Abstract
Background
Some unanswered questions persist regarding the effectiveness of corticosteroids for severe coronavirus disease 2019 (COVID-19) patients. We aimed to assess the clinical effect of corticosteroids on intensive care unit (ICU) mortality among mechanically ventilated COVID-19-associated acute respiratory distress syndrome (ARDS) patients.
Methods
This was a retrospective study of prospectively collected data conducted in 70 ICUs (68 Spanish, one Andorran, one Irish), including mechanically ventilated COVID-19-associated ARDS patients admitted between February 6 and September 20, 2020. Individuals who received corticosteroids for refractory shock were excluded. Patients exposed to corticosteroids at admission were matched with patients without corticosteroids through propensity score matching. Primary outcome was all-cause ICU mortality. Secondary outcomes were to compare in-hospital mortality, ventilator-free days at 28 days, respiratory superinfection and length of stay between patients with corticosteroids and those without corticosteroids. We performed survival analysis accounting for competing risks and subgroup sensitivity analysis.
Results
We included 1835 mechanically ventilated COVID-19-associated ARDS, of whom 1117 (60.9%) received corticosteroids. After propensity score matching, ICU mortality did not differ between patients treated with corticosteroids and untreated patients (33.8% vs. 30.9%; p = 0.28). In survival analysis, corticosteroid treatment at ICU admission was associated with short-term survival benefit (HR 0.53; 95% CI 0.39–0.72), although beyond the 17th day of admission, this effect switched and there was an increased ICU mortality (long-term HR 1.68; 95% CI 1.16–2.45). The sensitivity analysis reinforced the results. Subgroups of age < 60 years, severe ARDS and corticosteroids plus tocilizumab could have greatest benefit from corticosteroids as short-term decreased ICU mortality without long-term negative effects were observed. Larger length of stay was observed with corticosteroids among non-survivors both in the ICU and in hospital. There were no significant differences for the remaining secondary outcomes.
Conclusions
Our results suggest that corticosteroid treatment for mechanically ventilated COVID-19-associated ARDS had a biphasic time-dependent effect on ICU mortality. Specific subgroups showed clear effect on improving survival with corticosteroid use. Therefore, further research is required to identify treatment-responsive subgroups among the mechanically ventilated COVID-19-associated ARDS patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-021-00951-0.
Keywords: Corticosteroids, COVID-19-associated acute respiratory distress syndrome, Intensive care unit, Mortality, Invasive mechanical ventilation
Background
It has been more than a year since the devastating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak occurred, which emerged in Wuhan (China) on December 2019, and the global health issue is still a concern to resolve. As of July 2021, the World Health Organization reported more than 180 million cases and four million deaths worldwide due to coronavirus disease 2019 (COVID-19) [1]. The clinical presentation ranges from asymptomatic or mild-to-severe pneumonia in which the most critical cases develop life-threatening acute respiratory distress syndrome (ARDS), requiring admission to the intensive care unit (ICU) with high rates of invasive mechanical ventilation and mortality [2].
Worldwide, corticosteroids have become the standard of care (SOC) for severe COVID-19 patients, especially in mechanically ventilated patients since the results of the RECOVERY trial [3] and the subsequent meta-analysis [4] from seven randomized clinical trials (RCTs) revealed a reduction in 28-day mortality. However, several questions remain unanswered regarding the effectiveness of corticosteroids [5, 6]. It is unclear if there are particular subsets of COVID-19 patients under mechanical ventilation with different severity of illness or ARDS degree in whom corticosteroids perhaps had less pronounced effect and caution in the use of corticosteroids should be exercised. Clinical effectiveness of corticosteroid treatment in COVID-19 patients with ARDS is still limited and conflicted by the results from some meta-analysis [7, 8] and observational data [9–11].
We hypothesize that corticosteroid use may have to be individualized for corticosteroid-responsiveness COVID-19-associated ARDS patients, recognizing patient heterogeneity according to patient characteristics, severity of the disease, timing of the illness and other complications that occur during mechanical ventilation. Therefore, the aim of this study was to determine the association of corticosteroid treatment and ICU mortality among mechanically ventilated COVID-19-associated ARDS patients.
Methods
Study design and participants
This was a retrospective analysis of prospectively collected data of consecutive subjects admitted to 70 ICUs (68 Spanish, one Andorran, and one Irish) from February 6 to September 20, 2020. Data were collected through a registry created by the Spanish Society of Intensive Care Medicine and Coronary Units (SEMICYUC). Data were recorded using a case report form which included all information gathered from medical records, radiologic findings, and laboratory results during ICU admission. All data were de-identified, allowing the waiver of informed consent. The study was approved by the Ethics Committee board of Joan XXIII University Hospital (IRB# CEIM/066/2020). The study was registered in ClincalTrials.gov (NCT04948242) and followed the Strobe guidelines (see Additional file 1: Table S1). The end of follow-up was completed when patients were discharged alive or deceased in ICU.
Consecutive patients older than 16 years of age were eligible for participation if admitted to one of the participating ICUs and had received invasive mechanical ventilation for COVID-19 within the first day of ICU admission (Additional file 1: Fig. S1). A COVID-19 diagnosis had to be confirmed by a positive reverse transcriptase-polymerase chain reaction for SARS-CoV-2 from upper or lower respiratory tract samples [12]. The diagnosis of ARDS was fulfilled if patients had acute timing of respiratory failure within a week due to viral pneumonia and bilateral opacities on chest imaging not fully explained by cardiac failure or overload according the Berlin definition [13]. The severity of ARDS was classified as mild, moderate and severe accordingly with partial pressure of oxygen in arterial blood to fraction of inspired oxygen (PaO2/FiO2) ratio of < 300 to 200, < 200 to 100, and < 100 mmHg with levels of positive end-expiratory pressure (PEEP) ≥ 5 cmH2O, respectively.
For the current analysis, we excluded patients with: (1) ICU length of stay less than 2 days, and (2) corticosteroid use for confounding factors (patients with corticosteroids for refractory shock).
Data collection
Extracted data included demographic characteristics, comorbidities, time course of illness, treatments, laboratory, microbiologic and radiological findings, ventilatory parameters on day one of ICU admission, complications during ICU stay, and outcomes (Additional file 1: Table S2). Type of corticosteroid and duration of treatment were also recorded. Illness severity was determined with the Acute Physiology and Chronic Health Evaluation (APACHE) II score calculated at 24 h of ICU admission and organ failure was assessed using the Sequential Organ Failure Assessment (SOFA) score at ICU admission. Ventilatory management strategies were not standardized between centres and were left to the discretion of the attending clinician and based on SEMICYUC and National Ministry of Health recommendations, supported by international guidelines [14].
Corticosteroid treatment was defined as the administration of systemic corticosteroids within the first day of ICU admission, prescribed as adjuvant treatment for pneumonia. Patients who started corticosteroids within the 2 days before ICU admission were also considered exposed. Most patients were admitted during the first wave of the pandemic when corticosteroids were not SOC yet for severe COVID-19 patients, reason that explained why almost 40% of patients did not receive corticosteroids (Additional file 1: Fig. S2). Therefore, the decision to prescribe corticosteroids at ICU admission was based on the criteria of the attending physician based on clinical markers as well as arterial blood gas measurements. Ventilator-associated pneumonia (VAP) was diagnosed as the microbiologic confirmed pneumonia developed in ICU patients who have been mechanically ventilated for at least 48 h [15]. The remaining definitions are summarized in Additional file 1.
Outcomes
The primary outcome was all-cause ICU mortality. Secondary outcomes were in-hospital mortality, ventilator-free days at 28 days, respiratory superinfection, and length of stay (LOS) between patients with corticosteroids compared with those without corticosteroids.
