Significance
TNF receptor–associated factor 6 (TRAF6) is a key signaling molecule in innate immune response. Here, we identify a previously unrecognized role of protein phosphatase 4 in the regulation of TRAF6 activation through dephosphorylation of a native TRAF6 inhibitor, somatic nuclear autoantigenic sperm protein, to resolve the inflammatory response initiated by Toll-like receptor 4.
Keywords: Toll-like receptor 4, TRAF6, kinase, phosphatase, signaling
Abstract
Recognition of invading pathogens by Toll-like receptors (TLRs) activates innate immunity through signaling pathways that involved multiple protein kinases and phosphatases. We previously demonstrated that somatic nuclear autoantigenic sperm protein (sNASP) binds to TNF receptor–associated factor 6 (TRAF6) in the resting state. Upon TLR4 activation, a signaling complex consisting of TRAF6, sNASP, interleukin (IL)-1 receptor–associated kinase 4, and casein kinase 2 (CK2) is formed. CK2 then phosphorylates sNASP to release phospho-sNASP (p-sNASP) from TRAF6, initiating downstream signaling pathways. Here, we showed that protein phosphatase 4 (PP4) is the specific sNASP phosphatase that negatively regulates TLR4-induced TRAF6 activation and its downstream signaling pathway. Mechanistically, PP4 is directly recruited by phosphorylated sNASP to dephosphorylate p-sNASP to terminate TRAF6 activation. Ectopic expression of PP4 specifically inhibited sNASP-dependent proinflammatory cytokine production and downstream signaling following bacterial lipopolysaccharide (LPS) treatment, whereas silencing PP4 had the opposite effect. Primary macrophages and mice infected with recombinant adenovirus carrying a gene encoding PP4 (Ad-PP4) showed significant reduction in IL-6 and TNF-α production. Survival of Ad-PP4–infected mice was markedly increased due to a better ability to clear bacteria in a sepsis model. These results indicate that the serine/threonine phosphatase PP4 functions as a negative regulator of innate immunity by regulating the binding of sNASP to TRAF6.
Toll-like receptors (TLRs) are crucial for initiating the innate immune response and are initiated by recognizing pathogen-associated molecular patterns (PAMPs) such as bacterial lipopolysaccharide (LPS) (1–3). Upon detection of PAMPs, most TLRs (except TLR3), associate with the adaptor protein MyD88, which initiates the signaling process through interleukin 1 receptor–associated kinases and subsequently promotes the auto-ubiquitination of TNF receptor–associated factor 6 (TRAF6). Ubiquitinated TRAF6 then transmits intracellular signals through phosphorylation of transforming growth factor β-activated protein kinase 1 (TAK1), which results in the activation of mitogen-activated protein kinases (MAPKs) and nuclear factor kappa B (NF-κB) and subsequent production of a wide range of immune stimulatory cytokines and chemokines to aid against invading microorganisms (1, 4, 5). Dysregulation of TLRs signaling was implicated in human diseases, including sepsis, autoimmune diseases, cancer, and pulmonary fibrosis (6–13). Thus, tight regulation of TLRs signaling is necessary for homeostasis of an appropriate immune response.
Reversible protein phosphorylation and dephosphorylation serve as regulatory switches by regulating the activation and deactivation of multiple TLR-dependent signaling molecules, such as extracellular signal–related kinase (ERK), c-jun N-terminal kinase (JNK), p38α MAPK, IκB kinase (IKK), and interferon regulatory factor 3 (IRF3) (4, 14). The role of kinases in regulating the response of macrophages to TLR signaling has been extensively studied. Phosphorylation of both p38α and JNK directly mediated by TAK1 is critical for TLR-induced MAPK activation (15). The NF-κB subunit precursor protein p105 undergoes phosphorylation, which is necessary for K48-linked ubiquitylation and proteasome-mediated proteolysis of the IKK complex (16). Moreover, casein kinase 2 (CK2) was found to phosphorylate IκBα and the p65 unit to regulate NF-κB–mediated inflammation (17, 18). Our previous study has also indicated that CK2-mediated phosphorylation of somatic nuclear autoantigenic sperm protein (sNASP) is a key element activating TRAF6 downstream signaling during LPS stimulation, but it is still unknown how the sNASP/TRAF6-mediated pathway is negatively regulated (19).
Many proteins were identified that limit TLR signaling through regulating protein dephosphorylation, such as SH2-containing protein tyrosine phosphatase 2 (SHP-2) (20), NLR family member X1 (21), NLRP6 (22), and protein phosphatase 2A (PP2A) (23). As an example, PP2A suppresses TLRs-triggered type I IFN signaling by deactivation of IRF3 via dephosphorylation. Furthermore, PP2A-deficient macrophages showed enhanced type I IFN signaling upon stomatitis virus infection (23). SHP-2 negatively regulated TLR4- and TLR3-activated IFN-β and production of proinflammatory cytokines, interleukin (IL)-6 and TNF-α, by directly binding TANK binding kinase (20). Here, we report that the serine and threonine protein phosphatase 4 (PP4) is the phosphatase for phosphorylated sNASP and that after TLR4 stimulation, phospho-sNASP (p-sNASP) is dephosphorylated to return to a resting state. Our results also suggested that dephosphorylation of sNASP plays an essential role in the regulation of the LPS signaling cascade. The interaction between sNASP and PP4 is mediated by phosphorylation of sNASP by CK2. The phosphorylated sNASP/PP4 complex was found to transiently form upon LPS challenge. In vivo, PP4 overexpression alleviates the expression of proinflammatory cytokine in macrophages and protects mice from endotoxic shock. Thus, we present a critical role of PP4 in TLR signaling that targets phosphorylated sNASP, which contributes to homeostasis of the TLR/sNASP/TRAF6 axis in the innate immune response.
PP4 is a serine/threonine protein phosphatase that is comprised of a catalytic subunit (PP4C) and regulatory subunits PP4R (24, 25). PP4 has been highly conserved during eukaryote evolution, suggesting that PP4, like PP2A and PP6, might have critical functions in vivo (26). Although previous studies showed that PP4 was implicated in many cellular processes, including organelle assembly (27, 28), DNA damage repair (29), embryo development (30), pro-B-cell development (31), and regulatory T cell functions (32), its roles in regulation of proinflammatory signaling and innate immunity have not been demonstrated. In the present study, we identified serine/threonine phosphatase PP4 as a specific phosphatase for sNASP that is essential for the termination of TRAF6 activation. This knowledge may help in the developing therapeutic strategies for deregulating proinflammatory cytokine.
Results
PP4 Mediates Dephosphorylation of sNASP and Inhibits Activation of NF-κB.
