Abstract
Brewer’s spent grain (BSG) is the largest by-product originated from the brewery industry with a high potential for producing carbohydrases by solid-state fermentation. This work aimed to test the efficacy of a carbohydrases-rich extract produced from solid-state fermentation of BSG, to enhance the digestibility of a plant-based diet for European seabass (Dicentrarchus labrax). First, BSG was fermented with A. ibericus to obtain an aqueous lyophilized extract (SSF-BSG extract) and incorporated in a plant-based diet at increasing levels (0—control; 0.1%, 0.2%, and 0.4%). Another diet incorporating a commercial carbohydrases-complex (0.04%; Natugrain; BASF) was formulated. Then, all diets were tested in in vitro and in vivo digestibility assays. In vitro assays, simulating stomach and intestine digestion in European seabass, assessed dietary phosphorus, phytate phosphorus, carbohydrates, and protein hydrolysis, as well as interactive effects between fish enzymes and dietary SSF-BSG extract. After, an in vivo assay was carried out with European seabass juveniles fed selected diets (0—control; 0.1%, and 0.4%). In vitro digestibility assays showed that pentoses release increased 45% with 0.4% SSF-BSG extract and 25% with Natugrain supplemented diets, while amino acids release was not affected. A negative interaction between endogenous fish enzymes and SSF-BSG extract was observed in both diets. The in vivo digestibility assay corroborated in vitro data. Accordingly, the dietary supplementation with 0.4% SSF-BSG increased the digestibility of dry matter, starch, cellulose, glucans, and energy and did not affect protein digestibility. The present work showed the high potential of BSG to produce an added-value functional supplement with high carbohydrases activity and its potential contribution to the circular economy by improving the nutritional value of low-cost and sustainable ingredients that can be included in aquafeeds.
Subject terms: Animal physiology, Ichthyology
Introduction
Fish meal is one of the most expensive ingredients of aquafeeds and is still used as the main protein source for some particular fish species, namely for carnivorous fish. Several more sustainable and economical alternative protein sources to fish meal have been used, including plant ingredients1. However, the presence of antinutritional factors, low digestibility, and insufficient levels of some essential nutrients (namely amino acids and fatty acids) has limited the incorporation of plant ingredients in aquafeeds1. The presence of significant levels of indigestible carbohydrates, mainly non-starch polysaccharides (NSPs), is one of the main restrictions on their use in aquafeeds.
NSPs are structural constituents of the cell walls of plant feedstuffs, and their presence and composition vary according to the plant source2. Depending on their composition, dietary NSPs differently impact fish physiology, generally impairing feed utilization and growth performance3. It has been reported that supplementation of plant-based diets with exogenous carbohydrases, namely non-starch carbohydrases, increases diet digestibility, reduces nutrient excretion, and increases feed efficiency4–6. Carbohydrases are a wide group of enzymes, including cellulases, xylanases, and hemicellulases, which can hydrolyze NSPs into lower molecular weight polysaccharides and oligosaccharides7.
More than 80% of the global economic market of carbohydrases is dominated by xylanase and glucanase, but other enzymes are also available4,8. Special attention has been given to cellulose hydrolyzing enzymes, i.e., exoglucanases and endoglucanases, releasing cellobiose and glucose, respectively9. The potential of these enzymes to enhance the digestibility of carbohydrates in plant-based diets is well documented in terrestrial animals10,11 but it still needs further research in aquaculture nutrition4.
Solid-state fermentation (SSF) is a white biotechnological process that may be used to obtain carbohydrases through the hydrolysis of adequate solid substrates by selected microorganisms. SSF differs from submerged fermentation (SmF) as it occurs with little or no free water, is suitable for filamentous fungi growth, and is a cost-effective and eco-friendly process12. SmF is a liquid-state fermentation, commonly uses bacteria and allows easy final product recovery through filtration or centrifugation13. Even though SmF is the preferred process for producing enzymes at an industrial scale14, SSF presents some advantages over SmF, including lower susceptibility to contamination, microorganisms less vulnerable to inactivation by the substrate, and higher enzymatic productivity with higher specificity and activity15.
The production of enzymes with commercial interest through SSF, such as proteases, cellulases, xylanases, lipases, and phytases, can be modulated mainly by the microorganism species and type of substrate used in the fermentative process16. Carbohydrases are usually produced from lignocellulosic-rich by-products of agricultural origin17, as brewer’s spent grain (BSG). BSG is the main by-product resulting from the brewery industry, averaging 20 kg of BSG per 100 L of beer18. BSG is produced during the malting stage of beer manufacturing and is composed of barley grain husks, smaller parts of the pericarp, and endosperm. BSG possesses high hemicellulose and cellulose content (30–50% w/w), relatively high protein levels (19–30% w/w), residual levels of starch and hops, and a variable quantity of phenolic compounds18,19. Even though these nutritional characteristics make BSG a low-value feedstuff only suitable for ruminants nutrition18, BSG is an excellent substrate for SSF. Indeed, during SSF, the lignocellulosic structure and the free sugars available of BSG are used as a nutrient source and solid porous support matrix for fungal growth20. BSG has already been used to produce enzymes such as laccases21, xylanases22–24, cellulases24,25, and multi-complex cocktails of enzymes26. However, the potential application of a carbohydrases-enriched extract produced by SSF of BSG in aquafeeds has not yet been studied.
Although some research has been made to assess how plant-based diets supplementation with carbohydrases affects fish growth and feed utilization, to the best of our knowledge, there are no studies regarding the efficacy of enzymes produced through SSF of BSG in aquafeeds. The present work aimed to produce a carbohydrases-enriched extract by SSF of BSG and to evaluate its potential as an enzymatic feed additive using European seabass (Dicentrarchus labrax) as model species.
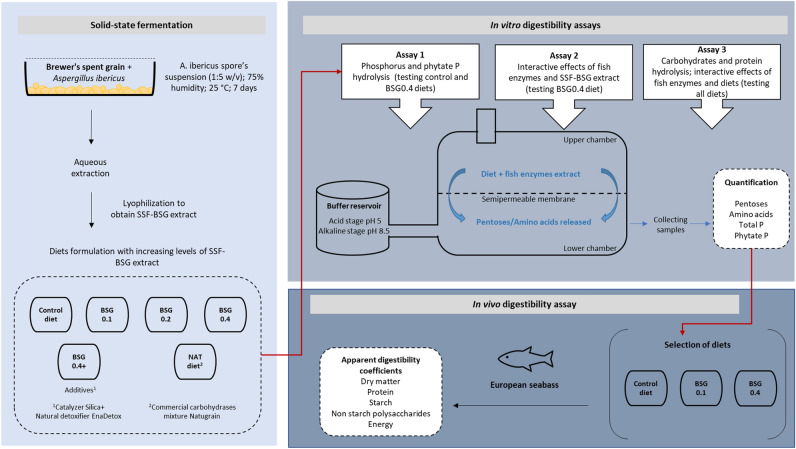
Firstly, BSG was fermented with A. ibericus to obtain an aqueous lyophilized extract (SSF-BSG extract) and incorporated in a plant-based diet at increasing levels. Then, the in vitro digestibility assays were performed to assess, the efficacy of SSF-BSG extract to improve the bioavailability of dietary phosphorus (P), phytate P, carbohydrates, and protein, as well as the interactions between fish digestive enzymes (endoenzymes) and dietary SSF-BSG extract enzymes. In vitro techniques that mimic in vivo fish digestion are methods based on the 3R’s approach (animal reduced use), successfully used to predict nutritional responses and to explain interactions between the feed matrix, additives, and fish enzymes27,28. Finally, using the results of in vitro assays as a reference, some of the most interesting diets were selected and tested in an in vivo digestibility trial. Results of both types of assays were correlated and discussed.
Materials and methods
This study was approved by the CIIMAR Animal Welfare Committee (ORBEA) and by the Portuguese National Authority for Animal Health (DGAV); reference ORBEACIIMAR_27_2019) and was carried out according to ARRIVE guidelines29. Experiments were directed by trained scientists (Functions A, B, C & D defined in article 23 of European Union Directive 2010/63) and conducted following the Federation of Laboratory Animal Science Association (FELASA) recommendations for experiments in laboratory animals30 and the EU Directive (2010/63/EU) on the protection of animals for scientific purposes.
Solid-state fermentation of brewer’s spent grain
Brewer’s spent grain (BSG) was provided by UNICER S.A. (Porto, Portugal). BSG proximal composition is presented in Table 1. SSF of BSG was performed with Aspergillus ibericus (MUM 03.49), maintained at 4 °C in Potato Dextrose Agar medium until utilization. This fungus was selected as it has GRAS (Generally Regarded As Safe) status and a high ability to produce carbohydrases, namely xylanase and cellulase. The SSF was carried out using 4 trays (43 × 33 × 7 cm) as bioreactors. In each tray, 400 g of dried BSG (bed height 2.5 cm) was inoculated with a spore’s suspension of A. ibericus with 106 spores’ concentration. BSG humidity was then adjusted to 75%, followed by incubation at 25 °C for 7 days. At the end of SSF, water-soluble compounds of fermented BSG were recovered. For that, the fermented BSG was mixed with distilled water (1:5 w/v) under constant agitation for 30 min31, sieved (0.45 µm pore-size filter), centrifuged (7000g for 5 min), and the supernatant (crude extract) was recovered and lyophilized for 48 h. The xylanase and cellulase activities of the lyophilized fermented BSG extract (SSF-BSG extract) are presented in Table 1.
