Introduction
For over two years now, the world has not looked the same. Indeed, in December 2019, the first cases of infection by a new virus named severe acute respiratory syndrome coronavirus 2 (SARS-COV2) were recorded in Wuhan, Hubei Province of China ( 1 ). Since then, the virus has continued to spread throughout the world despite the efforts of all countries to limit that. The clinical manifestations of COVID 19 are diverse. they range from respiratory manifestations, to cardiac, cutaneous, digestive, neurologic and musculoskeletal manifestations ( 2 ). Most musculoskeletal manifestations were myalgia in 80% of cases, followed by back pain 6.67% of cases, muscle weakness, skeletal muscle injury, arthralgia and facial muscle pain ( 3 ). Osteoporosis and osteonecrosis were rarely reported: there are only few studies that focused on these manifestations ( 4 ). To the best of our knowledge, only one previous study has been reported on vertebral fracture (VF) prevalence in patients affected by COVID-19 ( 5 ). We report here a case of a man who developed osteoporosis complicated by lumbar vertebral fracture following infection with COVID 19.
Case report
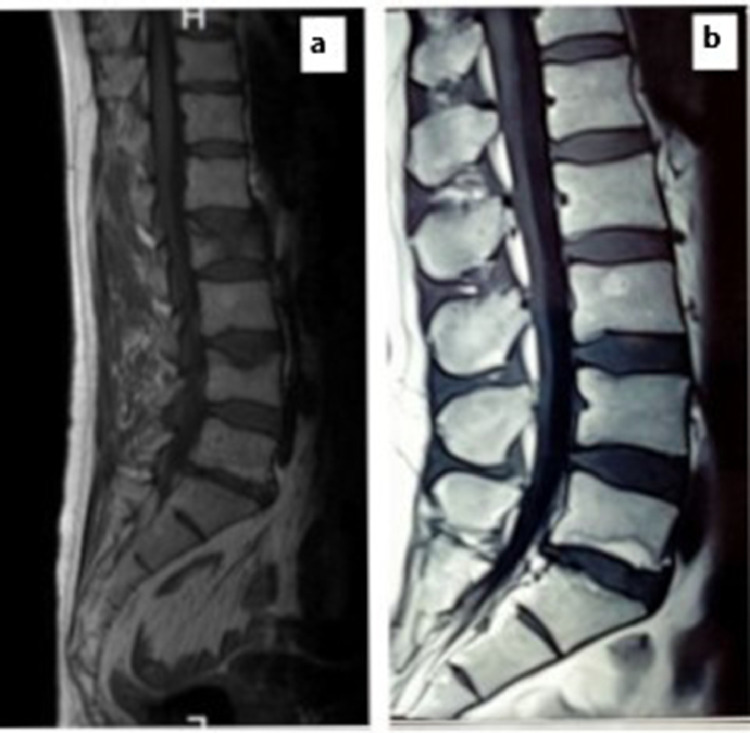
A 53-year-old man with no significant previous medical history, presented to our rheumatology department in March 2021 with chill and fever of unknown cause. On physical examination, chest auscultation was normal. Pulse oximetry showed an Sp02 at 92%. The diagnosis of COVID-19 was made by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) from a pharangeal swab with lung impairment estimated at 25%. The patient was therefore hospitalized and was treated with oxygen therapy, Azithromycin, vitamin therapy (D and C) and preventive anticoagulation. He received corticosteroid therapy for four days. The outcome was favorable one week later (O2 saturation at 98% on room air) and the patient returned home. Four days after COVID 19 infection, he developed an acute low back pain without fever, that didn't improve by level 1 analgesics. The X-ray of the lumbar spine showed vertebral fractures (VF) of L2 and L4 confirmed by Magnetic resonance imaging (MRI) of the spine(Fig. 1 ). Bone Mineral Density (BMD) was then performed revealing osteoporosis of the lumbar spine with a T score of -2.9 DS (T score at the femoral neck was -1.6 DS). Laboratory results did not show any abnormalities, in particular, normal blood count cell, and normal phosphocalcic balance: calcemia at 2.37 mmol/l, phosphoremia at 1 mmol/l and PAL at 120 UI/L. Creatinine level was within normal range: 10.8 mg/l (normal range: 7-14 mg/l). Acute phase reactants were also normal: C-reactive protein level at 0.1 mg/l and erythrocyte sedimentation rate at 37 mm/1st hour. The assays of FT4, TSH, testosteroneemia, cortisolemia, serum protein electrophoresis were all without abnormalities. Vitamin D level was normal.
Fig. 1.
Magnetic resonance imaging of the spine showing vertebral fractures of L2 and L4.
