TO THE EDITOR:
The prognosis of COVID-19 infection is poor in allogeneic hematopoietic stem cell transplant (HSCT) recipients.1 Immunocompromised patients have been excluded from initial trials evaluating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA (mRNA) vaccines,2, 3 and there is a crucial need to assess vaccine efficacy among these patients. In several reports from solid organ transplant (SOT) recipients4, 5, 6 as well as from patients with hematologic malignancies,7, 8 a high proportion mounted a negative antibody response after 2 doses of mRNA vaccine, and a third booster dose improved the response rate.4, 9, 10, 11 These results prompted the French National Authority of Health to recommend the use of a third dose in immunocompromised patients.12 However, regarding allogeneic HSCT recipients, data remains limited to a small monocentric report of 88 patients.13, 14 We therefore conducted a multicentric retrospective nationwide study, aiming to determine serologic response to 2-dose SARS-CoV-2 mRNA vaccines among allogeneic HSCT recipients and the effect of a third dose in patients with undetectable or weak serologic response.
We evaluated humoral response to 2-dose SARS-CoV-2 mRNA vaccines (BNT162b2 or mRNA-1273) among 687 consecutive HSCT recipients from 15 French centers belonging to the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). All included patients completed the 2-dose SARS-CoV-2 mRNA vaccine between 1 January and 15 July 2021 and had an available semiquantitative antispike serologic testing after the second dose (see supplemental Materials for details, available on the Blood Web site; supplemental Tables 1 and 2). In France, guidelines from the SFGM-TC recommended the vaccination for all allogeneic HSCT recipients, except for patients within 3 months of transplantation or in the case of uncontrolled graft-versus-host disease (GVHD).15 We excluded patients with a history of COVID-19 confirmed by serology or polymerase chain reaction. All patients had given written consent before transplant for data collection for future research in accordance with the Declaration of Helsinki. The SFGM-TC scientific council approved this study.
Patients were mainly male (59%), with a median age of 59 years old (interquartile range [IQR] 46 to 66), most transplanted for myeloid (69%) or lymphoid (26%) malignancies (supplemental Table 3). The median delay between the transplantation and the initiation of vaccination was 27 months (IQR 14 to 56) and was <12 months for 144 patients (21%). Donors were HLA-matched unrelated for 51%, HLA-identical sibling for 29%, and haplo-identical for 20%. Results for 81 patients from 1 center have been previously partly published.13, 14
The first 2 doses of the vaccine (96% with BNT162b2) were administered 1 month apart. At a median of 33 days after dose 2 (IQR 27 to 52), an antibody response was detectable in 538 patients (78%; 95% confidence interval [CI], 75% to 81%) with a median antibody level of 749 binding antibody units per mL16 (BAU/mL) (IQR 250 to 2500). Detectable antibody responses were classified as “weak” (<250 BAU/mL) in 118 patients (17%) and as “good” (≥250 BAU/mL) in 420 (61%), with a threshold of 250 BAU/mL, which has been associated to ∼90% of mRNA-1273 efficacy in the COVE trial17 (supplemental Table 2). The serologic response rate increased with time from HSCT (supplemental Figure 1): 32% (95% CI, 15% to 50%) within the 6 months from transplantation, 50% (95% CI, 42% to 61%) between 6 and 12 months, and 87% (95% CI, 84% to 89%) after 1 year.
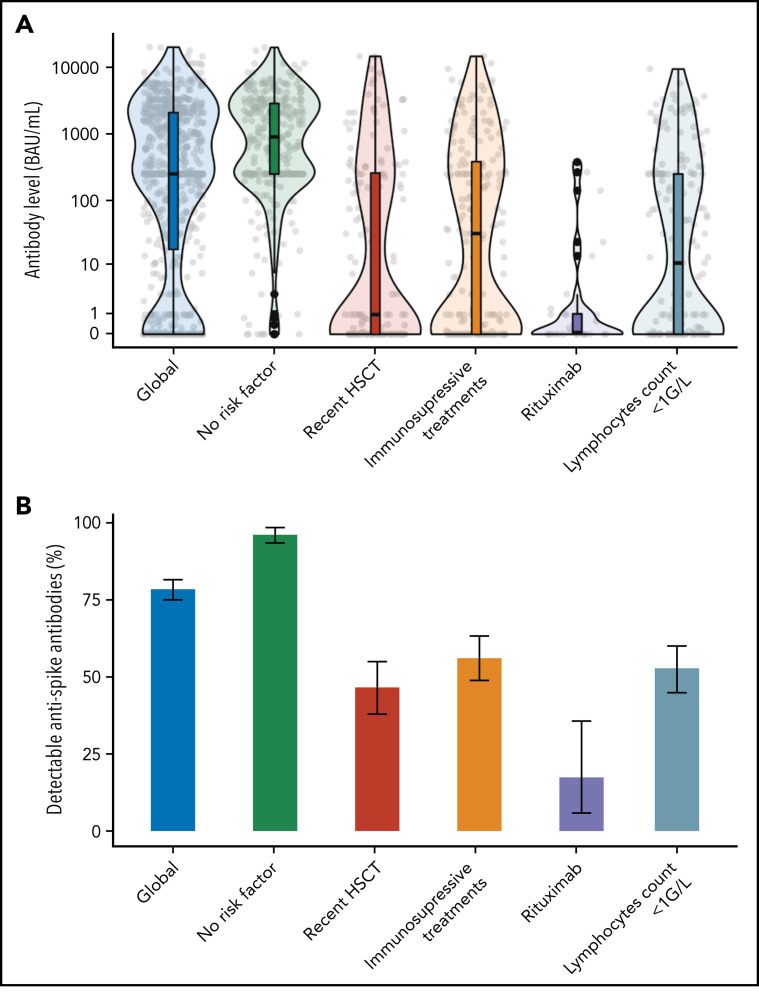
