Abstract
Bovine respiratory disease (BRD) is a multifactorial disease involving complex host immune interactions shaped by pathogenic agents and environmental factors. Advancements in RNA sequencing and associated analytical methods are improving our understanding of host response related to BRD pathophysiology. Supervised machine learning (ML) approaches present one such method for analyzing new and previously published transcriptome data to identify novel disease-associated genes and mechanisms. Our objective was to apply ML models to lung and immunological tissue datasets acquired from previous clinical BRD experiments to identify genes that classify disease with high accuracy. Raw mRNA sequencing reads from 151 bovine datasets (n = 123 BRD, n = 28 control) were downloaded from NCBI-GEO. Quality filtered reads were assembled in a HISAT2/Stringtie2 pipeline. Raw gene counts for ML analysis were normalized, transformed, and analyzed with MLSeq, utilizing six ML models. Cross-validation parameters (fivefold, repeated 10 times) were applied to 70% of the compiled datasets for ML model training and parameter tuning; optimized ML models were tested with the remaining 30%. Downstream analysis of significant genes identified by the top ML models, based on classification accuracy for each etiological association, was performed within WebGestalt and Reactome (FDR ≤ 0.05). Nearest shrunken centroid and Poisson linear discriminant analysis with power transformation models identified 154 and 195 significant genes for IBR and BRSV, respectively; from these genes, the two ML models discriminated IBR and BRSV with 100% accuracy compared to sham controls. Significant genes classified by the top ML models in IBR (154) and BRSV (195), but not BVDV (74), were related to type I interferon production and IL-8 secretion, specifically in lymphoid tissue and not homogenized lung tissue. Genes identified in Mannheimia haemolytica infections (97) were involved in activating classical and alternative pathways of complement. Novel findings, including expression of genes related to reduced mitochondrial oxygenation and ATP synthesis in consolidated lung tissue, were discovered. Genes identified in each analysis represent distinct genomic events relevant to understanding and predicting clinical BRD. Our analysis demonstrates the utility of ML with published datasets for discovering functional information to support the prediction and understanding of clinical BRD.
Subject terms: Machine learning, Molecular medicine, Bioinformatics, Gene expression analysis, RNA sequencing, Infection, Respiratory tract diseases
Introduction
Bovine respiratory disease (BRD) is the most important disease complex in beef cattle production. Although extensively researched, BRD remains the leading cause of infectious disease and economic loss in post-weaned beef cattle worldwide1–4. Due to the multifactorial and polymicrobial nature of BRD, effort has been made to illustrate host factors, management schema, etiological associations, and stressful environmental factors associated with disease development and progression1,2,4. Recent research has been focused on predicting BRD susceptibility and outcomes over time5–8. Unfortunately, clinical diagnostic and prognostic prediction models remain contested, and mechanistic information regarding host–pathogen interactions and the development of clinical BRD is not fully understood.
Clinical BRD is often linked with a select number of bacterial and viral etiologies. Bacteria, such as Histophilus somni, Mannheimia haemolytica, Mycoplasma bovis, and Pasteurella multocida, and viruses, such as bovine respiratory syncytial virus (BRSV), bovine viral diarrhea virus (BVDV), bovine herpesvirus-1 (IBR), and bovine parainfluenza type 3 virus (PI3), are well studied regarding their pathological capacity and disease association9–15. However, the clinical presentation of BRD is highly variable and antemortem diagnosis is often made without accompanying etiological identification9,13,16,17. Additionally, cattle experimentally exposed to these agents often fail to develop severe clinical BRD, demonstrating the underlying complexity of the disease and the requirement of implied predisposing factors18,19. Consequentially, current vaccination protocols possess varying effects in reducing ongoing rates of morbidity and mortality associated with BRD, and targeted antimicrobial usage and antimicrobial resistance is of particular public interest20–25. Therefore, research is needed to elucidate underlying host mechanisms associated with infectious BRD that represent biological components and regulatory functions amendable to manipulation to improve disease response and clinical diagnosis.
High-throughput RNA sequencing (RNA-Seq) is a highly sensitive methodology used to comprehensively evaluate functional mechanisms and molecular heterogeneity through global gene expression analysis26–29. Because of the high sensitivity of the technology, growing technological applications in research, and decreasing costs, RNA-Seq has become an excellent method of evaluating cellular transcriptomes and functionality at a given point in time. Several RNA-Seq studies performed with samples from post-weaned beef cattle have identified underlying genes and host mechanisms associated with both naturally occurring and experimentally induced BRD30–35. However, the results are highly dependent on the experimental design, sequencing technology, and selected data analysis technique, which may be highly conservative in nature28,36–39. Therefore, the use of supervised machine learning models with previously published RNA-Seq data could identify additional gene expression and mechanistic information related to clinical presentation of BRD.
Supervised machine learning (ML) models used in biological research aid in the discover of molecules and establishment of dynamic models that recognize, classify, and predict disease outcomes40–44. In recent years, studies have employed the use of ML framework to identify candidate biomarkers for disease classification, cell and tumor expression signatures, and novel protein mechanisms within publicly available RNA-Seq datasets45–49. However, to our knowledge, the use of ML-based methodology has not been explored with BRD-associated datasets. Therefore, we combined mRNA-Seq data from lung and immunological tissue of cattle experimentally challenged with causative agents of BRD, and tested the classification performance of ML methodology and selected gene classifiers. Our objective for this study was to integrate three publicly available datasets and utilize ML methodology, in order to both corroborate findings previously discovered through differential gene expression analysis and to potentially identify novel genes and mechanisms associated with experimentally induced BRD. Our overarching hypothesis is that ML methodology, when applied to previously published datasets, is capable of identifying genes which distinctly classify cattle challenged with etiological components of BRD, when compared to sham controls.
Materials and methods
Dataset acquisition
One hundred and sixty high throughput mRNA sequencing datasets were acquired from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO)50,51. The datasets originated from lymphoid and homogenized lung (healthy and diseased) tissue harvested during peak clinical signs in cattle that were experimentally challenged with isolated BRD pathogens (n = 35), or their sham controls (n = 10). Analyses of these datasets has been previously reported30–32. Details of sample sizes for challenged and control cattle, isolated BRD pathogens used for challenge, and tissue samples that were subjected to mRNA sequencing are summarized in Table 1.
Table 1.
