Abstract
Loneliness is defined as the subjective feeling that one’s social needs are not satisfied by both quantity and quality of one’s social relationships. Loneliness has been linked to a broad range of adverse physical and mental health consequences. There is an interest in identifying the neural and molecular processes by which loneliness adversely affects health. Prior imaging studies reported divergent networks involved in cognitive, emotional, and social processes associated with loneliness. Although loneliness is common among both younger and older adults, it is experienced differently across the lifespan and has different antecedents and consequences. The current study measured regional cerebral blood flow (CBF) using pulsed arterial spin labeling imaging. Forty-five older (Mage = 63.4) and forty-four younger adults (Mage = 20.9) with comparable degrees of loneliness were included. Whole-brain voxel-wise analysis revealed a main effect of age (in superior temporal and supramarginal gyri), but no main effect of loneliness. Furthermore, the age effect was only observed among people who reported higher level of loneliness. These regions have previously been implicated in social- and attention-related functions. The moderation of loneliness on age and regional CBF suggests that younger and older individuals present differential neural manifestations in response to loneliness, even with comparable levels of loneliness.
Keywords: Loneliness, Aging, Cerebral blood flow, Arterial spin labeling, Magnetic resonance imaging
Loneliness is the subjective experience of perceived social isolation, in which one’s social needs are not satisfied by both quantity and quality of one’s social relationships (Hawkley & Cacioppo, 2010). Longitudinal studies revealed that persistent feelings of loneliness can have significant adverse physical and mental health consequences (J. T. Cacioppo, Hawkley, & Thisted, 2010; Caspi, Harrington, Moffitt, Milne, & Poulton, 2006; Holwerda et al., 2016). For example, loneliness has been linked to disturbances in sleep, immune dysregulation, cardiovascular diseases, and increased mortality (Caspi et al., 2006; Harris, Qualter, & Robinson, 2013; Holwerda et al., 2016; Pressman et al., 2005). Loneliness has also been linked to cognitive and emotional dysfunction, including anxiety, depression, negative mood, less optimism, lower self-esteem, and lower perceived social support (Beutel et al., 2017; J. T. Cacioppo et al., 2010; Ge, Yap, Ong, & Heng, 2017). Gene expression studies of peripheral blood (Cole, Hawkley, Arevalo, & Cacioppo, 2011; Cole et al., 2007) and postmortem brain regions (Canli et al., 2017, 2018) have begun to identify the molecular signaling pathways by which loneliness may affect health.
Given the subjective nature of loneliness, as well as its potential psychosomatic cost, noninvasive imaging studies in humans began to probe the neural basis of perceived social isolation. In principle, such neuroimaging studies could identify neural systems that contribute to the subjective experience of social isolation, which could then be targeted with psychological interventions. The first task-based functional magnetic resonance imaging (fMRI) study reported reduced activation in the ventral striatum in lonely individuals in response to positive social, relative to non-social, stimuli (J. T. Cacioppo, Norris, Decety, Monteleone, & Nusbaum, 2009). This result was interpreted as reflecting impaired reward processing of social stimuli. However, this finding did not replicate across other studies using similar tasks (e.g., D’Agostino et al., 2018; Inagaki et al., 2016; Powers et al., 2013). An alternative to task-based imaging is task-free, resting-state imaging. Several resting-state functional connectivity studies reported that loneliness is associated with diverse networks involved in cognitive control, emotional processing, and social cognition (Feng, Wang, Li, & Xu, 2019; Nakagawa et al., 2015; Tian et al., 2014). In addition, studies also reported that loneliness associated with attentional, perceptual, and executive related networks (Layden et al., 2017; Mwilambwe-Tshilobo et al., 2019; Tian et al., 2017). Thus, multiple and functionally distinct neural circuits may contribute to the subjective experience of social isolation, and these circuits may further be differentially activated as a function of individual differences.
One individual difference that impacts loneliness across several lifespan studies is age (Beutel et al., 2017; Hawthorne, 2008; Luhmann & Hawkley, 2016; Perissinotto, Stijacic Cenzer, & Covinsky, 2012; Pinquart & Sorensen, 2001). For instance, among older adults, loneliness has been associated with poor self-rated health and quality of life, cognitive decline, and increased risk of developing Alzheimer’s disease (J. T. Cacioppo, Hughes, Waite, Hawkley, & Thisted, 2006; Canli et al., 2017; Donovan et al., 2017; Gerino, Rollè, Sechi, & Brustia, 2017; Hawkley, Thisted, & Cacioppo, 2009; Wilson et al., 2007). Furthermore, loneliness is experienced differently across the lifespan (Qualter et al., 2015) and has different antecedents and consequences (Böger & Huxhold, 2018). Thus, even at comparable levels of loneliness, older adults may have different neural manifestations associated with subjective social isolation than do younger adults. Identifying the neural circuitry that is associated with loneliness as a function of age could contribute to the development of future interventions that target the neural circuitry contributing to age-specific subjective social isolation.
Therefore, in the current study we compared the resting-state regional cerebral blood flow (CBF) between two cohorts of younger and older adults. Our chosen imaging modality was arterial spin labeling (ASL) magnetic resonance imaging (MRI). ASL measures CBF by using magnetically labeled arterial blood water as an endogenous tracer. It is therefore a quantitative and absolute measure that is not dependent on contrasting two conditions, as is the case with BOLD-based signals (F. Liu et al., 2018). In addition, whereas the BOLD signal is derived from the multiple factors that include CBF and oxygen consumption rate (Kim & Uǧurbil, 1997), the ASL signal is derived from a single physiological parameter (i.e., CBF) (F. Liu et al., 2018). Resting BOLD and ASL signals are correlated in functional networks, such as the default mode network and executive control network (Liang, Zou, He, & Yang, 2013; Tak, Polimeni, Wang, Yan, & Chen, 2015; Tak, Wang, Polimeni, Yan, & Chen, 2014). ASL has been increasingly used in CBF studies with various populations, including healthy younger and older adults (Xu et al., 2010; K. Zhang et al., 2014), autism spectrum disorder (Jann et al., 2015; Yerys et al., 2018), posttraumatic stress disorders (Yang Liu et al., 2016; Schuff et al., 2011), and Alzheimer’s disease (N. Zhang et al., 2017), and studies also demonstrate the correlation between ASL-derived resting CBF and behavioral measurements (Allen et al., 2016; Y. Liu et al., 2016; Schneider et al., 2019; Vasic et al., 2015), suggesting its usefulness in studying broad topics.
Although the majority of previous loneliness studies used task or resting-state BOLD-based signals, based on previous studies and considering the closely related nature of ASL and BOLD signals, we expected to observe main effects of age and loneliness on resting-state CBF. We hypothesized a negative association of CBF with age and with loneliness. In addition, we also expected to observe an interaction effect between age and loneliness, particularly in regions associated with cognitive, emotional, and social processes like middle and superior temporal, medial prefrontal, orbitofrontal, and anterior cingulate cortices, as well as anterior insula, amygdala, and ventral striatum. However, since the loneliness is experienced differently and individual responses to loneliness vary by age, we did not predict a particular direction for the interaction effect.
Method
Participants
Fifty-two older adults (53 – 81 years old, 33 females, Mage = 63.63 ± 6.54) were included in the current study. We created a matched cohort of fifty-two younger adults (18 – 36 years old, 36 females, Mage = 20.85 ± 3.18), who were selected out of an available dataset of 132 younger participants. Matching was based on a two-variable (loneliness, sex) nearest-neighbor matching algorithm (MatchIt package in R) (Ho, Imai, King, & Stuart, 2011), which calculated the regression distance using both variables and selected the closet (smallest distance) match. Older participants were recruited from the local community through flyers and internet advertisement. Younger participants were recruited either from the local community through flyers and internet advertisement or through the Department of Psychology at Stony Brook University and completed the study for course credit. All participants were prescreened to exclude history of psychiatric diagnoses, use of mood altering or psychoactive medication, substance abuse, neurological or serious health problems (e.g., thyroid disease, diabetes, stroke, brain surgery, head trauma or injury, heart attack), and MRI contraindications. The Institutional Review Board at Stony Brook University approved the study and all participants gave informed, written consent. Participants were compensated at a rate of $20 per hour or given course credit.
All participants completed the MRI scan. Eight younger and seven older participants were excluded because their ASL images showed incomplete coverage of the cortex. The final sample consisted of 44 younger (18 – 36 years old, 34 females, Mage = 20.89 ± 3.21) and 45 older adults (53 – 81 years old, 29 females, Mage = 63.40 ± 6.60).
Loneliness Measurement
Perceived social isolation was measured by the UCLA Loneliness Scale (Third Version) (Russell, 1996). The 20-item 4-point scale measures individual’s subjective feelings of loneliness from 1 (Never) to 4 (Often) for a range of 20 – 80. The higher the score indicates the higher level of loneliness. The questionnaire has high internal consistency (Cronbach’s α = 0.89 – 0.94) and it is commonly used in both young and older adults (J. T. Cacioppo et al., 2010; Russell, 1996). Participants whose total score was 41 or higher was then categorized in high lonely group, and participants whose total score was less than 41 was categorized in low lonely group (S. Cacioppo, Balogh, & Cacioppo, 2015).
