Abstract
Obsessive Compulsive Disorder (OCD) is a highly prevalent and severe neuropsychiatric disorder, with an incidence of 1.5–3% worldwide. However, despite the clear public health burden of OCD and relatively well-defined symptom criteria, effective treatments are still limited, spotlighting the need for investigation of the neural substrates of the disorder. Human neuroimaging studies have consistently highlighted abnormal activity patterns in prefrontal cortex (PFC) regions and connected circuits in OCD during both symptom provocation and performance of neurocognitive tasks. Because of recent technical advances, these findings can now be leveraged to develop novel targeted interventions. Here we will highlight current theories regarding the role of the prefrontal cortex in the generation of OCD symptoms, discuss ways in which this knowledge can be used to improve treatments for this often disabling illness, and lay out challenges in the field for future study.
Subject terms: Anxiety, Anxiety, Cognitive control, Human behaviour
Introduction
Obsessive Compulsive Disorder (OCD) is a prevalent psychiatric disorder, with an incidence of between 1.5–3% across all ethnic and cultural groups studied (Kessler et al., 2005). The average age of onset is in adolescence or early adulthood with a separate peak in childhood, contributing to significant lifetime disease burden (Kessler et al., 2005). In addition, OCD can be disabling, with a significant impact on quality of life [1–4]. Despite this severity and prevalence, effective treatments for OCD are still limited. Only 10–15% of people experience remission with serotonin reuptake inhibitors, the only proven pharmacological monotherapy for OCD. While exposure-therapy with response prevention (ERP) shows significant efficacy, it can be challenging for patients to complete even if they are able to find experienced treatment providers. A greater understanding of the neural substrates of OCD is therefore necessary to develop improved treatments. Traditional circuitry models of OCD have focused on frontal cortical-striatal pathways, with significant emphasis on the role of the basal ganglia in the generation of compulsive behavior. However, accumulated evidence, described in detail throughout this review, supports an important role for particular prefrontal cortical networks in both the pathophysiology and treatment of this severe mental illness. Here we review current theories regarding how dynamic prefrontal cortical networks capable of highly distributed parallel processing and their connected subcortical circuits may contribute to OCD symptoms, and highlight areas for future research.
Clinical characteristics of OCD
Compared to other neuropsychiatric disorders, OCD has relatively clearly defined diagnostic criteria. According to DSM-5, OCD consists of obsessions, which are recurrent, intrusive thoughts, images, or impulses, and/or compulsions, which are repetitive behaviors or mental rituals that patients tend to perform in order to reduce the often severe distress associated with the compulsions [5]. Importantly, although it is not necessary to have both obsessions and compulsions to be diagnosed with OCD, most patients do have temporal linking of both of these broad categories of symptoms—i.e., obsession/ compulsion pairs are often triggered together by an external or internal stimulus.
Models of OCD symptoms highlight importance of prefrontal cortical networks
Though there is spirited and active debate in the field as to whether obsessions drive compulsions or vice versa [6–8], there is little question that these symptoms are related to each other, with support for the idea that they are linked by anxiety and avoidance behavior [9]. In turn, this linkage provides testable hypotheses regarding possible underlying neural substrates. Specifically, the temporal association of obsessions and compulsions points toward a neurobiological model in which obsessions may be generated cortically, and may mediate subcortical selection of compulsive behaviors in the context of anxiety. For example, one potential model of OCD suggests that excessive anticipation of dreaded consequences coupled with an erroneous Bayesian determination of action outcomes leads to symptoms [10]. This pathological process is proposed to stem from abnormalities in prefrontal cortical networks, which play an important role in anticipating probabilistic outcomes and formulating goal-directed behavior to deal with contextual contingencies. Importantly, work described in more detail below suggests that specific prefrontal cortical networks may be particularly responsible for the generation of obsessive thoughts in OCD. In addition, several important features and/or endophenotypes of OCD have been localized to prefrontal cortical areas (see below for details), suggesting a prominent role in the pathophysiology of OCD. It is therefore imperative that we discover the potential roles of abnormal prefrontal cortex (PFC) structure and function in the generation of OCD pathology and associated endophenotypes, to facilitate development of new treatments. Note, it is clear that prefrontal cortical networks do not act in isolation; rather, they are connected to various cortical and subcortical brain regions and nuclei that in concert mediate key functions such as action selection and execution. In addition, techniques that permit identification of individual cell types in both animal model systems and humans have demonstrated significant heterogeneity within the PFC that is reflected within complex microcircuit functions. However, here we will focus primarily on roles of PFC regions in OCD that can be delineated through human imaging studies.
Neuroimaging literature highlights prefrontal cortex hyperactivity associated with OCD symptoms
Over the past 30 years, convergent findings from human neuroimaging studies have highlighted abnormal structure and function of cortical-basal ganglia-thalamic loops in people with OCD. Though much translational work has focused on abnormalities in the striatum for reasons discussed below, findings in prefrontal cortical networks—particularly within the orbitofrontal cortex (OFC)—have been especially convergent across studies. In pioneering work, early positron emission tomography (PET) studies highlighted hyperactivity in medial and lateral OFC both at baseline and after symptoms were provoked either using real sensory stimulation with physical objects [11–14] [15] or evoked with imaginal/word exposures [16–21], suggesting that excessive OFC activity may generate obsessions and/or compulsions [meta-analyses of these results in [16, 22, 23]. Consistent with this theory, effective OCD treatment with either serotonin reuptake inhibitors, ERP, or deep brain stimulation (DBS) consistently normalizes OFC hyperactivity observed with fMRI [20, 21, 24–30]. Though it is not possible to directly test causality in people due to ethical concerns, together these results provide strong support for the idea that abnormal activity patterns in OFC networks can cause OCD symptoms. Note, however, that contrasting findings have been observed. For example, in [31], VMPFC hypoactivation and pre-SMA hyperactivation was observed in an fMRI study designed to provoke symptoms using autobiographical stimuli in OCD patients.
Though less thoroughly studied, similar findings have been seen in other prefrontal cortical brain regions. In particular, significant evidence points to a role for anterior cingulate cortex (ACC) in the generation of OCD symptoms. ACC hyperactivity is seen at baseline [32] and after symptom provocation [11–13, 24, 25, 33]. In addition, similar to findings in OFC, successful treatment of OCD symptoms is associated with normalization of this hyperactivity [32]. Moreover, anterior cingulotomy, a neurosurgical procedure involving destruction of ACC gray matter and adjacent white matter tracts, has been associated with reduction of OCD symptoms in treatment refractory cases [34–36]. Thus, while there has been less attention to these findings in the translational literature, ACC remains an important area of focus for pathophysiology and treatment studies.
Hyperactivity during symptom provocation has also been observed in fMRI studies of ventromedial prefrontal cortex (VMPFC) [37]. In addition, VMPFC hyperactivity has been observed in response to threatening shock during a Pavlovian fear task [38]. Hyperactivity in VLPFC has likewise been observed in OCD patients with high sensitivity to disgust when exposed to pictures of disgusted facial expressions [39]. Finally, although it is not formally considered to be part of the PFC, hyperactivity in supplementary motor area (SMA) has been associated with abnormal repetitive behaviors in Tourette Syndrome, which is highly comorbid with OCD [40–42]. PET studies in people with Tourette’s show increased tic-associated activity in SMA [43]. In addition, fMRI demonstrates that SMA is activated prior to tic onset [44, 45], and has different activity patterns during tics vs. normal intentional movements [44]. Together, these data indicate that hyperactivity in many different prefrontal cortical regions is associated with OCD symptoms, raising several important questions. First, are any of these findings directly responsible for generation of obsessions and compulsions? If so, which PFC abnormalities most contribute to symptoms? Alternatively, is this broad hyperactivity seen across many different PFC regions an epiphenomenon reflective of an entirely different upstream pathologic process? If so, compared to other cortical regions, what makes prefrontal cortical regions as a group inherently and potentially uniquely vulnerable in the context of OCD? Answering these and other questions will help develop new avenues for improved treatments.
Note, when synthesizing these findings, it is important to recognize limitations of neuroimaging research that can impact data interpretation. First, significant heterogeneity is present within patient samples, particular with respect to comorbidities, and some studies do not assess for the presence of obsessive compulsive personality disorder (OCPD). Thus, it can be difficult to definitively assign observed behavioral or neural activity differences to OCD vs. highly comorbid disorders such as OCPD [46], anxiety disorders, and major depressive disorder [47]. Second, it is challenging to separate the contributions of state vs. trait in both neuroimaging studies and neurocognitive testing. For example, performance on neurocognitive tasks can be impacted by anxiety levels and interference from ongoing obsessions and compulsions, which may lead to heterogeneity within the clinical sample that can either mask or create differences between people with OCD and unaffected comparison subjects. Finally, fMRI is inherently limited in its ability to distinguish the cell types that are responsible for BOLD activation. Thus, a finding of BOLD signal in a particular PFC region could be accounted for by activation of excitatory and/or inhibitory neuron populations. This distinction has important implications for determining appropriate clinical targets. Preclinical studies in animal models can therefore be a useful bridge to dissect contributions of particular cell types to OCD-relevant behaviors.
How might disruptions in classic PFC-dependent functions lead to symptoms of OCD?
Though delineating the diverse functions of the PFC is beyond the scope of this review, here we will discuss executive functions classically thought to be PFC-regulated whose disruption may lead to the generation of obsessions and compulsions. Such a perspective is consistent with NIMH’s Research Domain Criteria (RDOC) framework, which aims to identify dimensions that cut across current diagnostic categories in order to facilitate translational investigations and identification of new treatment targets [48]. In particular, current theories suggest that abnormalities in the following domains may be key contributors to dysfunction in OCD: cognitive flexibility, threat processing/harm avoidance, habit formation/goal-directed planning, error monitoring, and response inhibition. However, whether and how abnormalities in these constructs actually generate the symptoms of OCD is still a matter of active investigation. Below, we will delineate the clinical and psychological evidence supporting these theories.
Cognitive flexibility
Based both on clinical observations and epidemiological studies demonstrating significant comorbidity of OCD and OCPD, OCD has been classically associated with impaired cognitive flexibility. These impairments are thought to be central to OCD pathology, in part due to the clinical perspective of obsessions and compulsions as fixed and rigid thoughts and behavioral patterns that are resistant to change. Many different tasks and variants within these tasks fall under the overarching rubric of cognitive flexibility, including reversal learning, set shifting, and cued task switching, leading to some difficulties in standardization across studies. In addition, it has at times been challenging to identify consistent abnormalities in tasks measuring cognitive flexibility vs. rigidity due to patient heterogeneity. However, impaired behavioral performance and/or altered neural activity patterns are generally seen on many neurocognitive tasks designed to test cognitive flexibility, with effect sizes typically in the medium range as reported in a relatively recent meta-analysis [49].
Several studies have identified abnormalities in reversal learning, task switching, and extra-dimensional set shifting [50, 51] in people with OCD, though it can be difficult to compare findings across studies because of differences in tasks. In some cases, differences in behavioral performance [52, 53] and reaction times [54–56] are noted between groups [though see [57–59]], but often neural activity differences are seen despite equivalent performance, suggesting potential compensatory processes (Chamberlain et al., 2007; Chamberlain et al., 2006; Gottwald et al., 2018). Specifically, evidence from neuroimaging studies conducted while OCD patients are performing reversal learning (Chamberlain et al., 2008; Remijnse et al., 2006; Remijnse et al., 2009; Remijnse et al., 2013) or a task switching paradigm (Gu et al., 2008) shows impaired recruitment of OFC, potentially due to excessive baseline activity. One study has additionally demonstrated that this OFC hypoactivity is seen in unaffected first-degree relatives of OCD patients. Notably, the abnormal activity during reversal learning [53, 54, 60, 61] occurs in the same orbitofrontal-striatal circuits that show abnormal activity during symptom provocation [11–13]. However, it is unknown whether the same abnormalities in OFC activity underlie both impaired reversal learning and compulsive behaviors. Dorsolateral PFC (DLPFC) also shows robust hypoactivity in OCD patients during reversal learning and task switching [53, 61–64], and reduced resting-state DLPFC-putamen functional connectivity was recently associated with impaired extra-dimensional set-shifting in people with OCD [65]. Finally, reduced ACC and VMPFC activity are also seen in people with OCD (Gu et al., 2008) during task switching. Together, these findings suggest a pattern in which people with OCD display less recruitment of multiple PFC structures compared to unaffected controls when faced with changing task conditions. It remains to be determined whether these decreases in activity represent primary pathologic changes, or are compensating for alterations in activity in other structures.