Initial training datasets identified for ML testing. A total of 160 mRNA-Seq datasets were derived from lymph node and lung tissue of 31 cattle challenged with isolated BRD pathogens and 10 sham challenged controls. Asterisk (*) indicates different tissues collected from the same animals. Specifically, transcriptomes from tissues reported by Behura et al.31 are from the same cattle from which Tizioto et al. analyzed bronchial lymph node transcriptomes (2015) except that P. multocida infected cattle reported by Tizioto et al.30 are not included in the cohort reported by Behura et al.31.
| NCBI BioProject ID | Animal breed | Animal age | Number of animals | Tissue types | Etiological agents used in challenge | Sequencing platform | Publication |
|---|---|---|---|---|---|---|---|
| PRJNA272725 | Angus × Hereford (steers) | 6–8 mon | n = 23 challenge; n = 4 control | Bronchial lymph node | BRSV* (n = 4), BVDV* (n = 4), IBR* (n = 4), M. haemolytica* (n = 4), P. multocida* (n = 4), M. bovis* (n = 3), control* (n = 4) | Illumina HiSeq 2500; 50 bp PE | Tizioto et al., 2015 (n = 27) |
| PRJNA272725 | Angus × Hereford (steers) | 6–8 mon | n = 19 challenge; n = 4 control | Lung (healthy), lung (lesion), retropharyngeal lymph node, nasopharyngeal lymph node, pharyngeal tonsil | BRSV* (n = 4), BVDV* (n = 4), IBR* (n = 4), M. haemolytica* (n = 4), M. bovis* (n = 3), control* (n = 4) | Illumina HiSeq 2500; 50 bp PE | Behura et al., 2017 (n = 115) |
| PRJNA543752 | Holstein–Friesian (bulls) | ~ 4 mon | n = 12 challenge; n = 6 control | Bronchial lymph node | BRSV (n = 12), control (n = 6) | Illumina NextSeq 500; 75 bp PE | Johnston et al., 2019 (n = 18) |
Read processing and gene count matrix generation
Paired-end read files for each dataset were concatenated to their corresponding forward and reverse direction. To eliminate potential variations induced by differing workflow toolkits, all reads were processed identically. Quality assessment, read trimming, and adapter contamination removal was performed with FastQC v0.11.952 and Trimmomatic v0.3953. Briefly, reads were trimmed by removing leading and trailing bases if base quality scores were less than 3, scanning each read with a 4-base pair sliding window and removing read segments below a minimum base quality score of 15, and retaining reads above a minimum length of 36 bases. Read quality analysis was summarized and evaluated for each study with MultiQC v0.3754. Read survival and quality assessment information are provided in Supplemental file 1. Trimmed reads were mapped to the bovine reference assembly ARS-UCD1.2 using HISAT2 v2.2.055. Reference-guided transcript/gene assembly and quantification was performed with StringTie v2.1.256,57. A gene-level raw count matrix was generated for each dataset with the program prepDE.py58. Five datasets [86684_Retrop_LN (control), 86688_Retrop_LN (BRSV), 86710_Retrop_LN (BVDV), 86698_dlung (M. bovis), and SRR1956908 (control)] were removed from further analysis due to low read count quantity and technical variability. Additionally, the four datasets related to Pasteurella multocida infection (SRR1952370, SRR1952371, SRR1952372, and SRR1952373) were removed to avoid unbalanced classification. The resulting compiled ML dataset was composed of 151 mRNA-Seq datasets.
Supervised machine learning analysis
A total of 151 mRNA-Seq datasets, spanning six tissue types, constituted the compiled ML dataset for further classification and feature selection. Raw gene counts generated for each dataset were processed and analyzed in R v4.0.2 with the Bioconductor package MLSeq v2.6.0 (https://github.com/dncR/MLSeq)59. The 151 mRNA-Seq libraries were allocated into 9 classes based on the nature of the experimental pathogen challenge: (1) sham-challenged controls (Control; n = 28), (2) challenged with any BRD pathogen (BRD; n = 123), (3) challenged with a BRD viral pathogen (Virus; n = 82), (4) challenged with a BRD bacterial pathogen (Bacteria; n = 41), and categories 5–9 for each of the 5 independent challenge pathogens (BRSV; n = 35, BVDV; n = 23, IBR; n = 24, M. haemolytica; n = 24, and M. bovis; n = 17). The objectives of the ensuing ML analysis were to develop ML models that would (1) accurately “classify” an mRNA-Seq dataset within the 9 experimental pathogen challenge classes and (2) extract genes and gene sets or “features” that accurately assign an mRNA-Seq dataset to its experimental pathogen challenge class. These objectives were pursued by comparisons of the 8 pathogen challenge classes and the control challenge class. The raw gene count matrix used for this approach is available in Supplemental file 2. Briefly, offset values of one were added to the count matrix to reduce the likelihood of convergence in model fitting and to reduce bulk sparsity60,61. Genes with a minimum count-per-million of 0.5 in three or more mRNA-Seq libraries were retained for analysis. Library normalization was performed with the DESeq median ratio approach, using default settings62. The resulting ML dataset was stratified into a training and testing set (70% and 30%, respectively), using controls as the comparative baseline (i.e., class statement).
Model validation and parameter optimization were evaluated using fivefold, 10 repeats with non-exhaustive cross validation. Six ML models were utilized for classification and/or significant gene selection: sparse Poisson linear discriminant analysis, with and without a power transformation (PLDA, PLDA2)63, negative binomial linear discriminant analysis (NBLDA)64, sparse voom-based nearest shrunken centroids (VNSC)65, support vector machine (SVM) (https://cran.r-project.org/web/packages/caret/caret.pdf), and nearest shrunken centroids provided by the pamr package (PAM) (https://cran.r-project.org/web/packages/pamr/pamr.pdf). Models were evaluated with confusion matrices and performance metrics provided by the MLSeq package. Feature selection from sparse classifier models was set to a maximum of 2000 genes, based on maximum variance filtering. Sparse classifier models (PLDA, PLDA2, VNSC, and PAM), which generate lists of a select number of significant genes used for model decision and classification, were manually designated as the top models for each test set based on highest associated balanced accuracy and Kappa statistic; if two or more models were equal, gene lists would be merged. Performance metric calculations are defined by Goksuluk and colleagues59. Balanced accuracy, the combined average of sensitivity and specificity, was a prioritized metric due to imbalance between challenged and control cattle and potential for skewed results when evaluating sensitivity and specificity alone. Further information regarding workflow parameters, model building, and optimization are found in the package vignette and associated GitHub repository mirror (https://bioconductor.org/packages/release/bioc/html/MLSeq.html; https://github.com/dncR/MLSeq).
Exploration and functional analysis of test set gene classifiers
Visual relationships of the genes identified by the top sparse classifiers was performed with UpSetR v1.4.066, utilizing the interactive interface Intervene67. Multidimensional scaling was applied to the gene count matrix with plotMDS, using pairwise distances of the top 500 genes based on variance68. Heatmaps of the unique gene classifiers identified across etiologic test sets were generated with the Bioconductor package pheatmap v1.0.1269, utilizing Ward’s method of unsupervised hierarchical clustering on Euclidean distances and Pearson correlation coefficients for samples and genes, respectively. Color scaling for all packages was performed with the Bioconductor package viridis v0.5.170 to allow ease of visual interpretation for individuals with color blindness.
Functional association and biological significance of genes from each test set was assessed. Gene Ontology (GO) terms and pathway analysis of DEGs was performed with WebGestalt 2019 (WEB-based GEne SeT AnaLysis Toolkit), utilizing human orthologs and functional databases71. Pathway analysis performed within WebGestalt 2019 utilized the pathway database Reactome72. Overrepresentation analysis parameters within WebGestalt 2019 included between 5 and 3000 genes per category, Benjamini–Hochberg procedure for multiple hypothesis correction, and FDR cutoff of 0.05 for significance.
