Abstract
Purpose
Limited studies have been published on the association between the urinary biomarkers of Polycyclic Aromatic Hydrocarbons (PAHs) and risk of Metabolic Syndromes (MetS) and blood cell levels in adults in the Middle East. The present study aimed to evaluate the exposure to PAHs and the distribution of urinary OH-PAH levels in the general population of Shiraz, Iran, as well as, the association between OH-PAHs and the prevalence of MetS and blood cell levels.
Methods
In this study, 200 participants were randomly selected from the adult population, and their first-morning void urine samples were collected.
Results
The mean concentrations of 1-OHNap, 2-OHNap, 2-OHFlu, 9-OHPhe, and 1-OHP were 639.8, 332.1, 129, 160.3, and 726.9 ng/g creatinine, respectively. The prevalence of MetS was 26% according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria. The results showed that urinary OH-PAHs, especially 1-OHP, were positively and significantly associated with higher waist circumstance (p < 0.001), triglyceride level (p < 0.001), systolic blood pressure (p < 0.001), diastolic blood pressure (p < 0.001), number of white blood cells (p = 0.041) and red blood cells (p < 0.001). It also caused lower levels of High Density Lipoprotein-Cholesterol (HDL-C). In conclusion, the results emphasized the adverse health effects of PAHs on human health, including obesity, hypertension, dyslipidemia, and decreased number of blood cells.
Conclusion
Therefore, in order to identify the PAHs sources and to develop methods for decreasing the amount of emissions to the environment, broader researches are needed.
Keywords: Organic chemicals, Polycyclic aromatic hydrocarbons, 1-hydroxypyrene, Metabolic disease, Metabolic syndrome
Introduction
As one of the most important organic pollutants, Polycyclic Aromatic Hydrocarbons (PAHs) can be found in the environment in large amounts [1–3]. They are toxic, mutagenic, and carcinogenic compounds with the highest priority among pollutants, according to the United States Environmental Protection Agency (USEPA), and have been considered as priority pollutants by the United States Environmental Protection Agency (USEPA) [4–6]. They are also known as Endocrine-Disrupting Chemicals (EDCs) [7]. Various epidemiological studies have shown that PAHs could produce Reactive Oxygen Species (ROS) [8–10]. Moreover, a positive and significant relationship has been found between PAHs exposure and the presence of oxidative stress biomarkers in different populations and age groups [11, 12]. The possible effect of PAHs on Metabolic Syndromes (MetS) (obesity, diabetes, cholesterol, and hypertension) has also been reported in some studies on different age groups [7, 13–15].
However, there is not much information about the effect of urinary OH-PAHs on MetS. Humans are exposed to PAHs in different ways, including breathing in the polluted air, smoking, consuming PAHs through diets, and absorbing them via soil, air, or particles on the skin [16]. Two main routes of public exposure to these compounds include inhalation and dietary PAHs intake [6, 7].
Human biomonitoring is a reliable tool for measuring human exposure to chemicals, and biomarkers can be used to identify the effects in an individual organism [17–20]. In this way, exposure to new chemicals, trends and changes in rates of exposure, exposure distribution among the general population, vulnerable groups and populations at higher exposure, as well as environmental hazards in contaminated areas can be identified. Human biomonitoring programs are often used for metals, phthalates, dioxins, pesticides, volatile organic compounds, and polycyclic aromatic hydrocarbons [20, 21]. Biomonitoring has many advantages compared to environmental monitoring because the total amount of internal dose from all pathways is measured in this method [22]. Different matrices such as urine, nails, hair, and breast milk are used depending on the type of pollutant and the detection limit (LOD) of the analysis method in biomonitoring [23]. Urine is the most common matrix used in human biomonitoring programs [24]. When PAHs enter the human body and after biological transformation processes, they are metabolized through two stages [25] and mainly excreted through urine or stool as hydroxylated metabolites [26, 27]. Thus, these urinary metabolites have been designated as an exposure biomarker or as the substitutes for assessing the exposure. 1-hydroxypyrene (1-OHP) has been utilized as the most usual metabolite among them [16, 28]. Other metabolites used to measure the exposure to PAHs include 1-naphthalene (1-OHNap), 2-naphthalene (2-OHNap), 2-fluorine (2-OHFlu), 3-fluorine (3-OHFlu), and 9-phenanthrene (9-OHPhe) [29].
The global MetS prevalence has been assessed to be around one-fourth of the global population. Put differently, more than one billion individuals experience this syndrome globally [30]. Various studies showed that the prevalence of MetS was about 15.5% in China [31] and 35% in the US [30]. The corresponding value in Iran has been reported to be about 25% of the adult population [32, 33]. MetS is a chronic disease that increases the risk of many diseases, including cardiovascular disease, stroke, and type II diabetes. MetS can be diagnosed based on at least three of the five following medical conditions: high blood sugar (glucose levels ˃100 mg/dl), hypertension (Systolic Blood Pressure (SBP) ≥ 130 mmHg and Diastolic Blood Pressure (DBP) ≥ 85 mmHg), abdominal obesity (Waist Circumference (WC) ˃ 102 and 88 cm in males and females, respectively), high serum Triglyceride (TG levels ˃ 150 mg/dl), and High Density Lipoprotein Cholesterol (HDL-C < 50 and 40 mg/dl for males and females, respectively) [34].
As PAHs are mutagenic and carcinogenic [35] they may affect on the prevalence of MetS in exposed individuals in different age groups [15, 36, 37]. Although some researches have been conducted to determine the exposure to PAHs and their health effects, such as diabetes, obesity, and dyslipidemia, in different age groups [38–41], limited data are available to reflect the effects of all urinary OH-PAHs on all MetS parameters. Thus, various urinary OH-PAHs are considered as useful biomarkers for evaluating the prevalence of MetS among the general population. Additionally, this issue has not been completely examined through the simultaneous measurement of exposure biomarkers and all components of MetS. Furthermore, limited epidemiological studies have been performed to evaluate these issues in Middle Eastern countries. The current study aimed to determine the exposure of the general population of Shiraz, Iran to PAHs through measurement of the urinary OH-PAHs and to evaluate the association between exposure to these compounds and the prevalence of MetS and blood cell levels.
Materials and methods
Study population, design, and sampling
This cross-sectional study was performed on adult population of Shiraz in autumn 2020. Shiraz is the capital of Fars province (29°36′37″ N and 52°31′52″ E; 1486 m above the sea level) and the fifth megacity in Iran, with a community over 1869,000 according to the 2016 census and about 17,889 ha. This city is restricted on the west and north by Zagros Mountains. The climate of this city is classified as a cold semi-arid. In the recent years, the air quality of Shiraz has deteriorated due to rapid population growth, increase in fuel consumption, increase in gasoline use, increased utilization of diesel motor vehicles, excessive emissions from their exhausts, and insufficient infrastructures. Besides, industrial development and changes in people’s lifestyles have led to changes in dietary habits. Hence, higher amounts of PAHs can be generated through food processing and cooking at high temperatures, such as drying, smoking, grilling, and roasting [42], which can eventually enter human bodies.
The following formula was used to determine the sample size.
| Formula 1 |
Where,
- N
number of samples
- Cr
regression correlation
- Zα
95% confidence level which is equal to 1.96
- Zβ
power of study which is equal to 80%
- β
error rate equal to 20%
Sampling was done randomly from the 200 adult population (aging 30–50 years). By referring to the SIB system (an integrated health system under the supervision of the Vice Chancellor for Health of Shiraz University of Medical Sciences), 200 national codes of Shiraz residents were randomly selected and their contact numbers were provided. Then, the selected people were contacted and asked to attend a medical diagnostic laboratory to perform the relevant tests on a specific day. First-morning void samples were gathered at the beginning of the day. These samples were gathered in polypropylene tubes with the volume of 50 ml covered in the aluminum foil that included the date and the ID code of the participants. Then, the samples were immediately transferred to the laboratory at about 0–4 °C and were stored at −18 °C until analysis. The inclusion criteria of the study were A) fasting for at least 12–14 h, B) presence in the town three days before the test, C) not having consumed acetaminophen, adult cold pills, and nutrient supplements (vitamins, minerals, iron, etc.) at least three days before the test, D) not consuming the drugs that affect the metabolism of glucose, lipids, and blood pressure such as steroids, nonsteroidal anti-inflammatory drugs, male or female hormones, thyroid hormone, aspirin, and dipyridamole, E) having no history of chronic diseases such as hypertension, heart, lung, kidney, liver, and thyroid diseases, and recurrent chronic diarrhea, and F) not having the history of occupational exposure to PAHs.
Before the tests, all participants were requested to sign written informed consent forms. The research was approved by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (ethical code: ir.sums.rec.1397.157).