Statistical analysis
No statistical sample size calculation was performed and sample size was equal to the number of patients admitted to the participating ICUs during the study period. Discrete variables were expressed as counts (percentages), while continuous variables were expressed as medians with interquartile ranges (IQR). For baselines characteristics, differences between groups were assessed using Chi-squared and Fisher’s exact tests for categorical variables or the Mann–Whitney U test for continuous variables. Significant differences were considered if p values were < 0.05 for a two-tailed test. Missing data were handled with multiple imputation by chained equations (Additional file 1: Table S3). All patients in the corticosteroid group initiated the treatment within the first day of ICU stay at the latest. Time zero of follow-up was ICU admission, but we discarded all patients censored within 48 h of ICU admission to avoid immortal time bias. Propensity score through Genetic matching algorithm was used to reduce treatment selection bias and balance the covariance matrix for both groups [16]. The matching was 1:1 with replacement and ties, and without calipers. Variables selected for the inclusion into the matching model were those baseline variables related to the outcome [17]. Those variables covered demographic characteristics and comorbidities, disease severity, laboratory data, organ failures and potential treatments affecting the survival (tocilizumab). Logistic regression analysis was performed to investigate the association of corticosteroids and ICU mortality in the matched cohort. These results were presented as odds ratios (OR) and 95% confidence intervals (CI). As a complementary analysis to the main outcome, we conducted a survival analysis to investigate whether survival times were related to covariates, and estimating the effect size of a corticosteroid treatment after adjusting for potential confounders. Owing to the fact that being discharged alive has been identified as a competitive event for ICU mortality [18], the survival analysis with Cox regression was performed using a competing risks analysis through a cause-specific hazard model [19, 20]. A competing risk (discharged alive) is an event whose occurrence precludes the occurrence of the primary event of interest (ICU mortality). In survival analysis in which competing risks are present, censoring patients who have been discharge alive may be misleading as it may violate the assumption of non-informative censoring. Thus, for etiological research the proportional cause-specific hazard model is a suitable method in case of competing risks analysis. In such case, the Cox regression analysis is applied for each of the specific types of events (both ICU mortality and discharged alive) and it directly quantify the estimates hazard ratios among subjects who are actually at risk of developing the event of interest.
When the Cox model was accomplished, the proportional hazards assumption was strongly violated for corticosteroids (Additional file 1: Fig. S3). Proportional hazards may not hold over the entire time axis, but may hold approximately over shorter time periods. The effect of a time-varying covariate (corticosteroid treatment) becomes stronger or weaker over time, which can be explored via stratification by time. Therefore, we carried out a time-dependent Cox regression using a step function to deal with non-proportional hazards. The step function consisted in a partitioning of the time axis dividing the follow-up into shorter time periods, hence the proportional hazard assumption was met within each interval of the partition [21]. We established to divide the study time frame in two intervals (at 17th of follow-up) when the proportional hazards assumption was met. The rationale behind the 17-day-step term was based on the pronounced change of case-fatality rates according with both the life-tables and the survival curves in this timeframe. With this methodology, we modelled the effect of corticosteroids on mortality in two ranges: the short and long-term. The results of time-to-event data were expressed as hazard ratios (HR) and 95% CI.
Prespecified subgroup sensitivity analysis with exploratory nature was performed with propensity score matching for each study subgroup to evaluate whether the observed effect of corticosteroids on ICU mortality was consistent across subgroups, and to assess the robustness of our findings. ICU mortality was investigated either by comparing proportions in the matched subsets and survival analysis with cause-specific hazard model. Subgroups were based on previous research as well as clinical relevance and categorized as: age (< 60, ≥ 60), severity of ARDS (mild, moderate and severe), and time since the symptom onset to the initiation of corticosteroids (< 7 days, ≥ 7 days). We also evaluated the subgroups of corticosteroid treatment duration (< 7 days, ≥ 7 days) and tocilizumab (yes, no) as post hoc analysis. To account for multiplicity and avoid the potential inflation of the type I error rate as a result of multiple testing in the subgroup analysis, we used the Benjamini–Hochberg method for controlling the false discovery rate.
Ventilator-free days at 28 days were calculated as the number of days alive and free from mechanical ventilation for at least 48 h without reintubation (successful liberation) in patients who have survived 28 days after ICU admission, and for patients ventilated 28 days or more, or who died before 28 days (irrespective of ventilated status), the number of ventilator-free days was recorded at zero. Results are expressed as means and standard deviation (SD) and with a competing risks analysis using the Fine and Gray model [22].
Centre effect for ICU mortality was investigated by multilevel logistic regression analysis through a conditional random intercept model using inter-hospital variation as a random-effects variable. Regression coefficients were summarized as the variance with standard deviation (SD) and the interclass correlation coefficient (ICC). Data analysis were done with SPSS version 24 (IBM Corp. Armonk, NY, USA) and R v.3.6 (cran.r-project.org).
Results
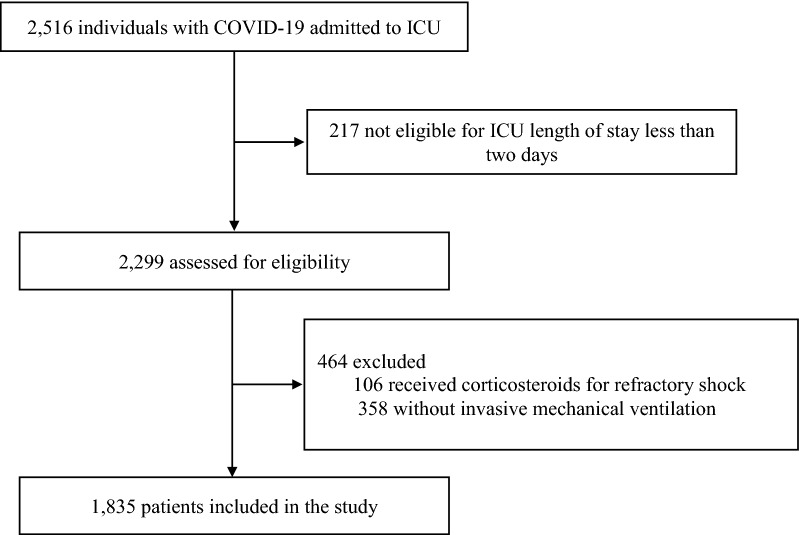
Between February 6 and September 20, 2020, data from 2516 critically ill COVID-19 patients were collected. We included 1835 mechanically ventilated COVID-19-associated ARDS patients in this analysis, of whom 1117 (60.9%) received corticosteroid treatment (Fig. 1).
Fig. 1.
Study flowchart
Demographics and characteristics
The median age was 65 years (IQR 56–72) and 1290 (70.3%) were male. The median baseline APACHE II and SOFA scores were 15 (IQR 11–19) and 6 (IQR 4–8), respectively. The median time from symptom onset to COVID-19 diagnosis was 6 (IQR 4–8) days and the time from hospital to ICU admission was 2 (IQR 0–4) days. Almost three out of four (n = 1361; 74.2%) patients had at least one comorbidity. The most frequent underlying condition was hypertension (n = 852; 46.4%). All patients were intubated for acute respiratory failure, after the failure of conventional oxygen therapy (n = 278, 15.1%), high-flow nasal cannula (n = 260; 14.2%), or non-invasive mechanical ventilation (n = 64; 3.5%), while the remaining (n = 1234; 67.2%) needed invasive mechanical ventilation as the first line respiratory support due to promptly failure of the initial oxygen therapy. Within the first day of ICU admission, 476 (25.9%) were classified as mild, 933 (50.8%) as moderate, and 426 (23.2%) as severe ARDS, respectively (Additional file 1: Table S4). Patients were profoundly hypoxaemic with a median value of PaO2/FiO2 of 141 (IQR 98–188) mmHg and prone position was frequently used (n = 1186; 64.6%). Lung-protective ventilation was widely implemented, with tidal volume of 6.5 (IQR 6–7) ml/kg/predicted body weight, moderate levels of PEEP (median 12 [IQR 10–14] cmH2O), and targeted plateau pressures (median 25 [IQR 22–29] cmH2O).
After propensity score matching, 1117 patients with corticosteroid treatment and 463 patients without corticosteroids were matched (Table 1). Several covariates were well balanced between groups and the summaries of balanced data of variables included into the matching model are shown in Additional file 1: Fig. S4. The matched cohort was appropriately well-adjusted in terms of demographic characteristics, illness severity, comorbidities (such as cardiovascular risk factors and immunosuppression status), time course of the disease, laboratory data, complications at admission (such as organ failures, myocardial dysfunction and co-infection) as well as in co-adjuvant treatments.
Table 1.