Results
Supervised machine learning model performance
Mapping and alignment of reads to the ARS-UCD1.2 reference assembly identified 33,129 genes across all 151 libraries (n = 28 controls from 10 animals, n = 123 BRD from 32 animals; Supplemental file 2); the corresponding count matrix resulted in a total library size of 5,132,593,936, with a median library size of approximately 32.7 million counts per library. The count matrix was partitioned into nine pathogen challenge classes; overall testing performance for each ML algorithm is provided in Supplemental file 3. Support vectors machine (SVM) modeling, a non-sparse classifier, performed best in terms of balanced accuracy for all testing groups except for BVDV, which the nearest shrunken centroids model provided by the pamr package (PAM) outperformed all other models (86.7%). Because sparse classifiers select a subset of genes for classification59, genes were acquired and compiled from the top sparse models (PLDA, PLDA2, VNSC, or PAM) within each experimental challenge comparison. PAM performed best in terms of balanced accuracy when classifying Virus (89.9%), BRSV (100.0%), BVDV (86.7%), M. bovis (71.4%), and M. haemolytica (73.3%) against controls. Poisson linear discriminant analysis (PLDA) performed best when classifying Bacteria (70.0%). Both power-transformed Poisson linear discriminant analysis (PLDA2) and PAM performed identically when classifying IBR (100.0%). BVDV was less accurate (PAM; 86.7%), which most likely affected classification accuracy when evaluating all viral samples (PAM; 89.9%). Bacteria-challenged classes performed worse overall, with accompanying top balanced accuracies of 80.0%, 71.4%, and 80.0% for M. haemolytica (SVM), M. bovis (SVM/PAM), and Bacteria (SVM) classification, respectively. Combination of all challenge classes (BRD) possessed poor balanced classification accuracy, with the highest non-sparse classifier at 65.0% (SVM) and sparse classifiers (VNSC) at 60.8%.
Visualization of gene expression variation
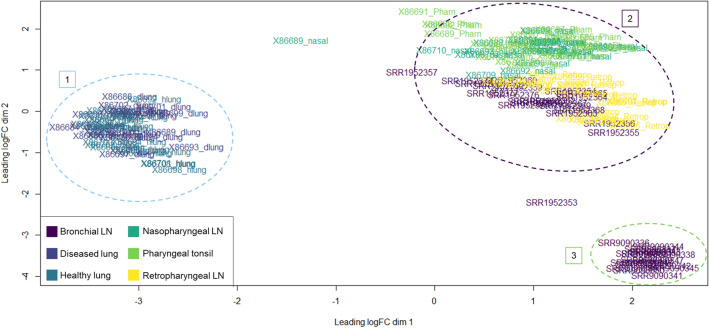
Multidimensional scaling (MDS) was applied to the integrated ML dataset, to discern dissimilarities between its individual mRNA-Seq libraries based on gene variation. Each point on x- and y-axes represents a different individual dataset and subsequent transformed Euclidean distance in two dimensions. Patterns from the top 500 genes based on log2-normalized standard deviation revealed that there was an overall similarity in gene expression across specific tissue types. While differences can be appreciated for each dataset with distinction to tissue site, lung (cluster 1; light blue) and lymphoid tissues (cluster 2; purple) were the most evident in terms of dissimilarity (Fig. 1). Notably, bronchial lymph node tissue from Johnston et al.32 (cluster 3; green) demonstrated equivalent dissimilarity from lung tissue as the bronchial lymph node tissue from Tizioto et al.30. However, the bronchial lymph node tissue from the two different studies were distinct from one-another when evaluated in the second dimensional space. Tissue-level gene expression differences (e.g., lung versus all other tissue types) were more pronounce compared to disease or etiological differences.
Figure 1.
MDS plot of 151 datasets utilized for ML classification. Clustering was performed with Euclidian distances across the top 500 genes based on log2 standard deviation. Datasets are demarked by color, representing the tissue site of sampling. Labels 1, 2, and 3 demark distinct gene expression clusters across tissue types, regardless of etiological component, based on expressional variation. Label 1 consists of healthy (non-consolidated) and diseased (consolidated) lung tissue. Label 2 consists of lymphoid tissue from Tizioto and colleagues30 and Behura and colleagues31. Label 3 consists of lymphoid (bronchial) tissue from Johnston and colleagues32.
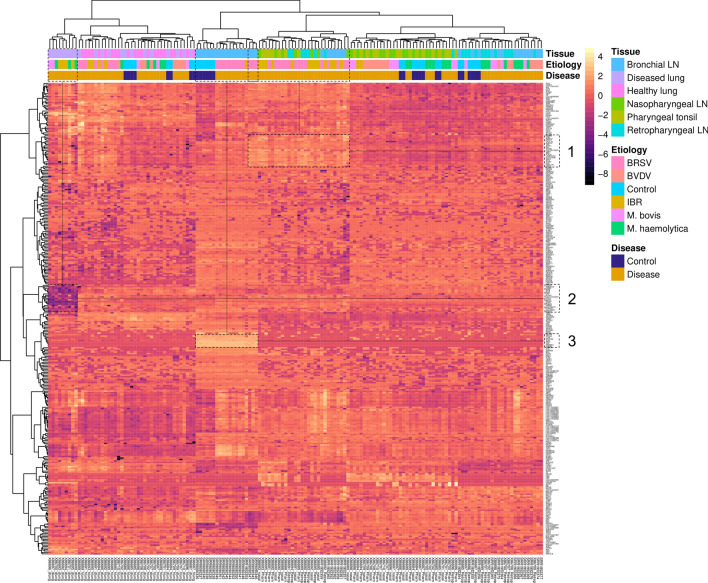
A heat map was generated for each dataset using the gene classifiers identified through the top ML sparse model in each etiologic-specific test group (Fig. 2). A total of 572 genes were identified across the five etiological test groups, 357 of which were uniquely identified after overlapping (Supplemental file 4). Expression patterns within each column are accompanied by unsupervised hierarchical clustering, visualizing likeness in tissue type, etiology, and disease classification. Similar to the MDS plot (Fig. 1), distinction based on gene expression can be appreciated across lung and lymphoid tissue types, as opposed to etiology or disease classification. This distinction in gene expression across tissues corroborates the findings of Behura and colleagues31. Pearson correlation coefficients clustering of genes (rows) allowed for the visualization of distinct expression patterns. Particularly, three visual expression modules were identified, and labeled as 1, 2, and 3. Visual expression module 1 contained the genes PSMB8, PPA1, PARP12, EPSTI1, CXCL10, CLEC4F, TIFA, ZNFX1, MX1, DHX58, LOC100139670, GBP4, ZBP1, PLAC8, LOC618737, LOC512486, ISG15, IFIT2, IFITM1, PML, FAM111B, and CD274, which were distinctly higher in expression in lymphatic tissue sampled from cattle experimentally challenged with BRSV and IBR compared to all remaining. Visual expression module 2 contained the genes CPSF6, TMEM123, CIRBP, ATP6, ATP8, ND4L, LPP, IFITM2, LOC112444847, DTX3L, LDHA, RPS26, STIP1, PSME2, PARP9, LOC786372, PTP4A2, CDC42SE1, and NLRC5, which were distinctly decreased in disease lung tissue sampled from cattle experimentally challenged with Mycoplasma bovis, Mannheimia haemolytica, and IBR. Visual expression module 3 contained the genes WDFY4, OTUD4, LCP2, OCDC69, TLN1, RPS7, VPC, HNRNPU, and HMGB2, which were distinctly increased in bronchial lymph node tissue sampled from cattle in the control group and experimentally challenged BRSV.
