Abstract
The internal organization of the hippocampal formation has been studied for more than a century. While early accounts emphasized its subfields along the medial-lateral axis, findings in recent decades have highlighted also the anterior-to-posterior (i.e., longitudinal) axis as a key contribution to this brain region’s functional organization. Hence, understanding of hippocampal function likely demands characterizing both medio-to-lateral and anterior-to-posterior axes, an approach that has be concretized by recent advances in in-vivo parcellation and gradient mapping techniques. Following a short historical overview, we here review the evidence provided by these approaches in brain mapping studies, as well as the perspectives they open for addressing the behavioral relevance of the interacting organizational axes in healthy and clinical populations.
Keywords: parcellation, gradients, connectivity, microstructure, phenotype, disease
The question of hippocampal organization
The hippocampal formation is a core brain region from a phylogeny standpoint (cfr., the dual origin hypothesis of brain evolution [1]), as well as a key node of the human memory system, but also a target of research in brain disorders, such as Alzheimer’s disease and temporal lobe epilepsy. In that context, the hippocampal formation has enjoyed a vivid and constant interest from various research fields. Consequently, numerous models and theories have been proposed regarding its organization, function, and relationship to behavior in the healthy and diseased brain. The traditional anatomical model subdivides the hippocampal formation into discrete subfields arranged along its medio-lateral and ventro-dorsal axis, including the Cornu Ammonis (CA1–4) subfields, the dentate gyrus and the subiculum. The implementation of this model in human research, in particular in neuroimaging studies, is supported by the availability of cytoarchitecture maps (e.g. [2–4]), extensive guidelines for manual segmentation (e.g. [5]), as well as automated in-vivo segmentation tools (e.g. [6, 7]). Through these easily available anatomical maps and segmentation tools, the issue of subfield distinction has achieved a prominent role in the study of behavioural functions and pathophysiological processes.
More recently, however, the organization of the hippocampus along its longitudinal axis has gained considerable interest. Several hypotheses on this organizational pattern have been proposed. Among the most popular models are a tripartite model, differentiating a head from body and tail, and the distinction between the anterior vs posterior parts [8]. Based on anatomical, genetic, and functional data, the relevance of this long-axis organizational for behaviour has been subject to vivid discussion. In that context, it appeared that the literature on human long-axis neuroanatomy was initially less developed than the analogous animal literature [9]. Addressing this point, we here review how advances in neuroimaging markers of functional and anatomical connectivity, together with the development of parcellation and gradient-mapping approaches, have recently provided substantial empirical support for a differentiation of the hippocampal formation along the long-axis [10–19]. In the same vein, it was pointed out a few years ago [20] that in humans discrete changes in molecular or anatomical organization along the hippocampus long axis have yet to be examined. Accordingly, we also review how recent resources, such as BigBrain [21–24] and genetic mapping have spurred progress in that respect [25, 26].
Considering the interacting nature of these organizational dimensions, we will show how they can be synergistically probed by both discrete (with subfield-to-subfield and head-body-tail differentiation) or dimensional approaches (with gradients running in medio-lateral and long-axis directions). We will also illustrate how such synergy may lead to a unified model of hippocampus organization, function and pathology.
Two main dimensions of hippocampal organization
Hippocampal subfields organization
Ex-vivo investigations in both animal models and humans, as well as in vivo experiments in animal models have converged to the distinction of several hippocampal subfields. Despite many subfields and their finer subdivisions still being debated (see Box 1), it seems generally acknowledged that the hippocampal formation (consisting of the hippocampus itself and the adjacent subiculum) can be subdivided into at least three parts: the CA fields comprising three or even four subfields (CA1 – CA4), the dentate gyrus, and the subiculum (or subicular complex) as illustrated in Figure I [2, 4, 27]. Evidence for this organizational model is provided by differences in cytoarchitectonic profiles (see Box 1), as well as physiological findings revealing different intrinsic connections of these subfields (i.e. within the hippocampal formation) or local ones (within the medial temporal lobe), such as the trisynaptic circuit [28]. This circuit encompasses the performant path system connecting the entorhinal cortex with the hippocampus, mossy fibers and Schaffer collaterals. Recent studies have shown that all these pathways consist of multiple components: superficial sheets of fibers emanating from the entorhinal cortex project to the presubiculum and parasubiculum; intermixed transverse and long-axis angular bundle fibers perforate the subiculum and then project to the CA fields and dentate molecular layer; while a significant alvear component runs from the angular bundle to the CA fields [29]. These pathways are relevant not only in terms of information processing, but also for understanding disease progression. For instance, it is assumed that the perforant path system is often the first white matter pathway degenerating in Alzheimer’s disease [30].
Box 1: Hippocampal subfields.
The hippocampal formation can be divided into at least three different anatomical parts: the CA fields, the subiculum and the dentate gyrus. Beyond this broad distinction, finer subdivisions into specific subfields are likely possible but remain debated. Within the CA subregion, Lorente de Nó [35] identified four regions, i.e., CA1, CA2, CA3 and CA4. However, CA2 and CA4 are both not universally accepted as distinct subfields (e.g. [36–38]). One practical consequence of such scientific disagreements is that while a probabilistic map of CA2 can be found, for example, in the SPM Anatomy Toolbox, a corresponding segment is not available in the Freesurfer automated segmentation of the hippocampus (Figure IB). These theoretical and practical discrepancies evidently complicate comparison and integration across studies. In the context of segmentation, different protocols may also have different reliability [39, 40]. Furthermore, finer subdivisions within specific CA subfields, such as subfields within CA1 or the subiculum have been proposed (see e.g. [4]) but are still a matter of debates. Acknowledging these diverging viewpoints, one of the most recent mapping of hippocampal subfields can be found in [4]. Resulting from a combination of cyto- and receptor-architectonic features, the proposed map includes nine distinct regions: fascia dentata (a subregion of the dentate gyrus), CA1, CA2, CA3, CA4, prosubiculum, subiculum proper, presubiculum and parasubiculum (Figure IA). All in all, microstructural investigations within the hippocampal formation suggest that one can differentiate distinct subfields, whose number diverges across studies but that are typically aligned along the medio-lateral and ventro-dorsal axis in humans.
