Abstract
Systems neuroscience approaches with a focus on large-scale brain organization and network analysis are advancing foundational knowledge of how cognitive control processes are implemented in the brain. Over the past decade, technological and computational innovations in the study of brain connectivity have led to advances in our understanding of how brain networks function, inspiring new conceptualizations of the role of prefrontal cortex (PFC) networks in the coordination of cognitive control. In this review, we describe six key PFC networks involved in cognitive control and elucidate key principles relevant for understanding how these networks implement cognitive control. Implementation of cognitive control in a constantly changing environment depends on the dynamic and flexible organization of PFC networks. In this context, we describe major empirical and theoretical models that have emerged in recent years and describe how their functional architecture and dynamic organization supports flexible cognitive control. We take an overarching view of advances made in the past few decades and consider fundamental issues regarding PFC network function, global brain dynamics, and cognition that still need to be resolved. We conclude by clarifying important future directions for research on cognitive control and their implications for advancing our understanding of PFC networks in brain disorders.
Subject terms: Neuroscience, Psychology
Introduction
Cognitive control, the coordination of mental processes and action in accordance with current goals and future plans, is a primary function of the prefrontal cortex (PFC) [1]. The term “cognitive control” is meant to capture the same abilities labeled in the clinical literature as “executive function”. The coordination of cognitive processes is implemented by multiple functional circuits anchored in the PFC, and systems neuroscience approaches with a focus on large-scale brain organization and network analysis have become influential in advancing knowledge of how cognitive control processes are implemented in the brain.
Much of our current knowledge of the role of PFC in cognitive control has been derived from the modular paradigm, in which specific functions have been ascribed to localized subdivisions of the PFC with the underlying assumption that they act as independent processors for specific cognitive functions [2]. However, the notion that PFC functions should be considered in conjunction with distributed and heavily interconnected neurons with long-range axons has now grown into an important paradigm for investigation of the role of the PFC in cognitive control. Such a view, is, of course, not new, as formulations of dedicated brain circuits for working memory, spatial attention, and cognition-action plans have been central to the proposals of Goldman-Rakic, Mesulam, Fuster and others [3–5]. Over the past decade, technological and computational innovations in the study of brain connectivity have advanced our understanding of how brain regions function together, inspiring new conceptualizations of the role of PFC networks in cognitive control.
The PFC is a highly heterogenous brain structure consisting of anatomical units each with its own cytoarchitectonic, neurochemical, and microstructural properties [6]. The function of a specific PFC area depends on its intrinsic properties as well as its extrinsic connections [7]. Each PFC area has a unique pattern of cortico-cortical and cortico-subcortical connections—a “connectional fingerprint” that distinguishes it from other areas (Fig. 1a). Although no two brain areas share identical connection patterns, their dense interconnections lead to a network organization with shared functional characteristics. Given the central role of the PFC in adaptive goal- directed behaviors, characterization of its large-scale functional networks and their dynamic spatiotemporal properties has the potential to provide foundational knowledge of their role as cognitive hubs for coordinating mental processes.
Fig. 1. From connectivity fingerprints to large-scale brain networks.
a Each PFC area has a unique pattern of cortico-cortical and cortico-subcortical connections—a “connectional fingerprint” that distinguishes it from other Brodmann’s areas. Adapted from [7]. b Large-scale networks in the human brain, each consisting of a distinct set of cortical and subcortical areas linked by temporally synchronous neural activity. Fourteen intrinsic connectivity networks identified using independent component analysis. (A) Auditory, (B) Basal Ganglia, (C) Posterior Cingulate Cortex (PCC)/Medial Prefrontal Cortex (MPFC), (D) Secondary Visual Cortex (V2), (E) Language, (F) Left Dorsolateral Prefrontal Cortex (DLPFC)/Left Parietal Lobe, (G) Sensorimotor, (H) Posterior Insula, (I) Precuneus, (J) Primary Visual Cortex (V1), (K) Right Dorsolateral Prefrontal Cortex (DLPFC)/Right Parietal Lobe, (L) Insula/Dorsal Anterior Cingulate Cortex (dACC), (M) Retrosplenial Cortex (RSC)/Medial Temporal Lobe (MTL), (N) Intraparietal Sulcus (IPS)/Frontal Eye Field (FEF). Adapted from [20].
A large-scale functional network is a collection of interconnected brain areas, or nodes, that are linked together to perform circumscribed functions [8]. The nodes of a network share dense interconnections among its constituent nodes when compared to connections with nodes that form other brain networks. A large body of research using multiple methodologies has established that the human brain is intrinsically organized into networks, each consisting of a distinct set of cortical and subcortical areas linked by temporally synchronous neural activity (Fig. 1b). The intrinsic connectivity of brain networks displays close correspondence with task-related co-activation of brain regions, and this correspondence has allowed intrinsic and task-related connectivity to be demarcated and studied under a common systems neuroscience framework. While brain networks impose constraints on signaling, their role in cognition is far from static and flexible integration with other networks lies at the core of the adaptive functions of PFC networks. Crucially, by virtue of their unique “connectional fingerprints”, component brain areas of large-scale functional networks may perform different roles, some acting as controllers that switch on the engagement of other areas, others contributing specific sensory or semantic content to network operations.
Six PFC networks that play central roles in cognitive control are the focus of this review (Fig. 2): the fronto-parietal (FPN) network, the salience network (SN),
Fig. 2. Six PFC networks that are the focus of this review.
a Fronto-parietal network (FPN), with key nodes in dorsolateral PFC (dlPFC) and posterior parietal cortex (PPC). b Salience network (SN), with key nodes in anterior insula (AI) and dorsal anterior cingulate cortex (ACC). c Cingulo-opercular network (CON, black) with key nodes in anterior insula/frontal operculum (aI/fO), dorsal ACC and medial superior frontal cortex (dACC/msFC), anterior PFC (aPFC) and thalamus, as distinguished from the FPN (yellow). d Ventral attention network (VAN), with key nodes in insula (Ins), inferior frontal junction (IFJ), supramarginal gyrus (SMG), and superior temporal gyrus (STG). e Dorsal attention network (DAN), with key nodes in frontal eye fields (FEF), inferior frontal junction (IFJ), intra-parietal sulcus and superior parietal lobule (IPS/SPL), angular gyrus (AG), visual area 3 A (V3A), and middle temporal visual area (MT). f Default mode network (DMN), with key nodes in ventromedial PFC (vmPFC) and posterior cingulate cortex (PCC). Adapted from [20, 32, 62, 93].
cingulo-opercular network (CON), the default mode network (DMN), and the dorsal and ventral attention networks (DAN and VAN). While these networks are proposed to serve as dedicated and specialized functional units, they do not function in isolation, and they display complex patterns of context-dependent dynamic interactions amongst each other. Moreover, dynamic integrative between-network communication is crucial for efficient cognitive control and adaptive behaviors [9–11], and models of intra- and inter-network dynamics are central to our understanding of how the PFC orchestrates cognitive control.
This chapter is organized as follows. We briefly describe multimodal imaging and computational techniques that are commonly used to identify PFC networks and provide an essential background for assessing demarcation of PFC networks involved in cognitive control. We then describe the anatomical and functional organization of PFC networks and review the role PFC networks that have been widely used for probing cognitive control. We next highlight basic principles of PFC network organization, followed by a review of potential network mechanisms implementing cognitive control. Finally, we present future directions for research on PFC network function and clinical implications of a network view of cognitive control for individuals with psychiatric and neurological disorders.
Identification of PFC networks
Distributed patterns of PFC connectivity associated with cognitive control were initially identified by Pandya, Petrides, Mesulam and others using tract tracing studes in non-human primates [4, 12, 13]. These studies established the presence of dense white matter tracts linking individual PFC areas with distinct cortical and subcortical areas. Two distinct PFC networks each dedicated to distinct cognitive functions, a working memory–executive function network and a spatial attention network were among the first to be identified [4]. The electrophysiological basis of these networks was also clarified on the basis of similar neuronal response profiles during cognitive tasks involving working memory and spatial attention [3]. Subsequently, studies using non-invasive diffusion tensor imaging provided robust support for homologous pathways in the human brain which can now be quantified in each individual and linked to other multimodal characterizations of PFC networks [14]. With rapid improvements in the spatial and temporal resolution of in vivo brain imaging, computational analysis of functional, structural and diffusion MRI now permit the investigation of the anatomical and functional connectivity in a reliable and robust manner across time within individuals.
Characterization of functional brain networks requires principled and computationally rigorous tools for specifying PFC nodes and edges, or inter-regional connectivity. We summarize some of the key methods below, providing essential background for assessing demarcation of PFC networks in cognitive control. For additional methodological details, readers are referred to excellent reviews on brain network analysis [15]. Connectivity analyses of task-induced and task-free (resting-state) fMRI (rs-fMRI) data have over the past decade become the mainstay for PFC network identification. Independent component analysis, clustering and modularity analysis of inter-regional fMRI connectivity are three of the most commonly used methods. Independent component analysis is a blind deconvolution technique which decomposes fMRI signals into spatially distinct components without requiring pre-specification of the nodes that constitute individual networks [16]. This approach has led to the identification of multiple spatially segregated PFC networks [17–19]. Importantly, spontaneous coupling between spatially distributed regions can be reliably identified at the individual subject level and the PFC networks identified in this manner also share consistent features across individuals [18–20].