Table 1.
Brewer’s spent grain composition and enzymatic activities of SSF-BSG extract and experimental diets.
| Composition (% dry matter) | Moisture | Reducing sugars (mg sugars g−1) | Protein | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|---|---|---|
| BSG | 4.3 | 6.7 | 25.8 | 21.2 | 23.8 | 15.0 |
| Enzymatic activity (U kg−1 diet) | Cellulase | Xylanase | β-glucosidase | Phytase | Protease | |
|---|---|---|---|---|---|---|
| SSF-BSG extract | 1343 | 15,885 | – | – | – | |
| Experimental diets | ||||||
| Control | 270 | 309 | 330 | 280 | 92 | |
| BSG0.1 | 1110 | 1590 | 330 | 652 | 646 | |
| BSG0.2 | 1910 | 5538 | 510 | 717 | 1824 | |
| BSG0.4 | 5075 | 10,153 | 830 | 1050 | 1686 | |
| BSG0.4 + | 4420 | 9955 | 820 | 989 | 1740 | |
| NAT | 1220 | 4590 | 710 | 1036 | – | |
BSG: Brewer’s spent grain.
SSF-BSG extract: lyophilized extract obtained after solid-state fermentation of BSG with Aspergillus ibericus.
Diets formulation
Six diets were formulated to be isoproteic (48% crude protein) and isolipidic (16% crude lipids), containing 15% of fishery products as protein source (fish meal and fish protein concentrate) and fish oil as the main lipid source. Chromium oxide was used as a digestibility marker. SSF-BSG extract was included in the diets at 0, 0.1, 0.2, and 0.4% of dry matter (control, BSG0.1, BSG0.2, and BSG0.4 diets, respectively), corresponding to a cellulase supplementation of 0, 1000, 2000, and 4000 U g−1 of diet (DM basis). Another BSG0.4 diet was formulated, including a commercial catalyzer (Silica+; CERESCO NUTRITION; inclusion level 0.02% of the diet) and a plant-derived detoxifier (Ena-Detox; EUROPEAN NATURAL ADDITIVES, inclusion level 0.07% of the diet) (diet BSG0.4+). For comparison purposes, a diet supplemented with a commercial carbohydrases-complex (endo-1,4-β-xylanase, and endo-1,4-β-glucanase complex; Natugrain TS, BASF, Germany) was formulated with a cellulase activity of 4000 U g−1 of diet (DM basis; NAT diet). The diets were produced as pellets according to Magalhães et al.5. The proximate composition of the control diet is presented in Table 2.
Table 2.
Composition and proximate analysis of all experimental diets.
| Diets | Control |
|---|---|
| Ingredients (% DM) | |
| Fish meala | 9.8 |
| Soluble fish protein concentrateb | 4.9 |
| Wheat gluten mealc | 18.6 |
| Soybean meald | 9.8 |
| Rice bran meale | 9.8 |
| Sunflower mealf | 8.8 |
| Rapeseed mealg | 7.8 |
| Wheat mealh | 5.4 |
| Hemoglobin AP310i | 4.9 |
| Taurinej | 0.5 |
| l-Lysinej | 0.6 |
| dl-Methioninej | 0.2 |
| Fish oil | 12.3 |
| Vitamin premixk | 1 |
| Choline chloride (50%) | 0.5 |
| Mineral premixl | 1 |
| Binderm | 1 |
| Betainn | 0.2 |
| Shrimp hydrolysedo | 1.2 |
| CaHPO4 | 1 |
| Chromium oxide | 0.5 |
| SSF-BSG extractp | 0.1–0.4 |
| Ena-detoxq | 0.07 |
| Silicon dioxider | 0.02 |
| Natugrain TSs | 0.04 |
| Proximate composition (%) | |
| Moisture | 7.1 |
| Protein | 47.9 |
| Lipids | 16.3 |
| Ash | 6.8 |
| Energy (kJ g−1) | 24.1 |
| Starch | 13.9 |
| Hemicellulose | 5.8 |
| Cellulose | 4.3 |
| Lignin | 2.6 |
CP: Crude protein; CL: Crude lipids; DM: Dry matter.
aSteam Dried LT-FM, Pesquera Centinela, Chile (CP: 71.2%; CL 11.1%).
bSopropèche G, France (CP: 79.7%; CL: 18.4%DM).
cSorgal, S.A. Ovar, Portugal (CP: 86.9%; CL: 1.0%).
dSorgal, S.A. Ovar, Portugal (CP: 53.8%; CL: 1.8%).
eSorgal, S.A. Ovar, Portugal (CP: 14.1%; CL: 1.0%).
fSorgal, S.A. Ovar, Portugal. (CP: 29.0%; CL: 1.8%).
gSorgal, S.A. Ovar, Portugal. (CP: 41.5%; CL: 1.9%).
hSorgal, S.A. Ovar, Portugal. (CP: 12.4%; CL: 0.9%).
iAPC Europe S.A.S.A. (CP: 94.5%; CL: 0.4%).
jFeed-grade taurine, l-lysine and dl-Methionine, Sorgal, S.A. Ovar, Portugal.
kVitamins (mg kg−1 diet): retinol, 18,000 (IU kg−1 diet); calciferol, 2000 (IU kg−1 diet); alpha tocopherol, 35; menadion sodium bis., 10; thiamin, 15; riboflavin, 25; Ca pantothenate, 50; nicotinic acid, 200; pyridoxine, 5; folic acid, 10; cyanocobalamin, 0.02; biotin, 1.5; ascorbyl monophosphate, 50; inositol, 400.
lMinerals (mg kg−1 diet): cobalt sulphate, 1.91; copper sulphate, 19.6; iron sulphate, 200; sodium fluoride, 2.21; potassium iodide, 0.78; magnesium oxide, 830; manganese oxide, 26; sodium selenite, 0.66; zinc oxide, 37.5; dicalcium phosphate, 8.02 (g kg−1 diet); potassium chloride, 1.15 (g kg−1 diet); sodium chloride, 0.4 (g kg−1 diet).
mLIPTOSA, Spain.
nSorgal, S.A. Ovar, Portugal.
oSorgal, S.A. Ovar, Portugal. (CP: 69.8%; CL: 12.1%).
pLyophilized extract obtained after solid-state fermentation of BSG with Aspergillus ibericus (xylanase and cellulase activities are, respectively, 15885 and 1343 U g−1 lyophilized extract). Incorporation levels: BSG0.1 diet—0.1%; BSG0.2 diet—0.2%; BSG0.4 diet—0.4%; BSG0.4 + diet—0.4%.
qPlant extract additive EUROPEAN NATURAL ADDITIVES, Madrid, Spain. Included in BSG0.4 + diet.
rSilica+, CERESCO NUTRITION, Quebec, Canada. Included in BSG0.4+ diet.
sNatugrain TS, BASF, Germany (2500 and 5600 U g−1 of cellulase and xylanase activity, commercial information). Included in NAT diet.
In vitro and in vivo trials performed are schematically indicated in Fig. 1 and described below.
Figure 1.
Flow chart of the experimental design.
In vitro digestibility assays
Three different in vitro assays were carried out to determine the:
Assay 1 > effect of SSF-BSG extract on phosphorus (P) availability (total and phytate P) of control and BSG0.4 diets, measured by total P and phytate P release.
Assay 2 > interactive effects of SSF-BSG extract and fish endoenzymes on the carbohydrates hydrolysis of BSG0.4 diet, measured by pentoses release. The conditions tested included: (i) active BSG0.4 diet and inactive fish enzymes; (ii) inactive BSG0.4 diet and active fish enzymes; and (iii) inactive BSG0.4 diet and inactive fish enzymes.
Assay 3 > effect of SSF-BSG extract, SSF-BSG extract plus catalyzer and detoxifier, or Natugrain on carbohydrates and protein hydrolysis of control, BSG0.1, BSG0.2, BSG0.4, BSG0.4+, and NAT diets, measured by pentoses and amino acids release with active or inactive fish enzymes, to estimate the interaction between fish enzymes and dietary SSF-BSG extract or NAT activity.
The fish enzyme extracts used in all the in vitro digestibility assays were obtained from full-fed European seabass juveniles as described by Morales and Moyano27. Briefly, fish were starved for 6 h prior to sampling and sacrificed by immersion in ice-cold water. Fish extracts were obtained after individual homogenization of the stomach and proximal intestine with pyloric caeca with distilled water (1:10 w/v), followed by centrifugation (12,000 g, 3 °C, 15 min). Fish extracts were lyophilized and resuspended when needed. The fish enzymes extract:diet ratio used was 13,000 U g−1 and 6800 U g−1 for acid and alkaline digestion. This ratio was defined considering the average total acid and basic proteases produced by European seabass (with circa 50 g body weight) fed a 45% crude protein diet at a ration level of 3% of body weight.