Figure I (for Box 1). Subfields within the human hippocampus and subiculum.
A. Multimodal mapping based on cytoarchitectonie and receptorarchitectonic features [4]. B. Automated segmentation of an anatomical scan provided by Freesurfer [81].
The subfield model has hence been well corroborated across techniques and species, using a variety of ex-vivo analyses and invasive investigations. The practical implementation of this model for neuroimaging research became crucial in the context of PET and MRI studies. Targeted examinations of imaging characteristics for hippocampal subfields became possible by the availability of cytoarchitectonic maps in stereotaxic MNI space [2, 3], the continuous development of guidelines for manual delineation of subfields, in particular by the Hippocampal Subfield Group [5], as well as the development of automated segmentation tools (see Box 1 and [31] for a review). These resources and developments drove a vast literature of subfields-based studies in the healthy brain, e.g., with regards to genetic profiles (e.g. [32]), influence of environmental factors (e.g.[33]) and aging (e.g. [34]), but also in the diseased brain (cf. below).
The long-axis organization
The most robust support for an anterior-posterior division in the hippocampal formation comes from anatomical and electrophysiological recordings in rodents, showing a differentiation between ventral and dorsal subregions (corresponding to the anterior and posterior human hippocampus, respectively). These include differences in connectivity (for a review see [20]) as well as in functional profiles (for a review see [9]), leading, for instance, to an emotion vs cognition segregation hypothesis [41]. Evidence for an organizational pattern along the long-axis is also further supported by gene expression studies in rodents and non-human primates (for reviews, see [20, 42]).
In humans, neuroimaging studies using PET and later fMRI have led to the “encoding vs retrieval” hypothesis for the differentiation along the long axis of the hippocampus (the HIPER model, [43, 44]). The encoding and retrieval components of this hypothesis were later linked to engagement of the dorsal attention and the default model networks, respectively, leading to the HERNET model [45]. In this hypothesis-driven framework, MRI was used to compare the connectivity profiles of a-priori defined anterior vs posterior subregions. In particular, differences in connectivity profiles along the long axis of the hippocampus were observed when examining functional connectivity based on resting-state fMRI [12, 46], but also when probing anatomical connectivity through diffusion MRI [10].
Hence, the long-axis organization pattern has gathered increasing support from evidence in rodents and from hypothesis-driven activation and connectivity neuroimaging studies in humans. Nevertheless, to achieve a level of scientific validation comparable to the subfields model, the long-axis organizational pattern should be supported by data-driven investigations in humans and across different neurobiological features tapping into large-scale functional integration and behavioral systems organization. As described in the next section, this became possible by capitalizing on parcellation and gradient approaches.
Capturing hippocampal organization using data-driven approaches
Parcellation and gradient-mapping approaches provide summary representations of spatial variability in a dataset, and can hence reveal the topographical patterns of large-scale integration provided by estimates of structural or functional connectivity (see Box 2). They thus allow disentangling the differentiation of hippocampus (or subiculum) voxels with regards to their whole brain connectivity profiles (which represent a multivariate pattern). Parcellation provides a spatial map of parcels (regions) that are internally as homogeneous as possible but at the same time maximally different from each other, whereas gradient mapping will reveal continuous dimensions in a latent space along which the connectivity profiles vary. Below, we first review studies that have applied parcellation approaches to connectivity data of the human hippocampal formation, followed by those that have used gradient methods on similar data. For the sake of comparison and discussion, we also report the few studies that have applied similar, data-driven approaches to markers of local hippocampal structure rather than connectivity patterns.
Box 2: parcellation and gradient mapping techniques.
In neuroimaging analyses, parcellation is an umbrella term that covers a variety of approaches to delineate brain regions. For the sake of brevity, here we focus on parcellation using decomposition approaches (i.e. clustering or factorization, in contrast to border detection algorithms; for a review see [47]). Gradient mapping techniques, on the other hand, often refer to ‘dimensional decompositions (such as principal component analysis or non-linear variants such as diffusion map embedding [48, 49]). Overall, parcellation and gradient mapping techniques can be applied to the same type of data and simplify spatial variations in a data-driven way. Notably, parcellation approaches focus on discretization into distinct but internally homogeneous regions whereas gradient mapping identifies continuous axes of feature variation. For example, the pattern of functional connectivity of hippocampal voxels can be summarized either by defining groups of voxels that show a similar pattern of connectivity, or alternatively, it can be summarized as principal gradients along which the connectivity profile of the voxels varies. Generally, the first principal dimensions along which the voxels vary (explaining most variance) will determine the first levels of subdivisions. These approaches are often used in a data-driven framework, that is, without specifying a-priori the number of clusters or principal gradients to be extracted from the data. Different metrics can evaluate the quality of fit, in addition to assessments of reproducibility and correspondence with other neural features [50]. Additionally, a model selection approach based on statistical testing against a null model that preserves the geometrical features of the region of interest has been recently promoted to delineate potential borders and gradual transitions [19, 51].
Hippocampal parcellations
Connectivity-based hippocampal parcellations
Different MRI-based measures can probe large-scale functional integration between distant brain regions and are hence commonly 1abelled as “connectivity” markers. Early parcellation studies that probed functional connectivity in the human hippocampus/subiculum used estimates of co-activation across experiments (based on an approach known as meta-analytic connectivity modelling [52]). These studies strikingly highlighted a pattern of differentiation along the long-axis, either when including both the hippocampus and the subiculum [15, 18] or when focusing on the subiculum solely [11]. This long-axis organization appears to be a general feature of hippocampal functional integration, as a similar pattern was also found when using a different marker of functional interactions, namely resting-state connectivity [15, 53, 54] as illustrated in Figure 1. This organizational dimension hence reflects the differential functional integration into whole brain networks of hippocampal anterior and posterior regions as illustrated in Figure 3 (see also [55]).