Questionnaire and physical examination
To collect the participants’ socio-demographic information, activities, PAHs dietary intake, and smoking history, a questionnaire consisting of 58 questions was given to each person on the day of sampling, which contained information such as age, lifestyle, characteristics of the living environment, occupational exposure, eating habits, Environmental Tobacco Smoke (ETS), and the length of time spent outdoors. The information obtained from this questionnaire was used to examine other effective factors in the increase of OH-PAHs levels in the body. The individuals were asked to enter the information about the past 72 h.
All participants also participated in face-to-face interviews. Blood and urine samples were also collected on the same day. Anthropometric measurements, including body weight, height, and WC, were performed on the sampling day, as well. Weight and height were measured in accordance with the National Health and Nutrition Examination Survey (NHANES) procedure with minimum clothing and without shoes [43]. Blood pressure was measured on the right hand after ten minutes of rest (OMRON, M2 Japan). Blood pressure was measured twice with a ten-minute interval and the mean value was considered as the final blood pressure. Complete Blood Count (CBC) and blood biochemical parameters, including TG, Total Cholesterol (TC), HDL-C, Low-Density Lipoprotein Cholesterol (LDL-C), and Fasting Blood Sugar (FBS), were measured by an automated biochemistry analyzer (PocH-100i, Sysmex, Kobe, Japan) in the clinical laboratory. Finally, the risk of MetS was evaluated according to the NCEP-ATP III criteria [34].
Measurements of urinary OH-PAHs
The samples were analyzed for OH-OHP at the Department of Environmental Health laboratory, Shiraz University of Medical Sciences. In doing so, 12 ml of sodium acetate buffer (0.1 M and pH = 5) was added to 6 ml of the sample. Hydrolysis was performed by adding 80 μl of β-glucuronidase/arylsulfatase to the solution in a water bath at 37 °C for five hours. Then, the mixture was placed on a shaker at 3000 rpm for 15 min, and the metabolite was separated. The separated supernatant was put in a GC vial, and finally, 3 ml of this supernatant was injected into the Gas Chromatography–Mass Spectrometry (GC-MS) device (Agilent, 7890, USA) equipped with the HP5-MS capillary column to measure OH-PAHs. In addition, bisphenol A solution was used as an internal standard to obtain a recovery percentage, which was initially added to the urine samples at a specified concentration. In this study, five urinary metabolites, including, 1-naphthalene (1-OHNap), 2-naphthalene (2-OHNap), 2-fluorine (2-OHFlu), 1-hydroxypyrene (1-OHP), and 9-phenanthrene (9- OHPhe), were measured [29].
The creatinine level in the samples was determined immediately after sampling according to the Jaffe method using a spectrophotometer. Finally, the concentrations were expressed in nanograms per gram of creatinine (ng/g creatinine) and nanograms per mmol of creatinine (ng/mmol creatinine). Creatinine was measured to adjust the effect of urine dilution in different individuals.
Quality control and quality assurance
When the device is set up, a few number of samples were prepared and injected into the GC-MS device to make sure that they are readable. In aggregate, ten samples were made with various concentration (from 10 to 10 × 106 ng/L) through solvating the OH-PAHs standards in dichloromethane. Then, the calibration curve was planned. The Limit of Detection (LOD) was considered as the blank sample’s mean concentration, plus three Standard Deviations (SDs). The Limits of Quantification (LOQ) were specified to be six or ten times the SDs of the blank determinations mean. Values less than the LOD were considered to be half of the LOD. The instrumental LOD, LOQ, Relative Standard Deviation (RSD), and R2 have been listed in Table 1.
Table 1.
The LOD, LOQ, RSD, R2 and % Recovery of the urinary OH-PAHs
| Compound | LOD (ng/g) | LOQ (ng/g) | RSD | R2 | % Recovery |
|---|---|---|---|---|---|
| 1-hydroxy naphthalene | 0.15 | 0.5 | 8.9 | 0.991 | 73 |
| 2-hydroxy naphthalene | 0.18 | 0.60 | 6.2 | 0.996 | 79 |
| 2-Hydroxy Florene | 0.21 | 0.69 | 3.8 | 0.994 | 86 |
| 9-Phenanthrenol | 0.20 | 0.66 | 4.6 | 0.991 | 92 |
| 1-Hydroxy Pyrene | 0.21 | 0.69 | 5.1 | 0.997 | 88 |
Using the spike sample and the duplicate sample methods, the quality control was done. Specific volumes of the standard solution were added to a sample in the spike sample method. Consequently, the recovery rates for compounds ranged from 73 to 92%. Notwithstanding, out of every ten samples was prepared twice and analyzed in the same way as the duplicate sample method.
Statistical analysis
SPSS version 21 and R software package for Windows were used for all analyses. Descriptive analysis was performed using median, mean, and SD. The normality test (Kolmogorov-Smirnov) was conducted to test the data distribution. Since the excretion of urinary OH-PAHs had a non-normal distribution, the OH-PAHs levels were log-transformed and then statistical analysis were done. Independent t-tests, ANOVA, and Pearson’s correlation analysis were used where appropriated. In addition, linear regression models were applied to determine the relationship between PAHs exposure and MetS indicators (i.e., FBS, SBP, DBP, TG, TC, HDL-C, and LDL-C). Both unadjusted and adjusted regression analyses were used for gender.
Results and discussion
Characteristics of the participants
Demographic characteristics and clinical visit results of the participants have been shown in Tables 2 and 3. According to Tables 2, 90 participants (45%) were male and 110 (55%) were female, and their mean age was 40.16 ± 6.89 years. In addition, 85% of the participants were non-smokers and 32.5% were exposed to ETS. Moreover, 68%, 23.5%, and 17.5% of the participants consumed fried, grilled, and burnt food, respectively at least once a week. The results presented in Table 3 showed a significant difference between males and females with regard to Red Blood Cell (RBC), platelet, and TG values (p < 0.05).
Table 2.
Characteristics of the study participants (n = 200)
| Characteristics | Questionnaire |
|---|---|
| Age (years, mean ± SD) | 40.16 ± 6.89 |
| Sex (male/female, n, %) | 90/110 (45/55) |
| Education (≤diploma, bachelor’s and master, ˃master, n, %) | 103/89/8 (51.5/44.5/4) |
| Active smoker (yes/no, n, %) | 30/170 (15/85) |
| Passive smoker (yes/no, n, %) | 65/135 (32.5/67.5) |
| Outdoor time in 48 h (hours, mean ± SD) | 3.24 ± 1.10 |
| Outdoor time in 24 h (hours, mean ± SD) | 8.18 ± 5.21 |
| Transportation device (bus/taxi/motorcycle/metro/private Car, n, %) | 35/42/12/25/86 (17.5/21/6/12.5/43.5) |
| Living traffic status (low/median or high, n, %) | 94/106 (47/53) |
| Eating fried food frequency (time in a week, never/once/twice/three times and more, %) | 42/68/53/37 (21/34/26.5/18.5) |
| Eating barbecue food frequency (time in a week, never/once and more, %) | 153/47 (76.5/23.5) |
| Eating burnt food frequency (time in a week, never/once and more, %) | 165/35 (82.5/17.5) |
| Exposure to fire (yes/no, n, %) | 24/176 (12/88) |
| Sleeping quality (very good/good/bad or very bad, n, %) | 34/126/40 (17/63/20) |
| Sleeping time (hours, mean ± SD) | 7.28 ± 2.12 |
| Family life quality (quiet/relatively quiet/sometimes tense/often tense, n, %) | 31/83/64/22 (15.5/41.5/32/11) |
| Metabolic Syndrome (yes/no, n %) | 52/148 (26/74) |
Table 3.
