Abstract
Background
Serum leptin has been considered as an important measurable diagnostic and prognostic biomarker for polycystic ovarian syndrome (PCOS), although its evidence for use in clinical practice is limited. We aim to synthesize the available evidence on the clinical use of serum leptin values in PCOS by doing a systematic review and meta-analysis of studies.
Objective
To conduct a meta-analysis to determine the pooled effect size of the association of leptin levels in patients with PCOS.
Methods
We searched electronic databases, i.e., PubMed, Google Scholar, Web of Science, ClinicalTrials.gov, and Medline from inception to September 2020, keeping filters for human studies and published in the English language. We used the random-effects model if heterogeneity between the studies was > 50%; otherwise, a fixed-effect model was applied to determine the standardized mean difference with 95% CI for comparison of leptin level between cases and controls. All the statistical analyses were completed using software STATA version 13.
Results
The meta-analysis included a total of 35 studies involving 2015 cases and 1767 controls that suggested statistically significantly higher leptin levels in the women with PCOS as compared to controls (SMD, 1.76, 95% CI 1.28 to 2.23, P < 0.001). In the stratified analysis when only high methodological quality studies were included, we did not observe a statistically significant difference in the leptin level between PCOS and controls (SMD 0.68, 95% CI −0.09 to 1.46). Analysis restricted to low methodological quality studies observed statistically significant high leptin levels in PCOS women as compared to controls (SMD 2.24, 95% CI 1.65 to 2.83).
Conclusion
The available evidence suggests that elevated leptin levels may be associated with risk of PCOS as compared to controls; however, failure to observe the similar association in high methodological quality studies demands further well-designed adequately powered studies to validate the findings.
Keywords: Leptin, PCOS, Polycystic ovary syndrome, Adipokine
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine abnormalities in women with controversial diagnosis and management [1]. Its prevalence varies from 5 to 10% in women of reproductive age group [2]. Leptin was the first fat cell-derived hormone (adipokine) which plays an important role in several metabolic processes. It is important in regulating energy homeostasis and has an impact on the reproductive systems in diverse ways. Leptin has been observed to affect the pathogenesis of PCOS in women with obesity. It is important to assess the inter-regulatory phenomena between leptin and ovarian function for its use in clinical settings. Evidence exists in the literature which suggests high leptin levels may also be associated with oxidative stress. Several studies have shown that oxidative stress markers are increased in women with PCOS compared to normal women and play an important role in the pathogenesis of PCOS. Leptin has been commonly identified as a marker for higher oxidative stress and is often increased in case of obesity which may be associated with PCOS.
Literature suggests mixed results in association between the level of leptin in patients with PCOS [4]. Few of the studies have shown increased levels of leptin in patients with PCOS as compared to controls [5]; in contrast to this, some studies have shown a lower level of leptin in women with PCOS[6, 7]. A study has shown that an optimal level of leptin in the follicular fluid may have a good correlation with embryo quality, fertilization rate, pregnancy rate, and successful live births [3].
The differences between the methodologies used in the leptin biochemical measurement, selection of cases and control, differences in the study settings may explain the inconsistent results shown in the literature. It is essential to determine the precise association of leptin level in PCOS women as it could serve as an important biomarker for PCOS diagnosis and also help in the optimal treatment of women with PCOS. Meta-analysis provides precise evidence that combines the findings of multiples studies addressing the same research question using robust statistical power. Therefore, we have conducted a systematic review and meta-analysis to determine the pooled effect size of the association between leptin levels and women with PCOS.
Methods
Literature Search: Relevant literature was searched using the electronic database in PubMed, Google Scholar, Web of Science, ClinicalTrials.gov, and Medline from the inception to September 2020, keeping filters for human studies and published in the English language. Search terms included “PCOS” or “polycystic ovary syndrome” and “leptin”. References of published relevant articles were also manually searched to obtain additional relevant studies.
Selection Criteria
Inclusion criteria for review of studies were 1. studies using Rotterdam or standard diagnostic criteria for defining PCOS; 2. studies having sufficient information for the extraction of relevant data in women with PCOS and controls; 3. studies reporting mean and standard deviation or sufficient information was available to extract mean and standard deviation in both cases and controls. Exclusion criteria for review of studies were 1. review articles; 2. case reports, commentary, or preclinical studies; 3. single study group without controls; 4. group which included the study subjects had conditions other than PCOS.
