In January 2021, New York’s Northwell Health hospital system launched a clinical trial to learn whether the over-the-counter drug famotidine (also known as Pepcid) reduces the severity of COVID-19 in symptomatic patients who do not require hospitalization. The randomized trial began in response to anecdotal reports along with clinical studies showing that Pepcid benefited COVID-19 patients (1). But the trial had a twist: It was completely virtual; no visits to a research site required (2). In fact, it was the first fully virtual clinical trial for the health system. Northwell leaders say it won’t be the last.
“COVID has sped up this process, and for the better,” says Christina Brennan, VP for clinical research at Northwell’s Feinstein Institutes for Medical Research. Brennan says that the initial design for the trial called for on-site visits, and that trial leaders pivoted upon realizing that prospective participants preferred to recuperate at home. The virtual model has enabled Northwell to recruit a more diverse set of participants than in its other trials, Brennan says, perhaps because on-site visits were not a barrier. Drugs (either Pepcid or placebo) were mailed to participants, and all laboratory draws were done at their home. The trial, which Northwell conducted in partnership with the Cold Spring Harbor Laboratory, NY, has concluded enrollment as of September 2021. Data analysis is ongoing.
Smartwatches are among the technologies that can facilitate large, decentralized trials. Patients monitor aspects of their health as they go about their daily lives. Image credit: Shutterstock/Andrey_Popov.
Northwell is not alone in direct mailing—the practice became widespread during the pandemic. “That was basically ubiquitous. For all of our trials with oral therapy, we kept patients at home,” says Keith Flaherty of Massachusetts General Hospital in Boston. Flaherty says a high priority in the early days of the pandemic was maintaining access to experimental treatments, including among those patients who were already enrolled in a clinical trial.
Direct mailing aside, some clinicians have also allowed participants to sign consent forms remotely rather than on-site, and the researchers are remotely monitoring how people progressed during a trial [see Fig. 1 (3)]. This could, for example, involve using a smartwatch that measures someone’s heart rate or oxygen saturation levels, or a telemedicine visit via video, or a phone call in which participants report how they are responding to an investigational treatment.
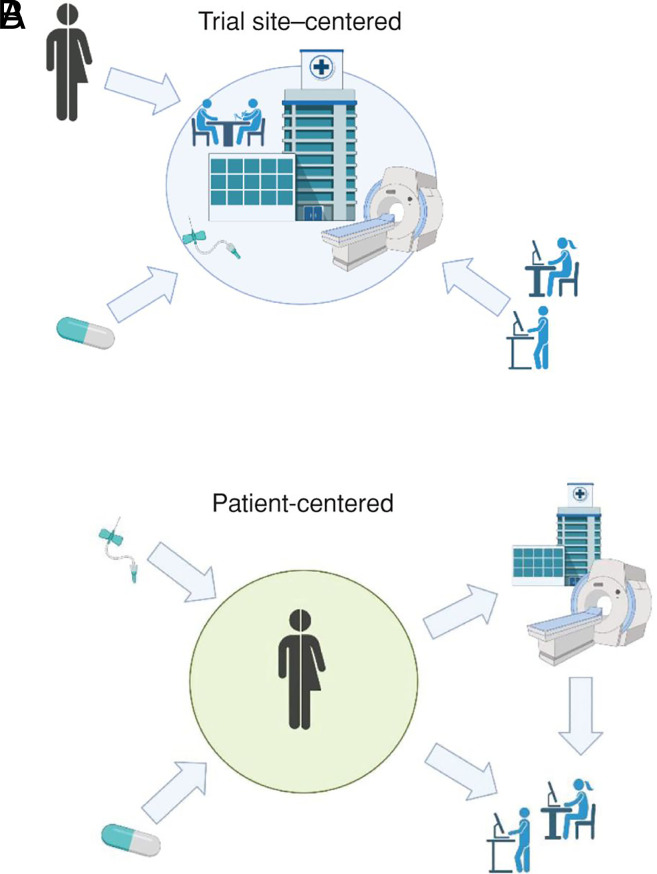
Fig. 1.
Many trials have moved to decentralized formats as physicians, researchers, and patients attempt to adapt amid the COVID-19 pandemic. Decentralized trials put the patient, not the clinical trial site, at the center of the study, with counseling, consent, and testing conducted remotely. Image credit: Reprinted from ref. 3, with permission from AACR.
Such changes collectively constitute “decentralized clinical trials.” Advocates say that the approach enables faster and more diverse enrollments in research studies, whereas skeptics warn that conducting clinical trials in uncontrolled environments could lead to faulty data and flawed conclusions. Regardless, the COVID-19 pandemic has prompted debate about the role and ramifications of these unconventional studies.
Increasing Participation
Like traditional trials, a decentralized trial can assess the impact of a new medication or of a lifestyle modification—say, exercising more regularly. It can either be fully remote, such as the Northwell Pepcid study, or combine in-person and remote activities.