Clinical visit and distributions of selected parameters in study participants (n = 200)
| Clinical visit | sex | Mean | Std. Deviation | P value |
|---|---|---|---|---|
| Height (cm) | male | 174.66 | 6.65 | 0.141 |
| female | 159.43 | 5.98 | ||
| Weight (kg) | male | 81.41 | 12.13 | 0.630 |
| female | 69.69 | 12.02 | ||
| W.C (cm) | male | 91.34 | 8.81 | 0.740 |
| female | 83.03 | 9.38 | ||
| SBP (mm Hg)* | male | 12.39 | 1.29 | 0.325 |
| female | 11.38 | 1.41 | ||
| DBP (mm Hg,)* | male | 8.41 | 1.05 | 0.849 |
| female | 7.86 | 1.02 | ||
| Heart Beat (n) | male | 72.26 | 11.11 | 0.308 |
| female | 77.04 | 9.64 | ||
| W.B.C (mm3)* | male | 7062.22 | 1704.82 | 0.725 |
| female | 6801.81 | 1487.80 | ||
| R.B.C (Mill/mm3)* | male | 5.55 | 0.56 | 0.023 |
| female | 4.83 | 0.430 | ||
| Hemoglobin (gm/dl) | male | 15.71 | 1.37 | 0.074 |
| female | 13.46 | 1.11 | ||
| Platelet (%) | male | 238,144.44 | 48,600.07 | 0.049 |
| female | 260,209.09 | 61,928.05 | ||
| FBS (mg/dl)* | male | 104.20 | 35.22 | 0.181 |
| female | 97.60 | 19.60 | ||
| TG (mg/dl)* | male | 159.57 | 83.63 | 0.028 |
| female | 127.76 | 74.10 | ||
| TC (mg/dl)* | male | 184.98 | 39.10 | 0.359 |
| female | 181.84 | 34.76 | ||
| HDL-C (mg/dl)* | male | 41.55 | 5.56 | 0.633 |
| female | 41.73 | 6.13 | ||
| LDL-C (mg/dl)* | male | 115.72 | 27.19 | 0.543 |
| female | 111.94 | 24.29 | ||
| TG (mg/dl, mean ± SD, median, IQR**) | 142.08 ± 79.92, 116 (85,176) | |||
| TC (mg/dl, mean ± SD, median, IQR) | 183.26 ± 36.72, 180 (157.25,205.75) | |||
| FBS (mg/dl, mean ± SD, median, IQR) | 100.57 ± 27.86, 96 (91,103) | |||
* WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; RBC, red blood cell; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein; FBS, fasting blood sugar; **IQR, interquartile range for general population
Distribution of urinary OH-PAHs levels
The distribution of creatinine-corrected urinary OH-PAHs has been presented in Table 4. Accordingly, the mean concentrations of 1-OHNap, 2-OHNap, 2-OHFlu, 9-OHPhe, and 1-OHP were 639.8, 332.1, 129, 160.3, and 726.9 ng/g creatinine, respectively. In addition, among the metabolites, the highest mean concentration were related to 1-OHP (726.9 ng/g creatinine) followed by 1-OHNap. The lowest concentrations was related to 2-OHFlu. The short half-life of 1-OHP (mean about 18–20 h) that results in the rapid excretion of urine might be a reason for the domination concentration [44]. In many studies, 1-OHNap has been introduced as the predominant metabolite [4, 45, 46], because it is rapidly excreted through urine due to its low molecular weight [46]. Motorykin et al. also demonstrated that the concentration of 1-OHP was higher than that of other species [47].
Table 4.
Urinary OH-PAHs levels of study participants (n = 200)
| Parameters | (1-OHNap)** ng/g cr (ng/mmol cr)*** |
(2-OHNap)** ng/g cr (ng/mmol cr) |
(2-OHFlu)** ng/g cr/ (ng/mmol cr) |
(9-OHPhe)** ng/g cr (ng/mmol cr) |
(1-OHP)** ng/g cr (ng/mmol cr) |
Createnine g/l |
|
|---|---|---|---|---|---|---|---|
| Mean | 639.8 (71.8) | 332.1 (37.4) | 129 (14.4) | 160.3 (18) | 726.9 (81.5) | 1.62 | |
| Std. Deviation | 840.2 (94.9) | 315.4 (35.7) | 234.9 (26.4) | 151.8 (17.1) | 517.4 (58.3) | 0.65 | |
| Median | 440 (50) | 280 (31.7) | 10 (1.8) | 120 (14.1) | 570 (64) | 1.65 | |
| Minimum | 80 (10) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.25 | |
| Maximum | 829 (940) | 316 (360) | 210 (240) | 122 (140) | 262 (300) | 3.90 | |
| Percentiles | 25 | 320 (35.7) | 170 (19.1) | 0.00 (0.00) | 80 (9.1) | 360 (40.4) | 1.10 |
| 50 | 445 (50) | 280 (31.7) | 15 (1.8) | 125 (14.1) | 575 (64) | 1.65 | |
| 75 | 677.5 (75.8) | 410 (46.5) | 187 (20.3) | 190 (21.3) | 937.5 (104.4) | 2.10 | |
| P value* | <0.001 (<0.001) | 0.166 (0.139) | 0.978 (0.927) | 0.290 (0.222) | 0.106 (0.167) | 0.007 | |
*Mann-Whitney U test was used to compare non-normal distribution of continuous variables, Grouping variable: sex
**1-OHNap: 1-hydroxynaphthalene 2-OHNap: 2-hydroxynaphthalene; 2-OHFlu: 2-hydroxyfluorene; 9-OHPhe: 2-hydroxyphenanthrene and 1-OHP: 1-hydroxypyrene
***ng/g createnine (ng/mmol createnine)
The levels of urinary OH-PAHs were also reported in terms of ng/mmol creatinine and a similar trend was observed for the concentration of urinary metabolites. The mean concentration of urinary ΣOH-PAHs was 223.1 ng/mmol creatinine, which was almost similar to the concentrations reported by Hoseini et al. in Tehran [48]. However, the OH-PAHs concentration was lower than that reported by other researchers [49–51] and was significantly lower compared to the survey performed in the United States [36]. On the other hand, Shahsavani et al. conducted a research on the biological monitoring of PAHs between school children in Shiraz and indicated that the mean 1-OHP concentration was 1460 ng/g creatinine, which was higher than those reported in present study [16]. The higher concentration could be related to the higher susceptibility of children to PAHs in comparison to adults [52].
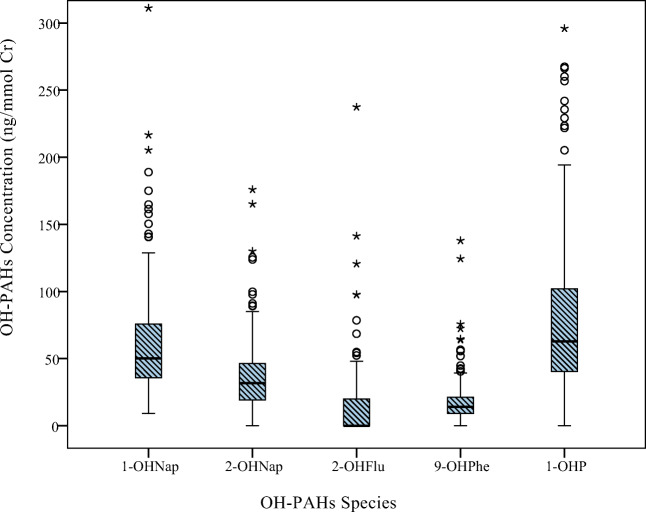
The distribution of urinary OH-PAHs (ng/mmol createnine) in the study participants has been depicted in Fig. 1. The results demonstrated a significant difference between males and females only regarding the urinary level of 1-OHNap (p < 0.001).
Fig. 1.
Urinary OH-PAHs levels in the study participants
The distribution of the studied factors and their relationship with 1-OHP and other OH-PAHs have been shown in Table 5. Based on the results, older, shorter, and less educated people, smokers or those exposed to ETS, individuals who lived in medium to heavy traffic areas, near a bus station, parking lot, restaurant, or repair shop or spent more time outdoors, as well as those who consumed more dietary PAHs were PAHs exposed more than others and had 1-OHP higher levels. The results also showed that high exposure to PAHs could significantly reduce hemoglobin levels.
Table 5.