All in vitro digestibility assays were performed using bioreactors modified from those described by Morales and Moyano27. The mixture of fish enzyme extracts and the desired diet is placed in the upper chamber, and the released products of hydrolysis (amino acids, pentoses, P, and others) pass through the membrane into the lower chamber, being recovered at different time intervals during the reaction time. Acid and alkaline stages of the hydrolysis were carried out at pH 5 and 8.5, respectively, according to the average pH measured in the stomach and intestine of fed European seabass (50 g average weight)32.
All in vitro digestibility assays were run in triplicate for each diet. At the beginning of each run, the diet, fish acid enzymatic extract, and acid buffer (0.2 M citrate buffer with NaCl 50 mM, pH 5) were placed in the upper chamber and maintained under continuous agitation (270 rpm) for 2 h. During this time, three samples were taken from the upper and lower chambers at 30 min, 1 h, and 2 h. Samples taken from the upper chamber were centrifuged (14,000 g, 4 °C, 15 min), and the supernatant was stored at − 20 °C for determination of residual enzyme activities. After acid digestion, the pH of the upper chamber was adjusted to 8.5 with NaOH 6 N, and the crude alkaline enzymatic extract was added. Subsequently, an alkaline buffer (0.01 M Borax-Boric buffer with 20 mM CaCl2 and 45 µM taurocholate, pH 8.5) was added in the lower chamber to begin the alkaline digestion. Alkaline hydrolysis was maintained for 4 h, and a sample was taken every hour from the lower chamber. Moreover, at the end of the digestion, a sample was also collected from the upper chamber. The entire digestion process (6 h) was carried out at 25 °C, being stopped by adding trichloroacetic acid (20%) before the last sample collection.
Specific assays aimed to evaluate the effect of SSF-BSG extract or commercial product (NAT) in the absence of active fish enzymes were carried out after inactivating fish enzyme extracts by heating at 100 °C for 30 min.
In vivo digestibility assay
Based on the in vitro assays results and to reduce the number of fish used in in vivo assays, the control, BSG0.1, and BSG0.4 diets were selected to be tested in an in vivo digestibility assay with European seabass juveniles. The assay was performed in a recirculating aquaculture system (RAS) equipped with 9 fiberglass tanks (60 l water capacity each) provided with a feces settling column connected to the outlet, designed according to Cho et al.33. During the assay, a constant water flow of 5 l min−1 was kept, the water temperature was regulated to 22 ± 1 °C, salinity averaged 32 ± 1 ‰, dissolved oxygen averaged 92% of saturation, ammonia and nitrites levels were kept below 0.02 mg ml−1, and photoperiod was adjusted to 12 L: 12 D.
Nine groups of 15 fish (initial body weight of 22 ± 1 g) were randomly distributed to each tank, and each experimental diet was randomly assigned to triplicate groups. Fish were fed by hand, until apparent satiety, twice a day, 7 days a week, for 64 days. During the last 30 days of feeding, feces accumulated in each settling column were collected daily before the first meal. Feces were then centrifuged at 3000 g for 15 min, pooled for each tank, and stored at − 20 °C until further analysis. After the last daily meal, the tanks, pipes, and settlings columns were cleaned to remove remaining feces and uneaten feed. Apparent digestibility coefficients (ADC) of dry matter, protein, gross energy, starch, cellulose, hemicellulose, glucans, xylan, arabinan, and lignin of the experimental diets were calculated as follows:
Physical–chemical analysis
Enzymatic activity analysis
Acid protease activity on fish stomach extracts used in the in vitro assays was measured34 using 0.5% w/v hemoglobin in a glycine–HCl buffer (100 mM, pH 2.0) as substrate. Alkaline protease activity in intestinal extracts was measured35 using 1% w/v casein dissolved on Tris–HCl buffer (100 mM + 10 mM CaCl2, pH 8) as substrate. For both assays, one unit of activity was defined as 1 µg of tyrosine released per minute. Xylanase and cellulase activities were measured in SSF-BSG extract and experimental diets, while β-glucosidase, phytase, proteases, and lipase activities were measured only in the experimental diets. Xylanase (endo-1,4-β-xylanase), cellulase (endo-1,4-β-glucanase), and β-glucosidase were measured according to Fernandes et al.36.
Phytase activity was assessed37 using sodium phytate in acetate buffer (0.2 M, pH 4.5) as substrate, and the activity was defined as the enzyme needed to release 1 µM of inorganic phosphate.
Protease and lipase activities were measured according to Charney and Tomarelli38 and Gomes et al.39, respectively.
Analysis of products released during in vitro assays
In the in vitro digestibility assays, variations in phytate P, pentoses, and amino acids were measured as indicators of the hydrolysis of phytic acid, non-starch polysaccharides, and protein, respectively.
Phytate P was determined according to Haug and Lantzsch40. Pentoses were measured according to Douglas41. Total amino acids were quantified according to Church et al.42.
Chemical analyses of ingredients, experimental diets, and feces
Chemical analyses were performed according to the Association of Official Analytical Chemists methods (AOAC79). Cellulose, hemicellulose, and lignin contents were determined according to Fernandes et al.36.
Statistical analysis
Before analysis, normality and homogeneity of variances were verified using the Shapiro–Wilk test. When necessary, data were normalized using log-transformation or arc-sin square-root transformation for percentage values.
In vitro assays data of total and phytate P release were evaluated using two-way ANOVA, with digestion stage (acid, alkaline, or total) and diets as factors. In vitro assay data of pentoses and amino acids release for each digestion stage (acid, alkaline, or total) were analyzed by two-way ANOVA, with diets and fish digestive enzymes as factors, followed by Bonferroni’s multiple range test to detect significant differences among means (p < 0.05).
Data from in vivo digestibility assay was analyzed by one-way ANOVA, followed by Bonferroni’s multiple range test to detect significant differences among means (p < 0.05). All statistical analyses were conducted using IBM SPSS Statistics software version 26 (IBM, NY, USA; https://www.ibm.com/products/spss-statistics).
Results
Enzyme production by SSF of BSG
SSF of BSG with Aspergillus ibericus allowed the production of 50 g of lyophilized SSF-BSG extract per kg of BSG. The lyophilized SSF-BSG extract had 15,885 and 1343 U g−1 of xylanase and cellulase activities, respectively. The enzyme activity of the experimental diets reflected the amount of lyophilized SSF-BSG extract added to the diets. The NAT supplemented diet had no protease activity, and cellulase and xylanase activities of this diet were within the range values of the BSG0.2 diet, while β-glucosidase and phytase activities were within the range values of the BSG0.4 diet (Table 1).
In vitro assays
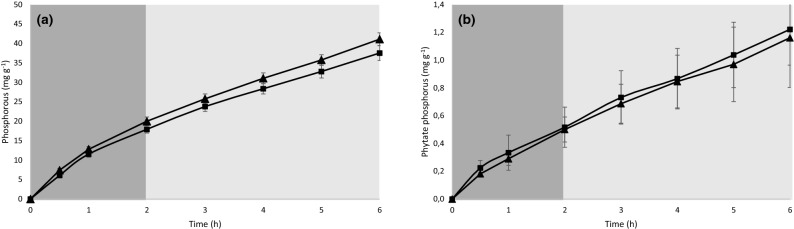
In Assay 1, aimed to evaluate differences in bioavailability of total and phytate P for control and BSG0.4 diets, a steady release of total and phytate P was observed with time for both diets. However, the inclusion of 0.4% of SSF-BSG extract in the diet significantly increased the release of total P (Fig. 2, Table 3).
Figure 2.
Total phosphorus (a) and phytate phosphorus (b) release (mg g−1 of diet) from control (filled black square) and BSG0.4 (filled black triangle) diets under the simulated conditions of European seabass digestive tract. Stomach and intestinal digestion are represented in dark and light grey, respectively. Values are presented as the mean of the cumulative values ± standard deviation.
Table 3.
Total and phytate phosphorus release of control and BSG0.4 diets during in vitro digestion (assay 1).
| Diet | Digestion stage | Phosphorus (mg g−1 diet−1) | Phytate phosphorus (mg g−1 diet−1) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||||
| Control | Acid | 17.9 ± 0.68 | 0.52 ± 0.10 | |||
| Alkaline | 19.6 ± 0.72 | 0.36 ± 0.03 | ||||
| Total digestion | 37.5 ± 1.40 | 1.22 ± 0.18 | ||||
| BSG0.4 | Acid | 20.0 ± 0.77 | 0.50 ± 0.06 | |||
| Alkaline | 21.1 ± 0.40 | 0.32 ± 0.13 | ||||
| Total digestion | 41.1 ± 1.20 | 1.16 ± 0.25 | ||||
| Two-way ANOVA* | Phosphorus | Phytate phosphorus | ||||
|---|---|---|---|---|---|---|
| Diet | Digestion stage | Interaction | Diet | Digestion stage | Interaction | |
| * | n.s. | n.s. | n.s. | n.s. | n.s. | |
Values presented as mean (n = 3) and standard deviation (SD).
Two-way ANOVA testing for the effect of the diet (control and BSG0.4) and digestion stage (acid, alkaline and total).
*p < 0.05; **p < 0.01; ***p < 0.001; n.s.: not significant.
