Abstract
Context:
Accelerating evidence of endocrine-related morbidity has raised alarm about the ubiquitous use of phthalates in the human environment, but studies have not directly evaluated mortality in relation to these exposures.
Objectives:
To evaluate associations of phthalate exposure with mortality, and quantify attributable mortality and lost economic productivity in 2013–4 among 55–64 year olds.
Design, setting and participants:
This nationally representative cohort study included 5,303 adults aged 20 years or older who participated in the US National Health and Nutrition Examination Survey 2001–2010 and provided urine samples for phthalate metabolite measurements. Participants were linked to mortality data from survey date through December 31, 2015. Data analyses were conducted in July 2020.
Main Outcome Measures:
Mortality from all causes, cardiovascular disease, and cancer.
Results:
Multivariable models identified increased mortality in relation to high-molecular weight (HMW) phthalate metabolites, especially those of di-2-ethylhexylphthalate (DEHP). Hazard ratios (HR) for continuous HMW and DEHP metabolites were 1.14 (95% CI 1.06–1.23) and 1.10 (95% CI 1.03–1.19), respectively, with consistently higher mortality in the third tertile (1.48, 95% CI 1.19–1.86; and 1.42, 95% CI 1.13–1.78). Cardiovascular mortality was significantly increased in relation to a prominent DEHP metabolite, mono-(2-ethyl-5-oxohexyl)phthalate. Extrapolating to the population of 55–64 year old Americans, we identified 90,761–107,283 attributable deaths and $39.9–47.1 billion in lost economic productivity.
Conclusions:
In a nationally representative sample, phthalate exposures were associated with all-cause and cardiovascular mortality, with societal costs approximating $39 billion/year or more. While further studies are needed to corroborate observations and identify mechanisms, regulatory action is urgently needed.
Keywords: Phthalates, mortality, costs, attributable burden
Graphical Abstract
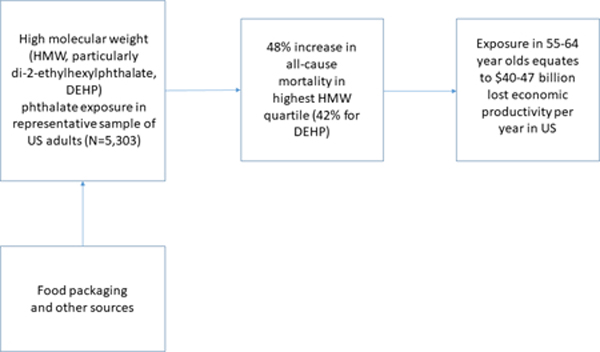
Introduction
Accelerating evidence of endocrine disruption has raised alarm about the ubiquitous use of phthalates in the human environment.1 Low-molecular weight (LMW) phthalates are frequently added to shampoos, lotions and other personal care products to preserve scent,2 while high-molecular (HMW) weight phthalates are ingredients in manufacturing of many plastics for flooring, food wraps and intravenous tubing.3,4 Di-2-ethylhexylphthalate (DEHP) is a HMW phthalate of particular interest because industrial food processes frequently use products containing DEHP.4 As a result of their broad use, metabolites of these chemicals are regularly found in nearly all Americans.5–8
Laboratory and animal studies have found that phthalates induce oxidative stress,9,10 estrogenicity,11 antagonism to androgens,8,12,13 and effects on expression of peroxisome-proliferator activated receptors,14 which are crucial to lipid and carbohydrate metabolism. Human studies have confirmed increases in prematurity,15–17 gestational diabetes,18–20 childhood21–23 and adult obesity,24 adult diabetes,25–27 cardiovascular disease,28 breast and thyroid cancers, 29–31 endometriosis,32 and infertility33 in relation to phthalate exposure.34 In 2015, an expert panel identified a 40–69% probability that phthalates contribute to cardiovascular mortality by decreasing testosterone levels in adult men.35 Yet, the contribution of phthalate exposures to mortality has not been systematically assessed.
Estimates of the burden of disease and disability, and the costs of environmentally attributable disease, have proven extremely useful to translate findings and inform policymaking. They are grounded in rigorous methodology first described by the US National Academy of Sciences (NAS) in a report led by the Nobel Laureate economist Kenneth Arrow.36 These can also be leveraged to document the potential economic benefits of policy actions, such as the phase-out of lead in gasoline.37,38 In addition to the import of mortality as an endpoint in policy-making, the additional lost economic productivity due to early mortality should be considered in tradeoffs against the additional societal costs of safer alternatives. We therefore leveraged data from a nationally representative cohort to examine the association of urinary phthalates with all-cause and cause-specific mortality in US adults. We also estimated the lost economic productivity due to phthalate-associated mortality.
Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative survey of civilian, noninstitutionalized residents, sampling rural, urban and suburban populations across the United States. It is administered by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC). NHANES collects questionnaire data through in-person interviews in Mobile Examination Centers and collects specimens for laboratory testing.
NHANES protocols are approved by the NCHS Ethics Review Board, with written informed consent obtained from all participants. The present study leverages publicly available, already collected and deidentified data, and Dr. Trasande completed a self-attestation form developed an NYU Grossman School of Medicine Institutional Review Board confirming that the present study represents non-human subjects research.
For the present analysis, we included adults aged 40 years or older who participated in NHANES during the period from 2001 to 2010 and had available urinary phthalate metabolite data. We linked all participants to mortality data through 2015, which enabled approximately 10 years of observation for mortality outcomes. We followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline for cohort studies.39
Measurement of Phthalate Metabolites
We provide a brief summary here, and refer the reader to a detailed description of the methods of phthalate monoester metabolite measurement in NHANES.40 Glucuronidated phthalate monoesters in spot urine samples were enzymatically deconjugated, and solid phase extraction was coupled with reversed phase high performance liquid chromatography-electrospray ionization-tandem mass spectrometry.41,42 Isotopically-labeled internal standards for each of the phthalate metabolites and 4-methyl umbelliferone glucuronide were added to improve assay precision, and monitor deconjugation of the monoesters. Levels of phthalate metabolites (12 in 2001–2002 samples, 15 in 2009–2010 samples) in urine were measured in one-third of randomly selected NHANES participants using at the Division of Laboratory Sciences, National Center for Environmental Health, CDC. Lower limits of detection (LLOD) varied from 0.1527–0.8 μg/L for the 2001 to 2002 samples and 0.2–0.84 μg/L for the 2009 to 2010 samples. The average percentage of samples below the LLOD was high for mono-cyclohexyl phthalate (93.3%), mono-isononyl phthalate (86.1%), mono-n-octyl phthalate (98.7%), mono-n-methyl phthalate (46.1%). Mono-(carboxyoctyl) phthalate and mono-(carboxynonyl) phthalate were only available in NHANES 2005–2006 to 2009–2010. Therefore, these metabolites were not included in this study. For levels below the LLOD (0–37.6% across the 9 phthalates), NHANES staff assigned a value of the LLOD divided by the square root of 2, following suggested practice.43
We grouped urinary biomarkers according to their use in product categories, calculating molar sums for LMW, HMW and DEHP metabolites, following the stratification used by previous authors, and their uses in consumer products.6 These are also produced in greater detail in the Supplement; briefly, LMW phthalates are generally used in personal care products and cosmetics, while HMW phthalates are found in flooring as well as food packaging materials, with DEHP particularly known for its contamination through food consumption and medical devices. All metabolite concentrations were log (base e)-transformed to account for skewed distribution. Our primary exposure variables were tertiles of metabolite groupings, though secondary analyses also examined the log-transformed total molar concentrations of LMW, HMW and DEHP metabolites and analyzed individual metabolites.
