Abstract
A hematologist receives a call from a maternal-fetal medicine (MFM) physician about a previously healthy patient who became ill at 25 weeks’ gestation. Her mental status is deteriorating. There are signs of fetal distress. Platelet count and hemoglobin are falling. The MFM physician is considering the hemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome. For the hematologist, everything seems unfamiliar. Our goal is to provide hematologists with the fundamental knowledge required for understanding and managing these patients who become suddenly and seriously ill during pregnancy and in whom thrombocytopenia and microangiopathic hemolytic anemia are part of their presentation.
1 |. INTRODUCTION
Most pregnancies are uncomplicated, however, most complicated pregnancies are effectively managed by obstetricians specializing in maternal-fetal medicine. Among the most critical complications of pregnancy are preeclampsia with severe features, eclampsia and hemolysis, elevated liver function tests and low platelets (HELLP) syndrome. Hemolysis in HELLP syndrome is caused by red blood cell fragmentation that results from microvascular thrombosis, described as microangiopathic hemolytic anemia (MAHA). In some pregnant women, the presenting clinical features may seem inconsistent with these familiar syndromes. Thrombocytopenia and MAHA may seem too severe. Neurologic abnormalities may seem more severe than the expected symptoms and signs associated with obstetric complications: headaches, dizziness, visual disturbances, impaired consciousness, and seizures. The occurrence may seem too early in gestation to be preeclampsia (PE), eclampsia or HELLP syndrome. If the diagnosis is preeclampsia with severe features, eclampsia or HELLP syndrome then urgent delivery is often required. However, if the diagnosis is not one of these obstetric complications, different management is urgently required. Therefore, the maternal-fetal medicine physician consults a hematologist. This consultation may also be difficult for the hematologist because their training did not include the evaluation and management of critical obstetric complications. These two specialists, maternal-fetal medicine (MFM) and hematologist, must share their knowledge to manage the extreme urgency of this patient’s illness.
Our goal is to prepare hematologists for this consultation. We review the multiple potential causes of severe thrombocytopenia and MAHA, occurring together, in pregnancy (Table 1). We distinguish the pregnancy-specific obstetric complications, for which the principal management is delivery of the fetus, from the disorders that can occur in all women, but may occur more commonly during pregnancy or postpartum.
TABLE 1.
Etiologies of severe thrombocytopenia and microangiopathic hemolytic anemia occurring together during pregnancy
| Disorders that only occur during pregnancy |
| Preeclampsia with severe featuresa |
| Eclampsia |
| Hemolysis, elevated liver function tests, low platelets (HELLP) syndrome |
| Acute fatty liver of pregnancy (AFLP) |
| Disorders that can occur in all women |
Thrombotic thrombocytopenic purpura (TTP)
|
| Severe hypertension |
| Complement-mediated thrombotic microangiopathy (C-TMA, also described as atypical hemolytic-uremic syndrome, aHUS) |
| Antiphospholipid syndrome |
| Drug-induced thrombotic microangiopathy (DITMA) |
Note: This table lists the disorders that can present with both of severe thrombocytopenia (platelet count <100 000/μL) and microangiopathic hemolytic anemia. We focus on the disorders highlighted by italics, because their pathogenesis and clinical features overlap. In the text we consolidate preeclampsia with severe features, eclampsia, and the HELLP syndrome into a single term, PE/HELLP.
The American College of Obstetricians and Gynecologists has replaced the term “severe preeclampsia” with the term “preeclampsia with severe features”.
Among the obstetric complications, we focus on preeclampsia with severe features, eclampsia and HELLP syndrome.1 Because the clinical features of these three obstetric complications overlap, we consolidate and describe them as a single disorder, designated PE/HELLP. Acute fatty liver of pregnancy (AFLP) can also have clinical features similar to PE/HELLP, but we do not include it in our discussion because of its rare occurrence, estimated to be approximately one patient per 8000 pregnancies.2 Other obstetric complications, such as placental abruption with vaginal hemorrhage and postpartum hemorrhage, are not included in our discussion even though they may cause disseminated intravascular coagulation with severe thrombocytopenia and MAHA.
Among the multiple causes of severe thrombocytopenia and MAHA that are not pregnancy-specific, some may occur more frequently during pregnancy and some can have clinical features similar to PE/HELLP. We focus on thrombotic thrombocytopenic purpura (TTP), which has a close association with pregnancy. The clinical features of TTP and PE/HELLP are similar; neither is associated with acute kidney injury.3,4 The placental pathology of TTP and PE/HELLP is also similar;5,6 both are described as maternal vascular malperfusion, which is characterized by decidual arteriolar thrombosis and infarction.5 The occurrence of acute episodes of acquired TTP may be increased during pregnancy. Patients with hereditary TTP almost always have severe complications with pregnancy.7 To understand and distinguish these clinically similar disorders, PE/HELLP and TTP, we focus on their pathophysiology, evaluation, management, and risk with future pregnancies.
We do not consider gestational thrombocytopenia in our discussion. It is a physiologic change that occurs during all pregnancies.8 Platelet counts in all women gradually decrease throughout pregnancy, beginning in the first trimester and continuing until delivery. At delivery, the platelet count has decreased by 15%–20%. A major cause of the decreased platelet counts is pooling of platelets in the intervillous spaces of the placenta.9 At the time of delivery, approximately 10% of women will have platelet counts < 150 × 103/μL. These will be the women whose platelet counts are always in the lower range of normal; their platelet counts during pregnancy will become less than the lower limit of normal (< 150 × 103/μL). Although gestational thrombocytopenia is the most common cause of thrombocytopenia during pregnancy, platelet counts < 100 × 103/μL (our definition for severe thrombocytopenia in this review) at any time during pregnancy, including at delivery, occur in only 0.7% of uncomplicated pregnancies.8 Therefore, a platelet count < 100 × 103/μL should not be attributed to gestational thrombocytopenia. Pregnant women with a platelet count < 100 × 103/μL require additional investigation.
2 |. SEVERE THROMBOCYTOPENIA AND MAHA CAUSED BY COMPLICATIONS OF PREGNANCY
The disorders of PE/HELLP are the principal cause of a platelet count < 100 × 103/μL during pregnancy.1 When this occurs in a patient with preeclampsia, the diagnosis becomes preeclampsia with severe features. Other abnormalities that define preeclampsia with severe features are high blood pressure (> 160/110 mm Hg), abnormal liver function, renal insufficiency, pulmonary edema, refractory headache and visual disturbances.1 Eclampsia is defined as preeclampsia with generalized seizures.1 The occurrence of eclampsia increases the risk for even more severe obstetric complications, such as cardiomyopathy and pancreatitis.10,11 The HELLP syndrome is defined as a platelet count < 100 × 103/μL, MAHA, and liver function abnormalities.1 New onset hypertension, the defining feature of preeclampsia, occurs in 85% of patients with HELLP syndrome.12 All of these obstetric complications primarily occur after 34 weeks’ gestation. By definition, they never (or almost never) occur before 20 weeks’ gestation. The syndromes of PE/HELLP are frequently associated with acute kidney injury (AKI), but rarely associated with severe AKI. In a review of 1 918 789 consecutive pregnancies in Ontario, only 188 (approximately 1/10 000) were complicated by AKI treated with renal replacement therapy.4 Therefore severe AKI is evidence against the diagnosis of PE/HELLP and hematologists should consider other etiologies. However, it is important to note that there is no widely accepted pregnancy specific definition for AKI because of the physiologic changes to the renal system in pregnancy. Thus, GFR increases 40%–50% in pregnancy and therefore even in a pregnant patient with injured kidneys, a normal creatinine could occur.13 Most of the literature on pregnancy related AKI uses the RIFLE14 or AKIN15 scoring systems. Stage 3 of either classification system would merit consideration of something beyond what would be expected in PE/HELLP.
