Abstract
The prefrontal cortex (PFC) has emerged as one of the regions most consistently impaired in major depressive disorder (MDD). Although functional and structural PFC abnormalities have been reported in both individuals with current MDD as well as those at increased vulnerability to MDD, this information has not translated into better treatment and prevention strategies. Here, we argue that dissecting depressive phenotypes into biologically more tractable dimensions – negative processing biases, anhedonia, despair-like behavior (learned helplessness) – affords unique opportunities for integrating clinical findings with mechanistic evidence emerging from preclinical models relevant to depression, and thereby promises to improve our understanding of MDD. To this end, we review and integrate clinical and preclinical literature pertinent to these core phenotypes, while emphasizing a systems-level approach, treatment effects, and whether specific PFC abnormalities are causes or consequences of MDD. In addition, we discuss several key issues linked to cross-species translation, including functional brain homology across species, the importance of dissecting neural pathways underlying specific functional domains that can be fruitfully probed across species, and the experimental approaches that best ensure translatability. Future directions and clinical implications of this burgeoning literature are discussed.
Subject terms: Prefrontal cortex, Depression
Introduction
Major depressive disorder (MDD) has become the leading cause of disease burden globally [1, 2]. Clinically, MDD is characterized by feelings of sadness and helplessness, loss of pleasure (anhedonia) and lack of motivation, cognitive deficits, and vegetative symptoms. Despite its profound impact, the etiology and pathophysiology of MDD – and consequently its treatment and prevention – remain poorly understood. There are multiple possible reasons for this modest progress, including inattention to the vast heterogeneity of MDD, imprecise translational models, and lack of cross-species integration [3–6].
Here, we argue that dissecting depressive phenotypes into biologically more tractable dimensions allows the development of preclinical models relevant to depression to more strongly align across species, and provides unique opportunities for improving our understanding of MDD. Additionally, we propose that embracing a systems-level approach to the study of the neurobiological underpinnings of depressive phenotypes promises to provide novel insights into personalized treatments. Within this framework, we review clinical and preclinical animal literatures, highlighting the critical role of the prefrontal cortex (PFC) in depressive states. In particular, we focus on the functional heterogeneity of PFC contributions which we suggest is key to understanding the marked individual variation in the etiology, pathophysiology and treatment of MDD. In doing so, we prioritize three abnormalities most productively investigated in preclinical models [3, 6–10]: anhedonia, negative processing biases, and despair-like behavior (learned helplessness). Despite the focus on these domains, they could be a product, in part, of executive dysfunction. Thus, the contribution of PFC dysregulation to executive deficits in MDD will also be discussed. When reviewing treatment effects, a variety of interventions will be integrated; nevertheless, findings with the NMDA antagonist ketamine will be emphasized owing to its rapid antidepressant effects as well as robust parallel findings across species. Before reviewing the literature pertinent to these core phenotypes, we discuss several key issues linked to cross-species translation, including (1) functional brain homology or analogy across species, (2) the importance of parsing out neural pathways that underlie specific domains of functioning that can be investigated across species, and (3) the experimental approaches that best ensure translatability.
Issues of translatability
Prefrontal organization
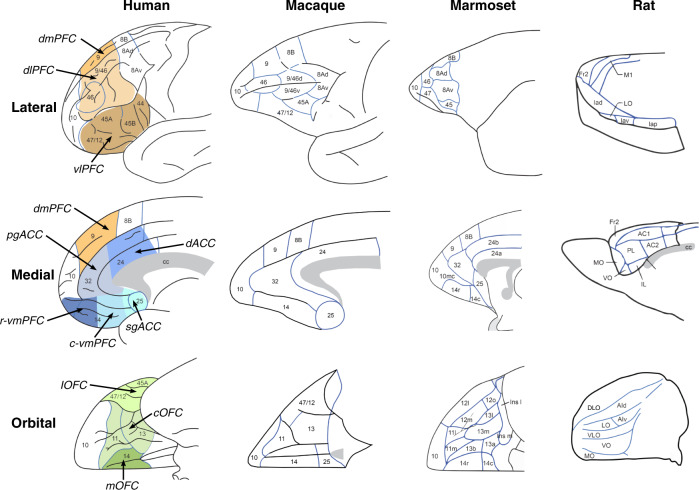
A key consideration particularly pertinent to cross-species translatability is recognition of anatomical and functional boundaries within prefrontal and anterior cingulate cortices of humans and other animals. Prefrontal structural and functional organization of non-human primates (NHP) is far similar to humans than that of rodents [11–14], but the majority of preclinical studies relevant to MDD are performed in rodents. Since our current understanding of functional homology or analogy between the PFC of rodents and humans is still in its infancy, it is important not to overstate it, as it may inadvertently hinder translational opportunities. This highlights the importance of NHP research, where back translation to rodents and forward translation into the clinic can bridge the gap. As we shall see, evidence in humans implicates orbitofrontal (OFC), dorsolateral/ventrolateral (dl/vl), as well as medial PFC (mPFC)/anterior cingulate cortex (ACC) in MDD [15–17], dl/vlPFC, in particular, having no known homologous or analogous regions in rodents. Indeed, even for those regions where greater anatomical similarity exists (e.g., mPFC/ACC), the extent to which anatomical similarity reflects functional homology or analogy is unclear [9, 18]. Translational opportunities can also be hampered when comparing across human studies because of the use of different nomenclatures to describe distinct prefrontal regions e.g., cytoarchitectonic description e.g., areas 11, 47 and 46 (not always easily translated to MR images) vs. gross regional positioning e.g., ventromedial, subgenual, medial orbital. With this in mind, Fig. 1 clearly lays out the nomenclature used in this review for humans, NHPs and rats (see also Box 1).
Fig. 1. Schematics of the medial, lateral and orbital views of the prefrontal and anterior cingulate cortex in humans, the NHPs, macaques and marmosets, and rats.
Specific areas are labeled based on the parcellation maps of Petrides and Pandya for humans and macaques [273], Paxinos et al. for marmoset [274] and Palomero-Gallagher and Zilles for lateral and medial views [275] and Uylings et al. [276] for the orbital view in rats. Note that an alternative parcellation of the orbital and medial views of human and macaques is provided by Price et al. [11] in which area 13, 14 and 12/47 are further subdivided, area 10 extends further caudally on the medial surface, replacing medial area 14 and area 25 in humans is smaller, with the addition of an adjacent subregion of area 32 (32/PL) and area 25 in macaques does not extend onto the orbital surface. See also Box 1. Caudal ventromedial (c-vm)PFC; central (c)OFC; dorsal (d)ACC; dorsomedial (dm)PFC; dorsolateral (dl)PFC; lateral (l)OFC; medial (m)OFC; perigenual (pg)ACC; rostral ventromedial (r-vm)PFC; subgenual (sg)ACC; ventrolateral (vl)PFC.
Box 1 Cross-species prefrontal nomenclature.
The detailed architectural-based parcellations [273–275] summarized in Fig. 1 are often replaced by another nomenclature which is based on the overall spatial location of regions, e.g., ventromedial (vm)PFC, dorsolateral (dl)PFC, perigenual (pg)ACC, medial OFC, which are variably used across both human and animal studies. This can lead to confusion in the literature both between human imaging studies and across human and animals studies, not only because the precise spatial location of these descriptors can vary between studies e.g., medial OFC in monkeys is commonly used to indicate area 14 [115] but recently used to describe areas 11 and 13 instead [151] but also, because in some cases the spatially defined area is very extensive and includes multiple, functionally heterogenous regions, e.g., vmPFC variably includes areas 14, 10, 32, 25, 24, and rarely do activations in imaging studies or interventions in animals include the entire region. Consequently, we have divided what is often traditionally referred to as vmPFC into three subregions, subgenual cingulate cortex (sgACC), caudal vmPFC and rostral vmPFC and OFC into lateral, central and medial regions and these, along with the other descriptors e.g., dlPFC, pgACC, are illustrated in color on the medial, lateral and orbital views of the human brain.
Symptom complexity
The complexity of depression-associated symptoms makes them difficult to assess with simple questionnaires in the clinic or single behavioral tests in animals. Recognition of the importance of parsing out distinct neural pathways underlying specific cognitive processes led to the Research Domain Criterion (RDoC) initiative [19]. Key to this initiative is a fundamental understanding of the neurocognitive processes regulating adaptive behavior to understand maladaptive behavior. For example, studies of the neurobiology of reward processing have highlighted the variety of distinct pathways involved in its different aspects resulting in the recognition of separate subtypes of anhedonia, including consummatory, anticipatory, motivational and decisional [20, 21]. Whilst traditionally the focus in depression-relevant rodent studies has been on consumption (e.g., sucrose preference test [22–24]), this has been re-assessed due to evidence indicating preserved consummatory reward in functionally analogous tests (e.g., sweet taste test) in MDD [25, 26]. Thus, there is growing interest in developing functionally analogous tasks that probe subdomains of reward processing, including reward learning [27–29] and effort-based decision making [30].
Negative processing biases are prevalent in MDD [31] and implicated in its etiology and treatment [31–33]. They exist across domains, including perception, attention, memory and decision making. Among the most widely used perceptual paradigms to probe negative biases in MDD are backward masking tasks, which allow experimenters to assess non-conscious responses to emotional stimuli (e.g., facial expressions) or tasks requiring implicit (e.g., judging the sex of a facial expression) vs. explicit (e.g., judging a facial expression) processing. These paradigms have been complemented by tasks involving self-referential processing (e.g., does a negative attribute apply to the self?). Whilst the latter is clearly specific to humans, the former have received little attention in animal studies of depression. Recently though, tests to measure other aspects of negative bias linked to decision making have been developed that are suitable for both humans and other animals, including probabilistic discrimination, approach-avoidance and ambiguous cue tasks. Probabilistic discrimination tasks are related to reward learning paradigms but focus particularly on the responsivity to misleading negative feedback [34–37]. In approach-avoidance decision making tasks responding for reward in the presence of punishment introduces conflict [38–42]. In ambiguous cue tests reward and punishment [43, 44] or different amounts of reward [45, 46] are associated with two different sensory cues lying along a continuum, e.g., low or high frequency tones. Subsequent presentation of an ambiguous cue (e.g., a tone equidistant in frequency between the high and low frequency tones), assesses the tendency to predict the more positive or negative of the two potential outcomes.
Behavioral despair is often associated with symptoms of helplessness and hopelessness in MDD in which a perceived lack of cognitive control is a core feature [47]. It has been a major focus in preclinical animal studies but has received relatively little attention in humans and when it has, there has been little cross-species overlap in responses measured. Despair is purported measured in rodents using the forced swim (FST) and tail suspension (TST) tests [48–50]. The quicker animals stop swimming or stop struggling, respectively, is proposed to reflect behavioral despair [51, 52]. Alternative accounts suggest instead that these tests measure adaptive learned responses to acute stress and reflect a natural shift from active to passive coping strategies [53, 54] as distinct from behavioral despair. Thus, they may be more relevant to studying brain mechanisms underlying acute stress rather than a chronic state, like depression. Moreover, increases in escape behavior, hypothesized to reflect an antidepressant effect, may reflect instead an increase in anxiety [55]. Accordingly, caution is required when equating enhanced or reduced immobility on these tests, respectively, with depressant and antidepressant behavior.
Behavioral despair may be more readily associated with concepts of learned helplessness whereby animals, having experienced uncontrollable negative events, subsequently display impaired instrumental learning in otherwise, controllable, contexts involving similar or different negative and positive reinforcers to that used in the uncontrollable context [56–59]. The finding that animals that had control over those same negative events do not subsequently show impaired instrumental actions demonstrates the critical factor of controllability. While superficially, the FST and TST appear to link to concepts of learned helplessness in that animals learn that their actions are ineffective, they do not capture the central tenet of learned helplessness [60], which is, that having experienced the ineffectiveness of actions when in an uncontrollable context, animals subsequently fail to learn actions even in a situation that is controllable. Aspects of controllability, per se, have been studied in the context of depression in humans although, as shall be seen, such studies are limited.
Experimental approaches
Neuroimaging studies have provided important insight into the neural correlates of MDD by identifying alterations in functional activity, resting state and structural integrity. Although findings from other imaging modalities will be mentioned, we focus primarily on fMRI because they allow (1) direct evaluation of brain responses related to environmental stimuli and behavioral performance and (2) a network-level analysis. Critically, imaging findings not only reveal changes associated with MDD but also determine markers that may act as predictors of disease onset as well as predictors of efficacious treatment. For example, with respect to risk, a growing number of studies have compared unaffected (never-depressed and symptom-free) first-degree relatives (e.g., children, monozygotic twins) of individuals with lifetime MDD (“high-risk” group) to “low-risk” groups (e.g., children without parental depression). Similarly, some studies have evaluated fully remitted individuals with past MDD to identify putative trait-like markers of MDD vulnerability.
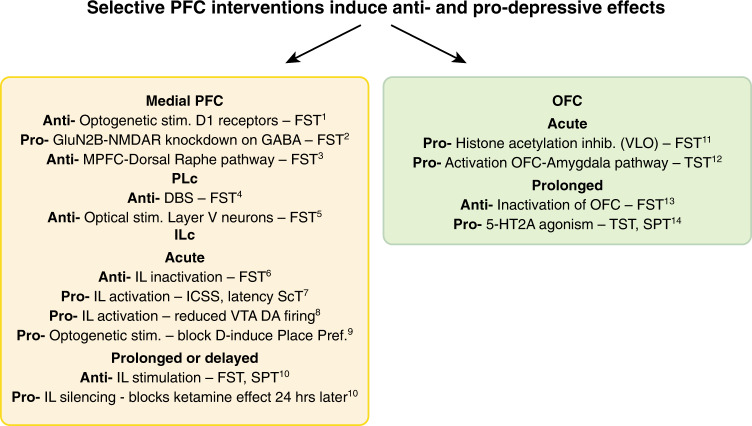
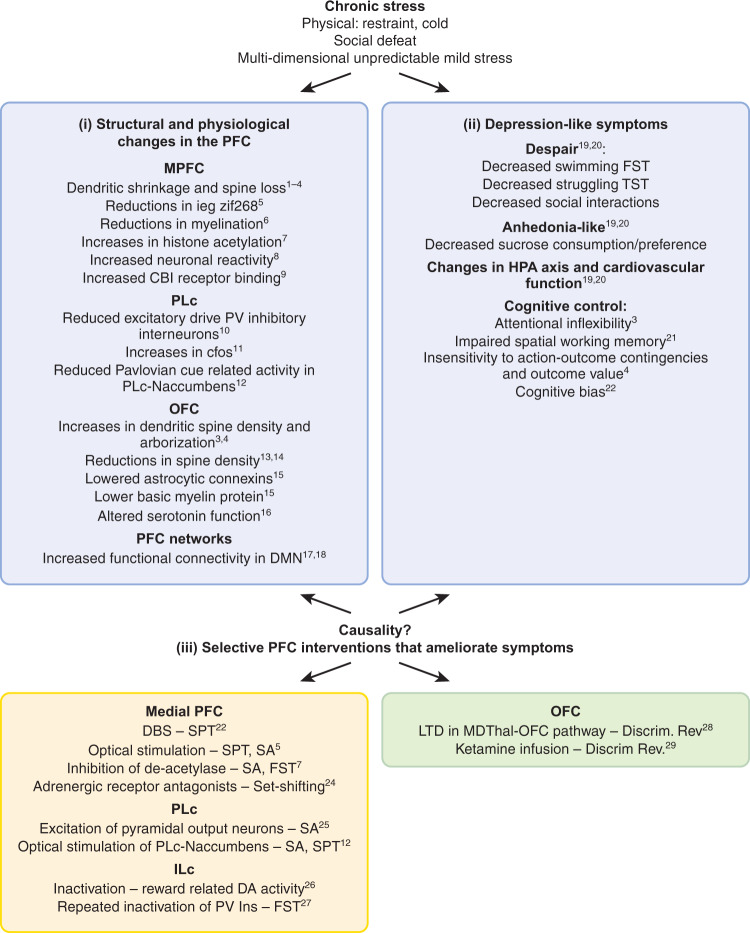
To provide insight into the role of the PFC in the etiology and treatment of MDD in preclinical animal studies, a range of experimental approaches have been adopted from basic science to more clinically relevant animal models. Specific to the former (Fig. 2) are studies determining the depression-related behavioral effects of central interventions within specific prefrontal subregions and circuits shown to be dysregulated in depression. In some cases, the ability of known antidepressants to ameliorate these intervention-induced effects have also been assessed [61, 62]. This approach allows independent study of specific depression-related alterations in prefrontal activity to determine not only their causal role in symptoms of depression but also their potential differential sensitivity to treatments. A second, related approach has identified prefrontal interventions that induce so-called ‘antidepressant’ effects in otherwise healthy animals or alternatively identified prefrontal interventions that block the behavioral effects of known systemically administered ‘antidepressants’, again, in the otherwise healthy animal [63]. Alternatively, as summarized in Fig. 3, a persistent depressive-like phenotype has been induced by exposing experimental animals to uncontrollable, chronic physical and/or psychological stress [64, 65], a known risk factor for depression [66]. Associated changes in prefrontal physiology and function are then determined, alongside the ability of targeted prefrontal manipulations to reverse/ameliorate these effects. Persistent depressive-like phenotypes are also produced with genetic modifications or other direct physiological-inducers, such as corticosterone or immune activation [67], but of these, chronic stress models have provided the majority of insight into PFC contributions and are discussed here.
Fig. 2. Summary of putative pro-depressant (pro) or antidepressant (anti) effects of interventions within the medial prefrontal cortex (medial PFC) and orbitofrontal cortex (OFC) of otherwise, normal, healthy rodents.