Thus, after ruling out secondary causes, the diagnosis of osteoporotic fractures was made. The imputability of SARS COV-2 infection was probable as the patient had a recent MRI a month prior to the infection without any fractures. The patient was put on bisphosphonate treatment (Zoledrenic acid).
Discussion
This is a unique case of a patient who developed osteoporosis with lumbar VF post-COVID-19 infection. Even though inflammatory conditions are known to induce bone fragility, data on bone loss and Covid-19 are scarces. Some authors suggested a higher increase of VF in general due to inadequacy of care at the expense of chronic diseases, mainly osteoporosis ( 6 ). However, other authors incriminate the COVID-19 itself in inducing bone fragility ( 5 ). The pathophysiological mechanism of bone involvement during COVID-19 is still debated; several hypotheses have been advanced ( 4 ). SARS-COV2 might promote osteoclastogenesis by direct and indirect mechanisms. On one hand, COVID-19 targets T lymphocytes resulting in pro-inflammatory cytokine storm with subsequent exhaustion of the immune response. The main incriminated cytokines were CXCL10, IL-17, IL-1, IL-6, and TNF-α, responsible for osteoclast activity, favoring bone resorption through the RANK-RANKL system ( 7 ). On the other hand, Covid-19 also targets type-II pneumocytes that line the respiratory epithelium. These cells express two genes: ACE2 and TMPRSS2 ( 8 ) that are also found in musculoskeletal tissues; such as: skeletal, muscle, cartilage, meniscus and synovium ( 9 ). Furthermore, the incidence of osteoporosis and fracture risk may increase upon recovery from Covid-19 due to the presence of multiple predisposing factors (diabetes mellitus, immobilization, steroid treatment, hypovitaminosis D, lean mass loss,etc) ( 7 ).
Our patient was formerly healthy with no history of bone disease, fracture nor endocrine diseases that affect bone metabolism. Laboratory sample ruled out other causes of secondary osteoporosis. He did not receive corticosteroid therapy, neither did he suffer from vitamin D deficiency. More importantly, an MRI of the spine performed one month prior to the infection did not reveal any VF. In the only study addressing this issue, Di Filippo et al supported our theory ( 5 ). In his recent retrospective study, lateral chest X-rays were performed in 114 COVID-19 patients. The prevalence of VF was 36% of cases, although only 3% of patients had a history of osteoporosis ( 5 ). In this study, COVID-19 patients with VF more frequently required noninvasive mechanical ventilation and had higher morality (p = 0.04) compared with those without VF ( 5 ). In light of these results, authors suggested that VF may be a cardiorespiratory risk of COVID-19 patients, and could be a useful clinical marker for poor prognosis. They also suggested that morphometric thoracic vertebral evaluation should be performed in all suspected COVID-19 patients ( 5 ). In this context, further studies should focus on investigating fracture risks and factors predicting of VF in SARS-Cov-2 patients.
In our case, VF was found in a male patient. Eventhough the incidence of osteoporosis is more frequent in post menopausal women, it may be underdiagnosed in males. Indeed, the European Vertebral Osteoporosis Study showed that at least one VF was found in 8% to 20% of men aged between 50 and 79 years ( 10 ). Similarly, male COVID patients were as affected by VF as females (35% vs 37% respectively) ( 5 ). This gender discrepancy may be explained by a higher exposure to risk factors such as smoking and a higher rate of mortality from COVID-19 in male gender (7). This also may be explained by the difference in the innate immune response between males and females. Indeed, estrogen modulates the innate immune cell response by decreasing the expression of IL-6 which is highly secreted in COVID-19 infection and an important factor for bone resorption in inflammatory conditions ( 11 ). Thus, men would express higher levels of IL-6 due to a lack of suppression by estrogen and suffer more from bone alterations during Covid-19 ( 11 ). Further studies are needed in order to validate this theory.
Although there is no consensus treatment regarding osteoportic fracture post Covid-19, it seems appropriate to treat osteoporosis with bisphosphonates as a first-line treatment. It is still unkown whether the severity of Covid-19 is associated with higher bone loss. It is also still unclear if this successfully treated infection would result in reversal of bone fragility. In a study evaluating bone loss and risk fracture in patients with HIV and hepatitis C, Bedimo et al, showed that the choice of the antiresorptive agent depends on the underlying mechanism and infection ( 12 ).
