Dual antiplatelet therapy (DAPT), a combination of aspirin and a P2Y12 inhibitor, remains the cornerstone therapy after percutaneous coronary intervention (PCI). Following the guideline recommended treatment intervals of 6-months in stable ischemic heart disease (SIHD) and 1-year in acute coronary syndrome (ACS), much debate remains around the optimal antiplatelet therapy regimen and duration (1,2). This debate centers on balancing ischemic risk, often defined by myocardial infarction or stent thrombosis, with bleeding harm (3). Ostensibly, longer or more intense DAPT may favor those patients with predominantly high ischemic risk while shorter or less intense DAPT may benefit those patients with predominantly high bleeding risk. Much of the effort thus far has focused on identifying patients at higher bleeding risk and shortening DAPT duration in such patients. However, high correlation between bleeding and ischemic risk may lead to the curtailing of DAPT in patients at high risk for coronary thrombotic events when evaluating bleeding alone. So how can clinicians assess bleeding and ischemic risk together?
The DAPT score emerged as one of the first clinical risk scores to predict and, more importantly, uncouple ischemic and bleeding risk, providing clinicians with a practical tool to guide DAPT duration that accounts for both factors (4). It subsequently was incorporated into contemporary guidelines (1,2). Derived from the DAPT study, which demonstrated ischemic benefit at a bleeding cost with 30-months of DAPT, the DAPT score stratifies patients into high score (DAPT Score≥2) or low score (DAPT Score<2) groups based on nine clinical variables (5). High score patients have a higher calculated ischemic risk and lower bleeding risk and were found to benefit the most from longer or more intense DAPT. Conversely, low score patients have a higher bleeding risk and lower ischemic risk and were found to benefit from shorter or less intense DAPT therapy in the DAPT Study.
Following the DAPT score’s development, several studies have assessed the score’s validity across different study populations and raised some questions about its external validity (6–11). However, to be clear, despite the wide variation in populations and definitions of endpoints, low DAPT score patients have demonstrated a higher cumulative incidence of bleeding in patients with low DAPT scores compared with high scores across all published validation studies. And, in all but one study (9), high DAPT score patients have demonstrated a higher incidence of ischemic events when compared to a low DAPT score (Figure). In other words, despite the high correlation between ischemic and bleeding risk seen with most conventional risk scores, the DAPT score can uniquely uncouple these risks across a broad range of patient populations.
Figure:
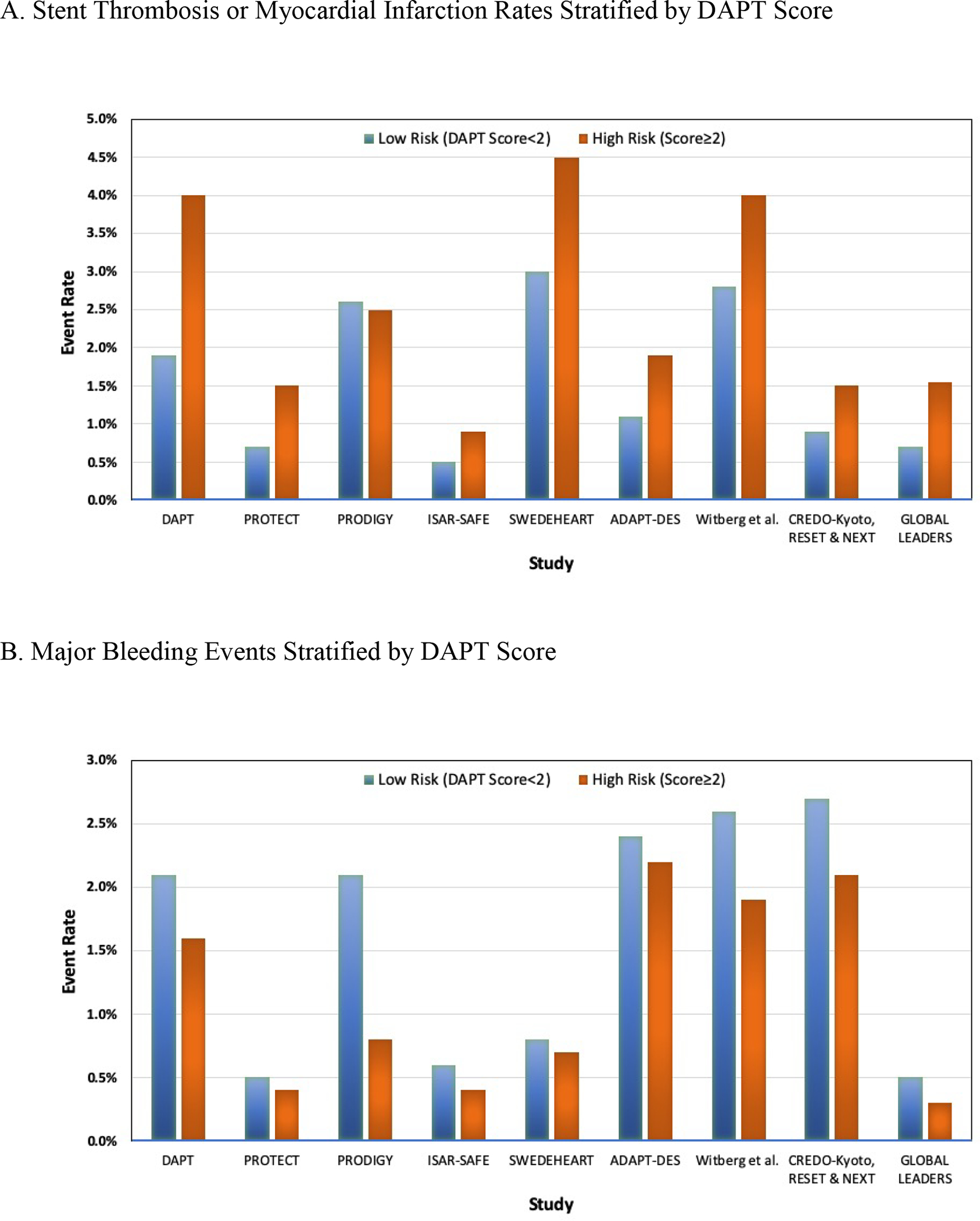
Ischemic (myocardial infarction and/or stent thrombosis; Panel A) and bleeding (Panel B) event rates across DAPT score validation studies stratified by high and low DAPT score.