Distributions of the selected parameters and log-transformed values of OH-PAHs
| Parameters | 1-OHNap (ng/mmol cr) |
2-OHNap (ng/mmol cr) |
2-OHFlu (ng/mmol cr) |
9-OHPhe (ng/mmol cr) |
1-OHP (ng/mmol cr) |
|
|---|---|---|---|---|---|---|
| Agec | Pearson C. | 0.189** | 0.096 | 0.200* | 0.128 | 0.148* |
| P value | 0.007 | 0.195 | 0.047 | 0.074 | 0.036 | |
| Sex (male/female)a | 0.95% CI |
(−0.238) (−0.085) |
(−0.175) (−0.005) |
(−0.116) (0.113) |
(−0.144) (0.056) |
(−0.019) (0.149) |
| P value | 0.000 | 0.037 | 0.729 | 0.390 | 0.132 | |
| Height (cm)c | Pearson. C. | −0.301** | −0.189** | −0.203* | −0.150* | −0.078 |
| P value | <0.001 | 0.010 | 0.043 | 0.037 | 0.273 | |
| Weight (kg)c | Pearson. C. | 0.100 | 0.115 | 0.136 | 0.067 | 0.098 |
| P value | 0.157 | 0.120 | 0.177 | 0.350 | 0.170 | |
| BMI (kg/m2)b | P value | 0.224 | 0.436 | 0.407 | 0.590 | 0.465 |
| Hemoglobin (gm/dl)c | Pearson. C. | −0.336* | −0.112 | −0.012 | −0.041 | −0.072* |
| P value | <0.001 | 0.130 | 0.909 | 0.569 | 0.031 | |
| Educationc | Pearson C. | −0.213** | −0.113 | −0.017 | −0.036 | −0.052 |
| P value | 0.002 | 0.127 | 0.868 | 0.620 | 0.469 | |
| number of cigarettes smokedc | Pearson C. | 0.025 | 0.013 | 0.004 | 0.048 | 0.194** |
| P value | 0.724 | 0.866 | 0.968 | 0.505 | 0.006 | |
|
time of exposure, ETSc (passive smoker) |
Pearson C. | 0.027 | 0.086 | 0.019 | 0.019 | 0.170* |
| P value | 0.708 | 0.246 | 0.848 | 0.789 | 0.016c | |
| Mean outdoor time in the last 72 hc | Pearson C. | 0.052 | 0.158* | 0.054 | 0.058 | 0.167* |
| P value | 0.463 | 0.031 | 0.593 | 0.419 | 0.018 | |
| Type of Transportation deviceb | P value | 0.913 | 0.542 | 0.515 | 0.760 | 0.377 |
|
Living traffic status (low/ medium or high)a |
0.95% CI |
(−0.079) (0.080) |
(−0.117) (0.054) |
(−0.334) (−0.699) |
(−0.1680) (0.315) |
(−0.074) (−0.095) |
| P value | 0.988 | 0.470 | 0.003 | 0.179 | 0.808 | |
| Dietary PAH intake in the last 72 hb | P value | 0.821 | 0.17 | 0.727 | 0.455 | 0.003 |
| Living Nearby to the bus station (yes/no)a | 0.95% CI |
(−0.119) (0.041) |
(−0.065) 0.109) |
(−0.225) (0.047) |
(−0.120) (0.082) |
(0.004) (0.174) |
| P value | 0.343 | 0.622 | 0.197 | 0.713 | 0.040 | |
| Living Nearby to the Car parking (yes/no)a | 0.95% CI | (−0.022)(0.139) |
(−0.003) (0.170) |
(−0.152) (0.139) |
(−0.084) (0.120) |
(0.108)(0.273) |
| P value | 0.157 | 0.059 | 0.928 | 0.724 | <0.001 | |
|
Living Nearby to the Restaurant (yes/no)a |
0.95% CI |
(−0.074) (0.085) |
(−0.091) (0.080) |
(−0.182) (0.099) |
(−0.146) (0.054) |
(0.010) (0.179) |
| P value | 0.892 | 0.905 | 0.562 | 0.370 | 0.027 | |
| Living Nearby to the car repair shop (yes/no)a | 0.95% CI |
(−0.078) (0.139) |
(−0.111) (0.125) |
(−0.224) (0.110) |
(−0.151) (0.118) |
(0.122) (0.344) |
| P value | 0.582 | 0.910 | 0.501 | 0.805 | 0.000 | |
| Sleeping qualityb | P value | 0.070 | 0.061 | 0.554 | 0.034* | 0.468 |
| Sleeping time in a dayc | Pearson C. | −0.064 | −0.087 | −0.056 | −0.016 | −0.034 |
| P value | 0.370 | 0.237 | 0.578 | 0.825 | 0.632 | |
| Family life qualityb | P value | 0.036 | 0.904 | 0.614 | 0.914 | 0.994 |
*correlation is significant at the 0.05 level
**correlation is significant at the 0.01 level
aindependent sample t-test: compare differences between the means of two groups based on log transformed OH-PAHs
b ANOVA: compare differences between the means of two or more groups (category variables) based on log transformed OH-PAHs.
cPearson correlation based on log transformed OH-PAHs
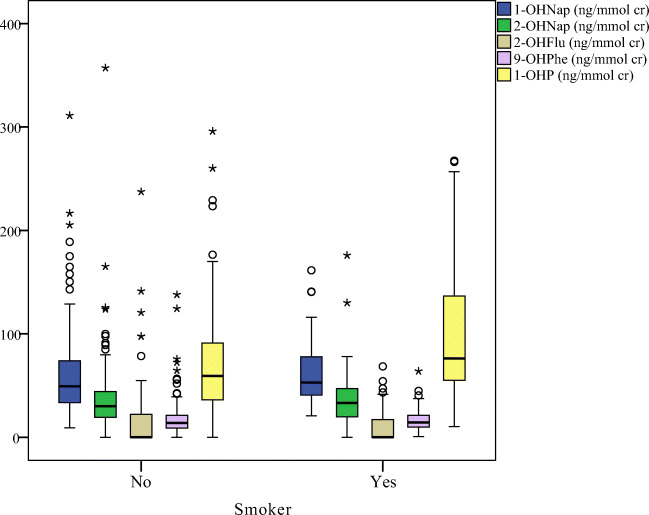
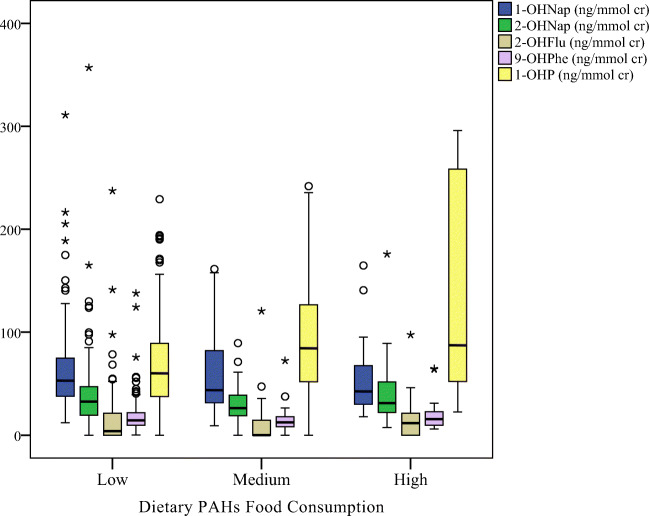
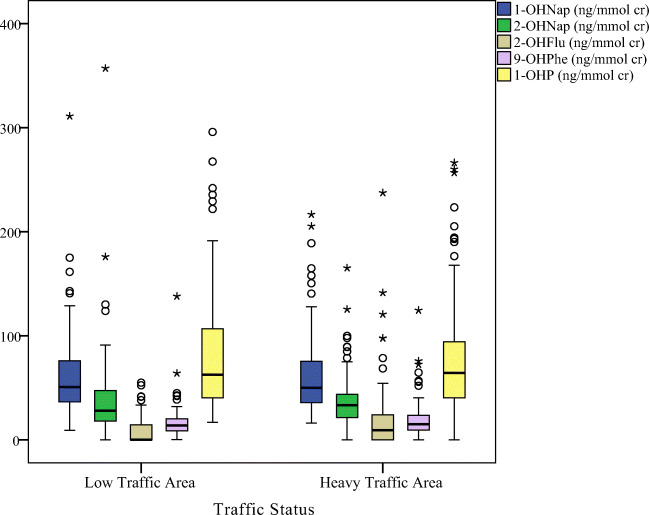
Figures 2, 3, 4, demonstrated the distribution of OH-PAHs by traffic status, dietary PAHs intake and smoking, respectively. Among the measured urinary OH-PAHs, 1-OHP is the most usual metabolite. Indoor sources of pollution, such as tobacco or cigarette combustion and ETS, significantly increase the urinary 1-OHP [1, 53]. According to the reports, about 0.1 mg benzo[a]pyrene and 0.5 mg pyrene are generated through smoking one cigarette [54]. The results of epidemiological researchs have shown that ETS exposure affects the health of the respiratory system and leads to acute and chronic diseases of the respiratory system [16]. In this study, smoking status (active or passive), total number of cigarettes smoked in active smokers (p = 0.006), and time of ETS exposure (p = 0.016) were significantly associated to the urinary 1-OHP concentrations. Previous studies also confirmed the effect of the number of cigarettes smoked [55], smoking habits, and ETS on increasing the urinary 1-OHP concentration [16, 56].
Fig. 2.
The box plot of urinary OH-PAHs levels distribution by smoking status
Fig. 3.
The box plot of urinary OH-PAHs levels distribution by different dietary PAHs intake
Fig. 4.
The box plot of urinary OH-PAHs levels distribution by traffic status
The results of current study indicated a relationship between the dietary PAHs intake and higher urinary 1-OHP concentrations. Correspondingly, a significant increase in the concentration of the urinary 1-OHP (p = 0.003) was considered in participants with high contents of PAHs in their daily diets. Many researches realized that daily diets and consuming burnt food or grilled meats can lead to an increase in the concentration of 1-OHP [57, 58]. As a matter of fact, diet has been announced as the most critical factor (approximately 90%) for exposure to PAHs [16]. Fried and grilled dishes comprise most of the Iranian diet, one of which is kebab or the grilled meat. Due to the incomplete combustion of charcoal, high amounts of PAHs can be produced as a result of grilling the meats while making kebab [59].