Figure 1. Parcellation and gradient patterns in the human hippocampal formation and its subcomponents.
Parcellation and gradients studies probing functional connectivity, be it during task or at rest, primarily highlight a long-axis differentiation (left/blue panel). In contrast, studies probing local microstructure (such as laminar pattern and white matter microstructural features) primarily highlight a subfields-like differentiation along the medial-lateral and ventro-dorsal axes (right/yellow panel). Interestingly, both dimensions of differentiation (long-axis and medial-lateral) markedly appear in the pattern of hippocampal covariance with whole brain structure. Notes: [11] focuses on the subiculum.[54] specifically examined a two-clusters subdivision of the right and left hippocampus, but only the right hippocampus is illustrated here. [82] did not use a fully data-driven parcellation approach, but a semi-supervised clustering technique.[16] examined RSFC and T1/T2 ratio gradients separately in distinct subfields, but only the left subiculum is illustrated here.
Figure 3. Relationships between hippocampal organization and functional brain organization at the macroscale.
Anterior and posterior subregions (see Figure 1) show different preferential coupling across the brain (top left panel, from [11]). In particular, the differentiation along the anterior-posterior axis is primarily related to a transmodal to sensory primary gradient of cortical organization (bottom left panel, from [23]). In contrast, iso to allocortical organization (i.e. latero-medial differentiation) within the medial temporal lobe (MTL) is strongly related to a cortical “multiple-demand” functional gradient (right panel, from [23]). The main organizational dimensions of the hippocampal formation are hence differentially related to the organizational dimensions of the rest of the human brain.
Despite some technical challenges (see Box 3), a primary pattern of subdivision into three subregions reminiscent of the hippocampus’s head-body-tail partitions can be found throughout the current literature of parcellation studies based on functional connectivity. Only secondly, higher parcellation levels further subdivide this pattern into medial vs lateral parcels. Thus, parcellation studies confirmed that functional connectivity of the hippocampal formation differs across the long-axis with a stable and relatively robust subdivision into a head, body and tail (i.e. the tripartite model). Further subdivisions along the long-axis could also be evidenced ([15] Figure 1), in particular with higher resolution [19] and it could be speculated that they partly reflect hippocampus’s gyrification along the long-axis (see [24] and hippocampus’s unfolding at high resolution in Figure 1). This emerging hypothesis should be further evaluated in future studies.
Box 3: challenges raised by hippocampus’s parcellation and gradients.
The elongated shape of the hippocampal formation and the intrinsic smoothness of MRI data are two technical factors that could potentially contribute to a primary gradient and clustering pattern along the longitudinal axis in MRI data [50]. It should be noted that when comparable parcellation techniques are applied to MRI data probing local microstructural features, the pattern of organization was not characterized along the longitudinal axis, but along the medial-lateral axis [59]. Additionally, this issue can be directly investigated with a model selection approach based on statistical testing against a null model taken into the hippocampus geometry [18, 50]. Such framework confirmed the long-axis organization in functional connectivity [18] and this pattern was replicated at higher spatial resolution (7 Tesla data [18]). These recent findings suggest that the pattern of organization along the long-axis, and in particular the tripartite subdivision, is genuinely related to large-scale functional connectivity as measured by fMRI and not primarily driven by technical factors.
Nevertheless, when considering a range of different neurobiological features, it is likely that the hippocampus comprise a mixture of continuous gradients and discrete boundaries as suggested in Figure 2. To examine that hypothesis, the robust identification in multimodal data of hybrid topographical representations that combine gradients and parcels would need a proper model selection. In that perspective, model selection could be used to determine whether a gradient or hard boundary provides the best fit to the data across different points of the topography. Such an approach would provide a potential path towards reconciling gradients and parcels in the hippocampus.
While the primary long-axis gradient is consistently seen across different connectivity features, and to an important extent reproduces a head-body-tail model [16] as well as gene expression gradients [25], the second gradient is less easily characterized. It partly corresponds to the differentiation revealed by microstructural mapping, in particular when using MRI-based estimates of myelin [16] or laminar features [23]. Nevertheless, the overlap remains partial, being either limited by noise in the data or reflecting the influence of additional, yet unknown, neurobiological factors. Furthermore, despite most studies focusing on the first or first and second gradient, additional gradients could be extracted from the data and their neurobiological interpretation remains to be established.
Finally, inter-hemispheric asymmetry represents an important open question for future research. Despite a relatively similar pattern being generally observed in the right and left hippocampal formation with both parcellation or gradient mapping (see Figure 1), some differences across hemispheres have also been noted. For instance, different levels of partitions have been suggested to represent the left and right hippocampal organization optimally [18]. Furthermore, the left hippocampus often shows a more consistent topographical organization than the right across subjects or samples [13, 16]. Such observations of hemispheric asymmetry currently remain anecdotal but call for future systematic investigations of the potential neurobiological factors, and the related implications for hippocampus’s/subiculum’s relationship to cognitive functions and behavioral phenotypes.
While functional connectivity markers are popular estimates of large-scale integration, the latter can also be probed by examining structural covariance reflecting shared neurobiological properties (e.g. regions that are influenced by similar genetic, environmental or metabolic factors) and/or contribution to the same behavioral systems [56, 57]. This hybrid nature of structural covariance (neurobiological vs behavioural) remarkably appeared when the hippocampal formation was parcellated into different subregions based on structural covariance patterns [14, 58]. Although a primary differentiation between the head (or anterior) and the body-tail (two-thirds) region was revealed by these patterns, the latter region is further subdivided into medial and lateral segments strikingly resembling the subiculum vs CA fields (Figure 1). This pattern contrasts with the functional tripartite model and demonstrates that structural variations to an important extent also follow microstructural territories despite being primarily segregated according to anterior vs posterior networks.
