Abstract
The visible light induced, photocatalysts or photoabsorbing EDA complexes mediated cleavage of pyridinium C-N bond were reported in the past years. Here, we report an ionic compound promote homolytic cleavage of pyridinium C-N bond by exploiting the photonic energy from visible light. This finding is successfully applied in deaminative hydroalkylation of a series of alkenes including naturally occurring dehydroalanine, which provides an efficient way to prepare β-alkyl substituted unnatural amino acids under mild and photocatalyst-free conditions. Importantly, by using this protocol, the deaminative cyclization of peptide backbone N-terminals is realized. Furthermore, the use of Et3N or PPh3 as reductants and H2O as hydrogen atom source is a practical advantage. We anticipate that our protocol will be useful in peptide synthesis and modern peptide drug discovery.
Subject terms: Photochemistry, Synthetic chemistry methodology
To address the shortcomings in the application of bioactive peptides as drugs, incorporation of unnatural amino acids (UAAs) has been used. Here, the authors report an ionic compound-promoted C-N cleavage of alkyl pyridinium to generate alkyl radicals upon excitation by visible light, and apply it for deaminative hydroalkylation of alkenes to synthesise diverse β-alkyl substituted UAAs.
Introduction
Peptides are indispensable bioactive components for exerting biological function normally in various cells. Thus, therapeutic peptide is deemed to be an exquisite substitution of an endogenous molecule which possesses high affinity and selectivity against a pharmacologically diverse set of biological targets1,2. Although remarkable achievements have been made in peptide research, poor membrane permeability, low metabolic resistance, and bioavailability still are insurmountable barriers to endogenous bioactive peptide therapeutic3. In this context, several methods have been developed and applied to solve these problems, such as incorporation of unnatural amino acids (UAAs) or macrocyclizations of linear peptides4.
In recent years, with the rapid development of photochemistry, visible light promoted radical coupling reactions have become important pathway for building chemical bonds5. Owing to mild reaction conditions and excellent functional group tolerance, photoinduced chemical transformations provided an excellent strategy for chemoselective biomolecule modification, which are widely applied in the modification of amino acids, peptides, and proteins6–12. The modification of amino acids is an important strategy for preparing UAAs, such as the modification of glycine, cysteine, tyrosine, tryptophan, histidine, etc. Dehydroalanine (Dha) is a naturally occurring amino acid13,14, and also can be easily prepared from Ser, Cys, and selenocystein, which is pre-inserted at the position of interest in peptides and proteins15,16. In recent years, Dha has been used as a versatile backbone for synthesis of UAAs. Although these methods provided efficient ways for preparation of various UAAs, the accessing of β-alkyl substituted UAAs were still limited, and the reactions required either transition metal catalysts or stoichiometric amount of metal reagents17–32. Furthermore, the compatible methods for modification of Dha unit in peptides are still rare33,34, and the related peptide cyclization protocol based on functionalization of Dha is still underdeveloped.
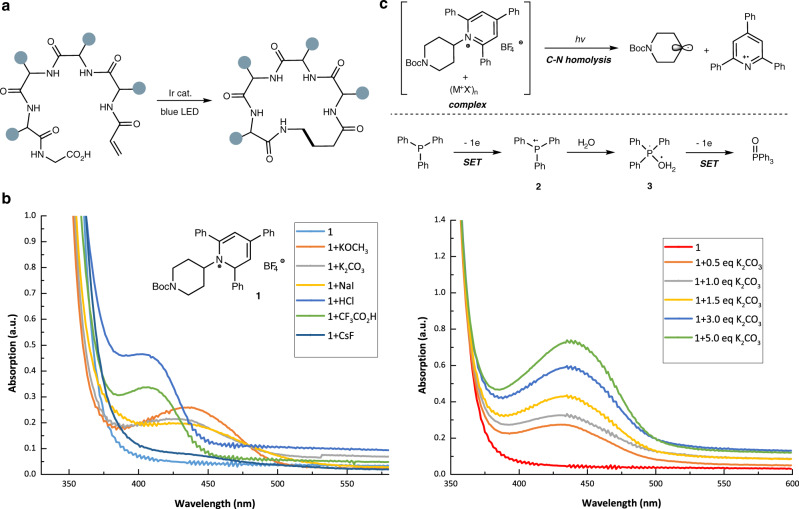
The traditional peptide cyclization methods are based on lactamization and disulfide bond formation. In the past decades, with the advent of new generations of peptide pharmaceuticals, varying the nature of ring-forming linkage in peptide macrocycles became a necessity35. In this context, transition metal-catalyzed peptide macrocyclization strategies are gaining increasing popularity, and include C–H activation, oxidative cross-couplings, heteroatom ligation, and radical reactions, etc36–39. MacMillan and co-workers recently reported a visible-light-promoted decarboxylative Giese reaction, which provided an efficient way for macrocyclization of peptide backbone C-terminal in an alternative manner besides lactam bond (Fig. 1a)40,41.
Fig. 1. Design plan.
a Photoredox decarboxylative macrocyclization. b UV-Vis absorption spectrum of 1 with ionic compounds. c Visible light induced ionic compound promoted homolytic fragmentation of pyridinium C–N bond.
