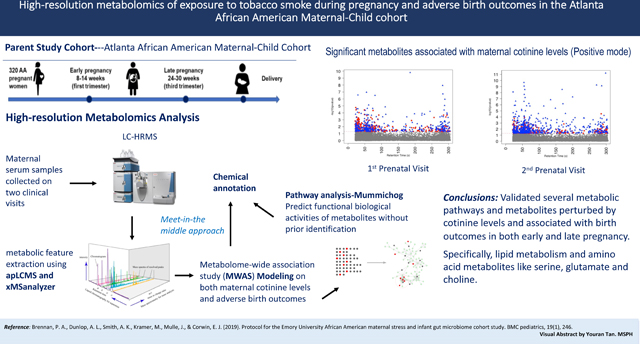
Abstract
Exposure to tobacco smoke during pregnancy has been associated with a series of adverse reproductive outcomes; however, the underlying molecular mechanisms are not well-established. We conducted an untargeted metabolome-wide association study to identify the metabolic perturbations and molecular mechanisms underlying the association between cotinine, a widely used biomarker of tobacco exposure, and adverse birth outcomes.We collected early and late pregnancy urine samples for cotinine measurement and serum samples for high-resolution metabolomics (HRM) profiling from 105 pregnant women from the Atlanta African American Maternal-Child cohort (2014–2016). Maternal metabolome perturbations mediating prenatal tobacco smoke exposure and adverse birth outcomes were assessed by an untargeted HRM workflow using generalized linear models, followed by pathway enrichment analysis and chemical annotation, with a meet-in-the-middle approach.The median maternal urinary cotinine concentrations were 5.93 μg/g creatinine and 3.69 μg/g creatinine in early and late pregnancy, respectively. In total, 16,481 and 13,043 metabolic features were identified in serum samples at each visit from positive and negative electrospray ionization modes, respectively. Twelve metabolic pathways were found to be associated with both cotinine concentrations and adverse birth outcomes during early and late pregnancy, including tryptophan, histidine, urea cycle, arginine, and proline metabolism. We confirmed 47 metabolites associated with cotinine levels, preterm birth, and shorter gestational age, including glutamate, serine, choline, and taurine, which are closely involved in endogenous inflammation, vascular reactivity, and lipid peroxidation processes.The metabolic perturbations associated with cotinine levels were related to inflammation, oxidative stress, placental vascularization, and insulin action, which could contribute to shorter gestations. The findings will support the further understanding of potential internal responses in association with tobacco smoke exposures, especially among African American women who are disproportionately exposed to high tobacco smoke and experience higher rates of adverse birth outcomes.
Keywords: Tobacco smoke exposure, high-resolution metabolomics, cotinine, preterm birth, shorter gestational age
Graphical Abstract
INTRODUCTION
Exposure to tobacco smoke during pregnancy is associated with a series of adverse reproductive outcomes, including preterm birth, various adverse placental conditions, and intrauterine growth restriction.(Salihu & Wilson, 2007) Maternal tobacco smoking during pregnancy was shown to be associated with a nearly two-fold higher risk of preterm birth and a lower birth weight in a dose-depending fashion.(Aliyu, Saidu, Alio, Marty, & Salihu, 2010; Harrod et al., 2014; Mei-Dan et al., 2015; Nabet, Ancel, Burguet, & Kaminski, 2005; Wagijo, Sheikh, Duijts, & Been, 2017) More severe adverse reproductive outcomes reported to be associated with maternal tobacco smoking included higher risk of infant morbidity and mortality, and sudden infant death syndrome (SIDS).(Dietz et al., 2010; Salihu et al., 2008; Shah, Sullivan, & Carter, 2006) Second-hand tobacco smoke exposure during pregnancy has also been associated with a lower birthweight and higher risk of small-for-gestation age and stillbirth infants.(Leonardi-Bee, Britton, & Venn, 2011; Rashid & Rashid, 2003) Notably, pregnant African American (AA) women and their infants are more likely to experience adverse health effects, as they are at higher risk of preterm birth delivery and experience disproportionately high rates of low birthweight,(Ahern, Pickett, Selvin, & Abrams, 2003; Giscombé & Lobel, 2005; Mukherjee, Velez Edwards, Baird, Savitz, & Hartmann, 2013) even with relatively lower prevalence of smoking during pregnancy among AA women compared to white women.(Bell, Zimmerman, Mayer, Almgren, & Huebner, 2007)
However, most of these previous studies used subject self-reporting data to assess the amount of tobacco exposure, which are subject to recall and reporting bias.(Janakiraman, Gantz, Maynard, & El-Mohandes, 2009) A more objective approach for measuring exposure involves quantifying biomarkers of tobacco exposure directly in biological samples. Nicotine, the primary addictive component in tobacco smoke, is metabolized into cotinine, which is the most widely used biomarker for evaluating tobacco exposure due to its higher stability and longer half-life in body fluids when compared with nicotine.(Neal L Benowitz, Hukkanen, & Jacob, 2009; Huang, Han, & Li, 2013; Hukkanen, Jacob, & Benowitz, 2005) Maternal cotinine concentrations have been shown to be associated with adverse birth outcomes including low birthweight (Peacock et al., 1998) and preterm birth.(Kharrazi et al., 2004; Tikkanen et al., 2010) However, the endogenous biological mechanisms underlying the association between cotinine levels and adverse birth outcomes remain unclear.(Fischer et al., 2017)
High-resolution metabolomics has emerged as an innovative analytical platform that may improve assessment of internal exposure and contribute to our understanding of biological responses to complex environmental mixtures through the identification and quantification of thousands of metabolic features associated with exogenous exposure and endogenous processes.(Athersuch, 2016; Cacciatore & Loda, 2015; D. Liang et al., 2018) Along with gaps in previous exposure assessment for tobacco smoke, there is interest in clarifying the role of tobacco-related metabolic alterations and corresponding reproductive health. Several metabolomics studies of maternal serum cotinine concentrations identified significantly perturbed metabolic pathways, including the benzoate, caffeine, vitamin groups (vitamin A, vitamin E and vitamin B6), steroid, amino acid, and carbohydrate pathways.(Fischer et al., 2017; Gu et al., 2016; Lefèvre, Palin, & Murphy, 2011; Rolle-Kampczyk et al., 2016) The metabolic signals associated with birthweight have also been examined, and include changes in the pathways related to tryptophan, carnitine shuttle, fatty acid, and glycerophospholipid metabolism.(Robinson et al., 2018) However, only a few of these studies examined whether the effects of maternal smoke exposure on adverse birth outcomes are mediated by metabolic perturbations,(Fischer et al., 2017; Rolle-Kampczyk et al., 2016) and none of the studies have focused on pregnant African American women, who are at higher risk for adverse birth outcomes.
To address the current research gaps, we performed a metabolome-wide association study (MWAS) using untargeted high-resolution metabolomics to identify metabolic perturbations associated with maternal cotinine level and adverse birth outcomes among pregnant women within the Atlanta African American Maternal-Child Cohort.(Brennan et al., 2019; Corwin et al., 2017) Ultimately, our results can build a foundation for further studies of biological mechanism of cotinine-birth outcome association and promote the development of sensitive biomarkers regarding maternal smoke exposure-related risk in this minor population.
METHODS
Study Population
The current analysis included study participants within the Atlanta African American Maternal-Child Cohort.(Brennan et al., 2019; Corwin et al., 2017) Pregnant women who self-identified as African American were recruited to participate in the study from two prenatal care clinics, Emory Midtown Hospital (privately funded) and Grady Hospital (publicly funded). In this study, prenatal enrollment was restricted to women with singleton pregnancies and without chronic medical conditions, who presented for their first prenatal clinic visit between 8- and 14-week gestation. Data collection occurred at two time points during pregnancy (visit 1-between 8–14 weeks gestation and visit 2-between 24–30 weeks gestation) and included a questionnaire on basic demographic information, biological samples (blood and urine), and medical records abstraction for clinical conditions. Collection of birth outcome data from medical records was done post-delivery. In this analysis, we included a total of 105 participants with paired urinary (for measurement on cotinine concentrations) and serum (for metabolic profiling) samples available from at least one clinic visit. Specifically, 97 participants had paired urinary and blood samples collected at the first clinical visit and 81 participants had the paired samples collected at the second clinic visit (73 women had paired samples from both visits and 32 women had paired samples from either first or second clinic visit). This study was approved by the Emory University Internal Review Board (IRB ID 1017) and signed informed consent was obtained from all study participants.
Measurement of Maternal Cotinine Concentrations
Maternal cotinine concentrations were measured from individual spot urine samples collected during the first and second clinical visits, separately, and quantified using liquid chromatography combined with mass spectrometry (LC-MS-MS).The limit of quantification was 2.5 ng/mL with relative standard deviations: 1.58–6.21% for the within-day precision and 1.71–6.26% for the between-day precision. The relative recoveries were in a range of 82.5–97.1%. The analysis of NIST reference materials showed good accuracy (86.8–95.3%). The method was certified by successful participation in the German External Quality Assessment Scheme (G-EQUAS) proficiency testing scheme (http://gequas.de/). Here, we used total cotinine concentration (the sum of cotinine and its glucuronide adduct; TCOT) in urine samples to represent the exposure level to tobacco smoke.(N. L. Benowitz, 1996; Burstyn et al., 2009) TCOT concentrations below the limit of detections (LODs) were imputed with LOD/√2 (Hornung & Reed, 1990). To correct for urine dilution, we also quantified the creatinine level in the urine samples and adjusted urinary cotinine level via two normalization approaches:(O’Brien, Upson, & Buckley, 2017) a standardization approach using the ratio of cotinine concentration to the creatinine levels measured in the same sample; and, the covariate adjustment approach with creatinine concentration as a covariate included in the regression model. We chose to use the standardization approach for our main analysis and a covariate adjustment approach for later sensitivity analysis. Unadjusted and creatinine-adjusted analyte concentrations were provided in ng cotinine/ml of urine and μg cotinine/g creatinine, respectively. The difference of maternal cotinine concentration in two visits was compared using the Mann-Whitney U test after examining the distribution of cotinine concentration at the two visits. We also calculated the intraclass correlation value of the cotinine level within the same subject across two visits to account for the potential variation in sample collections.
