Abstract
Prior studies have reported increased cortical excitability in people with Alzheimer’s disease (AD), but findings have been inconsistent, and how excitability relates to dementia severity remains incompletely understood. The objective of this study was to investigate the association between a transcranial magnetic stimulation (TMS) measure of motor cortical excitability and measures of cognition in AD. A retrospective cross-sectional analysis tested the relationship between resting motor threshold (RMT) and the Alzheimer’s Disease Assessment Scale - Cognitive Subscale (ADAS-Cog) across two independent samples of AD participants (a discovery cohort, n=22 and a larger validation cohort, n=129) and a control cohort of cognitively normal adults (n=26). RMT was correlated with ADAS-Cog in the discovery-AD cohort (n=22, β=−.70, p<0.001) but not in the control cohort (n=26, β=−0.13, p=0.513). This relationship was confirmed in the validation-AD cohort (n=129, β=−.35, p <0.001). RMT can be a useful neurophysiological marker of progressive global cognitive dysfunction in AD. Future translational research should focus on the potential of RMT to predict and track individual pathophysiological trajectories of aging.
Keywords: Transcranial magnetic stimulation, Cortical excitability, Hyperexcitability, Resting motor threshold, Cognitive dysfunction, Dementia, Neurophysiological marker, Alzheimer’s Disease Assessment Scale - Cognitive Subscale
1. Introduction
Dementia due to Alzheimer’s Disease (AD) affects 47 million people worldwide (Alzheimer’s Disease International (ADI), London, 2016). Cognitive decline in AD is preceded by a series of pathological changes, including amyloid-beta (Aβ) plaque deposition, tau accumulation, hypometabolism, and hippocampal and cortical atrophy (Jack and Holtzman, 2013). AD is also associated with neurophysiologic changes, including shifts in ongoing brain oscillations from higher to lower frequencies (Benwell et al., 2020), breakdown of network integrity (Palop and Mucke, 2016) that can be captured by EEG connectivity (Smailovic et al., 2019), alterations in synaptic plasticity (Brem et al., 2020; Di Lorenzo et al., 2018, 2016; Li and Selkoe, 2020), and loss of inhibitory interneurons (Verret et al., 2012) with transitions in cortical excitability from inhibition to facilitation (Vossel et al., 2013). Progressive increases in excitability in AD may be a consequence of neurotoxicity (Trebbastoni et al., 2016), inflammatory cascades (Newcombe et al., 2018), or a potentiating factor that can further accelerate neurodegeneration (Palop and Mucke, 2010). In any case, a progressive increase in excitability could act as a prognostic marker for AD severity.
Transcranial magnetic stimulation (TMS) is a non-invasive tool used for assessing the neurophysiology of the human brain in vivo (Rossi et al., 2009). The TMS resting motor threshold (RMT)–the lowest intensity needed to elicit corticospinal responses when TMS is applied to the contralateral motor cortex–provides a reliable index of motor cortical excitability, with lower RMT values representing greater motor cortical excitability (Cavaleri et al., 2017). TMS measures such as RMT reflect a combination of local and global (i.e., brain-wide) neurophysiologic properties and can be a sensitive measure of local neurocircuitry changes not captured by magnetic resonance imaging (MRI) and positron emission tomography (PET) (Kobayashi and Pascual-Leone, 2003). TMS evidence for hyperexcitability in AD includes lower RMT than similarly aged cognitively normal controls (Ferreri et al., 2003) and disrupted excitation-inhibition balance from paired-pulse TMS measures (Freitas, 2011). Specifically, a meta-analysis of TMS studies found that motor cortical excitability measures such as short-latency afferent inhibition (SAI), short-interval intracortical inhibition (SICI), long-interval intracortical inhibition (LICI), and RMT differ significantly between AD and age-matched cognitively intact healthy controls (Mimura et al., 2021). Altered cortical excitability is also present in earlier clinical stages of AD: RMT and SAI were also significantly reduced in mild cognitive impairment (MCI) compared to controls (Mimura et al., 2021). RMT, SAI, and intermittent theta-burst stimulation (iTBS)-measured long-term potentiation (LTP) are known to accurately discriminate between AD and controls (Motta et al., 2018). Limited but growing TMS-EEG literature shows hyperexcitability of sensory-motor cortex in mild AD, mirroring findings from TMS-EMG literature (Ferreri et al., 2016). In non-motor areas, TMS-EEG evoked P30 amplitude in the superior parietal cortex showing a correlation with disease severity (Bagattini et al., 2019). However, TMS and TMS-EEG findings have varied across studies due to differences in methodology, confounding effects of individual differences in the scalp-to-cortex distance (List et al., 2013) and cortical atrophy, and heterogeneity of disease severity (see (Freitas, 2011) for review).
It is not entirely clear why motor cortex excitability, assayed by RMT, would be related to cognition in AD, although there are several possible explanations. RMT measured cortical hyperexcitability in progressive AD could be due to decreased intercortical inhibition due to synapse and cellular loss in associative cortical and motor areas (Alagona et al., 2001; Pennisi et al., 2002). It could also be due to altered cellular energy metabolism leading to the decreased membrane potential of the pyramidal neurons and other neurons in the intracortical excitatory circuits that are recorded by RMT (Khedr et al., 2011; Pepin et al., 1999). Hence, these mechanisms could lead RMT to be associated with global cognitive dysfunction. Consistent with this hypothesis, lower RMT has been associated with worse cognitive function in AD studies (Khedr et al., 2011; Pennisi et al., 2002), but the finding has lacked replication (Wang et al., 2016) and the specificity of the relationship with different cognitive domains remains untested. A rigorous investigation of the relationship between RMT and cognitive dysfunction has the potential to advance our understanding of how motor cortical excitability is linked to clinical disease severity in AD and promote RMT as a translatable target for interventions aimed at restoring cerebral function.
This study investigated the relationship between RMT and cognitive dysfunction in two distinct cohorts of participants with mild-to-moderate AD using cross-sectional retrospective analysis. The discovery cohort was a well-characterized group of patients with mild AD with comprehensive neuropsychological testing, structural magnetic resonance imaging (MRI), and neuronavigated TMS measures, accompanied by comparative data from a control cohort of cognitively normal (CN) older participants. The validation cohort was a distinct multi-center cohort of mild-to-moderate AD participants enrolled in a TMS clinical trial (Sabbagh et al., 2019), with only basic pre-intervention neuropsychological testing and demographic data available for analysis. We hypothesized that RMT would be negatively correlated with cognitive dysfunction in AD (i.e., higher excitability associated with greater cognitive dysfunction). Our first aim was to test the association between RMT and measures of global cognitive dysfunction in AD and CN participants and to test the influence of potential confounding variables. Our second aim was to replicate the overall relationship between RMT and measures of cognitive dysfunction in a larger independent validation cohort of AD participants. Finally, our exploratory post hoc aim tested whether the relationship between RMT and global cognitive dysfunction was driven by memory or strategic thinking impairments.
2. Methods
2.1. Discovery and control cohorts
2.1.1. Participants
The discovery and control cohorts included 22 AD and 26 CN adults, respectively, who participated in research conducted at the Berenson-Allen Center for Noninvasive Brain Stimulation at Beth Israel Deaconess Medical Center between 2012 and 2015. Details regarding the recruitment procedure, research integrity, and participant inclusion have been previously published (Brem et al., 2020; Fried et al., 2017). All participants underwent comprehensive neuropsychological testing, neurological examination, and medical history evaluation. The AD participants had a clinical diagnosis of mild AD, with a Clinical Dementia Rating Scale (CDR)=1.0, and Mini-Mental State Examination (MMSE) scores between 18 and 23. The CN participants were cognitively intact with MMSE scores between 27 and 30, a normal neurologic exam, and denied a history of neuropsychiatric disorders or subjective cognitive complaints. Exclusion criteria were severe agitation, significant medical or psychiatric comorbidity, presence of any other probable forms of dementia, use of psychoactive medications, or anatomical MRI abnormality other than atrophy. For a subset of participants (nAD=12, nCN=21), genotypic testing was conducted to assess Apolipoprotein E (APOE) polymorphisms.