Mortality Data
We used the NHANES Public-Use Linked Mortality File through December 31, 2015, created by the NCHS by matching NHANES participants to the National Death Index with a probabilistic matching algorithm to determine mortality status.44 Underlying causes of death are categorized according to the International Classification of Diseases, Tenth Revision.45 NCHS classifies cardiovascular mortality as death from heart disease (codes I00-I09, I11, I13, and I20-I51) or cerebrovascular disease (codes I60-I69) and cancer mortality as death from malignant neoplasms (codes C00-C97).
Assessment of Covariates
NHANES collects questionnaire data on age, sex, race/ethnicity, educational level, family income, smoking status, alcohol drinking, physical activity, and dietary intake. Race/ethnicity was categorized into Hispanic (including Mexican and non-Mexican Hispanic), non-Hispanic White, non-Hispanic Black, and other. Family income was categorized as the ratio of family income to the federal poverty level (<1.0, 1.0–1.9, 2.0–3.9, and ≥4.0). Self-reported educational status was grouped as lower than high school, high school, and college or higher. In accordance with NCHS classifications, individuals who smoked <100 cigarettes in their lifetime were defined as never smokers; those who had smoked >100 cigarettes but did not smoke at the time of survey were considered former smokers; and those who had smoked >100 cigarettes in their lifetime and smoked cigarettes at the time of survey were considered current smokers. Alcohol intake was categorized as none (0 g/d), moderate drinking (0.1 to 27.9 g/d for men and 0.1 to 13.9 g/d for women), and heavy drinking (≥28 g/d for men and ≥14 g/d for women). Participants were asked an array of questions related to daily activities in the questionnaire, from which metabolic equivalent of task (MET) minutes per week were calculated, following previous practice.46 METs/week were categorized as below (less than 600 MET min/wk or 150 min/wk of moderate-intensity exercise); meeting (600 to 1200 MET min/wk or 150 to 300 min/wk of moderate-intensity exercise); or exceeding (at least 1200 MET min/wk or 300 min/wk of moderate-intensity exercise) 2008 guidelines. Dietary information was collected by 24-hour dietary recall interviews, from which total energy intake was calculated using the US Department of Agriculture Automated Multiple-Pass Method. We used the Healthy Eating Index-2010 (HEI-2010) to indicate overall quality of diet (ranging from 0, poor, to 100, optimal).47 Body weight and height were measured by trained health technicians following the NHANES Anthropometry Procedures Manual. Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared. To correct for urinary dilution, we controlled for urinary creatinine in all multivariable models, following usual practice.48,49
Statistical Analysis
Appropriately-weighted univariable, bivariable and multivariable analyses were conducted in a fashion that reflects the NHANES complex survey sampling design, following NCHS guidelines.50 All analyses applied two-sided tests, using a cutoff of p=0.05 for significance. All statistical analyses were conducted using the survey modules of SAS software, version 9.4 (SAS Institute: Cary, NC, USA).
We used Cox proportional hazards regression models to estimate hazard ratios (HR) and 95% CIs for the associations between each phthalate metabolite or category of metabolites and risk of mortality. Follow-up time was calculated as the difference between the NHANES examination date and the last known date alive or censored from the linked mortality file. Multivariable models sequentially added covariates as follows: (1) age, race/ethnicity, and creatinine; (2) sex, educational level, family income level, smoking status, alcohol intake, physical activity, total energy intake, and overall diet quality indicated by HEI-2010 score, and (3) BMI. To avert possible systematic bias introduced by a change in the urinary creatinine measurement method in 2007, we added a categorical variable representing NHANES wave to all models.
Analysis for Specificity of Association and Sensitivity to Design
We performed multiple analyses to interrogate the rigor of our associations. First, we used the E-value method51,52 to test whether and how our results were robust to potential unmeasured confounding. Second, we also performed unweighted analyses to confirm that findings were not artifacts of statistical weighting. Third, we repeated main analyses stratified by age, sex, race/ethnicity, diet quality, physical activity, and obesity status and confirmed interactions when P values for interaction were less than 0.05. Fourth, individuals with CVD or cancer at baseline were excluded to evaluate persistence of any observed associations. Fifth, because mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP) was not available in NHANES 2001–2002 we excluded MECPP from analyses to evaluate the effect of data availability on the observed associations.53 Sixth, we also examined associations with mono-(carboxyoctyl) phthalate and mono-(carboxynonyl) phthalate in NHANES 2005–2010 in separate models. Finally, as we also previously identified associations of bisphenol A (BPA) with mortality in a previous publication, we queried whether the associations of phthalates with mortality persisted despite addition of BPA to final models.
Estimates of Attributable Mortality and Lost Economic Productivity
We quantified mortality among 55–64 year olds, as this population was the basis for prior estimates of phthalate-attributable mortality,35 permitting closer comparison across methods. We computed these estimates using results from categorical and continuous models of phthalate exposure in multivariable regression analyses of all-cause mortality. Base-case estimates applied hazard ratios to the highest tertile of exposure derived from main statistical analyses with exposure as categorized in tertiles. As a sensitivity analysis, we applied the hazard ratios from multivariable models continuous exposure to the median exposure in each of the two higher tertiles to estimate increases in mortality in the US population.
For each of these two estimates, we first identified the baseline age-standardized mortality rate for 55–64 year olds in the US in 2014 from the CDC Wonder database.54 To generate the increment in death due to phthalates, we multiplied the age-standardized rate by the relative risk due to phthalate exposure, and subtracted the age-standardized rate. The resulting increment in death rate due to phthalate exposure was multiplied by the population of 55–64 year olds according to the Census Bureau55 to generate the attributable number of annual deaths. To generate estimates of lifetime economic productivity (LEP) loss due to death, we multiplied phthalate-attributable deaths by LEP estimates produced by Max et al.56 for 55–59 and 60–64 year olds from US sources in 2009 dollars, updating to 2014 using trends in general consumer prices from the US.57
Results
The median age of the study population (n=5,303) was 56.6 years (Table 1). Consistent with the demographics of the United States, 76.0% (SE 1.4) were non-Hispanic White, 9.1% (SE 1.0) were Hispanic and 10.2% (SE 0.8) were non-Hispanic Black. Nearly three-fifths of the sample were college-educated or higher, 52.6% (SE 0.9) were female and almost one tenth met the federal poverty definition. One-third were obese, while another third were overweight. Nearly half were past or current smokers, and nearly one-fifth reported moderate or heavy drinking. Levels of low-molecular weight phthalates were higher in younger participants, men, non-Hispanic Blacks and Hispanics as well as lower education subpopulations. DEHP metabolites were higher in males, more highly educated and moderately drinking populations (Table S1).
Table 1.