Threat processing/harm avoidance
Anxiety is often a prominent feature of OCD, despite its classification apart from other anxiety disorders in DSM-5 due to differences in underlying circuit abnormalities [66]. Whether anxiety is a primary pathologic driver in OCD or is simply a stressor that can exacerbate pre-existing obsessions and compulsions is still a matter of active debate. However, abnormalities in threat processing systems and aversive learning processes [38, 67, 68] have been documented in OCD. Impaired VMPFC recruitment in a subset of OCD patients with deficits in extinction recall is seen both during fear extinction training and recall deficits [68], consistent with its importance for flexible updating of stimuli that are no longer threatening [69, 70]. In addition, as described briefly above, OCD patients show impaired ability to compute safety signals in the VMPFC when they are required to update task contingencies with new information [38]. Specifically, using a threat reversal learning paradigm paired with fMRI, fear responses (as measured by skin conductance) were compared between people with OCD and unaffected comparison subjects in response to either a threatening (paired with shock) or safe (not paired with shock) visual stimulus, followed by contingency reversal. Excessive VMPFC activity predicted impaired adaptation on the task after reversal of neutral and shock-paired cues [38], suggesting negative functional consequences due to impaired generation of safety signals during value updating of previously-threatening cues. This may have important implications for delivery of exposure therapy, as particular OCD subpopulations may have worse response rates due to inherent difficulties with threat vs. safety processing. Normalizing these threat-associated circuits may therefore help facilitate behavioral treatments for OCD, by decreasing the propensity of patients to engage in harm avoidance strategies.
Habit formation/goal-directed planning
The conceptualization of OCD as a shift from normal goal-directed behavior to pathologic habits [described in [71] reflected the emerging recognition that striatal pathology plays an important role in OCD. Since this theory was first proposed [11, 33, 72], several investigations have tested this idea using a variety of paradigms designed to probe different features of these constructs. Using a novel outcome devaluation-based task (The Fabulous Fruit Game) to test whether actions are habitual or goal-directed, Gillan et al. [73] found that people with OCD were less likely than controls to respond appropriately to devaluation by changing their performance, suggesting an overreliance on habits. In addition, when explicit knowledge of contingencies was tested in a separate section of the task, OCD patients showed decreased understanding of the associations between actions and outcomes compared to controls. Increased habit formation in OCD has also been inferred using contingency degradation paradigms, with OCD patients demonstrating greater levels of responding for reward than controls when the causal link between a particular action and the outcome is diminished [74]. Note, however, that the OCD subjects in this study did explicitly report accurate assessments of the contingencies, raising questions about whether these behavioral patterns could instead be construed as an atypical version of goal-directed behavior. In addition, the constructs of model-based and model-free learning, which can be loosely mapped onto goal-directed and habitual strategies, have been examined in OCD using a two-step task [see [75] for detailed task description]. Here again OCD patients showed a propensity for habitual behavior, as manifested in a bias toward model-free learning, which was associated with a decrease in mOFC and caudate gray matter volume [76].
Extending this work to investigate devaluation of negative outcomes using an avoidance-based task, [67] also found that people with OCD continued to avoid a negative stimulus (i.e., shock), even when this outcome had been clearly devalued–i.e., the shocker was unplugged and therefore acknowledged as non-functional by the patients when their explicit knowledge was tested. This impairment in adjusting behavior to an updated negative contingency was found to be associated with hyperactivation of the medial OFC during the learning of the avoidance behavior [77]. Further supporting these results, a more recent study used a novel task with a milder aversive outcome– needing to prevent simulated “machines” from breaking as opposed to avoiding shock delivery [78]. Though no overall group differences were seen between patients with OCD and controls on this task, there was an association between unsuccessful devaluation of cues and reduced activity in pre-motor cortex and left inferior frontal gyrus, areas which have been linked to cognitive and response inhibition (Aron et al., 2007; Hung et al., 2018; Picton et al., 2007). Reduced mOFC activity in response to valued cues was also observed in OCD patients, consistent with [77]. However, during presentation of valued cues, unsuccessful devaluers within the group of OCD patients had greater mOFC activity than successful devaluers, suggesting a complex relationship in which either hyperactivity or hypoactivity in mOFC can lead to impaired devaluation. Together, these findings may yield insight into why OCD patients are prone to incorporating avoidance rituals into their compulsions, and thus inform the genesis of avoidance-related compulsive behaviors. However, it is important to note that the extent of avoidance in [77] did not correlate with compulsive behaviors as measured by the Yale Brown Obsessive Compulsive Scale (YBOCS), raising important questions about whether there is a direct relationship between abnormal performance on neurocognitive tasks and OCD symptoms.
Goal-directed planning has also been assessed independently of the investigation of habits in OCD. Using a self-paced version of the visuo-spatial planning Tower of London task, van den Heuvel et al. [79] found significant planning impairments in people with OCD that were associated with decreased activation of the DLPFC and caudate. Similarly, Den Braber et al. [80] found hypoactivation of DLPFC during planning in a sample of people at high risk for OCD, though behavioral deficits were not observed in this less severe clinical sample. Consistent with these findings, Vaghi et al. (2017) found that both OCD patients and their asymptomatic relatives showed both slower reaction times and hypoactivation of the DLPFC during goal-directed planning on the Tower of London task, suggesting this may be an endophenotype of the disorder [64].
Impairments in goal-directed planning in OCD are also supported by the fact that patient self-report in studies assessing habit formation yields accurate assessments about action-outcome contingencies. These findings suggest that patients are performing the “habitual” behavior intentionally, despite explicit knowledge that this is not necessary to achieve the desired outcome. Similarly, in an “illusion of control” task, people with OCD had more accurate assessments of their amount of control in the task [67], in keeping with the idea that they have the ability to correctly estimate the value of performing particular actions, but may have other competing value assignments that weight performance of compulsive behaviors more heavily. Thus, performance of a compulsive behavior may actually be goal-directed, but lead to pathology since the compulsions rank higher in the value hierarchy than other goal-directed behaviors necessary for health and well-being. This is consistent with metacognitive theories suggesting that compulsive behaviors are goal-directed [81]—i.e., performed consciously to achieve a valued outcome, such as anxiety reduction, neutralization of negative thoughts, or confirmation of uncertain priors, potentially based on overestimation of credibility of obsessive thoughts. For example, an attractive possibility consistent with clinical experience is that performance of compulsions can in some cases provide temporary anxiety relief via harm avoidance, which may be assigned a high value.
A similar pattern was seen in a predictive inference task, in which people with OCD displayed excessive actions that only took recent evidence into account, despite reporting similar confidence as controls in the fact that performing these actions was not the ideal strategy [82]. While other studies have also described decreased confidence in correctness of decisions in people with OCD [83] or non-diagnosed people with high levels of compulsive traits [84], these findings suggest that complexity beyond the dichotomy of goal-directed vs. habitual behavior must be integrated into theories of decision-making in OCD. Recent theories integrate these ideas by suggesting that OCD symptoms result from dysfunction in the process of arbitration between goal-directed and hierarchical systems, with a specific suggestion that the selection of goal-directed actions is hierarchically organized under a goal-directed controller—i.e., once a particular sequence of well-practiced actions (i.e., a “chunk” that could correspond to a compulsive behavior) is selected, the individual actions are insensitive to outcome, completed in an automated manner, and not individually monitored by the goal-directed system. Hyperactivity in mOFC could therefore drive repeated retrieval of unobservable but anxiety-provoking outcomes, as suggested by the findings in [85, 86] and proposed in [87], leading to repeated performance of chunked sequences of behavior like handwashing.
Together, these studies have found evidence consistent with the theory that people with OCD have impairments in the balance between goal-directed behavior and habits [87, 88]. However, depending on the task used, distinct abnormalities are seen that point to either excessive habit formation or maladaptive goal-directed behavior. Future studies testing these paradigms longitudinally within the same subjects will help to dissociate which set of impairments predominates in OCD.
Error monitoring
Impairments in error monitoring have also been observed in OCD, and suggested as the basis of a subtype of OCD obsessions often termed a “just not quite right” feeling. Across multiple studies, increased error-related negativity (ERN) [89] is observed as a negative deflection of the event-related potential on EEG with an ACC source in OCD patients compared to controls [90, 91] [also see [92, 93] for reviews]. Stability of these findings independent of treatment [94] and presence of increased ERN in unaffected first-degree relatives [95] suggests that impaired performance monitoring could be an OCD endophenotype. A recent quantitative meta-analysis indicates a strong effect size across 38 studies, specifically in tasks that create response conflicts [96]. Consistent with this, rostral ACC hyperactivity is seen in OCD patients during error commission on a cognitive task specifically designed to generate errors, and the extent of hyperactivity was positively correlated with symptom severity [97]. In addition, greater activation of the rostral ACC in the post-error trial period in OCD patients was found to positively correlate with severity of compulsions. Increased functional anisotropy was also seen in cingulum bundle underlying rACC, potentially serving as a substrate for an increase in the communication between anterior and posterior cingulate cortex that is necessary for error processing [98]. Combined fMRI and EEG studies have also observed a positive correlation between activity in the preSMA and ERN, which could reflect attempts to exert proactive control [99]. Further investigation of the relationship between ERN changes and other prefrontal cortical nodes of the error processing network (e.g., ventromedial PFC, DLPFC, anterior insula) is needed to gain greater understanding of mechanism [100]. Notably, an initial study has started to examine the therapeutic potential of attention bias modification in people with OCD [101], finding significantly reduced ERN in OCD subjects, but not in controls undergoing training. Future work is needed to determine if this reduction in ERN leads to symptom improvement.
Response inhibition
A prominent theory in the OCD literature suggests that response inhibition deficits may contribute to the symptomatology of OCD. This theory has been attractive because it makes some intuitive sense—i.e., compulsions are often colloquially considered as an inability to restrain oneself from performing particular actions. A variety of tasks have been used to formally test this idea, including Go/NoGo(Snyder et al., 2015), Stop Signal Reaction Time (SSRT) [60, 102, 103], and Stroop [104–107]. Though a meta-analysis has not demonstrated impairments on the Go/NoGo task (Snyder et al., 2015), OCD patients demonstrate moderate performance deficits on the Stroop task, with the strongest impact on interference scores and time on incongruent Stroop [108]. One study provides evidence that Stroop abnormalities in children and adolescents with OCD are correlated with decreased functional anisotropy in the cingulum bundle [105], but further work is needed to examine the role of PFC regions in mediating these phenotypic changes in OCD. Impairments in inhibition on the SSRT have been specifically associated with abnormalities in the PFC, including alterations in gray matter volume (decreased in OFC and inferior frontal; increased in anterior cingulate [109]). In addition, fMRI demonstrated functional differences in PFC during SSRT performance, including increased activity in preSMA during successful inhibition trials in OCD patients compared to controls, and decreased activity in the inferior frontal cortex [110], a region that has been consistently linked to response inhibition on this task [111]. However, note that though there is evidence that response inhibition is impaired in OCD and may even serve as an endophenotype (i.e., impairments are also observed in first-degree relatives [60]), a comprehensive meta-analysis has suggested a more complex picture [49], consistent with the fact that there is also significant evidence of normal performance on many of these tasks in OCD (Stop signal–[109, 112]; Go/No-go (Snyder et al., 2015); Stroop [104–107]. Although abnormal interference in the Stroop task was associated with a medium effect size across 23 studies, only a small effect size was seen for commission errors across 15 studies of multiple response inhibition tasks (Go/No-Go; continuous performance task; SSRT), with the upper limit of the confidence intervals approaching zero. Thus, there may be challenges in generalizing these findings due to heterogeneity of patient populations and/or differences in task parameters across testing sites [49, 113]). These findings highlight a potential need to identify subgroups of OCD patients in order to identify abnormalities in particular neurocognitive domains. In addition, performing longitudinal assessments of the same subjects on different response inhibition tasks while undergoing functional imaging would clarify whether particular tasks are more sensitive for detecting OCD-relevant response inhibition abnormalities in certain patient subgroups.
Emerging OCD postmortem literature also highlights molecular abnormalities in OFC that could be consistent with abnormal function
Although the etiology of OCD is unknown, both twin studies [114] and emerging genome-wide association studies (GWAS) support a genetic contribution. In particular, though OCD GWAS studies are not yet powered to detect significant common variants, preliminary evidence suggests that genes encoding proteins found at or around excitatory synapses may play an important role [115, 116]. More recent exome sequencing studies have identified rare damaging coding variants (CHD8, SCUBE1, and SLITRK5) that also provide support for synaptic abnormalities [117, 118]. Until relatively recently, however, there has been little direct investigation of molecular abnormalities in OCD.
