Abstract
Yorkshire swine were fed standard diet (n = 7) or standard diet containing applesauce rich in caffeic acid with Lactobacillus plantarum (n = 7) for 3 wk. An ameroid constrictor was next placed around the left coronary circumflex artery, and the dietary regimens were continued. At 14 wk, cardiac function, myocardial perfusion, vascular density, and molecular signaling in ischemic myocardium were evaluated. The L. plantarum-applesauce augmented NF-E2-related factor 2 (Nrf2) in the ischemic myocardium and induced Nrf2-regulated antioxidant enzymes heme oxygenase-1 (HO-1), NADPH dehydrogenase quinone 1 (NQO-1), and thioredoxin reductase (TRXR-1). Improved left ventricular diastolic function and decreased myocardial collagen expression were seen in animals receiving the L. plantarum-applesauce supplements. The expression of endothelial nitric oxide synthase (eNOS) was increased in ischemic myocardial tissue of the treatment group, whereas levels of asymmetric dimethyl arginine (ADMA), hypoxia inducible factor 1α (HIF-1α), and phosphorylated MAPK (pMAPK) were decreased. Collateral-dependent myocardial perfusion was unaffected, whereas arteriolar and capillary densities were reduced as determined by α-smooth muscle cell actin and CD31 immunofluorescence in ischemic myocardial tissue. Dietary supplementation with L. plantarum-applesauce is a safe and effective method of enhancing Nrf2-mediated antioxidant signaling cascade in ischemic myocardium. Although this experimental diet was associated with a reduction in hypoxic stimuli, decreased vascular density, and without any change in collateral-dependent perfusion, the net effect of an increase in antioxidant activity and eNOS expression resulted in improvement in diastolic function.
NEW & NOTEWORTHY Colonization of the gut microbiome with certain strains of L. Plantarum has been shown to convert caffeic acid readily available in applesauce to 4-vinyl-catechol, a potent activator of the Nrf2 antioxidant defense pathway. In this exciting study, we show that simple dietary supplementation with L. Plantarum-applesauce-mediated Nrf2 activation supports vascular function, ameliorates myocardial ischemic diastolic dysfunction, and upregulates expression of eNOS.
Keywords: antioxidant, chronic myocardial ischemia, diastolic function, endothelial function, probiotic
INTRODUCTION
Availability of ambient oxygen for aerobic respiration has likely been the basis for evolution of complex forms of life. However, given that oxygen exists in the atmosphere at toxic concentrations, an inability to regulate the intake and transport of oxygen or neutralize oxygen free radicals would culminate in irreversible cell injury (1).
Oxidative homeostasis entails precise control systems that are capable of responding to various forms of oxidative stress by mounting self-limiting effector responses. NF-E2-related factor 2 (Nrf2) is a redox-sensitive transcription factor that is well recognized as a key regulator of antioxidant mechanisms (2). Upon “sensing” redox stress, Nrf2 translocates to the nucleus and binds to enhancer sequences in promoter regions known as antioxidant response elements (AREs), inducing the expression of nearly 250 genes. This battery of genes encodes a myriad of antioxidant, cytoprotective, and immunomodulatory enzymes that coordinate the cellular response to a variety of stressors (3, 4).
Although interest in Nrf2 as a potential therapeutic target was initially limited by theoretical concerns over specificity, further insight into the structural basis of Nrf2 complex stimulation demonstrated targeted responsiveness to oxidative stimuli (5). This provided impetus for the development of many Nrf2 activating drugs, primarily used for chronic autoimmune/inflammatory diseases for which curative therapy remains nonexistent (6). However, these activators are synthetic compounds whose adverse effects and toxicity profiles continue to be problematic (7).
Interestingly, certain Lactobacillus species found in traditionally fermented foods and beverages provide a sustainable source of alkyl catechol that are natural inducers of Nrf2. In particular, L. plantarum expresses the enzyme phenolic acid decarboxylase, which converts caffeic acid or 3,4-dihydroxybenzoic (found in applesauce, vegetables, and coffee) to 4-vinyl-catechol and catechol, respectively. Indeed, alkyl catechol-mediated activation of Nrf2 would explain the putative antioxidant properties and overall health benefits attributed to traditional diets (8).
It is now widely recognized that Nrf2 directs a comprehensive response to oxidative stress and inflammation that confers protection against many pathological conditions including cancer, autoimmune disorders, and neurodegenerative diseases (9). Nrf2 activators were shown to be protective in small animal models of ischemia-reperfusion injury, heart failure, and atherosclerosis (10). However, the scope of Nrf2 signaling and the implications of potential therapeutic activation in the chronically ischemic myocardium are yet to be elucidated. The objectives of this study were twofold. First, we aimed to determine if dietary supplementation of applesauce (rich in caffeic acid) (11, 12) and L. plantarum probiotics would generate sufficient quantities of alkyl-catechol to augment Nrf2 signaling in a translational swine model of chronic myocardial ischemia. Second, we sought to examine the effects of Nrf2 activation on the overall cardiac function and collateral-dependent perfusion in the ischemic myocardium. Importantly, gross functional outcomes were assessed by studying cardiovascular hemodynamic parameters.
MATERIALS AND METHODS
Animal Model and Dietary Regimen
Fourteen Yorkshire swine (castrated males n = 6, females n = 8) aged 8 to 9 wk were separated into two groups; controls (n = 7; males n = 3 and females n = 4) received a standard chow diet (Envigo Teklad mini-swine diet 8753) with 10 mL of 10% ethanol vehicle daily, whereas treatment animals (n = 7; females n = 4 and males n = 3) were fed organic, unsweetened applesauce (365, 0.7% of total body wt) and L. plantarum supplements [Latarum-1 capsule for <50 kg body wt, 2 capsules/day (>10 million organisms/capsule) when animals exceeded 50 kg] containing a strain verified specifically for expression of active phenolic acid decarboxylase and conversion of caffeic acid to 4-vinyl-catechol. The daily amount of applesauce that was fed to the animals was determined as the minimum quantity sufficient to provide a source of caffeic acid while not imposing a caloric burden that would significantly alter body weight and metabolic features (13). Dosing of L. plantarum supplements was done by approximating human dosage indications provided by the manufacturer to the body weight of experimental animals.
Surgical Procedures and Hemodynamic Studies
The animals were allowed 2 wk on the dietary regimens mentioned above before having an ameroid constrictor surgically placed around their left circumflex coronary artery (LCx) as previously described (14–16). This induces chronic myocardial ischemia by slowly occluding the artery over a period of 10–20 days (17). Subsequently, the chest was entered through a lateral thoracotomy at the level of the fourth intercostal space. The lung tissue was retracted and the pericardium opened to expose the left atrioventricular (A-V) groove. The LCx was next dissected free in the left A-V groove and tracked to its proximal take-off from the left main coronary artery. The LCx was then secured with vessel loops before drawing 10 mL of arterial blood from the left atrium using a sterile 10-gauge syringe. We then tightened the vessel loops, commencing a 2-min occlusion of the LCx which produced observable ST-elevations on electrocardiographic monitoring. Concomitantly, 5 mL of gold microspheres (BioPal, Worcester, MA) were injected into the left atrial appendage. The vessel loops were then released and 1 mL of nitroglycerine was applied locally over the LCx to relieve spasm and allow for accurate sizing of an ameroid constrictor (Research Instruments SW, Escondido, CA). The constrictor was carefully applied to the circumference of the artery and the incision was closed in layers.
