchanger isoform 3; NKCC2, Na+-K+-2Cl− cotransporter; PEPCK, phosphoenolpyruvate carboxykinase; Rhbg, Rhesus B glycoprotein; Rhcg, Rhesus C glycoprotein; W, Western blot.
Protein Preparation
Animals were anesthetized with inhalant isoflurane, and kidneys were rinsed by in vivo cardiac perfusion with PBS (pH 7.4) containing 6,000 U/L of Na-heparin and 120 mg/L of lidocaine. The right kidney was removed rapidly, and the cortex, outer medulla, and inner medulla were isolated on a cold stage under a dissecting microscope. Samples were snap frozen in liquid nitrogen and stored frozen at −80°C until use. Tissues were homogenized in T-PER tissue protein extraction reagent (Pierce Biotechnology, Rockford, IL) using microtube pestles (USA Scientific, Ocala, FL), and protein was extracted according to the manufacturer’s recommendations. An aliquot was used for total protein quantification using a bicinchoninic acid (BCA) assay, and the remainder was stored frozen at −80°C until use.
Immunoblot Procedures
Ten micrograms of renal protein were electrophoresed on a 10% PAGE ReadyGel (Bio-Rad, Hercules, CA). Gels were transferred electrophoretically to nitrocellulose membranes, blocked with 5 g/dL nonfat dry milk diluted in Blotto buffer (50 mM Tris, 150 mM NaCl, 5 mM Na2EDTA, and 0.05% Tween 20, pH 7.6), and incubated at 4°C overnight with primary antibody in nonfat dry milk. Loading and transfer equivalence were assessed with Ponceau S staining and by evaluating expression of the housekeeping protein β-actin. After being washed, membranes were exposed to secondary antibody (goat anti-rabbit IgG, Cell Signaling Technology, Beverly, MA) conjugated to horseradish peroxidase at a dilution of 1:5,000. Sites of antibody-antigen reaction were visualized using enhanced chemiluminescence (SuperSignal West Pico Substrate, Pierce) and a Kodak Image Station 440CF digital imaging system. In selected experiments, blots were stripped. Band density was normalized such that the mean density in a region (cortex or outer medulla) in male wild-type (WT) tissue was 100. Both sexes (male and female) and both genotypes (WT and KO) were loaded on the same gel for these experiments. The absence of saturation was confirmed by examining pixel intensity distribution in all immunoblots.
Tissue Preparation for Immunohistochemistry
Mice were anesthetized with inhalant isoflurane. The kidneys were preserved by in vivo cardiac perfusion with PBS (pH 7.4) containing 6,000 U/L of Na-heparin and 120 mg/L of lidocaine followed by periodate-lysine-2% paraformaldehyde, cut transversely into several 2- to 3-mm-thick slices, and then immersed for 24–30 h at 4°C in the same fixative. Kidney samples from each animal were embedded in polyester wax made with of polyethylene glycol 400 distearate (Polysciences, Warrington, PA) with 10% 1-hexadecanol and 2-µm-thick sections were cut and mounted on gelatin-coated slides.
Immunohistochemistry
Immunolocalization for Rh family B glycoprotein (Rhbg), Rh family C glycoprotein (Rhcg), Na+/H+ exchanger isoform 3 (NHE3), glutamine synthetase (GS), NKCC2, and AR was accomplished using previously described immunoperoxidase procedures (20, 22, 34–37). Briefly, sections were dewaxed in ethanol, rehydrated, heated in Trilogy (Cell Marque, Rocklin, CA) to 88°C for 30 min and then to 96°C for 30 min, cooled for 30 min, and rinsed in PBS. Endogenous peroxidase activity was blocked by incubating sections in 3% H2O2 in distilled water for 45 min. Sections were blocked for 15 min with serum-free protein block (Dako Cytomation) and then incubated overnight at 4°C with primary antibody. Sections were washed in PBS, incubated for 30 min with polymer-linked, peroxidase-conjugated horse anti-rabbit IgG (ImmPRESS, Vector Laboratories, Burlingame, CA), washed again with PBS, and then exposed to diaminobenzidine for 5 min. Sections were washed with distilled water, dehydrated with xylene, mounted, and observed by light microscopy. Comparisons of labeling were made between sections from the same immunohistochemistry experiment. Sections were examined on a Zeiss Axio Imager A2 microscope equipped with DIC optics and photographed using Axiocam 305 color digital camera and Zen 2.3 software (Zeiss).
Immunolocalization for phosphoenolpyruvate carboxykinase (PEPCK) was accomplished using modified immunoperoxidase procedures as previously described (20, 22, 38–40). Briefly, sections were dewaxed in ethanol, rehydrated, heated in Trilogy (Cell Marque, Rocklin, CA) to 96°C for 60 min, cooled for 30 min, and rinsed in PBS. Endogenous peroxidase activity was blocked by incubation of the sections in 3% H2O2 in methanol for 45 min. Sections were treated with 0.5% Triton X-100 in PBS for 15 min. Sections then underwent several washes in PBS containing 1% BSA, 0.05% saponin, and 0.2% gelatin, followed by blocking for 15 min with serum-free protein block (DAKO Cytomation) and then incubated overnight at 4°C with primary antibody. Sections were washed in PBS containing 0.1% BSA, 0.05% saponin, and 0.2% gelatin followed by PBS and incubated for 60 min with polymer-linked, peroxidase-conjugated goat anti-rabbit IgG (MACH2, Biocare Medical, Concord, CA), washed again in PBS, and then exposed to diaminobenzidine for 5 min.
Negative Control
Each immunohistochemistry experiment included a section that was exposed to the immunolabeling procedures without primary antibody to ensure that the label was due to primary antibody binding only.
Morphometric Analysis
The volume density of the proximal tubule in the cortex and outer stripe of the outer medulla as well as the collecting duct in the cortex and inner stripe of the outer medulla was determined using standard point-counting techniques as previously described (20–22, 41).
Quantitative Immunohistochemistry
Quantitative immunohistochemistry was performed as we have previously described and validated (20, 22, 33, 37, 42). Proximal tubule segments were studied in sections labeled under identical conditions in the same immunolabeling experiment by an observer blinded to the treatment group. The specific proximal tubule segments measured were the initial proximal convoluted tubule (PCT), defined as PCT segments continuous with Bowman’s capsule, the proximal straight tubule (PST) in the medullary ray, and the PST in the outer stripe of the outer medulla. We used high-resolution digital micrographs taken of randomly selected fields of the renal cortex and outer stripe of the outer medulla using a Zeiss Axio Imager A2 microscope equipped with a Axiocam 305 color digital camera and Zen 2.3 software (Zeiss) using no imaging enhancement techniques. Using ImageJ software (v, 1.34j, National Institutes of Health, Bethesda, MD), we measured pixel intensity across a straight line drawn from the tubule lumen through an individual cell. These data were then analyzed using custom-written software executed in Microsoft Excel 2016. Net intensity at each pixel on the line was then determined as the difference between absolute intensity and mean background pixel intensity measured outside the cell. Total cellular expression was determined by integrating net pixel intensity across the entire cell. Cell height was determined as the distance in pixels between the apical and basolateral edges of the cells and concerted to absolute length using calibrated determination of individual pixel size. A minimum of 15 individual cells from at least four photomicrographs from each kidney were analyzed. Data from all cells examined of a given proximal tubule segment type were averaged to yield a single data point per animal for statistical analysis.
We determined type A intercalated cell size and cell-specific protein expression as previously described (20, 22). Briefly, we obtained high-power digital micrographs of the inner stripe of the outer medulla of tissue sections that had undergone immunohistochemistry for the intercalated cell-specific marker Rhbg or Rhcg. Individual intercalated cells were circumscribed using ImageJ software (v. 1.34j, National Institutes of Health). Cell size was determined as the number of pixels within the outlined regions and converted to area using calibrated measurement of pixels per micrometer. Immunolabel intensity at each pixel was determined as the difference between absolute pixel intensity and mean background intensity, and single cell immunolabel intensity was determined by integrating net pixel intensity within the cell using custom-written software executed in Microsoft Excel 2016.
Statistical Analysis
Results are presented as means ± SE; n refers to the number of animals studied. For quantitative immunohistochemistry, all measurements of a specific cell type in a mouse kidney were averaged and used as a single measurement for statistical analysis. Statistical analyses were performed using two-way ANOVA to analyze differences between the four groups. Tukey multiple comparisons were used for post hoc analysis. A Student’s t test was used to compare two groups. P < 0.05 was considered statistically significant. We performed statistical analysis using SPSS software (26.2) and Microsoft Excel 2016.
RESULTS
Verification of Kidney-Specific AR Deletion
AR pharmacological blockage or global deletion induces a wide variety of phenotypic changes (43), which could indirectly alter renal structural and ammonia responses. To avoid these issues, we generated KS-AR-KO mice using Cre-loxP techniques. We confirmed the effectiveness of renal AR deletion using immunohistochemistry (Fig. 1). In WT mice, immunohistochemistry showed nuclear AR immunolabel limited to the proximal tubule; this was similar to our findings in the normal mouse kidney (22). In the KO kidney, there was no detectable AR immunolabel present in either sex (Fig. 1). These findings show the generation of mice with renal AR deletion.
Figure 1.
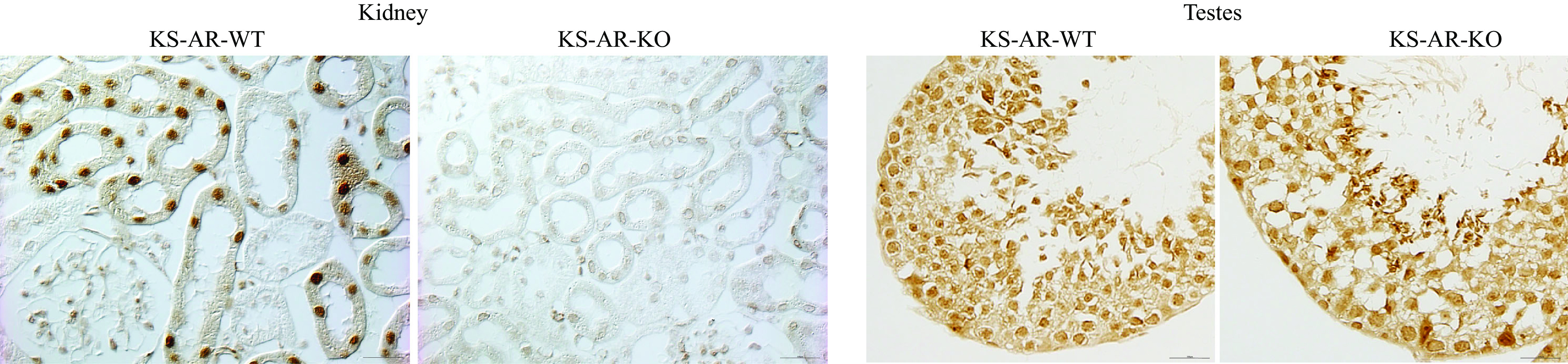
Verification of androgen receptor (AR) deletion. A: high-power photomicrographs of AR immunolabel in the cortex of wild-type (WT) mice and mice with kidney-specific AR deletion [KS-AR-knockout (KO)]. Strong nuclear AR immunoreactivity was present in the proximal tubules throughout the cortex in the WT kidney, where no detectable immunolabel was present in the KO kidney. Images are representative of findings in 24 mice. B: high-power photomicrographs of AR immunolabel in the testes of WT and KS-AR-KO mice. AR immunoreactivity was present and did not differ between WT and KO testes. Scale bars = 100 µm.
