Abstract
Resilience to stressful life events has received considerable attention in both clinical and preclinical studies. A number of neural substrates have been identified as putatively mediating resilience to stress. However, there remains considerable diversity in how resilience is defined and studied. This article aims to examine how resilience is defined and conceptualized in social psychology, public health, and related fields, to better inform the understanding of stress resilience in the neurobiological context and to differentiate resilience from other patterns of response to stressful experiences. An understanding of resilience through the lens of clinical and applied sciences is likely to lead to the identification of more robust and reproducible neural substrates, though many challenges remain.
Keywords: PTSD, Brain, Inflammation, neural substrates, sex differences
Challenges to current understanding of resilience to stress
How can an individual who experiences stressful life events that are traumatic, enduring or intense return to a healthy and balanced life? This question remains a foundational one for the field of stress research. Understanding how such resilience to stressful life events can be achieved has been a long-standing driver of research into the neurobiology of stress. The impact of stress on health can be stark, with stressful life events precipitating onset of symptoms or relapse of symptoms in a range of psychiatric and other disorders, including posttraumatic stress disorder (PTSD), obesity, inflammatory bowel disease and relapse to drug seeking [1–8]. Certain mental disorders have exposure to a traumatic or stressful event listed explicitly as a diagnostic criterion (in the DSM-5 category “Trauma- and Stressor-Related Disorders”), including PTSD, acute stress disorder, and adjustment disorder [9]. Additionally, stressful life events are well recognized as precipitants of major depressive episodes, and impaired coping with stressful events is important in the context of alcohol use disorders [9]. However, several factors hamper progress towards better understanding of resilience. These include the heterogeneity of stressful experiences, the highly individualized responses to those experiences, and the large number of neural substrates associated with resilience. Further, there is a lack of agreement about how to define resilience or even whether it is a useful term, partly because the term is overused and often used inappropriately. Despite these obstacles, there is a strong clinical need to understand how some individuals can bounce back from stressful life experiences while others cannot. Because of this clinical impetus, it is important to more clearly define the term resilience, so that one can make better sense of the rapid pace of various neural substrates being associated with resilience. The goal of this article is to examine how resilience is defined and understood in a number of fields, including social psychology, public health and related disciplines, and thereby better inform the understanding of stress resilience in the neurobiological context.
Our understanding of the neural substrates linked to stress resilience has accelerated in the last 10–15 years. These neural substrates may reflect pre-existing genetic and other factors that influence an individual’s resilience. Neural substrates linked to resilience have been identified in multiple stress-related brain regions including limbic areas, the hypothalamus, and brainstem structures. However, a bigger-picture understanding of these contributions is still limited: for instance, do these various resilience substrates normally work together, or independently from one another? Are they stressor-specific? Do different neural substrates control the initial response compared to the more enduring resilient response? These are some of the questions that need to be addressed in the next 10–15 years.
Defining Resilience
Resilience is a term long used in the social psychology, clinical psychology, and public health literatures. This literature, broadly speaking, has defined resilience as positive adaptation to adversity, requiring that there be both the presence of adversity and positive adaptation [10–12]. At its core, resilience is the concept that an individual can bend to threats from the environment but does not break. It is not usually meant to reflect that there is minimal disruption or a lack of disruption for an individual, rather, a resilient response is the ability to bounce back from disruption. It is both a process and an outcome [13, 14]. For example, there can be an impact on mental and physiological health soon after a traumatic experience. With time, there is a return to functioning closer to pre-trauma levels that is measurable as a resilient outcome (Figure 1) [13]. A resilient outcome may be hard to differentiate from two alternative scenarios: (i) a lack of an initial and ensuing response to disruptive events (resistance to stress); and (ii) complete inability to mount a response. These are very different processes measurable as similar outcomes and can be difficult to parse out, but are likely to have different biological underpinnings.
Figure 1. Hypothetical patterns of responses to an intense or traumatic stressful event.
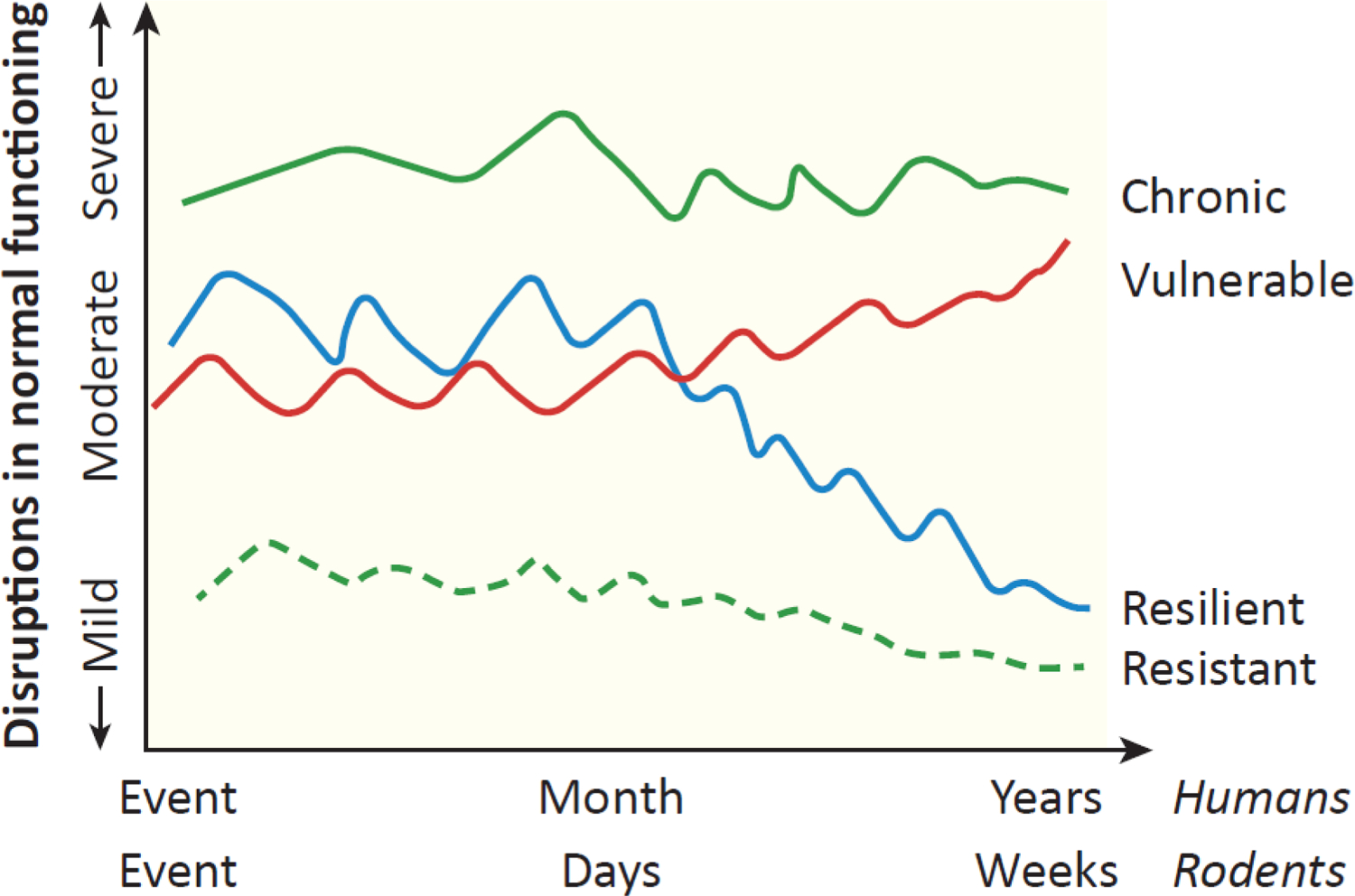
Multiple possible patterns of responses to stressful and traumatic stimuli exist. The vulnerable trajectory is one in which moderate levels of stress or distress persist and may increase further over time. The chronic trajectory is characterized by elevated responses to the trauma and those symptoms remain highly elevated. Resistance is one in which few symptoms develop either initially or later on. The resilient category is one in which there is moderate disruption and symptoms initially, followed by a return towards baseline. Cross-sectional studies of the response in stressed individuals at periods of time after patterns have stabilized are unable to differentiate resilient from resistant individuals or chronically responsive from vulnerable individuals. As a result, neural substrates underlying resilience may not be reproducibly identified. An approach that examines the trajectory of individuals’ responses improves the ability to identify underlying discrete and stress-specific neural substrates. Adapted from [13] with permission.
