To the Editor:
The association between immune thrombocytopenia (ITP) and the Covid‐19 vaccine drew national attention when The New York Times reported in January 2021 that a Florida physician died from a hemorrhagic stroke after developing ITP within 2 weeks of vaccination. 1 Shortly thereafter, a review of the Vaccine Adverse Event Reporting System database described 20 cases of Covid‐19 vaccine‐associated thrombocytopenia, including 1 with a prior history of known ITP. 2
These observations raised concerns regarding risks of ITP recrudescence with immunization and furthered hesitancy among patients with chronic ITP. Adherence to vaccinations against influenza and pneumococcus are known to be suboptimal in patients with autoimmune disease, and a recent survey of 1266 patients with autoimmune or inflammatory rheumatic disease reported that only 54.2% were willing to be vaccinated against Covid‐19. 3 In this context, hematologists have been tasked with providing clear messaging regarding benefits of vaccination and guidance on peri‐immunization platelet count monitoring. However, little data exist on platelet counts and rates of ITP exacerbation in the post‐immunization period among those with a history of chronic or persistent ITP. This single‐center retrospective observational study evaluates the platelet trends and clinical outcomes of 34 patients with chronic or persistent ITP after receiving a 2‐dose vaccination series against Covid‐19.
Potential subjects were identified among patients evaluated at the Seattle Cancer Care Alliance, the outpatient hematology site for the University of Washington hospital system, with an ICD‐10 diagnosis code of D69.3 for “Immune thrombocytopenic purpura.” Eligible subjects were ≥ 18 years old with a diagnosis of ITP (established by both the patient's primary hematologist and the clinician performing chart review based on a negative workup for other causes of thrombocytopenia) for at least 3 months and chart documentation of Covid‐19 vaccination. Patients were excluded if no platelet counts were available within the year prior to vaccination, between dose 1 and dose 2 of vaccination, or within 6 weeks after dose 2. Medical records were abstracted for baseline demographics, ITP treatment history, Covid‐19 vaccine manufacturer and administration dates, and clinical events and medication changes occurring within 6 weeks after the second Covid‐19 vaccine dose. Platelet counts from the last check performed before vaccination (baseline) to 6 weeks after completion of the vaccination series were recorded. If multiple post‐vaccination platelet counts were obtained, the lowest value was used for analysis. Subjects were contacted to confirm vaccination details if these were not completely recorded within the electronic medical record. One subject was described in a previously published case report. 4 We also contributed data from this subject and 12 others to a multicenter retrospective observational study 5 ; outcomes in these subjects are described in additional detail and with further follow‐up data here.
A total of 34 subjects were included. The average age was 60.3 years and 70.6% were female. The median baseline platelet count was 86 × 109/L [interquartile range (IQR) = 56.8–137.0 × 109/L] and 76.5% were receiving ITP‐directed therapy at the time of vaccination. Patients had been treated with a median of two second‐line therapies (IQR = 1.0–3.8) and 20.6% had a history of splenectomy. All received a 2‐dose vaccination series: 58.8% received Pfizer‐BioNTech Covid‐19 Vaccine and 41.2% received Moderna Covid‐19 Vaccine. On average, patients had their platelet count checked 2.8 times [standard deviation (SD) = 1.7] between dose 1 and dose 2 and 3.6 times (SD = 2.6) in the 6 weeks after dose 2.
Following dose 1, there was a trend toward a decrease in platelet count with a median platelet nadir of 60 × 109/L (IQR = 36.0–126.5 × 109/L, p = .09). Platelet counts decreased by 20% or greater from baseline in 47.1%, stayed the same in 41.2%, and increased by 20% or greater in 11.8% (Figure 1A). After dose 2, median platelet nadir was not significantly different from baseline [80.5 × 109/L (IQR = 42.3–118.8 × 109/L), p = .29]. Overall, 44.1% had a decrease, 38.2% had no change, and 17.6% had an increase after dose 2 (Figure 1B). Platelet trends were not significantly different depending on the vaccine manufacturer.
FIGURE 1.