Measure of Adverse Birth Outcomes and Other Factors
The main birth outcome of interest for this analysis was gestational age, which was based upon the date of delivery in relation to the estimated date of conception established during the first clinical visit, obtained from medical records that considers last menstrual period and first visit ultrasound.(Obstetricians, Gynecologists, & Practice, 2017) We assessed this variable both continuously and categorically. For the categorical variable, women were classified into three groups: preterm birth (> 20 and < 37 weeks), early term birth (≥ 37 and < 39 weeks), and full term birth (≥ 39 weeks).(Lumley, 2003) We also used questionnaire and medical record to collect information on potential confounders including individual-level demographic characteristics (age, marital status, education, and income level), behavioral risk factors (alcohol drinking and marijuana and tobacco use during pregnancy), and prenatal characteristic (gestational age at two prenatal care visits, parity, and body mass index (BMI) at first prenatal visit).(Brennan et al., 2019; Corwin et al., 2017)
Untargeted High-Resolution Metabolomics Analysis
Metabolic profiling was completed using maternal non-fasting serum samples collected at the first and second visits from each participant using previously established protocols.(Go et al., 2015; Ladva et al., 2018; Z. Li et al., 2021; D. Liang et al., 2019) Each sample was analyzed in triplicate using liquid chromatography with high-resolution mass spectrometry (LC-HRMS) techniques (Thermo Scientific™ Q- Exactive™ HF). To enhance the coverage of metabolic feature detection, the sample was performed using both polar and nonpolar analytical columns and analysis modes, hydrophilic interaction liquid chromatography (HILIC) with positive electrospray ionization (ESI) and C18 hydrophobic reversed-phase chromatography with negative ESI. Detected signals (referred to as metabolic features) were extracted using apLCMS with modifications by xMSanalyzer, which performed peak detection, mass-to-charge ratio (m/z), retention time (RT) alignment, feature quantification, and data quality filtering.(Uppal et al., 2013; Yu, Park, Johnson, & Jones, 2009) To filter out the noise signals and optimize the metabolomics data quality, only metabolic features detected in >15% of serum samples with median coefficient of variation (CV) among technical replicates < 30% and Pearson correlation ρ > 0.7 were included in further analyses. The resulting analytic data contained individual features defined by m/z, retention time, and ion intensities. Then, we averaged the replicate samples that had at least one non-zero intensity and performed log 2 transformation to normalize the metabolomics data for statistical analysis.
Statistical Analyses
To identify metabolic signals associated with cotinine concentrations and/or adverse birth outcomes, we conducted a series of metabolome-wide association studies (MWAS) models. Specifically, to evaluate the association of maternal cotinine concentrations with metabolic features, a generalized linear model was constructed for the first and second prenatal visit separately, respectively, using the following form:
| (1) |
where refers to the log base 2 intensity of metabolic feature j for participant i, is the intercept, and Cotininei is total maternal cotinine concentration for participant i. We also included covariates in the model to control for potential confounding, including maternal age (Agei), corresponding gestational week at each of the two clinical visits (Gestational Agei), parity (Nulliparousi, categorical), first visit prenatal BMI (BMIi), alcohol use during pregnancy (Alcoholi, categorical) and measures of socioeconomic status (individual income (Incomei)). Finally, we included sex of fetus (Sexi), as an a priori biological variable. represents residual random error. Separate models were conducted for each column (HILIC positive ESI and C18 negative ESI) at each clinical visit. Since we had repeated measures of metabolomics and cotinine from the participants at two time points, we also investigated the trend of the cotinine level and changes in metabolic profile during the pregnancy using linear mixed effect models in the sensitivity analysis. However, since the main goal of the study is to identify metabolic signals associated with both cotinine level and adverse birth outcomes using meet-in-the-middle approach, and that the birth outcome was only assessed at one time point, we decided to use generalized linear model in the main analysis.
To identify metabolic features associated with adverse birth outcomes, we conducted analyses using a similar generalized linear model for features detected at each clinic visit, controlling for similar covariates except gestational age at prenatal visits (Gestational Agei).
| (2) |
where Birthoutcomei refers to 1) gestational age at birth (continuous) and 2) preterm/early term/full term birth (categorical), with full term birth as reference group; separate models were conducted for these two different types of birth outcomes.
In total, we conducted and analyzed 12 sets of MWAS models (i.e., cotinine levels and two adverse birth outcomes, with features detected in two chromatograph columns from samples collected at two prenatal clinic visits). Results were presented using Manhattan plots, with the retention time of each metabolic feature on the x-axis against the −log10(p)for β1 from each equation above on the y-axis. All analyses were completed in R (version 3.6).
Metabolic Pathway Enrichment Analysis and Metabolite Annotation
To predict the functional activity of metabolic features from LC-HRMS output, we conducted pathway enrichment and metabolite annotation analyses. Pathway enrichment was performed using mummichog (v. 1.0.9), a novel bioinformatics platform to predict functional biological activities of metabolites without prior identification.(Shuzhao Li et al., 2013) We used two strategies to select eligible metabolic features for pathway analysis: raw p-value at 0.05 and multiple testing corrected p-value at 0.05 using Benjamini-Hochberg method for multiple comparison correction. In the first approach, to minimize the chance of false positive discovery, we excluded pathways with pathway size less than 4 of the number of features matched in pathway enrichment and identified by mummichog with p-value greater than 0.05. We also conducted a sensitivity analysis by using 0.5th and 1th percentile of raw p-values to perform pathway enrichment and examine whether the significant pathways would be largely different under different raw p-values.(Jeong et al., 2018) Furthermore, the metabolic features significantly associated with both maternal cotinine level and adverse birth outcomes, and also enriched in a relevant pathway were annotated by matching mass m/z value to common adducts using METLIN, ChemSpider, Human Metabolome Database (HMDB), and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases, using a mass error threshold of 10 ppm. To minimize further false positive matches, each matched feature was screened on their retention time, isotope patterns, and spectrum peak quality by examining the extracted ion chromatographs (EICs). Finally, a select number of annotated metabolites were confirmed with level one evidence (Morrison et al., 2007) by comparing their m/z, retention time and ion dissociation patterns to analytical standards in an in-house library that contains a list of exogenous or endogenous metabolites analyzed under identical experimental conditions.
Meet-in-the-middle Analysis
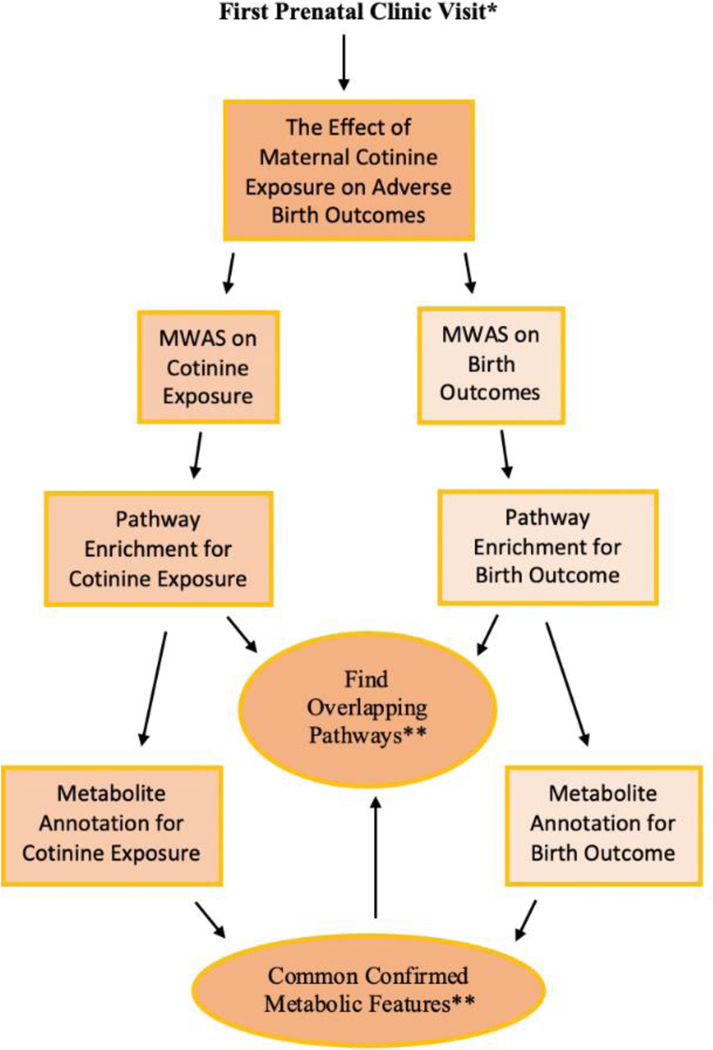
We conducted a meet-in-the-middle (Chadeau-Hyam et al., 2011) analysis to explore the role of the maternal metabolome in potentially mediating the association between maternal cotinine level and adverse birth outcomes. For these analyses, we separately conducted pathway enrichment analysis and chemical annotation for exposure and birth outcomes, and then searched if there were any common pathways or metabolites significantly associated with both exposure and the outcome. The analytical flow is summarized and presented in Figure 1.
Figure 1. The flow chart of meet-in-the-middle approach in our study.
* The second prenatal clinic visit was also conducted in the same process and we compare the metabolic perturbation in both visits.
** Meet in the middle approach: If no identified features on common, then switched to pathways identification.
RESULTS
Among 105 pregnant women who participated in at least one prenatal clinical visit, 43 (41%) were enrolled from the Emory University prenatal clinics and 62 (59%) were enrolled at the Grady Hospital prenatal clinics (Table 1). Overall, the average age of pregnant women at their first visit was 25.9±4.6 years, 16% of study participants were married, 44% had a college degree or above, and 40% had been pregnant before. 47.6% had a full-term birth, 30.5% had an early term birth, and 12.4% had a preterm birth. Only a small proportion of women reported using alcohol and tobacco during the month prior to visit. Among them, 7.2% reported alcohol usage and 14.4% reported tobacco usage in their initial visit, and none reported usage in alcohol and 3.7% reported usage of tobacco in their second visit. The maternal cotinine concentrations of study participants are presented in Table 2. Both unadjusted and creatinine adjusted cotinine level varied considerably, with adjusted cotinine concentration ranging from 0.113 μg/g to 3260 μg/g during the first visit and from 0.140 μg/g to 2650 μg/g in the second visit, respectively, indicative of substantial variation in magnitude of exposure to tobacco smoke among study participants. Notably, the cotinine level within the same subject across two visit points were very consistent, with intraclass correlation value greater than 0.9. Approximately 10% and 33% of urinary cotinine measurements were less than LOD for the first and second visits respectively. Moreover, it was found that around 20% of pregnant women who self-reported no use of tobacco had a high urinary cotinine concentration (>=30 μg/g) (Rolle-Kampczyk et al., 2016) in both clinic visits (Table S1).
Table 1.