Corresponding diets were then administered for a period of 12–14 wk. Finally, invasive hemodynamic assessment and perfusion studies were undertaken before the animals were euthanized. Animal care and procedures were conducted alongside veterinary staff, per Principles of Laboratory Animal Care guidelines by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals (18) and in accordance with the approved protocol by the Institutional Animal Care and Use Committee (IACUC) of Rhode Island Hospital (Lifespan, CMTT No. 505318).
Measures of Myocardial Blood Flow
Ten small segments of left ventricular tissue obtained from each animal were categorized as ischemic (4 segments from the territorial distribution of the LCx), nonischemic (4 segments from the territorial distribution of the LAD), or transitional (2 segments from a borderline territory between the LAD and LCx). Biophysics Assay Laboratory (Biophysics, Worcester, MA) protocols were followed for preparation of tissue and blood specimens; briefly, the wet weight of ventricular specimens was recorded and whole blood samples were collected throughout the experiments before heating the specimens in a 65°C oven overnight. The samples were then delivered to Biophysics Assay Laboratory for quantification of microsphere content. Ischemic territories were confirmed to contain the least amount of gold microspheres. Collateral-dependent perfusion to ischemic territories was assessed as both absolute blood flow (ABF) and ratio of ischemic territory perfusion to that of nonischemic ventricle (AAR/NV).
Analysis of Endothelial and Arterial Smooth Muscle Density by Immunofluorescence
Ischemic ventricular tissue was fixed in 10% formalin for 24 h before being transferred to 70% ethanol. The tissue was then paraffinized, sectioned into 5-μm segments, and prepared on charged slides. Following deparaffinization, the slides were stained for α-SMA (Cell Signaling, Danvers, MA), endothelial-marker CD31 (Abcam, Cambridge, UK), isolectin B4 (Vector Laboratories, Burlingame, CA), and vimentin (Abcam, Cambridge, UK).
Six randomly selected RGB images per slide (8 bit) were acquired with a Nikon E800 microscope (Nikon Inc. Mellville, NY) using a ×20 Plan Apo objective. Images were acquired with a Spot RT3 digital camera (Diagnostic Instruments, Sterling Heights, MI). Each channel was acquired separately and merged to an RGB image using Spot RT3 advanced software version 4.7. Image processing and analysis was performed in ImageJ software (National Institutes of Health). The split channel command was used to look at each stain separately. Positive staining was defined through intensity thresholding, and the resulting binary images were analyzed for total area. Images were calibrated so that the results are expressed as either individual CD31, α-SMA, isolectin B4, or vimentin area per total square millimeters. Vessel counts were performed on the RGB image in Adobe Photoshop CS6.
PicroSirius Red Stain
Paraffin-fixed tissue sections (5 μm thick) were prepared and mounted on slides for PicroSirius red staining (Polysciences, Inc., Warrington, PA). The slides were deparaffinized, baked, and rehydrated. Weigert’s hematoxylin stain was applied next, and the slides were washed and rinsed. We then applied PicroSirius stain for 1 h and washed the slides using acidified water. Then 100% ethanol was used to dehydrate the tissue samples that were subsequently cleared in xylene and mounted in a resinous medium. Three red, green, and blue images were acquired per specimen with a Nikon E800 microscope (Nikon, Inc., Melville, NY) using a ×10 Plan Apo objective and a Spot RT3 camera (Diagnostic Instruments, Sterling Heights, MI). Image processing was performed on QuPath software (19) where five areas were randomly selected at 15×, then transferred to ImageJ (20) for analysis. Positive staining was defined by thresholding the red component of the stain, and percentage area was calculated for five randomly selected areas of each sample.
Quantification of Protein Expression
Protein (40 μg) was extracted from flash-frozen and homogenized ischemic left ventricular tissue lysates, separated using 4%–20% mini-PROTEAN TGX gels (Biorad, Hercules, CA), and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). Subsequently, the membranes were immersed in blocking buffer (Thermo Fisher Scientific, Waltham, MA) for 1 h, washed, and incubated overnight at 4°C with a 1:1,000 dilution of primary antibodies against Nrf2 (Cell signaling technology, Danvers, MA, Cat. No. 12721), heme-oxygenase 1 (HO-1; Cell Signaling Technology, Danvers, MA, Cat. No. 43966), glutathione peroxidase (GPX-1; Cell Signaling Technology, Danvers, MA, Cat. No. 3206), thioredoxin reductase (TRXR1; Cell Signaling Technology, Danvers, MA, Cat. No. 15140), NADPH dehydrogenase quinone 1 (NQO-1; Cell Signaling Technology, Danvers, MA, Cat. No. 62262), copper zinc superoxide dismutase (Cu ZnSOD; Cell Signaling Technology, Danvers, MA, Cat. No. 2770), manganese superoxide dismutase (MnSOD or SOD2; Cell Signaling Technology, Danvers, MA, Cat. No. 13141), endothelial nitric oxide synthase (eNOS; Cell Signaling Technology, Danvers, MA, Cat. No. 9572), phosphorylated endothelial nitric oxide synthase (p-eNOS; Cell Signaling Technology, Danvers, MA, Cat. No. 9570), asymmetric dimethyl arginine (ADMA; Cell Signaling Technology, Danvers, MA, Cat. No. 13522), hypoxia inducible factor 1α (HIF-1α; Cell Signaling Technology, Danvers, MA, Cat. No. 14179), protein kinase B (AKT; Cell Signaling Technology, Danvers, MA, Cat. No. 9272), phosphorylated protein kinase B (pAKT; Cell Signaling Technology, Danvers, MA, Cat. No. 4060), mitogen activated protein kinase (MAPK; Cell Signaling Technology, Danvers, MA, Cat. No. 8690), phosphorylated MAPK (p-MAPK; Cell Signaling Technology, Danvers, MA, Cat. No. 5759), and transforming growth factor β (TGF-β; Cell Signaling Technology, Danvers, MA, Cat. No. 3711). GAPDH antibody (1:3,000 dilution, Cell Signaling Technology, Danvers, MA, Cat. No. 5174) was used as a loading control for all studies. The following day, primary antibody solution was rinsed off and either Goat anti-rabbit or anti-mouse-horseradish peroxidase (HRP) secondary antibody (1:10,000 dilution, Cell Signaling Technology, Danvers, MA, Cat. No. 7076) was applied to the membranes and left for 1 to 2 h. Immune complexes were visualized using Clarity Western ECL Blotting Substrate (BioRad, Hercules, CA) and validated according to the molecular weight, and the band intensity was quantified by ChemiDoc MP Imaging System.