FST forced swim test, ICSS intracranial self-stimulation, Place Pref place preference test, ScT sucrose consumption test, SPT sucrose preference test, TST tail suspension test, VLO ventrolateral orbital cortex, VTA DA ventral tegmental area dopamine neurons. 1. Hare, B.D. et al. Nat. Commun. 10, 1–12 (2019). 2. Gerhard, D.M. et al. J. Clin. Invest. 130, 1336–1349 (2020). 3. Warden, M.R. et al. Nature 492, 428–432 (2012). 4. Hamani, C. et al. Biol. Psychiatry 71, 30–35 (2012). 5. Kumar, S. et al. J. Neurosci. 33, 1116–1129 (2013). 6. Slattery, D.A., Neumann, I. & Cryan, J.F. J. Psychopharmacol. 25, 1295-1303 (2011). 7. John, C.S. et al. Neuropsychopharmacology 37, 2467–2475 (2012). 8. Moreines, J.L., Owrutsky, Z.L. & Grace, A.A. Neuropsychopharmacology 42, 904–913 (2017). 9. Ferenczi, E.A. et al. Science 351, aac9698 (2016). 10. Fuchikami, M. et al. Proc. Natl. Acad. Sci. 112, 8106–8111 (2015). 11. Zhao, Y. et al. PLoS One 8, e52698 (2013). 12. Kuniishi, H., Yamada, D., Wada, K., Yamada, M. & Sekiguchi, M. Transl. Psychiatry 10, 1–11 (2020). 13. Kuniishi, H. et al. Front. Behav. Neurosci. 10, 250 (2017). 14. Xu, C. et al. Neuropharmacology 109, 7–17 (2016).
Fig. 3. Summary of findings in rodents subject to chronic stress as adults revealing (i) structural and physiological changes in the medial prefrontal cortex (medial PFC) and orbitofrontal cortex (OFC), (ii) associated behavioral changes and (iii) interventions within the medial PFC and OFC that ameliorate the behavioral changes.
Both (i) and (iii) implicate dysregulation within medial PFC and OFC in relation to the behavioral changes associated with chronic stress. ieg immediate early gene, CB1 endocannabinoid presynaptic receptor, DA dopamine, DBS deep brain stimulation, Discrim Rev discrimination reversal task, DMN default mode network, FST forced swim test, LTD long-term depression, MDThal mediodorsal thalamus, NAccumbens nucleus accumbens, PV parvalbumin neurons, SA social avoidance (interaction) test, SPT sucrose preference test. 1. Cook, S.C. & Wellman, C.L. J. Neurobiol. 60, 236–248 (2004). 2. Radley, J.J. et al. Exp. Neurol. 196, 199–203 (2005). 3. Liston, C. et al. J. Neurosci. 26, 7870–7874 (2006). 4. Dias-Ferreira, E. et al. Science (80-.). 325, 621–625 (2009). 5. Covington, H.E. et al. J. Neurosci. 30, 16082–16090 (2010). 6. Lehmann, M.L., Weigel, T.K., Elkahloun, A.G. & Herkenham M. Sci. Rep. 7, 46548. 7. Covington, H.E., Maze, I., Vialou, V. & Nestler, E.J. Neuroscience 298, 329–335 (2015). 8. Kumar, S. et al. Nat. Commun. 5, 1–9 (2014). 9. McLaughlin, R.J. et al. Behav. Brain Res. 237, 333–337 (2013). 10. Perova, Z., Delevich, K. & Li, B. J. Neurosci. 35, 3201–3206 (2015). 11. Vialou, V. et al. J. Neurosci. 34, 3878–3887 (2014). 12. Spring, M.G. et al. J. Neurosci. JN-RM-1869-20 (2021). 13. Xu, C. et al. Neuropharmacology 109, 7–17 (2016). 14. Gourley, S.L., Swanson, A.M. & Koleske, A.J. J. Neurosci. 33, 3107–3112 (2013). 15. Miguel-Hidalgo, J.J., Moulana, M., Deloach, P.H. & Rajkowska, G. Chronic Stress (Thousand Oaks, Calif.) 2, 247054701881418 (2018). 16. Lapiz-Bluhm, M.D.S., Soto-Piña, A.E., Hensler, J.G. & Morilak, D.A. Psychopharmacology (Berl). 202, 329–341 (2009). 17. Henckens, M.J.A.G. et al. Neuroimage 105, 312–322 (2015). 18. Grandjean, J. et al. Neuroimage 142, 544–552 (2016). 19. Gururajan, A., Reif, A., Cryan, J.F. & Slattery, D.A. Nat. Rev. Neurosci. 20, 686–701 (2019). 20. Willner, P. Neurobiol. Stress 6, 78–93 (2017). 21. Mizoguchi, K. et al. J. Neurosci. 20, 1568–1574 (2000). 22. Papciak, J., Popik, P., Fuchs, E. & Rygula, R. Behav. Brain Res. 256, 305–310 (2013). 23. Hamani, C. et al. Biol. Psychiatry 71, 30–35 (2012). 24. Jett, J.D. & Morilak, D.A. Neuropsychopharmacology 38, 585–595 (2013). 25. Kumar, S. et al. J. Neurosci. 33, 1116–1129 (2013). 26. Moreines, J.L., Owrutsky, Z.L. & Grace, A.A. Neuropsychopharmacology 42, 904–913 (2017). 27. Nawreen, N. et al. eNeuro 7, (2020). 28. Adler, S.M., Girotti, M. & Morilak, D.A. Neurobiol. Stress 13, 100258 (2020). 29. Patton, M.S., Lodge, D.J., Morilak, D.A. & Girotti, M. Neuropsychopharmacology 42, 1220–1230 (2017).
There are advantages and disadvantages with all of these approaches with respect to translatability. The insight gained into the potential contribution to depressive symptoms of a particular brain region by studying the effects of a selective intervention within that region may be limited, especially if those interventions are acute, since any such changes in depression do not occur in isolation and are not acute. In contrast, although depressive phenotypes induced by e.g., uncontrollable stressors appear more clinically relevant, the state they produce may, nevertheless, be as variable in their etiology and treatment as human depression, limiting the level of insight gained. Finally, caution should be employed when interpreting findings from a study describing antidepressant effects in a context in which there is no explicit ‘depressant’ effect to ameliorate. In sum, advances in our understanding of the role of the PFC in depression will best be achieved by the synthesis of results from these varied approaches, especially when the results are convergent, as highlighted below.
MDD and chronic stress models in rodents
Resting state (task-free) functional connectivity findings in MDD
The literature probing resting state functional connectivity (rsFC) in MDD is vast, and generally points to abnormalities (1) between discrete PFC regions, (2) within networks anchored on different PFC regions (e.g., central executive network, default mode network), and (3) between PFC and other networks (for reviews, see [68–70]). Due to space limitation, the following sections present a selected summary of recent findings that are particularly relevant.
A recent voxel-wise meta-analysis compared MDD (N = 1399) and healthy controls (N = 1332) in their amplitude of low frequency fluctuations (ALFF), which represents an indirect measure of spontaneous brain activity [70]. Compared to controls, individuals with MDD showed increased ALFF in a region spanning the medial orbitofrontal cortex (OFC) and perigenual ACC (pgACC). In a coordinate-based meta-analysis [69], increased rsFC in the pgACC predicted response to a variety of treatments (e.g., repetitive transcranial magnetic stimulation (rTMS), pharmacotherapy, cognitive behavior therapy, electroconvulsive therapy (ECT)) (see also [16]). In a sample of 421 individuals with MDD and 488 healthy controls, reduced rsFC emerged in MDD between the central OFC (BA13) and memory-related regions (e.g., parahippocampal gyrus), with increasingly weaker rsFC correlating with higher depression severity [71]. In light of prior data [72], the authors speculated that reduced central OFC-memory regions rsFC might weaken reward-related information to be consolidated in memory.
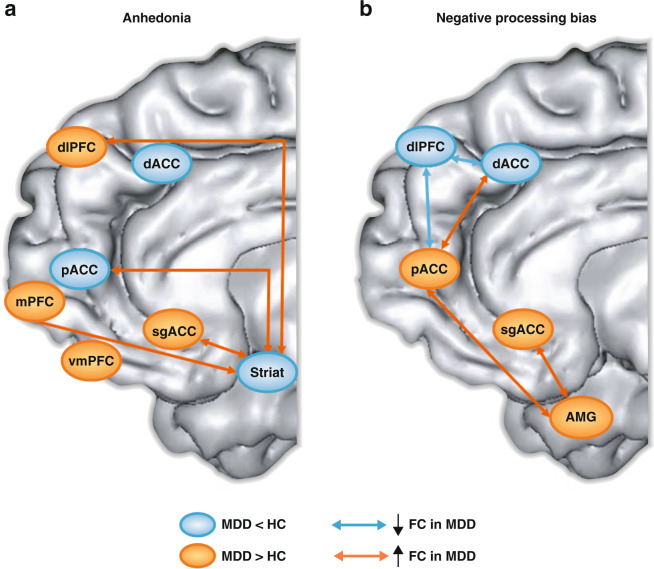
These findings were extended by a recent study, which compared individuals with current MDD, unaffected first-degree relatives, and healthy controls in their rsFC between OFC and mPFC sub-regions and the rest of the brain [73]. Current MDD and increased risk for MDD shared several abnormalities, including dysregulated coupling involving the pgACC, dmPFC and dorsal ACC (dACC) to other cortical regions (Fig. 4a). However, current MDD was uniquely characterized by abnormal rsFC between various orbital and mPFC sub-regions (pgACC, dACC, lateral and central OFC) and PFC (dlPFC), subcortical (e.g., dorsal striatum), as well as cortical (e.g., precuneus, inferior temporal lobes) regions. Based on these findings, the authors speculated that dmPFC plays a pivotal role in MDD vulnerability, with additional OFC and mPFC abnormalities emerging with the onset of MDD.
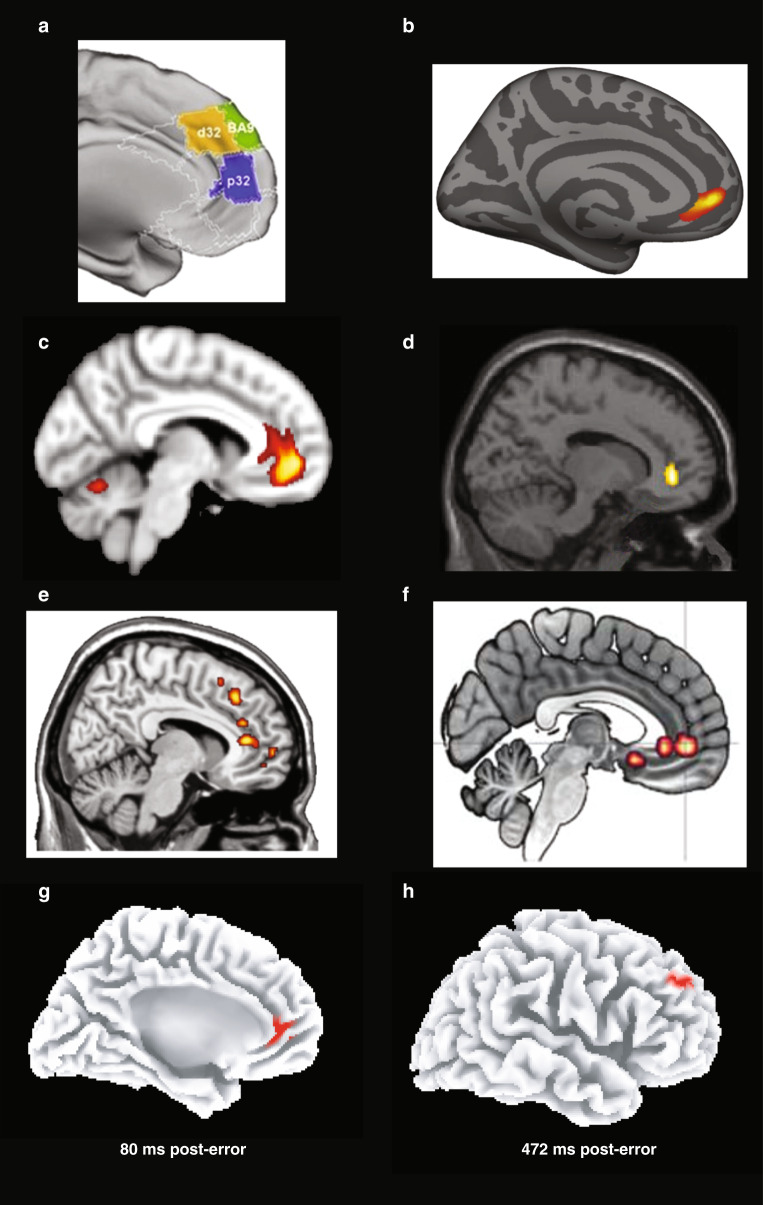
Fig. 4. Functional and structural findings implicating the perigenual anterior cingulate cortex (and other mPFC regions) in major depressive disorder (MDD).
a Individuals with current MDD and at-risk individuals (unaffected first-degree relatives) showed abnormal coupling involving the pgACC (see “p32”; decreased coupling with posterior cingulate), dmPFC (see “BA9”; decreased coupling with ventral intraparietal sulcus), and dACC (see “d32”; increased coupling with the occipital cortex) [73]. b pgACC region showing a positive correlation between cortical thickness change and improvement in depression symptoms with repetitive transcranial magnetic stimulation of the left dlPFC [90]. c pgACC region in which pre- to post-treatment changes in gray matter volume correlated with antidepressant response to transcranial magnetic stimulation of the left dlPFC [91]. d pgACC region showing higher activation to masked sad faces in MDD than healthy controls [169]. e Greater activation in the pgACC (and other mPFC regions) in response to a sad mood manipulation predicted relapse among fully remitted individuals 18 months later [155]. f Increased functional connectivity between the sgACC/pgACC and the amygdala during an implicit emotional processing task in individuals with increased MDD vulnerability [189]. g Increased pgACC activation 80 ms after committing a mistake in a speeded reaction time task in MDD vs. healthy controls [242]; and h Decreased left dlPFC activation 472 ms after committing a mistake in MDD vs. healthy controls [242]. Adapted with permission from publishers.
Importantly, rsFC abnormalities have emerged also in youth with increased MDD vulnerability but can be ameliorated by treatment. Thus, relative to healthy children (8–17 years old), children at increased vulnerability due to parental MDD history (as well as children with current MDD) had lower rsFC between the right dlPFC (BA6/8/9) and the amygdala [74] – a pathway previously linked to inhibition of emotional responses in adult MDD [75]. Treatment-resistant depression (TRD) was characterized by decreased global connectivity within the right dlPFC and bilateral dmPFC (including the dACC) [76]. Notably, ketamine infusion increased global connectivity in all three regions. In a similar study [77], TRD was characterized by higher pre-treatment resting state activity in a region between the pgACC and sgACC, and a course of ECT treatment normalized activity in this region and its connectivity with the vmPFC; moreover, higher pre-treatment sgACC activity predicted better response to ECT. Together, these findings indicate downregulation of sgACC activity and connectivity is key for ECT response.
Structural findings in MDD
An early meta-analysis (64 structural MRI studies) reported that frontal regions showed the largest volumetric reductions in MDD [78], particularly in the ACC and bilateral OFC, although distinct subregions were not investigated (see also [79]). A link between MDD and gray matter volume (GMV) reduction in the ACC (at the juncture between the pgACC and dACC) was confirmed in a later meta-analysis [80]. In the largest ever conducted structural MRI study, relative to controls (N = 7658), adults with MDD (N = 1902) showed thinner cortical gray matter in the ACC (with reduction spanning subgenual, perigenual and dorsal regions) and OFC/vmPFC [81]. In a 3-year prospective study, MDD patients with larger volumes in similar ACC regions (sgACC, pgACC and dACC) had better outcomes (fewer hospitalizations) than those with smaller volumes [82]. Similarly, in a 5-year naturalistic prospective study, GMV in the right dACC and right inferior frontal gyrus contributed to disease trajectory predictions; specifically, larger volume in these areas predicted better clinical outcome and lower depressive symptoms over 5 years [83]. Altogether, these findings point to structural reductions in mPFC regions, particularly various ACC subdivisions. Additional important insight has emerged from this literature.
First, recent meta-analyses have yielded surprisingly few structural abnormalities linked to MDD [79]. We believe one reason is the large heterogeneity of “MDD”; accordingly, studies linking structural abnormalities to biologically more homogenous phenotypes of depression might provide key insights. Fitting this, in the UK Biobank sample, polygenic risk for anhedonia was linked to smaller OFC volumes [84].
Second, some of these abnormalities appear early in the course of the disorder and might, in fact, represent risk markers. Thus, relative to healthy children, children (7–12 years) with a history of preschool-onset depression (age of onset: 3–6 years) had thinner right sgACC cortical thickness [85]. Importantly, decreased right sgACC cortical thickness at age 7–12 was predicted by depression severity at age 3–6 (and age 7–12). Thus, structural abnormalities spanning the sgACC and OFC emerge early in MDD and affect regions playing a key role in the regulation of negative emotion, value representation, and self-referential processing [17]. The laterality effect was intriguing in light of rodent data that the right vmPFC/ACC (the putative homolog to the human sgACC) is critically implicated in the regulation of HPA axis [86], which points to a possible risk mechanism. Similarly, adolescents with MDD (N = 213) had lower cortical thickness in the medial OFC/vmPFC and superior frontal gyrus than adolescents without MDD history (N = 294) [81]. Notably, similar regions emerged from a 5-year prospective study assessing never-depressed daughters (10–15 years old) with a mother with depression. Specifically, lower cortical thickness in the right medial OFC/vmPFC (but larger pgACC cortical thickness) emerged as two of the top three neural features predicting the first episode of MDD [87].
