Abstract
Background:
Meropenem is a broad-spectrum carbapenem antibiotic approved by the U.S. Food and Drug Administration for use in pediatric patients, including treating complicated intra-abdominal infections (cIAIs) in infants < 3 months of age. The impact of maturation in glomerular filtration rate (GFR) and tubular secretion by renal transporters on meropenem pharmacokinetics, and the effect on meropenem dosing, remains unknown. We applied physiologically based pharmacokinetic (PBPK) modeling to characterize meropenem’s disposition in preterm and term infants.
Methods:
An adult meropenem PBPK model was developed in PK-Sim® (v. 8) and scaled to infants accounting for renal transporter ontogeny and GFR maturation. The PBPK model was evaluated using 645 plasma concentrations from 181 infants (gestational age 23–40 weeks; postnatal age 1–95 days). PBPK model-based simulations were performed to evaluate meropenem dosing in the product label for infants < 3 months of age treated for cIAIs.
Results:
Our model predicted plasma concentrations in infants in agreement with the observed data (average fold error of 0.90). The PBPK model-predicted clearance in a virtual infant population was successfully able to capture the post hoc estimated clearance of meropenem in this population, estimated by a previously published model. For 90% of virtual infants, a 4 mg/L target plasma concentration was achieved for > 50% of the dosing interval following product label–recommended dosing.
Conclusion:
Our PBPK model supports the meropenem dosing regimens recommended in the product label for infants < 3 months of age.
1. Introduction
Meropenem is a broad-spectrum carbapenem antibiotic that inhibits cell wall synthesis by binding to penicillin-binding proteins and exerting bactericidal activity against both Gram-positive and Gram-negative bacteria [1–3]. It is approved by the U.S. Food and Drug Administration (FDA) for use via intravenous (IV) infusion in complicated intra-abdominal infections (cIAIs) (adult and pediatric patients of all ages), complicated skin and skin structure infections (adult and pediatric patients > 3 months of age), and bacterial meningitis (pediatric patients > 3 months of age) [1].
Meropenem pharmacokinetics (PK) in adults is well defined. In multiples studies that included European and Asian adult healthy subjects and patients without systemic infection, meropenem’s median (range) weight-normalized clearance was 0.22 L/hr/kg (0.16–0.36), the volume of distribution was 18.2 liters (11.0–28.7), and mean half-life was approximately 0.8 to 1.5 hours [4–8]. Meropenem is primarily cleared renally by both glomerular filtration and renal tubular secretion [8]. About 70% of the drug is cleared unchanged in the urine, 24%–30% is metabolized by dehydropeptidase I (DHP-1 or DPEP1) into its only known inactive metabolite, and ~2% of the drug is eliminated in feces [1]. Organic anion transporters 1 and 3 (OAT1 and OAT3) present in the proximal renal tubule are known to transport meropenem, and OAT inhibitor probenecid has been shown to reduce meropenem’s renal clearance [8,9]. Renal function, estimated using creatinine clearance, is the primary determinant of meropenem dosing in adults [1].
Meropenem PK has also been reported in pediatric subjects of different ages. In pediatric subjects ranging from newborns to 12 years of age, including premature infants, creatinine clearance, age, and body weight were significant covariates determining meropenem clearance [10–13]. Weight-normalized meropenem clearance was found to be lower in neonates [13,14] compared to pediatric subjects > 2 years of age [10]. Previous studies also suggested that weight- normalized dosing of meropenem, ranging from 20 to 40 mg/kg given every 8 hours, would be sufficient to achieve efficacy in pediatric subjects. One previous study characterized meropenem’s population PK (PopPK) in 200 infants < 91 days of age [15]. This study reported that serum creatinine and postmenstrual age (PMA) were significant covariates for meropenem clearance in infants, and an age-dependent dosing scheme for the treatment of complicated intra-abdominal infection in infants < 91 days of age was suggested [15].
Conducting PK studies in infants is challenging, thus often limiting evaluation of dosing regimens for newer indications and increasing the off-label or unlicensed use of drugs in this vulnerable patient population [16–18]. Physiologically based pharmacokinetic (PBPK) modeling can be useful in predicting PK and dose selection in the pediatric population [19]. In neonates, meropenem is currently only approved for cIAIs. A PBPK modeling approach combines drug-, physiology-, and clinical trial design–related information and can be used to predict PK and optimize dosing in vulnerable patient populations [19–21]. Thus, we sought to apply a PBPK modeling approach to characterize meropenem’s disposition in adults and then scale the model to infants. Once the model was verified, we sought to use a PBPK modeling approach to evaluate recommended meropenem dosing in infants with cIAIs.
2. Materials and Methods
2.1. Clinical Data Used for PBPK Model Development and Evaluation
Meropenem plasma concentration vs. time profiles from the literature were digitized using Graph Grabber software (Version 2.0, Quintessa, quintessa.org) to develop and evaluate the adult and pediatric PBPK models. For adult PBPK model development, plasma concentration vs. time profiles after a single or multiple dosing of meropenem IV infusion in healthy subjects or hospitalized patients were used (Table S1). After scaling the adult model to the pediatric population, the pediatric PBPK model was evaluated using digitized and available individual-level concentration vs. time data (Table S2).
2.2. Adult PBPK Model Development
A whole-body PBPK model for meropenem was developed for adults using the software PK-Sim® (v. 8.0, Open Systems Pharmacology Suite, open-systems-pharmacology.com). The PBPK model was developed using physicochemical and in vitro properties of meropenem as listed in Table 1. To match a previous publication focused on meropenem PK in adults [8], an average European male virtual individual with an age, weight, and height of 26 years, 74 kg and 179 cm, respectively, was created to simulate a mean plasma concentration vs. time profile following a 500 and 1000 mg meropenem IV infusion. Based on the published information, glomerular filtration, renal secretion, biliary clearance, and enzymatic metabolism via the DPEP1 enzyme were considered for the disposition of meropenem [8,22,23]. Meropenem’s lipophilicity (logP) was optimized, as was the method for calculation of partition coefficients (Kp). A renal tubular secretion process was defined by OAT3 mediated uptake of meropenem in renal epithelial cells and efflux of intracellular meropenem into urine by hypothetical efflux transporter. The reported maximum rate of transport (Vmax) by the OAT3 transporter, expressed on human embryonic kidney 293 (HEK293) cells, was converted from pmol/min/mg protein to μmol/L/min using single HEK293 cell protein concentration [24], and this concentration was assumed to be equivalent to OAT3 transporter expressed on the cell membrane. A hypothetical efflux transporter, entirely expressed in the kidney, was added on the renal tubule’s apical side to account for tubular secretion. The hypothetical efflux transporter was assumed to have a low affinity for meropenem (Michaelis-Menten constant [Km] 1500 μM, two-fold higher than the Km for the OAT3 transporter), and the Vmax value was optimized. A 25% coefficient of variation (CV) was assumed, informed by previously reported values, for the expression of the OAT3 and the hypothetical efflux transporter. Variability in the OAT3 transporter kinetic parameters was added based on the literature-reported values (20% CV on Km, 15% CV on Vmax) [9].
Table 1.
Meropenem physiologically based pharmacokinetic model parameters
| Parameter | Reported Values | Final values |
|---|---|---|
| Physicochemical properties | ||
| Molecular weight | 383.5 g/mol | 383.5 g/mol |
| Solubility | 5.63 mg/ml at pH 7 [51] 14 mg/ml [52] |
5.63 mg/ml |
| pKa | 3.47 [40] 7.4 [52] |
3.47 |
| Lipophilicity (logP) | −4.35 [40] | −1.39* |
| −3.28 [23] | - | |
| −0.69 [51] | - | |
| Partition coefficients | Rodgers and Rowland [40,53] | Rodgers and Rowland [53] |
| Cellular permeabilities | - | PK-Sim® Standard |
| Pharmacokinetic properties | ||
| Biliary Clearance | 0.0422 L/h [22] | 6.5E-04 L/h/kg |
| OAT3 K m | 847 ± 160 μM [9] | 850 μM |
| OAT3 V max | 48.5 ± 7.5 pmol/min/mg [9] | 600 μmol/L/min [24] |
| Hypothetical renal efflux transport protein V max | - | 200 μmol/L/min* |
| Hypothetical renal efflux transport protein K m | - | 1500 μM |
| DPEP1 V max | 8.58 ± 0.21 Units/mg protein [23] | - |
| DPEP1 K m | 3.56 ± 0.15 mM [23] | First order intrinsic clearance, 0.02 L/mina |
| f u | 0.98 [6,40] | - |
| Protein binding partner | Albumin | - |
DPEP1, dehydropeptidase 1 or renal dipeptidase 1; Km, Michaelis-Menten constant; OAT3, organic anion transporter subfamily 3; pKa, acid dissociation constant; Vmax, maximum velocity of transport/reaction; fu, unbound fraction
Optimized values
Optimized based on the reported in vivo non-renal clearance of meropenem.