The defining clinical features of these three complications of pregnancy are similar. They are all severe and they are usually indications for urgent delivery. Patients who have preeclampsia with severe features before 34 weeks’ gestation may be managed without delivery, allowing the pregnancy to progress to a safer gestational age for the fetus (described as “expectant management”) if they are clinically stable, the hypertension is controlled and fetus well-being is assured. After 34 weeks’ gestation, delivery is performed immediately. These data support the use of a composite term, PE/HELLP, to describe all three syndromes. For the hematologist, the distinction among the three components of PE/HELLP is unimportant.
We have previously published data for 15 723 deliveries, 2011–2014, at the University of Oklahoma Medical Center, a tertiary referral hospital.8 For this analysis, we excluded 2238 deliveries that were subsequent pregnancies in the same woman or were missing platelet counts at delivery. Among the 13 488 remaining women, 774 (5.74%) had preeclampsia, 39 (0.40%) had HELLP syndrome and 10 (0.07%) had eclampsia (Table 2). Altogether, 838 (6.2%) women had one of these three complications of pregnancy. Among all 13 488 women, 335 (2.5%) women had platelet counts < 100 × 103/μL at delivery. Among all 774 patients with preeclampsia, only 52 (6.7%) had platelet counts < 100 × 103/μL, indicating preeclampsia with severe features by this criterion. Among the 54 patients with HELLP syndrome, 39 (72%) had platelet counts < 100 × 103/μL. Among the 10 patients with eclampsia, only two had platelet counts < 100 × 103/μL. The sum of these three disorders with platelet counts < 100 × 103/μL, 93 patients, is the frequency of our consolidated term, PE/HELLP, among all pregnancies. Therefore, among all 335 women with platelet counts < 100 × 103/μL at delivery, 242 had etiologies other than preeclampsia with severe features, HELLP syndrome and eclampsia. These 242 women would include 94 (0.7% of 13 488) women with gestational thrombocytopenia, women who had preexisting disorders associated with thrombocytopenia (e.g., immune thrombocytopenia [ITP], systemic lupus erythematosus [SLE]), and women with other complications of pregnancy.8
TABLE 2.
Frequency of preeclampsia, HELLP syndrome and eclampsia in all women and in women with platelet counts <100 × 103/μL
| Pregnancy complications | All women | Women with a platelet count < 100 000/μL |
|---|---|---|
| All women | 13 488 | 335 (2.5%) |
| Preeclampsia | 774 (5.74%) | 52 (6.7%)a |
| HELLP syndrome | 54 (0.40%) | 39 (72%)a |
| Eclampsia | 10 (0.07%) | 2 (20%)a |
Note: Data are from women, ages 15–45, who delivered at the University of Oklahoma Medical Center between January 1, 2011 and August 19, 2014.8 Of all 15 723 deliveries during this time, 2238 deliveries were deleted because they were susbsequent pregnancies in the same woman or were missing platelet counts at delivery. Therefore the data for 13 488 women were analyzed. Column two presents the occurrence of preeclampsia, HELLP syndrome and eclampsia among all 13 488 women. To identify women with a platelet count <100 × 103/μL, we used the lowest count at delivery, ±2 days. Column three presents the occurrence of platelet counts <100 × 103/μL in all women, and also the occurrence of preeclampsia, HELLP syndrome and eclampsia among women with a platelet count of <100 × 103/μL.
These percent data are the frequencies of women with preeclampsia, HELLP syndrome or eclampsia who had platelet counts <100 × 103/μL.
If there is any doubt about the diagnosis of PE/HELLP, hematology consultation is appropriate. Common causes for doubt are the severity of thrombocytopenia, the severity of neurologic complications (such as transient ischemic attacks [TIA] or stroke), the presence of AKI that is not recovering and trending towards requiring dialysis, the severity of abnormal liver function, gestational age < 20 weeks, and/or failure of recovery with delivery.
2.1 |. Causes of severe thrombocytopenia and MAHA that can occur in all women
The Figure 1 illustrates the sequence for evaluation of a pregnant patient whose platelet count is < 100 × 103/μL and the pregnancy-related etiologies seem unlikely. First, we exclude the disorders that can commonly cause severe thrombocytopenia (immune thrombocytopenia [ITP], pseudothrombocytopenia caused by in vitro platelet clumping, and drug-induced thrombocytopenia) because they are not associated with MAHA. Next, the disorders that can cause severe thrombocytopenia and MAHA are considered. Most of these disorders (severe hypertension, complement-mediated thrombotic microangiopathy [c-TMA], antiphospholipid syndrome, and drug-induced TMA) also cause AKI. The presence of AKI is evidence against the diagnosis of TTP.3 Table 1 lists these disorders. This section describes their clinical features, their association with complications of pregnancy, and their similarities to the clinical features of PE/HELLP.
FIGURE 1.