Third, some of these PFC abnormalities predicted clinical response and were ameliorated by treatment. Accordingly, before starting TMS stimulation over the left dlPFC, eventual non-remitting TRD patients had lower structural integrity within the central executive network (anchored on the dlPFC) relative to both eventual remitters and healthy controls. Moreover, higher structural integrity within the central executive network predicted greater symptom reduction [88]. Similarly, ECT treatment was associated with increased cortical thickness across PFC (dlPFC) and dACC [89]. Changes in the dACC emerged only in treatment responders, and early increases in dACC cortical thickness 48 h after the second ECT administration predicted eventual clinical response. Finally, reduction in depressive symptoms after left dlPFC rTMS treatment was associated with increases in pgACC thickness (Fig. 4b) [90]. These findings replicated prior results showing that left dlPFC rTMS treatment increased GMV in the pgACC (Fig. 4c) and such volumetric increases correlated with symptom reduction [91].
Structural changes in chronic stress models in rodents
Chronic stress (e.g., social defeat, restraint stress, unpredictable mild stress) over a period of days to weeks induces a variety of depression-related symptoms (Fig. 3, top right-hand panel), including behavioral despair, blunted reward-related behaviors, negative biases and cognitive dysfunction [67, 92]. Specifically, animals display decreased swimming in the FST or struggling in the TST, reductions in sucrose preference or consumption and social interactions, changes in the HPA axis and cardiovascular function, negative judgment biases in ambiguous cue tests [43, 44] and impaired cognitive control [93, 94]. The latter includes response and attentional inflexibility [95], spatial working memory (WM) deficits [96] and insensitivity of goal-directed actions to action-outcome contingency and outcome value [93], although these may vary depending on the nature of the stressor. For example, chronic cold stress induces cognitive inflexibility specific to reversal learning, while chronic mild unpredictable stress or restraint stress disrupts higher-order attentional set-shifting more consistently than lower-order associative reversal learning [94, 97].
Accompanying these behavioral changes are marked physiological and structural alterations, not only in the amygdala and hippocampus but, importantly, the PFC [98, 99], that are similar to those seen in MDD. In rodents, the most abundant and consistent changes are in the mPFC (anterior cingulate, prelimbic and infralimbic) with somewhat more variable, mixed direction effects in the OFC (Fig. 3, top left-hand panel). The contrasting patterns between mPFC and OFC in dendritic spine density are particularly intriguing given similar contrasting effects pertaining to hyper- or hypo-activity in MDD, although differences in measures and precise regions involved render comparison challenging. Outstanding questions include (1) whether there are comparable increases in activity within sgACC (putative ILc in rodents) as seen in MDD; (2) whether there are structural/functional changes in dACC in rodents as reported in MDD; (3) what factors may determine whether increases or decreases in dendritic spine density are seen in the OFC in rats and whether there are differences between medial and lateral OFC regions, which we shall see below show contrasting effects in functional activity in MDD.
Anhedonia
Major depressive disorder
Recent reviews and meta-analyses have elucidated the neural correlates of reward processing dysfunctions in MDD [3, 100–104]. In general, these syntheses have highlighted that MDD-related deficits in different sub-domains of reward processing – including incentive motivation (reward anticipation), valuation (reward consumption), and reward learning – are associated with frontostriatal abnormalities. In the following sections, we summarize the main patterns, highlight recent findings (particularly those from large and prospective studies), and provide a synthesis of reward-related frontostriatal abnormalities linked to MDD.
Consummatory anhedonia
In tasks probing consummatory anhedonia (e.g., receipt of secondary rewards such as money), MDD has been associated with reduced activation in ventral (nucleus accumbens) and dorsal (caudate, putamen) striatum, ACC (rostral, dorsal), and OFC but hyperactivation in the mPFC, vmPFC (including sgACC) and dlPFC [100, 102, 104]. In some studies, blunted ventral striatal activation to rewards correlated with anhedonic symptoms [102]. In an instrumental reinforcement learning task, unmedicated MDD subjects showed lower reward value encoding in the pgACC but higher sgACC activation during the decision phase than healthy controls [105]. Among unmedicated individuals with MDD, FC between the caudal vmPFC and various reward hubs (nucleus accumbens, ventral tegmental area (VTA), OFC) in response to pleasant music correlated negatively with anhedonia [106]. Finally, although some abnormalities persist after remission (e.g., OFC hypo-responses to rewards [107], inability to sustain ventral striatal responses to positive cues [108]), acute striatal responses to rewards appear to normalize in individuals with past MDD [109].
Evidence of disrupted frontostriatal activation during reward consumption has been reported also in children with MDD, suggesting that such dysfunctions emerge early. In general, blunted striatal activation coupled with over-recruitment of PFC regions – including the dlPFC during reward outcome – has emerged [104]. In a subclinical sample, adolescents with elevated depressive symptoms showed blunted pgACC and rostral vmPFC activation during reward consumption, with the former correlating with higher anhedonia [110]. Across studies, over-recruitment of PFC regions (mPFC, dlPFC) during reward processing has been interpreted as suggesting over-compensation for reduced striatal responses to rewards [104, 111, 112].
Anticipatory anhedonia
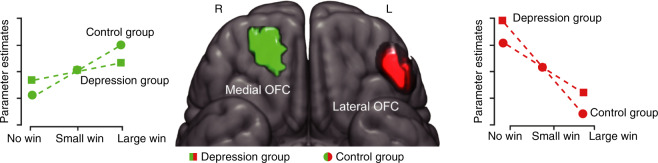
In tasks probing reward anticipation, MDD has been linked to decreased activation in ventral and dorsal striatum as well as middle frontal gyrus (BA8), but hyperactivation in the dlPFC and mPFC including pgACC and caudal vmPFC [3, 102, 113]. In the IMAGEN study (N = 1877 at age 14, N = 1140 at age 19), during reward anticipation, individuals with higher depression severity had reduced sensitivity to differing reward values in the central regions of OFC (areas 11 and 13) but higher lateral OFC (area 12) activation to non-rewards (no-win outcome) at both age 14 and 19 (Fig. 5) [114] (This paper refers to areas 11 and 13 as medial OFC but this nomenclature can be confusing as medial OFC is often used to refer to area 14 which lies on the gyrus rectus, and spans both the medial OFC and vmPFC surfaces. Thus, here, we refer to area 11 and 13 as central OFC and area 14 as medial OFC [115]). Moreover, at age 19, blunted reward-related central OFC activation correlated with more anhedonic symptoms, whereas potentiated non-reward lateral OFC activation correlated with sadness. Critically, depressive symptoms at ages 16 and 19 could be predicted by potentiated lateral OFC activation to non-rewards, but not central OFC activation to rewards, at age 14. Based on these findings, the authors suggested that “hypersensitivity to non-reward of the lateral OFC is an indicator for both current and future depression and that hyposensitivity to reward of the [central] OFC is an indicator for the current, but not future, status of depression” (p. 266). In this context, two recent findings are particularly interesting. First, among patients with TRD, a single ketamine infusion rapidly reduced anhedonic symptoms, and reduction in anhedonia correlated with decreased metabolism in the right lateral OFC (as well as increased glucose metabolism in dACC) [116]. Second, if MDD is characterized by excessive activation within a “non-reward pathway” centered on the right lateral OFC, inhibition of such a system might be beneficial. A case study indeed showed that disruption of such a system (through inhibitory 1 Hz rTMS over the right OFC) reduced both anhedonic symptoms and FC between the right lateral OFC and the ventral striatum [117]. Building on this evidence, the same group recently showed that inhibitory (1 Hz) TMS over the right OFC in TRD was associated with a 30% response rate [118].
Fig. 5. Abnormal orbitofrontal activation in depression during reward anticipation.
Central orbitofrontal cortex (see green) and lateral orbitofrontal cortex (see red) region in which individuals with elevated depression severity (see squares) had reduced sensitivity to differing reward values and potentiated activation to non-rewards (no-win outcome) during reward anticipation compared to the control group (see circles) [114]. Adapted with permission from publishers.
Imaging studies in children and adolescents with MDD have also highlighted blunted ventral striatal activation during reward anticipation, which was often accompanied by over-recruitment of PFC regions (e.g., pgACC/vmPFC) [104]. In a large youth sample (N = 1576; mean age: ~14 years), blunted reward-related ventral striatum correlated with anhedonia (but not low mood) and predicted transition to subthreshold or clinical depression 2 years later among previously healthy youths ([119]; see also [120]). Finally, among TRD patients, sgACC hyper-activation to positive feedback correlated with more anticipatory anhedonia (but not anxiety symptoms) [121]. Importantly, sgACC hyper-activation to positive (but not negative) incentives was normalized (decreased) by a single ketamine infusion. These findings fit recent evidence that a single-dose of ketamine reduced sgACC hyper-activity and improved an autonomic measure of anhedonia (anticipatory arousal to receipt of a primary reinforcer in marmosets (see below) [61]).
Reward learning
Studies probing reward learning have typically implemented computational modeling to estimate expected value and reward prediction errors (RPE) during Pavlovian, instrumental, and reversal learning tasks. These studies have generally linked MDD to blunted RPE in the ventral and dorsal striatum [122–124] (but see [125, 126] for important lack of replication), pgACC [127] and medial OFC [126], and greater RPE in the ventral striatum predicted reductions in anhedonia 6 months later [128]. In an instrumental reinforcement learning task, unmedicated individuals with MDD showed increasingly blunted medial OFC and ventral striatal RPE that correlated with anhedonia [126]. Moreover, in a community-based sample (N = 475) tested with a probabilistic reward learning task, increasing depressive symptoms were related to blunted (1) directional connectivity from the mPFC to the striatum, (2) RPE in the dACC, and (3) reward signal magnitude in the nucleus accumbens and caudal vmPFC [129]. Moreover, although fully remitted individuals showed normative ventral striatum RPE, they had greater RPE in the VTA [109], suggesting that some reward-related learning abnormalities do not normalize after recovery. Similarly, fully remitted MZ twins with a past history of mood disorders had blunted expected value signal in the frontal pole extending into middle frontal gyrus (BA10/46) as well as lower RPE signal in the left frontal pole and superior frontal gyrus (BA9/6) [130]. Interestingly, such dysfunctions did not emerge in their unaffected co-twins (high-risk group), suggesting that they might represent scars (as opposed to pre-existing vulnerabilities) of depression. Together, these data suggest that reduced valuation of expected rewards and blunted reward learning represent key vulnerabilities to future MDD.
Reward-related decision making
Several studies have used a variety of tasks to probe complex decision making in MDD, including gambling tasks that require weighting probabilities and amount of rewards and losses, and approach-avoidance decision making tasks. In general, these studies have highlighted abnormal task-related activation in various PFC regions.
In a reward-related decision making task parsing decision making (anticipation) and outcome, relative to healthy youths, youths with current MDD (9–17 years) showed potentiated responses in the left OFC (during anticipation), but decreased responses in the right OFC and dACC during both phases [131]. In a study using a card-guessing task, depressed adolescents had potentiated mPFC (BA10) activation (coupled with blunted ventral striatal activation) to rewards [111]. In studies using a “wheel-of-fortune” task, which involves making choices about varying amounts of monetary rewards with varying levels of risk, adults with MDD showed greater left OFC activation but reduced pgACC activation during the selection phase relative to controls; in addition, they showed greater activation to wins in the left inferior frontal gyrus (BA9/45) than controls [132]. In a sample tested with the same paradigm, adolescents with MDD had higher left OFC and dACC activation but lower right lateral OFC during high-risk trials (trials with only 25% chance of winning a higher amount) relative to controls [133]. In light of the functional role of these regions, the authors speculated that depressed adolescents might be characterized by reduced inhibitory control (right lateral OFC) but potentiated conflict monitoring (dACC) during risky decision making. Finally, initial evidence exists that abnormal reward-related decision making might index MDD vulnerability. Specifically, relative to healthy controls, unaffected individuals with a family history of MDD had significantly lower dlPFC (BA6) activation to risky choices in the Iowa Gambling task [134].
Recently, a study implemented a task modeled after a paradigm used in NHP [39] to probe neural correlates underlying approach-avoidance decision making in MDD [42]. In each trial, participants had to decide whether to approach a trial (which would be associated with the delivery of both a parametrically varying reward and punishment) or avoid a trial (which would be associated with no incentives). Relative to controls, unmedicated adults with MDD were less sensitive to reward. Moreover, they showed reduced approach-related activation in the dlPFC and, surprisingly, reduced avoidance-related pgACC activation. In MDD, reduced approach-related dlPFC activation correlated with increasing perceived stress.
Interim summary
Findings emerging from studies probing different subdomains of reward processing converge in highlighting disrupted activation within and functional connectivity across nodes of the brain reward pathways. As summarized in Fig. 6a and Table 1, across reward subdomains, MDD has been generally linked to blunted activation to reward-related cues within ventral and dorsal striatal regions, perigenual and dorsal ACC regions, and central and medial OFC. In contrast, MDD has been associated with hyper-activation in the medial frontal pole (BA10), vmPFC (including sgACC) and dlPFC to reward-related cues, as well as hyper-activation in the lateral OFC to non-rewards. Of note, many of these markers correlated with anhedonia, predicted future anhedonia and depressive symptoms, and predated MDD. Moreover, several markers (e.g., blunted frontostriatal rsFC and reward-related striatal activation) emerged also in unaffected, never-depressed offspring with parental depression and/or predicted the new onset of depressive disorder years later, suggesting that they represent markers of MDD vulnerability (and thus possible targets for prevention). Of relevance, disrupted coupling within reward-related corticostriatal pathways has emerged also from resting state studies, with reports of increased rsFC between (1) vlPFC and dorsal striatum [135], and (2) dmPFC and various striatal regions [136]. Critically, in a community-based sample of 6–12 years children (N = 637), rsFC strength between the ventral striatum and other reward-related regions (vmPFC, dACC, VTA) predicted the new onset of depressive disorder 3 years later [112]. Highlighting specificity, this metric did not predict the emergence of ADHD, anxiety or substance abuse over the same time period. These findings are important because they highlight that disruptions within brain reward pathways are not only present in the acute phase of MDD but predates its emergence (for similar conclusions, see [137, 138]).
Fig. 6. Summary of functional abnormalities in tasks probing (a) reward-related processes and (b) negative processing biases in major depressive disorder (MDD).
Regions highlighted in orange and blue show higher activation and lower activation, respectively, in MDD samples than healthy controls (HC). Orange and blue arrows denote higher and lower functional connectivity, respectively, in MDD samples than healthy controls. AMG amygdala, dACC dorsal anterior cingulate cortex, dlPFC dorsolateral prefrontal cortex, mPFC medial prefrontal cortex, pgACC perigenual anterior cingulate cortex, sgACC subgenual anterior cingulate cortex, Striat Striatum vmPFC ventromedial prefrontal cortex.
Table 1.
Summary of frontostriatal abnormalities in MDD in tasks probing different reward-related processes.
| Reward consumption | Reward anticipation | Reward learning | |
|---|---|---|---|
| A. Hypo-activation | |||
| Ventral and Dorsal Striatum |
↓ (greater ↓, higher anhedonia) |
↓ (greater ↓, higher anhedonia & more transition to MDD) |
↓ (greater ↓, higher anhedonia & higher anhedonia 6 months later) |
| pgACC |
↓ (greater ↓, higher depressive symptoms) |
↓ | ↓ |
| dACC | ↓ | ||
| Medial OFC | ↓ |
↓ (greater ↓, higher anhedonia) |
↓ (greater ↓, higher anhedonia) |
| B. Hyper-activation | |||
| mPFC | ↑ | ↑ | |
| vmPFC (subgenual PFC) |
↑ (greater ↑, higher anhedonia & more future MDD) |
||
| dlPFC | ↑ | ↑ | |
| Lateral OFC | ↑ to non-rewards (greater ↑, more future MDD) |
Preclinical animal studies
By far the majority of depression-focused studies in rodents have investigated consummatory aspects of reward-elicited behaviors including sucrose preference and consumption and conspecific social interactions. Many of these studies have implicated the mPFC, although not distinguishing between PLc and ILc, in chronic-stress-induced anhedonia-like behaviors. They have shown that various interventions (as listed in detail in Fig. 3) can reverse the associated deficits, in some cases within PLc specifically. In the case of a fosB-CCK interaction underlying susceptibility to chronic social defeat stress, it was explicitly related to PLc and not ILc. In contrast, few studies have attempted to reverse the blunting of consummatory reward-related behaviors through interventions specifically within ILc or OFC. In one case of ILc, its inactivation selectively blocked the effects of chronic mild stress on reward-related dopamine activity [139].
Medial regions, in particular, have also been implicated in the consummatory aspects of reward-related behavior following interventions in healthy rodents although here, the majority have focused on ILc and the effects have been mixed. For example, increasing the excitability of ILc by blockade of the glutamate transporter (GLT1) had an anhedonia-like effect, including an increase in the latency to drink sucrose and an increased threshold for intracranial self-stimulation [140]. Consistent with the latter, NMDA infusions into ILc reduced spontaneous firing of DA neurons in the medial VTA [139]. Moreover, optogenetic stimulation of ILc reduced the BOLD response to optogenetic-induced dopamine stimulation and blocked DA-stimulation-induced place preference [62]. In contrast, prolonged ILc pyramidal stimulation for 60 min mirrored the putative antidepressant effects of ketamine on sucrose preference, while neuronal silencing of ILc (and not PLc), using the GABA-A agonist, muscimol, blocked the actions of ketamine, 24 h later [63]. The latter suggests that the delayed actions of ketamine are dependent upon an intact ILc at the time of the ketamine infusion and may be more consistent with the hypothesis that the long-term effects of ILc activation are antidepressant-like. Altered performance on sucrose preference tests has also been associated with abnormal activity within the OFC. Specifically, a reduction in sucrose preference followed weekly infusions of a 5-HT2a agonist into lateral OFC, mirroring the effects seen following chronic stress [141].