Recent work from our group and others has begun to address this hole in the OCD literature, specifically focusing on examination of gene expression in the PFC and striatum. The first study to examine postmortem brain tissue from people with OCD did so in a trans-diagnostic way across several disorders characterized by obsessions and compulsions (eating disorders, OCD, OCPD, and tic disorder) (Jaffe et al., [119]. Using a microarray, 286 differentially-expressed genes were detected in the DLPFC of people with OCD/OCPD/tic disorder compared to unaffected comparison subjects. Gene set analyses indicated significant enrichment for genes associated with Alzheimer’s, nuclear phosphoproteins, and oxidative phosphorylation. However, this study did not examine gene expression in prefrontal cortical regions shown to be abnormal in OCD. To address this gap, we performed targeted qPCR of excitatory and inhibitory synaptic genes [120] and RNAseq [121] in the OFC. Strikingly, despite having only eight OCD subjects and eight comparison subjects, our initial qPCR findings showed a significant downregulation of a group of genes associated with glutamatergic synapse gene expression in OFC. In contrast, there was little change in expression of transcripts associated with GABAergic synapses. To follow up on this work, we performed unbiased RNAseq and gene set enrichment analysis to identify large scale changes in gene expression in the same subjects. A combined analysis of expression in OFC and striatal regions demonstrated downregulation of synaptic transmission gene sets, consistent with our previous findings, as well as upregulated gene sets associated with cardiovascular system development and transmembrane tyrosine kinase activity. In addition, analysis of cell-type fractions using a deconvolutional approach [121] showed an increase in the vascular cell-type fraction and decrease in the interneuron fraction in the OFC. Both of these findings could be consistent with the previous observations of hypermetabolism of the OFC in OCD patients [28, 33, 122, 123].
Combined with data from our lab [120, 121] and others [124] indicating similar downregulation of excitatory synaptic genes in the striatum, these findings support a model of impaired excitatory synaptic transmission in two key circuit nodes implicated in OCD pathophysiology. It remains to be seen whether: (1) similar gene expression changes are observed in other prefrontal cortical regions, or are unique to the OFC; (2) if decreases in excitatory synaptic gene expression are also observed in non-prefrontal cortical areas; and (3) whether these gene expression changes correspond to downregulation of expression vs. synapse loss. However, out of the 904 genes that were differentially expressed in OFC and striatum in our RNAseq study [121], only 13 were identified as differentially expressed by Jaffe et al. [119], suggesting that OCD gene expression abnormalities in DLPFC may be distinct from those observed in the adjacent OFC. Answering these questions will refine our understanding of the molecular pathology associated with OCD by helping us determine if these abnormalities stem from widespread brain changes or pathology localized to specific circuits.
Theories to link these findings together
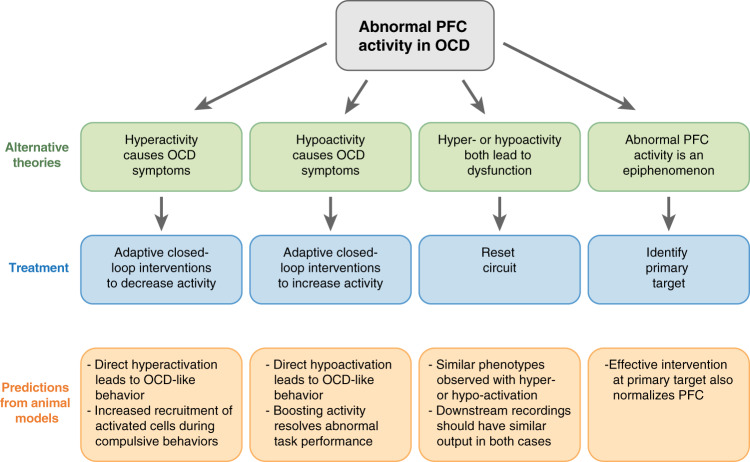
Together, the accumulated data described above provide significant support for the idea that abnormal PFC function plays an important role in the pathogenesis of OCD. However, because of the inherent difficulty of assessing causality in human studies, as well as the lack of cellular resolution in human imaging studies, this cannot be readily tested in humans. Despite this fact, synthesis of these data can lead to several alternative theories that can potentially be supported or refuted by evidence from human intervention studies and animal models (Fig. 1). Here, in order to ground the theories in the previously described findings from neuroimaging studies, we first focus on four broad alternatives describing how findings of either hyperactivity or hypoactivity in prefrontal cortical networks could lead to OCD symptoms through network dysfunction in the context of a signal processing framework. We believe this is a useful lens for considering novel treatment interventions, as even the most advanced currently available neuro-interventional treatments (i.e., focused TMS, DBS) are only able to broadly tune activity up or down within particular PFC regions, leading to impacts in their downstream connected networks. Within each of these Alternatives, we then highlight detailed theories of how dysfunction in particular PFC networks consistent with that broad scenario could produce obsessions and/or compulsions, generating testable hypotheses.
Fig. 1.
Alternative theories of how abnormal PFC function could lead to OCD symptoms. Treatments for each of these scenarios and testable predictions from animal models are provided for each theory.
Alternative 1: Hyperactivity in prefrontal cortical regions directly causes OCD symptoms
Functional neuroimaging studies showing hyperactivity at baseline and after symptom provocation in prefrontal cortical regions including OFC, ACC, VMPFC, and DMPFC provide strong support for the idea that hyperactivity in these regions (potentially generated through a conserved synaptic abnormality that impacts these areas in concert; see section on postmortem findings) may directly lead to OCD symptoms. Though not a comprehensive list, possible theories that would be consistent with this Alternative include the following examples.
Anticipatory autonomic arousal upon viewing a subjectively threatening stimulus could drive hyperactivity in either OFC or ACC. In turn, this OFC/ACC hyperactivity could lead to overvaluation of actions that would generate decreases in autonomic arousal, such as leaving the room to avoid the perceived potential threat or performing a compulsive behavior that subjectively “neutralizes” the perceived threat (e.g., checking the stove, handwashing, etc.).
Hyperactivity in OFC could lead to impaired action-outcome monitoring, such that performance of a particular behavior does not lead to the expected sense of completion. Its repeated selection would therefore be continuously assigned a high value, making it difficult—and potentially even illogical given their priors and decision-making framework—for the patient to choose an alternative, less valued outcome.
Increased activity in ACC could lead to enhanced error-related signals, which has been observed in patients with OCD [91, 97, 125, 126], resulting in increased error aversion. In turn, this could cause a corresponding increase in harm avoidance strategies to avoid the perception of committing an error—e.g., performing compulsions despite consciously understanding that the perceived negative outcome is unlikely to happen, as seen in [67].
If Alternative 1 is correct, hypoactivity in PFC regions during neurocognitive tasks may result from impaired ability to recruit these structures due to baseline circuit hyperactivity. Thus, while PFC hypoactivity may lead to cognitive dysfunction, this would not be the primary driver of symptoms in OCD in this scenario. This concept is supported by the fact that a comprehensive meta-analysis suggests that the effect sizes observed for neurocognitive deficits in OCD are relatively moderate in size, and are therefore unlikely to yield a major contribution to the significant symptom burden experienced by people with OCD [49]. In this model, the observed neurocognitive abnormalities would be considered an epiphenomenon of the other pathologic factors that are more central to symptom production in OCD. Thus, while it might be inherently valuable to improve these neurocognitive deficits if they have an impact on patients’ quality of life, such a therapeutic strategy would not be expected to lead to resolution of obsessions and compulsions.
Alternative 2: Hypoactivity in prefrontal cortical regions directly causes OCD symptoms
Hypoactivity in PFC regions during performance of neurocognitive tasks is quite consistent across multiple tasks and PFC regions, providing support for the idea that this hypoactivity is a cause, and not a consequence, of OCD symptoms. Possible theories that would be consistent with this Alternative include the following examples.
Deficits in the PFC cognitive control network [including ACC/pre-SMA (ACC/pSMA), dorsolateral prefrontal cortex (DLPFC), inferior frontal junction, anterior insular cortex, dorsal pre-motor cortex, and posterior parietal cortex [127, 128]] could directly lead to compulsive behaviors. For example, humans and monkeys with prefrontal damage display stimulus-bound behaviors—that is, salient cues lead to reflexive actions due to strong associations between the sensory stimulus and a particular action. Though not formally tested (but suggested in [129]), this phenomenon is commonly seen in people with OCD, who anecdotally report reacting to particular trigger stimuli using harm avoidance strategies before they can consciously engage in response prevention strategies learned through EX/RP (exposure therapy with response prevention). Findings of PFC hypoactivity could thus be consistent with impairments in inhibitory control of prepotent responses seen in OCD, which may lead to more automatic engagement in compulsive behaviors, particularly in stressful circumstances.
Hypoactivity in ACC/OFC could also be consistent with findings that people with OCD have less capacity to engage in online integration of changing values and application of this information to behavior selection in complex environments or emotionally stressful situations, thus reverting to established strong stimulus-outcome relationships. This is consistent with the clinical phenomenon of exposures being easier to perform in the relatively sparse environment of the clinician’s office, but harder to achieve in more complex “real-world” environments with changing and unpredictable contingencies and emotional states [130].
Because ACC plays an important role in error detection, evaluation of the degree of the error, and selection of the subsequent most appropriate actions [131, 132], ACC hypofunction may lead to impairments in updating values and altering decision-making strategies in response to errors, leading instead to persistent selection of maladaptive behaviors.
Hypofunction of prefrontal cortical networks including OFC and ACC could contribute to impairments in learning of new associations between cues, actions, and outcomes (i.e., reward vs. punishment), which could be detrimental for efficacy of exposure therapy.
If Alternative 2 is correct, hyperactivity in PFC regions at baseline and during symptom provocation may be compensatory responses from connected PFC regions and intraregional microcircuits that are attempting to boost performance of critical PFC executive control functions, including decision making and goal-directed planning.
Alternative 3: Either hyperactivity or hypoactivity in prefrontal cortical regions can lead to similar dysfunctional states
As described above, both hyperactivity and hypoactivity of PFC regions are commonly observed in OCD, though typically in different task states. If Alternative 3 is correct, both PFC hyperactivity or hypoactivity could have a similar negative impact on circuit dynamics in the PFC, thus effectively “jamming the system” and impacting downstream output in a similar way. For example, it has been proposed that OCD is associated with impairments in value-based decision making—specifically, with assignment of excessive value to unlikely negative outcomes associated with obsessions [133, 134], or inability to imagine positive outcomes through impaired metacognition [85, 87, 135]. Either OFC hyperactivity or hypoactivity could therefore lead to impairments in this key executive function.
Alternative 4: Altered prefrontal cortical activity is an epiphenomenon resulting from other primary pathologic sources
Although PFC abnormalities are prominent in OCD, leading theories suggest that symptoms result from abnormal activity within parallel vs. interconnected cortico-basal ganglia-thalamic loops [71, 136, 137]. Thus, pathologic disruptions at any node in the circuit could lead to downstream abnormalities in any connected node. Though disruptions in these extended networks connected to PFC regions are not the focus of this review, one example is that thalamic hyperactivity is also very consistently observed in OCD [12, 138–142], and could therefore be a primary pathologic feature. In turn, hyperactivity in medial thalamic nuclei could result in the observed pattern of PFC hyperactivity/ hypoactivity in OCD through long-range projections acting either directly on PFC glutamatergic pyramidal neurons or indirectly on inhibitory interneurons in a state-specific or task-specific way. Another example is that dysfunction in dopaminergic projections to PFC could cause abnormal representation of reward-related information via either positive or negative prediction error [143–145], leading to either overvaluation of the impact a compulsive behavior will have on decreasing the likelihood of a negative outcome, or undervaluation of the value of refraining from performing the compulsion ([146–149]. In addition, dopamine release in PFC can directly affect capacity for plasticity through its effects on NMDA-receptor mediated glutamatergic transmission [150] or facilitation of activity-dependent plasticity [151], thus impacting capacity to form new associations based on updated action values. Thus, it is possible that signal integration and readout within PFC regions is working correctly, but they are receiving faulty information from their inputs. In the case of Alternative 4, although therapeutic interventions can potentially be made at any point in the circuit, understanding the location of the primary pathology may help to develop new, more targeted treatments that directly attack the source of the problem—e.g., restoring normal thalamic rhythmicity or tuning function of dopaminergic neurons.