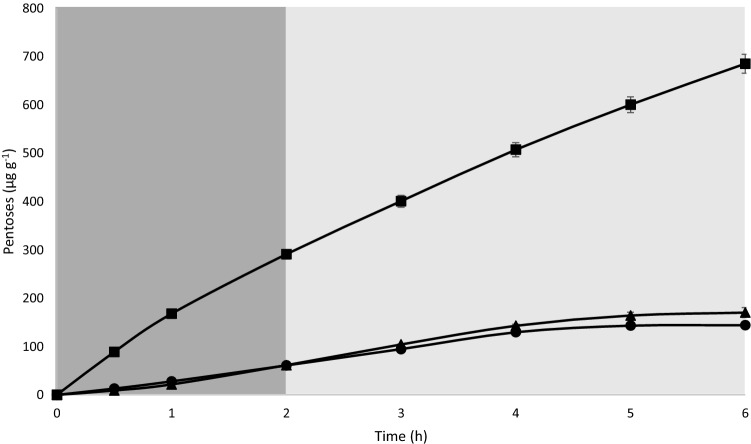
In Assay 2, designed to assess the interaction effect between endogenous (fish) and exogenous (SSF-BSG extract) enzymes on BSG0.4 diet carbohydrates hydrolysis, it was observed (i) a basal release of pentoses from the diet in the absence of any active enzymes; (ii) no significant effect of fish enzymes on such release; (iii) a fourfold increase in pentoses release with active relative to inactive BSG0.4 diet and; (iv) a 14% reduction on pentoses release when BSG0.4 diet was mixed with active fish enzymes compared to the amount released when fish enzymes were inactivated (Table 4). The dynamic of pentoses release during the in vitro hydrolysis is shown in Fig. 3.
Table 4.
Pentoses release (µg g−1 diet h−1) of BSG0.4 diet during the gastric, intestinal, and total in vitro digestion.
| Digestion stage | Inactive diet BSG0.4 plus inactive fish enzymes | Inactive diet BSG0.4 plus active fish enzymes | Active diet BSG0.4 plus inactive fish enzymes | Active diet BSG0.4 plus active fish enzymes |
|---|---|---|---|---|
| Acid | 61.6 ± 0.55b | 61.0 ± 1.64b | 291.2 ± 6.50a | 262.0 ± 27.5a |
| Alkaline | 108.8 ± 9.54c | 83.2 ± 1.39c | 394.3 ± 7.52a | 328.3 ± 15.1b |
| Total digestion | 170.4 ± 10.1b | 144.2 ± 3.03b | 685.5 ± 13.84a | 590.3 ± 42.0a |
Values presented as mean (n = 3) ± standard deviation (SD).
Values in the same row with different subscripts are significantly different (p < 0.05).
Figure 3.
Pentoses release (µg g−1 of diet) from BSG0.4 diet with inactivate fish enzymes (filled black square), inactive BSG 0.4 diet with active fish enzymes (filled black triangle) and both inactive BSG 0.4 diet and fish enzymes (filled black cirle), under the simulated conditions of European seabass digestive tract. Stomach and intestinal digestion are represented in dark and light grey, respectively. Values are presented as the mean of the cumulative values ± standard deviation.
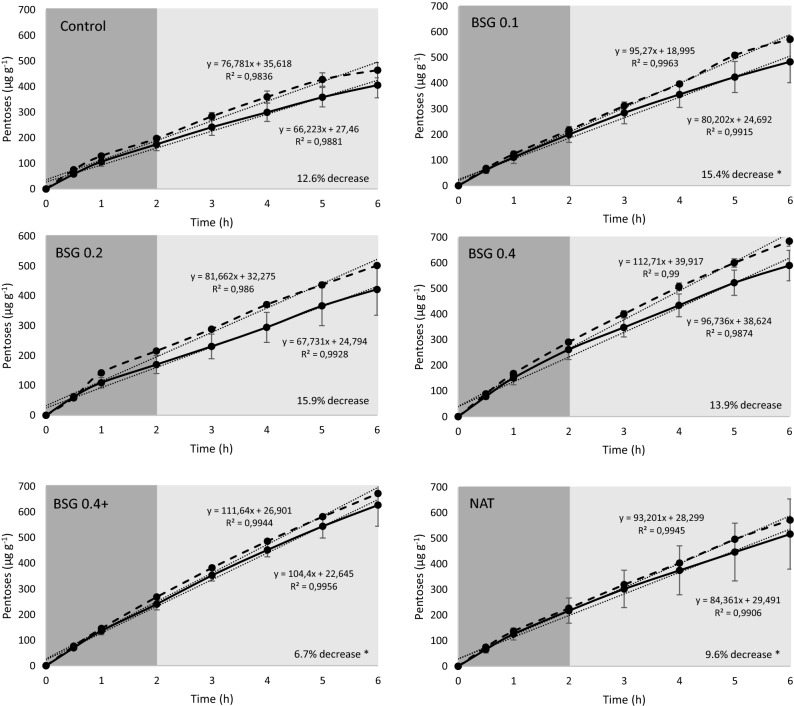
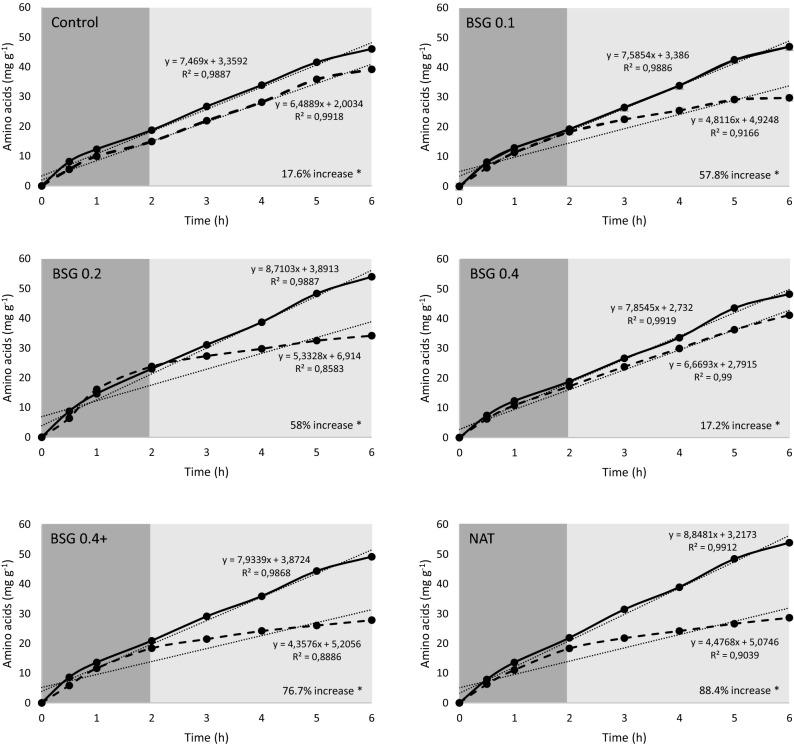
In Assay 3, designed to evaluate the effect of dietary inclusion of SSF-BSG extract or Natugrain on the hydrolysis of carbohydrates and protein in all experimental diets, it was observed (i) a positive effect of the supplementation with SSF-BSG extract on the release of pentoses from the control diet; (ii) a linear increase of pentoses release with the increase of dietary SSF-BSG extract supplementation level (R = 0.75; P < 0.05); (iii) no additional improvement on carbohydrates or protein hydrolysis when the diet containing 0.4% of SSF-BSG extract was combined with Silica+ and a plant-based detoxifier Ena-Detox; (iv) pentoses or amino acids release of Natugrain supplemented diet was similar to that measured in the control and SSF-BSG extract supplemented diets; (v) an interactive effect of active fish enzymes and SSF-BSG extract, decreasing carbohydrates hydrolysis by 7% (BSG0.4+ diet) and 16% (BSG0.2 diet); vi) an interactive effect of active fish enzymes and diets, increasing protein hydrolysis by 18% (control and BSG0.4% diets), 58% (BSG0.1 and BSG0.2 diets), 77% (BSG0.4+ diet), and 88% (NAT diets) (Tables 5 and 6; Figs. 4 and 5).
Table 5.
Pentoses release (µg g−1 diet h−1) during the gastric, intestinal, and total in vitro digestion.
| Diet | Fish enzymes | Acid digestion | Alkaline digestion | Total digestion | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Active | 174.3 ± 17.5 | 231.5 ± 18.0 | 405.8 ± 35.2 | |||||
| Inactive | 197.2 ± 6.3 | 267.0 ± 15.9 | 464.2 ± 21.2 | ||||||
| BSG0.1 | Active | 200.1 ± 22.0 | 284.1 ± 36.8 | 484.1 ± 58.1 | |||||
| Inactive | 217.1 ± 4.3 | 355.2 ± 5.8 | 572.3 ± 9.2 | ||||||
| BSG0.2 | Active | 170.0 ± 21.5 | 251.8 ± 41.7 | 421.8 ± 61.4 | |||||
| Inactive | 215.5 ± 0.8 | 286.2 ± 3.6 | 501.6 ± 3.8 | ||||||
| BSG0.4 | Active | 262.0 ± 27.5 | 328.3 ± 15.1 | 590.3 ± 42.0 | |||||
| Inactive | 291.2 ± 6.5 | 394.3 ± 7.6 | 685.5 ± 13.8 | ||||||
| BSG0.4+ | Active | 240.8 ± 16.0 | 386.8 ± 42.6 | 627.6 ± 58.6 | |||||
| Inactive | 268.7 ± 3.0 | 404.3 ± 3.8 | 673.0 ± 6.5 | ||||||
| NAT | Active | 217.4 ± 34.8 | 299.3 ± 62.4 | 516.7 ± 97.0 | |||||
| Inactive | 227.1 ± 1.4 | 344.4 ± 3.8 | 571.5 ± 3.8 |
| Two-way ANOVA | Variance source | Diets | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Diet | Fish enzymes | Interaction | Control | BSG0.1 | BSG0.2 | BSG0.4 | BSG0.4+ | NAT | |
| Acid | *** | * | n.s. | c | bc | c | a | ab | abc |
| Alkaline | *** | * | n.s. | c | abc | bc | ab | a | abc |
| Total | *** | * | n.s. | b | ab | b | a | a | ab |
Values presented as mean (n = 3) and standard deviation (SD).