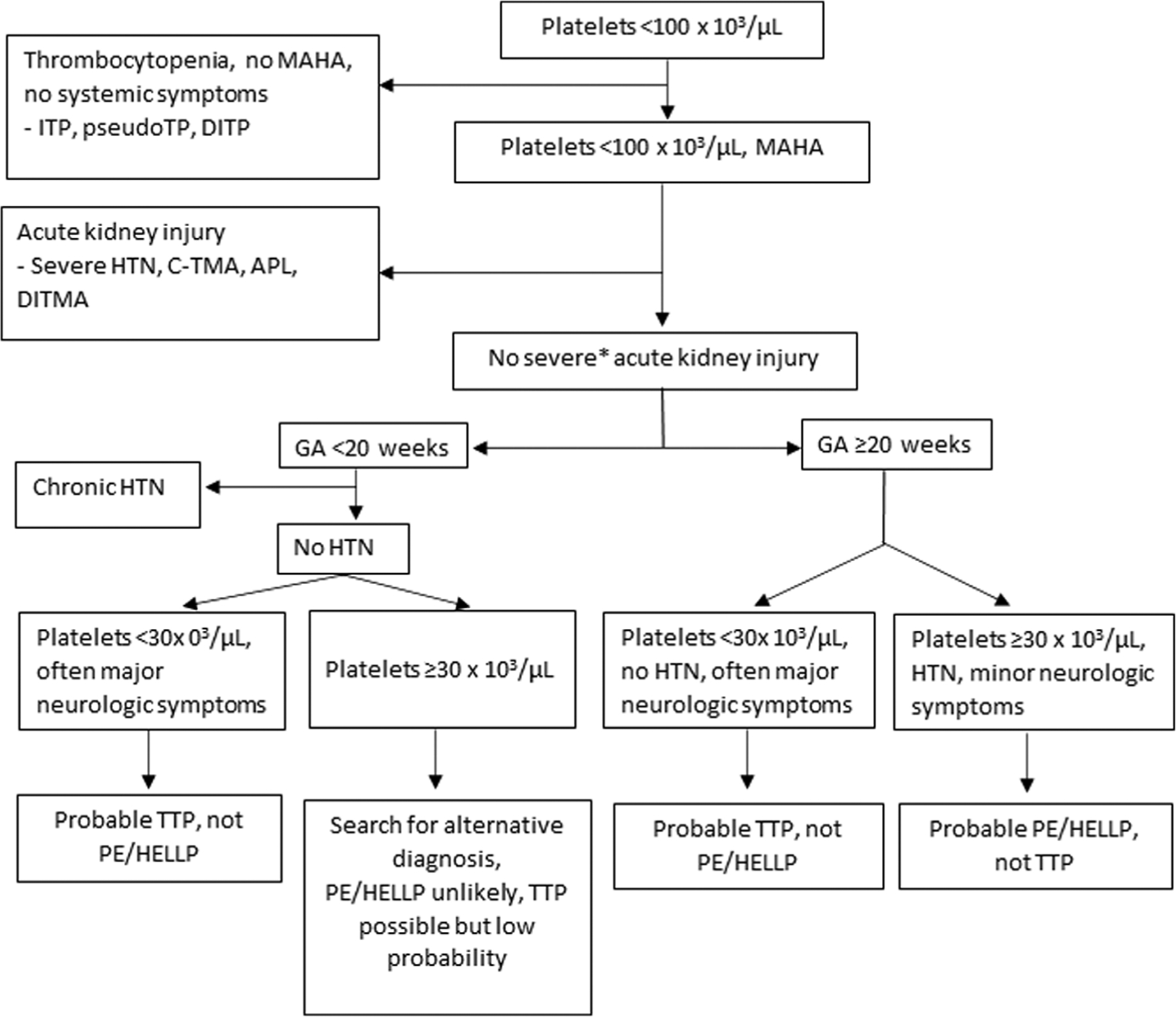
Distinguishing PE/HELLP syndrome from TTP and other causes of severe thrombocytopenia and MAHA
2.1.1 |. Thrombotic thrombocytopenic purpura (TTP)
The clinical features of TTP, both acquired and hereditary, and PE/HELLP have many similarities. One important difference is that the initial episode of acquired TTP is not associated with hypertension.16 The data for hereditary TTP are less certain; these patients may have increased risk for hypertension which may begin in childhood.17 When TTP in a pregnant patient presents with minimal symptoms or with only MAHA and thrombocytopenia,18 the diagnosis of PE/HELLP may be assumed and the diagnosis of TTP could be missed. The assumption of PE/HELLP is supported by its much greater frequency during pregnancy.19
Our experience with TTP is from the Oklahoma TTP Registry, an inception cohort of all consecutive patients with suspected TTP, 1995–2018.3 During the 5 years of our studies on pregnancy outcomes, 2011–2014, we diagnosed 13 women acquired TTP and three siblings with hereditary TTP, but in only one of these women was acquired TTP diagnosed during pregnancy. In spite of this infrequency, obstetricians should have a low threshold for considering the diagnosis of TTP and consulting a hematologist because of the high risk for death without urgent treatment. The hematologist’s responsibility is to confirm or exclude the diagnosis of TTP, and to consider the distinction between acquired and hereditary TTP. Because it is rare, physicians often initially misdiagnose hereditary TTP as acquired TTP, causing the inappropriate assumption that remission of the TTP episode equals resolution of the disorder.
The incidence of acquired, autoimmune TTP is two to three per million population/year.20 The occurrence of hereditary TTP is much less; it’s estimated prevalence is one per million population.21 However, since many women with hereditary TTP have their initial TTP symptoms during their first pregnancy,22 and 97% of women with hereditary TTP have severe complications of pregnancy,7 the relative frequency of hereditary TTP compared to acquired TTP increases remarkably during pregnancy. Among the 32 women in the French Reference Center for Thrombotic Microangiopathies who had their initial episode of TTP during their first pregnancy, 10 (31%) had hereditary TTP.23 Therefore, when physicians diagnose TTP in a pregnant woman, they should consider both acquired and hereditary TTP.
In the Oklahoma TTP Registry, 1995–2018, 21 pregnant/postpartum women have been initially diagnosed with TTP and treated with plasma exchange. In seven (33%) women, the diagnosis of acquired TTP was confirmed by severe ADAMTS13 deficiency (activity < 10%) and the presence of a functional ADAMTS13 inhibitor (autoantibody) and/or recovery of ADAMTS13 activity during remission. In the remaining 14 (67%) women, the appropriate diagnosis was PE/HELLP. The distinction between TTP and PE/HELLP is clearer now than when the Oklahoma Registry began 25 years ago. We diagnosed 13 of these 21 women between 1996 and 2003, before the availability of ADAMTS13 activity measurements and when the diagnostic features of TTP were less clear. Only two (15%) of these 13 women had TTP confirmed by severe ADAMTS13 deficiency (measured on stored serum samples). After 2003, the diagnosis of acquired TTP was confirmed in five (63%) of the eight women with clinically suspected TTP by documentation of severe ADAMTS13 deficiency. Even with the improved diagnostic accuracy in recent years, these data document the continuing difficulty of distinguishing PE/HELLP and TTP.
2.1.2 |. Severe hypertension
We describe chronic hypertension during pregnancy as preexisting hypertension if it began prior to pregnancy or occurs before 20 weeks’ gestation. We describe it as gestational hypertension when it first occurs after 20 weeks’ gestation. Gestational hypertension, defined by blood pressure > 140/90 mm Hg, is distinguished from preeclampsia only by the absence of proteinuria or a severe feature. The frequency of gestational hypertension is similar to the frequency of preeclampsia.1 Increased severity of chronic hypertension can cause AKI. Severe chronic hypertension causes the same placental pathology as PE/HELLP, described as maternal vascular malperfusion.5 Therefore, severe hypertension and PE/HELLP may not be easily distinguished.
Severe hypertension alone can also cause microangiopathic hemolytic anemia and thrombocytopenia. In the report that created and defined the term “microangiopathic hemolytic anemia”, severe hypertension was the cause of MAHA in five of the 25 reported patients.24 Four of the five patients were women; three of the women were ages 26–34; none were described as pregnant. Severe hypertension alone, defined by diastolic blood pressure > 120 mm Hg and the presence of retinal hemorrhages and ischemic organ injury, can cause the clinical features of TTP.25 Neurologic symptoms are common, including seizures, posterior reversible encephalopathy syndrome (PRES) and stroke. Patients with severe hypertension commonly have severe AKI, which rarely occurs in PE/HELLP4 or TTP.3
The clinical similarity of severe hypertension and TTP is illustrated by the experience of the Oklahoma TTP Registry with eight patients, from 1997 to 2018, who had severe hypertension with thrombocytopenia and MAHA and were initially suspected to have TTP and treated with plasma exchange. The diagnosis of TTP was excluded in these eight patients when their ADAMTS13 activity was reported to be 40%–100%.
2.1.3 |. Complement-mediated thrombotic microangiopathy (C-TMA, also described as atypical hemolytic-uremic syndrome [aHUS])
Note, C-TMA is associated with gene mutations that cause increased complement activation by the alternative pathway.26 The diagnosis of C-TMA became prominent when eculizumab, a drug that can block the complement alternative pathway, became available in 2012.27 Therefore, C-TMA is associated with pregnancy, most commonly occurring post-partum.19,28 It presents with thrombocytopenia and MAHA that are typically not as severe as in patients with TTP. The principal clinical feature of C-TMA is progressively severe AKI, which is not a clinical feature of either PE/HELLP or TTP. Physicians should consider C-TMA in a woman who was delivered because of presumed PE/HELLP with renal injury that does not resolve in the first 48–72 h postpartum.