Meropenem is metabolized via hydrolysis of the beta-lactam ring by the DPEP1 enzyme to its major inactive metabolite ICI 213689 [23], constituting 20%–30% of the total clearance [7]. Due to a lack of information on the expression of DPEP1, a first-order clearance by this enzyme was assumed and optimized based on in vivo non-renal clearance values in adults [7,8,25,26]. Optimization of renal and non-renal clearance was based on achieving the literature-reported total systemic clearance, which includes ~70% renal elimination and 20%−30% metabolism via DPEP1.
2.3. Adult PBPK Model Evaluation
An adult, healthy male virtual individual and a virtual adult population of 100 subjects (50% female) were created based on each publication’s average demographic information (Table S1 and Table S3). The model’s predictive performance was assessed based on a visual comparison of the observed vs. simulated data. From the simulated concentration vs. time profiles, we also calculated the area under the concentration vs. time curve (AUC) extrapolated to infinity and the clearance by noncompartmental analysis in Phoenix® WinNonlin® (v. 8.0, Certara, Princeton, NJ) and compared this result with the literature-reported result. The tidyverse collection of R packages was used for data processing and visualization [27]. Additional information is available in the Electronic Supplementary Material.
2.4. Pediatric PBPK Model Development
2.4.1. Physiological Parameters in the Pediatric Population
The algorithms implemented in PK-Sim® were used to generate a virtual pediatric population of ages 3 months to 12 years [28] and < 3 months of age [29]. As previously described [29], the gestational age (GA) for the infants < 3 months of age varied between 24 weeks preterm to 40 weeks term neonates. The default physiological parameters implemented in PK-Sim® were considered for the current model.
2.4.2. Scaling Unbound Fraction
The default albumin ontogeny in PK-Sim®, defined as the ratio of protein concentration in a child of a specific age to that in adults, was used to account for age-dependent changes in the unbound fraction of meropenem in the pediatric population, including infants < 3 months of age.
2.4.3. Scaling Meropenem Renal Clearance
The renal clearance processes for meropenem were assumed to be similar between adult and pediatric subjects and included both glomerular filtration and tubular secretion. Age-dependent changes in glomerular filtration have been previously described by Rhodin et al. [30] and implemented in PK-Sim® as described by Claassen et al. [29]. Tubular secretion was modeled as OAT3 and a hypothetical efflux transporter–mediated saturable transport process. Ontogeny information for the OAT3 transporter expression in the kidney has been previously described, and the mean and 95% confidence interval for the time to half-maximal OAT3 expression (TM50) and a Hill coefficient was based on OAT3 transporter protein expression at different postnatal ages (PNA) [31]. Standard deviation was calculated from the reported 90% confidence interval limits of TM50 and Hill, as described in Equation 1.
| (1) |
Based on this information, an ontogeny function with variability for OAT3 was generated at different PNAs using the software R (version 3.5.3 [32]) and R studio (version 1.1. 463; RStudio, Boston, MA) and Equation 2.
| (2) |
For the age-dependent change in hypothetical efflux transporter expression, an ontogeny function for tubular secretion developed by Hayton [33,34] was used.
Pediatric renal clearance contributed by the glomerular filtration and tubular secretion is scaled from their corresponding adult values using Equations 3 and 4 as previously described [35],
| (3) |
| (4) |
where CLR, (child) and CLR, (adult) represent the renal clearance via glomerular filtration in a child and an adult, respectively; fu,p (child) and fu,p (adult) are the unbound fraction in plasma in a child and an adult, respectively; CLTS, (child) and CLTS, (adult) are renal clearance via tubular secretion (mediated by OAT3 and the hypothetical TS efflux protein) in a child and an adult, respectively; GFRchild and GFRadult are glomerular filtration rate in a child and an adult, respectively; and TSchild and TSadult are tubular secretion in a child and an adult, respectively.
2.4.4. Scaling Meropenem Non-Renal Clearance
Non-renal clearance of meropenem in the pediatric population was assumed similar to adults and mediated by DPEP1. Due to a lack of information on DPEP1 expression as a function of age, no ontogeny was considered. Thus, non-renal clearance was scaled by age-based maturation of organ weight.
2.4.5. Pediatric PBPK Model Evaluation
The scaled PBPK model was first verified using digitized meropenem plasma concentration vs. time data from the literature (Table S2). The PBPK model was further evaluated using opportunistically collected clinical data obtained from 200 infants with a GA ranging from 22 to 40 weeks and a PNA ranging from 1 to 95 days, treated with meropenem for cIAIs. The detailed demographic characteristics for these subjects were previously published [36], and the data were previously analyzed using a nonlinear mixed-effect modeling approach implemented in NONMEM (version 7.4.1; Icon Development Solutions, Ellicott City, MD) [15]. The final dataset used for model evaluation had 181 subjects and 645 plasma concentrations. Out of 645 plasma samples, 15% were from infants that were GA < 32 weeks and PNA < 14 days (group 1); 53% from infants that were GA < 32 weeks and PNA ≥ 14 days (group 2); 13% from infants that were GA ≥ 32 weeks and PNA < 14 days (group 3); and 19% from infants that were GA ≥ 32 weeks and PNA ≥ 14 days (group 4). All concentrations were dose-normalized and separated into groups based on their age and the corresponding FDA-recommended dosing regimen for meropenem in infants < 3 months of age (Table S3). We created a virtual population comprising 4000 virtual infants matching the age and dosing groups from the clinical data, as described in Table S3. Simulated concentration vs. time data were compared with the observed data by visual and numeric inspection. The average fold error (AFE) for the PBPK model prediction was obtained based on Equation 5.
| (5) |
AFE within each age group was calculated by summarizing AFE for individual subjects by their postmenstrual age in weeks. Overall, the PBPK model performance was deemed acceptable if the AFEs were between 0.5 and 2. We also compared the PBPK model-predicted steady-state clearance with the post hoc empirical Bayesian estimates of clearance obtained from a previously published PopPK analysis [15]. Additional information is available in the Electronic Supplementary Material.
2.4.6. Evaluation of Recommended Dosing Regimens in Infants < 3 Months of Age
The final pediatric PBPK model was used to assess the probability of target attainment in virtual infants < 3 months of age who were administered the meropenem product label dosing recommendations for cIAIs [1]. From the dosing simulation results, the 10th percentile of the percentage of the dosing interval where the unbound concentration in plasma was above minimum inhibitory concentration (T>MIC) [3,37,38] for minimum inhibitory concentration (MIC) values of 0.25 to 16 mg/L was calculated and plotted against respective MICs. The 10th percentile corresponds to 90% of virtual infants. Based on previously described criteria, we evaluated if a 50% T>MIC was achieved for a MIC of 4 mg/L and if a 75% T>MIC was achieved for a MIC of 2 mg/L [15].
3. Results
3.1. PBPK Model Development
The physicochemical properties of meropenem were kept mostly unchanged, except for the optimization of logP. Similar to previous reports, meropenem was found to have a low logP value of −1.39, supporting its high water solubility and negative base-10 logarithm of acid dissociation constant (pKa) of 3.47. The kinetic parameters of the OAT3 transporter were mostly kept unchanged except for unitary correction of Vmax utilizing protein concentration of HEK293 cells as previously described. Affinity (Km) of the hypothetical efflux transport protein was considered lower than OAT3, and only Vmax was adjusted to reflect the urinary excretion of meropenem. Non-renal clearance by DPEP1 enzyme was considered a first-order process with a fixed value of 0.02 L/min, as obtained from literature and reflecting ~30% metabolism of meropenem in adults. The final PBPK model parameters are presented in Table 1.
3.2. Adult PBPK Model
The adult PBPK model of meropenem characterized the adult plasma PK data within the acceptable range for both 500 mg and 1000 mg IV infusions. Observed concentrations ± standard deviation from published adult data [8] were in good agreement with the 90% prediction interval of the simulated meropenem plasma concentration (Fig. 1a, Fig. 1b). A similar observation was made (Fig. 1c, Fig. 1d) when the model was evaluated using observed data from four additional publications (Table S1), including multiple-dose administration of meropenem in healthy subjects (Fig. S1a) and hospitalized patients (Fig. S1b and S1c). The simulated fraction excreted in urine 8 h after infusion ranged from 0.52 to 0.76, with a median value of 0.67. Simulated AUC and clearance were within a two-fold range of the published values (Table 2).
Fig. 1.