Approaches for testing these alternative theories
It is important to develop approaches to distinguish between these Alternatives, as different treatments may be required depending on regional localization of pathology, directionality of abnormal activity (e.g., hyper- vs. hypoactivity), and cell type origins of pathologic activity patterns. Specifically, determining the causal relationship between activity in PFC regions and clinically-important behaviors may help to direct novel, more specific pharmacologic, behavioral, and neurostimulation-based interventions.
One path forward toward this goal is to test these alternative theories in preclinical models using a combination of sophisticated behavioral paradigms and optogenetic and chemogenetic techniques for precise manipulation of activity in particular neural circuits. However, it is also increasingly clear that recent advances now allow for greater levels of precision and causality testing in humans than previously achievable. For example, Alternative 1 is consistent with our recent study in humans, in which we randomized individuals with compulsive behaviors (a majority of whom had OCD) to either intermittent TBS, which is expected to potentiate the OFC, or continuous TBS (cTBS), which is expected to depotentiate the OFC. In both conditions, TBS was paired with a “habit override” computer task, in which participants were trained to override an overlearned shock avoidance behavior. We found that cTBS paired with the habit override task led to a decrease in both urges and compulsive behaviors that persisted for at least a week after the intervention [152], consistent with findings from another recent study [153]. These findings are now being followed up with a sham-control randomized clinical trial.
It is attractive to try to develop a unified theory of the pathologic origins of OCD that will aid the field in the development of new treatments. However, the examples described above demonstrate that many different theories could be consistent with current neuroimaging findings, particularly given the inherent limitations of neuroimaging that prevent us from assigning BOLD activation to excitatory neurons, inhibitory neurons, or nonneuronal subtypes (e.g., astrocytes, microglia). In addition, it is important to recognize that there are likely many different pathological paths that can lead to similar circuit and symptom outputs, highlighting the need for patient stratification into subgroups based on differences in underlying biological mechanisms that may require different treatment approaches. For example, [154] recently compared the efficacy of two different DBS sites—ventral capsule/ventral striatum (VC/VS) and anteromedial STN—in the same patients with treatment refractory OCD, coupled with neurocognitive testing and tractography. They found that stimulation at either site within an individual patient led to similar improvements in OCD symptoms. However, amSTN DBS also led to significant improvements in cognitive flexibility, while VC/VS DBS had a greater impact on mood symptoms; these findings were associated with connectivity to the lateral OFC and medial OFC, respectively. Thus, a patient’s individual symptoms and network function may dictate the most effective treatment targets.
Interventions in PFC circuits can be effective OCD treatments
Consistent with the accumulated findings from the studies described above, there is evidence that interventions in prefrontal cortical circuits can lead to therapeutic effects in people with OCD. Some of these surgical interventions predated modern neuroimaging technology highlighting functional and structural abnormalities in the targeted regions. Other more recent interventions have taken advantage of neuroimaging findings to guide treatment approaches. Importantly, effective interventions, be they exposure therapy, pharmacotherapy, or DBS, appear to share the feature of ultimate normalization of hyperactivity in OFC, ACC, and mPFC [20, 26, 155–158]. Though therapeutic approaches for OCD will be covered in more detail in another article in this volume (see Rasmussen and Goodman, 2021), here we will briefly describe several different therapeutic PFC interventions in OCD that may help us to test the four alternative theories described above. Note that although the efficacy of interventions in prefrontal cortical circuits implies that the targeted PFC regions are involved in pathology, we acknowledge that this is not necessarily the case, because compensatory brain changes are likely to occur during the intersection of the disease process with normal development as OCD evolves. However, this does indicate that interventions in PFC circuits can improve functional outcomes, implying that restoration of normal PFC functions can override, if not correct, abnormal pathology.
Ablative surgical procedures
Several different types of ablation procedures have been used to treat refractory OCD, based on the theory that disruption of connections between hyperactive prefrontal cortical regions and their downstream basal ganglia circuits may lead to a decrease in transmission of abnormal neural signals [159]. These moderately efficacious procedures include anterior cingulotomy, capsulotomy, subcaudate tractotomy, and limbic leucotomy [34]. In anterior cingulotomy, the cingulum bundle is lesioned bilaterally to disrupt connections between cingulate cortex and subcortical regions. Similarly, white matter tracts in the anterior limb of the internal capsule (IC) are transected in capsulotomy, resulting in presumptive disconnection of the OFC and mediodorsal thalamus. Subcaudate tractotomy, a lesion below and anterior to the head of the caudate, is also thought to disrupt frontothalamic circuits, as is limbic leucotomy, which combines cingulotomy and subcaudate tractotomy.
Transcranial magnetic stimulation (TMS) in PFC
Though results from ablative neurosurgical procedures and DBS have been promising (see Rasmussen and Goodman, 2021 in this volume; [160]), nonsurgical interventions are preferable if they are equally efficacious. In this context, recent studies have highlighted the potential utility of transcranial magnetic stimulation (TMS) in OCD treatment. Early work suggested possible efficacy of low frequency rTMS of SMA [161, 162]. More recently, high-frequency (20 Hz) deep TMS of the mPFC-ACC has been found to yield clinical improvement on the YBOCS in a multi-center randomized placebo controlled trial, which correlated with increased ERN in the Stroop task in an initial study [163, 164]. Improvements in YBOCS were maintained at 1 month follow up, with 45.2% in the active TMS group and 17.8% in the sham group showing >30% reduction in YBOCS. This treatment has now been FDA approved [165] and investigations of predictors of response are ongoing, with some initial evidence that older adults and people with less severe illness may have greater responses [166]. In addition, as described above, work from our group [152] highlights the potential efficacy of deep TMS of OFC using continuous theta-burst stimulation (cTBS) for OCD treatment.
Investigation of prefrontal cortex in animal models for OCD-relevant behaviors and treatment
Studies in animal models can provide an important bridge to gain insight into pathophysiology. To date, mechanistic dissection of OCD-related behaviors in animal models has tended to focus on repetitive and/or compulsive behaviors, in part because it is not possible to assay obsessions in species that cannot verbally report their experiences. In addition, the designation of PFC subregions in rodents is quite controversial due to the absence of dysgranular cortex [167], highlighting the importance of including primate models in the study of psychiatric disorders with significant PFC pathology [168]. Thus, much of the OCD animal literature has focused on dissecting the contributions of the basal ganglia to abnormal repetitive and compulsive behaviors. With these caveats in mind, efforts have more recently been made to dissect contributions of PFC-homologous regions to OCD-relevant behaviors in rodents. Because we have recently performed comprehensive reviews of the OCD animal literature [169, 170], here we will focus on studies that may yield mechanistic insights into the proposed alternative theories described above.
Several studies in mice have recently focused on the role of both medial and lateral OFC in the generation and treatment of abnormal repetitive behaviors. In two studies using transgenic mice, constitutive knockout of synaptic proteins—SAPAP3 [171] or Slitrk5 [172]—led to compulsive grooming behavior, anxiety-like behavior, and OFC abnormalities. A corresponding increase in mOFC activity was inferred in Slitrk5-KOs by increased levels of the neural activity marker FosB in KOs compared to littermate controls [172]. In contrast, while SAPAP3-KOs had no evidence of baseline differences in lOFC activity using in vivo electrophysiology [173], our subsequent work using in vivo microscopy has shown that compulsive grooming is associated with an increased number of inhibited cells in the lOFC [174]. Consistent with these findings, prior work has shown that acute stimulation of lOFC-centromedial striatum projections normalized compulsive grooming in SAPAP3-KOs, potentially by boosting activity in striatal parvalbumin positive interneurons to decrease overall striatal activity [173]. In addition, we [175] and others [176–178] have identified reversal learning deficits in SAPAP3-KOs, with evidence of associated abnormal neural activity patterns in both mPFC [175] and lOFC (Manning et al., bioRxiv). Most recently, [178] found that impaired function of OFC GABAergic neurons tagged via expression driven by the non-specific Dlx GABAergic promoter is responsible for reversal learning abnormalities in SAPAP3-KOs, due to lack of inhibition of OFC pyramidal neurons during value updating following reversal. Furthermore, our new work indicates baseline deficits in the strength of encoding of both the correct lever press and reward cue in LOFC neurons from SAPAP3-KOs [174]. Thus, while the data from Slitrk5-KOs supports the idea that mOFC hyperactivity may play a role in the generation of compulsive grooming behavior (Alternative 1), the data from SAPAP3-KOs suggests that lOFC hypoactivity may be important in the generation of impaired reversal learning and potentially in compulsive grooming (Alternative 2). These seemingly disparate results could be synthesized either by Alternative 3, in which any disruption of signaling in a particular brain region interferes with normal processing and has the same impact on downstream neural signaling, or by the fact that these two constitutive transgenic models may have very different developmental compensatory effects, leading to distinct neural activity patterns associated with the same behavioral abnormalities. Two other sets of studies also highlight the potential role of OFC hyperactivity in the generation of abnormal repetitive behaviors. First, 5-HT1B receptor activation in ventrolateral OFC leads to perseverative locomotion and PPI disruption [179], which has translational relevance to OCD [180–185]. In addition, consistent with Alternative 1, repeated optogenetic stimulation of mOFC-striatal projections to simulate OFC hyperactivity in OCD patients led to a progressive increase in grooming accompanied by an increase in evoked firing rate [186]. Together, these findings are potentially consistent with the idea that a medial-lateral gradient exists, in which medial OFC and its connected circuits are responsible for “goal-directed” repetitive behaviors such as grooming, while lateral OFC and its downstream targets are more important for cognitive flexibility impairments seen in OCD. Though at first glance this idea seems inconsistent with our recent finding of recruitment of more grooming-associated inhibited cells in LOFC of SAPAP3-KOs (Manning et al., bioRxiv), decreased activity in the lOFC may lead to abnormal encoding of the stimulus→outcome associations necessary for Pavlovian instrumental transfer (PIT) or impaired contingency degradation, leading to grooming that appears “habit-like” due to its resistance to devaluation (Ostlund 2007, Balleine, Leung, Ostlund, 2011, Bradfield 2017. This view could be consistent with behavioral findings from a water-drop induced grooming test in [173].
Recent preclinical studies have also focused on the role of prefrontal cortical circuits in OCD treatment. One of the most effective treatments for OCD is EX/RP, but it can be quite challenging for patients to complete treatment due to associated distress and difficulties in achieving extinction. To investigate mechanisms underlying this process that might help guide development of improved treatments, [187] modified a previously developed platform avoidance task [188, 189]. Briefly, after rats were trained to press a lever to receive reward, they learned that a tone predicted an upcoming shock. After tone onset, they had 30 s in which they could either continue pressing the lever to receive reward, or escape to a plexiglass platform where they couldn’t be shocked. After 10 days, rats were exposed to a 3-day “EX-RP” phase, during which access to the avoidance platform was blocked. Rats that were resistant to EX-RP (i.e., displayed high freezing throughout extinction) also showed persistent avoidance on a probe test during which the platform was no longer blocked. Interestingly, while this persistent avoidance in EX-RP resistant rats was reduced after lOFC inactivation, persistent avoidance was actually increased after lOFC inactivation in rats that had successful EX-RP. This is reminiscent of differential effects of optogenetic stimulation of OFC circuits in wild-type [186] and mutant [173] mice, and highlights the importance of examining the mechanisms underlying individual variation in both healthy and pathological model systems. Interestingly, DBS of the dorsal portion of the ventral striatum (dVS) (thought to be comparable to the VC/VS target used in DBS for OCD [190–192]) also led to a decrease in persistent avoidance in this model. Based on previous findings, it is possible that these therapeutic effects result from plasticity in PFC regions including mOFC, lOFC, and mPFC (PL/IL) [193–195] through antidromic activation [196–198]. A recent study examining the mechanism of action of DBS in SAPAP3-KO mice also found that DBS of either the IC or the dVS reduced compulsive grooming, with IC stimulation being more effective but also causing increased locomotion [199]. Note, both IC and dVS DBS led to c-fos induction in PL, mOFC, and lOFC (only in IC), but it remains to be tested whether these changes in PFC activation were related to DBS mechanism of action. Finally, in our recent study, we found that both behavioral deficits (impaired reversal learning, compulsive grooming) and abnormal lOFC activity patterns were improved by treatment with chronic fluoxetine, a first-line OCD treatment (Manning et al., bioRxiv). Though causality remains to be determined, this suggests that increased serotonin in the lOFC may be an important treatment mechanism in this animal model.