2.1.2. Neuropsychological testing
Comprehensive neuropsychological testing was administered by a trained psychometrist. Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) (“A new rating scale for Alzheimer’s disease,” 1984) was used as the primary outcome measure, given its sensitivity as a measure of global cognitive impairment AD (Balsis et al., 2015). Secondary measures included MMSE (Folstein et al., 1975), composite scores reflecting memory (composite memory score; CMS) and strategic thinking (composite strategic thinking score; CSTS), assessing attention, working memory, set-shifting, verbal production, strategy allocation, and psychomotor processing speed). The composite scores were created using assessments from the National Alzheimer’s Coordinating Center – Uniform Data Set battery (Morris et al., 2006), following an approach from the Alzheimer’s Disease Neuroimaging Initiative (for the Alzheimer’s Disease Neuroimaging Initiative et al., 2012a, 2012b) and used in prior investigations by our group into the relationship of cognition to cortical atrophy (Buss et al., 2018), cortical plasticity (Buss et al., 2020), and resting-state EEG oscillatory power (Benwell et al., 2020) in early-AD participants. Z-scores were calculated for each neuropsychological measure by subtracting an individual’s score from published normative mean and standard deviation scores for each test (Besser et al., 2018; Gale et al., 2007; Graham et al., 2004). Sub-scores used from the Trail Making Tests (TMT) and ADAS-Cog were inverted so that lower scores reflected worse performance across all tests. The CMS was calculated by averaging z-scores for ADAS-Cog Immediate Recall, ADAS-Cog Delayed Recognition, Rey Auditory Verbal Learning Test (RAVLT) (Schmidt, 1996) Delayed Recall, RAVLT Delayed Recognition, Logical Memory Story-A (LMS) Immediate Recall, and LMS Delayed Recall. The CSTS was calculated by averaging z-scores for TMT-A and B, Digit Span Backward, Semantic Fluency, and Digit Symbol Substitution Test. Additional assessments included the Geriatric Depression Scale (GDS) (Yesavage and Sheikh, 1986), Wechsler-Test of Adult Reading (W-TAR) (Holdnack, 2001), and Activities of Daily Living (ADL) (Kreutzer et al., 2011).
2.1.3. TMS-RMT measures
TMS was applied using a monophasic figure-of-eight focal coil attached to a Nexstim stimulator (Nexstim Inc, Finland). The coil was placed tangentially on the scalp surface and the Navigated Brain Stimulation system (Nexstim Inc., Finland) was used to individually target stimulation in the left hemisphere motor cortex using each participant’s high-resolution T1-MRI image. Motor-evoked potentials (MEPs) were recorded from the right first dorsal interosseous (FDI) muscle using surface electrodes and electromyogram (EMG). The motor hotspot was determined to be the cortical site where MEPs showed higher amplitude in FDI than in either of two reference muscles (abductor digiti minimi and abductor pollicis brevis). Following the guidelines of the International Federation for Clinical Neurophysiology (IFCN), cortical RMT was defined as the minimum stimulation intensity measured as percent maximum stimulator output (% MSO) that was required to elicit MEPs of peak-to-peak amplitude ≥50 μV in at least five of consecutive 10 trials (Rossini et al., 2015).
2.1.4. Neuroimaging measures
An anatomical T1-weighted MRI scan was obtained in all participants on a 3T scanner (GE Healthcare, Ltd., UK) employing a 3D spoiled gradient-echo sequence: 162 axial-oriented slices for whole-brain coverage; 240-mm isotropic field-of-view; 0.937-mm x 0.937-mm x 1-mm native resolution; flip angle = 15°; TE/TR ≥ 2.9/6.9 ms; duration ≥ 432 s. Freesurfer 6.0 (freely available and documented online at http://surfer.nmr.mgh.harvard.edu/) was used to obtain cortical thickness measurements of left M1 within a region-of-interest (ROI) (Desikan et al., 2006) consisting of the pre-central gyrus and rostral bank of the central sulcus, encompassing the entire motor strip.
T1-weighted MRI scans were employed during TMS for neuronavigation as well as to assess the scalp-to-cortex distance (SCD) to control for individual differences in the physical distance between the TMS coil and the underlying hand-knob area of the motor cortex that could influence RMT values. Following a previously reported approach (Brem et al., 2020), SCD was measured as the Euclidian distance between coordinates representing the center of the coil on the participant’s scalp and the coordinates representing the outer edge of the cortical surface along the plane perpendicular to the scalp tangent (i.e., orthogonal to the plane of the TMS coil) on each individual’s brain MRI using Brainsight™ (Rogue Research Inc., Canada).
2.1.5. Statistical analysis
All analyses were performed in R v3.4.3 (https://www.r-project.org) using publicly available and verified packages (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2018). Significance was determined as α ≤ 0.05 (two-tailed). In the multiple regression models, Holm-Bonferroni correction was used to correct for multiple comparisons.
Participant characteristics between the AD and CN cohorts were compared using Welch’s t-test and a chi-squared test for numerical and categorical variables, respectively. To test whether RMT was significantly different between the cohorts, values were entered into a fixed-effects linear regression model with the primary effect of diagnosis, with age, SCD, and ROI-based left motor cortex thickness as covariables. For post hoc comparison, AD participants were classified as mild (MMSE≥21) or moderate (MMSE < 21). The difference between mild and moderate AD subgroups was tested using the nonparametric Wilcoxon rank-sum test (numerical) or Boschloo’s exact test (categorical).
The primary hypothesis that ADAS-Cog is correlated to RMT was tested using a simple linear regression in AD and CN cohorts separately. Given the small sample size (n=22) of the AD discovery cohort, we chose to pursue a 2-step analysis to investigate the effect of covariables: the primary linear model was followed by separate multiple regression models to measure the effect of each covariate and assess whether any covariables acted as mediators or moderators.
For the multiple regression models, we assessed factors that might affect the association between ADAS-Cog and RMT. Age, SCD, gender, handedness, and ROI-based left motor cortex thickness were included since they are known to be associated with cognition and/or TMS measurements of cortical excitability (Danner et al., 2012; Sollmann et al., 2017). APOE polymorphisms were included since having one or more ε4 alleles increases the risk of developing AD (Petersen et al., 1996). Separate single covariate linear regression models were used to assess the individual influence of each covariate on the relationship between RMT and ADAS-Cog. In cases where the effect of the covariate was observed to be above-tolerance (i.e., change in %βRMT ≥10), the covariate was assessed for mediation using nonparametric bootstrapping by percentile method (number of simulations = 20 0 0) and for moderation and confounding using ordinary least squares regression models. Additionally, to test if the correlations were significantly different across AD and CN cohorts, individual correlation coefficients were Fisher Z-transformed and compared by Zou’s 95% Confidence Interval. The inclusion of 0 in Zou’s 95% CI is interpreted as a statistically non-significant difference between correlations (Diedenhofen and Musch, 2015).
For post-hoc exploratory analysis, we tested if cortical excitability was correlated with memory and strategic thinking by entering CMS and CSTS into separate simple linear regression models. Finally, given the ubiquity of MMSE as a measure of global cognition, we also tested its relationship with RMT using nonparametric correlation measured by Spearman’s Rho (a nonparametric test was chosen since MMSE is not considered a continuous measure).
2.2. Validation cohort
2.2.1. Participants and neuropsychological testing
One hundred and twenty-nine participants with the clinical diagnosis of mild-to-moderate AD aged 60-90 were enrolled from 2013–2016 in a multicenter randomized parallel interventional trial titled, “Effect of NeuroAD on the Cognitive Function of Alzheimer Patients,” for which our center was one of the study sites. Details and results of this trial have been recently published (Sabbagh et al., 2019). AD participants were diagnosed using DSM-IV criteria (Bell, 1994) and were required to have a global CDR of 1-2, ADAS-Cog >17, and an MMSE score of 18–26. The cohort for analysis consisted of all 129 AD participants with both RMT and ADAS-Cog scores available. There was no participant overlap between test and validation cohorts. Only pre-intervention data of AD participants from this trial was used for analysis, including participants’ age, gender, ADAS-Cog, and MMSE scores. For full trial details with inclusion and exclusion criteria, see Clinicaltrials.gov identifier: NCT01825330.