Because testosterone has numerous extrarenal effects, which could possibly indirectly alter renal acid-base mechanisms, we considered the possibility that KS-AR-KO could alter plasma testosterone or estradiol levels. However, plasma testosterone and estradiol concentrations did not differ significantly between WT and KO mice in either sex (Table 2).
Table 2.
Physiological parameters
| Parameter | Males |
Females |
||
|---|---|---|---|---|
| AR-WT | AR-KO | AR-WT | AR-KO | |
| Testosterone (plasma), ng/dL | 67 ± 74 (8) | 239 ± 471 (11) | 27 ± 10 (8) | 29 ± 11 (10) |
| Estradiol (plasma), pg/mL | 4.4 ± 1.4 (8) | 5.1 ± 1.7 (11) | 8.4 ± 2.4(8)* | 7.4 ± 2.1 (10) |
| Food intake, g | 11.5 ± 0.2 (8) | 11.9 ± 0.5 (8) | 12.5 ± 1.9 (8) | 12.9 ± 1.7 (8) |
| Body weight, g | 25.6 ± 3.0 (8) | 25.7 ± 1.7(8) | 20.8 ± 1.7 (8)* | 21.3 ± 1.9 (8) |
| Plasma Na+, mmol/L | 151 ± 2 (7) | 150 ± 2 (7) | 152 ± 2 (8) | 148 ± 2 (7) |
| Plasma K+, mmol/L | 4.8 ± 0.8 (7) | 4.6 ± 0.5 (7) | 4.8 ± 0.4 (7) | 4.5 ± 0.2 (7) |
| Plasma , mmol/L | 20 ± 4 (7) | 20 ± 3 (7) | 22 ± 3 (7) | 18 ± 3 (7) |
| Titratable acid excretion, µmol/day | 43 ± 13 (5) | 65 ± 24 (5) | 100 ± 35 (5) | 133 ± 66 (5) |
| Urine pH | 6.44 ± 0.14 (8) | 6.18 ± 0.18 (8)† | 6.34 ± 0.17 (8) | 6.15 ± 0.18 (8) |
| Urea clearance, mL/min | 0.19 ± 0.12 (9) | 0.12 ± 0.04 (9) | 0.13 ± 0.07 (5) | 0.11 ± 0.02 (5) |
Results are reported as means ± SE; numbers of mice are shown in parentheses. AR, androgen receptor; KO, knockout (deletion); WT, wild-type. *P < 0.05 for sex; †P < 0.05 for genotype.
Physiological Characterization of Kidney-Specific AR Deletion
Body weight was greater in WT male mice than in WT female mice despite no sex difference in food intake, as previously reported (22), consistent with known extrarenal effects of sex on body weight. However, KS-AR-KO did not alter daily food intake or body weight in either sex (Table 2).
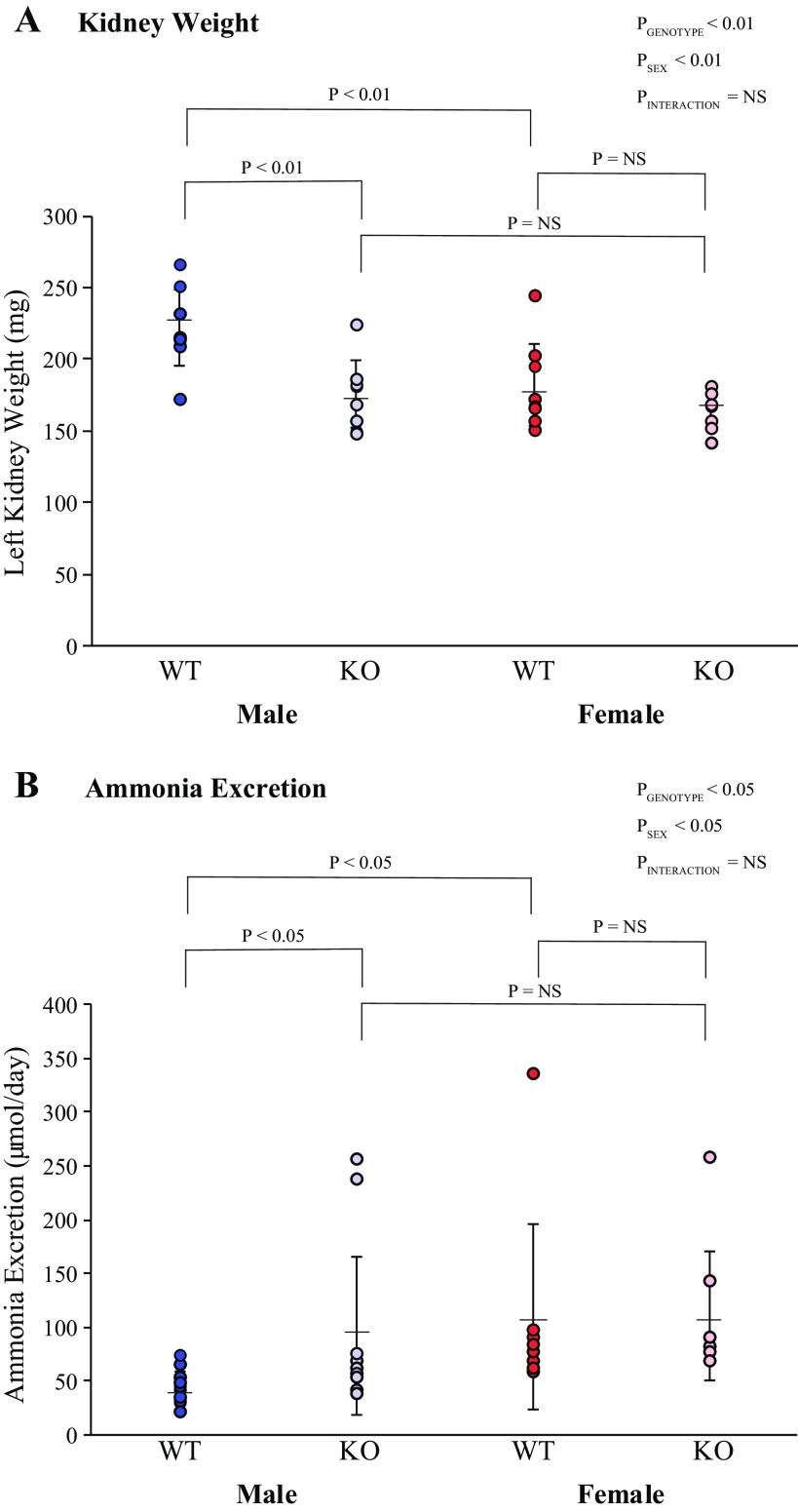
Kidney weight was significantly greater in male WT mice than in female WT mice (Fig. 2), similar to what we have previously reported in normal mice (22). KS-AR-KO significantly decreased kidney weight in male KS-AR-KO mice, to the extent that male KS-AR-KO kidney weight did not differ significantly from female WT or KS-AR-KO kidney weight (Fig. 2). In female mice, KS-AR-KO did not alter kidney weight significantly. Thus, the effect of sex on kidney weight requires AR expression in the male kidney.
Figure 2.
Kidney weight and ammonia excretion in mice with androgen receptor (AR) deletion. A: left kidney weight in male (left) and female (right) wild-type (WT) mice and mice with kidney-specific AR deletion [KS-AR-knockout (KO)]. Kidney weight was significantly greater in male WT mice than in female WT mice. KS-AR-KO significantly decreased kidney weight in male KS-AR-KO mice, to the extent that male KS-AR-KO kidney weight did not differ significantly from female WT or KS-AR-KO kidney weight. In female mice, KS-AR-KO did not alter kidney weight significantly. N = 8 mice per group. B: ammonia excretion in male (left) and female (right) WT and KS-AR-KO mice. In male mice, KS-AR-KO increased ammonia excretion significantly, which eliminated the sex difference in ammonia excretion. Ammonia excretion was not significantly altered in female mice by KS-AR-KO. P values indicate comparison of genotype and sex by two-way ANVOA or Student’s t test. n = 8 mice per group. NS, not significant.
Neither plasma Na+, K+, nor bicarbonate concentrations differed significantly between WT and KS-AR-KO mice for either sex (Table 2). Urea clearance, used as a marker of glomerular filtration rate, did not differ significantly between WT and KS-AR-KO mice in either sex (Table 2).
Net Acid Excretion
Net acid excretion involves two processes: ammonium () excretion and titratable acid excretion. In male mice, KS-AR-KO increased ammonia excretion significantly, which eliminated the sex difference in ammonia excretion (Fig. 2). Ammonia excretion was not significantly altered in female mice by KS-AR-KO. Titratable acid excretion was not significantly altered by KS-AR-KO in either sex (Table 2). Urine pH was significantly decreased by KS-AR-KO in male mice but not in female mice (Table 2); however, the effect of KS-AR-KO on ammonia excretion persisted after statistical consideration of urine pH (P < 0.001 by ANOVA). Thus, proximal tubule AR mediates sex differences in ammonia excretion in male mice, but its deletion had no significant effect on ammonia excretion in female mice.