MRI Acquisition
All MRI scans were performed on a Siemens 3T Trio with 12-channel head coil at the Social, Cognitive and Affective Neuroscience (SCAN) Center at Stony Brook University. ASL perfusion images were acquired using a 2D PASL sequence using PICORE (E. C. Wong, Buxton, & Frank, 1997) and Q2TIPS (Luh, Wong, Bandettini, & Hyde, 1999) with parameters set as TR = 2500 ms, TE = 11 ms, TI1 = 700 ms, TI2 = 1800 ms, flip angle = 90°, matrix = 64 × 64, FOV = 256 × 256, slice thickness = 8 mm with 1.92 mm gap, number of slices = 12, 7/8 partial Fourier, iPAT = 2. With ascending slice-order, each ASL series contained 91 images, including the first control image as reference image and 45 label/control pairs. High-resolution anatomical T1-weighted 3D magnetization-prepared rapid gradient-echo (MPRAGE) (TR = 1900 ms, TE = 2.53 ms, TI = 900 ms, flip angle = 9°, matrix = 256 × 256, FOV = 256 × 256, slice thickness = 1 mm, slice = 176, iPAT = 2) was acquired for segmentation of gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF), and spatial normalization.
MRI Imaging Processing
ASL images were processed using the ASLtoolbox (Wang et al., 2008) and Statistical Parametric Mapping Version 12 (SPM12) (Penny, Friston, Ashburner, Kiebel, & Nichols, 2011). The processing pipeline consisted of the following: realignment of all ASL images for motion correction (using the first control image as reference image); segmentation of the T1w-MPRAGE image into GM, WM, and CSF probability maps; co-registration of the T1w-MPRAGE image, GM, WM, and CSF probability maps to ASL images; smoothing of ASL images with a 10 mm full-width at half maximum (FWHM) isotropic Gaussian kernel; CBF quantification using the single compartment model (Buxton et al., 1998) with partial volume correction (PVC) (Asllani, Borogovac, & Brown, 2008). Partial volume effect mainly derives from the limited spatial resolution of the neuroimaging data and the mixed tissue-specific signals (i.e., GM, WM, CSF) and could result in incorrect estimation of the CBF signals, and is has been showed that the voxel-wise CBF signals are more consistent by correcting for partial volume effect (Asllani et al., 2008). The simple subtraction of label/control pairs was used to generate 45 perfusion images. The 45 perfusion images were averaged and used to generate a mean PVC-CBF map for each participant in units of milliliters of blood per 100 grams of tissue per minute (mL/100g/min). Due to the heterogeneity of brain structures and structural changes of normal aging (Matsuda, 2013), a study-specific template (SST) was created using Diffeomorphic Anatomical Registration through Exponentiated Lie algebra (DARTEL) (Ashburner, 2007). Unlike a standard template, which is optimized for studies of healthy young adults, a SST is recommended to account for the potential confounds of age-related brain structural differences to allow for less biased registration and normalization of images (Matsuda, 2013). To create a customized SST, individual GM and WM probability tissue maps were used to produce mean GM and WM maps across all participants. The final template was created through an iterative procedure, and it was registered to the Montreal Neurological Institute (MNI) standard space using affine registration. The individual PVC-CBF maps were co-registered to individual T1w images, upsampled to 2 × 2 × 2 mm3 and normalized to MNI space using flow fields derived from the DARTEL process. The co-registration (by inspecting whether the co-registered PVC-CBF maps matched with individual’s T1w anatomical images) and normalization (by inspecting whether the normalized PVC-CBF maps matched with the MNI template) procedure were based on visual inspection using SPM12 Check Reg function. We restricted all analyses within GM.
A major challenge in ASL imaging is the low signal-to-noise ratio (SNR), because the average blood flow delivered to the GM tissue at the imaging location accounts for approximately one percent of the water flowing into the tissue per minute. Extreme outliers resulting from low SNR and vascular artifacts could significantly distort the mean CBF value (Li et al., 2018; T. T. Liu & Brown, 2007). We therefore used an a priori outlier cleaning process that included the following steps. First, a voxel was defined as an outlier if its CBF value was either smaller than 5 ml/100g/min, or larger than 97.5% of the voxel values in the individual CBF map. Second, CBF values of the outlier voxels were set to zero and replaced with the mean value from the six nearest neighboring voxels through an iterative process.
Statistical Analysis
Statistical analyses of demographic and loneliness measures were performed in R version 3.4.3. We used SPM12 to analyze CBF data. We used the mean PVC-CBF map after outlier cleaning for CBF analysis comparing the two age cohorts. We conducted both global mean CBF and whole-brain voxel-wise CBF group analyses.
Global Mean CBF
For global mean CBF, a whole-brain GM mask conjoining with individual’s GM probability map at a threshold of 0.8 (Asllani et al., 2008) was used to restrict the analysis within GM. We examined whether global mean CBF was different with age (dichotomous, younger cohort as reference group) and UCLA loneliness (dichotomous, low lonely group as reference group) using two-way ANCOVA, controlling for sex.
Whole-Brain Voxel-Wise CBF
We first examine whether there was a main effect of loneliness within each age cohort, as the majority of loneliness neuroimaging studies included either only younger (e.g., J. T. Cacioppo et al., 2009; S. Cacioppo, Balogh, & Cacioppo, 2015; Feng et al., 2019; Kanai et al., 2012; Layden et al., 2017; Nakagawa et al., 2015; Tian et al., 2017b) or only older people (e.g., Lan et al., 2016; N. M. L. Wong et al., 2016). We conducted whole-brain voxel-wise two-sample t-test analyses separately for younger and older adults with loneliness levels (dichotomous, Low lonely group as reference group) as the independent variable of interest and controlling for sex. Next, to examine the main and interaction effects of age and loneliness on CBF across two age cohorts, we conducted a whole-brain voxel-wise 2 (age: Young versus Older) x 2 (loneliness: Low versus High) factorial analysis for both age cohorts combined and with age (dichotomous, younger cohort as reference group), UCLA loneliness score (dichotomous, Low lonely group as reference group), the interaction between the two variables as the independent variables of interest, and controlling for sex. We set the statistical significance at voxel-level family-wise error (FWE) corrected p < 0.05, with a minimum cluster size of 10 contiguous voxels. We applied an explicit GM mask created from the MNI template, and excluded the cerebellum from our analyses, because it was not entirely covered for most of the participants.
To provide a quantitative estimation of the magnitude and precision of the moderation effect of loneliness on age and regional CBF, we extracted regional mean CBF from the significant regions in which age differences on regional CBF were revealed among high lonely group and conducted simple ANCOVAs controlling for sex to compare the effect sizes (considering the small sample size of our data, we used partial ω2) and confidence intervals of age across and separately for low and high lonely groups. In the case of the potential multivariate outliers, we examined the outliers using global influence index, DFFITS, which measures whether the overall result changed if one case was removed and used the cutoff value at absolute value of 1. We then conducted a separate set of ANCOVAs removing outliers to examine whether the results changed.
Sex Effect
Studies have reported the sex differences in CBF (e.g., Yinan Liu et al., 2012; Parkes et al., 2004). We observed a significant sex difference in global mean CBF (Appendix Table A1), however, no sex difference was revealed on whole-brain voxel-wise factorial CBF analysis at FWE corrected p < .05, while controlling for age, loneliness, and interaction between age and loneliness. Considering the disproportional number of males and females in our sample, we controlled for sex in all group analyses reported in the current study.
Results
Table 1 lists demographics and descriptive statistics for loneliness and CBF results for both age cohorts from the final sample.
Table 1.
Descriptive statistics for loneliness and CBF measures for younger and older cohorts (N = 89).
| Younger | Older | t/X2 | df | p | |
|---|---|---|---|---|---|
| Mean ± SD (Range) | Mean ± SD (Range) | ||||
|
| |||||
| n | 44 | 45 | |||
| Age | 20.89 ± 3.21 (18–36) | 63.40 ± 6.60 (53–81) | |||
| Sex | 1.20 | 1 | 0.272 | ||
| Male (n (%)) | 10 (22.7) | 16 (35.6) | |||
| Female (n (%)) | 34 (77.3) | 29 (64.4) | |||
| UCLAa | 39.89 ± 8.34 (23–56) | 38.06 ± 8.16 (22–54) | 1.05 | 87 | 0.298 |
| Lowb (n (%)) | 23 (52.3) | 24 (53.3) | |||
| Highc (n (%)) | 21 (47.7) | 21 (46.7) | |||
| Global mean CBF | 39.83 ± 6.78 | 33.22 ± 8.80 | |||
| Low UCLA | 39.94 ± 7.46 | 34.39 ± 11.17 | |||
| High UCLA | 39.71 ± 6.13 | 31.89 ± 4.86 | |||
| Regional mean CBF (mL/100g/min) | |||||
| R STG | 56.66 ± 8.01 | 80.60 ± 23.63 | |||
| Low UCLA | 58.41 ± 9.06 | 77.42 ± 22.43 | |||
| High UCLA | 54.74 ± 6.35 | 84.23 ± 24.97 | |||
| L STG | 62.98 ± 11.41 | 79.20 ± 16.16 | |||
| Low UCLA | 64.25 ± 13.10 | 75.66 ± 15.53 | |||
| High UCLA | 61.60 ± 9.34 | 83.24 ± 16.28 | |||
| L SMG | 70.02 ± 19.02 | 110.86 ± 52.52 | |||
| Low UCLA | 69.46 ± 22.69 | 98.65 ± 36.55 | |||
| High UCLA | 70.64 ± 14.52 | 124.82 ± 64.41 | |||
Notes.
UCLA: UCLA Loneliness Scale scores, and total score range 20 – 80;
Low lonely group whose UCLA loneliness score was less than 41;
High lonely group whose UCLA loneliness score was 41 or higher. R: right hemisphere; L: left hemisphere; STG: superior temporal gyrus; SMG: supramarginal gyrus.