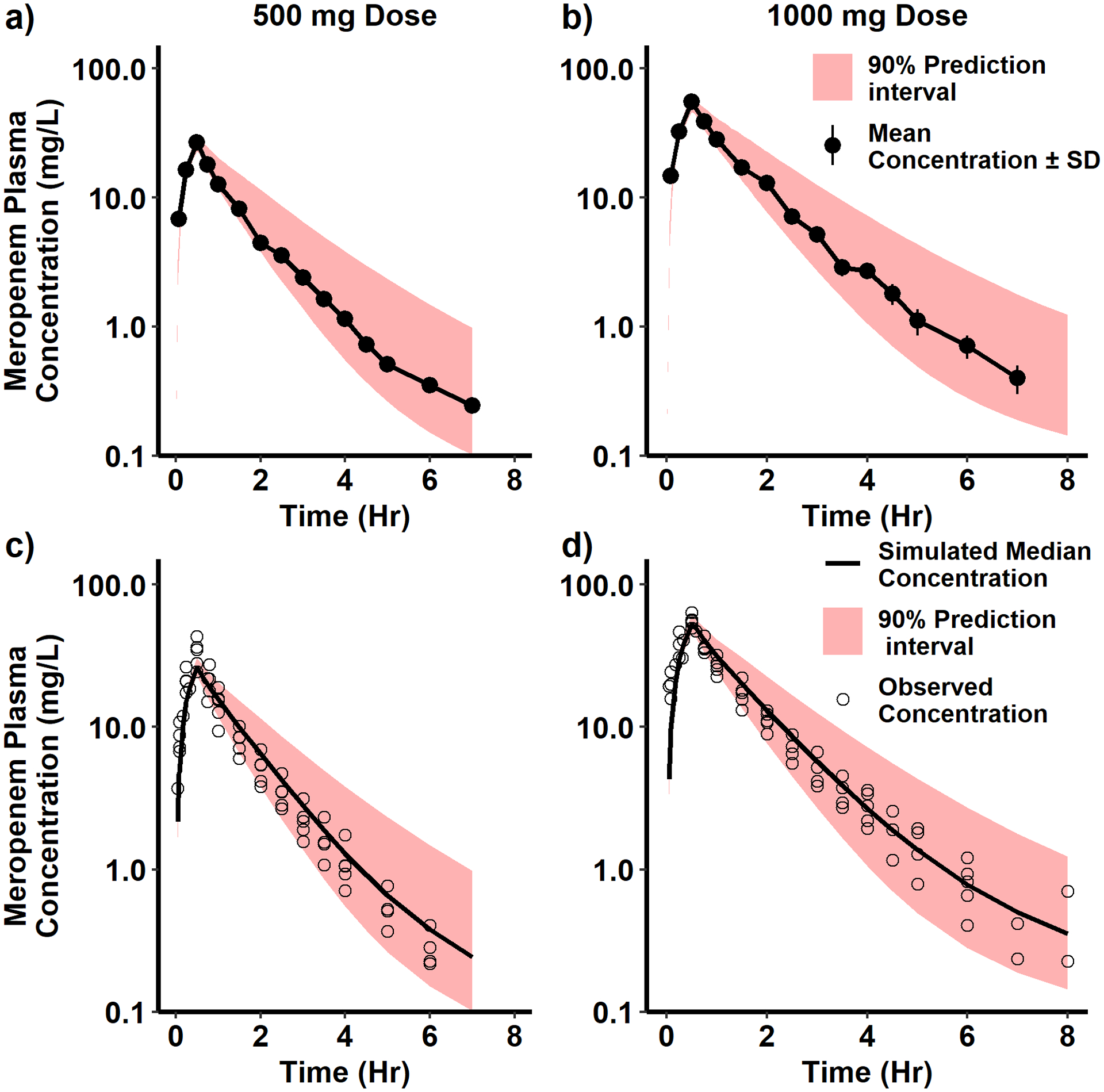
Simulation of meropenem concentration vs. time for one hundred virtual adults. The adult meropenem PBPK model was developed and evaluated using published plasma meropenem concentration vs. time data digitized for a 0.5 and 1 g intravenous (IV) infusion administered over 30 minutes. In a) and b), digitized observed mean plasma concentration vs. time data (black circles with ± standard deviation as black lines) [8] were compared with the 90% prediction interval of the simulated meropenem plasma concentration vs. time profile (red shaded region). In c) and d), digitized observed plasma concentration vs. time data (black circles) [6,7,25,26] were compared with the median and 90% prediction interval of the simulated meropenem plasma concentration vs. time profile (red shaded region).
Table 2.
Comparison of observed and physiologically based pharmacokinetic (PBPK) model simulated area under the plasma concentration vs. time curve (AUC) and clearance following single-dose administration in adults
| AUC (mg*h/L)a | Clearance (L/h) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Simulated | Observed | Simulated | |||||||||
| Dose (mg) | Mean | SD | Mean | SD | Ratiob | Mean | SD | Mean | SD | Ratiob | Reference | |
| 500 | 30.1 | 4.69 | 37.92 | 9.82 | 1.26 | 16.98 | 2.68 | 13.94 | 3.13 | 0.82 | [8] | |
| 1000 | 66.9 | 11.27 | 76.28 | 19.85 | 1.14 | 15.24 | 2.35 | 13.86 | 3.08 | 0.91 | ||
| 500 | 28.1 | 4.3 | 38.35 | 8.50 | 1.36 | 18.12 | 2.70 | 13.66 | 2.93 | 0.75 | [7] | |
| 1000 | 77.2 | 11.8 | 77.39 | 17.09 | 1.00 | 13.44 | 1.98 | 13.53 | 2.89 | 1.01 | ||
| 500 | 39.6 | 6.8 | 40.75 | 10.36 | 1.03 | - | - | 13.01 | 3.11 | - | [26] | |
| 500 | 28 | 15 | 48.86 | 16.01 | 1.75 | - | - | 11.18 | 3.21 | - | [25] | |
| 1000 | 77.5 | 11.5 | 80.62 | 18.23 | 1.04 | 11.28 | 1.86 | 13.03 | 2.95 | 1.16 | [6] | |
SD, standard deviation.
The area under the plasma concentration vs. time curve (AUC) for the physiologically based pharmacokinetic (PBPK) simulated data was calculated by extrapolation to infinity.
The ratio is calculated as the ratio of the mean simulated/mean observed.
3.3. Pediatric PBPK Model
3.3.1. PBPK Model Evaluation with Digitized Published Data
The scaled pediatric PBPK model was first evaluated with published plasma concentration vs. time data (Table S2) using a virtual pediatric population of 200 subjects that mimics the respective publications’ demographic characteristics. A simulated concentration vs. time profile adequately captured the observed data (Fig. 2, Fig. S2). We also compared the simulated PK parameters (AUC and clearance) with the values reported in each publication (when available) (Table 3), and the PBPK model-predicted parameters were found within two-fold of the reported parameters.
Fig 2.
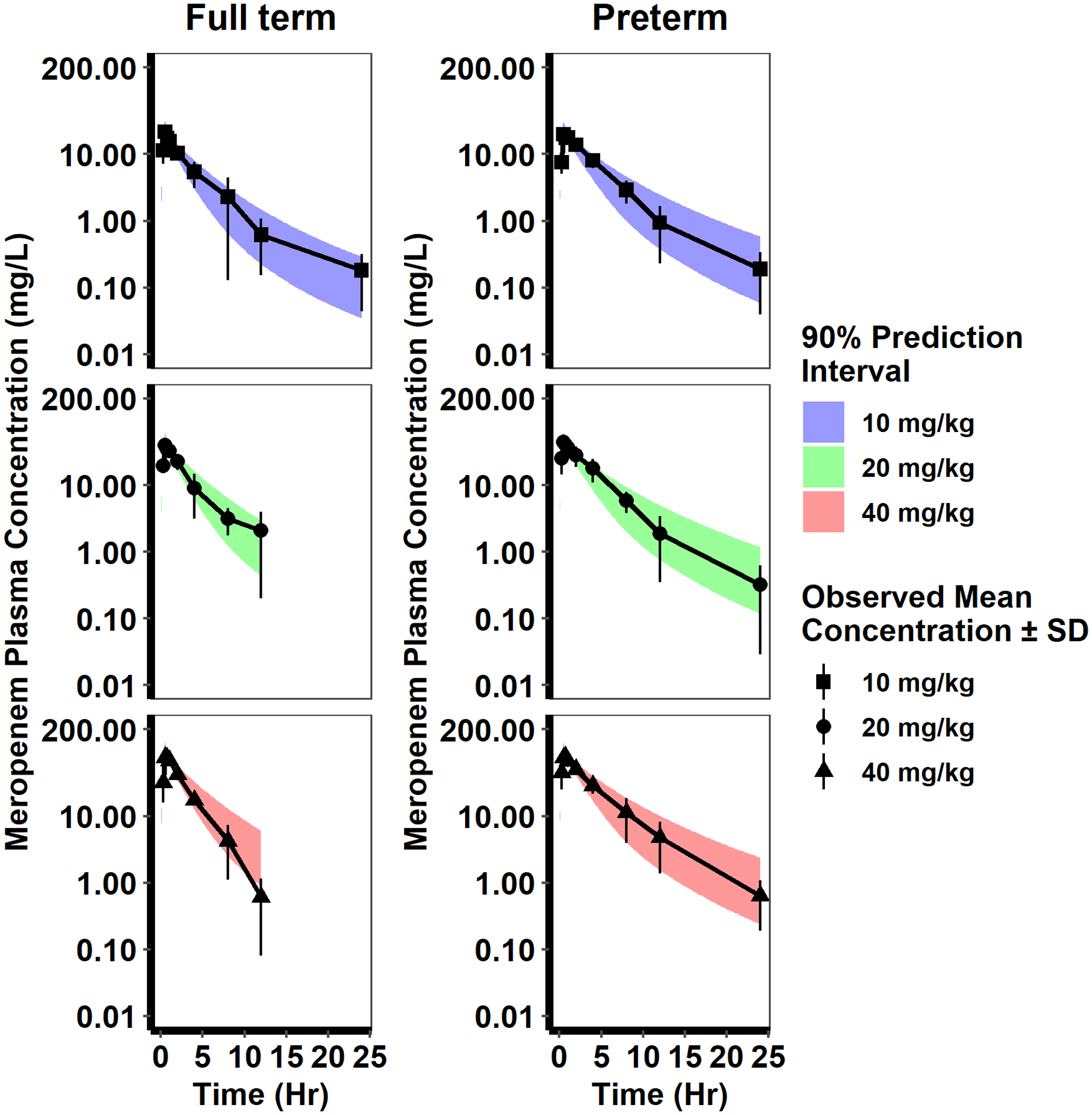
Pediatric PBPK model evaluation. The adult PBPK model was scaled to infants and evaluated using digitized published data for preterm and term infants [13]. A virtual population (n=200) was generated based on published demographic information, simulations were performed using the PBPK model, and the results plotted as the 90% prediction interval (shaded region) overlaid with mean (± standard deviation [SD]) observed meropenem plasma concentration vs. time data after a single IV infusion (30 minutes duration). The colors and symbols represent different body weight-based dosing regimens. For the full-term infant simulations, the virtual infants’ gestational age and postnatal age were 37 to 42 weeks and one day, respectively. For the preterm infant simulations, the virtual infants’ gestational age and postnatal age was 29 to 36 weeks and one day, respectively.