Phthalate Exposures and Associations with Sociodemographic and Behavioral Factors, NHANES 2001–2010 Linked Mortality File.
| Characteristic | No. | LMW | HMW | DEHP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | P | Tertile 1 | Tertile 2 | Tertile 3 | P | Tertile 1 | Tertile 2 | Tertile 3 | P | ||
| Number of participants | 5303 | 1768 | 1765 | 1769 | 1766 | 1768 | 1769 | 1766 | 1768 | 1769 | |||
| Age, years, mean ± SE | 57.9±0.4 | 56.2±0.3 | 55.4±0.4 | <0.0001 | 57.7±0.4 | 56.3±0.3 | 55.7±0.3 | <0.0001 | 57.4±0.4 | 56.5±0.4 | 55.8±0.4 | 0.001 | |
| Sex, % (SE) | |||||||||||||
| Male | 2663 | 45.6 (1.5) | 47.1 (1.4) | 49.7 (1.5) | 0.10 | 40.0 (1.3) | 51.2 (1.5) | 51.0 (1.5) | <0.0001 | 39.4 (1.4) | 52.2 (1.5) | 50.6 (1.5) | <0.0001 |
| Female | 2640 | 54.4 (1.5) | 52.9 (1.4) | 50.3 (1.5) | 60.0 (1.3) | 48.8 (1.5) | 49.0 (1.5) | 60.6 (1.4) | 47.8 (1.5) | 49.4 (1.5) | |||
| Race/ethnicity, % (SE) | |||||||||||||
| Hispanic | 1220 | 6.5 (1.0) | 8.7 (1.2) | 12.8 (1.3) | <0.0001 | 8.0 (1.0) | 10.2 (1.3) | 9.3 (1.1) | 0.001 | 7.7 (1.0) | 10.4 (1.4) | 9.4 (1.1) | 0.001 |
| Non-Hispanic whites | 2842 | 82.5 (1.4) | 76.6 (1.7) | 67.2 (2.1) | 78.6 (1.5) | 74.6 (1.8) | 74.6 (1.7) | 79.2 (1.4) | 73.4 (1.9) | 75.2 (1.8) | |||
| Non-Hispanic black | 1047 | 4.6 (0.5) | 10.9 (1.0) | 16.3 (1.4) | 7.5 (0.7) | 11.1 (1.1) | 12.0 (1.1) | 7.8 (0.7) | 11.2 (1.0) | 11.7 (1.1) | |||
| Other | 194 | 6.5 (0.9) | 3.7 (0.6) | 3.7 (0.6) | 5.9 (0.8) | 4.2 (0.7) | 4.0 (0.7) | 5.4 (0.7) | 5.0 (0.8) | 3.8 (0.7) | |||
| Education, % (SE) | |||||||||||||
| Less than high school | 1634 | 16.5 (1.0) | 19.7 (1.1) | 21.7 (1.4) | 0.001 | 18.6 (1.2) | 19.3 (1.2) | 19.4 (1.5) | 0.84 | 19.3 (1.2) | 19.7 (1.2) | 18.3 (1.4) | 0.89 |
| High school | 1274 | 24.0 (1.2) | 25.8 (1.3) | 27.1 (1.6) | 24.7 (1.2) | 26.0 (1.5) | 26.0 (1.3) | 25.3 (1.2) | 25.1 (1.5) | 26.2 (1.3) | |||
| College or higher | 2395 | 59.5 (1.6) | 54.6 (1.5) | 51.2 (2.0) | 56.7 (1.6) | 54.7 (1.8) | 54.6 (1.6) | 55.3 (1.6) | 55.2 (1.7) | 55.5 (1.7) | |||
| Family income to poverty ratio, % (SE) | |||||||||||||
| <1 | 797 | 8.6 (0.9) | 9.6 (0.8) | 10.7 (0.9) | 0.08 | 8.9 (0.8) | 10.0 (0.9) | 9.7 (0.9) | 0.9 | 9.3 (0.8) | 10.3 (0.9) | 9.1 (0.8) | 0.34 |
| 1.−1.9 | 1267 | 15.2 (1.0) | 17.2 (1.2) | 19.4 (1.2) | 18.0 (1.2) | 17.1 (1.2) | 16.2 (1.1) | 19.0 (1.2) | 16.8 (1.2) | 15.5 (1.0) | |||
| 2–3.9 | 1333 | 28.4 (1.7) | 26.4 (1.4) | 25.2 (1.2) | 26.1 (1.4) | 27.6 (1.3) | 26.8 (1.3) | 26.4 (1.4) | 27.2 (1.2) | 26.8 (1.4) | |||
| ≥4 | 1492 | 41.5 (1.9) | 39.8 (1.6) | 37.8 (1.7) | 40.0 (1.8) | 38.7 (1.9) | 40.8 (1.5) | 38.2 (1.9) | 39.4 (1.8) | 42.0 (1.5) | |||
| missing | 414 | 6.3 (0.7) | 6.9 (0.8) | 3.9 (0.7) | 7.0 (0.8) | 6.6 (0.7) | 6.5 (0.7) | 7.1 (0.8) | 6.3 (0.8) | 6.7 (0.7) | |||
| Alcohol drink, %(SE) | |||||||||||||
| Non-drinker | 3111 | 57.6 (1.4) | 57.3 (2.0) | 54.4 (1.6) | 0.74 | 54.6 (1.7) | 56.5 (1.8) | 58.6 (1.7) | 0.0001 | 52.3 (1.7) | 57.0 (1.7) | 60.4 (1.7) | <0.0001 |
| Moderate drinking | 362 | 6.9 (0.7) | 7.2 (0.9) | 7.2 (0.8) | 5.5 (0.6) | 6.9 (0.8) | 9.0 (0.8) | 4.8 (0.6) | 7.6 (0.8) | 9.0 (0.8) | |||
| Heavy drinking | 623 | 15.1 (1.3) | 14.7 (1.3) | 15.2 (1.1) | 14.7 (1.4) | 14.0 (1.0) | 16.2 (1.3) | 14.5 (1.3) | 14.1 (1.2) | 16.3 (1.3) | |||
| Missing | 1207 | 20.4 (1.4) | 20.8 (1.7) | 23.2 (1.4) | 25.3 (1.8) | 22.5 (1.6) | 16.2 (1.4) | 28.4 (1.8) | 21.2 (1.6) | 14.4 (1.5) | |||
| Smoke, % (SE) | |||||||||||||
| Never smoker | 2554 | 51.4 (1.5) | 49.7 (1.4) | 46.1 (1.6) | 0.06 | 49.2 (1.4) | 49.0 (1.4) | 49.6 (1.5) | 0.72 | 48.7 (1.5) | 49.1 (1.5) | 49.9 (1.5) | 0.21 |
| Ever smoker | 1696 | 30.7 (1.4) | 31.3 (1.5) | 31.2 (1.5) | 30.3 (1.3) | 30.9 (1.4) | 31.9 (1.4) | 30.0 (1.4) | 30.9 (1.3) | 32.4 (1.4) | |||
| Current smoker | 1053 | 17.9 (1.0) | 19.0 (1.5) | 22.7 (1.4) | 20.5 (1.3) | 20.1 (1.2) | 18.5 (1.0) | 21.5 (1.4) | 20.2 (1.2) | 17.7 (1.2) | |||
| Physical activity, % (SE) | |||||||||||||
| Below | 2531 | 41.8 (1.6) | 38.7 (1.2) | 43.3 (1.7) | 0.23 | 39.3 (1.7) | 43.0 (1.5) | 41.4 (1.5) | 0.44 | 41.2 (1.6) | 41.0 (1.8) | 41.5 (1.5) | 0.75 |
| Meet | 690 | 14.0 (1.0) | 15.8 (1.1) | 14.0 (1.1) | 14.2 (1.2) | 14.8 (1.0) | 14.7 (1.3) | 13.5 (1.2) | 15.7 (1.2) | 14.6 (1.3) | |||
| Exceed | 2082 | 44.2 (1.6) | 45.6 (1.3) | 42.8 (1.7) | 46.4 (1.6) | 42.2 (1.5) | 43.9 (1.7) | 45.3 (1.6) | 43.4 (1.7) | 43.9 (1.7) | |||
| BMI, % (SE) | |||||||||||||