2.2.2. TMS measures
TMS procedures were standardized across all clinical trial sites. TMS was delivered using an air-cooled figure-of-eight coil connected to a biphasic Magstim Rapid2 system (Magstim, Inc., Eden Prairie, MN, USA). The motor hotspot was chosen using scalp landmarks and systematic search to identify the scalp location with the most robust and specific visible twitch of FDI. The RMT was defined as the lowest intensity to elicit a visible muscle twitch of the FDI or thenar in five of 10 trials.
2.2.3. Statistical Analysis
The relationship between RMT and ADAS-Cog was tested using simple linear regression in R as described above. Single covariate linear regression models were then used to assess potential confounding effects of age, gender, and medication status. Participants were classified as mild (MMSE≥21) or moderate (MMSE<21) and the subgroup difference was tested using Welch’s t-test (numerical) and a chi-squared test (categorical). We also tested the relationship of MMSE with RMT using nonparametric correlation measured by Spearman’s Rho.
2.3. Standard protocol approvals, registrations, and patient consents
This study is a retrospective analysis of data on human subjects collected and reported previously. The procedures were approved by an ethical standards committee on human experimentation (institutional or regional). For discovery and control cohorts, details regarding the recruitment procedure and research integrity have been previously published (Fried et al., 2017). For validation cohort, full trial details with the recruitment procedure, and research integrity, see Clinicaltrials.gov identifier: NCT01825330. The details and results of this trial have been recently published (Sabbagh et al., 2019).
2.4. Data availability
Data not provided in the article because of space limitations must be made available at the request of other qualified investigators for purposes of replicating procedures and results.
3. Results
3.1. Discovery and control cohorts
3.1.1. Demographic and baseline measures
Table 1A shows the demographics for the participants in the discovery and control cohorts. The AD cohort showed significantly lower performance on the ADAS-Cog, MMSE, CMS, and CSTS compared to the CN cohort (p<0.05). Compared to the CN cohort, participants in the AD cohort were slightly older, reported higher levels of depression, had lower ADLs, increased cortical thinning in the left motor cortex, and a greater proportion of APOE-ε4 allele (all p values<0.05). RMT, though lower in AD participants than CN, did not differ significantly. A linear model showed no significant impact of the diagnosis on RMT (F1,42=.89, p=0.352, partialη2=.02) after controlling for age, SCD, and left motor cortex thickness; however, the covariable SCD was a significant predictor of RMT in this model (β=.40, p=0.009). Further, RMT was statistically significantly lower in the moderate AD subgroup compared to the mild AD subgroup (W=87, p=0.005, rW=.61), suggesting the lack of a stronger difference between AD and CN groups may be due to the inclusion of milder AD patients. The subgroup differences are presented in Appendix Table B.1.
Table 1 A & B:
Participant characteristics for (A) discovery and control cohorts, (B) validation cohort
| (a) Discovery and Control Cohorts | ||||||
|---|---|---|---|---|---|---|
| Alzheimer’s Disease Participants (AD) Measures - Mean (SD) or no. (%) |
Cognitively Normal Participants (CN) |
Group Comparison# |
||||
| Df | t or χ2 test statistic | Cohen’s d or ϕ Coefficient |
p (uncorrected) | |||
| Demographic Measures | ||||||
| Total Number | 22 | 26 | - | - | - | |
| Females (%) | 13 (59.1) | 12 (46.2) | - | 0.37 | −0.13 | 0.546 |
| Right-hand-dominants (%)* | 18 (81.8) | 26 (100) | - | 1.94 | −0.29 | 0.164 |
| Age | 69.64 (7.36) | 61.92 (8.97) | 45.97 | −3.27 | −0.93 | 0.002 |
| Years of Education | 16.59 (3.72) | 15.81 (2.42) | 34.91 | −0.85 | −0.25 | 0.403 |
| GDS† | 2.36 (2.3) | 0.5 (0.91) | 26.49 | −3.57 | −1.10 | 0.001 |
| ADL‡ | 69.91 (6.77) | 75.81 (2.87) | 27.35 | 3.81 | 1.17 | 0.001 |
| Genotypic Measures | ||||||
| ApoE- ε4 ≥ 1 (%)* | 11 (91.7) | 4 (20.0) | - | 12.72 | 0.70 | 3.61E-04 |
| Neuropsychological testing Measures | ||||||
| ADAS-Cog§ | 23.27 (10.49) | 3.92 (1.82) | 24.49 | 14.27 | −2.68 | 2.29E-13 |
| MMSE¶ | 21.77 (2.43) | 29.46 (0.76) | 22.07 | −8.54 | 4.43 | 1.90E-08 |
| Composite Memory Score (CMS) (Z-values) | −2.03 (0.87) | 0.48 (0.60) | 36.36 | 11.41 | 3.41 | 1.41E-13 |
| Composite Strategic Thinking Score (CSCS) (Z-values)* | −2.13 (1.00) | 0.55 (0.70) | 36.72 | 10.55 | 3.15 | 1.14E-12 |
| Neuroanatomical Measures | ||||||
| Scalp-to-cortex Distance (SCD) (mm) | 16.12 (2.25) | 16.12 (3.06) | 45.21 | −0.005 | −0.001 | 0.996 |
| Cortical Thickness (CT) - Left | 2.25 (0.14) | 2.39 (0.10) | 36.51 | 3.90 | 1.17 | < 0.001 |
| Motor Cortex ROI (mm)* | ||||||
| TMS Measures | ||||||
| Resting Motor Threshold (RMT) -Monophasic (% MSO) | 60.77 (14.70) | 64.42 (14.21) | 44.15 | 0.87 | 0.25 | 0.389 |
| Cognitive Enhancing Medications in AD Discovery Cohort (n = 21) (%) | ||||||
| Donepezil | 6 (28.57) | - | - | - | - | - |
| Rivastigmine | 1 (4.76) | - | - | - | - | - |
| Donepezil & Memantine | 9 (42.86) | - | - | - | - | |
| Rivastigmine & Memantine | 2 (9.52) | - | - | - | - | - |
| No medications | 3 (14.29) | - | - | - | - | - |
| b) Validation Cohort | ||||||
| Measures - Mean (SD) or no. (%) | Alzheimer’s Disease Participants | |||||
| Total Number | 129 | |||||
| Females (%) | 59 (45.74) | |||||
| Age (yrs) | 76.77 (6.89) | |||||
| Cognitive enhancing medication (%) | 103 (79.84) | |||||
| ADAS-Cog§ | 23.83 (5.44) | |||||
| MMSE¶ | 21.55 (2.47) | |||||
| Resting Motor Threshold (RMT) - Biphasic (%MSO) | 75.12 (12.93) | |||||
A&B: Demographic and experimental measures for the (a) Discovery and Control Cohorts and (b) Validation Cohort. All the measures are presented as mean (SD) or absolute number (%).
Missing data: 1 AD participant for right-hand dominants; 10 AD and 6 CN participants for ApoE- ε4; 1 CN participant Cortical Thickness (CT) - Left Motor Cortex ROI; For CSTS (Z-scores) – 1 AD participant for TMT-A, 6 AD participants for TMT-B, 1 AD participant for DSST, 1 AD participant for medication.
Geriatric Depression Scale
Activities of Daily Living
Alzheimer’s Disease Assessment Scale - Cognitive Subscale
Mini-Mental State Examination
For the Discovery and Control cohorts (1a), the AD and CN groups were compared using a chi-squared test for categorical variables and Welch’s t-test for numerical variables.