Authors showed that previous SARS-CoV had the potential to act directly on bone resorption units, thus the RANK RANKL system may represent the best therapeutic target. As Denosumab (monoclonal antibody that inhibits RANKL) was not available, we chose bisphosphonate treatment instead. Another feature of Denosumab is its immune system modulator effect ( 13 ). Regarding osteoporosis management in the era of COVID-19, authors recommend transitioning from Denosumab to oral bisphosphonate if continued treatment with denosumab is not possible within 7 months of the first inhection ( 13 ). Moreover, caution should be taken as Denosumab induces high turn over and increases the risk of VF ( 13 ). Bisphosphonates such as Alendronate may provide protection and decreases the rebound effect from Denosumab discontinuation ( 13 ).
Recently, it has been reported that MicroRNA-4485 (miR-4485), which is upregulated in COVID-19 patients, reduces toll-like receptor 4 (TLR-4) and thus, suppresses osteogenic differentiation imparing fracture healing ( 4 ). This provides a promising advance in the treatment of osteoporotic fractures in Covid-19 patients ( 4 ).
Given the protective role of vitamin D in the prevention and treatment of Covid-19, experts have published guidance on its supplementation ( 14 ). A recent systematic review of the literature revealed that Calcifediol supplementation may have a protective effect on COVID-19 related complications ( 14 ). However, there is no solid evidence that high doses of vitamin D are efficient ( 14 ).
In conclusion, to our knowledge, this is the first case report of osteoporosis complicated by lumbar vertebral fracture following infection with COVID 19. Our case and others reported in the literature suggest that osteoporotic VF may complicate the course of Covid-19. A close surveillance for the occurrence of this phenomena should be worthy for adequate clinical management.
References
- 1.WHO | Pneumonia of unknown cause – China [Internet]. WHO. World Health Organization; [cité 12 mai 2021]. Disponible sur Available at: http://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en
- 2.Da Rosa Mesquita R, Francelino Silva Junior LC, Santos Santana FM, et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr. 2021;133(7-8):377–382. doi: 10.1007/s00508-020-01760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdullahi A, Candan SA, Abba MA, et al. Neurological and Musculoskeletal Features of COVID-19: A Systematic Review and Meta-Analysis. Front Neurol. 2020;26(11):687. doi: 10.3389/fneur.2020.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mi B, Xiong Y, Zhang C, et al. SARS-CoV-2-induced Overexpression of miR-4485 Suppresses Osteogenic Differentiation and Impairs Fracture Healing. Int J Biol Sci. 2021;17(5):1277‑1288. doi: 10.7150/ijbs.56657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.di Filippo L, Formenti AM, Doga M, et al. Radiological Thoracic Vertebral Fractures are Highly Prevalent in COVID-19 and Predict Disease Outcomes. J Clin Endocrinol Metab. 2021;106(2):e602–e614. doi: 10.1210/clinem/dgaa738. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu EW, Tsourdi E, Clarke BL, et al. Osteoporosis management in the era of COVID-19. J Bone Miner Res. 2020;35(6):1009–1013. doi: 10.1002/jbmr.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvio G, Gianfelice C, Firmani F, et al. Bone Metabolism in SARS-CoV-2 Disease: Possible Osteoimmunology and Gender Implications. Clin Rev Bone Miner Metab. 2020;1:1. doi: 10.1007/s12018-020-09274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng H, Wang Y, Wang G. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92(7):726‑730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Micheli AJ, Spector JA, Elemento O, Cosgrove BD. A reference single-cell transcriptomic atlas of human skeletal muscle tissue reveals bifurcated muscle stem cell populations. Skelet Muscle. 2020;10(1):19. doi: 10.1186/s13395-020-00236-3. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Neill TW, Felsenberg D, Varlow J, et al. The prevalence of vertebral deformity in european men and women: the European Vertebral Osteoporosis Study. J Bone Miner Res. 1996;11(7):1010–1018. doi: 10.1002/jbmr.5650110719. [DOI] [PubMed] [Google Scholar]
- 11.Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2019;56(3):308–321. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 12.Bedimo RJ, Adams-Huet B, Poindexter J, et al. The Differential Effects of Human Immunodeficiency Virus and Hepatitis C Virus on Bone Microarchitecture and Fracture Risk. Clin Infect Dis. 2018;66(9):1442–1447. doi: 10.1093/cid/cix1011. 17. [DOI] [PubMed] [Google Scholar]
- 13.Formenti AM, Pedone E, di Filippo L, et al. Are women with osteoporosis treated with denosumab at risk of severe COVID-19? Endocrine. 2020;70(2):203–205. doi: 10.1007/s12020-020-02500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassatne A, Basbous M, Chakhtoura M, et al. The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metabolism. 2021;119 doi: 10.1016/j.metabol.2021.154753. [DOI] [PMC free article] [PubMed] [Google Scholar]