In this issue of JACC: Cardiovascular Interventions, Chichareon et al. use the GLOBAL LEADERS study population to add to the collection of studies assessing DAPT score validity in a randomized trial of DAPT strategy (12). GLOBAL LEADERS tested, in a randomized fashion, a novel antiplatelet strategy of 1-year of DAPT (aspirin plus clopidogrel or ticagrelor) followed by 1-year of aspirin monotherapy and compared it to 1-month of DAPT (aspirin plus ticagrelor) with 23-months of ticagrelor among patients undergoing PCI for SIHD or ACS. This DAPT validation analysis, the largest randomized study population to externally validate the DAPT score to date, included only those patients who remained free of ischemic and bleeding events at 1-year. Thus, it effectively evaluates the DAPT score’s ability to discern ischemic benefit from bleeding harm with aspirin monotherapy versus ticagrelor monotherapy at 1-year following PCI, adding yet another antiplatelet strategy to test the DAPT score.
Chichareon et al. reaffirm that DAPT score’s ability to predict ischemic events, observing a more than 2-fold greater incidence of ischemic events in high score patients. Ticagrelor monotherapy was associated with an overall decreased ischemic event rate compared to aspirin, a reduction observed in both low and high DAPT score groups.
In assessing bleeding risk, despite the overall very low bleeding rates in the population, there was a numerically higher event rate with low DAPT score compared with high (0.54% vs. 0.30%), narrowly missing statistical significance (p = 0.058). Similarly, there was a higher rate of bleeding in the low DAPT group with ticagrelor compared to aspirin, although the interaction between the high and low DAPT group and treatment arm for bleeding was not significant. This observation, in combination with the absence of an expected difference in major bleeding between ticagrelor and aspirin and the lower than expected GLOBAL LEADERS primary event rates, suggests that the trial is underpowered in its ability of assess the DAPT score’s ability to stratify the treatment benefit of these different regimens on bleeding events. Therefore, conclusions around optimal antiplatelet strategies guided by the DAPT score would be premature, as the authors acknowledge.
In an effort to improve the score’s bleeding discernment in this population, the authors undertook an additional analysis performed utilizing a DAPT score cut-off of 1. In truth, dichotomizing the DAPT score at any point represents a statistical sacrifice in order to enhance the usability of the score in practice. We suggest that users of the DAPT score incorporate other clinical factors gleaned from the in-person evaluation of patients as well as patient preferences to help guide decisions, particularly for those individuals whose DAPT scores are in the intermediate range near the cutoff value.