Primary amines are naturally occurring and chemically diverse starting materials, and deaminative reaction of primary amines has emerged as an important strategy for generating alkyl radicals. Katritzky salts derived from α-1° and α-2° amines, as well as imines derived from sterically encumbered α-3° primary amines are important alkyl radical precusors42,43. In line with our interests in photocatalysis and peptide synthesis44–46, we here report a visible light induced, ionic compounds promoted C–N cleavage of Katritzky salts, which is successfully used in the preparation of β-alkyl substituted UAAs and macrocyclization of peptide. Notably, Et3N and PPh3 are effective to act as single-electron reductants for the reactions.
Results
Design plan
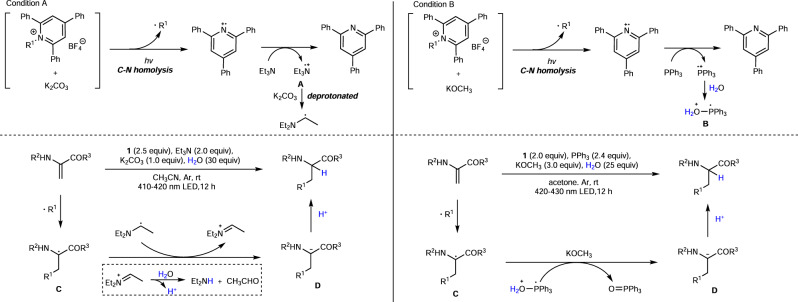
The Katritzky-type alkyl substituted pyridinium (1) can generate an alkyl radical through a photoinduced dissociative electron transfer47. However, the light absorption of alkyl pyridinium 1 is <400 nm wavelength. To exploit the photonic energy from visible light (wavelength > 400 nm) for cleavage of pyridinium C–N bond, photocatalysts48 or photoabsorbing EDA complexes are essentially required44,49–53. For example, Aggarwal and co-workers recently reported a visible light induced Giese-type alkylation reaction, in which pyridinium and Hantzsch esters in situ formed EDA complex and mediated the photoredox process52. Interestingly, in our recent studies, we found that some ionic compounds (M+X-), such as K2CO3, KOCH3, NaI, HCl, CF3CO2H, CsF, etc., could dramatically increase the absorption of 1 in visible light (400-480 nm) region (Fig. 1b, see the “Supplementary information” for details). The loading amount of ionic compound is directly proportional to the light absorption intensity, indicating the key role of ionic additives for increasing the visible light absorption (The reason that ionic additives increasing visible light absorption of Katritzky salt was unclear at this stage. The mechanistic studies and further applications are underway in our lab).
These findings let us wonder whether visible light can be directly used for homolytic alkyl pyridinium (1) C–N bond without assistance of photosensitizers by addition of ionic compounds. If so, upon irradiation, C–N homolysis of 1 can promote the release of alkyl radical. Compared with reported EDA complexes triggered SET to alkyl pyridinium, which were highly dependent on a suitable electron donor (e.g., indoles, Hantzsch esters, or aryl amines) to form a light-absorbing aggregator44,49–53, these ionic compounds promoted photocatalytic C–N homolysis would unveil more rich photochemistry.
To validate our proposal in Fig. 1c, a suitable single-electron reductant (SER) is required. Tertiary amines are previously widely used as SERs. Besides tertiary amines, we speculated that electron-rich tertiary phosphines, such as PPh3, could also be used in our reaction for electron transfer. We assume phosphine could provide one electron to pyridinium radical cation and form a phosphine radical cation 2, which would then react with H2O to give the intermediate 3. The latter could act as an SER again by supplying a second electron and yield phosphine oxide. Overall, the proposed photochemical mechanism to generate C(sp3)-centered radicals by using phosphine and H2O as SER was not realized before54.
Investigation of reaction conditions
For our initial explorations (Table 1), we selected N-Ac-Dha methyl ester (4a) (0.1 mmol) as Michael acceptor and N-Boc-protected cyclic pyridinium salt 1 as alkyl radical precursor. Most of the tested tertiary alkyl amines and trivalent tertiary phosphines could serve as SER for the reaction, while Et3N (entry 1) and PPh3 (entry 2) gave the best results, respectively. Notably, in the case when PPh3 was used, stoichiometric amount of triphenylphosphine oxide (Ph3PO) was formed as a byproduct, indicating that PPh3 and H2O were all involved in SET process. 5a was obtained in 50% yield in the absence of K2CO3 under condition A, which might be attributed to the formation of the EDA complex between Katritzky salt 1 and Et3N (entry 3)52. It is worth noting that the yield of 5a dramatically increased to 97% in the presence of K2CO3 (entry 1). The yield of 5a decreased to 13% in the absence of KOCH3 under condition B (entry 4). In addition to K2CO3 and KOCH3, fluoride additives could also increase the outcome (entries 5 and 6, respectively). Without Et3N or PPh3, only a trace amount of desired product formed (entry 7). The reaction gave diminished yield in the absence of H2O (entry 8). We assumed that H2O might play two roles in the reaction. One is increasing the solubility of an ionic compound; the other is involving in the SET process when PPh3 is used as an SER reagent (Fig. 1c). Without light irradiation, the reaction did not proceed at room temperature or 60 °C(entries 9 and 10, respectively).
Table 1.