Traffic is yet another potential source of PAHs exposure. The current study findings demonstrated that the 1-OHP concentration was higher in the individuals who lived in a higher level of ambient air pollution and higher congestion of traffics. These results were agree with previous studies in China [52] and Mongolia [57], which showed that traffic emissions could increase urinary 1-OHP levels in exposed population. Moreover, urinary 1-OHP levels were higher in the people who had spent more time outdoors in the last 72 h or lived near restaurants, parking lots, car repair shops, and bus stations.
These results may be partly associated with the relatively higher PAHs exposure because of the higher ambient air pollution produced from these shops. Some researches in developing countries have also exhibited a higher risk of PAHs exposure resulted by the emissions from the vehicle [51, 60].
Distribution of MetS and association between the OH-PAHs levels and risk of MetS
The results of the participants’ clinical visits have been shown in Table 2. Accordingly, only the mean level of TG was significantly higher in males compared to females. Nevertheless, there was no evidence of any significant difference between the two groups considering other parameter means. Based on the criteria of NCEP-ATP III, the MetS prevalence was 26% [34], which was not significantly different between men and women (p = 0.402, CI = −0.175, 0.070).
The associations between the MetS components and other factors and urinary log-transformed levels of OH-PAHs have been presented in Table 6. Based on the results, people with higher levels of OH-PAHs had significantly higher TG levels, SBP, DBP, and WC, larger number of White Blood Cells (WBC) and RBC, and higher heartbeat. They also had lower levels of HDL-C, but the relationship was not statistically significant. The OH-PAHs were significantly related to the three of the five mentioned medical conditions abut MetS (blood pressure, Waist Circumference and high serum Triglyceride). This implied that urinary OH-PAHs could affect some MetS parameters, and individual exposed to these compounds can increase the MetS prevalence. The results presented in Table 4 also indicated that exposure to increased levels of urinary metabolites could significantly reduce hemoglobin levels and ultimately affect the prevalence of anemia.
Table 6.
The associations between MetS components and other factors and urinary log-transformed levels of OH-PAHs
| parameters | 1-OHP (ng/mmol cr) |
1-OHNap (ng/mmol cr) |
2-OHNap (ng/mmol cr) |
9-OHFlu (ng/mmol cr) |
1-OHPhe (ng/mmol cr) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | Adj. Rb | P value | R | Adj. R | P value | R | Adj. R | P value | R | Adj. R | P value | R | Adj. R | P value | |
| FBS a | 0.013 | 0.003 | 0.251 | 0.084 | 0.003 | 0.249 | 0.023 | 0.013 | 0.094 | 0.014 | 0.004 | 0.243 | 0.017 | 0.007 | 0.168 |
| TG a | 0.069 | 0.060 | <0.001 | 0.040 | 0.030 | 0.017 | 0.042 | 0.032 | 0.014 | 0.043 | 0.033 | 0.012 | 0.044 | 0.034 | 0.011 |
| SBP a | 0.150 | 0.141 | <0.001 | 0.156 | 0.147 | <0.001 | 0.120 | 0.111 | <0.001 | 0.123 | 0.114 | <0.001 | 0.122 | 0.114 | 0.001 |
| DBP a | 0.070 | 0.061 | <0.001 | 0.076 | 0.067 | <0.001 | 0.065 | 0.055 | 0.001 | 0.101 | 0.920 | <0.001 | 0.079 | 0.070 | <0.001 |
| HDL-C a | 0.025 | −0.015 | 0.082 | 0.000 | −0.009 | 0.930 | 0.003 | −0.006 | 0.704 | 0.023 | −0.013 | 0.092 | 0.014 | −0.004 | 0.234 |
| LDL-C a | 0.011 | 0.001 | 0.320 | 0.012 | 0.002 | 0.285 | 0.006 | −0.003 | 0.540 | 0.005 | −0.004 | 0.566 | 0.006 | −0.003 | 0.535 |
| W.C a | 0.178 | 0.170 | <0.001 | 0.176 | 0.168 | <0.001 | 0.172 | 0.163 | <0.001 | 0.175 | 0.167 | <0.001 | 0.171 | 0.163 | <0.001 |
| TC | 0.009 | 0.001 | 0.408 | 0.004 | −0.005 | 0.623 | 0.003 | −0.006 | 0.701 | 0.003 | −0.006 | 0.699 | 0.004 | −0.005 | 0.643 |
| W.B.C | 0.031 | 0.022 | 0.041 | 0.036 | 0.026 | 0.025 | 0.026 | 0.016 | 0.072 | 0.016 | 0.006 | 0.202 | 0.029 | 0.019 | 0.041 |
| R.B.C | 0.355 | 0.348 | <0.001 | 0.357 | 0.351 | <0.001 | 0.353 | 0.347 | <0.001 | 0.378 | 0.372 | <0.001 | 0.366 | 0.359 | <0.001 |
| Heart Beat | 0.054 | 0.044 | 0.004 | 0.051 | 0.041 | 0.005 | 0.050 | 0.041 | 0.005 | 0.055 | 0.046 | 0.003 | 0.052 | 0.043 | 0.004 |
aMetS components
bAdjusted model: linear regression models adjusted for gender
PAHs have been identified as being of most significant concern about potential exposure and adverse health effects on humans. Thus, they threaten the health and well-being of humans in a seriously. In the present study, various urinary OH-PAHs had significant deleterious associations with several health risks by considering the interfering factor of sex. However, based on the OH-PAHs and the health factor, these associations were evidently both positive and negative.
In this study, the mean ± SD of Body Mass Index (BMI) was 26.51 ± 4.08 kg/m2. Additionally, 19.5% of the people were obese and 39% overweight. Evidently, higher OH-PAHs concentrations were significantly related to an increase in WC (non-central obesity). In addition, despite the positive correlation between BMI and OH-PAHs, this relationship was not statistically significant (Table 5).
This finding was consistent with other epidemiological researches, indicating an association between the exposure to PAHs and obesity [14, 35, 61]. PAHs are considered as Endocrine-Disrupting Chemicals (EDCs) [61]. Being exposed to EDCs and other types of chemicals has been mentioned as a notable obesity risk factor. Such chemical can affect adipocytes through changing the metabolism or lipid homeostasis by activating the Peroxisome Proliferator-Activated Receptor (PPAR); it marks the pre-adipocyte cells in the fat tissue as different [7]. In addition, EDCs might affect adipogenesis by acting on the thyroid hormone and hindering lipolysis in adipocytes [62]. Therefore, the exposure to PAHs disables the adipose tissue lipolysis and causes a weight gain increasing and the formation of a fat mass.
According to the regression analysis results, a significant association was observed between hypertension and PAHs (Table 5). Accordingly, higher OH-PAHs concentrations (all five type) were related to a higher hypertension risk (both systole and diastole) after modifying the confounding variable; i.e., sex. Numerous studies have found that air pollutants, such as PAHs, tended to increase the risk of hypertension [37, 63, 64], which was consistent with the present study findings. According to the reports, oxidative stress caused by various pollutants also significantly affected hypertension. [13]. Oxidative stress causes vasoconstriction, endothelial damage, and plaque promotion in arteries. It can also act on the autonomic nervous system and significantly influence blood pressure, which may explain the research findings [13]. A study in rodents suggested that PAHs may cause carcinogenesis and atherogenesis through inflammation and increasing the plaque size [65]. Additionally, this association may be modified by the imbalance of the autonomic nervous system and the arterial vascular dysfunction or vasoconstriction [66].
According to NCEP, dyslipidemia has been defined as serum TG levels ≥200 mg/dl, TC ≥ 200 mg/dl, and HDL-C < 35 mg/dl for males and < 40 mg/dl for females [67]. The results of this study suggested significant association between the levels of TG and OH-PAHs. They also pointed to a significant positive association between 1-OHP and the level of TC. Furthermore, there was a negative association between the levels of HDL-C and OH-PAHs. However, this association did not have any statistical value. These results were in line with the results of a study done previously, since both indicated that PAHs were related to a disadvantaged lipid profile [65, 68]. Another research argued that the high fluorene biomarkers levels are associated with unfavorable levels of HDL-C [68]. The fundamental mechanisms related to the effects that exposure to air pollutants, like PAHs, has on HDL–C are yet to be specified. Based on an experimental research, the enzymatic activity of paraoxonase may have an effect related to this. [69]. Furthermore OH-PAHs might affect dyslipidemia by increasing the adipose tissue mass through the cessation of lipolysis β-adrenergic stimulation [70]. Evidently, the increasing of adipose tissue mass affects the insulin resistance, dyslipidemia, and systemic metabolic homeostasis, increasingly [71]. Moreover, several studies have shown that exposure (directly or indirectly) to certain pollutants, such as PAHs, could increase the concentration of harmful elements in the blood, which could potentially increase the risk of cancer (e.g., leukemia) and other inflammatory diseases [46, 72, 73].