Future directions and challenges for the field
A wealth of behavioral, neuroimaging, and treatment studies in humans highlight the importance of the PFC in OCD pathology and treatment, with most evidence pointing to important roles for the OFC and ACC, and more recent meta- and mega-analyses highlighting the emerging roles of pre-supplementary and SMAs. However, several key questions remain to be addressed that will facilitate transforming these findings into improved treatment approaches.
First, how do we make sense of the pattern of hyperactivity of PFC regions at rest and during symptomatic states that are normalized with successful treatment, and hypoactivity during performance of specific neuropsychological/cognitive tasks? One potential explanation for this apparent contradiction is that by virtue of technical limitations, neuroimaging studies in OCD have often treated subregions of the PFC as homogenous structures. However, as discussed above, distinct cell-types within cortical regions can be defined not only by their neurotransmitter composition (e.g., glutamatergic vs. GABAergic), but also by their unique patterns of projections and inputs (e.g., PT and IT neurons [200]. For example, based on work in animal models it is conceivable that lOFC neurons that project to dorsal/central striatum are more involved in generation of OCD symptoms [173], while lOFC neurons projecting to BLA are more involved in mediating reversal learning [201, 202]. Different activity patterns in these distinct projections could therefore lead to hyperactivity vs. hypoactivity in different behavioral states. Assessing the same subjects in both symptom provocation and neurocognitive tasks could potentially help test this idea. However, it will also be important to further isolate postmortem abnormalities to particular circuits and cell types, and then take advantage of the capacity of animal models to dissect the contributions of specific PFC cell-types and projections to OCD-relevant behaviors using techniques such as optogenetics and chemogenetics [8]. In turn, this information could be used to develop more targeted neurostimulation-based treatments as suggested by [203–205].
In addition, to date, subregions of the PFC in OCD human imaging studies have been treated as fairly unitary structures–e.g., mOFC, lOFC, and ACC. However, there is evidence from humans, primates, and rodents supporting functional distinctions within these regions. For example, there are indications from rat studies that anterior and posterior mOFC may play distinct roles, with anterior mOFC being responsible for retrieval of unobservable outcomes [85], while posterior mOFC is involved in delay discounting [206]. This is consistent with findings from a meta-analysis of human neuroimaging studies indicating that more abstract and complex reinforcers are represented in activity patterns in the anterior, and not posterior, mOFC [207]. Higher resolution 7T neuroimaging methods may allow further parcellation of PFC subregions to determine if differential functions across anterior-posterior gradients contribute to the distinct PFC activity patterns seen during symptom provocation vs. neurocognitive tasks. This delineation could help develop more localized TMS protocols for particular OCD symptoms.
A final important question for the refinement of OCD treatment is how symptom subtypes should be delineated. Though there have been many proposed subtype designations, with some suggestions that particular subtypes may be differentially responsive to treatment [e.g., hoarding is generally less responsive to treatment [208–210], while tic-related OCD may be more responsive to antipsychotic augmentation [211]], large scale application of current symptomatic designations based on the five-factor designation [212, 213] have not yet led to development of targeted treatments. An example from the field of depression may provide a model for success [214]. Here, fMRI was performed on a large multisite sample (n = 1188), which afforded the statistical power to subdivide patients with depression into four neurophysiological subtypes (‘biotypes’) defined by distinct patterns of dysfunctional connectivity in limbic and frontostriatal networks. Though these biotypes couldn’t be differentiated solely on the basis of clinical features, they were associated with differing clinical symptom profiles. Importantly, in a subset of the patients that were treated with repetitive TMS of DMPFC (n = 154), these biotypes predicted treatment responsiveness. Similar large-scale studies in OCD could therefore begin to develop treatment-defining biotypes for OCD. Concerted investment of resources in OCD research could therefore realistically lead to the development of new, more tailored and effective treatments for this severe psychiatric illness.
Author contributions
SEA and SLR conceptualized the review and wrote the paper. Both authors approved the final paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perez-Vigil A, Fernandez de la Cruz L, Brander G, Isomura K, Jangmo A, Feldman I, et al. Association of Obsessive-Compulsive Disorder With Objective Indicators of Educational Attainment: a Nationwide Register-Based Sibling Control Study. JAMA Psychiatry. 2018;75:47–55. doi: 10.1001/jamapsychiatry.2017.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meier SM, Mattheisen M, Mors O, Schendel DE, Mortensen PB, Plessen KJ. Mortality Among Persons With Obsessive-Compulsive Disorder in Denmark. JAMA Psychiatry. 2016;73:268–74. doi: 10.1001/jamapsychiatry.2015.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lochner C, Fineberg NA, Zohar J, van Ameringen M, Juven-Wetzler A, Altamura AC, et al. Comorbidity in obsessive-compulsive disorder (OCD): a report from the International College of Obsessive-Compulsive Spectrum Disorders (ICOCS) Compr Psychiatry. 2014;55:1513–9. doi: 10.1016/j.comppsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Murray CJL, Lopez AD. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Global burden of disease and injury series. Cambridge, MA: Published by the Harvard School of Public Health on behalf of the World Health Organization and the World Bank; 1996. p. 990. [Google Scholar]
- 5.Leckman JF, Denys D, Simpson HB, Mataix-Cols D, Hollander E, Saxena S, et al. Obsessive-compulsive disorder: a review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM-V. Depress Anxiety. 2010;27:507–27. doi: 10.1002/da.20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillan CM, Robbins TW. Goal-directed learning and obsessive-compulsive disorder. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130475. doi: 10.1098/rstb.2013.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillan CM, Robbins TW, Sahakian BJ, van den Heuvel OA, van Wingen G. The role of habit in compulsivity. Eur Neuropsychopharmacol. 2016;26:828–40. doi: 10.1016/j.euroneuro.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piantadosi SC, Ahmari SE. Using Optogenetics to Dissect the Neural Circuits Underlying OCD and Related Disorders. Curr Treat Options Psychiatry. 2015;2:297–311. doi: 10.1007/s40501-015-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geramita MA, Yttri EA, Ahmari SE. The two-step task, avoidance, and OCD. J Neurosci Res. 2020;98:1007–19. doi: 10.1002/jnr.24594. [DOI] [PubMed] [Google Scholar]
- 10.Fradkin I, Adams RA, Parr T, Roiser JP, Huppert JD. Searching for an anchor in an unpredictable world: a computational model of obsessive compulsive disorder. Psychol Rev. 2020;127:672–99. doi: 10.1037/rev0000188. [DOI] [PubMed] [Google Scholar]
- 11.Baxter LR, Jr, Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. A comparison with rates in unipolar depression and in normal controls. Arch Gen Psychiatry. 1987;44:211–8. doi: 10.1001/archpsyc.1987.01800150017003. [DOI] [PubMed] [Google Scholar]
- 12.Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- 13.Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- 14.Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res. 2000;34:317–24. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- 15.Hendler T, Goshen E, Tzila Zwas S, Sasson Y, Gal G, Zohar J. Brain reactivity to specific symptom provocation indicates prospective therapeutic outcome in OCD. Psychiatry Res. 2003;124:87–103. doi: 10.1016/s0925-4927(03)00091-x. [DOI] [PubMed] [Google Scholar]
- 16.Simon D, Kaufmann C, Musch K, Kischkel E, Kathmann N. Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation. Psychophysiology. 2010;47:728–38. doi: 10.1111/j.1469-8986.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 17.Baioui A, Pilgramm J, Merz CJ, Walter B, Vaitl D, Stark R. Neural response in obsessive-compulsive washers depends on individual fit of triggers. Front Hum Neurosci. 2013;7:143. doi: 10.3389/fnhum.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert AR, Mataix-Cols D, Almeida JR, Lawrence N, Nutche J, Diwadkar V, et al. Brain structure and symptom dimension relationships in obsessive-compulsive disorder: a voxel-based morphometry study. J Affect Disord. 2008;109:117–26. doi: 10.1016/j.jad.2007.12.223. [DOI] [PubMed] [Google Scholar]
- 19.Schienle A, Schafer A, Stark R, Walter B, Vaitl D. Neural responses of OCD patients towards disorder-relevant, generally disgust-inducing and fear-inducing pictures. Int J Psychophysiol. 2005;57:69–77. doi: 10.1016/j.ijpsycho.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:901–10. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–76. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 22.Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, et al. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry. 2009;65:75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Rotge JY, Langbour N, Guehl D, Bioulac B, Jaafari N, Allard M, et al. Gray matter alterations in obsessive-compulsive disorder: an anatomic likelihood estimation meta-analysis. Neuropsychopharmacology. 2010;35:686–91. doi: 10.1038/npp.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxena S, Brody AL, Maidment KM, Dunkin JJ, Colgan M, Alborzian S, et al. Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology. 1999;21:683–93. doi: 10.1016/S0893-133X(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 25.Benkelfat C, Nordahl TE, Semple WE, King AC, Murphy DL, Cohen RM. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. Patients Treat Clomipramine Arch Gen Psychiatry. 1990;47:840–8. doi: 10.1001/archpsyc.1990.01810210048007. [DOI] [PubMed] [Google Scholar]
- 26.van der Straten AL, Denys D, van Wingen GA. Impact of treatment on resting cerebral blood flow and metabolism in obsessive compulsive disorder: a meta-analysis. Sci Rep. 2017;7:17464. doi: 10.1038/s41598-017-17593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Res. 2004;132:69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Swedo SE, Pietrini P, Leonard HL, Schapiro MB, Rettew DC, Goldberger EL, et al. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Revisualization Pharmacother Arch Gen Psychiatry. 1992;49:690–4. doi: 10.1001/archpsyc.1992.01820090018003. [DOI] [PubMed] [Google Scholar]
- 29.Baxter LR, Jr, Saxena S, Brody AL, Ackermann RF, Colgan M, Schwartz JM, et al. Brain Mediation of Obsessive-Compulsive Disorder Symptoms: evidence From Functional Brain Imaging Studies in the Human and Nonhuman Primate. Semin Clin Neuropsychiatry. 1996;1:32–47. doi: 10.1053/SCNP00100032. [DOI] [PubMed] [Google Scholar]
- 30.Mataix-Cols D, Cullen S, Lange K, Zelaya F, Andrew C, Amaro E, et al. Neural correlates of anxiety associated with obsessive-compulsive symptom dimensions in normal volunteers. Biol Psychiatry. 2003;53:482–93. doi: 10.1016/s0006-3223(02)01504-4. [DOI] [PubMed] [Google Scholar]
- 31.Banca P, Voon V, Vestergaard MD, Philipiak G, Almeida I, Pocinho F, et al. Imbalance in habitual versus goal directed neural systems during symptom provocation in obsessive-compulsive disorder. Brain. 2015;138:798–811. doi: 10.1093/brain/awu379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perani D, Colombo C, Bressi S, Bonfanti A, Grassi F, Scarone S, et al. [18F]FDG PET study in obsessive-compulsive disorder. A clinical/metabolic correlation study after treatment. Br J Psychiatry. 1995;166:244–50. doi: 10.1192/bjp.166.2.244. [DOI] [PubMed] [Google Scholar]
- 33.Baxter LR, Jr, Schwartz JM, Mazziotta JC, Phelps ME, Pahl JJ, Guze BH, et al. Cerebral glucose metabolic rates in nondepressed patients with obsessive-compulsive disorder. Am J Psychiatry. 1988;145:1560–3. doi: 10.1176/ajp.145.12.1560. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg BD, Rauch SL, Haber SN. Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 2010;35:317–36. doi: 10.1038/npp.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rauch SL, Kim H, Makris N, Cosgrove GR, Cassem EH, Savage CR, et al. Volume reduction in the caudate nucleus following stereotactic placement of lesions in the anterior cingulate cortex in humans: a morphometric magnetic resonance imaging study. J Neurosurg. 2000;93:1019–25. doi: 10.3171/jns.2000.93.6.1019. [DOI] [PubMed] [Google Scholar]