Baseline characteristics of groups according to corticosteroid treatment before and after propensity score matching
| Unmatched cohort (n = 1835) | Matched cohort (n = 1580) | |||||||
|---|---|---|---|---|---|---|---|---|
| Corticosteroids (n = 1117) | No corticosteroids (n = 718) | Smd | P value | Corticosteroids (n = 1117) | No corticosteroids (n = 463) | Smd | P value | |
| Demographic characteristics | ||||||||
| Age (years) | 64 [57–71] | 65 [55–72] | − 0.03 | 0.31 | 64 [57–71] | 66 [57–72] | − 0.06 | 0.33 |
| Gender | 0.04 | 0.44 | 0.05 | 0.38 | ||||
| Male | 793 (71%) | 497 (69.2%) | 793 (71%) | 339 (73.2%) | ||||
| Female | 324 (29%) | 221 (30.8%) | 324 (29%) | 124 (26.8%) | ||||
| BMI (kg/m2) | 28 [26–31] | 28 [26–31] | 0.02 | 0.45 | 28 [26–31] | 28 [26–31] | 0.03 | 0.82 |
| Time course of disease (days) | ||||||||
| Diagnosis gapa | 6 [4–8] | 6 [4–8] | 0.78 | 6.2 [4–8] | 6.5 [5–8] | 0.33 | ||
| Hospital gapb | 7 [5–8] | 6 [4–8] | 0.06 | 0.09 | 7 [5–8] | 6.2 [5–8] | 0.05 | 0.37 |
| ICU gapc | 2 [0–4] | 1 [0–3] | 0.06 | 0.02 | 2 [0–4] | 1 [0–3] | 0.14 | 0.11 |
| Comorbidities | ||||||||
| Any comorbidity | 825 (73.9%) | 536 (74.6%) | − 0.02 | 0.74 | 825 (73.9%) | 339 (73.2%) | 0.01 | 0.84 |
| Hypertension | 512 (45.8%) | 340 (47.3%) | − 0.03 | 0.55 | 512 (45.8%) | 213 (46%) | − 0.01 | 0.99 |
| Prior ACE inhibitors | 173 (15.5%) | 128 (17.8%) | 0.20 | 173 (15.5%) | 87 (18.8%) | 0.12 | ||
| Prior ARBs | 201 (18%) | 126 (17.5%) | 0.85 | 201 (18%) | 68 (14.8%) | 0.13 | ||
| Diabetes mellitus | 236 (21.1%) | 143 (20%) | 0.03 | 0.57 | 236 (21.1%) | 85 (18.4%) | 0.06 | 0.25 |
| Dyslipidaemia | 120 (10.7%) | 68 (9.5%) | 0.04 | 0.42 | 120 (10.7%) | 40 (8.7%) | 0.06 | 0.27 |
| Ischaemic heart disease | 73 (6.5%) | 49 (6.8%) | − 0.01 | 0.88 | 73 (6.5%) | 25 (5.5%) | 0.04 | 0.49 |
| Asthma | 80 (7.2%) | 41 (5.7%) | 0.06 | 0.25 | 80 (7.2%) | 28 (6%) | 0.04 | 0.47 |
| COPD | 79 (7.1%) | 61 (8.5%) | − 0.05 | 0.30 | 79 (7.1%) | 25 (5.4%) | 0.06 | 0.77 |
| Chronic kidney disease | 46 (4.1%) | 31 (4.3%) | − 0.01 | 0.92 | 46 (4.1%) | 15 (3.2%) | 0.04 | 0.48 |
| Immunosuppression | 40 (3.6%) | 24 (3.3%) | 0.01 | 0.88 | 40 (3.6%) | 15 (3.3%) | 0.01 | 0.90 |
| Haematological disease | 34 (3%) | 33 (4.6%) | − 0.09 | 0.10 | 34 (3%) | 14 (3%) | 0.01 | 0.99 |
| Autoimmune disease | 47 (4.2%) | 30 (4.2%) | 0.01 | 0.99 | 47 (4.2%) | 17 (3.7%) | 0.02 | 0.78 |
| Neuromuscular disease | 9 (0.8%) | 9 (1.2%) | − 0.05 | 0.47 | 9 (0.8%) | 4 (0.8%) | 0 | 0.99 |
| Hypothyroidism | 32 (2.8%) | 25 (3.5%) | − 0.04 | 0.54 | 32 (2.8%) | 8 (1.6%) | 0.07 | 0.20 |
| Disease severity | ||||||||
| APACHE II score | 15 [11–18] | 15 [11–19] | − 0.07 | 0.51 | 15 [11–18] | 15 [11–18] | 0.03 | 0.87 |
| SOFA score | 6 [4–8] | 6 [4–8] | − 0.04 | 0.74 | 6 [4–8] | 6 [4–8] | 0.04 | 0.49 |
| Pulmonary infiltrates | 0.02 | 0.78 | 0.07 | 0.20 | ||||
| ≤ 2 | 355 (31.8%) | 223 (31.1%) | 355 (31.8%) | 131 (28.4%) | ||||
| > 2 | 762 (68.2%) | 495 (68.9%) | 762 (68.2%) | 332 (71.6%) | ||||
| ARDS | 0.005 | 0.22 | ||||||
| Mildd | 267 (23.9%) | 209 (29.1%) | − 0.12 | 266 (23.8%) | 110 (23.8%) | 0 | ||
| Moderatee | 566 (50.7%) | 367 (51.1%) | − 0.01 | 566 (50.7%) | 253 (54.6%) | − 0.08 | ||
| Severef | 284 (25.4%) | 142 (19.8%) | 0.13 | 285 (25.5%) | 100 (21.6%) | 0.09 | ||
| Laboratory data | ||||||||
| C-reactive protein (mg/dl) | 15.9 [9.1–24.6] | 17.1 [10.1–24.8] | − 0.09 | 0.09 | 16 [9.0–24.3] | 17.4 [10.3–24.7] | − 0.06 | 0.11 |
| Procalcitonin (ng/ml) | 0.3 [0.2–0.8] | 0.3 [0.2–0.9] | − 0.05 | 0.22 | 0.3 [0.2–0.8] | 0.3 [0.2–0.7] | 0.06 | 0.28 |
| D-dimer (ng/ml) | 1810 [790–4845] | 1600 [742–4193] | 0.09 | 0.03 | 1801 [790–4817] | 1650 [774–4237] | 0.13 | 0.24 |
| Complications | ||||||||
| Shock | 545 (48.8%) | 370 (51.5%) | − 0.05 | 0.27 | 545 (48.8%) | 242 (52.2%) | − 0.07 | 0.24 |
| Acute kidney injury | 0.01 | 0.81 | ||||||
| RIFLE I | 94 (8.4%) | 84 (11.7%) | − 0.12 | 94 (8.4%) | 38 (8.2%) | 0.01 | ||
| RIFLE II | 81 (7.3%) | 67 (9.3%) | − 0.08 | 81 (7.3%) | 31 (6.6%) | 0.02 | ||
| RIFLE III | 147 (13.2%) | 101 (14.1%) | − 0.03 | 147 (13.2%) | 54 (11.7%) | 0.04 | ||
| Myocardial dysfunction | 89 (7.8%) | 91 (12.7%) | − 0.17 | 0.001 | 89 (7.8%) | 37 (8.1%) | − 0.01 | 0.99 |
| Bacterial co-infection | 128 (11.4%) | 74 (10.3%) | 0.48 | 128 (11.4%) | 49 (10.6%) | 0.67 | ||
| Treatments | ||||||||
| Remdesivir | 24 (2.2%) | 19 (2.7%) | 0.59 | 24 (2.2%) | 12 (2.6%) | 0.72 | ||
| Tocilizumab | 392 (35.1%) | 156 (21.7%) | 0.28 | < 0.001 | 392 (35.1%) | 147 (31.7%) | 0.07 | 0.21 |
Data are presented as numbers (%) or medians [interquartile range]. Smd < 0.01 are expressed as 0.01
Smd standardized mean differences, BMI body mass index, ICU intensive care unit, ACE angiotensin-converting enzyme, ARBs angiotensin receptor blockers, COPD chronic obstructive pulmonary disease, APACHE Acute Physiology And Chronic Health Evaluation, SOFA Sequential Organ Failure Assessment, ARDS acute respiratory distress syndrome, RIFLE criteria Risk, Injury, Failure, Loss, End stage
aDiagnosis gap means the time from disease onset to the confirmation of the diagnosis of SARS-CoV-2 infection
bHospital gap means the time from disease onset to hospital admission
cICU gap means the time from hospital to ICU admission
dClassified as the worst value of PaO2/FiO2 ratio < 300 within the first day of ICU admission
eClassified as the worst value of PaO2/FiO2 between 200 and 300 within the first day of ICU admission
fClassified as the worst value of PaO2/FiO2 < 100 within the first day of ICU admission
Details in corticosteroid use
Among patients treated with corticosteroids, methylprednisolone was the most frequently administered (n = 856/1117, 76.6%), followed by dexamethasone (n = 247/1117, 22.1%). The time from onset of symptoms to corticosteroids initiation was 9 (IQR 7–12) days. The duration of treatment with methylprednisolone was 5 (IQR 3–10) days, whereas dexamethasone treatment was 10 (IQR 5–10) days (Table 2). In the multivariable analysis, factors independently associated with corticosteroid use were severe ARDS (OR 1.29; 95% CI 1.00–1.65; p = 0.04) and tocilizumab therapy (OR 1.88; 95% CI 1.50–2.35; p < 0.001; Additional file 1: Table S5). The rate of co-adjuvant therapies was similar between groups, except for lopinavir plus ritonavir and interferon which were more used in the No corticosteroid group (Additional file 1: Table S6).