Clustering of intrinsic functional connectivity of each brain area is another widely used approach for network identification [21]. In this approach, boundaries of functionally distinct cortical regions are defined on the basis of differences in patterns of functional connectivity across regions distributed throughout the cerebral cortex. Sets of cortical regions with similar profiles of cortico-cortical and cortico-subcortical connectivity are deemed to constitute a network [21–24].
Network analysis based on graph theory has been central to our understanding of complex biological systems [25], and the human brain is no exception. Graph-theoretical analysis of inter-regional connectivity have identified network modules associated with the PFC [15, 26]. This approach is dependent on pre-defined nodes and researchers have used both anatomical atlases and functional parcellations for defining network nodes. Each module in this formulation is characterized by a high clustering coefficient with specialized hubs that bind nodes with a module, as well as connector hubs which link modules and are therefore anchors for dynamically reconfiguring networks.
None of the techniques described here currently dominates the study of PFC network function, and no single atlas of functional networks has emerged dominant. One reason for this is that each technique makes assumptions about stationarity of fMRI data, whose validity is hard to establish, and each involves estimation of model parameters, such as the number of independent components, the number of clusters, or the number of modules, for which there is no unique solution. Thus, for example, the FPN might be described by distinct networks in the left and right hemispheres in some studies and by a joint network encompassing both hemispheres in others. Moreover, individual networks can also be subdivided at different levels of spatial granularity [21]. Challenges of matching anatomical boundaries in native MRI space and imprecision from the use of normalized MRI-templates impose additional uncertainty in this regard. Nevertheless, while the PFC networks identified using these disparate approaches are not identical, they have revealed common underlying patterns of organization which make them powerful tools for probing PFC circuit function in a principled manner. The techniques discussed here are useful not only for network identification but also for probing their flexible reconfiguration during cognitive control [27].
Anatomy and taxonomy of PFC networks in cognitive control
Six PFC networks proposed to be involved in cognitive control have been consistently identified using computational approaches for network identification described in the previous section (Fig. 2). In this section we consider the anatomical anchors of these networks in the PFC and briefly consider their taxonomy in the context of task-based activation studies of cognition. It should be noted that networks identified using intrinsic functional connectivity are labeled on the basis of their relations to task-based functional activation profiles and, our current understanding of the cognitive processes these networks subserve. These networks have been historically assigned either anatomical or functional labels, however, the field has begun to grapple with a universal taxonomy for functional networks that will hopefully provide consistency for comparison of findings across studies [28].
Fronto-parietal network
The fronto-parietal network (FPN) is comprised of the dorsolateral (BA 9/46) and dorsomedial PFC (BA 6), the supramarginal gyrus (BA 40) in posterior parietal cortex and subcortical regions including the dorsal caudate and anterior thalamus [18]. A tight link between lateral PFC and posterior parietal cortex is supported by the demonstration of strong bidirectional anatomical connections with each other [4], as well as similar profiles of neuronal responses [3].
Salience network
The salience network (SN) is comprised of the anterior insula, adjoining fronto-insular cortex, and dorsal anterior cingulate cortex (BA 24), with prominent subcortical nodes including the amygdala, substantia nigra, ventral tegmental area, dorsomedial thalamus, hypothalamus, and periaqueductal gray [18].
Cingulo-opercular network
The cingulo-opercular network (CON) is comprised of the anterior insula, adjoining fronto-insular cortex and the anterior cingulate cortex and adjacent dorsomedial PFC [29]. The CON was identified using graph-based modularity analysis of resting-state fMRI data extracted from 39 brain areas that showed consistent activation across a wide range of tasks [30]. Although the SN and CON networks show considerable overlap in the insula and anterior cingulate cortex, they also diverge in specification of other cortical and subcortical network nodes due possibly due to differing methodologies used for network identification. However, it has been suggested the SN and CON may be distinct networks based on the finding that nodes within insular and anterior cingulate cortex may be anatomically distinct [27, 31]. Also, this anatomical divergence may explain differences in the hypothesized functions of the SN and CON in models of cognitive control, which we will discuss further later in this chapter.
Ventral attention network
The ventral attention network (VAN) is comprised of the ventral-posterior aspects of the inferior frontal gyrus and the temporoparietal junction in posterior cortex [32]. VAN nodes show significant anatomical overlap with the SN in the fronto-insular cortex. A crucial distinction however is that VAN comprises fronto-insular cortex connectivity with the temporoparietal junction whereas the SN (and similarly the CON) comprises connectivity with the dorsal anterior cingulate cortex and subcortical regions.
Dorsal attention network
The dorsal attention network (DAN) is comprised of the frontal eye fields (FEF) and intra-parietal sulcus (IPS). The network was initially identified in tract-tracing studies [4, 33], and subsequently further characterized and investigated using lesion [34] and resting-state fMRI studies [20, 35].
Default mode network
The default mode network (DMN) is comprised of the medial prefrontal cortex, the posterior cingulate cortex, medial temporal lobe, and angular gyrus [17, 18, 36]. The DMN was first identified using independent components analysis and seed-based connectivity of rs-fMRI and confirmed with multiple approaches including coactivation analysis and DTI studies showing a strong link between the medial PFC and posterior-medial nodes of the network [37].
Basic principles of PFC network organization
Despite differences in methodology yielding differing levels of granularity and anatomical boundaries, most approaches have converged on the FPN, SN, CON, DAN, VAN, and DMN as robust and reliably identifiable functional networks anchored in the PFC. An important feature of each of these networks is that they span association areas across frontal, parietal, temporal, and cingulate cortices. In this section, we highlight several principles of PFC network organization that are relevant for understanding how they may implement cognitive control.
First, PFC networks are presumed to operate as distinct and independent functional units, such that, nodes that comprise these networks will coactivate across a wide range of cognitive tasks, presumably supporting a specific cognitive process. Thus, for example, the middle frontal gyrus (BA 9/46) and supramarginal gyrus (BA 40) nodes of the FPN are almost always coactivated during tasks that engage working memory [38, 39]. Moreover, there is a strong spatial correspondence between functional networks derived from rs-fMRI data and the task-evoked activity maps of cognitive components derived from the BrainMap database (comprising 9208 experiments of 77 cognitive tasks), supporting the idea that specific networks perform distinct cognitive functions [40]. Indirect evidence for the notion that PFC networks are functionally distinct and independent derives from rs-fMRI data showing that these networks are anatomically distinct components when analyzed as a brain graph, and are anti- or weakly-correlated, that is, there is greater connectivity between regions within a network than between networks [29]. Direct evidence for functional independence has come from rs-fMRI studies of patients with focal lesions demonstrating that damage to the FPN and CON, thought to support different components of cognitive control, alters connectivity within the damaged network but leaves the other network preserved [41]. It is important to note, however, that the finding that these networks are functionally distinct units does not preclude the possibility that they flexibly interact to carry out cognitive control, which will be discussed later in this review.
Second, each PFC node includes a distinct reciprocally connected node in the parietal cortex, for example, the supramarginal gyrus in the case of the FPN and posterior cingulate cortex in the case of the DMN. Thus, each PFC network implements a unique pattern of dedicated signaling between anatomically distinct subdivisions of the PFC and parietal cortex. This anatomical realization highlights the importance of the parietal cortex in models of cognitive control as another critical source of top-down control signals, in addition to the PFC [42].
Third, the functional organization of PFC networks is aligned along a dorsal-ventral gradient with dorsal PFC regions linked to dorsal parietal cortical regions, and ventral PFC regions linked to more ventral parietal cortical regions. Thus, the FEF and dorsal IPS nodes of the DAN are dorsal to the dorsolateral PFC and SMG nodes of the FPN, which in turn are dorsal to the fronto-insular and temporal parietal junction nodes of the VAN. This functional segregation reflects anatomical segregation of pathways associated with three major fiber tracts that connect PFC and parietal cortex: the dorsal, middle, and ventral superior longitudinal fasciculi [43]. Notably, an outcome of this architecture is that adjacent PFC regions belong to entirely different networks.
Fourth, segregation of PFC networks also extends to distinct subcortical regions. Notably, the SN includes distinct limbic areas including the amygdala, ventral striatum, dorsomedial thalamus, hypothalamus, and substantia nigra/ventral tegmental area [18] (Fig. 3). In contrast, the FPN includes the dorsal caudate and anterior thalamus but lacks connectivity with limbic, hypothalamic, and midbrain structures. In general, subcortical nodes of PFC networks, and the interaction between PFC networks and basal ganglia/thalamic networks are often ignored in empirical studies of cognitive control. However, important models have been put forth postulating a critical role for the basal ganglia [44, 45] and thalamus [46] in cognitive control networks. A comprehensive view of the role of PFC networks in cognitive control will undoubtedly require an understanding of PFC-basal ganglia-thalamic interactions.
Fig. 3. Cortical and subcortical nodes of the salience and frontoparietal networks.