Table 3.
Comparison of observed and physiologically based pharmacokinetic (PBPK) model simulated area under the plasma concentration vs. time curve (AUC) and clearance after single and multiple-dose administration of meropenem in the pediatric populationa
| AUC (mg*h/L)b | Clearance (L/h/kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Simulated | Observed | Simulated | ||||||||
| Dose (mg/kg) | Mean | SD | Mean | SD | Ratioc | Mean | SD | Mean | SD | Ratioc | Reference |
| 10 | - | - | 42.36 | 9.83 | - | 0.312 | 0.08 | 0.24 | 0.06 | 0.77 | |
| 20 | - | - | 85.50 | 19.79 | - | 0.312 | 0.1 | 0.24 | 0.06 | 0.77 | [10] |
| 40 | - | - | 173.80 | 40.06 | - | 0.39 | 0.12 | 0.24 | 0.06 | 0.62 | |
| 15, Day 1 | 99.1 | 28.9 | 110.83 | 22.34 | 1.12 | 0.16 | 0.005 | 0.14 | 0.03 | 0.88 | [14]d |
| 15, Day 5 | 99.8 | 48.5 | 115.30 | 29.68 | 1.16 | 0.16 | 0.07 | - | - | ||
Pediatric population included subjects with postnatal age (PNA) ranging from 0.23 to 12.3 years[10]; and infants with GA of 27 to 32 weeks and PNA of 5 to 44 days [14].
Area under the plasma concentration vs. time curve (AUC) for the PBPK simulated data is reported from time 0 hr to the last observed time point.
The ratio is calculated as the ratio of mean simulated/mean observed
Subjects received 15 mg/kg meropenem dosed as an intravenous infusion over 1 min every 12 hours for five days. AUC was calculated from 0 h to 12 h for the first dose and from 108 hr to 120 hr for the last dose. Clearance at steady state was reported based on a noncompartment analysis of the simulated data.
3.3.2. PBPK Model Evaluation in Infants < 3 Months of Age
The final PBPK model was evaluated with 645 plasma concentrations from 181 infants < 3 months of age first by visual comparison of the simulated vs. observed data (Fig. 3). The overall model AFE was 0.9. If samples collected from infants with a GA < 24 weeks are removed (due to the lower limit of GA in PK-Sim® being 24 weeks), the overall model AFE is 0.96. The median AFE, including all samples, was 0.67, 0.57, 0.92, and 2.25 for group 1 (GA < 32 weeks, PNA < 14 days), group 2 (GA < 32 weeks, PNA ≥ 14 days), group 3 (GA ≥ 32 weeks, PNA < 14 days) and group 4 (GA ≥ 32 weeks, PNA ≥ 14 days), respectively. The median AFE for GA ≥ 24 weeks was 0.72, 0.59, 0.92, and 2.25 for group 1, group 2, group 3, and group 4. Thus, although for group 4, the AFE is slightly greater than 2, the overall AFE indicates good model performance in infants. Overall, comparable weight-normalized clearance estimates were obtained using PBPK and PopPK modeling approaches [15] (Fig. 4).
Fig. 3.
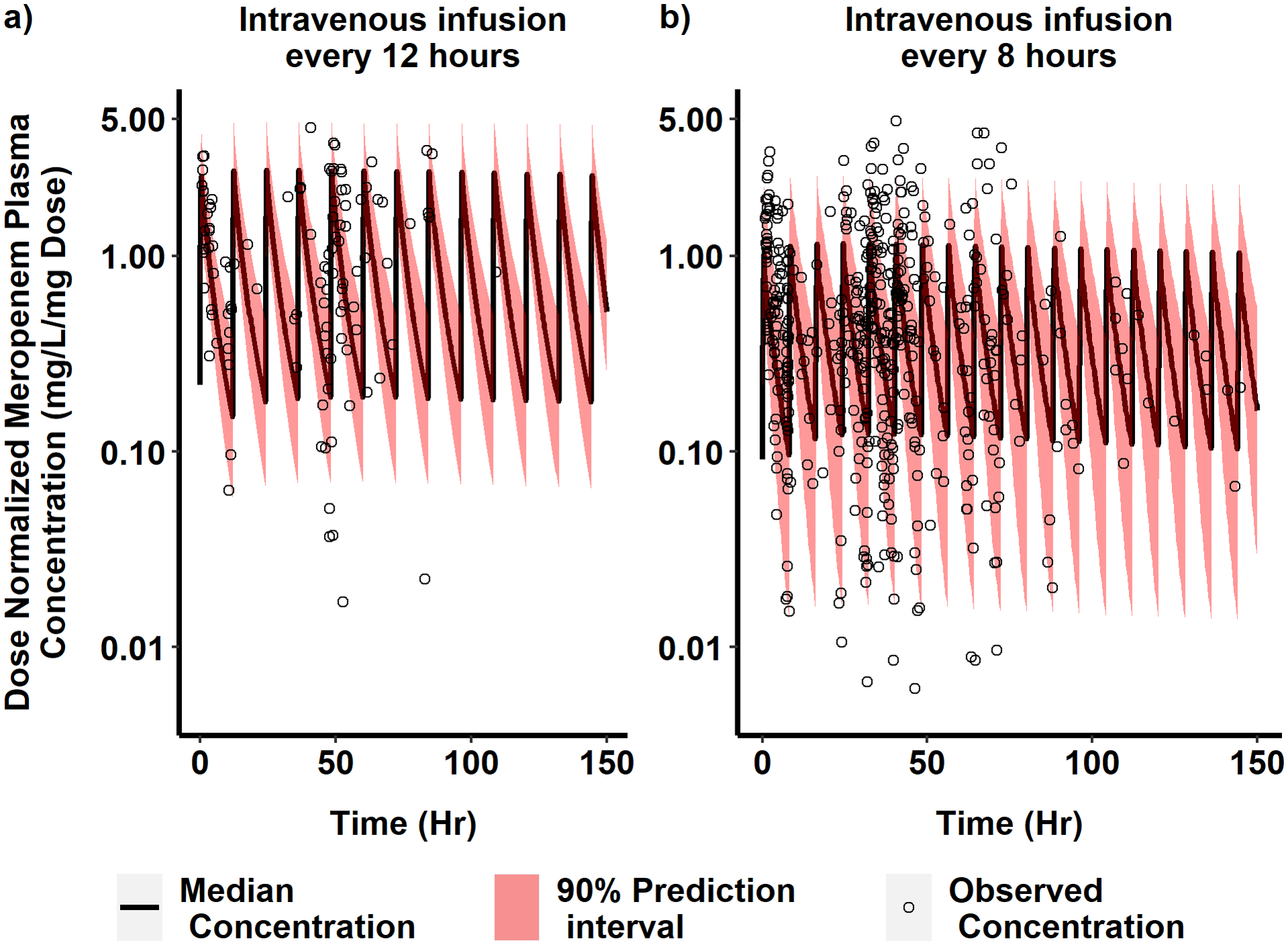
Observed vs PBPK model simulated dose-normalized meropenem concentration vs. time in infants. Dose-normalized and time-corrected observed plasma concentrations of meropenem (black circles) were compared with the 90% prediction interval (red shaded region) and the median (solid black line) simulated meropenem plasma concentration vs. time profile for a virtual neonatal population dosed 1 mg/kg of meropenem by intravenous (IV) infusion over 30 minutes. In a), 1000 virtual infants with gestational age (GA) < 32 weeks and postnatal age (PNA) < 14 days received meropenem every 12 hours. In b), 3000 virtual infants (1000 virtual infants per each of the following age groups: GA < 32 weeks and PNA ≥ 14 days; GA ≥ 32 weeks and PNA < 14 days; and GA ≥ 32 weeks and PNA ≥ 14 days) received meropenem every 8 hours. For the ease of visualization, x-axis is restricted up to 150 h, and the lower limit of y-axis is restricted up to 0.005 mg/L. These axes restrictions removed six concentrations from a) after 150 h time, and 29 concentrations from b) (19 of them were after 150 h and ten were below 0.005 mg/L/mg Dose).