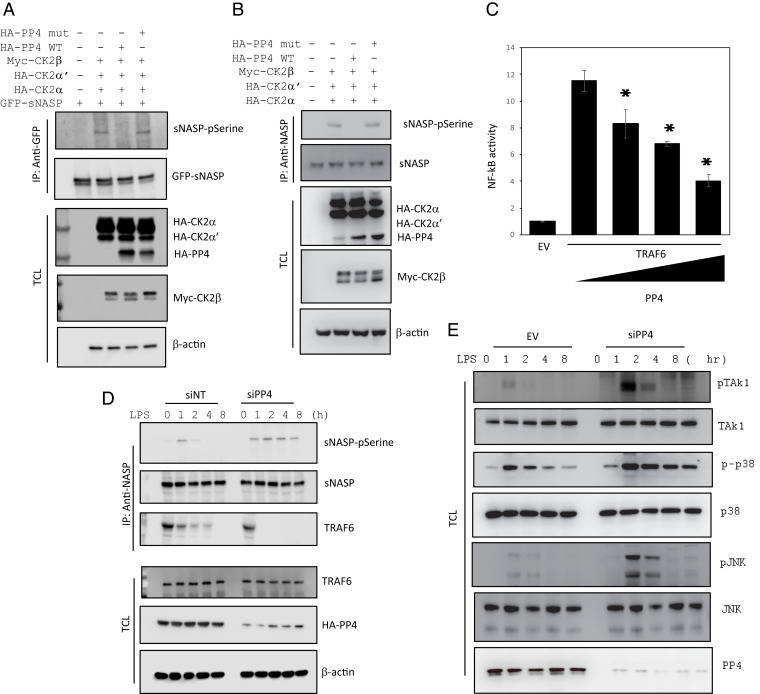
Phosphorylation of sNASP by CK2 is a critical step in activating innate immune responses (19). However, the underlying mechanism behind termination of sNASP-mediated immune responses is still unclear. Here, we showed that PP4 binds to and dephosphorylates p-sNASP to regulate TRAF6 signaling. To determine whether sNASP is a direct target of PP4, we transfected GFP-sNASP and CK2 subunits composed of two catalytic subunits (HA-CK2α and HA-CK2α’) and one regulatory subunit (Myc-CK2β) into HEK293 cells with PP4 wildtype (WT) or mutant and then performed coimmunoprecipitation experiments. Consistent with our previous results, overexpression of the CK2 subunits resulted in a marked increase in serine-phosphorylation of sNASP. However, HEK293 cells expressing WT PP4, but not the PP4 phosphatase-inactive mutant, exhibited markedly reduced serine-phosphorylation of sNASP (Fig. 1A). Similar results were confirmed in THP-1 cells with dephosphorylation of CK2-mediated endogenous p-sNASP by PP4 WT, not mutant (Fig. 1B). LPS-induced phosphorylation of endogenous sNASP was decreased when PP4 overexpressed in THP-1 cells (SI Appendix, Fig. S1A). Moreover, phosphorylation of sNASP was not affected by PP2A or PP6 (SI Appendix, Fig. S2). Taken together, these experiments indicate that p-sNASP is a selective substrate for PP4 in vivo.
Fig. 1.
PP4 dephosphorylates sNASP and inhibits LPS-induced NF-κB activation. (A) IP of sNASP (with anti-GFP) from HEK293 cells transfected with GFP-tagged sNASP in the presence (+) or absence (−) of Myc-tagged CK2β, HA-tagged CK2 catalytic subunits (CK2α and CK2α’), HA-tagged WT PP4 (HA-PP4), or mutant (mut), assessed by IB with antibodies against phosphorylated serine (pSerine) or anti-GFP after IP with anti-GFP. TCL was IB with anti-HA, anti-Myc, or anti–β-actin. (B) Cell lysates from THP-1 cells transduced with indicated CK2 subunits, HA-PP4 WT or mut was immunoprecipitated using anti-sNASP and immunoblotting conducted as in A. (C) Luciferase activity in HEK293 cells transfected with a luciferase reporter vector driven by an NF-κB-responsive promoter, plus EV or vector encoding TRAF6 and increasing concentrations of a vector encoding PP4. Results were standardized to EV (set as 1). Data are the mean ± SE for each group. *P < 0.05 (by Student’s t test). (D) THP-1 cells were transfected with siRNA negative control (siNT) or siPP4, followed by IB with antibodies against phosphorylated serine (pSerine), TRAF6, or sNASP after IP with anti-sNASP. TCL IB was done with anti-TRAF6, anti-HA, or anti–β-actin. (E) IB of indicated antibodies in LPS-stimulated THP-1 cells transduced with siNT or siPP4. Data represent a minimum of three independent experiments.
Because the phosphorylation status of sNASP regulates interaction between TRAF6 and sNASP (19), we asked whether PP4 also regulates interaction between TRAF6 and sNASP following LPS stimulation. SI Appendix, Fig. S1A also shows that overexpression of PP4 retains sNASP interaction with TRAF6 following LPS stimulation, suggesting that dephosphorylation of sNASP by PP4 regulates the interaction of TRAF6 and sNASP during TLR4 signaling. To further test whether PP4 is important in regulating TLR4 signaling, endogenous TAK1/p38 MAPK/JNK activation was examined in THP-1 cells for up to 8 h after LPS treatment. SI Appendix, Fig. S1B shows that LPS-induced phosphorylation of TAK1, p38 MAPK and JNK was significantly decreased when PP4 was overexpressed in THP-1 cells, compared to empty vector (EV). Furthermore, PP4 was found to inhibit TRAF6-mediated NF-κB activation in a dose-dependent manner (Fig. 1C). Collectively, these results suggest that PP4 is a negative regulator of the LPS-induced innate immune response.
PP4 Knockdown Enhanced Proinflammatory Cytokine Production by LPS.
We further demonstrated the biochemical and biological effects of PP4 by knocking-down PP4. PP4 small interfering (si) RNA (siPP4) was transfected into THP-1 cells, which significantly reduced the endogenous protein level of PP4, while the control siRNA (siNT) had no effect on PP4 expression (Fig. 1D). As shown in Fig. 1D, PP4 knockdown prolonged the phosphorylation status of sNASP for up to 8 h following LPS stimulation. Interestingly, endogenous sNASP dissociated from TRAF6 in cells transfected with siPP4 faster than siNT, suggesting that increased phosphorylation of sNASP by siPP4 in cells also facilitates its disassociation from TRAF6.
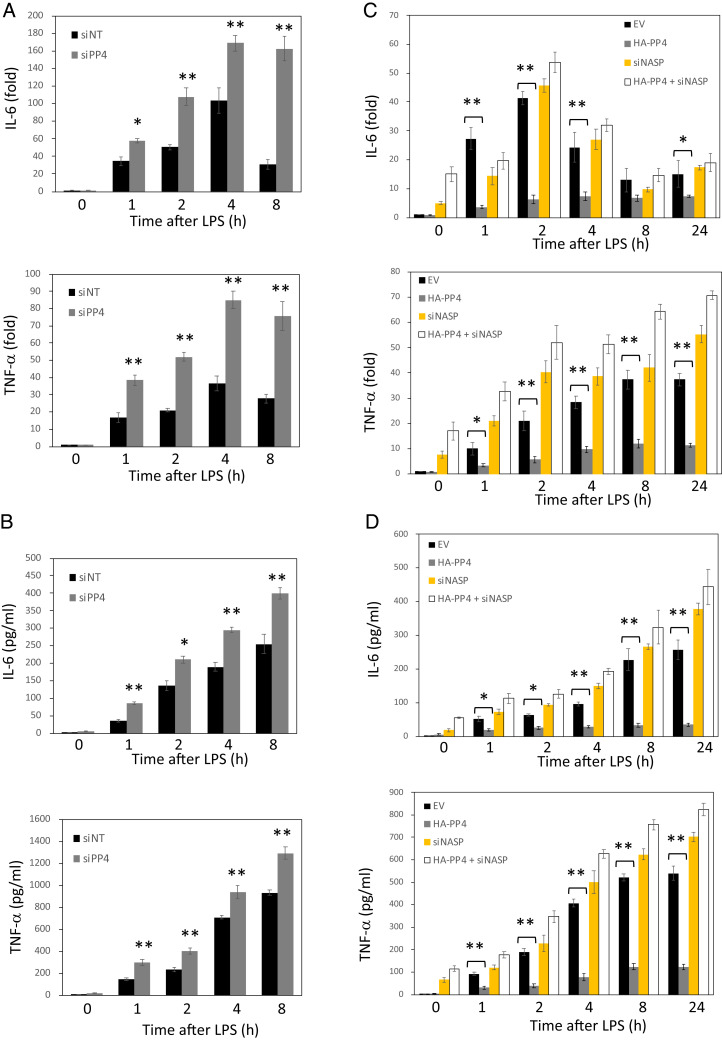
Next, we examined whether silencing of endogenous PP4 affected the LPS/TLR4 signaling cascade in a time-dependent manner. The Western blot analysis confirmed that phosphorylation of endogenous TAK1, p38 MAPK, and JNK was increased when PP4 was knocked down (Fig. 1E). Furthermore, knockdown of PP4 significantly increased the production of IL-6 and TNF-α at the level of both mRNA and protein (Fig. 2 A and B). Significantly, enhancement of proinflammatory cytokines was initially observed from 4 h to 8 h after LPS treatment in siPP4-expressing cells. These findings further confirmed that PP4 negatively regulates TLR4-induced proinflammatory cytokine responses.