Metabolic Parameters
Before the final harvest procedure, the weight and length of the animals were measured and a glucose tolerance test was done where 0.5 g/kg of dextrose 50% was injected, and blood glucose measurements were recorded at 30 and 60 min. Blood (5 mL) was next collected from the left atrium in ethylenediaminetetraacetic acid (EDTA) tubes. The whole blood specimens were then centrifuged, and the plasma supernatant was extracted and sent to IDEXX BioAnalytics laboratory (Atlanta, GA) for a comprehensive metabolic analysis, which includes levels of glucose, lipids, and hepatic enzymes.
Statistical Analysis
Analysis of previous protocols using a two-tailed α-level of 0.01, a B-error level of 0.10, and the known standard deviation −0.15 determined that the minimal sample size needed to yield statistically significant results to be seven animals per group. Datasets from the two cohorts were analyzed by means of a Mann–Whitney U test using GraphPad Prism 7.04 Software (GraphPad Software, Inc., San Diego, CA). Results are presented as means ± SE of each group (controls-NDC; L. plantarum-caffeic acid dietary group-ND-LP).
RESULTS
Metabolic Parameters
There were no significant differences in body mass index nor glucose tolerance between the two cohorts. Similarly, serum levels of albumin, alanine transferase (ALT), blood urea nitrogen (BUN), γ-glutamyl transferase (GGT), total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglycerides (TG), and thyroxine (T4) were not significantly affected by the experimental dietary regimen (Table 1).
Table 1.
Lactobacillus plantarum caffeic acid dietary regimen compared with the standard control diet
| Control | L. Plantarum-Caffeic Acid Treated | P Value | |
|---|---|---|---|
| Glucose, mg/dL* | 246 ± 40.60 | 246.67 ± 48.56 | 0.53 |
| Total cholesterol, mg/dL | 87.71429 ± 12.34868 | 88.33333 ± 5.374838 | 0.70 |
| LDL cholesterol, mg/dL | 42 ± 6.68 | 42.67 ± 2.43 | 0.86 |
| HDL cholesterol, mg/dL | 39.14 ± 4.82 | 39.33 ± 3.14 | 0.86 |
| Triglycerides, mg/dL | 20.43 ± 7.91 | 18.83 ± 8.19 | 0.81 |
| Albumin, g/dL | 3.1 ± 0.32 | 3.1 ± 0.29 | 0.86 |
| ALT, U/L | 53.43 ± 7.29 | 52.5 ± 8.52 | 0.75 |
| GGT, U/L | 29.83 ± 8.29 | 39.4 ± 12.47 | 0.45 |
| BUN, mg/dL | 9.29 ± 1.39 | 10.5 ± 3.64 | 0.56 |
| Thyroxine, µg/dL | 3.44 ± 0.57 | 3.42 ± 0.33 | 0.81 |
Values are means ± SE. ALT, alanine transferase; BUN, blood urea nitrogen; GGT, γ-glutamyl transferase; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol. *Glucose measured following administration of 0.5 mg/kg-glucose tolerance test. There were no significant differences in metabolic parameters attributable to the L. plantarum-caffeic acid dietary regimen compared with the standard (control) diet.
Cardiac Function and Myocardial Perfusion
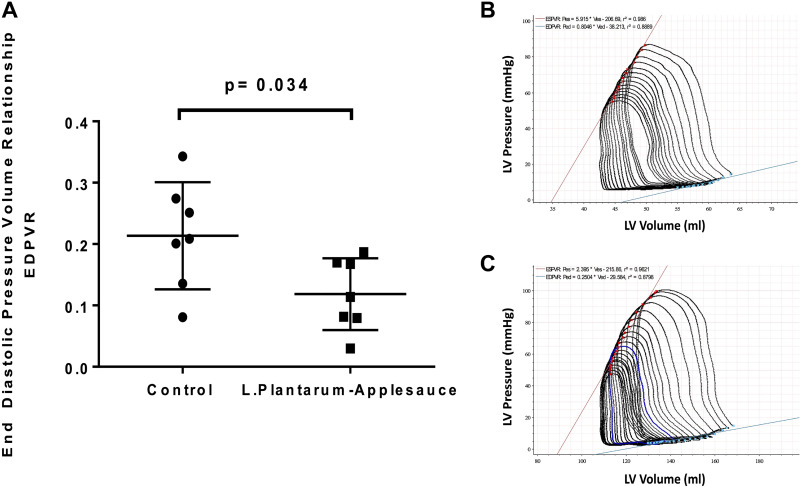
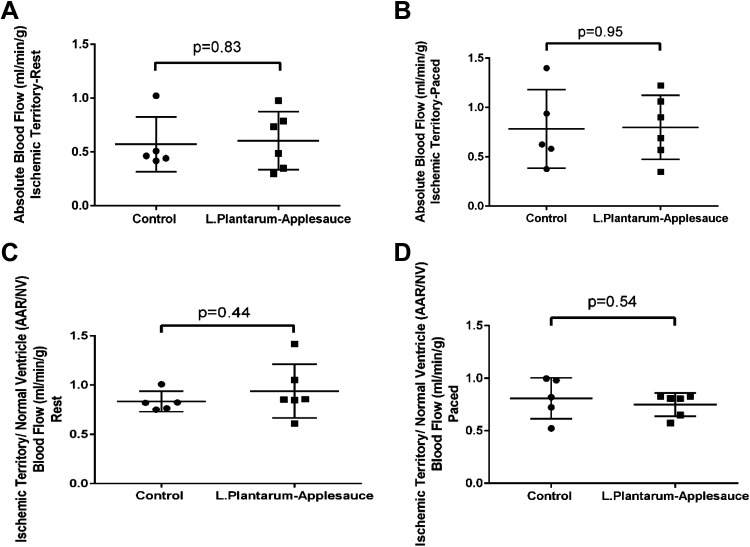
A significant (P = 0.03) reduction in end-diastolic pressure-volume relationship (EDPVR) was observed in animals that received the L. plantarum-caffeic acid diet (Fig. 1, A–C), reflecting improved myocardial relaxation in this cohort. EDPVR is a measure of the myocardium’s elasticity or stiffness when the left ventricle is maximally filled, that is, relaxed; it is measured as the slope of the pressure-volume relationship at end diastole. However, indices of contractility including stroke volume, cardiac output, and end-systolic pressure-volume relationship (ESPVR) were not significantly altered by this treatment. We did not find a significant change in absolute blood flow (ABF) or ratio of ischemic territory perfusion to that of nonischemic ventricle (AAR/NV) (Fig. 2, A–D) between cohorts that would support an effect of the L. plantarum-caffeic acid diet on collateral-dependent perfusion to ischemic territories.