Control experiments.
 | ||
|---|---|---|
| Entry | Changes from condition A or B | Yield of 5a (%)a |
| 1 | no change from condition A | 97% (94%) |
| 2 | no change from condition B | 88% (84%) |
| 3 | no K2CO3 under condition A | 50% |
| 4 | no KOCH3 under condition B | 13% |
| 5 | KF instead of K2CO3 under condition A | 63% |
| 6 | CsF instead of KOCH3 under condition B | 41% |
| 7 | without Et3N or PPh3 | trace |
| 8 | without H2O | <54% |
| 9 | no light | N.D. |
| 10 | no light, 60 oC | N.D. |
aYield was determined by 1H NMR using 4-bromobenzaldehyde as an internal standard. The value within parentheses refers to isolated yield.
Substrate scopes
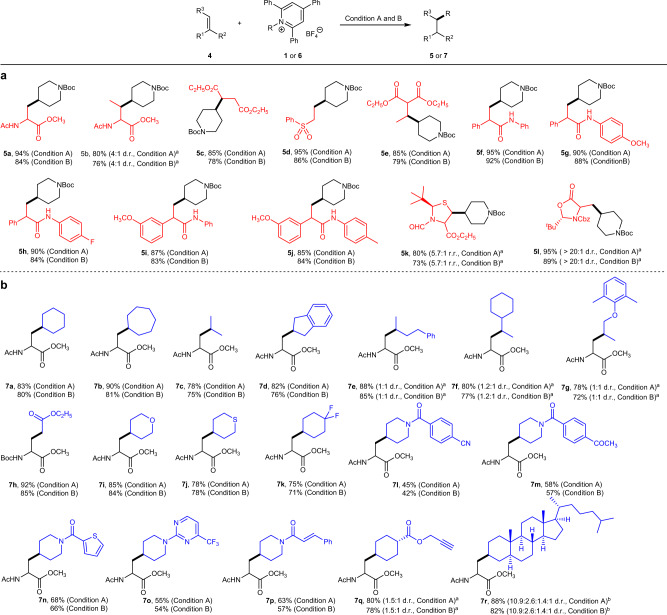
Having established the optimal reaction conditions (Table 1, entries 1 and 2), we examined a series of alkenes (4b–4l) as shown in Fig. 2a. Similar to Dha, another naturally occurring dehydroamino acid derived alkene, namely dehydrobutyrine, also reacted smoothly to give the corresponding product 5b in 80% yield (4:1 d.r.) under condition A and 76% yield (4:1 d.r.) under condition B. Other alkenes, such as diethyl fumarate, vinyl sulfone, or dimethyl malonate derived alkene were all compatible in the reaction with good yields (5c–5e, 78–95%). The Michael acceptors bearing amide groups also worked well under optimal conditions to give the desired products in uniformly good yields (5f–5j). Furthermore, we expanded the reaction scope to β-thiolated amino acid derivative (4k) which was readily prepared from L-cysteine and dehydroalanine derived Karady-Beckwith alkene (4l). The corresponding hydroalkylation products 5k and 5l were obtained in high yields and good r.r. (rotamers mixture ratio)55 or d.r.
Fig. 2. Investigation of reaction substrate scope with respect to the alkenes and Katritzky salts.
Reaction condition A: alkene (0.2 mmol), Katritzky salt (0.5 mmol, 2.5 equiv), Et3N (0.4 mmol, 2.0 equiv), K2CO3 (0.2 mmol, 1.0 equiv), and H2O (6.0 mmol. 30 equiv) in CH3CN (3.0 mL), violet LED (24 W, 410-420 nm), argon atmosphere, 12 h, room temperature. Reaction condition B: alkene (0.2 mmol), Katritzky salt (0.4 mmol, 2.0 equiv), PPh3 (0.48 mmol, 2.4 equiv), KOCH3 (0.6 mmol, 3.0 equiv), and H2O (5.0 mmol. 25 equiv) in acetone (3.0 mL), violet LED (24 W, 420–430 nm), argon atmosphere, 12 h, room temperature. Isolated yields after chromatographic purification. aRegio- or diastereomers were measured by1H NMR. bDiastereomers were measured by HPLC. a Substrates with alkenes (Condition A and B). b Substrates with Katritzky salts (Condition A and Condition B).
Subsequently, we shifted our attention to the scope of Katritzky salts for the synthesis of unnatural α-amino acids using a series of secondary alkyl substituted pyridinium (Fig. 2b). Uniformly good yields were obtained (7a–7h, up to 92%). Importantly, this reaction could be extended to glycine derived Katritzky salt (7h, 92 and 85%), which provided the possibility for deaminative N-terminal macrocyclization of the linear peptide backbone. Furthermore, more complex pyridinium radical precursors bearing various functional groups and structural motifs were tested under condition A and B, which provided corresponding products 7i–7r in moderate to excellent yields. Unfortunately, unactivated primary alkyl substrates had failed to give the desired hydroalkylation products (When the unactivated primary alkyl substrate was used in the reaction, significant amounts of byproduct from radical–radical coupling of primary alkyl dihydropyridine radical with unactivated primary alkyl radical was obtained. See supplementary information for details.).