Quality Assessment of Included Studies
The Newcastle–Ottawa Scale methodological quality assessment tool [8] was used to assess the quality of studies included in the meta-analysis. The criteria for the assessment of quality were (1) diagnosis of PCOS based on Rotterdam or standard criteria; (2) representativeness of cases; (3) recruitment method of controls if from the same community or hospital; (4) availability of sufficient information to assess the PCOS free status of controls; (5) matched controls or multivariable analysis was used; (6) if 1 or more factors, such as BMI or smoking was matched; (7) if the leptin level assessment was under blind measurement; and (8) if a similar method was used for assessment of both groups. For each item, a score of 0 or 1 was allocated based on the adequately satisfied condition. The highest score 9 represents the best quality, while the lowest score 0 represents worse quality. The study quality was categorized into two subgroups high quality (7 or more), low to moderate quality (6 or less) for the subgroup analysis.
Results
Characteristics of Studies
The study flow diagram represents the extensive steps of the literature search (Fig. 1). After thorough screening of articles, we obtained 35 articles meeting the inclusion criteria of the present meta-analysis and included 2015 cases and 1767 controls for quantitative analysis. The characteristics of the included studies have been shown in Table 1. The circulating levels of leptin in women with PCOS versus controls were measured in all the studies included in the meta-analysis. Out of 35 studies, seven studies were from the USA [9–15], six studies from Turkey [16–21], four studies from Iran [22–25], three from Italy [26–28], two from Bulgaria [29, 30], two from India [31, 32], two from Egypt [33, 34], one from Greece [35], one from Pakistan [36], one from the UK [37], one from Israel [38], one from Germany [39], one from Qatar [40], one from Korea[41], one from Saudi Arabia [42] and one from Taiwan [43]. The methodological quality of studies in individual studies is shown in Table 2.
Fig. 1.
Study flow diagram for screening and inclusion of studies
Table 1.
Study characteristics of included case control studies
| S. No | Authors | Country/year | Mean age in case | Population | Biomarker assessed |
|---|---|---|---|---|---|
| 1 | Baig M[36] | Pakistan/2014 | 29.9 ± 2.5 | 62/90 | FSH, LH, E2, FT4, FT3, TSH |
| 2 | Baldani DP [37] | UK/2018 | 26.5 ± 6 | 151/95 | DHEAS, (A),17-OH- (17-OHP), glucose, insulin, leptin, adiponectin, resistin, and ghrelin. Serum LH, FSH, TSH, PRL, and TT |
| 3 | Jahromi BN[23] | Iran/2017 | 27.6 ± 4.34 | 99/90 | LH, FSH, TSH, and prolactin level. Testosterone FI, FBS, and lipid profile |
| 4 | Hahn S [39] | Germany/2006 | 27.3 ± 5.7 | 122/81 | LH, testosterone, cortisol, TSH, cholesterol, triglycerides (TG) and blood glucose (G) |
| 5 | Iliana Tsouma [35] | Greece/2014 | 32 ± 4 | 70/76 | Total cholesterol, LDL cholesterol, TG, apolipoprotein B, lipoprotein(a), and homocysteine |
| 6 | Yildizhan R [17] | Turkey/2011 | 26.4 ± 2.75 | 57/27 | Dimethyl arginine, RBP4, leptin, LH, FSH, DHEAS, total T, E2, total cholesterol, HDL cholesterol, low-density, LDL cholesterol, TG |
| 7 | Peter R [15] | USA/2015 | 19 to 44 | 58/70 | Fasting glucose, leptin |
| 8 | Iuorno MJ [9] | USA/2007 | 27.3 ± 2.1 | 9/9 | Serum insulin, serum testosterone, and leptin |
| 9 | Saleh HA [33] | EGYPT/ 2004 | 28.1 ± 4.6 | 50/20 | Serum prolactin, FSH and LH, testosterone, and DHEA |
| 10 | Jeon YE [41] | KOREA/2013 | 23.68 ± 5.4 | 54/36 | Sex hormone-binding globulin (SHBG), glucose, insulin, leptin, RBP4, and kisspeptin |
| 11 | Saray S[42] | Saudi Arabia/2015 | 28.6 ± 6.1 | 211/215 | FSH, LH, prolactin, testosterone, 17 a-hydroxy, (TSH) |