Figure 1.
The Lactobacillus plantarum-caffeic acid diet was associated with significant decline in end-diastolic pressure-volume resistance (A) reflective of enhanced myocardial relaxation and diastolic performance in the treated group. These data were acquired by assessment of multiple indices of hemodynamic performance and extrapolation of pressure-volume loops for each of the control controls (n = 7; males n = 3 and females n = 4) (B) and treatment controls (n = 7; males n = 3 and females n = 4) (C) animal groups. Mann–Whitney t test was used for statistical analysis.
Figure 2.
Lactobacillus plantarum-caffeic acid dietary regimen does not have a significant effect on absolute blood flow to the ischemic myocardial region. Conditions shown are under resting (A) and paced (B). Similarly, the perfusion ratio to ischemic territory vs. nonischemic ventricle were unchanged at basal heart rate (C) and while pacing at 150 beats/min (D) between experimental cohorts (n = 7; males n = 3 and females n = 4) and controls (n = 7; males n = 3 and females n = 4) for each group; P values shown for each panel. Mann–Whitney t test was used for statistical analysis.
Myocardial Vascular Density and Fibrosis
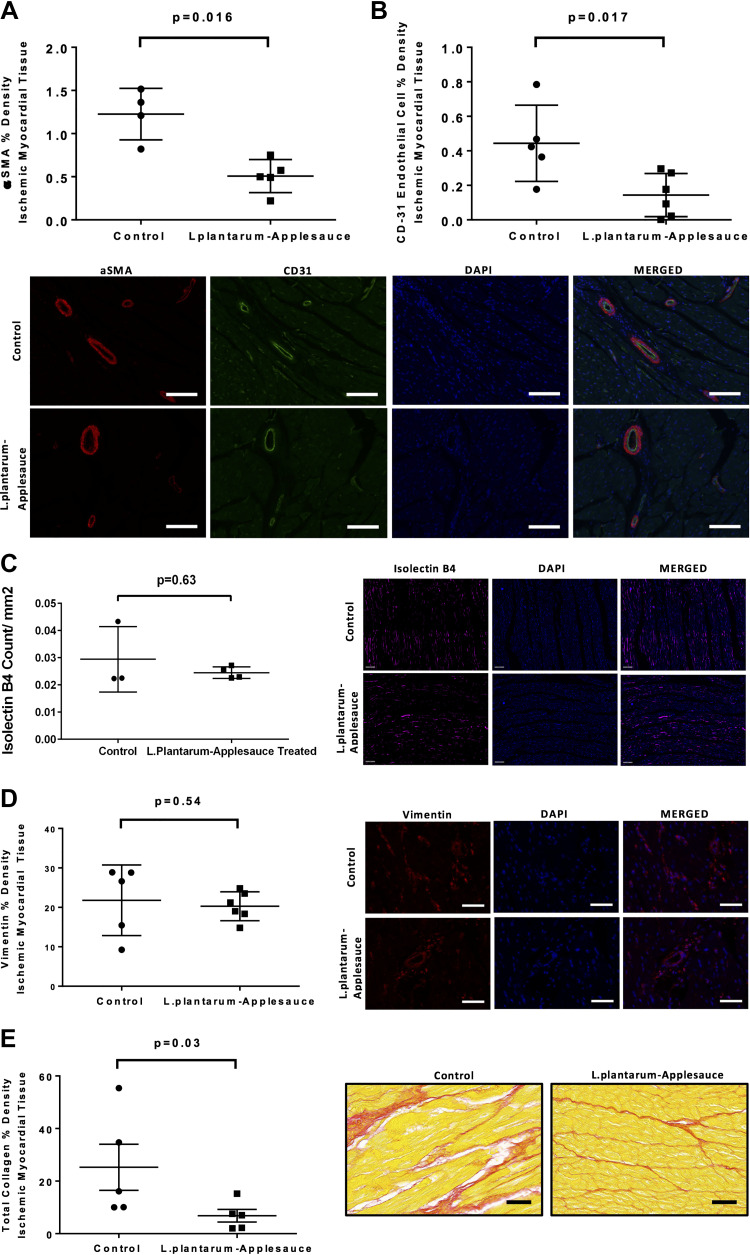
The L. plantarum and caffeic acid dietary regimen was associated with a significant reduction in the density of α-SMA (P = 0.016; Fig. 3A) and CD31 (P = 0.017; Fig. 3B) endothelial cells in ischemic myocardial tissue. Animals in the experimental dietary cohort exhibited decreased myocardial collagen expression in ischemic myocardial territories as compared with controls (P = 0.03; Fig. 3E). Density of isolectin B4 (P = 0.63; Fig. 3C), vimentin (P = 0.54; Fig. 3D), and expression of TGF-β (Supplemental File; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.15069885.v1) in ischemic myocardial tissue were not significantly altered between the two groups.
Figure 3.
Quantification of ischemic myocardial densities of endothelial α-SMA and CD-31 by immunofluorescence. α-SMA (A) and CD31 (B) endothelial cell densities were significantly less in ischemic myocardial tissue of animals that received the Lactobacillus plantarum-caffeic acid diet. Animals that received the experimental diet showed no significance in isolectin B4 density (C) nor the density of vimentin to be significantly altered between the two groups (D), whereas myocardial collagen expression was significantly reduced. Scale bars for representative images are 100 μm (A and B) and 50 μm (C–E).
