Abstract
Laboratory and epidemiological researches have indicated that ambient air particulate matter have a plays critical role in causing diseases. The current research evaluated the chemical attributes of PM2.5 in the ambient air of the cities of Karaj and Fardis and determined its toxicological effects on human lung epithelial cells (A549). In the study city, 16 points were selected from the two high-traffic and low-traffic points for sampling. A sampling of ambient air was carried out in spring, summer, autumn, and winter 2018–19. Air sampling was performed for 24 h according to the EPA-TO/13A guidelines. To analyze of toxic metals and polycyclic aromatic hydrocarbons (PAHs), ICP-OES and GC-MS were used, respectively, and for cell toxicity analysis, an ELISA reader was used. Then from SPSS, Excel and R software were used for statistical analysis. The results of the current study indicated that the concentration of PAHs carcinogenic in the autumn season in high-traffic stations was the highest and equal to 9.3 ng/m3, and in the spring season in the low-traffic stations, it was the lowest and equal to 5.82 ng/m3. In general, during the period of study, Heavy metals including Zn, Fe, Pb, Cu, and Al had the highest concentration compared to other metals. However, Hg, Cr, As, Pb, Cu, Cd, and Zn were higher concentration in the winter and autumn seasons than in the spring and summer seasons. Cell viability measurements by using MTT showed that low-traffic and high-traffic stations had the highest toxicity in autumn season compared to other seasons. (p < 0.05). In general, high-traffic stations had the highest toxicity than low-traffic stations. The general conclusion of the present study was that PM2.5–bound PAHs and toxic metals, due to their high concentration, were toxic pollutants in air for residents of Karaj and Fardis. Also, the high concentration of PM2.5 caused the mitochondrial activity of A549 cells to stop and this stop was more significant in cold seasons and high-traffic areas.
Keywords: Cytotoxicity, Lung epithelial cells, MTT, Heavy metals, PAHs
Introduction
Among the pollutants in the air, fine particulate is an environmental destructive effect, especially in u developing countries endangering human and atmosphere health [1–3]. Various epidemiological studies have examined the association between particles in the atmosphere with adverse health effects [4, 5]. In recent years, oxidative stress has been recognized as one of the important mechanisms by which PM exerts its adverse impacts on cellular systems and living organisms [6, 7]. According to the report of World Health Organization (WHO) in 2016, air pollution poses serious health risks worldwide, and the death rate in middle-income countries is higher than in other countries [8, 9]. Several epidemiological studies have demonstrated a relationship between mortality and particles in the air [10, 11]. Particulate matters play a critical role in urban ambient air pollution, affecting environmental ecosystems and human health significantly [12, 13]. Particles due to chemical non-uniformity is behaves differently from gases in the air [14]. Due to changes in the concentration, size, and chemical nature, some particles have more substantial effects on human health [15–17]. It comprises a mixture of several molecules, including inorganic ions, metals, PAHs, endotoxins, proteins, and microorganisms [18, 19]. Epidemiological research has indicated a relationship between high concentrations of inhaled particles and increased respiratory problems, mortality, and morbidity [20–22]. When inhaling fine particles, they can penetrate into the lungs and may even affect the alveolar region. In the long term, every 10 μg/m3 increase in the concentration of fine particles is associated with a 6% increase in mortality, a 12% increase in cardiovascular diseases, and a 14% increase in lung cancer [23, 24]. Evidence suggests that PM2.5 particles are the most common cause of respiratory and cardiovascular complications and premature death among different classes of particles size that playing an essential role in causing these diseases [25, 26].
Mortality from heart and lung disease due to exposure to PM2.5 particles is high worldwide. Furthermore, deaths from PM2.5 in East Asia and North America have recently increased due to climate changes [27–29]. Laboratory studies have shown that PM2.5 particles can damage the cell membrane, oxidative damage, antioxidant function, which can cause inflammation and impair the immune system. As a result, the toxicity of fine-fraction PM is higher than that of course –fraction [30–32]. PM2.5 particles can be deep in the alveoli region of the lung owing to their small size. Therefore, these fine particles can enter the blood and be distributed throughout the body [33, 34]. A549 cell lines are employed mostly as a transfection host and as an in-vitro model for drug metabolism. The cells, when cultured in vitro, stick to the vessel wall and create a monolayer. The in vitro examination of the impact of airborne pollutants on the cells can offer helpful insights into the real health implications of the substances [35].
Furthermore, the cell lines offer fixed phenotypic and genetic characteristics, causing them to be a convenient tool to examine the impacts of drugs in preliminary studies [36]. A549 epithelial cells are derived from alveolar cell carcinoma of human lung cancer cells with both malignant tumor cell characteristics and type-II alveolar cells. Thus, A549 cells are extensively used in cancer research, diagnosis, treatment, or differentiation of type II alveolar cells from normal human alveolar cells and functional structures [37, 38].
However, to the best of our knowledge and based on searches in databases, no study has examined the no cytotoxic effects of PM2.5 on the respiratory system in the cities of Karaj and Fardis. For the first time, this study aimed to investigate the amounts of heavy metals and polycyclic aromatic hydrocarbons bonded with PM2.5 particles in an industrial city and to evaluate the cytotoxic effects of PM2.5 particles on human lung epithelial cells (A549).
Material and methods
Study area and sampling
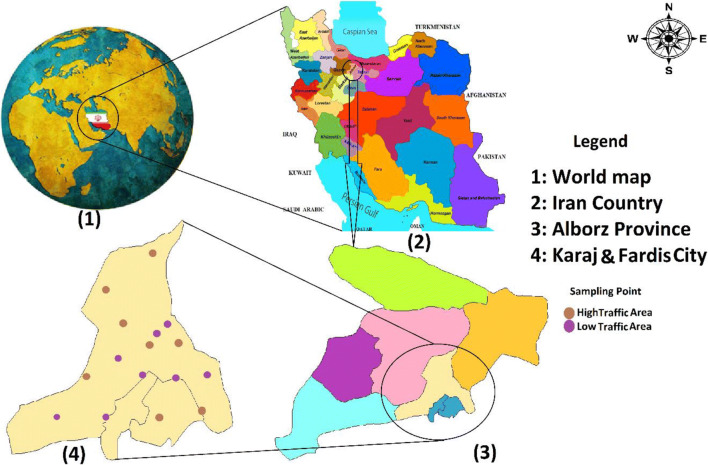
Karaj City is the capital of Alborz Province. This population is approximately 1.9 million, making it the fourth-largest city in Iran. Its coordinates are 50° 55′15˝E longitude and 35° 45´ 50˝N latitude. Its area is approximately 858 km2. Also, the annual rainfall of this city is 246 mm on average. Another feature of this city is the moderate temperature. [39] (Fig. 1).
Fig. 1.
Location of the study area and sampling station
The typical sampling time was 24 h. According to the 16 sampling stations per season, the number of samples equals 16 samples in each season, which is a total of 64 samples were collected from 21 March 2018 to 20 March 2019. PM2.5 particles was collected by personal Modular Impactor (PMI) on polytetrafluoroethylene (PTFE) filters with a diameter of 37 mm and pore size of 1 μm for 24 h by a sampler at a flow rate of 3 l per minute. Filters were 48 h equilibrated before and after sampling, and were weighted with an electronic balance (Mettler Toledo AB204-N). In addition, all sampling filters were maintained in the dark at temperatures of −20 °C [40].