Global Mean CBF
The two-way ANCOVA revealed a significant age effect, F(1, 85) = 16.61, p = .0001, partial ω2 = .15, 95% CI [.04, .29], in which older, relative to younger, adults showed lower global mean CBF, as shown in Table 1. No global mean CBF difference in terms of loneliness levels (F(1, 85) = 0.65, p = .423, partial ω2 = .00, 95% CI [.00, .00], global mean CBF across age cohorts: Low lonely 37.11 ± 9.84; High lonely 35.80 ± 6.75 mL/100g/min)).
Whole-Brain Voxel-Wise CBF
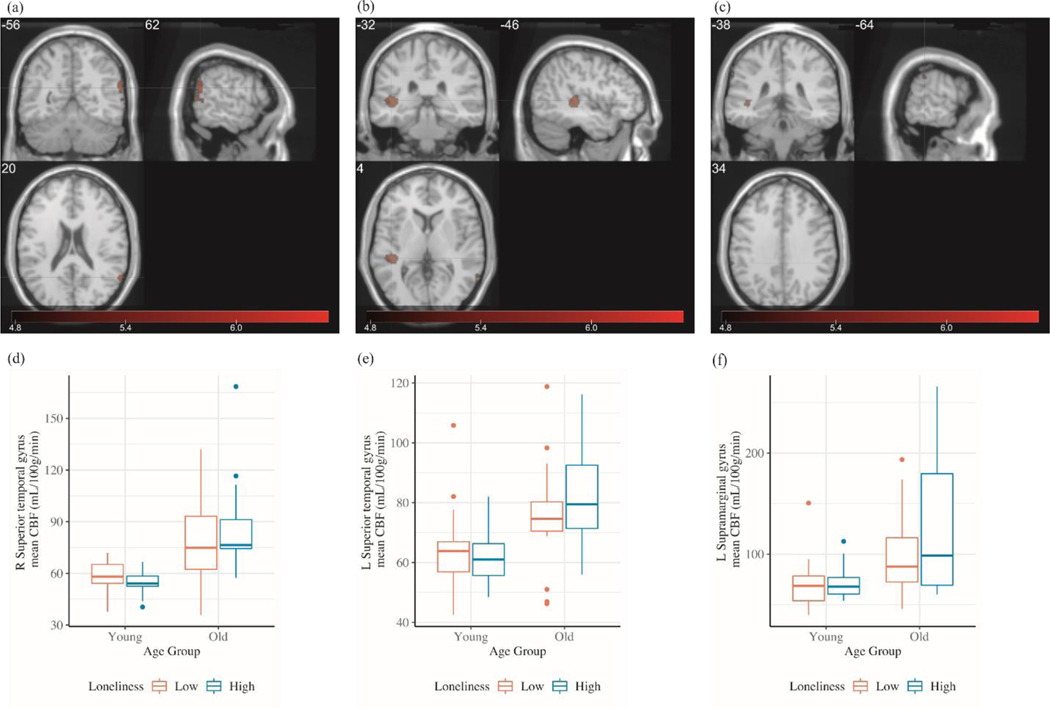
We first examined the main effect of loneliness on CBF separately within each age cohort. Whole-brain voxel-wise results revealed no significant clusters from neither age cohorts at corrected (FWE p < .05) threshold. Next, we examined the main and interaction effects of age and loneliness on CBF across the two age cohorts. Whole-brain voxel-wise results revealed a main effect of age, as shown in Table 2, such that the older adults showed greater regional CBF than did younger adults in frontal (superior, middle, inferior frontal gyri, and olfactory sulcus), temporal (superior, middle, and inferior temporal gyri), parietal (postcentral, supramarginal, and angular gyri), occipital (superior occipital gyrus), anterior cingulate, and subcortical (caudate) regions. We observed no clusters in which there was significantly greater CBF in younger than in older adults at corrected threshold (FWE p < .05). We observed no clusters in which CBF was different regarding the levels of loneliness, neither did we observe cluster from age x loneliness interaction. To further explore whether the levels of loneliness have impact on age-related CBF differences, we examined the age effect separately for low and high lonely groups. Results revealed that age-related CBF differences were only observed among high lonely group (UCLA ≥ 41). Among people who reported higher level of loneliness, as shown in Table 2 and Figure 1, we observed a main effect of age, such that older adults showed greater regional CBF than did younger adults in left and right superior temporal gyrus, and left supramarginal gyrus.
Table 2.
Significant clusters from whole-brain voxel-wise 2 × 2 factorial analysis with age and loneliness (controlled for sex) at family-wise error corrected p < .05, with a minimum cluster size of 10 contiguous voxels.
| Brian regions | k | Peak T | Peak Z | MNI (mm) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
|
| ||||||
| Age Old > Young | ||||||
| R Angular | 236 | 7.03 | 6.22 | 62 | −58 | 28 |
| R Inferior frontal gyrus | 52 | 6.72 | 5.99 | 62 | 26 | 16 |
| L Middle frontal gyrus | 11 | 6.31 | 5.69 | −46 | 56 | 0 |
| L Middle temporal gyrus | 287 | 6.06 | 5.51 | −46 | −32 | 0 |
| L Caudate | 43 | 6.01 | 5.46 | −6 | 26 | 8 |
| R Postcentral gyrus | 239 | 6.00 | 5.46 | 62 | −4 | 38 |
| L Inferior frontal gyrus | 14 | 5.99 | 5.45 | −54 | 44 | 6 |
| R Inferior temporal gyrus | 54 | 5.98 | 5.45 | 68 | −48 | −12 |
| R Superior occipital gyrus | 24 | 5.91 | 5.39 | 34 | −82 | 44 |
| L Superior temporal gyrus | 177 | 5.81 | 5.31 | −66 | −10 | 4 |
| L Supramarginal gyrus | 19 | 5.35 | 4.95 | −66 | −44 | 22 |
| R Olfactory sulcus | 12 | 5.12 | 4.77 | 4 | 26 | −2 |
| Anterior cingulate cortex | 13 | 5.12 | 4.77 | −4 | 28 | 12 |
| L Superior frontal gyrus | 10 | 5.10 | 4.74 | −12 | 42 | 54 |
|
| ||||||
| Within High Loneliness: Age Old > Young | ||||||
| R Superior temporal gyrus | 132 | 6.28 | 5.67 | 62 | −56 | 20 |
| L Superior temporal gyrus | 242 | 5.93 | 5.40 | −46 | −32 | 4 |
| L Supramarginal gyrus | 14 | 5.00 | 4.67 | −64 | −38 | 34 |
Notes. R: Right hemisphere; L: Left hemisphere; k: cluster size (voxel).
Figure 1.
Clusters from age effect among high lonely group from whole-brain voxel-wise 2 × 2 factorial analysis at family-wise error corrected p < .05, with a minimum cluster size of 10 contiguous voxels. (a) Right superior temporal gyrus; (b) left superior temporal gyrus; (c) left supramarginal gyrus. Color bar: T value. Bottom panel (d), (e), and (f) showed the bar graphs of the regional mean CBF values extracted from each region
Regional Mean CBF Extracted from Age Effect among High Lonely Group
To further estimate the magnitude and precision of age-related clusters among high lonely group, we extracted the regional mean CBF from each of the three significant clusters in which age differences were observed among high lonely group (i.e., left and right superior temporal gyrus, and left supramarginal gyrus) and conducted ANCOVAs. Table 3 lists the ANCOVA results of age effect across and separately for low and high lonely groups. As shown in Table 3 and Figure 1, age was significant in all three two-way ANCOVA with age and loneliness for three clusters. From one-way ANCOVA with age, the effect sizes of age were quantitatively larger in models among high lonely group, relative to low lonely group. To further examine the potential effect from brain structural difference, we extracted mean gray matter volume (GMV) from three significant clusters and added into respective ANCOVAs as covariate (in addition to sex) (see Appendix for voxel-based morphometry method). All results shown in Table 3 remained unchanged. Regarding the potential multivariate outliers, one older participant (in high lonely group) was identified as an outlier using DFFIT criterion as described in the Method in two of the three two-way ANCOVAs. Removing this outlier data point did not alter any of the data (Appendix Table A3).
Table 3.
ANCOVA results of age effect using regional mean CBF extracted from three age-related regions among high lonely group.
| Predictor | SS | df | MS | F | Partial ω2 | Partial ω2 95% CI [LL, UL] |
|---|---|---|---|---|---|---|
|
| ||||||
| Across Loneliness Groups (N = 89) | ||||||
| R STG | ||||||
| Age | 12765.5 | 1 | 12765.5 | 40.70 | .31 | [.16, .45] |
| Residual | 26346.1 | 84 | 313.6 | |||
| L STG | ||||||
| Age | 5866.2 | 1 | 5866.2 | 30.19 | .25 | [.10, .39] |
| Residual | 16320.8 | 84 | 194.3 | |||
| L SMG | ||||||
| Age | 37370 | 1 | 37370 | 24.68 | .21 | [.08, .36] |
| Residual | 127185 | 84 | 1514 | |||
|
| ||||||
| Within Low Loneliness Group (n = 47) | ||||||
| R STG | ||||||
| Age | 4245 | 1 | 4245 | 13.97 | .22 | [.04, .41] |
| Residual | 13369 | 44 | 304 | |||
| L STG | ||||||
| Age | 1529 | 1 | 1529.3 | 7.37 | .12 | [.00, .31] |
| Residual | 9132 | 44 | 207.5 | |||
| L SMG | ||||||
| Age | 10005 | 1 | 10005 | 10.68 | .17 | [.02, .37] |
| Residual | 41236 | 44 | 937 | |||
|
| ||||||
| Within High Loneliness Group (n = 42) | ||||||
| R STG | ||||||
| Age | 9129 | 1 | 9129 | 27.77 | .39 | [.16, .57] |
| Residual | 12820 | 39 | 329 | |||
| L STG | ||||||
| Age | 4917 | 1 | 4917 | 27.26 | .38 | [.15, .57] |
| Residual | 7035 | 39 | 180 | |||
| L SMG | ||||||
| Age | 30829 | 1 | 30829 | 13.99 | .24 | [.04, .44] |
| Residual | 85938 | 39 | 2204 | |||
Notes. Two-way ANCOVA was conducted across loneliness groups with age (dichotomous, younger cohort as the reference group), UCLA Loneliness score (dichotomous, low loneliness as the reference group), age x loneliness interaction, and controlled for sex, separately for three regions from age effect on regional CBF among high lonely group (regional mean CBF was extracted from each region). SS: Sum of Squares; MS: Mean Square; CI: confidence interval; LL: lower limit; UL: upper limit. R: right hemisphere; L: left hemisphere; STG: superior temporal gyrus; SMG: supramarginal gyrus.