Effect of Kidney-Specific AR Deletion on Proximal Tubule Structure
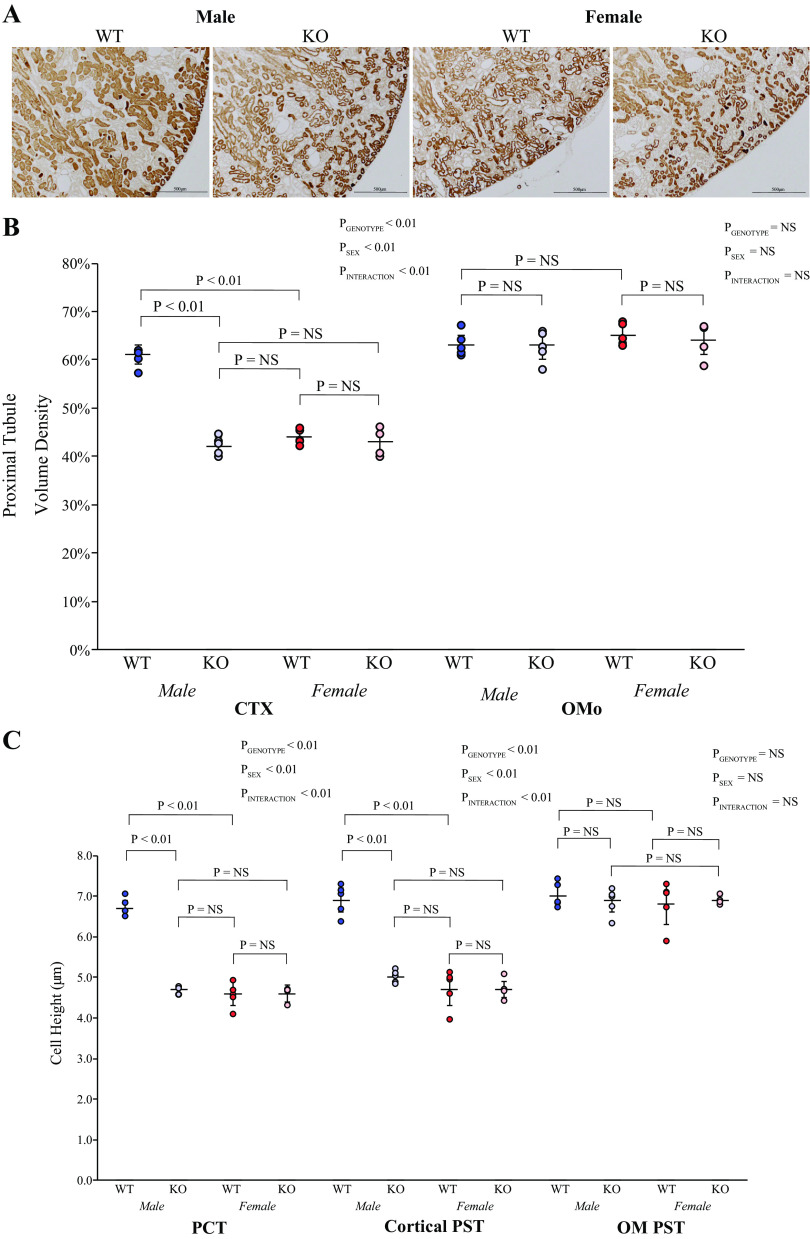
Proximal tubules account for a greater proportion of cortical volume in the male kidney than in the female kidney, and this is testosterone dependent (20, 22). Thus, we determined the effects of KS-AR deletion in male and female mice on proximal tubule structure. Using PEPCK immunolabel as a proximal tubule-specific marker, immunohistochemistry showed qualitatively that KS-AR-KO decreased the proportion of the cortex that was proximal tubule (Fig. 3A). Quantitative analysis, using formal point counting techniques (41), showed that KS-AR-KO significantly decreased proximal tubule volume density in the cortex of males, which parallels the change in kidney weight (Fig. 3B). In contrast, in the outer stripe of the outer medulla, KS-AR-KO did not alter proximal tubule volume density in male mice significantly (Fig. 3B). In female mice, KS-AR-KO did not qualitatively alter the proportion of the cortex that was proximal tubule (Fig. 3A) and quantitatively did not alter proximal tubule volume density in either the cortex or outer medulla (Fig. 3B). The sex difference in cortical proximal tubule volume density present in WT mice was not present in KS-AR-KO mice. Thus, in the male kidney, AR mediates the sex differences in proximal tubule volume density in the cortex. In the outer medulla, AR expression does not appear to influence proximal tubule volume density in either sex.
Figure 3.
Proximal tubule structure in mice with androgen receptor (AR) deletion. A: low-power photomicrographs of immunolabel for the proximal tubule-specific protein phosphoenolpyruvate carboxykinase (PEPCK) in the cortex (CTX) of male (left) and female (right) wild-type (WT) mice and mice with kidney-specific AR deletion [KS-AR-knockout (KO)]. In males, proximal tubules appeared sparser in KS-AR-KO kidneys compared with WT kidneys. In females, proximal tubule density appeared similar in WT and KO kidneys. B: quantitative analysis of proximal tubule volume density in the CTX and outer stripe of the outer medulla (OMo). In the CTX of male but not female mice, KS-AR-KO decreased proximal tubule volume density significantly. Volume density in male WT mice was greater than in female WT mice, but in male KO mice volume density was not significantly different than in female WT or KO mice. In the Omo, proximal tubule volume density did not differ between WT and KO mice in either sex. C: proximal tubule cell height. In both the proximal convoluted tubule (PCT) and cortical proximal straight tubule (PST), proximal tubule cell height was significantly decreased in male mice by KS-AR-KO. In female mice, cell height was not significantly altered by KO in either the PCT or cortical PST. Cell height in male WT mice was greater than in female WT mice, whereas male KO cell height in the PCT and cortical PST did not differ significantly from that in female WT or KO mice. In the outer medulla (OM), PST cell height did not differ significantly between WT and KO mice in either sex, nor did it differ between male WT and female WT mice. P values indicate comparison of genotype and sex by two-way ANVOA or Student’s t test. Scale bars = 500 µm. N = 5 mice per group. NS, not significant.
These changes in proximal tubule structure in male KS-AR-KO mice appear to involve changes in cell size. In male mice, KS-AR-KO decreased proximal tubule cell height significantly in both the PCT and cortical PST, i.e., throughout the cortex (Fig. 3C). Cell height in the PST in the outer medulla was not altered significantly (Fig. 3C). These results parallel the volume density findings. Kidney-specific AR deletion in female mice did not alter proximal tubule cell height in either the PCT, cortical PST, or PST in the outer medulla, which parallels the lack of effect on volume density and total kidney size in the female kidney (Fig. 2B).
Kidney weight, cortical proximal tubule volume density, and cell height in the PCT and cortical PST were all greater in the WT male kidney than in the WT female kidney, which parallels our previous observations (20, 22). In male KS-AR-KO mice, this sex difference no longer existed, and each of these parameters did not differ significantly from that observed in either WT or KS-AR-KO female mice. Thus, sex differences in kidney weight, cortical proximal tubule volume density, and cell height in the PCT and cortical PST are dependent on AR expression.
Effect of Kidney-Specific AR Deletion on Collecting Duct Structure
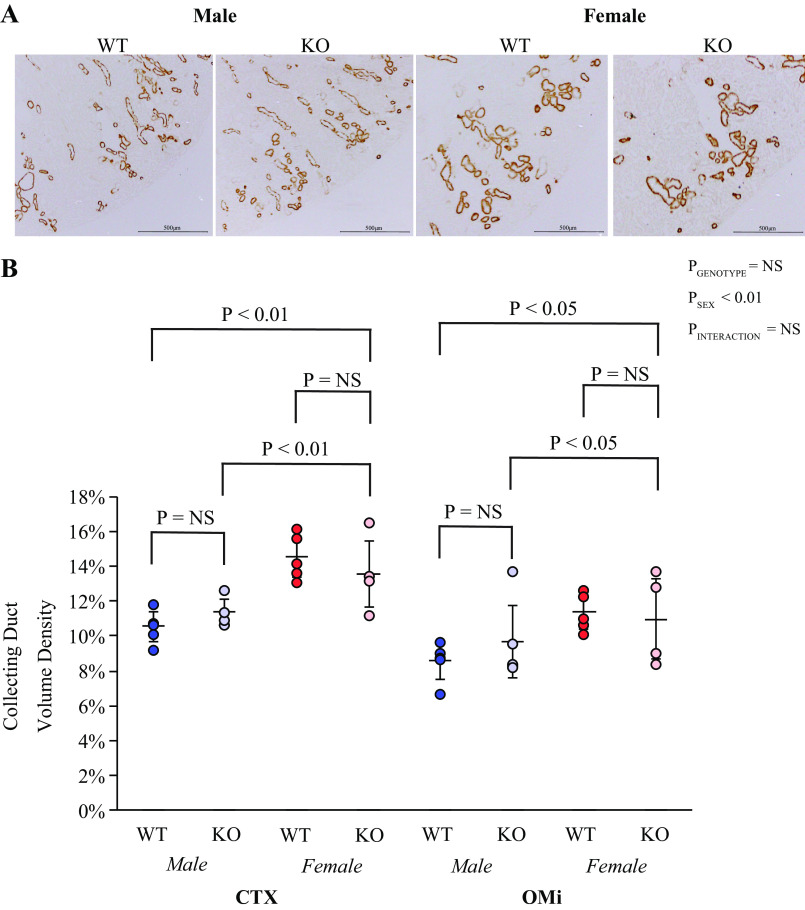
There are important sex differences in the collecting duct, with collecting duct volume density being greater in the female kidney than in the male kidney (20). Thus, we next evaluated the role of AR in these differences. To identify the collecting duct, we used Rhbg immunolabel. Low-power micrographs showed no detectable effect of KS-AR-KO on the proportion of the cortex or outer medulla that was collecting duct in either male or female mice (Fig. 4A). Quantitative morphometric analysis showed that KS-AR-KO did not alter collecting duct volume density significantly in either the cortex or outer medulla in either sex (Fig. 4B). Thus, renal AR does not mediate the sexual dimorphism in collecting duct volume density.
Figure 4.
Collecting duct structure in mice with androgen receptor (AR) deletion. A: low-power photomicrographs of the collecting duct marker Rhesus B glycoprotein (Rhbg) in the cortex (CTX) of male (left) and female (right) wild-type (WT) mice and mice with kidney-specific AR deletion [KS-AR-knockout (KO)]. KS-AR-KO did not detectably alter collecting duct abundance in either sex. B: morphometric analysis of collecting duct volume density in the CTX and inner stripe of the outer medulla (OMi). Collecting duct volume density was not significantly altered by KS-AR-KO in either sex. P values indicate comparison of genotype and sex by two-way ANVOA or Student’s t test. Scale bars = 500 µm. n = 5 mice per group. NS, not significant.
Ammoniagenic Enzyme Expression
The results in Net Acid Excretion show that kidney-specific AR deletion increases ammonia excretion in male mice even though it decreases proximal tubule volume density and cell height in the cortex. This indicates that the effects on ammonia excretion cannot be attributed simply to proximal tubule hypertrophy leading to increased ammoniagenesis and instead are the result of separate and specific effects on ammoniagenesis and/or ammonia transport. Thus, we next examined key proteins involved in ammoniagenesis and in ammonia transport.
PEPCK is a key proximal tubule enzyme required for ammoniagenesis and is found exclusively in the proximal tubule in the kidney (16, 44). In male mice, KS-AR-KO increased cortical PEPCK expression significantly; there was no effect of KS-AR-KO in female mice (Fig. 5A). The increase in PEPCK expression in male KS-AR-KO mice eliminated the sex difference in WT mice (Fig. 5A). Examination of PEPCK immunolabel expression showed that male KS-AR-KO mice had greater PEPCK immunolabel intensity in the PCT and cortical PST than did WT mice (Fig. 5B). In the outer stripe of the outer medulla, there was no detectable difference in PEPCK immunolabel intensity (Fig. 5B). Quantitative immunohistochemistry of male mice showed that KS-AR-KO increased PEPCK immunolabel intensity significantly in both the PCT and cortical PST but not in the outer stripe of the outer medulla (Fig. 5C). Thus, in male mice, but not in female mice, proximal tubule AR expression decreases PEPCK expression in cortical proximal tubule segments, but not in the PST in the outer medulla.
Figure 5.