Figure 2.
Heatmap of the 357 unique genes identified by top ML sparse classifier across the five etiology classes (BRSV, IBR, BVDV, M. bovis, and M. haemolytica). Ward clustering of datasets and gene expression was performed with Euclidian distance and Pearson correlation coefficients, respectively. Visual expression modules (1, 2 or 3) were empirically identified by class dissimilarity. Clustering of samples (datasets) is more apparent for tissue, compared to etiology and disease status.
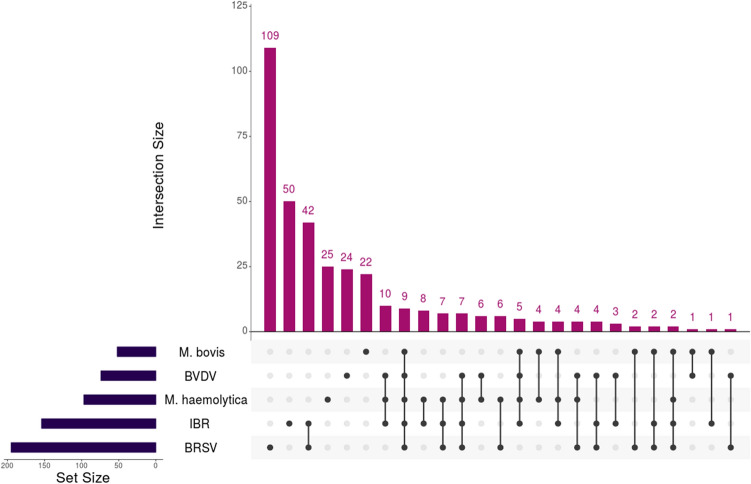
To explore the complex overlap of gene classifiers between etiological groups, we employed an UpSetR matrix intersection technique (Fig. 3). Among the genes identified through the top sparse classifiers, BRSV was the most distinct with 109 unique genes. There was an apparent separation of viral-related genes, whereas BRSV and IBR possessed the highest overlap (42), BVDV possessed 24 unique genes, and only four genes were shared across all three viruses. Similarly, the bacterial datasets possessed minor overlap, with 25 and 22 genes identified uniquely for M. haemolytica and M. bovis, respectively, and only four genes shared between both bacterial analyses.
Figure 3.
Matrix intersection of significant gene classifiers identified for each etiological class. Overlap of the 572 genes identified by top ML sparse classifiers, across the five etiology classes, were visualized for determining functional relevance and comparative uniqueness. BRSV possessed the highest number of uniquely identified genes (109), followed by IBR (50), M. haemolytica (25), BVDV (24), and M. bovis (22). BRSV and IBR shared the highest number of genes between all comparisons (42), primarily involved in type-I interferon production and signaling. The two bacterial classes (M. haemolytica and M. bovis) only shared four genes without any viral overlap (LOC787803, MTDH, NECAP2, and TCAF1).
Functional analysis of gene classifiers
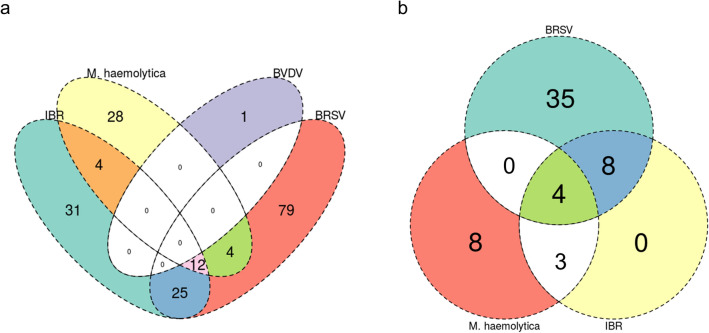
Gene Ontology (GO) terms for biological processes and Reactome pathway enrichment analyses were performed with WebGestalt (FDR ≤ 0.05). One hundred and twenty, 72, one, and 48 GO-BP terms were significantly enriched by gene classifiers identified for BRSV, IBR, BVDV, and M. haemolytica, respectively; no significant GO-BP terms were enriched for M. bovis. Forty-seven, 15, and 15 pathways were enriched by gene classifiers identified for BRSV, IBR, and M. haemolytica, respectively; no pathways were enriched for BVDV and M. bovis. All GO-BP terms and pathways identified are found in Supplemental file 5. Overlap of the GO-BP terms and pathways identified for each etiological group is shown in Fig. 4A,B. BRSV and IBR possessed the highest overlap of functional associations, with 37 GO-BP terms and 12 pathways shared between the two. GO-BP terms and pathways between BRSV and IBR were primarily related to type I interferon production and signaling, cellular metabolism and ATP production, unfolded protein response, antigenic cross presentation, and IL-8 secretion. Between BRSV, IBR, and M. haemolytica, 12 GO-BP terms and 4 pathways were shared across all three. GO-BP terms and pathways between BRSV, IBR, and M. haemolytica were related to innate immune response, apoptosis, and unfolded protein response. M. haemolytica differed in functional enrichment with processes and pathways related to neutrophilic activation and degranulation, classical and alternative complement activation, and immunoglobulin-mediated humoral immunity. All five etiological groups shared genes involved in heat-shock protein response. The complete list of overlapping significant genes, GO-BP terms, and enriched pathways is found in Supplemental file 6.
Figure 4.
Venn diagram of GO-BP term (a) and pathways (b) enriched by genes identified by top ML sparse classifiers across all etiological testing sets. (a) Twenty-five enriched GO-BP terms were shared specifically for BRSV and IBR, primarily consisting of apoptotic processes, type 1 interferon signaling, IL-8 secretion, and leukocyte degranulation. BVDV possessed only one enriched GO-BP term (anatomical structure homeostasis) and no GO-BP terms were enriched for M. bovis. (b) Eight enriched pathways were shared specifically across BRSV and IBR, primarily consisting of antigen cross presentation, uptake of ligands by scavenger receptors, and interferon alpha/beta signaling. The four pathways shared across BRSV, IBR, and M. haemolytica involved the innate immune system, stress response element binding via ATF6-alpha, and signal recognition protein-dependent protein translation. The eight enriched pathways specific to M. haemolytica involved alternative complement activation, MHC class I antigen presentation, cellular response to heat stress, and IRE1-alpha-dependent chaperone activation. No pathways were enriched for BVDV or M. bovis.
Discussion
Over the past several years, RNA-Seq analysis has been utilized in bovine disease research to evaluate gene expression related to risk of BRD development, stress response, and viral lesion development30–35,73,74. Primarily, studies that generate RNA-Seq data utilize statistical platforms and techniques for the detection of differentially expressed genes and subsequent construction of functional networks or modules. Many RNA-Seq studies are thus limited in extrapolatory capacity, as analyses are often performed through subsampling single populations and fitting fixed statistical models, which may be conservative when analyzing gene expression datasets with overdispersion75–77. Fortunately, publicly available gene expression repositories, such as the NCBI Gene Expression Omnibus, make it possible to acquire, integrate, and analyze datasets related to a particular field or disease. Such studies have been performed in mammalian species, including cattle, to better characterize genomic mechanisms and protein production related to a particular disease or condition49,78,79. Additionally, with the dynamic capacity for analysis that supervised ML models allow, it is possible to explore and characterize gene expression patterns associated with clinical BRD with profound sensitivity42,79. In this study, we integrated gene expression data from controlled BRD experiments and determine expression patterns and classification potential through supervised ML analysis.