In summary, the rodent mPFC and OFC have been implicated in the control of consummatory aspects of reward-related behavior, although their precise contribution to such behaviors remain unclear (Figs. 2 and 3). The weight of evidence so far though does support the hypothesis that acute ILc activation blunts reward processing, potentially through its projections into the nucleus accumbens which would appear consistent with the heightened activation of sgACC in MDD reported above. Left unanswered from rodent studies are the role of these regions in the other aspects of reward-related behaviors shown to be disrupted in MDD, including reward anticipation, reward learning, and reward-related decision making. These have received far less attention in rodent studies although considerable insight has been gained from basic science studies, particularly of OFC function in both rodents and monkeys. Much of this is covered in another chapter in this Special Issue (e.g., Izquierdo and Rudebeck) so we will only touch upon this briefly here, focusing particularly on those issues highly pertinent to our understanding of MDD. Also, left unanswered, though, especially with respect to mPFC, is the functional analogy/homology between rodents and humans and NHPs in relation to this region’s control of reward-related processing.
Whilst the rodent mPFC is most commonly parcellated into ACC, ILc and PLc, an alternative parcellation that directly parallels that used for the caudal aspects of primate mPFC/ACC, is the one by Vogt and Paxinos [14] which described these regions in terms of areas 24, 25 and 32, respectively. With this in mind, we will now consider a series of studies that have investigated the roles of area 25 and 32 in primates, specifically marmosets, in the consummatory and anticipatory aspects of reward-related behaviors, specifically addressing the hypothesis that heightened activation in sgACC reported in MDD is associated with anhedonia symptomatology. Temporary infusions of the glutamate transporter blocker, dihydrokainic acid (DHK), which heightens the excitability of neurons, induced the blunting of both anticipatory and motivational arousal when infused into area 25. Specifically, area 25 overactivation reduced the cardiovascular and behavioral Pavlovian conditioned arousal in anticipation of marshmallow reward and also decreased the number of responses an animal was willing to perform for reward on a progressive ratio task [61]. In contrast, consummatory responses, including behavioral and cardiovascular arousal during reward consumption itself and performance on a sucrose preference test remained relatively unaffected. Of particular relevance to MDD treatments, systemic ketamine ameliorated area 25 overactivation-induced reductions in Pavlovian anticipatory positive arousal if administered systemically 24 h earlier. The selectivity of these findings was illustrated by the lack of such effects following activation or inactivation of area 32; interestingly, a similar blunting of anticipatory but not consummatory arousal was also seen following over-activation of neighboring area 14 within primate medial OFC/caudal vmPFC [142]. However, only inactivation of area 14 (and not area 25) induced marked increases (Fig. 7) in cardiovascular and behavioral arousal. Thus, whereas area 14 appears to play a fundamental role in regulating such appetitive arousal, area 25 seems to have its effects only when activated; artificially, in the case of the experimental study in marmosets and perhaps under conditions of stress under naturally-occurring conditions. Consistent with the latter is the finding that area 25 activity positively correlates with cortisol levels during anxiety-provoking contexts in macaques [143].
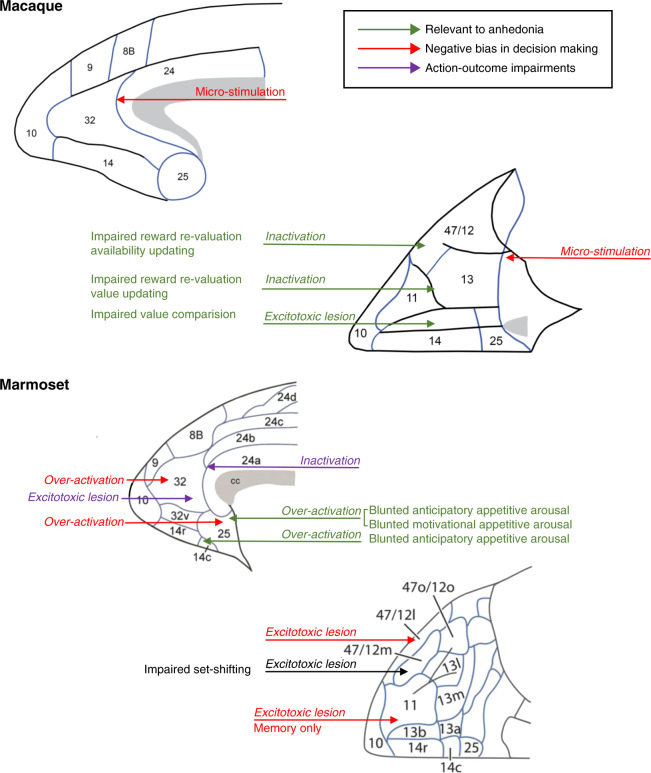
Fig. 7. Schematic of medial and orbital views of macaque and marmoset prefrontal and anterior cingulate cortex illustrating regions in which reductions or increases in activity induce behavioral deficits of relevance to symptoms of anhedonia, negative bias and despair/helplessness in MDD.
Relevant to anhedonia: Areas 47/12, 11/13, and 14 in macaques [115]; Areas 25 [61] and 14 [142] in marmosets; Negative bias in decision making: Area 32/24 border [39] and caudal OFC [202] in macaques; Areas 32 and 25 [41] and areas 47/12 and 11 [38] in marmosets; Action-outcome impairments: Areas 24 [206] and 32 [204] in marmosets; Attentional set-shifting: Area 47/12 in marmosets [207].
These blunted appetitive responses following area 25 over-activation appear comparable to reductions in reward responsivity associated with excitation of the putative homolog of area 25, namely ILc in rats, including increased threshold for intracranial stimulation [140] as well as reduced firing of DA neurons [139] and blocked DA-induced place preference [62]. The precise role of area 25 in reward processing, however, has yet to be fully determined. Its over-activation not only blunted appetitive processing but also enhanced responsivity to both uncertain threat and Pavlovian conditioned, certain threat [144], consistent with the high comorbidity of anxiety with MDD [145, 146]; but inconsistent with the role of ILc in inhibiting conditioned fear responses in rats [147]. (See [9] for a detailed discussion of the opposing effects in rats and marmosets on extinction of Pavlovian conditioned threat). It also reduced basal heart rate variability and increased sympathetic activity [144], consistent with sympathetically-driven alterations in cardiovascular activity in MDD [148, 149]. Thus, overall, this region in a primate may have a primary role in generating/maintaining negative affect which acts to dampen responsivity to reward.
In contrast to the relative paucity of information regarding mPFC and reward processing, the specializations for reward-guided learning and decision making within distinct regions of the OFC have been extensively studied and recently reviewed in [115, 150] and discussed in Rudebeck & Izquierdo (this volume). In summary, detailed parcellation studies in macaques have shown the importance of central regions of OFC in updating the valuations of expected outcomes on the basis of an animal’s current state (area 13) and translating that information into goals for action selection (area 11). Conversely, area 14 in the medial OFC/caudal vmPFC appears more important for comparing values of alternative options. It has been proposed that together these contribute to decisions based on desirability whilst the lateral OFC, extending onto the lateral surface of the vlPFC appears important for determining outcome availability. An alternative view for the lateral OFC, proposed by Rolls, stresses instead its importance for processing of non-reward [151]. The distinctions between lateral, central and medial OFC (summarized in Fig. 7) are particularly relevant to our understanding of depression since MDD appears primarily associated with reduced activity in central OFC but increased activity in lateral OFC and also in the caudal aspects of medial OFC, including area 14 (see above).
Negative processing biases
Major depressive disorder
In the following sections, the neuroimaging literature highlighting the role of several PFC regions in negative processing biases will be summarized. Although several studies have investigated negative biases in self-referential processing in MDD (e.g., [152–155]; Fig. 4e), these findings are not discussed due to our focus on cross-species integration. Treatment effects as well as evaluation of state vs. trait effects will be emphasized.
Negative processing biases: attention, perception and emotional processing
Studies using backward masking tasks or tasks requiring implicit vs. explicit emotional processing converge in implicating hypo-activation in various PFC regions, most prominently the dlPFC and dACC, as well as hyper-activation in sgACC, pgACC, and vlPFC regions in negative processing biases (for reviews, see [156–158]).
Relative to healthy controls, individuals with MDD showed reduced dlPFC activation during tasks involving matching negative facial expressions [159], presenting negative stimuli [156, 160], requiring to ignore negative faces [161], or inducing emotional interference [162]. In an emotional face-matching paradigm, currently depressed adults (as well as unaffected first-degree relatives of individuals with MDD) showed reduced dlPFC activation to negative faces compared to controls without familial risk [163]. Additionally, current MDD – but not familial MDD risk – was associated with potentiated medial OFC activation to negative faces. Among adolescents, blunted dlPFC to negative social status words mediated the relationship between social risk (peer victimization and fear of negative evaluation) and depressive symptoms [164]. Using a region-of-interest approach targeting the central OFC, Frodl et al. found that, relative to controls, unmedicated individuals with MDD showed decreased FC between the OFC and dACC [165] during implicit and explicit emotional tasks but increased connectivity between the OFC and various PFC regions (including the right dlPFC). Based on prior literature, reduced coupling between the central OFC and dACC might index impaired cognitive control of emotion processing, which could result in negative processing biases. These findings were largely confirmed in a meta-analysis of fMRI studies (N = 44) probing emotional processing, which found that MDD was characterized by reduced activation in the left dlPFC in response to negative stimuli [166].
Of note, deficient recruitment of PFC (particularly, dlPFC) regions has been coupled with hyperactivity in paralimbic (e.g., pgACC, sgACC) and limbic (e.g., amygdala) regions critically implicated in automatic processing of emotional cues (for review, see [16, 157]). Thus, blunted dlPFC activation has been accompanied by exaggerated amygdalar activation when (1) viewing and reappraising negative pictures [167], (2) processing subliminal negative stimuli [168, 169] (Fig. 4d) and negative words [170], (3) ignoring negative distractors [161], and (4) receiving negative feedback [171] and criticisms [172]. Highlighting an early and persistent course, amygdalar hyperactivity to negative stimuli has been reported in depressed children as young as 4–6 years old [173], persisted among adolescents with MDD [174], and emerged in fully remitted adults with past MDD [168]. Further highlighting insufficient cortical regulation, MDD has been linked to reduced cortico-limbic functional connectivity (e.g., mPFC↔amygdala) when presented with negative cues [175–178]. Critically, these patterns were linked to greater depression severity and a longer disease [175, 176, 178], highlighting possible exacerbations.
Similarly, hyperactivation of, or a failure to deactivate, the pgACC has been reported in MDD across disparate affective tasks [16, 179], including (1) affective evaluation and cognitive reappraisal of negative stimuli; (2) self-referential processing of negative attributes; (3) inhibition of negative words; and in response to (4) sad words and commission errors. Greater pgACC (and amygdalar) activation in response to negative stimuli, coupled with reduced activation in bilateral inferior frontal regions, emerged also among women with subthreshold depressive symptoms [180], suggesting that such abnormalities precede the onset of MDD. Among MDD subjects, a failure to deactivate the pgACC (and adjacent dmPFC) during cognitive or emotional processing tasks correlated with more depression severity and feelings of hopelessness [181, 182]. Critically, whereas 8 weeks of SSRI treatment normalized dlPFC (i.e., increased) and amygdalar (i.e., decreased) activation in trials requiring to ignore sad faces, sgACC hyperactivity and dACC (as well as pgACC) hypoactivity persisted after treatment response [183]. Findings of potentiated pgACC activation to negative cues in MDD are intriguing in light of evidence that, among healthy controls, a negativity bias correlated with pgACC activation for “sad” vs. “happy” choices in response to ambiguous faces [184].
Negative processing biases: state vs. trait?
Relative to control children (8–14 years), unaffected high-risk children (due to parental depression) showed higher responses to fearful vs. neutral faces in the dlPFC (BA10/46) and amygdala [185]. Whereas amygdalar hyper-activation to negative faces was reported in a similar offspring sample (mean age: 14 years) [186], the PFC findings contrast with reports of blunted dlPFC activation (and normative amygdalar responses) to fearful faces [187] and a sad mood induction [188] among asymptomatic offspring of MDD patients (16–21 years old and 9–14 years old, respectively). Similarly, in an emotional face-matching paradigm, unaffected first-degree relatives of acutely depressed patients with MDD (as well as individuals with current MDD) had reduced dlPFC activation in response to negative faces compared to healthy controls without familial risk [163].
Besides regional effects, evidence of network dysfunction linked to risk for MDD has emerged. Reduced FC between dmPFC regions (superior (BA6) and medial (BA10) frontal gyrus) and the amygdala was observed in healthy first-degree relatives of MDD patients during an implicit emotional processing task involving presentation of negative faces (Fig. 4f) [189]. Notably, relative to young adults without a family history of MDD, the high-risk group was also characterized by increased task-related FC between vmPFC regions (sgACC (BA25) and pgACC (BA24/32)) and the amygdala [189]. Importantly, both patterns – reduced dmPFC-amygdala FC but potentiated vmPFC-amygdala FC to negative faces – have been reported in adults with current MDD [176, 190, 191]. Collectively, these findings highlight that MDD and vulnerability to depression in youth samples are characterized by (1) dlPFC hypo-activation to emotional (mostly, negative) stimuli, (2) hyper-activity in limbic regions implicated in responding to emotional cues (pgACC, amygdala), (3) increased coupling between PFC regions implicated in processing negative self-referential information (vmPFC) and the amygdala, and (4) blunted coupling between PFC regions implicated in cognitive control of emotion (dmPFC) and the amygdala.
Interestingly, studies in middle-aged twins of individuals with MDD yielded different patterns. For example, in an fMRI study comparing never-depressed dizygotic twins with vs. without co-twin with a history of MDD, high-risk twins were characterized by negative functional connectivity between BA10/BA24 and the amygdala [192]. Although these findings appear counterintuitive, it is important to emphasize that the sample was, on average, 48–50 years old (i.e., well beyond the most vulnerable period for MDD onset). Thus, although speculative, these findings suggest that negative PFC-limbic coupling in response to emotional cues might represent a potential protective factor (potentiated top-downregulation of negative emotional processing) against the emergence of MDD in spite of familial MDD (see also [193]).
Negative processing bias: treatment effects
Growing evidence indicates that PFC abnormalities (as well as corticolimbic coupling) associated with negative processing biases are ameliorated by antidepressant treatments. For example, an early study reported that SSRI treatment (sertraline) reduced activation in the pgACC (BA24/32) (as well as left medial OFC and left vlPFC) in response to masked sad vs. happy faces [169]. Of note, such change was driven by a reduction in pgACC activation to sad faces and increased activation to happy faces, suggesting that SSRI treatment reduced negative processing biases. These findings were complemented by data showing that 8 weeks of sertraline treatment normalized amygdalar hyper-activation to sad faces in MDD [194], replicating prior findings [195, 196]. In line with the hypothesis that antidepressant medications might exert their effects by normalizing negative emotional biases before changes in symptoms, eventual week 6 responders showed greater reduction in neural responses to fearful vs. happy faces after 7 days of escitalopram treatment in the pgACC, dACC and amygdala [197].
A recent meta-analysis including 60 studies further clarified the role of PFC regions in treatment-related effects [198]. Thus, antidepressant treatment (mostly SSRI and SNRI) was associated with decreased activation to negative stimuli or emotions in the middle frontal gyrus (BA10 and pgACC) (as well as limbic regions, including the amygdala) but increased activation in response to positive stimuli or emotions in the rostral vmPFC (BA10), dlPFC (BA9) and pgACC (BA32) (as well as the amygdala). In addition, antidepressant treatments increased dlPFC (BA9) activation during both positive and negative emotions. Collectively, these findings suggest that, in MDD, pharmacological antidepressant treatments normalize brain activation in regions implicated in mood-congruent processing biases (vmPFC, pgACC, mPFC) as well as those implicated in emotion regulation and cognitive control (dlPFC).
Findings related to first-line treatments (e.g., SSRI) have been complemented by recent evidence from ketamine studies. Specifically, ketamine infusion reduced (1) hyperactivation in the sgACC (BA25) to positive feedback [121], (2) FC between the pgACC and dACC [199], and (3) FC between the sgACC and limbic regions (e.g., hippocampus) [121]. Critically, increased pre-treatment FC between the sgACC and amygdala predicted non-response to ketamine [200] and reduced dACC-pgACC FC correlated with a reduction in suicidal ideation [199].