The SN (red) shows stronger connectivity to amygdala, ventral striatopallidum, hypothalamus, dorsomedial thalamus (dmThal), periaqueductal gray (PAG) and ventral tegmental area (VTA). In contrast, the FPN (blue) shows extensive parietal connectivity, involving the supramarginal gyrus and superior parietal lobule, and more limited subcortical connectivity, mainly involving the dorsal caudate and anterior thalamus. Adapted from [18].
Fifth, PFC networks exhibit a high degree of symmetry reflecting strong intrinsic functional connectivity between homologous regions across hemispheres [47]. This symmetry reflects both direct monosynaptic inter-hemispheric cross-callosal tracts anatomical links as well as multi-synaptic links via cortico-thalamic tracts. During cognition, however, this intrinsic symmetry can be shifted with greater right hemisphere response and connectivity associated with visuo-spatial attention [32] and inhibitory control processes [48].
Dynamic mechanisms of PFC network function
In this section, we review several models that have emerged regarding dynamic network mechanisms that implement cognitive control. Importantly, we highlight the interplay between PFC networks and global brain dynamics necessary for integration of perceptual, cognitive, and motor plans during the implementation of cognitive control.
Top-down and bottom-up spatial attention
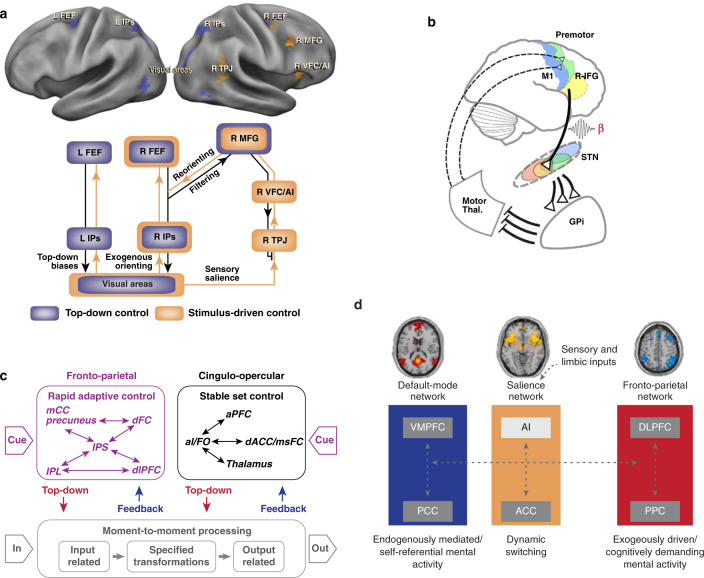
PFC networks are central to guiding attention in space and involves dynamic interactions between the segregated DAN and VAN, with each playing distinct and dissociable roles [49]. DAN nodes are also consistently coactivated by a wide range of tasks involving manipulation of overt or covert spatial attention [32]. The FEF and IPS contain retinotopically organized maps of contralateral space, which makes them candidate regions for the maintenance of spatial priority maps for covert spatial attention and saccade planning [34, 50]. Specifically, it is proposed that the DAN is primarily involved in applying top-down selection for stimuli and responses, whereas the VAN detects behaviorally relevant stimuli and might act as a “circuit breaker” for the DAN [49] (Fig. 4a). A further distinction is drawn between the PFC and parietal nodes of the VAN, with the temporoparietal junction consistently activated by stimulus-driven reorienting, whereas the fronto-insular cortex/inferior frontal gyrus is preferentially activated during reorientation to unexpected, surprising, stimuli which require a change in behavioral response via cognitive control [51, 52], analogous to the anterior insula node of the SN [53].
Fig. 4. Dynamic mechanisms of PFC network function.
a A circuit breaker for spatial attention. The VAN is hypothesized to act as a “circuit breaker” on the DAN, directing attention to salient events. Adapted from [32]. b Hypothetical model of different hyperdirect cortico-STN pathways for stopping and conflict processing. A, Stopping is initiated via right inferior frontal gyrus (R-IFG) in concert with the pre-SMA and implemented via projections to the subthalamic nucleus (STN) adapted from [157]. c Dual-network model of cognitive control which comprises parallel rapid-adaptive (FPN) and set-maintenance (CON) networks. Thin arrows schematize strong functional connections and boxed arrows schematize putative flow of information. The CON is hypothesized to maintain task-sets while the FPN rapidly adjusts adaptive control. Adapted from [62]. d Network switching model in which the SN is hypothesized to initiate dynamic switching between the FPN and DMN and regulate attention to endogenous and exogenous events. Sensory and limbic inputs are processed by the anterior insula (AI), which detects salient events and initiates appropriate control signals for (i) access to resources for working memory in FPN and (ii) action selection via the anterior cingulate cortex. Adapted from [8, 93].
A circuit breaker for response inhibition
The ability to suppress unwanted or inappropriate actions and impulses is essential for cognitive control and a crucial component of flexible and goal-directed behavior in general [54–56]. Reviewing evidence from behavioral studies of patients with unilateral PFC lesions, Aron and colleagues put forth the hypothesis that the right inferior frontal cortex is necessary for response inhibition, a cognitive process required to cancel an intended movement [55, 57, 58]. Ten years later, based on subsequent empirical work with the stop-signal task and fMRI, Aron and colleagues modified their view to conceptualize the right inferior frontal cortex’s function as a “brake” that “could be turned on in different modes (totally, to outright suppress a response; or partially to pause a response) and in different contexts (externally, by stop or salient signals; or internally, by goals).” [48] (Fig. 4b). The precise localization within the PFC of this function was proposed to be the inferior frontal gyrus, through interactions with the anterior insula, anterior cingulate cortex, pre-supplementary motor area, and subthalamic nucleus of the basal ganglia [11, 48, 54]. PFC regions involved in response inhibition show significant overlap with the SN, CON and VAN; their differential functional roles and circuit dynamics remain unresolved [11, 33, 59–61]. One challenge here is that the inferior frontal gyrus and anterior insula are typically coactivated during tasks involving response inhibition [59]. Quantitative analyses have, however, revealed that the anterior insula has stronger intrinsic and inhibition-task-related functional connectivity with the anterior cingulate cortex, whereas the inferior frontal cortex has stronger intrinsic and task-evoked functional connectivity with pre-supplemental motor area, dorsomedial PFC and FPN nodes [59]. An emerging view based on this pattern of functional circuity is that the right anterior insula is important for detecting behaviorally salient events, such as stop signals, whereas the right inferior frontal cortex is more involved in the motor implementation of inhibitory control via links with the pre-supplemental motor area.
Rapid-adjustments vs. Set-maintenance
In a cross-task analysis of 10 different tasks in 183 subjects, Dosenbach and colleagues [29, 30] identified a number of regions that were consistently active during cognitive control tasks. In a follow-up study, these brain regions obtained from the task data served as seeds in a correlation analysis of resting-state fMRI data [29] where graph theory and hierarchical clustering was applied to the correlation matrices. These analyses identified two distinct networks that were labeled as the FPN and the CON. Based on their role in cognitive tasks, they proposed a dual-network model of top-down control in which the FPN comprises brain regions that provide signals that act on a rapid time scale to initiate and adjust control whereas the CON comprises brain regions that provide signals that allow for set-maintenance over a longer time scale [62] (Fig. 4c). Other empirical studies utilizing both EEG and fMRI has led to the hypothesis that the FPN and CON modulate alertness, rather than specific task-set control processes. It is proposed that the FPN is involved in phasic control of alertness and the CON network with maintenance of tonic alertness [63, 64].
Although the SN and CON show considerable anatomical overlap in the anterior insula and anterior cingulate cortex, their precise role in cognitive control differs across functional models of cognitive control. In contrast to other formulations of CON function as described above that proposed a role in “tonic” processes, Menon and colleagues have argued that the anterior insula node of the SN is a fast-acting flexible hub [10, 65, 66] which facilitates the detection of the most biologically and cognitively relevant events for adaptively guiding attention and behavior [53]. Sadaghiani and colleagues have argued that while salience detection describes the monitoring and evaluation of homeostatic importance, tonic alertness maintenance is the sustained process of ensuring engagement (that may be a result of and action upon detection of homeostatically salient stimuli) [64]. An important avenue for future research will be to determine if the different proposed functions of the CON and SN—task-set maintenance, tonic alertness, and saliency detection—are explained by one network subserving different functions, or anatomically distinct subnetworks with different functions.
Network segregation and integration
Dynamic cooperation and competition between PFC networks likely plays a crucial role in cognitive control [67], which can be implemented via network segregation and integration. For example, when humans are engaged in a demanding working memory task, global measures of network integration increase, while global measures of network segregation decrease [9, 68] (Fig. 5a). Specifically, it was demonstrated that two cognitive control networks (FPN and CON) became more integrated with each other during conditions of increased cognitive control, and further, increased integration of these two cognitive control networks with a task-related, non-cognitive control network, was related to increased accuracy on the task [69]. Other studies have reported similar results [70, 71]. Moreover, time-varying functional connectivity analyses of fMRI data have demonstrated that network integration and segregation fluctuate even within trials, in a behaviorally meaningful manner. For example, it was found that a more integrated state leads to faster and more accurate performance on demanding cognitive tasks [72, 73], and the degree of network segregation/integration during the trial period before the target is presented during an auditory detection task predicts whether subjects heard or missed that target [74]. PFC networks are also highly dynamic even when not performing cognitive tasks. For example, hidden Markov models of state dynamics have revealed that temporal coupling between SN, FPN and DMN nodes fluctuates considerably over time and these networks exist in a state of complete segregation only intermittently with relatively short mean lifetimes [75].