| 12 | Demirel F[18] | TURKEY/2007 | 15.3 ± 1.1 | 44/31 | Insulin, FSH, LH, total testosterone (TT), DHEAS and E2 |
| 13 | Telli MH [19] | TURKEY/2002 | 21.4 ± 2.7 | 50/32 | FSH, LH, androstenedione, DHEAS, and fasting insulin |
| 14 | Sepilian VP [11] | AMERICA/2006 | 28.4 ± 0.9 | 40/15 | Fasting glucose, insulin, leptin, sOB-R, T, and DHEAS levels |
| 15 | Chen CI [43] | Taiwan/ 2015 | 26.05 ± 5.7 | 224/198 | Total testosterone, triglyceride, and low-density lipoprotein (LDL) |
| 16 | Savastano S[28] | Italy/ 2011 | 24.4 ± 4.3 | 90/40 | 25-hydroxyvitamin D, PED/PEA-15, leptin-to-adiponectin |
| 17 | Remsberg KE [13] | USA/2002 | 39.8 ± 6.4 | 46/46 | Leptin, insulin, glucose, SHBG, |
| 18 | Jalilian N [25] | IRAN /2016 | 27.15 ± 4.31 | 40/36 | Fasting blood glucose, (LH), (FSH), thyroid stimulating hormone (TSH), prolactin |
| 19 | Pusalkar M [32] | India /2010 | 31.19 ± 0.64 | 100/100 | Testosterone, androstenedione, 17-OHP, DHEAS, |
Table 2.
Methodological quality assessment of included studies using Newcastle–Ottawa quality (NOS) scale
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Overall |
|---|---|---|---|---|---|---|---|---|---|
| Gargari BP [22] | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 |
| Baldani DP [37] | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 1 | 6 |
| Jahromi BN[23] | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 4 |
| Hahn S [39] | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 1 | 6 |
| Iliana Tsouma [35] | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 1 | 6 |
| Yildizhan R [17] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Peter R [15] | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 5 |
| Iuorno MJ [9] | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 5 |
| Saleh HA [33] | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 5 |
| Jeon YE [41] | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 4 |
| Saray S[42] | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 1 | 6 |
| Demirel F[18] | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 4 |
| Telli MH [19] | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 5 |
| Sepilian VP [11] | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 5 |
| Chen CI [43] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Savastano S[28] | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 |
| Remsberg KE [13] | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 7 |
| Jalilian N [25] | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 5 |
| Pusalkar M [32] | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 5 |
Q1, Case Defined With Independent Validation; Q2, Representativeness of the Cases; Q3, Selection of Controls From Community; Q4, Statement That Controls Have No History of Outcome; Q5, Cases and Controls Matched and/or Adjusted by Factors; Q6, Ascertain Exposure by Blinded Structured Interview; Q7, Same Method of Ascertainment for Cases and Controls; Q8, Same Response Rate for Both Groups
Meta-Analysis Findings
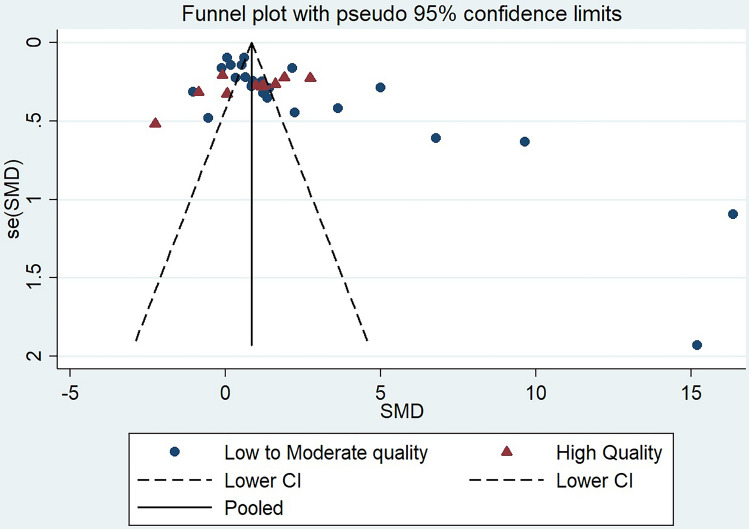
The meta-analysis provides precise evidence by providing the common effect size by combining the results of multiple studies conducted with similar study questions. The meta-analysis involving a total of thirty-five studies suggested leptin level is significantly higher in women with PCOS as compared to controls (SMD, 1.76, 95% CI 1.28 to 2.23, P < 0.001) (Fig. 2). The random-effect model was used due to the presence of significant heterogeneity between the studies. Further, we conducted the stratified analysis based on the methodological quality of studies in which studies were classified as low quality if the score was less than or equal to 6 and > 6 was considered a high-quality study. Interestingly, we did not observe a