As Fig. 1 shows, the stations were divided into two parts 1) low-traffic (crimson spots) and 2) high-traffic (gray spots) sites. This hybrid policy was used to obtain the appropriate final concentration of suspended particulate matter for exposure to the cells under study and obtain better results than the analyses results. Finally, three concentrations and three exposure times of the particles in the cells were considered. In this study, high-traffic stations are used for the effects of each of the studied pollutants on the exposed population. High-traffic stations monitored the level of pollution on the streets near crowded areas. These stations experienced the direct effects of busy highways and streets. The low-traffic stations were located in areas such as close to residential areas and busy streets. After sampling, the filters were transferred to the laboratory and their weight was measured using a digital scale (reading precision: 10 μg), and the PM concentration was calculated by Eq. 1.
| 1 |
Where Wf is the filter final weights (g), and Wi is the filter initial weights (g), V represents the sampled air volume (m3), and 106 is a factor to convert g to μg [41].
Preparation of samples for analysis in the laboratory
Filters were prepared to perform chemical and biological analyses. Each filter (a one-third of filter each) was cut: 2 pieces to be used for chemical characterization (metals and polycyclic aromatic hydrocarbons), and one-third used for biological suspension preparation for in vitro tests.
Analysis of heavy metals
Each sampling filter was divided into two equal parts, and it was then mixed in a Teflon tank with 2.5 ml nitric acid, 2.5 ml Perchloric acid (HClO4), and 5 ml hydrofluoric acid [42]. The resulting mixture was heated at 170 °C for 4 h. Then, after cooling, the mixture were dried at 95 °C. Afterward, it was mixed with distilled water (the ratio 1:9) and concentrated 2.5 ml nitric acid when it was cooled. After shaking for approximately 15 min, the solution was prepared to be injected into the device. It should be noted that during the preparation (conversion of sampling filters to solution), the samples were not passed through the syringe filter. Because if the soluble samples are passed through the filter, the concentration of heavy metals will be lower and will cause an error when leaving the ICP device. An ICP measured the solution, and then Cadmium, Iron, Vanadium, Nickel, Chromium, Lead, Aluminum, Copper, and Zinc contents were measured by an ICP-OES instrument. Since the ICP-OES device performs three analyzes for each metal sample and in addition to the output of the metal concentration, it also automatically calculates the amount of LOD [43]. By employing high purity standards, calibration curves were obtained. The Blank sample was analyzed by the same method. The on-screen calibration curve exhibited after each analytical run. Afterward, a visual examination was performed for reproducibility and linearity. The chemicals purchased from Merck, Germany, were of the analytical form [44].
Analysis of PAHs
The organic solvent-soluble suspension was required to measured of PAHs. One third of the sampling filters was mixed in a 10 mL-Erlenmeyer flask containing acetone and dichloromethane (3:1). After that, the mixture was mixed for about 30 min in an ultrasonic bath, and the obtained mixture was shaked on a shaker for about 60 min and filtered by a 0.45 μm syringe -filter. In addition, 16 PAHs were analyzed and measured by gas chromatography-mass spectrometry (GC-MS) using an Agilent Capillary HP-5MS Column [45, 46].
Cell line
In the present research, A549 cells were purchased from the Pasteur Institute of Iran cell bank. Target cells were cultivated in DMEMF12 medium containing 10% FBS, 1% penicillin/streptomycin, and two mM glutamine and were kept in a humid environment containing 95% air and 5% CO2 at 37 °C. Afterward, the cultures were transferred to 96-well plates (1 × 104 cells/well) and incubated for 1 day until the cells stuck to the bottom of the plate [47]. Then, the cells were observed by a microscope; the supernatant was emptied when it was deemed appropriate for the analysis. They were treated with PM2.5 extracts at final concentrations of 25, 50, and 100 μg/mL for 12, 24, or 48 h [48].
Treatment of A549 cells with PM2.5 extract
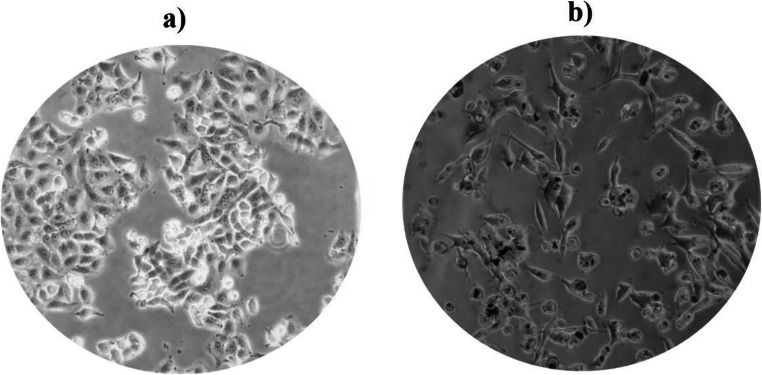
After 24 h of the culture of A549 cells (104 in each well) on 96-well plates, they were treated with PM2.5 at three concentrations, including low (25 μg/mL), medium (50 μg/mL), and high (100 μg/mL) and three stages of time (12, 24 and 48 h). As mentioned above, after preparing the cells in 96-well plates for 24 h, 100 μL of the PM2.5 sample and 100 μL of the fresh culture medium (DMEMF12) and dissolved at three concentrations low, medium, and high, were passed through the 0.25 μm filter and added to the wells under sterile conditions. The wells were incubated at three-time intervals (12, 24, and 48 h), and finally, they were subjected to MTT. For each incubation duration and more precision, we utilized a separate 96-well plate, and each stage of time incubated was repeated three times [48, 49]. Figure 2 shows the contrast-phase microscopy images of A549 cells before (a) and after (b) exposure to PM2.5.
Fig. 2.
Phase- contrast microscopy (PCM) images of A549 cells (a) before and (b) after exposure to PM2.5
Cell viability
The MTT assay was used to determine cell viability. The A549 cells were cultured in a 96-well plate. After exposure to extracted PM2.5, in the last step, MTT was added to each of the wells and incubated for 4 h. After removal of the supernatant, dark blue formazan crystals were dissolved in DMSO. The absorbance of suspension was prepared at 570 nm, and measured values were reported as a percentage of control [49]. Eqs determined the toxicity and viability. [2] and [3]:
| 2 |
| 3 |
Statistical analysis
The toxicity data charts and graphs were plotted using the Graph Pad Prism8 software, and a comparison between low-traffic and high-traffic stations was made by non-parametric tests (Mann-Whitney U test). The Kruskal-Wallis test was employed to compare the data of more than two groups.
Results and discussion
Concentration of PM2.5 on different stations and different seasons
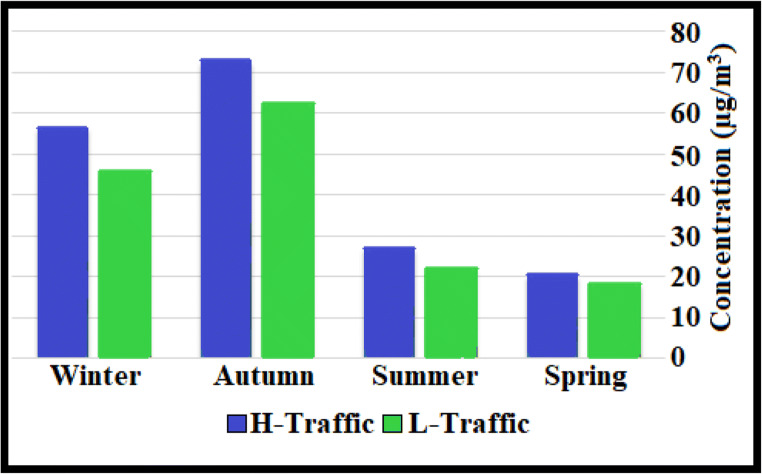
Table 1 and Fig. 3 show the concentration of PM2.5 in seasonally during the period of sampling. Significant differences were observed in winter and autumn concentrations with the guidelines of the WHO (25 μg/m3). In the two seasons of winter and autumn, the amount of concentrations was much higher than the other two seasons, namely summer and spring. The concentrations of PM2.5 for the autumn in low-traffic and high-traffic were 62.3 μg/m3 and 72.7 μg/m3, respectively. The lowest concentrations of PM2.5 for the spring in low-traffic and high-traffic were 18.3 μg/m3 and 20.3 μg/m3, respectively. Several studies also reported considerably higher aerosol concentrations in cold seasons than in other seasons and showed that with increasing concentrations in the cold season of the year, air conditions become worse and more polluted [40].