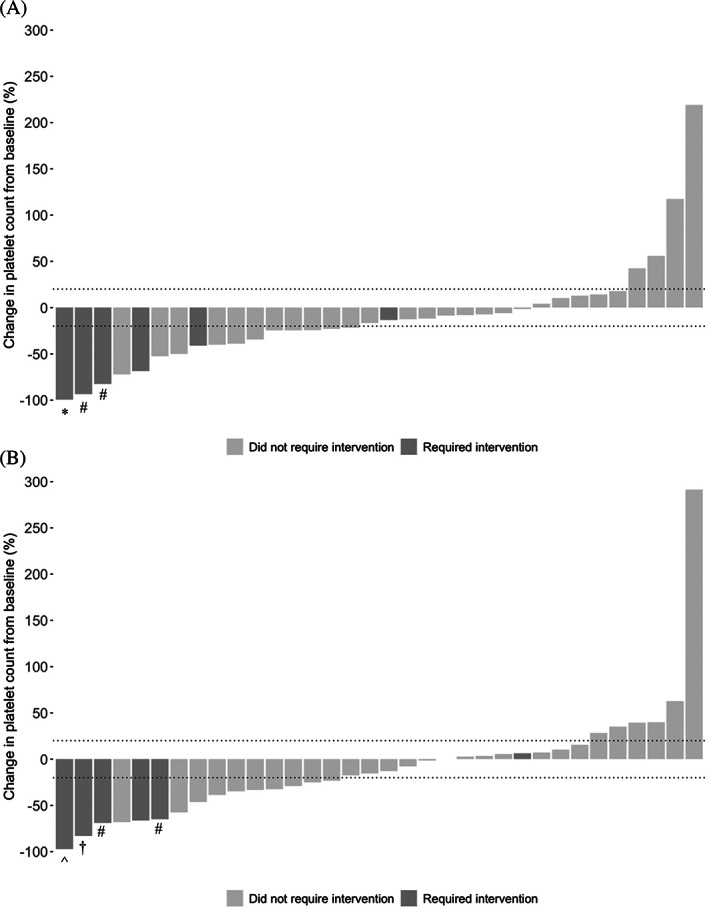
Waterfall plot of percent change in platelet count following Covid‐19 vaccination. Dotted lines demarcate 20% increase and decrease from baseline. (A) Three patients required intervention for thrombocytopenia occurring after dose 1. *Required rescue therapy with IVIg and increased dosing of avatrombopag and prednisone. #Received increased dosing of TPO‐RA. (B) Four patients required intervention for thrombocytopenia occurring after dose 2. ^ Treated with IVIg, increase in prednisone dose, and addition of second TPO‐RA. †Received anti‐D and increase in prednisone dose. #Received increased dosing of TPO‐RA
Fourteen patients had a post‐vaccination platelet decrease resulting in a count of less than 50 × 109/L and four had a count less than 10 × 109/L. Platelet nadir below 50 × 109/L occurred after dose 1 in 50% (7/14), after dose 2 in 28.6% (4/14), and after both doses of vaccine in 21.4% (3/14). Six of the 14 and 3 of the 4 with platelet counts less than 50 × 109/L and 10 × 109/L, respectively, received intervention for post‐vaccination thrombocytopenia. The eight patients who did not receive intervention had a significantly lower median baseline compared to the overall cohort (50.5 × 109/L vs 86.0 × 109/L, p = .01) and platelet counts at last follow up remained stable from baseline at a median of 53 × 109/L (IQR 41.0–104.8 × 109/L). The patient who did not receive intervention for platelet count less than 10 × 109/L after vaccination had pre‐vaccination counts consistently in the single digits, with a baseline count of 5 × 109/L.
Six subjects required adjustment of their existing ITP therapy or addition of new therapy because of post‐vaccination thrombocytopenia. Two patients had received dose‐reduction of their ITP therapy within 3 weeks prior to platelet nadir while the other four had been on stable dosing for several months. There were no significant differences in demographic or clinical characteristics between patients who required intervention and the overall cohort. The median time to platelet nadir was 5 days (IQR = 3.0–19.0 days). Two subjects developed World Health Organization Grade 1 mucocutaneous bleeding at platelet counts of 3 × 109/L and 15 × 109/L, respectively. None of the other patients noted symptoms and none were hospitalized during the study period. All were treated with increased intensity of existing ITP therapy: 4/6 received increase in thrombopoietin receptor agonist (TPO‐RA) dosing and 3/6 received increase in corticosteroid dosing (Figure 1A,B). In addition, one received intravenous immune globulin (IVIg), one received anti‐D, and one received IVIg with introduction of a second TPO‐RA. After a median follow‐up of 113.5 days from vaccination, two patients have returned to pre‐vaccination dosing, three are receiving their prior ITP‐directed therapy at slightly increased intensity, and one is receiving less intense therapy.