3.1.2. Correlation between RMT and global cognitive dysfunction in AD
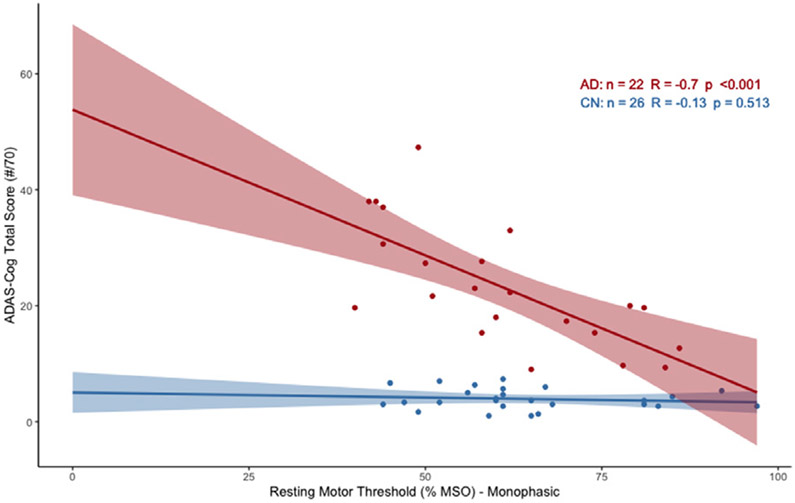
There was a significant relationship between lower RMT and worse performance on the ADAS-Cog for the AD cohort (β=−0.70, Adj.R2=.47, p<0.001), but not for the CN cohort (β=−0.13, Adj.R2=−0.02, p=0.513) (Fig. 1). The statistical comparison of the correlations revealed that the relationship between RMT and ADAS-Cog varied between the two cohorts (Zou’s 95% CI = [−1.004, −0.096]). The AD cohort showed a significant association between RMT and MMSE (Spearman’s ρ=.53, p=0.010), which was not present in the CN cohort (Spearman’s ρ=.19, p=0.352) (see Appendix Figure A.1). These results indicate that higher excitability is associated with greater cognitive dysfunction in AD, but not CN participants.
Fig. 1.
Relationship between RMT and ADAS-Cog (raw scores) in Discovery and Control Cohorts. Scatterplot portrays correlation as found by simple linear regression for AD (red) and CN (blue) participants (95% CI overlaid).
3.1.3. Effect of covariables on the relationship between RMT and ADAS-Cog in AD Discovery Cohort
Table 2A shows the results of multiple regression models testing the influence of each covariate on the relationship between RMT and ADAS-Cog in the AD discovery cohort. SCD and left motor cortex thickness showed an above-tolerance influence on the relationship between RMT and ADAS-Cog, with motor thickness weakening and SCD strengthening the relationship. Neither SCD (β=.33, p=0.130) nor left motor cortex thickness (β=.28, p=0.210) was significantly associated with RMT individually. Additionally, left motor cortex thickness was a significant covariate predictor for ADAS-Cog (β=−0.48, p=0.024), while SCD was not (β=.10, p=0.665). Neither variable showed statistically significant mediation effects (see Appendix Table B.2).
Table 2 A&B:
Multiple regression for the relationship between RMT and ADAS-Cog in AD participants in (A) discovery and (B) validation cohorts
| A) Discovery Cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Models with covariates* | df | β RMT | %βRMT† | Adj.R2model | partial R2covar‡ | pmodel | pRMT | pcovar | Corrected pmodel | Corrected pRMT | Corrected pcovar |
| RMT & Age | 2,19 | −0.73 | −4.29 | 0.46 | 0.03 | < 0.001 | < 0.001 | 0.446 | < 0.001 | < 0.001 | 1.000 |
| RMT & Gender | 2,19 | −0.67 | 4.29 | 0.51 | 0.11 | < 0.001 | < 0.001 | 0.140 | < 0.001 | < 0.001 | 0.700 |
| RMT & Hand-dominance | 2,18 | −0.7 | 0 | 0.43 | 0.00 | 0.001 | 0.001 | 0.960 | 0.002 | 0.003 | 1.000 |
| RMT & ApoE- ε4 | 2,9 | −0.68 | 2.86 | 0.34 | 0.31 | 0.022 | 0.022 | 0.924 | 0.022 | 0.022 | 1.000 |
| RMT & AD Medication Status | 2,18 | −0.69 | 1.43 | 0.48 | 0.06 | < 0.001 | < 0.001 | 0.301 | < 0.001 | < 0.001 | 1.000 |
| RMT & SCD | 2,19 | −0.83 | −18.57 | 0.58 | 0.25 | < 0.001 | < 0.001 | 0.022 | < 0.001 | < 0.001 | 0.154 |
| RMT & left motor cortex CT | 2,19 | −0.62 | 11.43 | 0.54 | 0.17 | < 0.001 | 0.001 | 0.060 | < 0.001 | 0.003 | 0.360 |
| B) Validation Cohort | |||||||||||
| RMT & Age | 2,125 | −0.35 | 0 | 0.13 | 0.02 | < 0.001 | < 0.001 | 0.095 | < 0.001 | < 0.001 | 0.190 |
| RMT & Gender | 2,126 | −0.35 | 0 | 0.11 | 0.00 | < 0.001 | < 0.001 | 0.829 | < 0.001 | < 0.001 | 0.829 |
| RMT & AD Medication Status | 2,126 | −0.36 | −2.86 | 0.13 | 0.03 | < 0.001 | < 0.001 | 0.048 | < 0.001 | < 0.001 | 0.144 |
Covariables were used in separate models to check their individual influence on the relationship.
%Δβ > 10 is the tolerance threshold for influence.
Partial R2covar measures the additional covariance explained by each factor.
3.1.4. Relationship between RMT and composite cognitive-domain scores
The exploratory analysis of the discovery and control cohorts revealed a significant correlation between RMT and CMS in the AD (R20=.61, p=0.002) and the CN (R24=.52, p=0.006) cohorts. By comparison, there was a moderate, but nonsignificant correlation between RMT and CSTS for the AD cohort (R20=.25, p=0.263), which was not present for the CN cohort (R24=−0.03, p=0.872). These results indicate that a nonspecific relationship between RMT and learning and memory might be driving RMT’s association with overall cognitive function.
3.2. Validation cohort
3.2.1. Demographic and baseline measures
Table 1B shows the demographics for the participants in the validation cohort.
3.2.2. Correlation between RMT and global cognitive dysfunction in AD
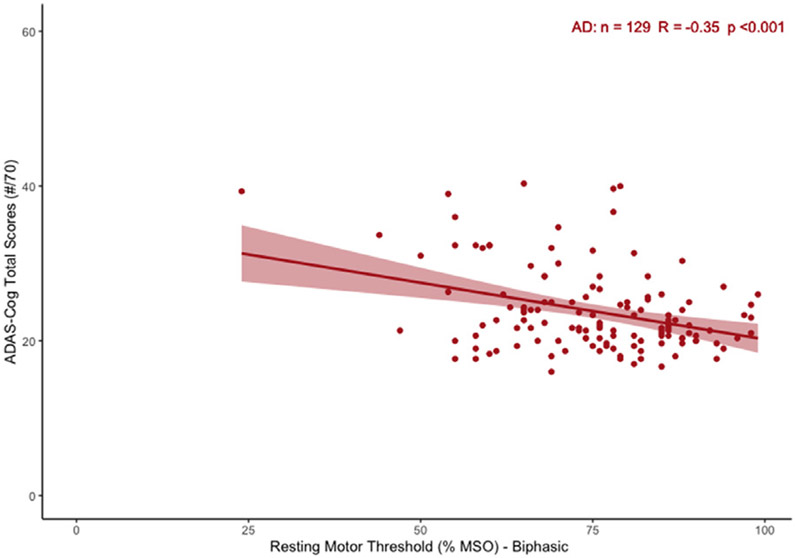
Fig. 2 shows that in the AD validation cohort, RMT was significantly associated with ADAS-Cog (β=−0.35, Adj.R2=.11, p<0.001). We also found a significant relationship between RMT and MMSE (Spearman’s ρ=.24, p=0.006) (see Appendix Figure A.2). Correspondingly, RMT was significantly lower in the moderate AD (MMSE<21) subgroup compared to the mild AD (MMSE≥21) subgroup (t91=2.72, p=0.008, Cohen’s d=.51). The subgroup differences are presented in Appendix Table B.1.
Fig. 2.
Relationship between RMT and ADAS-Cog (raw scores) in Validation Cohort. Scatter plot represents the correlation as found by simple linear regression (95% CI overlaid).
3.2.3. Effect of covariables on the relationship between RMT and ADAS-Cog
Table 2B shows multiple regression models for the validation cohort. None of the covariables had an above-tolerance influence on the relationship between RMT and ADAS-Cog.