In the social psychology and public health literature, the types of resilience being studied can range from those relating to an individual person to whole communities. Many of the factors that promote positive adaptation originate outside the individual – in their families, communities, cultures and societies. Although these levels of focus vary greatly from those of neurobiologists, there is much to be learnt from these other perspectives. In the following, I highlight briefly four central themes that emerge from this body of literature (based on [12–17]). First is the idea that resilience in one domain may co-exist with dysfunction in another domain. As a result, an individual who is resilient to the effects of stress in one domain may be vulnerable to them in another. Despite the dysfunctions in some domains, there is evidence to argue that resilient individuals tend to sustain an overall high quality of life [18, 19]. In animal models, a multidimensional approach can identify the impact of stress in multiple domains, including behavioral and physiological health, in order to determine the overall impact on the individual. Second is the idea that resilience is a process that individuals progress through, rather than a fixed trait of those individuals. Individuals may possess the capacity to engage protective factors (traits), but resilience only occurs when those factors come together to initiate specific processes to promote resilience. Thus, resilience is not an absolute characteristic of an individual but influenced by traits specific to the individual. Such traits may include low anxiety, low novelty-seeking, and characteristics such as optimism, cognitive flexibility and cognitive reappraisal strategies that serve to reduce stress responsiveness [20–23]. Third, resilience may be specific to certain contexts. A behavior in one context may signal positive adaptation of an individual to a specific disruptive environment. However, in another context, that same behavior may not confer resilience, and may be even maladaptive. Studies in animals support this concept, as adaptations to stressful stimuli are influenced by contextual cues [24]. Fourth, there may be new growth in resilient individuals after stressful experiences, not just a return to some prior level of homeostasis.
What resilience is not
Resilience is not simply reductions in stress responses compared to those of a control group. By definition (as noted above), the term resilience implies that there is a response, a bending, but that there is a return to a pre-stress state or perhaps to a new level of homeostasis (allostasis). For example, a neural substrate that reduces the hypothalamic-pituitary-adrenal (HPA) response to stress is not necessarily a substrate of resilience to stress but may just be a substrate that has inhibitory control over HPA activity. Resilience is best understood in conditions in which subgroups of divergent response types emerge. That is, understanding resilience requires a comparison group of individuals in whom the stress response remains elevated (i.e. a vulnerable or susceptible group). Resilient individuals may not be better off in all domains impacted by the stress exposure; however, their quality of life or day-to-day functioning is overall better [61]. Indeed, an individual may not return to the same baseline that preceded the stress exposure. They may return to a shifted baseline, a “new normal”, as reflected in the concept of allostasis. Finally, it is widely recognized that domains in which resilience is manifested can vary between individuals [10–12, 15] further emphasizing the need for measures across multiple domains.
The trajectory of resilience
The neuroscience literature has typically studied resilience in a cross-sectional manner, at a single time point after the stress exposure in order to determine whether resilience is observed compared to a control group or some type of vulnerable group. This can lead to potential misinterpretation of findings. For example, a lower response at a later time after stress could reflect resilience in that individual but it could also reflect a lack of an initial response to the stressful stimulus, without bending which is the sine qua non of resilience. That latter individual is better defined as a resistant individual. Thus, both resistant and resilient individuals can exhibit a similar outcome while their initial response and their trajectory are different. Examining only the outcome means that there is an inability to fully differentiate a resistant from a resilient individual. It is possible that there are few relevant differences between these two types of individuals. However, a lack of response to an intense stressor is not necessarily adaptive. In fact, the importance of having a response (neuroendocrine, autonomic, behavioral) to intense and life-threatening stimuli has been well demonstrated [25, 26]. Further, different neural substrates are likely to be engaged in an individual that responds to the initial stressor compared to an individual that does not respond. This suggests that these two individuals ultimately reach the same physiological or behavioral outcomes through different neural paths. It is important to note that individuals that are exposed to stress early in life, whether pre-natally, post-natally or during adolescence, may display a relative lack of stress responses to subsequent stressors at a different timepoint in life, and this may reflect resilience as well.