In the multivariate analysis (Figure 1 ; supplemental Table 4), factors associated with the absence of humoral responses were a time-interval from HSCT <12 months (adjusted odds ratio [aOR] 2.7, 95% CI, 1.6 to 4.6), an absolute lymphocyte count <1 G/L (aOR 3.1; 95% CI, 1.8 to 5.1), and systemic immunosuppressive treatments within 3 months of vaccination (aOR 3.4; 95%, CI, 2.1 to 5.6), together with the use of rituximab within 6 months (aOR 13.7; 95% CI, 4.1 to 45.2). In a subsequent multivariate analysis conducted on a subset of 352 patients with available gammaglobulinemia, B-CD19+, and T−CD4+ lymphocytes counts (supplemental Table 4), only low B-lymphocytes count (aOR 5.7; 95% CI, 2.8 to 11.9), time-interval from HSCT <12 months (aOR 4.7; 95% CI, 2.5 to 13.9), and ongoing immunosuppressive treatments (aOR 2.8; 95% CI, 1.4 to 5.5) remained independently associated with the absence of antibody response. These risk factors are largely consistent with studies conducted in SOT recipients as well as patients with hematological malignancies5, 6, 7, 8 and could be used to stratify the risk of negative response among HSCT recipients (supplemental Figure 2). In particular, patients receiving immunosuppressive treatments had a 56% serologic response rate (supplemental Table 1), consistent with reports from SOT recipients (ranging from 36% to 54% after 2 doses).4, 5, 6 As immunodepression decreases with distance from HSCT, we specifically analyzed patients vaccinated within the first year from transplantation (supplemental Tables 5 and 6). In this subgroup, absolute lymphocyte count <1 G/L, use of rituximab, and history of GVHD necessitating systemic treatment were found to be independently associated with the absence of antibody response. Specifically, within this subgroup, no independent association was found with time-interval from HSCT (<6 months vs 6 to 12 months) in multivariate analysis, although our study is likely underpowered to assess this point.
Figure 1.
Antispike response by risk factors associated with immunization after 2 vaccine doses. Serologic response to a 2-dose vaccination according to main factors associated with immunization after dose 2 (identified in multivariate analysis; see text and supplemental Tables 3 and 4). (A) Antispike antibody level. The violin plots contain interior box plots with upper and lower horizontal edges the 25th and 75th percentiles of antibody level and middle line the 50th percentile. The shape of the violin plots shows the smoothed probability density of the data. (B) Proportion of detectable antispike antibodies with 95% CI. The positivity threshold was given by the manufacturer for each used serological assay as detailed in supplemental Materials.
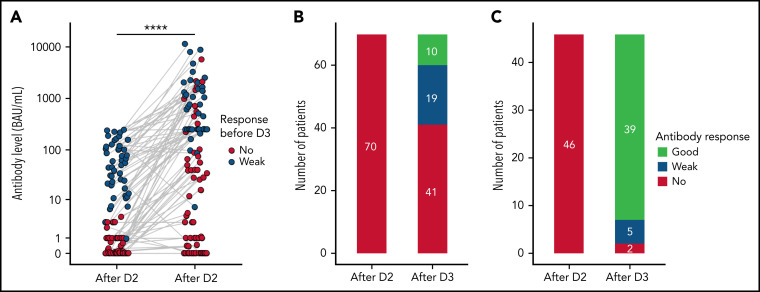
A systematic third dose was not recommended during the study period and remained at the discretion of the attending physician. In this cohort, 181 allogeneic HSCT recipients received a third dose of mRNA vaccine at a median of 54 days after dose 2, with subsequent semiquantitative antispike serological testing (Figure 2 ; supplemental Table 5). Among 70 patients with no prior detectable response (supplemental Table 6), 29 (41%; 95% CI, 30% to 54%) mounted a detectable response after dose 3, with a median level of 65.6 BAU/mL (IQR 34.4 to 551). Among 46 patients with a detectable but weak (<250 BAU/mL) response before the third dose, antibody level significantly increased from a median of 52.3 BAU/mL (IQR 20 to 112.9) to 477.4 BAU/mL (IQR 250 to 1497), and 39 (85%) reached a good serologic response (≥250 BAU/mL). In all 65 patients who received a third dose while having a good (≥250 BAU/mL) serologic response, the antibody level either increased or remained the highest possible expressed by the used serologic assay (data not shown). Sixty-five patients vaccinated within the first year after HSCT received a third vaccine dose with similar results to the whole sample (supplemental Figure 3). Taken together, these elements support the systematic use of a third booster dose in non- or weakly responding allogeneic HSCT recipients.
