Abstract
We present a rare case of myopericarditis developing one day after the injection of the second dose of the MODERNA mRNA-1273 vaccine (Cambridge, MA, USA). The patient complained of typical positional chest pain with initial laboratory results significant for elevated troponin, erythrocyte sedimentation rate, and C-reactive protein. Autoimmune predisposition was suggested by elevated anti-nuclear antibodies and anti–Sjögren's-syndrome-related antigen A autoantibodies titers. Subsequent cardiac magnetic resonance imaging (cMRI) revealed mild global hypokinesis with an ejection fraction of 48%, diffuse pericardial hyperenhancement suggestive of acute pericarditis, and T2-weighted short tau inversion recovery apical septal hyperenhancement suggestive of myocardial edema. Based on clinical, laboratory, and cMRI findings, a diagnosis of acute myopericarditis was made and the patient was treated with colchicine and ibuprofen with prompt resolution of symptoms. Vaccine-associated myopericarditis is rare, however, there have been reports of myocarditis developing after smallpox vaccination. The American College of Rheumatology has expressed concern about flaring or development of autoimmune inflammatory rheumatic disease (AIIRD) after COVID vaccination. Further studies are required to quantify AIIRD flaring/development including myopericarditis after mRNA-1273 vaccination.
<Learning objective: With the recent reports of myocarditis developing after COVID vaccination, our case report highlights the need for further studies to investigate a possible link between the mRNA-1273 vaccine and autoimmune inflammatory rheumatic disease development including myopericarditis. In addition to clinical findings and laboratory workup, our case also assesses the clinical utility of cardiac magnetic resonance imaging for the diagnosis of myopericarditis.>
Keywords: Myopericarditis, mRNA-1273, Covid vaccination
Introduction
Throughout history, widespread community vaccination has been one of the most effective tools in combating pandemics. The same has been shown in the ongoing COVID-19 pandemic, and both efficacious and relatively safe vaccines were developed in record-breaking time. In particular, vaccination with the MODERNA mRNA-1273 vaccine (Cambridge, MA, USA) has been shown to minimize the severity of disease or prevent infection entirely. However, like all vaccinations, vaccination against COVID-19 has also been shown to have side effects, sometimes with serious consequences. These side effects most commonly include localized reactions such as pain, erythema, swelling, and lymphadenopathy, as well as systemic side effects such as fever, headache, fatigue, myalgias, and arthralgias [1]. However, more severe side effects have also been reported. Specifically, cases of vaccine-associated myocarditis have occurred following the development of myocarditis in a vaccinated cohort in Israel [2]. Furthermore, rheumatologic societies, including the American College of Rheumatology (ACR), have expressed concern about symptomatic flares of autoimmune rheumatic disease following vaccination [3]. Here, we present the case of a young woman who presented to our facility with cardiac magnetic resonance imaging (cMRI) confirmed myopericarditis shortly after receiving the second dose of the MODERNA mRNA-1273 vaccination.
Case report
A 22-year-old female presented to our facility from an outpatient clinic with a two-day history of sharp substernal chest pain and shortness of breath. Her chest pain was positional, worse with laying down, and improved when leaning forward. Associated symptoms included subjective fever, chills, and myalgias. She denied any sick contact including COVID-19 exposure or recent travel. She also denied other preceding symptoms suggestive of an upper respiratory tract infection, joint stiffness or pain, personal cardiac or autoimmune disease history. She denied recent medication changes other than receiving the second dose of the MODERNA COVID-19 vaccine series the day before the onset of symptoms. She also denied fevers or any other symptoms after reception of previous vaccinations including childhood vaccinations. Past medical and social history were unremarkable. She endorsed a family history of autoimmune disorders (her mother has autoimmune thyroid disease, but she does not recall the exact diagnosis). No pericardial rub was noted on examination.
