The perception that cannabis can be safely consumed without health risks or that use is safer than alternative recreational drugs is perhaps an unintended consequence of recent marijuana legalization campaigns in the United States and other countries. Often missing in the debates about cannabis legalization is the potential harm caused to the next generation. These would include not just teenagers, for which laws are often written to exclude, but those still in utero who are exposed to everything their mothers consume or are exposed to. Cannabis is becoming one of the most prevalent recreational drugs of abuse used during pregnancy, with an estimated prevalence of 3 to 16% among pregnant women in the United States (1). An increase of 64% in maternal cannabis use from 2001 to 2014 was observed in one study (2). Unlike for in utero tobacco and alcohol exposures, there is currently a major gap in research on the effects of maternal cannabis use on child outcomes and mechanisms of action, particularly in humans. In PNAS, Rompala et al. (3) publish a study that adds important insights to both the outcomes and the possible mechanisms of maternal cannabis use during pregnancy.
What was previously known is that the psychoactive component in cannabis, tetrahydrocannabinol (THC), passes freely from maternal blood through the placenta to the fetus (4). Maternal cannabis use is associated with complications in pregnancy, including low birth weight and preterm birth (5). THC is known to target the endocannabinoid system, which is involved in the body’s homeostatic mechanisms of balancing internal systems (e.g., mood, immune, temperature, etc.) with energy needs, particularly important during pregnancy and brain development (6). In a few studies, maternal cannabis use has been associated with adverse cognitive and mental health outcomes in children, including autism, depression, and psychosis (7–9). Animal studies have confirmed some behavioral changes associated with prenatal cannabis exposure, including increased anxiogenic and drug-seeking behaviors in offspring (4).
Leveraging a longitudinal cohort of mother–child dyads from the New York metropolitan area, Rompala et al. (3) first examined the effect of maternal cannabis use in relation to offspring development. Cannabis use was self-reported, and both pre- and postnatal uses were combined as “maternal cannabis use.” From a cohort of 322 mother–child dyads, 71 (22%) were classified as maternal cannabis users. Maternal cannabis users were slightly more likely to be African American, younger, and single and had higher rates of anxiety, depression, and cigarette use than cannabis nonusers, but these confounding variables were adjusted for in outcome data. Maternal cannabis use was significantly associated with increased anxiety in the child (3 to 6 y old) according to three separate measures: increased cortisol levels from hair, elevated anxiety traits measured on the Behavioral Assessment System for Children survey, and reduced heart rate variability at rest and during auditory startle.
The authors importantly made use of term placenta samples frozen within 1 h after birth in a subset of pregnancies from this cohort (∼40%) to provide an important mechanistic link to cannabis use and child anxiety (3). Term placenta is a normal by-product of birth that is usually disposed with medical waste after hospital deliveries, but if collected and promptly frozen, it can be incredibly useful as a “time capsule” of molecular signatures of in utero exposures. Placenta is a complex tissue of fetal origin predominantly composed of trophoblast and immune cells. In this study, placentas were biopsied equidistant from the umbilical cord on the fetal side of the placenta. Placental transcriptomes were analyzed from RNA sequencing, comparing those from mothers who self-reported cannabis use vs. the nonusers. While none of the 480 differentially expressed genes passed false discovery correction, as a group they were significantly enriched for gene pathways involved in immune responses coming from monocytes, specifically type I interferon and other cytokine signaling, neutrophil functions, and inflammatory response. The majority of these immune pathway genes were at lower levels in placenta from mothers who used cannabis, indicating immunosuppression.
The authors also complemented the standard bioinformatic approach for placental transcriptome comparisons with a systems biology approach based on weighted gene correlation network analyses (10). This approach condenses groups of genes that vary together into a smaller number of coexpressed modules for which a single “eigengene” value can then be examined for associations with other measurements in the dataset: in this case, the child behavior scores of anxiety, hyperactivity, aggression, and depression (3). Three gene modules from the placental transcriptome were significantly associated with child anxiety, and these contained genes enriched for functions in type I interferon signaling and immune response. These anxiety-associated modules included genes encoding the histocompatibility genes HLA-A, -B, and -F, which are involved in adaptive immune responses, as well as the interferon-responsive IFI-35, which is involved in regulation of innate immunity. Like the results of the individual gene analyses, these immune response genes were suppressed in the placenta samples from pregnancies with self-reported cannabis use.