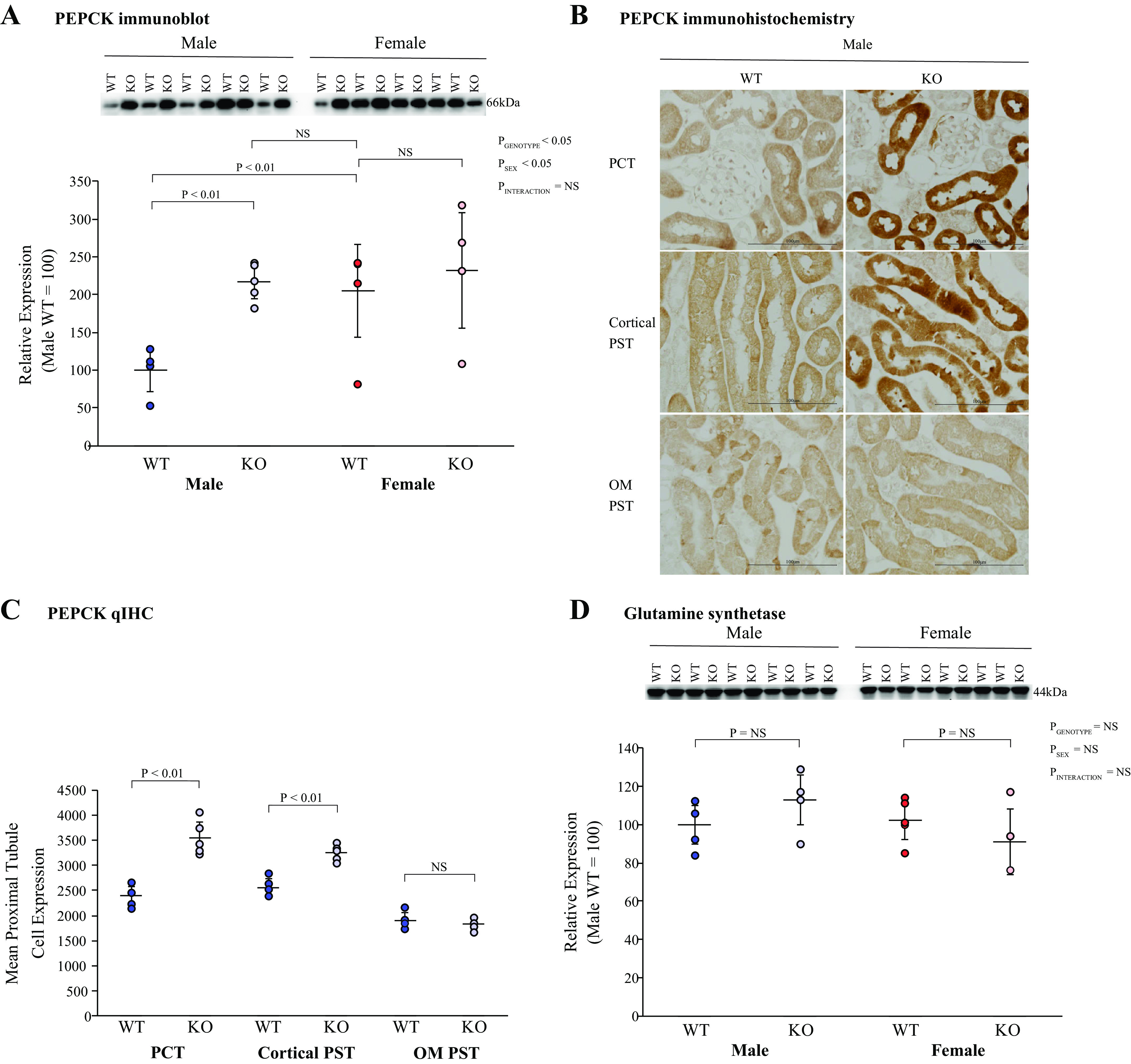
Effect of androgen receptor (AR) deletion on ammoniagenesis and recycling. A: immunoblot analysis of renal cortical phosphoenolpyruvate carboxykinase (PEPCK) abundance. In male mice (left), kidney-specific AR deletion [KS-AR-knockout (KO)] increased PEPCK protein expression significantly, whereas in female mice (right), KS-AR-KO did not. PEPCK protein expression was significantly less in male WT mice than in female WT mice. KS-AR-KO in male mice resulted in expression that did not differ significantly from either female WT or KS-AR-KO mice. B: PEPCK immunohistochemistry in male mice in the proximal convoluted tubule (PCT; top), cortical proximal straight tubule (PST; middle), and PST in the outer medulla (OM PST; bottom) in male WT mice (left) and male KS-AR-KO mice (right). PEPCK immunolabel appeared more intense in both PCT and cortical PST segments in KS-AR-KO mice than in WT mice. C: quantitative immunohistochemistry (qIHC) of the male kidney. KS-AR-KO increased PEPCK expression significantly in the PCT and cortical PST but not in the OM PST. N = 6 mice/group. D: immunoblot analysis of renal cortical glutamine synthetase (GS) abundance. KS-AR-KO did not significantly alter GS abundance in either sex. P values indicate comparison of genotype and sex by two-way ANVOA or Student’s t test. Scale bars = 100 µm. n = 5 mice per group. NS, not significant.
The proximal tubule has an ammonia recycling capability involving GS (37, 42, 45, 46). In neither male nor female mice did KS-AR-KO significantly alter GS expression (Fig. 5D). Thus, renal AR does not appear to modulate GS expression.
Effect of Kidney-Specific AR Deletion on NBCe1 Expression
NBCe1 is a proximal tubule integral membrane protein with a major role in filtered bicarbonate reabsorption that also regulates ammonia metabolism (47–49). Two splice variants of NBCe1, NBCe1-A and NBCe1-B, are present in the mouse kidney (50, 51). Using a pan-NBCe1 antibody, which recognizes both NBCe1 variants, we found that KS-AR-KO significantly decreased cortical NCBe1 expression in the male, but not female, kidney (Fig. 6A).
Figure 6.
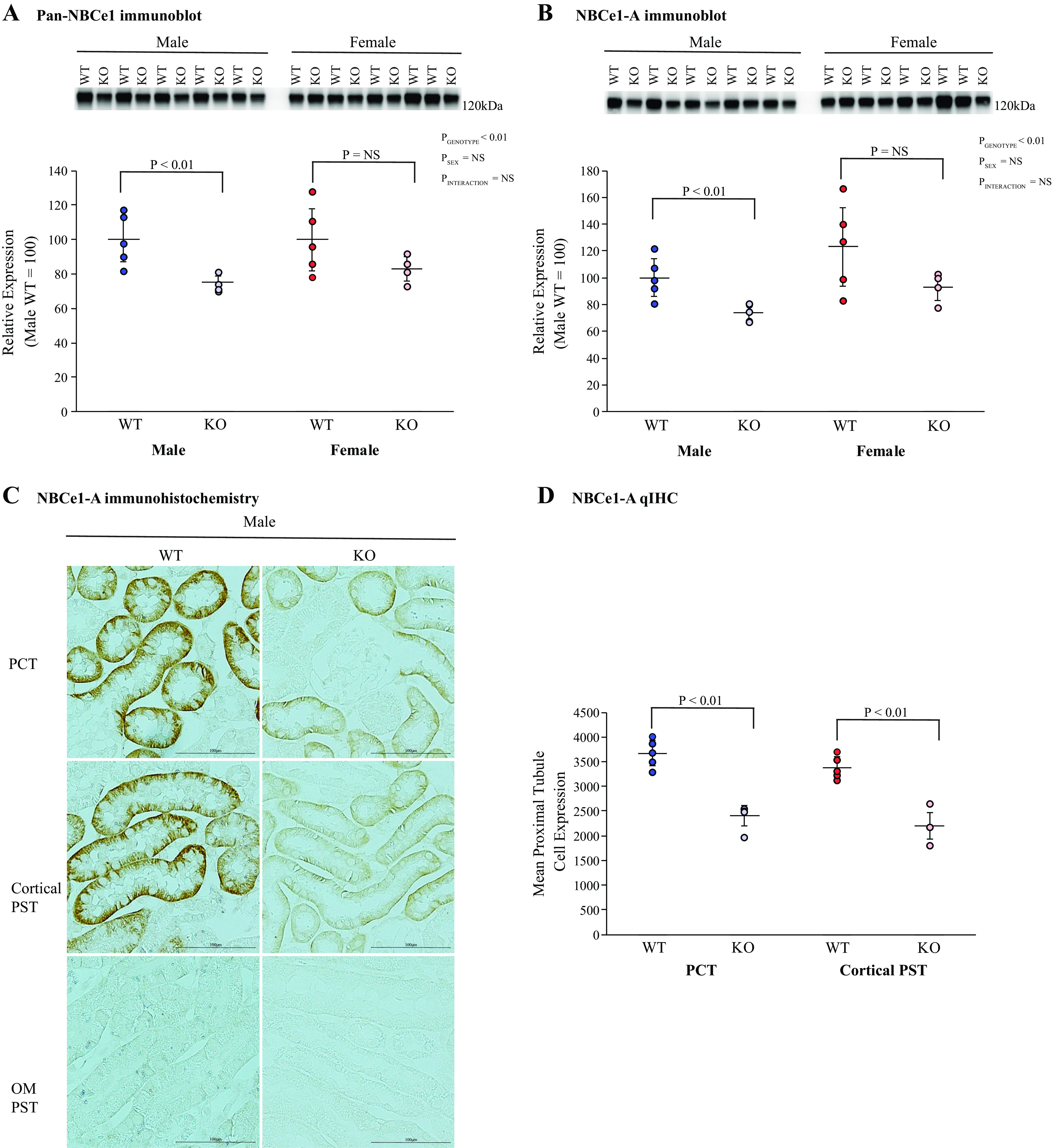
Effect of androgen receptor (AR) deletion on electrogenic Na+-bicarbonate cotransporter 1 (NBCe1) expression. A: immunoblot analysis using a pan-NBCe1 antibody in the cortex of male (left) and female (right) wild-type (WT) mice and mice with kidney-specific AR deletion [KS-AR-knockout (KO)]. Total NBCe1 protein expression was significantly less in male KS-AR-KO mice than in male WT mice. Total NBCe1 abundance did not change significantly in female mice. B: immunoblot analysis using a NBCe1-A-specific antibody. NBCe1-A expression was significantly less in male KS-AR-KO mice than in WT mice. KS-AR-KO did not change NBCe1-A abundance significantly in female mice. C: NBCe1-A immunohistochemistry in the proximal convoluted tubule (PCT; top), cortical proximal straight tubule (PST; middle), and PST outer medulla (PST OM; bottom) in male WT mice (left) and male KS-AR-KO mice (right). NBCe1-A immunolabel intensity was less in cortical proximal tubule segments in KS-AR-KO mice than in WT mice. NBCe1-A immunolabel was not detectable in the OM PST, as previously reported (47, 48). D: quantitative immunohistochemistry (qIHC) for NBCe1-A. Male KS-AR-KO mice had significantly less NBCe1-A expression in the PCT and cortical PST than did WT mice. P values indicate comparison of genotype and sex by two-way ANVOA or Student’s t test. Scale bars = 100 µm. n = 5 mice per group. NS, not significant.
The A variant of NBCe1 is the predominant variant present in the proximal tubule (50), and NBCe1-A has been shown to regulate ammonia metabolism (47, 48). In male mice, KS-AR-KO decreased cortical NBCe1-A expression significantly; in female mice, expression was unaltered (Fig. 6B). Immunohistochemistry qualitatively showed that in males, KS-AR-KO decreased NBCe1-A immunolabel intensity in cortical proximal tubule segments (PCT and cortical PST). NBCe1-A immunolabel was not detectable in outer medullary proximal tubule segments (Fig. 6C), as previously reported (48). We then used quantitative immunohistochemistry analysis to quantify segment-specific changes in NBCe1-A expression. This showed that KS-AR-KO in male mice decreased NBCe1-A expression in the PCT and cortical PST, i.e., throughout the cortex (Fig. 6D). Thus, AR expression regulates cortical proximal tubule NBCe1-A expression in the male kidney but not in the female kidney.