Table 1.
Concentration of PM2.5 in during the study period (L-T: Low traffic, H-T: High traffic) (μg/m3)
| Season | Site | Mean | Maximum | Minimum | S. D |
|---|---|---|---|---|---|
| Autumn | L-T | 62.3 | 102.4 | 45 | 18.3 |
| H-T | 72.7 | 110.2 | 54.3 | 19.2 | |
| Winter | L-T | 45.6 | 97.5 | 11.6 | 24.1 |
| H-T | 56.4 | 95.8 | 18.5 | 27.2 | |
| Spring | L-T | 18.9 | 31.9 | 10.1 | 7.6 |
| H-T | 20.3 | 36.42 | 11.38 | 8.1 | |
| Summer | L-T | 22 | 41.9 | 11.2 | 9.3 |
| H-T | 26.9 | 48.6 | 11.2 | 13.5 |
Fig. 3.
Seasonal and condition impact on the concentration of particulates
The results of our study are similar to studies conducted in the world that have shown that the concentration of these pollutants is lower in the hot season than in the cold season [50, 51]. Different sources of PM2.5 compounds may be different in each region [52, 53]. Terrain, emissions, and meteorological factors affected the PM2.5 concentration [47].
Concentration of heavy metal on different stations and different seasons
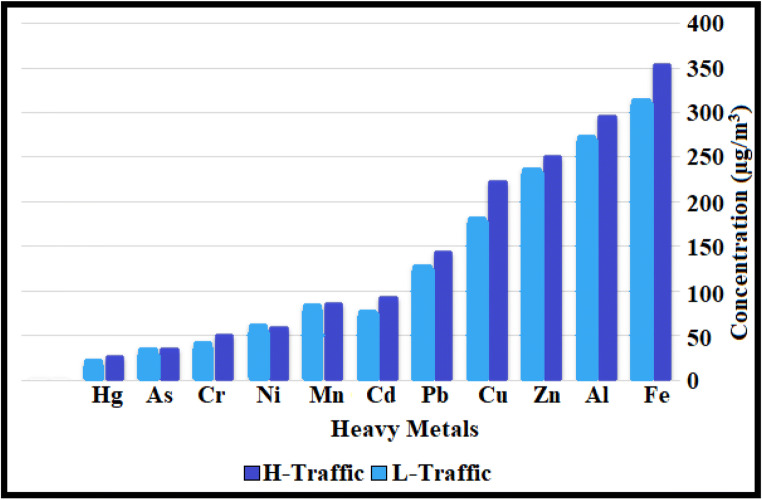
Table 2 shows the variations in the heavy metals concentration in the atmospheres of the study area during the investigated seasons. Figure 4 depicts the average of heavy metals for low-traffic and high-traffic. Heavy metal concentrations varied in different seasons. In high-traffic stations, high concentrations of heavy metals can be associated with high concentrations of PM2.5, as a result of which more heavy metals bond with these particles in the air. For both stations, Al and Fe had the highest concentrations. The average concentrations of Al and Fe in low-traffic and high-traffic stations were 257.1 ± 138.5 and 297.1 ± 134.5 ng/m3 as well as 306.8 ± 189.3 and 354.6 ± 172 ng/m3, respectively. This table shows the highest concentration of Al and Fe in high traffic with an average concentration of 450.6, 528.5 ng/m3 in summer, followed by Zn and Fe in high traffic with an average concentration of 365.9, 360 ng/m3 it is in the autumn. The High concentrations of iron can be associated with the natural nature of this element in the Earth’s crust [54, 55]. Table 2 shows that the ratio of heavy metal concentrations in high-traffic stations is higher than in low-traffic stations.
Table 2.
Statistical summary of heavy metal concentrations (ng/m3)
| Metal | Condition | Season | Ratio | ||||
|---|---|---|---|---|---|---|---|
| Autumn | Winter | Spring | Summer | Overall | (H-T/L-T) | ||
| Hg | L-T | 20 ± 16 | 25.5 ± 21.2 | 9.6 ± 14.5 | 9.3 ± 17.5 | 16.1 ± 18 | 1.27 |
| H-T | 20.3 ± 13.2 | 40.2 ± 39.7 | 10.5 ± 12.8 | 11.2 ± 14.5 | 20.5 ± 25.1 | ||
| Cr | L-T | 53.2 ± 21.7 | 39.3 ± 40.4 | 17.5 ± 20.1 | 18.4 ± 21 | 32.1 ± 30.1 | 1.31 |
| H-T | 77.2 ± 31.5 | 45.6 ± 32.2 | 25.2 ± 22.4 | 20 ± 18.7 | 42 ± 34.2 | ||
| As | L-T | 22.4 ± 10.9 | 32.7 ± 28.5 | 22.3 ± 23.6 | 25.3 ± 21.4 | 27.6 ± 23.2 | 1.05 |
| H-T | 30 ± 22 | 40 ± 35.8 | 29.2 ± 16.2 | 27 ± 20 | 29.1 ± 22.4 | ||
| Pb | L-T | 245.4 ± 137.7 | 116.1 ± 56.4 | 57.4 ± 38.1 | 83.4 ± 67 | 125.6 ± 108.3 | 1.14 |
| H-T | 270 ± 126.7 | 127.1 ± 71.4 | 79 ± 29 | 98.5 ± 59 | 143.6 ± 107.4 | ||
| Ni | L-T | 36.7 ± 67.3 | 44.6 ± 64.5 | 30.1 ± 34.5 | 49.3 ± 39.1 | 43.1 ± 44.3 | 1.14 |
| H-T | 48.2 ± 39.2 | 54 ± 51.1 | 43 ± 28.4 | 63 ± 47.6 | 49.1 ± 38.8 | ||
| Cd | L-T | 60 ± 28.7 | 97 ± 38.1 | 42.7 ± 28 | 91.2 ± 55 | 72.6 ± 43.5 | 1.21 |
| H-T | 66.7 ± 24.9 | 107.5 ± 82.5 | 79.7 ± 47.7 | 98.2 ± 54.1 | 88 ± 55.8 | ||
| Cu | L-T | 175.2 ± 67.3 | 209.2 ± 86.3 | 76.6 ± 61.4 | 167.1 ± 86.3 | 177.7 ± 103.2 | 1.26 |
| H-T | 255 ± 97 | 237.5 ± 104 | 110.8 ± 52 | 372.1 ± 232 | 224 ± 160.8 | ||
| Zn | L-T | 280.6 ± 171.4 | 185 ± 126.1 | 212.9 ± 94.1 | 224.1 ± 133.3 | 238 ± 130.4 | 1.06 |
| H-T | 365.9 ± 174 | 227.6 ± 72 | 219.2 ± 141.8 | 242.7 ± 146.5 | 251.6 ± 149 | ||
| Al | L-T | 178.3 ± 105.4 | 231.4 ± 74.8 | 196.3 ± 103.1 | 422.4 ± 120 | 257.1 ± 138.5 | 1.16 |
| H-T | 251.2 ± 94.6 | 243.2 ± 99.2 | 243.6 ± 88.7 | 450.6 ± 132.3 | 297.1 ± 134.5 | ||
| Fe | L-T | 241 ± 52.7 | 220.2 ± 119.6 | 225.4 ± 116.7 | 528.5 ± 160.5 | 306.8 ± 189.3 | 1.16 |
| H-T | 360 ± 127.2 | 287 ± 139.1 | 243 ± 124.4 | 540.7 ± 210.7 | 354.6 ± 172 | ||
| Mn | L-T | 33.1 ± 28.1 | 86.4 ± 44.7 | 38.2 ± 29.2 | 88.4 ± 75.5 | 77.3 ± 66.7 | 1.05 |
| H-T | 47.5 ± 25.4 | 104.6 ± 42 | 49 ± 33.4 | 125.3 ± 37.9 | 81.6 ± 48.2 | ||
Fig. 4.