2.1.4 |. Antiphospholipid syndrome
This is an uncommon systemic autoimmune disorder defined by thrombosis and severe obstetrical complications. It is diagnosed by the persistent presence of antiphospholipid antibodies.29 The clinical characteristics are venous, arterial, and/or microvascular thrombosis. A severe form, described as catastrophic antiphospholipid syndrome (CAPS), includes the occurrence of ischemic injury of ≥ 3 ischemic organs in addition to the presence of antiphospholipid antibodies. Thrombocytopenia, with platelet counts of 50–150 × 103/μL, and MAHA are common; platelet counts < 20 × 103/μL are uncommon. Stroke and TIA are common arterial thrombotic events. The clinical criteria for diagnosis of antiphospholipid syndrome are the presence of either thrombosis or the occurrence of ≥ 1 of three pregnancy morbidities: [1] ≥ 1 unexplained deaths of a normal fetus at or beyond 10 weeks’ gestation, [2] ≥ 1 births at gestational age < 34 weeks’ gestation because of preeclampsia with severe features or eclampsia, or recognized features of placental insufficiency, or [3] ≥ 3 consecutive, unexplained fetal losses before 10 weeks’ gestation.30 Note, AKI caused by thrombotic microangiopathy is common in patients with antiphospholipid syndrome. The antiphospholipid syndrome is often associated with systemic lupus erythematosus,29 a feature of poly-autoimmunity.31 Similarly, acquired TTP is also commonly associated with other autoimmune disorders.32 From 1995–2018, 10 (11%) of 90 patients in the Oklahoma TTP Registry have been diagnosed with systemic lupus erythematosus.32
2.1.5 |. Drug-induced thrombotic microangiopathy (DITMA)
Almost all occurrences of DITMA are caused by dose-dependent drug toxicity, such as with oxymorphone (Opana ER), oxycodone or immunosuppressive or chemotherapy agents.33,34 So, DITMA often causes severe AKI in addition to thrombocytopenia and MAHA. Dose-dependent DITMA may begin suddenly, as with intravenous oxymorphone or oxycodone toxicity, or gradually, as with gemcitabine.35 The causative drug is usually apparent. When immune-mediated DITMA occurs, it is sudden and severe. It can mimic TTP, with severe thrombocytopenia and MAHA. However, AKI is also common.36 Quinine, including quinine in beverages such as tonic water, is a common cause of immune-mediated DITMA.36,37
2.2 |. Pathogenesis of PE/HELLP and TTP
The exact pathophysiology of PE/HELLP still remains unknown. However, the pathologic features of the placenta are similar in PE/HELLP and hereditary TTP.6 In both disorders, the placental pathology is described as maternal vascular malperfusion, characterized by thrombosis of decidual (maternal portion of the placenta) arterioles causing placental infarction and villous hypoplasia.5 In both PE/HELLP and hereditary TTP, the thrombi are composed primarily of platelets and von Willebrand factor (VWF),6 rather than the typical dominant fibrin of arterial thrombi.38 Maternal vascular malperfusion occurs because of failure of remodeling of the maternal uterine spiral arterioles as they penetrate into the developing placenta during the first trimester. Normal remodeling is caused by endovascular trophoblasts that transform the muscular walls and narrow lumens of the spiral arterioles into vessels with thinner, flexible walls and larger lumens. The remodeling allows increased blood flow with slower velocity.5 This is critical for effective perfusion of the intervillous spaces and transfer of maternal nutrients to the fetal circulation within the villi. With failure of remodeling, the spiral arterioles maintain their high velocity, turbulent blood flow which increases risk for decidual arteriolar thrombosis.
Why spiral arterioles fail to remodel in women with preeclampsia is unknown. In patients with acquired and hereditary TTP, obstruction within the arterioles by the ultra-large multimers of von Willebrand factor (ULVWF) may cause the failure of spiral arterioles to remodel. The increased shear stress of the high-velocity, turbulent blood flow in the spiral arterioles can cause unfolding of the ULVWF and exposure of platelet binding sites.39 Platelet binding to the ULVWF increases the risk of thrombosis. This is illustrated by the ability of caplacizumab, which blocks platelet binding to VWF, to promptly stop the thrombotic symptoms of TTP.40 These observations suggest a similar pathogenesis of PE/HELLP and TTP. Although the cause of the failure of maternal spiral arterioles to adapt to the placenta may be different, the placental injury is the same.
2.3 |. Evaluation of women with severe thrombocytopenia and microangiopathic hemolytic anemia during pregnancy
A platelet count < 100 × 103/μL does not define severe thrombocytopenia for a hematologist. For a hematologist, a common defining platelet count for severe thrombocytopenia is < 30 × 103/μL. For example, patients with immune thrombocytopenia (ITP) who have a platelet count ≥ 30 × 103/μL may not require treatment.41 Also, a platelet count < 30 × 103/μL is a defining diagnostic feature for an acute episode of acquired TTP.42,43
However, for a pregnant woman, a platelet < 100 × 103/μL is an appropriate definition for severe thrombocytopenia. During pregnancy, a platelet < 100 × 103/μL is rare. Platelet counts < 100 × 103/μL occur in < 1% of women with uncomplicated pregnancies.8 Therefore, a platelet count < 100 × 103/μL is a severe feature for a woman with preeclampsia, creating urgency for delivery.1 A platelet count < 100 × 103/μL is also a diagnostic criterion for the HELLP syndrome.1
2.4 |. Clinical presentation
The most important distinguishing clinical feature for the etiology of MAHA and severe thrombocytopenia during pregnancy is the gestational age of occurrence. Table 3 illustrates gestational age at delivery of all 838 women with preeclampsia, HELLP syndrome and eclampsia, regardless of platelet count, in the University of Oklahoma Medical Center cohort.8 No women delivered before 10 weeks’ gestation. Only one (0.1%) woman delivered at 10–19 weeks’ gestation; nine (1.1%) women delivered before 25 weeks’ gestation; 46 (5.6%) women delivered before 30 weeks’ gestation. Since immediate delivery is necessary in these women, these data document that the occurrence of these pregnancy complications rarely occur before 30 weeks’ gestation. A woman with chronic hypertension who has an exacerbation before 20 weeks’ gestation may mimic the clinical features of PE/HELLP, but the focus of her management is control of her hypertension. After 30 weeks’ gestation, the risk for preeclampsia, HELLP syndrome and eclampsia increases with increasing gestational age.
TABLE 3.