Interim summary
Imaging studies have clarified that negative processing bias are accompanied by hyper-activation of various limbic/paralimbic regions, most prominently the pgACC and sgACC (as well as the amygdala) (Fig. 6b). Such hyper-responses are coupled with deficits in recruiting regions critically implicated in cognitive control and emotional regulation, such as the dlPFC and dACC, and are also reflected in disrupted coupling between PFC regions implicated in (1) cognitive control of emotion (dmPFC) and the amygdala (reduced coupling) and (2) processing negative self-referential information (vmPFC) and the amygdala (increased coupling). These findings are important since, among healthy controls, appraisal of negative vs. positive stimuli elicits stronger functional coupling between the amygdala and both the dACC and dlPFC [201]. Additionally, in individuals without MDD, increased top-down attentional control over emotional stimuli was associated with dlPFC activation as well as increased connectivity between dlPFC and dACC. Similarly, in healthy controls, resolution of emotional conflict has been linked to increased dACC and potentiated functional connectivity between the dACC and both the dlPFC and amygdala. Thus, MDD has been associated with multiple PFC abnormalities that can give rise and maintain negative processing biases. Critically, many of these abnormalities precede the onset of MDD, since they are present in never-depressed youth with increased MDD vulnerability. Finally, treatment studies have generally shown that successful antidepressant treatment normalizes brain activation in regions implicated in mood-congruent negative processing biases (sgACC, pgACC, dmPFC) as well as those implicated in emotion regulation and cognitive control (dlPFC).
Preclinical studies in animals
A somewhat different set of behavioral tests have been used to study negative biases in animals. In rodents, negative biases in the context of the PFC have received little attention despite their presence in chronically stressed animals. However, one study employing acute restraint stress to induce a negative bias revealed that the effectiveness of systemic ketamine to reduce such a bias in the ambiguous cue test was dependent upon the mPFC [45] and that similar bias reductions followed ketamine infusions directly into the mPFC (although PLc or ILc were not distinguished). A greater understanding of the network of PFC regions contributing to negative biases has emerged from studies in monkeys employing the approach-avoidance decision-making task; in particular, altered activity in the PFC of both macaques and marmosets was found to induce negative biases akin to those reported in depression (summarized in Fig. 7). Thus, over-activation of area 25 in marmosets enhanced the avoidance of punishment [41], alongside the already described blunting of anticipatory and motivational appetitive arousal and increased responsivity to uncertain threat and Pavlovian conditioned, certain threat. This effect was attributed to a likely overall enhancement of threat reactivity, consistent with increased activation in this region associated with enhanced negative reactivity in MDD [157, 183]. In contrast, over-activation of area 32 reduced the bias away from punishment, whilst area 32 inactivation had no effect, altogether suggesting that area 32 is engaged and only contributes to approach-avoidance conflict under particular contexts. A region bordering area 32 within the pgACC of macaques [39] has also been implicated in negative bias in decision making using a task subsequently adapted to assess negative biases in MDD [42]. In macaques, a cluster of neurons was identified that displayed activity in the decision period, which was negatively correlated with the overall value; notably, when microstimulated, these neurons enhanced the avoidance response and this effect was ameliorated by the anxiolytic, diazepam. Indeed, stimulation of the striosomes of the striatum to which this region projects also induces a negative bias [202] suggesting the importance of this frontostriatal circuit in the generation of negative biases. Using an analogous task in rats, a similar pathway projecting from PLc to the striatal striosomes has been implicated in approach-avoidance decision making, with its inactivation increasing approach [203].
Both area 32 and area 24 within pgACC have been implicated in goal-directed actions. Excitotoxic lesion studies of area 32 in marmosets [204], similar to lesions of PLc in rodents [205], have implicated this region in the learning, but not expression of action-outcome associations in the appetitive domain, particularly with respect to the contingent relationship between actions and their outcomes. More recently, area 24 and projection target region in the caudate nucleus in marmosets have also been implicated in the detection/expression of changes in action-outcome contingencies for reward [206]. If these same regions also process action-outcome information in the punishment domain as suggested by [39], at least for pgACC, then altered action-outcome learning and expression could likely play an important role in modulating one’s actions in response to varying reward and punishment in an approach-avoidance task, and hence underlie the reported negative biases. Indeed, it should be noted that impaired action-outcome learning and expression was disrupted by chronic stress [93]. We shall see below that it has also been suggested to contribute to aspects of learned helplessness [59].
Such hypothesis is unlikely to explain the negative biases that have also been reported following manipulations in the central OFC and vlPFC. Akin to microstimulation in pgACC, microstimulation of the caudal OFC in macaques also induces a negative bias [202]. Since this region not only has projections into pgACC but also has marked input onto the striosomes of the striatum, it has been proposed to form an integrated network involved in modulating decision making under conflict; nevertheless, its precise contribution has yet to be determined. Inactivation of more rostral regions of the central OFC (primarily area 11) and vlPFC in marmosets also induce a negative bias in decision making [38], although likely the result of distinct underlying psychological mechanisms. Specifically, vlPFC, like area 25, contributes to decision making when it is based upon experiencing rewarding and punishing outcomes at the time of one’s actions, the only difference being that negative biases are induced by a loss of activity in vlPFC but over-activity in area 25. Conversely, inactivation of area 11 only induces negative biases based on the memory of those experiences, memories dependent on the OFC’s interaction with the amygdala and anterior hippocampus [38]. Thus, it has been hypothesized that the negative bias following OFC inactivation may be due to the loss of the ability to update the value of expected rewarding and punishing outcomes [115], likely leading to uncertainty and thus anxiety-induced negative bias. In contrast, the attentional inflexibility induced by excitotoxic lesions of vlPFC [207] may lead to persistent attention to intrinsically salient negative outcomes that, through bottom up mechanisms, naturally attract our attention, but through a loss of top down attentional control cause increased negative bias. The contribution of cognitive dysfunction will be addressed in more detail below.
Learned helplessness
Major depressive disorder
Few studies have investigated the neural correlates of learned helplessness (and the associated construct of controllability) and despair in MDD. Using an electrophysiological marker hypothesized to reflect a PFC-based evaluation of stressor uncontrollability (post-imperative negative variation (PINV)), Diener et al. reported that, relative to controls, unmedicated individuals with MDD had potentiated frontal PINV during settings in which control over an aversive outcome was removed and then restored [208]. Additionally, higher levels of perceived helplessness and PINV magnitude correlated with rumination. Although the precise cortical sources of these effects are unclear, these data suggest that individuals with MDD are more susceptible to stressor uncontrollability, and such experience affects subsequent interaction with the environment even when control over the stressor is re-established. In a study using graph analyses, rsFC strength within a module involving several PFC regions (e.g., dorsal and medial superior frontal gyrus; OFC; middle frontal gyrus, anterior cingulum gyrus) correlated positively with self-reported helplessness in MDD [209]. Finally, in a task that manipulated the perception of control over monetary rewards or losses, unlike controls, depressed individuals failed to show greater striatal activation to self-attributed vs. externally attributed monetary gains [210].
Although not focused on patients, additional studies have yielded important insights into PFC regions (and corticostriatal pathways) that might mediate the emergence of despair and learned helplessness in MDD. First, fitting the notion that being able to exert control over outcome is beneficial [211], studies in healthy controls have shown that personally chosen vs. passively received rewards elicit stronger activation in the striatum (ventral and dorsal) and PFC (inferior frontal gyrus) [212–215], which is inversely related to depressive symptoms [212]. Notably, perception of control increased the reward value of the chosen option by 30% and caudal vmPFC activation was linked to such value inflation [215]. Second, among healthy controls, subjective (but not objective) uncontrollability over an aversive outcome reduced FC between dlPFC and the parahippocampal gyrus (and more generally, between the frontoparietal executive and salience networks) and impaired WM performance [216]. Third, probing individual differences in how perceived controllability might modulate pain perception, it was found that pgACC activation in response to noxious stimulation tracked whether uncontrollable pain was experienced as more aversive than controllable pain [217]. Conversely, greater vlPFC activation during the anticipation of uncontrollable pain correlated with lower pain ratings.
Altogether, these findings point to an important role of PFC regions in perception of controllability and the impact this has on the valence of the event, which moderates the depressogenic effects of stressors [3]. Owing to this evidence, one could expect that the opposite scenario – increased controllability and expectancy of a positive outcome – would engage similar PFC-based networks. Consistent with this conjecture, neuroimaging studies of placebo analgesia have revealed that dlPFC, vlPFC, rostral vmPFC (as well as pgACC) and central OFC show the most consistent placebo-related activation increases in response to pain. In particular, dlPFC regions appear especially important for exerting top-downregulation of treatment and placebo expectancies, whereas mPFC regions play a key role in encoding value of expected outcomes [218, 219]. Further highlighting a central role of the dlPFC in these processes, transient dlPFC inhibition (via TMS) reduced placebo analgesia [220].
Preclinical animal studies
In contrast to studies in MDD, purported despair-like behaviors have been one of the major focuses in depression-related studies in rodents, although as discussed above, decreases and increases in active responding on FST and TST more likely reflect, respectively, shifts in active to passive coping, and vice versa, rather than being related to chronic despair or learned helplessness per se. Findings from studies using either chronic stress models or healthy animals combined with PFC and OFC interventions are summarized in detail in Figs. 2 and 3. Very often, studies in the mPFC have not discriminated between PLc and ILc, although all have implicated the region as a whole in the regulation of active and passive coping strategies [221–225]. However, where these regions have been discriminated, regardless of the model, PLc tends to promote active coping [226, 227] whilst ILc appears to have the opposite effect, promoting passive coping [228]. However, as seen for reward processing (described above) there appear to be opposing acute and chronic effects within ILc, with prolonged ILc pyramidal stimulation for 60 min, mirroring the actions of ketamine to promote active coping on FST [63]. Conversely, neuronal silencing of IL (and not PL) blocks the actions of ketamine, 24 h later [63], suggesting that the delayed actions of ketamine are dependent upon an intact IL at the time of the ketamine infusion.
Altered performance on FST and TST are also associated with abnormal activity within the OFC including promotion of passive coping behavior following inhibition of histone acetylation in ventrolateral regions [229], activation of the OFC-amygdala pathway [230] and weekly infusions of a 5-HT2a agonist into lateral OFC [141]. In contrast, continuous inactivation of OFC for two weeks using muscimol [231] increased active coping. In the absence, however, of a greater understanding of the different interventions on overall activity in the OFC, its precise role, alongside the PLc and ILc, remains unclear. What is clear, however, is that in all studies described so far interventions in PLc, ILc and OFC can all impact on responsivity to acute, uncontrollable stress as measured by the FST and TST.
The involvement of both ILc and PLc in the regulation of behavioral responses to stress is also seen in the immunization effect of learned control over a stressor, arguably more relevant to our understanding of MDD. This effect of controllability is dependent upon the mPFC [232], in particular the PLc, since PLc inactivation during exposure to controllable stress, disrupts subsequent behavioral immunization effects across a range of contexts, such that the animal acts as if the stress had been inescapable or uncontrollable. In contrast, the effects of ILc inactivation appear more restricted to social behavior [233]. Moreover, only neurons in the PLc (but not ILc) projecting to the dorsal raphe nucleus show selective activation to escapable stress [234] and thus are in a position to inhibit the prolonged release of serotonin associated with uncontrollability. This contribution of PLc to controllability fits with its involvement in action-outcome learning, albeit only reported so far for the appetitive domain [205]. Together, these findings are consistent with the hypothesis that PLc actively protects behavior from the deleterious consequences of such stress.
Integrating the MDD and preclinical animal studies, it is apparent that a number of regions within the mPFC may contribute either directly in the learning and maintenance of action-outcome associations that underlie the ability of humans and other animals to control their environment or indirectly through, for example, the updating of reward value, as a consequence of the level of controllability available to them [215]. Regions of area 32 and area 24 appear directly involved whilst medial OFC/vmPFC may be more related to the valuation process. Different again may be the contribution of regions within central OFC, with their links to the updating of predictive stimuli which may be involved in the mechanisms by which predictive stimuli can influence goal-directed actions, known as Pavlovian-to-Instrumental transfer [206, 235]. Disruption of any of these mechanisms could distort the perception of controllability apparent in MDD.
PFC and executive dysfunction
Major depressive disorder
The role of PFC abnormalities in executive dysfunction in MDD has been reviewed [16, 236]. Thus, the following sections provide updates and a synopsis of the most widely replicated findings. Studies using various executive tasks (e.g., Stroop, Flanker, N-Back) have highlighted reduced activation in dACC and dlPFC regions in experimental settings in which individuals with MDD show impaired behavioral performance. Interestingly, in studies in which MDD subjects performed at the same level as controls, MDD has been linked to potentiated pgACC, dACC and left dlPFC [16, 237, 238] and pgACC hyperactivation persisted after remission [239]. These findings point to cortical (PFC) inefficiency in MDD as well as a reduced ability to deactivate the pgACC (and DMN more generally) and recruit dlPFC-based cognitive control regions as task demands increase [16, 240–243] (Fig. 4g, h). Similarly, MDD was associated with reduced recruitment of the dlPFC [160, 161, 170, 244–246] and dACC [160, 161, 246] in contexts requiring task-irrelevant negative cues to be ignored or behavioral adjustments after errors.
Additional evidence that recovery from depression is linked to an ability to deactivate the pgACC (and DMN) during cognitive tasks emerges from recent studies. Among medication-free patients with MDD, greater deactivation of an anteromedial PFC (amPFC) region encompassing the pgACC and mPFC (BA10) during a WM task predicted greater symptom reduction with escitalopram (and greater WM improvements) [247]. Moreover, stronger pre-treatment dlPFC activation – coupled with weaker dlPFC-amPFC functional connectivity – predicted depression recovery. Notably, a reduced ability to deactivate the amPFC along with increased dlPFC-amPFC functional connectivity during the same WM task characterized remitted individuals with past history of MDD. Moreover, reduced amPFC deactivation was associated with more rumination [248]. Finally, greater DMN (specifically, dmPFC) deactivation during an emotional WM task 24 h before ketamine infusion predicted greater reduction of depressive (especially, cognitive) symptoms in TRD patients [249]. Together, these findings indicate that the ability to deactivate key nodes within the DMN (pgACC, dmPFC) during an executive task is essential for symptom reduction and that reduced ability to do so might represent a marker of increased MDD vulnerability and a more ruminative style.
Preclinical animal studies
Cognitive dysfunction has received less attention in depression-related animal studies although, as described above, response and attentional inflexibility [95], spatial WM disruption [96] and insensitivity of goal-directed actions to action-outcome contingency and outcome value [93] have been induced by chronic stress exposure. Importantly, cognitive dysfunction is likely to contribute to the deficits seen in the other domains, including despair, negative bias and blunted reward processing. We have already highlighted how attentional inflexibility, dependent upon vlPFC [207] could lead to difficulty disengaging from threat-related stimuli and a resulting negative bias in monkeys [9] including a disruption in approach-avoidance decision making. Similarly, a failure to detect changes in the contingencies between one’s actions and their outcome (associated with disrupted function in both areas 32 and 24 in monkeys [206] and PLc in rodents [205, 250]) could contribute not only to negative biases in approach-avoidance decision making but also despair/helplessness [232]. Clearly, disrupted WM, associated with lateral prefrontal regions may also contribute to impaired decision making (see Menon and D’Esposito, Shenhav and Collins, this volume) and certainly dysfunction in these higher-order cognitive regions have been implicated in cognitive re-appraisal of emotion [251, 252] although studies have generally shown no or few differences between MDD and healthy groups in emotion regulation tasks that provided clear cognitive reappraisal instructions [253, 254].
Where cognitive dysfunction has been studied in the context of models of chronic stress it has given us the clearest indication of the separable contributions of distinct PFC regions to different behaviors associated with chronic stress. Originally identified in primates [207], different forms of cognitive inflexibility are associated with dysfunction within distinct PFC regions. Subsequent studies in rats revealed a similar dissociation, whereby the OFC contributed specifically to lower-order reversal learning of stimulus-reward associations whilst the mPFC (as opposed to the vlPFC in marmosets) contributed selectively to higher-order attentional set-shifting [255, 256]. Both forms of inflexibility have been reported following chronic stress but only stress-induced attentional set-shifting deficits were prevented by localized infusions of a cocktail of alpha 1, beta 1 and beta 2 adrenergic receptor agonists into the mPFC before each stress session of the chronic treatment period [257], whilst stress-induced reversal learning deficits remain. Conversely, the latter was prevented by LTD in the mediodorsal thalamo-orbitofrontal pathway [258] and locally infused ketamine [259]. These differential effects contrast with those described for despair, blunting of appetitive consummatory responses and social interaction, associated with changes in both mPFC and OFC (see Figs. 2 and 3), thereby supporting the hypothesis that the behaviors measured by these tests are multi-determined, dependent upon a variety of functions including those associated with PLc, ILc and OFC. Negative biases are also multi-determined contributed to by altered activity in primate areas 24, 32, OFC and vlPFC.
Conclusions, future directions and clinical implications
A vast literature has strongly implicated PFC dysfunction in MDD, revealing functional, structural, and systems-level abnormalities that span many PFC regions. By focusing on intermediate phenotypes that map onto neurobiological abnormalities more lawfully than the syndrome-based conceptualizations of “MDD,” PFC abnormalities in MDD show opposite patterns depending on the functional domains under investigation. Thus, although blunted pgACC activation in MDD has emerged from studies probing reward-related processes (e.g., reward anticipation, consumption and learning), potentiated pgACC (or a general inability to deactivate the pgACC) in MDD has been observed in studies assessing negative processing biases and cognitive control. Similarly, although the dlPFC has been found to be over-activated in reward-related processes (likely to compensate for hypoactive striatal responses to reward cues), the same region has shown hypo-activation in affective and cognitive tasks requiring emotional or stress regulation, cognitive control, and/or shifting attention to external task demands. Although the neurobiological mechanisms underlying context-dependent direction of abnormalities remain to be elucidated, it is likely that different inputs to PFC regions (e.g., weakened input from reward-related regions vs. heightened input from threat-related regions) as well as different neuromodulators (e.g., dopamine vs. serotonin) are implicated. A clear need for future research will be to evaluate such context-dependent patterns in the same sample by using tasks probing different RDoC domains. Collectively, this literature highlights disrupted frontostriatal, frontolimbic, and cortico-cortical disruptions in MDD that appear to be critically implicated in the emergence and maintenance of anhedonic phenotypes, negative processing biases, and learned helplessness (despair). These phenotypes are not independent, with reductions in sensitivity to reward and loss of subjective control over negative events, for example, likely to induce negative processing biases. Thus, ultimately the individual variation in MDD may arise from the interaction between overlapping dysfunctional domains.