statistically significant difference in the leptin level between PCOS and controls in a pooled analysis of high methodological quality of studies (SMD 0.68, 95% CI -0.09 to 1.46) (Fig. 2). On the other hand, the pooled analysis on the low methodological quality of studies observed statistically significantly high leptin levels in PCOS women as compared to controls (SMD 2.24, 95% CI 1.65–2.83) (Fig. 2). Since there are huge variations in the methodological qualities in the studies involved in the present meta-analysis, we did the meta-regression analysis to check whether the methodological quality of studies has a significant effect on the leptin level. Our meta-regression analysis demonstrated a negative linear relationship in the decrease in effect size with an increase in the methodological quality of studies (Beta coefficient -0.80, P = 0.13) (Fig. 3). In the meta-regression analysis, keeping year of publication as a moderator variable, we did not observe the effect of year of publication in effect size associated with leptin level (beta coefficient 0.13, P = 0.24) (Fig. 4). Due to a high degree of heterogeneity observed in the present study, we did the sensitivity analysis to verify the reliability of the results, in which one study was removed subsequently to see if removal of a single study may change the effect size significantly. Our sensitivity analysis did not observe a significant change in effect size after the removal of a single study in the meta-analysis although Sarray et al. had some excessive effect (Fig. 5). The funnel plot shows asymmetry suggesting significant publication bias. This finding is also consistent with Begg's (0.014); however, egger test did not observe statistically significant difference (P = 0.28) (Fig. 6).
Fig. 2.
Forest plot showing an association between leptin level and risk of PCOS
Fig. 3.

Influence of methodological quality analysis in effect size associated with leptin level in women with PCOS (Coefficient = −0.80, p = 0.13)
Fig. 4.

Meta-regression analysis keeping year of publication as moderator variable Coefficient = 0.13, p = 0.24
Fig. 5.
Sensitivity analysis
Fig. 6.
Funnel plot showing publication bias
Heterogeneity
Statistical Heterogeneity: In the present meta-analysis, significant high heterogeneity was observed in the overall studies analysis (I2 = 97.3%, P < 0.001). In the subgroup analysis, the significant heterogeneity remained present in both high methodological quality studies (I2 = 95.3%, P < 0.001) and low methodological quality studies ( I2 = 97.7%, P < 0.001). Differences in the methodological quality may explain the large degree of heterogeneity between studies.
Clinical Heterogeneity: Examination of clinical heterogeneity is imperative to examine the meta-analysis results as it significantly affects the observed findings. Differences in the study participants, treatment received by the patients, and research settings, clinical setup, differences in the measurement methods of leptin, could have introduced the clinical heterogeneity between the studies.
Discussion
The present study observed that high leptin levels may be associated with women having PCOS; however, the association was observed only in the low-quality study. Analysis including high methodological quality failed to observe the statistically significant association of leptin with the risk of PCOS. Several studies have shown a statistically significant higher level of serum leptin in women with PCOS [15, 20, 44]; however, some of the studies failed to derive such an association [18, 21]. A meta-analysis reported in 2016 which included a total of 19 studies, observed that leptin levels are higher (statistically significant) for women with PCOS as compared to controls (SMD, 1.62, 95% CI 1.01–2.23).