Fig. 2.
PP4 negatively regulates proinflammatory cytokine production through sNASP. (A and C) RNA expression level of TNF-α and IL-6 in RAW264.7 cells transduced with (A) siNT or siPP4, (C) EV, HA-tagged PP4 with or without siNASP, and stimulated with LPS. Results were normalized to the expression of ACTB (encoding β-actin) and are presented relative to those of untreated cells. (B and D) Production of TNF-α and IL-6 by RAW264.7 cells transduced as in A and C and stimulated with LPS. Data are the mean ± SE for each group. *P < 0.05, **P < 0.01 (by one-way ANOVA). Data represent a minimum of three independent experiments.
PP4 Negatively Regulates Proinflammatory Cytokine Responses That Are Mediated by sNASP.
To investigate how PP4 functions in the negative regulation of TLR4 signaling activation, we performed real-time PCR and ELISA by transfection with PP4-expressing plasmid in the absence or presence of small interfering RNA NASP (siNASP) and stimulated these cells with LPS. Overexpression of PP4 resulted in a dramatic reduction of LPS-induced TNF-α and IL-6 at the level of both mRNA and protein, compared to WT (Fig. 2 C and D). However, loss of sNASP completely eliminated the effects of PP4 on TNF-α and IL-6 production (Fig. 2 C and D), suggesting that sNASP is required for PP4 to suppress the LPS-triggered proinflammatory cytokines. The Western blot analysis confirmed the appropriate overexpression of PP4 and down-regulation of sNASP (SI Appendix, Fig. S3). In additional, silencing NASP alone exhibited spontaneous IL-6 and TNF-α productions independent of LPS stimulation, which is similar to our previous data (19). These results suggested that PP4 negatively regulates the activation of LPS-triggered TLR4 pathway by regulating the phosphorylation status of sNASP.
PP4 Directly Interacts With Phosphorylated sNASP.
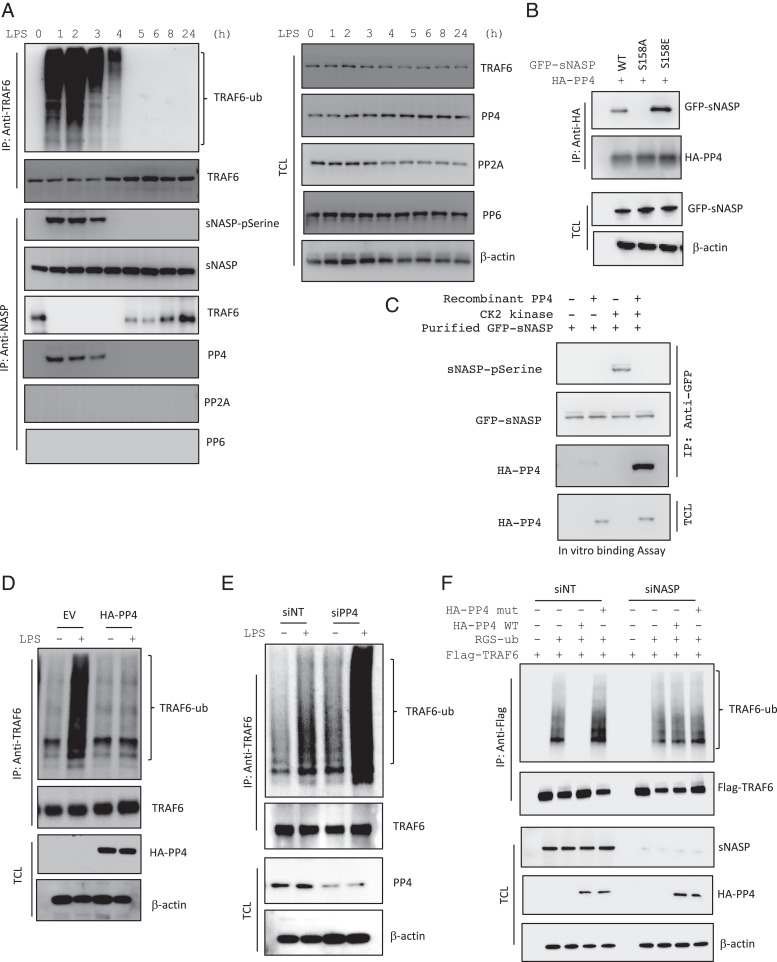
Next, we investigated whether PP4 could interact with sNASP during the regulation of TLR4 signaling. Immunoprecipitation assays showed that endogenous PP4 was associated with phosphorylated endogenous sNASP in macrophages 1 h after LPS treatment (Fig. 3A). The interaction between PP4 and p-sNASP was observed 1 to 3 h following LPS stimulation and was correlated with dissociation of sNASP from TRAF6 and TRAF6 ubiquitination (Fig. 3A). This is specific because p-sNASP did not interact with PP2A or PP6 (Fig. 3A). To further determine whether phosphorylation of sNASP directly associated with PP4, THP-1 cells were cotransfected PP4 with sNASP WT or various phosphor mutants and then coimmunoprecipitated with PP4. Two sNASP mutants were used in this study: one is sNASP S158E, a phosphomimetic mutant (serine-to-glutamic acid substitution) to mimic constitutive sNASP phosphorylation on serine 158 residue, and the other is sNASP S158A (serine-to-alanine acid substitution), which is a mutant serine 158 to phosphorylation-deficient mutant (19). There was a clear interaction between PP4 WT and the phosphomimetic sNASP S158E mutant but not phosphorylation-deficient sNASP S158A mutant (Fig. 3B). Furthermore, PP4 had a much higher affinity for the sNASP S158E mutant than to the WT (Fig. 3B). Similar results were found in an in vitro assay. Purified green fluorescent protein (GFP)-tagged sNASP was incubated with recombinant CK2 in the presence or absence of the PP4 recombinant protein. Phosphorylation of sNASP was detected in the presence of CK2 but not found when coincubated with the PP4 recombinant protein in vitro (Fig. 3C). Furthermore, GFP-tagged sNASP interacted with the PP4 recombinant protein only in the presence of recombinant CK2 (Fig. 3C), suggesting that PP4 can directly interact with phosphorylated sNASP to regulate its phosphorylation status.
Fig. 3.
PP4 interacts with phosphorylated sNASP and inhibits TRAF6 ubiquitination through sNASP. (A) THP-1 cells were stimulated with LPS for different time points and assessed by IB with indicated antibodies after IP with anti-sNASP or anti-TRAF6. TCL IB was done with the indicated antibodies. (B) 293T cells were transfected with GFP-sNASP WT or S158A or S158E mutants, followed by IB with antibody against GFP or HA after IP with anti-HA. TCL IB was done with anti-GFP or anti–β-actin. (C) Purified GFP-tagged sNASP protein was incubated with CK2 kinase with or without recombinant PP4 protein. The reaction products were followed by IB with antibodies against phosphorylated serine (pSerine), GFP or HA after IP with anti-GFP. TCL IB was done with anti-HA. (D) IP of TRAF6 (with anti-TRAF6) from THP-1 cells transfected with EV or HA-tagged PP4 (HA-PP4), simulated with LPS and followed by IB with antibody against TRAF6 or Ub. TCL IB was done with anti-HA and β-actin. (E) siNT or siPP4-treated THP-1 cells were stimulated with LPS, followed by IP and IB with indicated antibodies. (F) IP of Flag-TRAF6 (with anti-Flag agarose) from HEK293 cells transfected with siNT or siNASP in the presence (+) or absence (–) of Flag-TRAF6, RGS-Ub, HA-PP4 WT, or mutant, followed by IB with antibodies against Flag or Ub. TCL IB was done with anti-sNASP, anti-HA, and β-actin. Data represent a minimum of three independent experiments.