Comparison of metals concentrations in different sites
On the other hand, in this study, no significant relationship was observed at the two stations (L-T, H-T) for heavy metals. In the study of Shilira et al., Iron metal had the highest annual average of concentration [38].
The amount and type of metal adsorbed in the Schilirò study was different from our study, which could be due to the type of particles and the topographic conditions of the study area. The ratio of the concentration of the heavy metals in high-traffic (H-T) and low-traffic (L-T) (H-T/L-T ratio) ranged from 1.31 to 1.05. The highest H-T/L-T ratio was obtained for Cr, and the lowest proportion was obtained for Mn and As (Table 2). Figure 5 indicates that the weight ratio of all metals was higher than in high-traffic stations than in low-traffic ones. This could be due to man-made resources such as the burning of fossil fuels by factories inside the city [56]. Each of the heavy metals can be of one origin, so that oils emit Cu, Mn, Pb, vehicle tires emit Zn, vehicle exhaust gases also emit the metals Mn, Fe, Pb, Cu and Zn [57]. Another study [58] showed that Pb, Ni, Zn, and Cd could be partially due to vehicular exhausts.
Fig. 5.
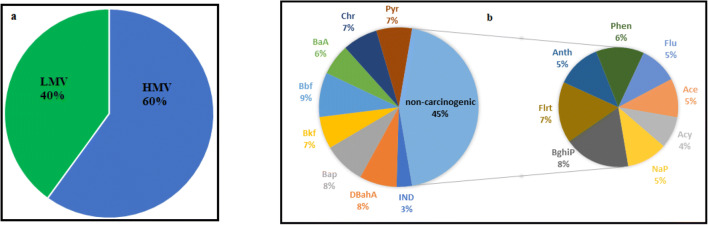
(a) The percentage of (LMW) and (HMW) PAHs, (b) the percentage of non-carcinogenic and carcinogenic PAHs
Furthermore, coal combustion in the industry was also observed to be an essential source of Cd, Pb, and As [59]. Industrial processes and operations can emit the metals Pb, Cu, Cd, Co, Se, Mo and Ag. [60]. Zn, Cr, As, Hg, Ni, and Pb concentrations in the winter and fall were higher than the respective concentrations in the summer and spring. In the winter and autumn, the highest metal concentration in PM2.5 was related to Pb and Zn with a value of 199 and 275 ng/m3, and the lowest belonged to Hg (24 ng/m3). The highest concentrations in the summer and spring also belonged to these metals, including Al, Fe, and Cu, and the lowest levels also belonged to Hg. The high rate of these metals in the cold season is due to the stable conditions of the Karaj atmosphere, and repeated temperature inversions caused air stability in this city by controlling the pollution dispersions and dilutions.
Concentration of PAH compounds on different stations and different seasons
In this study, the properties of PAHs compounds were evaluated. Figure 5(a) shows that approximately 40% of them are LMW-PAHs, indicating that their emissions are caused by man-made resources [60, 61]. Man-made resources in this study are mostly cement factories, sand factories, small and large industries that exist around and inside the city of Karaj and Fardis. Furthermore, according to the carcinogenicity, PAHs can be divided into 2 classes: non-human carcinogen compounds and human carcinogen compounds. Carcinogenic PAHs, including BaA, Chr, BbF, BkF, BaP, DahA, Pyr, and Ind, constitute approximately 55% of the ∑PAHs in PM2.5, and non-carcinogenic PAHs constituted 45% of the total concentration. As Fig. 5(b) shows, the quantity for (LMW) and (HMW) in the autumn and high-traffic stations is in the highest value and equal to 0.68 and 0.94 (ng/m3), respectively.
As the data in Table 3 shows, the amount for non-human carcinogen compounds and human carcinogen compounds in the autumn and the high-traffic is in the highest value and equal to 7.74, 7.7, 9.07, and 9.3 ng/m3, respectively. Owing to the partitioning of LMWs into the gas phase, their abundance was relatively low. On the contrary, HWMs, which tended to be more carcinogenic, were basically at higher concentrations and particle-bound [62]. In the present study, 60% of the total PAHs were made of [3–5] rings. In general, concentration was higher than in the high-traffic areas in the low-traffic ones, and the highest quantity was associated with the autumn season. A study has demonstrated that PAHs are produced mainly in warm seasons, but higher amounts are produced in cold seasons due to residential and factory heating. As the aforementioned studies revealed, industrial and high traffic cities have higher PAHs concentrations when temperature inversion occurs, particularly in cold seasons [63]. The amount and type of PAHs compounds vary in different seasons and places [64]. In this study, there was a significant difference between the particle concentrations and the studied seasons. Temperature inversion can also be one of the effective factors to increase the concentration of PAHs compounds in winter compared to summer season. While high traffic density and increased fossil fuels consumption by automobiles are considered the leading causes of ambient air pollution. One of the stations of this study was in the hospital, where the hospital burned the waste after modernizing the waste with modern and advanced methods. Also, in a station that was in a high-traffic area near the doctors’ building, waste decontamination was done in new ways. These factors were the origin of PAHs compounds. Other possible PAHs origins are incinerators used for the incineration of industrial waste, wood-burning, electric power plants, diesel vehicles, and iron and steel industries [65, 66]. Combustion PAHs (e.g., vehicle and domestic heating emissions), including Flu, Pyr, BaA, Chr, BbF, BkF, BaP, BghiP, and Ind, play a crucial role in air pollution. In our study, these PAHs are equal to 60%.
Table 3.