The idea of a decentralized trial dates back to the early 2000s, according to Craig Lipset, who led the first fully remote trial for Pfizer in 2011 (4). “And a lot of the methods that we’re bundling together to call decentralized date back longer than that, such as using electronic diaries,” adds Lipset, who cochairs a consultancy aimed at speeding adoption of decentralized trials. Lipset points to a feasibility study about conducting decentralized trials in 2003 (5) and a fully decentralized study of anxiety and insomnia treatments in 2005 (6).
But it took COVID-19 to finally move the needle in a fundamental way, says Lipset. Shortly before the pandemic, fewer than half of representatives of pharmaceutical companies, or of the organizations pharma companies contract with to conduct clinical trials, expected remote trials to be a major part of their portfolios, according to the New York consulting firm McKinsey. When McKinsey revisited the question one year later, that number was 100% (7).
“Everyone was like, ‘Oh, we’ll do this over the next five to ten years. It’s really hard,’” says Andy Coravos, CEO of the digital health consultancy HumanFirst in San Francisco, CA. “All of a sudden COVID hit, and it wasn’t that hard.”
Coravos has long been an advocate of decentralized clinical trials. When she wrote about the prospect in 2018, Coravos emphasized that less than 5% of the US population participates in clinical research and that more than 70% of the population lives more than two hours from an academic medical center. Plus, Coravos says, clinical trial sites are artificial environments that don’t reflect the daily realities of how people live with a disease or health challenge. Connected sensors such as smartwatches, wireless blood pressure cuffs, or home-based sleep monitors could, in theory, bridge this gap (8).
The Heartline trial, launched in February 2020 just before the pandemic started to take hold, demonstrates just how valuable a decentralized approach can be (9). Thanks to the decentralized model, trial investigators successfully continued recruitment throughout the pandemic without needing to change their study protocol. Recruitment is ongoing, and researchers hope to enroll 150,000 participants 65 or older.
The goal of Heartline is to learn whether earlier alerts of the irregular heartbeat pattern, and stroke warning sign, known as atrial fibrillation (AFib), can inspire heart-healthy behaviors that reduce risk of stroke or death. All Heartline participants have access to the iPhone’s Heartline app, which reminds and advises people to practice heart health. Some participants have an Apple Watch, which can alert them of possible instances of AFib. Heartline leaders want to assess whether the group who receives these alerts engages in healthier behaviors than those who do not, as measured by diverging incidence of death or stroke in the two groups over time.
In a traditional trial, people would only learn about potential AFib periodically, during visits to a study site. The decentralized model, says study leader C. Michael Gibson, brings to light previously invisible instances of AFib. It also allows clinicians to “collect hard outcomes, like death and stroke,” adds Gibson, a Harvard University cardiologist in Cambridge, MA. That said, Gibson does think that tracking more “subtle end points,” such as fluctuations in a patient’s lung capacity, will continue to require visits to clinics. Because the study is ongoing, it’s not yet clear whether the smartwatch alerts will in fact improve health outcomes.
Pragmatics, Privacy, and Data Integrity
Logistics can be challenging when running a decentralized trial. Writing in the June issue of Applied Clinical Trials, Ken Getz of the Tufts Center for the Study of Drug Development in Boston, MA, forecasts “retrenchment and more conservative application of adaptive and innovative practices as we move into a post-pandemic world” (10). Getz points to survey data showing that the people who run such trials often spend an “inordinate” amount of time learning new software or coordinating disparate activities in far-flung locations.
And then there’s the task at the heart of any trial: data collection. “I do have some concerns, mainly related to the integrity and the validity of the data collection,” says Mary McDermott, a professor of medicine at Northwestern University in Chicago, IL, who treats and studies vascular diseases that can make it hard for older people to walk easily.
McDermott points to, for example, the six-minute walk test, which measures how people with peripheral artery disease are responding to exercise strategies meant to increase their walking capacity. The proper way to do this test to ensure reliable and comparable results, McDermott says, is always along a 100-foot-long hallway that is always the same surface; people walk back and forth as much as they can for six minutes. At home, people might do the test on carpet one time and a hard floor the next, McDermott notes, or not have 100 feet of unobstructed space to begin with. All such variables are controlled at a research site.
Always a concern, ensuring patient privacy presents special challenges for decentralized trials. Without strong privacy protections that specify how frequently data collected from a health sensor can be used, and for what purpose, insurers could potentially discriminate against people whose, say, smartwatch indicates labored breathing or frequent heart flutters. The hacking of such health data, as with any online information, is another concern. “I really think we need some version of the Genetic Information Nondiscrimination Act for connected sensors and digital data,” says Coravos.