Gestational age at delivery of women with preeclampsia, HELLP syndrome and eclampsia, and the gestational age at diagnosis of women with acquired TTP and hereditary TTP
| Complications of Pregnancy | TTP | ||||||
|---|---|---|---|---|---|---|---|
| Acquired | Hereditary | ||||||
| GA(weeks) | Preeclampsia (755) | HELLP (54) | Eclampsia (9) | FR (22) | OK (7) | FR (10) | PUB (38) |
| <10 | 0 | 0 | 0 | 2 (9%) | 2 (29%) | 0 | 1 (3%) |
| 10–19 | 1 (0.1%) | 0 | 0 | 4 (18%) | 0 | 2 (20%) | 9 (24%) |
| 20–24 | 7 (1%) | 2 (4%) | 0 | 4 (18%) | 0 | 0 | 12 (31%) |
| 25–29 | 38 (5%) | 7 (13%) | 1 (12%) | 4 (18%) | 0 | 3 (30%) | 8 (21%) |
| 30–34 | 144 (19%) | 15 (28%) | 4 (44%) | 2 (9%) | 0 | 2 (20%) | 5 (13%) |
| ≥35 | 565 (75%) | 30 (55%) | 4 (44%) | 2 (9%) | 1 (14%) | 3 (30%) | 3 (8%) |
| PP | 0 | 0 | 0 | 4 (18%) | 4 (57%) | 0 | 0 |
Note: Complications of pregnancy. Compared to the numbers of women reported in Table 2, the gestational age at delivery was not reported in 19 women with preeclampsia and one woman with eclampsia. Occurrence of TTP during pregnancy and postpartum. The data from the French Reference Center for Thrombotic Microangiopathy (FR) are from 32 women who had their firstt episode of TTP during their first pregnancy; 22 had acquired TTP, 10 had hereditary TTP. The gestational age of the occurrence of the TTP episode is reported23 Seven women from the Oklahoma TTP Registry (OK) had their first episode of acquired TTP with their firstt pregnancy. A systematic review of published case reports of women with hereditary TTP (PUB) identified 38 pregnancies that occurred in 29 women before their diagnosis of hereditary TTP, complicated by a severe episode of TTP.7
Abbreviations: GA, gestational age at delivery or diagnosis; PP, postpartum.
Table 3 also the gestational age of diagnosis for both acquired and hereditary TTP. In a report of 32 women from the French Reference Center for TMA who had their first episode of acquired TTP during pregnancy or postpartum, the episodes appeared to occur evenly across all time periods of pregnancy and postpartum.23 Among the 67 women with acquired TTP in the Oklahoma Registry, seven (10%) had their first episode of TTP during pregnancy or postpartum. In four of the seven patients, TTP occurred postpartum.
In contrast to acquired TTP, women with hereditary TTP almost always have severe complications with pregnancy. In our review and analysis of published reports of 61 pregnancies in 35 women with previously undiagnosed hereditary TTP, 34 (97%) women had severe complications; two women died during pregnancy.7 The gestational age of acute exacerbations of hereditary TTP during pregnancy were similar to the gestational age of occurrence of PE/HELLP, but with more occurrences earlier during pregnancy, < 30 weeks’ gestation (Table 3). The earlier gestational age of exacerbations of hereditary TTP, compared to the occurrence of PE/HELLP, may be related to earlier occurrence of placental thrombosis.
The symptoms of PE/HELLP and TTP may be similar. Women with PE/HELLP commonly have symptoms of severe headache, visual abnormalities, such as unclear vision or scotomata, and confusion. Patients with TTP may also have these symptoms. If hypertension is severe in women with PE/HELLP, serous retinal detachments can occur, causing vision loss. More severe neurologic symptoms, such as transient cerebral ischemic attacks (TIA) and stroke, are rare in PE/HELLP but common in TTP. Generalized seizures may occur in women with PE/HELLP, defining the diagnosis of eclampsia. Severe hypertension in eclampsia can cause posterior reversible encephalopathy syndrome (PRES).11 Severe hypertension is not a clinical feature of TTP. Physical examination may not distinguish PE/HELLP from TTP. Purpura, indicating severe thrombocytopenia, is one clinical feature that would strongly suggest that the diagnosis is TTP and not PE/HELLP. Vaginal bleeding suggests a pregnancy complication, such as placental abruption, which commonly occurs in women with PE/HELLP.
2.5 |. Laboratory evaluation
The most important laboratory value for distinguishing PE/HELLP from TTP is the platelet count. Table 4 presents the lowest platelet count at delivery (±2 days) of the 838 women with preeclampsia, HELLP syndrome and eclampsia in the University of Oklahoma Medical Center cohort.8 Among these women, only 93 (11%) patients had platelet counts < 100 × 103/μL, 35 (4%) patients had platelet counts < 60 × 103/μL, and 10 (1%) patients had platelet counts < 30 × 103/μL.
TABLE 4.
Platelet counts in women with preeclampsia, HELLP syndrome or eclampsia compared to platelet counts in patients with acquired or hereditary TTP
| Pregnancy complications | TTP | ||||
|---|---|---|---|---|---|
| Platelet counts/μL | Preeclampsia (N = 774) | HELLP (N = 54) | Eclampsia (N = 10) | Acquired (N = 90) | Hereditary (N = 23) |
| ≥100 000 | 722 (93%) | 15 (28%) | 8 (80%) | 1 (1%) | 0 |
| 80–99 000 | 18 (2%) | 12 (22%) | 2 (20%) | 0 | 0 |
| 60–79 000 | 15 (2%) | 11 (20%) | 0 | 2 (2%) | 1 (4%) |
| 30–59 000 | 13 (2%) | 12 (22%) | 0 | 1 (1%) | 6 (26%) |
| 10–29 000 | 5 (1%) | 4 (8%) | 0 | 46 (51%)3 | 16 (70%) |
| <10 000 | 1 (0.1%) | 0 | 0 | 40 (45%)4 | 0 |
Note: The total number of patients in each column is presented in parentheses. Data for the women with pregnancy complications are also presented in Tables 2 and 3. For women with pregnancy complications, the platelet counts are the lowest count at delivery, ±2 days. Data for acquired TTP are from the 90 consecutive patients (23 men, 67 women) enrolled in the Oklahoma TTP Registry, 1995–2018. The data are from the patients’ first episode, the most abnormal value among platelet counts measured on the day of the first plasma exchange treatment ±7 days. Seven of the 67 women whose initial episode of TTP occurred during pregnancy or postpartum (Table 3) are indicated by the superscript numbers.3 Data for hereditary TTP are from 23 women who had severe complications of pregnancy, described in our review of case reports.7
The platelet counts of patients with acquired TTP are distinctly lower than in patients with PE/HELLP. Only 2 (2%) of patients with acquired TTP had platelet counts ≥ 30 × 103/μL. Even though the complications during pregnancy in patients with hereditary TTP were consistently severe, their platelet counts were not as low as the platelet counts in acquired TTP. The course of disease in patients with hereditary TTP is not as distinct as in patients with acquired TTP. Instead of discrete, acute episodes of disease, that occur in patients with acquired TTP, patients with hereditary TTP have less discrete exacerbations and recoveries of their continuous disease. Hematologic and neurologic abnormalities may appear and resolve spontaneously; neurologic abnormalities may occur without thrombocytopenia.21
2.6 |. Management of women with severe thrombocytopenia and microangiopathic hemolytic anemia during pregnancy
When PE/HELLP occurs at gestational age ≥ 34 weeks, immediate delivery is appropriate because risks to the mother far outweigh the small risks to the fetus that are associated with a late pre-term delivery. When PE/HELLP occurs at gestational age is < 34 weeks, it may be appropriate to delay delivery as long as maternal stability is assured. When thrombocytopenia is severe and TTP is the probable diagnosis, delivery may also be appropriate depending on maternal and fetal stability; if the mother’s condition is stable and fetal monitoring is reassuring, then the physician may manage the pregnancy expectantly. For both PE/HELLP and TTP the decision between expectant management and delivery is a discussion that requires evaluation of many factors. However, because maternal vascular malperfusion of the placenta also occurs in women with TTP and PE/HELLP, fetal growth restriction may occur and is also a factor that would influence delivery. Because of the complexity of these types of decisions, these conversations are typically multidisciplinary in nature and must balance maternal risk and fetal benefit both for expectant management and delivery.