As shown in Tables 5 and 6, a significant positive association was observed between OH-PAHs and number of RBC. Besides, 1-OHNap, 1-OHPhe, and 1-OHP had a significant positive effect on increasing the number of WBC. The results presented in Table 5 also indicated a significant decrease in hemoglobin level due to an increase in the levels of 1-OHNap and 1-OHP. These results were consistent with the previous studies disclosing that direct contact with industrial pollution increased the number of white [46, 72] and red blood cells [72]. However, some researches have obtained different results [74]. A study done in 2015 demonstrated that benzene exposure reduced the number of RBC, but had no effects on the number of WBC [75]. The difference between the results could be due to the difference in the nature of the pollutants. Another research indicated that exposure to low-dose benzene caused a decrease in the number of WBC [76]. The fundamental mechanism of the blood cell interaction with air pollution is yet to be revealed. One mechanism might be that PAHs lead to an abnormal bone marrow function, which causes an abnormal increase in the production of WBC and RBC and a decrease in hemoglobin level.
Current study faces some restrictions that must be mentioned. First and major, this research was a cross-sectional study. Whole data were collected at a certain time, the causal relationships between the study parameters and exposure to PAHs could not be determined. In addition, only five OH-PAHs were considered although there are several OH-PAHs metabolites that may cause MetS.
Ultimately, despite the consideration of various potential confounders, other confounders, like genetic factors, were not considered. Current study, however, had several strengths. Current study evaluated five different OH-PAHs in the adolescents (30–50 years old) as an active group. On the other hand, the novelty of the current study was determining the association between OH-PAHs and MetS and blood cell risk factors, which has never been done in Shiraz, Iran. In addition, all multivariate statistical models were adjusted for sex. Furthermore, high concentrations of these metabolites and high prevalence of MetS (in comparison with other researches) can be an alarm accounting for the demand for more stringent laws to be imposed on PAHs compounds in developing countries.
Conclusion
The mean concentration of urinary ΣOH-PAHs in the present study was similar to the concentrations reported in Tehran, Iran, but was lower than those reported in the United States and China. The results indicated that the total number of cigarettes consumed, ETS exposure, dietary PAHs intake, and living in high traffic areas near restaurants, parking lots, car repair shops, and bus stations had a significant positive effect on urinary OH-PAHs and were the major predictors of exposure to PAHs among the participants. Moreover, the prevalence of MetS was 26% according to the NCEP-ATP III criteria, which was higher than the values reported in China and lower than those found in the United States. The study findings demonstrated that some OH-PAHs were associated with a greater risk of MetS parameters. Adults with high levels of OH-PAHS might have a greater risk of obesity, hypertension, and dyslipidemia, higher WBC and RBC levels, and higher heartbeat.
Regarding the harmful PAHs effects on humans’ health, broader researches are needed in order to found the sources that help the creation of PAHs and find methods for decreasing the emissions of this compound into the environment. Future studies should also investigate the way that the physiological mechanisms related to every biomarker affects the human health.
Acknowledgments
This article was extracted from the thesis written by Mrs. Samaneh Shahsavani, PhD candidate of Environmental Health engineering. The authors would like to thank the Research Vice-chancellor of Shiraz University of Medical Sciences for financially supporting the research (Proposal No. 19157). They would also like to appreciate Ms. A. Keivanshekouh at the Research Improvement Center of Shiraz University of Medical Sciences for improving the use of English in the manuscript.
Code availability
SPSS version 21 and R software package for Windows were used for all analyses.
Authors’ contributions
Samaneh Shahsavani: Data curation, Writing- Original draft preparation, Writing-Reviewing and Editing.
Mansooreh Dehghani: Supervision, Conceptualization, Reviewing and Editing, Project administration.
Mohammad Hoseini: Supervision, Methodology, Reviewing and Editing, Project administration.
Mohammad Fararouei: Software, Validation.
Mahmood Soveid: Investigation.
Funding
Research Vice-chancellor of Shiraz University of Medical Sciences (Proposal No. 19157).
Data availability
Not applicable.
Declarations
Ethics approval
The Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran, approved the research (ethical code: ir.sums.rec.1397.157).
Consent to participate
All of the authors have read and approved the paper and they are consent to participate.
Consent for publication
All of the authors have read and approved the paper and they are consent to publication.
Conflicts of interest/competing interests
None declared.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad Hoseini, Email: mohhoseini@sums.ac.ir.
Mansooreh Dehghani, Email: mdehghany@sums.ac.ir.
References
- 1.Freire C, Abril A, Fernández M, Ramos R, Estarlich M, Manrique A, et al. Urinary 1-hydroxypyrene and PAH exposure in 4-year-old Spanish children. Sci Total Environ. 2009;407(5):1562–1569. doi: 10.1016/j.scitotenv.2008.10.068. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari S, Mandel F, Berset J. Quantitative determination of 1-hydroxypyrene in bovine urine samples using high-performance liquid chromatography with fluorescence and mass spectrometric detection. Chemosphere. 2002;47(2):173–182. doi: 10.1016/s0045-6535(01)00302-2. [DOI] [PubMed] [Google Scholar]
- 3.Shahsavani S, Hoseini M, Dehghani M, Fararouei M. Characterisation and potential source identification of polycyclic aromatic hydrocarbons in atmospheric particles (PM10) from urban and suburban residential areas in Shiraz, Iran. Chemosphere. 2017;183:557–564. doi: 10.1016/j.chemosphere.2017.05.101. [DOI] [PubMed] [Google Scholar]
- 4.Chou C-W, Chen Y-Y, Wang C-C, Kao T-W, Wu C-J, Chen Y-J, Zhou YC, Chen WL. Urinary biomarkers of polycyclic aromatic hydrocarbons and the association with hearing threshold shifts in the United States adults. Environ Sci Pollut Res. 2020;27(1):562–570. doi: 10.1007/s11356-019-06883-4. [DOI] [PubMed] [Google Scholar]
- 5.Grainger J, Huang W, Patterson DG, Jr, Turner WE, Pirkle J, Caudill SP, Wang RY, Needham LL, Sampson EJ. Reference range levels of polycyclic aromatic hydrocarbons in the US population by measurement of urinary monohydroxy metabolites. Environ Res. 2006;100(3):394–423. doi: 10.1016/j.envres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Ramesh A, Walker SA, Hood DB, Guillén MD, Schneider K, Weyand EH. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int J Toxicol. 2004;23(5):301–333. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- 7.Scinicariello F, Buser MC. Urinary polycyclic aromatic hydrocarbons and childhood obesity: NHANES (2001–2006) Environ Health Perspect. 2014;122(3):299–303. doi: 10.1289/ehp.1307234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 9.Boström C-E, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110(Suppl 3):451. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araujo JA, Nel AE. Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part Fibre Toxicol. 2009;6(1):24. doi: 10.1186/1743-8977-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh VK, Patel D, Ram S, Mathur N, Siddiqui M. Blood levels of polycyclic aromatic hydrocarbons in children and their association with oxidative stress indices: an Indian perspective. Clin Biochem. 2008;41(3):152–161. doi: 10.1016/j.clinbiochem.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Yoon H-S, Lee K-M, Lee K-H, Kim S, Choi K, Kang D. Polycyclic aromatic hydrocarbon (1-OHPG and 2-naphthol) and oxidative stress (malondialdehyde) biomarkers in urine among Korean adults and children. Int J Hyg Environ Health. 2012;215(4):458–464. doi: 10.1016/j.ijheh.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Li Z, Ma Y, Qiu X, Ren A. Association of polycyclic aromatic hydrocarbons in housewives' hair with hypertension. Chemosphere. 2016;153:315–321. doi: 10.1016/j.chemosphere.2016.03.067. [DOI] [PubMed] [Google Scholar]
- 14.Jung KH, Perzanowski M, Rundle A, Moors K, Yan B, Chillrud SN, Whyatt R, Camann D, Perera FP, Miller RL. Polycyclic aromatic hydrocarbon exposure, obesity and childhood asthma in an urban cohort. Environ Res. 2014;128:35–41. doi: 10.1016/j.envres.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou J, Sun H, Huang X, Zhou Y, Zhang Y, Yin W, Xu T, Cheng J, Chen W, Yuan J. Exposure to polycyclic aromatic hydrocarbons and central obesity enhanced risk for diabetes among individuals with poor lung function. Chemosphere. 2017;185:1136–1143. doi: 10.1016/j.chemosphere.2017.07.056. [DOI] [PubMed] [Google Scholar]
- 16.Shahsavani S, Dehghani M, Hoseini M, Fararouei M. Biological monitoring of urinary 1-hydroxypyrene by PAHs exposure among primary school students in Shiraz, Iran. Int Arch Occup Environ Health. 2017;90(2):179–187. doi: 10.1007/s00420-016-1184-9. [DOI] [PubMed] [Google Scholar]