Although the original GLOBAL LEADERS trial found no difference in bleeding or ischemic events between its two treatment arms, the differential treatment effect observed at 1-year in the original analysis and a separate prespecified landmark analysis of GLOBAL LEADERS corroborates a risk transition point between high upfront ischemic risk and downstream bleeding harm following PCI that continue to support interval risk re-evaluation and therapy modification (13,14). The optimal time point for this evaluation remains unknown but objective assessment of bleeding and ischemic risk remains critical in informing this decision. The DAPT score continues to show promise in its ability to stratify ischemic benefit now across nine study populations (4,6–12). While the bleeding analysis in this study is underpowered, the results remain similarly consistent. The score’s ultimate validation to guide treatment would entail the prospective randomization of patients to a score-based personalized DAPT regimen compared with usual care. Until then, however, a large and growing body of evidence supports the notion that clinicians can use the DAPT score to uncouple ischemic and bleeding risks in PCI patients - identifying high ischemic risk patients with lower bleeding risk, as well as high bleeding risk patients with lower ischemic risk.
CONFLICT OF INTEREST STATEMENT:
RWY has served on scientific advisory boards, consulted for, and received research support from Abbott Vascular, AstraZeneca, Boston Scientific, and Medtronic. He also receives funding from the National Heart, Lung, and Blood Institute (grant R01HL136708) and the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. NM receives funding from the National Institutes of Health (grant T32HL007208).
References:
- 1.Levine GN, Bates ER, Bittl JA, et al. Focused Update Writing Group, 2016 ACC/AHA Guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol 2016;68(10):1082–115. [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39(3):213–60. [DOI] [PubMed] [Google Scholar]
- 3.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115(17):2344–51. [DOI] [PubMed] [Google Scholar]
- 4.Yeh RW, Secemsky EA, Kereiakes DJ, et al. Development and Validation of a Prediction Rule for Benefit and Harm of Dual Antiplatelet Therapy Beyond 1 Year After Percutaneous Coronary Intervention. JAMA 2016;315(16):1735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371(23):2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda P, Jernberg T, James S, et al. External Validation of the DAPT Score in a Nationwide Population. J Am Coll Cardiol 2018;72(10):1069–78. [DOI] [PubMed] [Google Scholar]
- 7.Brener SJ, Kirtane AJ, Rinaldi MJ, et al. Prediction of ischemic and bleeding events using the dual antiplatelet therapy score in an unrestricted percutaneous coronary intervention population: analysis from the ADAPT-DES registry. Circ Cardiovasc Interv 2018;11(10):e006853. [DOI] [PubMed] [Google Scholar]
- 8.Harada Y, Michel J, Lohaus R, et al. Validation of the DAPT score in patients randomized to 6 or 12 months clopidogrel after predominantly second-generation drug-eluting stents. Thromb Haemost 2017;117(10):1989–99. [DOI] [PubMed] [Google Scholar]
- 9.Piccolo R, Gargiulo G, Franzone A, et al. Use of the Dual-Antiplatelet Therapy Score to Guide Treatment Duration After Percutaneous Coronary Intervention. Ann Intern Med 2017;167(1):17–25. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa Y, Shiomi H, Watanabe H, et al. Validating Utility of Dual Antiplatelet Therapy Score in a Large Pooled Cohort From 3 Japanese Percutaneous Coronary Intervention Studies. Circulation 2018;137(6):551–62. [DOI] [PubMed] [Google Scholar]
- 11.Witberg G, Zusman O, Bental T, et al. Validation of the DAPT score in real-world patients undergoing coronary stent implantation. Int J Cardiol 2019. Doi: 10.1016/j.ijcard.2019.08.044. [DOI] [PubMed] [Google Scholar]
- 12.Ply Chichareon et al. on behalf of the GLOBAL LEADERS investigators. Ischemic and bleeding risk stratification during the second year after contemporary PCI with DAPT score. JACC Cardiovasc Interv 2020. [Google Scholar]
- 13.Vranckx P, Valgimigli M, Jüni P, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet 2018;392(10151):940–9. [DOI] [PubMed] [Google Scholar]
- 14.Franzone A, McFadden E, Leonardi S, et al. Ticagrelor Alone Versus Dual Antiplatelet Therapy From 1 Month After Drug-Eluting Coronary Stenting. J Am Coll Cardiol 2019;74(18):2223–34. [DOI] [PubMed] [Google Scholar]