Concentration of PAHs compounds (ng/m3)
| Season | site | ΣLMW PAHs | ΣHMW PAHs | Not human carcinogens ΣPAHs | Human carcinogens ΣPAHs | Overall average |
|---|---|---|---|---|---|---|
| Autumn | L-T | 0.64 | 0.8 | 7.74 | 7.7 | 14.8 |
| H-T | 0.68 | 0.94 | 9.07 | 9.3 | 17.95 | |
| Winter | L-T | 0.25 | 0.68 | 5.4 | 6.56 | 11.6 |
| H-T | 0.64 | 0.75 | 5.91 | 7.3 | 12.94 | |
| Spring | L-T | 0.2 | 0.44 | 3.97 | 5.82 | 7.7 |
| H-T | 0.60 | 0.45 | 4.66 | 6.33 | 10 | |
| Summer | L-T | 0.16 | 0.46 | 4.81 | 6.11 | 8.5 |
| H-T | 0.38 | 0.91 | 4.96 | 8.1 | 12.3 |
Cytotoxicity analysis on different sites and different seasons
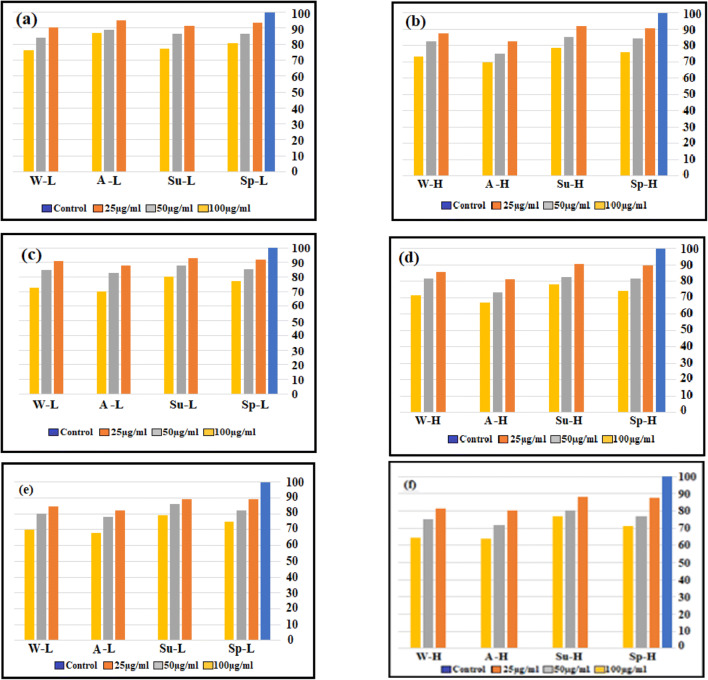
In this research, the viability of A549 cells was evaluated over three incubation periods (12, 24, and 48 h) for exposure to water-soluble extracts of PM2.5 at three levels (25, 50, and 100 μg / mL) in 4 seasons of the year, and the results are shown in Fig. 6 (a-f). The cytotoxicity findings obtained by the MTT assay test are reported as the decrease percentage in viable cells concerning negative controls. An extensive range of cytotoxicity was obtained for two stations (low-traffic and high-traffic) and four seasons ranging from 63.8 to 94.9% for water-soluble in different treatment periods. Figure 6 (a, b) shows the effect exerted by particle extracts for the concentrations of 25, 50, and 100 μg/mL for 12 h in low-traffic and high-traffic sites in four seasons. Furthermore, as Fig. 6(c, d, e, and f) shows, the influence of the time of incubation (24 and 48 h) on the proliferation of the studied cells (cell viability) was investigated.
Fig. 6.
Effect of PM2.5 exposures on cell viability in A549 cells by MTT assay.(low-traffic(L) and high-traffic(H)(sp:spring, su:summer, A:Autumn, w:winter)
Figure 6 (a-f) indicates that both extracts of two sites and four seasons of the year inhibit cell proliferation and decrease cell viability. The cell viability for water-soluble in the autumn was significantly reduced compared to the control cell at high concentrations (p < 0.05). However, the decrease in cell viability in the summer compared to control cells in other seasons showed the lowest value (p < 0.05). Various substances attached on the PM surface, such as PAHs, nitro-PAHs, and metals, may cause reduced cell viability [40, 67]. As shown in the metals and PAH sections, these materials have the highest amount in the autumn. Perrone et al. observed that the chemical elements of PM samples, such as Cr, As Cu, and Zn, were highly associated with the reduction of A549 cell viability. Metal components were demonstrated to exert adverse outcomes by generating oxidative stress and inflammatory factors, resulting in cell membrane lipids, protein and DNA damage, and then cell death [68, 69]. At two stations with low particle concentrations, there was a statistically significant difference (P < 0.05) between cell viability at12-hour incubation time for cytotoxicity. PM2.5 from the low-traffic site samples exhibited a relatively lower decrease in the viability of cells. It was observed that a reduction in target-cell viability after treatment with water-soluble fractions of high-traffic station samples at a concentration of 100 μg/mL was 20% higher than that at a concentration of 25 μg/mL for an incubation time of 12 h. Snanchez-Sobernon et al. found that in cases where A549 cells were exposed to higher PM2.5 doses, they had reduced A549 cell viability [70]. After 24 and 48 h exposure, cell viabilities show that the treatments induced a concentration-dependent decrease in MTT-reduction activity [62, 71, 72]. Low-traffic and high-traffic stations in the fall (with an incubation time of 24 h and a concentration of 100 μg/mL) were 70% and 66.8%, respectively (with an incubation time of 48 h and a concentration of 100 μg/mL) were 68.1% and 63.8%, respectively. Figure 6 (c, d, e, and f) shows that the cell viability rate decreased further with increasing incubation time. Yang et al. found that at the same treatment time, the A549-cell viability was reduced with the increase of PM2.5 concentration, and at the same treatment concentration, it was decreased with the increase of exposure time [73]. Statistical analysis, a significant difference between low-traffic and high-traffic station samples in cell viability (% of control) was not observed, but between 4 season year samples in a significant difference was there (p < 0.05). Contrary to the control group, the viability of PM2.5-treated A549 cells significantly decreased in a dose-dependent manner for samples of different seasons [31, 74].
Conclusion
This study was the first precise study in Karaj and Fardis that simultaneously measured PM2.5 particles, polycyclic aromatic hydrocarbons, and heavy metals bonded with PM2.5 particles, with a high number of samples. We also exposed human lung epithelial cells (A549) to particles for the first time to determine the toxicity of those particles. The results obtained in this study are very valuable and can be used for comprehensive planning for these two cities by relevant organizations and agencies. Some metals, including Al and Fe, had the highest concentrations among other metals. Carcinogenic PAHs constituted approximately 55% of the total PM2.5. Due to the high concentration of Chr, BbF and DBahA compounds in this study, they were introduced as an indicator of carcinogenicity in humans. The combustion indices were also Pyr and BbF. The results demonstrated that water-soluble extracts remarkably inhibited cell proliferation when the concentration and time were increased. The cytotoxicity of PM2.5 may be attributed to the presence of PAH fractions as well as heavy metals. The PAHs contents and toxicity parameters indicated that the organic components derived from motor vehicle exhaust and coal combustion were related to the cell toxicity.
On the other hand, this study also had some limitations, including the fact that only the particulate phase of PAHs and heavy metals was studied. Also, scattering modeling with more samples was performed to obtain more information about the weather in Karaj and Fardis. It should be noted that the limitations mentioned in this study should be considered in other studies.
Acknowledgments
This article is the result of the MSc approved thesis, research project no. 13062. Thus, the authors are thankful for the funding provided by the Iran University of Medical Sciences.
Funding
Support of this work by Iran University of Medical Sciences is gratefully acknowledged (Grant number: 97–3–2-13062).
Declarations
Ethical approval
The authors of this article have covered all the ethical points, including non-plagiarism, duplicate publishing, data distortion, and data creation in this article. This project has been registered in the Iran University of Medical Sciences with the code of ethics of IR.IUMS.REC.1397.639.
Conflict of interest
The authors of this article declare that they have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Majid Kermani, Email: kermani.m@iums.ac.ir, Email: majidkermani@yahoo.com.
Farzad Fanaei, Email: farzadfanaei37@gmail.com.
