Significance
Lung cancer is the leading cause of cancer-related deaths in the United States. PD-L1 is often overexpressed on cancer cells, driving immune-independent, cell-intrinsic functions that increase resistance to DNA-damaging therapies such as IR or cisplatin. FBXO22, a known ubiquitin E3 ligase, decreases the level of PD-L1 in NSCLC cells and increases their sensitivity to DNA-damaging therapies. Furthermore, targeting cyclin-dependent kinase 5 (CDK5), which is highly expressed in NSCLC, increases FBXO22 levels, leading to decreased expression of PD-L1. Thus, we identify an innovative strategy for treating NSCLC by targeting CDK5 to enhance the efficacy of immunotherapy alone or in combination with DNA-damaging therapies.
Keywords: lung cancer, CDK5, FBXO22, PD-L1
Abstract
High expression of programmed death-ligand 1 (PD-L1) in cancer cells drives immune-independent, cell-intrinsic functions, leading to resistance to DNA-damaging therapies. We find that high expression of the ubiquitin E3 ligase FBXO22 sensitizes nonsmall cell lung cancer (NSCLC) cells to ionizing radiation (IR) and cisplatin, and that activation of FBXO22 by phosphorylation is necessary for this function. Importantly, FBXO22 activates PD-L1 ubiquitination and degradation, which in turn increases the sensitivity of NSCLC cells to DNA damage. Cyclin-dependent kinase 5 (CDK5), aberrantly active in cancer cells, plays a crucial role in increasing the expression of PD-L1 in medulloblastoma [R. D. Dorand et al., Science 353, 399–403 (2016)]. We show in NSCLC cells that inhibiting CDK5 or reducing its expression increases the level of FBXO22, decreases that of PD-L1, and increases the sensitivity of the cells to DNA damage. We conclude that FBXO22 is a substrate of CDK5, and that inhibiting CDK5 reduces PD-L1 indirectly by increasing FBXO22. Pairing inhibitors of CDK5 with immune checkpoint inhibitors may increase the efficacy of immune checkpoint blockade alone or in combination with DNA-damaging therapies.
Lung cancer is the leading cause of cancer-related deaths in the United States, where nonsmall cell lung cancer (NSCLC) represents about 85% of all lung cancer. Despite significant advances in targeted therapies, DNA-damaging cytotoxic therapies, including ionizing radiation (IR) and cisplatin, remain the mainstay for the majority of patients with NSCLC (1, 2). However, intrinsic and acquired resistance present major limitations to these therapies. To obtain improved clinical responses in NSCLC, it is crucial to develop new therapeutic strategies to overcome this resistance. We have developed a powerful validation-based insertional mutagenesis (VBIM) method that identifies proteins whose overexpression confers resistance to chemotherapeutic drugs (3, 4). Utilizing this method, we found that overexpression of a dominant-negative truncated form of the ubiquitin E3 ligase FBXO22 (5) confers resistance to IR in NSCLC cells. Importantly, full-length, wild-type (WT) FBXO22 has the opposite effect, sensitizing NSCLC cells to both IR and cisplatin.
FBXO22 is a member of the F-box family, which constitutes subunits of the ubiquitin E3 ligase complex SCF (SKP1–cullin–F-box) (5). When overexpressed F-box proteins can function as tumor suppressors if their substrates are oncoproteins or as oncoproteins if their substrates are tumor suppressors (6). Mammals have about 70 F-box proteins, which can be divided into three families, F-box and WD40 domain (FBXW), F-box and Leu-rich repeat (FBXL), and F-box only (FBXO) (7). Recent studies showed that FBXO22 targets key regulators of cellular activators of ubiquitination and protein degradation, and that it plays important roles in several different types of cancer. It inhibits breast cancer metastasis by targeting HDM2 (8), by suppressing the epithelial–mesenchymal transition (EMT), and by promoting the proteasomal degradation of SNAIL (9). In renal cell carcinoma, FBXO22 suppresses cell motility and EMT by inhibiting the expression of both metalloproteinase-9 (MMP-9) and VEGF (10). Suppression of FBXO22-dependent degradation of the transcription regulatory protein BACH1 leads to NSCLC metastasis (11). In contrast, a tumorigenic role of FBXO22 has also been reported: it degrades the tumor suppressor PTEN in colorectal cancer (12) and liver kinase B1 (LKB1) in NSCLC (13). However, our studies, performed in cells having either wild-type LKB1, or a loss of function mutation of LKB1, indicate that LKB1 activity is not likely to be required for the CDK5–FBXO22–PD-L1 pathway. Consistent with an earlier report that FBXO22 sensitizes NSCLC cells to cisplatin (14), we observe here that its overexpression and activation sensitizes NSCLC cells to DNA-damaging therapies, and that this sensitivity was reversed in cells depleted of FBXO22.