Local structure-based hippocampal parcellations
Recently, data-driven clustering has also been applied to features representing local structural properties of the human hippocampal formation. Typical markers of local microstructure offered by MRI techniques are T1/T2 ratio as a proxy of myelin, as well as estimates of white matter microstructure from diffusion weighted imaging. Applying a non-negative matrix factorization approach to the combination of these features in the hippocampus revealed four stable components mainly organized along the medial-lateral and ventro-dorsal axis in a subfield-like fashion [59] (Figure 1). Nevertheless, a clear one-to-one mapping between the derived component and the traditional, histologically defined subfields was not evident, preventing a straightforward link to classical neurobiological models.
In addition to MRI-based estimates, more direct investigations of hippocampal microstructure with automated approaches has been facilitated by the BigBrain initiative, which, for example, allows the characterization of laminar features [21]. A recent application of a clustering approach to laminar and morphological features (such as thickness, curvature, and gyrification) in the BigBrain’s hippocampal formation reveals again subregions arranged along the medial-lateral and ventro-dorsal axis [24] (Figure 1). This replicated the classical subfield model to some extent. Importantly, though, some changes in features along the long-axis were also observed. In particular, anterior-posterior differences were qualitatively observed in gyrification in CA1, and in density in CA1 and CA4, hence raising the hypothesis of a relationship between gyrification and functional properties along the long-axis.
Hippocampal gradients
Connectivity-based hippocampal gradients
In contrast to clustering approaches aiming for discrete subdivisions, gradient mapping techniques reveal continuous dimensions of brain organization [60] and can likewise be applied to MRI-derived connectivity estimates. As in a traditional eigenvector decomposition, the first gradient reflects the main dimension that captures the highest part of variance in the features (the connectivity profiles), while subsequent gradients explain successively lower amounts of variance. Several studies have applied gradient mapping approaches to connectivity profiles of the hippocampal formation using the same features as discussed above for parcellation studies, i.e., resting-state functional connectivity [13, 16, 17, 19], task co-activation profiles (with MACM) [13, 16] and structural covariance [13, 25] (Figure 1). Across these studies, and regardless of the connectivity features, the emerging first organizational dimension systematically corresponds to a differentiation along the long-axis, resonating well with the distinctions revealed by parcellation studies.
In turn, the topographical pattern of the second gradient is generally distributed along the medial-lateral and ventro-dorsal axes. It is worth mentioning that this second dimension of connectivity variance was not only revealed when the hippocampus and subiculum were analysed together, but also when focussing on specific subfields [13, 16]. Moreover, when probing its correspondence with microstructural differentiation patterns, a partial overlap with the spatial distribution of myelin estimates and subfield differentiation was shown [16] (see Box 3 for further discussion). Along the same line, the within subfield functional gradient in humans resonates with intrinsic functional gradients evidenced using invasive methods in rodents. For instance, a spatial signal gradient along the transverse axis of CA1 has been described in place-cell firing within CA1 [61]. However, cross-species comparisons of within-subfields functional gradients is currently limited by the spatial resolution of methods for human studies (primarily MRI in this context).
Local structure-based hippocampal gradients
A recent study capitalized on the BigBrain data to assess cytoarchitectural transitions in the broader mesiotemporal lobe (MTL) [23]. The analyses support a consistent medio-lateral gradient of cytoarchitecture differentiation, from parahippocampal isocortex towards hippocampal allocortex, and within the hippocampal formation, a cytoarchitecture-based gradient that recapitulates subfield-to-subfield variations in microstructure (Figure 1). Interestingly, this medio-lateral organization was found to also reflect patterns of intrinsic functional signal within the hippocampal circuitry (assessed using co-registered functional MRI data). This study hence highlights again the local organization of information processing represented by this medio-lateral pattern [23]. Further advancing our understanding of the relationships between topographical gradients and brain information processing, the iso-to-allocortical and anterior-to-posterior axes differentially contributed to distinct dimensions of the macroscale functional systems as illustrated in Figure 3 [23]. Even though there was no apparent one-to-one mapping between organizational dimensions of the MTL and cortical networks or gradients, distinct hippocampal organizational dimensions differently mapped onto cortical gradients. In particular, the anterior-to-posterior progression along the hippocampal complex primarily correlated with the sensory to transmodal functional dimension. In contrast, and importantly, the iso-to-allocortical positions (i.e. medio-lateral and ventro-dorsal organization) was related to the relative contribution of the multiple-demand system, a system supporting complex tasks whose execution cannot rely on a pre-determined schema (cf. Figure 3). Hence, recent developments have opened new perspectives to relate the hippocampal organizational dimensions to functional dimensions of information processing.
In sum, when parcellation and gradient mapping approaches are applied to connectivity patterns of the hippocampal formation, different superimposed organization patterns can be observed as synthetized in Figure 2. Generally, two main organizational dimensions appear: a primary pattern of subregions or gradients along the long-axis, and a secondary one, along the medial-lateral and ventro-dorsal axes. This latter organizational dimension appears primarily when these approaches are used to probe hippocampal local microstructure, be it estimated from MRI data or extracted from high-resolution ex-vivo data. This pattern, in line with its local microstructure origin, is assumed to serve local information processing flows possibly corresponding to different processing requirements at the macroscale. In turn, the long-axis organizational pattern maps more directly into brain large-scale behavioral systems [55].
Figure 2. Schematic of superimposed differentiation patterns in human hippocampal formation.
A tripartite subdivision corresponding to a head, body and tail along the longitudinal axis is suggested by task-based and resting-state functional connectivity patterns. MRI-based estimates of local microstructural and macrostructural features in turn highlight a medial-lateral and ventro-dorsal differentiation separating the CA complex from the subiculum complex, as well as intrinsic medial-lateral gradients (within subfield gradients). Finally, the head shows a specific whole brain structural covariance in population cohorts. Superimposing these multiple differentiation patterns provide a holistic model to better understand hippocampal function and implications for clinical phenotypes.