Some of the limitations of this study are evident. First, data were integrated from three studies, two of which utilized the same animals for their transcriptomic analysis30,31. While a clear separation in gene expression patterns was appreciated across tissue types, corroborating the findings Behura and colleagues31, utilizing datasets from a limited number of animals and at single time points may not account for the heterogenous nature of gene expression related to BRD development and clinical presentation75,80. Additionally, these datasets were acquired from samples of cattle experimentally challenged with single pathogens. BRD challenge models using single etiological components often fail to elicit severe disease, as described by the three studies used here and may not fully represent the complex nature of the disease process seen with naturally occurring BRD81,82. Accordingly, future studies applying ML methodology in BRD research should prioritize natural disease models for improved discovery adaptation within beef production systems. Moreover, RNA-Seq analysis remains a relatively new modality in BRD research, and publicly available data are limited at this time. Nonetheless, this study, which to our knowledge is the first to evaluate host transcriptomes related to BRD with integrated supervised ML methodology, substantiating many of the gene expression findings previously identified, and may serve as a template for modern data analysis in bovine health research.
Between all testing groups and the six models utilized in this study, the support vector machines (SVM) model typically performed the best in terms of classification capacity. While originally utilized in microarray experiments, this algorithm is popular for genomic classification research in RNA-Seq, as it has been used to discover cancer biomarkers in humans, classify genes related to early conception in cattle, and automate single-cell RNA-Seq identification49,83,84. While this algorithm was capable of accurately classifying BRSV and IBR challenged datasets, compared to controls, this model is a non-sparse classifier and therefore does not have a built-in process for feature selection and gene extraction within MLSeq. Therefore, particular interest was placed on the PLDA, PLDA2, PAM, and VNSC algorithms, as subsets of genes used to drive classification decisions could be obtained. The compiling of datasets for classifying total BRD, viral, and bacterial challenge yielded mixed results. For total BRD, sparse classifiers PAM and VNSC yielded high classificational accuracy for identifying the challenged cattle, but performed poorly in discerning them from the controls, as illustrated by the accompanying sensitivity and balanced accuracy. This finding may be related to the complexity and distinction of infection processes associated with each etiological component, and highlights that an all-encompassing approach may be inappropriate for determining relevant gene expression and pathways in BRD. To a lesser extent, this is similarly found when compiling bacterial datasets, as discernment from controls was relatively poor. Viral datasets yielded much higher overall balanced accuracies, compared to the bacterial counterparts. Regarding sparse classifiers, BRSV, IBR, and BVDV were capable of being classified with high balanced accuracy (100%, 100%, 86.67%, respectively) through the PAM model; IBR was also identified with 100% balanced accuracy with PLDA2.
Generally, viruses were independently the most well classified, followed by M. haemolytica. Collectively, BRSV and IBR were well defined by genes involved in the production and response to type I interferons. More specifically, these genes were seen to be driven primarily by lymphoid tissue, as opposed to lung tissue (expression module 1, Fig. 2). This result, coupled with the subsequent lack of type I interferon genes from the BVDV class, corroborates findings previously described30,31. Biologically, the lack of this innate interferon response has been described as a potential immunosuppressive response driven by BVDV, allowing for persistent infection and viral shedding85–88. Notably, non-cytopathic BVDV strains used in experimental infection models, such as the one utilized in this project, have been shown to reduce proinflammatory signaling31,89. While the functional enrichment of the genes classified for BVDV were largely non-specific, several have been previously identified and have known immunological functionality30,31. Related to M. haemolytica, there was apparent overlap in functionality of genes identified through ML (Fig. 4). Largely, this was driven by genes encoding for heat shock proteins, calreticulin, talin-1, and X-box binding protein. These proteins are involved in apoptotic and cell stress events, and may ultimately impact immunoglobulin production and cellular homeostasis90–93. Additionally, genes classified in M. haemolytica were unique to the activation of classical and alternative pathways of complement. While complement-related genes were identified across all viruses in previously reported gene expression studies and here, the alternative complement component CFB was only identified in M. haemolytica. This may be an indication that the presence and activation of the alternative complement pathway is more indicative of inflammation and disease associated with lipopolysaccharide, often caused by extracellular Gram-negative bacteria such as M. haemolytica14,94. Regarding Mycoplasma bovis, our findings here are similar to that of Behura and colleagues31, in that we identified the fewest number of significant genes in MB, with regard to all other classes, and failed to define significantly enriched processes and pathways. As discussed by Behura and colleagues31, these infected cattle may have been euthanized and sample collected too early in the course of BRD to detect immunological changes. Additionally, Mycoplasma bovis is capable of evading the host immune response, specifically neutrophilic responses, and may lead to the development of T-cell “exhaustion” that eventually culminates in severe clinical disease95. Future transcriptomic evaluation of single cells or the sub-cellular space, instead of bulk tissue samples, may better elucidate mechanisms associated with Mycoplasma bovis.
Lastly, novel findings were identified through visual expression modules found in Fig. 2. Expression module 2 possessed 19 genes with reduced expression in disease lung tissue sampled from cattle experimentally challenged with Mycoplasma bovis, Mannheimia haemolytica, and IBR. While often assumed that the oxygenating capability of consolidated lung space during acute respiratory disease is substantially decreased, this expression module provides evidence of this event, as these genes largely possess aerobic ATP synthase and mitochondrial function96–99. Expression module 3 had nine genes with increased expression in bronchial lymph node tissue sampled from cattle in the control group and BRSV. These genes play important roles in T-cell proliferation, integrin activation and antigenic presentation through actin/tubulin reorganization100–103. Potentially, this serves as an underlying mechanism of immunological stimulation unique to lymph nodes of the lower airway.
Conclusion
This study was conducted to integrate and analyze mRNA-Seq datasets with supervised ML methodology. This approach allowed for a novel and comprehensive analysis of lung and immunological tissues in to experimentally induced BRD. ML enabled the classification of viral-induced BRD, specifically with BRSV and IBR, with 100% balanced accuracy, against sham controls, regardless of the tissue type. This experimental investigation illustrates a novel and powerful approach to the investigation of host response mechanisms in BRD through the use of mRNA-Seq and supervised ML analysis.
Supplementary Information
Acknowledgements
The authors would acknowledge the support for this work from the Mississippi State University Department of Pathobiology and Population Medicine and Texas A&M University Department of Large Animal Clinical Sciences. We would like to thank the support staff of NCBI GEO, who organize, maintain, and assure free distribution of sequence data and necessary metadata which made this project possible.
Author contributions
Experimental design: M.A.S., A.D.P., B.N. Data collection: M.A.S. Computational analysis: M.A.S., C.E.S., A.D.P., B.N. Project supervision: M.A.S., A.R.W., C.E.S., A.D.P., B.N. Composed the original draft: M.A.S. All authors contributed to review and editing of the final manuscript.