Discussion
Main Effect of Age
Loneliness is commonly experienced by both younger and older adults, and its association with adverse physical and mental health has been established in both age populations (J. T. Cacioppo, Hawkley, et al., 2006; Hawkley et al., 2009). To our knowledge, this is the first study to compare two age cohorts matched for loneliness using resting-state ASL MRI. Global mean CBF was significantly lower in the older than in the younger cohort, consistent with prior reports of age-related global mean reduction in CBF (Ambarki et al., 2015; Chen, Rosas, & Salat, 2011; Leoni, Oliveira, Pontes-Neto, Santos, & Leite, 2017; Y. Liu et al., 2012; Parkes, Rashid, Chard, & Tofts, 2004). Group differences as a function of age based on whole-brain voxel-wise analysis at corrected threshold revealed regions of greater resting-state CBF in older adults, consistent with prior studies that reported greater regional CBF in older, compared to younger, adults in temporal, parietal, occipital, and subcortical regions (Galiano et al., 2019; Preibisch et al., 2011).
Main Effect of Loneliness
The most surprising result from this study, given the prior resting-state imaging studies (Feng et al., 2019; Layden et al., 2017; Mwilambwe-Tshilobo et al., 2019; Tian et al., 2017), was the absence of a main effect for loneliness, whether measured by global mean CBF or whole-brain voxel-wise CBF. Our null result of a main effect of loneliness is consistent with our earlier task-based BOLD fMRI study, in which we presented cohorts of younger and older adults with positive social and non-social stimuli and no main effect of loneliness was observed across two age cohorts or within each age cohort (D’Agostino et al., 2018). However, the results from these two studies are not independent, because 14 individuals from the younger cohort, and all individuals from the older cohort, also took part in the D’Agostino et al. study. The absence of a main effect of loneliness may have reflected lacking statistical power due to a small sample size and/or a restricted range of reported loneliness scores. To address the power issue, we conducted a second-level Bayesian Inference analysis using SPM12 and we set the significance threshold at logBF (natural logarithm of Bayes factor) greater than 3 (Han & Park, 2018; Kass & Raftery, 1995). The Bayesian analysis also revealed null result of loneliness main effect. We then concluded that our data did not provide evidence of supporting loneliness effect on resting CBF.
When comparing to the previous resting-state imaging studies, those studies reported regions whose functional connectivity in BOLD signal varied as function of loneliness, whereas our study reported CBF in individual clusters that varied as a function of loneliness. The outcome measures of our study are not directly comparable to prior resting-state BOLD work. It is possible that a connectivity approach is more suitable compared to single voxel/region-based approach. However, the low SNR inherent to the specific form of ASL (pulsed ASL) used here prevented us from conducting functional connectivity analyses that would have allowed us a more direct comparison to prior resting-state studies. Future studies may address this by using a pseudo-continuous ASL approach with higher SNR, and possibly the concurrent collection of resting-state BOLD data.
Moderation of Loneliness on Age and Regional CBF
Although we did not observe a significant interaction between age and loneliness in our whole-brain voxel-wise analyses, we did note that the age-related differences in regional CBF were limited to individuals who reported feeling high levels of loneliness. The first and second cluster are located in superior temporal regions which covered temporo-parietal junction (TPJ). Superior temporal and temporo-parietal regions have been suggested to be key regions crucially involved in social cognition, including social information processing (e.g., Herold, Spengler, Sajonz, Usnich, & Bermpohl, 2016), theory of mind (ToM) and mentalizing processes (e.g., Lombardo et al., 2010; Völlm et al., 2006). Previous studies also suggested that superior temporal region is association with social and emotional processing among people reported higher loneliness (J. T. Cacioppo et al., 2009) and experienced social exclusion (Beyer, Münte, Krämer, Munte, & Kramer, 2014). The third cluster was located in supramarginal gyrus. Both superior temporal and supramarginal regions have been suggested to be a part of attention network (Tian et al., 2017; Vossel, Geng, & Fink, 2013). Tian and colleague (2017) also demonstrated that among high lonely people (UCLA > 45), the association between dorsal attention network and ventral attention network was characterized by hypo-connectivity, such that higher loneliness scores associated with lower functional connectivity between the two networks. The authors suggested that attention processing and vigilance towards the environment might be altered in people with high loneliness. Thus, our data revealed that loneliness has a moderation effect on age and regional CBF in regions associated with social- and attention-related processes, such that age-related differences in those processes were more pronounced among people with high loneliness. Our data is line with the observations that older adults generally performed more poorly in ToM tasks (Duval, Piolino, Bejanin, Eustache, & Desgranges, 2011; Moran, Jolly, & Mitchell, 2012) and poor ToM performance was associated with higher loneliness (Beadle, Brown, Keady, Tranel, & Paradiso, 2012). Furthermore, greater regional CBF in older, relative to younger, adults in all three clusters and such age-related increased regional CBF could be a compensatory mechanism. For example, older adults tended to show greater regional activations during higher cognitive or emotional load tasks (e.g., Ebner et al., 2012; Huang et al., 2012; Zheng et al., 2018) and it is suggested that the increased neural activity is to compensate the age-related neuronal degeneration and to fulfill the comparable behavioral functioning with younger adults. It is possible that age-related differences in social and attention processes and behaviors depend partly on their subjective perceived social isolation. Alternatively, it is possible that younger and older adults adopt different social and attention strategies in response to loneliness.
Limitations
Our study has several limitations. First, our cross-sectional study design does not allow us to draw conclusions regarding causal links, if any, between loneliness, age and regional CBF changes, nor can we delineate whether the observed age-related differences represent consequence of compensation or maladaptation (for example, studies have suggested that both hypo- and hyper-CBF associated with cognitive (e.g., Mak et al., 2012) and social (e.g., Jann et al., 2015) functioning impairment, respectively). Future studies using longitudinal designs could address causal relations. Second, the use of a resting-state paradigm prevents us from drawing conclusions about the functional role of the clusters we identified. Future work using task designs that probe the presumed underlying cognitive, emotional, and social processes as a function of loneliness would fill this gap. In addition, some studies reported the association between personality (especially neuroticism and extraversion) and loneliness (Beadle et al., 2012; Mwilambwe-Tshilobo et al., 2019) and the association between personality and loneliness-related resting-state network was also reported (Feng et al., 2019). A subset of participants from the present study had personality data (measured by NEO-Personality Inventory-Revised (Costa & McCrae, 1992)). We observed that neuroticism and extraversion were associated with loneliness among this subset sample. We then conducted separate ANCOVAs and additionally controlled for neuroticism and extraversion with this subset sample and reported the results in Appendix. As shown in Table A5, when taking personality into account, the age effect remained significant for all three two-way ANCOVAs. Future work is recommended to take personality into account for the potential effect on loneliness. Third, the reported loneliness scores in either cohort did not represent the full range of the scale, although the levels of loneliness from our sample were comparable to previous neuroimaging studies (e.g., Feng et al., 2019; Layden et al., 2017; Nakagawa et al., 2015) and comparable to the populations (including college students, hospital-based nurses, and older people) recruited in the UCLA Loneliness Scale psychometric study (Russell, 1996). This may reflect some degree of subject self-selection, because the same factors that may render some individuals to experience extreme levels of loneliness may have also prevented them from joining our study. On the other hand, it is possible that older people who participated in our study have more active social engagement and/or higher cognitive and social competence than age-matched non-participants, and may therefore not be representative of all older individuals. Future studies may address this by refining methods for recruiting highly lonely individuals. Fourth, the cerebrovascular abnormalities should be considered. This study pre-screened the participants and excluded people with a history of neurological and/or serious medical conditions, including diabetes and stroke. Future work may consider to include diffusion-weighted imaging and/or fluid-attenuated inversion recovery imaging, as these methods are able to detect cerebrovascular abnormalities (Donahue, Strother, & Hendrikse, 2012), and to add abnormalities as a confounding factor.