As noted earlier, it is common in the neuroscience literature to assess resilience via outcomes at a single timepoint after stress exposure. A more informative and translationally relevant approach, consistent with the origins of the term resilience (as discussed above) is to study the response to stress over time, thinking of it as a trajectory. The clinical literature, particularly of studies examining PTSD in the military, has identified different categories of PTSD symptoms reflecting the trajectory of responses to trauma. Although there are some variations in terminology, the categories generally include acute, chronic, vulnerable, resilient and resistant groups [13, 27, 28]. The acute category is one in which posttraumatic stress symptoms subside within a month after the traumatic experience. The vulnerable trajectory is one in which moderate levels of distress emerge and persist, and may even increase further over time. The chronic trajectory is characterized by elevated responses to the trauma and those symptoms remain highly elevated. Resistance is one in which few symptoms develop either initially or later on. Lastly, the resilient category is one in which there is some disruption in response to the trauma, and display of symptoms, followed by a return to close to pre-trauma levels. These trajectories are depicted in Figure 1. Not all of these are necessarily relevant to preclinical studies, amenable to study in animal models or necessarily exhibited by rodents. However, evidence supports the idea that stressed rodents can exhibit resilient, resistant and vulnerable trajectories [29–31].
A temporally cross-sectional approach has been useful for identifying the behavioral and physiological outcomes in stressed animals segregated into resilient or vulnerable subgroups. The segregation has been based on different factors, including coping strategies, social anxiety, anhedonia and urine scent marking [29, 30, 32, 33]. These methods of segregation have identified several neural substrates that promote resilience. These include the peptides Neuropeptide Y (NPY) and orexins, the sphingosine-1-phosphate 3 receptor (S1PR3) and the glucocorticoid receptor [34–37]. However, the cross-sectional approach is unable to differentiate a resilient individual from one that doesn’t respond to the stressful stimulus (i.e. a resistant individual). Similarly, it fails to differentiate a pattern of vulnerability that develops over time from a pattern of chronic high symptoms patterns of vulnerability (Figure 1). More simply put, a cross sectional approach fails to distinguish between different trajectories that culminate in the same outcome. These limitations have detrimental effects on the ability to develop strategies to promote resilience: identified neural substrates may not replicate across stress paradigms; and neural substrates may only reflect a temporary stage but not enduring resilience. These caveats may obscure the identification of unique substrates underlying the different trajectories in the response to stress.
Resilience is best measured by multi-dimensional or multimodal constructs to provide the most integrated study of how stress has impacted an individual. The results may indicate resilience in one domain, but not in another. For example, an animal resilient to the anxiety-inducing effects of stress may exhibit increased heart rate variability or elevated blood pressure. Thus, a more accurate way of referring to the potential resilience or vulnerability of stressed individuals would be to identify the domain in which the investigators consider the individuals to be resilient or vulnerable, and to note the specific stressor that was experienced. This narrower approach to labeling individuals as resilient or vulnerable is better aligned with the original definitions of resilience. It is also more translationally relevant and may help alleviate concerns about inappropriate use of the term resilience, which muddies our understanding of the neural substrates that underlie resilience to stress. In practical terms, assessment of trajectories in rodent models requires assessment of dependent variables that are amenable to repeated measurement. This could range from relatively simple measures such as body weight and food intake, to more complex assessments of hormones and other circulating factors, core temperature and heart rate variability, EEG, local field potentials, single unit activity in relevant brain structures and some behavioral assessments. Rodent models are increasingly amenable to the assessment of trajectories using both new and traditional technologies that allow monitoring at high resolution and specificity the activity of neuronal and other cell populations, as well as detailed and semi-automated behavioral assessment.
Beyond neuronal activity: Are neuroinflammatory processes key?
Much of the literature on the substrates of stress resilience or vulnerability has focused on the activity and function of neurons. However, considerable evidence suggests stress-related psychiatric diseases are heavily interconnected with inflammatory processes and that these processes contribute to pathology [38–41]. In the brain, inflammatory processes are governed by the interaction among neurons, microglia, astrocytes, endothelial cells lining blood vessels and other cell types that collectively constitute the functional element known as the ‘neurovascular unit’ as well as by neuroinflammatory processes at other brain barriers [40–44] Elevations in the pro-inflammatory cytokines IL-6, IL-1β and TNFα are observed in the circulation of individuals with depression [45–51], and PTSD patients display enhanced cytokine release from mononuclear cells [52, 53]. These peripheral changes are aligned with microglial activation in the brains of individuals with atypical depression or with anxiety [54–57]. Antidepressant treatments can improve cytokine-induced sickness behavior [58]. Conversely, anti-inflammatory medications given to individuals with depression or cancer patients undergoing chemotherapy improve mood symptoms [59–61], and pro-inflammatory cytokines (or stimuli that induce them) can produce symptoms of depression [58, 62–64]. However, lowering inflammatory responses in a disorder as heterogeneous as depression is not universally effective in treating symptoms, likely because inflammatory markers are only elevated in subgroups of patients [65, 66]. These results underscore the need to better understand the involvement of neuroinflammatory processes in the response to stress. Neuroinflammatory processes may be more broadly activated in stressed individuals than previously thought. Studies in rodents suggest that neuroinflammatory processes are activated in the ventral hippocampus, medial prefrontal cortex and nucleus accumbens in defeated rodents and in rodents exposed to other types of psychosocial stressors [36, 67–69]. However, significant gaps in our knowledge about the role of neuroinflammatory processes remain. These include understanding whether specific pro- and anti-inflammatory substrates are important for responses to specific types of stressors, and determining the extent to which neuroinflammatory substrates are activated by local mechanisms such as activation of microglia or by peripheral cytokines that cross the blood brain barrier to impact brain substrates. Finally, determining how gut microbiota or microbial populations in other tissues are influenced by stress and can contribute to resilience will be important to our understanding of how neuroinflammatory processes control resilience.
Substrates that promote resilience are not the same as those that reduce vulnerability.