- 36.Rauch SL, Dougherty DD, Cosgrove GR, Cassem EH, Alpert NM, Price BH, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for obsessive compulsive disorder. Biol Psychiatry. 2001;50:659–67. doi: 10.1016/s0006-3223(01)01188-x. [DOI] [PubMed] [Google Scholar]
- 37.An SK, Mataix-Cols D, Lawrence NS, Wooderson S, Giampietro V, Speckens A, et al. To discard or not to discard: the neural basis of hoarding symptoms in obsessive-compulsive disorder. Mol Psychiatry. 2009;14:318–31. doi: 10.1038/sj.mp.4002129. [DOI] [PubMed] [Google Scholar]
- 38.Apergis-Schoute AM, Gillan CM, Fineberg NA, Fernandez-Egea E, Sahakian BJ, Robbins TW. Neural basis of impaired safety signaling in Obsessive Compulsive Disorder. Proc Natl Acad Sci USA. 2017;114:3216–21. doi: 10.1073/pnas.1609194114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrence NS, An SK, Mataix-Cols D, Ruths F, Speckens A, Phillips ML. Neural responses to facial expressions of disgust but not fear are modulated by washing symptoms in OCD. Biol Psychiatry. 2007;61:1072–80. doi: 10.1016/j.biopsych.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 40.Swedo SE, Leonard HL. Childhood movement disorders and obsessive compulsive disorder. J Clin Psychiatry. 1994;55:32–7. [PubMed] [Google Scholar]
- 41.Sheppard DM, Bradshaw JL, Purcell R, Pantelis C. Tourette’s and comorbid syndromes: obsessive compulsive and attention deficit hyperactivity disorder. A common etiology? Clin Psychol Rev. 1999;19:531–52. doi: 10.1016/s0272-7358(98)00059-2. [DOI] [PubMed] [Google Scholar]
- 42.Deeb W, Malaty IA, Mathews CA. Tourette disorder and other tic disorders. Handb Clin Neurol. 2019;165:123–53. doi: 10.1016/B978-0-444-64012-3.00008-3. [DOI] [PubMed] [Google Scholar]
- 43.Stern E, Silbersweig DA, Chee KY, Holmes A, Robertson MM, Trimble M, et al. A functional neuroanatomy of tics in Tourette syndrome. Arch Gen Psychiatry. 2000;57:741–8. doi: 10.1001/archpsyc.57.8.741. [DOI] [PubMed] [Google Scholar]
- 44.Hampson M, Tokoglu F, King RA, Constable RT, Leckman JF. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65:594–9. doi: 10.1016/j.biopsych.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–37. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- 46.Gordon OM, Salkovskis PM, Oldfield VB, Carter N. The association between obsessive compulsive disorder and obsessive compulsive personality disorder: prevalence and clinical presentation. Br J Clin Psychol. 2013;52:300–15. doi: 10.1111/bjc.12016. [DOI] [PubMed] [Google Scholar]
- 47.Sasson Y, Zohar J, Chopra M, Lustig M, Iancu I, Hendler T. Epidemiology of obsessive-compulsive disorder: a world view. J Clin Psychiatry. 1997;58:7–10. [PubMed] [Google Scholar]
- 48.Pittenger C, Pushkarskaya H, Gruner P. Animal models of OCD-relevant processes: an RDoC perspective. J Obsessive Compuls Relat Disord. 2019;23:100433. doi: 10.1016/j.jocrd.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin Psychol Rev. 2013;33:1163–71. doi: 10.1016/j.cpr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Veale DM, Sahakian BJ, Owen AM, Marks IM. Specific cognitive deficits in tests sensitive to frontal lobe dysfunction in obsessive-compulsive disorder. Psychol Med. 1996;26:1261–9. doi: 10.1017/s0033291700035984. [DOI] [PubMed] [Google Scholar]
- 51.Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164:335–8. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Endrass T, Koehne S, Riesel A, Kathmann N. Neural correlates of feedback processing in obsessive-compulsive disorder. J Abnorm Psychol. 2013;122:387–96. doi: 10.1037/a0031496. [DOI] [PubMed] [Google Scholar]
- 53.Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:1225–36. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- 54.Remijnse PL, Nielen MM, van Balkom AJ, Hendriks GJ, Hoogendijk WJ, Uylings HB, et al. Differential frontal-striatal and paralimbic activity during reversal learning in major depressive disorder and obsessive-compulsive disorder. Psychol Med. 2009;39:1503–18. doi: 10.1017/S0033291708005072. [DOI] [PubMed] [Google Scholar]
- 55.Szabó C, Németh A, Kéri S. Ethical sensitivity in obsessive-compulsive disorder and generalized anxiety disorder: the role of reversal learning. J Behav Ther Exp Psychiatry. 2013;44:404–10. doi: 10.1016/j.jbtep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Valerius G, Lumpp A, Kuelz AK, Freyer T, Voderholzer U. Reversal learning as a neuropsychological indicator for the neuropathology of obsessive compulsive disorder? A behavioral study. J Neuropsychiatry Clin Neurosci. 2008;20:210–8. doi: 10.1176/jnp.2008.20.2.210. [DOI] [PubMed] [Google Scholar]
- 57.Chamberlain SR, Fineberg NA, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. A neuropsychological comparison of obsessive-compulsive disorder and trichotillomania. Neuropsychologia. 2007;45:654–62. doi: 10.1016/j.neuropsychologia.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Kim HW, Kang JI, Namkoong K, Jhung K, Ha RY, Kim SJ. Further evidence of a dissociation between decision-making under ambiguity and decision-making under risk in obsessive-compulsive disorder. J Affect Disord. 2015;176:118–24. doi: 10.1016/j.jad.2015.01.060. [DOI] [PubMed] [Google Scholar]
- 59.Morein-Zamir S, Papmeyer M, Pertusa A, Chamberlain SR, Fineberg NA, Sahakian BJ, et al. The profile of executive function in OCD hoarders and hoarding disorder. Psychiatry Res. 2014;215:659–67. doi: 10.1016/j.psychres.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–2. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- 61.Remijnse PL, van den Heuvel OA, Nielen MM, Vriend C, Hendriks GJ, Hoogendijk WJ, et al. Cognitive inflexibility in obsessive-compulsive disorder and major depression is associated with distinct neural correlates. PLoS ONE. 2013;8:e59600. doi: 10.1371/journal.pone.0059600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, et al. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry. 2005;62:301–9. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- 63.Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, et al. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008;131:155–64. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- 64.Vaghi MM, Hampshire A, Fineberg NA, Kaser M, Brühl AB, Sahakian BJ, et al. Hypoactivation and Dysconnectivity of a Frontostriatal Circuit During Goal-Directed Planning as an Endophenotype for Obsessive-Compulsive Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:655–63. doi: 10.1016/j.bpsc.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaghi MM, Vertes PE, Kitzbichler MG, Apergis-Schoute AM, van der Flier FE, Fineberg NA, et al. Specific Frontostriatal Circuits for Impaired Cognitive Flexibility and Goal-Directed Planning in Obsessive-Compulsive Disorder: evidence From Resting-State Functional Connectivity. Biol Psychiatry. 2017;81:708–17. doi: 10.1016/j.biopsych.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein DJ, Fineberg NA, Bienvenu OJ, Denys D, Lochner C, Nestadt G, et al. Should OCD be classified as an anxiety disorder in DSM-V? Depress Anxiety. 2010;27:495–506. doi: 10.1002/da.20699. [DOI] [PubMed] [Google Scholar]
- 67.Gillan CM, Morein-Zamir S, Urcelay GP, Sule A, Voon V, Apergis-Schoute AM, et al. Enhanced avoidance habits in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:631–8. doi: 10.1016/j.biopsych.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, et al. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70:608–18. doi: 10.1001/jamapsychiatry.2013.914. [DOI] [PubMed] [Google Scholar]
- 69.Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Avila-Parcet A, et al. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry. 2016;21:500–8. doi: 10.1038/mp.2015.88. [DOI] [PubMed] [Google Scholar]
- 70.Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14:268–76. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–7. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 72.Modell JG, Mountz JM, Curtis GC, Greden JF. Neurophysiologic dysfunction in basal ganglia/limbic striatal and thalamocortical circuits as a pathogenetic mechanism of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 1989;1:27–36. doi: 10.1176/jnp.1.1.27. [DOI] [PubMed] [Google Scholar]
- 73.Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW, et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry. 2011;168:718–26. doi: 10.1176/appi.ajp.2011.10071062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vaghi MM, Cardinal RN, Apergis-Schoute AM, Fineberg NA, Sule A, Robbins TW. Action-Outcome Knowledge Dissociates From Behavior in Obsessive-Compulsive Disorder Following Contingency Degradation. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:200–9. doi: 10.1016/j.bpsc.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron. 2011;69:1204–15. doi: 10.1016/j.neuron.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voon V, Derbyshire K, Ruck C, Irvine MA, Worbe Y, Enander J, et al. Disorders of compulsivity: a common bias towards learning habits. Mol Psychiatry. 2015;20:345–52. doi: 10.1038/mp.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gillan CM, Apergis-Schoute AM, Morein-Zamir S, Urcelay GP, Sule A, Fineberg NA, et al. Functional neuroimaging of avoidance habits in obsessive-compulsive disorder. Am J Psychiatry. 2015;172:284–93. doi: 10.1176/appi.ajp.2014.14040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chase HW, Graur S, Versace A, Greenberg T, Bonar L, Hudak R, et al. Neural mechanisms of persistent avoidance in OCD: a novel avoidance devaluation study. NeuroImage: Clin. 2020;28:102404. doi: 10.1016/j.nicl.2020.102404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, et al. Frontal-Striatal Dysfunction During Planning in Obsessive-Compulsive Disorder. Arch Gen Psychiatry. 2005;62:301. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- 80.Den Braber A, Van T, Ent D, Cath DC, Wagner J, Boomsma DI. & De Geus, E. J. C. Brain activation during cognitive planning in twins discordant or concordant for obsessive-compulsive symptoms. Brain. 2010;133:3123–40. doi: 10.1093/brain/awq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salkovskis PM. Obsessional-compulsive problems: a cognitive-behavioural analysis. Behav Res Ther. 1985;23:571–83. doi: 10.1016/0005-7967(85)90105-6. [DOI] [PubMed] [Google Scholar]
- 82.Vaghi MM, Luyckx F, Sule A, Fineberg NA, Robbins TW, De Martino B. Compulsivity Reveals a Novel Dissociation between Action and Confidence. Neuron. 2017;96:348–54. doi: 10.1016/j.neuron.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Banca P, Vestergaard MD, Rankov V, Baek K, Mitchell S, Lapa T, et al. Evidence accumulation in obsessive-compulsive disorder: the role of uncertainty and monetary reward on perceptual decision-making thresholds. Neuropsychopharmacology. 2015;40:1192–202. doi: 10.1038/npp.2014.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hauser TU, Allen M, Consortium N, Rees G, Dolan RJ. Metacognitive impairments extend perceptual decision making weaknesses in compulsivity. Sci Rep. 2017;7:6614. doi: 10.1038/s41598-017-06116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bradfield LA, Hart G, Balleine BW. Inferring action-dependent outcome representations depends on anterior but not posterior medial orbitofrontal cortex. Neurobiol Learn Mem. 2018;155:463–73. doi: 10.1016/j.nlm.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 86.Bradfield LA, Dezfouli A, van Holstein M, Chieng B, Balleine BW. Medial Orbitofrontal Cortex Mediates Outcome Retrieval in Partially Observable Task Situations. Neuron. 2015;88:1268–80. doi: 10.1016/j.neuron.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 87.Bradfield L, Morris R, Balleine BW. OCD as a failure to integrate goal-directed and habitual action control, in Obsessive Compulsive Disorder. Oxford, England: Oxford University Press; 2017. pp. 343–52. [Google Scholar]
- 88.Gruner P, Vo A, Ikuta T, Mahon K, Peters BD, Malhotra AK, et al. Arbitration between Action Strategies in Obsessive-Compulsive Disorder. Neuroscientist. 2016;22:188–98. doi: 10.1177/1073858414568317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 90.Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- 91.Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Res. 2002;110:63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- 92.Endrass T, Ullsperger M. Specificity of performance monitoring changes in obsessive-compulsive disorder. Neurosci Biobehav Rev. 2014;46:124–38. doi: 10.1016/j.neubiorev.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 93.Gillan CM, Fineberg NA, Robbins TW. A trans-diagnostic perspective on obsessive-compulsive disorder. Psychol Med. 2017;47:1528–48. doi: 10.1017/S0033291716002786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stern ER, Liu Y, Gehring WJ, Lister JJ, Yin G, Zhang J, et al. Chronic medication does not affect hyperactive error responses in obsessive-compulsive disorder. Psychophysiology. 2010;47:913–20. doi: 10.1111/j.1469-8986.2010.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: evidence from unaffected first-degree relatives. Am J Psychiatry. 2011;168:317–24. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- 96.Riesel A. The erring brain: error-related negativity as an endophenotype for OCD-A review and meta-analysis. Psychophysiology. 2019;56:e13348. doi: 10.1111/psyp.13348. [DOI] [PubMed] [Google Scholar]