Two-way ANOVA testing for the effect of the diet (control, BSG0.1, BSG0.2, BSG0.4, BSG0.4 + ; NAT) and fish enzymes (active and inactive): *p < 0.05; ***p < 0.001; n.s.: not significant.
Table 6.
Amino acids release (mg g−1 diet h−1) during the gastric, intestinal, and total in vitro digestion.
| Diet | Fish enzymes | Acid digestion | Alkaline digestion | Total digestion | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Active | 18.7 ± 0.06 | 27.3 ± 0.29 c | 46.1 ± 0.36c | |||||
| Inactive | 14.9 ± 0.09 | 24.3 ± 0.13 A | 39.2 ± 0.05AB | ||||||
| BSG0.1 | Active | 19.2 ± 0.16 | 27.7 ± 0.31 c | 47.0 ± 0.15bc | |||||
| Inactive | 18.3 ± 0.21 | 11.4 ± 0.13B | 29.7 ± 0.21BC | ||||||
| BSG0.2 | Active | 23.0 ± 0.02 | 31.0 ± 0.22ab | 54.0 ± 0.20a | |||||
| Inactive | 23.8 ± 3.5 | 10.4 ± 0.45B | 34.2 ± 3.00ABC | ||||||
| BSG0.4 | Active | 18.9 ± 0.01 | 29.4 ± 0.07bc | 48.3 ± 0.06bc | |||||
| Inactive | 17.3 ± 0.17 | 23.9 ± 0.37A | 41.2 ± 0.20A | ||||||
| BSG0.4+ | Active | 20.9 ± 0.3 | 28.3 ± 0.21c | 49.2 ± 0.53b | |||||
| Inactive | 18.4 ± 0.51 | 9.4 ± 0.06B | 27.8 ± 0.58C | ||||||
| NAT | Active | 21.9 ± 0.04 | 32.0 ± 0.36a | 53.9 ± 0.32a | |||||
| Inactive | 18.3 ± 0.12 | 10.3 ± 0.26B | 28.6 ± 0.13C |
| Two-way ANOVA | Variance source | Diets | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Diet | Fish enzymes | Interaction | Control | BSG0.1 | BSG0.2 | BSG0.4 | BSG0.4+ | NAT | |
| Acid | *** | * | n.s. | b | ab | b | ab | ab | ab |
| Alkaline | *** | * | ** | ||||||
| Total | ** | *** | *** | ||||||
Values presented as mean (n = 3) and standard deviation (SD).
Two-way ANOVA testing for the effect of the diets (control, BSG0.1, BSG0.2, BSG0.4, BSG0.4 + ; NAT) and fish enzymes (active and inactive): *p < 0.05; **p < 0.01; ***p < 0.001; n.s.: not significant. If the interaction was significant, one-way ANOVA was performed testing the effect of the diets during digestion with active (lower letters) or inactive fish enzymes (upper letters).
Figure 4.
Pentoses release (mg g−1 of diet) from the different experimental diets under the simulated conditions of the European seabass digestive tract with active (continuous line) and inactive fish enzymes (dashed line). The slope of linear fitting provides the rate of release of pentoses per hour (mg g−1 of diet h−1). Stomach and intestinal digestions are represented in dark and light grey, respectively. Values expressed as mean ± standard deviation. Asterisks indicate significant differences between active and inactive fish enzymes (p < 0.05).
Figure 5.
Amino acids release (mg g−1 of diet) from the different experimental diets under the simulated conditions of the European seabass digestive tract with active (continuous line) and inactive fish enzymes (dashed line). The slope of linear fitting provides the rate of release of amino acids per hour (mg g−1 of diet h−1). Stomach and intestinal digestions are represented in dark and light grey, respectively. Values expressed as mean ± standard deviation. Asterisks indicate significant differences between active and inactive fish enzymes (p < 0.05).
In vivo digestibility assay
The digestibility of dry matter, starch, cellulose, and glucans was significantly higher with the BSG0.4 diet than with the BSG0.1 or control diets. Energy digestibility was higher with the BSG0.4 diet than the control diet, but it was not significantly different from the BSG0.1 diet. The digestibility of hemicellulose, xylan, and arabinan tended to be higher with the BSG0.4 diet than in the other dietary treatments, though differences were not statistically significant (Table 7).
Table 7.
Apparent digestibility coefficients (ADC) of dietary nutrients and energy in European seabass juveniles fed with the experimental diets.
| Diets | Control | BSG 0.1 | BSG 0.4 |
|---|---|---|---|
| Dry matter | 67.7 ± 0.04b | 68.0 ± 1.61b | 72.8 ± 2.08a |
| Protein | 96.2 ± 0.26 | 96.4 ± 0.43 | 96.4 ± 0.46 |
| Starch | 75.8 ± 3.17b | 80.1 ± 0.31b | 82.4 ± 1.88a |
| Energy | 78.3 ± 1.69b | 79.9 ± 1.28ab | 83.4 ± 1.12a |
| Hemicellulose | 49.9 ± 2.09 | 49.7 ± 6.70 | 58.0 ± 2.04 |
| Cellulose | 16.4 ± 1.14b | 14.7 ± 2.85b | 25.5 ± 4.79a |
| Lignin | 0.0 | 0.0 | 0.0 |
| Glucan | 16.4 ± 1.14b | 14.7 ± 2.85b | 25.5 ± 4.79a |
| Xylan | 46.1 ± 3.49 | 45.4 ± 6.50 | 56.0 ± 4.50 |
| Arabinan | 56.3 ± 0.20 | 57.1 ± 8.71 | 63.1 ± 1.03 |
Values presented as mean (n = 3) and standard deviation (SD).
Values in the same row with different subscripts are significantly different (p < 0.05).
Discussion
Enzyme production by SSF of BSG
BSG is an underutilized agro-industrial by-product with the potential to replace high carbon footprint conventional feedstocks, given its high production rate, acquisition price, and supply chain42. However, for the moment, no “established market function” for BSG exist, and its high moisture content and low sugars content hinder the development of economic models for its valorisation43. Considering costs associated with BSG disposal, new and promising utilizations for this by-product must be envisioned to meet circular economy guidelines and ensure sustainability and food security. New and alternative applications for BSG are increasing, as construction materials, alternative fuels, textile applications, human food, and animal feeds44.
The reutilization of BSG towards the obtention of value-added compounds following eco-friendly and circular economy approaches, such as SSF, is of utmost importance. BSG is a lignocellulosic by-product with 24% of hemicellulose and 21% of cellulose, with a high potential to produce carbohydrases by SSF, an under-explored process. In the present study, the biotechnological treatment of BSG through SSF with A. ibericus resulted in high xylanase and cellulase production (15,885 and 1343 U g−1, respectively). Previously, SSF of BSG with Penicillium janczewskii produced 371 U xylanase g−1 BSG23, a lower value than obtained in the present study. These results confirm the potential of agro-industrial by-products to produce added-value compounds, such as enzymes, through SSF processes45–47.
The production of enzymes through SSF processes presents several advantages compared to SmF, including higher enzymatic productivity and stability within a broader range of conditions, and lower susceptibility to inactivation by the substrate15. The present study confirmed that the enzymes of SSF-BSG extract remained functional throughout the acid and alkaline digestion. Indeed, even though endogenous fish enzymes may negatively interact with the SSF-BSG extract activity, the in vitro carbohydrates bioavailability increased fourfold when comparing an active versus inactive SSF-BSG extract supplemented diet (BSG0.4 diet). Accordingly, Vasconcellos et al. compared the production of endoglucanase and β-glucosidase through SSF or SmF and concluded that SSF produced enzymes less susceptible to inactivation by the substrate's phenolic compounds and that were highly thermostable, maintaining 80% to 100% of activity after incubation at 50 °C for 24 h48.
In vitro and in vivo assays
Experimental approaches based on in vitro models simulating biological functions are increasingly encouraged to reduce the number of animals used for experimental purposes27,49 and are in line with the eco-friendly approach of the present study. In vitro assays simulating the European seabass gut biochemical conditions and previously optimized for this species27,28, allowed a detailed assessment of the effect of SSF-BSG extract and Natugrain on nutrients bioavailability, the interactive effects of SSF-BSG extract and Natugrain with fish enzymes during digestion, and the fine-tune selection of the most potential experimental diets to be further evaluated during an in vivo digestibility trial.