Data availability
The data utilized in this study were downloaded from the National Center for Biotechnology Information Gene Expression Omnibus (NCBI-GEO), Bioproject numbers PRJNA272725 and PRJNA543752.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-02343-7.
References
- 1.Griffin D, Chengappa MM, Kuszak J, McVey DS. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. North Am. Food Anim. Pract. 2010;26:381–394. doi: 10.1016/j.cvfa.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Delabouglise A, et al. Linking disease epidemiology and livestock productivity: The case of bovine respiratory disease in France. PLoS One. 2017;12:e0189090. doi: 10.1371/journal.pone.0189090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timurkan MO, Aydin H, Sait A. Identification and molecular characterisation of bovine parainfluenza virus-3 and bovine respiratory syncytial virus—First report from Turkey. J. Vet. Res. 2019;63:167–173. doi: 10.2478/jvetres-2019-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray GM, et al. Pathogens, patterns of pneumonia, and epidemiologic risk factors associated with respiratory disease in recently weaned cattle in Ireland. J. Vet. Diagn. Investig. 2017;29:20–34. doi: 10.1177/1040638716674757. [DOI] [PubMed] [Google Scholar]
- 5.Blakebrough-Hall C, Hick P, González LA. Predicting bovine respiratory disease outcome in feedlot cattle using latent class analysis. J. Anim. Sci. 2020;98:skaa381. doi: 10.1093/jas/skaa381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baruch J, et al. Performance of multiple diagnostic methods in assessing the progression of bovine respiratory disease in calves challenged with infectious bovine rhinotracheitis virus and Mannheimia haemolytica. J. Anim. Sci. 2019;97:2357–2367. doi: 10.1093/jas/skz107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glover ID, Barrett DC, Reyher KK. Little association between birth weight and health of preweaned dairy calves. Vet. Rec. 2019;184:477. doi: 10.1136/vr.105062. [DOI] [PubMed] [Google Scholar]
- 8.Dutta E, et al. Development of a multiplex real-time PCR assay for predicting macrolide and tetracycline resistance associated with bacterial pathogens of bovine respiratory disease. Pathogens. 2021;10:64. doi: 10.3390/pathogens10010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cusack PM, McMeniman N, Lean IJ. The medicine and epidemiology of bovine respiratory disease in feedlots. Aust. Vet. J. 2003;81:480–487. doi: 10.1111/j.1751-0813.2003.tb13367.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, et al. The pulmonary virome, bacteriological and histopathological findings in bovine respiratory disease from western Canada. Transbound. Emerg. Dis. 2020;67:924–934. doi: 10.1111/tbed.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, et al. Respiratory viruses identified in western Canadian beef cattle by metagenomic sequencing and their association with bovine respiratory disease. Transbound. Emerg. Dis. 2019;66:1379–1386. doi: 10.1111/tbed.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klima CL, et al. Lower respiratory tract microbiome and resistome of bovine respiratory disease mortalities. Microb. Ecol. 2019;78:446–456. doi: 10.1007/s00248-019-01361-3. [DOI] [PubMed] [Google Scholar]
- 13.Taylor JD, Fulton RW, Lehenbauer TW, Step DL, Confer AW. The epidemiology of bovine respiratory disease: What is the evidence for predisposing factors. Can. Vet. J. 2010;51:1095–1102. [PMC free article] [PubMed] [Google Scholar]
- 14.Rice JA, Carrasco-Medina L, Hodgins DC, Shewen PE. Mannheimia haemolytica and bovine respiratory disease. Anim. Health Res. Rev. 2007;8:117–128. doi: 10.1017/S1466252307001375. [DOI] [PubMed] [Google Scholar]
- 15.Woolums AR, et al. Multidrug resistant Mannheimiahaemolytica isolated from high-risk beef stocker cattle after antimicrobial metaphylaxis and treatment for bovine respiratory disease. Vet. Microbiol. 2018;221:143–152. doi: 10.1016/j.vetmic.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 16.White BJ, Renter DG. Bayesian estimation of the performance of using clinical observations and harvest lung lesions for diagnosing bovine respiratory disease in post-weaned beef calves. J. Vet. Diagn. Investig. 2009;21:446–453. doi: 10.1177/104063870902100405. [DOI] [PubMed] [Google Scholar]
- 17.Timsit E, Dendukuri N, Schiller I, Buczinski S. Diagnostic accuracy of clinical illness for bovine respiratory disease (BRD) diagnosis in beef cattle placed in feedlots: A systematic literature review and hierarchical Bayesian latent-class meta-analysis. Prev. Vet. Med. 2016;135:67–73. doi: 10.1016/j.prevetmed.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Shahriar FM, Clark EG, Janzen E, West K, Wobeser G. Coinfection with bovine viral diarrhea virus and Mycoplasmabovis in feedlot cattle with chronic pneumonia. Can. Vet. J. 2002;43:863–868. [PMC free article] [PubMed] [Google Scholar]
- 19.Allen JW, Viel L, Bateman KG, Rosendal S. Changes in the bacterial flora of the upper and lower respiratory tracts and bronchoalveolar lavage differential cell counts in feedlot calves treated for respiratory diseases. Can. J. Vet. Res. 1992;56:177–183. [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor AM, et al. A systematic review and network meta-analysis of bacterial and viral vaccines, administered at or near arrival at the feedlot, for control of bovine respiratory disease in beef cattle. Anim. Health Res. Rev. 2019;20:143–162. doi: 10.1017/S1466252319000288. [DOI] [PubMed] [Google Scholar]
- 21.Griffin CM, et al. A randomized controlled trial to test the effect of on-arrival vaccination and deworming on stocker cattle health and growth performance. Bov. Pract. (Stillwater) 2018;52:26–33. [PMC free article] [PubMed] [Google Scholar]
- 22.Richeson JT, Falkner TR. Bovine respiratory disease vaccination: What is the effect of timing. Vet. Clin. North Am. Food Anim. Pract. 2020;36:473–485. doi: 10.1016/j.cvfa.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Richeson JT, et al. Effects of on-arrival versus delayed clostridial or modified live respiratory vaccinations on health, performance, bovine viral diarrhea virus type I titers, and stress and immune measures of newly received beef calves. J. Anim. Sci. 2009;87:2409–2418. doi: 10.2527/jas.2008-1484. [DOI] [PubMed] [Google Scholar]
- 24.Richeson JT, et al. Effects of on-arrival versus delayed modified live virus vaccination on health, performance, and serum infectious bovine rhinotracheitis titers of newly received beef calves. J. Anim. Sci. 2008;86:999–1005. doi: 10.2527/jas.2007-0593. [DOI] [PubMed] [Google Scholar]
- 25.Coetzee JF, et al. Association between antimicrobial drug class for treatment and retreatment of bovine respiratory disease (BRD) and frequency of resistant BRD pathogen isolation from veterinary diagnostic laboratory samples. PLoS One. 2019;14:e0219104. doi: 10.1371/journal.pone.0219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Gerstein M, Snyder M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong M, et al. RNA sequencing: New technologies and applications in cancer research. J. Hematol. Oncol. 2020;13:166. doi: 10.1186/s13045-020-01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stark R, Grzelak M, Hadfield J. RNA sequencing: The teenage years. Nat. Rev. Genet. 2019;20:631–656. doi: 10.1038/s41576-019-0150-2. [DOI] [PubMed] [Google Scholar]