Table 2.
Description of corticosteroid use for COVID-19-associated acute respiratory distress syndrome patients
| Corticosteroid group (n = 1117) | |
|---|---|
| Type of corticosteroid | |
| Methylprednisolone | 856 (76.6%) |
| Dexamethasone | 247 (22.1%) |
| Hydrocortisone | 10 (0.9%) |
| Methylprednisolone plus hydrocortisone | 2 (0.2%) |
| Methylprednisolone plus dexamethasone | 2 (0.2%) |
| Timeline of corticosteroids use (days) | |
| Time from symptom onset to corticosteroid initiation | 9 [7–12] |
| Duration of corticosteroid treatment | 6 [3–10] |
| Methylprednisolone | 5 [3–10] |
| Dexamethasone | 10 [5–10] |
| ARDS severity when corticosteroid initiation | |
| Milda ARDS | 267 (23.9%) |
| PaO2/FiO2 (mmHg) | 240 [217–266] |
| Moderateb ARDS | 566 (50.7%) |
| PaO2/FiO2 (mmHg) | 145 [121–169] |
| Severec ARDS | 284 (25.4%) |
| PaO2/FiO2 (mmHg) | 80 [69–91] |
Data are expressed as numbers (%) or medians [interquartile range]
ARDS acute respiratory distress syndrome, PaO2/FiO2 partial pressure of oxygen to fractional inspired oxygen
aClassified as the worst value of PaO2/FiO2 ratio ≤ 300 to 200 mmHg within the first day of ICU admission
bClassified as the worst value of PaO2/FiO2 between < 200 and 100 mmHg within the first day of ICU admission
cClassified as the worst value of PaO2/FiO2 < 100 mmHg within the first day of ICU admission
Mortality analysis
Overall, ICU mortality was 33.5% (n = 615/1835). In the multilevel logistic regression, no centre effect was observed for ICU mortality as between-centre variability was negligible (inter-hospital variance 0.17, SD ± 0.41, ICC 0.05). In the matched cohort, the ICU mortality did not differ between groups (corticosteroids n = 378/1117 [33.8%] vs. no corticosteroids n = 143/463 [30.9%]; p = 0.28). After adjusting for confounding factors (Additional file 1: Table S7), corticosteroid treatment was not independently associated with ICU mortality in the matched cohort (OR 1.26, 95% CI 0.96–1.65; p = 0.09; Additional file 1: Table S8).
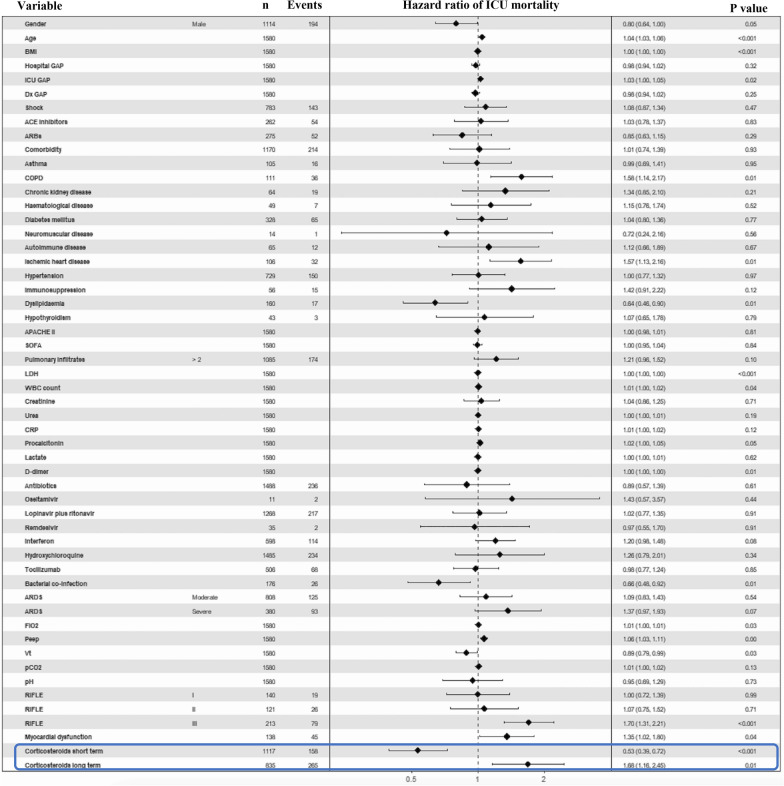
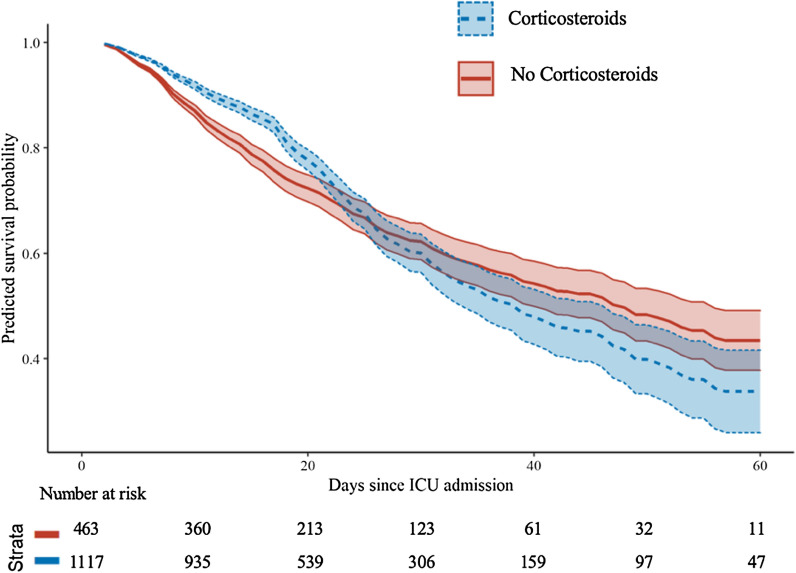
To evaluate the ICU mortality over time, a survival analysis was performed though the cause-specific hazard model using the step function. After adjusting for several confounding factors (Fig. 2), the time-dependent Cox regression showed that corticosteroids were associated with decreased ICU mortality (short-term HR 0.53; 95% CI 0.39–0.72; p < 0.001) within the 17th day of ICU admission but, beyond this timeframe the effect switched and corticosteroids were associated with increased ICU mortality thereafter (long-term HR 1.68; 95% CI 1.16–2.45; p = 0.01; Fig. 3). The Cox model for the competitive event (ICU discharged alive) is shown in the Additional file 1: Fig. S5.
Fig. 2.
Forest plot of the cause-specific hazard model (time-dependent Cox regression) for ICU mortality. The time-dependent Cox regression included all the variables and those were adjusted as potential confounding factors. The interaction with time in this case was modelled using the step function to deal with non-proportional hazards of the covariate of interest (corticosteroid treatment). Through the step function, a partitioning of the time axis was made at day 17th, when the effect of the covariate changed over time. Consequently, the model allowed to estimate the effect of the treatment covariate before (corticosteroid treatment short-term) and after (corticosteroid treatment long-term) the 17th day of ICU admission. Notably, the regression model showed the protective effects of corticosteroids on survival up to day 17 of admission to the ICU, although these effects changed over time, as after the 17th day corticosteroid treatment at ICU admission was associated with higher mortality. Diagnosis GAP means the time (days) from disease onset to the confirmation of the diagnosis of SARS-CoV-2 infection; Hospital GAP (days) means the time from disease onset to hospital admission. ICU GAP (days) means the time from hospital to ICU admission. BMI body mass index, ACE angiotensin-converting enzyme, ARBs angiotensin receptor blockers, COPD chronic obstructive pulmonary disease, APACHE Acute Physiology And Chronic Health Evaluation, SOFA Sequential Organ Failure Assessment, LDH lactate dehydrogenase, WBC white blood cells count, CRP C-reactive protein, ARDS acute respiratory distress syndrome, FiO2 fraction of inspired oxygen, Peep positive end-expiratory pressure, Vt tidal volume, pCO2 partial pressure of carbon dioxide, RIFLE criteria Risk, Injury, Failure, Loss, End stage
Fig. 3.