Antioxidant and Proangiogenic Signaling in the Ischemic Myocardium
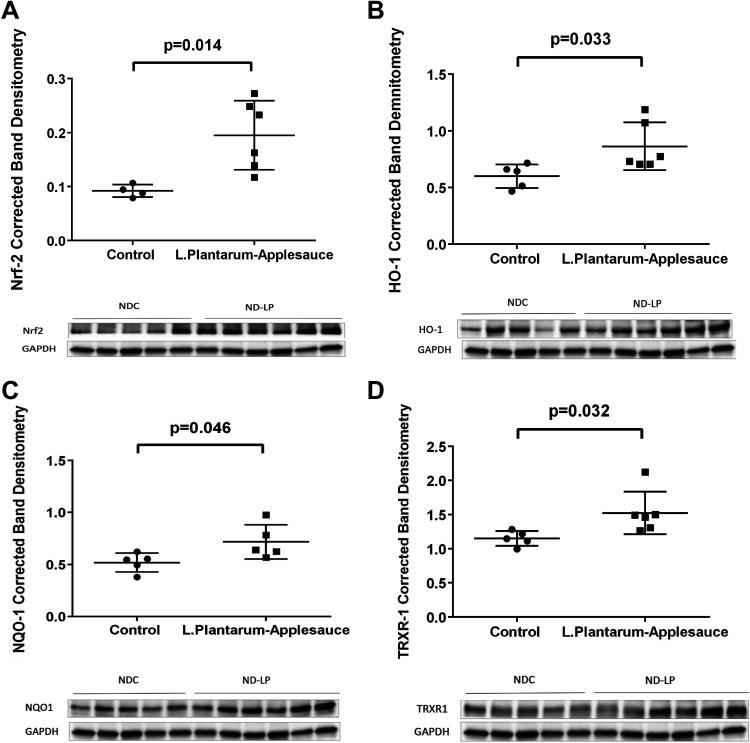
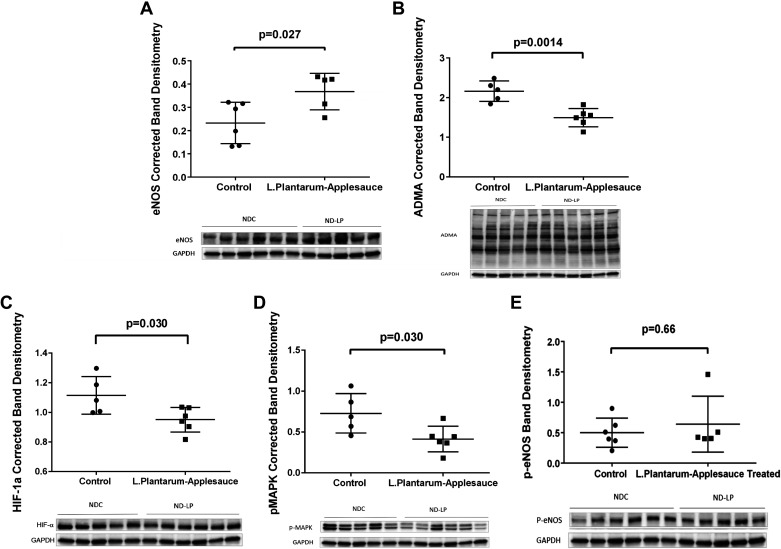
Dietary supplementation with L. plantarum and caffeic acid produced robust activation of the Nrf-2 pathway in ischemic myocardial tissue. In previous work by our collaborator Dr. Senger and colleagues (8), it was shown that human coronary artery endothelial cells (HCAEs) incubated with only this specific strain of L. plantarum did not show appreciable increase in the levels of 4-vinyl-catechol or downstream Nrf2 activity. Alternatively, addition of caffeic acid to the aforementioned culture media saw significant expression of Nrf2 target proteins which was associated with a sixfold increase in levels of 4-vinyl-catechol (8). Based on these data, we deemed that it was in the interest of cost effectiveness and avoidance of unnecessary experimentation with large animal to exclude a third control cohort examining the effects of only L. plantarum this model which would not undermine the scientific rationale of the findings presented herein. Expressions of Nrf2 (P = 0.014; Fig. 4A), HO-1 (P = 0.033; Fig. 4B), NQO-1 (P = 0.046; Fig. 4C), and TRXR-1 (P = 0.032; Fig. 4D) were all upregulated in ischemic myocardial lysates. Heightened Nrf2 signaling was associated with greater expression of total eNOS (P = 0.027; Fig. 5A). The expression of HIF-1α (P = 0.03; Fig. 5C) and pMAPK (P = 0.03; Fig. 5D) and the concentration of ADMA (P = 0.0014; Fig. 5B) were reduced in ischemic myocardial lysates from animals that received the L. plantarum-caffeic acid diet. Levels of phosphorylated e-NOS (P = 0.66; Fig. 5E), protein kinase B (Akt), and phosphorylated Akt (data not shown) in ischemic myocardial lysates were not affected by this dietary regimen.
Figure 4.
Nrf-2-mediated expression of antioxidant proteins in ischemic myocardium. Significant upregulation of Nrf2 signaling is seen in the Lactobacillus plantarum-caffeic acid cohort (n = 7; males n = 3 and females n = 4). Ischemic myocardial lysates from treated animals exhibit greater expression of Nrf2 (A), as well as important downstream antioxidant enzymes including HO-1 (B), NQO-1 (C), and TRXR-1 (D) with respect to controls (n = 7; males n = 3 and females n = 4). Mann–Whitney t test was used for statistical analysis. HO-1, heme oxygenase 1; NDC, normal-diet control; NQO-1, NADPH dehydrogenase quinone 1; Nrf2, nuclear factor erythroid 2-related factor 2; TRXR-1, thioredoxin reductase.
Figure 5.
Increased Nrf2 activity was associated with increased expression of eNOS (A) and reduced levels of ADMA (B) in the treatment (ND-LP) group (n = 7; males n = 3 and females n = 4) compared with normal-diet control (NDC) group (n = 7; males n = 3 and females n = 4). The treatment group demonstrated decreased expression of HIF-1α (C), suggesting decreased hypoxic stress in ischemic myocardium of the treated (ND-LP) group compared with control (NDC), plausibly due to better oxygenation of the ischemic myocardium. Lastly, decreased p-MAPK (D) suggests reduction in proliferative/angiogenic signaling in ischemic myocardium of ND-LP group (n = 6). There was no significant difference between the groups (E) (NDC: n = 6; males n = 3 and females n = 3 and ND-LP: n = 5; males n = 2 females and n = 3) and with respect to phosphorylation of e-NOS in ischemic myocardial lysates. ADMA, asymmetric dimethyl arginine; eNOS, endothelial nitric oxide synthase; HIF-1α, hypoxia inducible factor-1α; Nrf2, nuclear factor erythroid 2-related factor 2; p-eNOS, phosphorylated endothelial nitric oxide synthase; p-MAPK, phosphorylated mitogen activated protein kinase.
DISCUSSION
We present data suggesting that dietary supplementation with L. plantarum and caffeic acid induced a robust Nrf2 response that may have supported endothelium-dependent tissue oxygenation, thereby leading to improved myocardial diastolic function. We initially hypothesized that L. plantarum and caffeic acid treatment may increase vascular density and improve collateral-dependent myocardial perfusion, as observed with other interventions (15, 16). However, there was no change in myocardial perfusion; in fact, we found a slight reduction in vascular density. Consistently, density of CD31 and α-SMA and the expression of pMAPK were decreased in ischemic myocardial tissue, although we note no significance in isolectin B4 as a less important marker of endothelial cells (21). These findings, combined with antioxidant, eNOS, and ADMA levels, collagen content, and EDPVR, support improved cardiac function through relaxation rather than revascularization.
Nrf2 indeed appeared to direct an integrated antioxidant response via induction of HO-1, NQO-1, and TRXR-1 plausibly resulting in mitigation of oxidative injury, which in turn supported myocardial viability. HO-1 is a prominent downstream target of Nrf2 induction and catalyzes the degradation of heme, generating carbon monoxide (CO) and biliverdin while releasing iron. This is particularly important in heme-rich tissues such as the heart where clearance of heme by HO-1 was shown to mitigate apoptosis and resolve inflammation following ischemic injury (22). Importantly, HO-1 serves as a source of CO, which has several well-characterized roles in supporting endothelial cell (EC) viability under conditions of stress including promoting cGMP-derived vasodilation, prosurvival signaling, and targeting eNOS for increased production of NO (23).