Prenatal characteristics of study participants overall (n=105) and by recruitment site, the Atlanta African American Maternal-Child cohort (2014–2016)
| Characteristic | Emory Midtown Hospital | Grady Hospital | Overall |
|---|---|---|---|
| N (%) | 43 (41) | 62 (59) | 105 (100) |
| Age, years, mean (SD) | 27.6 (4.8) | 24.6 (4.1) | 25.8 (4.6) |
| Sex of the baby, n (%)a | |||
| Female | 22 (51.2) | 27 (45.8) | 49 (48.0) |
| Male | 19 (44.2) | 27 (45.8) | 46 (45.1) |
| Gestational age at 1st visit, wks, mean (SD) | 12.1 (2.1) | 11.5 (2.2) | 11.7 (2.2) |
| Gestational age at 2nd visit, wks, mean (SD) | 26.0 (1.7) | 25.6 (1.5) | 25.8 (1.6) |
| Maternal BMI, kg/m2, mean (SD)b | 29.3 (6.9) | 29.6 (8.3) | 29.5 (7.7) |
| Birth outcomes, wks, n (%)a,c | |||
| Full Term (≥ 39) | 24 (55.8) | 26 (41.9) | 50 (47.6) |
| Early Term (≥ 37 and < 39) | 16 (37.2) | 16 (25.8) | 32 (30.5) |
| Preterm (> 20 and < 37) | 1 (2.3) | 12 (19.4) | 13 (12.4) |
| Marital status, n (%) | |||
| Single | 30 (69.8) | 58 (93.5) | 88 (83.8) |
| Married | 13 (30.2) | 4 (6.5) | 17 (16.2) |
| Educational attainment, n (%) | |||
| Less than high school | 1 (2.3) | 16 (25.8) | 17 (16.2) |
| High school graduate | 10 (23.3) | 34 (54.8) | 44 (41.9) |
| College or higher | 32 (74.4) | 12 (19.4) | 44 (41.9) |
| Income, n (%) | |||
| <$26,000 | 14 (32.6) | 53 (85.5) | 67 (63.8) |
| $26,000-$60,000 | 11 (25.6) | 7 (11.3) | 18 (17.1) |
| >$60,000 | 18 (41.9) | 2 (3.2) | 20 (19.0) |
| Nulliparous, n (%)a | |||
| Yes | 17 (39.5) | 25 (40.3) | 42 (40.0) |
| No | 26 (60.5) | 33 (53.2) | 59 (56.2) |
| Insurance type, n (%) | |||
| Medicaid | 20 (46.5) | 61 (98.4) | 81 (77.1) |
| Private | 23 (53.5) | 1 (1.6) | 24 (22.9) |
| Alcohol use at 1st visit, n (%)a | |||
| Not used last month | 28 (77.8) | 53 (86.9) | 81 (83.5) |
| Used last month | 2 (5.6) | 5 (8.2) | 7 (7.2) |
| Alcohol use at 2nd visit, n (%)a | |||
| Not used last month | 32 (94.1) | 45 (95.7) | 77 (95.1) |
| Tobacco use at 1st visit, n (%)a | |||
| Not used last month | 28 (77.8) | 48 (78.8) | 76 (78.4) |
| Used last month | 2 (5.6) | 12 (19.7) | 14 (14.4) |
| Tobacco use at 2nd visit, n (%)a | |||
| Not used last month | 32 (94.1) | 42 (89.4) | 74 (91.4) |
| Used last month | 0 (0.0) | 3 (6.4) | 3 (3.7) |
Abbreviations: BMI, body mass index; wks, weeks.
The total percentage doesn’t sum to 100 as missing information on these variables.
Maternal BMI was measured at the pregnant women’s first prenatal clinic visit.
Elective abortion and spontaneous abortion were not included here.
Table 2.
Unadjusted and creatinine-adjusted maternal cotinine concentrations by clinic visits, the Atlanta African American Maternal-Child cohort (2014–2016).
| First visit (N=97) | Second visit (N=81) | P-valuea | |
|---|---|---|---|
| Unadjusted cotinine, ng/mL urine | |||
| Geometric mean (SD) | 11.78 (12.57) | 6.50 (15.28) | 0.089 |
| Median [Min, Max] | 11.0 [0.354, 6050] | 4.80 [0.354, 5310] | |
| Adjusted cotinine, μg/g Creatinine | |||
| Geometric mean (SD) | 7.15 (11.95) | 4.79 (12.62) | 0.275 |
| Median [Min, Max] | 5.93 [0.113, 3260] | 3.69 [0.140, 2650] |
Mann-Whitney U test was conducted to test the difference of cotinine levels between two visiting stages.
MWAS Model
After data quality assurance and quality control (See method: Untargeted High-Resolution Metabolomics Analysis), 16,481 and 13,043 metabolic features in HILIC positive ESI and C18 negative ESI, respectively, were included for final analyses. The number of statistically significant (raw and FDR-corrected) metabolic features associated with cotinine concentrations or birth outcomes are shown in Table S2. 701 features were significantly associated with maternal cotinine levels for positive ion mode, and 478 features for negative ion mode at the 1st clinic visit, after adjusting for covariates (p-value<0.05, Figure S1a). A slightly larger number of significant features (HILIC: 903 and C18: 619, p-value<0.05, Figure S1a) were found to be associated with cotinine levels at the 2nd visit. The number of significant metabolic features associated with birth outcomes in each column are similar to each other in the two clinic visits, although fewer significant features (N=451, p-value<0.05, Figure S1b) were associated with early term birth in positive ion mode in the second clinic visit compared to preterm birth (N=1522, p-value<0.05, Figure S1b) and gestational age at birth (N=1113, p-value<0.05, Figure S1b).
Pathway Enrichment Analysis
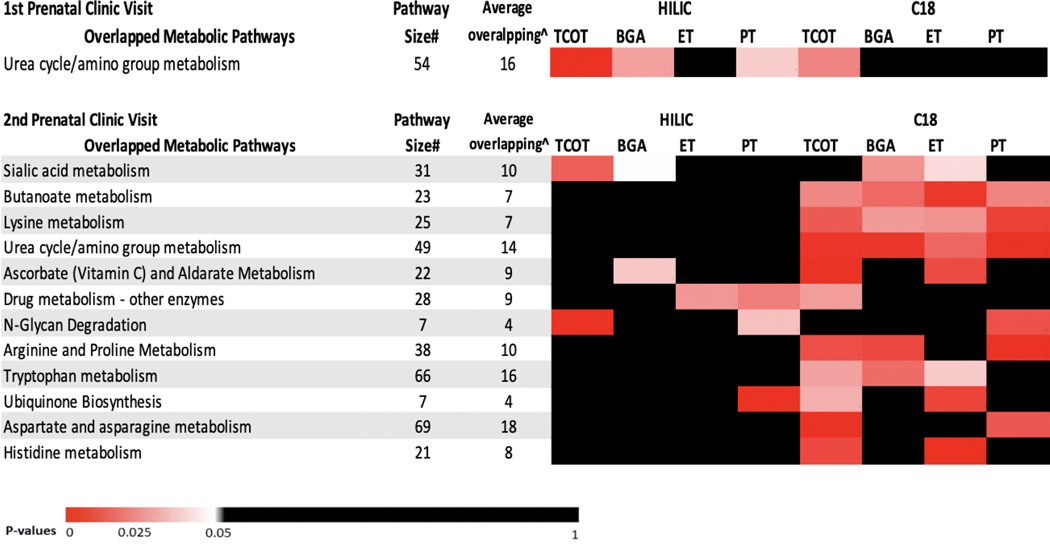
Using significant metabolic features from the two ionization modes (raw p-value<0.05) in each clinic visit as input in the pathway enrichment analysis, total of 27 and 36 significant pathways in two ionization modes from two visits were associated with cotinine levels and birth outcomes, respectively. The metabolic features from the two analytical columns significantly associated with cotinine concentrations at the first and second visits mapped to three and 23 unique pathways, respectively (Figure S2). Urea cycle and amino group metabolism appeared as the only common pathway for both clinical visits. For birth outcome associated metabolic profiling, 9 and 20 unique pathways were associated with at least two birth outcomes at clinic visits 1 and 2, respectively (Figure S3). Amino acid metabolism (arginine and proline metabolism, lysine metabolism, and urea cycle and amino group metabolism), nucleic acids damage and repair pathways (pyrimidine metabolism) and lipid metabolism (linoleate metabolism and glycosphingolipid biosynthesis) were found as common for two clinic visits.
In the meet-in-the-middleanalysis, urea cycle and amino group metabolism was found as the overlapping pathway at the first visit (Figure 2). Twelve overlapping pathways were identified for the second clinic visit and included pathways related to amino acid, lipid, carbohydrate and xenobiotics metabolism. Specifically, butanoate, lysine and urea cycle and amino group metabolism were found to be associated with cotinine levels and all birth outcomes.
Figure 2. The heatmap of overlapping metabolic pathways in two prenatal clinic visits.
Those pathways are associated with both maternal cotinine levels and at least one adverse birth outcome. Each cell represents the association between each metabolic pathway and each exposure/birth outcome and the colors were shaded according to the p-value of this association. The pathways were ordered according to the total number of the significant associations (p-value<0.05) in HILIC mode and C18 mode.
Abbreviations: TCOT: total cotinine concentration in urine samples; BGA: gestational age at birth; ET: early term birth (≥ 37 and < 39 weeks); PT: preterm birth (>20 and <37 weeks).
* For HILIC positive ion mode, only the following adducts were considered: M[1+], M+H[1+], M-H2O+H[1+], M+Na[1+], M+K[1+], M+2H[2+], and M(C13)+2H[2+]. For C18 negative ion mode, only the following adducts were considered: M-H[-], M+Cl[-], M+ACN-H[-], M+HCOO[-], M(C13)-H[-], M-H2O-H[-], and M+Na-2H[-].
# The total number of features in the specific metabolic pathway across the significant metabolism-exposure or metabolism-birth outcomes association in two analytical columns.
^ The average number of metabolic features with m/z matched within the specific metabolic pathway across the significant metabolism-exposure or metabolism-birth outcomes association in two chromatography columns.
Metabolite Annotation and Confirmation
In total, we confirmed 47 metabolites with level 1 evidence, with 28 and 20 significantly associated with cotinine levels and birth outcomes, respectively (Table 3 and Table S3). Generally, we validated more metabolites associated with cotinine levels in late pregnancy and most of them are involved in the common amino acid metabolism and lipid metabolism. Specifically, inflammation and oxidation related amino acids were identified, including tyramine, serine, glutamate, choline and taurine, and they were all positively associated with cotinine levels. Other oxidative stress related metabolites associated with gestation outcomes were also identified including valine, serine and pantothenic acid. All of these endogenous metabolites have important roles in numerous physiological pathways and metabolic functions with potential perturbation by smoke exposure or related to gestational health responses. We also identified 7 exogenous metabolites from xenobiotics metabolism associated with either cotinine levels or birth outcomes in the confirmed chemicals, including nicotine,pirimicarb, methyl ecgonine, diisopropyl phthalate, and other environmental chemicals and toxicants.
Table 3.