4. Discussion
TMS can be used to assess cortical excitability and synaptic dysfunction in AD (Di Lazzaro, 2018). In this study, we tested the relationship between RMT and global cognition in a well-characterized cohort of participants with mild-to-moderate AD and validated the findings in an independent, larger cohort. Our results show that higher motor cortical excitability (lower RMT) is associated with greater global cognitive dysfunction (higher ADAS-Cog score) in AD. Additionally, RMT was significantly lower in the moderate AD subgroup compared to the mild AD subgroup, indicating hyperexcitability may worsen with disease progression.
Our results suggest that TMS measures of motor cortical excitability should be corrected for anatomical factors: SCD increased the negative correlation between RMT and ADAS-Cog while left-motor CT influenced the relationship in the opposite direction. Prior literature has highlighted the importance of accounting for SCD and other anatomical factors to interpret RMT (Stokes et al., 2007) since the strength of the magnetic field generated by TMS decays exponentially with distance (Deng et al., 2013). While the relationship between RMT and SCD was not statistically significant in our AD cohort, the direction of the effect was in line with prior literature (List et al., 2013). Additionally, controlling for SCD increased the strength of the relationship between RMT and cognition in the AD group, suggesting that anatomical factors should be included in the analysis of TMS-based measures since there is a risk that local atrophy could otherwise mask differences in motor cortical excitability in neurodegenerative disorders. On the other hand, accounting for CT within the motor strip weakened the observed relationship between RMT and ADAS-Cog. This may be due to the independent relationship of CT with ADAS-Cog since both are expected to worsen with disease progression. Indeed, we found that CT was an independent predictor of ADAS-Cog in AD in line with studies showing an association between cortical thinning and cognitive deterioration (for the Alzheimer’s Disease Neuroimaging Initiative et al., 2012c; Ossenkoppele et al., 2019). CT was not a significant predictor for RMT in AD, hence, did not act as a mediator for RMT’s relationship with ADAS-Cog.
We demonstrate that RMT is related to global cognition in AD across two distinct cohorts, clarifying prior literature. Most prior studies have used MMSE as a primary outcome measure (Hoeppner et al., 2012; Khedr et al., 2011; Olazarán et al., 2010; Pennisi et al., 2002; Wang et al., 2016), relied on smaller cohorts (Hoeppner et al., 2012; Olazarán et al., 2010; Pennisi et al., 2002), included heterogeneous disease stages (Khedr et al., 2011; Olazarán et al., 2010; Pennisi et al., 2002) or stimulated non-focally using a circular TMS coil (Olazarán et al., 2010; Pennisi et al., 2002). We found a large effect size (Adj.R2=.47) in our discovery cohort and medium effect size (Adj.R2=.11) in the validation cohort. The difference in effect size between the two cohorts is likely attributable to several key differences in TMS application. The discovery cohort utilized MRI-based neuro navigation and EMG, while the validation cohort relied on visual twitch observation for determination of the RMT and did not employ MRI guidance for TMS coil placement. In the analysis of data from the discovery cohort, we controlled for SCD. Furthermore, data in the validation cohort came from 10 different sites. Considering these differences in approach, the replication of the results across both cohorts supports the generalizability of our findings by indicating that the relationship between motor cortical excitability and dementia severity is not limited to a specific methodological approach (e.g., including use of neuronavigation, presence of EMG, or TMS pulse waveform, etc.). While age was neither a significant predictor of RMT or ADAS-Cog in either group nor did it modify the relationship between RMT and ADAS-Cog, we cannot rule out the possibility that the slightly older age of the Validation Cohort played a role in the smaller observed effect size.
Exploratory analyses showed that RMT was strongly related to learning and memory in both the AD discovery and CN control cohorts, but not strategic thinking. Overall, these findings demonstrate that increased motor cortical excitability is associated with more severe dementia in AD. Taken together, these findings highlight the need for future research to investigate the extent to which RMT and other TMS measures may offer valuable metrics of disease progression and indicators of risk of cognitive decline longitudinally. Our findings held true despite the differences between the discovery and validation cohorts for the age of AD participants, neuronavigation, EMG, and TMS pulse phase, strengthening their generalizability. As previously shown, RMT demonstrates very high intra-individual reliability and reproducibility (Fried et al., 2017), making it an attractive tool to follow changes in motor cortical excitability longitudinally.
Past evidence from meta-analysis suggests that RMT is significantly lowered in AD and MCI groups compared to healthy controls (Mimura et al., 2021). RMT has also been shown to accurately classify AD patients and controls (Motta et al., 2018). Here, we found that RMT is significantly correlated with cognitive dysfunction. The three findings: group-level differences, ability to classify AD vs. CN at individual-level, and correlation with cognitive dysfunction within AD, together make RMT an important candidate as a neurophysiological AD biomarker. Recent studies have investigated the classifier models built using SICI, LICI, and SAI to distinguish among various dementia types (Benussi et al., 2020). Including RMT in such models would further make a case towards its clinical utility.
Preclinical animal model studies have repeatedly shown a link between AD pathology and increased cortical excitability. Oligomeric Aβ can induce remodeling of inhibitory circuits and promotes epileptiform discharges (Palop et al., 2007). Tau deposits (Roberson et al., 2011) may further contribute to abnormally increased cortical excitability. The multiple factors leading to increased excitability likely interact non-linearly and may progress at different rates in different stages of AD. As such, TMS-based measures of excitability, such as RMT, show strong potential as markers to index and longitudinally follow motor cortical excitability changes in humans with AD. These measures can further our understanding of the neurophysiologic consequences of regional amyloid deposition, tau accumulation, and cortical atrophy, and may be useful as a prognostic marker or as a measure of target engagement in AD clinical trials.
Since our study investigated cortical excitability in the hand knob region, this study is not able to disentangle whether RMT in AD primarily reflects global cortical excitability or local excitability within motor circuits. Our finding of an association between RMT and memory function raises the possibility that RMT may reflect global cortical excitability in AD, which should be confirmed with future multimodal studies. For example, if RMT reflects global cortical excitability, it should be related to hippocampal hyperactivity seen on task-based fMRI (Dickerson et al., 2005; Zott et al., 2018) and to cortical excitability in frontal and parietal brain regions using TMS with electroencephalography (TMS-EEG) (Bagattini et al., 2019; Ferreri et al., 2016). Alternatively, RMT could simply reflect local motor cortex pathology in AD. Although the motor cortex is relatively preserved compared to other brain regions until late in disease pathogenesis, amyloid deposition is detectible in later pathological stages of AD (Grothe et al., 2017). If the association between RMT and disease severity is due to soluble amyloid concentrations in the motor cortex, lower RMT could act as a proxy for overall disease burden and therefore be only indirectly related to global cognition. This hypothesis is potentially supported by our finding of lower RMT in the moderate AD subgroup compared to the mild AD subgroup. If this hypothesis is supported by future research, it could suggest a role for TMS-EEG in non-motor brain regions as a future theragnostic marker in pre-symptomatic or prodromal AD. For example, if TMS-EEG is found to be sensitive to local soluble amyloid concentrations, it could be employed in future clinical trials aiming to modify abnormal cortical excitability related to amyloid deposition in parietal or frontal regions early in disease pathogenesis. Alternatively, increased motor cortical excitability could be mediated by frontal and premotor region atrophy, which could lead to disinhibition of motor regions. However, our finding that RMT was more strongly related to memory than strategic thinking makes this hypothesis less likely.
Our exploratory analyses showed an association between memory function and RMT in both the AD and CN participants. The finding in our control cohort is unexpected. While it is likely that our control cohort contains several individuals with pre-symptomatic AD (with 10%–30% expected to show Aβ positivity (Chetelat et al., 2013)), levels of Aβ and tau deposition in the motor cortex are very low in pre-symptomatic AD (Thal et al., 2002). As discussed above, RMT could be capturing a release phenomenon in the motor cortex related to a loss of inhibitory input from other brain regions, such as frontal and temporal regions, which are affected in the earliest stages of AD (Chételat et al., 2013). Another possibility could be that the RMT-measured motor cortical excitability may progress along a U-shaped curve, with the most abnormal excitability seen early- and late- in disease progression due to different mechanisms at each stage (initially related to oligomeric Aβ, later reflecting tau deposition, neuroinflammation, and neurodegeneration) (Menardi et al., 2018). In any scenario, these results highlight the importance of utilizing highly sensitive measures of memory function in cognitively normal adults, which may capture subtle cognitive decline not assessed by the ADAS-Cog, and of confirming the absence of AD biomarkers in cognitively normal individuals. Future longitudinal studies are needed to directly assess how cortical excitability changes over time in older adults, including those with and without evidence of Aβ deposition, and how those changes track with cognition in the aging brain.