- 29.Westermann AJ, Gorski SA, Vogel J. Dual RNA-seq of pathogen and host. Nat. Rev. Microbiol. 2012;10:618–630. doi: 10.1038/nrmicro2852. [DOI] [PubMed] [Google Scholar]
- 30.Tizioto PC, et al. Immunological response to single pathogen challenge with agents of the bovine respiratory disease complex: An RNA-sequence analysis of the bronchial lymph node transcriptome. PLoS One. 2015;10:e0131459. doi: 10.1371/journal.pone.0131459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behura SK, et al. Tissue tropism in host transcriptional response to members of the bovine respiratory disease complex. Sci. Rep. 2017;7:17938. doi: 10.1038/s41598-017-18205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston D, et al. Experimental challenge with bovine respiratory syncytial virus in dairy calves: Bronchial lymph node transcriptome response. Sci. Rep. 2019;9:14736. doi: 10.1038/s41598-019-51094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott MA, et al. Whole blood transcriptomic analysis of beef cattle at arrival identifies potential predictive molecules and mechanisms that indicate animals that naturally resist bovine respiratory disease. PLoS One. 2020;15:e0227507. doi: 10.1371/journal.pone.0227507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott MA, et al. Comprehensive at-arrival transcriptomic analysis of post-weaned beef cattle uncovers type I interferon and antiviral mechanisms associated with bovine respiratory disease mortality. PLoS One. 2021;16:e0250758. doi: 10.1371/journal.pone.0250758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun HZ, et al. Longitudinal blood transcriptomic analysis to identify molecular regulatory patterns of bovine respiratory disease in beef cattle. Genomics. 2020;112:3968–3977. doi: 10.1016/j.ygeno.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Rehrauer H, Opitz L, Tan G, Sieverling L, Schlapbach R. Blind spots of quantitative RNA-seq: The limits for assessing abundance, differential expression, and isoform switching. BMC Bioinform. 2013;14:370. doi: 10.1186/1471-2105-14-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conesa A, et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016;17:13. doi: 10.1186/s13059-016-0881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang Z, Martin J, Wang Z. Statistical methods for identifying differentially expressed genes in RNA-Seq experiments. Cell Biosci. 2012;2:26. doi: 10.1186/2045-3701-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajkumar AP, et al. Experimental validation of methods for differential gene expression analysis and sample pooling in RNA-seq. BMC Genomics. 2015;16:548. doi: 10.1186/s12864-015-1767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu C, Jackson SA. Machine learning and complex biological data. Genome Biol. 2019;20:76. doi: 10.1186/s13059-019-1689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cascianelli S, Molineris I, Isella C, Masseroli M, Medico E. Machine learning for RNA sequencing-based intrinsic subtyping of breast cancer. Sci. Rep. 2020;10:14071. doi: 10.1038/s41598-020-70832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Xi Y, Sung S, Qiao H. RNA-seq assistant: Machine learning based methods to identify more transcriptional regulated genes. BMC Genomics. 2018;19:546. doi: 10.1186/s12864-018-4932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma C, Xin M, Feldmann KA, Wang X. Machine learning-based differential network analysis: A study of stress-responsive transcriptomes in Arabidopsis. Plant Cell. 2014;26:520–537. doi: 10.1105/tpc.113.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bzdok D, Altman N, Krzywinski M. Statistics versus machine learning. Nat. Methods. 2018;15:233–234. doi: 10.1038/nmeth.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song X, et al. Identification of risk genes related to myocardial infarction and the construction of early SVM diagnostic model. Int. J. Cardiol. 2021;328:182–190. doi: 10.1016/j.ijcard.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Wang C, Xue W, Zhang H, Fu Y. Identification of candidate genes encoding tumor-specific neoantigens in early- and late-stage colon adenocarcinoma. Aging (Albany NY) 2021;13:4024–4044. doi: 10.18632/aging.202370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmer D, Fabris F, Doherty A, Freitas AA, de Magalhães JP. Ageing transcriptome meta-analysis reveals similarities and differences between key mammalian tissues. Aging (Albany NY) 2021;13:3313–3341. doi: 10.18632/aging.202648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moon M, Nakai K. Stable feature selection based on the ensemble L 1-norm support vector machine for biomarker discovery. BMC Genomics. 2016;17:1026. doi: 10.1186/s12864-016-3320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rabaglino MB, Kadarmideen HN. Machine learning approach to integrated endometrial transcriptomic datasets reveals biomarkers predicting uterine receptivity in cattle at seven days after estrous. Sci. Rep. 2020;10:16981. doi: 10.1038/s41598-020-72988-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrett T, et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013;41:D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Online at http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (2010).
- 53.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pertea M, et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kovaka S, et al. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019;20:278. doi: 10.1186/s13059-019-1910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pertea G. prepDE.py. https://github.com/gpertea/stringtie/blob/master/prepDE.py (2019).
- 59.Goksuluk D, et al. MLSeq: Machine learning interface for RNA-sequencing data. Comput. Methods Programs Biomed. 2019;175:223–231. doi: 10.1016/j.cmpb.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Silverman JD, Roche K, Mukherjee S, David LA. Naught all zeros in sequence count data are the same. Comput. Struct. Biotechnol. J. 2020;18:2789–2798. doi: 10.1016/j.csbj.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaul A, Mandal S, Davidov O, Peddada SD. Analysis of microbiome data in the presence of excess zeros. Front. Microbiol. 2017;8:2114. doi: 10.3389/fmicb.2017.02114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buttenschoen K, et al. Endotoxemia and endotoxin tolerance in patients with ARDS. Langenbecks Arch. Surg. 2008;393:473–478. doi: 10.1007/s00423-008-0317-3. [DOI] [PubMed] [Google Scholar]
- 64.Dong K, Zhao H, Tong T, Wan X. NBLDA: Negative binomial linear discriminant analysis for RNA-Seq data. BMC Bioinform. 2016;17:369. doi: 10.1186/s12859-016-1208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zararsiz G, et al. voomDDA: Discovery of diagnostic biomarkers and classification of RNA-seq data. PeerJ. 2017;5:e3890. doi: 10.7717/peerj.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conway JR, Lex A, Gehlenborg N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics. 2017;33:2938–2940. doi: 10.1093/bioinformatics/btx364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khan A, Mathelier A. Intervene: A tool for intersection and visualization of multiple gene or genomic region sets. BMC Bioinform. 2017;18:287. doi: 10.1186/s12859-017-1708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kolde R. pheatmap: Pretty Heatmaps. https://cran.r-project.org/web/packages/pheatmap/index.html (2019).
- 70.Garnier S. et al. viridis: Default Color Maps from 'matplotlib'. https://cran.r-project.org/web/packages/viridis/index.html (2018).