Survival analysis with the cause-specific hazard model and step function (time-dependent Cox regression) to investigate the association of corticosteroids and ICU mortality over time. The estimated survival curves showed the estimated effect of exposure of corticosteroids on ICU mortality over time. Survival curves cross each other implying time-dependency of corticosteroid exposure; hence step function approach was used to handle non-proportional hazards assumption by stratification of time on day 17 of follow-up when hazards changed the direction statistically significant. Then, the proportional hazards assumption was met for both periods. Models were adjusted for gender, age, body mass index, hospital GAP, ICU GAP, diagnosis GAP, shock, ACE inhibitors, ARBs, Comorbidity, asthma, COPD, chronic kidney disease, haematological disease, diabetes mellitus, neuromuscular disease, autoimmune disease, ischaemic heart disease, hypertension, immunosuppression, dyslipidaemia, hypothyroidism, APACHE II, SOFA, pulmonary infiltrates, lactate dehydrogenase, white blood cells count, creatinine, urea, C-reactive protein, procalcitonin, Lactate, d-dimer, antibiotics, oseltamivir, lopinavir plus ritonavir, remdesivir, interferon, hydroxychloroquine, Tocilizumab, bacterial co-infection, ARDS severity, fractional of inspired oxygen (FiO2), positive end-expiratory pressure, tidal volume, partial pressure of carbon dioxide, pH, RIFLE criteria, myocardial dysfunction and corticosteroid treatment (short and long-term). ICU intensive care unit, ACE angiotensin-converting enzyme, ARBs angiotensin receptor blockers, COPD chronic obstructive pulmonary disease, APACHE Acute physiology and chronic health evaluation, SOFA sequential organ failure assessment, ARDS acute respiratory distress syndrome
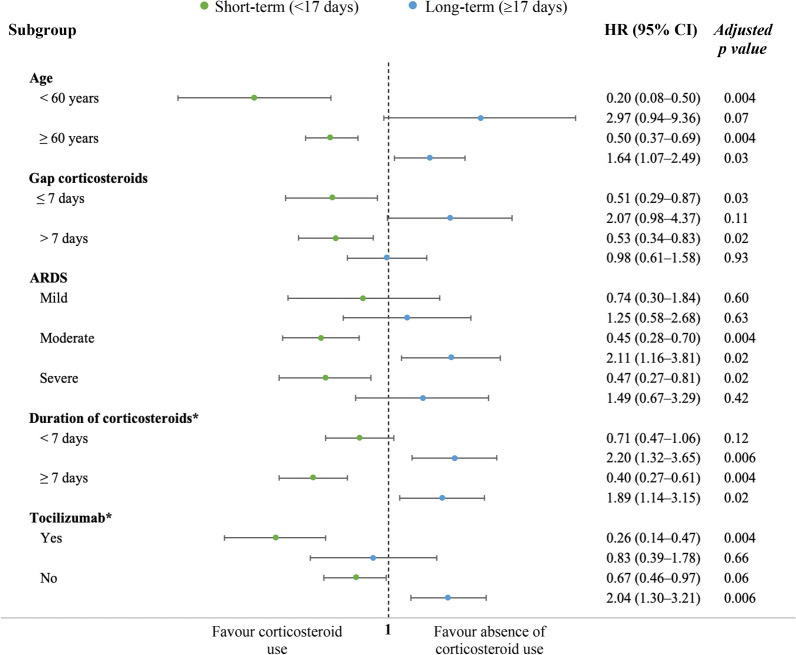
The sensitivity subgroup analysis with comparison of proportions of ICU mortality yielded similar finding as in the primary analysis, without finding significant differences between mortality rates across all the subgroups (Additional file 1: Table S9). In the survival analysis of subgroups, the time-dependent effect of corticosteroid treatment was also observed among the subgroups in the sensitivity analysis (Fig. 4 and Additional file 1: Table S10). Nonetheless, corticosteroids were associated with short-term survival benefit without long-term negative effects in subgroups of age < 60 years and severe ARDS, whereas no survival effects were found in mild ARDS (Additional file 1: Fig. S6). We further investigate the association of corticosteroids with mortality according with the duration of treatment (post hoc subgroup analysis in Additional file 1: Fig. S7). With shorter course of treatment (< 7 days), no survival effects were found in short-term, but associated with increased ICU mortality in long-term. When corticosteroid treatment lasted 7 days or longer, time-dependent effects were also found as in the main analysis. In a second post hoc analysis, patients who received corticosteroids plus tocilizumab presented short-term benefit on survival without long-term negative effects (Additional file 1: Fig. S8).
Fig. 4.
Subgroup sensitivity analysis. Propensity score matching was performed for each study subgroup as in the primary analysis to evaluate the estimated effect of corticosteroids on ICU mortality over time with cause-specific hazard model. Post hoc analysis was made for subgroups of duration of corticosteroid treatment and Tocilizumab. GAP corticosteroids mean the time (in days) since onset of symptoms to corticosteroid initiation. Models were adjusted for gender, age, body mass index, hospital GAP, ICU GAP, diagnosis GAP, shock, ACE inhibitors, ARBs, Comorbidity, asthma, COPD, chronic kidney disease, haematological disease, diabetes mellitus, neuromuscular disease, autoimmune disease, ischaemic heart disease, hypertension, immunosuppression, dyslipidaemia, hypothyroidism, APACHE II, SOFA, pulmonary infiltrates, lactate dehydrogenase, white blood cells count, creatinine, urea, C-reactive protein, procalcitonin, Lactate, d-dimer, antibiotics, oseltamivir, lopinavir plus ritonavir, remdesivir, interferon, hydroxychloroquine, Tocilizumab, bacterial co-infection, ARDS severity, fractional of inspired oxygen (FiO2), positive end-expiratory pressure, tidal volume, partial pressure of carbon dioxide, pH, RIFLE criteria, myocardial dysfunction and corticosteroid treatment (short and long-term). ICU intensive care unit, ACE angiotensin-converting enzyme, ARBs angiotensin receptor blockers, COPD chronic obstructive pulmonary disease, APACHE acute physiology and chronic health evaluation, SOFA sequential organ failure assessment, ARDS acute respiratory distress syndrome, CRP C-reactive protein
Secondary outcomes
Secondary outcomes are shown in Table 3. In-hospital mortality was 38.3% in the corticosteroid group and 33.0% in the no corticosteroid group (p = 0.05). The number of ventilator-free days at 28 days did not achieve statistical differences and the cumulative incidence of ventilator-free days was also similar between groups after competing risks analysis (Additional file 1: Fig. S9). No differences were found in median ICU LOS in survivors. However, among non-survivors, the time to death was significantly larger in those who received corticosteroids compared with the non-corticosteroid users both in ICU (median 19 [IQR 11–29] vs. 12 [6–23], p < 0.001) and in hospital (median 24 [IQR 15–33] vs. 14 [IQR 9–24], p < 0.001). Regarding respiratory superinfection, corticosteroids were not associated with the development of VAP in the multivariable analysis (OR 1.05; 95% CI 0.83–1.34; Additional file 1: Table S11).
Table 3.