Resilience and vulnerability to stress likely exist on a continuum and are dependent on the response domain being studied. Examples of this concept come from rodent studies employing chronic social defeat paradigms. Dominance rankings and coping strategies (active vs. passive coping) displayed during such experiments are a key determinant of resilience or vulnerability to the social anxiety, anhedonia, passive coping and disrupted neuroendocrine function produced by defeat [36, 37, 68, 70–72]. Active coping strategies include upright boxing postures and lack of submission to a dominant conspecific animal, and passive coping is associated with more rapid submission. These coping strategies exist on a continuum. Do the underlying processes also exist on a continuum? Some substrates certainly do, but there is evidence that certain substrates are uniquely engaged in resilient animals whereas others are uniquely engaged in vulnerable animals. For example, actively coping rats exhibit increased expression of the S1pr3 gene in the medial prefrontal cortex, whereas this expression is similar between passively coping and control animals [36]. When S1PR3 expression is reduced, characteristics of the passive phenotype emerge. Does this mean that S1PR3 reductions induce vulnerability, or alternatively, that they reduce resilience? We would argue that the latter is the case since, initially, S1PR3 expression is similar between the passively coping and control rats. Similarly, whereas changes in neurovascular unit function in the ventral hippocampus of passive coping rats do occur, actively coping rats are not different from controls in that regard. Reducing neuroinflammatory processes pushes passively coping rats towards an active coping phenotype [68] but should this be interpreted as increased resilience or reduced vulnerability? Again, we would argue the latter, since the actively coping group is not different from controls, suggesting that this substrate is not important for resilience. Such interpretations are complex and must be tested with other stress experiences and in other behavioral and physiological domains.
Developing a cohesive picture of how resilience can be promoted
The last 10–15 years have identified a plethora of substrates that are thought to underlie vulnerability to stress or resilience to stress in a variety of stress paradigms, and many excellent reviews of these substrates are available (including citations described above and [30, 73]). Making sense of these varied substrates is amongst the biggest challenges moving forward, especially since the aim is to translate these findings into clinically relevant treatments. The range of stress paradigms being used seems, at first pass, a disadvantage, because it is a common occurrence that substrates identified in a specific model are not tested using other paradigms or replicated by other labs. Substrates common across multiple paradigms are potentially high value targets for translation because their validity is broad. But even substrates identified in only one category of paradigm (eg. social or physical stress [74]) can have notable value. For example, substrates identified only in social stress models may be valuable because they underlie the effects of a specific type of stress limited to the social domain. It is possible that resilience substrates differ across types of stressors. Lack of replication of a resilience substrate in a paradigm different from the one in which the original finding was made may suggest that the substrate is limited to a specific stress type, and thereby has value for understanding how stressors originating in specific domains are regulated.
Putting aside this issue of stress-specific substrates, how can one make sense of the multitude of resilience substrates that have been identified? It seems likely that there are multiple substrates that function in parallel, rather than serially, to subserve unique physiological and behavioral aspects of resilience (Figure 2). Studying each substrate in isolation can help better understand the specifics of the substrate and identify its functions, but sheds limited light on the broader relevance of these functions and their interactions with those of other substrates. The existence of multiple substrates likely does not reflect redundancy in the brain but rather specificity. Some substrates may emerge because they are responsive to early life events, some to epigenetic modulation, some to social experiences, some to interoceptive flux – each substrate providing value to the organism, allowing it to respond to a rich environment. To the extent that all individuals possess the capacity for resilience, these multiple substrates may reflect the different pathways and circuits that can be recruited to achieve resilience. In an intact organism, many substrates are likely to work simultaneously and in concert, to enable an organism to cope with the challenges of a complex environment. As an example from our own work, increases in S1PR3 in the mPFC induced by social defeat reduce expression of the pro-inflammatory cytokine TNFa. These gene expression changes also promote active coping and prevent the reductions in social interaction produced by social defeat. At the same time, these resilient rats also exhibit reduced prepro-orexin (hypocretin) mRNA in the lateral hypothalamus [37] compared to passively coping and novel cage control rats. Orexins mediate arousal and wakefulness, and this reduction in effects of social defeat may underlie the reduced rapid awakenings from REM sleep exhibited by these actively coping/resilient animals compared to the passively coping/vulnerable and control rats [75] that we have observed. Thus, reductions in orexin mRNA may help protect against the effects of social defeat on sleep disruptions while increases in S1PR3 in the mPFC buffer against neuroinflammatory processes which impact social anxiety.
Figure 2. Schematic illustration of some of the neural substrates involved in stress resilience in the rodent brain, and how their relative predominance may shift depending on modifying factors.