Notably, several of these disruptions have emerged also in never-depressed, symptom-free, first-degree relatives of individuals with MDD, suggesting that they represent markers of vulnerability. Specifically, increased vulnerability to depression (as well as MDD) has been linked to (1) blunted striatal activation and frontostriatal coupling during reward tasks; (2) dlPFC hypo-activation to negative cues; (3) hyper-activity in limbic regions implicated in responding to emotional cues (pgACC, sgACC, amygdala); (4) increased coupling between PFC regions implicated in processing negative self-referential information (vmPFC) and the amygdala; and (5) reduced coupling between PFC regions implicated in cognitive control of emotion (dmPFC/dACC) and the amygdala. This information is important because it highlights possible targets for prevention/intervention, which could involve mindfulness-based psychotherapy [260], neurofeedback targeting the amygdala or dlPFC [261, 262], or neurostimulation [263]. An important area of further inquiry will be to determine the specificity of the presented findings vis-à-vis MDD, especially since psychiatric comorbidity is the rule in MDD. Although human neuroimaging studies typically report the degree of comorbidity, few formally test whether findings are truly specific to MDD. An important exception is the recent study by Pan and colleagues [112], who found that resting state functional connectivity within the brain reward pathway predicted the new onset of depressive disorder – but not ADHD, anxiety or substance abuse – 3 years later.
In parallel, results from chronic stress models and interventions in otherwise healthy animals provide causal evidence for the induction of these intermediate phenotypes of MDD by dysregulation in the PFC, including the OFC and ACC. Whilst the former have provided limited insight into the marked heterogeneity in MDD and importantly the marked individual variation in treatment efficacy, there is some evidence from the latter (e.g., functional differences between interventions in NHP areas 25, 14, 11 and 12 and differential effects of ketamine on area 25-induced anxiety and anhedonia-like marmoset behavior). We propose that, in part, variation in etiology and treatment response may come from differences in the form that prefrontal dysregulation takes in individuals e.g., over-activation in sgACC and/or lateral OFC and under-activation of central OFC and/or dlPFC. These regions do not only have distinct functions but are also modulated by distinct neurochemical transmitter systems, acting through distinct receptor subtypes, associated with distinct cellular and molecular pathways (see Arnsten and Cools, this volume). Thus, their differential dysregulation may have a marked impact on inter-individual differences in sensitivity to pharmacotherapies making our ability to distinguish between them, essential. New directions should include (i) viral-mediated strategies that induce more chronic changes in selected MDD-related prefrontal circuitry, more akin to that reported in MDD, e.g., chronic activation of area 25, (ii) use of e.g., calcium imaging to monitor chronic phenotypes longitudinally in living brain tissue [264] and where possible combined with (iii) animal MRI [265] and PET [142, 144] to reveal whole-brain activity changes related to experimental interventions and facilitate human-animal comparisons.
Finally, we believe embracing a computational psychiatry approach – including the use of deep learning and artificial neural network to diagnose MDD [266, 267] and predict treatment response [268] – offers unprecedented opportunities, particularly when coupled with improved experimental design to isolate precise psychological constructs. Specifically, although many studies point to, for example, negative processing biases, “traditional” behavioral or neural variables typically fail to disentangle whether such biases are due to reduced reward sensitivity vs. increased avoidance, or both. Computational modeling allows such differentiation, including regressing the same computational parameters to, for example, fMRI activation in humans and neuronal recordings in non-human primates or rodents (e.g., [39, 42, 269]. We argue that, even if tasks across species are not identical due to practical limitations, showing that an intervention (e.g., a given pharmacological manipulation) affects a computational process in similar ways across species greatly reduces the translational divide (for recent examples, see [29, 270–272]). Eventually, we believe these approaches and those highlighted in the current review will help reduce the translational divide and accelerate the development of much-needed novel treatments for depression.
Acknowledgments
Funding and Disclosure
DAP was partially supported by R37 MH068376, R01 MH108602, P50 MH119467 and R01 MH095809. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. ACR was supported by a MRC Programme grant (M023990/1) and WT Investigator award (108089/Z/15/Z). The funding organizations had no role in the preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Over the past 3 years, DAP has received consulting fees from Albright Stonebridge Group, BlackThorn Therapeutics, Boehringer Ingelheim, Compass Pathway, Concert Pharmaceuticals, Engrail Therapeutics, Neurocrine Biosciences, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals; one honorarium from Alkermes, and research funding from NIMH, Dana Foundation, Brain and Behavior Research Foundation, and Millennium Pharmaceuticals. In addition, he has received stock options from BlackThorn Therapeutics. ACR within the last 3 years undertook a neuropharmacological study funded by Shionogi Inc. There are no conflicts of interest with the work conducted in this study. All views expressed are solely those of the authors.
Author contributions
The authors made equal contributions to drafting and finalizing this review.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised due to wrong supplementary information.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Diego A. Pizzagalli, Angela C. Roberts.
Change history
8/19/2021
A Correction to this paper has been published: 10.1038/s41386-021-01160-w
Contributor Information
Diego A. Pizzagalli, Email: dap@mclean.harvard.edu
Angela C. Roberts, Email: acr4@cam.ac.uk
References
- 1.Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–79. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–8. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 3.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyman SE. Revolution stalled. Sci Transl Med. 2012;4:155cm11. doi: 10.1126/scitranslmed.3003142. [DOI] [PubMed] [Google Scholar]
- 5.Hyman SE. Can neuroscience be integrated into the DSM-V? Nat Rev Neurosci. 2007;8:725–32. doi: 10.1038/nrn2218. [DOI] [PubMed] [Google Scholar]
- 6.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–81. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 7.Der-Avakian A, Pizzagalli DA. Translational assessments of reward and anhedonia: a tribute to Athina Markou. Biol Psychiatry. 2018;83:932–9. doi: 10.1016/j.biopsych.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein BL, Klein DN. A review of selected candidate endophenotypes for depression. Clin Psychol Rev. 2014;34:417–27. doi: 10.1016/j.cpr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts A. Prefrontal regulation of threat-elicited behaviors: a pathway to translation. Annu Rev Psychol. 2020;71:357–87. doi: 10.1146/annurev-psych-010419-050905. [DOI] [PubMed] [Google Scholar]
- 10.Oikonomidis L, Santangelo AM, Shiba Y, Clarke FH, Robbins TW, Roberts AC. A dimensional approach to modeling symptoms of neuropsychiatric disorders in the marmoset monkey. Dev Neurobiol. 2017;77:328–53. doi: 10.1002/dneu.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 12.Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert programme reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogt BA, Paxinos G. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct Funct. 2014;219:185–92. doi: 10.1007/s00429-012-0493-3. [DOI] [PubMed] [Google Scholar]
- 15.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiser J, Koenigs M. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol Psychiatry. 2018;83:638–47. doi: 10.1016/j.biopsych.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts AC, Clarke HF. Why we need nonhuman primates to study the role of ventromedial prefrontal cortex in the regulation of threat- And reward-elicited responses. PNAS. 2019;116:26297–304. doi: 10.1073/pnas.1902288116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 20.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–55. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharm Sci. 2002;23:238–45. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 23.Katz RJ. Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharm Biochem Behav. 1982;16:965–8. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- 24.Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology. 1991;104:255–9. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- 25.Dichter GS, Smoski MJ, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar depression does not moderate responses to the Sweet Taste Test. Depress Anxiety. 2010;27:859–63. doi: 10.1002/da.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–9. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- 27.Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–27. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kangas BD, Wooldridge LM, Luc OT, Bergman J, Pizzagalli DA. Empirical validation of a touchscreen probabilistic reward task in rats. Transl Psychiatry. 2020;10:285. doi: 10.1038/s41398-020-00969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Der-Avakian A, D’Souza MSS, Pizzagalli DAA, Markou A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry. 2013;3:e297. doi: 10.1038/tp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009;4:e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–77. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- 33.Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37:117–36. doi: 10.1038/npp.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, et al. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol Med. 2003;33:455–67. doi: 10.1017/s0033291702007018. [DOI] [PubMed] [Google Scholar]
- 36.Rygula R, Clarke HF, Cardinal RN, Cockcroft GJ, Xia J, Dalley JW, et al. Role of central serotonin in anticipation of rewarding and punishing outcomes: effects of selective amygdala or orbitofrontal 5-HT depletion. Cereb Cortex. 2015;25:3064–76. doi: 10.1093/cercor/bhu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke HF, Cardinal RN, Rygula R, Hong YT, Fryer TD, Sawiak SJ, et al. Orbitofrontal dopamine depletion upregulates caudate dopamine and alters behavior via changes in reinforcement sensitivity. J Neurosci. 2014;34:7663–76. doi: 10.1523/JNEUROSCI.0718-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke HF, Horst NK, Roberts AC. Regional inactivations of primate ventral prefrontal cortex reveal two distinct mechanisms underlying negative bias in decision making. PNAS. 2015;112:4176–81. doi: 10.1073/pnas.1422440112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amemori K, Graybiel AM. Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nat Neurosci. 2012;15:776–85. doi: 10.1038/nn.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amemori KI, Amemori S, Graybiel AM. Motivation and affective judgments differentially recruit neurons in the primate dorsolateral prefrontal and anterior cingulate cortex. J Neurosci. 2015;35:1939–53. doi: 10.1523/JNEUROSCI.1731-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallis CU, Cockcroft GJ, Cardinal RN, Roberts AC, Clarke HF. Hippocampal interaction with area 25, but not area 32, regulates marmoset approach-avoidance behavior. Cereb Cortex. 2019;29:4818–30. doi: 10.1093/cercor/bhz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ironside M, Amemori KI, McGrath CL, Pedersen ML, Kang MS, Amemori S, et al. Approach-avoidance conflict in major depressive disorder: congruent neural findings in humans and nonhuman primates. Biol Psychiatry. 2020;87:399–408. doi: 10.1016/j.biopsych.2019.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harding EJ, Paul ES, Mendl M. Cognitive bias and affective state. Nature. 2004;427:312. doi: 10.1038/427312a. [DOI] [PubMed] [Google Scholar]
- 44.Papciak J, Popik P, Fuchs E, Rygula R. Chronic psychosocial stress makes rats more ‘pessimistic’ in the ambiguous-cue interpretation paradigm. Behav Brain Res. 2013;256:305–10. doi: 10.1016/j.bbr.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 45.Hales CA, Robinson ESJ, Houghton CJ. Diffusion modelling reveals the decision making processes underlying negative judgement bias in rats. PLoS One. 2016;11:e0152592. doi: 10.1371/journal.pone.0152592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aylward J, Hales C, Robinson E, Robinson OJ. Translating a rodent measure of negative bias into humans: the impact of induced anxiety and unmedicated mood and anxiety disorders. Psychol Med. 2019;50:237–46. doi: 10.1017/S0033291718004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark DA, Beck AT. Cognitive theory and therapy of anxiety and depression: convergence with neurobiological findings. Trends Cogn Sci. 2010;14:418–24. doi: 10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 49.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 51.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 52.Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2011;55:8.10A.1–8.10A.14. doi: 10.1002/0471142301.ns0810as55. [DOI] [PubMed] [Google Scholar]
- 53.De Kloet ER, Molendijk ML. Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast. 2016;2016:6503162. doi: 10.1155/2016/6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem Neurosci. 2017;8:955–60. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anyan J, Amir S. Too depressed to swim or too afraid to stop? A reinterpretation of the forced swim test as a measure of anxiety-like behavior. Neuropsychopharmacology. 2018;43:931–3. doi: 10.1038/npp.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seligman MEP. Depression and learned helplessness. In The psychology of depression: contemporary theory and research. New York: Winston-Wiley; 1974, p. 83–113.
- 57.Seligman ME, Beagley G. Learned helplessness in the rat. J Comp Physiol Psychol. 1975;88:534–41. doi: 10.1037/h0076430. [DOI] [PubMed] [Google Scholar]
- 58.Maier SF, Seligman MEP. Learned helplessness at fifty: Insights from neuroscience. Psychol Rev. 2016;123:1–19. doi: 10.1037/rev0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffiths KR, Morris RW, Balleine BW. Translational studies of goal-directed action as a framework for classifying deficits across psychiatric disorders. Front Syst Neurosci. 2014;8:101. doi: 10.3389/fnsys.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vollmayr B, Gass P. Learned helplessness: unique features and translational value of a cognitive depression model. Cell Tissue Res. 2013;354:171–8. doi: 10.1007/s00441-013-1654-2. [DOI] [PubMed] [Google Scholar]
- 61.Alexander L, Gaskin PLR, Sawiak SJ, Fryer TD, Hong YT, Cockcroft GJ, et al. Fractionating blunted reward processing characteristic of anhedonia by over-activating primate subgenual anterior cingulate cortex. Neuron. 2019;101:307–320.e6. doi: 10.1016/j.neuron.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351:aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ, et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci USA. 2015;112:8106–8111. doi: 10.1073/pnas.1414728112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Leary OF, Cryan JF. Towards translational rodent models of depression. Cell Tissue Res. 2013;354:141–53. doi: 10.1007/s00441-013-1587-9. [DOI] [PubMed] [Google Scholar]
- 65.Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 67.Gururajan A, Reif A, Cryan JF, Slattery DA. The future of rodent models in depression research. Nat Rev Neurosci. 2019;20:686–701. doi: 10.1038/s41583-019-0221-6. [DOI] [PubMed] [Google Scholar]
- 68.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–11. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Long Z, Du L, Zhao J, Wu S, Zheng Q, Lei X. Prediction on treatment improvement in depression with resting state connectivity: a coordinate-based meta-analysis. J Affect Disord. 2020;276:62–68. doi: 10.1016/j.jad.2020.06.072. [DOI] [PubMed] [Google Scholar]
- 70.Gong J, Wang J, Qiu S, Chen P, Luo Z, Wang J, et al. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: voxel-based meta-analysis. Transl Psychiatry. 2020;10:353. doi: 10.1038/s41398-020-01036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng W, Rolls ET, Qiu J, Liu W, Tang Y, Huang CC, et al. Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain. 2016;139:3296–309. doi: 10.1093/brain/aww255. [DOI] [PubMed] [Google Scholar]
- 72.Rolls ET. The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia. 2019;128:14–43. doi: 10.1016/j.neuropsychologia.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 73.Samara Z, Evers EAT, Peeters F, Uylings HBM, Rajkowska G, Ramaekers JG, et al. Orbital and medial prefrontal cortex functional connectivity of major depression vulnerability and disease. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:348–57. doi: 10.1016/j.bpsc.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh MK, Leslie SM, Packer MM, Weisman EF, Gotlib IH. Limbic intrinsic connectivity in depressed and high-risk youth. J Am Acad Child Adolesc Psychiatry. 2018;57:775–785.e3. doi: 10.1016/j.jaac.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu Q, Li H, Luo G, Wang Y, Tang H, Han L, et al. Impaired prefrontal-amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: a dynamic causal modeling study on MEG. Neurosci Lett. 2012;523:125–30. doi: 10.1016/j.neulet.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 76.Abdallah CG, Averill CL, Salas R, Averill LA, Baldwin PR, Krystal JH, et al. Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:566–74. doi: 10.1016/j.bpsc.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Argyelan M, Lencz T, Kaliora S, Sarpal DK, Weissman N, Kingsley PB, et al. Subgenual cingulate cortical activity predicts the efficacy of electroconvulsive therapy. Transl Psychiatry. 2016;6:e789. doi: 10.1038/tp.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gray JP, Müller VI, Eickhoff SB, Fox PT. Multimodal abnormalities of brain structure and function in major depressive disorder: a meta-analysis of neuroimaging studies. Am J Psychiatry. 2020;177:422–34. doi: 10.1176/appi.ajp.2019.19050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lai CH. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Res Neuroimaging. 2013;211:37–46. doi: 10.1016/j.pscychresns.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 81.Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–9. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frodl T, Jager M, Born C, Ritter S, Kraft E, Zetzsche T, et al. Anterior cingulate cortex does not differ between patients with major depression and healthy controls, but relatively large anterior cingulate cortex predicts a good clinical course. Psychiatry Res. 2008;163:76–83. doi: 10.1016/j.pscychresns.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 83.Serra-Blasco M, de Diego-Adeliño J, Vives-Gilabert Y, Trujols J, Puigdemont D, Carceller-Sindreu M, et al. Naturalistic course of major depressive disorder predicted by clinical and structural neuroimaging data: a 5-year follow-up. Depress Anxiety. 2016;33:1055–64. doi: 10.1002/da.22522. [DOI] [PubMed] [Google Scholar]