Relating hippocampus organization to phenotypes
Understanding hippocampal function and its relationship to behavior has been a longstanding topic of interest in neuroscience ( e.g. [62–64]). A substantial part of the related scientific literature has focused on developing theories of hippocampus function based on the subfield-model, with the pattern separation theory (e.g. [65]) being a prominent example. Such computational models (in terms of pattern separation/completion processes) are typically anchored in local circuitry between subfields and build on experimental work in animal models. Nevertheless, they often fall short on accounting for the variety and complexity of behavioral functions or psychological aspects in which the hippocampal formation is engaged [63, 66]. Addressing this complexity requires a systemic view in which large-scale integration of the hippocampal formation into brain-wide networks holds key information.
As the long-axis axis organization increasingly gathered empirical support, several hypotheses have been proposed to explain its relevance for behavior, both in terms of a behavioral gradient or as a behavioral segregation (for reviews see [9, 20]). As noted, most of these theories live within a hypothesis-driven framework. For example, the famous encoding vs retrieval model was initially based on a selective review of the PET and fMRI activation literature [43] and further reinforced by a targeted meta-analysis [45]. Nevertheless, examining behavioral functions associated with activation along the long-axis in a hypothesis-free context did not result in additional support to this theory. In contrast, the hypothesis of an emotion vs cognition gradient could be corroborated in a data-driven manner [15, 16]. However, we would argue that it does not appear as an optimal conceptualization of functional differentiation given a relative specificity for emotion processing in the anterior hippocampus but more widespread, i.e., unselective, associations for cognitive functions. Alternative conceptualizations based on large-scale behavioral decoding propose either a self-centric vs world-centric mode of processing [15], or a social and motivational system vs visuo-spatial cognition distinction [25]. These recent frameworks hence highlight the benefit of data-driven approaches of brain-behavior associations for generating new hypotheses.
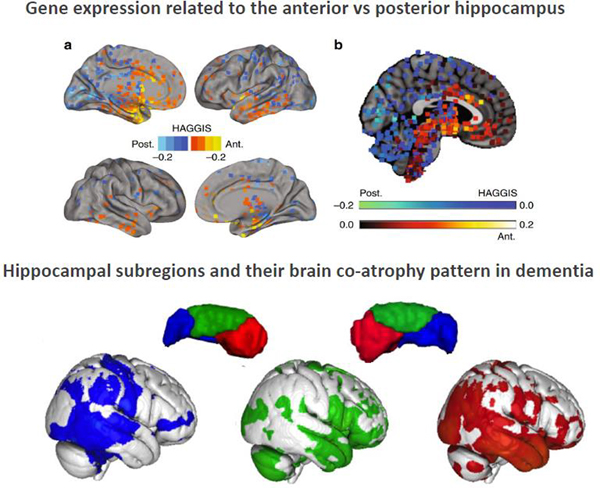
The additional insight offered by such approaches might be particularly relevant when aiming to relate hippocampal organization to behavioral phenotypes in healthy and clinical populations. About a decade ago, a pathophysiological framework was proposed to relate specific diseases (corresponding to common diagnostic categories) to specific hippocampal subregions [67]. Importantly and in line with the nosology of the previous decades, this framework postulated that current diagnostic categories indeed reflect distinct brain pathologies. It was hence suggested that specific regional vulnerability within the hippocampus differentiate Alzheimer’s disease, vascular disease, aging, depression and PTSD. Nevertheless, evidence for this view is inconsistent and specific patterns of subfield alterations for different disorders do not clearly emerge from the (substantial) literature. In turn, a few studies have highlighted the role of the long-axis organization in interindividual variability, in particular in relation to behavioral phenotypes across childhood development (e.g. [68, 69]) and aging (e.g.[70]). Furthermore, in young adults, different dimensions of the schizotypy risk phenotype were differently related to anterior vs posterior volume of hippocampal subfields [71]. This preclinical feature resonates well with differential alterations of anterior and posterior hippocampus in schizophrenia where the anterior region is preferentially affected in the early stage [72–74]. Preferential atrophy in the hippocampus’s head have also been reported in temporal lobe epilepsy [75, 76] and functional decoupling of the anterior and posterior subregions have been evidenced in specific subtypes thereof [77]. Along the same line, the pattern of co-atrophy in dementia was recently shown to follow a differentiation along the long-axis [14] (Figure 4). This suggests that the pattern of hippocampal-cortical co-degeneration follows to a large extent functional coupling. In that regards, hippocampal anterior (self-oriented) and posterior (goal-oriented) networks may show opposite alterations in dementia, as increased medial temporal lobe connectivity to anterior networks has been associated with a decreased connectivity to posterior networks in AD patients [78].
Figure 4. The relevance of hippocampal long-axis organization in relation to phenotype.
The top panel illustrates the molecular gradient along the long-axis reported by [25] using a Hippocampal Axis Genomic Gradient Index of Similarity (HAGGIS), a value representing the degree to which the genomic signature of the hippocampal long-axis is represented in the gene expression profile of a given non-hippocampal sample. Larger positive values represent greater genomic similarity to the anterior hippocampus, while smaller negative values represent greater genomic similarity to the posterior hippocampus. The bottom panel illustrates the differentiation of the left and right hippocampal formation into three subregions along the long-axis based on their pattern of brain co-atrophy in Alzheimer’s disease reported in [14].
Finally, providing hints for the underlying neurobiological factors of interindividual differences along the long-axis and their relationships with behavioral phenotype, a long-axis molecular gradient has been recently demonstrated (Figure 4), and has been related to large-scale behavioral systems [25, 26]. In particular, cell-type specific expression of genes related to mood and affect have been found in the anterior hippocampus, whereas expression of genes related to cognitive functions have been identified in the posterior hippocampus [26]. Together, these observations highlight the relevance of the differentiation along the long-axis for understanding interindividual differences in physiological phenotypes, but also in pathology and associated behavioral dysfunction.