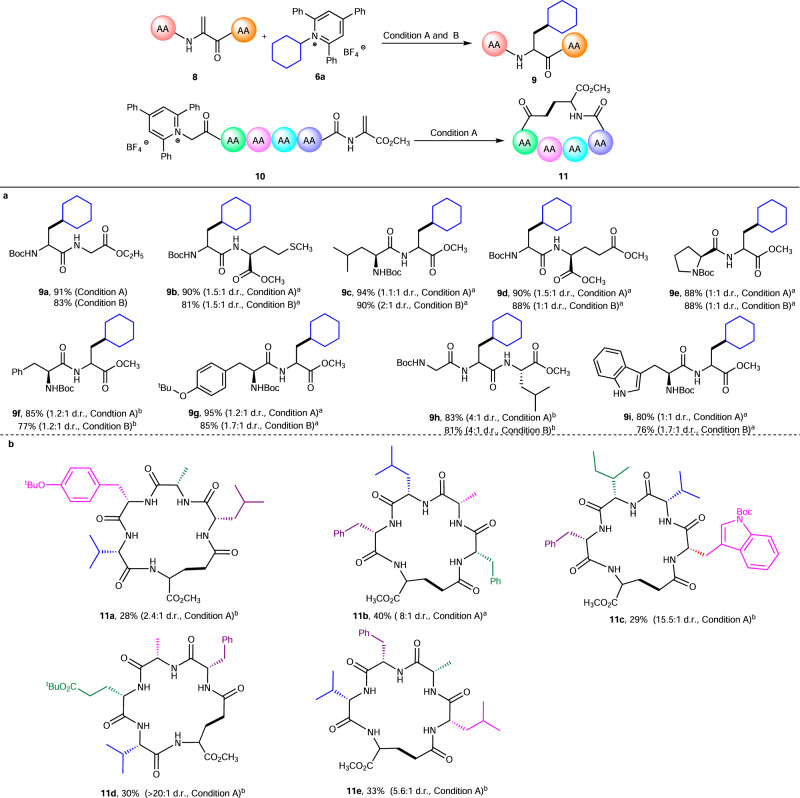
Next, a variety of Dha containing peptides was prepared to test the functional group tolerance of this reaction with various amino acid residues incorporated. To our delight, peptides bearing glycine, proline, leucine, tryptophan, phenylalanine, or tyrosine units were able to participate in the deaminative conjugation to furnish the desired adducts in yields between 76 and 95% with other amino acid residues untouched (Fig. 3a, 9a–9i). Based on excellent performance on modification of peptides, we turned our attention to deaminative N-terminals macrocyclization of linear peptides. As shown in Fig. 3b, a series of peptides that incorporate a structurally diverse set of amino acids can be successfully cyclized using this deaminative method (11a–11e, 28–40% isolated yields after HPLC purifications). Regrettably, the attempt to macrocyclize Dha contained peptide “on resin” during solid-phase peptide synthesis procedure has failed.
Fig. 3. Modification and macrocyclization of peptides.
aDiastereomers were measured by 1H NMR. bDiastereomers were measured by HPLC. a Isolated yield on 0.2 mmol scale (condition A and condition B). b For 11a and 11c–11e, isolated yields on 0.02 mmol under condition A; for 11b, isolated yield on 0.05 mmol under condition A.
Mechanistic studies
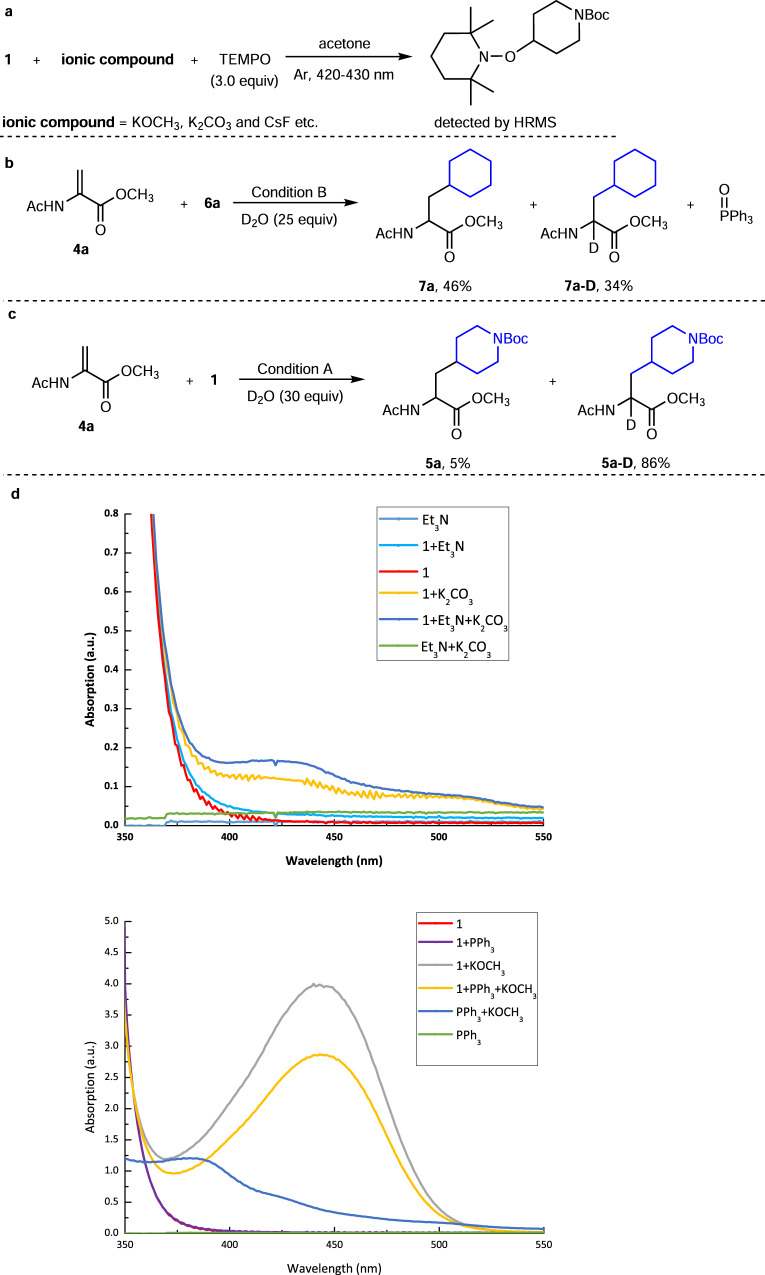
To gain some detailed information for the reaction, a series of mechanistic studies were carried out (see Supplementary information for details). As shown in Fig. 4a, radical trapping experiments indicated that the combination of ionic compounds (such as K2CO3, KOCH3, and CsF) and pyridinium were able to harness photonic energy from visible light and promoted C–N homolysis of Katritzky salt to generate an alkyl radical. In isotope tracking experiments (Fig. 4b, c), when D2O was used instead of H2O under Condition A and B, deuterated product 5a–D and 7a–D were obtained in 86 and 34% yield, respectively, revealing that H2O acted as hydrogen atom source to provide an H+ for the hydroalkylation process. Furthermore, stoichiometric amount of triphenylphosphine oxide (Ph3PO) was formed as the byproduct, which suggested that PPh3 could possibly provide two electrons in a stepwise way during the SET process (Fig. 4b). The UV-Vis absorption of individual reagents or mixtures were shown in Fig. 4d. None of Et3N, PPh3, pyridinium (1), or the mixture of [PPh3 + 1] showed significant absorption over 400 nm. However, basic ionic compounds, namely K2CO3 and KOCH3, could dramatically increase the absorption of pyridinium (1) in the visible light region, respectively. Interestingly, the combination [Et3N + 1] showed a slight bathochromic shift, which might be due to the formation of EDA complex between 1 and Et3N52. Although this EDA complex could possibly mediate the alkylation process (Table 1, entry 3), the yield was significantly lower than for K2CO3 promoted reaction (50% vs 97%). Some ionic compounds, such as Cs2CO3, K3PO4, and NaOCH3, provide higher levels of visible light absorption; however, only moderate yields were obtained (Table S3 and Fig. S8). The use of KBF4 and NaBF4 as ionic additives did not give any desired product, which indicated that BF4− counter anion in Katritzky salt was not crucial for the transformation (Table S3, Table S13, and Fig. S8).