Effect of Kidney-Specific AR Deletion on Ammonia Transporters
Renal ammonia excretion involves coordinated NH3/ transport by specific membrane proteins (10, 16, 52). This involves transporters in the proximal tubule (NHE3), TAL of the loop of Henle (NKCC2), and collecting duct (Rhbg and Rhcg). Sex differences in ammonia excretion correlate with differences in the expression of each of these (6, 20), and testosterone regulates expression of both NHE3 and NKCC2 (22).
NHE3 is the primary transporter mediating proximal tubule preferential luminal ammonia secretion (53–55). In male mice, KS-AR-KO decreased NHE3 protein expression significantly compared with WT mice; in female mice, KS-AR-KO did not significantly alter NHE3 expression (Fig. 7A). Immunohistochemistry showed decreased NHE3 immunolabel intensity in the cortical proximal tubule of male KS-AR-KO mice compared with male WT mice (Fig. 7B). Quantitative analysis showed that KS-AR-KO in male mice significantly decreased NHE3 immunolabel intensity in the PCT and cortical PST (Fig. 7C). In the outer stripe of the outer medulla, KS-AR-KO did not detectably alter NHE3 immunolabel intensity (Fig. 7C). Thus, renal AR regulates cortical proximal tubule NHE3 expression in male mice but not in female mice.
Figure 7.
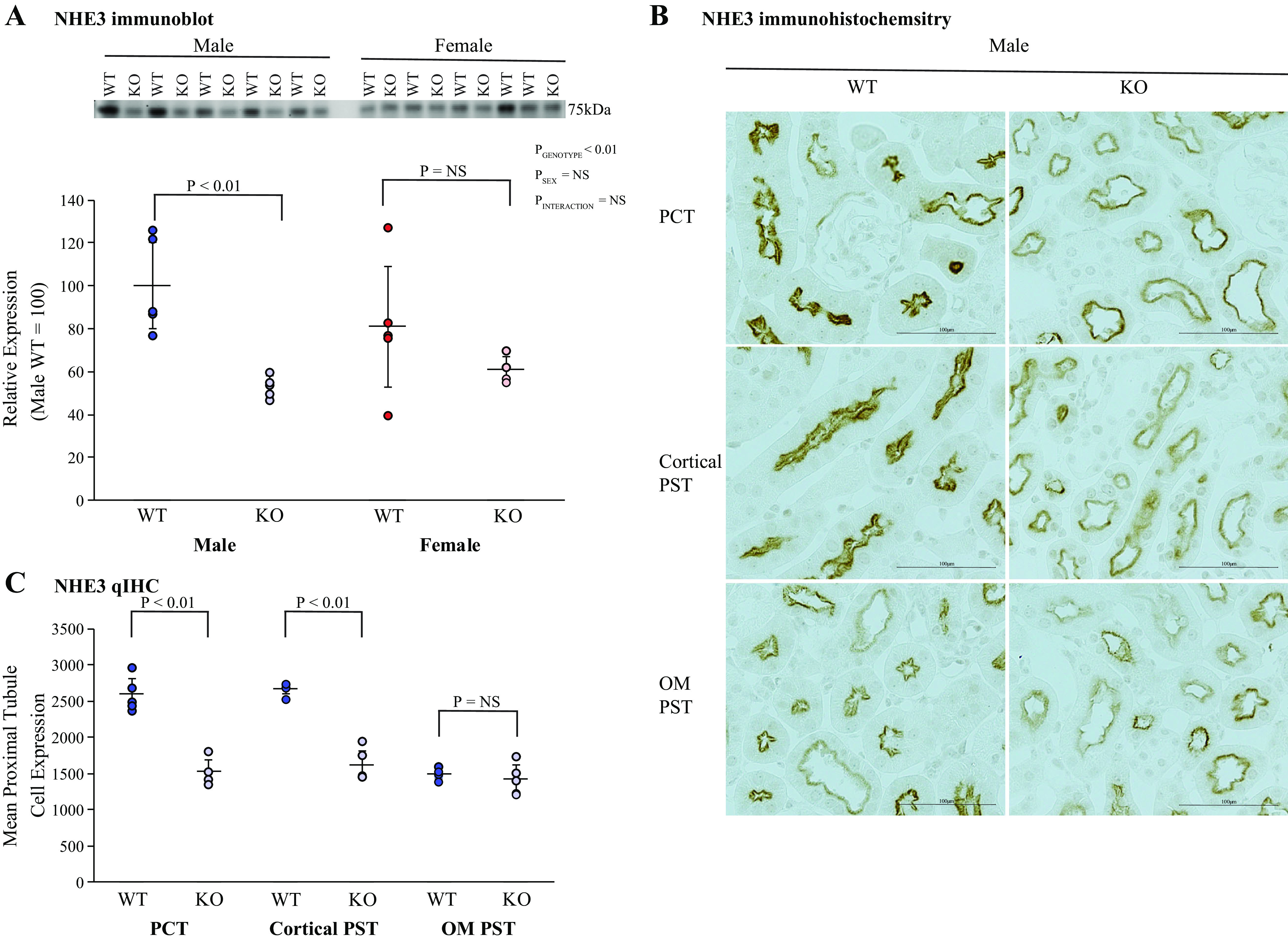
Effect of androgen receptor (AR) deletion on proximal tubule Na+/H+ exchanger isoform 3 (NHE3) expression. A: immunoblot analysis of NHE3 expression. Kidney-specific AR deletion [KS-AR-knockout (KO)] significantly decreased NHE3 expression in male mice. In female mice, NHE3 expression was not significantly altered. B: NHE3 immunohistochemistry. NHE3 immunolabel was less intense in the proximal straight tubule (PST) and cortical proximal convoluted tubule (PCT) of male KS-AR-KO mice compared with male WT mice. C: quantitative immunohistochemistry (qIHC). In male mice, PCT and cortical PST NHE3 expression was decreased significantly by KS-AR-KO, and in the outer medullary PST (OM PST) it was not significantly altered. P values indicate comparison of genotype and sex by two-way ANVOA or Student’s t test. Scale bars = 100 µm. n = 5 mice per group. NS, not significant.
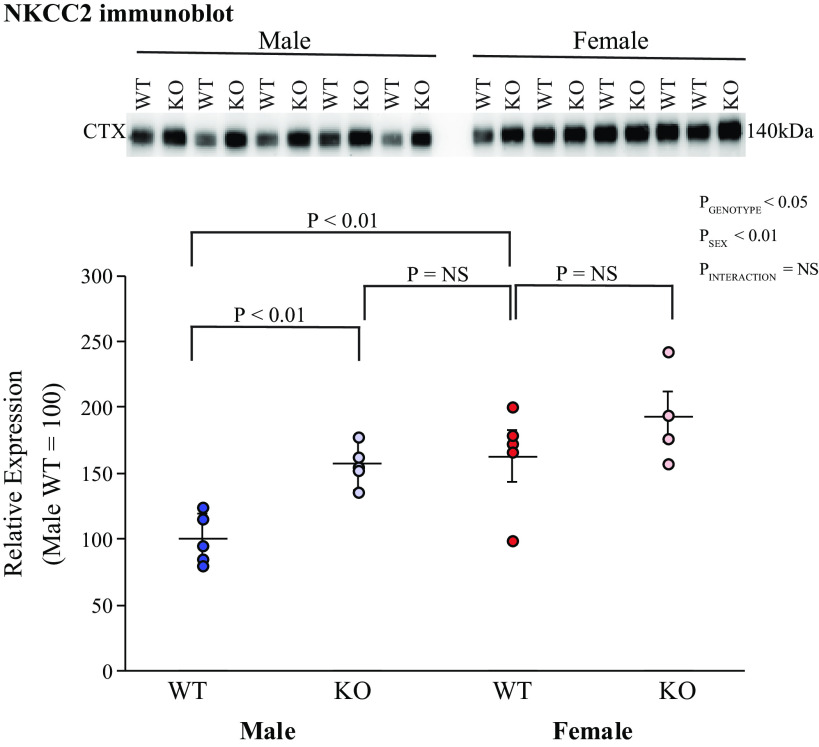
NKCC2 mediates TAL ammonia reabsorption (10, 16, 52). In male mice, KS-AR-KO increased NKCC2 protein expression using immunoblot analysis significantly; in female mice, there was no significant change (Fig. 8). This change in expression in male mice likely contributes to altered medullary ammonia shunting and thereby facilitates the effect of KS-AR-KO to increase ammonia excretion in male mice.
Figure 8.
Effect of androgen receptor (AR) deletion on thick ascending limb Na+-K+-2Cl− cotransporter (NKCC2). A: immunoblot analysis of cortical NKCC2 expression. Male mice with kidney-specific AR deletion [KS-AR-knockout (KO)] had significantly greater NKCC2 expression than WT mice. There was no detectable change in NKCC2 expression between the groups of female mice. NKCC2 expression was significantly less in male WT mice than in female WT mice. In male KO mice, expression did not differ significantly from either female WT or KO mice. P values indicate comparison of genotype and sex by two-way ANVOA or Student’s t test. n = 5 mice per group. NS, not significant.
Rh glycoproteins Rhbg and Rhcg are the primary proteins that mediate collecting duct ammonia secretion, and their expression generally parallels changes in ammonia excretion (10, 16, 52). However, KS-AR-KO did not alter significantly Rhbg protein expression in either male or female KS-AR-KO mice in either the cortex or inner stripe of the outer medulla (Fig. 9). The effects on Rhcg expression were similar. Rhcg immunohistochemistry, evaluated qualitatively and using quantitative immunohistochemistry, showed no significant changes in Rhcg immunolabel intensity in intercalated cells of the outer stripe of the outer medulla in either sex (Fig. 10). Thus, renal AR does not appear to modulate either Rhbg or Rhcg expression.
Figure 9.