Two studies have revealed that PP4 can inhibit NF-κB activation through suppressing the ubiquitination of TRAF6 (33, 34), which is similar to our NF-κB activity assay shown in Fig. 1C. Next, we thought to evaluate whether increase or reduction of PP4 protein level would affect the TRAF6 activation. Results indicated that PP4 overexpression reduced autoubiquitination of TRAF6 in both HEK293 and THP-1 cells (Fig. 3D and SI Appendix, Fig. S4A). Conversely, knockdown of PP4 significantly increased the ubiquitination levels of TRAF6 (Fig. 3E and SI Appendix, Fig. S4B). These results also provided supportive evidence that PP4 inhibits TRAF6-dependent activation of NF-κB (Fig. 1C) by negatively regulating the TRAF6 polyubiquitination. The presence of sNASP was shown to be important for PP4-mediated down-regulation of proinflammatory cytokine production in response to LPS (Fig. 2 C and D). So, we hypothesized that sNASP is a key element in reducing TRAF6 autoubiquitination by PP4, which in turns leads to the inhibition of IL-6 and TNF-α cytokines production. Moreover, ubiquitination of TRAF6 was markedly reduced by overexpression of PP4 WT, but not PP4 mutant (Fig. 3F and SI Appendix, Fig. S4A), suggesting that the phosphatase activity of PP4 is critical for suppressing TRAF6 polyubiquitination. On the other hand, ubiquitination of TRAF6 was not affected by either PP4 WT or mutant in the absence of sNASP (Fig. 3F). These results clearly indicated that sNASP is a necessary component involved in the PP4-mediated negative regulation of the TLR4/TRAF6 pathway.
PP4 Suppresses Inflammatory Cytokine and Chemokine Production Triggered by LPS in Primary Bone Marrow–Derived Macrophage.
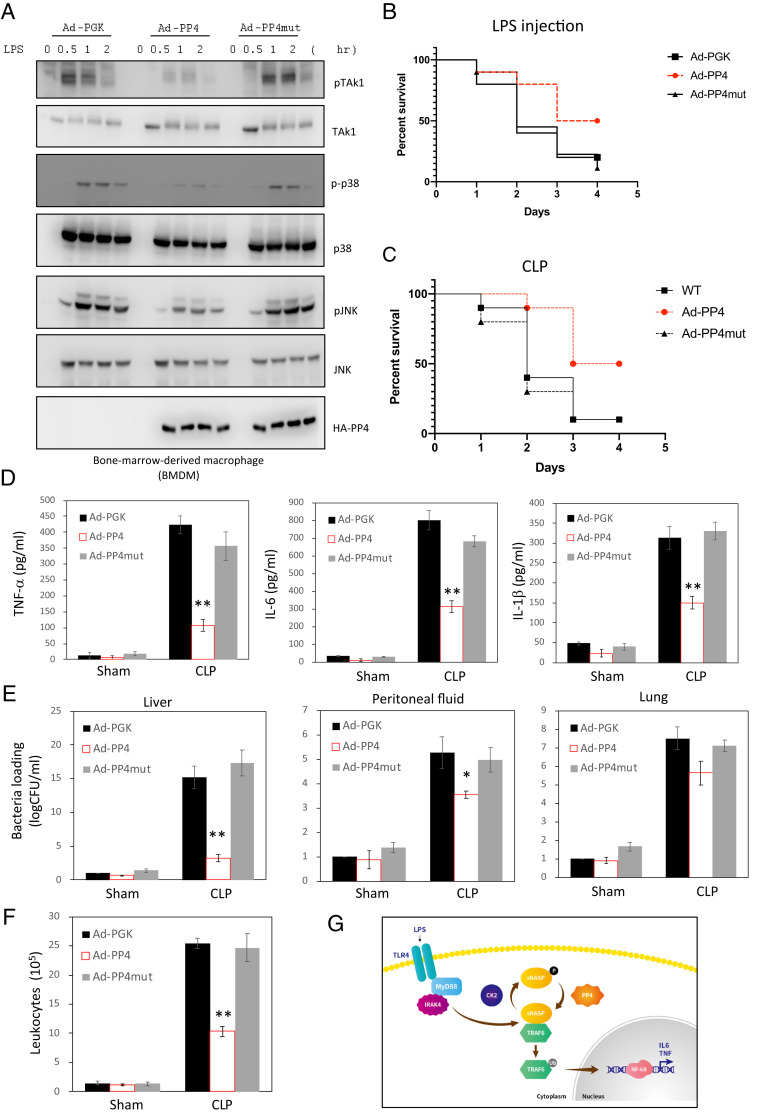
As PP4 overexpression resulted in inhibition of TRAF6 signaling and lower production of proinflammatory cytokines in cell lines (Fig. 2 C and D), we sought to assess if those changes also occurred in primary bone marrow–derived macrophages (BMDMs) with LPS. We isolated primary BMDMs from WT B6 mice and then infected with either adenoviruses expressing HA-tagged PP4 WT (Ad-PP4) or mutant (Ad-PP4mut). Adenoviruses expressing EV (Ad-PGK) were used as a negative control. Consistent with the results obtained with THP-1 (SI Appendix, Fig. S1B), inhibition of phosphorylation levels of TAK1, p38, and JNK was also observed in PP4 WT-overexpressed primary BMDMs but not PP4 mutant (Fig. 4A). There were much lower expression of the inflammatory cytokine-encoding genes, IL-6 and TNF-α, in PP4 WT-expressed cells than EV or PP4 mutant expressing cells after stimulation with LPS (SI Appendix, Fig. S5A). However, we found no difference in the anti-inflammatory cytokine IL-10 production (SI Appendix, Fig. S5B). Additionally, primary BMDMs with Ad-PP4 secreted much fewer chemokines such as CXCL-1, CXCL-9, and IL-15 than with either control vector or Ad-PP4mut in response to LPS (SI Appendix, Fig. S5C). Taken together, our results further demonstrated that PP4 can influence proinflammatory and chemokine production depending on its phosphatase activity in primary BMDMs.
Fig. 4.
PP4 protected mice from CLP-induced polymicrobial sepsis. (A) BMDM cells infected with adenoviruses expressing EV (Ad-PGK) or gene encoding HA-PP4 WT (Ad-PP4) or HA-PP4 mutant (Ad-PP4mut) were stimulated with LPS for different time points and assessed by IB analysis with indicated antibodies. (B and C) Survival curves of Ad-PGK, Ad-PP4, or Ad-PP4mut infected mice following LPS injection (B) or CLP (C) (n = 10 per group per experiment). (D–F) Serum cytokines (D) and bacterial load (as CFU) in the lung, liver, and peritoneal fluid (E) and circulating leukocytes (CD11b+) (F) in Ad-PGK, Ad-PP4 or Ad-PP4mut infected mice were measured 24 h after sham or CLP (n = 10 per group per experiment). Data are the mean ± SE for each group. *P < 0.05, **P < 0.01 (by one-way ANOVA). (G) Model of TLR4/TRAF6/sNASP axis regulated by PP4. After bacterial infection (such as LPS), phosphorylation of sNASP dissociated from TRAF6 results in TRAF6 autoubiquitination and proinflammatory cytokine release. Then, PP4 is recruited to dephosphorylate sNASP to prevent persistent TRAF6 autoubiquitination and overwhelm proinflammatory cytokines production.