Five patients required dose decreases during the study period: four underwent dose reduction of TPO‐RA and one was tapered off romiplostim.
With the advent of widespread Covid‐19 vaccination, sporadic cases of immunization‐related ITP recrudescence raised safety concerns among those with a history of ITP. We report that among a cohort of patients managed at a tertiary care center for chronic or persistent ITP, half had a decrease in platelet count of 20% or more from baseline while half had no change or an increase. There was a trend toward a decrease in platelet count following dose 1 and no difference in platelet count following dose 2. Six patients (17.6%) required intervention, but for two of the six, worsening thrombocytopenia may have been related to recent dose adjustments. Nearly the same number received a reduction in the intensity of their ITP therapy during the study period. No patients experienced severe adverse events or required hospitalization during the study period.
The study was limited by sample size given the rarity of ITP and the small number of patients (typically of older age) who had access to Covid‐19 vaccination in early 2021 and received post‐vaccination platelet count monitoring. In addition, this cohort consisted primarily of patients with active ITP and the close monitoring may have led to prompt intervention for post‐vaccination thrombocytopenia and curtailed the rate of adverse events.
Little is known regarding the effect of vaccination in those with a history of ITP. Case reports and anecdotal accounts of vaccine‐associated ITP flare have been reported and are thought to result from an augmentation of a prior immune response. 4 A subset of 13 patients in this cohort were included in a multicenter retrospective observational study evaluating platelet trends following Covid‐19 immunization among those with a history of ITP. 5 Here, we corroborate findings that approximately half had a drop in platelet count following vaccination, but that intervention was required in a minority of cases. Further, with additional follow‐up data, our study demonstrates that adverse events were rare and platelet decreases were mostly transient. All six patients who received intervention in this study were successfully managed through a combination of increased intensity of existing ITP therapy and rescue therapy; half have returned to pre‐vaccination dosing or less intense therapy, while half are receiving prior ITP‐directed therapy at a slightly increased intensity. Among eight patients with moderate to severe baseline thrombocytopenia who experienced platelet decreases to a nadir below 50 × 109/L, all were successfully managed with close monitoring and had a median post‐vaccination platelet count that returned to baseline.
CONFLICT OF INTEREST
TG has received honoraria from Amgen, Dova Pharmaceuticals, and Novartis; has acted as a consultant for the Platelet Disorder Support Association, Amgen, Dova Pharmaceuticals, Biogen, Momenta, Sanofi, Vertex, Cellphire, Fujifilm, Rigel, Shionogi, and Principia; and has received research support from the National Heart, Lung, and Blood Institute, Principia, Rigel, and Cellphire. DJ, AJP, AW, and DG have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Data extraction was performed by Debbie Jiang and Amanda Weatherford. All authors contributed to the study design and interpretation of results. Debbie Jiang was responsible for drafting the manuscript and data analysis. Andrew Jay Portuguese, Amanda Weatherford, David Garcia, and Terry Gernsheimer contributed to critical revision.
FUNDING INFORMATION
National Heart, Lung, and Blood Institute, T32 HL007093.
ACKNOWLEDGMENT
DJ receives support from an institutional training grant from the National Heart, Lung, and Blood Institute (T32 HL007093).
Funding information National Heart, Lung, and Blood Institute, Grant/Award Number: T32 HL007093
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Grady D, Mazzei P. Doctor's death after covid vaccine is being investigated. The New York Times. Published January 12, 2021. Accessed May 18, 2021. https://www.nytimes.com/2021/01/12/health/covid-vaccine-death.html
- 2. Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS‐CoV‐2 vaccination. Am J Hematol. 2021;96(5):534‐537. doi: 10.1002/10.1002/ajh.26132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Felten R, Dubois M, Ugarte‐Gil MF, et al. Vaccination against COVID‐19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021;3(4):e243‐e245. doi: 10.1016/10.1016/S2665-9913(21)00039-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Portuguese AJ, Sunga C, Kruse‐Jarres R, Gernsheimer T, Abkowitz J. Autoimmune‐ and complement‐mediated hematologic condition recrudescence following SARS‐CoV‐2 vaccination. Blood Adv. 2021;5(13):2794‐2798. doi: 10.1182/10.1182/BLOODADVANCES.2021004957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee EJ, Beltrami Moreira M, Al‐Samkari H, et al. SARS‐CoV‐2 vaccination and immune thrombocytopenia in de novo and pre‐existing ITP patients. Blood. 2021. Published online September 29, 2021. doi: 10.1182/blood.2021013411 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