References
- 1.Xu X, Yu X, Mo L, Xu Y, Bao L, Lun X. Atmospheric particulate matter accumulation on trees: a comparison of boles, branches and leaves. J Clean Prod. 2019;226:349–356. [Google Scholar]
- 2.Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- 3.Nabizadeh R, Yousefi M, Azimi F. Study of particle number size distributions at Azadi terminal in Tehran, comparing high-traffic and no traffic area. MethodsX. 2018;5:1549–1555. doi: 10.1016/j.mex.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fathi Fathabadi MK, Abdolahnejad A, Teiri H, Hajizadeh Y. Spatio-seasonal variation of airborne asbestos concentration in urban areas of shiraz, Iran. Int J Occup Environ Health. 2017;23(2):143–150. doi: 10.1080/10773525.2018.1436016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masroor K, Fanaei F, Yousefi S, Raeesi M, Abbaslou H, Shahsavani A, et al. Spatial modelling of PM2. 5 concentrations in Tehran using kriging and inverse distance weighting (IDW) methods. J Air Pollut Health. 2020;5(2):89–96. [Google Scholar]
- 6.Li S, Tan H-Y, Wang N, Zhang Z-J, Lao L, Wong C-W, Feng Y. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16(11):26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Øvrevik J. Oxidative potential versus biological effects: a review on the relevance of cell-free/abiotic assays as predictors of toxicity from airborne particulate matter. Int J Mol Sci. 2019;20(19):4772. doi: 10.3390/ijms20194772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heydari G, Taghizdeh F, Fazlzadeh M, Jafari AJ, Asadgol Z, Mehrizi EA, Moradi M, Arfaeinia H. Levels and health risk assessments of particulate matters (PM 2.5 and PM 10) in indoor/outdoor air of waterpipe cafés in Tehran, Iran. Environ Sci Pollut Res. 2019;26(7):7205–7215. doi: 10.1007/s11356-019-04202-5. [DOI] [PubMed] [Google Scholar]
- 9.Masjedi MR, Taghizadeh F, Hamzehali S, Ghaffari S, Fazlzadeh M, Jafari AJ, et al. Air pollutants associated with smoking in indoor/outdoor of waterpipe cafés in Tehran, Iran: concentrations, affecting factors and health risk assessment. Sci Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-39684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo W-C, Shie R-H, Chan C-C, Lin H-H. Burden of disease attributable to ambient fine particulate matter exposure in Taiwan. J Formos Med Assoc. 2017;116(1):32–40. doi: 10.1016/j.jfma.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Zeng Q, Ni Y, Jiang G, Li G, Pan X. The short term burden of ambient particulate matters on non-accidental mortality and years of life lost: a ten-year multi-district study in Tianjin, China. Environmental pollution. 2017;220:713–719. doi: 10.1016/j.envpol.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 12.Kermani M, Jafari AJ, Gholami M, Arfaeinia H, Shahsavani A, Fanaei F. Characterization, possible sources and health risk assessment of PM2. 5-bound Heavy Metals in the most industrial city of Iran. J Environ Health Sci Eng. 2021;19:151–163. [DOI] [PMC free article] [PubMed]
- 13.Hajizadeh Y, Jafari N, Fanaei F, Ghanbari R, Mohammadi A, Behnami A, et al. Spatial patterns and temporal variations of traffic-related air pollutants and estimating its health effects in Isfahan city, Iran. J Environ Health Sci Eng 2021;19:781–791. [DOI] [PMC free article] [PubMed]
- 14.Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manage Assoc. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 15.Farrokhzadeh H, Jafari N, Sadeghi M, Talesh Alipour M, Amin MM, Abdolahnejad A. Estimation of spatial distribution of PM10, lead, and radon concentrations in Sepahanshahr, Iran using geographic information system (GIS) J Mazandaran Univ Med Sci. 2018;27(159):84–96. [Google Scholar]
- 16.Kermani M, Arfaeinia H, Masroor K, Abdolahnejad A, Fanaei F, Shahsavani A, et al. Health impacts and burden of disease attributed to long-term exposure to atmospheric PM10/PM2. 5 in Karaj, Iran: effect of meteorological factors. International Journal of Environmental Analytical Chemistry. 2020. 10.1080/03067319.2020.1807534
- 17.Arfaeinia H, Moradi M, Sharafi K, Mahdi Esfahan N, Dobaradaran S. Evaluation of public health impacts related to urban air pollution in shiraz and Bushehr, Iran.Int J PharmTechnol. 2015;7:9811–9824.
- 18.Hetland R, Cassee F, Refsnes M, Schwarze P, Låg M, Boere A, Dybing E. Release of inflammatory cytokines, cell toxicity and apoptosis in epithelial lung cells after exposure to ambient air particles of different size fractions. Toxicol in Vitro. 2004;18(2):203–212. doi: 10.1016/s0887-2333(03)00142-5. [DOI] [PubMed] [Google Scholar]
- 19.Kim W, Jeong S-C, Shin C-Y, Song M-K, Cho Y, Lim J-H, et al. A study of cytotoxicity and genotoxicity of particulate matter (PM 2.5) in human lung epithelial cells (A549) Mol Cell Toxicol. 2018;14(2):163–172. [Google Scholar]
- 20.Hajizadeh Y, Jafari N, Mohammadi A, Momtaz SM, Fanaei F, Abdolahnejad A. Concentrations and mortality due to short-and long-term exposure to PM 2.5 in a megacity of Iran (2014–2019) Environ Sci Pollut Res. 2020;27(30):38004–38014. doi: 10.1007/s11356-020-09695-z. [DOI] [PubMed] [Google Scholar]
- 21.Fanaei F, Ghorbanian A, Shahsavani A, Jafari AJ, Abdolahnejad A, Kermani M. Quantification of mortality and morbidity in general population of heavily-industrialized city of Abadan: effect of long-term exposure. J Air Pollut Health. 2020;5(3):171–180. [Google Scholar]
- 22.Ghasemi FF, Dobaradaran S, Saeedi R, Nabipour I, Nazmara S, Abadi DRV, et al. Levels and ecological and health risk assessment of PM 2.5-bound heavy metals in the northern part of the Persian Gulf. Environ Sci Pollut Res. 2020;27(5):5305–5313. doi: 10.1007/s11356-019-07272-7. [DOI] [PubMed] [Google Scholar]
- 23.Afroz R, Hassan MN, Ibrahim NA. Review of air pollution and health impacts in Malaysia. Environ Res. 2003;92(2):71–77. doi: 10.1016/s0013-9351(02)00059-2. [DOI] [PubMed] [Google Scholar]
- 24.Zarasvandi A, Carranza E, Moore F, Rastmanesh F. Spatio-temporal occurrences and mineralogical–geochemical characteristics of airborne dusts in Khuzestan Province (southwestern Iran) J Geochem Explor. 2011;111(3):138–151. [Google Scholar]
- 25.Santamouris M, Cartalis C, Synnefa A. Local urban warming, possible impacts and a resilience plan to climate change for the historical center of Athens, Greece. Sustain Cities Soc. 2015;19:281–291. [Google Scholar]