Fig. 5. Models of network segregation and integration.
a Global network integration increases, while network segregation decreases during a working memory task. Adapted from [9]. b Brain-wide functional connectivity patterns of FPN nodes shift more than those of other PFC networks across different cognitive task conditions. Adapted from [158].
Cross-network integration is necessary for cognitive control, and may be implemented via symmetry breaking, which refers to qualitative changes in brain states, induced by relatively small perturbations within a homogeneous system of inter-connected nodes [76]. Symmetry breaking is often accompanied by formation of segregated modules with different phase relationships [77, 78], a process that underlies the emergence of dynamic cell assemblies with functional ordering of interacting components in space and time [79].
Network switching
A shift between networks involved in cognitive control may be a distinct mechanism from network integration and segregation. It is proposed that switching between networks is implemented by flexible hubs in the SN [10] (Fig. 4d). For example, the SN is hypothesized to play a prominent role in network switching between context dependent engagement and disengagement of the FPN and DMN [53]. Crucially, switching between DMN and FPN would allow for disengaging from internal mental processes in order to respond to current goals. The anterior insula node of the SN is proposed to play a key role in identifying relevant stimuli from the vast and continuous stream of sensory stimuli that impact the senses. The anterior insula may facilitate task-related information processing by initiating appropriate transient control signals to engage the FPN while disengaging the DMN. The SN is thus proposed to be a fast-acting system rather than a system for maintenance of information over a longer time scale as posited by the CON-based dual-network model of top-down control [11, 61, 80]. Consistent with this switching role, the SN demonstrates some of the highest levels of flexibility in time-varying connectivity compared with other PFC brain networks, and SN flexibility is predictive of individual differences in cognitive flexibility [65, 66].
Flexible hubs
The adaptability of PFC networks necessary for cognitive control is proposed to be made possible by flexible hubs: brain regions that rapidly update their pattern of global functional connectivity according to task demands [81] (Fig. 5b). Supporting this notion, brain-wide functional connectivity patterns of FPN nodes shift more than nodes in other PFC networks across a wide variety of cognitive task states [31]. In contrast, CON nodes are proposed to play a complementary role to FPN nodes during cognitive control [82]. CON nodes exhibit a reduction of within-network connections from rest to task resulting in a switch in connectivity to other networks during tasks, whereas FPN exhibits extensive between-network reconfiguration along with maintenance of within-network connections. This proposed flexible “shifter” mechanism for the CON, which complements a flexible “coordinator mechanism” for the FPN may allow the CON to lend processing resources to other goal-relevant networks. This idea is similar to the proposal that the SN is involved in network switching and transient engaging of other task relevant networks, most notably the FPN [53].
Graph theoretical analyses applied to rs-fMRI data have led to the proposal that there are groups of highly interconnected nodes in brain networks, referred to as the “rich club” [83] or “diverse club” [84], which are critical for global communication. Moreover, in these highly connected brain regions, activity increases as the number of cognitive functions engaged in a task increases [40]. This finding suggests that these “connector hubs” are potentially integrating information across networks and coordinating connectivity between networks. Also, individuals with diversely connected hubs and consequent modular brain networks exhibit increased cognitive performance, regardless of the task [85].
Self-referential monitoring
A distinguishing feature of the DMN is that, unlike the FPN, SN, CON, DAN, and VAN, nodes of this network are typically suppressed or deactivated during a wide range of cognitively demanding tasks [17, 86, 87]. Importantly, this response profile is not an artifact of global signal changes found in fMRI data, as suppression of local field potentials in DMN nodes has been found during performance of cognitive tasks [88]. The DMN is proposed to play a crucial role in self-referential information processing and monitoring of the internal mental landscape [17, 89], processes that need to be suppressed during external-stimulus dependent cognition. The medial PFC node is particularly sensitive to value judgment and social evaluative processes related to the self and others [90–92]. Although the DMN may not be as directly involved in cognitive control in the same manner as the other PFC networks, it likely exerts a profound indirect influence on cognitive function. For example, inability to disengage the DMN during external stimulus driven cognition significantly impairs task performance [87, 93].
PFC networks, global brain dynamics and cognition: open questions and challenges
Our discussion of PFC networks in the previous sections has highlighted the tight interplay between PFC networks and global brain dynamics, raising broader questions regarding the role of PFC networks in cognition. Much as individual PFC areas do not function in isolation, PFC networks also do not function in isolation. The role of individual brain areas involved in cognition is constrained by the specific network it is embedded in. PFC networks involve segregated brain areas that are engaged during cognition and also show a consistent pattern of intrinsic connectivity in the absence of overt tasks. Dedicated PFC networks create a functional architecture for efficient processing without the persistent need for reconfiguring circuits, while at the same time flexible enough for reconfiguration when more flexible behaviors are needed based on perceptual, mnemonic, linguistic, and motoric demands.
Most empirical research on the role of PFC networks in cognitive control has attempted to assign the function of specific networks to specific cognitive control processes. Clearly, the goal of this research has been to identify the “specialized processors” in the brain that are involved in, and perhaps, necessary, for cognitive control. On the one hand, one may consider this approach to be “phrenological” at the network level and not different from most previous studies of cognitive control that have attributed specific cognitive control processes to specific brain PFC regions. And this is perhaps not surprising since the empirical studies of brain networks using brain imaging evolved from the empirical studies that have mapped a cognitive process to a specific brain region. A problem with this empirical approach for determining the neural mechanisms that implement cognitive control is that it is faced with the same questions that have challenged researchers studying cognitive control through behavioral approaches, such as: What is the ontology of cognitive control? What are the essential and most meaningful components of cognitive control? Can we describe all aspects of cognitive control by limited set of cognitive processes? These are clearly important topics for further investigation.
There are two further issues regarding simply assigning a potential cognitive function to a specific brain network for developing a mechanistic understanding of cognitive control. First, if a brain region is assigned the same function as the network as a whole (e.g., response inhibition, or set-maintenance), what is the role of the other brain regions in the network? Second, how do specialized brain networks interact to carry out cognitive control? Our review of empirical work in the previous sections has provided evidence that specialized, modular network processes do exist in the brain, and that cognitive control is implemented through both segregation and integration of these networks. Beyond this, we have also emphasized that although the nodes in any particular PFC network can function generally as a unit, it is not the case that each node functions identically. Our understanding of these differential processes is still in its infancy—identification of the response profile of a particular brain node within a cognitive control network will require exquisite manipulation of task exigencies, such as manipulation of sensory load, which differentially modulates parietal cortex, or increasing complexity of task rules, action selection and motor plans acting on the same input, which differentially modulates individual PFC regions [94–97]. Also, as we have discussed, causal manipulation of each node’s role in a particular network may lead to further understanding of the role that network plays in cognitive control [27]. With such manipulations, it is possible that different response profiles will be observed in different nodes within the same network, since no two brain areas are identical in their connectivity patterns—each have distinct “fingerprints” with inputs and outputs that are also not identical, allowing each node to function dissimilarly depending on task demands and contexts. For example, within the network thought to subserve directed spatial attention, it is proposed a parietal node provides an internal sensory map, a cingulate node regulates the spatial distribution of motivational valence, a frontal node coordinates the motor programs for exploration, scanning, reaching, and fixating; and a reticular activating system node provides the underlying level of arousal and vigilance [98].
In addition to the important role that each brain region within a network may play for a particular type of cognitive control process, it is possible that the same brain region may be a member of different networks, with different roles depending on the network engaged. Common methodological approaches for identifying networks using fMRI data do not allow for nodes to be members of more than one network. However, approaches do exist, such as the mixed-membership algorithm [99], which allows for a particular node to participate in multiple networks. The notion that a single brain region can subserve multiple functions is consistent with numerous human brain mapping studies that have found that one brain region is engaged in seemingly disparate tasks. For example, Aron and colleagues noted that the “right inferior frontal cortex is not only involved in response inhibition, but is also involved in category learning, visuomotor conditional learning, memory retrieval, memory encoding” [57]. An understanding of how the same brain region is a member of different networks yet serves different functions is far from understood and requires careful future consideration.
Importantly, cognitive control is not implemented by one individual PFC network, but rather by dynamic interactions among PFC networks embedded in a global brain architecture. The importance of network interactions required for complex cognition more broadly has been discussed elsewhere [73, 100]. Although the empirical studies we have reviewed illustrate what we have learned about which individual brain networks are involved in cognitive control, considerable work is needed to elucidate the way in which these networks interact during cognitive control. Assigning a particular function to a particular brain region, or a particular brain network, does not reveal a mechanism. A complete mechanistic understanding of cognitive control remains to be uncovered [101].
