Abstract
Objectives:
To quantify myocardial blood flow (MBF) and myocardial perfusion reserve (MPR) in dilated cardiomyopathy (DCM) and examine the relationship between myocardial perfusion and adverse left ventricular (LV) remodeling.
Background:
Although regarded as a non-ischemic condition, DCM has been associated with microvascular dysfunction, which is postulated to play a role in its pathogenesis. However, the relationship of the resulting perfusion abnormalities to myocardial fibrosis and the degree of LV remodeling is unclear.
Methods:
Sixty-five patients and 35 healthy controls underwent adenosine (140ug/kg/min) stress perfusion cardiovascular magnetic resonance with late gadolinium enhancement imaging. Stress and rest MBF and MPR were derived using a modified Fermi-constrained deconvolution algorithm.
Results:
Patients had significantly higher global rest MBF compared to controls (1.73±0.42 vs 1.14±0.42 mL/g/min, p<0.001). In contrast, global stress MBF was significantly lower versus controls (3.07±1.02 vs 3.53±0.79 mL/g/min, p=0.02), resulting in impaired MPR in the DCM group (1.83±0.58 vs 3.50±1.45, p<0.001). Global stress MBF (2.70±0.89 vs 3.44±1.03 mL/g/min, p=0.017) and global MPR (1.67±0.61 vs 1.99±0.50, p=0.047) were significantly reduced in DCM patients with LVEF≤35% compared to those with LVEF>35%. Segments with fibrosis had lower rest MBF (mean difference −0.12 mL/g/min, 95% confidence interval: −0.23 to −0.01 mL/g/min, p=0.035) and lower stress MBF (mean difference −0.15 mL/g/min, 95% confidence interval: −0.28 to −0.03 ml/g/min, p=0.013).
Conclusions:
Patients with DCM exhibit microvascular dysfunction, the severity of which is associated with the degree of LV impairment. However, rest MBF is elevated rather than reduced in DCM. If microvascular dysfunction contributes to the pathogenesis of DCM, then the underlying mechanism is more likely to involve stress-induced repetitive stunning rather than chronic myocardial hypoperfusion.
Keywords: Cardiovascular Magnetic Resonance Imaging, Myocardial Blood Flow, Myocardial Perfusion Imaging, Dilated Cardiomyopathy, Microvascular Dysfunction
Background
Dilated cardiomyopathy (DCM) is characterized by left ventricular (LV) cavity enlargement and impaired contraction in the absence of significant coronary artery disease (CAD) (1). Although traditionally regarded as a “non-ischemic” cardiomyopathy, several studies have identified myocardial perfusion abnormalities in DCM patients with moderate or severe adverse remodeling (2–11). In particular, positron emission tomography (PET) and invasive angiographic assessment have demonstrated impaired myocardial perfusion reserve (MPR) (4–7,10,11), and coronary flow reserve (2,3,9). In the absence of obstructive epicardial CAD, these findings may indicate underlying microvascular dysfunction, providing support for a “microvascular ischemia hypothesis”, whereby chronic or recurrent myocardial hypoperfusion may drive fibrosis and adverse remodeling in DCM (4,6,12).
Cardiovascular Magnetic Resonance (CMR) facilitates detailed phenotyping of DCM and assessment of myocardial perfusion in a single investigation (1). As with PET, CMR allows the absolute quantification of myocardial blood flow (MBF), but with the added advantages of offering superior spatial resolution and concomitant evaluation of myocardial fibrosis (13,14). Such advantages are of particular relevance to DCM, in which LV wall thinning frequently accompanies adverse remodeling and myocardial replacement fibrosis is an important prognosticator (15). While the assessment of myocardial perfusion in DCM has been an area of active study, the relationship between myocardial perfusion and markers of adverse remodeling and fibrosis has not been fully elucidated. In this prospective study, we used CMR to evaluate absolute MBF and MPR in a broad spectrum of patients with DCM, and to examine the relationship between myocardial perfusion, LV morphology, function and replacement fibrosis.
Methods
Patients
Consecutive patients (n=65) referred for CMR with a clinical diagnosis of DCM of at least 6 months duration were prospectively enrolled (16). The diagnosis of DCM was confirmed by CMR on the basis of: 1) increased left ventricular end-diastolic volume indexed to body surface area and reduced left ventricular ejection fraction (LVEF) compared to published reference ranges normalized for age and gender (17); and 2) absence of significant CAD or subendocardial late gadolinium enhancement (LGE) indicative of previous myocardial infarction. No patient had a history of previous myocardial infarction or coronary revascularization. Additional exclusion criteria included diabetes, hypertensive heart disease and significant primary valvular heart disease. Significant CAD, defined as ≥50% luminal stenosis in one or more epicardial vessels was excluded by coronary angiography in 45 (69%) patients, negative stress echo/SPECT in 12 (18%) patients, and CT coronary angiography in 5 (8%) patients. The remaining 3 patients were ≤40 years of age, with no history of angina, and no atherosclerotic risk factors. Healthy volunteers, of similar age and gender distributions, without cardiovascular disease, hypertension, diabetes, or smoking history, were recruited as controls (n=35). The study was approved by the local ethics committee and all patients and controls gave written informed consent.
CMR Protocol
CMR was performed on a 1.5T Siemens Avanto using a 12-element phased-array coil (Siemens Medical Solutions, Erlangen, Germany). Steady-state free precession cine imaging was first performed in standard long- and short-axis views with full myocardial coverage (18). All subjects were instructed to avoid caffeine ingestion for 24 hours prior to the CMR study. Adenosine was infused at 140μg/kg/min for 4 minutes to induce hyperemia. In subjects who did not exhibit an adequate hemodynamic response (increase in heart rate>10 beats per minute and/or fall in blood pressure>10mmHg), adenosine was increased in a step-wise fashion to a maximum dose of 210μg/kg/min (18). After injection of 0.1 mmol/kg gadobutrol (Gadovist, Schering AG, Berlin, Germany), stress first-pass perfusion images were then acquired in 3 short-axis imaging planes (basal, mid-ventricular and apical slices) using a hybrid echo planar imaging sequence (19), together with a low-resolution image for the arterial input function, as previously described (20). Typical perfusion sequence pulse parameters were as follows: echo time 1.12ms, repetition time 5.6ms, voxel size 2.3 × 2.3 × 8.0 mm3, and flip angle 28°.