In this context, we observed higher levels of eNOS likely due to activation of upstream transcriptional pathway or by enhanced stability of the enzyme itself by Nrf2-mediated signaling (24). Reduced levels of ADMA in ischemic myocardial tissue may reflect greater bioavailability of nitric oxide (NO) (25). In theory, a healthy endothelium equates to better tissue oxygenation, which would abolish the hypoxic stimulus for angiogenesis such as endothelial cell proliferation and migration (26). Indeed, we found that levels of HIF-1α were reduced in the ischemic tissue of the L. plantarum-treated animals. This key finding further substantiated that Nrf2 antioxidant mechanisms effectively reduced oxidative stress, thereby improving tissue oxygenation, possibly by augmenting microvascular endothelial-dependent vasodilation which would not manifest in gross perfusion measures used in this study. Our study did not find a significant difference in phosphorylation of eNOS (p-eNOS) between the groups, which does not constitute a prerequisite for activity of this enzyme and would, therefore, not discredit the premise that higher levels of total eNOS may have contributed to improved endothelial-dependent vasodilation. However, whether there was an alteration in p-eNOS levels induced by L. plantarum at an early time point during myocardial ischemia will require further studies.
NQO-1, a well-known antioxidant that functions in the reduction of quinones, has also been found to contribute to endothelium-dependent relaxation. Kim and collaborators (27) stimulated NQO-1 in a hypertensive rat model and found improved blood pressure mediated by the activation of arterial eNOS. They proceeded to investigate the underlying mechanisms where plasma angiotensin converting enzyme (ACE) activity and angiotensin II (ANG II) levels were reduced via Ca2+/calmodulin-dependent protein kinase II (CaMKII) activation, which was dependent on NQO-1-mediated effect on the NAD+/NADH ratio and hence Ca2+ levels (28).
In addition, previous findings have shown that induction of the NQO-1 pathway prevented arterial restenosis via suppression of vascular smooth muscle cell (VSMC) proliferation, which was mediated by the AMPK pathway (27). HO-1/CO pathway has similarly been shown to inhibit hyperproliferation of VSMCs by means of nuclear factor-κB and p38 MAPK signaling, which prevented neointimal hyperplasia and stenosis in vascular grafts (29, 30). Indeed, inhibition of inflammatory proliferation and migration of VSMCs would be expected to hinder collateral vessel formation in the ischemic myocardium (31, 32). Conversely, this inhibition would support improved blood flow in the myocardium through existing vessels. We note that this connection between stimulation of NQO-1 or HO-1/CO and suppression of VSMC hyperproliferation is consistent with our finding of decreased α-SMA density.
We also report improved measures of myocardial relaxation in the treated animals that is attributable to a lesser degree of postischemic fibrosis. Despite earlier reports showing inhibitory effects on transforming growth factor β1(TGF-β1) signaling and the expression of proinflammatory cytokines (IL-6 and IL-1β) in animal models of parenchymal organ fibrosis (33, 34), the Nrf2 response did not significantly affect ischemic myocardial expression of TGF-β1 nor fibroblast density in our model. No change in fibroblast density combined with reduced α-SMA density may support less fibroblast activation to a myofibroblast phenotype.
Furthermore, many of the proteins that we found to be induced by Nrf2 have well-documented cardioprotective roles in the failing heart by reversing mitochondrial damage (35). Through restoration of mitochondrial biogenesis in cardiomyocytes, HO-1 improves contractility and reduces cardiac fibrosis (23). Similarly, modulation of mitochondrial ROS balance by thioredoxin has been associated with preservation of ventricular geometry and functional performance when subject to stress (36). In fact, cardiac overexpression of TRXR1 was shown to promote mitochondria biogenesis and mitophagy to improve myocardial dysfunction in septic mice (37). It is plausible that Nrf2-induced antioxidants may have inhibited fibrous scarring in the ischemic myocardium through mitigation of oxidative impairment of mitochondrial processes.
The spectrum of cytoprotective mechanisms linked to Nrf2 signaling have prompted interest to search for novel Nrf2 activators. One could argue, however, that compounds potentiating Nrf2 have long existed in nature; curcumin, phenylisothiocyanates in broccoli, and anthyocyanins in berries all have antioxidant effects linked to Nrf2 activity (38). In addition, ancient diets and traditionally fermented foods contain L. plantarum in abundance (8). The results presented in the current study support a positive role of supplementation with L. plantarum in the improvement of cardiac function in chronically ischemic myocardium. Natural supplements appear to be a safe and reliable method of augmenting Nrf2 antioxidant mechanisms while avoiding the metabolic and other side effects of pharmacological intervention.
Future studies exploring the effects of Nrf2 in endothelial cells at a global and subcellular level will be valuable, particularly regarding redox levels, metabolism, and ion homeostasis. As we described, evaluation of the mitochondria structure and function may reveal key insights into the therapeutic effects of Nrf2. Furthermore, it may be worthwhile to substantiate a reduced angiogenic capacity in vitro in response to hypoxia, through common proliferation, migration, and tube formation assays. Simultaneously, it will be valuable to elucidate the underlying pathway related to Nrf2-mediated relaxation of the endothelium.
Conclusions
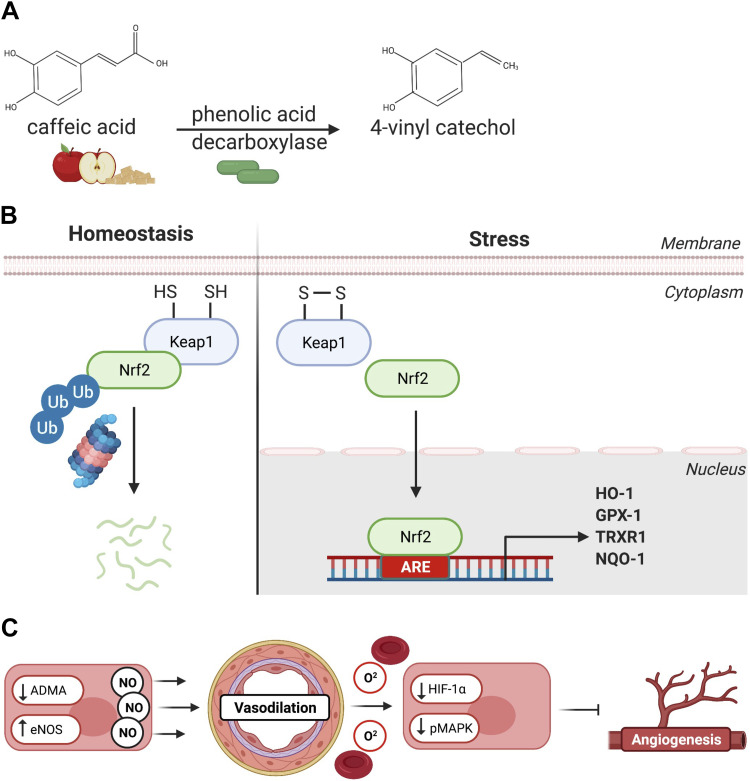
In this study, we demonstrate that dietary supplementation with applesauce in combination with L. plantarum probiotic is an effective natural method for inducing Nrf2 activity in vivo. Such activation is consistent with previous findings in vitro that L. plantarum enzyme phenolic acid decarboxylase efficiently converts caffeic acid, found abundantly in applesauce, to the potent Nrf2 activator 4-vinyl-catechol (Fig. 6A) (8). The data presented herein demonstrate that increased Nrf2 activity upregulated several cardioprotective antioxidant enzymes while reducing fibrosis, which in turn contributed to improved diastolic function in the chronically ischemic myocardium (Fig. 6B). Finally, we provide interesting perspective proposing that Nrf2 signaling, while improving diastolic function, may abort the process of hypoxic tissue injury needed to mobilize angiogenic signaling cascades (Fig. 6C) (39).