Strength
The present study also has several strengths. First, to our knowledge, this is the first ASL-based imaging study of aging and loneliness, which allowed us to quantify resting-state regional CBF in a biologically meaningful measure as a function of these two variables. Furthermore, ASL, compared to a BOLD-based signal, is not sensitive to low-frequency signal drift and noise due to subtractive quantification, and CBF reflects a more localized surrogate of cerebral metabolism (F. Liu et al., 2018; T. T. Liu & Brown, 2007; Wang et al., 2008). Second, we controlled for age-related atrophy and individual-level tissue-specific differences in perfusion between GM and WM, using voxel-wise PVC. Third, we addressed a common concern about age-related structural brain differences by creating a SST to reduce bias in the comparison of the two age cohorts. Fourth, we avoided confounds by matching the two age cohorts for loneliness as some population-based studies suggested that younger adults reported higher loneliness (e.g., Beutel et al., 2017; Hawthorne, 2008). Our data highlights the age-related neural manifestation as a function of loneliness, even with comparable levels of loneliness. Our data also encourages the future research to study both variables (i.e., age and loneliness) simultaneously.
Conclusion
In summary, we examined the effect of age and loneliness on CBF at rest by comparing younger and older adults. We observed a cross-sectional reduction in resting-state global mean CBF in older adults. Our two age cohorts were matched for levels of perceived social isolation, and we observed three regions in which participants differed by age and moderated by the levels of loneliness. These regions have previously been implicated in social cognition and attention. The differential associations between age and regional mean CBF between two levels of loneliness potentially support the view of loneliness is experienced differently across the lifespan (Qualter et al., 2015) and different cognitive and emotional processes and strategies used in response to those negative experiences in different ages (Böger & Huxhold, 2018). The neural manifestation of loneliness and age, even with comparable levels of loneliness, observed from our data highlights that the neural findings could be informative and complement behavioral studies and may guide the development of future age-specific behavioral interventions aimed to reduce feelings of social isolation.
Acknowledgments
The work was supported by the National Institute on Aging under Grant R01 AG094578-01 and the National Science Foundation under Grant BCS-0843346 to T.C.
Appendix.
Sex Effect
We conducted two-sample t-test to compare the sex effect on global mean CBF across and separating the age cohorts. Females showed greater global mean CBF, compared to males across age cohorts (Appendix Table A1), consistent with the previous studies (Liu et al., 2012; Parkes, Rashid, Chard, & Tofts, 2004). When separating the age cohorts, the sex difference on global CBF was only among older adults (Appendix Table A1), which also consistent with the previous report (Chen, Rosas, & Salat, 2011). Furthermore, we conducted a factorial ANOVA to examine the main effect of age and sex and the interaction of the two variables on global mean CBF, and only the age was significantly associated with global mean CBF (Age: F(1, 85) = 16.50, p = .0001, partial ω2 = .15, 95% CI [.04, .29]; Sex: F(1, 85) = 6.48, p = .0127, partial ω2 = .06, 95% CI [.00, .18]; Age x Sex: F(1, 85) = 0.04, p = .8377, partial ω2 = .00, 95% CI [.00, .00]).
We examined whether there is sex difference in regional CBF from the whole-brain voxel-wise factorial analyses, while controlling for age, loneliness, and interaction between age and loneliness. No cluster was revealed at family-wise error (FWE) corrected p < .05, while controlling for age, loneliness, and their interaction.
Voxel-Based Morphometry Analysis
To further examined the potential effect from brain structural difference, we conducted a voxel-base morphometry (VBM) analysis to measure gray matter volume (GMV). Individual gray matter images, which were obtained from the segmentation of the T1w-MPRAGE image were normalized to the SST created using DARTEL (as described in the Method section). Absolute GMVs were modulated by multiplying the Jacobian determinants derived from the normalization step to preserve the volumes of different structures (Ashburner & Friston, 2000). Finally, the modulated GMV images were smoothed with a 10 mm FWHM isotropic Gaussian kernel. To exclude the potential background noise, a threshold of absolute value of 0.2 was applied (i.e., excluding voxels with absolute value < 0.2) for further statistical analysis.
We first examined whether age, loneliness, or their interaction yielded significant effect on GMV. We conducted a 2 (age: Young versus Old) x 2 (loneliness: Low versus High) factorial analysis among three significant clusters (i.e., left and right superior temporal gyrus, and left supramarginal gyrus, a binary mask containing three functional clusters was created using MarsBaR (Brett, Anton, Valabregue, & Poline, 2002)). No main effect of age and loneliness, nor the interaction effect on GMV were observed at voxel-level FWE p < .05, k > 10 voxels. Next, we extracted mean GMV from each of three significant clusters. For all three clusters, younger cohort showed greater mean GMVs compared to older cohort (Table A2). We then added the mean GMV into respective ANCOVAs as covariate (in addition to sex) and all results shown in Table 3 remained unchanged, in which that age was the only significant IV in all two-way and one-way ANCOVAs and that the effect sizes (partial ω2) of age were quantitatively larger in models among high lonely group, relative to low lonely group.
Personality Effect
From the included participants in the present study, a subset of fifty-eight individuals (Table A4) had personality trait data, measured by NEO-Personality Inventory-Revised (Costa & McCrae, 1992). We observed that loneliness was associated with neuroticism (b = 0.27, p = .0009) and extraversion (b = −0.38, p = .0011) (all five traits (continuous variables) were entered in the same regression). When additionally controlling for Neuroticism and Extraversion, the age effect remained significant for all three two-way ANCOVAs.
Table A1.
Sex effect on global mean CBF, separating the age cohorts.
| Males | Females | t | df | p | |
|---|---|---|---|---|---|
|
|
|||||
| Mean ± SD | Mean ± SD | ||||
|
| |||||
| N | |||||
| Younger | 10 | 34 | |||
| Older | 16 | 29 | |||
| Age | |||||
| Younger | 21.60 ± 3.66 | 20.68 ± 3.10 | 0.80 | 42 | .4310 |
| Older | 62.96 ± 7.56 | 63.64 ± 6.15 | −0.32 | 43 | .7489 |
| UCLAa | |||||
| Younger | 42.50 ± 8.72 | 39.12 ± 8.19 | 1.13 | 42 | .2643 |
| Low UCLA (n (%)) | 4 (40.0) | 19 (55.9) | |||
| High UCLA (n (%)) | 6 (60.0) | 15 (44.1) | |||
| Older | 39.97 ± 8.65 | 37.00 ± 7.83 | 1.17 | 43 | .2470 |
| Low UCLA (n (%)) | 7 (43.8) | 17 (58.6) | |||
| High UCLA (n (%)) | 9 (56.3) | 12 (41.4) | |||
| Global mean CBF (mL/100g/min) | |||||
| Across age (N = 89) | 32.57 ± 5.79 | 38.11 ± 8.94 | −3.46 | 70.83 | .0009*** |
| Younger (n = 44) | 36.61 ± 4.19 | 40.78 ± 7.15 | −1.75 | 42 | .0875 |
| Older (n = 45) | 30.05 ± 5.26 | 34.97 ± 9.90 | −2.18 | 42.98 | .0351* |
Note.
UCLA: UCLA Loneliness Scale scores, and total score range 20 – 80. The variances of global mean CBF between females and males from the across age cohorts and among older cohort were identified as unequal and the t-test was performed with two variances being treated as unequal.
p < .05;
p < .01;
p < .001
Table A2.
Descriptive statistics for mean GMV from three significant clusters for younger and older cohorts (N = 89)
| Younger | Older | t | df | p | |
|---|---|---|---|---|---|
|
| |||||
| Mean ± SD | Mean ± SD | ||||
|
| |||||
| N | 44 | 45 | |||
| Regional mean GMV (L) | |||||
| R STG | 0.36 ± 0.03 | 0.30 ± 0.03 | 7.97 | 87 | 5.78e-12*** |
| L STG | 0.35 ± 0.03 | 0.30 ± 0.03 | 8.30 | 87 | 1.24e-12*** |
| L SMG | 0.36 ± 0.06 | 0.29 ± 0.04 | 5.95 | 87 | 5.50e-08*** |
Note. GMV: gray matter volume; R: right hemisphere; L: left hemisphere; STG: superior temporal gyrus; SMG: supramarginal gyrus.
p < .05;
p < .01;
p < .001
Table A3.
Results of age from ANCOVAs using regional mean CBF extracted from three age-related regions among high lonely group after removing one outlier identified by DFFIT (N = 88).
| Predictor | SS | df | MS | F | Partial ω2 | Partial ω2 95% CI [LL, UL] |
|---|---|---|---|---|---|---|
|
| ||||||
| Across Loneliness Groups (N = 88) | ||||||
| R STG | ||||||
| Age | 10574.1 | 1 | 10574.1 | 45.77 | .34 | [.18, .48] |
| Residual | 19174 | 83 | 231 | |||
| L STG | ||||||
| Age | 5311 | 1 | 5311 | 28.50 | .24 | [.10, .38] |
| Residual | 15469.2 | 83 | 186.4 | |||
| L SMG | ||||||
| Age | 31007 | 1 | 31006.7 | 23.88 | .21 | [.07, .35] |
| Residual | 107754 | 83 | 1298.2 | |||
|
| ||||||
| Within Low Loneliness Group (n = 47) | ||||||
| R STG | ||||||
| Age | 4245 | 1 | 4245 | 13.97 | .22 | [.04, .41] |
| Residual | 13369 | 44 | 304 | |||
| L STG | ||||||
| Age | 1529 | 1 | 1529.3 | 7.37 | .12 | [.00, .31] |
| Residual | 9132 | 44 | 207.5 | |||
| L SMG | ||||||
| Age | 10005 | 1 | 10005 | 10.68 | .17 | [.02, .37] |
| Residual | 41236 | 44 | 937 | |||
|
| ||||||
| Within High Loneliness Group (n = 41) | ||||||
| R STG | ||||||
| Age | 6544 | 1 | 6544 | 42.85 | .51 | [.27, .66] |
| Residual | 5803 | 38 | 153 | |||
| L STG | ||||||
| Age | 4212 | 1 | 4212 | 25.56 | .37 | [.14, .56] |
| Residual | 6263 | 38 | 165 | |||
| L SMG | ||||||
| Age | 22765 | 1 | 22765 | 13.05 | .23 | [.04, .44] |
| Residual | 66292 | 38 | 1745 | |||
Notes. Two-way ANCOVA was conducted across loneliness groups with age (dichotomous, younger cohort as the reference group), UCLA Loneliness score (dichotomous, low loneliness as the reference group), age x loneliness interaction, and controlled for sex, separately for three regions from age effect on regional CBF among high lonely group (regional mean CBF was extracted from each region). SS: Sum of Squares; MS: Mean Square; CI: confidence interval; LL: lower limit; UL: upper limit. R: right hemisphere; L: left hemisphere; STG: superior temporal gyrus; SMG: supramarginal gyrus.