Fig. 4.
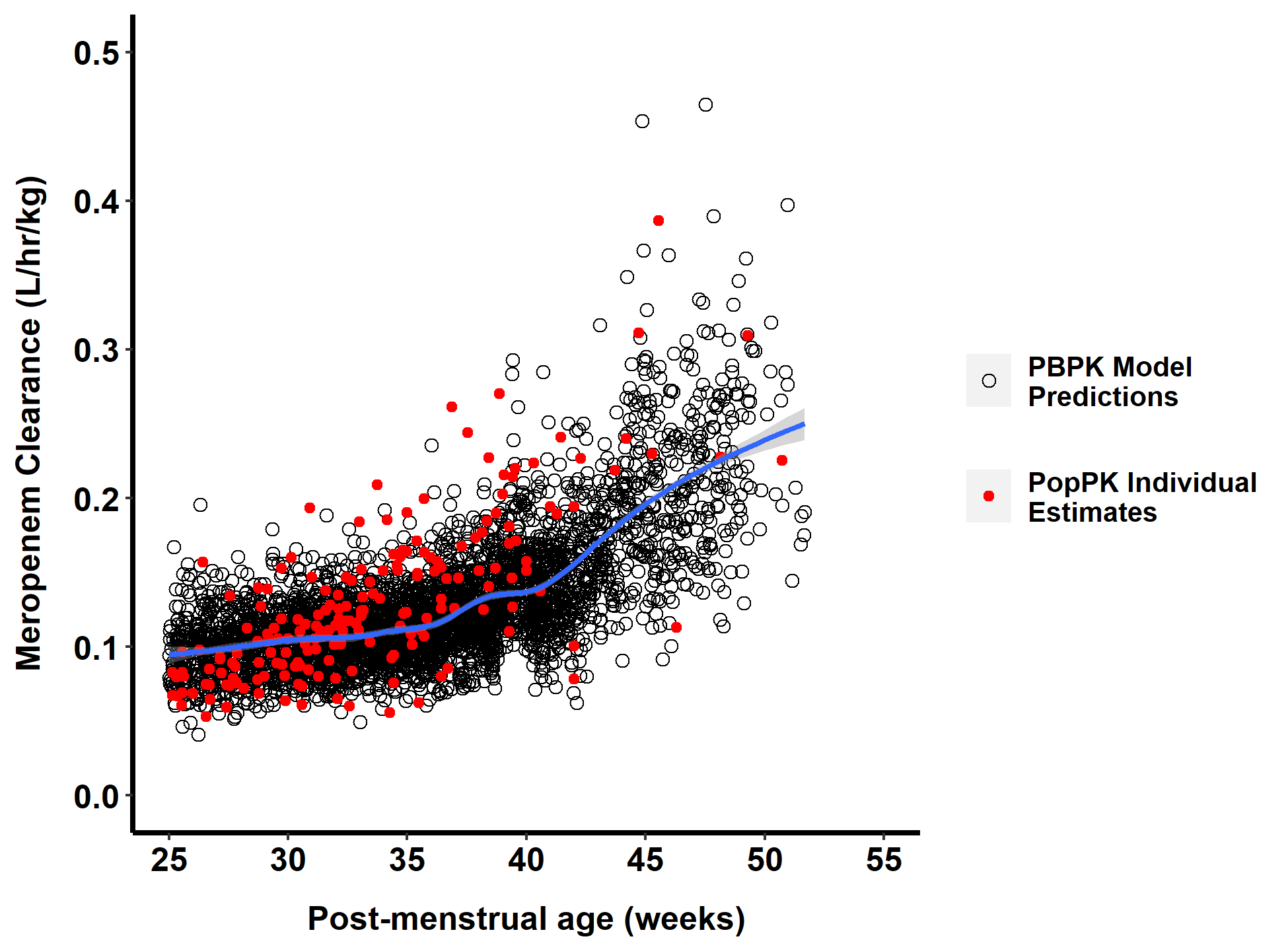
Comparison of body weight-normalized meropenem clearance in a virtual population of 4000 infants using the final physiologically based pharmacokinetic (PBPK) model (black open circles) and individual estimates of body weight-normalized clearance obtained from a previously published population pharmacokinetic (PopPK) analysis (red solid circles) [15] by postmenstrual age. The blue line indicates the smooth line based on a generalized additive model (default when sample size >1000) using the geom smooth function in the R package ggplot2. The virtual population for PBPK model simulations is comprised of 1000 virtual infants for each of the following age groups: gestational age (GA) < 32 weeks and postnatal age (PNA) < 14 days; GA < 32 weeks and PNA ≥ 14 days; GA ≥ 32 weeks and PNA < 14 days; and GA ≥ 32 weeks and PNA ≥ 14 days of age. The body weight and height range for each age group were selected to match the observed data’s demographic characteristics (Table S3).
3.4. Dosing Simulations in Infants < 3 Months of Age
Across all dosing groups, greater than 90% of virtual infants achieve at least 50% T>MIC for a MIC of 4 mg/L or 75% T>MIC for a MIC of 2 mg/L (Fig. 5).
Fig. 5.
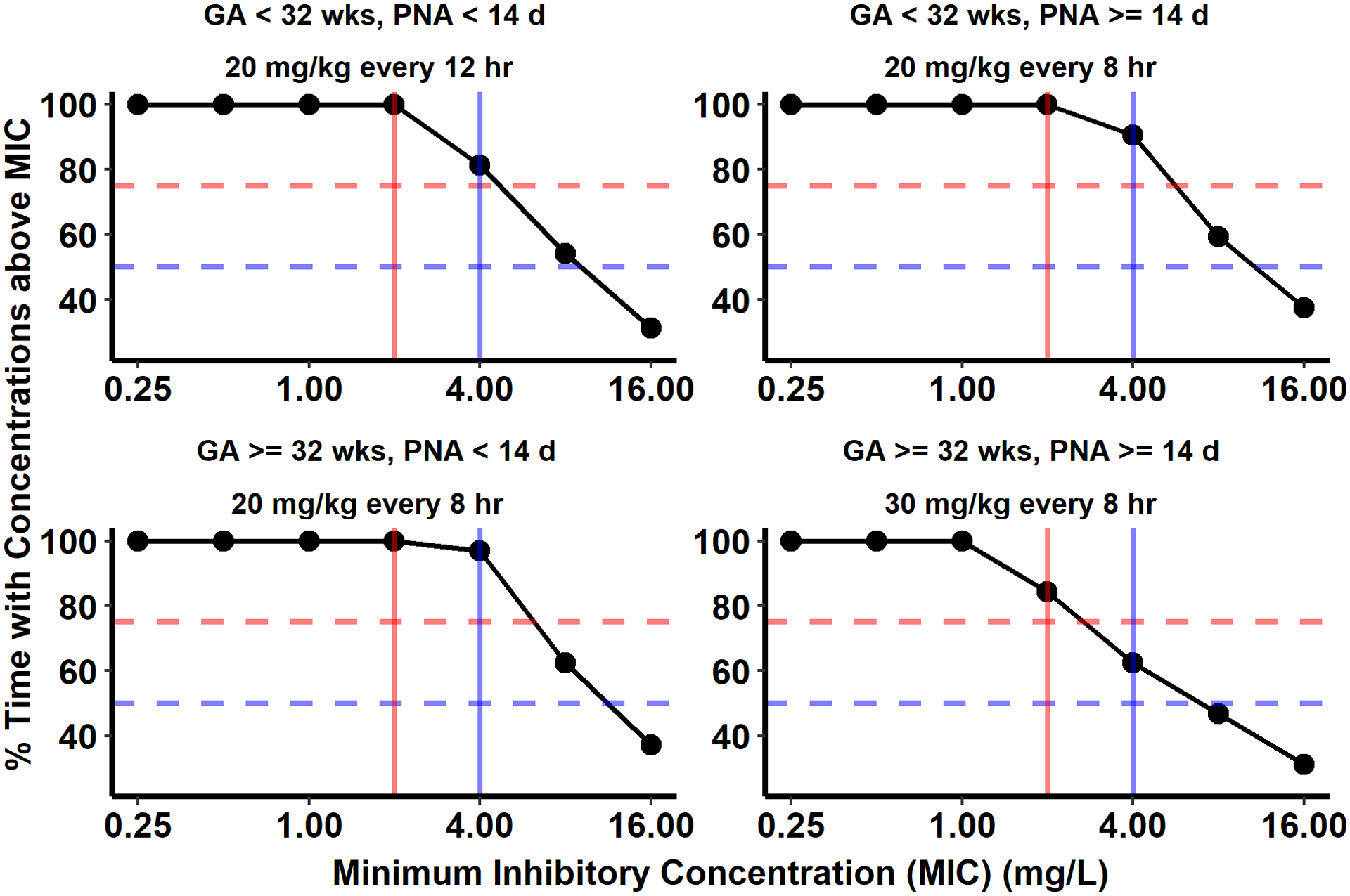
Target attainment analysis performed using the final physiologically based pharmacokinetic (PBPK) model to evaluate the product label recommended meropenem dosing for infants < 3 months of age with complicated intra-abdominal infections. One thousand virtual infants for each age cohort (stratified into four gestational age [GA] and postnatal age [PNA] groups) were dosed with the label recommended meropenem intravenous (IV) dosing. The percentage of the dosing interval for which the meropenem steady-state unbound plasma concentration was above the minimum inhibitory concentrations (MIC) of 0.25 to 16 mg/L was calculated for 90% of virtual infants (solid black line) and plotted against MIC on the x-axis. The horizontal dotted blue and red lines indicate the achievement of unbound plasma concentration higher than MICs for 50% and 75% of the dose interval, respectively. The two vertical lines indicate 2 mg/L (red) and 4 mg/L (blue) MICs. The intersection of the blue horizontal line and vertical lines indicates unbound plasma concentration achievement above 2 and 4 mg/L for 50% of the dosing interval.