These results raise important potential implications for the mechanisms of cannabis exposure during pregnancy. Cannabis use in adulthood is a known modulator of immune responses and is being considered for medical use in treating some autoimmune disorders (11). Placental immunosuppression associated with maternal cannabis use may, therefore, have detrimental effects on the ability to fight off viral infections, such as Zika virus or severe acute respiratory syndrome coronavirus 2 (12). However, perhaps less obvious are the nondefense functions of the innate immune system that have been repurposed by the recent evolution of placentation in eutherian mammals (13). The invasion of fetal trophoblast cells of the placenta into maternal tissue evokes an innate immune response from uterine natural killer cells and macrophages in the placental bed. A functioning innate immune system is required for the steps of successful placentation, including trophoblast invasion that brings in a blood supply, called angiogenesis. Disruptions in these immune processes are characteristic of poor placentation observed in preeclampsia and other pregnancy complications (14). Recently, evidence for a protective role of macrophages in preventing preterm birth was observed in both human pregnancies and a mouse model (15). This raises the interesting question that perhaps the link between maternal cannabis use and preterm birth may be through suppression of placental monocytes and macrophages.
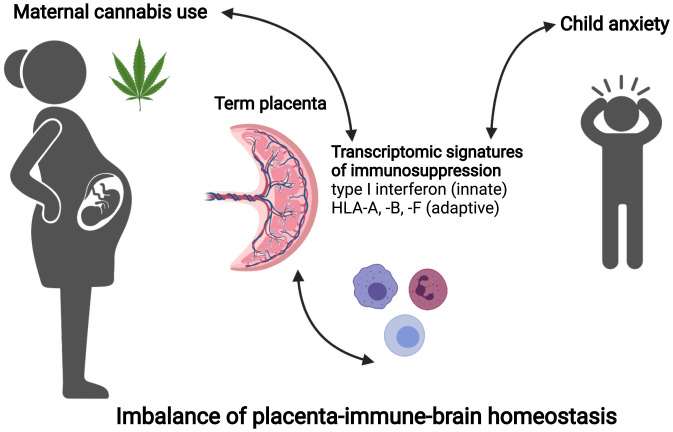
However, how may placental immunosuppression associated with cannabis exposure be linked to anxiety in the child? This is somewhat puzzling since most of the clinical and preclinical evidence to date linking immune responses with mental health conditions in offspring is due to an activated maternal immune system (16, 17). The limitations of this human study are that correlations are not necessarily causation, so we do not know if the elevated child anxiety observed in cannabis-using pregnancies was a result of direct exposure in utero, immune differences present in the placenta at the time of birth, or differences in the home environment. Also, the authors could not distinguish prenatal from postnatal cannabis use from their self-reporting data. However, the placental transcriptional modules linking the reduced placental immune responses to child anxiety suggest a possible homeostatic imbalance of the maternal immune response reflected in the placenta (Fig. 1)—in other words, a “Goldilocks effect.” Too much of an immune response likely drains energy and resources that could be directed to the developing brain, increasing the risk for schizophrenia or autism, but too little immune response will likely compromise the placental blood supplying nutrients and oxygen to the fetus early in pregnancy, as well as the precise timing of labor and delivery at the end.
Fig. 1.
Placental immune suppression links maternal cannabis and child anxiety. In the study by Rompala et al. (3), a systems biology approach to transcriptomic analyses revealed clusters of genes involved in innate and adaptive immune responses that distinguished placenta samples from mothers who used cannabis from those who did not. Suppression of these immune response genes was also significantly associated with elevated anxiety in the child (age 3 to 6 y). Immune responses are beneficial for healthy placentation and the timing of labor, so cannabis-induced immunosuppression in the placenta may impact child behavior by direct or indirect homeostatic mechanisms in the placental–immune–brain interface.
Like all early research into a relatively new field, this study raises more questions than it answers, leaving much room for future research. More animal models are needed, but because of the recent evolution of the mammalian placenta and higher brain functions, nonhuman primate models may turn out to be the most informative for human relevance. Human pregnancy cohorts that collect and store term placentas as well as measure actual cannabis use prenatally will be critical for moving understanding forward. Furthermore, use of improved statistical and bioinformatic methods that adjust for the many potential confounding variables present in this and other human studies will be important. Sex differences in the response to in utero cannabis exposure are expected and will be important to investigate in future human and preclinical models (18). In addition to future investigations of the placental transcriptome, extending investigation into epigenetic marks, such as the placental methylome, is expected to improve understanding of the placental–brain axis in maternal cannabis exposures (19). While some rodent studies have shown alterations to DNA methylation or histone modifications, there has been a paucity of human epigenetic studies on in utero cannabis exposure. Because of the striking landscape of the human placental methylome, where DNA methylation levels over genes reflect past and present expression (20), human epigenomic studies may reveal additional insights not revealed by placental transcriptomics.