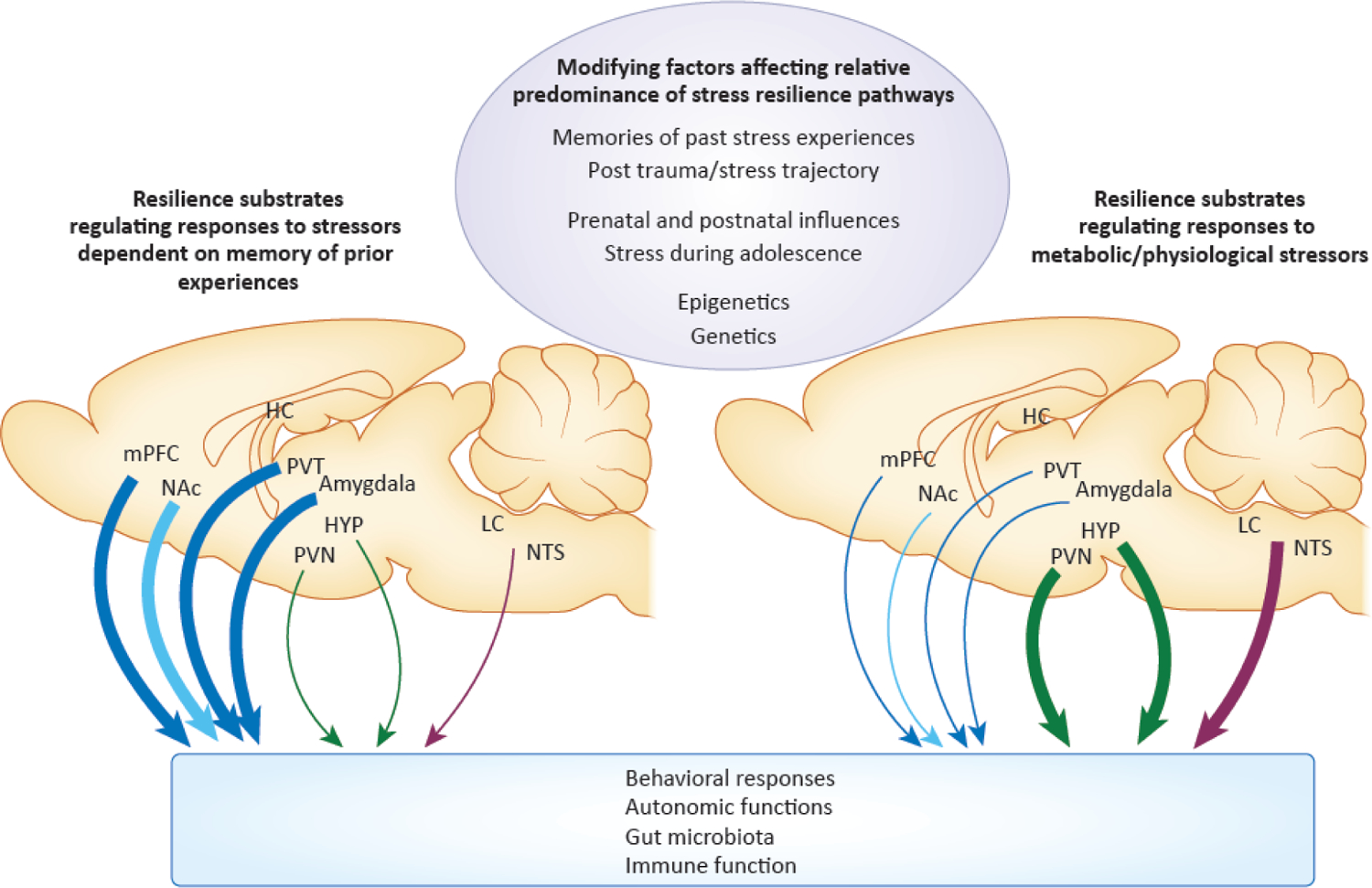
Multiple substrates and circuits exist in parallel that can promote resilience. Which substrates or circuits predominate may be determined by modifying factors. These include experiences in early life; prenatal or postnatal disruptions; stress during adolescence; epigenetic or genetic influences; prior experience with the ongoing, current stressors; and the time point being examined during the post-stress trajectory. These modifying factors can shift the weight of resilience circuits and substrates, so that some circuits/substrates predominate in promoting resilience. For example, resilience substrates in limbic structures may predominate to control responses to stress when memories for prior experiences are relevant, such as when familiar stressors are experienced or when the same stressors are experienced over time (heavier blue lines on the left). With more metabolic or physiological types of stressors, resilience substrates in hypothalamic structures may predominate (heavier green lines on the right). The structures, responses, and modifying factors depicted in the figure are not intended to be all inclusive.
Abbreviations: mPFC: medial prefrontal cortex, NAc: nucleus accumbens, HC: hippocampus, HYP: hypothalamus, PVT: paraventricular nucleus of the thalamus, PVN: paraventricular nucleus of the hypothalamus, LC: locus coeruleus, NTS: nucleus tractus solitarius.
Sex differences in stress responses are well-established [76–79]. The majority of resilience substrates identified to date have been studied and identified in male rodents. However, sex differences in the neural substrates of resilience are apparent, and the sources of these differences are complex [75, 80]. Some substrates may be similar but work on different timescales in males compared to females or be influenced to a greater degree by genetic or epigenetic influences or by gonadal hormones. While the activational effects of gonadal hormones in females are typically studied when sex differences in stress responses or resilience substrates are identified, organizational influences of gonadal hormones at various developmental stages have received much less attention. Examining a specific neural substrate of resilience in both males and females can reinforce the relevance of that particular substrate as a shared regulator of resilience in both males and females or highlight important differences in regulation of that substrate between males and females. Alternatively, a substrate might be identified as being unique to males or unique to females. Determining whether a substrate is shared between males and females or is unique to one sex is critical for directing the development of strategies that promote resilience.
More broadly, rather than seeing the variety of substrates as a problem, one should seek to identify commonalities in the conditions that recruit these substrates to determine how the various substrates may work together and interact with one another in a whole organism. To aid in this, as discussed earlier, longitudinal approaches are likely to be helpful, as is a consideration of outcomes across multiple domains controlled by the neural substrates. To address the complexity of multiple domains, inventories or libraries that establish the aggregate impact of a stress exposure in individuals of different ages, sexes or exposed to specific types of stressors will become increasingly important and informative.
Concluding remarks and future perspectives
The psychology, public health and psychiatry literatures offer a rich foundation for understanding stress resilience, including insights based on clinical observations. For neurobiological studies to focus on translational relevance, our understanding of resilience needs to be well-aligned with this complementary literature and guided by it. Resilience is both a process and an outcome, and these two aspects are tightly interconnected: it is difficult to understand the outcome without a clear picture of the underlying processes that lead to it. With regards to behavioral outcomes, it is also important to keep in mind that some aspects of resilience and vulnerability may be best viewed on a continuum, rather than approaching them as an either/or process.
A principal challenge moving forward is to understand the extent to which the varied putative neural substrates controlling resilience are integrated or conversely work independently to regulate features that are unique to each individual (see also Outstanding Questions). These substrates and their interactions can be influenced by early life events, genetic or epigenetic processes or distinctive stressful environments and experiences. To determine whether a specific substrate is important in resilience, it should be studied under different stress conditions. For example, if an anti-inflammatory substrate is expressed in higher concentrations in animals resilient to social stress, is that same substrate also important in response to predator odor or physical stress? Is that same substrate important in both sexes? Is that same substrate important for outcomes in multiple response domains? In addition, future research on resilience should emphasize interactions between neuronal and non-neuronal cell types and interactions between peripheral and central inflammatory processes. Ultimately, a common conceptual understanding of resilience, regardless of the terminology being used, is necessary to drive research in this field forward.
Outstanding Questions Box.