Fig. 4. Mechanistic studies.
a Radical trapping experiments. b Isotope tracking experiments of Condition B. c Isotope tracking experiments of Condition A. d Analysis of UV-Vis absorption spectra.
On the basis of these preliminary results, a possible mechanism is proposed (Fig. 5) (Although the mechanistic investigations suggested that a visible light promoted C-N homolysis of Katritzky salts in the presence of ionic compounds is highly possible in our reaction, the EDA complex pathway cannot be completely ruled out at this stage. See supplementary information for details.). Initially, C–N homolysis of Katritzky salt occurred in the presence of basic ionic compounds under visible light irradiation, followed by the formation of an alkyl radical (•R1) and pyridinium radical cation. Et3N (PPh3) was oxidized to nitrogen radical cation A52 (phosphine radical cation) with the formation of 2,4,6-triphenylpyridine. Notably, under condition A, nitrogen radical cation A is deprotonated to yield α-amino radical, and under condition B, phosphine radical cation reacted with H2O to give intermediate B54. Radical addition of •R1 to Michael acceptor 4 gives radical intermediate C. According to the previous report56, under condition A, intermediate C was reduced by α-amino radical to give the carbon anion species D and an immonium ion. Subsequently, the hydrolysis of immonium ion promoted the generation of diethylamine and acetaldehyde, followed by the release of a proton. Under condition B, intermediate C was reduced by B to form a carbon anion species D alone with the formation of triphenylphosphine oxide. The final protonation of D provided our desired deaminative hydroalkylation product. In condition B, PPh3 served as the single-electron reductant and provided two electrons in a stepwise way.
Fig. 5. Proposed mechanism.
Visible-light-mediated catalyst-free synthesis of unnatural α-amino acids.
Discussion
In summary, we report ionic compounds promoted homolytic cleavage of pyridinium C–N bond by exploiting the photonic energy from visible light. This finding was successfully applied in deaminative hydroalkylation of a series of alkenes including naturally occurring Dha, which provided an efficient way to prepare β-alkyl substituted UAAs under mild and catalyst-free conditions. Importantly, by using this protocol, the deaminative cyclization of peptide backbone N-terminals was realized. Furthermore, the use of Et3N or PPh3 as reductants and H2O as hydrogen atom source is a significantly practical advantage. Further investigating the mechanism of ionic compounds improved visible light absorption of pyridinium and other synthetic applications are currently underway and will be reported in due course.