Current initial management of TTP is to assume an acquired etiology and to begin plasma exchange and corticosteroids. Although rituximab has become standard initial treatment for patients with acquired TTP,44 beginning rituximab is not urgent. It is appropriate to wait until the confirmation of severe ADAMTS13 deficiency before beginning rituximab. If a functional ADAMTS13 inhibitor is present, then a physician should diagnose acquired TTP. If a functional ADAMTS13 inhibitor is not present, the diagnosis of hereditary TTP becomes possible, but continued treatment for acquired TTP remains appropriate. Determining the distinction between acquired and hereditary can be deferred until clinical remission occurs and ADAMTS13 activity recovers, confirming the diagnosis of acquired TTP, or remains severely deficient, which can occur in either acquired or hereditary TTP.
Rituximab is safe during the first trimester since immunoglobulins do not cross the placenta until after gestational week 13.45 Observational data is reassuring for the use of rituximab throughout pregnancy, if it is the most appropriate treatment.46 Caplacizumab is an effective treatment for acquired TTP and its use as initial treatment is increasing.47–49 Increasing case reports have described that caplacizumab can provide effective treatment without plasma exchange.40,50–52 The administration of caplacizumab would be more convenient than plasma exchange for management during pregnancy, but the Food and Drug Administration (FDA) has not approved it for use during pregnancy and there are no reports of the use of caplacizumab in pregnancy yet.
Although hereditary TTP is rare, physicians should suspect it in all women whose initial episode of TTP occurs with their first pregnancy. A previous uncomplicated pregnancy is evidence against hereditary TTP.7 The French Reference Center for TMA documented the relative frequency of hereditary and acquired TTP during pregnancy. They reported that among 32 women who had their first episode of TTP during their first pregnancy (excluding postpartum occurrences), 10 (31%) had hereditary TTP.23 Hereditary TTP may be suspected if the mother has a history of severe jaundice when she was a newborn infant or if she had repeated episodes of thrombocytopenia and hemolysis during childhood. If the diagnosis of hereditary TTP is supported by a sibling who has been diagnosed with hereditary TTP, then plasma infusion without corticosteroids is appropriate, and simpler, treatment.
2.7 |. Anticipating a pregnancy
What is the risk for the occurrence of TTP with pregnancy? The answer is different for acquired and hereditary TTP. For women who have recovered from an episode of acquired TTP, the risk for recurrent TTP with a subsequent pregnancy is low. The Oklahoma Registry has documented the outcomes of 23 pregnancies in 13 women following their recovery from acquired TTP (Table 5).53 Only two (15%) of 13 women (9% of 23 pregnancies) had a relapse of TTP with a subsequent pregnancy; both occurred postpartum, at 9 and 29 days, following a pregnancy complicated by preeclampsia. One of these women had had two uncomplicated pregnancies preceding her initial episode of TTP. Among these 13 women, the risk for preeclampsia following TTP was higher than expected. Four (31%) women had preeclampsia in all six of their subsequent pregnancies. The increased occurrence of preeclampsia may be related to the similar placental pathology in both TTP and preeclampsia6 and the similar risk factors for preeclampsia and TTP. For both TTP and preeclampsia, the risk is greater with obesity and in Black women.54–56 Three of these four women were Black; one was obese.
TABLE 5.
Outcomes of 23 pregnancies in 13 women following recovery from acquired TTP
| Pregnancy outcome | Women | Pregnancy | Normal infants | Comment |
|---|---|---|---|---|
| Normal | 6a | 11 | 11 | None |
| SLE flare | 1 | 1 | 1 | At 23 weeks, controlled with rituximab |
| Preeclampsia | 2 | 3 | 3 | One woman had two pregnancies; TTP relapse occurred 29 days postpartum, second pregnancy |
| Preeclampsia with severe features | 2 | 3 | 3 | One woman had one pregnancy; TTP relapse occurred 9 days postpartum |
| 1st trimester fetal death | 1 | 2 | 0 | Gestational ages, 12 and 13 weeks |
| 2nd trimester fetal/infant death | 2a | 3 | 0 | Both women had a spontaneous pregnancy loss at 20 weeks. One also had IUGR at 26 weeks, infant delivered, died at 10 days |
Note: Data are from the Oklahoma TTP Registry, 2000–2018. Data for 10 of these women with 16 pregnancies have been previously reported.53 Relapse of TTP occurred in two (15%) of 13 women, two (9%) of 23 pregnancies.
Abbreviations: IUGR, intrauterine growth restriction; Preg, pregnancy; SLE, systemic lupus erythematosus.
One woman had spontaneous pregnancy loss at 20 weeks, then a normal pregnancy.
We do not discourage pregnancy following recovery from acquired TTP. We prescribe aspirin, 81 mg/day, throughout pregnancy and postpartum. This is effective for prevention of preeclampsia57 and may also decrease risk for thrombosis associated with TTP. Also, because ADAMTS13 deficiency during remission increases the risk for relapse58 and stroke,59,60 we measure the woman’s ADAMTS13 activity before becoming pregnant. If ADAMTS13 activity is < 20%, we recommend rituximab to increase ADAMTS13 activity prior to pregnancy. We follow these women throughout their pregnancies and postpartum, together with their obstetrician or maternal-fetal medicine specialist.
For women with hereditary TTP, the risk for a severe exacerbation of TTP with pregnancy is high.7 We also prescribe daily aspirin, 81 mg/day throughout pregnancy and postpartum.57 Our practice for plasma prophylaxis is to begin promptly when pregnancy is documented. Our experience with multiple patients supports this practice. Targeting a trough ADAMTS13 activity > 10% is ideal, however using the patient’s baseline (pre-pregnancy) platelet count as a surrogate is also a reasonable strategy when ADAMTS13 activity testing is not readily available.61 We describe one patient as an example. She had been asymptomatic since she had had severe jaundice at birth. She developed severe thrombocytopenia and systemic symptoms at 7 weeks’ gestation, which was the day after documentation of her pregnancy and before we had arranged for plasma infusion. Her subsequent course, managed with plasma prophylaxis, was uncomplicated. Our practice is to begin with a standard prophylactic regimen of 15 mL/kg of plasma (three 250 mL bags for a 50 kg woman) every 2 weeks. Our goal is to maintain the woman’s platelet count at her unique normal level.62 For example, if her platelet count is normally 250–300 × 103/μL, we increase the plasma amount and/or decrease the interval between infusions if her platelet count decreases to < 200 × 103/μL. Our experience is that increasing the frequency of plasma infusions to weekly is required, beginning at gestational age 26–33 weeks. We then continue plasma prophylaxis with the initial regimen for 6 weeks postpartum. With this regimen, we have avoided complications in three subsequent pregnancies in the woman described above and in multiple pregnancies in several other women.