LGE images were subsequently obtained using an inversion-recovery prepared gradient echo sequence. Inversion times were optimized to null normal myocardium with images repeated in two orthogonal phase-encoding directions to exclude artefact. First-pass perfusion imaging was repeated at least twenty minutes after stress image acquisition in identical slice locations to obtain resting images.
Image Analysis
Ventricular volumes, ejection fraction, and LV mass were quantified using dedicated semi-automated software (CMRtools, Cardiovascular Imaging Solutions, London, UK) (17). For perfusion quantification, after non-rigid in-plane image registration (21), the endocardial and epicardial contours were manually traced to generate the myocardial regions of interest (ROI) for the three short-axis perfusion slices. Each slice was then subdivided into four segments corresponding to the anterior, lateral, inferior and septal walls. Proton-density weighted images were used for surface coil intensity bias correction (22). Time-signal intensity curves were generated from the mean signal intensity in each of the twelve myocardial segments during first-pass perfusion. Perfusion quantification was performed by an observer blinded to all other CMR and clinical data. Absolute rest and stress MBF were quantified for each segment by a modified Fermi-function constrained deconvolution of the time-signal intensity curves from the arterial input function measured in the LV blood pool, as previously described (20,22). Rest MBF values were corrected for rate-pressure product in order to eliminate the effect of inter-individual differences in cardiac workload at rest (rest MBFcorrected=rest MBF/rate-pressure-productx10,000) (23). MPR was calculated as the ratio of stress MBF to rest MBFcorrected. Global rest MBF, stress MBF and MPR were derived by calculating the mean of the corresponding values for the twelve segments. For each patient, the presence and segmental location of mid-wall and/or epicardial fibrosis on LGE short-axis images corresponding to the slice location of the perfusion images was adjudicated by 2 experienced observers.
Statistical Analysis
Categorical data are presented as frequencies (%). Continuous data are expressed as mean ± standard deviation or as medians with interquartile ranges as appropriate. Baseline characteristics were compared using Fisher’s exact test for categorical data and the Wilcoxon rank-sum test for continuous data. Patients with DCM were subdivided into two binary sub-groups: 1) LVEF >35% or LVEF ≤35%, and 2) with or without fibrosis. Comparisons of rest MBF, stress MBF and MPR between these sub-groups were performed using the Wilcoxon rank-sum test. Mixed-effects linear regression was used to investigate within patients whether segments with fibrosis had significantly different MBF to segments without fibrosis.
Linear regression analysis was performed to examine the relationships between perfusion measurements and LVEF in the entire study population. Univariable regression was used to investigate the association between perfusion measurements and clinical/CMR characteristics within the DCM cohort. Multivariable models were used to adjust for age, sex, LVEF, New York Heart Association (NYHA) functional class and LV mass index, as well as any other variables that were significant on univariable analysis, unless two variables were collinear in which case only one was included. Two-tailed values of p<0.05 were considered significant. Where multiple comparisons were necessary, a Bonferroni correction was applied to the p-values. All statistical analyses were conducted using Stata Version 15 (StataCorp, College Station, Texas, USA).
Results
Sixty-five patients with DCM and 35 healthy control volunteers were studied. The baseline characteristics of the study population are summarized in Table 1. Patients and controls were comparable with respect to age and sex. There was no significant difference in baseline systolic blood pressure between patients and controls, but DCM patients had a higher weight, resting heart rate, and rate pressure product. Among the patients with DCM, 33 subjects (51%) had an LVEF≤35% and 32 (49%) subjects had an LVEF>35%. Patients in the LVEF≤35% and >35% subgroups had similar cardiovascular risk factor profile, heart failure duration, and treatment rates with angiotensin converting enzyme inhibitors/angiotensin II receptor blockers and beta-blockers, as well as a similar prevalence of mid-wall fibrosis (Table 2). The subgroup of patients with LVEF≤35% were older than the DCM LVEF>35% subgroup and were also more likely to receive treatment with loop diuretics, with a trend towards higher NYHA functional class.
Table 1:
Baseline Demographic and Imaging Data for DCM and Control Groups
| DCM n=65 | Controls n=35 | P- value | |
|---|---|---|---|
| Age, years | 50 [44, 62] | 45 [38, 54] | 0.055 |
| Male, n (%) | 48 (73.8) | 21 (60.0) | 0.18 |
| Height, m | 1.74 [1.69, 1.80] | 1.73 [1.63, 1.82] | 0.50 |
| Weight, kg | 80 [69, 95] | 73 [62, 83] | 0.009 |
| Rest heart rate, min−1 | 73 [67, 85] | 64 [57, 70] | <0.001 |
| Stress heart rate, min−1 | 93 [80, 103] | 90 [80, 103] | 0.76 |
| Rest Systolic Blood Pressure, mmHg | 124 [108, 134] | 117 [112, 133] | 0.57 |
| Stress Systolic Blood Pressure, mmHg | 128 [114, 139] | 122 [107, 134] | 0.28 |
| Rate-Pressure Product, mmHg min−1 | 8829 [7560, 10500] | 7606 [6669, 8970] | 0.002 |
| Imaging Data | |||
| Cardiac Index | 3570 [3039, 4104] | 3460 [3125, 3852] | 0.58 |
| LVEDVi, mL/m2 | 129 [112, 171] | 79 [74, 88] | <0.001 |
| LVESVi, mL/m2 | 84 [62, 124] | 26 [21, 31] | <0.001 |
| LVEF, % | 35 [26, 46] | 68 [64, 71] | <0.001 |
| LV mass index, g/m2 | 82 [74, 95] | 57 [49, 63] | <0.001 |
| RVEDVi, mL/m2 | 90 [69, 108] | 80 [70, 89] | 0.045 |
| RVESVi, mL/m2 | 42 [29, 55] | 31 [25, 37] | 0.002 |
| RVEF, % | 54 [41, 61] | 61 [57, 69] | 0.001 |
| Mid-wall fibrosis, n (%) | 16 (24.6) | 0 (0.0) | <0.001 |
DCM=Dilated cardiomyopathy; LVEDVi=Left Ventricular End-Diastolic Volume index; LVESVi=Left Ventricular End-Systolic Volume index; LVEF=Left Ventricular Ejection Fraction; LV=Left ventricular; RVEDV=Right Ventricular End-Diastolic Volume index; RVESVi=Right Ventricular End-Systolic Volume index; RVEF=Right Ventricular Ejection Fraction.