There are certainly ethical considerations for future research on maternal use of recreational drugs, such as cannabis. Best practices should include removing the blame and stigma placed on expecting mothers (including referral to child protection agencies in some states) in favor of education and support. Listening to the concerns of birthing people from underserved and underresourced communities is particularly important in human research endeavors to avoid misconceptions, psychological harm, and further minoritization of research participants. Ultimately, a goal is the discovery of molecular biomarkers from placenta that may be sampled from maternal blood in the form of cell-free fetal DNA during pregnancy. The difference would be a specific diagnostic test for personalized fetal changes that may be counteracted, rather than simpler tests of drug use that may be used to establish maternal blame without direct proof of harm to the child.
Acknowledgments
I thank Leigh Ann Simmons for a critical read and comments on the manuscript.
Footnotes
The author declares no competing interests.
See companion article, “Maternal cannabis use is associated with suppression of immune gene networks in placenta and increased anxiety phenotypes in offspring,” 10.1073/pnas.2106115118.
References
- 1.Volkow N. D., Han B., Compton W. M., McCance-Katz E. F., Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA 322, 167–169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown Q. L., et al. , Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002-2014. JAMA 317, 207–209 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rompala G., Nomura Y., Hurd Y. L., Maternal cannabis use is associated with suppression of immune gene networks in placenta and increased anxiety phenotypes in offspring. Proc. Natl. Acad. Sci. U.S.A., (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bara A., Ferland J. N., Rompala G., Szutorisz H., Hurd Y. L., Cannabis and synaptic reprogramming of the developing brain. Nat. Rev. Neurosci. 22, 423–438 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Memedovich K. A., Dowsett L. E., Spackman E., Noseworthy T., Clement F., The adverse health effects and harms related to marijuana use: An overview review. CMAJ Open 6, E339–E346 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowe H., Toyang N., Steele B., Bryant J., Ngwa W., The endocannabinoid system: A potential target for the treatment of various diseases. Int. J. Mol. Sci. 22, 9472 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corsi D. J., et al. , Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat. Med. 26, 1536–1540 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Fine J. D., et al. , Association of prenatal cannabis exposure with psychosis proneness among children in the Adolescent Brain Cognitive Development (ABCD) Study. JAMA Psychiatry 76, 762–764 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray K. A., Day N. L., Leech S., Richardson G. A., Prenatal marijuana exposure: Effect on child depressive symptoms at ten years of age. Neurotoxicol. Teratol. 27, 439–448 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Langfelder P., Horvath S., WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giorgi V., et al. , Cannabis and autoimmunity: Possible mechanisms of action. ImmunoTargets Ther. 10, 261–271 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maggirwar S. B., Khalsa J. H., The link between cannabis use, immune system, and viral infections. Viruses 13, 1099 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao Y., et al. , Baby genomics: Tracing the evolutionary changes that gave rise to placentation. Genome Biol. Evol. 12, 35–47 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faas M. M., De Vos P., Innate immune cells in the placental bed in healthy pregnancy and preeclampsia. Placenta 69, 125–133 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Lopez N., et al. , Macrophages exert homeostatic actions in pregnancy to protect against preterm birth and fetal inflammatory injury. JCI Insight 6, e146089 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quagliato L. A., de Matos U., Nardi A. E., Maternal immune activation generates anxiety in offspring: A translational meta-analysis. Transl. Psychiatry 11, 245 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han V. X., Patel S., Jones H. F., Dale R. C., Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 17, 564–579 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Bara A., et al. , Sex-Dependent Effects of In Utero Cannabinoid Exposure on Cortical Function (Elife, Cambridge, UK, 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wanner N. M., Colwell M. L., Faulk C., The epigenetic legacy of illicit drugs: Developmental exposures and late-life phenotypes. Environ. Epigenet. 5, dvz022 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroeder D. I., et al. , The human placenta methylome. Proc. Natl. Acad. Sci. U.S.A. 110, 6037–6042 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]