Phytate P is present in a wide range of plant feedstuffs but is unavailable for most fish species as they lack intestinal phytases, impairing mineral utilization, growth, and feed utilization. Phytate P can bind with minerals, such as calcium, zinc, sodium, and others, decreasing its solubility and, concomitantly, minerals bioavailability50. Dietary supplementation with phytase has been used as a nutritional strategy to increase phosphorus bioavailability for fish and other animals50. Even though A. ibericus used in the present study was selected based on its high capacity to produce carbohydrases, phytases were also produced during SSF of BSG. In vitro assay 1 confirms that supplementation of a plant-based diet with 0.4% of SSF-BSG extract significantly increased total P release but not that of phytate P. This result may be interpreted as an increase in the potential bioavailability of this element.
In vitro digestibility assay 2 confirms the potential of SSF-BSG extract to hydrolyze dietary carbohydrates. Dietary inclusion of 0.4% SSF-BSG extract (BSG0.4 diet) increased more than fourfold the pentoses release during in vitro digestion compared to the same diet in which the enzymatic activity was previously inactivated by heat. Moreover, combining active or inactive BSG0.4 diet with inactive fish enzymes during in vitro digestion did not affect pentoses release, corroborating the main role of SSF-BSG extract on carbohydrates hydrolysis. However, combining active BSG0.4 diet with active fish enzymes during in vitro acid or alkaline digestion decreased pentoses release by about 10% to 16%, respectively, suggesting a negative interaction between endogenous fish enzymes and SSF-BSG extract carbohydrases. The efficacy of feed enzymes is affected by different factors, including pH specificity and gastrointestinal inhibitors, limiting the potential benefits of dietary supplementation with exogenous enzymes4,50. For example, Morales et al. observed that both activity and stability of a fungal phytase (from Peniophora lycii) were significantly compromised when evaluating the in vitro acid digestion of rainbow trout (Oncorhynchus mykiss)51.
Moreover, in assay 2, the in vitro digestion of inactive BSG0.4 diet combined with inactive endogenous fish enzymes also resulted in pentoses release. Pentoses release without the presence of active carbohydrases indicates that some non-starch glucosidases may be present in plant feedstuffs and retained some activity after pelleting and digestion processes. In fact, cellulases have been found in various plants, being involved in the defense mechanisms, lignification process, cell-wall development, and symbiotic activities of the plant52,53.
The in vitro digestibility assay 3 confirms a dose–response effect of SSF-BSG extract on carbohydrates hydrolysis, as pentoses release linearly increased with dietary SSF-BSG extract inclusion level (R = 0.75; P < 0.05, including total digestion data with active or inactive fish enzymes). Moreover, dietary supplementation with 0.4% SSF-BSG extract combined with the commercial catalyzer, Silica+, and a plant-based detoxifier, Ena-Detox, promoted similar carbohydrates or protein hydrolysis than to the diet supplemented with 0.4% SSF-BSG extract alone. Contrarily to the present results, silicon dioxide has been utilized in poultry and pigs diets with promising results on growth performance and feed utilization54,55.
Additionally, the in vitro digestibility assay 3 confirmed that SSF-BSG extract enzymes exhibited carbohydrates and protein hydrolytic activity at both acid and alkaline pH (5 and 8.5, respectively), being more efficient during the alkaline than the acid stage. Although optimum enzyme activity occurs under certain pH and temperature conditions8, the higher carbohydrates hydrolysis observed during the alkaline digestion is not aligned to the optimum pH for maximum activity of carbohydrases produced in SSF by filamentous fungi. In general, cellulases produced by filamentous fungi have maximum activities at acidic pH (3.6–5)56. For example, xylanases produced by A. fumigatus showed higher activity at pH between 3 and 657, and xylanases and β-glucosidases produced through the SSF of Nopalea cochenillifera with A. niger and Rhizopus sp. presented optimum activities at pH between 4 and 758. Besides the pH and temperature effect, enzymes activity may also be affected by enzymes accessibility to the substrate. In the present study, the higher pentoses and amino acids release observed in the alkaline stage may have been triggered by the previous stomach digestion, which facilitated protein and carbohydrate hydrolysis during intestinal digestion by increasing substrate accessibility to the enzymes. Similarly, other authors also observed that the acid pre-digestion of fishmeal and plant feedstuffs increased intestinal protein hydrolysis27,59, reflecting the significant contribution of alkaline digestion in the total protein hydrolysis60.
In vitro trials are well suited to evaluate differences in the bioaccessibility of nutrients and the effect of diverse factors that may influence their potential bioavailability upon intestinal absorption. For this reason, in vitro trials provide a pre-absorption estimation of nutrient bioavailability, while in vivo digestibility measures are considered a post-absorption estimation of nutrients bioavailability, being affected by other factors such as microbial biomass or products of their metabolism present in fecal mass28. This could explain why not always both types of determinations present a good correlation. Nevertheless, the results of the in vivo digestibility trial of the present study corroborated to a great extent the results obtained in vitro. In both trials, dietary SSF-BSG extract supplementation did not significantly affect protein hydrolysis, whereas bioavailability of carbohydrates increased with 0.4% SSF-BSG extract incorporation. Dietary supplementation with 0.4% of SSF-BSG extract increased the pentoses release by circa 45% in the in vitro digestibility trial. Accordingly, in the in vivo digestibility trial, 0.4% SSF-BSG extract supplementation increased cellulose and hemicellulose digestibility by circa 52% and 16%, respectively, while xylan, glucan, and arabinan digestibility increased 20%, 16%, and 11%, respectively. For carnivorous fish, as European seabass, the digestibility of NSPs was never evaluated. Nevertheless, the digestion of some NSPs fractions has been reported for some non-carnivorous fish species. For example, in Nile tilapia (Oreochromis niloticus), depending on dietary fiber composition, NSPs digestibility averaged 24%61, ranging between 23 and 73%62,63, with soluble NSPs digestibility being 60% and insoluble NSPs undigested64. The beneficial effect of dietary supplementation with SSF-BSG extract on carbohydrates bioavailability of European seabass is probably not only related to NSPs digestion. The effect of SSF-BSG extract carbohydrases on the digesta physical properties, decreasing its viscosity, releasing entrapped nutrients by facilitating the fish enzymes action4,7, and promoting microbiota fermentation processes3,61,65 may also have contributed to the present results. Fish microbiota plays a significant role in digestion and metabolism66 and is modulated by dietary supplementation with exoenzymes, supporting the development of a more favorable microbiota community for nutrient hydrolysis6,67.
Present results confirmed that dietary incorporation of SSF-BSG extract is a promising nutritional tool to increase nutrient digestibility by hydrolyzing NSPs. The use of carbohydrases in fish feeds was revised by Castillo and Gatlin and Zheng et al. with promising results4,8. For example, in European seabass, dietary supplementation with a commercial carbohydrases complex increased dry matter, protein, lipids, energy, and phosphorus digestibilities, decreased chyme pH along the intestine but did not affect digestive enzymes activity68. In white seabream (Diplodus sargus), the same carbohydrases complex increased feed, nitrogen, and energy utilization efficiency as well as intestinal amylase and lipase activities5. This carbohydrases complex also increased dry matter and some amino acids digestibility, intestinal lipase, and protease activity, and modulated intestinal microbiota in turbot (Scophthalmus maximus)6. Dietary supplementation with cellulase in a Chlorella-based diet increased dry matter, energy, and protein digestibility, digestive enzymes activity, and growth performance of crucian carp (Carassius auratus)69. In juvenile yellow croaker (Larimichthys crocea), dietary supplementation with xylanase improved zootechnical performance, intestinal morphology structure, and microbiota constitution70. An enzymatic-rich extract produced from SSF of agro-industrial by-products with Aspergillus niger and A. oryzae increased protein, mineral, energy, and lipids digestibility of Nile tilapia71.
Conclusions
SSF of BSG with Aspergillus ibericus allowed the production of highly active enzymatic enriched extract. The in vitro assays confirmed that the inclusion of 0.4% SSF-BSG extract in a plant-based diet for European seabass increases phosphorus and carbohydrates hydrolysis, being more effective during the alkaline than the acid stage of digestion. Fish endogenous enzymes reduce the efficacy of SSF-BSG extract by about 7% to 16%. The in vivo digestibility assay results corroborated the data obtained in in vitro assays. Dietary supplementation with 0.4% SSF-BSG extract increased digestibility of dry matter, energy, starch, cellulose, and glucans. This study also highlighted the partial digestibility of NSPs in plant-based diets for carnivorous fish, requiring further research.
Overall, the results showed the potential of SSF of BSG to produce an added-value enzymatic supplement that increases the bioavailability of plant ingredients. Additionally, the use of BSG, an underutilized agro-industrial by-product, as substrate for filamentous fungi enzymes production, represents a synergy between economic and environmental efforts under the circular economy guidelines. Further research is needed to develop technological strategies to incorporate the SSF-BSG extract into aquafeeds, including coating, to reduce the negative interaction with fish enzymes, and assess the economic and environmental impact of SSF-BSG extract utilization.