Table 2:
Baseline Clinical and Imaging Characteristics stratified by LV Function
| DCM LVEF ≤35% n=33 (51%) | DCM LVEF >35% n=32 (49%) | P value | |
|---|---|---|---|
| Age, years | 55 [46, 66] | 47 [35, 58] | 0.018 |
| Male, n (%) | 26 (78.8) | 22 (68.8) | 0.41 |
| Hypertension, n (%) | 5 (15.2) | 6 (18.8) | 0.75 |
| Hyperlipidemia, n (%) | 11 (33.3) | 5 (15.6) | 0.15 |
| Smoker, n (%) | 5 (15.2) | 5 (15.) | 1.00 |
| Left Bundle Branch Block, n (%) | 10 (30.3) | 10 (31.3) | 1.00 |
| Rate-Pressure Product, mmHg min−1 | 9672 [8100, 10578] | 8364 [7336, 10464] | 0.13 |
| New York Heart Association Class, n (%) | |||
| I | 11 (33.3) | 20 (62.5) | |
| II | 15 (45.5) | 9 (28.1) | 0.057 |
| III | 7 (21.2) | 3 (9.4) | |
| Medical therapy, n (%) | |||
| ACE inhibitor or A2RB | 31 (93.9) | 29 (90.6) | 0.67 |
| Beta blocker | 28 (84.8) | 23 (71.9) | 0.24 |
| Aldosterone antagonist | 16 (48.5) | 12 (37.5) | 0.46 |
| Loop diuretic | 19 (57.6) | 6 (18.8) | 0.002 |
| Imaging Data | |||
| Cardiac Index | 3158 [2900, 3765] | 3834 [3349, 4202] | 0.005 |
| LVEDVi, mL/m2 | 171 [129, 199] | 123 [108, 134] | <0.001 |
| LVESVi, mL/m2 | 124 [92, 153] | 64 [54, 79] | <0.001 |
| LVEF, % | 26 [22, 32] | 47 [42, 52] | <0.001 |
| LV mass index, g/m2 | 85 [75, 116] | 78 [69, 88] | 0.033 |
| RVEDVi, mL/m2 | 85 [66, 112] | 98 [79, 107] | 0.43 |
| RVESVi, mL/m2 | 52 [29, 70] | 41 [29, 48] | 0.16 |
| RVEF, % | 43 [28, 56] | 59 [54, 63] | <0.001 |
| Midwall fibrosis, n (%) | 11 (33.3) | 5 (15.6) | 0.15 |
DCM=Dilated Cardiomyopathy; ACE=Angiotensin Converting Enzyme; A2RB=Angiotensin II Receptor Blocker; LVEDVi= Left Ventricular End-Diastolic Volume index; LVESVi=Left Ventricular End-Systolic Volume index; LVEF=Left Ventricular Ejection Fraction; LV=Left Ventricular; RVEDVi=Right Ventricular End-Diastolic Volume index; RVESVi=Right Ventricular End-Systolic Volume index; RVEF=Right ventricular ejection fraction.
Global Myocardial Blood Flow and Myocardial Perfusion Reserve
DCM vs Controls:
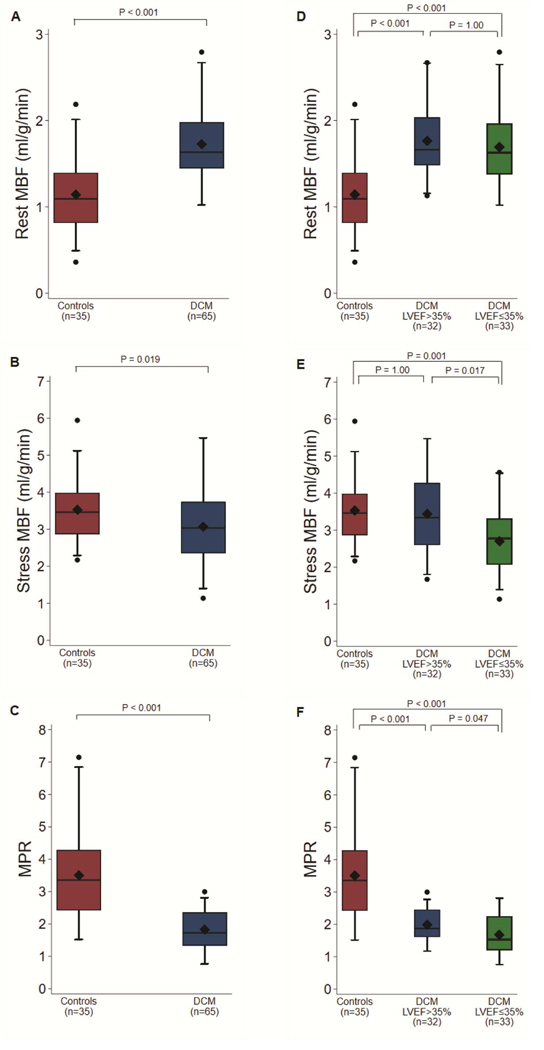
Patients with DCM had a significantly higher global rest MBF compared to controls (1.73±0.42 vs 1.14±0.42 mL/g/min, p<0.001) (Figure 1A). In contrast, global stress MBF was significantly lower in DCM patients than in controls (3.07±1.02 vs 3.53±0.79 mL/g/min, p=0.02) (Figure 1B). Consequently, global MPR was severely impaired in the DCM group (1.83±0.58 vs 3.50±1.45, p<0.001) (Figure 1C). The differences in global rest MBF and MPR remained significant when rest MBF without RPP correction was substituted in the analysis (uncorrected rest MBF: 1.56±0.38 vs 0.95±0.52 mL/g/min, p<0.001; MPR 2.01±0.65 vs 5.02±3.20 mL/g/min, p<0.001).