| <25 kg/m2 | 1366 | 33.0 (1.9) | 28.9 (1.4) | 22.8 (1.3) | <0.0001 | 35.9 (1.6) | 27.8 (1.9) | 21.2 (1.3) | <0.0001 | 35.4 (1.7) | 28.8 (1.7) | 20.7 (1.3) | <0.0001 |
| 25–29.9 kg/m2 | 1899 | 34.3 (1.4) | 34.5 (1.6) | 34.3 (1.4) | 35.1 (1.4) | 33.2 (1.4) | 34.8 (1.4) | 35.4 (1.2) | 33.1 (1.5) | 34.5 (1.4) | |||
| ≥30 kg/m2 | 1934 | 31.4 (1.4) | 35.9 (1.6) | 41.2 (1.5) | 28.0 (1.4) | 37.2 (1.4) | 42.4 (1.6) | 28.1 (1.6) | 36.3 (1.3) | 43.1 (1.7) | |||
| Missing | 104 | 1.3 (0.4) | 1.5 (0.4) | 1.8 (0.4) | 1.1 (0.3) | 1.9 (0.4) | 1.7 (0.4) | 1.1 (0.3) | 1.9 (0.4) | 1.7 (0.4) | |||
| HEI2010 score, mean ± SE | 51.9±0.5 | 50.1±0.4 | 48.5±0.5 | <0.0001 | 52.6±0.6 | 49.6±0.5 | 48.5±0.5 | <0.0001 | 52.0±0.6 | 49.9±0.5 | 48.8±0.5 | <0.0001 | |
| Total energy intake, kcal/d, mean ± SE | 2043.2±27.8 | 2047.5±31.3 | 2141.2±37.0 | 0.04 | 1937.9±29.7 | 2129.1±31.5 | 2145.8±34.1 | <0.0001 | 1940.7±30.5 | 2114.9±31.3 | 2149.0±35.4 | <0.0001 | |
Multivariable models (Table 2) identified increased all-cause mortality in relation to higher tertiles of HMW (HR for highest quartile 1.48, 95% CI 1.19–1.86) and DEHP metabolites (HR for highest quartile 1.42, 95% CI 1.13–1.78). All-cause mortality was also significantly related to continuous, log (base e)-transformed measures of HMW (HR 1.14, 95% CI 1.06–1.23) and DEHP (HR 1.10, 95% CI 1.03–1.19). When cardiovascular mortality was the outcome (Table S2), continuous models were nearly significant for DEHP with significant increases in the second quartile that persisted until BMI was added in full models. There were no significant associations with cancer mortality as the outcome (Table S3).
Table 2.
Association of Urinary Phthalate Metabolites with All-cause Mortality, NHANES 2001–2010 Linked Mortality File. (n=5,303)
| Continuous | Tertiles |
|||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| LMW | ||||
| Median level (SE), µmol/L | 0.90 (0.03) | 0.28 (0.01) | 1.02 (0.02) | 4.53 (0.16) |
| Deaths/person-years | 1014/46825 | 379/15026 | 326/15629 | 309/16169 |
| HR (95% CI) | ||||
| Model 1 | 1.03 (0.98–1.08) | 1.0 (ref) | 1.01 (0.83–1.24) | 1.16 (0.98–1.39) |
| Model 2 | 1.01 (0.96–1.06) | 1.0 (ref) | 0.95 (0.77–1.17) | 1.10 (0.92–1.33) |
| Model 3 | 1.01 (0.96–1.07) | 1.0 (ref) | 0.96 (0.78–1.18) | 1.11 (0.91–1.34) |
|
| ||||
| HMW | ||||
| Median level (SE), µmol/L | 0.22 (0.006) | 0.08 (0.002) | 0.22 (0.003) | 0.63 (0.02) |
| Deaths/person-years | 1014/46825 | 324/15780 | 346/15533 | 344/15512 |
| HR (95% CI) | ||||
| Model 1 | 1.14 (1.05–1.23) | 1.0 (ref) | 1.33 (1.09–1.61) | 1.55 (1.23–1.96) |
| Model 2 | 1.15 (1.06–1.24) | 1.0 (ref) | 1.30 (1.08–1.58) | 1.50 (1.20–1.88) |
| Model 3 | 1.14 (1.06–1.23) | 1.0 (ref) | 1.28 (1.05–1.55) | 1.48 (1.19–1.86) |
|
| ||||
| DEHP | ||||
| Median level (SE), µmol/L | 0.16 (0.004) | 0.05 (0.001) | 0.16 (0.002) | 0.47 (0.01) |
| Deaths/person-years | 1014/46825 | 340/15917 | 344/15471 | 330/15437 |
| HR (95% CI) | ||||
| Model 1 | 1.09 (1.01–1.17) | 1.0 (ref) | 1.19 (0.98–1.45) | 1.38 (1.09–1.75) |
| Model 2 | 1.12 (1.04–1.20) | 1.0 (ref) | 1.21 (0.98–1.48) | 1.44 (1.14–1.82) |
| Model 3 | 1.10 (1.03–1.19) | 1.0 (ref) | 1.18 (0.97–1.45) | 1.42 (1.13–1.78) |
Model 1: adjustment for age, race/ethnicity, and urinary creatinine.
Model 2: model 1 + education levels, family income status, smoking, alcohol use, physical activity, total energy intake, HEI2010 score, survey year.
Model 3: model 2 + BMI.
When specific metabolites were examined (Table 3), increased cardiovascular mortality was identified in relation to the highest tertile of monoethylphthalate (MEP, a LMW metabolite; HR 1.64, 95% CI 1.07–2.51), though continuous MEP was not significantly associated. The consistent associations were for two DEHP metabolites (mono-(2-ethyl-5-hydroxyhexyl) phthalate, MEHHP, and MECPP) with all-cause mortality, and one DEHP metabolite, mono-(2-ethyl-5-oxohexyl)-phthalate (MEOHP), with all-cause and cardiovascular mortality. Log-transformed MEHHP (1.09, 95% CI 1.02–1.17) and MEOHP (1.02, 95% CI 1.02–1.17) was significantly associated with greater all-cause mortality, while MECPP approached significance (1.09, 95% CI 0.997–1.18). Highest tertiles of MEHHP (1.27, 95% CI 1.01–1.589), MEOHP (1.32, 95% CI 1.08–1.62) and MECPP (1.31, 95% CI 1.04–1.64) were associated with all-cause mortality, and MEOHP was associated with cardiovascular mortality whether examined continuously (1.18, 95% CI 1.04–1.35) or by tertiles (highest: 1.74, 95% CI 1.05–2.88).