PP4 Protects Mice Against Endotoxin Challenge.
To verify the functional role of PP4 as an attenuator of LPS stimulation in an animal model, we injected either Ad-PP4 or Ad-PP4mut into mice. Four days later, mice with Ad-PP4 or Ad-PP4mut injection both displayed a marked increase in the PP4 protein level in the liver and moderate increases in the kidneys, intestines, and lungs compared to the control group (SI Appendix, Fig. S6). Then, we evaluated the effects of Ad-PP4 in two different models of endotoxemia [injection with LPS and cecal ligation puncture (CLP)] in mice. In mice challenged with LPS, which is a common component of Gram-negative bacteria, we observed that 80% of Ad-PGK (control group) and Ad-PP4mut mice died within 4 d of treatment, whereas 50% of the Ad-PP4 WT mice were alive at 4 d, and ultimately survived (Fig. 4B). After treatment with LPS, Ad-PP4 mice produced less IL-6, TNF-α, and IL-1β than Ad-PGK and Ad-PP4 mutant mice (SI Appendix, Fig. S7). Similar results were found in CLP model, where more than 90% of Ad-PGK and Ad-PP4mut mice died within 72 h, compared to 50% of Ad-PP4 WT mice that lived (Fig. 4C). The concentrations of IL-6, TNF-α, and IL-1β were also significantly lower in Ad-PP4 WT serum than in Ad-PGK and Ad-PP4 mutant serum (Fig. 4D). In addition, bacterial counts in the lung, liver, and peritoneal fluid after CLP were lower in Ad-PP4 mice than in Ad-PGK and Ad-PP4mut mice (Fig. 4E). Flow cytometry indicated that the number of circulating CD11b+ leukocytes was much lower in the peritoneal fluid of Ad-PP4 WT mice than in Ad-PGK or Ad-PP4mut mice after CLP challenge (Fig. 4F). These results demonstrated that PP4 plays an essential role in negatively regulating inflammation in response to endotoxemia in vivo.
Discussion
Bacterial infection initiates a series of signaling cascades that lead to the production of proinflammatory cytokines to eliminate the bacteria (35). It is widely believed that kinases and phosphatases cooperatively control protein activities (14, 36). Our previous publication has revealed that phosphorylation of sNASP by CK2 is essential for proinflammatory cytokines production, especially in bacterial infection (19). Therefore, regulation of sNASP phosphorylation is a key step in antimicrobial innate immunity because excessive proinflammatory cytokines result in immune disorders (6, 37). However, the phosphatase responsible for dephosphorylation of sNASP has been elusive. In this study, we identified PP4 as an sNASP phosphatase that dephosphorylates sNASP in response to LPS and subsequently inhibits TRAF6 autoubiquitination, which leads to suppression of IL-6 and TNF-α induced by TLR4 and restrains the innate immune response. Thus, we propose that dephosphorylation is one of major mechanisms in controlling or terminating of TLR4/sNASP/TRAF6 signaling.
Serine/threonine-specific phosphatases that catalyze the dephosphorylation of serine and threonine residues are divided into four major subtypes: PP1, PP2A, PP2B, and PP2C (38). PP4 belongs to the PP2A subfamily, which includes PP2A, PP4, PP5, and PP6 (24, 25). Activation of PP2A was shown to have a suppressive effect on the inflammatory response via enhanced phosphorylation of the NF-κB p65 subunit, and PP6 is involved in negatively regulating the activity of TAK1 (39, 40). However, the role of PP4 in regulating NF-κB signaling is still controversial, since PP4 was reported to activate or inhibit NF-κB signaling due to different pathways or cell types. In HEK293 (nonimmune) cells, overexpression of PP4C can activate NF-κB transcription through stimulating the binding of c-Rel to NF-κB specific sequences (41, 42). On the contrary, PP4 can inhibit NF-κB activity by dephosphorylating of TRAF2 Ser11 and inhibiting TRAF6 polyubiquitination (34, 43). In RAW264.7 (immune) cells, PP4 negatively regulated LPS-induced NF-κB activation by inhibiting the ubiquitination of TRAF6 (33). Our results supported that PP4 as a negative regulator of LPS-induced and TRAF6-mediated NF-κB activation in both nonimmune and immune cells. Overexpression of PP4 inhibited TRAF6-mediated NF-κB activation was shown in Fig. 1C. Knockdown of PP4 and suppression of PP4 activity promoted NF-κB–mediated proinflammatory cytokines production, whereas overexpression of PP4 ameliorated proinflammatory cytokines production (Figs. 2 and 4D and SI Appendix, Figs. S5A and S7). Thus, our findings may provide insights into PP4 activities in the innate immune system.
Control of NF-κB through a decrease in the ubiquitination of TRAF6 is a critical step in maintaining homeostasis of TLR responses (44). Previous studies showed that the suppressive role of PP4 in LPS-induced NF-κB activation was directly associated with TRAF6 and negatively regulated its polyubiquitination of TRAF6 (33, 34), but the detail mechanism was still unclear. Here, we propose an alternative mechanism of how PP4 negatively regulates the activation of TLR4/TRAF6 cascade. In our experiments, we detected the physical interaction between phosphorylated sNASP and PP4 following LPS stimulation. After serine-phosphorylation removed from p-sNASP, PP4 dissociated from sNASP, allowing sNASP to inhibit TRAF6 activation. Moreover, TRAF6 polyubiquitination and proinflammatory cytokines production were not suppressed in the sNASP-deficient cells by the overexpression of PP4, which led us to propose that PP4 cannot inactivate TRAF6 in the absence of sNASP. Thus, our work has elucidated a regulatory function of PP4 in fine tunings of TRAF6 signaling.
p-sNASP was found to associate with PP4 but not PP2A and PP6 (Fig. 3A). Thus, PP2A and PP6 could not dephosphorylate p-sNASP (SI Appendix, Fig. S2). PP4 functions as a heterodimer composed of a catalytic subunit and variable regulatory subunits. Five regulatory subunits (PP4R1, PP4R2, PP4R3α, PP4R3β, and PP4R4) have been identified in mammalian cells. The interplay between different regulatory subunits is likely to be central to the substrate specificity of PP4 family of phosphatase. For example, histone deacetylase 3 activity is regulated by interaction with PP4C and PP4R1 complex (45). Previous studies also revealed that FxxP motifs is a specific binding motif for PP4R3 to regulate meiosis (46). Moreover, the PP2A regulatory B56 subunit binds to RacGAP1 and FOXO3 through a conserved LxxIxE motif (47). PP6R1 contains ankyrin repeat domains, which is crucial to the degradation of IκBε in response to TNFa stimulation (48).
The physiological importance of the PP4 in the TLR4/sNASP/TRAF6/TAK1/p38 MAPK/JNK pathway was supported by adenovirus-mediated gene delivery experiments with PP4 overexpression in primary BMDMs and mice. In primary BMDMs, overexpression of PP4 WT, but not mutant, decreased TAK1 and p38 MAPK phosphorylation as well as proinflammatory cytokines and chemokines expression (Fig. 4 A and D and SI Appendix, Figs. S5 and S7), consistent with results from cell line studies (Fig. 2 and SI Appendix, Fig. S1B). In endotoxic shock animal models, PP4 also reduced the production of inflammatory cytokines and the numbers of bacteria in vivo and improved the survival rate, compared to PP4mut. Thus, PP4-overexpressed mice displayed a protective effect following bacterial invasion. Our data also suggest that the phosphatase activity of PP4 is critical for regulating TLR4 pathway in vitro and in vivo. Furthermore, PP4 overexpression did not affect the anti-proinflammatory cytokine IL-10 expression, implying that the PP4 is specifically involved in the sNASP/TRAF6 axis, which mediates its proinflammatory but not anti-inflammatory cytokines.