Figure 1: Myocardial Perfusion Measurements in Dilated Cardiomyopathy and Healthy Controls.
Stress and Rest Global Myocardial Blood Flow and Myocardial Perfusion Reserve in Dilated Cardiomyopathy Patients versus Controls (A-C) and stratified by Left Ventricular Ejection Fraction (D-F). MBF=Myocardial blood flow; DCM=Dilated cardiomyopathy; MPR=Myocardial perfusion reserve; LVEF=Left ventricular ejection fraction.
DCM LVEF≤35% vs DCM LVEF>35%:
At rest, global MBF was comparable between the DCM subgroups (1.69±0.43 vs 1.77±0.41 mL/g/min, p=1.00) (Figure 1D). However, both global stress MBF (2.70±0.89 vs 3.44±1.03 mL/g/min, p=0.017) and global MPR (1.67±0.61 vs 1.99±0.50, p=0.047) were significantly reduced in DCM patients with LVEF≤35% compared to those with LVEF>35% (Figures 1E and 1F). Although the DCM subgroup with LVEF>35% and healthy controls had similar global stress MBF (3.44±1.03 vs 3.53±0.79 mL/g/min, p=1.00) (Figure 1E), global rest MBF was significantly increased in the LVEF>35% subgroup (1.77±0.41 vs 1.14±0.42 mL/g/min, p<0.001) (Figure 1D). Accordingly, DCM patients with LVEF>35% still demonstrated a significantly depressed global MPR compared to controls (1.99±0.50 vs 3.50±1.45, p<0.001) (Figure 1F).
Myocardial Perfusion and Clinical/CMR Parameters
Global Rest MBF:
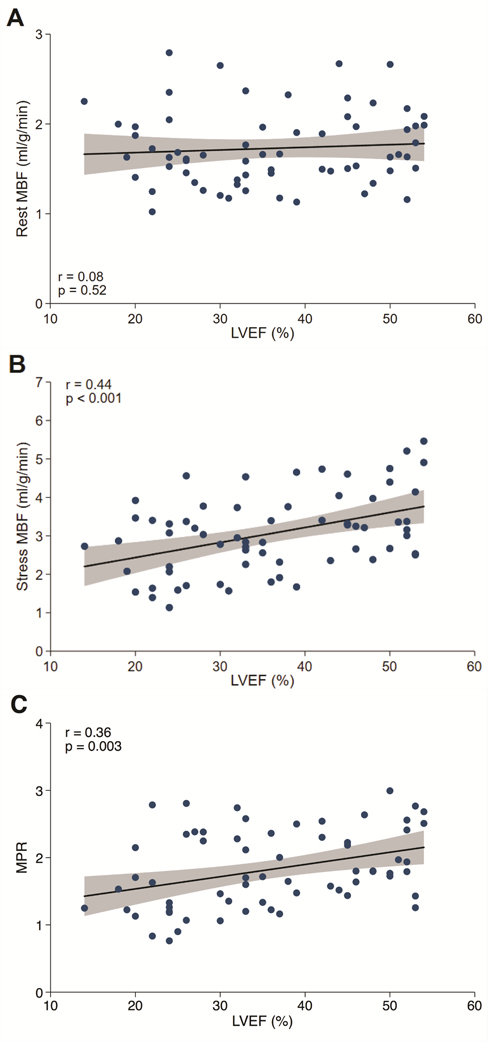
In DCM patients, univariable analysis revealed that weight (β=−0.08, 95% confidence interval [CI] −0.13 to −0.02, p=0.008), resting systolic blood pressure (β=−0.15, 95% CI −0.20 to −0.10, p<0.001), resting diastolic blood pressure (β=−0.13, 95% CI −0.20 to −0.07, p<0.001), and indexed LV mass (β =−0.04, 95% CI −0.08 to 0.00, p=0.032) were significantly associated with global rest MBF (Online Figure 1A). However, only weight retained borderline significance on multivariable analysis (β=−0.67, 95% CI −0.13 to −0.00, p=0.049). Evaluating DCM patients, there was no correlation between rest MBF and LVEF (Figure 2A).
Figure 2: Myocardial Perfusion and Left Ventricular Function.
Relationship of Rest MBF (A), Stress MBF (B), and MPR (C) to LVEF in Dilated Cardiomyopathy. MBF=Myocardial blood flow; LVEF=Left Ventricular Ejection Fraction; MPR=Myocardial Perfusion Reserve.
Global Stress MBF:
Significant univariable predictors of stress MBF in DCM patients included stress heart rate (β=0.23, 96% CI 0.09 to 0.37, p=0.002), NYHA functional class (β=−0.26, 95% CI −0.50 to −0.02, p=0.036), LVEF (β=0.39, 95% CI 0.19 to 0.59, p<0.001), LV end-diastolic volume index (β =−0.09, 95% CI −0.14 to −0.03, p=0.004), LV end-systolic volume index (β=−0.10, 95% CI −0.16 to −0.04, p<0.001), indexed LV mass (β=−0.14, 95% CI −0.23 to −0.05, p=0.003), RVEF (β=0.20, 95% CI 0.03 to 0.37, p=0.020), and the presence of midwall fibrosis (β=−0.62, 95% CI −1.20 to −0.05, p=0.034) (Online Figure 1B). Multivariable regression analysis demonstrated that stress heart rate (β=0.20, 95% CI 0.06 to 0.34, p=0.007) and LVEF (β=0.28, 95% CI 0.05 to 0.51, p=0.018) were independently associated with stress global MBF. Figure 2B illustrates the significant positive association between stress MBF and LVEF in DCM patients.