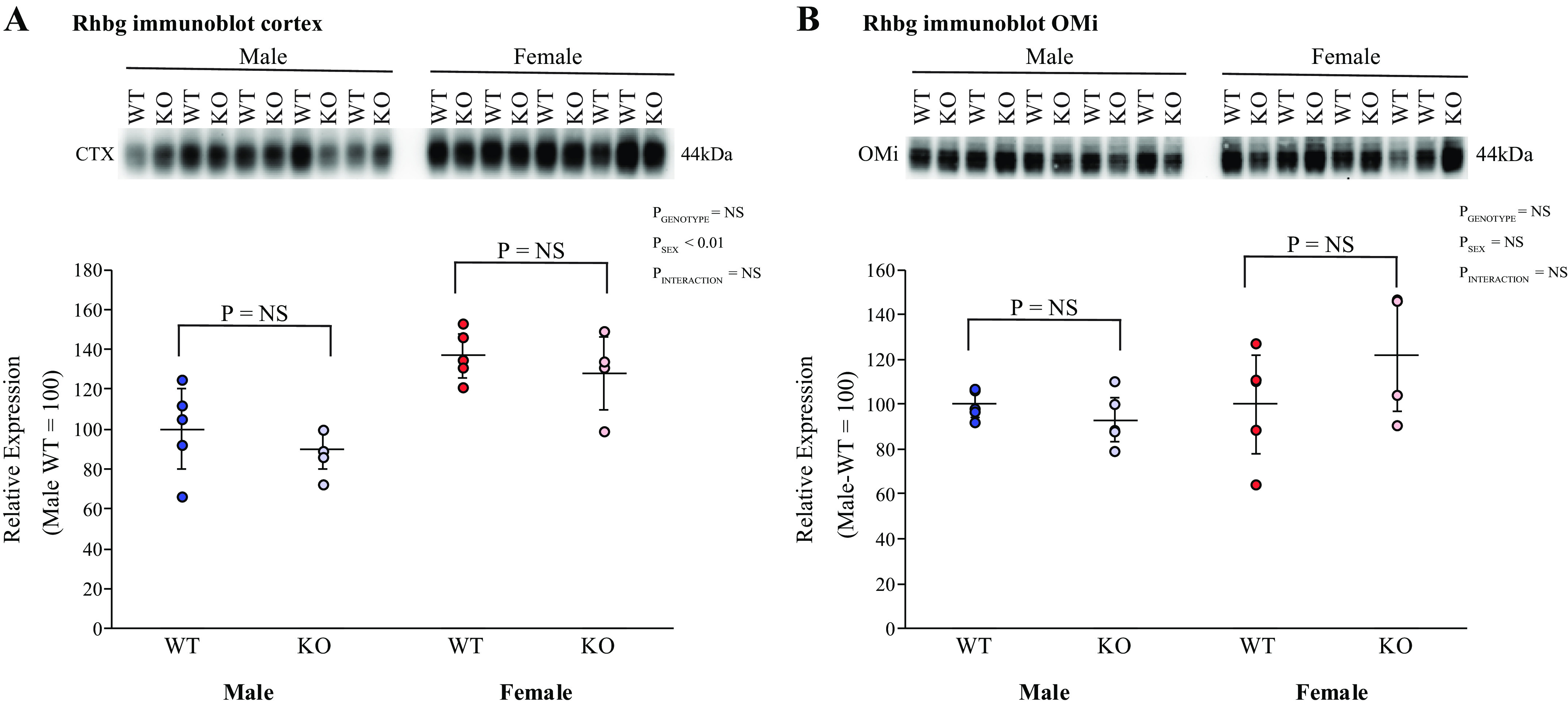
Effect of kidney-specific androgen receptor deletion [KS-AR-knockout (KO)] on Rhesus B glycoprotein (Rhbg) expression. A: cortical Rhbg expression. Cortical Rhbg abundance did not differ significantly between male (left) or female (right) wild-type (WT) and KS-AR-KO mice. B: inner stripe of the outer medulla (OMi) Rhbg expression. OMi Rhbg abundance was not significantly altered by KS-AR-KO in either male (left) or female (right) mice. P values indicate comparison of genotype and sex by two-way ANVOA or Student’s t test. n = 5 mice per group. NS, not significant.
Figure 10.
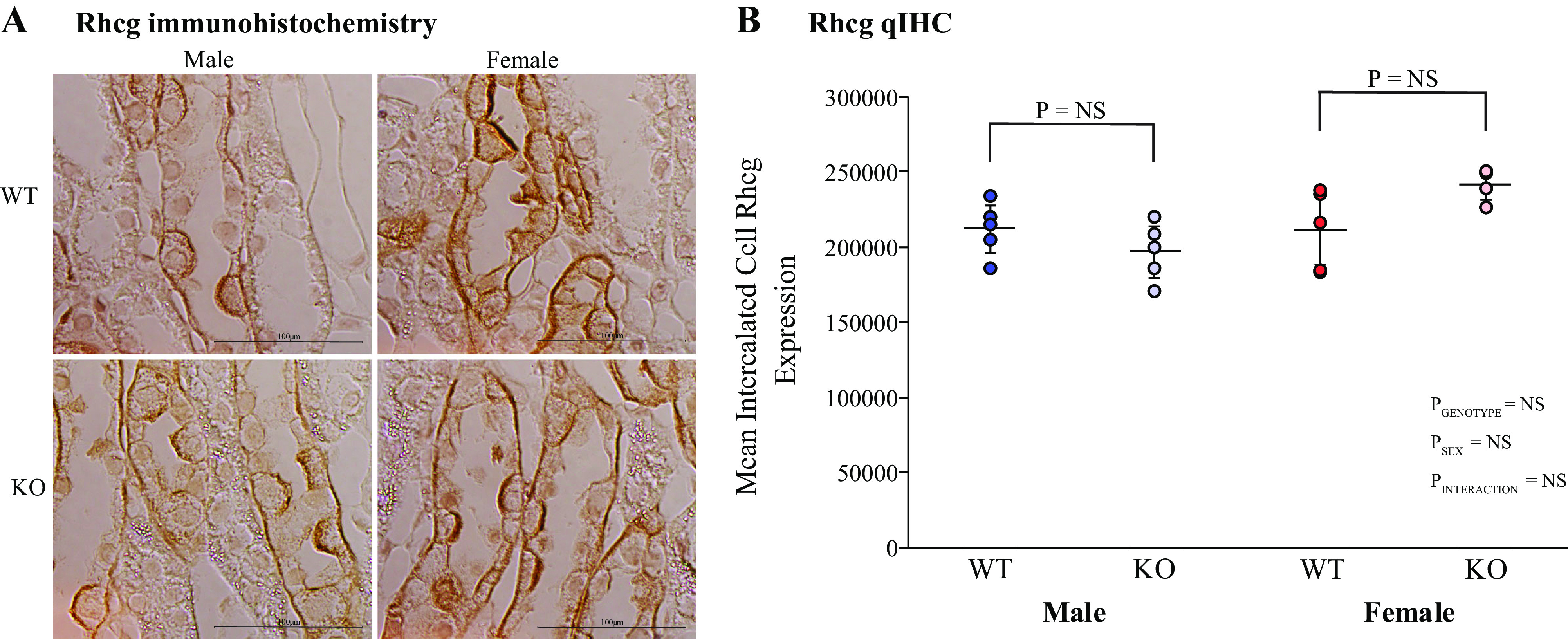
Effect of kidney-specific androgen receptor deletion [KS-AR-knockout (KO)] on Rhesus C glycoprotein (Rhcg) expression. A: inner stripe of the outer medulla (OMi) Rhcg immunohistochemistry. There was no detectable effect of KS-AR-KO on Rhcg immunolabel in either male or female mice. B: single cell quantitative immunohistochemistry (qIHC) Rhcg expression. Mean single cell Rhcg expression in OMi intercalated cells was determined using qIHC techniques. KS-AR-KO did not alter Rhcg expression significantly in either sex. P values indicate comparison of genotype and sex by two-way ANVOA or Student’s t test. Scale bars = 100 µm. n = 5 mice per group. NS, not significant; WT, wild type.
DISCUSSION
The present study provides important new information regarding the mechanism of how AR modulates sexual dimorphisms in the kidney. Because AR has numerous extrarenal roles, which could complicate understanding its role in the kidney, we created the first KS-AR-KO mouse. KS-AR-KO in male mice increased ammonia excretion, yet decreased total kidney size, proximal tubule volume density, and proximal tubule cell height, eliminating previously identified sex differences. The expression of multiple key proteins (PEPCK and NKCC2) involved in ammonia handling was increased by KS-AR-KO, likely contributing to the increased ammonia excretion. Simultaneously, expression of other proteins (NHE3 and NBCe1-A) were decreased, which will tend to blunt changes in ammonia excretion. KS-AR-KO had no detectable effect in female mice or in the collecting duct of either sex. Table 3 shows these effects. These findings suggest that renal ammonia handling and proximal tubule structure involve renal AR-dependent signaling pathways in the cortex of the male kidney but not the female kidney and not in the outer medulla of either sex.
Table 3.
Summary of renal AR deletion effects
| Parameter | Male Findings | Female Findings |
|---|---|---|
| Ammonia excretion | Increased by KO | No effect of KS-AR-KO |
| Kidney size | Decreased by KO | No effect of KS-AR-KO |
| Cortical proximal tubule volume density | Decreased by KO | No effect of KS-AR-KO |
| Collecting duct volume density | No effect of KS-AR-KO | No effect of KS-AR-KO |
| PEPCK expression | Increased KO | No effect of KS-AR-KO |
| Glutamine synthetase expression | No effect of KS-AR-KO | No effect of KS-AR-KO |
| NHE3 expression | Decreased by KO | No effect of KS-AR-KO |
| NKCC2 expression | Increased by KO | No effect of KS-AR-KO |
| NBCe1 and NBCe1-A expression | Decreased by KO | No effect of KS-AR-KO |
| Rhbg expression | No effect of KS-AR-KO | No effect of KS-AR-KO |
| Rhcg expression | No effect of KS-AR-KO | No effect of KS-AR-KO |
KS-AR-KO, kidney-specific androgen receptor (AR) knockout (deletion); NBCe1, electrogenic Na+-bicarbonate cotransporter; NHE3, Na+/H+ exchanger isoform 3; NKCC2, Na+-K+-2Cl− cotransporter; PEPCK, phosphoenolpyruvate carboxykinase; Rhbg, Rhesus B glycoprotein; Rhcg, Rhesus C glycoprotein.
The first major finding in this study is that AR expression mediates sex differences in renal size. Previous studies in humans, rats, and mice have shown that the male kidney is larger than the female kidney (3, 56, 57). The first evidence that this involved a testosterone-dependent signaling pathway was the observation that excess androgen administration qualitatively increased rat proximal tubule size (57). Further evidence in adult males suggests that there is a dose-dependent increase in kidney volume, with an estimated 4.04-cm3 increase per 100 mg of weekly testosterone supplementation (58). We have recently shown that male mice have greater kidney and proximal tubule size than do female mice (20, 22) and that these differences were testosterone dependent (22). Consistent with these previous findings, the present study demonstrates that in male mice, renal AR expression regulates both whole kidney and proximal tubule size. Moreover, KS-AR-KO results in male kidney size being the same as in females with intact AR expression. Thus, in male mice, testosterone acting through renal AR-dependent signaling pathways can entirely account for the sexual dimorphism in kidney and proximal tubule size.
The second major finding is that sex-based differences in ammonia excretion are mediated through AR-dependent pathways in male mice. Our previous studies showed that female mice excrete approximately twice as much urinary ammonia than do male mice (20–22), use different response pathways to respond to acid loading (21), and that the difference in basal ammonia excretion involves a testosterone-dependent mechanism (22). The present study extends these findings by showing that KS-AR-KO eliminates the sexual dimorphism in ammonia excretion. Alterations in dietary fixed acid loads can alter ammonia excretion, but changes in ammonia excretion in mice with AR deletion cannot be attributed to this mechanism as KS-AR-KO did not alter food intake. Most changes that occur in ammonia metabolism, such as occurs with metabolic acidosis or hypokalemia, result in changes in kidney and proximal tubule size that parallel ammonia excretion (59–61). However, male mice with renal AR deletion have increased ammonia excretion but decreased total kidney and proximal tubule size, indicating that the effect on ammonia excretion is not a nonspecific response to changes in renal mass. This incongruent renal size and ammonia response in response to renal AR deletion parallels findings in our previous studies using a gonadectomy model that evaluated the effect of testosterone (22). Thus, in male mice, testosterone acting through renal AR in males regulates ammonia excretion through pathways unrelated to effects on renal size, proximal tubule size, and endogenous acid loads related to food intake.
The majority of ammonia generation occurs in the proximal tubule. Our previous work has identified sexual dimorphisms in proximal tubule ammonia generation that are dependent on testis-derived testosterone (22). The results of the present study further extend these findings by showing that renal AR in male mice regulates expression of the ammonia-generating enzyme PEPCK but not the ammonia recycling enzyme GS. This effect on PEPCK expression is not a nonspecific effect related to changes in proximal tubule size, since the effects of renal AR deletion on proximal tubule size are the opposite of its effects on PEPCK expression. Moreover, the finding that renal AR deletion does not alter GS expression indicates that GS is regulated through mechanisms distinct from those used for PEPCK.