Table 3.
Association of Individual Urinary Phthalate Metabolite with Mortality, NHANES 2001–2010.
| All-cause mortality | CVD mortality | Cancer mortality | |
|---|---|---|---|
| mono-ethyl phthalate (MEP) | |||
| Continuous | 1.01 (0.96–1.06) | 1.09 (0.98–1.21) | 0.97 (0.88–1.07) |
| Tertile 1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Tertile 2 | 0.97 (0.79–1.18) | 1.34 (0.90–2.01) | 0.98 (0.65–1.48) |
| Tertile 3 | 1.12 (0.93–1.35) | 1.64 (1.07–2.51) | 0.97 (0.60–1.58) |
| mono-benzyl phthalate (MBzP) | |||
| Continuous | 1.11 (1.04–1.19) | 1.03 (0.88–1.20) | 1.19 (1.04–1.36) |
| Tertile 1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Tertile 2 | 1.13 (0.92–1.40) | 1.05 (0.66–1.67) | 1.17 (0.72–1.92) |
| Tertile 3 | 1.20 (0.96–1.50) | 0.86 (0.49–1.50) | 1.25 (0.76–2.05) |
| mono-n-butyl phthalate (MnBP) | |||
| Continuous | 1.10 (1.03–1.19) | 1.14 (0.96–1.35) | 1.17 (0.996–1.38) |
| Tertile 1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Tertile 2 | 1.31 (1.03–1.66) | 1.69 (0.97–2.96) | 1.43 (0.90–2.27) |
| Tertile 3 | 1.21 (0.94–1.54) | 1.32 (0.73–2.38) | 1.35 (0.80–2.25) |
| mono-isobutyl phthalate (MiBP) | |||
| Continuous | 1.07 (0.99–1.16) | 1.06 (0.88–1.27) | 1.04 (0.85–1.27) |
| Tertile 1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Tertile 2 | 0.97 (0.80–1.18) | 1.08 (0.71–1.64) | 0.96 (0.65–1.41) |
| Tertile 3 | 1.22 (0.95–1.57) | 0.86 (0.50–1.50) | 1.16 (0.65–2.09) |
| mono-(3-carboxypropyl) phthalate (MCPP) | |||
| Continuous | 1.07 (0.996–1.14) | 0.97 (0.84–1.12) | 0.99 (0.86–1.14) |
| Tertile 1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Tertile 2 | 0.92 (0.75–1.13) | 0.76 (0.47–1.24) | 0.86 (0.57–1.30) |
| Tertile 3 | 1.17 (0.95–1.45) | 0.98 (0.64–1.50) | 0.87 (0.57–1.33) |
| mono-2-ethyl hexyl phthalate(MEHP) | |||
| Continuous | 1.02 (0.94–1.09) | 0.95 (0.81–1.10) | 0.98 (0.86–1.11) |
| Tertile 1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Tertile 2 | 0.92 (0.78–1.10) | 1.37 (0.95–1.98) | 0.92 (0.61–1.39) |
| Tertile 3 | 1.07 (0.88–1.30) | 0.95 (0.60–1.48) | 1.03 (0.70–1.52) |
| mono-(2-ethyl-5hydroxyhexyl) phthalate (MEHHP) | |||
| Continuous | 1.09 (1.02–1.17) | 1.14 (0.98–1.32) | 1.03 (0.88–1.19) |
| Tertile 1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Tertile 2 | 1.01 (0.84–1.21) | 1.22 (0.76–1.97) | 0.88 (0.57–1.36) |
| Tertile 3 | 1.27 (1.01–1.59) | 1.31 (0.70–2.47) | 0.92 (0.57–1.48) |
| mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) | |||
| Continuous | 1.09 (1.02–1.17) | 1.18 (1.04–1.35) | 1.00 (0.86–1.17) |
| Tertile 1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Tertile 2 | 1.07 (0.89–1.28) | 1.39 (0.96–2.03) | 0.70 (0.45–1.09) |
| Tertile 3 | 1.32 (1.08–1.62) | 1.74 (1.05–2.88) | 0.84 (0.52–1.35) |
| mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP) | |||
| Continuous | 1.09 (0.997–1.18) | 1.12 (0.92–1.37) | 0.95 (0.80–1.12) |
| Tertile 1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Tertile 2 | 1.13 (0.92–1.39) | 1.56 (0.96–2.55) | 0.92 (0.52–1.61) |
| Tertile 3 | 1.31 (1.04–1.64) | 1.29 (0.72–2.32) | 1.11 (0.70–1.78) |
| mono-(carboxyoctyl)phthalate (MCOP) | |||
| Continuous | 1.03 (0.91–1.17) | 0.97 (0.71–1.33) | 1.01 (0.85–1.20) |
| Tertile 1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Tertile 2 | 1.11 (0.83–1.47) | 0.63 (0.28–1.44) | 1.02 (0.64–1.64) |
| Tertile 3 | 1.14 (0.78–1.66) | 1.05 (0.46–2.40) | 1.14 (0.64–2.03) |
| mono-(carboxynonyl) phthalate (MCNP) | |||
| Continuous | 1.06 (0.95–1.19) | 1.06 (0.81–1.40) | 0.99 (0.78–1.25) |
| Tertile 1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Tertile 2 | 1.12 (0.83–1.52) | 0.60 (0.34–1.06) | 0.89 (0.49–1.62) |
| Tertile 3 | 1.09 (0.79–1.49) | 0.91 (0.43–1.93) | 0.63 (0.30–1.34) |
Mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP) was available in NHANES 2003–2010 (n=4,271).
Mono-(carboxyoctyl)phthalate (MCOP) and mono-(carboxynonyl) phthalate (MCNP) are only available in NHANES 2005–2010 (n=3,310).
All models adjusted for age, race/ethnicity, urinary creatinine, education levels, family income status, smoking, alcohol use, physical activity, total energy intake, HEI2010 score, survey year and BMI.