Global MPR:
Several parameters were associated with MPR on univariable analysis including resting systolic blood pressure (β=0.14, 95% CI 0.07 to 0.22, p<0.001), resting diastolic blood pressure (β=0.10, 95% CI 0.00 to 0.20, p=0.048), stress heart rate (β=0.12, 95% CI 0.04 to 0.20,p=0.004), stress systolic blood pressure (β=0.08, 95% CI 0.02 to 0.14, p=0.012), LVEF (β=0.18, 95% CI 0.06 to 0.30, p=0.003), LV end-diastolic volume index (β=−0.04, 95% CI −0.07 to −0.00, p=0.026), LV end-systolic volume index (β=−0.05, 95% CI −0.08 to −0.01, p=0.007), and RVEF (β=0.10, 95% CI 0.01 to 0.20, p=0.036) (Online Figure 1C). In the multivariable model, only stress HR (β=0.14, 95% CI 0.06 to 0.22, p<0.001), stress systolic BP (β=0.08, 95% CI 0.02 to 0.14, p=0.008), and LVEF (β=0.14, 95% CI 0.01 to 0.28, p=0.042) were identified as independent determinants of MPR. The significant positive association between global MPR and LVEF in DCM patients is illustrated in Figure 2C.
LV Wall Stress:
there was no association between stress or rest MBF and the ratio of LVEDV to LV mass (a surrogate for LV wall stress) in patients with DCM (r=0.06, p=0.59 and r=0.12, p=0.34 respectively).
Myocardial Perfusion and Fibrosis
Overall, 16 (25%) DCM patients had midwall fibrosis, detected in 72/192 (38%) segments. Compared to patients without fibrosis, those with fibrosis were similar in terms of their baseline demographic characteristics, CMR parameters and medication history (Online Table 1). Patients with fibrosis had similar rest MBF (1.70±0.48 vs 1.74±0.40 mL/g/min, p=0.66) but lower stress MBF (2.60±1.04 vs 3.22±0.98 mL/g/min, p=0.04) and a trend towards lower MPR (1.60±0.63 vs 1.90±0.54, p=0.05), compared to those without fibrosis.
After adjustment for intracluster correlation within the 16 patients who had fibrosis, segments with fibrosis had lower rest MBF (mean difference −0.12 mL/g/min, 95% CI: −0.23 to −0.01 mL/g/min, p=0.035) and lower stress MBF (mean difference −0.15 mL/g/min, 95% CI: −0.28 to −0.03 ml/g/min, p=0.013) but similar MPR (mean difference 0.04, 95% CI: −0.07 to 0.15, p=0.44).
Discussion
We have performed the first study using fully quantitative first-pass perfusion CMR methods to evaluate rest and stress MBF in a large and broad cohort of patients with DCM. Our study highlights that patients with DCM not only have reduced MPR and absolute stress MBF, but also increased rest MBF, compared with matched controls. Furthermore, we show that reduced stress MBF is associated with both the degree of LV dysfunction and the presence of myocardial fibrosis.
It has been hypothesized that a myocardial oxygen deficit caused by microvascular dysfunction may contribute to deterioration of LV function in DCM (6,12). The ‘microvascular ischemia hypothesis’ postulates that chronic (rest) and/or repetitive (stress) myocardial hypoperfusion as a result of microvascular dysfunction may drive progressive LV systolic dysfunction and dilatation, which in turn may affect microvascular function in a vicious cycle (6,12). If proven, the microcirculation may in turn represent a novel therapeutic target to promote reverse remodeling.
Our results, which demonstrate a lower stress MBF in DCM, support the premise that microvascular dysfunction may exist at the level of the resistance vessels responsible for augmenting perfusion (4,6,10,24). The mean global absolute stress MBF of our DCM cohort is higher than previously reported (4,6,10,24), and not reduced to levels conventionally indicative of ischemia (25). This finding is consistent with the work of Dass et al who identified dissociation between microvascular dysfunction and tissue oxygenation in DCM (26). However, myocardial ischemia is a metabolic state that represents the mismatch between supply and demand, and assessment of perfusion alone is not therefore a perfect surrogate for this. Nevertheless, our finding that rest MBF is significantly higher in DCM patients compared to controls provides circumstantial evidence against chronic microvascular ischemia being the main driver of adverse LV remodeling. We therefore hypothesize that the failing myocardium requires higher rest MBF to compensate for the increased myocardial oxygen demands from increased work and potentially the need to overcome higher wall stress. However, in contrast to the significant difference in rest MBF between DCM patients and controls, we found no significant association between rest MBF and LVEF within the DCM group. We propose that this may be attributable to a primary myocardial problem of impaired substrate utilization, as documented by Neglia et al (27), with the result that the metabolic drive to increased rest MBF is attenuated. When MBF is uncoupled from metabolic demand by the vasodilator adenosine, stress MBF is intimately related to LVEF, and therefore stress MBF and MPR correlate with LVEF. If microvascular ischemia plays a role in the pathogenesis of DCM, we therefore speculate that the myocardial insult stems from transient repetitive episodes of myocardial ischemia, due to abnormal vasodilation and inadequate MBF augmentation during stress, rather than resting hypoperfusion.
In our study, DCM patients with severe LV dysfunction had greater reductions in stress MBF and MPR, compared to those with lesser degrees of contractile impairment. On univariable analysis, we observed that stress MBF was significantly associated with LVEF, LV volumes, and LV mass index. Of these LV indices, only LVEF was independently associated with stress MBF, supportive of a close relationship between stress perfusion and pump function, particularly in the pathophysiologic range.