Beside local inflammation, systemic sepsis is also characterized by profound leukocyte activation and mobilization throughout the circulation because organ damage is attenuated by inhibiting leukocyte-endothelial interactions and systemic leukocyte activation (49, 50). For example, endothelial P2RX7 activation plays an essential role in initiating leukocyte adhesion by enhanced the binding of leukocytes to adhesion molecular intercellular adhesion molecule-1 (ICAM-1) during septic encephalopathy (51). Inhibition of the P2RX7 pathway not only decreased endothelial ICAM-1 expression and leukocyte adhesion but also reduced organ injury and prolong the survival of septic mice. Pretreatment with anti-P-selectin antibodies can also significantly decrease CLP-induced neutrophils recruitment and tissue damage by inhibiting leukocyte-endothelial interactions (52). Our laboratory has previously demonstrated that P-selectin was induced only in the sNASP WT, but not mutant, in endothelial cells isolated from CLP-induced septic shock mice, suggesting that sNASP/TRAF6/PP4 axis could also be involved in regulating leukocyte recruitment (19). Phosphorylation of E-selectin and P-selectin were enhanced by the phosphatase inhibitors, okadaic acid, during leukocyte-endothelial adhesion, indicating that altering the phosphorylation state of adhesion protein may change signals in the leukocyte-endothelial adhesion cascade (53, 54). It is possible that much lower circulating CD11b+ leukocytes found in CLP-challenged Ad-PP4 WT mice (Fig. 4F) may be due to increase of PP4, which altered the phosphorylation status of adhesion molecules and changed leukocyte-endothelial interactions. Thus, PP4 could regulate multiple levels of cellular process in response to sepsis-induced inflammation, including leukocyte rolling in endothelial cells.
This report shows that PP4 is recruited by phospho-sNASP to negatively regulate the TLR4 pathway. Whole body PP4-deficient mice could not be obtained because ablation of PP4 leads to embryonic lethality (30). Previously, PP4 was shown to play important role in thymocyte development and B-cell lineage development through tissue-specific PP4-knockout mice (21, 31, 32). We are in the process of generating myeloid-specific PP4-knockout mice to further evaluate the physiologic function of sNASP in monocytic in response to bacterial infection.
In conclusion, our data demonstrate that PP4 is the phosphatase that specifically dephosphorylates p-sNASP to negatively regulate innate immunity against bacterial infection to protect the host from excessive immune responses. Our findings also showed that PP4 is recruited by phosphorylated sNASP after LPS stimulation to control TRAF6 polyubiquitination, suggesting that PP4 is part of a feedback loop of LPS/TLR4 pathway. Therefore, modulation of PP4 may be a promising target for regulating inflammation related to the LPS-induced activation of the TAK1/p38 MAPK/JNK pathway, and a better understanding of this pathway will benefit the design of immunotherapeutic strategies to ameliorate harmful immune response.
Methods
Cell Culture, Antibodies, and Reagents.
Primary BMDMs were isolated from murine femurs and maintained as previously described (55). The human monocyte THP-1 cell line (American Type Culture Collection [ATCC] TIB-202, ATCC) was propagated in RPMI-1640 medium (ATCC 30-2001, ATCC) containing 10% FBS, 2 mM glutamine, and 1% penicillin and streptomycin at 37 °C and 5% CO2 in air. The mouse macrophage RAW264.7 cell line (ATCC TIB-71, ATCC) and embryonic kidney epithelial HEK293 cell line (ATCC CRL-1573, ATCC) were both cultured in DMEM supplemented with 10% FBS and 1% penicillin and streptomycin at 37 °C and 5% CO2 in air.
The antibodies used for the immunoblot analysis were goat anti-NASP (SC-161915, Santa Cruz), rabbit anti-TRAF6 (SC-7221, Santa Cruz), mouse anti-ubiquitin (SC-8017, Santa Cruz), mouse anti-PPX (SC-374106, Santa Cruz), mouse anti-β-actin (A1544, Sigma-Aldrich), mouse anti–phospho-TAK1 (4531, Cell Signaling), mouse anti-TAK1 (4505, Cell Signaling), mouse anti–phospho-p38 MAPK (9211, Cell Signaling), anti-p38 MAPK (9212, Cell Signaling), mouse anti–phospho-JNK kinase (4671, Cell Signaling), mouse anti-JNK kinase (9252, Cell Signaling), mouse anti-GFP (632281, Clontech), mouse anti-phosphoserine (ab17465, Abcam), mouse anti-HA (H9658, Sigma-Aldrich), mouse anti-Flag (F1804, Sigma-Aldrich), and mouse anti-Myc (631206, Clontech). Antibody dilutions were according to the manufacturers’ instructions. LPS from the Salmonella enterica serotype abortus equi was obtained from Sigma-Aldrich.
Plasmids, siRNA, and Transfection.
Plasmids encoding human HA-PP4 and HA-PP4 R235L mutant were gifts from Tse-Hua Tan (Immunology Research Center, National Health Research Institutes, Zhunan, Taiwan) (41). The plasmids Flag-TRAF6, HA-CK2α, HA-CK2α’, Myc-CK2β, HA-Ub, RGS-Ub, GFP-sNASP WT, and mutants (S158A and S158E) were previously described (56, 57). In additional, the HA-PP2A and Flag-PP6 plasmids were generously provided by Chi-Wu Chiang (Institute of Molecular Medicine, National Cheng Kung University, Tainan, Taiwan) and Benjamin E. Turk (Department of Pharmacology, Yale School of Medicine, New Haven, CT) respectively (58, 59).
siRNAs were transfected by using the DharmaFECT 1 reagent (T-2001-02, Dharmacon) according to the manufacturer’s protocol. Sequences for siRNA are listed as follows: the siRNA specific for the gene encoding human NASP (siNASP, 5'-GGAACUGCUACCCGAAAUU-3'), PP4 (siPP4, 5'-CUGGUCGCUUACAUCACUUUA-3'), and siRNA specific for the gene encoding mouse NASP (the pool of siNASP no. 9, 5'-GGAUAUAAGUGAGCCUGAA-3', siNASP no. 10, 5'-GCAGGAGAAUUACAGUUAUU-3', siNASP no. 11, 5'-GGUAAGAAGUAUGGAGAAA-3' and siNASP no. 12, 5'-GAUGAAAGAGGGUGAAGAA-3'). All siRNAs were synthesized by Sigma-Aldrich. An siRNA Universal Negative control was purchased from Sigma-Aldrich (catalog S1C001).
THP-1 cells were transfected using X-tremeGene HP (06-365-752-001, Roche). Transfection of HEK293 and RAW264.7 cells was performed using Lipofectamine 3000 (L3000015, Invitrogen) according to the manufacturer’s protocol.
Coimmunoprecipitation and Immunoblotting.
Immunoprecipitation and Western blot analysis were carried out as previously described (60). Cells were lysed in modified RIPA buffer [25 mM Tris·HCl at pH 7.6, 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 5 mM EDTA, 1 mM EGTA, and protease inhibitors (P8340, Sigma)]. Sonication was followed by centrifugation at 13,000 g for 30 min at 4 °C, and the supernatant fraction was collected and analyzed by the following steps. For immunoprecipitation, the indicated antibody was incubated with supernatant fraction overnight at 4 °C. Immunocomplexes were subjected to protein G agarose (16-266, Millipore) for 1 h. After being washed twice with lysis buffer, immunoprecipitated products were separated from the beads by adding 2× protein sample buffer and analyzed for immunoblotting. The immunoblot analysis was performed using the indicated antibodies.