- 26.Velali E, Papachristou E, Pantazaki A, Choli-Papadopoulou T, Argyrou N, Tsourouktsoglou T, Lialiaris S, Constantinidis A, Lykidis D, Lialiaris TS, Besis A, Voutsa D, Samara C. Cytotoxicity and genotoxicity induced in vitro by solvent-extractable organic matter of size-segregated urban particulate matter. Environ Pollut. 2016;218:1350–1362. doi: 10.1016/j.envpol.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Silva RA, West JJ, Zhang Y, Anenberg SC, Lamarque J-F, Shindell DT, Collins WJ, Dalsoren S, Faluvegi G, Folberth G, Horowitz LW, Nagashima T, Naik V, Rumbold S, Skeie R, Sudo K, Takemura T, Bergmann D, Cameron-Smith P, Cionni I, Doherty RM, Eyring V, Josse B, MacKenzie IA, Plummer D, Righi M, Stevenson DS, Strode S, Szopa S, Zeng G. Global premature mortality due to anthropogenic outdoor air pollution and the contribution of past climate change. Environ Res Lett. 2013;8(3):034005. [Google Scholar]
- 28.Torkashvand J, Jafari AJ, Hopke PK, Shahsavani A, Hadei M, Kermani M. Airborne particulate matter in Tehran’s ambient air. J Environ Health Sci Eng. 2021;19:1179–1191. doi: 10.1007/s40201-020-00573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karimi H, Nikaeen M, Gholipour S, Hatamzadeh M, Hassanzadeh A, Hajizadeh Y. PM 2.5-associated bacteria in ambient air: is PM 2.5 exposure associated with the acquisition of community-acquired staphylococcal infections? J Environ Health Sci Eng. 2020;18(2):1007–1013. doi: 10.1007/s40201-020-00522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vuong NQ, Breznan D, Goegan P, O’Brien JS, Williams A, Karthikeyan S, Kumarathasan P, Vincent R. In vitro toxicoproteomic analysis of A549 human lung epithelial cells exposed to urban air particulate matter and its water-soluble and insoluble fractions. Particle and fibre toxicology. 2017;14(1):39. doi: 10.1186/s12989-017-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Luo X-S, Zhao Z, Chen Q, Wu D, Sun X, Wu L, Jin L. Summer–winter differences of PM2. 5 toxicity to human alveolar epithelial cells (A549) and the roles of transition metals. Ecotoxicol Environ Saf. 2018;165:505–509. doi: 10.1016/j.ecoenv.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 32.Haghnazari L, Mirzaei N, Arfaeinia H, Karimyan K, Sharafi H, Fattahi N. Speciation of as (ΙΙΙ)/as (V) and total inorganic arsenic in biological fluids using new mode of liquid-phase microextraction and electrothermal atomic absorption spectrometry. Biol Trace Elem Res. 2018;183(1):173–181. doi: 10.1007/s12011-017-1118-8. [DOI] [PubMed] [Google Scholar]
- 33.Palleschi S, Rossi B, Armiento G, Montereali MR, Nardi E, Tagliani SM, et al. Toxicity of the readily leachable fraction of urban PM2. 5 to human lung epithelial cells: role of soluble metals. Chemosphere. 2018;196:35–44. doi: 10.1016/j.chemosphere.2017.12.147. [DOI] [PubMed] [Google Scholar]
- 34.Kim K-H, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter. Environ Int. 2015;74:136–143. doi: 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Armand L, Biola-Clier M, Bobyk L, Collin-Faure V, Diemer H, Strub J-M, Cianferani S, van Dorsselaer A, Herlin-Boime N, Rabilloud T, Carriere M. Molecular responses of alveolar epithelial A549 cells to chronic exposure to titanium dioxide nanoparticles: a proteomic view. J Proteome. 2016;134:163–173. doi: 10.1016/j.jprot.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Schiliro T, Alessandria L, Degan R, Traversi D, Gilli G. Chemical characterisation and cytotoxic effects in A549 cells of urban-air PM10 collected in Torino, Italy. Environ Toxicol Pharmacol. 2010;29(2):150–157. doi: 10.1016/j.etap.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Jia Y-Y, Wang Q, Liu T. Toxicity research of PM2. 5 compositions in vitro. Intl J Environ Res Pub Health. 2017;14(3):232. doi: 10.3390/ijerph14030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavagadhi S, Betha R, Venkatesan S, Balasubramanian R, Hande MP. Physicochemical and toxicological characteristics of urban aerosols during a recent Indonesian biomass burning episode. Environ Sci Pollut Res. 2013;20(4):2569–2578. doi: 10.1007/s11356-012-1157-9. [DOI] [PubMed] [Google Scholar]
- 39.Vahidi MH, Fanaei F, Kermani M. Long-term health impact assessment of PM2. 5 and PM10: Karaj, Iran. Intl J Environ Health Eng. 2020;9(1):8. [Google Scholar]
- 40.Happo M, Markkanen A, Markkanen P, Jalava P, Kuuspalo K, Leskinen A, Sippula O, Lehtinen K, Jokiniemi J, Hirvonen MR. Seasonal variation in the toxicological properties of size-segregated indoor and outdoor air particulate matter. Toxicol in Vitro. 2013;27(5):1550–1561. doi: 10.1016/j.tiv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Cokic SM, Ghosh M, Hoet P, Godderis L, Van Meerbeek B, Van Landuyt KL. Cytotoxic and genotoxic potential of respirable fraction of composite dust on human bronchial cells. Dental Materials. 2020;36(2):270–83. [DOI] [PubMed]
- 42.Asadollahfardi G, Zangooei H, Aria SH. Predicting PM 2.5 Concentrations Using Artificial Neural Networks and Markov Chain, a Case Study Karaj City. Asian Journal of Atmospheric Environment (AJAE). 2016;10(2):67–79.
- 43.Naimabadi A, Ghadiri A, Idani E, Babaei AA, Alavi N, Shirmardi M, Khodadadi A, Marzouni MB, Ankali KA, Rouhizadeh A, Goudarzi G. Chemical composition of PM10 and its in vitro toxicological impacts on lung cells during the middle eastern dust (MED) storms in Ahvaz, Iran. Environ Pollut. 2016;211:316–324. doi: 10.1016/j.envpol.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Schilirò T, Bonetta S, Alessandria L, Gianotti V, Carraro E, Gilli G. PM10 in a background urban site: chemical characteristics and biological effects. Environ Toxicol Pharmacol. 2015;39(2):833–844. doi: 10.1016/j.etap.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Hoseini M, Yunesian M, Nabizadeh R, Yaghmaeian K, Ahmadkhaniha R, Rastkari N, Parmy S, Faridi S, Rafiee A, Naddafi K. Characterization and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in urban atmospheric particulate of Tehran, Iran. Environ Sci Pollut Res. 2016;23(2):1820–1832. doi: 10.1007/s11356-015-5355-0. [DOI] [PubMed] [Google Scholar]
- 46.Dehghani S, Fararouei M, Rafiee A, Hoepner L, Oskoei V, Hoseini M. Prenatal exposure to polycyclic aromatic hydrocarbons and effects on neonatal anthropometric indices and thyroid-stimulating hormone in a middle eastern population. Chemosphere. 2022;286:131605. doi: 10.1016/j.chemosphere.2021.131605. [DOI] [PubMed] [Google Scholar]
- 47.Yin X, Sun Z, Miao S, Yan Q, Wang Z, Shi G, Li Z, Xu W. Analysis of abrupt changes in the PM2.5 concentration in Beijing during the conversion period from the summer to winter half-year in 2006–2015. Atmos Environ. 2019;200:319–328. [Google Scholar]
- 48.Deng X, Zhang F, Rui W, Long F, Wang L, Feng Z, Chen D, Ding W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol in Vitro. 2013;27(6):1762–1770. doi: 10.1016/j.tiv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Zhang WJ, Xiong W, Lu WH, Zheng HY, Zhou X, Yuan J. PM2.5 stimulated the release of cytokines from BEAS-2B cells through activation of IKK/NF-kappaB pathway. Hum Exp Toxicol. 2019;38(3):311–320. doi: 10.1177/0960327118802628. [DOI] [PubMed] [Google Scholar]