4. Discussion
Meropenem is frequently used to treat bacterial infections (e.g., complicated skin and skin-structure infections, bacterial meningitis, and cIAIs) in pediatric patients [37,39]. It is currently approved by the FDA only to treat cIAIs in infants < 3 months of age. We developed a PBPK model of meropenem in infants < 3 months of age, which improves our understanding of meropenem disposition in this vulnerable age group. The PBPK model-derived clearance of meropenem in the virtual infant population is comparable with individual estimated clearance using standard PopPK methodology. The PopPK model was previously developed with extensive plasma concentration data from infants < 3 months of age treated with meropenem for cIAIs. Further, our analyses confirmed the existing FDA-approved label dosing regimen for this population. As there are logistical and ethical challenges associated with performing PK studies in infants, our model could be useful in investigating meropenem dosing in other infections for infants < 3 months of age without performing extensive PK studies.
Our PBPK model included elimination via glomerular filtration, OAT3-mediated uptake into renal proximal tubular cells, efflux via a hypothetical renal efflux transport protein, and non-renal clearance by DPEP1 as well as biliary clearance of meropenem informed by a previous publication. To predict meropenem’s PK in preterm and term infants, we leveraged the virtual populations in PK-Sim® that accounted for developmental changes, including age-dependent changes in glomerular filtration, organ weights, blood flow rates, and tissue composition [28,29,35]. There are previously published meropenem pediatric PBPK models [40,41], but these previous studies focused model evaluation on pediatric patients > 3 months of age. In this analysis, we leveraged extensive individual-level data collected from preterm and term infants < 3 months of age to evaluate meropenem’s PK early in life. Previously published PBPK models partitioned meropenem’s clearance into renal clearance and an additional/nonspecific clearance pathway [40,41]. Therefore, although we obtained comparable simulated meropenem clearance values for these two pathways, our model sought to account for the specific mechanistic processes that modulate meropenem PK.
Recently published data for OAT3 transporter ontogeny were used to account for the maturation of the OAT3 transporter [31]. The published OAT3 transporter ontogeny was developed based on protein concentration in term infants, where no GA information was available. Although no mammalian efflux transporter for meropenem has been identified yet, in vitro experiments using rat ileal segments suggested active secretion of meropenem in rat intestine [42]. A generic maturation function, developed by Hayton [33], for active renal secretion was used to model a hypothetical efflux transport protein’s age-dependent changes. It is important to note that the Hayton maturation function’s development included only term infants > 40 weeks GA [43]. Although in vitro data were available for DPEP1, ultimately, we assumed a first-order clearance process and optimized intrinsic clearance to match previously reported values for non-renal clearance (simulated vs. observed: 0.02 vs. 0.03–0.08 L/min [7,8,25,26]). Due to a lack of information on DPEP1 expression and ontogeny, we were unable to use any maturation function for metabolic clearance of meropenem. Although meropenem has been reported to have low protein binding (~ 1% to 2%) [1], a recent publication reported much higher protein binding (40% to 60%) of meropenem in patients [44]. As acknowledged by the study’s authors, this difference in protein binding could be due, at least in part, to differences in disease status [44]. Given that a clear consensus on the observed variability for meropenem protein binding has not been reached [45], we used the product label reported fraction unbound in plasma (0.98) in our model. But, we acknowledge that as meropenem is a drug with low extraction ratio, higher protein binding in patients could significantly reduce the clearance of meropenem and could be one reason for observed differences between PBPK model-predicted and observed data.
The adult and pediatric PBPK model-simulated concentration vs. time, AUC and clearance values were in good agreement with previously published data. Performance of the population simulations in infants < 3 months of age, evaluated using individual-level data, was assessed using AFE, ranging between 0.57 and 2.25. The highest AFE (suggesting slight overprediction) of 2.25 was observed for the group with a GA ≥ 32 weeks and PNA ≥ 14 days. The lowest AFE value of 0.57 (suggesting underprediction) was observed for the group with a GA < 32 weeks and PNA ≥ 14 days. Therefore, although the data were well captured overall, some trends with age may be related to the underlying model assumptions (e.g., maturation of key mechanisms in neonates as discussed previously) or patient-related variables (e.g., renal dysfunction) that are not captured in the model. Of note, AFE was calculated based on the differences between observed and median predicted concentration. Thus it does not account for the variability.
The current model also does not capture the possible effect of disease and co-medications on the observed concentration of meropenem in infants. Fourteen percent of patients had necrotizing enterocolitis (NEC) Grade I, 27% had NEC Grade II, and about 8% had spontaneous perforations. Such severe infection and inflammation can lead to changes in kidney function [46,47] that can affect meropenem exposure. Along with meropenem, these infants were also treated with, among other drugs, vancomycin (50% of subjects), gentamicin (46%), furosemide (38%), fentanyl (33%), and morphine (26%), alone or in combination. Some of these co-treatments are well known for their effect on renal function and OAT transporter-mediated tubular secretion process [48,49].
The PBPK model clearance predictions for infants were compared with the estimates obtained from a previously published PopPK analysis. When these data were visualized as a function of PMA, an age-dependent increase in clearance was observed as expected (Fig. 4). Overall, there was good agreement between simulated PBPK model predictions and the individual estimates obtained using the PopPK model that included creatinine and PMA as covariates. At PMA values < 30 weeks, the PopPK model individual estimates were on the lower end of the PBPK model range, whereas the opposite is true at PMA values > 35 weeks. This is consistent with the trends observed when AFE values were evaluated across age groups. Therefore, although there was generally good agreement between these two methodologies, some age-dependent trends can be observed [15].
The PBPK model was applied to evaluate suggested meropenem dosing for infants < 3 months of age with cIAIs. Using a criterion of 50% T>MIC for a MIC of 4 mg/L or 75% T>MIC for a MIC of 2 mg/L, favorable target attainment was observed across all dosing groups. These PBPK model simulation results are in agreement with the previously published PopPK analyses [15]. Therefore, these analyses suggest that both PBPK and PopPK modeling approaches were able to support similar meropenem dosing recommendations for infants < 3 months of age. Although in infants < 3 months of age meropenem is only indicated for complicated intra-abdominal infection, different dosing regimens of meropenem have been proposed for other indications (e.g., meningitis, sepsis, and pneumonia) [37,39]. While a 40% T>MIC is generally considered a bactericidal target for meropenem, the literature supports a higher %T>MIC (e.g. 64%, 80%) in critically ill patients, including infants and children [2,37,50]. Given that the individual-level data we used for model evaluation was collected from infants with suspected or confirmed intra-abdominal infections, we restricted our analysis to the FDA-approved drug label dosing regimens.
Our analysis is not without limitations. Although we sought to mechanistically account for the key variables that affect meropenem’s disposition, due to lack of data for some physiological processes, we optimized required model parameters and assumed a hypothetical efflux transporter in the kidney. Although we made these assumptions, there was still an acceptable agreement between the observed and simulated clearance and AUC values, and the fraction excreted unchanged in the urine. Second, we matched virtual subject demographic characteristics with those of the observed data. However, because the PK data available for infants < 3 months of age were collected from patients with suspected or confirmed intra-abdominal infections, there may be disease alterations in these patients that were not captured in our simulations.
5. Conclusion
Our meropenem PBPK model confirms that product label dosing regimens for infants < 3 months of age are expected to result in adequate drug exposure and supports the use of PBPK modeling to characterize drug disposition in infants.
Supplementary Material
Key Points.
Meropenem is a carbapenem antibiotic approved by the U.S. Food and Drug Administration for the treatment of complicated intra-abdominal infections in infants < 3 months of age.
Physiologically based pharmacokinetic modeling of meropenem disposition in infants < 3 months of age showed that age- and weight-based dosing recommendations in the product label are expected to result in adequate drug exposure.