Resilience includes a process of responding to a traumatic stimulus/stressor, engaging the biological stress response, but then bouncing back from that experience. What are some of the readily accessible measures from the fields of biology, physiology, hematology, immunology and related disciplines that can help characterize resilience and differentiate it from other response patterns?
In current understanding, resilient and vulnerable individuals are initially impacted in similar ways by stressful experiences but resilient individuals bounce back. Are similar substrates engaged in both groups in their initial response, or do resilient individuals recruit substrates that are inherently different from those of vulnerable individuals? After the initial response in resilient individuals, are new substrates recruited to produce resilience?
Peptides, neurotransmitters, receptors and other neuroactive substances have been identified as potential substrates of resilience. How can researchers extract a coherent picture from this wealth of information? Are there substrates of resilience that are specific to different types of stressors, or to stressors experienced at different phases of life? Are there substrates of resilience that differ between males and females? Based on these categories and the potential differences in resilience substrates between them, is it possible to develop a catalogue of resilience substrates?
Are neuroinflammatory substrates at the core of resilience or vulnerability to stress? Does stress exposure create an inflammatory milieu that overrides other substrates? How do neuroinflammatory substrates regulate previously identified substrates? A greater focus on cell types within the neurovascular unit is likely to help address these questions
Highlights.
Resilience to stress is characterized by an initial response to a stressful stimulus but a relative recovery towards baseline. It is, therefore, both a process that occurs over time and an outcome.
It is important to differentiate resilience from resistance or from other response patterns because different neural substrates are likely to mediate these different response patterns.
A focus on the trajectory of an individual’s response to stress is required to identify neural substrates that can be engaged to promote resilience.
Numerous putative neural substrates underlying resilience to stress have been identified, reflecting the complexity of the mammalian lifespan and environment.
To translate preclinical findings into meaningful clinical advances, efforts should focus on how divergent neural substrates of resilience work together to drive a trajectory of resilience.
Acknowledgements:
Some of the author’s work cited in this article was supported by grants from the NIMH, Cohen’s Veterans Foundation and DARPA. The author thanks Drs. Gordon Barr, Richard Ross and Phil Gehrman as well as lab members for discussions on the topic of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
The author declares no competing interests in relation to this work.
REFERENCES
- 1.Ehlert U, Gaab J, and Heinrichs M, Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biol Psychol, 2001. 57(1–3): p. 141–52. [DOI] [PubMed] [Google Scholar]
- 2.Yehuda R, Biology of posttraumatic stress disorder. J Clin Psychiatry, 2001. 62 Suppl 17: p. 41–6. [PubMed] [Google Scholar]
- 3.Scott KM, McLaughlin KA, Smith DA, and Ellis PM, Childhood maltreatment and DSM-IV adult mental disorders: comparison of prospective and retrospective findings. Br J Psychiatry, 2012. 200(6): p. 469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjet C, Borges G, and Medina-Mora ME, Chronic childhood adversity and onset of psychopathology during three life stages: childhood, adolescence and adulthood. J Psychiatr Res, 2010. 44(11): p. 732–40. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Aguilar-Gaxiola S, Alhamzawi AO, Alonso J, Angermeyer M, Benjet C, Bromet E, Chatterji S, de Girolamo G, Demyttenaere K, Fayyad J, Florescu S, Gal G, Gureje O, Haro JM, Hu CY, Karam EG, Kawakami N, Lee S, Lepine JP, Ormel J, Posada-Villa J, Sagar R, Tsang A, Ustun TB, Vassilev S, Viana MC, and Williams DR, Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry, 2010. 197(5): p. 378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein CN, Singh S, Graff LA, Walker JR, Miller N, and Cheang M, A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol, 2010. 105(9): p. 1994–2002. [DOI] [PubMed] [Google Scholar]
- 7.Collins SM, Stress and the Gastrointestinal Tract IV. Modulation of intestinal inflammation by stress: basic mechanisms and clinical relevance. Am J Physiol Gastrointest Liver Physiol, 2001. 280(3): p. G315–8. [DOI] [PubMed] [Google Scholar]
- 8.Kuroki T, Ohta A, Sherriff-Tadano R, Matsuura E, Takashima T, Iwakiri R, and Fujimoto K, Imbalance in the stress-adaptation system in patients with inflammatory bowel disease. Biol Res Nurs, 2011. 13(4): p. 391–8. [DOI] [PubMed] [Google Scholar]
- 9.Association, A.P., Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5. . 2013.
- 10.Luthar SS, Resilience in development: A synthesis of research across five decades. In Cicchetti D & Cohen DJ (Eds.), Developmental psychopathology: Risk, disorder, and adaptation. John Wiley & Sons, Inc.. 2006: p. 739–795. [Google Scholar]
- 11.Luthar SS, Cicchetti D, and Becker B, The construct of resilience: a critical evaluation and guidelines for future work. Child Dev, 2000. 71(3): p. 543–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming J and Ledogar RJ, Resilience, an Evolving Concept: A Review of Literature Relevant to Aboriginal Research. Pimatisiwin, 2008. 6(2): p. 7–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Litz BT, Resilience in the aftermath of war trauma: a critical review and commentary. Interface Focus, 2014. 4(5): p. 20140008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutter M, Resilience as a dynamic concept. Dev Psychopathol, 2012. 24(2): p. 335–44. [DOI] [PubMed] [Google Scholar]
- 15.Rutter M, Resilence: some conceptual considerations. Journal of Adolescent Health, 1993. 14(8): p. 626–631. [DOI] [PubMed] [Google Scholar]
- 16.Rutter M, Implications of resilience concepts for scientific understanding. Ann N Y Acad Sci, 2006. 1094: p. 1–12. [DOI] [PubMed] [Google Scholar]
- 17.Rutter M, Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry, 1987. 57(3): p. 316–331. [DOI] [PubMed] [Google Scholar]
- 18.Tory Toole J, Rice MA Jr., Cargill J, Craddock TJA, Nierenberg B, Klimas NG, Fletcher MA, Morris M, Zysman J, and Broderick G, Increasing Resilience to Traumatic Stress: Understanding the Protective Role of Well-Being. Methods Mol Biol, 2018. 1781: p. 87–100. [DOI] [PubMed] [Google Scholar]