The need for broader considerations of PFC networks and global circuit dynamics is further underscored by frameworks such as “cell assemblies”, “global workspace” and “multi-demand-systems”. The notion of dynamic cell assemblies first articulated by Hebb [102] has now been articulated in the context of modern neuroscientific discoveries. Baars proposed the concept of a ‘global workspace’, where information is integrated in a small group of “specialized processors” or “conductors” before being broadcast to the whole brain resulting in a hierarchical flow of information [103]. Dehaene and colleagues extended this model and proposed that effortful cognitive tasks require two main computational spaces: “a unique global workspace composed of distributed and heavily interconnected neurons with long-range axons, and a set of specialized and modular perceptual, motor, memory, evaluative, and attentional processors” [104]. These computational spaces were proposed to be implemented within a large-scale distributed brain network that spanned from the macro-level (e.g., brain regions) to the micro-level (e.g., cortical layers). Moreover, it was conceived that the connectivity profile of the “global workspace” allowed it to communicate, and presumably influence, multiple distributed and specialized processors. Although there is potentially an infinite number of “workspace representations”, it is proposed that only one can be active at any given time.
More recently, based on a review of monkey electrophysiology and human fMRI studies, Duncan highlighted a group of brain regions that includes the anterior insula, adjacent frontal operculum, dorsomedial and dorsolateral frontal cortex, dorsal anterior cingulate nodes, premotor cortex, intraparietal sulcus, basal ganglia, thalamus, and cerebellum, that are engaged by diverse cognitive demands [105]. These brain regions were collectively labeled as the “multi-demand” (MD) system and proposed to play a key role in achieving goals by assembling sub-tasks and creating structured mental programs. Moreover, the MD system is hypothesized to be involved in “defining and controlling the parts of such programs, with focus on the specific content of a current cognitive operation”, rapid reorganization as mental focus is changed, and robust separation of successive task steps. In a later formulation of this hypothesis [106], it was proposed that a core role of the MD system was the assembly of an “attentional episode”, which “drives linked processing in multiple other brain regions, configuring widespread brain activity for solution of the selected problem.” An important unresolved question is the relationship of the MD system functions with the PFC networks described in this review. Recently, this question was addressed by analyzing rs-fMRI data from the Human Connectome Project [107]. It was found that core MD regions were concentrated in the FPN. However, in another study that analyzed fMRI data collected during the performance of a cognitive control task, it was concluded that the MD system may be divided into two subnetworks - the FPN and SN/CON [108]. Thus, further work is still required to determine how PFC networks involved in cognitive control orchestrate global workspaces, such as the MD system, to engender complex cognitive functions. Computational modeling of multi-task data holds particular promise in this regard [109].
Future research directions
In this section we discuss future research needed to further clarify the role of PFC networks and their dynamic interactions in cognitive control and executive function. A summary of key open questions arising from the present review is presented in Box 1.
Box 1 Open questions raised by this review.
What are the essential and most meaningful components of cognitive control? Can we describe key aspects of cognitive control by a limited set of processes involving PFC networks?
Can a universal taxonomy for functional brain networks be defined?
Are the SN and CON distinct functional networks supporting different aspects of cognitive control?
A comprehensive view of the role of PFC networks in cognitive control will require an understanding of PFC-basal ganglia-thalamic interactions. How do subcortical interactions influence PFC network function?
How do brain networks evolve over time during cognitive control? How can we use intracranial EEG to advance understanding of the temporal dynamics of network function? How do they relate to fMRI models of network function?
What is the role of neuronal oscillations in PFC networks involved in cognitive control?
What are the computational mechanisms underlying PFC network function in cognitive control?
How do PFC networks involved in cognitive control orchestrate global workspaces to engender complex cognitive functions? How can computational modeling of multi-task data inform information flow?
What is the role of brainstem neuromodulatory systems, such as dopamine, norepinephrine and serotonin, on PFC networks involved in cognitive control?
How can knowledge of PFC network function be used to ameliorate behavioral and cognitive deficits in psychiatric and neurological disorders?
How can we better integrate translational neuroscience studies involving optogenetic manipulation in rodents to probe causal mechanisms of network function?
How can studies of PFC network function using fMRI lead to a better understanding of the pathophysiology of brain disorders with cognitive control deficits? Can fMRI biomarkers of PFC networks become clinically useful for diagnosis, clinical characterization and guidance of therapeutic interventions of brain disorders with cognitive control deficits?
Temporal dynamics on the cognitive timescale
Flexible and adaptive human cognition depends on dynamic PFC networks that transiently link distributed brain regions in response to moment-by-moment changes in task demands. To address this challenge, future studies will need to overcome limitations of the poor temporal resolution of fMRI and map dynamics over the scales of tens of milliseconds. While considerable progress is being made in decoding fast temporal dynamics in individual brain areas [110] their extensions to multiple distributed nodes remains a challenge. Recent studies using distributed intracranial EEG recordings have begun to address gaps in our understanding of interactions between PFC network nodes [80]. For example, in human intracranial electrocorticography recordings, it has been demonstrated that during performance of a cognitive control task, the need to apply progressively more abstract rules resulted in greater frontal network theta band phase encoding, which predicted trial-by-trial response times. Moreover, theta phase encoding was coupled with high gamma band activity, suggesting a potential mechanism by which frontal networks are dynamically involved in cognitive control [111]. Even though considerable technical and methodological challenges remain for human intracranial recording [112], progress in this area will be essential for probing how cognitive control processes involving PFC networks unfold over time in humans.
Latent brain dynamics, state transitions and optimal brain states
At both fast and slow time scales, dynamic brain states and functional connectivity have a significant influence on cognition. New computational tools for modeling brain state dynamics are beginning to address this challenge. For example, latent brain space models and Bayesian switching dynamical systems algorithms which can identify hidden brain states and their transition probabilities appear particularly promising for probing flexible reconfiguration of functional brain circuits and their influence on task performance [113]. A critical aspect of such approaches is that they help identify brain states that are optimal for cognitive task performance– failure to engage such states and weak transitions to them from other brain states can impair performance and decision-making dynamics [114]. For example, analyses of fMRI data have revealed during cognitively demanding tasks, there is a flow of activity embedded within a low-dimensional state space, that moves from engagement of an integrative core of brain regions that maximizes information-processing and performance, to a more segregated state when the task at hand concludes [73]. In another fMRI study of functional connectivity during multiple different cognitive tasks and rest, it was found that high-performing individuals exhibit smaller changes in functional network architecture between rest and task, suggesting that these individuals have more efficient network updates. These findings suggest that high performers on a given task may have a network configuration at rest that is already closer in state space to that task’s network configuration [115].
Future empirical studies implementing models based on unsupervised learning procedures for identifying latent brain states, their temporal evolution, lifetimes and occurrence of states, and their switching probabilities will allow us to uncover context-dependent latent brain dynamics associated with PFC networks. Progress in this area will be essential for addressing the challenge of the role of PFC networks in cognitive control and reconfiguration of cell assemblies to support adaptive cognitive functions.
Causal manipulation and control of PFC networks
Optogenetic techniques has revolutionized our understanding of the causal role of functional circuits in the rodent brain [116]. Although the cell-specific manipulation offered by optogenetic techniques is not a feasible approach in the human brain and has met with limited success in non-human primates, a number of tools to manipulate neural responses in individual brain areas are now available to investigate causal effects of PFC networks. These include transcranial magnetic stimulation (TMS) and direct current stimulation techniques as well as more invasive intracranial stimulation with simultaneous EEG recordings [117]. For example, two different frontal nodes in the CON and FPN network were disrupted with theta-burst TMS, followed by collection of fMRI data. Changes in the functional connectivity between and within these two PFC networks was observed after TMS [118]. Thus, brain stimulation in healthy individuals along with studies of patients with focal lesions [41], provide a promising convergent approach for studying the role of PFC networks in cognitive control.
In contrast to computational methods that assess functional connectivity, methods that assess the influence that one neuronal system exerts over another (sometimes referred to as effective connectivity) hold promise for providing convergent, albeit indirect, evidence for a causal role of PFC networks in cognition. Such methods include structural equation modeling [119], coherence [120], Granger causal analysis [121, 122], dynamic causal modeling [123], and multidimensional state space models [124]. The relative strengths and weaknesses of these approaches for estimating causal interactions has been the subject of some debate owing to the slow time course of fMRI signals [125–127]. Identification of causal interactions between brain regions using computational techniques has nevertheless remained a challenging problem for several reasons. First, fMRI measures a hemodynamic response to neural activity rather than neuronal activity directly. Second, regional variations in the hemodynamic response function can significantly influence estimation of causal interactions between brain regions. Third, causal interactions between brain regions can change with experimental context. State-space computational algorithms validated using neurophysiologically realistic simulations, and novel optogenetic fMRI manipulations hold promise for identifying context-dependent dynamic causal interactions [124, 128]. Notably, application of these techniques to high-temporal resolution fMRI data may allow identification of causal outflow and inflow hubs associated with cognition [129]. Crucially, analysis of causal control circuits has the potential to inform how asymmetries in directed influence allow individual networks or specific brain nodes to control others, and further advance our understanding of how dynamic causal interactions involving PFC network nodes play a fundamental role in cognition.