- 17.Freire C, Ramos R, Lopez-Espinosa M-J, Díez S, Vioque J, Ballester F, Fernández MF. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environ Res. 2010;110(1):96–104. doi: 10.1016/j.envres.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Rafiee A, Delgado-Saborit JM, Gordi E, Quémerais B, Moghadam VK, Lu W, et al. Use of urinary biomarkers to characterize occupational exposure to BTEX in healthcare waste autoclave operators. Sci Total Environ. 2018;631:857–865. doi: 10.1016/j.scitotenv.2018.03.090. [DOI] [PubMed] [Google Scholar]
- 19.Rafiee A, Delgado-Saborit JM, Sly PD, Amiri H, Hoseini M. Lifestyle and occupational factors affecting exposure to BTEX in municipal solid waste composting facility workers. Sci Total Environ. 2019;656:540–546. doi: 10.1016/j.scitotenv.2018.11.398. [DOI] [PubMed] [Google Scholar]
- 20.Faridi S, Naddafi K, Kashani H, Nabizadeh R, Alimohammadi M, Momeniha F, et al. Bioaerosol exposure and circulating biomarkers in a panel of elderly subjects and healthy young adults. Sci Total Environ. 2017;593:380–389. doi: 10.1016/j.scitotenv.2017.03.186. [DOI] [PubMed] [Google Scholar]
- 21.DHHS U. Toxicological profile for polycyclic aromatic hydrocarbons. Agency for Toxic Substances and Disease Registry, Atlanta. 1995. [PubMed]
- 22.Björkman L, Lundekvam BF, Lægreid T, Bertelsen BI, Morild I, Lilleng P, et al. Mercury in human brain, blood, muscle and toenails in relation to exposure: an autopsy study. Environ Health. 2007;6(1):1–14. doi: 10.1186/1476-069X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esteban M, Castaño A. Non-invasive matrices in human biomonitoring: a review. Environ Int. 2009;35(2):438–449. doi: 10.1016/j.envint.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Chai Z, Sun H. Human hair as a potential biomonitor for assessing persistent organic pollutants. Environ Int. 2007;33(5):685–693. doi: 10.1016/j.envint.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Alonso E, Cambra K, Martinez T. Lead and cadmium exposure from contaminated soil among residents of a farm area near an industrial site. Archives of Environmental Health: An International Journal. 2001;56(3):278–282. doi: 10.1080/00039890109604454. [DOI] [PubMed] [Google Scholar]
- 26.Nakao T, Aozasa O, Ohta S, Miyata H. Survey of human exposure to PCDDs, PCDFs, and coplanar PCBs using Hairas an indicator. Arch Environ Contam Toxicol. 2005;49(1):124–130. doi: 10.1007/s00244-004-0059-3. [DOI] [PubMed] [Google Scholar]
- 27.Schummer C, Appenzeller BM, Millet M, Wennig R. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in human hair by gas chromatography–negative chemical ionization mass spectrometry. J Chromatogr A. 2009;1216(32):6012–6019. doi: 10.1016/j.chroma.2009.05.068. [DOI] [PubMed] [Google Scholar]
- 28.Smolders R, Schramm K-W, Nickmilder M, Schoeters G. Applicability of non-invasively collected matrices for human biomonitoring. Environ Health. 2009;8(1):8. doi: 10.1186/1476-069X-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciarrocca M, Rosati MV, Tomei F, Capozzella A, Andreozzi G, Tomei G, Bacaloni A, Casale T, Andrè JC, Fioravanti M, Cuartas MF, Caciari T. Is urinary 1-hydroxypyrene a valid biomarker for exposure to air pollution in outdoor workers? A meta-analysis. J Expo Sci Environ Epidemiol. 2014;24(1):17–26. doi: 10.1038/jes.2012.111. [DOI] [PubMed] [Google Scholar]
- 30.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Mi J, Shan X, Wang QJ, Ge K. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes. 2007;31(1):177–188. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
- 32.Delavari A, Forouzanfar MH, Alikhani S, Sharifian A, Kelishadi R. First nationwide study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes Care. 2009;32(6):1092–1097. doi: 10.2337/dc08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharifi F, Mousavinasab S, Saeini M, Dinmohammadi M. Prevalence of metabolic syndrome in an adult urban population of the west of Iran. Exp Diabetes Res. 2009;2009:1–5. doi: 10.1155/2009/136501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Expert Panel on Detection E. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486. [DOI] [PubMed]
- 35.Poursafa P, Dadvand P, Amin MM, Hajizadeh Y, Ebrahimpour K, Mansourian M, Pourzamani H, Sunyer J, Kelishadi R. Association of polycyclic aromatic hydrocarbons with cardiometabolic risk factors and obesity in children. Environ Int. 2018;118:203–210. doi: 10.1016/j.envint.2018.05.048. [DOI] [PubMed] [Google Scholar]
- 36.Stallings-Smith S, Mease A, Johnson TM, Arikawa AY. Exploring the association between polycyclic aromatic hydrocarbons and diabetes among adults in the United States. Environ Res. 2018;166:588–594. doi: 10.1016/j.envres.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 37.Bangia KS, Symanski E, Strom SS, Bondy M. A cross-sectional analysis of polycyclic aromatic hydrocarbons and diesel particulate matter exposures and hypertension among individuals of Mexican origin. Environ Health. 2015;14(1):51. doi: 10.1186/s12940-015-0039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu S-W, Chan Y-J, Hsu H-T, Wu K-Y, ChangChien G-P, Shie R-H, Chan CC. Urinary levels of 1-hydroxypyrene in children residing near a coal-fired power plant. Environ Res. 2011;111(8):1185–1191. doi: 10.1016/j.envres.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Hemat H, Wittsiepe J, Wilhelm M, Müller J, Göen T. High levels of 1-hydroxypyrene and hydroxyphenanthrenes in urine of children and adults from Afghanistan. J Expo Sci Environ Epidemiol. 2012;22(1):46–51. doi: 10.1038/jes.2011.33. [DOI] [PubMed] [Google Scholar]
- 40.Cavanagh J-AE, Brown L, Trought K, Kingham S, Epton MJ. Elevated concentrations of 1-hydroxypyrene in schoolchildren during winter in Christchurch, New Zealand. Sci Total Environ. 2007;374(1):51–59. doi: 10.1016/j.scitotenv.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 41.Lee M-S, Eum K-D, Lee K, Kim H, Paek D. Seasonal and regional contributors of 1-hydroxypyrene among children near a steel mill. Cancer Epidemiol Prev Biomark. 2009;18(1):96–101. doi: 10.1158/1055-9965.EPI-08-0612. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Shafy HI, Mansour MS. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet. 2016;25(1):107–123. [Google Scholar]
- 43.Control CD. Prevention. National health and nutrition examination survey (nhanes): anthropometry procedures manual. Centers for Disease Control and Prevention: Atlanta; 2007. [Google Scholar]
- 44.Jeng HA, Pan C-H. 1-Hydroxypyrene as a biomarker for environmental health. General Methods in Biomarker Research and their Applications. 2014:1–15.
- 45.Alshaarawy O, Elbaz HA, Andrew ME. The association of urinary polycyclic aromatic hydrocarbon biomarkers and cardiovascular disease in the US population. Environ Int. 2016;89:174–178. doi: 10.1016/j.envint.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koukoulakis K, Kanellopoulos P, Chrysohou E, Koukoulas V, Minaidis M, Maropoulos G, et al. Leukemia and PAHs levels in human blood serum: preliminary results from an adult cohort in Greece. Atmos Pollut Res. 2020;11:1552–1565. [Google Scholar]
- 47.Motorykin O, Santiago-Delgado L, Rohlman D, Schrlau JE, Harper B, Harris S, Harding A, Kile ML, Massey Simonich SL. Metabolism and excretion rates of parent and hydroxy-PAHs in urine collected after consumption of traditionally smoked salmon for native American volunteers. Sci Total Environ. 2015;514:170–177. doi: 10.1016/j.scitotenv.2015.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoseini M, Nabizadeh R, Delgado-Saborit JM, Rafiee A, Yaghmaeian K, Parmy S, Faridi S, Hassanvand MS, Yunesian M, Naddafi K. Environmental and lifestyle factors affecting exposure to polycyclic aromatic hydrocarbons in the general population in a middle eastern area. Environ Pollut. 2018;240:781–792. doi: 10.1016/j.envpol.2018.04.077. [DOI] [PubMed] [Google Scholar]
- 49.Hou J, Sun H, Xiao L, Zhou Y, Yin W, Xu T, Cheng J, Chen W, Yuan J. Combined effect of urinary monohydroxylated polycyclic aromatic hydrocarbons and impaired lung function on diabetes. Environ Res. 2016;148:467–474. doi: 10.1016/j.envres.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 50.Yin W, Hou J, Xu T, Cheng J, Li P, Wang L, et al. Obesity mediated the association of exposure to polycyclic aromatic hydrocarbon with risk of cardiovascular events. Sci Total Environ. 2018;616:841–854. doi: 10.1016/j.scitotenv.2017.10.238. [DOI] [PubMed] [Google Scholar]
- 51.Huo X, Wu Y, Xu L, Zeng X, Qin Q, Xu X. Maternal urinary metabolites of PAHs and its association with adverse birth outcomes in an intensive e-waste recycling area. Environ Pollut. 2019;245:453–461. doi: 10.1016/j.envpol.2018.10.098. [DOI] [PubMed] [Google Scholar]
- 52.Fan R, Wang D, Mao C, Ou S, Lian Z, Huang S, Lin Q, Ding R, She J. Preliminary study of children's exposure to PAHs and its association with 8-hydroxy-2′-deoxyguanosine in Guangzhou, China. Environ Int. 2012;42:53–58. doi: 10.1016/j.envint.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 53.Petchpoung K, Kaojarern S, Yoovathaworn K, Sura T, Sirivarasai J. The influence of metabolic gene polymorphisms on urinary 1-hydroxypyrene concentration in Thai bus drivers. Environ Toxicol Pharmacol. 2011;31(1):160–164. doi: 10.1016/j.etap.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Health UDo, Services H. Environmental Protection Agency. Respiratory health effects of passive smoking: lung cancer and other disorders (Smoking and Tobacco Control Monograph 4): NIH publication. 1993;2:71–9.