- 19.Leppin AL, Bora PR, Tilburt JC, Gionfriddo MR, Zeballos-Palacios C, Dulohery MM, Sood A, Erwin PJ, Brito JP, Boehmer KR, and Montori VM, The efficacy of resiliency training programs: a systematic review and meta-analysis of randomized trials. PLoS One, 2014. 9(10): p. e111420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebara E, Zanoletti O, Ghosal S, Grosse J, Schneider BL, Knott G, Astori S, and Sandi C, Mitofusin-2 in the Nucleus Accumbens Regulates Anxiety and Depression-like Behaviors Through Mitochondrial and Neuronal Actions. Biol Psychiatry, 2021. 89(11): p. 1033–1044. [DOI] [PubMed] [Google Scholar]
- 21.Jaksic N, Brajkovic L, Ivezic E, Topic R, and Jakovljevic M, The role of personality traits in posttraumatic stress disorder (PTSD). Psychiatr Danub, 2012. 24(3): p. 256–66. [PubMed] [Google Scholar]
- 22.Weger M and Sandi C, High anxiety trait: A vulnerable phenotype for stress-induced depression. Neurosci Biobehav Rev, 2018. 87: p. 27–37. [DOI] [PubMed] [Google Scholar]
- 23.Yao ZF and Hsieh S, Neurocognitive Mechanism of Human Resilience: A Conceptual Framework and Empirical Review. Int J Environ Res Public Health, 2019. 16(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grissom N and Bhatnagar S, Habituation to repeated stress: get used to it. Neurobiol Learn Mem, 2009. 92(2): p. 215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sapolsky R, Romero L, Munck A, How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. . Endocr Rev, 2000. 21(1): p. 55–89. [DOI] [PubMed] [Google Scholar]
- 26.Sapolsky RM, Glucocorticoids, the evolution of the stress-response, and the primate predicament. Neurobiol Stress, 2021. 14: p. 100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonanno GA, Loss, trauma, and human resilience: have we underestimated the human capacity to thrive after extremely aversive events? Am Psychol, 2004. 59(1): p. 20–8. [DOI] [PubMed] [Google Scholar]
- 28.Layne CM, Warren JS, Watson PJ, and Shalev AY, Risk, Vulnerability, Resistance, and Resilience Following Disaster post-traumatic adjustment: Retraumatization and the roles of distressing reminders, secondary adversities. 2007, In Friedman TKM & Resick P (eds.), Handbook of PTSD: Science and Practice: New York, NY: Guilford Press. [Google Scholar]
- 29.Wood SK and Bhatnagar S, Resilience to the effects of social stress: evidence from clinical and preclinical studies on the role of coping strategies. . Neurobiol Stress, 2015. 1: p. 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han MH and Nestler EJ, Neural Substrates of Depression and Resilience. Neurotherapeutics, 2017. 14(3): p. 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleshner M, Maier SF, Lyons DM, and Raskind MA, The neurobiology of the stress-resistant brain. Stress, 2011. 14(5): p. 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Febbraro F, Svenningsen K, Tran TP, and Wiborg O, Neuronal substrates underlying stress resilience and susceptibility in rats. PLoS One, 2017. 12(6): p. e0179434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmann ML, Geddes CE, Lee JL, and Herkenham M, Urine scent marking (USM): a novel test for depressive-like behavior and a predictor of stress resiliency in mice. PLoS One, 2013. 8(7): p. e69822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji MJ, Zhang XY, Chen Z, Wang JJ, and Zhu JN, Orexin prevents depressive-like behavior by promoting stress resilience. Mol Psychiatry, 2019. 24(2): p. 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross M, Romi H, Miller A, and Pinhasov A, Social dominance predicts hippocampal glucocorticoid receptor recruitment and resilience to prenatal adversity. Sci Rep, 2018. 8(1): p. 9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbett BF, Luz S, Arner J, Pearson-Leary J, Sengupta A, Taylor D, Gehrman P, Ross R, and Bhatnagar S, Sphingosine-1-phosphate receptor 3 in the medial prefrontal cortex promotes stress resilience by reducing inflammatory processes. Nat Commun, 2019. 10(1): p. 3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grafe LA, Eacret D, Dobkin J, and Bhatnagar S, Reduced Orexin System Function Contributes to Resilience to Repeated Social Stress. eNeuro, 2018. 5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felger JC, Haroon E, and Miller AH, Risk and Resilience: Animal Models Shed Light on the Pivotal Role of Inflammation in Individual Differences in Stress-Induced Depression. Biol Psychiatry, 2015. 78(1): p. 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong S, Inflammation at the interface of physical and neuropsychiatric outcomes: Investigation of neuroendocrine regulatory pathways to inform therapeutics. Brain Behav Immun, 2020. 88: p. 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walchli T, Wacker A, Frei K, Regli L, Schwab ME, Hoerstrup SP, Gerhardt H, and Engelhardt B, Wiring the Vascular Network with Neural Cues: A CNS Perspective. Neuron, 2015. 87(2): p. 271–96. [DOI] [PubMed] [Google Scholar]
- 41.Thurgur H and Pinteaux E, Microglia in the Neurovascular Unit: Blood-Brain Barrier-microglia Interactions After Central Nervous System Disorders. Neuroscience, 2019. 405: p. 55–67. [DOI] [PubMed] [Google Scholar]
- 42.Kovacs GG, Cellular reactions of the central nervous system. Handb Clin Neurol, 2017. 145: p. 13–23. [DOI] [PubMed] [Google Scholar]
- 43.Marin IA and Kipnis J, Central Nervous System: (Immunological) Ivory Tower or Not? Neuropsychopharmacology, 2017. 42(1): p. 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demeestere D, Libert C, and Vandenbroucke RE, Therapeutic implications of the choroid plexus-cerebrospinal fluid interface in neuropsychiatric disorders. Brain Behav Immun, 2015. 50: p. 1–13. [DOI] [PubMed] [Google Scholar]
- 45.Howren MB, Lamkin DM, and Suls J, Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med, 2009. 71(2): p. 171–86. [DOI] [PubMed] [Google Scholar]
- 46.Miller AH, Maletic V, and Raison CL, Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry, 2009. 65(9): p. 732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maes M, Major depression and activation of the inflammatory response system. Adv Exp Med Biol, 1999. 461: p. 25–46. [DOI] [PubMed] [Google Scholar]