Programmed death-ligand 1 (PD-L1, also called B7-H1) is an immune checkpoint protein that is highly expressed in cancer cells, including lung cancer (15). PD-L1 binds to programmed cell death protein 1 (PD-1), inhibiting CD8 T cell activity, thus helping tumor cells to evade killing (16). High expression of PD-L1 in cancer cells also drives immune-independent cell intrinsic functions (17) that enhance survival and confer resistance to DNA-damaging therapies, including IR and cisplatin (18–20), thereby increasing cancer cell survival and tumor progression.
Cyclin-dependent kinase 5 (CDK5), a proline-directed serine-threonine kinase that is aberrantly active in many tumor types, plays a crucial role in increasing the expression level of PD-L1 in medulloblastoma (21). In contrast to other members of the CDK family, CDK5 is not activated by cyclins, and is instead activated by the noncyclin regulatory proteins p35 and p39 (22). Moreover, in contrast to other CDKs which require an activating phosphorylation on Thr-160 by CDK-activating kinase, binding to p35 or p39 is sufficient to fully activate CDK5 (22). High expression and deregulated activation of CDK5 correlates with poor prognosis and shorter survival for cancer patients, including those with NSCLC (23). CDK5 plays a role in the initiation of the DNA damage response in cancer (24), and increased expression levels and up-regulation of its activation follow exposure of cancer cells to DNA damage–inducing agents, including IR or chemotherapeutic drugs, leading to an increase in resistance to these treatments (25). Roscovitine (Seliciclib) inhibits CDKs through direct competition with adenosine trisphosphate (ATP) and has been used in several different cancers, including NSCLC (26). Here, we show that FBXO22 degrades PD-L1 in NSCLC cells, leading to increased sensitivity to DNA-damaging therapies, and that CDK5 increases PD-L1 expression by negatively regulating FBXO22.
Results
High Expression of FBXO22 Increases Sensitivity to IR and Cisplatin.
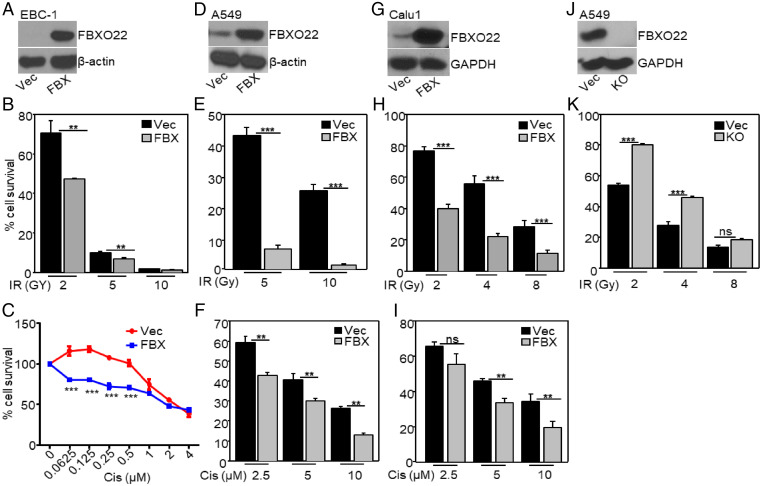
To identify regulators of resistance to IR, we used the VBIM method of insertional mutagenesis (3, 4, 27). EBC-1 squamous carcinoma cells were infected with VBIM lentiviruses and exposed cumulatively to three rounds of ionizing radiation (5, 5, and 10 Gy), with expansion of the surviving cells after each round. Since vector-specific RNA is covalently linked to RNA downstream of insertion sites that are activated by the vector’s CMV promoter, we were able to identify VBIM-targeted RNAs by high throughput sequencing (HTS) of pooled RNA from the entire surviving population. Two vector–cellular RNA fusions were very substantially enriched in this experiment. Nicotinamide phosphoribosyl transferase (NAMPT) and FBXO22 represented about 25 and 8%, respectively, of the total VBIM-tagged RNA sequences. NAMPT is the rate-limiting enzyme in NAD biosynthesis (28), and NAMPT inhibitors enhance sensitivity to IR in cancer (29), indicating that NAMPT mediates resistance to