Leptin is mainly produced by adipocytes and is considered a polypeptide hormone for the regulation of normal body weight. Several studies have observed a strong association of circulating leptin with obesity, which may be connected with PCOS. It plays an important role in regulating energy homeostasis and has an impact on the reproductive systems in diverse ways. PCOS, the common diovulatory infertility, is characterized by chronic anovulation, hyperandrogenaemia, insulin resistance, and a high incidence of obesity; therefore, leptin level may be related to the pathogenesis of PCOS. A study concluded that further studies are required to elucidate the independent correlations between serum leptin levels and phenotypic features in women with PCOS [39]. Our updated meta-analysis involving the largest number of studies so far including 35 studies, observed a statistically significant association between higher leptin level and risk of PCOS disease. Interestingly, the association was noted only when the analysis was restricted to low methodological quality studies. Analysis including well-conducted studies having good methodological quality scores failed to observe a statistically significant difference between PCOS and controls. These findings are reported for the first time by the present study, which raises a concern regarding the precise association of leptin in women with PCOS. Our meta-regression analysis also observed a non-significant negative relationship between effect size and methodological quality which further indicates the questionable association of high leptin with PCOS (P = 0.13). The year of publication of articles did not act as a significant moderator variable in the meta-regression analysis. Our sensitivity analysis did not observe the significant influence of a single study in altering the significant association between leptin level and risk of PCOS including overall studies.
Limitation of the Meta-Analysis
The present study has several limitations. Several confounding factors could not be extracted which potentially influence the outcome and exposure relationship like insulin resistance, BMI, diabetes, and age which may result in overestimation of the summary effect size, therefore, might undermine the generalization of the findings observed in the current meta-analysis. In addition to this, the high statistical heterogeneity among the studies further raises concern regarding the generalizability of finding observed in the current study. Interpretation of the summary effect size should be made with caution due to significant heterogeneity. We could not check the influence of visceral adiposity, waist circumference, LH/FSH ratio which may have a significant influence on the effect size. Unavailability of individual patient data is a limitation to conduct the individual patient data meta-analysis.
Conclusion
The findings of meta-analysis suggest that the association of elevated levels of leptin with PCOS is controversial due to the high degree of heterogeneity and failure to observe association in the studies including high methodological quality studies. Further, well-designed adequately powered prospective studies are needed to clarify the precise relationship between leptin levels and PCOS.
Take Home Message
A low energy diet therapy is associated with lower leptin concentration thereby slowing the metabolic rate. A healthy diet, exercise, and adequate sleep could be effective in maintaining leptin sensitivity and have a great potential to improve the outcome in patients with PCOS.
Acknowledgements
We acknowledge the Indian Council of Medical Research, India for providing technical support for conducting this review
Mahesh Kumar Seth
is working as Scientist D in Clinical Epidemiology Unit, All India Institute of Medical Sciences, New Delhi and is currently working in evidence based medicine.