- 48.Brambilla P, Bellani M, Isola M, Bergami A, Marinelli V, Dusi N, Rambaldelli G, Tansella M, Finardi AM, Martino G, Perlini C, and Furlan R, Increased M1/decreased M2 signature and signs of Th1/Th2 shift in chronic patients with bipolar disorder, but not in those with schizophrenia. Transl Psychiatry, 2014. 4: p. e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mostafavi S, Battle A, Zhu X, Potash JB, Weissman MM, Shi J, Beckman K, Haudenschild C, McCormick C, Mei R, Gameroff MJ, Gindes H, Adams P, Goes FS, Mondimore FM, MacKinnon DF, Notes L, Schweizer B, Furman D, Montgomery SB, Urban AE, Koller D, and Levinson DF, Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Mol Psychiatry, 2014. 19(12): p. 1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, and Lanctot KL, A meta-analysis of cytokines in major depression. Biol Psychiatry, 2010. 67(5): p. 446–57. [DOI] [PubMed] [Google Scholar]
- 51.Hannestad J, DellaGioia N, and Bloch M, The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology, 2011. 36(12): p. 2452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, Groettrup M, Elbert T, and Kolassa IT, Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry, 2013. 13: p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, Gillespie CF, and Ressler KJ, Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry, 2015. 172(4): p. 353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, and Meyer JH, Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry, 2015. 72(3): p. 268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, and Mechawar N, Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun, 2014. 42: p. 50–9. [DOI] [PubMed] [Google Scholar]
- 56.Rao JS, Harry GJ, Rapoport SI, and Kim HW, Increased excitotoxicity andneuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry, 2010. 15(4): p. 384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, and Bogerts B, Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res, 2008. 42(2): p. 151–7. [DOI] [PubMed] [Google Scholar]
- 58.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, and Miller AH, Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology, 2002. 26(5): p. 643–52. [DOI] [PubMed] [Google Scholar]
- 59.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, and Krishnan R, Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet, 2006. 367(9504): p. 29–35. [DOI] [PubMed] [Google Scholar]
- 60.Abbott R, Whear R, Nikolaou V, Bethel A, Coon JT, Stein K, and Dickens C, Tumour necrosis factor-alpha inhibitor therapy in chronic physical illness: A systematic review and meta-analysis of the effect on depression and anxiety. J Psychosom Res, 2015. 79(3): p. 175–84. [DOI] [PubMed] [Google Scholar]
- 61.Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, and Krogh J, Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry, 2014. 71(12): p. 1381–91. [DOI] [PubMed] [Google Scholar]
- 62.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, and Maes M, Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alphainduced changes in the serotonergic system. J Clin Psychopharmacol, 2002. 22(1): p. 86–90. [DOI] [PubMed] [Google Scholar]
- 63.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, and Pollmacher T, Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry, 2001. 58(5): p. 445–52. [DOI] [PubMed] [Google Scholar]
- 64.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, and Critchley HD, Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry, 2009. 66(5): p. 407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller AH and Raison CL, Are Anti-inflammatory Therapies Viable Treatments for Psychiatric Disorders?: Where the Rubber Meets the Road. JAMA Psychiatry, 2015. 72(6): p. 527–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, and Miller AH, A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry, 2013. 70(1): p. 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, and Howes OD, Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl), 2016. 233(9): p. 1637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pearson-Leary J, Eacret D, Chen R, Takano H, Nicholas B, and Bhatnagar S, Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress. Transl Psychiatry, 2017. 7(6): p. e1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB, Janssen WG, Labonte B, Parise EM, Lorsch ZS, Golden SA, Heshmati M, Tamminga C, Turecki G, Campbell M, Fayad ZA, Tang CY, Merad M, and Russo SJ, Social stress induces neurovascular pathology promoting depression. Nat Neurosci, 2017. 20(12): p. 1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wood SK, Walker HE, Valentino RJ, Bhatnagar S, Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology, 2010. 151(4): p. 1795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wood SK, Wood CS, Lombard CM, Lee CS, Zhang XY, Finnell JE, and Valentino RJ, Inflammatory Factors Mediate Vulnerability to a Social Stress-Induced Depressive-like Phenotype in Passive Coping Rats. . Biol Psychiatry 2015(78): p. 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Larrieu T, Cherix A, Duque A, Rodrigues J, Lei H, Gruetter R, and Sandi C, Hierarchical Status Predicts Behavioral Vulnerability and Nucleus Accumbens Metabolic Profile Following Chronic Social Defeat Stress. Curr Biol, 2017. 27(14): p. 2202–2210 e4. [DOI] [PubMed] [Google Scholar]
- 73.Southwick, C. DS, What is Resilience? In Resilience: The Science of Mastering Life’s Greatest Challenges 2018: Cambridge: Cambridge University Press. 1–34. [Google Scholar]
- 74.Herman JP and Cullinan WE, Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci, 1997. 20(2): p. 78–84. [DOI] [PubMed] [Google Scholar]
- 75.Grafe LA, O’Mara L, Branch A, Dobkin J, Luz S, Vigderman A, Shingala A, Kubin L, Ross R, and Bhatnagar S, Passive Coping Strategies During Repeated Social Defeat Are Associated With Long-Lasting Changes in Sleep in Rats. Front Syst Neurosci, 2020. 14: p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bangasser DA and Valentino RJ, Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol, 2014. 35(3): p. 303–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hokenson RE, Short AK, Chen Y, Pham AL, Adams ET, Bolton JL, Swarup V, Gall CM, and Baram TZ, Unexpected Role of Physiological Estrogen in Acute Stress-Induced Memory Deficits. J Neurosci, 2021. 41(4): p. 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fariborzi M, Park SB, Ozgur A, and Lur G, Sex-dependent long-term effects of prepubescent stress on the posterior parietal cortex. Neurobiol Stress, 2021. 14: p. 100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goel N, Workman JL, Lee TT, Innala L, and Viau V, Sex differences in the HPA axis. Compr Physiol, 2014. 4(3): p. 1121–55. [DOI] [PubMed] [Google Scholar]
- 80.Hodes GE and Epperson CN, Sex Differences in Vulnerability and Resilience to Stress Across the Life Span. Biol Psychiatry, 2019. 86(6): p. 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]