Chemical identity of the metabolites significantly associated with maternal cotinine levels (raw p<0.05).
| m/z | RT(s) | Identified Metabolite | Adduct Form | Associated with TCOT exposurea | Pathways | Metabolism | Visit Stage | ESI |
|---|---|---|---|---|---|---|---|---|
| 138.0914 | 22.5 | Tyramine | M+H | β=0.063 | Tyrosine metabolism & Methane metabolism | Amino acid metabolism & Energy metabolism | Visit 1 | ESI+ |
| 496.3397 | 29.9 | Lysopc(16:0) | M+H | β=0.025 | Fatty Acid Metabolism | Lipid metabolism | Visit 1 | ESI+ |
| 482.3243 | 31 | Lysope(18:0) | M+H | β=0.030 | Fatty Acid Metabolism | Lipid metabolism | Visit 1 | ESI+ |
| 524.3708 | 33.1 | Lysopc(18:0) | M+H | β=0.037 | Fatty Acid Metabolism | Lipid metabolism | Visit 1 | ESI+ |
| 480.3436 | 30.7 | Lysopc(o/p-16:1) | M+H | β=0.030 | Fatty Acid Metabolism | Lipid metabolism | Visit 1 | ESI+ |
| 142.0262 | 109.9 | Ethanolamine phosphate | M+H | β=0.148 | Glycerophospholipid metabolism; Glycosphingolipid metabolism | Lipid metabolism | Visit 1 | ESI+ |
| 331.2272 | 26.9 | 17-hydroxyprogesterone | M+H | β=−0.084 | C21-steroid hormone biosynthesis and metabolism | Lipid metabolism | Visit 1 | ESI+ |
| 239.1487 | 29.6 | Pirimicarb | M+H | β=−0.124 | An environmental contaminant, a xenobiotic and an insecticide | Xenobiotics biodegradation and metabolism | Visit 1 | ESI+ |
| 163.1231 | 39.3 | Nicotine | M+H | β1=0.122; β2=0.081 | Tropane, piperidine and pyridine alkaloid biosynthesis & Metabolism of xenobiotics by cytochrome P450 | Biosynthesis of other secondary metabolites & Xenobiotics biodegradation and metabolism | Visit 1/Visit 2 | ESI+ |
| 200.1282 | 37.1 | Methyl ecgonine | M+H | β1=0.097; β2=0.088 | Tropane, piperidine and pyridine alkaloid biosynthesis | Biosynthesis of other secondary metabolites | Visit 1/Visit 2 | ESI+ |
| 168.1019 | 26.1 | 3-methoxytyramine | M+H | β=0.065 | Tyrosine metabolism | Amino acid metabolism | Visit 2 | ESI+ |
| 104.0353 | 24.8 | l-serine | M-H | β=0.034 | Glycine, serine and threonine metabolism & Cysteine and methionine metabolism | Amino acid metabolism | Visit 2 | ESI- |
| 118.0509 | 26.4 | Homoserineb | M-H | β=0.033 | Glycine, serine and threonine metabolism & Cysteine and methionine metabolism & Lysine biosynthesis | Amino acid metabolism | Visit 2 | ESI- |
| 120.0656 | 67.7 | Homoserineb | M+H | β=0.031 | Glycine, serine, alanine and threonine metabolism & Lysine biosynthesis & Methionine and cysteine metabolism | Amino acid metabolism | Visit 2 | ESI+ |
| 148.0604 | 69.4 | l-glutamic acid | M+H | β=0.073 | Tryptophan metabolism & Alanine, aspartate and glutamate metabolism & Arginine and proline metabolism & Histidine metabolism & D-Glutamine and D-glutamate metabolism & Glutathione metabolism & Butanoate metabolism | Amino acid metabolism & Carbohydrate metabolism | Visit 2 | ESI+ |
| 146.046 | 20.9 | Glutamate | M-H | β=0.059 | Alanine, aspartate and glutamate metabolism & Arginine and proline metabolism & Histidine metabolism & D-Glutamine and D-glutamate metabolism & Glutathione metabolism & Butanoate metabolism & Nitrogen metabolism | Amino acid metabolism & Carbohydrate metabolism & Energy metabolism | Visit 2 | ESI- |
| 134.0448 | 74.7 | Aspartate | M+H | β=0.046 | Arginine biosynthesis & Alanine, aspartate and glutamate metabolism& Glycine, serine and threonine metabolism & Cysteine and methionine metabolism & Histidine metabolism & beta-Alanine metabolism | Amino acid metabolism | Visit 2 | ESI+ |
| 102.055 | 66.1 | 1-aminocyclopropane-1-carboxylate | M+H | β=0.031 | Cysteine and methionine metabolism | Amino acid metabolism | Visit 2 | ESI+ |
| 106.0497 | 76.7 | Serine | M+H | β=0.032 | Glycine, serine, alanine and threonine metabolism & Tyrosine metabolism & Methionine and cysteine metabolism | Amino acid metabolism | Visit 2 | ESI+ |
| 104.1071 | 46.5 | Choline | M+ | β=0.033 | Glycine, serine, alanine and threonine metabolism & Glycerophospholipid metabolism | Amino acid metabolism & Lipid metabolism | Visit 2 | ESI+ |
| 126.022 | 67.8 | Taurine | M+H | β=0.038 | Methionine and cysteine metabolism & Primary bile acid biosynthesis | Amino acid metabolism & Lipid metabolism | Visit 2 | ESI+ |
| 581.2418 | 25.4 | Biliverdin | M-H | β=−0.140 | Porphyrin and chlorophyll metabolism | Metabolism of cofactors and vitamins | Visit 2 | ESI- |
| 258.1094 | 76.2 | Sn-glycero-3-phosphocholine | M+H | β=0.106 | Glycerophospholipid metabolism | Lipid metabolism | Visit 2 | ESI+ |
| 171.0061 | 20.5 | Glycerol 2-phosphate | M-H | β=0.055 | Glycerophospholipid metabolism | Lipid metabolism | Visit 2 | ESI- |
| 308.0996 | 19.5 | N-acetylneuraminate | M-H | β=0.087 | Amino sugar and nucleotide sugar metabolism | Carbohydrate metabolism | Visit 2 | ESI- |
| 375.2892 | 26 | Chenodeoxycholate | M H2O+ H | β=0.111 | Primary bile acid biosynthesis | Lipid metabolism | Visit 2 | ESI+ |
| 377.0867 | 20.8 | Maltose | M+Cl | β=0.079 | Starch and Sucrose Metabolism | Carbohydrate metabolism | Visit 2 | ESI- |
| 251.1272 | 29.3 | Diisopropyl phthalate | M+H | β=0.043 | Role as a plasticizer | Xenobiotics biodegradation and metabolism | Visit 2 | ESI+ |
Note: Chemical identity of metabolic features was confirmed by matching peaks via accurate mass to charge ratio and retention time to authentic reference standards under the same conditions using tandem mass spectrometry.
Abbreviations: m/z: mass to charge ratio; RT: retention time; ESI+: positive electrospray ionization mode; ESI-: negative electrospray ionization mode; TCOT: total cotinine concentration in urine sample.
The beta coefficient represents the change in log-transformed metabolite intensity per 1-unit increase in urinary cotinine level.
Metabolites were identified in both HILIC+ with positive electrospray ionization mode and C18- with negative electrospray ionization mode.
No common metabolic feature associated with both exposure and outcome were found from the same clinic visit. One feature, serine--reported to be a part of metabolic network that support oxidant/antioxidant balance and cell proliferation--was found to have a positive association with cotinine levels in the 2nd clinic visit and a negative association with preterm birth compared to full term birth in the 1st clinic visit. (Kalhan et al., 2003) Although no overlapping metabolites have been confirmed, most of the identified metabolites were closely linked and connected within some metabolic pathways such as tyrosine metabolism, glycine, serine, alanine and threonine metabolism, and fatty acid metabolism, which can modulate physiological processes such as inflammation, vascular reactivity and lipid peroxidation with the potentially toxicant impact of cotinine levels.
Sensitivity Analysis
Several sets of sensitivity analyses were performed to reduce the possibility for false discoveries and test the consistency of the metabolomics analyses. First, we used different percentiles of p-values from each MWAS result as a cut-off point to perform pathway enrichment analysis.(Shuzhao Li et al., 2013) The related pathways such as urea cycle and amino group metabolism, aspartate and asparagine metabolism and glycosphingolipid metabolism shown in the main analysis remained as overlapping pathways linking cotinine levels and birth outcomes when we changed p-values to 0.01 and 0.005 (Figure S4a-S4b). Furthermore, we used two different methods to adjust for urine dilution and compared the results using exposure pathway enrichment. All significant metabolic pathways found in the first clinic visit and most of pathways found in the second visit in the original analysis were also identified in the sensitivity analysis, indicating our normalization approaches were consistent. Additionally, to evaluate the impact of study site and recorded prenatal medical complications (preeclampsia, gestational hypertension and gestational diabetes mellitus), we conducted the additional MWAS model by adding these two variables to account for the potential discrepancy (Table S4). Although more enriched pathways were identified compared to the main analysis, especially in the first prenatal visit, top significant pathways that are closely related to our study showed consistency, and most of identified chemicals in main analysis can be found in the enriched pathways in sensitivity analysis. Finally, we observed similar results when using the linear mixed effect models examining the associations between cotinine levels and metabolic feature intensities in the sensitivity analysis.
DISCUSSION
Using both targeted exposure assessment and untargeted high-resolution metabolomics, we detected numerous significant metabolic perturbations during early and late pregnancy associated with both cotinine levels and adverse birth outcomes among participants from the Atlanta African American Mother-Child cohort. We also verified metabolites that were closely linked and connected in several inflammation, oxidative stress, placental vascularization, and insulin action related pathways. Taken together, we believe these findings point to several potential biological mechanisms of prenatal exposure to tobacco smoke on adverse birth outcomes.