Funding
The author(s) received no financial support for this research.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Mahesh Kumar Seth is an Scientist D in Clinical Epidemiology Unit, All India Institute of Medical Sciences, New Delhi and is currently working in evidence based medicine.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lujan ME, Chizen DR, Pierson RA. Diagnostic criteria for polycystic ovary syndrome: pitfalls and controversies. J Obstet Gynaecol Can JOGC J Obstet Gynecol Can JOGC. 2008;30:671–679. doi: 10.1016/S1701-2163(16)32915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammadi M. Oxidative stress and polycystic ovary syndrome: a brief review. Int J Prev Med. 2019;10:86. doi: 10.4103/ijpvm.IJPVM_576_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anifandis G, Koutselini E, Stefanidis I, Liakopoulos V, Leivaditis C, Mantzavinos T, et al. Serum and follicular fluid leptin levels are correlated with human embryo quality. Reprod Camb Engl. 2005;130:917–921. doi: 10.1530/rep.1.00705. [DOI] [PubMed] [Google Scholar]
- 4.Murri M, Luque-Ramírez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19:268–288. doi: 10.1093/humupd/dms059. [DOI] [PubMed] [Google Scholar]
- 5.Jeelani H, Ganie MA, Masood A, Amin S, Kawa IA, Fatima Q, et al. Assessment of PON1 activity and circulating TF levels in relation to BMI, testosterone, HOMA-IR, HDL-C, LDL-C, CHO, SOD activity and TAC in women with PCOS: an observational study. Diabetes Metab Syndr. 2019;13:2907–2915. doi: 10.1016/j.dsx.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Masjedi F, Keshtgar S, Agah F, Karbalaei N. Association between sex steroids and oxidative status with vitamin D levels in follicular fluid of non-obese PCOS and healthy women. J Reprod Infertil. 2019;20:132–142. [PMC free article] [PubMed] [Google Scholar]
- 7.Enechukwu CI, Onuegbu AJ, Olisekodiaka MJ, Eleje GU, Ikechebelu JI, Ugboaja JO, et al. Oxidative stress markers and lipid profiles of patients with polycystic ovary syndrome in a Nigerian tertiary hospital. Obstet Gynecol Sci. 2019;62:335–343. doi: 10.5468/ogs.2019.62.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 9.Iuorno MJ, Islam LZ, Veldhuis PP, Boyd DG, Farhy LS, Johnson ML, et al. Leptin secretory burst mass correlates with body mass index and insulin in normal women but not in women with polycystic ovary syndrome. Metabolism. 2007;56:1561–1565. doi: 10.1016/j.metabol.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Mantzoros CS, Dunaif A, Flier JS. Leptin concentrations in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:1687–1691. doi: 10.1210/jcem.82.6.4017. [DOI] [PubMed] [Google Scholar]
- 11.Sepilian V, Nagamani M. Adiponectin levels in women with polycystic ovary syndrome and severe insulin resistance. J Soc Gynecol Investig. 2005;12:129–134. doi: 10.1016/j.jsgi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Kale-Gurbuz T, Akhan SE, Bastu E, Telci A, Iyibozkurt AC, Topuz S. Adiponectin, leptin and ghrelin levels in obese adolescent girls with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2013;26:27–30. doi: 10.1016/j.jpag.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Remsberg KE, Talbott EO, Zborowski JV, Evans RW, McHugh-Pemu K. Evidence for competing effects of body mass, hyperinsulinemia, insulin resistance, and androgens on leptin levels among lean, overweight, and obese women with polycystic ovary syndrome. Fertil Steril. 2002;78:479–486. doi: 10.1016/s0015-0282(02)03303-4. [DOI] [PubMed] [Google Scholar]
- 14.Laughlin GA, Morales AJ, Yen SS. Serum leptin levels in women with polycystic ovary syndrome: the role of insulin resistance/hyperinsulinemia. J Clin Endocrinol Metab. 1997;82:1692–1696. doi: 10.1210/jcem.82.6.4028. [DOI] [PubMed] [Google Scholar]
- 15.Brzechffa PR, Jakimiuk AJ, Agarwal SK, Weitsman SR, Buyalos RP, Magoffin DA. Serum immunoreactive leptin concentrations in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:4166–4169. doi: 10.1210/jcem.81.11.8923878. [DOI] [PubMed] [Google Scholar]