Table A4.
Descriptive statistics for personality sub-sample (N = 58).
| Younger | Older | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
|
| ||
| n | 30 | 28 |
| Age | 21.33 ± 3.76 | 63.18 ± 6.43 |
| Sex | ||
| Male (n (%)) | 7 (23.3) | 10 (35.7) |
| Female (n (%)) | 23 (76.7) | 18 (64.3) |
| UCLAa | 39.33 ± 8.26 | 37.69 ± 7.84 |
| Lowb (n (%)) | 16 (53.3) | 15 (53.6) |
| Highc (n (%)) | 14 (46.7) | 13 (46.4) |
| Regional mean CBF (mL/100g/min) | ||
| Right STG | 56.94 ± 7.38 | 76.14 ± 15.11 |
| Low UCLA | 58.16 ± 7.72 | 74.54 ± 18.24 |
| High UCLA | 55.55 ± 7.00 | 77.99 ± 10.93 |
| Left STG | 62.57 ± 11.15 | 78.80 ± 14.67 |
| Low UCLA | 64.93 ± 13.09 | 76.36 ± 15.13 |
| High UCLA | 59.88 ± 8.06 | 81.62 ± 14.19 |
| Left SMG | 68.87 ± 14.29 | 104.70 ± 47.43 |
| Low UCLA | 67.67 ± 13.82 | 100.45 ± 36.10 |
| High UCLA | 70.23 ± 15.22 | 109.60 ± 59.10 |
Note.
UCLA: UCLA Loneliness Scale scores, and total score range 20 – 80;
Low lonely group whose UCLA loneliness score was less than 41;
High lonely group whose UCLA loneliness score was 41 or higher. R: right hemisphere; L: left hemisphere; STG: superior temporal gyrus; SMG: supramarginal gyrus.
Table A5.
Personality sub-sample results of age from ANCOVAs using regional mean CBF extracted from three age-related regions among high lonely group, controlling for sex, Neuroticism, and Extraversion.
| Predictor | SS | df | MS | F | Partial ω2 | Partial ω2 95% CI [LL, UL] |
|---|---|---|---|---|---|---|
| Across Loneliness Groups (N = 58) | ||||||
|
| ||||||
| R STG | ||||||
| Age | 5341 | 1 | 5341 | 35.79 | .37 | [.17, .54] |
| Residual | 7610 | 51 | 149 | |||
| L STG | ||||||
| Age | 3815 | 1 | 3815 | 22.18 | .27 | [.08, .45] |
| Residual | 8772 | 51 | 172 | |||
| L SMG | ||||||
| Age | 18613 | 1 | 18613 | 14.45 | .19 | [.03, .37] |
| Residual | 65676 | 51 | 1288 | |||
|
| ||||||
| Within Low Loneliness Group (n = 31) | ||||||
|
| ||||||
| R STG | ||||||
| Age | 2078 | 1 | 2078 | 9.94 | .22 | [.01, .47] |
| Residual | 5438 | 26 | 209.2 | |||
| L STG | ||||||
| Age | 1011 | 1 | 1011 | 4.77 | .11 | [.00, .35] |
| Residual | 5508 | 26 | 211.9 | |||
| L SMG | ||||||
| Age | 8322 | 1 | 8322 | 10.92 | .24 | [.02, .49] |
| Residual | 19823 | 26 | 762 | |||
|
| ||||||
| Within High Loneliness Group (n = 27) | ||||||
|
| ||||||
| R STG | ||||||
| Age | 3394 | 1 | 1070.8 | 36.64 | .57 | [.27, .74] |
| Residual | 2038 | 22 | 93 | |||
| L STG | ||||||
| Age | 3188 | 1 | 3188 | 25.88 | .48 | [.17, .68] |
| Residual | 2711 | 22 | 123 | |||
| L SMG | ||||||
| Age | 10447 | 1 | 10447 | 5.40 | .14 | [.00, .41] |
| Residual | 42571 | 22 | 1935 | |||
Notes. Two-way ANCOVA was conducted across loneliness groups with age (dichotomous, younger cohort as the reference group), UCLA Loneliness score (dichotomous, low loneliness as the reference group), age x loneliness interaction, and controlled for sex, neuroticism, and extraversion, separately for three regions from age effect on regional CBF among high lonely group (regional mean CBF was extracted from each region). SS: Sum of Squares; MS: Mean Square; CI: confidence interval; LL: lower limit; UL: upper limit. R: right hemisphere; L: left hemisphere; STG: superior temporal gyrus; SMG: supramarginal gyrus.
Footnotes
Declaration of Interest Statement
No potential competing interest was reported by the authors.
Data Availability Statement
The un-thresholded statistical maps were uploaded to NeuroVault.org database and are available at http://neurovault.org/collections/8562/. The data that support the findings of this study are available from the senior author (T.C.) upon reasonable request.
References
- Allen P, Chaddock CA, Egerton A, Howes OD, Bonoldi I, Zelaya F, … McGuire P. (2016). Resting Hyperperfusion of the Hippocampus, Midbrain, and Basal Ganglia in People at High Risk for Psychosis. American Journal of Psychiatry, 173(4), 392–399. 10.1176/appi.ajp.2015.15040485 [DOI] [PubMed] [Google Scholar]
- Ambarki K, Wåhlin A, Zarrinkoob L, Wirestam R, Petr J, Malm J, & Eklund A. (2015). Accuracy of parenchymal cerebral blood flow measurements using pseudocontinuous arterial spin-labeling in healthy volunteers. American Journal of Neuroradiology, 36(10), 1816–1821. 10.3174/ajnr.A4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. 10.1016/J.NEUROIMAGE.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Asllani I, Borogovac A, & Brown TR (2008). Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magnetic Resonance in Medicine, 60(6), 1362–1371. 10.1002/mrm.21670 [DOI] [PubMed] [Google Scholar]
- Beadle JN, Brown V, Keady B, Tranel D, & Paradiso S. (2012). Trait empathy as a predictor of individual differences in perceived loneliness. Psychological Reports, 110(1), 3–15. 10.2466/07.09.20.PR0.110.1.3-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutel ME, Klein EM, Brähler E, Reiner I, Jünger C, Michal M, … Tibubos AN (2017). Loneliness in the general population: Prevalence, determinants and relations to mental health. BMC Psychiatry, 17(1), 97. 10.1186/s12888-017-1262-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer F, Münte TF, Krämer UM, Munte TF, & Kramer UM (2014). Increased neural reactivity to socio-emotional stimuli links social exclusion and aggression. Biological Psychology, 96(1), 102–110. 10.1016/j.biopsycho.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Böger A, & Huxhold O. (2018). Do the antecedents and consequences of loneliness change from middle adulthood into old age? Developmental Psychology, 54(1), 181–197. 10.1037/dev0000453 [DOI] [PubMed] [Google Scholar]
- Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, & Edelman RR (1998). A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magnetic Resonance in Medicine, 40(3), 383–396. 10.1002/mrm.1910400308 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Ernst JM, Burleson M, Berntson GG, Nouriani B, & Spiegel D. (2006). Loneliness within a nomological net: An evolutionary perspective. Journal of Research in Personality, 40(6), 1054–1085. 10.1016/J.JRP.2005.11.007 [DOI] [Google Scholar]
- Cacioppo JT, Hawkley LC, & Thisted RA (2010). Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychology and Aging, 25(2), 453–463. 10.1037/a0017216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, & Thisted RA (2006). Loneliness as a specific risk factor for depressive symptoms: Cross-sectional and longitudinal analyses. Psychology and Aging, 21(1), 140–151. 10.1037/0882-7974.21.1.140 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Norris CJ, Decety J, Monteleone G, & Nusbaum H. (2009). In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. Journal of Cognitive Neuroscience, 21(1), 83–92. 10.1162/jocn.2009.21007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo S, Balogh S, & Cacioppo JT (2015). Implicit attention to negative social, in contrast to nonsocial, words in the Stroop task differs between individuals high and low in loneliness: Evidence from event-related brain microstates. Cortex, 70, 213–233. 10.1016/J.CORTEX.2015.05.032 [DOI] [PubMed] [Google Scholar]
- Canli T, Wen R, Wang X, Mikhailik A, Yu L, Fleischman D, … Bennett DA (2017). Differential transcriptome expression in human nucleus accumbens as a function of loneliness. Molecular Psychiatry, 22(7), 1069–1078. 10.1038/mp.2016.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Yu L, Yu X, Zhao H, Fleischman D, Wilson RS, … Bennett DA (2018). Loneliness 5 years ante-mortem is associated with disease-related differential gene expression in postmortem dorsolateral prefrontal cortex. Translational Psychiatry, 8(1), 2. 10.1038/s41398-017-0086-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Moffitt TE, Milne BJ, & Poulton R. (2006). Socially Isolated Children 20 Years Later. Archives of Pediatrics & Adolescent Medicine, 160(8), 805. 10.1001/archpedi.160.8.805 [DOI] [PubMed] [Google Scholar]
- Chen JJ, Rosas HD, & Salat DH (2011). Age-associated reductions in cerebral blood flow are independent from regional atrophy. NeuroImage, 55(2), 468–478. 10.1016/j.neuroimage.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JMG, & Cacioppo JT (2011). Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 3080–3085. 10.1073/pnas.1014218108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, & Cacioppo JT (2007). Social regulation of gene expression in human leukocytes. Genome Biology, 8(9). 10.1186/gb-2007-8-9-r189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PTJ, & McCrae RR (1992). Revised NEO Personality Inventory (NEO-PI-R) and NEO Five Factor Inventory (NEO-FFI) Professional Manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- D’Agostino AE, Kattan D, & Canli T. (2018). An fMRI study of loneliness in younger and older adults. Social Neuroscience, 14(2), 136–148. 10.1080/17470919.2018.1445027 [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Strother MK, & Hendrikse J. (2012, March). Novel MRI approaches for assessing cerebral hemodynamics in ischemic cerebrovascular disease. Stroke, Vol. 43, pp. 903–915. 10.1161/STROKEAHA.111.635995 [DOI] [PubMed] [Google Scholar]