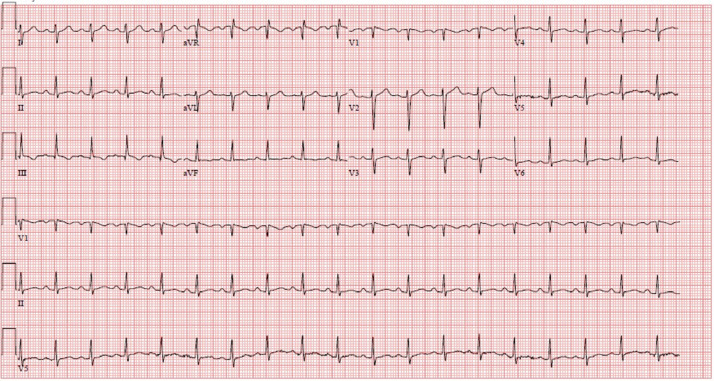
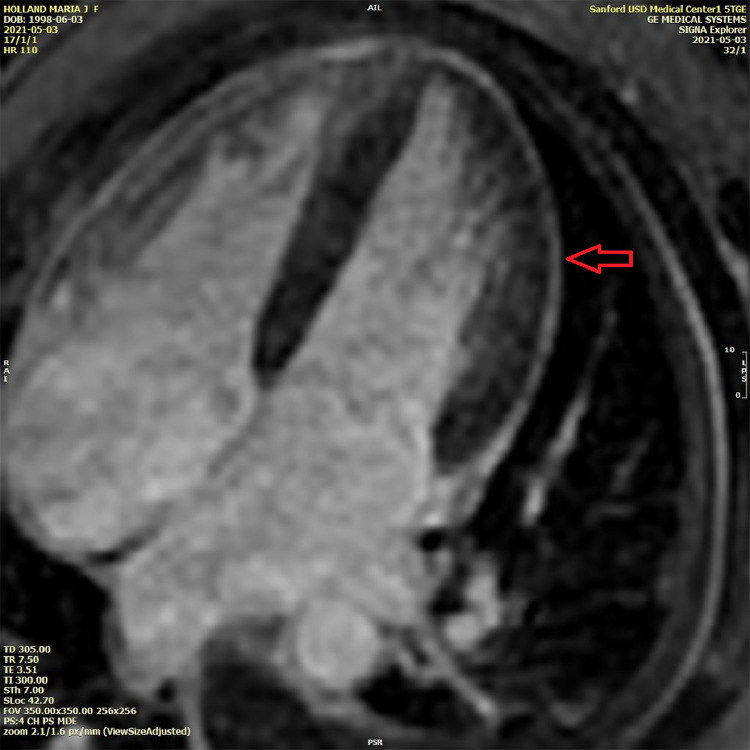
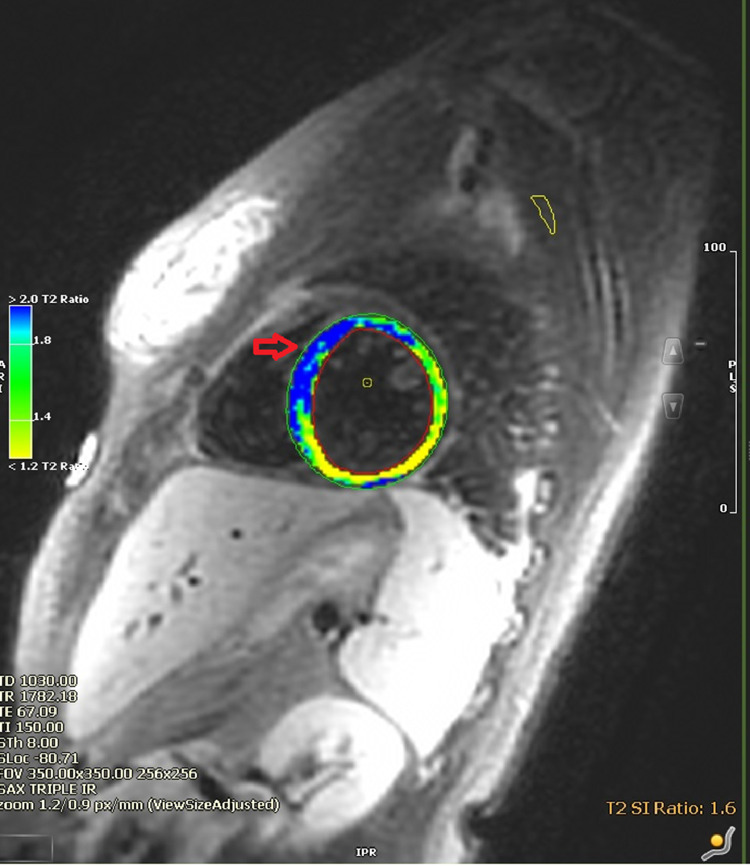
In the emergency room, she was febrile and tachycardiac with a temperature of 38.8 °C and a pulse of 127 beats/min. Initial troponin I level within one hour of admission was elevated at 0.164 ng/mL (reference 0.000–0.028 ng/mL) and was trended to a peak of 3.897 ng/mL 10 h after admission, with a subsequent downtrend to 3.186 ng/mL 15 h after admission. Erythrocyte sedimentation rate, C-reactive protein, and d-dimer levels were elevated at 50 mm/hr (reference 0–22 mm/hr), 129.1 mg/L (reference <5.0 mg/L), and 6.30 μg/mL (reference < 0.50 μg/mL), respectively. The electrocardiogram (Fig. 1) showed sinus tachycardia with borderline right axis deviation and non-specific T wave abnormalities in inferior leads without PR or ST-segment changes. Computed tomography angiography of the chest showed a trace left pleural effusion and opacities of lung bases suggestive of atelectasis without acute pulmonary embolism. She was then admitted for suspected myopericarditis. The following day, anti-nuclear antibody screening was positive with 1:1280 titer, and a speckled pattern and anti–Sjögren's-syndrome-related antigen A autoantibodies were also positive. A transthoracic echocardiogram showed a left ventricular ejection fraction of 60–65% and trace tricuspid regurgitation. No pericardial effusion or other acute findings were noted. A cMRI with gadolinium enhancement was performed to diagnose myopericarditis. cMRI showed a mildly reduced left ventricular ejection fraction of 48% with mild global hypokinesis. There was diffuse circumferential hyperenhancement of the pericardium suggestive of acute pericarditis (Fig. 2). On T2-weighted short tau inversion recovery images, hyperenhancement of the apical septal walls was noted as suggestive of myocardial edema (Fig. 3).
Fig. 1.
Electrocardiogram showing sinus tachycardia, borderline right axis deviation, and borderline T-wave abnormalities in leads II and III.