Meropenem weight-normalized clearance estimates in infants obtained using PBPK and population pharmacokinetic modeling were compared and, generally, both methodologies resulted in comparable estimates.
Acknowledgments
Pediatric Trials Network (PTN) Steering Committee Members: Daniel K. Benjamin Jr., Christoph Hornik, Kanecia Zimmerman, Phyllis Kennel, and Rose Beci, Duke Clinical Research Institute, Durham, NC; Chi Dang Hornik, Duke University Medical Center, Durham, NC; Gregory L. Kearns, Scottsdale, AZ; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; Janice Sullivan, University of Louisville, Louisville, KY; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA; Paula Delmore, Wichita Medical Research and Education Foundation, Wichita, KS
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): Perdita Taylor-Zapata and June Lee
The Emmes Company, LLC (Data Coordinating Center): Ravinder Anand, Gaurav Sharma, Gina Simone, Kim Kaneshige, and Lawrence Taylor
PTN Publications Committee: Chaired by Thomas Green, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL
Pediatric Trials Network Meropenem Study Team
Akron Children’s Hospital: Anand Kantak, MD and Judy Ohlinger, RN; Albany Medical Center: Mike Horgan, MD and Susan Boynton, RN, BSN; Baylor College of Medicine: Eric C. Eichenwald, MD and Karen Jones, RN, CCRC; Children’s Hospital of Oakland: David J. Durand, MD and Jeanette Asselin, RRT, MS; Children’s Hospital of Orange County: Antonio Arrieta, MD and Kathy Shea, BA; Children’s Hospital of Philadelphia: Kelly Wade, MD, PhD, MSCE and Tonia Morrison, BA, CCRC; Children’s Hospital of Pittsburgh of UPMC: Beverly S. Brozanski, MD and Robyn Baker, BSN, NNP; Children’s Hospital at Vanderbilt: Joern-Hendrik Weitkamp, MD and Millie Nannie, RN, BA, BS, CCRN, CLNC; Children’s Medical Center of Dallas: Pablo Sanchez, MD and Shirley Montanye, RN, ADN, CCRC; Children’s National Medical Center: John van den Anker, MD and Elaine Williams, RN, MSN; Duke University: P. Brian Smith, MD, MPH, MHS, Michael Cohen-Wolkowiez, MD, PhD, Margarita Bidegain, MD, MHS, Daniel K. Benjamin Jr., MD, PhD, MPH, and Sandy Grimes, RN, BSN; Evanston Northwestern Healthcare: William MacKendrick, MD and Sue Wolf, RNC-NIC, CCRP; Indiana University, James W. Riley Hospital for Children: Brenda Poindexter, MD, MS and Leslie Dawn Wilson, BSN, CCRC; Kansas City Children’s Mercy Hospital: Lisa M. Castro, MD and Ann Harris, RN, BSN, MBA; Kapiolani Medical Center for Women and Children: Venkataraman Balaraman, MD and Robyn Morse, BA, CCRC; Sharp Mary Birch Hospital for Women: Maynard Rasmussen, MD and Kathy Arnell, RNC; SUNY Downstate Medical Center: Gloria Valencia, MD and Sara Higgerson, BA; University Hospitals (Cleveland, OH): Michele Walsh, MD and Arlene Zadell, RN, BSN; University of Alabama at Birmingham: Claire M. Roane, RN, MSN; University of California-San Diego: Neil Finer, MD, Edmund V. Capparelli, PharmD, and Wade Rich, BSHS, RRT, CCRC; University of Florida: David Burchfield, MD and Cindy Miller, RN; University of Louisville and Kosair Children’s Hospital: Janice E. Sullivan, MD and Gwendolyn Pierce, RN, CCRC; University of Michigan-Ann Arbor: Varsha Bhatt-Mehta, MS, PharmD, FCCP and Ron Dechert, DPH, MS, RRT, FAARC; University of Utah, Primary Children’s Medical Center: Robert M. Ward, MD and JoAnn Narus, MS, JD, MD; Yale University: Mathew Bizzaro, MD and Monica Konstantino, RN, BSN.
Certara Academic Center of Excellence Program
Phoenix® WinNonlin® is generously provided to the authors by Certara through the Academic Center of Excellence Program.
Funding
This work was funded under the National Institute of Child Health and Human Development (NICHD) contract (HHSN275201000003I) for the Pediatric Trials Network (PI Danny Benjamin). J.G.G. received research support from a National Institute of General Medical Sciences (NIGMS) funded T32 program (T32GM122741), as well as the American Foundation for Pharmaceutical Education (AFPE). D.G. received research support from the NICHD (K23HD083465, R01HD096435, and HHSN275201000003I). The Best Pharmaceuticals for Children Act (BPCA) Data Coordinating Center was funded under HHSN275201700002C (PI: Ravinder Anand). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest
M.C.-W. receives support for research from the NIH [1U24-MD016258], National Institute of Allergy and Infectious Diseases [HHSN272201500006I, HHSN272201300017I, 1K24-AI143971], NICHD [HHSN275201000003I], U.S. Food and Drug Administration [5U18-FD006298], and industry for drug development in adults and children. The remaining authors have no relevant conflicts of interest to disclose.
Footnotes
Ethics approval
For the study protocol that allowed the collection of the PK data from infants with a suspected or confirmed intra-abdominal infection (ClinicalTrials.gov # NCT00621192), Institutional Review Board (IRB) approval was obtained at each participating site. For the PBPK analyses described in this publication, the Office of Human Research Ethics at the University of North Carolina at Chapel Hill determined that the research did not constitute human subjects research as defined under federal regulations [45 CFR 46.102 (d or f) and 21 CFR 56.102(c)(e)(l)] and did not require IRB approval.
Consent to participate
For the original study in infants with a suspected or confirmed intra-abdominal infection (ClinicalTrials.gov # NCT00621192), written permission (informed consent) was obtained from the parent or legal guardian.
Data availability
Meropenem infant PK data was accessed through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Data and Specimen Hub (DASH, https://dash.nichd.nih.gov/).
References
- 1.MERREM(R) IV (meropenem for injection), for intravenous use <https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/050706s041lbl.pdf>(2019). Accessed May 8, 2020.
- 2.Nicolau DP. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis. 2008;47 Suppl 1:S32–40 [DOI] [PubMed] [Google Scholar]
- 3.Turnidge JD. The pharmacodynamics of β-Lactams. Clin Infect Dis. 1998;27:10–22. [DOI] [PubMed] [Google Scholar]
- 4.Thyrum PT, Yeh C, Birmingham B, Lasseter K. Pharmacokinetics of meropenem in patients with liver disease. Clin Infect Dis. 1997;24 Suppl 2:S184–90. [DOI] [PubMed] [Google Scholar]
- 5.Burman LÅ, Nilsson-ehle I, Hutchison M, Haworth SJ, Norrby SR. Pharmacokinetics of meropenem and its metabolite ICI 213,689 in healthy subjects with known renal metabolism of imipenem. J Antimicrob Chemother. 1991;27:219–24. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson-Ehle I, Hutchison M, Haworth SJ, Norrby SR. Pharmacokinetics of meropenem compared to imipenem-cilastatin in young, healthy males. Eur J Clin Microbiol Infect Dis. 1991;10:85–8. [DOI] [PubMed] [Google Scholar]
- 7.Kelly HC, Hutchison M, Haworth SJ. A comparison of the pharmacokinetics of meropenem after administration by intravenous injection over 5 min and intravenous infusion over 30 min. J Antimicrob Chemother. 1995;36 Suppl A:35–41. [DOI] [PubMed] [Google Scholar]
- 8.Bax RP, Bastain W, Featberstone A, Wilkinson DM, Hutchison M, Haworth SJ. The pharmacokinetics of meropenem in volunteers. J Antimicrob Chemother. 1989;24:311–20. [DOI] [PubMed] [Google Scholar]
- 9.Shibayama T, Sugiyama D, Kamiyama E, Tokui T, Hirota T, Ikeda T. Characterization of CS-023 (RO4908463), a novel parenteral carbapenem antibiotic, and meropenem as substrates of human renal transporters. Drug Metab Pharmacokinet. 2007;22:41–7. [DOI] [PubMed] [Google Scholar]
- 10.Blumer JL, Reed MD, Kearns GL, Jacobs RF, Gooch WM, Yogev R, et al. Sequential, single-dose pharmacokinetic evaluation of meropenem in hospitalized infants and children. Antimicrob Agents Chemother. 1995;39:1721–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker EM, Hutchison M, Blumer JL. The pharmacokinetics of meropenem in infants and children: A population analysis. J Antimicrob Chemother. 1995;36:63–71. [DOI] [PubMed] [Google Scholar]
- 12.Du X, Li C, Kuti JL, Nightingale CH, Nicolau DP. Population pharmacokinetics and pharmacodynamics of meropenem in pediatric patients. J Clin Pharmacol. 2006;46:69–75. [DOI] [PubMed] [Google Scholar]
- 13.van den Anker JN, Pokorna P, Kinzig-Schippers M, Martinkova J, de Groot R, Drusano GL, et al. Meropenem pharmacokinetics in the newborn. Antimicrob Agents Chemother. 2009;53:3871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Enk JG, Touw DJ, Lafeber HN. Pharmacokinetics of meropenem in preterm neonates. Ther Drug Monit. 2001;23:198–201. [DOI] [PubMed] [Google Scholar]