DNA damage. The vector–FBXO22 RNA encodes a C-terminal fragment of the full-length protein that acts as a dominant negative and, consistently, increased expression of a cDNA encoding full-length FBXO22 (Fig. 1A) significantly reduces cell survival in response to IR (Fig. 1B). In summary, from a single screen in which we used RNA sequencing (RNA-seq) to identify vector–cellular RNA fusions, we identified two proteins that regulate radiosensitivity. Importantly, increased expression of FBXO22 also increases sensitivity to the DNA-damaging therapeutic agent cisplatin (Fig. 1C). Furthermore, we revealed by Kaplan–Meier analysis that high expression of FBXO22 is significantly associated with higher overall survival in lung cancer patients (SI Appendix, Fig. S1), showing the clinical significance of high FBXO22 expression. Since adenocarcinoma is the most common histologic subtype of NSCLC (30), we used the NSCLC adenocarcinoma cell line A549 to better understand the role of FBXO22 in mediating responses to IR or cisplatin. We also studied the NSCLC squamous cell carcinoma cell line Calu1. Both cell lines are resistant to IR (31, 32). In A549 and Calu1 cells, increasing the expression of FBXO22 increases their sensitivity to IR and cisplatin (Fig. 1 D–F for A549 and Fig. 1 G–I for Calu1). Consistently, in FBXO22-null A549 cells, generated by using CRISPR-Cas9 technology (Fig. 1J), the sensitivity to IR was decreased (Fig. 1K). Furthermore, overexpressing FBXO22 also increased sensitivity to IR in breast (MDAMB231, MCF7) and colon (HCT116) cancer cells (SI Appendix, Fig. S2). Interestingly, increased expression of FBXO22 did not increase sensitivity to IR in nontumorigenic human mammary epithelial (hTERT-HME) cells (SI Appendix, Fig. S3).
Fig. 1.
High expression of FBXO22 increases sensitivity to IR and cisplatin. (A) FBXO22 was overexpressed in EBC-1 cells (FBX). Controls were cells with empty vector (Vec). (B) The cells were exposed to IR (2, 5, or 10 Gy) or kept untreated. Seven days after exposure, the cells were lysed and the A260 was measured as an indication of the total amount of nucleic acid. The fraction of surviving cells was calculated relative to untreated cells. (C) Cells were treated with cisplatin and the survival was determined after 72 h. The fraction of surviving cells was calculated relative to vehicle controls. (D) FBXO22 was overexpressed in A549 cells. Cell survival was measured after treatment with IR (E) or cisplatin (F). (G) FBXO22 was overexpressed in Calu1 cells. Cell survival was measured after treatment with IR (H) or cisplatin (I). (J) The level of FBXO22 was analyzed in FBXO22 knockout (KO) A549 cells. The cells were exposed to IR, and their viability was determined 7 d later (K). Each cell survival assay was performed at least three times, and the data shown represent means ± SD of three experiments, in which each measurement was performed in triplicate. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
High Expression of FBXO22 Decreases the Levels of PD-L1.