Figure 6.
Activation of Nrf2-mediated antioxidant signaling and therapeutic outcomes. A: treated swine were fed an applesauce diet rich in caffeic acid supplemented with L. plantarum, a source of phenolic acid decarboxylase. This enzyme converts caffeic acid to 4-vinyl-catechol, an Nrf-2 activator. B: under normal conditions, Nrf2 is sequestered by Keap1 in the cytoplasm and proteolytically degraded. Oxidative challenge induces Nrf2 dissociation from Keap2 resulting in nuclear translocation of Nrf2. Nrf2 binds to antioxidant response elements (AREs) in the nucleus inducing the expression of a variety of antioxidant proteins. C: protein expression of ADMA was found to be reduced, whereas eNOS expression was increased, suggesting increased availability of nitric oxide (NO), which may have resulted in improved tissue oxygenation. On the contrary, reduction in HIF-1α and pMAPK signaling suggests inhibition of proliferative and angiogenic stimuli in ischemic myocardium of the treated group, which may have resulted in the lack of collateral vessel formation in the treated group. Image created with BioRender and published with permission. ADMA, asymmetric dimethyl arginine; eNOS, endothelial nitric oxide synthase; GPX-1, glutathione peroxidase 1; HIF-1α, hypoxia inducible factor-1α; HO-1, heme oxygenase 1; Keap1, kelch-like ECH-associated protein 1; NQO-1, NADPH dehydrogenase quinone 1; Nrf2, nuclear factor erythroid 2-related factor 2; pMAPK, phosphorylated mitogen activated protein kinase; TXRX1, thioredoxin reductase. Created with BioRender.com; published with permission.
Limitations
Oxidative stress and age-related decline in homeostatic functions are cardinal features of chronic disease encountered in patients and are conceivably the most appropriate triggers of the Nrf2 response (40). Despite incurring mechanical obstruction of blood flow, the tissue we studied came from young, otherwise healthy animals. It would be interesting to study the effects of Nrf2 activity in a model of chronic myocardial ischemia subject to metabolic stress. It is plausible that Nrf2 exerts its protective effects in the ischemic myocardium predominantly by supporting endothelial expression of eNOS. Although the primary focus of this study was to examine collateral-dependent blood flow and cardiac function, we will be using a methodology to directly assess the effects of Nrf2 on coronary endothelial function in future.
SUPPLEMENTAL DATA
Supplemental File: https://doi.org/10.6084/m9.figshare.15069885.v1.
GRANTS
This work was funded by National Institutes of Health Grants R01HL46716, R01HL128831-01A1, and P20 GM103652 (to F.W.S) and 1R01HL133624 (to M.R.A.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A., A.T., N.S., M.R.A., and F.W.S. conceived and designed research; A.A., C.K., Z.Z., M.S., G.S., A.T., and N.S. performed experiments; A.A., C.K., Z.Z., M.S., G.S., A.T., N.S., and F.W.S. analyzed data; A.A., C.K., Z.Z., M.S., A.T., N.S., and M.R.A. interpreted results of experiments; A.A., C.K., M.S., and A.T. prepared figures; A.A. and C.K. drafted manuscript; A.T., N.S., M.R.A., and F.W.S. edited and revised manuscript; A.A., C.K., Z.Z., M.S., G.S., A.T., N.S., M.R.A., and F.W.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Donald Senger, Dr. Richard Clements, Tiffany M. Borjeson, Jessica Johnston, Jessica Fernandes, Cindy Phun, Maria Veliz, and Virginia Hovanesian.
REFERENCES
- 1.Hsia CCW, Schmitz A, Lambertz M, Perry SF, Maina JN. Evolution of air breathing: oxygen homeostasis and the transitions from water to land and sky. Compr Physiol 3: 849–915, 2013. doi: 10.1002/cphy.c120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghunath A, Sundarraj K, Nagarajan R, Arfuso F, Bian J, Kumar AP, Sethi G, Perumal E. Antioxidant response elements: discovery, classes, regulation and potential applications. Redox Biol 17: 297–314, 2018. doi: 10.1016/j.redox.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284: 13291–13295, 2009. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal 29: 1727–1745, 2018. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liby KT, Sporn MB. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev 64: 972–1003, 2012. doi: 10.1124/pr.111.004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL, Attucks OC, Franklin S, Levonen AL, Kensler TW, Dinkova-Kostova AT. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov 18: 295–317, 2019. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Assmann JC, Krenz A, Rahman M, Grimm M, Karsten CM, Köhl J, Offermanns S, Wettschureck N, Schwaninger M. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate's protective effect in EAE. J Clin Invest 124: 2188–2192, 2014. doi: 10.1172/JCI72151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senger DR, Li D, Jaminet S-C, Cao S. Activation of the Nrf2 cell defense pathway by ancient foods: disease prevention by important molecules and microbes lost from the modern western diet. PLoS One 11: e0148042, 2016. doi: 10.1371/journal.pone.0148042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu W, Wang H, Li S, Liu Q, Sha H. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis 10: 637–651, 2019. doi: 10.14336/AD.2018.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen QM, Maltagliati AJ. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics 50: 77–97, 2018. doi: 10.1152/physiolgenomics.00041.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattila P, Kumpulainen J. Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J Agric Food Chem 50: 3660–3667, 2002. doi: 10.1021/jf020028p. [DOI] [PubMed] [Google Scholar]
- 12.Haghi G, Hatami A. Simultaneous quantification of flavonoids and phenolic acids in plant materials by a newly developed isocratic high-performance liquid chromatography approach. J Agric Food Chem 58: 10812–10816, 2010. doi: 10.1021/jf102175x. [DOI] [PubMed] [Google Scholar]
- 13.Reese DE, Thale RC, Brumm MC, Lewis AJ, Miller PS, Libal GW. Swine Nutrition Guide. Faculty Papers and Publications in Animal Science, Nebraska and South Dakota, 2000. https://digitalcommons.unl.edu/animalscifacpub/694
- 14.Sellke FW, Wang SY, Friedman M, Harada K, Edelman ER, Grossman W, Simons M. Basic FGF enhances endothelium-dependent relaxation of the collateral-perfused coronary microcirculation. Am J Physiol Heart Circ Physiol 267: H1303–H1311, 1994. doi: 10.1152/ajpheart.1994.267.4.H1303. [DOI] [PubMed] [Google Scholar]