- 50.Song Y, Zhang Y, Li R, Chen W, Chung CKA, Cai Z. The cellular effects of PM2. 5 collected in Chinese Taiyuan and Guangzhou and their associations with polycyclic aromatic hydrocarbons (PAHs), nitro-PAHs and hydroxy-PAHs. Ecotoxicol Environ Safety. 2020;191:110225. doi: 10.1016/j.ecoenv.2020.110225. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H-H, Li Z, Liu Y, Xinag P, Cui X-Y, Ye H, et al. Physical and chemical characteristics of PM 2.5 and its toxicity to human bronchial cells BEAS-2B in the winter and summer. J Zhejiang Univ-Sci B. 2018;19(4):317–326. doi: 10.1631/jzus.B1700123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang HH, Li Z, Liu Y, Xinag P, Cui XY, Ye H, Hu BL, Lou LP. Physical and chemical characteristics of PM2.5 and its toxicity to human bronchial cells BEAS-2B in the winter and summer. J Zhejiang Univ Sci B. 2018;19(4):317–326. doi: 10.1631/jzus.B1700123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang F, Guo Z, Lin T, Rose NL. Seasonal variation of carbonaceous pollutants in PM2. 5 at an urban ‘supersite’in Shanghai, China. Chemosphere. 2016;146:238–244. doi: 10.1016/j.chemosphere.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 54.Sowlat MH, Naddafi K, Yunesian M, Jackson PL, Shahsavani A. Source apportionment of total suspended particulates in an arid area in southwestern Iran using positive matrix factorization. Bull Environ Contam Toxicol. 2012;88(5):735–740. doi: 10.1007/s00128-012-0560-8. [DOI] [PubMed] [Google Scholar]
- 55.Hajizadeh Y, Mokhtari M, Faraji M, Abdolahnejad A, Mohammadi A. Biomonitoring of airborne metals using tree leaves: protocol for biomonitor selection and spatial trend. MethodsX. 2019;6:1694–1700. doi: 10.1016/j.mex.2019.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prabhakar G, Sorooshian A, Toffol E, Arellano AF, Betterton EA. Spatiotemporal distribution of airborne particulate metals and metalloids in a populated arid region. Atmos Environ. 2014;92:339–347. doi: 10.1016/j.atmosenv.2014.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang N, Liu X, Wang S, Yu X, Yin S, Duan S, Wang S, Zhang R, Li S. Pollution characterization, source identification, and health risks of atmospheric-particle-bound heavy metals in PM10 and PM2. 5 at multiple sites in an emerging megacity in the central region of China. Aerosol Air Qual Res. 2019;19:247–271. [Google Scholar]
- 58.Rai P, Chakraborty A, Mandariya AK, Gupta T. Composition and source apportionment of PM1 at urban site Kanpur in India using PMF coupled with CBPF. Atmos Res. 2016;178:506–520. [Google Scholar]
- 59.Liu J, Chen Y, Chao S, Cao H, Zhang A, Yang Y. Emission control priority of PM2. 5-bound heavy metals in different seasons: a comprehensive analysis from health risk perspective. Sci Total Environ. 2018;644:20–30. doi: 10.1016/j.scitotenv.2018.06.226. [DOI] [PubMed] [Google Scholar]
- 60.Bi C, Chen Y, Zhao Z, Li Q, Zhou Q, Ye Z, Ge X. Characteristics, sources and health risks of toxic species (PCDD/fs, PAHs and heavy metals) in PM2. 5 during fall and winter in an industrial area. Chemosphere. 2020;238:124620. doi: 10.1016/j.chemosphere.2019.124620. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Chen J, Yang H, Li R, Yu Q. Seasonal variation and potential source regions of PM2. 5-bound PAHs in the megacity Beijing, China: impact of regional transport. Environ Pollut. 2017;231:329–338. doi: 10.1016/j.envpol.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 62.Niu X, Ho SSH, Ho KF, Huang Y, Sun J, Wang Q, Zhou Y, Zhao Z, Cao J. Atmospheric levels and cytotoxicity of polycyclic aromatic hydrocarbons and oxygenated-PAHs in PM2.5 in the Beijing-Tianjin-Hebei region. Environ Pollut. 2017;231(Pt 1):1075–1084. doi: 10.1016/j.envpol.2017.08.099. [DOI] [PubMed] [Google Scholar]
- 63.Liu X, Li C, Tu H, Wu Y, Ying C, Huang Q, et al. Analysis of the effect of meteorological factors on PM2. 5-associated PAHs during autumn-winter in urban Nanchang. Aerosol Air Qual Res. 2016;16:3222–3229. [Google Scholar]
- 64.Lyu Y, Su S, Wang B, Zhu X, Wang X, Zeng EY, Xing B, Tao S. Seasonal and spatial variations in the chemical components and the cellular effects of particulate matter collected in northern China. Sci Total Environ. 2018;627:1627–1637. doi: 10.1016/j.scitotenv.2018.01.224. [DOI] [PubMed] [Google Scholar]
- 65.Yang H-H, Lai S-O, Hsieh L-T, Hsueh H-J, Chi T-W. Profiles of PAH emission from steel and iron industries. Chemosphere. 2002;48(10):1061–1074. doi: 10.1016/s0045-6535(02)00175-3. [DOI] [PubMed] [Google Scholar]
- 66.Yang H-H, Lee W-J, Chen S-J, Lai S-O. PAH emission from various industrial stacks. J Hazard Mater. 1998;60(2):159–174. [Google Scholar]
- 67.Chen Q, Luo XS, Chen Y, Zhao Z, Hong Y, Pang Y, et al. Seasonally varied cytotoxicity of organic components in PM2.5 from urban and industrial areas of a Chinese megacity. Chemosphere. 2019;230:424–431. doi: 10.1016/j.chemosphere.2019.04.226. [DOI] [PubMed] [Google Scholar]
- 68.Zhang K, Nie D, Chen M, Wu Y, Ge X, Hu J, et al. Chemical Characterization of Two Seasonal PM2.5 Samples in Nanjing and Its Toxicological Properties in Three Human Cell Lines. Environments. 2019;6(4):42.
- 69.Wang S, Ye J, Soong R, Wu B, Yu L, Simpson AJ, et al. Relationship between chemical composition and oxidative potential of secondary organic aerosol from polycyclic aromatic hydrocarbons. Atmospheric Chemistry & Physics. 2018;18(6):3987–4003.
- 70.Sanchez-Soberon F, Cuykx M, Serra N, Linares V, Belles M, Covaci A, et al. In-vitro metabolomics to evaluate toxicity of particulate matter under environmentally realistic conditions. Chemosphere. 2018;209:137–146. doi: 10.1016/j.chemosphere.2018.06.065. [DOI] [PubMed] [Google Scholar]
- 71.Chi Y, Huang Q, Lin Y, Ye G, Zhu H, Dong S. Epithelial-mesenchymal transition effect of fine particulate matter from the Yangtze River Delta region in China on human bronchial epithelial cells. J Environ Sci (China) 2018;66:155–164. doi: 10.1016/j.jes.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Zou Y, Wu Y, Wang Y, Li Y, Jin C. Physicochemical properties, in vitro cytotoxic and genotoxic effects of PM1.0 and PM2.5 from Shanghai, China. Environ Sci Pollut Res Int. 2017;24(24):19508–19516. doi: 10.1007/s11356-017-9626-9. [DOI] [PubMed] [Google Scholar]
- 73.Yang J, Huo T, Zhang X, Ma J, Wang Y, Dong F, Deng J. Oxidative stress and cell cycle arrest induced by short-term exposure to dustfall PM 2.5 in A549 cells. Environmental Science and Pollution Research. 2018;25(23):22408–19. [DOI] [PubMed]
- 74.Wang G, Zhang X, Liu X, Zheng J, Chen R, Kan H. Ambient fine particulate matter induce toxicity in lung epithelial-endothelial co-culture models. Toxicol Lett. 2019;301:133–145. doi: 10.1016/j.toxlet.2018.11.010. [DOI] [PubMed] [Google Scholar]