Summary of the secondary outcomes of the study
| Corticosteroids (n = 1117) | No corticosteroids (n = 463) | P value | |
|---|---|---|---|
| Secondary outcomes | |||
| In-hospital mortality | 428 (38.3%) | 153 (33.0%) | 0.05 |
| Hospital LOS (days) | |||
| Overall | 30 (21–46) | 30 (18–46) | 0.63 |
| Survivors | 37 (25–53) | 38 (25–52) | 0.70 |
| Non-survivors | 24 (15–33) | 14 (9–24) | < 0.001 |
| Ventilator-free days at 28 days | 8.2 ± 9.2 | 7.5 ± 8.6 | 0.17 |
| ICU LOS (days) | |||
| Overall | 19 (11–31) | 17 (10–31) | 0.04 |
| Survivors | 18 (12–33) | 20 (12–31) | 0.82 |
| Non-survivors | 19 (11–29) | 12 (6–23) | < 0.001 |
| Ventilator-associated pneumonia | 225 (20.1%) | 90 (19.4%) | 0.83 |
Ventilator-free days are expressed as mean and standard deviation using the Wilcoxon rank sum test with continuity correction. LOS (length of stay) is expressed as medians with interquartile ranges. Ventilator-associated pneumonia incidences are presented as numbers with percentages
Discussion
In this study, corticosteroid treatment did not reduce ICU mortality rates among mechanically ventilated COVID-19-associated ARDS. However, our findings suggested that corticosteroids at ICU admission had time-dependent effects based on the short-term benefit of survival in the first 2 weeks, but an increased risk of mortality thereafter. Subgroups of patients aged less than 60 years, severe ARDS and tocilizumab plus corticosteroids showed short-term protective effects without deleterious long-term effects on mortality suggesting that not all mechanically ventilated COVID-19-associated ARDS patients might be corticosteroid-responsiveness. These results have been observed in a large cohort of ICU patients after controlling for observational-related biases and competing risks, thereby the benefit or potential harm of corticosteroids should be taken into account.
Data regarding the effectiveness of corticosteroids in COVID-19-associated ARDS remain limited. A recent meta-analysis (18 RCTs enrolling 2826 patients) evaluating the use of corticosteroids in ARDS (due to COVID-19 and non-COVID-19) concluded that corticosteroids probably reduce mortality in patients with ARDS of any aetiology [23]. However, the pooled estimate data of COVID-19-associated ARDS either using the ARDS criteria (risk ratio 0.92, 95% CI 0.76–1.11) and data from mechanically ventilated patients (risk ratio 0.89, 95% CI 0.71–1.12) did not reach statistical significance for 28-day mortality in the random-effects model. Different meta-analysis of COVID-19 patients with high heterogeneity on illness severity reported beneficial effects on mortality with corticosteroid treatment [7, 24, 25]. It should be pointed out that in most meta-analysis, the weight of the RECOVERY trial [3] made important contribution to overall pooled data. Conversely, conflicting results from other meta-analysis did not report benefits on mortality with corticosteroid treatment [8, 26].
The results of the RECOVERY trial [3] indicate that moderate dose of dexamethasone reduces 28-day mortality among patients with COVID-19 that require oxygen support, with more pronounced effect in those receiving invasive mechanical ventilation (rate ratio 0.64; 95% CI 0.51–0.81). This landmark trial has changed clinical practice worldwide for severe COVID-19 patients. Nonetheless, some uncertain issues deserve discussion and they must to be accounted for [6]. One major concern is that at day 28 of treatment allocation, 75% of patients were still hospitalized and investigating long-term outcomes could impact on the observed results [27]. Several factors affecting the outcome such as the level of respiratory support and the degree of hypoxemia were not measured, for instance, we found that corticosteroids may have higher benefit among severe ARDS, whereas patients with mild ARDS were not corticosteroid-responders. Additionally, there were 1707 (15%) patients who were not considered suitable for randomization but reasons for exclusion were not adequately disclosed. Physicians may exclude those patients because observed contraindications in whom corticosteroids might have detrimental consequences, which constitutes an important selection bias. Likewise, centre effect was not evaluated and there might be some imbalance in group allocation, especially in the subset of ventilated patients in which an average of only six patients were included per centre. Also in the stratified analysis, there was no benefit from dexamethasone in some subgroups, even in those with higher baseline risk (≥ 45%) which involves one half of the study patients. Therefore, the beneficial effect of corticosteroids among whole mechanically ventilated patients may be questionable.
The CoDEX trial performed in 299 ventilated COVID-19 moderate-to-severe ARDS assigned to received dexamethasone plus SOC versus SOC alone, did not find significant differences on all-cause 28-day mortality [28]. A randomized double-blind placebo-controlled trial conducted in 393 COVID-19 patients allocated to receive methylprednisolone 0.5 mg/kg twice daily for 5 days or placebo found no differences in 28-day mortality between groups, whereas lower mortality with corticosteroids was observed in post hoc analysis among patients over 60 years old (HR 0.63, 95% CI 0.41–0.97) [29]. Nonetheless, the rate of invasive mechanical ventilation was low and information regarding the degree of respiratory support or ARDS severity are lacking.
Results from retrospective also reported survival benefit for critically ill patients with COVID-19. In a multicentre cohort including 882 patients and high rates of invasive mechanical ventilation, it was observed that early corticosteroid treatment (within the first 48 h of ICU admission) was associated with a reduction in ICU mortality compared with delayed use or none after inverse probability weighting, although competing risks were not accounted for [30]. Retrospectives studies with robust statistical approach also showed positive effects with corticosteroids on mortality in severe [31] or COVID-19-associated ARDS [32], albeit smaller sample sizes and lower rates of invasive mechanical ventilation were recognized and these results might not be applicable to all COVID-19-associated ARDS. On the contrary, conflicting data from multicentre studies in COVID-19-associated ARDS [9, 10] and critical cases [11] suggested that corticosteroid treatment might be associated even with higher mortality, after carefully controlling for biases.
All this controversy on the evidence may be due to the heterogeneity of patients leading to conflicting data and it is unclear whether the use of corticosteroids is adequate for overall mechanically ventilated COVID-19 patients. We focused on mechanically ventilated patients with COVID-19-associated ARDS and observed that corticosteroid treatment at ICU admission had time-dependent effects. While corticosteroids seemed to be protective within the first 2 weeks of severe illness, the likelihood of survival changes beyond this timeframe and corticosteroid exposure at ICU admission appeared to be harmful. The possible explanations for the observed long-term negative effects from corticosteroids must be evaluated in further research as data regarding side effects are still lacking, since evidence from a recent meta-analysis suggested that there is no association between corticosteroids and superinfections [33], although a trend toward higher rates of delayed viral shedding and venous thromboembolism have been observed in some data [7, 26, 34–36].
Our results have been observed in a representative cohort of COVID-19-associated ARDS, with close similarities from other studies in terms of severity degree of hypoxemia, ventilatory parameters, and respiratory support management [37, 38]. The survival benefit from dexamethasone in mechanically ventilated patients in the RECOVERY trial [3] might be due to the capacity of corticosteroids to dampen both inflammation and late-onset fibrosis [39] in some cases that leads to severe lung injury in ARDS. Actually, in the present study, the subgroup of severe ARDS could benefit from corticosteroids over time without experimenting detrimental effects in long-term. Conversely, in patients with mild ARDS, corticosteroids had no significant effect on mortality questioning the need to treat such subgroup. These short-term protective effects have also been found for the subgroup of patients aged < 60 years, similar findings as those observed in the RECOVERY trial which showed protective effects of corticosteroids for patients younger than 70 years. However, a multicentre study conducted in 303 critically ill COVID-19 patients found opposite results to ours, reporting that early corticosteroid administration was associated with a lower mortality rate in patients aged ≥ 60 years [40]. The contradictory results might be due to a different population than ours with only one-third of included patients under invasive mechanical ventilation.
Regarding the duration of corticosteroid treatment, we found that a shorter course of treatment did not have protective effects, similar results as reported in the Metcovid trial [29]. Indeed, a meta-analysis found that ARDS patients (COVID-19 and non-COVID-19) who received a longer course of corticosteroids (over 7 days) had higher survival rates compared with a shorter course of treatment [23]. Despite the short-term benefit on survival with a longer course of treatment, possible long-term negative effects could arise; albeit, these results would need confirmation.
The use of tocilizumab plus corticosteroids at ICU admission seem to have also short-term protective effects on survival without presenting long-term deleterious effects on mortality. These findings coincide with a recent RCT conducted in hospitalized COVID-19 patients with hypoxia and systemic inflammation, in which the allocation to tocilizumab was associated with a significant reduction in 28-day mortality compared with usual care alone (with corticosteroid use up to 80% with systemic corticosteroids in both arms) [41].