The increased requirement for plasma later in pregnancy is related to the increased risk for thrombosis in the third trimester.63 The increasing dose of plasma as pregnancy progresses is important because recent evidence suggests that a regimen of 10–15 mL/kg every 2 weeks does not decrease the frequency acute episodes of TTP in patients with hereditary TTP.64
2.8 |. A patient’s story
A patient’s clinical presentation is rarely as straightforward as described in the scientific literature and multiple etiologic diagnoses may coexist. This was the case of one of our patients who was 5 days postpartum following an uncomplicated pregnancy. She developed severe headaches and then had a grand mal seizure. Her blood pressure was 193/117 mm Hg. Her platelet count was 75 × 103/μL. The diagnosis of eclampsia was clear. Four days later her platelet count was 19 × 103/μL; her ADAMTS13 activity was < 3%. She also had TTP and we treated her with plasma exchange, corticosteroids and rituximab. Then her serum creatinine concentration increased to 6.1 mg/dL and dialysis was required. Neither PE/HELLP nor TTP cause severe AKI. Can both PE/HELLP and TTP together cause AKI? Did she also have C-TMA? Should we have treated her with eculizumab? Then she developed sudden, severe pulmonary edema. Her left ventricular ejection fraction was 25%. She had peripartum cardiomyopathy. Then she developed pancreatitis and sepsis, and she died.11 This woman’s tragic illness emphasizes how pregnancy-related disorders can rapidly become extremely complex. Her story illustrates the importance of multidisciplinary care.
3 |. CONCLUSION
Management of severe complications of pregnancy can be extremely complex. Our experience emphasizes the importance of a close collaboration of the maternal-fetal medicine specialist and the hematologist. Collaboration with nephrology and other specialists may also be critically important. Complications of pregnancy can be sudden and severe. Both mother and fetus are at risk. Physicians must make management decisions rapidly. Ideal management requires an established maternal-fetal medicine/hematology collaboration. This type of close collaboration simplifies complex care and improves patient outcomes.
At Froedtert/Medical College of Wisconsin, the combined MFM/Hematology clinic was created in 2018 by a MFM specialist with interest and expertise in hematologic disorders and a hematologist dedicated to caring for patients with non-malignant hematologist conditions. The goal was to address the specific needs of patients with pre-existing or new hematologic disorders that impact the course of pregnancy and the post-partum period. A year after, the participation of obstetric anesthesiology was added. In this monthly clinic, patients receive care for their high-risk pregnancy including prenatal care, consultation with the MFM/Heme team, ultrasound studies, laboratory studies and genetic counseling within the MFM clinic space. Patients are seen by the MFM specialist and the hematologist at the same time, allowing for all aspects of care to be addressed and coordinated in the same visit. This collaborative practice creates a space that facilitates clinical decision making, patient education and communication between members of the treatment team. It has greatly facilitated education for trainees and been the starting point for future research projects. The success of this clinic not only has lead to increased patient satisfaction and more coordinated care, but it has become an exciting and rewarding part of our clinical practice. We encourage anyone who frequently co-manages patients with other medical or surgical specialists, to consider establishing integrated and collaborative care through the creation of combined clinics. This combined practice has certainly elevated the care of hematologically complex pregnant women in our region and made some of the clinical conundrums mentioned in this manuscript easier to navigate.
CONFLICT OF INTEREST
Juliana Perez Botero, Jessica Reese, James George and Jennifer McIntosh have no conflicts of interest to report. Dr. Jennifer McIntosh receives funding through the NHLBI 1K08HL150340-01 grant.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Gestational Hypertension and Preeclampsia. ACOG Practice Bulletin No. 202. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;133:e1–e25. [Google Scholar]
- 2.Joueidi Y, Peoc’h K, Le Louis M, et al. Maternal and neonatal outcomes and prognostic factors in acute fatty liverof pregnancy. Eur J Obstet Gynecol Reprod Biol. 2020;252:198–205. [DOI] [PubMed] [Google Scholar]
- 3.Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Thrombotic thrombocytopenic purpura: diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv. 2017;1:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hildebrand AM, Liu K, Shariff SZ, et al. Characteristics and outcomes of AKI treated with dialysis during pregnancy and the postpartum period. J Am Soc Nephrol. 2015;26:3085–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ernst LM. Maternal vascular malperfusion of the placental bed. APMIS. 2018;126:551–560. [DOI] [PubMed] [Google Scholar]
- 6.Avery EJ, Kenney SP, Byers BD, et al. Thrombotic thrombocytopenic purpura masquerading as preeclampsia with severe features at 13 weeks’ gestation. Am J Hematol. 2020;95:1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasht R, Borogovac A, George JN. Frequency and severity of pregnancy complications in women with hereditary thrombotic thrombocytopenic purpura. Am J Hematol. 2020;95:E316–E318. [DOI] [PubMed] [Google Scholar]
- 8.Reese JA, Peck JD, Deschamps DR, et al. Platelet counts during pregnancy. N Eng J Med. 2018;379:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reese JA, Peck JD, Yu Z, et al. Platelet sequestration and consumption in the placental intervillous space contribute to lower platelet counts during pregnancy. Am J Hematol. 2019;94:E8–E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong A, Chau CT, Pan D, Ogunyemi DA. Clinical morbidities, trends, and demographics of eclampsia: a populatioin-based study. Am J Obstet Gynecol. 2013;209:229 e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayanambakkam A, Owens KC, McIntosh JJ, Nester CM, George JN. A postpartum perfect storm. Amer J Hematol. 2017;92:1105–1110. [DOI] [PubMed] [Google Scholar]
- 12.Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 2004;103:981–991. [DOI] [PubMed] [Google Scholar]
- 13.Conti-Ramsden FI, Nathan HL, De Greeff A, et al. Pregnancy-related acute kidney injury in preeclampsia: risk factors and renal outcomes. Hypertension. 2019;74:1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute dialysis quality initiative w. acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care. 2004;8:R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deford CC, Reese JA, Schwartz LH, et al. Multiple major morbidities and increased mortality during long-term follow-up after recovery from thrombotic thrombocytopenic purpura. Blood. 2013;122:2023–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borogovac A, Tarasco E, Kremer Hovinga JA, George JN. Hypertension in patients with hereditary TTP. eJHaem. 2020;1:342–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George JN. The remarkable diversity of thrombotic thrombocytopenic purpura. Blood Adv. 2018;2:1510–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George JN, Nester CM, McIntosh JJ. Syndromes of thrombotic microangiopathy associated with pregnancy. Hematology Am Soc Hematol Educ Program. 2015;2015:644–648. [DOI] [PubMed] [Google Scholar]
- 20.Page EE, Little DJ, Vesely SK, George JN. Quinine-induced thrombotic microangiopathy: a report of 19 patients. Am J Kid Dis. 2017;70: 686–695. [DOI] [PubMed] [Google Scholar]