This study has several limitations. First, for discovery and validation cohorts, the determination of AD was based on clinical diagnosis rather than Aβ PET or CSF measures (which were not available). Some prior studies have shown that clinical diagnosis of AD has an accuracy above 85 percent (Franklin et al., 2015), with alternative etiologies including vascular dementia, Lewy body dementia, or hippocampal sclerosis. Nonetheless, studying measures of cortical excitability in the context of well-characterized AD patients following the ATN framework (Jack et al., 2018) would be ideal.
Second, the absence of AD biomarker status in our control cohort is a limitation. This may have limited our ability to find a difference in RMT value between the discovery and control cohorts and could have impacted the interpretation of the relationship between RMT and memory scores for controls in the exploratory analysis. However, the likelihood of influence on the validity of our primary results is low, as misdiagnosis (e.g., including other/mixed dementia types in the AD cohort or including pre-symptomatic MCI in the control cohort) would have expectedly biased the data towards the null hypothesis. The robustness of our findings is further supported by replication of results in two independent cohorts of AD participants, despite differences between cohorts in demographics and the methodology of the TMS administration.
Third, we did not find a significant difference in RMT between AD and CN groups. This could be due to younger age among CN participants than AD. Age-dependent hypo-excitability exists is known in old healthy people compared to young healthy people (Bhandari et al., 2016). Hence, the inclusion of slightly younger CN participants combined with the presence of mild AD participants may have reduced the group-level differences in RMT between the two groups. Further, since the CN group was not characterized based on CDR or AD biomarkers, the nonsignificant RMT difference could be due to undetected preclinical AD in the CN group. Therefore, the inclusion of slightly younger CN participants combined with the presence of mild AD participants may have decreased our ability to detect group-level differences in RMT.
Fourth, we do not present test-retest measures of RMT in either the Discovery or Validation Cohorts. However, a prior study from our group (Fried et al., 2017) found RMT demonstrated high test-retest reliability in both AD and CN groups, which notably included many of the same participants in the current analysis.
Fifth, ADAS-Cog is not designed to measure cognition in cognitively normal/intact population has a limited sensitivity among CN participants. There is an evident ceiling effect for ADAS-Cog scores in the CN group that could result in a nonsignificant correlation. However, we also found a lack of correlation between MMSE and RMT in the CN group. Further, we included the CMS and CSTS in the post hoc analysis to provide measures that might be more sensitive in the CN group, which is exactly what we found with the CMS. Future studies could benefit from using sensitive outcomes like preclinical Alzheimer’s cognitive composite (PACC) that can capture subtle cognitive difficulties in ‘cognitively normal’ people (Papp et al., 2017). It can improve the validity of the comparison of RMT-cognition correlations between AD and CN groups.
Sixth, the sample size is small for AD discovery and control cohorts limiting the statistical power for comparisons, multiple regression, and mediation analyses.
Finally, our study focused on measures of excitability obtained from stimulating the primary motor cortex, which is thought to be involved relatively late in AD pathology (Jack et al., 2018). While this was appropriate in our dataset with substantial cognitive decline, future studies of cortical excitability in pre-symptomatic AD participants may require alternative assays of cortical excitability in non-motor brain regions, which show the earliest signs of Aβ and tau deposition. TMS-EEG can be used to measure the cortical response to stimulation in non-motor brain regions such as the frontal and parietal cortex involved in the earlier stage of pre-symptomatic AD.
5. Conclusions
We demonstrated that increased motor cortical excitability as measured by RMT is related to global cognitive dysfunction in participants with mild-to-moderate AD dementia. These noninvasive assessments of cortical excitability may be able to translate findings of increased cortical excitability seen in animal models into humans with AD. Potential applications include understanding the prognostic value of increased cortical excitability in AD, screening for novel therapeutic agents which normalized cortical excitability, selecting participants for clinical trials, and confirming target engagement of novel investigational treatments.
Supplementary Material
Acknowledgements
The authors thank Neuronix Ltd. for providing multicenter trial data, E. Seligson, S. Saxena, V. Chen (Beth Israel Deaconess Medical Center) for their assistance in data collection, A. Connor and J. Macone (Beth Israel Deaconess Medical Center) for assistance with evaluation of participant health and medical history, and Arianna Menardi (Beth Israel Deaconess Medical Center) for helpful discussions.
Funding
This study was primarily supported by grants from the National Institutes of Health (NIH; R21 NS082870, R21 AG051846) with support from the Harvard Catalyst ∣ The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). Siddhesh Zadey was supported by the Khorana Scholars Award from the Indo-US Science and Technology Forum (IUSSTF) and Kishore Vaigyanik Protsahan Yojana (KVPY) Fellowship from the Department of Science and Technology, Govt. of India. Dr. Buss was further supported by the Sidney R. Baer Jr. Foundation (01028951), the American Academy of Neurology (2016-0229), the Alzheimer’s Association (2019-AACSF-643094), and NeuroNEXT (U24NS107183). Dr. Pascual-Leone was also supported in part by the Sidney R. Baer Jr. Foundation and the NIH (R01HD069776, R01NS073601, R21 MH099196, R21 NS085491, R21 HD07616). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers, the National Institutes of Health, or the Sidney R. Baer Jr. Foundation.
Role of Funder/Sponsor
The sponsors did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations:
- AD
Alzheimer’s disease
- TMS
Transcranial magnetic stimulation
- RMT
Resting motor threshold
- ADAS-Cog
Alzheimer’s Disease Assessment Scale – Cognitive Subscale
- MMSE
Mini-Mental state examination
- CN
Cognitively normal (controls)
- CMS
Composite memory score
- CSTS
Composite strategic thinking score
- SCD
Scalp-to-cortex distance
Footnotes
Disclosure
Dr. Pascual-Leone serves on the scientific advisory boards for Starlab Neuroscience, Neuroelectrics, Neosync, NovaVision, and Cognito; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging. Dr. Buss serves as a consultant for Kinto Care. The authors declare no competing interests.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neurobiolaging.2021.06.007.