Maternal metabolic pathways associated with cotinine level and adverse birth outcomes
The most pronounced finding from the current analyses was the identification of several metabolic pathways and metabolites associated with both cotinine level and adverse birth outcomes. Most of these identified pathways, including tryptophan metabolism, alanine, aspartate and glutamate metabolism, arginine and proline metabolism, fatty acid metabolism, and glycerophospholipid metabolism, were closely linked to tobacco-smoke-exposure-related acute oxidative stress and systemic inflammatory responses, which cancontribute to shorter gestations.(Feng et al., 2014; Fischer et al., 2017; Gu et al., 2016; Kelly et al., 2017; Lefèvre et al., 2011) The identification of these specific pollution-mediated pathways mirror results reported recently. In an analysis of second-trimester amniotic fluid, Fischer, et al., found that low-level maternal nicotine exposure was associated with significant metabolic perturbations in aspartate and asparagine metabolism, arginine and proline metabolism.(Fischer et al., 2017) Similarly, in a large observational study of 892 men and women, Gu, et al., identified associations between cigarette smoking and metabolites involving in fatty acid metabolism and tryptophan metabolism.(Gu et al., 2016) An animal study on rat also showed that prenatal nicotine exposure can cause maternal metabolome alterations in fatty acid metabolism and some amino acid accumulation.(Feng et al., 2014)
Perturbations in pathways and metabolites enriched in lipid and fatty acid metabolism
Fatty acid metabolism, an active inflammatory mediatory pathway, was consistently among the most prominent pathways found perturbed by cotinine levels and was also shown to be significantly associated with gestational age at birth in our study. These results were consistent with the previous findings, where cigarette smoking was linked to alterations in fatty acid and glycerophospholipid metabolism.(Gu et al., 2016; Kelly et al., 2017; Robinson et al., 2018; Wang-Sattler et al., 2008) Specifically, maternal fatty acid metabolism has been closely associated with fetal intrauterine growth, thus, fatty acid deficiency could result in malnutrition of fetus, shorter gestational age, and preterm birth (Bobiński & Mikulska, 2015; Reece, McGregor, Allen, & Harris, 1997), indicating the potential effect of maternal smoking on endogenous metabolites and birth outcomes through lipid metabolism. Cigarette smoking is known to be a significant source of oxidative stress (Alberg, 2002; Orhon et al., 2009), and tobacco smoke exposure during pregnancy was reported to be linked to increased production of reactive oxygen species, resulting in oxidative damage to certain selected lipids.(Cross et al., 1993; Schwertner, 1998; Yoshie & Ohshima, 1997) In turn, the abnormality in lipid metabolism during pregnancy can also increase oxidative stress, (Herrera & Ortega-Senovilla, 2010) further disturbing the balance of lipid peroxidation and antioxidation processes. This can lead to the decomposition of some types of fatty acids that damages the function and structure of capillary endothelial cells,(Bukhari et al., 2011) as capillary endothelial cells are related to certain placental vascular functions and dyslipidemia-associated oxidative stress was shown to be associated with shorter gestational age.(Loy, Sirajudeen, & JM, 2013)
The metabolic feature annotation and validation findings in lipid metabolism offered further coherence to the pathway enrichment results. Among the confirmed metabolites significantly associated with cotinine levels in early pregnancy, most are involved in lipid metabolism. Specifically, lysophosphatidylcholines (LysoPCs), groups of bioactive pro-inflammatory lipids, were found to be positively associated with maternal cotinine levels, and have been reported to be linked with oxidative stress and inflammation.(Sevastou, Kaffe, Mouratis, & Aidinis, 2013) In particular, early gestational maternal lipid concentrations of lysophosphatidylcholine (LysoPC) and lysophosphatidylethanolamine (LysoPE) were positively associated with birthweight,(Hellmuth et al., 2017; LaBarre et al., 2020; Lu et al., 2018) and birthweight and gestational length are positively correlated.(Donahue, Kleinman, Gillman, & Oken, 2010) 17-hydroxyprogesterone is another identified lipid metabolism-related metabolite associated with cotinine levels. Previously, 17-hydroxyprogesterone, one of the metabolic clocks of blood metabolites, accurately predicts gestational age approaching the labor event.(L. Liang et al., 2020) We also observed a positive association between acylcarnitines, which are involved in fatty acid oxidation, and gestational age as well as preterm birth. The reduced level of acylcarnitines can lead to a reduced energy production and fat oxidation that could be the linkage in the mechanism leading to adverse birth outcomes in smoking mothers.(Melichar, Novak, Zoula, Hahn, & Koldovský, 1966)
Changes in pathways and metabolites in amino acid metabolism and carbohydrate metabolism
In addition to fatty acid metabolism, we also identified perturbations in pathways and metabolites from amino acid metabolism in both early and late pregnancy, which are closely involved in inflammation, oxidative stress, and placental vascularization. The pathways perturbed by cotinine levels, including aspartate and asparagine metabolism, arginine and proline metabolism, histidine metabolism, and tryptophan metabolism, are recognized as critical pathways in determining the potential intrauterine growth retardation.(Feng et al., 2014; Fischer et al., 2017; Lefèvre et al., 2011) Consistently, we observed positive associations between maternal cotinine level and various amino acid metabolites enriched in these pathways. In particular, glutamate is an amino acid that can reduce susceptibility to oxidative stress, control lipid peroxidation and prevent free radical-linked deleterious effects among pregnant women [70, 73–75]. In animal models, nicotine receptor activation was found to initiate the release of glutamate (Arneric, 2000) to compensate for the oxidative effect caused by smoke exposure. Glutamate was also found to be positively associated with gestational age,(Murgia et al., 2019; Wu, 2013) and negatively associated with intrauterine growth restriction (IUGR).(Cetin, 2001; Lin et al., 2014). Other cotinine-level-perturbed amino acids we identified include taurine and choline. Taurine has been associated primarily with nicotine-induced vascular adverse events and can ameliorate impairment of vascular reactivity(Abebe & Mozaffari, 2011). It was also reported to be involved in defense against oxygen free radical species, inhibiting apoptosis, inflammation, and cell death while increasing NO generation in endothelial cells.(Franconi, Loizzo, Ghirlanda, & Seghieri, 2006; Oliveira et al., 2010) We found positive associations between cotinine level and taurine intensities consistently in our analysis. A similar pattern was observed with choline, an amino acid closely linked to placental vascular function through modulating inflammation, angiogenesis and apoptosis and further downregulation of the placental pro-inflammatory cytokines at several gestational time points (S. Kwan, King, & Caudill, 2015). In particular, it was reported that increased concentration of choline can decrease the placental production and circulating concentration of placental oxidative stress factors during late pregnancy.(S. T. C. Kwan et al., 2017)
We also identified several inflammatory metabolites in amino acid metabolism related to our birth outcome of interest. 2-methylhippurate levels in early pregnancy was found to be higher among women with early term births, and it is closely related to tyrosine that, in turn, was reported to be associated with augmented placental oxidative stress and subsequent small for gestational age in the newborn.(Thadhani et al., 2004). Another confirmed amino acid was valine, a key moderator in gluconeogenesis and glucose tolerance related birth outcomes.(Park, Park, Lee, & Kim, 2015) This observation might help to build the linkage between tobacco smoke exposure, perturbations in the amino acid and carbohydrate metabolism, and adverse birth outcomes. Specifically, tobacco smoke exposure during pregnancy has been reported to be associated with decreased glucose tolerance and increased contribution to oxidative metabolism.(Troisi et al., 2003) In our results, metabolites enriched in carbohydrate metabolism showed significant association with gestational age at birth. In early pregnancy, D-mannose level have been shown to have a negative association with early term birth compared to full term birth, which was also shown to be related to spontaneous preterm labor in a previous study (Romero et al., 2010), while glucose 6-phosphate showed negative association, indicating that different carbohydrate metabolites functioned collectively to regulate the glucose metabolic pattern on birth outcomes. Notably, it was reported that young pregnant AA women (≤18 years) have higher insulin and lower glucose concentrations, highlighting that this vulnerable group might be more likely to experience the adverse birth outcome caused by cotinine levels.(Scholl, Chen, Khoo, & Lenders, 2004)
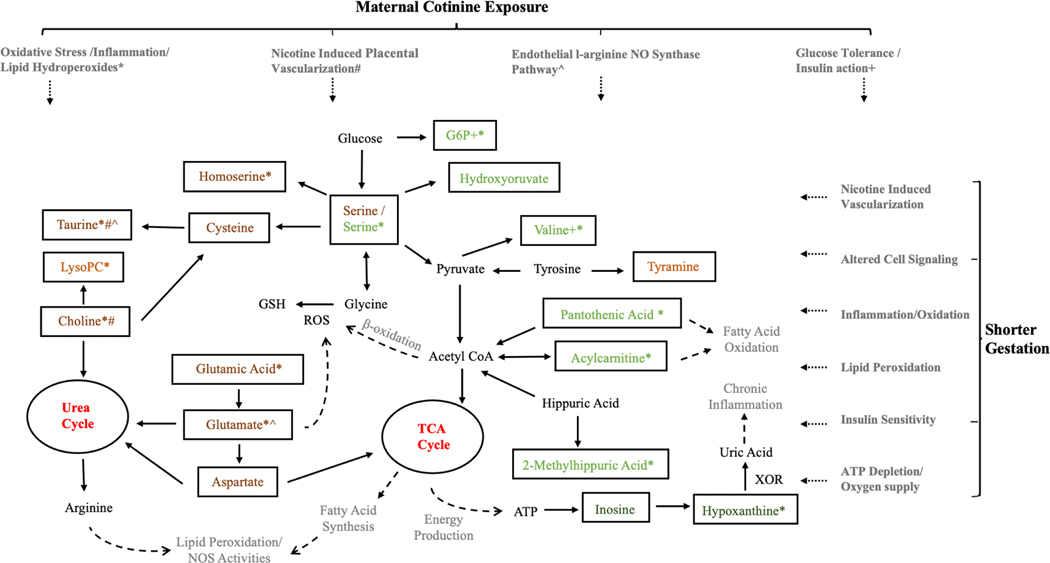
Urea cycle and tricarboxylic acid cycles (TCA) metabolic network
Although there were no overlapping metabolites identified in the same stage of pregnancy, our pathway analysis results consistently indicated the urea cycle and amino group metabolism as common response pathways in both early and late pregnant profiling. Based on those collective results, we propose that those identified molecules may be related to systemic changes under an interrelated metabolic network centered at urea cycle and tricarboxylic acid cycles (TCA) that linked the amino acid, lipid, carbohydrate and nucleotide metabolism together to response to external cotinine levels during the pregnancy (Figure 3). Maternal tobacco smoke exposure during pregnancy is related to oxidative stress, lipid hydroperoxides, DNA damage, inflammation, and it can also affect the endothelial l-arginine NO synthase pathway, resulting in reducing NO production and elevated oxidative stress.(Gonçalves et al., 2011; Harats et al., 1989; Polidori, Mecocci, Stahl, & Sies, 2003; J. Zhang, Liu, Shi, Larson, & Watson, 2002; W. Z. Zhang, Venardos, Chin-Dusting, & Kaye, 2006) The high levels of pro-inflammatory molecules can cause endothelial cell dysfunction and adversely affect placental vascularization,(Ali & Khalil, 2015) resulting in higher risk of various adverse birth outcomes.(Loy et al., 2013; Reynolds et al., 2010) Importantly, most of identified metabolites including glutamate, homoserine, taurine and choline in amino acid metabolism are positively associated with cotinine level, and those slight increases in these antioxidant biomarkers may represent a compensatory mechanism to reduce the adverse oxidative effect caused by cotinine levels. Additionally, we also identified inosine and hypoxanthine in nucleotide metabolism to be related to gestational age at delivery under this exposure-response network, both of which are considered to mediate energy production and chronic inflammation process that are important in fetal development in pregnancy.(Issel, Lun, Pohle, & Gross, 1988)
Figure 3. The potential molecular mechanisms under metabolic network for the effect of maternal cotinine levels on the gestational birth outcomes among pregnant AA women cohort.
Molecules in light orange and dark orange denote the confirmed metabolites significantly associated with cotinine levels in the first and second clinic visit, respectively. Molecules in light green and dark green denote the confirmed metabolites significantly associated with birth outcomes in the first and second clinic visit, respectively.
Abbreviations : ROS: reactive oxygen species; XOR: xanthine oxidoreductase; NOS: nitric oxide synthases; G6P: glucose 6-phosphate; ATP: adenosine triphosphate; LysoPC: Lysophosphatidylcholines; GSH:Glutathione.
*#^+ The maternal cotinine levels could be perturbed the metabolic response and further affect the gestation mainly through four mechanisms. The confirmed metabolites and related metabolism could be involved in one or several mechanisms indicated by those symbols.