- 71.Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019;47:W199–W205. doi: 10.1093/nar/gkz401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fabregat A, et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2017;46:D649–D655. doi: 10.1093/nar/gkx1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao H, et al. Transcriptome characterization of short distance transport stress in beef cattle blood. Front. Genet. 2021;12:616388. doi: 10.3389/fgene.2021.616388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barreto DM, Barros GS, Santos LABO, Soares RC, Batista MVA. Comparative transcriptomic analysis of bovine papillomatosis. BMC Genomics. 2018;19:949. doi: 10.1186/s12864-018-5361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lim WK, Mathuru AS. Design, challenges, and the potential of transcriptomics to understand social behavior. Curr. Zool. 2020;66:321–330. doi: 10.1093/cz/zoaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Conesa A, et al. Erratum to: A survey of best practices for RNA-seq data analysis. Genome Biol. 2016;17:181. doi: 10.1186/s13059-016-1047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cai G, Liang S, Zheng X, Xiao F. Local sequence and sequencing depth dependent accuracy of RNA-seq reads. BMC Bioinform. 2017;18:364. doi: 10.1186/s12859-017-1780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lagani V, Karozou AD, Gomez-Cabrero D, Silberberg G, Tsamardinos I. A comparative evaluation of data-merging and meta-analysis methods for reconstructing gene-gene interactions. BMC Bioinform. 2016;17(Suppl 5):194. doi: 10.1186/s12859-016-1038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Libbrecht MW, Noble WS. Machine learning applications in genetics and genomics. Nat. Rev. Genet. 2015;16:321–332. doi: 10.1038/nrg3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Y, Zhou J, White KP. RNA-seq differential expression studies: More sequence or more replication? Bioinformatics. 2013;30:301–304. doi: 10.1093/bioinformatics/btt688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Theurer ME, Larson RL, White BJ. Systematic review and meta-analysis of the effectiveness of commercially available vaccines against bovine herpesvirus, bovine viral diarrhea virus, bovine respiratory syncytial virus, and parainfluenza type 3 virus for mitigation of bovine respiratory disease complex in cattle. J. Am. Vet. Med. Assoc. 2015;246:126–142. doi: 10.2460/javma.246.1.126. [DOI] [PubMed] [Google Scholar]
- 82.Colby LA, Quenee LE, Zitzow LA. Considerations for infectious disease research studies using animals. Comp. Med. 2017;67:222–231. [PMC free article] [PubMed] [Google Scholar]
- 83.Huang S, et al. Applications of support vector machine (SVM) learning in cancer genomics. Cancer Genomics Proteomics. 2018;15:41–51. doi: 10.21873/cgp.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abdelaal T, et al. A comparison of automatic cell identification methods for single-cell RNA sequencing data. Genome Biol. 2019;20:194. doi: 10.1186/s13059-019-1795-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng Z, et al. Acute bovine viral diarrhea virus infection inhibits expression of interferon tau-stimulated genes in bovine endometrium. Biol. Reprod. 2017;96:1142–1153. doi: 10.1093/biolre/iox056. [DOI] [PubMed] [Google Scholar]
- 86.Peterhans E, Schweizer M. BVDV: A pestivirus inducing tolerance of the innate immune response. Biologicals. 2013;41:39–51. doi: 10.1016/j.biologicals.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 87.Peterhans E, Jungi TW, Schweizer M. BVDV and innate immunity. Biologicals. 2003;31:107–112. doi: 10.1016/s1045-1056(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 88.Alkheraif AA, et al. Type 2 BVDV Npro suppresses IFN-1 pathway signaling in bovine cells and augments BRSV replication. Virology. 2017;507:123–134. doi: 10.1016/j.virol.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 89.Lee SR, Pharr GT, Boyd BL, Pinchuk LM. Bovine viral diarrhea viruses modulate toll-like receptors, cytokines and co-stimulatory molecules genes expression in bovine peripheral blood monocytes. Comp. Immunol. Microbiol. Infect. Dis. 2008;31:403–418. doi: 10.1016/j.cimid.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 90.Liu H, Miller E, van de Water B, Stevens JL. Endoplasmic reticulum stress proteins block oxidant-induced Ca2+ increases and cell death. J. Biol. Chem. 1998;273:12858–12862. doi: 10.1074/jbc.273.21.12858. [DOI] [PubMed] [Google Scholar]
- 91.Kober L, Zehe C, Bode J. Development of a novel ER stress based selection system for the isolation of highly productive clones. Biotechnol. Bioeng. 2012;109:2599–2611. doi: 10.1002/bit.24527. [DOI] [PubMed] [Google Scholar]
- 92.Lenny N, Green M. Regulation of endoplasmic reticulum stress proteins in COS cells transfected with immunoglobulin mu heavy chain cDNA. J. Biol. Chem. 1991;266:20532–20537. [PubMed] [Google Scholar]
- 93.Xu Z, Jensen G, Yen TS. Activation of hepatitis B virus S promoter by the viral large surface protein via induction of stress in the endoplasmic reticulum. J. Virol. 1997;71:7387–7392. doi: 10.1128/jvi.71.10.7387-7392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paréj K, et al. Cutting edge: A new player in the alternative complement pathway, MASP-1 is essential for LPS-induced, but not for zymosan-induced, alternative pathway activation. J. Immunol. 2018;200:2247–2252. doi: 10.4049/jimmunol.1701421. [DOI] [PubMed] [Google Scholar]
- 95.Askar H, et al. Immune evasion of Mycoplasma bovis. Pathogens. 2021;10:297. doi: 10.3390/pathogens10030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mayr JA, et al. Reduced respiratory control with ADP and changed pattern of respiratory chain enzymes as a result of selective deficiency of the mitochondrial ATP synthase. Pediatr. Res. 2004;55:988–994. doi: 10.1203/01.pdr.0000127016.67809.6b. [DOI] [PubMed] [Google Scholar]
- 97.Sonawane AR, et al. Microbiome-transcriptome interactions related to severity of respiratory syncytial virus infection. Sci. Rep. 2019;9:13824. doi: 10.1038/s41598-019-50217-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fearnley IM, Walker JE. Two overlapping genes in bovine mitochondrial DNA encode membrane components of ATP synthase. EMBO J. 1986;5:2003–2008. doi: 10.1002/j.1460-2075.1986.tb04456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dautant A, et al. ATP synthase diseases of mitochondrial genetic origin. Front. Physiol. 2018;9:329. doi: 10.3389/fphys.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martín-Cófreces NB, Alarcón B, Sánchez-Madrid F. Tubulin and actin interplay at the T cell and antigen-presenting cell interface. Front. Immunol. 2011;2:24. doi: 10.3389/fimmu.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Erasmus JC, et al. Defining functional interactions during biogenesis of epithelial junctions. Nat. Commun. 2016;7:13542. doi: 10.1038/ncomms13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yago T, et al. Blocking neutrophil integrin activation prevents ischemia–reperfusion injury. J. Exp. Med. 2015;212:1267–1281. doi: 10.1084/jem.20142358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lichtenfels R, et al. A proteomic view at T cell costimulation. PLoS One. 2012;7:e32994. doi: 10.1371/journal.pone.0032994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized in this study were downloaded from the National Center for Biotechnology Information Gene Expression Omnibus (NCBI-GEO), Bioproject numbers PRJNA272725 and PRJNA543752.