- 84.Ward J, Lyall LM, Bethlehem RAI, Ferguson A, Strawbridge RJ, Lyall DM, et al. Novel genome-wide associations for anhedonia, genetic correlation with psychiatric disorders, and polygenic association with brain structure. Transl Psychiatry. 2019;9:327. doi: 10.1038/s41398-019-0635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marrus N, Belden A, Nishino T, Handler T, Tilak Ratnanather J, Miller M, et al. Ventromedial prefrontal cortex thinning in preschool-onset depression. J Affect Disord. 2015;180:79–86. doi: 10.1016/j.jad.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–40. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Foland-Ross LC, Sacchet MD, Prasad G, Gilbert B, Thompson PM, Gotlib IH. Cortical thickness predicts the first onset of major depression in adolescence. Int J Dev Neurosci. 2015;46:125–31. doi: 10.1016/j.ijdevneu.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ge R, Downar J, Blumberger DM, Daskalakis ZJ, Lam RW, Vila-Rodriguez F. Structural network integrity of the central executive network is associated with the therapeutic effect of rTMS in treatment resistant depression. Prog Neuro-Psychopharmacology. Biol Psychiatry. 2019;92:217–25. doi: 10.1016/j.pnpbp.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 89.Pirnia T, Joshi SH, Leaver AM, Vasavada M, Njau S, Woods RP, et al. Electroconvulsive therapy and structural neuroplasticity in neocortical, limbic and paralimbic cortex. Transl Psychiatry. 2016;6:e832. doi: 10.1038/tp.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boes AD, Uitermarkt BD, Albazron FM, Lan MJ, Liston C, Pascual-Leone A, et al. Rostral anterior cingulate cortex is a structural correlate of repetitive TMS treatment response in depression. Brain Stimul. 2018;11:575–81. doi: 10.1016/j.brs.2018.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lan MJ, Chhetry BT, Liston C, Mann JJ, Dubin M. Transcranial magnetic stimulation of left dorsolateral prefrontal cortex induces brain morphological changes in regions associated with a treatment resistant major depressive episode: an exploratory analysis. Brain Stimul. 2016;9:577–83. doi: 10.1016/j.brs.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress. 2017;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–5. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 94.Lapiz-Bluhm MDS, Soto-Piña AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology. 2009;202:329–41. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–74. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–31. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 98.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang B, Lin P, Shi H, Öngür D, Auerbach RP, Wang X, et al. Mapping anhedonia-specific dysfunction in a transdiagnostic approach: an ALE meta-analysis. Brain Imaging Behav. 2016;10:920–39. doi: 10.1007/s11682-015-9457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry. 2018;175:1111–20. doi: 10.1176/appi.ajp.2018.17101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borsini A, Wallis ASJ, Zunszain P, Pariante CM, Kempton MJ. Characterizing anhedonia: a systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cogn Affect Behav Neurosci. 2020;20:816–41. doi: 10.3758/s13415-020-00804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Admon R, Pizzagalli DA. Dysfunctional reward processing in depression. Curr Opin Psychol. 2015;4:114–8. doi: 10.1016/j.copsyc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O’Callaghan G, Stringaris A. Reward processing in adolescent depression across neuroimaging modalities: a review. Z Kinder Jugendpsychiatr Psychother. 2019;47:535–41. doi: 10.1024/1422-4917/a000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rupprechter S, Stankevicius A, Huys QJM, Series P, Steele JD. Abnormal reward valuation and event-related connectivity in unmedicated major depressive disorder. Psychol Med. 2020;2020:7. doi: 10.1017/S0033291719003799. [DOI] [PubMed] [Google Scholar]
- 106.Young CB, Chen T, Nusslock R, Keller J, Schatzberg AF, Menon V. Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl Psychiatry. 2016;6:e810. doi: 10.1038/tp.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord. 2012;136:1126–34. doi: 10.1016/j.jad.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Admon R, Pizzagalli DA. Corticostriatal pathways contribute to the natural time course of positive mood. Nat Commun. 2015;6:10065. doi: 10.1038/ncomms10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Geugies H, Mocking RJT, Figueroa CA, Groot PFC, Marsman JBC, Servaas MN, et al. Impaired reward-related learning signals in remitted unmedicated patients with recurrent depression. Brain. 2019;142:2510–22. doi: 10.1093/brain/awz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rzepa E, Fisk J, McCabe C. Blunted neural response to anticipation, effort and consummation of reward and aversion in adolescents with depression symptomatology. J Psychopharmacol. 2017;31:303–11. doi: 10.1177/0269881116681416. [DOI] [PubMed] [Google Scholar]
- 111.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pan PM, Sato JR, Salum GA, Rohde LA, Gadelha A, Zugman A, et al. Ventral striatum functional connectivity as a predictor of adolescent depressive disorder in a longitudinal community-based sample. Am J Psychiatry. 2017;174:1112–9. doi: 10.1176/appi.ajp.2017.17040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151:531–9. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 114.Xie C, Jia T, Rolls ET, Robbins TW, Sahakian BJ, Zhang J, et al. Reward versus nonreward sensitivity of the medial versus lateral orbitofrontal cortex relates to the severity of depressive symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;6:259–69. doi: 10.1016/j.bpsc.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 115.Murray EA, Rudebeck PH. Specializations for reward-guided decision-making in the primate ventral prefrontal cortex. Nat Rev Neurosci. 2018;19:404–17. doi: 10.1038/s41583-018-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lally N, Nugent AC, Luckenbaugh DA, Niciu MJ, Roiser JP, Zarate CA. Neural correlates of change in major depressive disorder anhedonia following open-label ketamine. J Psychopharmacol. 2015;29:596–607. doi: 10.1177/0269881114568041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fettes P, Peters S, Giacobbe P, Blumberger DM, Downar J. Neural correlates of successful orbitofrontal 1 Hz rTMS following unsuccessful dorsolateral and dorsomedial prefrontal rTMS in major depression: a case report. Brain Stimul. 2017;10:165–7. doi: 10.1016/j.brs.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 118.Feffer K, Fettes P, Giacobbe P, Daskalakis ZJ, Blumberger DM, Downar J. 1 Hz rTMS of the right orbitofrontal cortex for major depression: Safety, tolerability and clinical outcomes. Eur Neuropsychopharmacol. 2018;28:109–17. doi: 10.1016/j.euroneuro.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 119.Stringaris A, Belil PVR, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, et al. The brain s response to reward anticipation and depression in adolescence: Dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiatry. 2015;172:1215–23. doi: 10.1176/appi.ajp.2015.14101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiol Dis. 2013;52:66–74. doi: 10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morris LS, Costi S, Tan A, Stern ER, Charney DS, Murrough JW. Ketamine normalizes subgenual cingulate cortex hyper-activity in depression. Neuropsychopharmacology. 2020;45:975–81. doi: 10.1038/s41386-019-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–93. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- 123.Kumar P, Goer F, Murray L, Dillon DG, Beltzer ML, Cohen AL, et al. Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology. 2018;43:1581–8. doi: 10.1038/s41386-018-0032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751–64. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- 125.Rutledge RB, Moutoussis M, Smittenaar P, Zeidman P, Taylor T, Hrynkiewicz L, et al. Association of neural and emotional impacts of reward prediction errors with major depression. JAMA Psychiatry. 2017;74:790–7. doi: 10.1001/jamapsychiatry.2017.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rothkirch M, Tonn J, Köhler S, Sterzer P. Neural mechanisms of reinforcement learning in unmedicated patients with major depressive disorder. Brain. 2017;140:1147–57. doi: 10.1093/brain/awx025. [DOI] [PubMed] [Google Scholar]
- 127.Ubl B, Kuehner C, Kirsch P, Ruttorf M, Diener C, Flor H. Altered neural reward and loss processing and prediction error signalling in depression. Soc Cogn Affect Neurosci. 2014;10:1102–12. doi: 10.1093/scan/nsu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eckstrand KL, Forbes EE, Bertocci MA, Chase HW, Greenberg T, Lockovich J, et al. Anhedonia reduction and the association between left ventral striatal reward response and 6-month improvement in life satisfaction among young adults. JAMA Psychiatry. 2019;76:958–65. doi: 10.1001/jamapsychiatry.2019.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rupprechter S, Romaniuk L, Series P, Hirose Y, Hawkins E, Sandu AL, et al. Blunted medial prefrontal cortico-limbic reward-related effective connectivity and depression. Brain. 2020;143:1946–56. doi: 10.1093/brain/awaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Macoveanu J, Meluken I, Chase HW, Phillips ML, Kessing LV, Siebner HR, et al. Reduced frontostriatal response to expected value and reward prediction error in remitted monozygotic twins with mood disorders and their unaffected high-risk co-twins. Psychol Med. 2020;1–10. 10.1017/S0033291720000367. Online ahead of print. [DOI] [PubMed]
- 131.Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47:1031–40. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shad MU, Bidesi AP, Chen LA, Ernst M, Rao U. Neurobiology of decision making in depressed adolescents: a functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2011;50:612–.e2. doi: 10.1016/j.jaac.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ding Y, Pereira F, Hoehne A, Beaulieu MM, Lepage M, Turecki G, et al. Altered brain processing of decision-making in healthy first-degree biological relatives of suicide completers. Mol Psychiatry. 2017;22:1149–54. doi: 10.1038/mp.2016.221. [DOI] [PubMed] [Google Scholar]
- 135.Kerestes R, Harrison BJ, Dandash O, Stephanou K, Whittle S, Pujol J, et al. Specific functional connectivity alterations of the dorsal striatum in young people with depression. NeuroImage Clin. 2015;7:266–72. doi: 10.1016/j.nicl.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, et al. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013;52:628–.e13. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G. Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. Am J Psychiatry. 2016;173:1223–30. doi: 10.1176/appi.ajp.2016.15121524. [DOI] [PubMed] [Google Scholar]
- 138.Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50:74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- 139.Moreines JL, Owrutsky ZL, Grace AA. Involvement of infralimbic prefrontal cortex but not lateral habenula in dopamine attenuation after chronic mild stress. Neuropsychopharmacology. 2017;42:904–13. doi: 10.1038/npp.2016.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.John CS, Smith KL, Van’T Veer A, Gompf HS, Carlezon WA, Cohen BM, et al. Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology. 2012;37:2467–75. doi: 10.1038/npp.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu C, Ma XM, Chen HBin, Zhou MH, Qiao H, AN SC. Orbitofrontal cortex 5-HT2A receptor mediates chronic stress-induced depressive-like behaviors and alterations of spine density and Kalirin7. Neuropharmacology. 2016;109:7–17. doi: 10.1016/j.neuropharm.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 142.Stawicka ZM, Massoudi R, Horst NK, Koda K, Alexander L, Cockcroft GJ, et al. Ventromedial prefrontal area 14 provides opposing regulation of threat and reward-elicited responses in the common marmoset. PNAS. 2020;117:25116–27. doi: 10.1073/pnas.2009657117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jahn AL, Fox AS, Abercrombie HC, Shelton SE, Oakes TR, Davidson RJ, et al. Subgenual PFC Activity Predicts Individual Differences in HPA Activity Across Different Contexts. Biol Psychiatry. 2010;67:175–81. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alexander L, Wood CM, Gaskin PLR, Sawiak SJ, Fryer TD, Hong YT, et al. Over-activation of primate subgenual cingulate cortex enhances the cardiovascular, behavioral and neural responses to threat. Nat Commun. 2020;11:1–14. doi: 10.1038/s41467-020-19167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pollack MH. Comorbid Anxiety and Depression. J Clin Psychiatry. 2005;66(Suppl 8):22–9. [PubMed] [Google Scholar]
- 146.Melton TH, Croarkin PE, Strawn JR, Mcclintock SM. Comorbid anxiety and depressive symptoms in children and adolescents: a systematic review and analysis. J Psychiatr Pract. 2016;22:84–98. doi: 10.1097/PRA.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–51. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stapelberg NJ, Hamilton-Craig I, Neumann DL, Shum DH, McConnell H. Mind and heart: heart rate variability in major depressive disorder and coronary heart disease-A review and recommendations. Aust N. Z J Psychiatry. 2012;46:946–57. doi: 10.1177/0004867412444624. [DOI] [PubMed] [Google Scholar]
- 149.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67:1067–74. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 150.Zhou J, Gardner MP, Schoenbaum G. Is the core function of orbitofrontal cortex to signal values or make predictions? Curr Opin Behav Sci. 2021;41:1–9. doi: 10.1016/j.cobeha.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rolls ET, Cheng W, Feng J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. 2020;2:1–25. doi: 10.1093/braincomms/fcaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lemogne C, le Bastard G, Mayberg H, Volle E, Bergouignan L, Lehé;ricy S, et al. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Soc Cogn Affect Neurosci. 2009;4:305–12. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lemogne C, Mayberg H, Bergouignan L, Volle E, Delaveau P, Lehéricy S, et al. Self-referential processing and the prefrontal cortex over the course of depression: a pilot study. J Affect Disord. 2010;124:196–201. doi: 10.1016/j.jad.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 154.Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Ueda K, Suzuki SIchi, et al. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J Affect Disord. 2010;122:76–85. doi: 10.1016/j.jad.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 155.Farb NAS, Anderson AK, Bloch RT, Segal ZV. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biol Psychiatry. 2011;70:366–72. doi: 10.1016/j.biopsych.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–95. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1:1–10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roiser JP, Sahakian BJ. Hot and cold cognition in depression. CNS Spectr. 2013;18:139–49. doi: 10.1017/S1092852913000072. [DOI] [PubMed] [Google Scholar]
- 159.Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, et al. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol. 2011;88:233–42. doi: 10.1016/j.biopsycho.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 160.Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008;163:143–55. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chechko N, Augustin M, Zvyagintsev M, Schneider F, Habel U, Kellermann T. Brain circuitries involved in emotional interference task in major depression disorder. J Affect Disord. 2013;149:136–45. doi: 10.1016/j.jad.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 163.Opel N, Redlich R, Grotegerd D, Dohm K, Zaremba D, Meinert S, et al. Prefrontal brain responsiveness to negative stimuli distinguishes familial risk for major depression from acute disorder. J Psychiatry Neurosci. 2017;42:343–52. doi: 10.1503/jpn.160198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee KH, Oppenheimer CW, Siegle GJ, Ladouceur CD, Lee GE, Silk JS, et al. Prefrontal cortical response to negative social words links social risk to depressive symptoms in adolescence. J Res Adolesc. 2018;28:87–102. doi: 10.1111/jora.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Frodl T, Bokde ALW, Scheuerecker J, Lisiecka D, Schoepf V, Hampel H, et al. Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biol Psychiatry. 2010;67:161–7. doi: 10.1016/j.biopsych.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 166.Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2013;37:152–63. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 167.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Victor TA, Furey ML, Fromm SJ, Öhman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67:1128–38. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Victor TA, Furey ML, Fromm SJ, Öhman A, Drevets WC. Changes in the neural correlates of implicit emotional face processing during antidepressant treatment in major depressive disorder. Int J Neuropsychopharmacol. 2013;16:2195–208. doi: 10.1017/S146114571300062X. [DOI] [PubMed] [Google Scholar]
- 170.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 171.Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42:1118–26. doi: 10.1016/j.neuroimage.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hooley JM, Gruber SA, Parker HA, Guillaumot J, Rogowska J, Yurgelun-Todd DA. Cortico-limbic response to personally challenging emotional stimuli after complete recovery from depression. Psychiatry Res. 2009;172:83–91. doi: 10.1016/j.pscychresns.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 173.Gaffrey MS, Barch DM, Singer J, Shenoy R, Luby JL. Disrupted amygdala reactivity in depressed 4- to 6-year-uld children. J Am Acad Child Adolesc Psychiatry. 2013;52:737–46. doi: 10.1016/j.jaac.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: an fMRI study. Biol Psychiatry. 2006;60:966–73. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 175.Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, et al. Reduced amygdalaprefrontal coupling in major depression: Association with MAOA genotype and illness severity. Int J Neuropsychopharmacol. 2009;12:11–22. doi: 10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- 176.Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008;111:13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 177.Kong L, Chen K, Tang Y, Wu F, Driesen N, Womer F, et al. Functional connectivity between the amygdala and prefrontal cortex in medication-naive individuals with major depressive disorder. J Psychiatry Neurosci. 2013;38:417–22. doi: 10.1503/jpn.120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, et al. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3 T fMRI study. J Psychiatry Neurosci. 2007;32:423–9. [PMC free article] [PubMed] [Google Scholar]
- 179.Jaworska N, Yang XR, Knott V, Macqueen G. A review of fMRI studies during visual emotive processing in major depressive disorder. World J Biol Psychiatry. 2015;16:448–71. doi: 10.3109/15622975.2014.885659. [DOI] [PubMed] [Google Scholar]