- 21.Kremer Hovinga JA, George JN. Hereditary thrombotic thrombocytopenic purpura. N Eng J Med. 2019;381:1653–1662. [DOI] [PubMed] [Google Scholar]
- 22.Fujimura Y, Matsumoto M, Isonishi A, et al. Natural history of Upshaw-Schulman syndrome based on ADAMTS13 gene analysis in Japan. J Thromb Haemost. 2011;9(Suppl 1):283–301. [DOI] [PubMed] [Google Scholar]
- 23.Moatti-Cohen M, Garrec C, Wolf M, et al. French reference Center for Thrombotic Microangiopathies. Unexpected frequency of Upshaw-Schulman syndrome in pregnancy-onset thrombotic thrombocytopenic purpura. Blood. 2012;119:5888–5897. [DOI] [PubMed] [Google Scholar]
- 24.Brain MC, Dacie JV, Hourihane OB. Microangiopathic hemolytic anemia: the possible role of vascular lesions in pathogenesis. Br J Haematol. 1962;8:358–374. [DOI] [PubMed] [Google Scholar]
- 25.van den Born B-JH, van der Hoeven NV, Groot E, et al. Association between thrombotic microantiopathy and reduced ADAMTS13 activity in malignant hypertension. Hypertension. 2008;51:862–866. [DOI] [PubMed] [Google Scholar]
- 26.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Eng J Med. 2014;371:654–666. [DOI] [PubMed] [Google Scholar]
- 27.Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Eng J Med. 2013;368:2169–2181. [DOI] [PubMed] [Google Scholar]
- 28.Fakhouri F, Roumenina L, Provot F, et al. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J Am Soc Nephrol. 2010;21:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018;378:2010–2021. [DOI] [PubMed] [Google Scholar]
- 30.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an updata of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 31.Anaya J-M. The diagnosis and clinical significance of polyautoimmunity. Autoimmun Rev. 2014;13:423–436. [DOI] [PubMed] [Google Scholar]
- 32.Hassan A, Iqbal M, George JN. Additional autoimmune disorders in patients with acquired autoimmune thrombotic thrombocytopenic purpura. Am J Hematol. 2019;94:E172–E173. [DOI] [PubMed] [Google Scholar]
- 33.Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systematic review of published reports. Blood. 2015;125:616–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleem R, Reese JA, George JN. Drug-induced thrombotic microangiopathy: an updated systematic review, 2014–2018. Am J Hematol. 2018;93:E241–E243. [DOI] [PubMed] [Google Scholar]
- 35.Morton JM, George JN. Microangiopathic hemolytic anemia and thrombocytopenia in patients with cancer. J Oncol Pract. 2016;12: 523–530. [DOI] [PubMed] [Google Scholar]
- 36.George JN, Morton JM, Liles NW, Nester CM. After the party’s over. NEJM. 2017;371:74–80. [DOI] [PubMed] [Google Scholar]
- 37.Liles NW, Page EE, Liles AL, Vesely SK, Raskob GE, George JN. Diversity and severity of adverse reactions to quinine: a systematic review. Am J Hematol. 2016;91:461–466. [DOI] [PubMed] [Google Scholar]
- 38.Chernysh IN, Nagaswami C, Kosolapova S, et al. The distinctive structure and composition of arterial and venous thrombi and pulmonary emboli. Sci Rep. 2020;10:5112. 10.1038/s41598-020-59526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. [DOI] [PubMed] [Google Scholar]
- 40.Chander DP, Loch MM, Cataland SR, George JN. Caplacizumab therapy without plasma exchange for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;381:92–94. [DOI] [PubMed] [Google Scholar]
- 41.Neunert CE, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3:3829–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bendapudi PK, Hurwitz S, Fry A, et al. Derivation and extrenal validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017; 3026:1–8. [DOI] [PubMed] [Google Scholar]
- 43.Coppo P, Cuker A, George JN. Thrombotic thrombocytopenic purpura: toward targeted therapy and precision medicine. Res Pract Thromb Haemost. 2018;3:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18:2496–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pentsuk N, van der Laan JW. An interspecies comparison of placental antibody transfer: new insights into developmental toxicity testing of monoclonal antibodies. Birth Defects Res. 2009;85:328–344. [DOI] [PubMed] [Google Scholar]
- 46.Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania: estimates obtained using hospitalization data. Arthritis Rheum. 2007; 56:2092–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volker LA, Kaufeld J, Miesbach W, et al. Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura. Blood Adv. 2020;4:3085–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coppo P, Bubenheim M, Azoulay E, et al. A regimen with caplacizumab, immunosuppression and plasma exchange prevents unfavorable outcomes in immune-mediated TTP. Blood. 2021;137: 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dutt T, Shaw RJ, Stubbs M, et al. Real-world evidence of caplacizumab in the management of TTP. Blood. 2021;137:1731–1740. [DOI] [PubMed] [Google Scholar]
- 50.Sukumar S, Cataland SR, George JN. Shared decision making, thrombotic thrombocytopenic purpura, and caplacizumab. Am J Hematol. 2020;95:E76–E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Irani MS, Sanchez F, Friedman KD. Caplacizumab for treatment of thrombotic thrombocytopenic purpura in a patient with anaphylaxis to fresh-frozen plasma. Transfusion. 2020;60:1666–1668. [DOI] [PubMed] [Google Scholar]
- 52.Volker LA, Brinkkoetter PT, Knobl P, et al. Treatment of acquired thrombotic thrombocytopenic purpura without plasma exchange in selected patients under caplacizumab. J Thromb Haemost. 2020;18: 3061–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Y, McIntosh JJ, Reese JA, et al. Pregnancy outcomes following recovery from acquired thrombotic thrombocytopenic purpura. Blood. 2014;123:1674–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.George JN. Thrombotic thrombocytopenic purpura: long-term outcomes following recovery. Hematology Am Soc Hematol Educ Program. 2018;2018:548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203–212. [DOI] [PubMed] [Google Scholar]
- 56.Johnson JD, Louis JM. Does race or ethnicity play a role in the origin, pathophysiology, and outcomes of preeclampsia? An expert review of the literature. Am J Obstet Gynecol. 2020. 10.1016/j.ajog.2020.07.038. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 57.Committee on Obstetric Practice SfM-FM. Low-dose aspirin use during pregnancy, ACOG Committee opinion. Obstet Gynecol. 2018;132: e44–e52. [DOI] [PubMed] [Google Scholar]
- 58.Jestin M, Benhamou Y, Schelpe A-S, et al. Preemptive rituximab prevents long-term relapses in immune-mediated thrombotic thrombocytopenic purpura. Blood. 2018;132:2143–2153. [DOI] [PubMed] [Google Scholar]
- 59.Prodan CI, Vijayvarjiya P, Feisal JK, Khawandanah MO, Jiang Y, George JN. Embolic stroke of undermined source in a young woman. Am J Hematol. 2019;94:1044–1048. [DOI] [PubMed] [Google Scholar]
- 60.Upreti H, Kasmani J, Dane K, et al. Reduced ADAMTS13 activity during TTP remission is associated with stroke in TTP survivors. Blood. 2019;134:1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Epperla N, Hemauer K, Friedman KD, George JN, Foy P. Congenital thrombotic thrombocytopenic purpura related to a novel mutation in ADAMTS13 gene and management during pregnancy. Am J Hematol. 2016;91:644–646. [DOI] [PubMed] [Google Scholar]
- 62.Buckley MF, James JW, Brown DE, et al. A novel approach to the assessment of variations in the human platelet count. Thromb Haemost. 2000;83:480–484. [PubMed] [Google Scholar]
- 63.James AH. Pregnancy-associated thrombosis. Hematology Am Soc Hematol Educ Program. 2009;2009:277–285. [DOI] [PubMed] [Google Scholar]
- 64.Tarasco E, Butikofer L, Friedman KD, et al. Annual incidence and severity of acute episodes in hereditary thrombotic thrombocytopenic purpura. 2021;137(25):3563–3575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