Methods
General procedure of condition A
To an oven-dried 10 mL quartz test tube with a stirring bar was added alkene (0.2 mmol), alkyl pyridinium salt (0.5 mmol, 2.5 equiv), Et3N (0.4 mmol, 2 equiv), and K2CO3 (0.2 mmol, 1 equiv). Then, the air was withdrawn and backfilled with Ar (three times). CH3CN (3 mL) and H2O (6 mmol, 30 equiv) were added. The mixture was transferred to a violet LED photoreactor (24-W, 410–420 nm), where it was irradiated for 12 h. Then, the reaction was quenched with water (5 mL), extracted with ethyl acetate, washed with brine, dried over anhydrous sodium sulfate, concentrated in vacuo, and purified by column chromatography (hexane/acetone) to afford the product.
General procedure of condition B
To an oven-dried 10 mL quartz test tube with a stirring bar was added alkene (0.2 mmol), alkyl pyridinium salt (0.4 mmol, 2.0 equiv), PPh3 (0.48 mmol, 2.4 equiv), and KOCH3 (0.6 mmol, 3.0 equiv). Then, the air was withdrawn and backfilled with Ar (three times). Acetone (3 mL) and H2O (5 mmol, 25 equiv) were added. The mixture was transferred to a violet LED photoreactor (24-W, 420–430 nm), where it was irradiated for 12 h. Then, the reaction was quenched with water (5 mL), extracted with ethyl acetate, washed with brine, dried over anhydrous sodium sulfate, concentrated in vacuo, and purified by column chromatography (hexane/acetone) to afford the product.
Supplementary information
Acknowledgements
Supported by the National Natural Science Foundation of China (21971098), Innovation Project of Medicine and Health Science and Technology of Chinese Academy of Medical Sciences (2019-I2M-5-074), and the Funds for Fundamental Research Creative Groups of Gansu Province (20JR5RA310).
Author contributions
Z.X. conceived the idea and directed the project; Z.X. and C.W. designed the experiments; M.W., C.W., Y.H., H.X., L.L., H.C., X.X., Z.L., X.D., and D.L. performed the experiments; M.W. and C.W. analyzed the data; Z.X. and C.W. wrote the manuscript.
Data availability
The authors declare that the data supporting the findings of this study, including experimental details and compound characterization, are available within the article and its Supplementary information file. All data are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Markus Kärkäs and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mengran Wang, Chao Wang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-27086-x.
References
- 1.Lau JL, Dunn MK. Therapeutic peptide: historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018;26:2700–2707. doi: 10.1016/j.bmc.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 2.Kaspar AA, Reichert JM. Future direction for peptide therapeutics development. Drug Discov. Today. 2013;18:807–817. doi: 10.1016/j.drudis.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Qvit N, Rubin SJS, Urban TJ, Mochly-Rosen D, Gross ER. Peptidomimetic therapeutics: scientific approaches and opportunities. Drug Discov. Today. 2017;22:454–462. doi: 10.1016/j.drudis.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gentilucci L, De Marco R, Cerisoli L. Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr. Pharm. Des. 2010;16:3185–3203. doi: 10.2174/138161210793292555. [DOI] [PubMed] [Google Scholar]
- 5.Shaw MH, Twilton J, MacMillan DWC. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016;81:6898–6926. doi: 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Easton CJ. Free-radical reactions in the synthesis of α-amino acids and derivatives. Chem. Rev. 1997;97:53–82. doi: 10.1021/cr9402844. [DOI] [PubMed] [Google Scholar]
- 7.Bottecchia C, Noël T. Photocatalytic modification of amino acids, peptides, and proteins. Chem. Eur. J. 2019;25:26–42. doi: 10.1002/chem.201803074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J-Q, Shatskiy A, Matsuura BS, Kärkäs MD. Recent advances in photoredox catalysis enabled functionalization of α-amino acids and peptides: concepts, strategies and mechanisms. Synthesis. 2019;51:2759–2791. [Google Scholar]
- 9.Larionov VA, Stoletova NV, Maleev VI. Advances in asymmetric amino acid synthesis enabled by radical chemistry. Adv. Syn. Catal. 2020;362:4325–4367. [Google Scholar]
- 10.King TA, Kandemir JM, Walsh SJ, Spring DR. Photocatalytic methods for amino acid modification. Chem. Soc. Rev. 2021;50:39–57. doi: 10.1039/d0cs00344a. [DOI] [PubMed] [Google Scholar]
- 11.Troyano FJA, Merkens K, Anwar K, Gómez-Suárez A. Radical-based synthesis and modification of amino acids. Angew. Chem. Int. Ed. 2021;60:1098–1115. doi: 10.1002/anie.202010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kärkäs, M. D. & Shatskiy, A. Photoredox-enabled decarboxylative synthesis of unnatural α-amino acids. Synlett10.1055/a-1499-8679 (2021).