Moreover, different phenotypes of COVID-19 critically ill patients with different host response patterns and impact on outcomes have been recently reported [42, 43]. Indeed, a retrospective study conducted in 428 critically ill COVID-19 patients reported that corticosteroids had significant survival benefits only in hyperinflammatory phenotype (HR 0.51; 95% CI 0.34–0.78) compared with the hypoinflammatory phenotype [44]. The subgroup analysis in our study showed that these time-dependent effects of corticosteroids on survival could be present in different subsets suggesting that patients’ response to corticosteroids may vary depending on distinct baseline conditions, although the exploratory nature of the sensitivity analysis requires that these results should be evaluated in further clinical research.
To our knowledge, this is one of the largest multicentre observational study of mechanically ventilated COVID-19-associated ARDS evaluating the effectiveness of corticosteroids on ICU mortality and results pointed out the possible time-dependent pattern of corticosteroids with likely opposite short and long-term effects on mortality. These findings support the hypothesis that not all mechanically ventilated patients with COVID-19-associated ARDS treated with corticosteroids have survival benefit over time. Our approach sought to account for selection bias and confounding, immortal time bias, and competing risks. In addition, performing subgroup sensitivity analysis made our results to be robust. As corticosteroids have become the SOC for severe COVID-19 patients, to randomize patients allocated to receive or not corticosteroids to investigate knowledge gaps would entail some ethical issues. This study attempted to make causal effects between corticosteroids and mortality from observational data. Hence, it provides real-world evidence on this topic currently under debate and new insights for a better personalization of care among mechanically ventilated patients with COVID-19-associated ARDS.
Nevertheless, our study had some limitations. First, as it was a retrospective study and some biases can arise from the observational design. Although RCTs are known to be the “gold standard” for research in investigating the effectiveness of interventions, methodological tools applied to observational data can decrease bias and confounding caused by the lack of randomization and could provide data from usual clinical practice. However, unmeasured confounders may persist. Second, our study was based on mechanically ventilated COVID-19-associated ARDS patients. Therefore, results may not be generalized to other settings such us hospitalized or outpatients. Third, we could not assess the established dosage of corticosteroids, although we deemed that suggested recommendations were followed using moderate doses of methylprednisolone, as all included patients in this study were diagnosed with ARDS [45]. Preferably, we focused on the effect of the exposure to corticosteroids at ICU admission. Nonetheless, dosage should be also a point of discussion in further research. Likewise, the safety profile and side effects such as hyperglycaemia, secondary superinfections different from respiratory source, or myopathy could not be evaluated. Fourth, different treatment approaches were used during the first wave, in accordance with current guidance [46], which could affect outcomes. Nevertheless, to avoid confounding by indication, all treatments received at admission were included within the adjusted Cox model after propensity score matching. Fifth, we did not collect data regarding the causes of death and the possibility of withdrawal of therapy beyond the 17th day of ICU admission. The population potentially at risk for life support withdrawal could be larger among corticosteroids users, therefore, some residual confounding may exist. Intensivists are used to facing to ethical concerns in their daily practice [47], and our ARDS patients died under invasive mechanical ventilation and other life support therapies in both groups, meaning that the most probable cause of death was persistent organ failure, mainly terminal respiratory failure [48]. Indeed, therapeutic withdrawal decisions in such critical care patients usually are taken under refractory/irreversibility stages of the disease. Further, we found that among non-survivors, the time to death was significantly larger in those who received corticosteroids compared with the non-corticosteroid users, suggesting that withdrawal life support rates might be lower in patients who received corticosteroids. Sixth, although two hundred and fifty-five patients without corticosteroids were not retained after matching, a sub-analysis revealed that no significant differences were found regarding demographic characteristics, severity of the disease, comorbidities and mortality, compared with those patients without corticosteroids included in the matching model. Nonetheless, propensity score matching yielded well balanced groups in terms of several observed confounders and, therefore eliminating selection bias. Seventh, conducting multiple testing for subgroup analysis may result in multiplicity. However, the sensitivity subgroup analysis was performed with an exploratory rather than confirmatory nature and these results aimed to yield consistency to the primary analysis and also to generate new hypothesis on corticosteroid-responsive subgroups for future research. Likewise, we accounted for multiplicity with the Benjamini–Hochberg approach to avoid the potential inflation of type I error. Eight, we could not evaluate the impact on outcomes of the ICU demand in periods of inundated critical care system. Finally, we did not collect data about viral shedding. The possibility of delay in viral clearance due to corticosteroid administration is an area for concern and more data are required to address this issue.
Conclusion
In this study, corticosteroids treatment did not reduce ICU mortality rates among mechanically ventilated COVID-19-associated ARDS patients. However, our results suggested that corticosteroid treatment could present with biphasic time-dependent effects on survival with initial protective effects within the first 2 weeks of critical illness while beyond this timeframe there may be long-term negative effects. Subgroup of patients aged less than 60 years, severe ARDS and tocilizumab plus corticosteroids could be some of the subsets with the greatest benefit from corticosteroids, hence further research is needed to identify the treatment-responsive subgroups among mechanically ventilated COVID-19-associated ARDS patients.
Supplementary Information
Additional file 1. Additional data of the study.
Acknowledgements
We thank all the collaborators of the COVID-19/SEMICYUC Working Group who collected these data under challenging circumstances. We thank Alexis Garduno for English manuscript edition. COVID-19 SEMICYUC Working Group authors are listed in Additional file 1.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- ARDS
Acute respiratory distress syndrome
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- HR
Hazard ratio
- ICU
Intensive care unit
- IQR
Interquartile range
- LOS
Length of stay
- OR
Odds ratio
- PaO2/FiO2
Partial pressure of oxygen in arterial blood to fraction of inspired oxygen ratio
- PEEP
Positive end-expiratory pressure
- RCT
Randomized controlled trial
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SD
Standard deviation
- SEMICYUC
Spanish Society of Intensive Care Medicine and Coronary Units
- SOFA
Sequential Organ Failure Assessment
- SOC
Standard of care
- VAP
Ventilator-associated pneumonia
Authors’ contributions
GM is the guarantor of the content of the manuscript, including the data and the analysis. GM, RC, IML, JSV, MB, EC, and AR conceptualized the study. GM, ST, EC and AR accessed and verified the underlying data. GM, RC, ST, EC, JG, MR-B, and AR curated the data. GM, EC, RC, IML, and AR did the formal analysis and contributed to the methodology. MB, JSV, IML, and AR acquired funding and resources. GM, IML, JSV, and AR wrote the original draft of the manuscript. GM, RC, IML, JSV, EC, JG, MRB, ST, MB, JM, ED, PV, EP, AA, SS, LS, MCL, AL, RVA, MTR, JCB, RF, EF, MR, AE, AM, NG, LFR, JMC, and AR contributed substantially to the data analysis interpretation, review and edition of the manuscript. All authors read and approved the final manuscript.
Funding
Ricardo Barri Casanovas Foundation grant (RBCF2020) and SEMICYUC (Spanish Society of Intensive Care Medicine and Coronary Units). The study sponsors have no role in the study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit the paper for publication.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
After approval of the local ethics committee (Ethics Committee board of Joan XXIII University Hospital with registration number IRB# CEIM/066/2020), we prospectively collected data from critical COVID-19 patients admitted to any participating ICU. All data were de-identified, allowing the waiver of informed consent.
Consent for publication
Not applicable.
Competing interests
PV-C reports personal fees and non-financial support from Pfizer, personal fees and non-financial support from MSD, personal fees from Shionogi, personal fees from Menarini, outside the submitted work. RF reports personal fees from Jafron, personal fees from Toray, personal fees from ThermoFisher, personal fees from Grifols, personal fees from Pfizer, personal fees from MSD, personal fees from Becton-Dickinson, personal fees from Shionogi, outside the submitted work. All other authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int. Accessed 3 Mar 2021.
- 2.Quah P, Li A, Phua J, Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care. 2020;24:285. doi: 10.1186/s13054-020-03006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Group TRC Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marmor M, Jonas A. Corticosteroids for COVID-19-associated ARDS. Clin Pulm Med. 2020;27:165–167. [Google Scholar]
- 6.Matthay MA, Thompson BT. Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties. Lancet Respir Med. 2020;8:1170–1172. doi: 10.1016/S2213-2600(20)30503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24:696. doi: 10.1186/s13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarkar S, Khanna P, Soni KD. Are the steroids a blanket solution for COVID-19? A systematic review and meta-analysis. J Med Virol. 2021;93:1538–1547. doi: 10.1002/jmv.26483. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Meng Q, Rao X, Wang B, Zhang X, Dong F, et al. Corticosteroid therapy in critically ill patients with COVID-19: a multicenter, retrospective study. Crit Care. 2020;24:698. doi: 10.1186/s13054-020-03429-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Zhang S, Dong X, Li Z, Xu Q, Feng H, et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020;130:6417–6428. doi: 10.1172/JCI140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Huang J, Zhu G, Liu Y, Xiao H, Zhou Q, et al. Systemic corticosteroids and mortality in severe and critical COVID-19 patients in Wuhan, China. J Clin Endocrinol Metab. 2020;105:dgaa627. doi: 10.1210/clinem/dgaa627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim guidance. 2020. https://apps.who.int/iris/handle/10665/331446. Accessed 3 Mar 2021.