- 97.Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, et al. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry. 2005;57:287–94. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 98.Agam Y, Greenberg JL, Isom M, Falkenstein MJ, Jenike E, Wilhelm S, et al. Aberrant error processing in relation to symptom severity in obsessive-compulsive disorder: a multimodal neuroimaging study. Neuroimage Clin. 2014;5:141–51. doi: 10.1016/j.nicl.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grutzmann R, Endrass T, Kaufmann C, Allen E, Eichele T, Kathmann N. Presupplementary Motor Area Contributes to Altered Error Monitoring in Obsessive-Compulsive Disorder. Biol Psychiatry. 2016;80:562–71. doi: 10.1016/j.biopsych.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 100.Fitzgerald KD, Taylor SF. Error-processing abnormalities in pediatric anxiety and obsessive compulsive disorders. CNS Spectr. 2015;20:346–54. doi: 10.1017/S1092852915000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Klawohn J, Hajcak G, Amir N, Kathmann N, Riesel A. Application of attentional bias modification training to modulate hyperactive error-monitoring in OCD. Int J Psychophysiol. 2020;156:79–86. doi: 10.1016/j.ijpsycho.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 102.Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–49. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lei H, Zhu X, Fan J, Dong J, Zhou C, Zhang X, et al. Is impaired response inhibition independent of symptom dimensions in obsessive-compulsive disorder? Evidence from ERPs. Sci Rep. 2015;5:10413. doi: 10.1038/srep10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang ZM, Wang MY, Guo X, Miao X, Zhang T, Gao D, et al. Attentional avoidance of threats in obsessive compulsive disorder: an event related potential study. Behav Res Ther. 2017;97:96–104. doi: 10.1016/j.brat.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 105.Gruner P, Vo A, Ikuta T, Mahon K, Peters BD, Malhotra AK, et al. White matter abnormalities in pediatric obsessive-compulsive disorder. Neuropsychopharmacology. 2012;37:2730–9. doi: 10.1038/npp.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Snyder HR, Kaiser RH, Warren SL, Heller W. Obsessive-compulsive disorder is associated with broad impairments in executive function: a meta-analysis. Clin Psychol Sci. 2015;3:301–30. doi: 10.1177/2167702614534210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shin NY, Lee TY, Kim E, Kwon JS. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2014;44:1121–30. doi: 10.1017/S0033291713001803. [DOI] [PubMed] [Google Scholar]
- 108.Yun JY, Jang JH, Jung WH, Shin NY, Kim SN, Hwang JY, et al. Executive Dysfunction in Obsessive-Compulsive Disorder and Anterior Cingulate-Based Resting State Functional Connectivity. Psychiatry investigation. 2017;14:333–43. doi: 10.4306/pi.2017.14.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, del Campo N, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–36. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 110.de Wit SJ, de Vries FE, van der Werf YD, Cath DC, Heslenfeld DJ, Veltman EM, et al. Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry. 2012;169:1100–8. doi: 10.1176/appi.ajp.2012.12010073. [DOI] [PubMed] [Google Scholar]
- 111.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18:177–85. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 112.Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am J Psychiatry. 2006;163:1282–4. doi: 10.1176/ajp.2006.163.7.1282. [DOI] [PubMed] [Google Scholar]
- 113.Ahmari SE, Eich T, Cebenoyan D, Smith EE. & Blair Simpson, H. Assessing neurocognitive function in psychiatric disorders: a roadmap for enhancing consensus. Neurobiol Learn Mem. 2014;115:10–20. doi: 10.1016/j.nlm.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taylor S. Etiology of obsessions and compulsions: a meta-analysis and narrative review of twin studies. Clin Psychol Rev. 2011;31:1361–72. doi: 10.1016/j.cpr.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 115.Stewart SE, Yu D, Scharf JM, Neale BM, Fagerness JA, Mathews CA, et al. Genome-wide association study of obsessive-compulsive disorder. Mol Psychiatry. 2013;18:788–98. doi: 10.1038/mp.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mattheisen M, Samuels JF, Wang Y, Greenberg BD, Fyer AJ, McCracken JT, et al. Genome-wide association study in obsessive-compulsive disorder: results from the OCGAS. Mol Psychiatry. 2015;20:337–44. doi: 10.1038/mp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cappi C, Oliphant ME, Péter Z, Zai G, Conceição do Rosário M, Sullivan CAW, et al. De Novo Damaging DNA Coding Mutations Are Associated With Obsessive-Compulsive Disorder and Overlap With Tourette’s Disorder and Autism. Biol Psychiatry. 2020;87:1035–44. doi: 10.1016/j.biopsych.2019.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Halvorsen M, Samuels J, Wang Y, Greenberg BD, Fyer AJ, McCracken JT, et al. Exome sequencing in obsessive-compulsive disorder reveals a burden of rare damaging coding variants. Nat Neurosci. 2021;24:1071–6. doi: 10.1038/s41593-021-00876-8. [DOI] [PubMed] [Google Scholar]
- 119.Jaffe AE, Deep-Soboslay A, Tao R, Hauptman DT, Kaye WH, Arango V, et al. Genetic neuropathology of obsessive psychiatric syndromes. Transl Psychiatry. 2014;4:e432. doi: 10.1038/tp.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Piantadosi SC, Chamberlain BL, Glausier JR, Lewis DA, Ahmari SE. Lower excitatory synaptic gene expression in orbitofrontal cortex and striatum in an initial study of subjects with obsessive compulsive disorder. Mol Psychiatry. 2019;26:986–98. doi: 10.1038/s41380-019-0431-3. [DOI] [PubMed] [Google Scholar]
- 121.Piantadosi SC, McClain LL, Klei L, Wang J, Chamberlain BL, Springer SA, et al. Transcriptome alterations are enriched for synapse-associated genes in the striatum of subjects with obsessive-compulsive disorder. Transl Psychiatry. 2021;11:171. doi: 10.1038/s41398-021-01290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baxter LR, Jr, Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49:681–9. doi: 10.1001/archpsyc.1992.01820090009002. [DOI] [PubMed] [Google Scholar]
- 123.Saxena S, Brody AL, Ho ML, Alborzian S, Ho MK, Maidment KM, et al. Cerebral metabolism in major depression and obsessive-compulsive disorder occurring separately and concurrently. Biol Psychiatry. 2001;50:159–70. doi: 10.1016/s0006-3223(01)01123-4. [DOI] [PubMed] [Google Scholar]
- 124.Lisboa BCG, Oliveira KC, Tahira AC, Barbosa AR, Feltrin ASA, Gouveia G, et al. Initial findings of striatum tripartite model in OCD brain samples based on transcriptome analysis. Sci Rep. 2019;9:1–12. doi: 10.1038/s41598-019-38965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. Am J Psychiatry. 2008;165:116–23. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- 126.Nieuwenhuis S, Nielen MM, Mol N, Hajcak G, Veltman DJ. Performance monitoring in obsessive-compulsive disorder. Psychiatry Res. 2005;134:111–22. doi: 10.1016/j.psychres.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 127.Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–60. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 128.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 129.Hoehn-Saric R, McLeod DR, Zimmerli WD, Hipsley PA. Symptoms and physiologic manifestations in obsessive compulsive patients before and after treatment with clomipramine. J Clin Psychiatry. 1993;54:272–6. [PubMed] [Google Scholar]
- 130.Wise SP, Murray EA, Gerfen CR. The frontal cortex-basal ganglia system in primates. Crit Rev Neurobiol. 1996;10:317–56. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 131.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 132.Swick D, Turken AU. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci USA. 2002;99:16354–9. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giele CL, van den Hout MA, Engelhard IM, Dek EC, Hofmeijer FK. Obsessive-compulsive-like reasoning makes an unlikely catastrophe more credible. J Behav Ther Exp Psychiatry. 2011;42:293–7. doi: 10.1016/j.jbtep.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 134.Hezel DM, Stewart SE, Riemann BC, McNally RJ. Standard of proof and intolerance of uncertainty in obsessive-compulsive disorder and social anxiety disorder. J Behav Ther Exp Psychiatry. 2019;64:36–44. doi: 10.1016/j.jbtep.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 135.Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–28. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 136.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–30. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 137.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 138.Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998;35:26–37. [PubMed] [Google Scholar]
- 139.Saxena S, Bota RG, Brody AL. Brain-behavior relationships in obsessive-compulsive disorder. Semin Clin Neuropsychiatry. 2001;6:82–101. doi: 10.1053/scnp.2001.21833. [DOI] [PubMed] [Google Scholar]
- 140.Smith EA, Russell A, Lorch E, Banerjee SP, Rose M, Ivey J, et al. Increased medial thalamic choline found in pediatric patients with obsessive-compulsive disorder versus major depression or healthy control subjects: a magnetic resonance spectroscopy study. Biol Psychiatry. 2003;54:1399–405. doi: 10.1016/s0006-3223(03)00474-8. [DOI] [PubMed] [Google Scholar]
- 141.Lacerda AL, Dalgalarrondo P, Caetano D, Camargo EE, Etchebehere EC, Soares JC. Elevated thalamic and prefrontal regional cerebral blood flow in obsessive-compulsive disorder: a SPECT study. Psychiatry Res. 2003;123:125–34. doi: 10.1016/s0925-4927(03)00061-1. [DOI] [PubMed] [Google Scholar]
- 142.Rotge JY, Dilharreguy B, Aouizerate B, Martin-Guehl C, Guehl D, Jaafari N, et al. Inverse relationship between thalamic and orbitofrontal volumes in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:682–7. doi: 10.1016/j.pnpbp.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 143.Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–51. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- 144.Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–9. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- 145.Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- 146.Hesse S, Müller U, Lincke T, Barthel H, Villmann T, Angermeyer MC, et al. Serotonin and dopamine transporter imaging in patients with obsessive-compulsive disorder. Psychiatry Res. 2005;140:63–72. doi: 10.1016/j.pscychresns.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 147.McDougle CJ, Goodman WK, Price LH. Dopamine antagonists in tic-related and psychotic spectrum obsessive compulsive disorder. J Clin Psychiatry. 1994;55:24–31. [PubMed] [Google Scholar]
- 148.Perani D, Colombo C, Bressi S, Bonfanti A, Grassi F, Scarone S, et al. In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42:306–14. doi: 10.1016/j.neuroimage.2008.04.233. [DOI] [PubMed] [Google Scholar]
- 149.van der Wee NJ, Stevens H, Hardeman JA, Mandl RC, Denys DA, van Megen HJ, et al. Enhanced dopamine transporter density in psychotropic-naive patients with obsessive-compulsive disorder shown by [123I]{beta}-CIT SPECT. Am J Psychiatry. 2004;161:2201–6. doi: 10.1176/appi.ajp.161.12.2201. [DOI] [PubMed] [Google Scholar]
- 150.Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–80. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–5. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 152.Price RB, Gillan CM, Hanlon C, Ferrarelli F, Kim T, Karim HT, et al. Effect of Experimental Manipulation of the Orbitofrontal Cortex on Short-Term Markers of Compulsive Behavior: a Theta Burst Stimulation Study. Am J Psychiatry. 2021;178:459–68. doi: 10.1176/appi.ajp.2020.20060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Williams NR, Sudheimer KD, Cole EJ, Varias AD, Goldstein-Piekarski AN, Stetz P, et al. Accelerated neuromodulation therapy for Obsessive-Compulsive Disorder. Brain Stimul. 2021;14:435–7. doi: 10.1016/j.brs.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tyagi H, Apergis-Schoute AM, Akram H, Foltynie T, Limousin P, Drummond LM, et al. A Randomized Trial Directly Comparing Ventral Capsule and Anteromedial Subthalamic Nucleus Stimulation in Obsessive-Compulsive Disorder: clinical and Imaging Evidence for Dissociable Effects. Biol Psychiatry. 2019;85:726–34. doi: 10.1016/j.biopsych.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57:510–6. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 156.Le Jeune F, Verin M, N'Diaye K, Drapier D, Leray E, Du Montcel ST, et al. Decrease of prefrontal metabolism after subthalamic stimulation in obsessive-compulsive disorder: a positron emission tomography study. Biol Psychiatry. 2010;68:1016–22. doi: 10.1016/j.biopsych.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 157.Rauch SL, Shin LM, Dougherty DD, Alpert NM, Fischman AJ, Jenike MA. Predictors of fluvoxamine response in contamination-related obsessive compulsive disorder: a PET symptom provocation study. Neuropsychopharmacology. 2002;27:782–91. doi: 10.1016/S0893-133X(02)00351-2. [DOI] [PubMed] [Google Scholar]