References
- A new rating scale for Alzheimer’s disease, 1984. In: American Journal of Psychiatry, 141, pp. 1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Alagona G, Bella R, Ferri R, Carnemolla A, Pappalardo A, Costanzo E, Pennisi G, 2001. Transcranial magnetic stimulation in Alzheimer disease: motor cortex excitability and cognitive severity. Neurosci Lett 314, 57–60. doi: 10.1016/S0304-3940(01)02288-1. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Disease International (ADI), London, 2016. World Alzheimer Report 2016. [Google Scholar]
- Bagattini C, Mutanen TP, Fracassi C, Manenti R, Cotelli M, Ilmoniemi RJ, Miniussi C, Bortoletto M, 2019. Predicting Alzheimer’s disease severity by means of TMS–EEG coregistration. Neurobiol Aging 80, 38–45. doi: 10.1016/j.neurobiolaging.2019.04.008. [DOI] [PubMed] [Google Scholar]
- Balsis S, Benge JF, Lowe DA, Geraci L, Doody RS, 2015. How do scores on the ADAS-Cog, MMSE, and CDR-SOB correspond? Clin Neuropsychol 29, 1002–1009. doi: 10.1080/13854046.2015.1119312. [DOI] [PubMed] [Google Scholar]
- Bell CC, 1994. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. JAMA 272, 828. doi: 10.1001/jama.1994.03520100096046. [DOI] [Google Scholar]
- Benussi A, Grassi M, Palluzzi F, Koch G, Di Lazzaro V, Nardone R, Cantoni V, Dell’Era V, Premi E, Martorana A, Lorenzo F, Bonnì S, Ranieri F, Capone F, Musumeci G, Cotelli MS, Padovani A, Borroni B, 2020. Classification accuracy of transcranial magnetic stimulation for the diagnosis of neurodegenerative dementias. Ann Neurol 87, 394–404. doi: 10.1002/ana.25677. [DOI] [PubMed] [Google Scholar]
- Benwell CSY, Davila-Pérez P, Fried PJ, Jones RN, Travison TG, Santarnecchi E, Pascual-Leone A, Shafi MM, 2020. EEG spectral power abnormalities and their relationship with cognitive dysfunction in patients with Alzheimer’s disease and type 2 diabetes. Neurobiol Aging 85, 83–95. doi: 10.1016/j.neurobiolaging.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser L, Kukull W, Knopman DS, Chui H, Galasko D, Weintraub S, Jicha G, Carlsson C, Burns J, Quinn J, Sweet RA, Rascovsky K, Teylan M, Beekly D, Thomas G, Bollenbeck M, Monsell S, Mock C, Zhou XH, Thomas N, Robichaud E, Dean M, Hubbard J, Jacka M, Schwabe-Fry K, Wu J, Phelps C, Morris JC, 2018. Version 3 of the national alzheimer’s coordinating center’s uniform data set. Alzheimer Dis Assoc Disord 1. doi: 10.1097/WAD.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari A, Radhu N, Farzan F, Mulsant BH, Rajji TK, Daskalakis ZJ, Blumberger DM, 2016. A meta-analysis of the effects of aging on motor cortex neurophysiology assessed by transcranial magnetic stimulation. Clin Neurophysiol 127, 2834–2845. doi: 10.1016/j.clinph.2016.05.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem A-K, Di Iorio R, Fried PJ, Oliveira-Maia AJ, Marra C, Profice P, Quaranta D, Schilberg L, Atkinson NJ, Seligson EE, Rossini PM, Pascual-Leone A, 2020. Corticomotor plasticity predicts clinical efficacy of combined neuromodulation and cognitive training in Alzheimer’s. Disease. Front. Aging Neurosci 12, 200. doi: 10.3389/fnagi.2020.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss SS, Padmanabhan J, Saxena S, Pascual-Leone A, Fried PJ, 2018. Atrophy in distributed networks predicts cognition in alzheimer’s disease and type 2 diabetes. J. Alzheimers Dis 65, 1301–1312. doi: 10.3233/JAD-180570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss SS, Press DZ, McDonald K, Kitchener E, O’Connor M, Donohoe K, Shafi MM, Pascual-Leone A, Fried PJ, 2020. LTP-like plasticity is impaired in amyloid-positive amnestic MCI but independent of PET-amyloid burden. Neurobiol Aging 96, 109–116. doi: 10.1016/j.neurobiolaging.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaleri R, Schabrun SM, Chipchase LS, 2017. The number of stimuli required to reliably assess corticomotor excitability and primary motor cortical representations using transcranial magnetic stimulation (TMS): a systematic review and meta-analysis. System Rev 6. doi: 10.1186/s13643-017-0440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chételat G, La Joie R, Villain N, Perrotin A, de La Sayette V, Eustache F, Vandenberghe R, 2013. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. Neuroimage Clin 2, 356–365. doi: 10.1016/j.nicl.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner N, Könönen M, Säisänen L, Laitinen R, Mervaala E, Julkunen P, 2012. Effect of individual anatomy on resting motor threshold – Computed electric field as a measure of cortical excitability. J Neurosci Methods 203, 298–304. doi: 10.1016/j.jneumeth.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Deng Z-D, Lisanby SH, Peterchev AV, 2013. Electric field depth–focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimulation 6, 1–13. doi: 10.1016/j.brs.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, 2018. Emergence of neurophysiological biomarkers of Alzheimer disease. J Neurol, Neurosurg Psychiatry 89, 1235. doi: 10.1136/jnnp-2018-318867, –1235. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo F, Ponzo V, Bonnì S, Motta C, Negrão Serra PC, Bozzali M, Caltagirone C, Martorana A, Koch G, 2016. Long-term potentiation-like cortical plasticity is disrupted in Alzheimer’s disease patients independently from age of onset: Cortical Plasticity in Alzheimer’s Disease. Ann Neurol 80, 202–210. doi: 10.1002/ana.24695. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo F, Ponzo V, Motta C, Bonnì S, Picazio S, Caltagirone C, Bozzali M, Martorana A, Koch G, 2018. Impaired spike timing dependent cortico-cortical plasticity in Alzheimer’s disease patients. J Alzheimer’s Dis 66, 983–991. doi: 10.3233/JAD-180503. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA, 2005. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedenhofen B, Musch J, 2015. cocor: A Comprehensive solution for the statistical comparison of correlations. PLoS One 10, e0121945. doi: 10.1371/journal.pone.0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri F, Pauri F, Pasqualetti P, Fini R, Dal Forno G, Rossini PM, 2003. Motor cortex excitability in Alzheimer’s disease: a transcranial magnetic stimulation study. Ann Neurol 53, 102–108. doi: 10.1002/ana.10416. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Vecchio F, Vollero L, Guerra A, Petrichella S, Ponzo D, Määtta S, Mervaala E, Könönen M, Ursini F, Pasqualetti P, Iannello G, Rossini PM, Di Lazzaro V, 2016. Sensorimotor cortex excitability and connectivity in Alzheimer’s disease: A TMS-EEG Co-registration study: sensorimotor cortex excitability and connectivity in AD. Hum Brain Mapp 37, 2083–2096. doi: 10.1002/hbm.23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. Mini-mental state. J Psychiatr Res 12, 189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas Dfor the Alzheimer’s Disease Neuroimaging Initiative, 2012a. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imag Behav 6, 502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Curtis SM, Mungas D, Crane PKfor the Alzheimer’s Disease Neuroimaging Initiative, 2012b. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imag Behav 6, 517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Manly JJ, Pa J, Johnson JK, Park LQ, Mitchell MB, Melrose RJ, Inouye SK, McLaren DGfor the Alzheimer’s Disease Neuroimaging Initiative, 2012c. Cortical signatures of cognition and their relationship to Alzheimer’s disease. Brain Imag Behav 6, 584–598. doi: 10.1007/s11682-012-9180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin EE, Perrin RJ, Vincent B, Baxter M, Morris JC, Cairns NJ Alzheimer’s Disease Neuroimaging Initiative, 2015. Brain collection, standardized neuropathologic assessment, and comorbidity in Alzheimer’s Disease Neuroimaging Initiative 2 participants. Alzheimer’s Dementia 11, 815–822. doi: 10.1016/j.jalz.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C, 2011. Noninvasive brain stimulation in Alzheimer’s disease: Systematic review and perspectives for the future. Exp Gerontol 46 (8), 611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PJ, Jannati A, Davila-Pérez P, Pascual-Leone A, 2017. Reproducibility of single-pulse, paired-pulse, and intermittent theta-burst TMS measures in healthy aging, type-2 diabetes, and Alzheimer’s disease. Front Aging Neurosci 9. doi: 10.3389/fnagi.2017.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Baxter L, Connor DJ, Herring A, Comer J, 2007. Sex differences on the rey auditory verbal learning test and the brief visuospatial memory test–revised in the elderly: normative data in 172 participants. J Clin Exp Neuropsychol 29, 561–567. doi: 10.1080/13803390600864760. [DOI] [PubMed] [Google Scholar]
- Graham DP, Cully JA, Snow AL, Massman P, Doody R, 2004. The Alzheimer’s disease assessment scale-cognitive subscale: normative data for older adult controls. Alzheimer Dis Assoc Disord 18, 236–240. [PubMed] [Google Scholar]
- Grothe MJ, Barthel H, Sepulcre J, Dyrba M, Sabri O, Teipel SJFor the Alzheimer’s Disease Neuroimaging Initiative, 2017. In vivo staging of regional amyloid deposition. Neurology 89, 2031–2038. doi: 10.1212/WNL.0000000000004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeppner J, Wegrzyn M, Thome J, Bauer A, Oltmann I, Buchmann J, Teipel S, 2012. Intra- and inter-cortical motor excitability in Alzheimer’s disease. J Neural Transm 119, 605–612. doi: 10.1007/s00702-011-0738-y. [DOI] [PubMed] [Google Scholar]
- Holdnack JA, 2001. Psychological Corporation: Wechsler test of adult reading [WWW Document]. URL https://scholar.google.com/scholar_lookup?title=Manual%20for%20the%20Wechsler%20test%20of%20adult%20reading&publication_year=2001.