Overall, the current literature points to relevant roles for both subfields and long-axis differences in hippocampal organization for interindividual differences and pathology. Considering both organizational dimensions simultaneously thus offers the opportunity to account for properties inherent to the computational infrastructure (related to local microstructural features), as well as features reflecting network-integration. Such a view has already been shown to characterize hippocampus structural changes with age (e.g. [79, 80]). Further insight should be facilitated by parcellation and gradient-based representations that can be used to study pathophysiological aspects and the relationships between interindividual variability in hippocampal structure, function and connectivity and interindividual variability in behavioral phenotype.
Concluding remarks and future perspectives
Adding to the well-established histological subfields of the human hippocampal formation, parcellation and gradient approaches capture at least two dimensions of organization in this brain region (and additional dimensions could be relevant; see Outstanding Questions). Applying these approaches to local neurobiological features, such as local connectivity and microstructural properties, readily highlights a differentiation along the medial-lateral and ventro-dorsal axes. In turn, applying these approaches to neurobiological features that tap into the integration of the hippocampal formation into large-scale networks and its relation to behavioral systems reveals a differentiation along the long-axis. Accordingly, the medial-lateral and anterior-to-posterior axes could differentially contribute to distinct functional dimensions, with medial-lateral differentiation possibly relating to the relative contribution of the multiple-demand system while anterior-to-posterior position seemingly reflecting trade-offs between sensory/self-centric and transmodal function. When related to large-scale behavioral systems and related phenotypes, this latter dimension should reflect a differential involvement of emotional, motivational and self-centric systems vs more integrative and word-oriented cognition. Parcellation maps and decomposition techniques have opened new perspectives to further investigate how this organization relates to behavioral phenotype, in particular in a transdiagnostic approach linking the dimensions of hippocampal organization to behavioral dimensions spanning across the healthy brain and disease categories.
Outstanding Questions.
Can the human hippocampal organization be further characterized by additional gradients/dimensions, beyond the medial-lateral and longitudinal dimensions?
How do pathophysiological processes interact with the dimensions of hippocampal organization?
Can finer characterization of neurobiological alterations along both the medial-lateral and longitudinal dimensions improve diagnostics and/or treatment of brain disorders?
How do inter-individual differences in hippocampus’ organization relate to inter-individual differences in behavioral phenotype?
Are there inter-hemispheric differences in hippocampal organization?
How do fine long-axis subdivisions suggested by connectivity and structural features (such as gyrification) emerge across ontogenesis and phylogeny?
Highlights.
MRI-based parcellation of the hippocampal formation and gradient-mapping are data-driven techniques that can capture many dimensions of hippocampal organization and provide readily usable outcomes.
Features of cortical architecture, such as local connectivity and microstructure, reveal differentiation within the hippocampal formation along the medial-lateral axis. This organizational dimension seemingly reflects local information processing organization.
Neuroimaging markers taping into hippocampal integration into large-scale networks (i.e. whole brain connectivity) highlight the long-axis differentiation.
The long-axis organization corresponds to a molecular gradient and differential integration across distinct behavioral systems.
Capitalizing on gradients and parcellations maps, the long-axis organization of the hippocampal formation can be related to behavioral phenotypes in healthy and clinical populations.
Acknowledgments
SG, SBE and KA acknowledge research support from the Deutsche Forschungsgemeinschaft (DFG, GE 2835/2–1, EI 816/16-1 and EI 816/21-1), the National Institute of Mental Health (R01-MH074457), the Helmholtz Joint Lab “Supercomputing and Modeling for the Human Brain”, the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement 785907 (HBP SGA2), 945539 (HBP SGA3), and The Virtual Brain Cloud (EU H2020, no. 826421). BCB acknowledges research support from the National Science and Engineering Research Council of Canada (NSERC Discovery-1304413), CIHR (FDN-154298, PJT-174995), SickKids Foundation (NI17-039), Azrieli Center for Autism Research (ACAR-TACC), BrainCanada (Azrieli Future Leaders), the Helmholtz International BigBrain Analytics and Learning Laboratory (Hiball), and the Tier-2 Canada Research Chairs program. RL acknowledges support from the NIH (K99AG065501).
Footnotes
Declaration of interests
The authors declare no competing interests in relation to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pandya D et al. (2015) Cerebral cortex: architecture, connections, and the dual origin concept, Oxford University Press. [Google Scholar]
- 2.Amunts K et al. (2005) Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and embryology 210 (5–6), 343–352. [DOI] [PubMed] [Google Scholar]
- 3.Eickhoff SB et al. (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25 (4), 1325–1335. [DOI] [PubMed] [Google Scholar]
- 4.Palomero-Gallagher N et al. (2020) Multimodal mapping and analysis of the cyto-and receptorarchitecture of the human hippocampus. Brain Structure and Function, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wisse LE et al. (2017) A harmonized segmentation protocol for hippocampal and parahippocampal subregions: Why do we need one and what are the key goals? Hippocampus 27 (1), 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iglesias JE et al. (2015) A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115, 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeKraker J et al. (in press) Surface-based hippocampal subfield segmentation. Trends Neurosci. DOI: 10.1016/j.tins.2021.06.005 [DOI] [PubMed] [Google Scholar]
- 8.Duvemoy HM (2013) The human hippocampus: an atlas of applied anatomy, JF Bergmann-Verlag. [Google Scholar]
- 9.Poppenk J et al. (2013) Long-axis specialization of the human hippocampus. Trends in cognitive sciences 17 (5), 230–240. [DOI] [PubMed] [Google Scholar]