Several reports have also highlighted endothelial dysfunction as a potential cause of perfusion abnormalities in DCM (6,9,10,12). The reduced stress MBF and MPR observed in our study may additionally stem from failure of the coronary microcirculation to adapt to myocardial hypertrophy, since the increase in LV mass in DCM is not accompanied by an appropriate growth of coronary resistance vessels (28,29). Tsagalou et al found a reduction in myocardial capillary density, which correlated with depressed coronary flow reserve measured by left heart catheterization (30). Work by Laguens et al has identified a significant reduction in length density and loss of vessel wall smooth muscle in small resistance-arterioles (31). Although a reduction in intramyocardial capillaries was not found, their findings suggest that coronary microcirculatory remodeling may still contribute to decreased flow reserve (31). In our DCM cohort, indexed LV mass was higher compared to controls and correlated negatively with both rest and stress MBF. Although we do not have histological corroboration, our findings imply incomplete adaptation of the coronary microcirculation to eccentric LV hypertrophy and remodeling.
The decreased stress MBF and MPR observed in the DCM patients may relate to increases in wall stress and extravascular compression of microcirculatory vessels. Previous studies using intracoronary Doppler flow velocity found that hyperemic flow velocity positively correlated with LVEF in DCM patients, and exhibited a significant negative association with LV end-diastolic wall stress (29,32). Van den Heuval et al similarly observed a negative correlation in DCM patients between MPR measured by PET and LV systolic wall stress derived by echocardiography (6). Knaapen et al used CMR to measure the ratio of LVEDV to LV mass, an indirect measure of global end-diastolic wall stress (33), and found that this inversely correlated with stress MBF derived by PET (34). In contrast, Neglia et al found no correlation between coronary flow values or stress MBF values derived by perfusion PET between LVEF and LV wall stress measured by echocardiography and LVEDP on left heart catheterization (10). This observation is corroborated by the present study, in which we found no association between stress or rest MBF and the ratio of LVEDV to LV mass (33,34).
Although some previous PET studies have also suggested that rest MBF may also be impaired in DCM (5,7,10,35), others have found no difference between DCM patients and controls (4,6,34). It is likely that the lack of consensus on rest myocardial perfusion in the literature using nuclear methods is a reflection of the small sample sizes in these studies, but also the limited spatial resolution making detection of areas of MBF abnormality challenging to resolve in a pathology characterized by thin myocardial walls (36). The differences in MBF values between these PET studies may also be explained by the use of different acquisition protocols, PET tracers, and quantification models (37,38). We cannot exclude the possibility that the divergence in rest MBF between our results and PET data may partly reflect the qualitatively different approaches and limitations of the two modalities (25). Nevertheless, significant advantages of our study over the previous CMR perfusion studies include the absolute quantification of MBF at rest and stress with in-plane spatial resolution down to 2.3 mm, and the larger sample size with age- and sex-matched controls used (26,35).
Previous work by our group has highlighted the prognostic value of midwall fibrosis assessed by LGE-CMR in DCM patients (15,39). Although the pathophysiologic basis of midwall fibrosis remains unclear, perfusion and microvascular abnormalities have been implicated (12). Studies using PET to explore the hypothesis that fibrosis may lead to perfusion abnormalities (or vice versa) have failed to demonstrate a clear association (10,34,40). Knaapen et al quantified MBF and the perfusable tissue index as a measure of fibrosis (34), finding no association between MBF and perfusable tissue index, although histological validation for the latter in DCM is lacking. CMR is able to accurately identify myocardial fibrosis in-vivo with good histological correlation (15), in addition to allowing quantification of MBF in a single study. Our data show that stress and rest MBF in DCM patients are reduced in segments with LGE compared to those without, suggesting myocardial fibrosis is associated with impaired perfusion. These findings raise the possibility that segments with fibrosis exhibit microvascular abnormalities, exemplified by the inability to augment MBF at stress, although the impaired perfusion may simply reflect diminished demand secondary to the reduced number of myocytes. Alternatively, the presence of fibrosis may have confounded the evaluation of perfusion as reported in the setting of hypertrophic cardiomyopathy by Villa et al (41). Nevertheless, the observed differences in stress and rest MBF did not translate to a significant difference in the MPR in segments with LGE compared to those without. Only a minority of our cohort had LGE, and the patient subgroup without fibrosis still demonstrated depressed stress MBF and MPR. Hence, while fibrosis may contribute towards perfusion abnormalities in DCM, it is unlikely to be the principal cause.
Limitations
This is a single center study based in a large tertiary referral hospital and may therefore be subject to referral bias. The impact of this was minimized by enrolling consecutive patients who met CMR criteria for DCM. Patients in the LVEF>35% and LVEF≤35% groups differed in age. Although advanced age has been shown to affect myocardial blood flow (42), this was not found to be an independent determinant of MBF or MPR in our cohort.
A small proportion of our cohort did not have coronary artery disease excluded by coronary angiography. However, these patients were predominantly young (<40 years) and of low risk for ischemic heart disease. Invasive coronary angiography would not therefore have been ethical or in keeping with guidelines given the low pre-test probability for ischemic heart disease.
Furthermore, these patients had negative non-invasive imaging (CT coronary angiography or SPECT) and no evidence of infarction or indeed visually apparent inducible ischemia on their CMR scan.
A modified Fermi deconvolution model was used to derive myocardial blood flow values from the arterial input function. Severely impaired LV function, particularly when associated with right ventricular dysfunction and severe tricuspid regurgitation may theoretically cause dilution of the contrast agent compact bolus, leading to slow arterial enhancement and thereby rendering myocardial enhancement insensitive to blood flow. However, other studies have also demonstrated the feasibility of perfusion quantification in the setting of severe left ventricular dysfunction (43).
Conclusions
Our data demonstrate that patients with DCM exhibit a higher rest MBF but lower stress MBF and MPR, indicating partial consumption of vasodilatory reserve as a mechanism to augment MBF to the failing myocardium. Reductions of stress MBF and MPR significantly correlate with LVEF, and highlight the interplay between myocardial perfusion abnormalities and impaired pump function. Impaired stress MBF is also associated with fibrosis. These findings suggest that if microvascular dysfunction plays a role in the pathogenesis of DCM, then stress-induced repetitive stunning rather than chronic myocardial hypoperfusion is likely to be responsible. Further work, including longitudinal studies, is required to elucidate the temporal relationship between perfusion abnormalities and LV dysfunction in DCM, and to establish their prognostic implications.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
There is a large body of evidence to support the clinical role of LGE-CMR in the diagnosis and risk stratification of patients with heart failure. Quantitative CMR perfusion is now emerging as an adjunctive clinical tool. For the first time, we apply this quantitative approach to fully characterize perfusion abnormalities in DCM. We demonstrate that stress MBF and MPR are reduced in DCM and correlate with LVEF, supporting the premise that microvascular dysfunction exists in DCM. However, rest MBF is increased, suggesting that sustained hypoperfusion is unlikely to drive disease progression.