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N, 2018. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dementia 14, 535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Holtzman DM, 2013. Biomarker modeling of Alzheimer’s disease. Neuron 80, 1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Darwish ES, Ali AM, 2011. The relationship between motor cortex excitability and severity of Alzheimer’s disease: A transcranial magnetic stimulation study. Neurophysiol Clin Neurophysiol 41, 107–113. doi: 10.1016/j.neucli.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A, 2003. Transcranial magnetic stimulation in neurology. Lancet Neurol 2, 145–156. doi: 10.1016/S1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Kreutzer JS, DeLuca J, 2011. Alzheimer’s Disease co-operative study ADL scale for mild cognitive impairment (ADCS-ADL-MCI). In: Caplan B (Ed.), Encyclopedia of Clinical Neuropsychology. Springer, New York, New York, NY, p. 112. doi: 10.1007/978-0-387-79948-3_5028 –112. [DOI] [Google Scholar]
- Li S, Selkoe DJ, 2020. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Ab oligomers from Alzheimer brain. J Neurochem doi: 10.1111/jnc.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List J, Kübke JC, Lindenberg R, Külzow N, Kerti L, Witte V, Flöel A, 2013. Relationship between excitability, plasticity and thickness of the motor cortex in older adults. Neuroimage 83, 809–816. doi: 10.1016/j.neuroimage.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Menardi A, Pascual-Leone A, Fried PJ, Santarnecchi E, 2018. The role of cognitive reserve in Alzheimer’s disease and aging: a multi-modal imaging review. J Alzheimer’s Dis 66, 1341–1362. doi: 10.3233/JAD-180549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura Y, Nishida H, Nakajima S, Tsugawa S, Morita S, Yoshida K, Tarumi R, Ogyu K, Wada M, Kurose S, Miyazaki T, Blumberger DM, Daskalakis ZJ, Chen R, Mimura M, Noda Y, 2021. Neurophysiological biomarkers using transcranial magnetic stimulation in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Neuroscience & Biobehav Rev 121, 47–59. doi: 10.1016/j.neubiorev.2020.12.003. [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, DeCarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA, 2006. The uniform data set (UDS): clinical and cognitive variables and descriptive data from Alzheimer disease centers. Alzheimer Dis Assoc Dis 20, 210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Motta C, Di Lorenzo F, Ponzo V, Pellicciari MC, Bonnì S, Picazio S, Mercuri NB, Caltagirone C, Martorana A, Koch G, 2018. Transcranial magnetic stimulation predicts cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 89, 1237–1242. doi: 10.1136/jnnp-2017-317879. [DOI] [PubMed] [Google Scholar]
- Newcombe EA, Camats-Perna J, Silva ML, Valmas N, Huat TJ, Medeiros R, 2018. Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J Neuroinflamm 15. doi: 10.1186/s12974-018-1313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazarán J, Prieto J, Cruz I, Esteban A, 2010. Cortical excitability in very mild Alzheimer’s disease: a long-term follow-up study. J Neurol 257, 2078–2085. doi: 10.1007/s00415-010-5663-8. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Smith R, Ohlsson T, Strandberg O, Mattsson N, Insel PS, Palmqvist S, Hansson O, 2019. Associations between tau, Aβ, and cortical thickness with cognition in Alzheimer disease. Neurology 92, e601–e612. doi: 10.1212/WNL.0000000000006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu G-Q, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L, 2007. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55, 697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L, 2016. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci 17, 777–792. doi: 10.1038/nrn.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L, 2010. Synaptic depression and aberrant excitatory network activity in Alzheimer’s disease: two faces of the same coin? Neuromolecular Med 12, 48–55. doi: 10.1007/s12017-009-8097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp KV, Rentz DM, Orlovsky I, Sperling RA, Mormino EC, 2017. Optimizing the preclinical Alzheimer’s cognitive composite with semantic processing: The PACC5. Alzheimer’s & dementia. Trans Res Clin Intervent 3, 668–677. doi: 10.1016/j.trci.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi G, Alagona G, Ferri R, Greco S, Santonocito D, Pappalardo A, Bella R, 2002. Motor cortex excitability in Alzheimer disease: one year follow-up study. Neurosci Lett 329, 293–296. doi: 10.1016/S0304-3940(02)00701-2. [DOI] [PubMed] [Google Scholar]
- Pepin JL, Bogacz D, de Pasqua V, Delwaide PJ, 1999. Motor cortex inhibition is not impaired in patients with Alzheimer’s disease: evidence from paired transcranial magnetic stimulation. J Neurol Sci 170, 119–123. doi: 10.1016/S0022-510X(99)00206-3. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Waring SC, Smith GE, Tangalos EG, Thibodeau SN, 1996. Predictive value of APOE genotyping in incipient Alzheimer’s diseases. Ann N Y Acad Sci 802, 58–69. doi: 10.1111/j.1749-6632.1996.tb32599.x. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2018. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: [WWW Document]. URL https://www.R-project.org. [Google Scholar]
- Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu G-Q, Palop JJ, Noebels JL, Mucke L, 2011. Amyloid- /Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci 31, 700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120, 2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U, 2015. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126, 1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh M, Sadowsky C, Tousi B, Agronin ME, Alva G, Armon C, Bernick C, Keegan AP, Karantzoulis S, Baror E, Ploznik M, Pascual-Leone A, 2019. Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer’s disease. Alzheimer’s & Dementia doi: 10.1016/j.jalz.2019.08.197. [DOI] [PubMed] [Google Scholar]
- Schmidt Michael, 1996. Rey Auditory Verbal Learning Test : RAVLT : a handbook. Western Psychological Services, Los Angeles, CA. [Google Scholar]
- Smailovic U, Koenig T, Laukka EJ, Kalpouzos G, Andersson T, Winblad B, Jelic V, 2019. EEG time signature in Alzheimerś disease: Functional brain networks falling apart. NeuroImage 24, 102046. doi: 10.1016/j.nicl.2019.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollmann N, Tanigawa N, Bulubas L, Sabih J, Zimmer C, Ringel F, Meyer B, Krieg SM, 2017. Clinical factors underlying the inter-individual variability of the resting motor threshold in navigated transcranial magnetic stimulation motor mapping. Brain Topogr 30, 98–121. doi: 10.1007/s10548-016-0536-9. [DOI] [PubMed] [Google Scholar]
- Stokes MG, Chambers CD, Gould IC, English T, McNaught E, McDonald O, Mattingley JB, 2007. Distance-adjusted motor threshold for transcranial magnetic stimulation. Clin Neurophysiol 118, 1617–1625. doi: 10.1016/j.clinph.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Orantes M, Braak H, 2002. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Trebbastoni A, Pichiorri F, D’Antonio F, Campanelli A, Onesti E, Ceccanti M, de Lena C, Inghilleri M, 2016. Altered cortical synaptic plasticity in response to 5-Hz repetitive transcranial magnetic stimulation as a new electrophysiological finding in amnestic mild cognitive impairment converting to Alzheimer’s disease: results from a 4-year prospective cohort study. Front Aging Neurosci 7. doi: 10.3389/fnagi.2015.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AMI, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ, 2012. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, Henry ML, Nelson AB, Seeley WW, Geschwind MD, Gorno-Tempini ML, Shih T, Kirsch HE, Garcia PA, Miller BL, Mucke L, 2013. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol 70, 1158. doi: 10.1001/jamaneurol.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhang H, Han L, Zhou Y, 2016. Cortical function in Alzheimer’s disease and frontotemporal dementia. Trans Neurosci 7. doi: 10.1515/tnsci-2016-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Sheikh JI, 1986. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 5, 165–173. doi: 10.1300/J018v05n01_09. [DOI] [Google Scholar]
- Zott B, Busche MA, Sperling RA, Konnerth A, 2018. What happens with the circuit in Alzheimer’s disease in mice and humans? Annu Rev Neurosci 41, 277–297. doi: 10.1146/annurev-neuro-080317-061725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data not provided in the article because of space limitations must be made available at the request of other qualified investigators for purposes of replicating procedures and results.