In Vitro Binding Assay.
The recombinant GFP-sNASP protein prepared by immunoprecipitation using GFP-Trap_M (gtm-20, Chromotek) was phosphorylated by casein kinase II (P6010, New England Biolabs) in vitro for 60 min at 30 °C and followed by incubation with recombinant human PP4 protein (ORITP760519, Origene) for another 120 min at 4 °C. Samples were analyzed by immunoprecipitation and Western blotting with the indicated antibodies.
Animal Experiments and Recombinant Adenovirus.
Eight-week-old male C57BL/6JNarl mice were purchased from the National Laboratory Animal Center (Taipei, Taiwan). Mice were housed in a pathogen-free animal facility at the Taipei Medical University. Adenoviruses expressing a control vector (Ad-PGK), HA-PP4 (Ad-PP4), and HA-PP4mut (Ad-PP4mut) were generated by the Adenovirus Laboratory at the Institute of Biomedical Sciences in Academia Sinica (Taipei, Taiwan) as previously described (61). For animal experiments involving adenoviruses, mice were injected in the tail with recombinant adenovirus (0.1∼0.5 × 109 pfu per mice). Three days postinjection, LPS-induced endotoxemia model was administered by injecting 4.5 mg of LPS per kilogram body weight intraperitoneally or CLP-induced polymicrobial sepsis model was performed by aseptic surgical technique as previously described (62). In brief, C57BL/6JNarl mice (8 wk) were anesthetized. A 1 cm incision was cut along the midline of the abdomen and the cecum was isolated. The cecum was ligated between the ileocecal junction and the distal end of the cecum. The ligated cecum was then punctured through-and-through four times by using 18-gauge needles and a small amount of feces was squeezed out. Then, the cecum was placed back and the abdomen was closed. Sham animals underwent identical procedures, except for the ligation and puncture of the cecum. For survival studies, mice were monitored twice times daily for up to 4 d. For time-point studies, mice were killed and serum, peritoneal fluid, lung and liver samples were collected for further analyses. The animal protocol was approved by the animal and ethics review committee of the Laboratory Animal Center at Taipei Medical University (Taipei, Taiwan).
Luciferase Assays.
NF-κB luciferase reporter assays were conducted as previously described (63). HEK293 cells were seeded in a 24-well tissue culture plate at a density of 2 × 105 cells/well. The next day, the cells were transfected with various expression plasmids along with NF-κB luciferase and pRL-TK Renilla luciferase using Lipofectamine 3000 (L3000015, Invitrogen). Twenty-four hours later, the cells were lysed with lysis buffer, and firefly luciferase and Renilla luciferase activities in the lysates were determined using the Dual-Glo Luciferase Assay System (E2920, Promega). Results were normalized to internal Renilla luciferase activities. Data were obtained from at least three independent experiments.
Enzyme-Linked Immunosorbent Assay.
Serum and cell culture supernatants were collected and assayed for cytokines. Cytokine production was measured using Mouse ELISA MAX Deluxe Set for IL-6 (#431304), TNF-α (#430904), and IL-1β (#432604) were obtained from BioLegend and conducted according to the manufacturer’s protocol.
Real-time PCR.
RNA was extracted from whole cell lysates using RNeasy Mini Kit (74104, Qiagen) and then reverse transcribed into complementary DNA by Advantage RT-PCR Kit (639506, Clontech) according to the manufacturer’s instructions. Expression of mouse genes encoding IL-6, TNF-α, IL-1β, IL-10, CXCL-1, IL-15, and CXCL-9 (Mm00446190_m1, Mm00443258_m1, Mm000434228_m1, Mm01288386_m1, Mm04207460_m1, Mm00434210_m1, Mm00434946_m1, ThermoFisher Scientific) was assessed by real-time PCR with using TaqMan Gene Expression Master Mix (4369016, Applied Biosystems); results were normalized to expression of the gene encoding 18s and were quantified by the change-in-threshold method (ΔΔCT).
Statistical Analysis.
GraphPad Prism 6 software (GraphPad Software) was used for statistical analyses as determined by Student’s t test for two groups or one-way ANOVA for three or more groups. Values with P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
This work was financially supported of the Higher Education Sprout Project by the Ministry of Education in Taiwan. F.Y. is also supported by grants (TMU 108-AE1-B14, DP2-109-21121-01-T-04-02, and DP2-110-21121-01-T-04-02) from Taipei Medical University and grants (MOST 108-2320-B-038-069-MY2 and MOST 110-2320-B-038-065-MY3) from the Ministry of Science and Technology of Taiwan. E.Y. is supported by Nolan Family Endowed Chair of the Department of Internal Medicine and Scholar of Arkansas Research Alliance. H.M.C. and E.Y. are also supported by the Winthrop P. Rockefeller Cancer Institute of the University of Arkansas for Medical Sciences. The authors acknowledge the academic and science graphic illustration service provided by TMU Office of Research and Development.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. J.P.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2107044118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Aderem A., Ulevitch R. J., Toll-like receptors in the induction of the innate immune response. Nature 406, 782–787 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R., Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Poltorak A., et al. , Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 282, 2085–2088 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Arthur J. S., Ley S. C., Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13, 679–692 (2013). [DOI] [PubMed] [Google Scholar]
- 5.O’Shea J. J., Murray P. J., Cytokine signaling modules in inflammatory responses. Immunity 28, 477–487 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook D. N., Pisetsky D. S., Schwartz D. A., Toll-like receptors in the pathogenesis of human disease. Nat. Immunol. 5, 975–979 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Schneider M., et al. , The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat. Immunol. 13, 823–831 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J., et al. , Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z., et al. , Oxidative stress activates endothelial innate immunity via sterol regulatory element binding protein 2 (SREBP2) transactivation of microRNA-92a. Circulation 131, 805–814 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karampitsakos T., Woolard T., Bouros D., Tzouvelekis A., Toll-like receptors in the pathogenesis of pulmonary fibrosis. Eur. J. Pharmacol. 808, 35–43 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Villarino A. V., Kanno Y., O’Shea J. J., Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 18, 374–384 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCall K. D., Muccioli M., Benencia F., Toll-like receptors signaling in the tumor microenvironment. Adv. Exp. Med. Biol. 1223, 81–97 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Li Y., et al. , Preventing abnormal NF-κB activation and autoimmunity by Otub1-mediated p100 stabilization. Cell Res. 29, 474–485 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subbannayya Y., Pinto S. M., Bösl K., Prasad T. S. K., Kandasamy R. K., Dynamics of dual specificity phosphatases and their interplay with protein kinases in immune signaling. Int. J. Mol. Sci. 20, 2086 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai H., Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol. Sci. 33, 522–530 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Beinke S., Robinson M. J., Hugunin M., Ley S. C., Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105. Mol. Cell. Biol. 24, 9658–9667 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McElhinny J. A., Trushin S. A., Bren G. D., Chester N., Paya C. V., Casein kinase II phosphorylates I kappa B alpha at S-283, S-289, S-293, and T-291 and is required for its degradation. Mol. Cell. Biol. 16, 899–906 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chantôme A., et al. , Casein kinase II-mediated phosphorylation of NF-kappaB p65 subunit enhances inducible nitric-oxide synthase gene transcription in vivo. J. Biol. Chem. 279, 23953–23960 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Yang F. M., et al. , sNASP inhibits TLR signaling to regulate immune response in sepsis. J. Clin. Invest. 