Ammonia produced in the proximal tubule is secreted preferentially into the luminal fluid through an apical NHE3-dependent mechanism (54, 55). Previous studies have shown that loss of testosterone decreases NHE3 expression (22, 62). The findings of the present study further extend these findings by showing that renal AR deletion in male mice decreases expression of NHE3. Thus, testosterone acting through AR activation appears to increase cortical proximal tubule NHE3 expression. However, it is important to recognize that these effects do not parallel ammonia excretion and instead would be predicted to minimize the observed changes in ammonia excretion. Instead, because NHE3 is also critical for filtered NaCl and water reabsorption, these effects may contribute to the greater proximal tubule NaCl reabsorption present in males (6, 7).
Ammonia reabsorption in the TAL establishes the interstitial ammonia gradient necessary for collecting duct ammonia secretion (63, 64). Previous studies from our group have shown that NKCC2 expression is greater in female mice than in male mice (20–22), that there is a sexual dimorphism in the response of NKCC2 to acid loading (21), and that the difference in basal NKCC2 expression involves a testosterone-dependent mechanism (22). The present study shows that loss of renal AR in male mice increased NKCC2 expression. The magnitude of this effect was almost identical to the difference we have identified between male and female mice (20, 21) and in the current study eliminated the sexual dimorphism of NKCC2 expression. Furthermore, the identified difference between male mice with intact renal AR and AR deletion parallels our previous identified differences between male mice with orchiectomy compared with intact and sham-operated controls (24). Thus, testosterone acting through AR appears to mediate the sexual dimorphism in NKCC2 expression. However, this regulation appears to be indirect, as there was no detectable AR expression in the TAL in either our previous report (22) or in the present study. One possible indirect mechanism explaining this observation is that in males, AR deletion leads to decreased proximal tubule size that in combination with decreased proximal tubule NHE3 expression increases increased solute delivery to the TAL, which stimulates NKCC2 expression.
Collecting duct ammonia secretion is the major determinant of urinary ammonia excretion, and the Rh glycoproteins Rhbg and Rhcg are the primary collecting duct ammonia transporters (16, 65–68). We have previously shown sexual dimorphisms in collecting duct volume density and expression of the Rh glycoproteins, with female mice having greater collecting duct volume density, intercalated cell size, and Rhbg and Rhcg expression (20). The present study found no detectable changes in collecting duct volume density or Rhbg and Rhcg expression in response to KS-AR-KO in either male or female mice. This parallels the lack of effect of testosterone deficiency, induced by orchiectomy, on collecting duct volume density, intercalated cell size, and Rhbg and Rhcg expression (22). We conclude that the sexual dimorphism in the collecting duct occurs through mechanisms not involving testosterone and AR-dependent signaling pathways.
The outer medulla appears to respond differently to sex, testosterone, and AR than does the cortex. In contrast to the renal cortex, in the outer medulla there is no sexual dimorphism in proximal tubule volume density or proximal tubule height (20), no effect of orchiectomy-induced testosterone deficiency on these parameters (22), and no effect of renal AR deletion (present study). This lack of effect of sex, testosterone, and AR expression is not due to absence of AR expression, as we have shown AR expression throughout the entire proximal tubule, including in the outer medulla (22). Understanding the mechanisms underlying this axial difference in the proximal tubule response to sex, testosterone, and AR will be an important avenue for future investigation.
A final observation is that AR deletion did not have detectable effects on the female kidney. AR is present throughout the entire proximal tubule in both sexes, and although expressed at lower levels in females, the absolute difference in expression was only ∼50% (22). In part, the lack of effect of AR deletion could indicate decreased AR activation by circulating testosterone, which is, as shown in the present study, substantially less in females than in males. However, immunohistochemistry indicates substantial nuclear AR localization in the female kidney (Ref. 22 and present study), which typically indicates activation of AR-dependent signaling processes. These observations suggest the possibility that other sex-dependent differences impair AR-dependent signaling mechanisms in the female proximal tubule.
Perspectives and Significance
A better understanding of the mechanism and biological implications of sex’s effect on renal structure and ammonia metabolism is critical for optimizing our ability to care for both men and women with acid-base disturbances. Despite the recent advances in our understanding of the effect of sex on ammonia metabolism and role of renal AR, additional studies are needed. There are certainly testosterone-independent effects of sex, the mechanism of which is incompletely understood at present. Whether this involves direct effects of other sex steroid hormones, such as estrogens or progesterone, indirect effects of proximal tubule AR activation, or effects of sex chromosomes that are independent of gonadal hormones will be important issues for future studies to address.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK45788 (to I.D.W.), R01DK107798 (to I.D.W. and J.W.V.), and 5T32DK104721 and K08DK120873 (to A.N.H.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.N.H. and I.D.W. conceived and designed research; A.N.H., R.A.C., H.-W.L., and J.W.V. performed experiments; A.N.H., R.A.C., H.-W.L., J.W.V. and I.D.W. analyzed data; A.N.H., R.A.C., J.W.V. and I.D.W. interpreted results of experiments; A.N.H. prepared figures; A.N.H. drafted manuscript; A.N.H., R.A.C., H.-W.L., J.W.V. and I.D.W. edited and revised manuscript; A.N.H., R.A.C., H.-W.L., J.W.V. and I.D.W. approved final version of manuscript
ACKNOWLEDGMENTS
We thank Dr. Sharon W. Matthews and Chao Chen (University of Florida, College of Medicine Electron Microscopy Core Laboratory) for excellent tissue processing for our immunohistochemical experiments. We thank The University of Virginia School of Medicine Center for Research in Reproduction Ligand Assay and Analysis Core for measurement of serum testosterone levels. We thank Dr. Gail Prins for supplying anti-AR antibodies and for the expertise in AR.
Footnotes
Ammonia exists in two molecular forms, NH3 and , which are in equilibrium with each other. When we use the term “ammonia,” we are referring to both molecular forms. If referring to a specific molecular form, we explicitly state either NH3 or
REFERENCES
- 1.Institute of Medicine Committee on Understanding the Biology of Sex and Gender D; Wizemann TM, Pardue ML (Editors). Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington, DC: National Academies Press, 2001. doi: 10.17226/10028. [DOI] [PubMed] [Google Scholar]
- 2.Karp NA, Mason J, Beaudet AL, Benjamini Y, Bower L, Braun RE, et al. Prevalence of sexual dimorphism in mammalian phenotypic traits. Nat Commun 8: 15475, 2017. doi: 10.1038/ncomms15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabolić I, Asif AR, Budach WE, Wanke C, Bahn A, Burckhardt G. Gender differences in kidney function. Pflugers Arch 455: 397–429, 2007. doi: 10.1007/s00424-007-0308-1. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens 31: 1247–1254, 2018. doi: 10.1093/ajh/hpy148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Layton AT, Sullivan JC. Recent advances in sex differences in kidney function. Am J Physiol Renal Physiol 316: F328–F331, 2019. doi: 10.1152/ajprenal.00584.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28: 3504–3517, 2017. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, McDonough AA, Layton HE, Layton AT. Functional implications of sexual dimorphism of transporter patterns along the rat proximal tubule: modeling and analysis. Am J Physiol Renal Physiol 315: F692–F700, 2018. doi: 10.1152/ajprenal.00171.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitch WE. Metabolic and clinical consequences of metabolic acidosis. J Nephrol 19 Suppl 9: S70–S75, 2006. [PubMed] [Google Scholar]
- 9.Hamm LL, Nakhoul N, Hering-Smith KS. Acid-base homeostasis. Clin J Am Soc Nephrol 10: 2232–2242, 2015. doi: 10.2215/CJN.07400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiner ID, Mitch WE, Sands JM. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol 10: 1444–1458, 2015. doi: 10.2215/CJN.10311013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wrong O, Davies HE. The excretion of acid in renal disease. Q J Med 28: 259–313, 1959. [PubMed] [Google Scholar]
- 12.Baertl JM, Sancetta SM, Gabuzda GJ. Relation of acute potassium depletion to renal ammonium metabolism in patients with cirrhosis. J Clin Invest 42: 696–706, 1963. doi: 10.1172/JCI104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maher T, Schambelan M, Kurtz I, Hulter HN, Jones JW, Sebastian A. Amelioration of metabolic acidosis by dietary potassium restriction in hyperkalemic patients with chronic renal insufficiency. J Lab Clin Med 103: 432–445, 1984. [PubMed] [Google Scholar]
- 14.Shear L, Gabuzda GJ. Potassium deficiency and endogenous ammonium overload from kidney. Am J Clin Nutr 23: 614–618, 1970. doi: 10.1093/ajcn/23.5.614. [DOI] [PubMed] [Google Scholar]
- 15.Tannen RL, McGill J. Influence of potassium on renal ammonia production. Am J Physiol 231: 1178–1184, 1976. doi: 10.1152/ajplegacy.1976.231.4.1178. [DOI] [PubMed] [Google Scholar]
- 16.Weiner ID, Verlander JW. Ammonia transporters and their role in acid-base balance. Physiol Rev 97: 465–494, 2017. doi: 10.1152/physrev.00011.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant 24: 1232–1237, 2009. doi: 10.1093/ndt/gfn633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Wehbe E, Raina R, Simon JF, Srinivas TR, Jain A, Schreiber MJ Jr, Nally JV Jr. Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin J Am Soc Nephrol 6: 2395–2402, 2011. doi: 10.2215/CJN.03730411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raphael KL, Murphy RA, Shlipak MG, Satterfield S, Huston HK, Sebastian A, Sellmeyer DE, Patel KV, Newman AB, Sarnak MJ, Ix JH, Fried LF; Health ABC Study. Bicarbonate concentration, acid-base status, and mortality in the health, aging, and body composition study. Clin J Am Soc Nephrol 11: 308–316, 2016. doi: 10.2215/CJN.06200615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris AN, Lee H-W, Osis G, Fang L, Webster KL, Verlander JW, Weiner ID. Differences in renal ammonia metabolism in male and female kidney. Am J Physiol Renal Physiol 315: F211–F222, 2018. doi: 10.1152/ajprenal.00084.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris AN, Lee HW, Fang L, Verlander JW, Weiner ID. Differences in acidosis-stimulated renal ammonia metabolism in the male and female kidney. Am J Physiol Renal Physiol 317: F890–F905, 2019. doi: 10.1152/ajprenal.00244.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris AN, Lee HW, Verlander JW, Weiner ID. Testosterone modulates renal ammonia metabolism. Am J Physiol Renal Physiol 318: F922–f935, 2020. doi: 10.1152/ajprenal.00560.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter I, Hay CW, Esswein B, Watt K, McEwan IJ. Tissue control of androgen action: the ups and downs of androgen receptor expression. Mol Cell Endocrinol 465: 27–35, 2018. doi: 10.1016/j.mce.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol 20: 3001–3015, 2002. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Grimont A, Bloch-Faure M, El Abida B, Crambert G. Mapping of sex hormone receptors and their modulators along the nephron of male and female mice. FEBS Lett 583: 1644–1648, 2009. doi: 10.1016/j.febslet.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Ruizeveld de Winter JA. Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. J Histochem Cytochem 42: 125–126, 1994. doi: 10.1177/42.1.8263324. [DOI] [PubMed] [Google Scholar]