Exogenous Metabolites
Additionally, we also identified several exogenous toxicants and organic chemicals such as nicotine, methyl ecgonine, and diisopropyl phthalate associated with maternal cotinine levels. Nicotine and methyl ecgonine, biomarkers of tobacco smoke and crack cocaine respectively, both were found to be positively associated with cotinine levels in our MWAS results. Diisopropyl phthalate is widely used in household products and reported to have adverse toxicant effect on reproductive health, liver and kidney function, and to be associated with cancer.(Yost et al., 2019)
Study limitation
While our study provides many novel insights into potential biological mechanisms underlying the impact of maternal smoke exposure on adverse birth outcomes, it also has a number of limitations. First, due to the cross-sectional study design, causal relationship could not be established between maternal cotinine levels, metabolic perturbation, and adverse birth outcomes. However, the two-time metabolic profiling on women at different stages of pregnancy can help us compare the maternal metabolome perturbation in both early and late pregnancy. In addition, mapping out the endogenous metabolic networks could further our understanding of the potential biological mechanisms perturbed by cotinine that affect the birth outcomes. Further, it is possible that other exogenous environmental exposures could also result in similar metabolic perturbations as cotinine does, and thus further research could be required to test these potential effects. Another limitation is that while we can accurately quantify the maternal cotinine concentration in urine sample, we may only infer the true source of nicotine exposure. Our data showed that large proportion of women in our cohort who reported no use of the tobacco had a high urinary cotinine concentration (Table S1),(Rolle-Kampczyk et al., 2016) indicating that there was potential report bias among participants during the interview or there were potential alternative routes to tobacco exposure. The one-time exposure measurement may not reflect the long-term exposure status, and thus limits our interpretation of underlying mechanisms for effect of maternal smoke on birth outcomes. In addition, the choice of bio samples for measurement of cotinine levels needs to be considered. Although urinary cotinine is a shorter-term dosimeter of nicotine exposure that may result in exposure misclassification, most smokers and second-hand smokers have continual exposures resulting in steady state excretion so that a urine sample often accurately captures average nicotine exposure. Due to the limited statistical power, we did not adjust for some potential confounding factors, including dietary and some lifestyle variables. Nevertheless, to minimize the potential impact of non-fasting state on the metabolomics results, we used pool standards and internal references in the metabolic profiling and followed a comprehensive metabolomics workflow, which has been shown to successfully analyze many non-fasting samples previously.(Gaskins et al., 2021; Go et al., 2015) We also examined the associations between dietary intake, smoke exposures, and preterm birth, and found no associations. Thus, we did not control dietary variables in the MWAS models. We also conducted sensitivity analysis and adjusted for several potential confounding factors, including prenatal medical complications, and we observed consistent results in the MWAS model and pathway enrichment analysis. Therefore, we only included a basic set of covariates in the MWAS model. Finally, our study population of pregnant African Americans women may have a different nicotine metabolism pattern compared to other populations, (Pérez-Stable, Herrera, Jacob, & Benowitz, 1998) and experience a higher frequency of adverse birth outcomes, which may limit the generalizability of this study. Nevertheless, we observed consistent perturbation in similar metabolites and metabolic pathways related to smoke exposure or birth outcomes previously reported in other racial groups.(Fischer et al., 2017; S. Li, Dunlop, Jones, & Corwin, 2016; Rolle-Kampczyk et al., 2016)
Conclusion
By building on a unique minority birth cohort, capitalizing on existing resources, and applying high-resolution metabolomics, we identified and validated several metabolic pathways and metabolites perturbed by cotinine levels and associated with birth outcomes in both early and late pregnancy, and presented a potential disturbed metabolic network centered on urea cycle and TCA cycle that connects amino acid, lipid, carbohydrate and nucleotide metabolism together to response to cotinine levels. Specifically, lipid metabolism and a series of amino acid metabolites such as glutamate, serine, choline, and taurine play a crucial role in molecular mechanisms underlying cotinine-associated toxicity and its maternal health effect in the early and late pregnancy, respectively, and the birth outcome associated metabolic profiling further established the connection between those pathways and metabolites and potential adverse birth outcomes. Collectively, we anticipate this study will contribute to further understanding of the biological responses to nicotine exposures and development of sensitive and robust biomarkers to support future development of targeted interventions to reduce the adverse birth health effect caused by tobacco smoke exposures among minority pregnant women and newborns.
Supplementary Material
Metabolic perturbation associated with cotinine may contribute to shorter gestation
Urea cycle and amino metabolism associated with both cotinine and birth outcomes
47 metabolic features were confirmed at level 1 confidence
Metabolites mostly involved in oxidative stress and placental vascularization
ACKNOWLEDGEMENT
This work was supported by the National Institute of Health (NIH) research grants [R01NR014800, R01MD009064, R24ES029490, R01MD009746, R21ES032117], NIH Center Grants [P50ES02607, P30ES019776, UH3OD023318, U2CES026560], and Environmental Protection Agency (USEPA) center grant [83615301]. V. Fedirko is supported by the Cancer Prevention and Research Institute of Texas (CPRIT) Rising Stars Award (Grant ID RR200056). Additionally, we are grateful for our colleagues -Nathan Mutic, Cierra Johnson, Erin Williams, Priya D’Souza, Estefani Ignacio Gallegos, Nikolay Patrushev, Kristi Maxwell Logue, Castalia Thorne, Shirleta Reid, Cassandra Hall, and the clinical health care providers and staff at the prenatal recruiting sites for helping with data and sample collection and logistics and sample chemical analyses in the laboratory. We also thank the participants themselves for their additions to this project.
Footnotes
Declaration of competing financial interests (CFI): None
CRediT Authors Statement
Youran Tan: Formal Analysis, Investigation, Writing-Original Draft, Visualization. Dana Boyd Barr: Methodology, Validation, Resources, Data Curation, Writing-Review&Editing, Funding acquisition. P. Barry Ryan: Methodology, Resources, Data Curation, Writing-Review&Editing, Funding acquisition. Veronika Fedirko: Methodology, Resources, Writing-Review&Editing. Jeremy A. Sarnat: Methodology, Resources, Writing-Review&Editing. Audrey J. Gaskins: Methodology, Resources, Writing-Review&Editing. Che-Jung Chang: Data Curation, Writing-Review&Editing. Ziyin Tang: Data Curation, Writing-Review&Editing. Carmen J. Marsit: Resources, Writing-Review&Editing, Funding acquisition. . Elizabeth J. Corwin: Resources, Data Curation, Writing-Review&Editing, Funding acquisition. Dean P. Jones: Methodology, Resources, Writing-Review&Editing, Funding acquisition. Anne L. Dunlop: Investigation, Resources, Data Curation, Writing-Review&Editing, Project administration, Funding acquisition. Donghai Liang: Conceptualization, Methodology, Investigation, Writing-Original Draft, Writing-Review&Editing, Supervision, Funding acquisition.
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
REFERENCES
- Abebe W, & Mozaffari MS (2011). Role of taurine in the vasculature: an overview of experimental and human studies. American journal of cardiovascular disease, 1(3), 293. [PMC free article] [PubMed] [Google Scholar]
- Ahern J, Pickett KE, Selvin S, & Abrams B. (2003). Preterm birth among African American and white women: a multilevel analysis of socioeconomic characteristics and cigarette smoking. J Epidemiol Community Health, 57(8), 606–611. 10.1136/jech.57.8.606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberg AJ (2002). The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology, 180(2), 121–137. 10.1016/s0300-483x(02)00386-4 [DOI] [PubMed] [Google Scholar]
- Ali SM, & Khalil RA (2015). Genetic, immune and vasoactive factors in the vascular dysfunction associated with hypertension in pregnancy. Expert Opin Ther Targets, 19(11), 1495–1515. 10.1517/14728222.2015.1067684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliyu MH, Saidu R, Alio AP, Marty PJ, & Salihu HM (2010). Intrauterine exposure to tobacco and risk of medically indicated and spontaneous preterm birth. American journal of perinatology, 27(05), 405–410. 10.1055/s-0029-1243316 [DOI] [PubMed] [Google Scholar]
- Arneric SP (2000). Neurobiology and clinical pathophysiology of neuronal nicotinic acetylcholine receptors. Nicotine in Psychiatry, Psychopathology and Emerging Therapeutics, 3–35. [Google Scholar]
- Athersuch T. (2016). Metabolome analyses in exposome studies: profiling methods for a vast chemical space. Archives of biochemistry and biophysics, 589, 177–186. 10.1016/j.abb.2015.10.007 [DOI] [PubMed] [Google Scholar]
- Bell JF, Zimmerman FJ, Mayer JD, Almgren GR, & Huebner CE (2007). Associations between residential segregation and smoking during pregnancy among urban African-American women. Journal of Urban Health, 84(3), 372–388. 10.1007/s11524-006-9152-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL (1996). Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev, 18(2), 188–204. 10.1093/oxfordjournals.epirev.a017925 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, & Jacob P. (2009). Nicotine chemistry, metabolism, kinetics and biomarkers. In Nicotine psychopharmacology (pp. 29–60): Springer. 10.1007/978-3-540-69248-5_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobiński R, & Mikulska M. (2015). The ins and outs of maternal-fetal fatty acid metabolism. Acta Biochimica Polonica, 62(3). 10.18388/abp.2015_1067 [DOI] [PubMed] [Google Scholar]
- Brennan PA, Dunlop AL, Smith AK, Kramer M, Mulle J, & Corwin EJ (2019). Protocol for the Emory University African American maternal stress and infant gut microbiome cohort study. BMC pediatrics, 19(1), 246. 10.1186/s12887-019-1630-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari SA, Rajoka MI, Ibrahim Z, Jalal F, Rana SM, & Nagra SA (2011). Oxidative stress elevated DNA damage and homocysteine level in normal pregnant women in a segment of Pakistani population. Molecular biology reports, 38(4), 2703–2710. 10.1007/s11033-010-0413-7 [DOI] [PubMed] [Google Scholar]