Translational Outlook
Longitudinal studies are warranted to elucidate the relationship between microvascular dysfunction and outcome in DCM. In the absence of such data, our study suggests that the microcirculation is unlikely to represent a promising disease-modifying target in DCM.
Acknowledgments
Funding: This work was supported by the National Institute for Health Research Cardiovascular Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College, London, England. Dr Arai and Dr Hsu were funded by the Intramural Research Program of the National Heart, Lung and Blood Institute, and National Institutes of Health (Grant #HL 006137-07).
Disclosures: Dr Gulati reported receiving grant support from the National Institute for Health Research, CORDA, and Rosetrees Trust. Professor Mathur reported receiving grant support from the Heart Cells Foundation and Barts and the London Charity. Professor Pennell reported receiving grant support from the National Institute for Health Research and the British Heart Foundation; serving as a consultant to Siemens, Novartis, ApoPharma, AMAG, and Bayer; and serving as the director and owning stock in Cardiovascular Imaging Solutions. Dr Prasad reported receiving grant support from the British Heart Foundation, CORDA, and Rosetrees Trust, the Alexander Jansons Foundation and the National Institute for Health Research; and receiving speaking fees from Bayer Schering. Dr. Arai reported a research agreement with Siemens and a clinical trial agreement with Bayer. The remaining authors have no relevant conflicts of interest to disclose.
Abbreviations
- CAD
coronary artery disease
- CMR
cardiovascular magnetic resonance
- DCM
dilated cardiomyopathy
- LGE
late gadolinium enhancement
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- LVESVi
left ventricular end-systolic volume index
- MBF
myocardial blood flow
- MPR
myocardial perfusion reserve
- PET
positron emission tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Japp AG, Gulati A, Cook SA, Cowie MR, Prasad SK. The Diagnosis and Evaluation of Dilated Cardiomyopathy. J Am Coll Cardiol 2016;67:2996–3010. [DOI] [PubMed] [Google Scholar]
- 2.Skalidis EI, Parthenakis FI, Patrianakos AP, Hamilos MI, Vardas PE. Regional coronary flow and contractile reserve in patients with idiopathic dilated cardiomyopathy. Journal of the American College of Cardiology 2004;44:2027–32. [DOI] [PubMed] [Google Scholar]
- 3.Canetti M, Akhter MW, Lerman A et al. Evaluation of myocardial blood flow reserve in patients with chronic congestive heart failure due to idiopathic dilated cardiomyopathy. The American journal of cardiology 2003;92:1246–9. [DOI] [PubMed] [Google Scholar]
- 4.Weismuller S, Czernin J, Sun KT, Fung C, Phelps ME, Schelbert HR. Coronary vasodilatory capacity is impaired in patients with dilated cardiomyopathy. American journal of cardiac imaging 1996;10:154–62. [PubMed] [Google Scholar]
- 5.Morales MA, Neglia D, L’Abbate A. Reduction of myocardial blood flow reserve in idiopathic dilated cardiomyopathy without overt heart failure and its relation with functional indices: an echo-Doppler and positron emission tomography study. J Cardiovasc Med (Hagerstown) 2008;9:778–82. [DOI] [PubMed] [Google Scholar]
- 6.van den Heuvel AF, van Veldhuisen DJ, van der Wall EE et al. Regional myocardial blood flow reserve impairment and metabolic changes suggesting myocardial ischemia in patients with idiopathic dilated cardiomyopathy. Journal of the American College of Cardiology 2000;35:19–28. [DOI] [PubMed] [Google Scholar]
- 7.Neglia D, Michelassi C, Trivieri MG et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation 2002;105:186–93. [DOI] [PubMed] [Google Scholar]
- 8.L’Abbate A, Sambuceti G, Neglia D. Myocardial perfusion and coronary microcirculation: from pathophysiology to clinical application. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology 2002;9:328–37. [DOI] [PubMed] [Google Scholar]
- 9.Unverferth DV, Magorien RD, Lewis RP, Leier CV. The role of subendocardial ischemia in perpetuating myocardial failure in patients with nonischemic congestive cardiomyopathy. American heart journal 1983;105:176–9. [DOI] [PubMed] [Google Scholar]
- 10.Neglia D, Parodi O, Gallopin M et al. Myocardial blood flow response to pacing tachycardia and to dipyridamole infusion in patients with dilated cardiomyopathy without overt heart failure. A quantitative assessment by positron emission tomography. Circulation 1995;92:796–804. [DOI] [PubMed] [Google Scholar]
- 11.Lima MF, Mathias W Jr., Sbano JC et al. Prognostic value of coronary and microvascular flow reserve in patients with nonischemic dilated cardiomyopathy. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography 2013;26:278–87. [DOI] [PubMed] [Google Scholar]
- 12.Roura S, Bayes-Genis A. Vascular dysfunction in idiopathic dilated cardiomyopathy. Nature reviews Cardiology 2009;6:590–8. [DOI] [PubMed] [Google Scholar]
- 13.Ismail TF, Hsu LY, Greve AM et al. Coronary microvascular ischemia in hypertrophic cardiomyopathy - a pixel-wise quantitative cardiovascular magnetic resonance perfusion study. J Cardiovasc Magn Reson 2014;16:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sammut EC, Villa ADM, Di Giovine G et al. Prognostic Value of Quantitative Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc Imaging 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulati A, Jabbour A, Ismail TF et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013;309:896–908. [DOI] [PubMed] [Google Scholar]
- 16.Richardson P, McKenna W, Bristow M et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996;93:841–2. [DOI] [PubMed] [Google Scholar]
- 17.Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 2006;8:417–26. [DOI] [PubMed] [Google Scholar]