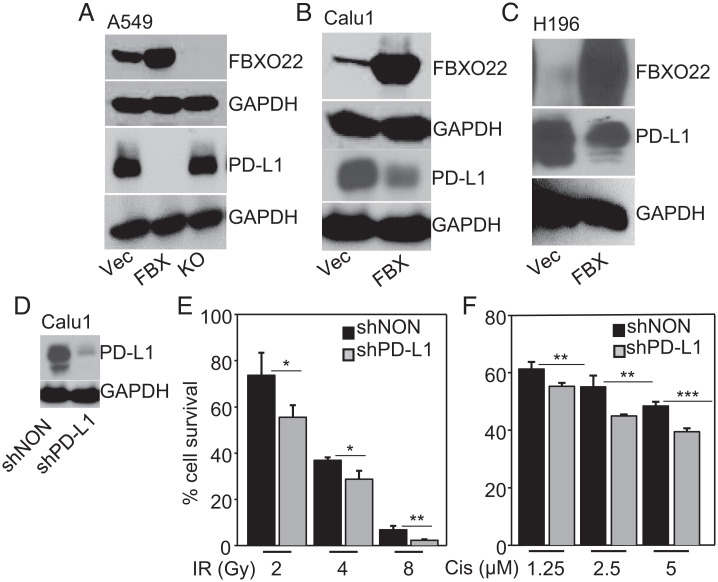
High expression of PD-L1 confers resistance to IR and increases intrinsic prosurvival signals in cancer cells, thus favoring tumor progression (19, 33). We find that overexpression of FBXO22 drastically decreased PD-L1 levels in A549 cells, and that this decrease was reversed when FBXO22 was depleted (Fig. 2A). FBXO22 overexpression also decreased PD-L1 levels in Calu1 cells (Fig. 2B) and in the small cell lung cancer cell line H196, both of which have very high basal levels of PD-L1 (Fig. 2C). When we knocked the expression of PD-L1 down in Calu1 cells, the sensitivity to IR or cisplatin was increased (Fig. 2 D–F). We conclude that overexpressing FBXO22 increases the sensitivity of cancer cells to IR and cisplatin by decreasing PD-L1 expression.
Fig. 2.
FBXO22 overexpression decreases PD-L1 and increases sensitivity to IR or cisplatin. (A) PD-L1 protein levels were determined by Western analysis of lysates of A549 cells either expressing FBXO22 (FBX) or depleted for this protein (KO), compared to vector controls (Vec). GAPDH was used as a loading control. (B and C) PD-L1 levels were determined in Calu1 (B) or H196 cells (C) overexpressing FBXO22, compared to vector controls. GAPDH was used as a loading control. (D) Whole-cell lysates of Calu1 cells expressing a shRNA targeting PD-L1 were analyzed by the Western method. (E) The cells were exposed to IR, and their viability was determined 7 d after exposure to IR. (F) The cells were treated with cisplatin and survival was measured 72 h after treatment. Data shown represent means ± SD of three experiments in which each measurement was performed in triplicate. *P < 0.05; **P < 0.01; ***P < 0.001.
FBXO22 Mediates the Ubiquitination and Degradation of PD-L1.
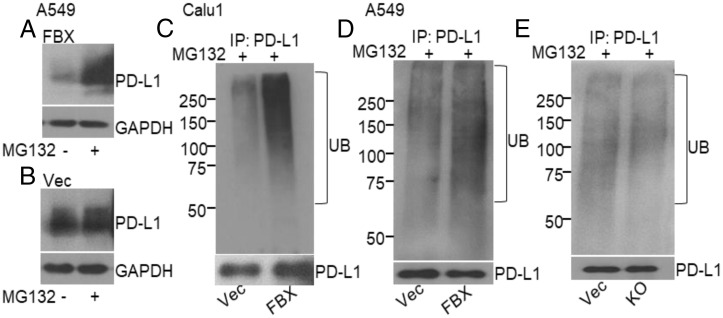
FBXO22 is known to target several cellular proteins for degradation (8, 9, 11, 14). To understand how FBXO22 decreases PD-L1 levels, we first showed that the ability of FBXO22 to reduce PD-L1 levels was reversed when the cells were treated with the proteasome inhibitor MG132 (Fig. 3A), indicating that the FBXO22-driven down-regulation of PD-L1 requires the activity of the 26S proteasome. The increase in PD-L1 level was not observed in control cells following treatment with MG132 (Fig. 3B). We next used in-cell assays for ubiquitination to investigate whether FBXO22 promoted the ubiquitination of PD-L1 as the basis of its enhanced degradation. Immunoprecipitation assays were performed with antibodies to PD-L1 in vector control or FBXO22 overexpressing cells treated with MG132, followed by Western analysis using anti-ubiquitin or anti–PD-L1. Increased expression of FBXO22 remarkably increased the polyubiquitination of endogenous PD-L1 in Calu1 cells (Fig. 3C). Moreover, increased expression of FBXO22 substantially promoted the ubiquitination of PD-L1 in A549 cells (pretreated with interferon-γ to increase PD-L1 expression), and this activity was reduced when FBXO22 expression was reduced by knockout (Fig. 3 D and E). In summary, these results support the hypothesis that FBXO22 mediates the ubiquitination and degradation of PD-L1 and thus sensitizes cells to DNA damage.