- Burstyn I, Kapur N, Shalapay C, Bamforth F, Wild TC, Liu J, & LeGatt D. (2009). Evaluation of the accuracy of self-reported smoking in pregnancy when the biomarker level in an active smoker is uncertain. Nicotine Tob Res, 11(6), 670–678. 10.1093/ntr/ntp048 [DOI] [PubMed] [Google Scholar]
- Cacciatore S, & Loda M. (2015). Innovation in metabolomics to improve personalized healthcare. Annals of the New York Academy of Sciences, 1346(1), 57. 10.1111/nyas.12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin I. (2001). Amino acid interconversions in the fetal-placental unit: the animal model and human studies in vivo. Pediatric research, 49(2), 148–154. 10.1203/00006450-200102000-00004 [DOI] [PubMed] [Google Scholar]
- Chadeau-Hyam M, Athersuch TJ, Keun HC, De Iorio M, Ebbels TM, Jenab M, . . . Vineis P. (2011). Meeting-in-the-middle using metabolic profiling - a strategy for the identification of intermediate biomarkers in cohort studies. Biomarkers, 16(1), 83–88. 10.3109/1354750x.2010.533285 [DOI] [PubMed] [Google Scholar]
- Corwin EJ, Hogue CJ, Pearce B, Hill CC, Read TD, Mulle J, & Dunlop AL (2017). Protocol for the Emory University African American vaginal, oral, and gut microbiome in pregnancy cohort study. BMC pregnancy and childbirth, 17(1), 161. 10.1186/s12884-017-1357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross CE, O’NEIL CA, Reznick AZ, HU ML, Marcocci L, Packer L, & Frei B. (1993). Cigarette Smoke Oxidation of Human Plasma Constituents a. Annals of the New York Academy of Sciences, 686(1), 72–89. 10.1111/j.17496632.1993.tb39157.x [DOI] [PubMed] [Google Scholar]
- Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, & Callaghan WM (2010). Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med, 39(1), 45–52. 10.1016/j.amepre.2010.03.009 [DOI] [PubMed] [Google Scholar]
- Donahue SM, Kleinman KP, Gillman MW, & Oken E. (2010). Trends in birth weight and gestational length among singleton term births in the United States: 1990–2005. Obstetrics and gynecology, 115(2 Pt 1), 357. 10.1097/AOG.0b013e3181cbd5f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J. h., Yan Y. e., Liang G, Liu Y. s., Li X. j., Zhang B. j., . . . Wang H. (2014). Maternal and fetal metabonomic alterations in prenatal nicotine exposure-induced rat intrauterine growth retardation. Molecular and cellular endocrinology, 394(1–2), 59–69. 10.1016/j.mce.2014.06.016 [DOI] [PubMed] [Google Scholar]
- Fischer ST, Lili LN, Li S, Tran VT, Stewart KB, Schwartz CE, . . . Fridovich-Keil JL (2017). Low-Level maternal exposure to nicotine associates with significant metabolic perturbations in second-trimester amniotic fluid. Environment international, 107, 227–234. 10.1016/j.envint.2017.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franconi F, Loizzo A, Ghirlanda G, & Seghieri G. (2006). Taurine supplementation and diabetes mellitus. Current Opinion in Clinical Nutrition & Metabolic Care, 9(1), 32–36. 10.1097/01.mco.0000196141.65362.46 [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Tang Z, Hood RB, Ford J, Schwartz JD, Jones DP, . . . Liang D. (2021). Periconception air pollution, metabolomic biomarkers, and fertility among women undergoing assisted reproduction. Environ Int, 155, 106666. 10.1016/j.envint.2021.106666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giscombé CL, & Lobel M. (2005). Explaining disproportionately high rates of adverse birth outcomes among African Americans: the impact of stress, racism, and related factors in pregnancy. Psychological bulletin, 131(5), 662. 10.1037/00332909.131.5.662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go Y-M, Walker DI, Liang Y, Uppal K, Soltow QA, Tran V, . . . Pennell KD (2015). Reference standardization for mass spectrometry and high-resolution metabolomics applications to exposome research. Toxicological Sciences, 148(2), 531–543. 10.1093/toxsci/kfv198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves RB, Coletta RD, Silvério KG, Benevides L, Casati MZ, da Silva JS, & Nociti FH Jr. (2011). Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res, 60(5), 409–424. 10.1007/s00011-011-03087 [DOI] [PubMed] [Google Scholar]
- Gu F, Derkach A, Freedman ND, Landi MT, Albanes D, Weinstein SJ, . . . Xiao Q(2016). Cigarette smoking behaviour and blood metabolomics. International journal of epidemiology, 45(5), 1421–1432. 10.1093/ije/dyv330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harats D, Ben-Naim M, Dabach Y, Hollander G, Stein O, & Stein Y. (1989). Cigarette smoking renders LDL susceptible to peroxidative modification and enhanced metabolism by macrophages. Atherosclerosis, 79(2–3), 245–252. 10.1016/0021-9150(89)90130-5 [DOI] [PubMed] [Google Scholar]
- Harrod CS, Reynolds RM, Chasan-Taber L, Fingerlin TE, Glueck DH, Brinton JT, & Dabelea D. (2014). Quantity and timing of maternal prenatal smoking on neonatal body composition: the Healthy Start study. The Journal of pediatrics, 165(4), 707–712. 10.1016/j.jpeds.2014.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmuth C, Uhl O, Standl M, Demmelmair H, Heinrich J, Koletzko B, & Thiering E. (2017). Cord blood metabolome is highly associated with birth weight, but less predictive for later weight development. Obesity facts, 10(2), 85–100. 10.1159/000453001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, & Ortega-Senovilla H. (2010). Maternal lipid metabolism during normal pregnancy and its implications to fetal development. Clinical Lipidology, 5(6), 899–911. 10.2217/clp.10.64 [DOI] [Google Scholar]
- Hornung RW, & Reed LD (1990). Estimation of average concentration in the presence of nondetectable values. Applied occupational and environmental hygiene, 5(1), 46–51. 10.1080/1047322x.1990.10389587 [DOI] [Google Scholar]
- Huang R, Han S, & Li XS (2013). Detection of tobacco-related biomarkers in urine samples by surface-enhanced Raman spectroscopy coupled with thin-layer chromatography. Analytical and bioanalytical chemistry, 405(21), 6815–6822. 10.1007/s00216-013-7107-7 [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, & Benowitz NL (2005). Metabolism and disposition kinetics of nicotine. Pharmacological reviews, 57(1), 79–115. 10.1124/pr.57.1.3 [DOI] [PubMed] [Google Scholar]
- Issel EP, Lun A, Pohle R, & Gross J. (1988). The relationship of hypoxia to hypoxanthine concentration during pregnancy and delivery. J Perinat Med, 16(2), 99–107. 10.1515/jpme.1988.16.2.99 [DOI] [PubMed] [Google Scholar]
- Janakiraman V, Gantz M, Maynard S, & El-Mohandes A. (2009). Association of cotinine levels and preeclampsia among African-American women. Nicotine & Tobacco Research, 11(6), 679–684. 10.1093/ntr/ntp049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong A, Fiorito G, Keski-Rahkonen P, Imboden M, Kiss A, Robinot N, . . . Probst-Hensch N. (2018). Perturbation of metabolic pathways mediates the association of air pollutants with asthma and cardiovascular diseases. Environ Int, 119, 334–345. 10.1016/j.envint.2018.06.025 [DOI] [PubMed] [Google Scholar]
- Kalhan SC, Gruca LL, Parimi PS, O’Brien A, Dierker L, & Burkett E. (2003). Serine metabolism in human pregnancy. American Journal of Physiology-Endocrinology and Metabolism, 284(4), E733–E740. 10.1152/ajpendo.00167.2002 [DOI] [PubMed] [Google Scholar]
- Kelly RS, Croteau-Chonka DC, Dahlin A, Mirzakhani H, Wu AC, Wan ES, . . . Al-Garawi A. (2017). Integration of metabolomic and transcriptomic networks in pregnant women reveals biological pathways and predictive signatures associated with preeclampsia. Metabolomics, 13(1), 1–15. 10.1007/s11306-0161149-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharrazi M, DeLorenze GN, Kaufman FL, Eskenazi B, Bernert JT Jr., Graham S, . . . Pirkle J. (2004). Environmental tobacco smoke and pregnancy outcome. Epidemiology, 15(6), 660–670. 10.1097/01.ede.0000142137.39619.60 [DOI] [PubMed] [Google Scholar]
- Kwan S, King J, & Caudill M. (2015). Choline and placental trophoblast development. Human Placental Trophoblasts; Duttaroy A, Basak S, Eds. 10.1201/b19151-18 [DOI] [Google Scholar]
- Kwan STC, King JH, Yan J, Jiang X, Wei E, Fomin VG, . . . Caudill MA (2017). Maternal choline supplementation during murine pregnancy modulates placental markers of inflammation, apoptosis and vascularization in a fetal sex-dependent manner. Placenta, 53, 57–65. 10.1016/j.placenta.2017.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarre JL, Puttabyatappa M, Song PX, Goodrich JM, Zhou L, Rajendiran TM, . . . Dolinoy DC (2020). Maternal lipid levels across pregnancy impact the umbilical cord blood lipidome and infant birth weight. Scientific reports, 10(1), 1–15. 10.1038/s41598-020-71081-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladva CN, Golan R, Liang D, Greenwald R, Walker DI, Uppal K, . . . Sarnat JA (2018). Particulate metal exposures induce plasma metabolome changes in a commuter panel study. PLoS One, 13(9), e0203468. 10.1371/journal.pone.0203468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre PL, Palin M-F, & Murphy BD (2011). Polyamines on the reproductive landscape. Endocrine reviews, 32(5), 694–712. 10.1210/er.2011-0012 [DOI] [PubMed] [Google Scholar]
- Leonardi-Bee J, Britton J, & Venn A. (2011). Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: a meta-analysis. Pediatrics, 127(4), 734–741. 10.1542/peds.2010-3041 [DOI] [PubMed] [Google Scholar]
- Li S, Dunlop AL, Jones DP, & Corwin EJ (2016). High-Resolution Metabolomics: Review of the Field and Implications for Nursing Science and the Study of Preterm Birth. Biol Res Nurs, 18(1), 12–22. 10.1177/1099800415595463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, . . . Pulendran B. (2013). Predicting network activity from high throughput metabolomics. PLoS Comput Biol, 9(7), e1003123. 10.1371/journal.pcbi.1003123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liang D, Ye D, Chang HH, Ziegler TR, Jones DP, & Ebelt ST (2021). Application of high-resolution metabolomics to identify biological pathways perturbed by traffic-related air pollution. Environ Res, 193, 110506. 10.1016/j.envres.2020.110506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Ladva CN, Golan R, Yu T, Walker DI, Sarnat SE, . . . Sarnat JA (2019). Perturbations of the arginine metabolome following exposures to traffic-related air pollution in a panel of commuters with and without asthma. Environ Int, 127, 503–513. 10.1016/j.envint.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, . . . Sarnat JA (2018). Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environ Int, 120, 145–154. 10.1016/j.envint.2018.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Rasmussen M-LH, Piening B, Shen X, Chen S, Röst H, . . . Lee NC(2020). Metabolic dynamics and prediction of gestational age and time to delivery in pregnant women. Cell, 181(7), 1680–1692. e1615. 10.1016/j.cell.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Wang X, Wu G, Feng C, Zhou H, Li D, & Wang J. (2014). Improving amino acid nutrition to prevent intrauterine growth restriction in mammals. Amino acids, 46(7), 1605–1623. 10.1007/s00726-014-1725-z [DOI] [PubMed] [Google Scholar]