- 13.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 14.Ministry of Health in Spain. Documento técnico Manejo clínico del COVID-19: unidades de cuidados intensivos. 2020. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Protocolo_manejo_clinico_uci_COVID-19.pdf. Accessed 7 Mar 2021.
- 15.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J. 2017;50:1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 16.Diamond A, Sekhon JS. Genetic matching for estimating causal effects: a general multivariate matching method for achieving balance in observational studies. Rev Econ Stat. 2013;95:932–945. [Google Scholar]
- 17.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resche-Rigon M, Azoulay E, Chevret S. Evaluating mortality in intensive care units: contribution of competing risks analyses. Crit Care. 2006;10:R5. doi: 10.1186/cc3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noordzij M, Leffondré K, Van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28:2670–2677. doi: 10.1093/ndt/gft355. [DOI] [PubMed] [Google Scholar]
- 21.Dey T, Mukherjee A, Chakraborty S. A practical overview and reporting strategies for statistical analysis of survival studies. Chest. 2020;158:S39–48. doi: 10.1016/j.chest.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200:828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudhuri D, Sasaki K, Karkar A, Sharif S, Lewis K, Mammen MJ, Alexander P, Ye Z, Lozano LEC, Munch MW, Perner A, Du B, Mbuagbaw L, Alhazzani W, Pastores SM, Marshall J, Lamontagne F, Annane D, Meduri GU, Rochwerg B. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47(5):521–537. doi: 10.1007/s00134-021-06394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cano EJ, Fonseca Fuentes X, Corsini Campioli C, O’Horo JC, Abu Saleh O, Odeyemi Y, et al. Impact of corticosteroids in coronavirus disease 2019 outcomes. Chest. 2021;159:1019–1040. doi: 10.1016/j.chest.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasin L, Navalesi P, Zangrillo A, Kuzovlev A, Likhvantsev V, Hajjar LA, et al. Corticosteroids for patients with coronavirus disease 2019 (COVID-19) with different disease severity: a meta-analysis of randomized clinical trials. J Cardiothorac Vasc Anesth. 2021;35:578–584. doi: 10.1053/j.jvca.2020.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tlayjeh H, Mhish OH, Enani MA, Alruwaili A, Tleyjeh R, Thalib L, Hassett L, Arabi YM, Kashour T, Tleyjeh IM. Association of corticosteroids use and outcomes in COVID-19 patients: a systematic review and meta-analysis. J Infect Public Health. 2020;13(11):1652–1663. doi: 10.1016/j.jiph.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Backer D, Azoulay E, Vincent JL. Corticosteroids in severe COVID-19: a critical view of the evidence. Crit Care. 2020;24:627. doi: 10.1186/s13054-020-03360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, Placebo-controlled trial. Clin Infect Dis. 2021;72:e373–e381. doi: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monedero P, Gea A, Castro P, Candela-Toha AM, Hernández-Sanz ML, Arruti E, et al. Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: a cohort study. Crit Care. 2021;25:2. doi: 10.1186/s13054-020-03422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Song Y, Wang L, Zhang Y, Han L, Liu J, et al. Corticosteroids treatment in severe patients with COVID-19: a propensity score matching study. Expert Rev Respir Med. 2021;15:543–552. doi: 10.1080/17476348.2021.1856659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu C, Hou D, Du C, Cai Y, Zheng J, Xu J, et al. Corticosteroid therapy for coronavirus disease 2019-related acute respiratory distress syndrome: a cohort study with propensity score analysis. Crit Care. 2020;24:643. doi: 10.1186/s13054-020-03340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulakurthi YS, Pederson JM, Saravu K, Gupta N, Balasubramanian P, Kamrowski S, Schmidt M, Vegivinti CTR, Dibas M, Reierson NL, Pisipati S, Joseph BA, Selvan PT, Dmytriw AA, Keesari PR, Sriram V, Chittajallu S, Brinjikji W, Katamreddy RR, Chibbar R, Davis AR, Malpe M, Mishra HK, Kallmes KM, Hassan AE, Evanson KW. Corticosteroid therapy for COVID-19: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2021;100(20):e25719. doi: 10.1097/MD.0000000000025719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Loeches I, Torres A. Corticosteroids for CAP, influenza and COVID-19: when, how and benefits or harm? Eur Respir Rev. 2021;30:200346. doi: 10.1183/16000617.0346-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarfraz A, Sarfraz Z, Razzack AA, Patel G, Sarfraz M. Venous thromboembolism, corticosteroids and COVID-19: a systematic review and meta-analysis. Clin Appl Thromb Hemost. 2021 doi: 10.1177/1076029621993573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martínez M, Care C, Martínez MM, Menchaca EPP, Nuvials FX, Roca O, et al. Risk factors and outcomes of ventilator-associated pneumonia in COVID-19 patients: a propensity score matched analysis. Crit Care. 2021;25:235. doi: 10.1186/s13054-021-03654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferreira JC, Ho YL, Besen BAMP, Malbouisson LMS, Taniguchi LU, Mendes PV, et al. Protective ventilation and outcomes of critically ill patients with COVID-19: a cohort study. Ann Intensive Care. 2021;11:92. doi: 10.1186/s13613-021-00882-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Investigators C-IG on behalf of the RN and the C-I Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mac Sweeney R, McAuley DF. Acute respiratory distress syndrome. Lancet. 2016;388:2416–2430. doi: 10.1016/S0140-6736(16)00578-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dupuis C, de Montmollin E, Buetti N, Goldgran-Toledano D, Reignier J, Schwebel C, et al. Impact of early corticosteroids on 60-day mortality in critically ill patients with COVID-19: a multicenter cohort study of the OUTCOMEREA network. PLoS ONE. 2021;16:e0255644. doi: 10.1371/journal.pone.0255644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodríguez A, Ruiz-Botella M, Martín-Loeches I, Herrera MJ, Solé-Violan J, Gómez J, et al. Deploying unsupervised clustering analysis to derive clinical phenotypes and risk factors associated with mortality risk in 2022 critically ill patients with COVID-19 in Spain. Crit Care. 2021;25:63. doi: 10.1186/s13054-021-03487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutiérrez-Gutiérrez B, Del Toro MD, Borobia AM, Carcas A, Jarrín I, Yllescas M, et al. Identification and validation of clinical phenotypes with prognostic implications in patients admitted to hospital with COVID-19: a multicentre cohort study. Lancet Infect Dis. 2021;21:783–792. doi: 10.1016/S1473-3099(21)00019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H, Xie J, Su N, Wang J, Sun Q, Li S, et al. Corticosteroid therapy is associated with improved outcome in critically ill coronavirus disease 2019 patients with hyperinflammatory phenotype. Chest. 2021;159:1793–1802. doi: 10.1016/j.chest.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2017;43:1751–1763. doi: 10.1007/s00134-017-4919-5. [DOI] [PubMed] [Google Scholar]
- 46.Díaz E, Amézaga Menéndez R, Vidal Cortés P, Escapa MG, Suberviola B, Serrano Lázaro A, et al. Pharmacological treatment of COVID-19: narrative review of the Working Group in Infectious Diseases and Sepsis (GTEIS) and the Working Groups in Transfusions and Blood Products (GTTH) Med Intensiva. 2021;45:104–121. doi: 10.1016/j.medin.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robert R, Kentish-Barnes N, Boyer A, Laurent A, Azoulay E, Reignier J. Ethical dilemmas due to the Covid-19 pandemic. Ann Intensive Care. 2020;10:84. doi: 10.1186/s13613-020-00702-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vincent JL, Taccone FS. Understanding pathways to death in patients with COVID-19. Lancet Respir Med. 2020;8:430–432. doi: 10.1016/S2213-2600(20)30165-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional data of the study.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.