Network controllability
Control theory techniques borrowed from engineering may also offer promising new approaches for probing how PFC networks can be manipulated to facilitate desirable or optimal brain states [130–133]. This latter question may be relevant for the treatment of psychiatric disorders such as depression where stimulation of the dorsolateral PFC node of the FPN is used to alter distal brain targets that influence mood [134]. Controllability, in the classical sense, measures the ability to perturb a system from a given initial state to specific target states, in finite time, by means of external control inputs [133, 135, 136]. Nodes with higher controllability require lower energy for perturbing a system from its current state [137, 138] and controllability measures are useful for identifying driver nodes which have the potential to influence overall system dynamics [136]. Control properties of complex systems can provide novel insights into how they can be perturbed to achieve desired behaviors [130–132]. A recent advance is the application of control theory to human neuroimaging based on structural brain connectivity derived using diffusion tensor imaging [139], with the suggestion that inputs to DMN nodes may facilitate transition to many other brain states. However, it has been also been suggested that structural topology by itself may be ill-suited for assessing brain- cognitive-state dependent control in complex functional brain networks [140] and researchers have drawn attention to the importance of state dynamics for the study of controllability in complex systems [138]. Incorporating such a perspective a recent study found that SN nodes have the highest functional network controllability and, more generally, functional network controllability is dependent on cognitive load [129]. Characterization of how intrinsic functional connectivity and structural connectivity constrain the temporal progression of brain states remains a challenge, resolution of which will likely yield insights into how PFC networks can be manipulated to desired outcomes.
Clinical implications for psychiatric and neurological disorders
Deficits in cognitive control have been observed in many psychiatric and neurological disorders, such as autism, anxiety disorders, mood disorders, schizophrenia, traumatic brain injury, stroke, and frontotemporal dementia [141, 142]. The brain’s cognitive control systems have been probed in these clinical disorders with multiple brain imaging techniques, and as expected, dysfunction in PFC networks is observed [61, 93, 143–148]. These empirical studies of describing functional connectivity changes in brain disorders complement decades of clinical studies of these disorders that have associated the location of pathology, or changes in brain activity in particular regions, with cognitive control deficits observed in patients with brain disorders. As we strive to use functional connectivity metrics to gain further understanding of the neural mechanisms of cognitive control, we must also strive to use these metrics in a way that provides meaningful clinical utility. In our view, there are several potential ways in which a network analysis of functional brain imaging data in patients with brain disorders could have a significant clinical impact.
Identification of network dysfunction using fMRI data has the potential to aid a clinician in reaching a diagnosis, categorizing clinical subtypes of a particular disorder or guiding therapeutic interventions. However, despite the introduction of fMRI almost 30 years ago, there is still no clinically approved use of fMRI. The significant challenge that must be overcome is that a functional brain imaging biomarker must have sufficient sensitivity and specificity within an individual patient, rather than a group of patients as compared to a group of healthy controls. Network approaches may have an advantage over previously failed univariate approaches that have measured more or less activity within individual brain regions without considering their interactions with other brain regions. Some progress has been made in the development of functional connectivity metrics that predict the response to various therapeutic interventions aimed at improving cognitive control deficits. For example, individual differences in baseline brain network architecture as measured with rs-fMRI predicts gains in cognitive control functions across several clinical populations and cognitive interventions [149–151].
Even if the goal of developing fMRI as a clinically useful tool is never achieved, functional connectivity metrics of fMRI data will still likely provide valuable insight into the pathophysiological mechanisms that underlie brain disorders. In fact, one may argue that fMRI is more optimally suited to achieve this goal. The explosion of network neuroscience over the past decade has undoubtedly led to a re-consideration of the pathophysiological mechanisms underlying neurological and psychiatric disorders [152]. For example, a triple network model of psychopathology posits that aberrant functional organization of the SN, FPN and DMN and their dynamic interactions contribute to a wide range of psychiatric symptoms [93, 141, 148, 151, 153]. Specifically, this model posits that aberrant salience assignment and mapping of external and internal events by the SN leads to altered dynamic temporal interactions with the FPN and DMN, resulting in cognitive control deficits. A key question to be answered for any network model of disease is why specific networks are targeted in specific diseases. One notion put forth is that highly connected brain regions, independent of their network membership, are the target of neuropathology because they are biologically costly due to their high metabolic demands. Empirical support for this hypothesis was found in a study of over 20,000 subjects with 26 different brain disorders. MRI lesions that were common across all brain disorders were more likely to be located in highly connected brain regions [154]. This network view of brain disorders is consistent with empirical findings that lesions to highly connected brain regions cause global changes in brain connectivity [155] and extensive cognitive deficits [156].
Acknowledgments
Funding information
Supported by NIH grants MH121069 and NS086085 to VM, and MH63901 to MDE.
Author contributions
VM and MDE wrote and edited the paper together.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vinod Menon, Email: menon@stanford.edu.
Mark D’Esposito, Email: despo@berkeley.edu.
References
- 1.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 2.T Egner, The Wiley Handbook of Cognitive Control (Wiley, New York, 2017).
- 3.Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–56. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 4.Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–52. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 5.Fuster JM. The cognit: a network model of cortical representation. Int J Psychophysiol. 2006;60:125–32. doi: 10.1016/j.ijpsycho.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 6.J Fuster, The prefrontal cortex (Elsevier Science, Amsterdam, 2015).
- 7.Passingham RE, Stephan KE, Kotter R. The anatomical basis of functional localization in the cortex. Nat Rev Neurosci. 2002;3:606–16. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- 8.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–90. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JR, D’Esposito M. The segregation and integration of distinct brain networks and their relationship to cognition. J Neurosci. 2016;36:12083–94. doi: 10.1523/JNEUROSCI.2965-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai W, Chen T, Ryali S, Kochalka J, Li CS, Menon V. Causal Interactions Within a Frontal-Cingulate-Parietal Network During Cognitive Control: Convergent Evidence from a Multisite-Multitask Investigation. Cereb cortex. 2016;26:2140–53. doi: 10.1093/cercor/bhv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MM Mesulam, Principles of behavioral and cognitive neurology (Oxford University Press, 2000).
- 13.Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex. 2012;48:46–57. doi: 10.1016/j.cortex.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Thiebaut de Schotten M, Ffytche DH, Bizzi A, Dell'Acqua F, Allin M, Walshe M, et al. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage. 2011;54:49–59. doi: 10.1016/j.neuroimage.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 15.A Fornito, A Zalesky, E Bullmore, Fundamentals of brain network analysis (Elsevier Science, Amsterdam, 2016).
- 16.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–52. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 17.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb cortex. 2012;22:158–65. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.BT Yeo, FM Krienen, J Sepulcre, MR Sabuncu, D Lashkari, M Hollinshead, et al., The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. [DOI] [PMC free article] [PubMed]
- 22.Cohen AL, Fair DA, Dosenbach NU, Miezin FM, Dierker D, Van Essen DC, et al. Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage. 2008;41:45–57. doi: 10.1016/j.neuroimage.2008.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA. 2004;101:13335–40. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.P Golland, Y Golland, R Malach (2007) Detection of spatial activation patterns as unsupervised segmentation of fMRI data. in International Conference on Medical Image Computing and Computer-Assisted Intervention (Springer), pp 110–8. [DOI] [PMC free article] [PubMed]
- 25.Bassett DS, Sporns O. Network neuroscience. Nat Neurosci. 2017;20:353–64. doi: 10.1038/nn.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 27.Gratton C, Sun H, Petersen SE. Control networks and hubs. Psychophysiology. 2018;55:e13032. doi: 10.1111/psyp.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uddin LQ, Yeo BT, Spreng RN. Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr. 2019;32:926–42. doi: 10.1007/s10548-019-00744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–8. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011;34:569–99. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu T, Wang X, Wu Q, Spagna A, Yang J, Yuan C, et al. Anterior insular cortex is a bottleneck of cognitive control. NeuroImage. 2019;195:490–504. doi: 10.1016/j.neuroimage.2019.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–9. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sestieri, C., Shulman, G. L., & Corbetta, M. Orienting to the environment: Separate contributions of dorsal and ventral frontoparietal attention networks. In G. R. Mangun Editor. The neuroscience of attention: Attentional control and selection (pp. 100–130). 10.1093/acprof:oso/9780195334364.003.0005 (Oxford University Press, 2012).
- 36.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 37.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, et al. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012;60:830–46. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Esposito M, Postle BR. The cognitive neuroscience of working memory. Annu Rev Psychol. 2015;66:115–42. doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertolero MA, Yeo BTT, D’Esposito M. The modular and integrative functional architecture of the human brain. Proc Natl Acad Sci. 2015;112:E6798–E6807. doi: 10.1073/pnas.1510619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nomura EM, Gratton C, D’Esposito M. Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad Sci USA. 2010;107:12017 12022. doi: 10.1073/pnas.1002431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallis G, Stokes M, Cousijn H, Woolrich M, Nobre AC. Frontoparietal and Cingulo-opercular Networks Play Dissociable Roles in Control of Working Memory. J Cogn Neurosci. 2015;27:2019–34. doi: 10.1162/jocn_a_00838. [DOI] [PubMed] [Google Scholar]
- 43.M Catani, M Thiebaut de Schotten, Atlas of human brain connections (Oxford University Press, Oxford; New York, 2012), pp. xii, 519 p.