Sensitivity analyses revealed robust associations with all-cause mortality (for HMW and DEHP exposures and individual metabolites) and cardiovascular mortality (for individual DEHP metabolites). E-values for the association of HMW and DEHP were 1.95 (lower limit of 95% CI 1.51) and 1.87 (lower limit of 95% CI 1.40). E-values for the estimate (and E-value for the lower limit of the 95% CIs) for third tertiles of MEHHP, MEOHP and MECPP with all-cause mortality were 1.64 (1.09), 1.72 (1.30) and 1.70 (1.20), higher than the observed primary HRs for association of 1.27, 1.32 and 1.31. E-values (and lower limits for 95% CI) for the third tertile of MEP and MEOHP with cardiovascular mortality were 2.66 (1.34) and 2.87 (1.28), compared to the observed HRs of 1.64 and 1.74. These HR were higher than the HRs of many of the known confounders that were measured in this study (e.g., age, which had an HR of 1.10 with all-cause mortality). Therefore, it is less likely that unmeasured confounding would otherwise explain all-cause mortality more than the known risk factors also evaluated. Unweighted models strengthened rather than diminished primary associations observed in weighted models (Tables S4-S7). When all-cause and cardiovascular mortality models were stratified by potential confounders, no interaction tests were significant for HMW and DEHP (Tables S8 and S9). Exclusion of CVD or cancer at baseline did not attenuate associations with all-cause mortality (Table S10). Exclusion of NHANES 2001–2002 participants attenuated DEHP associations with all-cause mortality to null, though associations with HMW remained significant (Table S11). Removal of MECPP from calculation of HMW and DEHP metabolites did not alter their relationshiops with all-cause mortality when exposure was measured either as a continuous or categorical variable (Table S12). Mono-(carboxyoctyl) phthalate and mono-(carboxynonyl) phthalate were not associated with mortality in NHANES 2005–2010 (Table S13). Addition of BPA to models failed to attenuate associations of HMW with mortality, though associations of DEHP did attenuate to near-significance (Table S14).
In main analyses assuming effects only in the most highly exposed quartile of DEHP metabolites, we identified 107,283 attributable deaths, costing $47.1 billion in lost economic productivity. This is an annual societal cost insofar as exposures continue at current levels. Using relative risks derived from continuous models of all-cause mortality, we identified 90,761 deaths and $39.9 billion in social costs (Table 4).
Table 4.
Phthalate-attributable mortality and associated lost economic productivity in the US, 2014.
| Base Case Estimate (Derived From Hazard Ratio for All-Cause Mortality of Highest Tertile) | |
|---|---|
| Mortality Rate, 55–64 year olds, 2013–14 | 965.2/100,000 |
| Hazard Ratio in Most Highly Exposed Tertile of DEHP metabolites, 2003–8 NHANES | 1.48 |
| Incremental Mortality Rate (Based upon Relative Risk) | 415/100,000 |
| Population in Highest Tertile, 55–64 year olds, 2013–14 | 26,464,671 |
| Attributable Deaths, 55–64 year olds | 107,283 |
| Lifetime Economic Productivity Per Adult, 55–64 year olds (2014 dollars) | $439,313 |
| Attributable Annual Cost of Phthalate Exposure, 2013–14 | $47.1 billion |
| Sensitivity Analysis (Hazard Ratios Derived From Continuous Models of All Cause Mortality) | Lowest tertile | Second tertile | Highest tertile |
|---|---|---|---|
| Level of DEHP Metabolite (Derived from Median of Tertile) | 0.05 µmolar | 0.16 µmolar | 0.47 µmolar |
| Mortality Rate, 55–64 year olds, 2013–14 | 965.2/100,000 | ||
| Hazard Ratio of Mortality | N/A | 1.12 | 1.24 |
| Incremental Mortality Rate (Based upon Relative Risk) | N/A | 113/100 000 | 230/100,000 |
| Population in Percentile of Exposure, 55–64 year olds, 2013–14 | 26,464,671 | 26,464,671 | 26,464,671 |
| Attributable Deaths, 55–64 year olds | N/A | 29,947 | 60,815 |
| Lifetime Economic Productivity Per Adult, 55–64 year olds (2014 dollars) | $439,313 | ||
| Attributable Annual Cost of Phthalate Exposure, 2013–14 | N/A | $13.2 billion | $26.7 billion |
Discussion
The present study potentially increase the probability that phthalates contribute to cardiovascular mortality in adult men, and suggest that increased risk also exists among adult women. The lost economic productivity due to these exposures is large, on the order of $40–47 billion annually for people 55–64 years of age, much greater than a previous estimate of phthalate-associated mortality, $8.8 billion/year, based upon decreases in testosterone in adult men.35
Strengths of this analysis include the use of a nationally representative dataset, with careful documentation of cause of death. We were able to control for an abundant array of potential confounders, including but not limited to diet, physical activity and adiposity. There are a number of limitations. We appreciate a spot urine is limited in its capacity to represent chronic, long-term cardiovascular mortality as identified here, given that the typical half-life for phthalates is on the order of one to three days.58 However, previous evidence has suggested that a single spot-sampling approach may adequately reflect average population exposure when urine samples are collected from a sufficiently large population with random meal ingestion and bladder emptying times.59 Despite the use of the National Death Index, we cannot rule out possible errors in cause of death. There may also be residual confounding, though sensitivity analysis using E-values showed that the association of phthalates could only be negated by unmeasured confounder that had associations both with phthalate exposure and mortality with a moderate HR, in the range of >1.30.
We were not surprised to find cardiovascular mortality in relation to phthalate exposures, and particularly phthalates used in food contact materials. Mono-(2-ethylhexyl) phthalate (MEHP), a DEHP metabolite, increases expression of three peroxisome proliferator-activated receptors (PPARs)14 which play key roles in lipid and carbohydrate metabolism, providing biological plausibility for DEHP metabolites in childhood and diabetes. Emerging animal evidence also suggests that DEHP may produce arrhythmia,60 change metabolic profiles and produce dysfunction in cardiac myocytes.61 Longitudinal studies of adults have confirmed weight gain and incident diabetes in exposed adults.34 One explanation of the observed results is that phthalates increase cardiovascular morbidity but not mortality, which requires further study in large longitudinal cohorts of adults with and without antecedent cardiovascular risks such as obesity and diabetes. That said, the findings suggest an opportunity to reduce cardiovascular disease by limiting or eliminating environmental contamination of phthalates, such as phthalates in food.
While some will argue that economic analyses are premature in the case of phthalate-induced cardiovascular mortality, it is important to consider the criteria first described by Sir Austin Bradford Hill for evaluating whether sufficient evidence exists to proscribe public health action.62 Leaving aside previous evidence for cardiovascular risks that further supports attribution, cardiovascular mortality is hardly the only endpoint known to be affected by this group of endocrine-disrupting chemicals (EDCs). We acknowledge that weighting by probability of causation should be considered in these and other costs of EDCs.35,63–65 We also acknowledge extrapolation from a single study is limited in calculating attributable deaths. We note that LEP is typically much lower than the value of a statistical life for the same age, and so we may have underestimated the costs of phthalate-induced mortality.66
Regulatory agencies have the power to require the use of safer alternatives in cosmetics, personal care products and food packaging. Already, the USA, Canada, Israel, Brazil, Hong Kong, Australia and China have all restricted or banned diethylhexylphthalate (DEHP), dibutylphthalate (DBP) and butylbenzylphthalate (BBP) in toys.63 However, there are fewer limits on phthalates in food contact materials and cosmetics. The European Food Safety Authority recently announced that current exposure to five phthalates commonly used in food contact materials “is not a concern for public health,”67 while the US Food and Drug Administration still allows the use of phthalates in food packaging under the Generally Recognized as Safe designation of the Federal Food Drug and Cosmetic Act (FFDCA).68 Europe has banned DBP69 and DEHP70 from cosmetics, but the FDA has not used its regulatory authority under FFDCA to limit their use in the US.71
Given the multiple phthalates, sources of exposure and potential adverse outcome pathways, the present study focuses substantial urgency in further limits on DEHP in food contact materials and other consumer products. Due to the lag between measurement and mortality, we were unable to evaluate replacements of DEHP that have emerged over the past decade, including diisononyl (DINP) and diisodecylphthalate (DIDP). They have been associated with adverse metabolic endpoints previously associated with DEHP.72,73 Alternatives to plastic in food packaging also exist (e.g., glass, stainless steel), and alternatives to plasticizers free of the hazards posed by antiandrogenic and oxidative stress may not be readily available, or may introduce other adverse effects. Given the body of evidence suggest ecological effects of plastic use, this study adds to the public health and business cases for reducing or eliminating the use of plastics. The latency of cardiovascular disease to mortality will require additional time before DIDP and DINP can be interrogated as risks, but the existing body of evidence suggests we will repeat history rather than learn from the failure to regulate other endocrine disruptors, such as bisphenols, as classes, rather than individual compounds.74
Conclusion
We identify substantial all-cause and cardiovascular mortality in a nationally representative sample in relation to phthalate exposures. The cost due to lost economic productivity are approximately $40 billion/year or more. While further studies are needed to examine the specific cancers driving the observed effects, the results are consistent with a large body of evidence supporting antiandrogenicity observed in laboratory and human studies. In the context of a broader body of evidence confirming broad effects of these ubiquitous contaminants, regulatory action is urgently needed to reduce these preventable exposures.