- Donovan NJ, Wu Q, Rentz DM, Sperling RA, Marshall GA, & Glymour MM (2017). Loneliness, depression and cognitive function in older U.S. adults. International Journal of Geriatric Psychiatry, 32(5), 564–573. 10.1002/gps.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval C, Piolino P, Bejanin A, Eustache F, & Desgranges B. (2011). Age effects on different components of theory of mind. Consciousness and Cognition, 20(3), 627–642. 10.1016/j.concog.2010.10.025 [DOI] [PubMed] [Google Scholar]
- Ebner NC, Johnson MK, & Fischer H. (2012). Neural mechanisms of reading facial emotions in young and older adults. Frontiers in Psychology, 3(JUL), 223. 10.3389/fpsyg.2012.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Wang L, Li T, & Xu P. (2019). Connectome-based individualized prediction of loneliness. Social Cognitive and Affective Neuroscience, 14(4), 353–365. 10.1093/scan/nsz020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiano A, Mengual E, García de Eulate R, Galdeano I, Vidorreta M, Recio M, … Fernández-Seara MA (2019). Coupling of cerebral blood flow and functional connectivity is decreased in healthy aging. Brain Imaging and Behavior. 10.1007/s11682-019-00157-w [DOI] [PubMed] [Google Scholar]
- Ge L, Yap CW, Ong R, & Heng BH (2017). Social isolation, loneliness and their relationships with depressive symptoms: A population-based study. PLOS ONE, 12(8), e0182145. 10.1371/journal.pone.0182145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerino E, Rollè L, Sechi C, & Brustia P. (2017). Loneliness, resilience, mental health, and quality of life in old age: A structural equation model. Frontiers in Psychology, 8, 2003. 10.3389/fpsyg.2017.02003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, & Park J. (2018). Using SPM 12’s second-Level Bayesian inference procedure for fMRI analysis: Practical guidelines for end users. Frontiers in Neuroinformatics, 12, 1. 10.3389/fninf.2018.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Qualter P, & Robinson SJ (2013). Loneliness trajectories from middle childhood to pre-adolescence: Impact on perceived health and sleep disturbance. Journal of Adolescence, 36(6), 1295–1304. 10.1016/J.ADOLESCENCE.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Hawkley LC, & Cacioppo JT (2010). Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Annals of Behavioral Medicine, 40(2), 218–227. 10.1007/s12160-010-9210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Thisted RA, & Cacioppo JT (2009). Loneliness predicts reduced physical activity: cross-sectional & longitudinal analyses. Health Psychology, 28(3), 354–363. 10.1037/a0014400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne G. (2008). Perceived social isolation in a community sample: Its prevalence and correlates with aspects of peoples’ lives. Social Psychiatry and Psychiatric Epidemiology, 43(2), 140–150. 10.1007/s00127-007-0279-8 [DOI] [PubMed] [Google Scholar]
- Herold D, Spengler S, Sajonz B, Usnich T, & Bermpohl F. (2016). Common and distinct networks for self-referential and social stimulus processing in the human brain. Brain Structure & Function, 221(7), 3475–3485. 10.1007/s00429-015-1113-9 [DOI] [PubMed] [Google Scholar]
- Ho DE, Imai K, King G, & Stuart EA (2011). MatchIt: Nonparametric preprocessing for parametric causal inference. Journal of Statistical Software, 42(8), 1–28. 10.18637/jss.v042.i08 [DOI] [Google Scholar]
- Holwerda TJ, van Tilburg TG, Deeg DJH, Schutter N, Van R, Dekker J, … Schoevers RA (2016). Impact of loneliness and depression on mortality: Results from the Longitudinal Ageing Study Amsterdam. British Journal of Psychiatry, 209(2), 127–134. 10.1192/bjp.bp.115.168005 [DOI] [PubMed] [Google Scholar]
- Huang CM, Polk TA, Goh JO, & Park DC (2012). Both left and right posterior parietal activations contribute to compensatory processes in normal aging. Neuropsychologia, 50(1), 55–66. 10.1016/j.neuropsychologia.2011.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Moieni M, Dutcher JM, Jevtic I, Irwin MR, & Eisenberger NI (2016). Yearning for connection? Loneliness is associated with increased ventral striatum activity to close others. Social Cognitive and Affective Neuroscience, 11(7), 1096–1101. 10.1093/scan/nsv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K, Hernandez LM, Beck-Pancer D, McCarron R, Smith RX, Dapretto M, & Wang DJJ (2015). Altered resting perfusion and functional connectivity of default mode network in youth with autism spectrum disorder. Brain and Behavior, 5(9), e00358. 10.1002/brb3.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Duchaine B, Janik A, Banissy MJ, & Rees G (2012). Brain structure links loneliness to social perception. Current Biology, 22(20), 1975–1979. 10.1016/J.CUB.2012.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass RE, & Raftery AE (1995). Bayes factors. Journal of the American Statistical Association, 90(430), 773–795. 10.1080/01621459.1995.10476572 [DOI] [Google Scholar]
- Kim SG, & Uǧurbil K. (1997). Comparison of blood oxygenation and cerebral blood flow effects in fMRI: Estimation of relative oxygen consumption change. Magnetic Resonance in Medicine, 38(1), 59–65. 10.1002/mrm.1910380110 [DOI] [PubMed] [Google Scholar]
- Lan C-C, Tsai S-J, Huang C-C, Wang Y-H, Chen T-R, Yeh H-L, … Yang AC (2016). Functional connectivity density mapping of depressive symptoms and loneliness in non-demented elderly male. Frontiers in Aging Neuroscience, 7, 251. 10.3389/fnagi.2015.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden EA, Cacioppo JT, Cacioppo S, Cappa SF, Dodich A, Falini A, & Canessa N. (2017). Perceived social isolation is associated with altered functional connectivity in neural networks associated with tonic alertness and executive control. NeuroImage, 145(Pt A), 58–73. 10.1016/j.neuroimage.2016.09.050 [DOI] [PubMed] [Google Scholar]
- Leoni RF, Oliveira IAF, Pontes-Neto OM, Santos AC, & Leite JP (2017). Cerebral blood flow and vasoreactivity in aging: An arterial spin labeling study. Brazilian Journal of Medical and Biological Research, 50(4). 10.1590/1414-431X20175670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Dolui S, Xie D-F, & Wang Z. (2018). Priors-guided slice-wise adaptive outlier cleaning for arterial spin labeling perfusion MRI. Journal of Neuroscience Methods, 307, 248–253. 10.1016/J.JNEUMETH.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, & Yang Y. (2013). Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proceedings of the National Academy of Sciences, 110(5), 1929–1934. 10.1073/pnas.1214900110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Duan Y, Peterson BS, Asllani I, Zelaya F, Lythgoe D, & Kangarlu A. (2018). Resting state cerebral blood flow with arterial spin labeling MRI in developing human brains. European Journal of Paediatric Neurology, 22(4), 642–651. 10.1016/J.EJPN.2018.03.003 [DOI] [PubMed] [Google Scholar]
- Liu TT, & Brown GG (2007). Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. Journal of the International Neuropsychological Society, 13(03), 517–525. 10.1017/S1355617707070646 [DOI] [PubMed] [Google Scholar]
- Liu Y, Li B, Feng N, Pu H, Zhang X, Lu H, & Yin H. (2016). Perfusion deficits and functional connectivity alterations in memory-related regions of patients with Post-Traumatic Stress Disorder. PLOS ONE, 11(5), e0156016. 10.1371/journal.pone.0156016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhu X, Feinberg D, Guenther M, Gregori J, Weiner MW, & Schuff N. (2012). Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magnetic Resonance in Medicine, 68(3), 912–922. 10.1002/mrm.23286 [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling JS, … Williams SC (2010). Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience, 22(7), 1623–1635. 10.1162/jocn.2009.21287 [DOI] [PubMed] [Google Scholar]
- Luh WM, Wong EC, Bandettini PA, & Hyde JS (1999). QUIPSS II with thin-slice TI1 periodic saturation: A method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magnetic Resonance in Medicine, 41(6), 1246–1254. [DOI] [PubMed] [Google Scholar]
- Luhmann M, & Hawkley LC (2016). Age differences in loneliness from late adolescence to oldest old age. Developmental Psychology, 52(6), 943–959. 10.1037/dev0000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak HKF, Chan Q, Zhang Z, Petersen ET, Qiu D, Zhang L, … Golay X. (2012). Quantitative assessment of cerebral hemodynamic parameters by QUASAR arterial spin labeling in Alzheimer’s disease and cognitively normal elderly adults at 3-Tesla. Journal of Alzheimer’s Disease, 31(1), 33–44. 10.3233/JAD-2012-111877 [DOI] [PubMed] [Google Scholar]