- 15.Ruel M, Wu GF, Khan TA, Voisine P, Bianchi C, Li J, Li J, Laham RJ, Sellke FW. Inhibition of the cardiac angiogenic response to surgical FGF-2 therapy in a Swine endothelial dysfunction model. Circulation 108 Suppl 1: II335–I1340, 2003. doi: 10.1161/01.cir.0000087903.75204.ad. [DOI] [PubMed] [Google Scholar]
- 16.Scrimgeour LA, Potz BA, Aboul Gheit A, Shi G, Stanley M, Zhang Z, Sodha NR, Ahsan N, Abid MR, Sellke FW. Extracellular vesicles promote arteriogenesis in chronically ischemic myocardium in the setting of metabolic syndrome. J Am Heart Assoc 8: e012617, 2019. doi: 10.1161/JAHA.119.012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radke PW, Heinl-Green A, Frass OM, Post MJ, Sato K, Geddes DM, Alton EWFW. Evaluation of the porcine ameroid constrictor model of myocardial ischemia for therapeutic angiogenesis studies. Endothelium 13: 25–33, 2006. doi: 10.1080/10623320600660128. [DOI] [PubMed] [Google Scholar]
- 18.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: The National Academies Press, 2011. [Google Scholar]
- 19.Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. QuPath: open source software for digital pathology image analysis. Sci Rep 7: 16878, 2017. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung WY, Jensen MB. Histological quantification of angiogenesis after focal cerebral infarction: a systematic review. ISRN Neurol 2013: 853737, 2013. doi: 10.1155/2013/853737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol 24: 449–455, 2003. doi: 10.1016/S1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 23.Otterbein LE, Foresti R, Motterlini R. Heme oxygenase-1 and carbon monoxide in the heart: the balancing act between danger signaling and pro-survival. Circ Res 118: 1940–1959, 2016. doi: 10.1161/CIRCRESAHA.116.306588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Münzel T, Camici GG, Maack C, Bonetti NR, Fuster V, Kovacic JC. Impact of oxidative stress on the heart and vasculature: part 2 of a 3-part series. J Am Coll Cardiol 70: 212–229, 2017. doi: 10.1016/j.jacc.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sibal L, Agarwal SC, Home PD, Boger RH. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev 6: 82–90, 2010. doi: 10.2174/157340310791162659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toffoli S, Roegiers A, Feron O, Van Steenbrugge M, Ninane N, Raes M, Michiels C. Intermittent hypoxia is an angiogenic inducer for endothelial cells: role of HIF-1. Angiogenesis 12: 47–67, 2009. doi: 10.1007/s10456-009-9131-y. [DOI] [PubMed] [Google Scholar]
- 27.Kim S-Y, Jeoung NH, Oh CJ, Choi Y-K, Lee H-J, Kim H-J, Kim J-Y, Hwang JH, Tadi S, Yim Y-H, Lee K-U, Park K-G, Huh S, Min K-N, Jeong K-H, Park MG, Kwak TH, Kweon GR, Inukai K, Shong M, Lee I-K. Activation of NAD(P)H:Quinone oxidoreductase 1 prevents arterial restenosis by suppressing vascular smooth muscle cell proliferation. Circ Res 104: 842–850, 2009. doi: 10.1161/CIRCRESAHA.108.189837. [DOI] [PubMed] [Google Scholar]
- 28.Kim Y-H, Hwang JH, Kim K-S, Noh J-R, Gang G-T, Kim S-W, Jang SP, Lee S-J, Her S-H, Jeong K-H, Kwak TH, Park WJ, Balyasnikova IV, Shong M, Lee C-H. NQO1 activation regulates angiotensin-converting enzyme shedding in spontaneously hypertensive rats. Cardiovasc Res 99: 743–750, 2013. doi: 10.1093/cvr/cvt147. [DOI] [PubMed] [Google Scholar]
- 29.Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Clinton Webb R, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med 7: 693–698, 2001. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 30.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AMK, Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med 9: 183–190, 2003. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 31.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res 49: 507–521, 2001. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 32.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui T-Y, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol 172: 3553–3563, 2004. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 33.Ryoo I-G, Ha H, Kwak M-K. Inhibitory role of the KEAP1-NRF2 pathway in TGFβ1-stimulated renal epithelial transition to fibroblastic cells: a modulatory effect on SMAD signaling. PLoS One 9: e93265, 2014. doi: 10.1371/journal.pone.0093265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, Ghezzi P, León R, López MG, Oliva B, Pajares M, Rojo AI, Robledinos-Antón N, Valverde AM, Guney E, Schmidt HHHW. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev 70: 348–383, 2018. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 35.Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol 61: 599–610, 2013. doi: 10.1016/j.jacc.2012.08.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conrad M, Jakupoglu C, Moreno SG, Lippl S, Banjac A, Schneider M, Beck H, Hatzopoulos AK, Just U, Sinowatz F, Schmahl W, Chien KR, Wurst W, Bornkamm GW, Brielmeier M. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol 24: 9414–9423, 2004. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez-Villamil JP, D'Annunzio V, Finocchietto P, Holod S, Rebagliati I, Pérez H, Peralta JG, Gelpi RJ, Poderoso JJ, Carreras MC. Cardiac-specific overexpression of thioredoxin 1 attenuates mitochondrial and myocardial dysfunction in septic mice. Int J Biochem Cell Biol 81: 323–334, 2016. doi: 10.1016/j.biocel.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 38.Rose P, Won YK, Ong CN, Whiteman M. Beta-phenylethyl and 8-methylsulphinyloctyl isothiocyanates, constituents of watercress, suppress LPS induced production of nitric oxide and prostaglandin E2 in RAW 264.7 macrophages. Nitric Oxide 12: 237–243, 2005. doi: 10.1016/j.niox.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer 2: 1117–1133, 2011. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenvinkel P, Meyer CJ, Block GA, Chertow GM, Shiels PG. Understanding the role of the cytoprotective transcription factor nuclear factor erythroid 2–related factor 2–lessons from evolution, the animal kingdom and rare progeroid syndromes. Nephrol Dial Transplant 35: 2036–2045, 2019. doi: 10.1093/ndt/gfz120. [DOI] [PMC free article] [PubMed] [Google Scholar]