- 13.Siodłak D. α,β-Dehydroamino acids in naturally occurring peptides. Amino Acids. 2015;47:1–17. doi: 10.1007/s00726-014-1846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Repka LM, Chekan JR, Nair SK, van der. Donk WA. Mechanistic understanding of lanthipeptide biosynthetic enzymes. Chem. Rev. 2017;117:5457–5520. doi: 10.1021/acs.chemrev.6b00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strumeyer DH, White WN, Koshland DE., Jr. Role of serine in chymotrypsin action-conversion of active serine to dehydroalanine. Proc. Natl Acad. Sci. U. S. A. 1963;50:931–935. doi: 10.1073/pnas.50.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernardes GJL, Chalker JM, Errey JC, Davis BG. Facile conversion of cysteine and alkyl cysteines to dehydroalanine on protein surfaces: versatile and switchable access to functionalized proteins. J. Am. Chem. Soc. 2008;130:5052–5053. doi: 10.1021/ja800800p. [DOI] [PubMed] [Google Scholar]
- 17.Navarre L, Darses S, Genet J-P. Tandem 1,4-addition/enantioselective protonation catalyzed by rhodium complexes: efficient access to α-amino acids. Angew. Chem. Int. Ed. 2004;43:719–723. doi: 10.1002/anie.200352518. [DOI] [PubMed] [Google Scholar]
- 18.Aycock RA, Vogt DB, Jui NT. A practical and scalable system for heteroaryl amino acid synthesis. Chem. Sci. 2017;8:7998–8003. doi: 10.1039/c7sc03612d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suárez RM, Pérez Sestelo J, Sarandeses LA. Practical and efficient enantioselective synthesis of α-amino acids in aqueous media. Org. Biomol. Chem. 2004;2:3584–3587. doi: 10.1039/b413017k. [DOI] [PubMed] [Google Scholar]
- 20.Larionov VA, et al. A general synthesis of unnatural α-amino acids by iron-catalysed olefin-olefin coupling via generated radicals. Org. Chem. Front. 2019;6:1094–1099. [Google Scholar]
- 21.Huang T-S, Li C-J. Novel synthesis of α-amino acids via catalysis in air and water. Org. Lett. 2001;3:2037–2039. doi: 10.1021/ol010079s. [DOI] [PubMed] [Google Scholar]
- 22.Kieffer ME, Repka LM, Reisman SE. Enantioselective synthesis of tryptophan derivatives by a tandem Friedel-Crafts conjugate addition/asymmetric protonation reaction. J. Am. Chem. Soc. 2012;134:5131–5137. doi: 10.1021/ja209390d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah AA, Kelly MJ, III, Perkins JJ. Access to unnatural α‑amino acids via visible-light-mediated decarboxylative conjugate addition to dehydroalanine. Org. Lett. 2020;22:2196–2200. doi: 10.1021/acs.orglett.0c00371. [DOI] [PubMed] [Google Scholar]
- 24.Zhang O, Schubert JW. Derivatization of amino acids and peptides via photoredox-mediated conjugate addition. J. Org. Chem. 2020;85:6225–6232. doi: 10.1021/acs.joc.0c00635. [DOI] [PubMed] [Google Scholar]
- 25.Merkens K, Troyano FJA, Djossou J, Gómez-Suárez A. Synthesis of unnatural α-amino acid derivatives via light mediated radical decarboxylative processes. Adv. Synth. Catal. 2020;362:2354–2359. [Google Scholar]
- 26.Aycock RA, Pratt CJ, Jui NT. Aminoalkyl radicals as powerful intermediates for the synthesis of unnatural amino acids and peptides. ACS Catal. 2018;8:9115–9119. [Google Scholar]
- 27.Sim J, Campbell MW, Molander GA. Synthesis of α‑fluoro-α-amino acid derivatives via photoredox catalyzed carbofluorination. ACS Catal. 2019;9:1558–1563. doi: 10.1021/acscatal.8b04284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandhofer T, Mancheño OG. Versatile Ru-photoredox-catalyzed functionalization of dehydro-amino acids and peptides. ChemCatChem. 2019;11:3797–3801. [Google Scholar]
- 29.Reich D, Trowbridge A, Gaunt MJ. Rapid syntheses of (-)-FR901483 and (+)-TAN1251C enabled by complexity-generating photocatalytic olefin hydroaminoalkylation. Angew. Chem. Int. Ed. 2020;59:2256–2261. doi: 10.1002/anie.201912010. [DOI] [PubMed] [Google Scholar]
- 30.Ji P, et al. Synthesis of enantioenriched α‑deuterated α‑amino acids enabled by an organophotocatalytic radical approach. Org. Lett. 2020;22:1557–1562. doi: 10.1021/acs.orglett.0c00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Chen Y, Song H, Liu Y, Wang Q. Synthesis of unnatural α‑amino acids via photoinduced decatungstate-catalyzed Giese reactions of aldehydes. Org. Lett. 2021;23:2199–2204. doi: 10.1021/acs.orglett.1c00345. [DOI] [PubMed] [Google Scholar]
- 32.Dai Z-Y, Nong Z-S, Song S, Wang PS. Asymmetric photocatalytic C(sp3)-H bond addition to α‑substituted acrylates. Org. Lett. 2021;23:3157–3161. doi: 10.1021/acs.orglett.1c00801. [DOI] [PubMed] [Google Scholar]
- 33.van Lier RCW, de Bruijn AD, Roelfes G. A water-soluble iridium photocatalyst for chemical modification of dehydroalanines in peptides and proteins. Chem. Eur. J. 2021;27:1430–1437. doi: 10.1002/chem.202002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vries RH, Viel JH, Kuipers OP, Roelfes G. Rapid and selective chemical editing of ribosomally synthesized and post-translationally modified peptides (RiPPs) via CuII-catalyzed β-borylation of dehydroamino acids. Angew. Chem. Int. Ed. 2021;60:3946–3950. doi: 10.1002/anie.202011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivera DG, Ojeda-Carralero GM, Reguera L, Eycken, van der EV. Peptide macrocyclization by transition metal catalysis. Chem. Soc. Rev. 2020;49:2039–2059. doi: 10.1039/c9cs00366e. [DOI] [PubMed] [Google Scholar]