- 44.Chatham CH, Badre D. Multiple gates on working memory. Curr Opin Behav Sci. 2015;1:23 31. doi: 10.1016/j.cobeha.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hazy TE, Frank MJ, O’Reilly RC. Banishing the homunculus: making working memory work. Neuroscience. 2006;139:105–18. doi: 10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- 46.Hwang K, Bertolero MA, Liu WB, D’Esposito M. The human thalamus is an integrative hub for functional brain networks. J Neurosci. 2017;37:5594 5607. doi: 10.1523/JNEUROSCI.0067-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryali S, Chen T, Supekar K, Menon V. Estimation of functional connectivity in fMRI data using stability selection-based sparse partial correlation with elastic net penalty. Neuroimage. 2012;59:3852–61. doi: 10.1016/j.neuroimage.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18:177–85. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 50.K Nobre, S Kastner, The Oxford handbook of attention, Oxford library of psychology (Oxford University Press, Oxford; New York, 2014), pp. xvi, 1242 p.
- 51.Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, McAvoy MP, et al. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29:4392–407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Todd JJ, Fougnie D, Marois R. Visual short-term memory load suppresses temporo-parietal junction activity and induces inattentional blindness. Psychological Sci. 2005;16:965–72. doi: 10.1111/j.1467-9280.2005.01645.x. [DOI] [PubMed] [Google Scholar]
- 53.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69:e55–68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–68. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 57.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–6. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 59.Cai W, Ryali S, Chen T, Li CS, Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J Neurosci. 2014;34:14652–67. doi: 10.1523/JNEUROSCI.3048-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ide JS, Shenoy P, Angela JY, Chiang-Shan RL. Bayesian prediction and evaluation in the anterior cingulate cortex. J Neurosci. 2013;33:2039–47. doi: 10.1523/JNEUROSCI.2201-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci. 2010;107:6106–11. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud AL, D'Esposito M, et al. Alpha-band phase synchrony is related to activity in the fronto-parietal adaptive control network. J Neurosci. 2012;32:14305–10. doi: 10.1523/JNEUROSCI.1358-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sadaghiani S, D’Esposito M. Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cereb cortex. 2015;25:2763–73. doi: 10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen T, Cai W, Ryali S, Supekar K, Menon V. Distinct global brain dynamics and spatiotemporal organization of the salience network. PLoS Biol. 2016;14:e1002469. doi: 10.1371/journal.pbio.1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krönke K-M, Wolff M, Shi Y, Kräplin A, Smolka MN, Bühringer G, et al. Functional connectivity in a triple-network saliency model is associated with real-life self-control. Neuropsychologia. 2020;149:107667. doi: 10.1016/j.neuropsychologia.2020.107667. [DOI] [PubMed] [Google Scholar]
- 67.Cocchi L, Zalesky A, Fornito A, Mattingley JB. Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn Sci. 2013;17:493–501. doi: 10.1016/j.tics.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Zippo AG, Rosa PAD, Castiglioni I, Biella GEM. Alternating dynamics of segregation and integration in human EEG functional networks during working-memory task. Neuroscience. 2018;371:191–206. doi: 10.1016/j.neuroscience.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Cohen JR, Gallen CL, Jacobs EG, Lee TG, D’Esposito M. Quantifying the reconfiguration of intrinsic networks during working memory. PloS One. 2014;9:e106636. doi: 10.1371/journal.pone.0106636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kitzbichler MG, Henson RN, Smith ML, Nathan PJ, Bullmore ET. Cognitive effort drives workspace configuration of human brain functional networks. J Neurosci. 2011;31:8259–70. doi: 10.1523/JNEUROSCI.0440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang X, Zou Q, He Y, Yang Y. Topologically reorganized connectivity architecture of default-mode, executive-control, and salience networks across working memory task loads. Cereb cortex. 2016;26:1501–11. doi: 10.1093/cercor/bhu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shine JM, Bissett PG, Bell PT, Koyejo O, Balsters JH, Gorgolewski KJ, et al. The dynamics of functional brain networks: integrated network states during cognitive task performance. Neuron. 2016;92:544–54. doi: 10.1016/j.neuron.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shine JM, Poldrack RA. Principles of dynamic network reconfiguration across diverse brain states. NeuroImage. 2018;180:396–405. doi: 10.1016/j.neuroimage.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 74.Sadaghiani S, Poline J-B, Kleinschmidt A, D’Esposito M. Ongoing dynamics in large-scale functional connectivity predict perception. Proc Natl Acad Sci. 2015;112:8463–8. doi: 10.1073/pnas.1420687112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryali S, Supekar K, Chen T, Kochalka J, Cai W, Nicholas J, et al. Temporal dynamics and developmental maturation of salience, default and central-executive network interactions revealed by variational bayes hidden markov modeling. PLoS Comput Biol. 2016;12:e1005138. doi: 10.1371/journal.pcbi.1005138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pillai AS, Jirsa VK. Symmetry breaking in space-time hierarchies shapes brain dynamics and behavior. Neuron. 2017;94:1010–26. doi: 10.1016/j.neuron.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 77.Fasoli D, Cattani A, Panzeri S. The complexity of dynamics in small neural circuits. PLoS Comput Biol. 2016;12:e1004992. doi: 10.1371/journal.pcbi.1004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh R, Menon SN, Sinha S. Complex patterns arise through spontaneous symmetry breaking in dense homogeneous networks of neural oscillators. Sci Rep. 2016;6:22074. doi: 10.1038/srep22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.JS Kelso, Dynamic patterns: the self-organization of brain and behavior (MIT press, Cambridge, 1997).
- 80.Das A, Menon V. Spatiotemporal integrity and spontaneous nonlinear dynamic properties of the salience network revealed by human intracranial electrophysiology: a multicohort replication. Cereb cortex. 2020;30:5309–21. doi: 10.1093/cercor/bhaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–51. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cocuzza CV, Ito T, Schultz D, Bassett DS, Cole MW. Flexible coordinator and switcher hubs for adaptive task control. J Neurosci. 2020;40:6949–68. doi: 10.1523/JNEUROSCI.2559-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heuvel MPvd, Sporns O. Rich-club organization of the human connectome. J Neurosci: Off J Soc Neurosci. 2011;31:15775 15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bertolero MA, Yeo BTT, D’Esposito M. The diverse club. Nat Commun. 2017;8:1277. doi: 10.1038/s41467-017-01189-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bertolero MA, Yeo BTT, Bassett DS, D’Esposito M. A mechanistic model of connector hubs, modularity and cognition. Nat Hum Behav. 2018;2:765–77. doi: 10.1038/s41562-018-0420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–92. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- 88.Dastjerdi M, Foster BL, Nasrullah S, Rauschecker AM, Dougherty RF, Townsend JD, et al. Differential electrophysiological response during rest, self-referential, and non-self-referential tasks in human posteromedial cortex. Proc Natl Acad Sci USA. 2011;108:3023–8. doi: 10.1073/pnas.1017098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–33. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 90.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 91.Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 92.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 94.Scott BB, Constantinople CM, Akrami A, Hanks TD, Brody CD, Tank DW. Fronto-parietal cortical circuits encode accumulated evidence with a diversity of timescales. Neuron. 2017;95:385–98 e385. doi: 10.1016/j.neuron.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akrami A, Kopec CD, Diamond ME, Brody CD. Posterior parietal cortex represents sensory history and mediates its effects on behaviour. Nature. 2018;554:368–72. doi: 10.1038/nature25510. [DOI] [PubMed] [Google Scholar]
- 96.Hanks TD, Kopec CD, Brunton BW, Duan CA, Erlich JC, Brody CD. Distinct relationships of parietal and prefrontal cortices to evidence accumulation. Nature. 2015;520:220–3. doi: 10.1038/nature14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19:2082–99. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- 98.Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol: Off J Am Neurological Assoc Child Neurol Soc. 1981;10:309–25. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- 99.Najafi M, McMenamin BW, Simon JZ, Pessoa L. Overlapping communities reveal rich structure in large-scale brain networks during rest and task conditions. NeuroImage. 2016;135:92–106. doi: 10.1016/j.neuroimage.2016.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sporns O. Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol. 2013;23:162–71. doi: 10.1016/j.conb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 101.RD Mill, T Ito, MW Cole, From connectome to cognition: the search for mechanism in human functional brain networks. NeuroImage. 2017;160:124–139. [DOI] [PMC free article] [PubMed]
- 102.DO Hebb, The organization of behavior: a neuropsychological theory (Psychology Press, Hove, 2005).
- 103.BJ Baars, A cognitive theory of consciousness (Cambridge University Press, 1993).