- 180.Li H, Wei D, Sun J, Zhang Q, Qiu J. Fronto-limbic alterations in negatively biased attention in young adults with subthreshold depression. Front Psychol. 2017;8:1354. doi: 10.3389/fpsyg.2017.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Korgaonkar MS, Grieve SM, Etkin A, Koslow SH, Williams LM. Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: first wave results from the iSPOT-D study. Neuropsychopharmacology. 2013;38:863–71. doi: 10.1038/npp.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:843–932. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- 183.Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112:206–11. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ito T, Yokokawa K, Yahata N, Isato A, Suhara T, Yamada M. Neural basis of negativity bias in the perception of ambiguous facial expression. Sci Rep. 2017;7:420. doi: 10.1038/s41598-017-00502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chai XJ, Hirshfeld-Becker D, Biederman J, Uchida M, Doehrmann O, Leonard JA, et al. Functional and structural brain correlates of risk for major depression in children with familial depression. NeuroImage Clin. 2015;8:398–407. doi: 10.1016/j.nicl.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, 3rd, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 187.Mannie ZN, Taylor MJ, Harmer CJ, Cowen PJ, Norbury R. Frontolimbic responses to emotional faces in young people at familial risk of depression. J Affect Disord. 2011;130:127–32. doi: 10.1016/j.jad.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 188.Joormann J, Cooney RE, Henry ML, Gotlib IH. Neural correlates of automatic mood regulation in girls at high risk for depression. J Abnorm Psychol. 2012;121:61–72. doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wackerhagen C, Wüstenberg T, Mohnke S, Erk S, Veer IM, Kruschwitz JD, et al. Influence of familial risk for depression on cortico-limbic connectivity during implicit emotional processing. Neuropsychopharmacology. 2017;42:1729–38. doi: 10.1038/npp.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.De Almeida JRC, Kronhaus DM, Sibille EL, Langenecker SA, Versace A, LaBarbara EJ, et al. Abnormal left-sided orbitomedial prefrontal cortical-amygdala connectivity during happy and fear face processing: a potential neural mechanism of female MDD. Front Psychiatry. 2011;2:69. doi: 10.3389/fpsyt.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wackerhagen C, Veer IM, Erk S, Mohnke S, Lett TA, Wüstenberg T, et al. Amygdala functional connectivity in major depression-disentangling markers of pathology, risk and resilience. Psychol Med. 2020;50:2740–50. doi: 10.1017/S0033291719002885. [DOI] [PubMed] [Google Scholar]
- 192.Miskowiak KW, Svendsen AMB, Harmer CJ, Elliott R, Macoveanu J, Siebner HR, et al. Differences in neural and cognitive response to emotional faces in middle-aged dizygotic twins at familial risk of depression. Psychol Med. 2017;47:2345–57. doi: 10.1017/S0033291717000861. [DOI] [PubMed] [Google Scholar]
- 193.Miskowiak KW, Glerup L, Vestbo C, Harmer CJ, Reinecke A, Macoveanu J, et al. Different neural and cognitive response to emotional faces in healthy monozygotic twins at risk of depression. Psychol Med. 2015;45:1447–58. doi: 10.1017/S0033291714002542. [DOI] [PubMed] [Google Scholar]
- 194.Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, et al. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 2012;7:e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 196.Fu CHY, Williams SCR, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–89. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 197.Godlewska BR, Near J, Cowen PJ. Neurochemistry of major depression: a study using magnetic resonance spectroscopy. Psychopharmacology. 2015;232:501–7. doi: 10.1007/s00213-014-3687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ma Y. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry. 2015;20:311–9. doi: 10.1038/mp.2014.24. [DOI] [PubMed] [Google Scholar]
- 199.Chen MH, Lin WC, Tu PC, Li CT, Bai YM, Tsai SJ, et al. Antidepressant and antisuicidal effects of ketamine on the functional connectivity of prefrontal cortex-related circuits in treatment-resistant depression: a double-blind, placebo-controlled, randomized, longitudinal resting fMRI study. J Affect Disord. 2019;259:15–20. doi: 10.1016/j.jad.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 200.Nakamura T, Tomita M, Horikawa N, Ishibashi M, Uematsu K, Hiraki T, et al. Functional connectivity between the amygdala and subgenual cingulate gyrus predicts the antidepressant effects of ketamine in patients with treatment‐resistant depression. Neuropsychopharmacol Rep. 2021;npr2:12165. doi: 10.1002/npr2.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Comte M, Schön D, Coull JT, Reynaud E, Khalfa S, Belzeaux R, et al. Dissociating bottom-up and top-down mechanisms in the cortico-limbic system during emotion processing. Cereb Cortex. 2016;26:144–55. doi: 10.1093/cercor/bhu185. [DOI] [PubMed] [Google Scholar]
- 202.Amemori S, Amemori K, Yoshida T, Papageorgiou GK, Xu R, Shimazu H, et al. Microstimulation of primate neocortex targeting striosomes induces negative decision‐making. Eur J Neurosci. 2020;51:731–41. doi: 10.1111/ejn.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Friedman A, Homma D, Gibb LG, Amemori K, Rubin SJ, Hood AS, et al. A corticostriatal path targeting striosomes controls decision-making under conflict. Cell. 2015;161:1320–33. doi: 10.1016/j.cell.2015.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jackson SAW, Horst NK, Pears A, Robbins TW, Roberts AC. Role of the perigenual anterior cingulate and orbitofrontal cortex in contingency learning in the marmoset. Cereb Cortex. 2016;26:3273–84. doi: 10.1093/cercor/bhw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–19. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 206.Duan LY, Horst NK, Cranmore SAW, Horiguchi N, Cardinal RN, Roberts AC, et al. Controlling one’s world: identification of sub-regions of primate PFC underlying goal-directed behavior. Neuron. 2021;109:1–14. doi: 10.1016/j.neuron.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 208.Diener C, Kuehner C, Brusniak W, Struve M, Flor H. Effects of stressor controllability on psychophysiological, cognitive and behavioural responses in patients with major depression and dysthymia. Psychol Med. 2009;39:77–86. doi: 10.1017/S0033291708003437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Peng D, Shi F, Shen T, Peng Z, Zhang C, Liu X, et al. Altered brain network modules induce helplessness in major depressive disorder. J Affect Disord. 2014;168:21–29. doi: 10.1016/j.jad.2014.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Späti J, Chumbley J, Doerig N, Brakowski J, Grosse Holtforth M, Seifritz E, et al. Valence and agency influence striatal response to feedback in patients with major depressive disorder. J Psychiatry Neurosci. 2015;40:394–400. doi: 10.1503/jpn.140225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rodin J. Aging and health: effects of the sense of control. Science. 1986;233:1271–6. doi: 10.1126/science.3749877. [DOI] [PubMed] [Google Scholar]
- 212.Romaniuk L, Sandu AL, Waiter GD, McNeil CJ, Xueyi S, Harris MA, et al. The neurobiology of personal control during reward learning and its relationship to mood. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:190–9. doi: 10.1016/j.bpsc.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Leotti LA, Delgado MR. The inherent reward of choice. Psychol Sci. 2011;22:1310–8. doi: 10.1177/0956797611417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Leotti LA, Delgado MR. The value of exercising control over monetary gains and losses. Psychol Sci. 2014;25:596–604. doi: 10.1177/0956797613514589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang KS, Delgado MR. Corticostriatal circuits encode the subjective value of perceived control. Cereb Cortex. 2019;29:5049–60. doi: 10.1093/cercor/bhz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wanke N, Schwabe L. Subjective uncontrollability over aversive events reduces working memory performance and related large-scale network interactions. Cereb Cortex. 2020;30:3116–29. doi: 10.1093/cercor/bhz298. [DOI] [PubMed] [Google Scholar]
- 217.Salomons TV, Johnstone T, Backonja MM, Shackman AJ, Davidson RJ. Individual differences in the effects of perceived controllability on pain perception: critical role of the prefrontal cortex. J Cogn Neurosci. 2007;19:993–1003. doi: 10.1162/jocn.2007.19.6.993. [DOI] [PubMed] [Google Scholar]
- 218.Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16:403–18. doi: 10.1038/nrn3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zunhammer M, Spisák T, Wager TD, Bingel U. Meta-analysis of neural systems underlying placebo analgesia from individual participant fMRI data. Nat Commun. 2021;12:1391. doi: 10.1038/s41467-021-21179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148:368–74. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 221.Covington HE, Maze I, Vialou V, Nestler EJ. Antidepressant action of HDAC inhibition in the prefrontal cortex. Neuroscience. 2015;298:329–35. doi: 10.1016/j.neuroscience.2015.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.McLaughlin RJ, Hill MN, Dang SS, Wainwright SR, Galea LAM, Hillard CJ, et al. Upregulation of CB1 receptor binding in the ventromedial prefrontal cortex promotes proactive stress-coping strategies following chronic stress exposure. Behav Brain Res. 2013;237:333–7. doi: 10.1016/j.bbr.2012.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun. 2019;10:1–12. doi: 10.1038/s41467-018-08168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Invest. 2020;130:1336–49. doi: 10.1172/JCI130808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–32. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hamani C, Diwan M, Macedo CE, Brandão ML, Shumake J, Gonzalez-Lima F, et al. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010;67:117–24. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 227.Kumar S, Black SJ, Hultman R, Szabo ST, Demaio KD, Du J, et al. Cortical control of affective networks. J Neurosci. 2013;33:1116–29. doi: 10.1523/JNEUROSCI.0092-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Slattery DA, Neumann I, Cryan JF. Transient inactivation of the infralimbic cortex induces antidepressant-like effects in the rat. J Psychopharmacol. 2011;25:1295–303. doi: 10.1177/0269881110368873. [DOI] [PubMed] [Google Scholar]
- 229.Zhao Y, Xing B, Dang Y, Qu C, Zhu F, Yan C. Microinjection of valproic acid into the ventrolateral orbital cortex enhances stress-related memory formation. PLoS One. 2013;8:e52698. doi: 10.1371/journal.pone.0052698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kuniishi H, Yamada D, Wada K, Yamada M, Sekiguchi M. Stress induces insertion of calcium-permeable AMPA receptors in the OFC–BLA synapse and modulates emotional behaviours in mice. Transl Psychiatry. 2020;10:1–11. doi: 10.1038/s41398-020-0837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kuniishi H, Ichisaka S, Matsuda S, Futora E, Harada R, Hata Y. Chronic inactivation of the orbitofrontal cortex increases anxiety-like behavior and impulsive aggression, but decreases depression-like behavior in rats. Front Behav Neurosci. 2017;10:250. doi: 10.3389/fnbeh.2016.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 233.Christianson JP, Flyer-Adams JG, Drugan RC, Amat J, Daut RA, Foilb AR, et al. Learned stressor resistance requires extracellular signal-regulated kinase in the prefrontal cortex. Front Behav Neurosci. 2014;8:348. doi: 10.3389/fnbeh.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur J Neurosci. 2009;30:1111–6. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in pavlovian but not instrumental conditioning. J Neurosci. 2007;27:4819–25. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Grahek I, Everaert J, Krebs RM, Koster EHW. Cognitive control in depression: toward clinical models informed by cognitive neuroscience. Clin Psychol Sci. 2018;6:464–80. [Google Scholar]
- 237.Gärtner M, Elisabetta Ghisu M, Scheidegger M, Bönke L, Fan Y, Stippl A, et al. Aberrant working memory processing in major depression: Evidence from multivoxel pattern classification. Neuropsychopharmacology. 2018;43:1972–9. doi: 10.1038/s41386-018-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang XL, Du MY, Chen TL, Chen ZQ, Huang XQ, Luo Y, et al. Neural correlates during working memory processing in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:101–8. doi: 10.1016/j.pnpbp.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 239.Schöning S, Zwitserlood P, Engelien A, Behnken A, Kugel H, Schiffbauer H, et al. Working-memory fMRI reveals cingulate hyperactivation in euthymic major depression. Hum Brain Mapp. 2009;30:2746–56. doi: 10.1002/hbm.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Vasic N, Walter H, Sambataro F, Wolf RC. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychol Med. 2009;39:977–87. doi: 10.1017/S0033291708004443. [DOI] [PubMed] [Google Scholar]
- 241.Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, et al. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biol Psychiatry. 2006;59:958–65. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 242.Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry. 2008;65:179–88. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Aizenstein HJ, Butters MA, Wu M, Mazurkewicz LM, Stenger VA, Gianaros PJ, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17:30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Holmes AJ, Pizzagalli DA. Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia. 2008;46:2904–13. doi: 10.1016/j.neuropsychologia.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mitterschiffthaler MT, Williams SC, Walsh ND, Cleare AJ, Donaldson C, Scott J, et al. Neural basis of the emotional Stroop interference effect in major depression. Psychol Med. 2008;38:247–56. doi: 10.1017/S0033291707001523. [DOI] [PubMed] [Google Scholar]
- 246.Wang L, Krishnan KR, Steffens DC, Potter GG, Dolcos F, McCarthy G. Depressive state- and disease-related alterations in neural responses to affective and executive challenges in geriatric depression. Am J Psychiatry. 2008;165:863–71. doi: 10.1176/appi.ajp.2008.07101590. [DOI] [PubMed] [Google Scholar]
- 247.Meyer BM, Rabl U, Huemer J, Bartova L, Kalcher K, Provenzano J, et al. Prefrontal networks dynamically related to recovery from major depressive disorder: a longitudinal pharmacological fMRI study. Transl Psychiatry. 2019;9:64. doi: 10.1038/s41398-019-0395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bartova L, Meyer BM, Diers K, Rabl U, Scharinger C, Popovic A, et al. Reduced default mode network suppression during a working memory task in remitted major depression. J Psychiatr Res. 2015;64:9–18. doi: 10.1016/j.jpsychires.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stippl A, Scheidegger M, Aust S, Herrera A, Bajbouj M, Gärtner M, et al. Ketamine specifically reduces cognitive symptoms in depressed patients: An investigation of associated neural activation patterns. J Psychiatr Res. 2021;136:402–8. doi: 10.1016/j.jpsychires.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 250.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N. Y. Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–90. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dillon DGDG, Pizzagalli DADA. Evidence of successful modulation of brain activation and subjective experience during reappraisal of negative emotion in unmedicated depression. Psychiatry Res. 2013;212:99–107. doi: 10.1016/j.pscychresns.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Loeffler LAK, Radke S, Habel U, Ciric R, Satterthwaite TD, Schneider F, et al. The regulation of positive and negative emotions through instructed causal attributions in lifetime depression – a functional magnetic resonance imaging study. NeuroImage Clin. 2018;20:1233–45. doi: 10.1016/j.nicl.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tait DS, Bowman EM, Neuwirth LS, Brown VJ. Assessment of intradimensional/extradimensional attentional set-shifting in rats. Neurosci Biobehav Rev. 2018;89:72–84. doi: 10.1016/j.neubiorev.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 256.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jett JD, Morilak DA. Too much of a good thing: blocking noradrenergic facilitation in medial prefrontal cortex prevents the detrimental effects of chronic stress on cognition. Neuropsychopharmacology. 2013;38:585–95. doi: 10.1038/npp.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Adler SM, Girotti M, Morilak DA. Optogenetically-induced long term depression in the rat orbitofrontal cortex ameliorates stress-induced reversal learning impairment. Neurobiol Stress. 2020;13:100258. doi: 10.1016/j.ynstr.2020.100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Patton MS, Lodge DJ, Morilak DA, Girotti M. Ketamine corrects stress-induced cognitive dysfunction through JAK2/STAT3 signaling in the orbitofrontal cortex. Neuropsychopharmacology. 2017;42:1220–30. doi: 10.1038/npp.2016.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yang CC, Barrós-Loscertales A, Pinazo D, Ventura-Campos N, Borchardt V, Bustamante JC, et al. State and training effects of mindfulness meditation on brain networks reflect neuronal mechanisms of its antidepressant effect. Neural Plast. 2016;2016:9504642. doi: 10.1155/2016/9504642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Keynan JN, Cohen A, Jackont G, Green N, Goldway N, Davidov A, et al. Electrical fingerprint of the amygdala guides neurofeedback training for stress resilience. Nat Hum Behav. 2019;3:63–73. doi: 10.1038/s41562-018-0484-3. [DOI] [PubMed] [Google Scholar]
- 262.Young KD, Misaki M, Harmer CJ, Victor T, Zotev V, Phillips R, et al. Real-time functional magnetic resonance imaging amygdala neurofeedback changes positive information processing in major depressive disorder. Biol Psychiatry. 2017;82:578–86. doi: 10.1016/j.biopsych.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Chrysikou EG, Wing EK, van Dam WO. Transcranial direct current stimulation over the prefrontal cortex in depression modulates cortical excitability in emotion regulation regions as measured by concurrent functional magnetic resonance imaging: an exploratory study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019; S2451-9022(19)30344-1. 10.1016/j.bpsc.2019.12.004. Online ahead of print. [DOI] [PubMed]
- 264.Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364:eaat8078. doi: 10.1126/science.aat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.McIntosh AL, Gormley S, Tozzi L, Frodl T, Harkin A. Recent advances in translational magnetic resonance imaging in animal models of stress and depression. Front Cell Neurosci. 2017;11:150. doi: 10.3389/fncel.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mumtaz W, Qayyum A. A deep learning framework for automatic diagnosis of unipolar depression. Int J Med Inform. 2019;132:103983. doi: 10.1016/j.ijmedinf.2019.103983. [DOI] [PubMed] [Google Scholar]
- 267.Su C, Xu Z, Pathak J, Wang F. Deep learning in mental health outcome research: a scoping review. Transl Psychiatry. 2020;10:116. doi: 10.1038/s41398-020-0780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Squarcina L, Villa FM, Nobile M, Grisan E, Brambilla P. Deep learning for the prediction of treatment response in depression. J Affect Disord. 2021;281:618–22. doi: 10.1016/j.jad.2020.11.104. [DOI] [PubMed] [Google Scholar]
- 269.Friedman A, Homma D, Bloem B, Gibb LG, Amemori K, Hu D, et al. Chronic stress alters striosome-circuit dynamics, leading to aberrant decision-making. Cell. 2017;171:1191–205. doi: 10.1016/j.cell.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Robble MA, Schroder HS, Kangas BD, Nickels S, Breiger M, Iturra-Mena AM, et al. Concordant neurophysiological signatures of cognitive control in humans and rats. Neuropsychopharmacology. 2021;21:2021. doi: 10.1038/s41386-021-00998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proc Natl Acad Sci USA. 2013;110:6145–50. doi: 10.1073/pnas.1214364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Robbins TW, Cardinal RN. Computational psychopharmacology: a translational and pragmatic approach. Psychopharmacol. 2019;236:2295–305. doi: 10.1007/s00213-019-05302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex. 2012;48:46–57. doi: 10.1016/j.cortex.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 274.Paxinos G. The marmoset brain in stereotaxic coordinates. London UK: Academic Press; 2012. [Google Scholar]
- 275.Palomero-Gallagher N, Zilles K. Isocortex. In: The Rat Nervous System. Paxinos G editor, pp. 601–25. Amsterdam: Elsevier; 2015.
- 276.Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]