Fig. 2.
Cardiac magnetic resonance imaging: late gadolinium enhancement showing circumferential pericardial hyper-enhancement (red arrow).
Fig. 3.
Cardiac magnetic resonance imaging: myocardial edema (red arrow) on T2 weighted short tau inversion recovery images.
A diagnosis of acute myopericarditis was made, prompting initiation of colchicine 0.6 mg daily (for three months) and 600 mg ibuprofen three times daily (for two weeks). She reported improvement in chest pain, followed by resolution by the time of a two-week outpatient follow-up visit.
Discussion
COVID vaccines serve as a powerful tool to curb the spread of the pandemic. The US Food and Drug Administration has given emergency use authorization to three vaccines in the USA: Pfizer BioNTech BNT162b2, MODERNA mRNA-1273, and J&J/Jansen Ad26.COV2. Vaccine-induced myocarditis is a rare phenomenon, however, cases of myocarditis have been reported, especially after smallpox vaccination [4].
The MODERNA mRNA-1273 vaccine trial was seen as a breakthrough showing 94.1% effectiveness against COVID infection, however, the trial also showed that the vaccine group was associated with more adverse events when compared to the placebo group. The adverse events included pain at the injection site and systemic adverse events. The severity of systemic* adverse events was worse after the second vaccine dose compared to the first dose (grade 2* events increased from 16.5% after the first dose to 38.1% after the second dose and grade 3* events increased from 2.9% after 1st dose to 15.8% after 2nd dose) [1]. What is interesting about this trial is that the adverse events occurred more frequently in the younger population (18–65 years) possibly indicating increased reactogenicity to the vaccine in the younger population. However, the ACR recommends COVID vaccination for autoimmune inflammatory rheumatic disease (AIIRD) patients due to worse COVID infection outcomes in these patients [3].