128, 2459–2472 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An H., et al. , SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity 25, 919–928 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Xia X., et al. , NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity 34, 843–853 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand P. K., et al. , NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488, 389–393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long L., et al. , Recruitment of phosphatase PP2A by RACK1 adaptor protein deactivates transcription factor IRF3 and limits type I interferon signaling. Immunity 40, 515–529 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Park J., Lee D. H., Functional roles of protein phosphatase 4 in multiple aspects of cellular physiology: A friend and a foe. BMB Rep. 53, 181–190 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen P. T., Philp A., Vázquez-Martin C., Protein phosphatase 4—From obscurity to vital functions. FEBS Lett. 579, 3278–3286 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Hu M. C., Shui J. W., Mihindukulasuriya K. A., Tan T. H., Genomic structure of the mouse PP4 gene: A developmentally regulated protein phosphatase. Gene 278, 89–99 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Sumiyoshi E., Sugimoto A., Yamamoto M., Protein phosphatase 4 is required for centrosome maturation in mitosis and sperm meiosis in C. elegans. J. Cell Sci. 115, 1403–1410 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Toyo-oka K., et al. , Protein phosphatase 4 catalytic subunit regulates Cdk1 activity and microtubule organization via NDEL1 dephosphorylation. J. Cell Biol. 180, 1133–1147 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee D. H., et al. , A PP4 phosphatase complex dephosphorylates RPA2 to facilitate DNA repair via homologous recombination. Nat. Struct. Mol. Biol. 17, 365–372 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shui J. W., Hu M. C., Tan T. H., Conditional knockout mice reveal an essential role of protein phosphatase 4 in thymocyte development and pre-T-cell receptor signaling. Mol. Cell. Biol. 27, 79–91 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su Y. W., Chen Y. P., Chen M. Y., Reth M., Tan T. H., The serine/threonine phosphatase PP4 is required for pro-B cell development through its promotion of immunoglobulin VDJ recombination. PLoS One 8, e68804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao F. H., et al. , Protein phosphatase 4 is an essential positive regulator for Treg development, function, and protective gut immunity. Cell Biosci. 4, 25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L., et al. , Protein phosphatase 4 negatively regulates LPS cascade by inhibiting ubiquitination of TRAF6. FEBS Lett. 582, 2843–2849 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Hadweh P., Habelhah H., Kieff E., Mosialos G., Hatzivassiliou E., The PP4R1 subunit of protein phosphatase PP4 targets TRAF2 and TRAF6 to mediate inhibition of NF-κB activation. Cell. Signal. 26, 2730–2737 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Diacovich L., Gorvel J. P., Bacterial manipulation of innate immunity to promote infection. Nat. Rev. Microbiol. 8, 117–128 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Liu J., Qian C., Cao X., Post-translational modification control of innate immunity. Immunity 45, 15–30 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Chen J. Q., Szodoray P., Zeher M., Toll-like receptor pathways in autoimmune diseases. Clin. Rev. Allergy Immunol. 50, 1–17 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Wang Y., et al. , Roles of phosphotase 2A in nociceptive signal processing. Mol. Pain 9, 46 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun S. C., Maggirwar S. B., Harhaj E., Activation of NF-kappa B by phosphatase inhibitors involves the phosphorylation of I kappa B alpha at phosphatase 2A-sensitive sites. J. Biol. Chem. 270, 18347–18351 (1995). [DOI] [PubMed] [Google Scholar]
- 40.Kajino T., et al. , Protein phosphatase 6 down-regulates TAK1 kinase activation in the IL-1 signaling pathway. J. Biol. Chem. 281, 39891–39896 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu M. C., et al. , Protein phosphatase X interacts with c-Rel and stimulates c-Rel/nuclear factor kappaB activity. J. Biol. Chem. 273, 33561–33565 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Yeh P. Y., Yeh K. H., Chuang S. E., Song Y. C., Cheng A. L., Suppression of MEK/ERK signaling pathway enhances cisplatin-induced NF-kappaB activation by protein phosphatase 4-mediated NF-kappaB p65 Thr dephosphorylation. J. Biol. Chem. 279, 26143–26148 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Abdul-Sada H., et al. , The PP4R1 sub-unit of protein phosphatase PP4 is essential for inhibition of NF-κB by merkel polyomavirus small tumour antigen. Oncotarget 8, 25418–25432 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walsh M. C., Lee J., Choi Y., Tumor necrosis factor receptor-associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol. Rev. 266, 72–92 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X., et al. , Histone deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine phosphatase 4. Genes Dev. 19, 827–839 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueki Y., et al. , A consensus binding motif for the PP4 protein phosphatase. Mol. Cell 76, 953–964.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hertz E. P. T., et al. , A conserved motif provides binding specificity to the PP2A-B56 phosphatase. Mol. Cell 63, 686–695 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Stefansson B., Ohama T., Daugherty A. E., Brautigan D. L., Protein phosphatase 6 regulatory subunits composed of ankyrin repeat domains. Biochemistry 47, 1442–1451 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Ploppa A., et al. , Mechanisms of leukocyte distribution during sepsis: An experimental study on the interdependence of cell activation, shear stress and endothelial injury. Crit. Care 14, R201 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zahr A., et al. , Endomucin prevents leukocyte-endothelial cell adhesion and has a critical role under resting and inflammatory conditions. Nat. Commun. 7, 10363 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H., et al. , P2RX7 sensitizes Mac-1/ICAM-1-dependent leukocyte-endothelial adhesion and promotes neurovascular injury during septic encephalopathy. Cell Res. 25, 674–690 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asaduzzaman M., Rahman M., Jeppsson B., Thorlacius H., P-selectin glycoprotein-ligand-1 regulates pulmonary recruitment of neutrophils in a platelet-independent manner in abdominal sepsis. Br. J. Pharmacol. 156, 307–315 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujimoto T., McEver R. P., The cytoplasmic domain of P-selectin is phosphorylated on serine and threonine residues. Blood 82, 1758–1766 (1993). [PubMed] [Google Scholar]
- 54.Yoshida M., Szente B. E., Kiely J. M., Rosenzweig A., Gimbrone M. A. Jr., Phosphorylation of the cytoplasmic domain of E-selectin is regulated during leukocyte-endothelial adhesion. J. Immunol. 161, 933–941 (1998). [PubMed] [Google Scholar]
- 55.Heo K. S., et al. , ERK5 activation in macrophages promotes efferocytosis and inhibits atherosclerosis. Circulation 130, 180–191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamitani T., Kito K., Nguyen H. P., Yeh E. T., Characterization of NEDD8, a developmentally down-regulated ubiquitin-like protein. J. Biol. Chem. 272, 28557–28562 (1997). [DOI] [PubMed] [Google Scholar]
- 57.Gong L., Kamitani T., Millas S., Yeh E. T., Identification of a novel isopeptidase with dual specificity for ubiquitin- and NEDD8-conjugated proteins. J. Biol. Chem. 275, 14212–14216 (2000). [DOI] [PubMed] [Google Scholar]
- 58.Chuang E., et al. , The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity 13, 313–322 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Cho E., Lou H. J., Kuruvilla L., Calderwood D. A., Turk B. E., PPP6C negatively regulates oncogenic ERK signaling through dephosphorylation of MEK. Cell Rep. 34, 108928 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang F. M., Lin Y. C., Hu M. C., Identification of two functional nuclear localization signals mediating nuclear import of liver receptor homologue-1. Cell. Mol. Life Sci. 68, 1241–1253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Juan S. H., et al. , Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation 104, 1519–1525 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Rittirsch D., Huber-Lang M. S., Flierl M. A., Ward P. A., Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang F. M., Feng S. J., Lai T. C., Hu M. C., A calreticulin-dependent nuclear export signal is involved in the regulation of liver receptor homologue-1 protein folding. Biochem. J. 471, 199–209 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.