Acknowledgements
The authors thank UNICER, a soft-drink company (Porto, Portugal), for providing the brewer’s spent grain used in this study. This study was supported by the project “SPO3-Development of innovative sustainable protein and omega-3 rich feedstuffs for aquafeeds, from local agro-industrial by-products”, reference POCI-01-0145-FEDER-030377, funded by European Regional Development Fund (ERDF) and Portuguese Foundation for Science and Technology (FCT) and by strategic funding of UIDB/04423/2020 by UIDB/04469/2020 unit, the BioTecNorte operation (NORTE-01-0145-FEDER-000004) through national funds provided by FCT, and under the scope of the strategic funding of UIDB/04469/2020 unit. The H.F. and C.C were supported by grants SFRH/BD/131219/2017 and SFRH/BPD/114942/2016, respectively, from FCT, MCTES, FSE, and UE under the North Portugal Regional Operational Programme (NORTE2020). José Manuel Salgado was supported by Beatriz Galindo contract of Ministry of Education (Spain).
Abbreviations
- BSG
Brewer’s spent grain
- SSF
Solid-state fermentation
- NAT
Natugrain
- NSPs
Non-starch polysaccharides
- ADC
Apparent digestibility coefficient
Author contributions
H.F. carried out the in vitro assays, data analysis, and writing of the manuscript. F.M. participated in research conceptualization, conceived in vitro assays, data analyses, and writing. F.M. and M.A. contributed to in vitro assays work and analysis; C.C. and N.F. carried out the in vivo digestibility trial and chemical analysis. P.F and M.G. carried out chemical analysis. J.M.S. and I.B. conceived and developed the solid-state fermentation optimization and obtention of the SSF-BSG extract. A.T. participated in research conceptualization and writing. H.P. participated in the funding acquisition, research conceptualization, supervision, and writing. All authors discussed the results, contributed to the final manuscript, and reviewed the manuscript.
Data availability
The datasets obtained and analyzed in the present study are not available for public consultation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Teles, A. O., Couto, A., Enes, P. & Peres, H. Dietary protein requirements of fish—A meta-analysis. Rev. Aquac. 12, 1–33 (2019).
- 2.Habte-Tsion HM, Kumar V. Enzymes in Human and Animal Nutrition. Andre Gerhard Wolff; 2017. Non-starch polysaccharide enzymes—general aspects; pp. 183–209. [Google Scholar]
- 3.Sinha AK, Kumar V, Makkar HPS, De Boeck G, Becker K. Non-starch polysaccharides and their role in fish nutrition—A review. Food Chem. 2011;127:1409–1426. [Google Scholar]
- 4.Castillo S, Gatlin DM. Dietary supplementation of exogenous carbohydrase enzymes in fish nutrition: A review. Aquaculture. 2015;435:286–292. [Google Scholar]
- 5.Magalhães R, et al. Carbohydrases supplementation increased nutrient utilization in white seabream (Diplodus sargus) juveniles fed high soybean meal diets. Aquaculture. 2016;463:43–50. [Google Scholar]
- 6.Diógenes AF, et al. Exogenous enzymes supplementation enhances diet digestibility and digestive function and affects intestinal microbiota of turbot (Scophthalmus maximus) juveniles fed distillers’ dried grains with solubles (DDGS) based diets. Aquaculture. 2018;486:42–50. [Google Scholar]
- 7.Adeola O, Cowieson AJ. Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011;89:3189–3218. doi: 10.2527/jas.2010-3715. [DOI] [PubMed] [Google Scholar]
- 8.Zheng, C. cai et al. Exogenous enzymes as functional additives in finfish aquaculture. Aquac. Nutr. 26, 1–12 (2019).
- 9.Steudler S, Werner A, Walther T. It is the mix that matters: Substrate-specific enzyme production from filamentous fungi and bacteria through solid-state fermentation. Adv. Biochem. Eng. Biotechnol. 2019;169:51–82. doi: 10.1007/10_2019_85. [DOI] [PubMed] [Google Scholar]
- 10.Dadalt JC, Velayudhan DE, Neto MT, Slominski BA, Nyachoti CM. Ileal amino acid digestibility in high protein sunflower meal and pea protein isolate fed to growing pigs with or without multi-carbohydrase supplementation. Anim. Feed Sci. Technol. 2016;221:62–69. [Google Scholar]
- 11.Bedford MR. Exogenous enzymes in monogastric nutrition - Their current value and future benefits. Anim. Feed Sci. Technol. 2000;86:1–13. [Google Scholar]
- 12.Leite P, et al. Recent advances in production of lignocellulolytic enzymes by solid-state fermentation of agro-industrial wastes. Curr. Opin. Green Sustain. Chem. 2021;27:100407. [Google Scholar]
- 13.Mussatto S, Teixeira J. Lignocellulose as raw material in fermentation processes. Appl. Microbiol. Microb. Biotechnol. 2010;2:897–907. [Google Scholar]
- 14.Singhania RR, et al. Industrial Enzymes Industrial. Biorefineries and White Biotechnology. Elsevier B.V.; 2015. [Google Scholar]
- 15.Soccol CR, et al. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017;1:52–71. [Google Scholar]
- 16.Lizardi-Jiménez MA, Hernández-Martínez R. Solid state fermentation (SSF): Diversity of applications to valorize waste and biomass. 3 Biotech. 2017;7:44. doi: 10.1007/s13205-017-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen GH, Lübeck M, Frisvad JC, Lübeck PS, Andersen B. Production of cellulolytic enzymes from ascomycetes: Comparison of solid state and submerged fermentation. Process Biochem. 2015;50:1327–1341. [Google Scholar]
- 18.Lynch KM, Steffen EJ, Arendt EK. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016;122:553–568. [Google Scholar]
- 19.Mussatto SI. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014;94:1264–1275. doi: 10.1002/jsfa.6486. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Kanwar S. Lipase production in solid-state fermentation (SSF): Recent developments and biotechnological applications. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2012;6:13–27. [Google Scholar]
- 21.Tišma M, Jurić A, Bucić-Kojić A, Panjičko M, Planinić M. Biovalorization of brewers’ spent grain for the production of laccase and polyphenols. J. Inst. Brew. 2018;124:182–186. [Google Scholar]
- 22.Panagiotou G, Granouillet P, Olsson L. Production and partial characterization of arabinoxylan-degrading enzymes by Penicillium brasilianum under solid-state fermentation. Appl. Microbiol. Biotechnol. 2006;72:1117–1124. doi: 10.1007/s00253-006-0394-6. [DOI] [PubMed] [Google Scholar]
- 23.Terrasan CRF, Carmona EC. Solid-state fermentation of brewer’s spent grain for xylanolytic enzymes production by Penicillium janczewskii and analyses of the fermented substrate|Fermentação em estado sólido com bagaço de cevada para produção de enzimas xilanolíticas por penicilli. Biosci. J. 2015;31:1826–1836. [Google Scholar]
- 24.Paz A, Outeiriño D, Pérez Guerra N, Domínguez JM. Enzymatic hydrolysis of brewer’s spent grain to obtain fermentable sugars. Bioresour. Technol. 2019;275:402–409. doi: 10.1016/j.biortech.2018.12.082. [DOI] [PubMed] [Google Scholar]
- 25.Sousa D, Venâncio A, Belo I, Salgado JM. Mediterranean agro-industrial wastes as valuable substrates for lignocellulolytic enzymes and protein production by solid-state fermentation. Sci. Food Agric. 2018;98:5248–5256. doi: 10.1002/jsfa.9063. [DOI] [PubMed] [Google Scholar]
- 26.Xiros C, Topakas E, Katapodis P, Christakopoulos P. Evaluation of Fusarium oxysporum as an enzyme factory for the hydrolysis of brewer’s spent grain with improved biodegradability for ethanol production. Ind. Crops Prod. 2008;28:213–224. [Google Scholar]
- 27.Morales GA, Moyano FJ. Application of an in vitro gastrointestinal model to evaluate nitrogen and phosphorus bioaccessibility and bioavailability in fish feed ingredients. Aquaculture. 2010;306:244–251. [Google Scholar]
- 28.Moyano FJ, Saénz de Rodrigáñez MA, Díaz M, Tacon AGJ. Application of in vitro digestibility methods in aquaculture: Constraints and perspectives. Rev. Aquac. 2015;7:223–242. [Google Scholar]
- 29.du Sert NP, et al. The arrive guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:1–12. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharp RJA, Ewald JA, Kenward RE. FELASA guidelines and recommendations. J. Am. Assoc. Lab. Anim. Sci. 2012;51:246–257. [PMC free article] [PubMed] [Google Scholar]
- 31.Salgado JM, Abrunhosa L, Venâncio A, Domínguez JM, Belo I. Integrated use of residues from olive mill and winery for lipase production by solid state fermentation with Aspergillus sp. Appl. Biochem. Biotechnol. 2014;172:1832–1845. doi: 10.1007/s12010-013-0613-4. [DOI] [PubMed] [Google Scholar]