- 27.Takeda H, Chodak G, Mutchnik S, Nakamoto T, Chang C. Immunohistochemical localization of androgen receptors with mono- and polyclonal antibodies to androgen receptor. J Endocrinol 126: 17–25, 1990. doi: 10.1677/joe.0.1260017. [DOI] [PubMed] [Google Scholar]
- 28.Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol 120: 51–57, 1996. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- 29.De Gendt K, Swinnen JV, Saunders PTK, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lécureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101: 1327–1332, 2004. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Gendt K, Verhoeven G. Tissue- and cell-specific functions of the androgen receptor revealed through conditional knockout models in mice. Mol Cell Endocrinol 352: 13–25, 2012. doi: 10.1016/j.mce.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Chen CV, Brummet JL, Jordan CL, Breedlove SM. Down, but not out: partial elimination of androgen receptors in the male mouse brain does not affect androgenic regulation of anxiety or HPA activity. Endocrinology 157: 764–773, 2016. doi: 10.1210/en.2015-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouchard M, Souabni A, Busslinger M. Tissue-specific expression of cre recombinase from the Pax8 locus. Genesis 38: 105–109, 2004. doi: 10.1002/gene.20008. [DOI] [PubMed] [Google Scholar]
- 33.Kim H-Y, Baylis C, Verlander JW, Han K-H, Reungjui S, Handlogten ME, Weiner DI. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293: F1238–F1247, 2007. doi: 10.1152/ajprenal.00151.2007. [DOI] [PubMed] [Google Scholar]
- 34.Bishop JM, Lee HW, Handlogten ME, Han KH, Verlander JW, Weiner ID. Intercalated cell-specific Rh B glycoprotein deletion diminishes renal ammonia excretion response to hypokalemia. Am J Physiol Renal Physiol 304: F422–F431, 2013. doi: 10.1152/ajprenal.00301.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HW, Verlander JW, Bishop JM, Handlogten ME, Han KH, Weiner ID. Renal ammonia excretion in response to hypokalemia: effect of collecting duct-specific Rh C glycoprotein deletion. Am J Physiol Renal Physiol 304: F410–F421, 2013. doi: 10.1152/ajprenal.00300.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HW, Verlander JW, Handlogten ME, Han KH, Weiner ID. Effect of collecting duct-specific deletion of both Rh B glycoprotein (Rhbg) and Rh C glycoprotein (Rhcg) on renal response to metabolic acidosis. Am J Physiol Renal Physiol 306: F389–F400, 2014. doi: 10.1152/ajprenal.00176.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verlander JW, Chu D, Lee HW, Handlogten ME, Weiner ID. Expression of glutamine synthetase in the mouse kidney: localization in multiple epithelial cell types and differential regulation by hypokalemia. Am J Physiol Renal Physiol 305: F701–F713, 2013. doi: 10.1152/ajprenal.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee H-W, Handlogten ME, Osis G, Clapp WL, Wakefield DN, Verlander JW, Weiner ID. Expression of sodium-dependent dicarboxylate transporter 1 (NaDC1/SLC13A2) in normal and neoplastic human kidney. Am J Physiol Renal Physiol 312: F427–F435, 2017. doi: 10.1152/ajprenal.00559.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han K-H, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim H.-Y, Handlogten ME, Weiner ID. Expression of the ammonia transporter, Rh C glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol 17: 2670–2679, 2006. doi: 10.1681/ASN.2006020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han K-H, Lee H-W, Handlogten ME, Whitehill F, Osis G, Croker BP, Clapp WI, Verlander JW, Weiner ID. Expression of the ammonia transporter family member, Rh B glycoprotein, in the human kidney. Am J Physiol Renal Physiol 304: F972–F981, 2013. doi: 10.1152/ajprenal.00550.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellhouse DR. Area estimation by point-counting techniques. Biometrics 37: 303–312, 1981. doi: 10.2307/2530419. [DOI] [Google Scholar]
- 42.Lee H-W, Osis G, Handlogten ME, Lamers WH, Chaudhry FA, Verlander JW, Weiner ID. Proximal tubule-specific glutamine synthetase deletion alters basal and acidosis-stimulated ammonia metabolism. Am J Physiol Renal Physiol 310: F1229–F1242, 2016. doi: 10.1152/ajprenal.00547.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto T, Sakari M, Okada M, Yokoyama A, Takahashi S, Kouzmenko A, Kato S. The androgen receptor in health and disease. Annu Rev Physiol 75: 201–224, 2013. doi: 10.1146/annurev-physiol-030212-183656. [DOI] [PubMed] [Google Scholar]
- 44.Curthoys NP, Moe OW. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol 9: 1627–1638, 2014. doi: 10.2215/CJN.10391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee HW, Osis G, Handlogten ME, Verlander JW, Weiner ID. Proximal tubule glutamine synthetase expression is necessary for the normal response to dietary protein restriction. Am J Physiol Renal Physiol 313: F116–F125, 2017. doi: 10.1152/ajprenal.00048.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conjard A, Komaty O, Delage H, Boghossian M, Martin M, Ferrier B, Baverel G. Inhibition of glutamine synthetase in the mouse kidney: a novel mechanism of adaptation to metabolic acidosis. J Biol Chem 278: 38159–38166, 2003. doi: 10.1074/jbc.M302885200. [DOI] [PubMed] [Google Scholar]
- 47.Lee H-W, Harris AN, Romero MF, Welling PA, Wingo CS, Verlander JW, Weiner ID. NBCe1-A is required for the renal ammonia and K+ response to hypokalemia. Am J Physiol Renal Physiol 318: F402–F421, 2020. doi: 10.1152/ajprenal.00481.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H-W, Osis G, Harris AN, Fang L, Romero MF, Handlogten ME, Verlander JW, Weiner ID. NBCe1-A regulates proximal tubule ammonia metabolism under basal conditions and in response to metabolic acidosis. J Am Soc Nephrol 29: 1182–1197, 2018. doi: 10.1681/ASN.2017080935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Handlogten ME, Osis G, Lee HW, Romero MF, Verlander JW, Weiner ID. NBCe1 expression is required for normal renal ammonia metabolism. Am J Physiol Renal Physiol 309: F658–F666, 2015. doi: 10.1152/ajprenal.00219.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang L, Lee H-W, Chen C, Harris AN, Romero MF, Verlander JW, Weiner ID. Expression of the B splice variant of NBCe1 (SLC4A4) in the mouse kidney. Am J Physiol Renal Physiol 315: F417–F428, 2018. doi: 10.1152/ajprenal.00515.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brandes A, Oehlke O, Schümann A, Heidrich S, Thévenod F, Roussa E. Adaptive redistribution of NBCe1-A and NBCe1-B in rat kidney proximal tubule and striated ducts of salivary glands during acid-base disturbances. Am J Physiol Regul Integr Comp Physiol 293: R2400–R2411, 2007. doi: 10.1152/ajpregu.00208.2007. [DOI] [PubMed] [Google Scholar]
- 52.Weiner ID, Verlander JW. Emerging features of ammonia metabolism and transport in acid-base balance. Semin Nephrol 39: 394–405, 2019. doi: 10.1016/j.semnephrol.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laghmani K, Preisig PA, Moe OW, Yanagisawa M, Alpern RJ. Endothelin-1/endothelin-B receptor-mediated increases in NHE3 activity in chronic metabolic acidosis. J Clin Invest 107: 1563–1569, 2001. doi: 10.1172/JCI11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagami GT. Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J Clin Invest 81: 159–164, 1988. doi: 10.1172/JCI113287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagami GT. Role of angiotensin II in the enhancement of ammonia production and secretion by the proximal tubule in metabolic acidosis. Am J Physiol Renal Physiol 294: F874–F880, 2008. doi: 10.1152/ajprenal.00286.2007. [DOI] [PubMed] [Google Scholar]
- 56.Mackay EM, Raulston BO. factors which determine renal weight: xi. renal function. J Exp Med 53: 109–113, 1931. doi: 10.1084/jem.53.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Selye H. The effect of testosterone on the kidney. J Urol 42: 637–641, 1939. doi: 10.1016/S0022-5347(17)71560-1. [DOI] [Google Scholar]
- 58.Gagliano-Jucá T, Tang ER, Bhasin S, Pencina KM, Anderson S, Jara H, Li Z, Melamud K, Coleman SL, Aakil A, Almeida RR, Huang G, Travison TG, Storer TW, Basaria S. Effects of testosterone administration (and its 5-alpha-reduction) on parenchymal organ volumes in healthy young men: findings from a dose-response trial. Andrology 5: 889–897, 2017. doi: 10.1111/andr.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Throssell D, Harris KP, Bevington A, Furness PN, Howie AJ, Walls J. Renal effects of metabolic acidosis in the normal rat. Nephron 73: 450–455, 1996. doi: 10.1159/000189109. [DOI] [PubMed] [Google Scholar]
- 60.Toback FG, Ordónez NG, Bortz SL, Spargo BH. Zonal changes in renal structure and phospholipid metabolism in potassium-deficient rats. Lab Invest 34: 115–124, 1976. [PubMed] [Google Scholar]
- 61.Wang Q, Domenighetti AA, Pedrazzini T, Burnier M. Potassium supplementation reduces cardiac and renal hypertrophy independent of blood pressure in DOCA/salt mice. Hypertension 46: 547–554, 2005. doi: 10.1161/01.HYP.0000178572.63064.73. [DOI] [PubMed] [Google Scholar]
- 62.Quan A, Chakravarty S, Chen J-K, Chen J-C, Loleh S, Saini N, Harris RC, Capdevila J, Quigley R. Androgens augment proximal tubule transport. Am J Physiol Renal Physiol 287: F452–F459, 2004. doi: 10.1152/ajprenal.00188.2003. [DOI] [PubMed] [Google Scholar]
- 63.Good DW. Ammonium transport by the thick ascending limb of Henle's loop. Annu Rev Physiol 56: 623–647, 1994. doi: 10.1146/annurev.ph.56.030194.003203. [DOI] [PubMed] [Google Scholar]
- 64.Kinne R, Kinne-Saffran E, Schütz H, Scholermann B. Ammonium transport in medullary thick ascending limb of rabbit kidney: involvement of the Na+,K+,Cl−-cotransporter. J Membr Biol 94: 279–284, 1986. doi: 10.1007/BF01869723. [DOI] [PubMed] [Google Scholar]
- 65.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007. doi: 10.1146/annurev.physiol.69.040705.142215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpire r J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008. doi: 10.1038/nature07518. [DOI] [PubMed] [Google Scholar]
- 67.Bourgeois S, Bounoure L, Christensen EI, Ramakrishnan SK, Houillier P, Devuyst O, Wagner CA. Haploinsufficiency of the ammonia transporter Rhcg predisposes to chronic acidosis: Rhcg is critical for apical and basolateral ammonia transport in the mouse collecting duct. J Biol Chem 288: 5518–5529, 2013. doi: 10.1074/jbc.M112.441782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim H-Y, Verlander JW, Bishop JM, Cain BD, Han K-H, Igarashi P, Lee H-W, Handlogten ME, Weiner ID . Basolateral expression of the ammonia transporter family member Rh C glycoprotein in the mouse kidney. Am J Physiol Renal Physiol 296: F543–F555, 2009. 5. doi: 10.1152/ajprenal.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]