- Loy S-L, Sirajudeen K, & JM HJ (2013). Increase in maternal adiposity and poor lipid profile is associated with oxidative stress markers during pregnancy. Preventive medicine, 57, S41–S44. 10.1016/j.ypmed.2012.11.021 [DOI] [PubMed] [Google Scholar]
- Lu Y-P, Reichetzeder C, Prehn C, Yin L-H, Yun C, Zeng S, . . . Hocher B. (2018). Cord blood lysophosphatidylcholine 16: 1 is positively associated with birth weight. Cellular Physiology and Biochemistry, 45(2), 614–624. 10.1159/000487118 [DOI] [PubMed] [Google Scholar]
- Lumley J. (2003). Defining the problem: the epidemiology of preterm birth. BJOG: An International Journal of Obstetrics & Gynaecology, 110, 3–7. 10.1046/j.1471-0528.2003.00011.x [DOI] [PubMed] [Google Scholar]
- Mei-Dan E, Walfisch A, Weisz B, Hallak M, Brown R, & Shrim A. (2015). The unborn smoker: association between smoking during pregnancy and adverse perinatal outcomes. Journal of Perinatal Medicine, 43(5), 553–558. 10.1515/jpm-2014-0299 [DOI] [PubMed] [Google Scholar]
- Melichar V, Novak M, Zoula J, Hahn P, & Koldovský O. (1966). Energy sources in the newborn. In Development of Metabolism as Related to Nutrition (pp. 298–306): Karger Publishers. 10.1159/000389888 [DOI] [Google Scholar]
- Morrison N, Bearden D, Bundy JG, Collette T, Currie F, Davey MP, . . . Rochfort S. (2007). Standard reporting requirements for biological samples in metabolomics experiments: environmental context. Metabolomics, 3(3), 203–210. 10.1007/s11306-007-0080-4 [DOI] [Google Scholar]
- Mukherjee S, Velez Edwards DR, Baird DD, Savitz DA, & Hartmann KE (2013). Risk of miscarriage among black women and white women in a US Prospective Cohort Study. American journal of epidemiology, 177(11), 1271–1278. 10.1093/aje/kws393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia F, Iuculano A, Peddes C, Santoru ML, Tronci L, Deiana M, . . . Monni G. (2019). Metabolic fingerprinting of chorionic villous samples in normal pregnancy and chromosomal disorders. Prenatal diagnosis, 39(10), 848–858. 10.1002/pd.5461 [DOI] [PubMed] [Google Scholar]
- Nabet C, Ancel PY, Burguet A, & Kaminski M. (2005). Smoking during pregnancy and preterm birth according to obstetric history: French national perinatal surveys. Paediatric and perinatal epidemiology, 19(2), 88–96. 10.1111/j.13653016.2005.00639.x [DOI] [PubMed] [Google Scholar]
- Obstetricians A. C. o., Gynecologists, & Practice C. o. O. (2017). Methods for estimating the due date. Obstet Gynecol, 129, 959–960. 10.1097/aog.0000000000002046 [DOI] [Google Scholar]
- Oliveira MW, Minotto JB, de Oliveira MR, Zanotto-Filho A, Behr GA, Rocha RF, . . . Klamt F. (2010). Scavenging and antioxidant potential of physiological taurine concentrations against different reactive oxygen/nitrogen species. Pharmacological Reports, 62(1), 185–193. 10.1016/s1734-1140(10)70256-5 [DOI] [PubMed] [Google Scholar]
- Orhon FS, Ulukol B, Kahya D, Cengiz B, Başkan S, & Tezcan S. (2009). The influence of maternal smoking on maternal and newborn oxidant and antioxidant status. European journal of pediatrics, 168(8), 975–981. 10.1007/s00431-008-0873-0 [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Upson K, & Buckley JP (2017). Lipid and creatinine adjustment to evaluate health effects of environmental exposures. Current environmental health reports, 4(1), 44–50. 10.1007/s40572-017-0122-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JY, Lee JH, & Kim SH (2015). Plasma levels of lysine, tyrosine, and valine during pregnancy are independent risk factors of insulin resistance and gestational diabetes. Metab Syndr Relat Disord, 13(2), 64–70. 10.1089/met.2014.0113 [DOI] [PubMed] [Google Scholar]
- Peacock JL, Cook DG, Carey IM, Jarvis MJ, Bryant AE, Anderson HR, & Bland JM (1998). Maternal cotinine level during pregnancy and birthweight for gestational age. International journal of epidemiology, 27(4), 647–656. 10.1093/ije/27.4.647. [DOI] [PubMed] [Google Scholar]
- Polidori MC, Mecocci P, Stahl W, & Sies H. (2003). Cigarette smoking cessation increases plasma levels of several antioxidant micronutrients and improves resistance towards oxidative challenge. Br J Nutr, 90(1), 147–150. 10.1079/bjn2003890 [DOI] [PubMed] [Google Scholar]
- Pérez-Stable EJ, Herrera B, Jacob P 3rd, & Benowitz NL (1998). Nicotine metabolism and intake in black and white smokers. Jama, 280(2), 152–156. 10.1001/jama.280.2.152 [DOI] [PubMed] [Google Scholar]
- Rashid M, & Rashid H. (2003). Passive maternal smoking and pregnancy outcome in a Saudi population. Saudi medical journal, 24(3), 248–253. [PubMed] [Google Scholar]
- Reece MS, McGregor JA, Allen KG, & Harris MA (1997). Maternal and perinatal long-chain fatty acids: possible roles in preterm birth. American Journal of Obstetrics and Gynecology, 176(4), 907–914. 10.1016/s0002-9378(97)70620-3 [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, . . . Redmer DA(2010). Uteroplacental vascular development and placental function: an update. Int J Dev Biol, 54(2–3), 355–366. 10.1387/ijdb.082799lr [DOI] [PubMed] [Google Scholar]
- Robinson O, Keski-Rahkonen P, Chatzi L, Kogevinas M, Nawrot T, Pizzi C, . . . Sunyer J. (2018). Cord blood metabolic signatures of birth weight: a population-based study. Journal of proteome research, 17(3), 1235–1247. 10.1021/acs.jproteome.7b00846 [DOI] [PubMed] [Google Scholar]
- Rolle-Kampczyk UE, Krumsiek J, Otto W, Röder SW, Kohajda T, Borte M, . . . von Bergen M. (2016). Metabolomics reveals effects of maternal smoking on endogenous metabolites from lipid metabolism in cord blood of newborns. Metabolomics, 12, 76. 10.1007/s11306-016-0983-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Chaiworapongsa T, Gomez R, . . . Beecher C. (2010). Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J Matern Fetal Neonatal Med, 23(12), 1344–1359. 10.3109/14767058.2010.482618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihu HM, Sharma PP, Getahun D, Hedayatzadeh M, Peters S, Kirby RS, . . . Gaafer-Ahmed H. (2008). Prenatal Tobacco Use and Risk of Stillbirth: A Case— Control and Bidirectional Case—Crossover Study. Nicotine & Tobacco Research, 10(1), 159–166. 10.1080/14622200701705431 [DOI] [PubMed] [Google Scholar]
- Salihu HM, & Wilson RE (2007). Epidemiology of prenatal smoking and perinatal outcomes. Early human development, 83(11), 713–720. 10.1016/j.earlhumdev.2007.08.002 [DOI] [PubMed] [Google Scholar]
- Scholl TO, Chen X, Khoo CS, & Lenders C. (2004). The dietary glycemic index during pregnancy: influence on infant birth weight, fetal growth, and biomarkers of carbohydrate metabolism. Am J Epidemiol, 159(5), 467–474. 10.1093/aje/kwh068 [DOI] [PubMed] [Google Scholar]
- Schwertner HA (1998). Association of smoking and low serum bilirubin antioxidant concentrations. Atherosclerosis, 136(2), 383–387. 10.1016/s00219150(97)00232-3 [DOI] [PubMed] [Google Scholar]
- Sevastou I, Kaffe E, Mouratis M-A, & Aidinis V. (2013). Lysoglycerophospholipids in chronic inflammatory disorders: the PLA2/LPC and ATX/LPA axes. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1831(1), 42–60. 10.1016/j.bbalip.2012.07.019 [DOI] [PubMed] [Google Scholar]
- Shah T, Sullivan K, & Carter J. (2006). Sudden infant death syndrome and reported maternal smoking during pregnancy. American journal of public health, 96(10), 1757–1759. 10.2105/AJPH.2005.073213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, . . . Karumanchi SA (2004). First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab, 89(2), 770–775. 10.1210/jc.2003-031244 [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Surcel HM, Bloigu A, Nuutila M, Ylikorkala O, Hiilesmaa V, & Paavonen J. (2010). Self-reported smoking habits and serum cotinine levels in women with placental abruption. Acta Obstet Gynecol Scand, 89(12), 1538–1544. 10.3109/00016349.2010.526187 [DOI] [PubMed] [Google Scholar]
- Troisi R, Potischman N, Roberts JM, Harger G, Markovic N, Cole B, . . . Hoover RN (2003). Correlation of serum hormone concentrations in maternal and umbilical cord samples. Cancer Epidemiology and Prevention Biomarkers, 12(5), 452–456. [PubMed] [Google Scholar]
- Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, & Jones DP (2013). xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC bioinformatics, 14(1), 15. 10.1186/1471-2105-14-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagijo M. a., Sheikh A, Duijts L, & Been JV (2017). Reducing tobacco smoking and smoke exposure to prevent preterm birth and its complications. Paediatric respiratory reviews, 22, 3–10. 10.1016/j.prrv.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Wang-Sattler R, Yu Y, Mittelstrass K, Lattka E, Altmaier E, Gieger C, . . . Hao P. (2008). Metabolic profiling reveals distinct variations linked to nicotine consumption in humans—first results from the KORA study. PloS one, 3(12), e3863. 10.1371/journal.pone.0003863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. (2013). Functional amino acids in nutrition and health. Amino Acids, 45(3), 407–411. 10.1007/s00726-013-1500-6 [DOI] [PubMed] [Google Scholar]
- Yoshie Y, & Ohshima H. (1997). Synergistic induction of DNA strand breakage by cigarette tar and nitric oxide. Carcinogenesis, 18(7), 1359–1363. 10.1093/carcin/18.7.1359 [DOI] [PubMed] [Google Scholar]
- Yost EE, Euling SY, Weaver JA, Beverly BEJ, Keshava N, Mudipalli A, . . . Makris SL (2019). Hazards of diisobutyl phthalate (DIBP) exposure: A systematic review of animal toxicology studies. Environ Int, 125, 579–594. 10.1016/j.envint.2018.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Park Y, Johnson JM, & Jones DP (2009). apLCMS—adaptive processing of high-resolution LC/MS data. Bioinformatics, 25(15), 1930–1936. 10.1093/bioinformatics/btp291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu Y, Shi J, Larson DF, & Watson RR (2002). Side-stream cigarette smoke induces dose-response in systemic inflammatory cytokine production and oxidative stress. Exp Biol Med (Maywood), 227(9), 823–829. 10.1177/153537020222700916 [DOI] [PubMed] [Google Scholar]
- Zhang WZ, Venardos K, Chin-Dusting J, & Kaye DM (2006). Adverse effects of cigarette smoke on NO bioavailability: role of arginine metabolism and oxidative stress. Hypertension, 48(2), 278–285. 10.1161/01.Hyp.0000231509.27406.42 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.