- 55.Talaska G, Thoroman J, Schuman B, Käfferlein HU. Biomarkers of polycyclic aromatic hydrocarbon exposure in European coke oven workers. Toxicol Lett. 2014;231(2):213–216. doi: 10.1016/j.toxlet.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Liu Y, Zhang H, Zhang H, Wang W, Fan Y. Urinary 1-hydroxypyrene and smoking are determinants of LINE-1 and AhRR promoter methylation in coke oven workers. Mutat Res/Genet Toxicol Environ Mutagen. 2018;826:33–40. doi: 10.1016/j.mrgentox.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y-T, Huang Y-K, Luvsan M-E, Gombojav E, Ochir C, Bulgan J, Chan CC. The influence of season and living environment on children's urinary 1-hydroxypyrene levels in Ulaanbaatar, Mongolia. Environ Res. 2015;137:170–175. doi: 10.1016/j.envres.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 58.Alghamdi MA, Alam MS, Stark C, Mohammed N, Harrison RM, Shamy M, Khoder MI, Shabbaj II, Göen T. Urinary metabolites of polycyclic aromatic hydrocarbons in Saudi Arabian schoolchildren in relation to sources of exposure. Environ Res. 2015;140:495–501. doi: 10.1016/j.envres.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 59.Gorji ME, Ahmadkhaniha R, Moazzen M, Yunesian M, Azari A, Rastkari N. Polycyclic aromatic hydrocarbons in Iranian Kebabs. Food Control. 2016;60:57–63. [Google Scholar]
- 60.Li Y, Jia Z, Wijesiri B, Song N, Goonetilleke A. Influence of traffic on build-up of polycyclic aromatic hydrocarbons on urban road surfaces: a Bayesian network modelling approach. Environ Pollut. 2018;237:767–774. doi: 10.1016/j.envpol.2017.10.125. [DOI] [PubMed] [Google Scholar]
- 61.Amin MM, Ebrahimpour K, Parastar S, Shoshtari-Yeganeh B, Hashemi M, Mansourian M, Poursafa P, Fallah Z, Rafiei N, Kelishadi R. Association of urinary concentrations of phthalate metabolites with cardiometabolic risk factors and obesity in children and adolescents. Chemosphere. 2018;211:547–556. doi: 10.1016/j.chemosphere.2018.07.172. [DOI] [PubMed] [Google Scholar]
- 62.Viguerie N, Millet L, Avizou S, Vidal H, Larrouy D, Langin D. Regulation of human adipocyte gene expression by thyroid hormone. J Clin Endocrinol Metab. 2002;87(2):630–634. doi: 10.1210/jcem.87.2.8200. [DOI] [PubMed] [Google Scholar]
- 63.Guo Y, Tong S, Zhang Y, Barnett AG, Jia Y, Pan X. The relationship between particulate air pollution and emergency hospital visits for hypertension in Beijing, China. Sci Total Environ. 2010;408(20):4446–4450. doi: 10.1016/j.scitotenv.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 64.Trasande L, Urbina EM, Khoder M, Alghamdi M, Shabaj I, Alam MS, Harrison RM, Shamy M. Polycyclic aromatic hydrocarbons, brachial artery distensibility and blood pressure among children residing near an oil refinery. Environ Res. 2015;136:133–140. doi: 10.1016/j.envres.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ranjbar M, Rotondi MA, Ardern CI, Kuk JL. Urinary biomarkers of polycyclic aromatic hydrocarbons are associated with cardiometabolic health risk. PLoS One. 2015;10(9):e0137536. doi: 10.1371/journal.pone.0137536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Neill MS, Diez-Roux AV, Auchincloss AH, Shen M, Lima JA, Polak JF, Barr RG, Kaufman J, Jacobs DR., Jr Long-term exposure to airborne particles and arterial stiffness: the multi-ethnic study of atherosclerosis (MESA) Environ Health Perspect. 2011;119(6):844–851. doi: 10.1289/ehp.0901524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Legro RS, Azziz R, Ehrmann D, Fereshetian AG, O’Keefe M, Ghazzi MN, et al. Minimal response of circulating lipids in women with polycystic ovary syndrome to improvement in insulin sensitivity with troglitazone. Obstetl Gynecol Survey. 2004;59(8):595–597. doi: 10.1210/jc.2003-030044. [DOI] [PubMed] [Google Scholar]
- 68.Patel CJ, Cullen MR, Ioannidis JP, Butte AJ. Systematic evaluation of environmental factors: persistent pollutants and nutrients correlated with serum lipid levels. Int J Epidemiol. 2012;41(3):828–843. doi: 10.1093/ije/dys003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin F, Lawal A, Ricks J, Fox JR, Larson T, Navab M, Fogelman AM, Rosenfeld ME, Araujo JA. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler Thromb Vasc Biol. 2013;33(6):1153–1161. doi: 10.1161/ATVBAHA.112.300552. [DOI] [PubMed] [Google Scholar]
- 70.Irigaray P, Ogier V, Jacquenet S, Notet V, Sibille P, Méjean L, et al. Benzo [a] pyrene impairs β-adrenergic stimulation of adipose tissue lipolysis and causes weight gain in mice: a novel molecular mechanism of toxicity for a common food pollutant. FEBS J. 2006;273(7):1362–1372. doi: 10.1111/j.1742-4658.2006.05159.x. [DOI] [PubMed] [Google Scholar]
- 71.Hu H, Kan H, Kearney GD, Xu X. Associations between exposure to polycyclic aromatic hydrocarbons and glucose homeostasis as well as metabolic syndrome in nondiabetic adults. Sci Total Environ. 2015;505:56–64. doi: 10.1016/j.scitotenv.2014.09.085. [DOI] [PubMed] [Google Scholar]
- 72.Ahmadiraad H, Hemmati M, Mahmodi M, SayadiAnari A, Mirzaee M, Khoshdel A, et al. Evaluating blood parameters, P53, and IL6 in personnel of copper complex: a comparison with control group. J Fasa Univ Med Sci. 2016;5(4):460–469. [Google Scholar]
- 73.Lee K-M, Ward MH, Han S, Ahn HS, Kang HJ, Choi HS, Shin HY, Koo HH, Seo JJ, Choi JE, Ahn YO, Kang D. Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk Res. 2009;33(2):250–258. doi: 10.1016/j.leukres.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu D, Chen Y, Sun P, Bai W, Gao A. STAT3 methylation in white blood cells as a novel sensitive biomarker for the toxic effect of low-dose benzene exposure. Toxicol Res. 2016;5(3):800–807. doi: 10.1039/c5tx00445d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koh D-H, Jeon H-K, Lee S-G, Ryu H-W. The relationship between low-level benzene exposure and blood cell counts in Korean workers. Occup Environ Med. 2015;72(6):421–427. doi: 10.1136/oemed-2014-102227. [DOI] [PubMed] [Google Scholar]
- 76.Angelini S, Maffei F, Bermejo JL, Ravegnini G, L’Insalata D, Cantelli-Forti G, Violante FS, Hrelia P. Environmental exposure to benzene, micronucleus formation and polymorphisms in DNA-repair genes: a pilot study. Mutat Res/Genet Toxicol Environ Mutagen. 2012;743(1–2):99–104. doi: 10.1016/j.mrgentox.2011.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.