- 16.Alper T, Kahraman H, Cetinkaya MB, Yanik F, Akcay G, Bedir A, et al. Serum leptin and body composition in polycystic ovarian syndrome. Ann Saudi Med. 2004;24:9–12. doi: 10.5144/0256-4947.2004.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yildizhan R, Ilhan GA, Yildizhan B, Kolusari A, Adali E, Bugdayci G. Serum retinol-binding protein 4, leptin, and plasma asymmetric dimethylarginine levels in obese and nonobese young women with polycystic ovary syndrome. Fertil Steril. 2011;96:246–250. doi: 10.1016/j.fertnstert.2011.04.073. [DOI] [PubMed] [Google Scholar]
- 18.Demirel F, Bideci A, Cinaz P, Camurdan MO, Biberoğlu G, Yesilkaya E, et al. Serum leptin, oxidized low density lipoprotein and plasma asymmetric dimethylarginine levels and their relationship with dyslipidaemia in adolescent girls with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2007;67:129–134. doi: 10.1111/j.1365-2265.2007.02849.x. [DOI] [PubMed] [Google Scholar]
- 19.Telli MH, Yildirim M, Noyan V. Serum leptin levels in patients with polycystic ovary syndrome. Fertil Steril. 2002;77:932–935. doi: 10.1016/S0015-0282(02)02995-3. [DOI] [PubMed] [Google Scholar]
- 20.Atamer A, Demir B, Bayhan G, Atamer Y, Ilhan N, Akkuş Z. Serum levels of leptin and homocysteine in women with polycystic ovary syndrome and its relationship to endocrine, clinical and metabolic parameters. J Int Med Res. 2008;36:96–105. doi: 10.1177/147323000803600113. [DOI] [PubMed] [Google Scholar]
- 21.Bideci A, Camurdan MO, Yeşilkaya E, Demirel F, Cinaz P. Serum ghrelin, leptin and resistin levels in adolescent girls with polycystic ovary syndrome. J Obstet Gynaecol Res. 2008;34:578–584. doi: 10.1111/j.1447-0756.2008.00819.x. [DOI] [PubMed] [Google Scholar]
- 22.PourghassemGargari B, Houjeghani S, Farzadi L, Houjeghani S, Safaeiyan A. Relationship between Serum Leptin, Ghrelin and Dietary Macronutrients in Women with Polycystic Ovary Syndrome. Int J Fertil Steril. 2015;9:313–321. doi: 10.22074/ijfs.2015.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Namavar Jahromi B, Dabaghmanesh MH, Parsanezhad ME, Fatehpoor F. Association of leptin and insulin resistance in PCOS: a case-controlled study. Int J Reprod Biomed. 2017;15:423–428. [PMC free article] [PubMed] [Google Scholar]
- 24.Houjeghani S, Pourghassem Gargari B, Farzadi L. Serum leptin and ghrelin levels in women with polycystic ovary syndrome: correlation with anthropometric, metabolic, and endocrine parameters. Int J Fertil Steril. 2012;6:117–126. [PMC free article] [PubMed] [Google Scholar]
- 25.Jalilian N, Haghnazari L, Rasolinia S. Leptin and body mass index in polycystic ovary syndrome. Indian J Endocrinol Metab. 2016;20:324–328. doi: 10.4103/2230-8210.180005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garruti G, de Palo R, Rotelli MT, Nocera S, Totaro I, Nardelli C, et al. Association between follicular fluid leptin and serum insulin levels in nonoverweight women with polycystic ovary syndrome. BioMed Res Int. 2014 doi: 10.1155/2014/980429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancini F, Cianciosi A, Reggiani GM, Facchinetti F, Battaglia C, de Aloysio D. Endothelial function and its relationship to leptin, homocysteine, and insulin resistance in lean and overweight eumenorrheic women and PCOS patients: a pilot study. Fertil Steril. 2009;91:2537–2544. doi: 10.1016/j.fertnstert.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Savastano S, Valentino R, Di Somma C, Orio F, Pivonello C, Passaretti F, et al. Serum 25-Hydroxyvitamin D Levels, phosphoprotein enriched in diabetes gene product (PED/PEA-15) and leptin-to-adiponectin ratio in women with PCOS. Nutr Metab. 2011;8:84. doi: 10.1186/1743-7075-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kedikova SE, Sirakov MM, Boyadzhieva MV. Leptin levels and adipose tissue percentage in adolescents with polycystic ovary syndrome. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2013;29:384–387. doi: 10.3109/09513590.2012.752455. [DOI] [PubMed] [Google Scholar]