- 104.Dehaene S, Kerszberg M, Changeux J-P. A neuronal model of a global workspace in effortful cognitive tasks. Proc Natl Acad Sci. 1998;95:14529–34. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172–9. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Duncan J. The structure of cognition: attentional episodes in mind and brain. Neuron. 2013;80:35–50. doi: 10.1016/j.neuron.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Assem M, Glasser MF, Van Essen DC, Duncan J. A domain-general cognitive core defined in multimodally parcellated human cortex. Cereb cortex. 2020;30:4361–80. doi: 10.1093/cercor/bhaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crittenden BM, Mitchell DJ, Duncan J. Task encoding across the multiple demand cortex is consistent with a frontoparietal and cingulo-opercular dual networks distinction. J Neurosci. 2016;36:6147–55. doi: 10.1523/JNEUROSCI.4590-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.G Deco, D Vidaurre, ML Kringelbach, Revisiting the Global Workspace orchestrating the hierarchical organization of the human brain. Nat Human Behav. 2021;5:497–511. [DOI] [PMC free article] [PubMed]
- 110.Vyas S, Golub MD, Sussillo D, Shenoy KV. Computation through neural population dynamics. Annu Rev Neurosci. 2020;43:249–75. doi: 10.1146/annurev-neuro-092619-094115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Voytek B, Kayser AS, Badre D, Fegen D, Chang EF, Crone NE, et al. Oscillatory dynamics coordinating human frontal networks in support of goal maintenance. Nat Neurosci. 2015;18:1318 1324. doi: 10.1038/nn.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parvizi J, Kastner S. Promises and limitations of human intracranial electroencephalography. Nat Neurosci. 2018;21:474–83. doi: 10.1038/s41593-018-0108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Taghia J, Cai W, Ryali S, Kochalka J, Nicholas J, Chen T, et al. Uncovering hidden brain state dynamics that regulate performance and decision-making during cognition. Nat Commun. 2018;9:2505. doi: 10.1038/s41467-018-04723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taghia J, Ryali S, Chen T, Supekar K, Cai W, Menon V. Bayesian switching factor analysis for estimating time-varying functional connectivity in fMRI. Neuroimage. 2017;155:271–90. doi: 10.1016/j.neuroimage.2017.02.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cole MW, Ito T, Bassett DS, Schultz DH. Activity flow over resting-state networks shapes cognitive task activations. Nat Neurosci. 2016;19:1718–26. doi: 10.1038/nn.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim CK, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci. 2017;18:222–35. doi: 10.1038/nrn.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parvizi J, Jacques C, Foster BL, Witthoft N, Rangarajan V, Weiner KS, et al. Electrical stimulation of human fusiform face-selective regions distorts face perception. J Neurosci. 2012;32:14915–20. doi: 10.1523/JNEUROSCI.2609-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gratton C, Lee TG, Nomura EM, D’Esposito M. The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Front Syst Neurosci. 2013;7:124. doi: 10.3389/fnsys.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mclntosh A, Gonzalez‐Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp. 1994;2:2–22. [Google Scholar]
- 120.Sun FT, Miller LM, D’Esposito M. Measuring interregional functional connectivity using coherence and partial coherence analyses of fMRI data. Neuroimage. 2004;21:647–58. doi: 10.1016/j.neuroimage.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 121.Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage. 2005;25:230–42. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 122.Seth AK. Causal connectivity of evolved neural networks during behavior. Network. 2005;16:35–54. doi: 10.1080/09548980500238756. [DOI] [PubMed] [Google Scholar]
- 123.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 124.Ryali S, Shih YY, Chen T, Kochalka J, Albaugh D, Fang Z, et al. Combining optogenetic stimulation and fMRI to validate a multivariate dynamical systems model for estimating causal brain interactions. Neuroimage. 2016;132:398–405. doi: 10.1016/j.neuroimage.2016.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol. 2009;7:e33. doi: 10.1371/journal.pbio.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roebroeck A, Formisano E, Goebel R. The identification of interacting networks in the brain using fMRI: Model selection, causality and deconvolution. NeuroImage. 2009;S1053-8119:01015–5. doi: 10.1016/j.neuroimage.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 127.Kayser AS, Sun FT, D’Esposito M. A comparison of Granger causality and coherency in fMRI-based analysis of the motor system. Hum brain Mapp. 2009;30:3475–94. doi: 10.1002/hbm.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ryali S, Chen T, Supekar K, Tu T, Kochalka J, Cai W, et al. Multivariate dynamical systems-based estimation of causal brain interactions in fMRI: Group-level validation using benchmark data, neurophysiological models and human connectome project data. J Neurosci Methods. 2016;268:142–53. doi: 10.1016/j.jneumeth.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.W Cai, S Ryali, R Pasumarthi, V Talasila, V Menon, Dynamic causal brain circuits during working memory and their functional controllability. Nat Commun. 2021;12:3314. [DOI] [PMC free article] [PubMed]
- 130.A Lombardi, M Hornquist, Controllability analysis of networks. Phys Rev E. 2007;75(5 Pt 2):056110. 10.1103/PhysRevE.75.056110. [DOI] [PubMed]
- 131.Ruths J, Ruths D. Control profiles of complex networks. Science. 2014;343:1373–6. doi: 10.1126/science.1242063. [DOI] [PubMed] [Google Scholar]
- 132.E Tang, DS Bassett, Colloquium: control of dynamics in brain networks. Rev Mod Phys. 2018;90:031003.
- 133.Yan G, Vértes PE, Towlson EK, Chew YL, Walker DS, Schafer WR, et al. Network control principles predict neuron function in the Caenorhabditis elegans connectome. Nature. 2017;550:519–23. doi: 10.1038/nature24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Holtzheimer PE, Mayberg HS. Deep brain stimulation for psychiatric disorders. Annu Rev Neurosci. 2011;34:289–307. doi: 10.1146/annurev-neuro-061010-113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.YY Liu, AL Barabasi, Control principles of complex systems. Rev Mod Phys. 2016;88:035006.
- 136.Liu YY, Slotine JJ, Barabasi AL. Controllability of complex networks. Nature. 2011;473:167–73. doi: 10.1038/nature10011. [DOI] [PubMed] [Google Scholar]
- 137.JP Hespanha, Linear systems theory (Princeton Press, Princeton, New Jersey, 2009).
- 138.Leitold D, Vathy-Fogarassy A, Abonyi J. Controllability and observability in complex networks - the effect of connection types. Sci Rep. 2017;7:151. doi: 10.1038/s41598-017-00160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gu S, Pasqualetti F, Cieslak M, Telesford QK, Yu AB, Kahn AE, et al. Controllability of structural brain networks. Nat Commun. 2015;6:8414. doi: 10.1038/ncomms9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tu C, Rocha RP, Corbetta M, Zampieri S, Zorzi M, Suweis S. Warnings and caveats in brain controllability. Neuroimage. 2018;176:83–91. doi: 10.1016/j.neuroimage.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Menon V. Brain networks and cognitive impairment in psychiatric disorders. World Psychiatry. 2020;19:309–10. doi: 10.1002/wps.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Szczepanski SM, Knight RT. Insights into human behavior from lesions to the prefrontal cortex. Neuron. 2014;83:1 17. doi: 10.1016/j.neuron.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McTeague LM, Goodkind MS, Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J Psychiatr Res. 2016;83:37–46. doi: 10.1016/j.jpsychires.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. 2017;174:676–85. doi: 10.1176/appi.ajp.2017.16040400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Filippi M, Agosta F, Scola E, Canu E, Magnani G, Marcone A, et al. Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex. 2013;49:2389–401. doi: 10.1016/j.cortex.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 147.Hayes JP, Bigler ED, Verfaellie M. Traumatic brain injury as a disorder of brain connectivity. J Int Neuropsych Soc. 2016;22:120–37. doi: 10.1017/S1355617715000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sha Z, Wager TD, Mechelli A, He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol Psychiatry. 2019;85:379–88. doi: 10.1016/j.biopsych.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 149.Gallen CL, D’Esposito M. Brain modularity: a biomarker of intervention-related plasticity. Trends Cogn Sci. 2019;23:293–304. doi: 10.1016/j.tics.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, et al. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70:869–79. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Supekar K, Cai W, Krishnadas R, Palaniyappan L, Menon V. Dysregulated brain dynamics in a triple-network saliency model of schizophrenia and its relation to psychosis. Biol Psychiatry. 2019;85:60–69. doi: 10.1016/j.biopsych.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 152.Stam CJ. Modern network science of neurological disorders. Nat Rev Neurosci. 2014;15:683 695. doi: 10.1038/nrn3801. [DOI] [PubMed] [Google Scholar]
- 153.Cai W, Chen T, Szegletes L, Supekar K, Menon V. Aberrant time-varying cross-network interactions in children with attention-deficit/hyperactivity disorder and the relation to attention deficits. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:263–73. doi: 10.1016/j.bpsc.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, et al. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014;137:2382–95. doi: 10.1093/brain/awu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gratton C, Nomura EM, Pérez F, D’Esposito M. Focal brain lesions to critical locations cause widespread disruption of the modular organization of the brain. J Cogn Neurosci. 2012;24:1275 1285. doi: 10.1162/jocn_a_00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Warren DE, Power JD, Bruss J, Denburg NL, Waldron EJ, Sun H, et al. Network measures predict neuropsychological outcome after brain injury. Proc Natl Acad Sci USA. 2014;111:14247 14252. doi: 10.1073/pnas.1322173111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aron AR, Herz DM, Brown P, Forstmann BU, Zaghloul K. Frontosubthalamic circuits for control of action and cognition. J Neurosci. 2016;36:11489–95. doi: 10.1523/JNEUROSCI.2348-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16:1348–55. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]