- Matsuda H. (2013). Voxel-based morphometry of brain MRI in normal aging and Alzheimer’s Disease. Aging and Disease, 4(1), 29–37. [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Jolly E, & Mitchell JP (2012). Social-cognitive deficits in normal aging. The Journal of Neuroscience, 32(16), 5553–5561. 10.1523/JNEUROSCI.5511-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwilambwe-Tshilobo L, Ge T, Chong M, Ferguson MA, Misic B, Burrow AL, … Spreng RN (2019). Loneliness and meaning in life are reflected in the intrinsic network architecture of the brain. Social Cognitive and Affective Neuroscience, 14(4), 423–433. 10.1093/scan/nsz021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Takeuchi H, Taki Y, Nouchi R, Sekiguchi A, Kotozaki Y, … Kawashima R. (2015). White matter structures associated with loneliness in young adults. Scientific Reports, 5, 17001. 10.1038/srep17001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, & Tofts PS (2004). Normal cerebral perfusion measurements using arterial spin labeling: Reproducibility, stability, and age and gender effects. Magnetic Resonance in Medicine, 51(4), 736–743. 10.1002/mrm.20023 [DOI] [PubMed] [Google Scholar]
- Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, & Nichols TE (2011). Statistical parametric mapping : the analysis of funtional brain images. Cambridge, Massachusetts: Academic Press. Retrieved from https://books.google.com/books?id=G_qdEsDlkp0C&lr=&source=gbs_navlinks_s [Google Scholar]
- Perissinotto CM, Stijacic Cenzer I, & Covinsky KE (2012). Loneliness in older persons: A predictor of functional decline and death. Archives of Internal Medicine, 172(14), 1078–1084. 10.1001/archinternmed.2012.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M, & Sorensen S. (2001). Influences on loneliness in older adults: A meta-analysis. Basic and Applied Social Psychology, 23(4), 245–266. 10.1207/S15324834BASP2304_2 [DOI] [Google Scholar]
- Powers KE, Wagner DD, Norris CJ, & Heatherton TF (2013). Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes. Social Cognitive and Affective Neuroscience, 8(2), 151–157. 10.1093/scan/nsr079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preibisch C, Sorg C, Förschler A, Grimmer T, Sax I, Wohlschläger AM, … Alexopoulos P. (2011). Age-related cerebral perfusion changes in the parietal and temporal lobes measured by pulsed arterial spin labeling. Journal of Magnetic Resonance Imaging, 34(6), 1295–1302. 10.1002/jmri.22788 [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, & Treanor JJ (2005). Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychology, 24(3), 297–306. 10.1037/0278-6133.24.3.297 [DOI] [PubMed] [Google Scholar]
- Qualter P, Vanhalst J, Harris R, Van Roekel E, Lodder G, Bangee M, … Verhagen M. (2015). Loneliness across the life span. Perspectives on Psychological Science, 10(2), 250–264. 10.1177/1745691615568999 [DOI] [PubMed] [Google Scholar]
- Russell DW (1996). UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. Journal of Personality Assessment, 66(1), 20–40. 10.1207/s15327752jpa6601_2 [DOI] [PubMed] [Google Scholar]
- Schneider K, Michels L, Hartmann-Riemer MN, Burrer A, Tobler PN, Stämpfli P, … Kaiser S. (2019). Cerebral blood flow in striatal regions is associated with apathy in patients with schizophrenia. Journal of Psychiatry and Neuroscience, 44(2), 102–110. 10.1503/jpn.170150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, … Neylan TC (2011). Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: An MRI study. NeuroImage, 54, S62–S68. 10.1016/J.NEUROIMAGE.2010.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak S, Polimeni JR, Wang DJJ, Yan L, & Chen JJ (2015). Associations of Resting-State fMRI Functional Connectivity with Flow-BOLD Coupling and Regional Vasculature. Brain Connectivity, 5(3), 137–146. 10.1089/brain.2014.0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak S, Wang DJJ, Polimeni JR, Yan L, & Chen JJ (2014). Dynamic and static contributions of the cerebrovasculature to the resting-state BOLD signal. NeuroImage, 84, 672–680. 10.1016/j.neuroimage.2013.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Liang S, Yuan Z, Chen S, Xu P, & Yao D. (2014). White matter structure in loneliness: Preliminary findings from diffusion tensor imaging. NeuroReport, 25(11), 843–847. 10.1097/WNR.0000000000000197 [DOI] [PubMed] [Google Scholar]
- Tian Y, Yang L, Chen S, Guo D, Ding Z, Tam KY, & Yao D. (2017). Causal interactions in resting-state networks predict perceived loneliness. PLOS ONE, 12(5), e0177443. 10.1371/journal.pone.0177443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasic N, Wolf ND, Grön G, Sosic-Vasic Z, Connemann BJ, Sambataro F, … Wolf RC (2015). Baseline brain perfusion and brain structure in patients with major depression: A multimodal magnetic resonance imaging study. Journal of Psychiatry and Neuroscience, 40(6), 412–421. 10.1503/jpn.140246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völlm BA, Taylor ANW, Richardson P, Corcoran R, Stirling J, McKie S, … Elliott R. (2006). Neuronal correlates of theory of mind and empathy: A functional magnetic resonance imaging study in a nonverbal task. NeuroImage, 29(1), 90–98. 10.1016/j.neuroimage.2005.07.022 [DOI] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, & Fink GR (2013). Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist, 20(2), 150–159. 10.1177/1073858413494269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernández-Seara MA, Childress AR, & Detre JA (2008). Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magnetic Resonance Imaging, 26(2), 261–269. 10.1016/J.MRI.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, … Bennett DA (2007). Loneliness and risk of Alzheimer disease. Archives of General Psychiatry, 64(2), 234. 10.1001/archpsyc.64.2.234 [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, & Frank LR (1997). Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR in Biomedicine, 10(4‐5), 237–249. [DOI] [PubMed] [Google Scholar]
- Wong NML, Liu H-L, Lin C, Huang C-M, Wai Y-Y, Lee S-H, & Lee TMC (2016). Loneliness in late-life depression: structural and functional connectivity during affective processing. Psychological Medicine, 46(12), 2485–2499. 10.1017/S0033291716001033 [DOI] [PubMed] [Google Scholar]
- Xu G, Rowley HA, Wu G, Alsop DC, Shankaranarayanan A, Dowling M, … Johnson SC (2010). Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15 O-water PET in elderly subjects at risk for Alzheimer’s disease. NMR in Biomedicine, 23(3), 289–293. 10.1002/nbm.1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Herrington JD, Bartley GK, Liu HS, Detre JA, & Schultz RT (2018). Arterial spin labeling provides a reliable neurobiological marker of autism spectrum disorder. Journal of Neurodevelopmental Disorders, 10(1). 10.1186/s11689-018-9250-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Herzog H, Mauler J, Filss C, Okell TW, Kops ER, … Shah NJ (2014). Comparison of cerebral blood flow acquired by simultaneous [15O]water positron emission tomography and arterial spin labeling magnetic resonance imaging. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 34(8), 1373–1380. 10.1038/jcbfm.2014.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Gordon ML, & Goldberg TE (2017). Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neuroscience & Biobehavioral Reviews, 72, 168–175. 10.1016/j.neubiorev.2016.11.023 [DOI] [PubMed] [Google Scholar]
- Zheng L, Gao Z, Xiao X, Ye Z, Chen C, & Xue G. (2018). Reduced fidelity of neural representation underlies episodic memory decline in normal aging. Cerebral Cortex, 28(7), 2283–2296. Retrieved from 10.1093/cercor/bhx130 [DOI] [PubMed] [Google Scholar]
Appendix References
- Ashburner J, & Friston KJ (2000). Voxel-based morphometry - The methods. NeuroImage, 11(6 I), 805–821. 10.1006/nimg.2000.0582 [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, & Poline J-B (2002, June). Region of interest analysis using an SPM toolbox [abstract]. Presented at The 8th International Conferance on Functional Mapping of the Human Brain, Sendai, Japan. [Google Scholar]
- Chen JJ, Rosas HD, & Salat DH (2011). Age-associated reductions in cerebral blood flow are independent from regional atrophy. NeuroImage, 55(2), 468–478. 10.1016/j.neuroimage.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PTJ, & McCrae RR (1992). Revised NEO Personality Inventory (NEO-PI-R) and NEO Five Factor Inventory (NEO-FFI) Professional Manual Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Liu Y, Zhu X, Feinberg D, Guenther M, Gregori J, Weiner MW, & Schuff N. (2012). Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magnetic Resonance in Medicine, 68(3), 912–922. 10.1002/mrm.23286 [DOI] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, & Tofts PS (2004). Normal cerebral perfusion measurements using arterial spin labeling: Reproducibility, stability, and age and gender effects. Magnetic Resonance in Medicine, 51(4), 736–743. 10.1002/mrm.20023 [DOI] [PubMed] [Google Scholar]