- 30.Pehlivanov B, Mitkov M. Serum leptin levels correlate with clinical and biochemical indices of insulin resistance in women with polycystic ovary syndrome. Eur J Contracept Reprod Health Care Off J Eur Soc Contracept. 2009;14:153–159. doi: 10.1080/13625180802549962. [DOI] [PubMed] [Google Scholar]
- 31.Chakrabarti J. Serum leptin level in women with polycystic ovary syndrome: correlation with adiposity, insulin, and circulating testosterone. Ann Med Health Sci Res. 2013;3:191–196. doi: 10.4103/2141-9248.113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pusalkar M, Meherji P, Gokral J, Savardekar L, Chinnaraj S, Maitra A. Obesity and polycystic ovary syndrome: association with androgens, leptin and its genotypes. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2010;26:874–882. doi: 10.3109/09513590.2010.487586. [DOI] [PubMed] [Google Scholar]
- 33.Saleh HA, El-Nwaem MA, El-Bordiny MM, Maqlad HME-S, El-Mohandes AA, Eldaqaq EM. Serum leptin elevation in obese women with PCOs: a continuing controversy. J Assist Reprod Genet. 2004 doi: 10.1023/b:jarg.0000046204.81682.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Orabi H, Ghalia AA, Khalifa A, Mahfouz H, El Shalkani A, Shoieb N. Serum leptin as an additional possible pathogenic factor in polycystic ovary syndrome. Clin Biochem. 1999;32:71–75. doi: 10.1016/s0009-9120(98)00091-5. [DOI] [PubMed] [Google Scholar]
- 35.Tsouma I, Kouskouni E, Gennimata V, Demeridou S, Boutsikou M, Grigoriou V, et al. Leptin levels in women with polycystic ovaries undergoing ovarian stimulation: relation to lipoprotein profiles. Vivo Athens Greece. 2014;28:989–992. [PubMed] [Google Scholar]
- 36.Baig M, Rehman R, Tariq S, Fatima SS. Serum Leptin Levels in Polycystic Ovary Syndrome and Its Relationship with Metabolic and Hormonal Profile in Pakistani Females. Int J Endocrinol. 2014 doi: 10.1155/2014/132908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baldani DP, Skrgatic L, Kasum M, Zlopasa G, Kralik Oguic S, Herman M. Altered leptin, adiponectin, resistin and ghrelin secretion may represent an intrinsic polycystic ovary syndrome abnormality. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2019;35:401–405. doi: 10.1080/09513590.2018.1534096. [DOI] [PubMed] [Google Scholar]
- 38.Pinhas-Hamiel O, Singer S, Pilpel N, Koren I, Boyko V, Hemi R, et al. Adiponectin levels in adolescent girls with polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2009;71:823–827. doi: 10.1111/j.1365-2265.2009.03604.x. [DOI] [PubMed] [Google Scholar]
- 39.Hahn S, Haselhorst U, Quadbeck B, Tan S, Kimmig R, Mann K, et al. Decreased soluble leptin receptor levels in women with polycystic ovary syndrome. Eur J Endocrinol. 2006;154:287–294. doi: 10.1530/eje.1.02078. [DOI] [PubMed] [Google Scholar]
- 40.Rizk NM, Sharif E. Leptin as well as free leptin receptor is associated with polycystic ovary syndrome in young women. Int J Endocrinol. 2015 doi: 10.1155/2015/927805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeon YE, Lee KE, Jung JA, Yim SY, Kim H, Seo SK, et al. Kisspeptin, leptin, and retinol-binding protein 4 in women with polycystic ovary syndrome. Gynecol Obstet Invest. 2013;75:268–274. doi: 10.1159/000350217. [DOI] [PubMed] [Google Scholar]
- 42.Sarray S, Madan S, Saleh LR, Mahmoud N, Almawi WY. Validity of adiponectin-to-leptin and adiponectin-to-resistin ratios as predictors of polycystic ovary syndrome. Fertil Steril. 2015;104:460–466. doi: 10.1016/j.fertnstert.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Chen C-I, Hsu M-I, Lin S-H, Chang Y-CI, Hsu C-S, Tzeng C-R. Adiponectin and leptin in overweight/obese and lean women with polycystic ovary syndrome. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2015 doi: 10.3109/09513590.2014.984676. [DOI] [PubMed] [Google Scholar]
- 44.Calvar CE, Intebi AD, Bengolea SV, et al. Leptin in patients with polycystic ovary syndrome. Direct correlation with insulin resistance. Medicina (Mex) 2003;63:704–710. [PubMed] [Google Scholar]