- 158.Ho Pian KL, van Megen HJ, Ramsey NF, Mandl R, van Rijk PP, Wynne HJ, et al. Decreased thalamic blood flow in obsessive-compulsive disorder patients responding to fluvoxamine. Psychiatry Res. 2005;138:89–97. doi: 10.1016/j.pscychresns.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 159.Rodman AM, Milad MR, Deckersbach T, Im J, Chou T, Dougherty DD. Neuroimaging contributions to novel surgical treatments for intractable obsessive-compulsive disorder. Expert Rev Neurother. 2012;12:219–27. doi: 10.1586/ern.11.189. [DOI] [PubMed] [Google Scholar]
- 160.Ahmari SE, Dougherty DD. Dissecting Ocd Circuits: from Animal Models to Targeted Treatments. Depression Anxiety. 2015;32:550–62. doi: 10.1002/da.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mantovani A, Lisanby SH, Pieraccini F, Ulivelli M, Castrogiovanni P, Rossi S. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS) Int J Neuropsychopharmacol. 2006;9:95–100. doi: 10.1017/S1461145705005729. [DOI] [PubMed] [Google Scholar]
- 162.Mantovani A, Rossi S, Bassi BD, Simpson HB, Fallon BA, Lisanby SH. Modulation of motor cortex excitability in obsessive-compulsive disorder: an exploratory study on the relations of neurophysiology measures with clinical outcome. Psychiatry Res. 2013;210:1026–32. doi: 10.1016/j.psychres.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Carmi L, Alyagon U, Barnea-Ygael N, Zohar J, Dar R, Zangen A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11:158–65. doi: 10.1016/j.brs.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 164.Carmi L, Tendler A, Bystritsky A, Hollander E, Blumberger DM, Daskalakis J, et al. Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. Am J Psychiatry. 2019;176:931–8. doi: 10.1176/appi.ajp.2019.18101180. [DOI] [PubMed] [Google Scholar]
- 165.Roth Y, Tendler A, Arikan MK, Vidrine R, Kent D, Muir O, et al. Real-world efficacy of deep TMS for obsessive-compulsive disorder: post-marketing data collected from twenty-two clinical sites. J Psychiatr Res. 2020;137:667–72. doi: 10.1016/j.jpsychires.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 166.Storch EA, Tendler A, Schneider SC, Guzick AG, La Buissonniere-Ariza V, Goodman WK. Moderators and predictors of response to deep transcranial magnetic stimulation for obsessive-compulsive disorder. J Psychiatr Res. 2020;136:508–14. doi: 10.1016/j.jpsychires.2020.10.023. [DOI] [PubMed] [Google Scholar]
- 167.Laubach M, Amarante LM, Swanson K, White SR. What, If Anything, Is Rodent Prefrontal Cortex? eNeuro. 2018;5:ENEURO.0315-18. doi: 10.1523/ENEURO.0315-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci. 2011;15:13–9. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Manning EE, Ahmari SE. How can preclinical mouse models be used to gain insight into prefrontal cortex dysfunction in obsessive-compulsive disorder? Brain Neurosci Adv. 2018;2:2398212818783896. doi: 10.1177/2398212818783896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chamberlain BL, Ahmari SE. Animal Models for OCD Research. Curr Topics Behav Neurosci. 2021;49:55–96. doi: 10.1007/7854_2020_196. [DOI] [PubMed] [Google Scholar]
- 171.Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shmelkov SV, Hormigo A, Jing D, Proenca CC, Bath KG, Milde T, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat Med. 2010;16:598–602. doi: 10.1038/nm.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Burguiere E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–6. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Manning EE, Geramita MA, Piantadosi SC, and Ahmari SE Independent and distinct patterns of abnormal lateral orbitofrontal cortex activity during compulsive grooming and reversal learning normalize after fluoxetine. bioRxiv 2021.03.02.433664, 2021. [DOI] [PubMed]
- 175.Manning EE, Dombrovski AY, Torregrossa MM, Ahmari SE. Impaired instrumental reversal learning is associated with increased medial prefrontal cortex activity in Sapap3 knockout mouse model of compulsive behavior. Neuropsychopharmacology. 2019;44:1494–504. doi: 10.1038/s41386-018-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.van den Boom BJG, Mooij AH, Miseviciute I, Denys D, Willuhn I. Behavioral flexibility in a mouse model for obsessive-compulsive disorder: Impaired Pavlovian reversal learning in SAPAP3 mutants. Genes Brain Behav. 2019;18:e12557. doi: 10.1111/gbb.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Benzina N, N'Diaye K, Pelissolo A, Mallet L, Burguiere E. A cross-species assessment of behavioral flexibility in compulsive disorders. Commun Biol. 2021;4:96. doi: 10.1038/s42003-020-01611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang Z, Wu G, Liu M, Sun X, Xu Q, Zhang C, et al. Dysfunction of Orbitofrontal GABAergic Interneurons Leads to Impaired Reversal Learning in a Mouse Model of Obsessive-Compulsive Disorder. Curr Biol. 2021;31:381–93. doi: 10.1016/j.cub.2020.10.045. [DOI] [PubMed] [Google Scholar]
- 179.Shanahan NA, Velez LP, Masten VL, Dulawa SC. Essential role for orbitofrontal serotonin 1B receptors in obsessive-compulsive disorder-like behavior and serotonin reuptake inhibitor response in mice. Biol Psychiatry. 2011;70:1039–48. doi: 10.1016/j.biopsych.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gross-Isseroff R, Cohen R, Sasson Y, Voet H, Zohar J. Serotonergic dissection of obsessive compulsive symptoms: a challenge study with m-chlorophenylpiperazine and sumatriptan. Neuropsychobiology. 2004;50:200–5. doi: 10.1159/000079970. [DOI] [PubMed] [Google Scholar]
- 181.Stein DJ, Van Heerden B, Wessels CJ, Van Kradenburg J, Warwick J, Wasserman HJ. Single photon emission computed tomography of the brain with Tc-99m HMPAO during sumatriptan challenge in obsessive-compulsive disorder: investigating the functional role of the serotonin auto-receptor. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:1079–99. doi: 10.1016/s0278-5846(99)00051-2. [DOI] [PubMed] [Google Scholar]
- 182.Pittenger C, Adams TG, Jr, Gallezot JD, Crowley MJ, Nabulsi N, James R, et al. OCD is associated with an altered association between sensorimotor gating and cortical and subcortical 5-HT1b receptor binding. J Affect Disord. 2016;196:87–96. doi: 10.1016/j.jad.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Prepulse Inhibition Deficits in Obsessive-Compulsive Disorder are More Pronounced in Females. Neuropsychopharmacology. 2016;41:2963–4. doi: 10.1038/npp.2015.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired Sensorimotor Gating in Unmedicated Adults with Obsessive-Compulsive Disorder. Neuropsychopharmacology. 2012;37:1216–23. doi: 10.1038/npp.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatry. 1993;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- 186.Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, et al. Repeated Cortico-Striatal Stimulation Generates Persistent OCD-Like Behavior. Science. 2013;340:1234–9. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rodriguez-Romaguera J, Greenberg BD, Rasmussen SA, Quirk GJ. An Avoidance-Based Rodent Model of Exposure With Response Prevention Therapy for Obsessive-Compulsive Disorder. Biol Psychiatry. 2016;80:534–40. doi: 10.1016/j.biopsych.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bravo-Rivera C, Roman-Ortiz C, Brignoni-Perez E, Sotres-Bayon F, Quirk G. J.Neural structures mediating expression and extinction of platform-mediated avoidance. J Neurosci. 2014;34:9736–42. doi: 10.1523/JNEUROSCI.0191-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bravo-Rivera C, Roman-Ortiz C, Montesinos-Cartagena M, Quirk GJ. Persistent active avoidance correlates with activity in prelimbic cortex and ventral striatum. Front Behav Neurosci. 2015;9:184. doi: 10.3389/fnbeh.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rauch SL, Dougherty DD, Malone D, Rezai A, Friehs G, Fischman AJ, et al. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J Neurosurg. 2006;104:558–65. doi: 10.3171/jns.2006.104.4.558. [DOI] [PubMed] [Google Scholar]
- 191.Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–93. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 192.Dougherty DD, Chou T, Corse AK, Arulpragasam AR, Widge AS, Cusin C, et al. Acute deep brain stimulation changes in regional cerebral blood flow in obsessive-compulsive disorder. J Neurosurg. 2016;125:1087–93. doi: 10.3171/2015.9.JNS151387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rodriguez-Romaguera J, Do Monte FH, Quirk GJ. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc Natl Acad Sci USA. 2012;109:8764–9. doi: 10.1073/pnas.1200782109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rodriguez-Romaguera J, Do-Monte FH, Tanimura Y, Quirk GJ, Haber SN. Enhancement of fear extinction with deep brain stimulation: evidence for medial orbitofrontal involvement. Neuropsychopharmacology. 2015;40:1726–33. doi: 10.1038/npp.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rothwell PE, Hayton SJ, Sun GL, Fuccillo MV, Lim BK, Malenka RC. Input- and Output-Specific Regulation of Serial Order Performance by Corticostriatal Circuits. Neuron. 2015;88:345–56. doi: 10.1016/j.neuron.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McCracken CB, Grace AA. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci. 2007;27:12601–10. doi: 10.1523/JNEUROSCI.3750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 2009;29:5354–63. doi: 10.1523/JNEUROSCI.0131-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haber SN, Heilbronner SR. Translational research in OCD: circuitry and mechanisms. Neuropsychopharmacology. 2013;38:252–3. doi: 10.1038/npp.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pinhal CM, et al. Differential Effects of Deep Brain Stimulation of the Internal Capsule and the Striatum on Excessive Grooming in Sapap3 Mutant Mice. Biol Psychiatry. 2018;84:917–25. doi: 10.1016/j.biopsych.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 200.Shepherd GM. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci. 2013;14:278–91. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Groman SM, Keistler C, Keip AJ, Hammarlund E, DiLeone RJ, Pittenger C, et al. Orbitofrontal Circuits Control Multiple Reinforcement-Learning Processes. Neuron. 2019;103:734–46. doi: 10.1016/j.neuron.2019.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Malvaez M, Shieh C, Murphy MD, Greenfield VY, Wassum KM. Distinct cortical-amygdala projections drive reward value encoding and retrieval. Nat Neurosci. 2019;22:762–9. doi: 10.1038/s41593-019-0374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Creed M. Current and emerging neuromodulation therapies for addiction: insight from pre-clinical studies. Curr Opin Neurobiol. 2018;49:168–74. doi: 10.1016/j.conb.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 204.Creed M, Pascoli VJ, Luscher C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347:659–64. doi: 10.1126/science.1260776. [DOI] [PubMed] [Google Scholar]
- 205.Creed MC. Toward a targeted treatment for addiction. Science. 2017;357:464–5. doi: 10.1126/science.aao1197. [DOI] [PubMed] [Google Scholar]
- 206.Izquierdo A. Functional Heterogeneity within Rat Orbitofrontal Cortex in Reward Learning and Decision Making. J Neurosci. 2017;37:10529–40. doi: 10.1523/JNEUROSCI.1678-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 208.Saxena S. Recent advances in compulsive hoarding. Curr Psychiatry Rep. 2008;10:297–303. doi: 10.1007/s11920-008-0048-8. [DOI] [PubMed] [Google Scholar]
- 209.Williams MT, Farris SG, Turkheimer EN, Franklin ME, Simpson HB, Liebowitz M, et al. The impact of symptom dimensions on outcome for exposure and ritual prevention therapy in obsessive-compulsive disorder. J Anxiety Disord. 2014;28:553–8. doi: 10.1016/j.janxdis.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bloch MH, Bartley CA, Zipperer L, Jakubovski E, Landeros-Weisenberger A, Pittenger C, et al. Meta-analysis: hoarding symptoms associated with poor treatment outcome in obsessive-compulsive disorder. Mol Psychiatry. 2014;19:1025–30. doi: 10.1038/mp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bloch MH, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry. 2006;11:622–32. doi: 10.1038/sj.mp.4001823. [DOI] [PubMed] [Google Scholar]
- 212.Pinto A, Greenberg BD, Grados MA, Bienvenu OJ, 3rd, Samuels JF, Murphy DL, et al. Further development of YBOCS dimensions in the OCD Collaborative Genetics study: symptoms vs. categories. Psychiatry Res. 2008;160:83–93. doi: 10.1016/j.psychres.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pinto A, Greenberg BD, Murphy DL, Nestadt G, Rasmussen SA. Using individual items to clarify OCD symptom structure: the case for five factors. Am J Psychiatry. 2009;166:728–9. doi: 10.1176/appi.ajp.2009.09020287. [DOI] [PubMed] [Google Scholar]
- 214.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]