- 36.Malins LR. Peptide modification and cyclization via transition-metal catalysis. Curr. Opin. Chem. Biol. 2018;46:25–32. doi: 10.1016/j.cbpa.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Lorion MM, Shah J, Kapdi AR, Ackermann L. Late-stage peptide diversification by position-selective C-H activation. Angew. Chem. Int. Ed. 2018;57:14700–14717. doi: 10.1002/anie.201806250. [DOI] [PubMed] [Google Scholar]
- 38.Noisier AFM, Brimble MA. C-H functionalization in the synthesis of amino acids and peptides. Chem. Rev. 2014;114:8775–8806. doi: 10.1021/cr500200x. [DOI] [PubMed] [Google Scholar]
- 39.Raynal L, Rose NC, Donald JR, Spicer CD. Photochemical methods for peptide macrocyclisation. Chem. Eur. J. 2021;27:69–88. doi: 10.1002/chem.202003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarver SJ, et al. Decarboxylative peptide macrocyclization through photoredox catalysis. Angew. Chem. Int. Ed. 2017;56:728–732. doi: 10.1002/anie.201608207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu L, Ohta C, Zuo Z, MacMillan DWC. Carboxylic acids as a traceless activation group for conjugate additions: a three-step synthesis of (±)-pregabalin. J. Am. Chem. Soc. 2014;136:10886–10889. doi: 10.1021/ja505964r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Correia JTM, et al. Photoinduced deaminative strategies: Katritzky salts as alkyl radical precursors. Chem. Commun. 2020;56:503–514. doi: 10.1039/c9cc08348k. [DOI] [PubMed] [Google Scholar]
- 43.Ashley MA, Rovis T. Photoredox-catalyzed deaminative alkylation via C-N bond activation of primary amines. J. Am. Chem. Soc. 2020;142:18310–18316. doi: 10.1021/jacs.0c08595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, et al. Visible-light-promoted C(sp3)-H alkylation by intermolecular charge transfer: preparation of unnatural α-amino acids and late-stage modification of peptides. Angew. Chem. Int. Ed. 2020;59:7462–7466. doi: 10.1002/anie.201914555. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, et al. Visible-light-driven, copper-catalyzed decarboxylative C(sp3)-H alkylation of glycine and peptides. Angew. Chem. Int. Ed. 2018;57:15841–15846. doi: 10.1002/anie.201809400. [DOI] [PubMed] [Google Scholar]
- 46.Xue H, et al. Photo-induced preparation of unnatural α-amino acids: synthesis and characterization of novel Leu5-enkephalin analogues. Org. Chem. Front. 2020;7:2426–2431. [Google Scholar]
- 47.Lorance ED, Kramer WH, Gould IR. Kinetics of reductive N-O bond fragmentation: the role of a conical intersection. J. Am. Chem. Soc. 2002;124:15225–15238. doi: 10.1021/ja020768e. [DOI] [PubMed] [Google Scholar]
- 48.Rössler SL, et al. Pyridinium salts as redox-active functional group transfer reagents. Angew. Chem. Int. Ed. 2020;59:9264–9280. doi: 10.1002/anie.201911660. [DOI] [PubMed] [Google Scholar]
- 49.Sandfort F, Strieth-Kalthoff F, Klauck FJR, James MJ, Glorius F. Deaminative borylation of aliphatic amines enabled by visible light excitation of an electron-donor-acceptor complex. Chem. Eur. J. 2018;24:17210–17214. doi: 10.1002/chem.201804246. [DOI] [PubMed] [Google Scholar]
- 50.Wu J, He L, Noble A, Aggarwal VK. Photoinduced deaminative borylation of alkylamines. J. Am. Chem. Soc. 2018;140:10700–10704. doi: 10.1021/jacs.8b07103. [DOI] [PubMed] [Google Scholar]
- 51.James MJ, et al. Visible-light-mediated charge transfer enables C-C bond formation with traceless acceptor groups. Chem. Eur. J. 2019;25:8240–8244. doi: 10.1002/chem.201901397. [DOI] [PubMed] [Google Scholar]
- 52.Wu J, Grant PS, Li X, Noble A, Aggarwal VK. Catalyst-free deaminative functionalizations of primary amines via photoinduced single-electron transfer. Angew. Chem. Int. Ed. 2019;58:5697–5701. doi: 10.1002/anie.201814452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crisenza GEM, Mazzarella D, Melchiorre P. Synthetic methods driven by the photoactivity of electron donor-acceptor complexes. J. Am. Chem. Soc. 2020;142:5461–5476. doi: 10.1021/jacs.0c01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan D, Nie G, Jiang S, Li T, Jin Z. Radical reactions promoted by trivalent tertiary phosphines. Org. Chem. Front. 2020;7:2349–2371. [Google Scholar]
- 55.Yin H, et al. Stereoselective and divergent construction of β-thiolated/selenolated amino acids via photoredox-catalyzed asymmetric Giese reaction. J. Am. Chem. Soc. 2020;142:14201–14209. doi: 10.1021/jacs.0c04994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis FD, Ho T-I, Simpson JT. Photochemical addition of tertiary amines to stilbene. Stereoelectronic control of tertiary amine oxidation. J. Org. Chem. 1981;46:1077–1082. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study, including experimental details and compound characterization, are available within the article and its Supplementary information file. All data are available from the corresponding author upon request.