- 10.Adnan A et al. (2016) Distinct hippocampal functional networks revealed by tractography-based parcellation. Brain Structure and Function 221 (6), 2999–3012. [DOI] [PubMed] [Google Scholar]
- 11.Chase HW et al. (2015) Evidence for an anterior-posterior differentiation in the human hippocampal formation revealed by meta-analytic parcellation of fMRI coordinate maps: Focus on the subiculum. NeuroImage 113, 44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton MA et al. (2019) Differences in functional connectivity along the anterior-posterior axis of human hippocampal subfields. NeuroImage 192, 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharabian Masouleh S et al. (2020) Characterizing the gradients of structural covariance in the human hippocampus. Neuroimage 218, 116972. [DOI] [PubMed] [Google Scholar]
- 14.Plachti A et al. (2020) Hippocampus co-atrophy pattern in dementia deviates from covariance patterns across the lifespan. Brain 143 (9), 2788–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plachti A et al. (2019) Multimodal Parcellations and Extensive Behavioral Profiling Tackling the Hippocampus Gradient. Cereb Cortex 29 (11), 4595–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Wael RV et al. (2018) Anatomical and microstructural determinants of hippocampal subfield functional connectome embedding. Proceedings of the National Academy of Sciences 115 (40), 10154–10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Przeździk I. et al. (2019) The functional organisation of the hippocampus along its long axis is gradual and predicts recollection. Cortex 119, 324–335. [DOI] [PubMed] [Google Scholar]
- 18.Robinson JL et al. (2015) Neurofunctional topography of the human hippocampus. Human brain mapping 36 (12), 5018–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian Y et al. (2020) Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat Neurosci 23 (11), 1421–1432. [DOI] [PubMed] [Google Scholar]
- 20.Strange BA et al. (2014) Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience 15 (10), 655–669. [DOI] [PubMed] [Google Scholar]
- 21.Amunts K et al. (2013) BigBrain: an ultrahigh-resolution 3D human brain model. Science 340 (6139), 1472–1475. [DOI] [PubMed] [Google Scholar]
- 22.Amunts K et al. (2020) Julich-Brain: A 3D probabilistic atlas of the human brain’s cytoarchitecture. Science 369 (6506), 988–992. [DOI] [PubMed] [Google Scholar]
- 23.Paquola C et al. (2020) Convergence of cortical types and functional motifs in the human mesiotemporal lobe. eLife 9, e60673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeKraker J et al. (2020) Hippocampal subfields revealed through unfolding and unsupervised clustering of laminar and morphological features in 3D BigBrain. Neuroimage 206, 116328. [DOI] [PubMed] [Google Scholar]
- 25.Vogel JW et al. (2020) A molecular gradient along the longitudinal axis of the human hippocampus informs large-scale behavioral systems. Nature Communications 11 (1), 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayhan F et al. (2021) Resolving cellular and molecular diversity along the hippocampal anterior-to-posterior axis in humans. Neuron 109 (13), 2091–2105 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding SL and Van Hoesen GW (2015) Organization and detailed parcellation of human hippocampal head and body regions based on a combined analysis of cyto- and chemoarchitecture. Journal of Comparative Neurology 523 (15), 2233–2253. [DOI] [PubMed] [Google Scholar]
- 28.Andersen P et al. (1971) Lamellar organization of hippocampal excitatory pathways. Experimental brain research 13 (2), 222–238. [DOI] [PubMed] [Google Scholar]
- 29.Zeineh MM et al. (2017) Direct visualization and mapping of the spatial course of fiber tracts at microscopic resolution in the human hippocampus. Cerebral cortex 27 (3), 1779–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gómez-Isla T et al. (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. Journal o f Neuroscience 16 (14), 4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dill V et al. (2015) Automated methods for hippocampus segmentation: the evolution and a review of the state of the art. Neuroinformatics 13 (2), 133–50. [DOI] [PubMed] [Google Scholar]
- 32.van der Meer D et al. (2020) Brain scans from 21,297 individuals reveal the genetic architecture of hippocampal subfield volumes. Molecular Psychiatry 25 (11), 3053–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marečková K et al. (2018) Perinatal stress and human hippocampal volume: Findings from typically developing young adults. Scientific Reports 8 (1), 4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Flores R et al. (2015) Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease. Neuroscience 309, 29–50. [DOI] [PubMed] [Google Scholar]
- 35.Lorente de Nó R (1934) Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. Journal für Psychologie und Neurologie. [Google Scholar]
- 36.Vogt C and Vogt O (1919) Allgemeine ergebnisse unserer hirnforschung, JA Barth. [Google Scholar]
- 37.Rose J (1938) Zur normalen und pathologischen Architektonik der Ammonsformation. J Psychol Neurol 49, 137–186. [Google Scholar]
- 38.Amaral D et al. (1990) The human nervous system. San Diego: Academic, 711–755. [Google Scholar]
- 39.Yushkevich PA et al. (2015) Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. NeuroImage 111, 526–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kulaga-Yoskovitz J et al. (2015) Multi-contrast submillimetric 3 Tesla hippocampal subfield segmentation protocol and dataset. Scientific data 2 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fanselow MS and Dong H-W (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65 (1), 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cembrowski MS et al. (2016) Spatial Gene-Expression Gradients Underlie Prominent Heterogeneity of CA1 Pyramidal Neurons. Neuron 89 (2), 351–68. [DOI] [PubMed] [Google Scholar]
- 43.Lepage M et al. (1998) Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus 8 (4), 313–322. [DOI] [PubMed] [Google Scholar]
- 44.Tulving E et al. (1996) Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cerebral Cortex 6 (1), 71–79. [DOI] [PubMed] [Google Scholar]
- 45.Kim H (2015) Encoding and retrieval along the long axis of the hippocampus and their relationships with dorsal attention and default mode networks: The HERNET model. Hippocampus 25 (4), 500–510. [DOI] [PubMed] [Google Scholar]
- 46.Qin S et al. (2016) Large-scale intrinsic functional network organization along the long axis of the human medial temporal lobe. Brain Structure and Function 221 (6), 3237–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eickhoff SB et al. (2018) Imaging-based parcellations of the human brain. Nat Rev Neurosci 19 (11), 672–686. [DOI] [PubMed] [Google Scholar]