More Inclusive Clinical Trials
The rationale for these trials is, in part, increasing participant diversity. Some public health experts argue, however, that diversity will only increase significantly with proactive outreach by clinical trial leaders. “Many lower-income individuals, many people of color, don’t access care at large academic research centers, so they’re not even aware that clinical trials occur,” says Bisola Ojikutu, a Harvard physician who is also the executive director of the Boston Public Health Commission.
“I do have some concerns, mainly related to the integrity and the validity of the data collection.”
—Mary McDermott
Ojikutu says that trial leaders should go into neighborhoods to explain that their clinical trial exists and how to participate. And because many decentralized trials will have an online component, Ojikutu suggests budgeting for data plans and any necessary equipment to make sure that nobody fails to participate in a trial owing to lack of Internet access. Also, to ensure that a confusing app or interface doesn’t stymie participation, Ojikutu suggests that trial designers include instruction in technological literacy so that all participants can engage equally.
Challenges aside, it seems likely that decentralized trials are here to stay, at least in some form. The recently proposed Cures 2.0 Act in Congress calls for the FDA Commissioner to offer guidance on how to formalize regulation of decentralized trials, including how to evaluate digitally collected health data (11). Cures 2.0 will likely be up for consideration in Congress before year’s end.
Such a framework could be essential to driving widespread adoption of decentralized trials. Flaherty says that drug companies are the most cautious about moving to decentralized trials—compared with trial participants or clinicians—out of concern that any new treatment studied with such a cutting-edge approach won’t ultimately receive FDA approval. And even decentralized trial advocates are moving cautiously. The Heartline trial does not involve any drugs, and Flaherty only began mailing drugs to patients as part of clinical trials taking place during the pandemic, via the emergency authority the FDA provided.
Meanwhile, the debate about the wisdom of decentralized trials continues. “People don’t want to travel. I feel stronger than ever that it just makes a ton more sense at home,” Coravos says. But others encourage clinicians to tread carefully. “There’s all kinds of things,” McDermott notes, “that could interfere with how the data are collected.”
Change History
November 24, 2021: The article text has been updated.
References
- 1.Freedberg D. E., et al. ; Famotidine Research Group, Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology 159, 1129–1131.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libassi M., Northwell, CSHL open virtual Covid-19 clinical trial for non-hospitalized patients (January 27, 2021). https://feinstein.northwell.edu/news/the-latest/-northwell-cshl-open-virtual-covid-19-clinical-trial-for-non-hospitalized-patients. Accessed 15 September 2021.
- 3.Flaherty K. T., et al. , Rethinking cancer clinical trial conduct induced by COVID-19: An academic center, industry, government, and regulatory agency perspective. Cancer Discov. 11, 1881–1885 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfizer, Pfizer conducts first “virtual” clinical trial allowing patients to participate regardless of geography (June 7, 2011). https://www.pfizer.com/news/press-release/press-release-detail/pfizer_conducts_first_virtual_clinical_trial_allowing_patients_to_participate_regardless_of_geography. Accessed 15 September 2021.
- 5.McAlindon T., Formica M., Kabbara K., LaValley M., Lehmer M., Conducting clinical trials over the internet: Feasibility study. BMJ 327, 484–487 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs B. P., Bent S., Tice J. A., Blackwell T., Cummings S. R., An internet-based randomized, placebo-controlled trial of kava and valerian for anxiety and insomnia. Medicine (Baltimore) 84, 197–207 (2005). [DOI] [PubMed] [Google Scholar]
- 7.McKinsey& Company, No place like home? Stepping up the decentralization of clinical trials (June 10, 2021). https://www.mckinsey.com/industries/life-sciences/our-insights/no-place-like-home-stepping-up-the-decentralization-of-clinical-trials. Accessed 15 September 2021.
- 8.Coravos A., Decentralized clinical trials (October 15, 2018). https://blog.andreacoravos.com/decentralized-clinical-trials-e9dbde90ea95. Accessed 24 September 2021.
- 9.Johnson & Johnson, Johnson and Johnson launches Heartline, the first-of-its-kind, virtual study designed to explore if a new iPhone app and Apple Watch can help reduce the risk of stroke (February 25, 2020). https://www.jnj.com/johnson-johnson-launches-heartline-the-first-of-its-kind-virtual-study-designed-to-explore-if-a-new-iphone-app-and-apple-watch-can-help-reduce-the-risk-of-stroke. Accessed 15 September 2021.
- 10.Getz K., Contemplating the full measure of pandemic response. Applied Clinical Trials (June 7, 2021). https://www.appliedclinicaltrialsonline.com/view/contemplating-the-full-measure-of-pandemic-response. Accessed 22 September 2021.
- 11.Discussion draft, Cures 2.0 Act (June 21, 2021). https://degette.house.gov/sites/degette.house.gov/files/Cures%202.0_DISCUSSION%20DRAFT.pdf. Accessed 15 September 2021.