Figure 2.
Antibody response after a third dose of SARS-CoV-2 vaccine. Antibody response before and after the third dose (D3). (A) Antibody levels (in BAU/mL) after the second and third dose of vaccine. Dots represent individual values and are filled according to the response after dose 2 (red for no response and blue for response weak response [<50 BAU/mL]). Antibody level significantly increased after dose 3 (P < .001, Mann-Whitney U test). (B-C) Antibody qualitative response to the third dose classified according antibody levels among patients with no (B) or weak (C) prior detectable response. “No” for undetectable response, “weak” for response <50 BAU/mL, and “good” for response ≥250 BAU/mL. The positivity threshold was given by the manufacturer for each used serological assay as detailed in supplemental Materials. D2, dose 2.
After a median follow-up of 156 days since dose 2 (IQR 141 to 191), COVID-19 was reported in 4 patients: 2 with no detectable antibodies and 2 with good serologic responses (324 and 2654 BAU/mL). Only 1 patient, who had no detectable antibodies, developed a severe COVID-19 requiring hospitalization.
Main limitations of this study include the lack of an immunocompetent control group, its retrospective and observational design leading to a risk of selection bias, and the absence of neutralizing antibody testing. However, 2 recent analyses of COVID-19 vaccine trials showed a similar correlation with vaccine efficacy for both neutralizing and binding antibodies17, 18 consistently with in vivo experimental studies on nonhuman primate.19 Also, we did not explore the absence of B-cell memory and T-cell functional responses. In particular, B-cell memory response may be critical to warrant the longevity of the vaccine-induced protection, which will be a fundamental issue in the close future. Also, we had no information about the severity of chronic GVHD, and, as only 28 patients were vaccinated within the 6 months after transplantation, this study is clearly underpowered to confidently assess serologic response rate early after HSCT.
To conclude, this study shows that the majority of allogeneic HSCT recipients developed an antibody response after 2 doses of SARS-CoV-2 vaccine and supports the use of a third vaccine booster dose for non- or weakly responding patients.
Acknowledgments
The authors thank Nicole Raus for her help in data collection and data management.
Footnotes
Data will be shared on demand by e-mails to the corresponding author.
The online version of this article contains a data supplement.
Three reports address the protection of the vulnerable population of patients with hematologic malignancies in the face of the ongoing COVID pandemic. The reports suggest that some patients who fail to mount a B-cell response to vaccine may nevertheless have protective T cell responses. As a group, these reports suggest that patients should continue to be immunized with additional doses to attempt to improve immune response but that they need to maintain the precautions recommended for the unvaccinated.
Authorship
Contribution: S.N. conceptualized the study; A.M. designed and performed the statistical analyses; A.M. and S.N. wrote the first draft of the manuscript. All authors participated in data collection and revised and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Supplementary Material
REFERENCES
- 1.Ljungman P, de la Camara R, Mikulska M, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35(10):2885–2894. doi: 10.1038/s41375-021-01302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rozen-Zvi B, Yahav D, Agur T, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27(8):1173.e1–1173.e4. doi: 10.1016/j.cmi.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583–e592. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Bello AD, Abravanel F, Marion O, et al. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant. 2021 doi: 10.1111/ajt.16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326(11):1063–1065. doi: 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.French Government,. Precisions sur la vaccination COVID-19: modalites d'administration des rappels et vaccination des personnes immunodeprimes et de leurs proches. https://solidarites-sante.gouv.fr/IMG/pdf/dgs_urgent_52_precisions_sur_la_vaccination_imd.pdf. Accessed 10 September 2021.
- 13.Redjoul R, Le Bouter A, Beckerich F, Fourati S, Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398(10297):298–299. doi: 10.1016/S0140-6736(21)01594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redjoul R, Le Bouter A, Parinet V, Fourati S, Maury S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021;8(10):e681–e683. doi: 10.1016/S2352-3026(21)00274-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baudoux E, Bay JO, Beguin Y, et al. Recommandations de la SFGM-TC Stratégie de vaccination pour les patients recevant une allogreffe de cellules souches hématopoïétiques. 2021. Accessed 10 September 2021.
- 16.Kristiansen PA, Page M, Bernasconi V, et al. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282):1347–1348. doi: 10.1016/S0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert PB, Montefiori DC, McDermott A, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial [published online ahead of print 23 November 2021] Science. 2021:eab3435. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021;27(11):2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbett KS, Nason MC, Flach B, et al. Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. Science. 2021;373(6561):eabj0299. doi: 10.1126/science.abj0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.