- 48.Margulies DS et al. (2016) Situating the default-mode network along a principal gradient of macroscale cortical organization. Proceedings of the National Academy of Sciences 113 (44), 12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Wael RV et al. (2020) BrainSpace: a toolbox for the analysis of macroscale gradients in neuroimaging and connectomics datasets. Communications biology 3 (1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eickhoff SB et al. (2015) Connectivity- based parcellation: Critique and implications. Human brain mapping 36 (12), 4771–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian Y and Zalesky A (2018) Characterizing the functional connectivity diversity of the insula cortex: Subregions, diversity curves and behavior. NeuroImage 183, 716–733. [DOI] [PubMed] [Google Scholar]
- 52.Langner R et al. (2014) Meta-analytic connectivity modeling revisited: controlling for activation base rates. NeuroImage 99, 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong Q et al. (2019) Functional parcellation of the hippocampus from resting-state dynamic functional connectivity. Brain research 1715, 165–175. [DOI] [PubMed] [Google Scholar]
- 54.Barnett AJ et al. (2019) Parcellation of the Hippocampus Using Resting Functional Connectivity in Temporal Lobe Epilepsy. Frontiers in Neurology 10 (920). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng A et al. (2021) Parallel hippocampal-parietal circuits for self-and goal-oriented processing. Proceedings of the National Academy of Sciences 118 (34). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alexander-Bloch A et al. (2013) The convergence of maturational change and structural covariance in human cortical networks. Journal of Neuroscience 33 (7), 2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valk SL et al. (2020) Shaping brain structure: Genetic and phylogenetic axes of macroscale organization of cortical thickness. Science advances 6 (39), eabb3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ge R et al. (2019) Parcellation of the human hippocampus based on gray matter volume covariance: Replicable results on healthy young adults. Human Brain Mapping 40 (13), 3738–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel R et al. (2020) Investigating microstructural variation in the human hippocampus using non-negative matrix factorization. Neuroimage 207, 116348. [DOI] [PubMed] [Google Scholar]
- 60.Huntenburg JM et al. (2018) Large-scale gradients in human cortical organization. Trends in cognitive sciences 22 (1), 21–31. [DOI] [PubMed] [Google Scholar]
- 61.Henriksen EJ et al. (2010) Spatial representation along the proximodistal axis of CA1. Neuron 68 (1), 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lisman J et al. (2017) Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci 20 (11), 1434–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Genon S et al. (2018) How to Characterize the Function of a Brain Region. Trends Cogn Sci 22 (4), 350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stachenfeld KL et al. (2017) The hippocampus as a predictive map. Nature neuroscience 20 (11), 1643. [DOI] [PubMed] [Google Scholar]
- 65.Kesner RP and Rolls ET (2015) A computational theory of hippocampal function, and tests of the theory: New developments. Neuroscience & Biobehavioral Reviews 48, 92–147. [DOI] [PubMed] [Google Scholar]
- 66.Quian Quiroga R (2020) No Pattern Separation in the Human Hippocampus. Trends Cogn Sci 24 (12), 994–1007. [DOI] [PubMed] [Google Scholar]
- 67.Small SA et al. (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature Reviews Neuroscience 12 (10), 585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Decker AL et al. (2020) Children’s family income is associated with cognitive function and volume of anterior not posterior hippocampus. Nature communications 11 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JK et al. (2020) Changes in anterior and posterior hippocampus differentially predict item-space, item-time, and item-item memory improvement. Developmental cognitive neuroscience 41, 100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Langnes E et al. (2020) Anterior and posterior hippocampus macro- and microstructure across the lifespan in relation to memory—A longitudinal study. Hippocampus 30 (7), 678–692. [DOI] [PubMed] [Google Scholar]
- 71.Sahakyan L et al. (2021) Anterior vs Posterior Hippocampal Subfields in an Extended Psychosis Phenotype of Multidimensional Schizotypy in a Nonclinical Sample. Schizophr Bull 47 (1), 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalmady SV et al. (2017) Clinical correlates of hippocampus volume and shape in antipsychotic-naïve schizophrenia. Psychiatry Research: Neuroimaging 263, 93–102. [DOI] [PubMed] [Google Scholar]
- 73.McHugo M et al. (2018) Regionally specific volume deficits along the hippocampal long axis in early and chronic psychosis. NeuroImage: Clinical 20, 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szeszko PR et al. (2003) Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. American Journal of Psychiatry 160 (12), 2190–2197. [DOI] [PubMed] [Google Scholar]
- 75.Bernasconi N et al. (2003) Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain 126 (2), 462–469. [DOI] [PubMed] [Google Scholar]
- 76.Bernhardt BC et al. (2015) Magnetic resonance imaging pattern learning in temporal lobe epilepsy: classification and prognostics. Ann Neurol 77 (3), 436–46. [DOI] [PubMed] [Google Scholar]
- 77.Bernhardt BC et al. (2016) The spectrum of structural and functional imaging abnormalities in temporal lobe epilepsy. Annals of Neurology 80 (1), 142–153. [DOI] [PubMed] [Google Scholar]
- 78.Dautricourt S et al. (2021) Longitudinal Changes in Hippocampal Network Connectivity in Alzheimer’s Disease. Ann Neurol 90 (3), 391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Malykhin NV et al. (2017) Differential vulnerability of hippocampal subfields and anteroposterior hippocampal subregions in healthy cognitive aging. Neurobiology of Aging 59, 121–134. [DOI] [PubMed] [Google Scholar]
- 80.Lowe AJ et al. (2019) Targeting age- related differences in brain and cognition with multimodal imaging and connectome topography profiling. Human brain mapping 40 (18), 5213–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown EM et al. (2020) Test-retest reliability of FreeSurfer automated hippocampal subfield segmentation within and across scanners. Neuroimage 210, 116563. [DOI] [PubMed] [Google Scholar]
- 82.Cheng H et al. (2020) Functional parcellation of the hippocampus by semi-supervised clustering of resting state fMRI data. Scientific Reports 10 (1), 16402. [DOI] [PMC free article] [PubMed] [Google Scholar]