Supplementary Material
Highlights.
Phthalate exposures were associated with all-cause and cardiovascular mortality.
Further studies are needed to corroborate observations and identify mechanisms.
Extrapolating to 55–64 year olds, we identified >90,000 attributable deaths/year.
The results suggest $39.9–47.1 billion in lost economic productivity/year.
Regulatory action is urgently needed.
Acknowledgments
We thank the participants and staff of the National Health and Nutrition Examination Survey and the National Center for Environmental Health for their valuable contributions.
The authors acknowledge support from the National Institutes of Health (grants R01ES022972, R01ES029779, R01ES032214, P30ES000260 and P30ES005605). Funding sources had no role in the study.
LT acknowledges honoraria from Houghton Mifflin Harcourt, Audible, Paidos and Kobunsha; travel support from the Endocrine Society, WHO, UNEP, Japan Environment and Health Ministries and the American Academy of Pediatrics; as well as scientific advisory board activities for Beautycounter, IS-Global and Footprint.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Gore AC, Chappell VA, Fenton SE, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015:er20151010. [DOI] [PMC free article] [PubMed]
- 2.Sathyanarayana S. Phthalates and children’s health. Curr Probl Pediatr Adolesc Health Care. 2008;38(2):34–49. [DOI] [PubMed] [Google Scholar]
- 3.Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29(1):134–139. [DOI] [PubMed] [Google Scholar]
- 4.Fromme H, Gruber L, Schlummer M, et al. Intake of phthalates and di(2-ethylhexyl)adipate: Results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environment International. 2007;33(8):1012–1020. [DOI] [PubMed] [Google Scholar]
- 5.Trasande L, Attina TM. Association of Exposure to Di-2-Ethylhexylphthalate Replacements with Increased Insulin Resistance in Adolescents from NHANES 2009–2012. J Clin Endocrinol Metab. 2015:jc20151686. [DOI] [PMC free article] [PubMed]
- 6.Trasande L, Spanier AJ, Sathyanarayana S, Attina TM, Blustein J. Urinary phthalates and increased insulin resistance in adolescents. Pediatrics. 2013;132(3):e646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weuve J, Hauser R, Calafat AM, Missmer SA, Wise LA. Association of exposure to phthalates with endometriosis and uterine leiomyomata: findings from NHANES, 1999–2004. Environmental health perspectives. 2010;118(6):825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodward MJ, Obsekov V, Jacobson MH, Kahn LG, Trasande L. Phthalates and Sex Steroid Hormones Among Men From NHANES, 2013–2016. J Clin Endocrinol Metab. 2020;105(4):e1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gourlay T, Samartzis I, Stefanou D, Taylor K. Inflammatory Response of Rat and Human Neutrophils Exposed to Di-(2-ethyl-hexyl)-phthalate-Plasticized Polyvinyl Chloride. Artificial Organs. 2003;27(3):256–260. [DOI] [PubMed] [Google Scholar]
- 10.Jepsen KF, Abildtrup A, Larsen ST. Monophthalates promote IL-6 and IL-8 production in the human epithelial cell line A549. Toxicology in Vitro. 2004;18(3):265–269. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Xu S, Tan T, et al. Toxicity and estrogenic endocrine disrupting activity of phthalates and their mixtures. International journal of environmental research and public health. 2014;11(3):3156–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urinary Phthalate Metabolites Are Associated With Decreased Serum Testosterone in Men, Women, and Children From NHANES 2011–2012. The Journal of Clinical Endocrinology & Metabolism. 2014;99(11):4346–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desdoits-Lethimonier C, Albert O, Le Bizec B, et al. Human testis steroidogenesis is inhibited by phthalates. Human reproduction (Oxford, England). 2012;27(5):1451–1459. [DOI] [PubMed] [Google Scholar]
- 14.Desvergne B, Feige JN, Casals-Casas C. PPAR-mediated activity of phthalates: A link to the obesity epidemic? Molecular and cellular endocrinology. 2009;304(1–2):43–48. [DOI] [PubMed] [Google Scholar]
- 15.Gao H, Wang Y-f, Huang K, et al. Prenatal phthalate exposure in relation to gestational age and preterm birth in a prospective cohort study. Environmental Research. 2019;176:108530. [DOI] [PubMed] [Google Scholar]
- 16.Latini G, De Felice C, Presta G, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environmental health perspectives. 2003;111(14):1783–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatrics. 2014;168(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martínez-Ibarra A, Martínez-Razo LD, Vázquez-Martínez ER, et al. Unhealthy Levels of Phthalates and Bisphenol A in Mexican Pregnant Women with Gestational Diabetes and Its Association to Altered Expression of miRNAs Involved with Metabolic Disease. International journal of molecular sciences. 2019;20(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaffer RM, Ferguson KK, Sheppard L, et al. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ Int. 2019;123:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James-Todd TM, Meeker JD, Huang T, et al. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ Int. 2016;96:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valvi D, Casas M, Romaguera D, et al. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect. 2015. [DOI] [PMC free article] [PubMed]
- 22.Deierlein AL, Wolff MS, Pajak A, et al. Longitudinal Associations of Phthalate Exposures During Childhood and Body Size Measurements in Young Girls. Epidemiology. 2016;27(4):492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harley KG, Berger K, Rauch S, et al. Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Pediatr Res. 2017;82(3):405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Y, Hauser R, Hu FB, Franke AA, Liu S, Sun Q. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. International journal of obesity (2005). 2014. [DOI] [PMC free article] [PubMed]
- 25.Radke EG, Galizia A, Thayer KA, Cooper GS. Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence. Environment International. 2019;132:104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Q, Cornelis MC, Townsend MK, et al. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environmental health perspectives. 2014;122(6):616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lind PM, Zethelius B, Lind L. Circulating Levels of Phthalate Metabolites Are Associated With Prevalent Diabetes in the Elderly. Diabetes Care. 2012;35(7):1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauser R, Skakkebaek NE, Hass U, et al. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European union. J Clin Endocrinol Metab. 2015;100(4):1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahern TP, Broe A, Lash TL, et al. Phthalate Exposure and Breast Cancer Incidence: A Danish Nationwide Cohort Study. Journal of Clinical Oncology. 2019;37(21):1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marotta V, Russo G, Gambardella C, et al. Human exposure to bisphenol AF and diethylhexylphthalate increases susceptibility to develop differentiated thyroid cancer in patients with thyroid nodules. Chemosphere. 2019;218:885–894. [DOI] [PubMed] [Google Scholar]