- 18.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E, Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized P. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson 2013;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding S, Wolff SD, Epstein FH. Improved coverage in dynamic contrast-enhanced cardiac MRI using interleaved gradient-echo EPI. Magn Reson Med 1998;39:514–9. [DOI] [PubMed] [Google Scholar]
- 20.Gatehouse PD, Elkington AG, Ablitt NA, Yang GZ, Pennell DJ, Firmin DN. Accurate assessment of the arterial input function during high-dose myocardial perfusion cardiovascular magnetic resonance. Journal of magnetic resonance imaging : JMRI 2004;20:39–45. [DOI] [PubMed] [Google Scholar]
- 21.Xue H, Guehring J, Srinivasan L et al. Evaluation of rigid and non-rigid motion compensation of cardiac perfusion MRI. Medical image computing and computer-assisted intervention : MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention 2008;11:35–43. [DOI] [PubMed] [Google Scholar]
- 22.Hsu LY, Groves DW, Aletras AH, Kellman P, Arai AE. A quantitative pixel-wise measurement of myocardial blood flow by contrast-enhanced first-pass CMR perfusion imaging: microsphere validation in dogs and feasibility study in humans. JACC Cardiovasc Imaging 2012;5:154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czernin J, Muller P, Chan S et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 1993;88:62–9. [DOI] [PubMed] [Google Scholar]
- 24.Watzinger N, Lund GK, Saeed M et al. Myocardial blood flow in patients with dilated cardiomyopathy: quantitative assessment with velocity-encoded cine magnetic resonance imaging of the coronary sinus. Journal of magnetic resonance imaging : JMRI 2005;21:347–53. [DOI] [PubMed] [Google Scholar]
- 25.Morton G, Chiribiri A, Ishida M et al. Quantification of absolute myocardial perfusion in patients with coronary artery disease: comparison between cardiovascular magnetic resonance and positron emission tomography. J Am Coll Cardiol 2012;60:1546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dass S, Holloway CJ, Cochlin LE et al. No Evidence of Myocardial Oxygen Deprivation in Nonischemic Heart Failure. Circ Heart Fail 2015;8:1088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neglia D, De Caterina A, Marraccini P et al. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 2007;293:H3270–8. [DOI] [PubMed] [Google Scholar]
- 28.Gil KE, Pawlak A, Frontczak-Baniewicz M, Gil RJ, Nasierowska-Guttmejer A. The proposed new classification of coronary microcirculation as the predictor of the heart failure progression in idiopathic dilated cardiomyopathy. Cardiovascular Pathology 2015;24:351–358. [DOI] [PubMed] [Google Scholar]
- 29.Inoue T, Sakai Y, Morooka S et al. Vasodilatory capacity of coronary resistance vessels in dilated cardiomyopathy. American heart journal 1994;127:376–81. [DOI] [PubMed] [Google Scholar]
- 30.Tsagalou EP, Anastasiou-Nana M, Agapitos E et al. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. Journal of the American College of Cardiology 2008;52:1391–8. [DOI] [PubMed] [Google Scholar]
- 31.Laguens R, Alvarez P, Vigliano C et al. Coronary microcirculation remodeling in patients with idiopathic dilated cardiomyopathy. Cardiology 2011;119:191–6. [DOI] [PubMed] [Google Scholar]
- 32.Opherk D, Schwarz F, Mall G, Manthey J, Baller D, Kubler W. Coronary dilatory capacity in idiopathic dilated cardiomyopathy: analysis of 16 patients. The American journal of cardiology 1983;51:1657–62. [DOI] [PubMed] [Google Scholar]
- 33.Gaasch WH. Left ventricular radius to wall thickness ratio. The American journal of cardiology 1979;43:1189–94. [DOI] [PubMed] [Google Scholar]
- 34.Knaapen P, Gotte MJ, Paulus WJ et al. Does myocardial fibrosis hinder contractile function and perfusion in idiopathic dilated cardiomyopathy? PET and MR imaging study. Radiology 2006;240:380–8. [DOI] [PubMed] [Google Scholar]
- 35.Jerosch-Herold M, Sheridan DC, Kushner JD et al. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 2008;295:H1234–H1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanza GA, Camici PG, Galiuto L et al. Methods to investigate coronary microvascular function in clinical practice. Journal of cardiovascular medicine 2013;14:1–18. [DOI] [PubMed] [Google Scholar]
- 37.Gerber BL. Quantification of myocardial perfusion and myocardial perfusion reserve by positron emission tomography and cardiovascular magnetic resonance imaging. Journal of the American College of Cardiology 2012;60:1556–7. [DOI] [PubMed] [Google Scholar]
- 38.Gerber BL, Melin JA, Bol A et al. Nitrogen-13-ammonia and oxygen-15-water estimates of absolute myocardial perfusion in left ventricular ischemic dysfunction. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 1998;39:1655–62. [PubMed] [Google Scholar]
- 39.Halliday BP, Gulati A, Ali A et al. Association Between Midwall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients With Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation 2017;135:2106–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parodi O, De Maria R, Oltrona L et al. Myocardial blood flow distribution in patients with ischemic heart disease or dilated cardiomyopathy undergoing heart transplantation. Circulation 1993;88:509–22. [DOI] [PubMed] [Google Scholar]
- 41.Villa AD, Sammut E, Zarinabad N et al. Microvascular ischemia in hypertrophic cardiomyopathy: new insights from high-resolution combined quantification of perfusion and late gadolinium enhancement. J Cardiovasc Magn Reson 2016;18:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uren NG, Camici PG, Melin JA et al. Effect of aging on myocardial perfusion reserve. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 1995;36:2032–6. [PubMed] [Google Scholar]
- 43.Sammut E, Zarinabad N, Wesolowski R et al. Feasibility of high-resolution quantitative perfusion analysis in patients with heart failure. J Cardiovasc Magn Reson 2015;17:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.