- 32.Nikolopoulou D, et al. Patterns of gastric evacuation, digesta characteristics and pH changes along the gastrointestinal tract of gilthead sea bream (Sparus aurata L.) and European sea bass (Dicentrarchus labrax L.) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011;158:406–414. doi: 10.1016/j.cbpa.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Cho CY, Slinger SJ, Bayley HS. Bioenergetics of salmonid fishes: Energy intake, expenditure and productivity. Comp. Biochem. Physiol. Part B Biochem. 1982;73:25–41. [Google Scholar]
- 34.Anson ML. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J. Gen. Physiol. 1938;22:79–89. doi: 10.1085/jgp.22.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter HE. Proteinases: methods with haemoglobin, casein and azocoll as substrates. In: Bergmeyer HU, Bermeyer J, editors. Methods of Enzymatic Analysis. Weinham; 1984. pp. 270–277. [Google Scholar]
- 36.Fernandes H, et al. Sequential bioprocessing of Ulva rigida to produce lignocellulolytic enzymes and to improve its nutritional value as aquaculture feed. Bioresour. Technol. 2019;281:277–285. doi: 10.1016/j.biortech.2019.02.068. [DOI] [PubMed] [Google Scholar]
- 37.Shivanna GB, Venkateswaran G. Dephytinization of cereals and pulses by phytase producing lactic acid bacteria. Int. J. Curr. Res. Acad. Rev. 2015;3:61–69. [Google Scholar]
- 38.Charney J, Tomarelli R. A colorimetric method for the determination of the proteolytic activity of duodenal juice. J. Biol. Chem. 1947;171:501–505. [PubMed] [Google Scholar]
- 39.Gomes N, Gonçalves C, García-Román M, Teixeira JA, Belo I. Optimization of a colorimetric assay for yeast lipase activity in complex systems. Anal. Methods. 2011;3:1008–1013. [Google Scholar]
- 40.Haug W, Lantzsch H-J. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 1983;34:1423–1426. [Google Scholar]
- 41.Douglas SG. A rapid method for the determination of pentosans in wheat flour. Food Chem. 1981;7:139–145. [Google Scholar]
- 42.Church FC, Swaisgood HE, Porter DH, Catignani GL. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983;66:1219–1227. [Google Scholar]
- 43.Buffington J. The economic potential of brewer’s spent grain (BSG) as a biomass feedstock. Adv. Chem. Eng. Sci. 2014;04:308–318. [Google Scholar]
- 44.Jackowski M, Niedzwiecki L, Jagiello K, Uchanska O, Trusek A. Brewer’s spent grains—Valuable beer industry by-product. Biomolecules. 2020;10:1–18. doi: 10.3390/biom10121669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nahid P, et al. Production of glucoamylase by Aspergillus niger under solid state fermentation. Int. J. Eng. Trans. B Appl. 2012;25:1–7. [Google Scholar]
- 46.Roy S, Dutta T, Sarkar TS, Ghosh S. Novel xylanases from Simplicillium obclavatum MTCC 9604: Comparative analysis of production, purification and characterization of enzyme from submerged and solid state fermentation. Springerplus. 2013;2:1–10. doi: 10.1186/2193-1801-2-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spalvins K, Zihare L, Blumberga D. Single cell protein production from waste biomass: Comparison of various industrial by-products. Energy Procedia. 2018;147:409–418. [Google Scholar]
- 48.Vasconcellos VM, Tardioli PW, Giordano RLC, Farinas CS. Production efficiency versus thermostability of (hemi)cellulolytic enzymatic cocktails from different cultivation systems. Process Biochem. 2015;50:1701–1709. [Google Scholar]
- 49.Rungruangsak-Torrissen K, et al. In vitro digestibility based on fish crude enzyme extract for prediction of feed quality in growth trials. J. Sci. Food Agric. 2002;82:644–654. [Google Scholar]
- 50.Morales, G. A., Marquez, L., Hérnández, A. J. & Moyano, F. J. Chapter 9—Phytase effects on protein and phosphorous bioavailability in fish diets. In Phytate Destruction—Consequences for Precision Animal Nutrition 129–165 (2016).
- 51.Morales GA, Moyano FJ, Marquez L. In vitro assessment of the effects of phytate and phytase on nitrogen and phosphorus bioaccessibility within fish digestive tract. Anim. Feed Sci. Technol. 2011;170:209–221. [Google Scholar]
- 52.Cairns JRK, Esen A. β-Glucosidases. Cell. Mol. Life Sci. 2010;67:3389–3405. doi: 10.1007/s00018-010-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Behera SS, Ray RC. Solid state fermentation for production of microbial cellulases: Recent advances and improvement strategies. Int. J. Biol. Macromol. 2016;86:656–669. doi: 10.1016/j.ijbiomac.2015.10.090. [DOI] [PubMed] [Google Scholar]
- 54.Tran ST, Bowman ME, Smith T. Effects of a silica-based supplement on performance, health, and litter quality of growing turkeys. Poult. Sci. 2015;94:1902–1908. doi: 10.3382/ps/pev158. [DOI] [PubMed] [Google Scholar]
- 55.Martel-Kennes Y, Lévesque J, Decaux C. Effect of crystalline silicon dioxide in piglet feed on growth performance with different levels of growth promoters. J. Anim. Sci. 2016;94:479. [Google Scholar]
- 56.Astolfi V, et al. Cellulolytic enzyme production from agricultural residues for biofuel purpose on circular economy approach. Bioprocess Biosyst. Eng. 2019;42:677–685. doi: 10.1007/s00449-019-02072-2. [DOI] [PubMed] [Google Scholar]
- 57.da Silva Delabona P, et al. Effect of initial moisture content on two Amazon rainforest Aspergillus strains cultivated on agro-industrial residues: Biomass-degrading enzymes production and characterization. Ind. Crops Prod. 2013;42:236–242. [Google Scholar]
- 58.dos Santos TC, et al. Production, optimisation and partial characterisation of enzymes from filamentous fungi using dried forage cactus pear as substrate. Waste Biomass Valorization. 2018;9:571–579. [Google Scholar]
- 59.Yasumaru F, Lemos D. Species specific in vitro protein digestion (pH-stat) for fish: Method development and application for juvenile rainbow trout (Oncorhynchus mykiss), cobia (Rachycentron canadum), and Nile tilapia (Oreochromis niloticus) Aquaculture. 2014;426–427:74–84. [Google Scholar]
- 60.Gilannejad N, Martínez-Rodríguez G, Yúfera M, Moyano FJ. Modelling digestive hydrolysis of nutrients in fish using factorial designs and desirability function. PLoS ONE. 2018;13:1–19. doi: 10.1371/journal.pone.0206556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maas RM, Verdegem MCJ, Wiegertjes GF, Schrama JW. Carbohydrate utilisation by tilapia: A meta-analytical approach. Rev. Aquac. 2020;12:1851–1866. [Google Scholar]
- 62.Haidar MN, Petie M, Heinsbroek LTN, Verreth JAJ, Schrama JW. The effect of type of carbohydrate (starch vs. nonstarch polysaccharides) on nutrients digestibility, energy retention and maintenance requirements in Nile tilapia. Aquaculture. 2016;463:241–247. [Google Scholar]
- 63.Maas RM, Verdegem MCJ, Schrama JW. Effect of non-starch polysaccharide composition and enzyme supplementation on growth performance and nutrient digestibility in Nile tilapia (Oreochromis niloticus) Aquac. Nutr. 2019;25:622–632. [Google Scholar]
- 64.Leenhouwers JI, Ortega RC, Verreth JAJ, Schrama JW. Digesta characteristics in relation to nutrient digestibility and mineral absorption in Nile tilapia (Oreochromis niloticus L.) fed cereal grains of increasing viscosity. Aquaculture. 2007;273:556–565. [Google Scholar]
- 65.Ghosh, K. & Ray, A. K. Aquafeed Formulation Using Plant Feedstuffs: Prospective Application of Fish-Gut Microorganisms and Microbial Biotechnology. Soft Chemistry and Food Fermentation (2017).
- 66.Ganguly S, Prasad A. Microflora in fish digestive tract plays significant role in digestion and metabolism. Rev. Fish Biol. Fish. 2012;22:11–16. [Google Scholar]
- 67.Ringø E, et al. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquac. Nutr. 2016;22:219–282. [Google Scholar]
- 68.Magalhães R, et al. Improved digestibility of plant ingredient-based diets for European seabass (Dicentrarchus labrax) with exogenous enzyme supplementation. Aquac. Nutr. 2018;24:1287–1295. [Google Scholar]
- 69.Shi X, et al. Effects of dietary cellulase addition on growth performance, nutrient digestibility and digestive enzyme activities of juvenile crucian carp Carassius auratus. Aquac. Nutr. 2017;23:618–628. [Google Scholar]
- 70.Luo J, et al. Effects of dietary exogenous xylanase supplementation on growth performance, intestinal health, and carbohydrate metabolism of juvenile large yellow croaker, Larimichthys crocea. Fish Physiol. Biochem. 2020;46:1093–1110. doi: 10.1007/s10695-020-00774-z. [DOI] [PubMed] [Google Scholar]
- 71.Novelli PK, et al. Enzymes produced by agro-industrial co-products enhance digestible values for Nile tilapia (Oreochromis niloticus): A significant animal feeding alternative. Aquaculture. 2017;481:1–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets obtained and analyzed in the present study are not available for public consultation.