- 15.Smith PB, Cohen-Wolkowiez M, Castro LM, Poindexter B, Bidegain M, Weitkamp JH, et al. Population pharmacokinetics of meropenem in plasma and cerebrospinal fluid of infants with suspected or complicated intra-abdominal infections. Pediatr Infect Dis J. 2011;30:844–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh E, Hornik C, Clark R, Laughon M, Benjamin D, Smith P. Medication use in the neonatal intensive care unit. Am J Perinatol. 2013;31:811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landwehr C, Richardson J, Bint L, Parsons R, Sunderland B, Czarniak P. Cross-sectional survey of off-label and unlicensed prescribing for inpatients at a paediatric teaching hospital in Western Australia. PLoS One. 2019;14(1):e0210237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magalhães J, Teixeira Rodrigues A, Roque F, Figueiras A, Falcão A, Herdeiro MT. Use of off-label and unlicenced drugs in hospitalised paediatric patients: A systematic review. Eur. J. Clin. Pharmacol 2015;71(1):1–13. [DOI] [PubMed] [Google Scholar]
- 19.Wagner C, Zhao P, Pan Y, Hsu V, Grillo J, Huang SM, et al. Application of physiologically based pharmacokinetic (PBPK) modeling to support dose selection: Report of an FDA public workshop on PBPK. CPT Pharmacometrics Syst Pharmacol. 2015;4:226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salerno SN, Burckart GJ, Huang S, Gonzalez D. Pediatric drug–drug interaction studies: barriers and opportunities. Clin Pharmacol Ther. 2019;105:1067–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dallmann A, Ince I, Solodenko J, Meyer M, Willmann S, Eissing T, et al. Physiologically based pharmacokinetic modeling of renally cleared drugs in pregnant women. Clin Pharmacokinet. 2017;56:1525–41. [DOI] [PubMed] [Google Scholar]
- 22.Ikawa K, Nakashima A, Morikawa N, Ikeda K, Murakami Y, Ohge H, et al. Clinical pharmacokinetics of meropenem and biapenem in bile and dosing considerations for biliary tract infections based on site-specific pharmacodynamic target attainment. Antimicrob Agents Chemother. 2011;55:5609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SW, We JS, Kim GW, Choi SH, Park HS. Stability of new carbapenem DA-1131 to renal dipeptidase (dehydropeptidase I). Antimicrob Agents Chemother. 2002;46:575–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milman G. Towards single cell protein analysis of cardiac progenitor cells [Internet]. Imperial College London; 2015. Available from: http://hdl.handle.net/10044/1/33760 [Google Scholar]
- 25.Leroy A, Fillastre JP, Borsa-Lebas F, Etienne I, Humbert G. Pharmacokinetics of meropenem (ICI 194,660) and its metabolite (ICI 213,689) in healthy subjects and in patients with renal impairment. Antimicrob Agents Chemother. 1992;36:2794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ljungberg B, Nilsson-Ehle I. Pharmacokinetics of meropenem and its metabolite in young and elderly healthy men. Antimicrob Agents Chemother. 1992;36:1437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wickham H, Averick M, Bryan J, Chang W, D’ L, Mcgowan A, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4:1686. [Google Scholar]
- 28.Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45:1013–34. [DOI] [PubMed] [Google Scholar]
- 29.Claassen K, Thelen K, Coboeken K, Gaub T, Lippert J, Allegaert K, et al. Development of a physiologically-based pharmacokinetic model for preterm neonates: evaluation with in vivo data. Curr Pharm Des. 2015;21:5688–98. [DOI] [PubMed] [Google Scholar]
- 30.Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: A quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24:67–76. [DOI] [PubMed] [Google Scholar]
- 31.Cheung KWK, van Groen BD, Spaans E, van Borselen MD, de Bruijn ACJM, Simons-Oosterhuis Y, et al. A comprehensive analysis of ontogeny of renal drug transporters: mRNA analyses, quantitative proteomics, and localization. Clin Pharmacol Ther. 2019;106:1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Internet]. 2020. Available from: https://www.r-project.org/ [Google Scholar]
- 33.Hayton WL. Maturation and growth of renal function: Dosing renally cleared drugs in children. AAPS J. 2000;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson EJ, Wu H, Maharaj A, Edginton AN, Balevic SJ, Cobbaert M, et al. Physiologically based pharmacokinetic modeling for trimethoprim and sulfamethoxazole in children. Clin Pharmacokinet. 2019;58:887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edginton AN, Schmitt W, Voith B, Willmann S. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet. 2006;45:683–704. [DOI] [PubMed] [Google Scholar]
- 36.Cohen-Wolkowiez M, Poindexter B, Bidegain M, Weitkamp JH, Schelonka RL, Randolph DA, et al. Safety and effectiveness of meropenem in infants with suspected or complicated intra-abdominal infections. Clin Infect Dis. 2012;55:1495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Germovsek E, Lutsar I, Kipper K, Karlsson MO, Planche T, Chazallon C, et al. Plasma and CSF pharmacokinetics of meropenem in neonates and young infants: Results from the NeoMero studies. J Antimicrob Chemother. 2018;73:1908–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lomaestro BM, Drusano GL. Pharmacodynamic evaluation of extending the administration time of meropenem using a Monte Carlo simulation. Antimicrob Agents Chemother. 2005;49:461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z-M, Chen X-Y, Bi J, Wang M-Y, Xu B-P, Tang B-H, et al. Reappraisal of the optimal dose of meropenem in critically ill infants and children: a developmental pharmacokinetic-pharmacodynamic analysis. Antimicrob Agents Chemother. 2020;64(8):e00760–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou W, Johnson T, Xu H, Cheung S, Bui K, Li J, et al. Predictive performance of physiologically based pharmacokinetic and population pharmacokinetic modeling of renally cleared drugs in children. CPT Pharmacometrics Syst Pharmacol. 2016;5:475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martins FS, Zhu P, Heinrichs MT, Sy SKB. Physiologically based pharmacokinetic-pharmacodynamic evaluation of meropenem plus fosfomycin in paediatrics. Br J Clin Pharmacol. 2021;87(3):1012–1023. [DOI] [PubMed] [Google Scholar]
- 42.Saito T, Sawazaki R, Ujiie K, Oda M, Saitoh H. Possible factors involved in oral inactivity of meropenem, a carbapenem antibiotic. Pharmacol Pharm. 2012;3:201–6. [Google Scholar]
- 43.Rubin MI, Bruck E, Rapoport M, Snively M, McKay H, Baumler A. Maturation of renal function in childhood: clearance studies. J Clin Invest. 1949;28(5 Pt 2):1144–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Shaer MH, Alghamdi WA, Graham E, Peloquin CA. Meropenem, cefepime, and piperacillin protein binding in patient samples. Ther Drug Monit. 2020;42:129–32. [DOI] [PubMed] [Google Scholar]
- 45.Liebchen U, Dorn C, Kees M, Schiesser S, Hitzenbichler F, Kees F, et al. Comment on “Meropenem, cefepime, and piperacillin protein binding in patient samples”. Ther Drug Monit. 2020;42:909–10. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez C, García MA, Valdés BD. Acute kidney injury in newborns with necrotizing enterocolitis: risk factors and mortality. Bol Med Hosp Infant Mex. 2019;76(5):210–214. [DOI] [PubMed] [Google Scholar]
- 47.Garg PM, Tatum R, Ravisankar S, Shekhawat PS, Chen Y-H. Necrotizing enterocolitis in a mouse model leads to widespread renal inflammation, acute kidney injury, and disruption of renal tight junction proteins. Pediatr Res. 2015;78(5):527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasannejad H, Takeda M, Taki K, Shin HJ, Babu E, Jutabha P, et al. Interactions of human organic anion transporters with diuretics. J Pharmacol Exp Ther. 2004;308:1021–9. [DOI] [PubMed] [Google Scholar]
- 49.McWilliam SJ, Antoine DJ, Smyth RL, Pirmohamed M. Aminoglycoside-induced nephrotoxicity in children. Pediatr Nephrol. 2017;32:2015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cies JJ, Moore WS, Enache A, Chopra A. Population pharmacokinetics and pharmacodynamic target attainment of meropenem in critically ill young children. J Pediatr Pharmacol Ther. 2017;22:276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DrugBank. Meropenem (DB00760) [Internet]. Available from: https://go.drugbank.com/drugs/DB00760
- 52.AstraZeneca. Environmental Risk Assessment Data: Meropenem [Internet]. 2017. Available from: https://www.astrazeneca.com/content/dam/az/our-company/Sustainability/2017/Meropenem.pdf
- 53.Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95:1238–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Meropenem infant PK data was accessed through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Data and Specimen Hub (DASH, https://dash.nichd.nih.gov/).