Although the ACR expresses theoretical concern for AIIRD flaring after COVID vaccination [3], to date, no causality has been established between COVID vaccination and AIIRD development. Although the exact mechanism of mRNA-vaccine associated AIIRD development specifically myopericarditis and myocarditis are not clear, possible mechanisms may include molecular mimicry between SARS-COV-2 spike protein and self-antigens, triggering and flaring of autoimmunity in patients with pre-existing AIIRD, or immune response to mRNA portion of the vaccine [5]. Nevertheless, in the case of mRNA 1273, there have been case reports of immune thrombocytopenic purpura (ITP) related to the MODERNA vaccine [6] and immune thrombotic thrombocytopenia is a known complication of Astra Zeneca ChAdOx1 vaccination [7]. A multinational case series study [8] also demonstrated 27 immuno-modulatory disease flares or new disease development after reception of COVID vaccination, including pericarditis after reception of BNT-162b2 vaccine (23/27 BNT-162b2, 2/27 mRNA-1273, and 2/27 ChAdOx1). Although rare compared to immune thrombotic thrombocytopenia, to 6/23/2021, there have been 252 reports of myocarditis and 174 reports of pericarditis reported to the US Vaccine Adverse Event Reporting System (VAERS), both after 1st and 2nd dose of mRNA-1273 vaccination (https://vaers.hhs.gov/). To date, there has been only one published case report of myocarditis developing after mRNA-1273 vaccination [9], there is a concern for myocarditis after 62 cases of myocarditis developed in patients after 5 million BNT-162b2 vaccines were administered in Israel [2].
In our case, although the patient did not undergo myocardial biopsy, clinical presentation, laboratory results, and MRI findings are suggestive of probable post-COVID vaccine myopericarditis triggered by an underlying, subclinical predisposition for autoimmunity [10].
*Adverse event grading derived from toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trial provided by US Department of Health and Human Services (DHHS 2007) per protocol in [1].
Declaration of Competing Interest
All authors declare that there is no conflict of interest.
References
- 1.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.https://www.timesofisrael.com/israel-said-probing-link-between-pfizer-shot-and-heart-problem-in-men-under-30/.
- 3.Curtis J.R., Johnson S.R., Anthony D.D., Arasaratnam R.J., Baden L.R., Bass A.R., et al. American College of Rheumatology Guidance for COVID-19 Vaccination in Patients With Rheumatic and Musculoskeletal Diseases: version 1. Arthritis Rheumatol. 2021;73:1093–1107. doi: 10.1002/art.41734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckart R.E., Love S.S., Atwood J.E., Arness M.K., Cassimatis D.C., Campbell C.L., Boyd S.Y., Murphy J.G., Swerdlow D.L., Collins L.C., Riddle J.R., Tornberg D.N., Grabenstein J.D., Engler R.J. Department of Defense Smallpox Vaccination Clinical Evaluation Team. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44:201–205. doi: 10.1016/j.jacc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malayala S.V., Mohan G., Vasireddy D., Atluri P. Purpuric rash and thrombocytopenia after the mRNA-1273 (Moderna) COVID-19 vaccine. Cureus. 2021;13:e14099. doi: 10.7759/cureus.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watad A., De Marco G., Mahajna H., Druyan A., Eltity M., Hijazi N., Haddad A., Elias M., Zisman D., Naffaa M.E., Brodavka M., Cohen Y., Abu-Much A., Abu Elhija M., Bridgewood C., et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel) 2021;9:435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert E., Aurigemma G., Saucedo J., Gerson D.S. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021;16:2142–2145. doi: 10.1016/j.radcr.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler Y., Charron P., Imazio M., Badano L., Barón-Esquivias G., Bogaert J., Brucato A., Gueret P., Klingel K., Lionis C., Maisch B., Mayosi B., Pavie A., Ristic A.D., M Sabaté Tenas, et al. ESC Guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2015;36:2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]