- 31.Miao H, Liu X, Li J, et al. Associations of urinary phthalate metabolites with risk of papillary thyroid cancer. Chemosphere. 2020;241:125093. [DOI] [PubMed] [Google Scholar]
- 32.Buck Louis GM, Peterson CM, Chen Z, et al. Bisphenol A and phthalates and endometriosis: the Endometriosis: Natural History, Diagnosis and Outcomes Study. Fertility and sterility. 2013;100(1):162–169.e161–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol A, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertility and sterility. 2014;101(5):1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: implications for human health. The Lancet Diabetes & Endocrinology. 2020;8(8):703–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attina TM, Hauser R, Sathyanarayana S, et al. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. The lancet Diabetes & endocrinology. 2016;4(12):996–1003. [DOI] [PubMed] [Google Scholar]
- 36.Institute of Medicine. Costs of Environment-Related Health Effects. Washington DC: National Academy Press. 1981. [Google Scholar]
- 37.Grosse SD, Matte TD, Schwartz J, Jackson RJ. Economic Gains Resulting from the Reduction in Children’s Exposure to Lead in the United States. Environmental Health Perspectives. 2002;110(6):563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai PL, Hatfield TH. Global benefits of phasing out leaded fuel. J Environ Health 2011;74(5):8–15. [Google Scholar]
- 39.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. [DOI] [PubMed] [Google Scholar]
- 40.Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/highperformance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77(9):2985–2991. [DOI] [PubMed] [Google Scholar]
- 41.Silva MJ, Barr DB, Reidy JA, et al. Urinary Levels of Seven Phthalate Metabolites in the U.S. Population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2003;112(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860(1):106–112. [DOI] [PubMed] [Google Scholar]
- 43.Trasande L, Attina TM, Sathyanarayana S, Spanier AJ, Blustein J. Race/Ethnicity–Specific Associations of Urinary Phthalates with Childhood Body Mass in a Nationally Representative Sample. 2013;121(4):501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention (National Center for Health Statistics). The Linkage of National Center for Health Statistics Survey Data to the National Death Index — 2015 Linked Mortality File (LMF): Methodology Overview and Analytic Considerations. 2019. Accessed 26 August, 2020.
- 45.Brämer GR. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat Q. 1988;41(1):32–36. [PubMed] [Google Scholar]
- 46.Bao W, Liu B, Rong S, Dai SY, Trasande L, Lehmler HJ. Association Between Bisphenol A Exposure and Risk of All-Cause and Cause-Specific Mortality in US Adults. JAMA network open. 2020;3(8):e2011620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental health perspectives. 2008;116(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environmental health perspectives. 2009;117(5):784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lauer RM, Clarke WR, Burns TL. Obesity in childhood: the Muscatine Study. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1997;38(6):432–437. [PubMed] [Google Scholar]
- 51.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational Studies. Jama. 2019;321(6):602–603. [DOI] [PubMed] [Google Scholar]
- 52.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 53.CDC. Phthalates, Phytoestrogens & PAHs - Urine (PHPYPA_B). Available at https://wwwn.cdc.gov/Nchs/Nhanes/2001-2002/PHPYPA_B.htm (Accessed 26 July 2021). 2021.
- 54.CDC WONDER Online Databases. Mortality Rates. Available at http://wonder.cdc.gov/ (Accessed 10 May 2016). 2010.
- 55.Census Bureau. Population estimates. Available at https://www.census.gov/popest/data/ (Accessed 21 January, 2016). 2010.
- 56.Max W. Present Value of Lifetime Earnings, 2009. Unpublished tables, Institute for Health and Aging, University of California, San Francisco. 2013. [Google Scholar]
- 57.Bureau of Labor Statistics. Consumer Price Index. Avaialable at http://www.bls.gov/cpi/ (Accessed 21 Jan 2016). 2010.
- 58.Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environmental health perspectives. 2002;110(5):515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye X, Pierik FH, Hauser R, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environ Res. 2008;108(2):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Posnack NG, Lee NH, Brown R, Sarvazyan N. Gene expression profiling of DEHPtreated cardiomyocytes reveals potential causes of phthalate arrhythmogenicity. Toxicology. 2011;279(1–3):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Posnack NG, Swift LM, Kay MW, Lee NH, Sarvazyan N. Phthalate Exposure Changes the Metabolic Profile of Cardiac Muscle Cells. Environ Health Perspect. 2012. [DOI] [PMC free article] [PubMed]
- 62.Hill A. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58(5):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kassotis CD, Vandenberg LN, Demeneix BA, Porta M, Slama R, Trasande L. Endocrine-disrupting chemicals: economic, regulatory, and policy implications. The lancet Diabetes & endocrinology. 2020;8(8):719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trasande L, Zoeller RT, Hass U, et al. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European union. J Clin Endocrinol Metab. 2015;100(4):1245–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trasande L, Zoeller RT, Hass U, et al. Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: an updated analysis. Andrology. 2016. [DOI] [PMC free article] [PubMed]
- 66.Kniesner TJ, Viscusi WK. The Value of a Statistical Life. In: Oxford University Press; 2019. [Google Scholar]
- 67.Authority EFS. FAQ: phthalates in plastic food contact materials. 2019.
- 68.USFDA. CFR - Code of Federal Regulations Title 21. 2020. Accessed 26 August, 2020.
- 69.European Chemicals Agency. EVALUATION OF NEW SCIENTIFIC EVIDENCE CONCERNING THE RESTRICTIONS CONTAINED IN ANNEX XVII TO REGULATION (EC) NO 1907/2006 (REACH). REVIEW OF NEW AVAILABLE INFORMATION FOR dibutyl phthalate (DBP). 2010. Accessed 26 August, 2020.
- 70.European Chemicals Agency. Substance Infocard: Bis(2-ethylhexyl) phthalate. 2010. Accessed August 26, 2020.
- 71.USFDA. Phthalates. 2020. Accessed August 26, 2020.
- 72.Attina TM, Trasande L. Association of Exposure to Di-2-Ethylhexylphthalate Replacements With Increased Insulin Resistance in Adolescents From NHANES 2009–2012. J Clin Endocrinol Metab. 2015;100(7):2640–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trasande L, Attina TM. Association of exposure to di-2-ethylhexylphthalate replacements with increased blood pressure in children and adolescents. Hypertension (Dallas, Tex : 1979). 2015;66(2):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trasande L. Exploring regrettable substitution: replacements for bisphenol A. The Lancet Planetary Health. 2017;1(3):e88–e89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