Fig. 3.
FBXO22 mediates ubiquitination and degradation of PD-L1. (A and B) PD-L1 levels were determined by Western analysis after treatment with MG132 (30 µM) for 6 h in A549 cells overexpressing FBXO22 (A) or transfected with the empty vector control (B). (C) Endogenous ubiquitination of PD-L1 was determined in Calu1 cells overexpressing FBXO22 (FBX) or the empty vector (Vec) after treatment with MG132 (30 µM for 6 h). Whole-cell lysates of MG132-treated cells were used for immunoprecipitation of PD-L1 and the levels of ubiquitination and PD-L1 were measured by the Western method. (D and E) Ubiquitination of PD-L1 in A549 cells, with or without manipulation of FBXO22 (overexpression in C; KO in D). The cells were stimulated with interferon-γ (1 ng/mL) for 16 h, followed by MG132 (30 µM for 6 h). Immunoprecipitation was followed by Western analysis.
Down-Regulation or Inhibition of CDK5 Increases the Levels of FBXO22 and Decreases Those of PD-L1, Thus Enhancing the Sensitivity of Cells to DNA Damage.
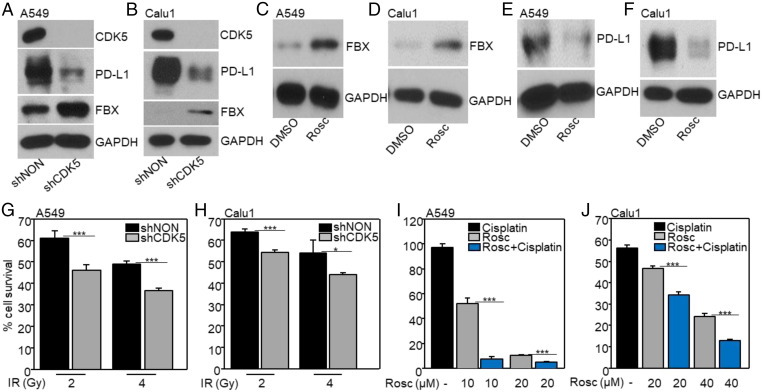
Independent of its immune-suppressive activity, high expression of PD-L1 in cancer cells promotes proliferation and survival (17). The expression of PD-L1 is regulated by a variety of mechanisms (34). A previous study showed that CDK5 up-regulates the expression of PD-L1 in medulloblastoma (21), prompting us to investigate whether CDK5 regulates PD-L1 levels in NSCLC cells. Down-regulation of CDK5 with a shRNA in A549 (Fig. 4A), Calu1 (Fig. 4B), H1299, or PC9 (SI Appendix, Fig. S4) cells decreased PD-L1 levels and, importantly, also increased the levels of FBXO22. Furthermore, inhibiting CDK5 with Roscovitine also increased the levels of FBXO22 and decreased those of PD-L1 (Fig. 4 C–F), indicating that CDK5-dependent phosphorylation of target protein increases the expression of PD-L1. Therefore, inhibiting CDK5 to suppress PD-L1 presents a plausible strategy for increasing the sensitivity of cells to IR or cisplatin in some cancers. Indeed, down-regulation of CDK5 or treatment with Roscovitine did increase the sensitivity of NSCLC cells to IR or cisplatin (Fig. 4 G–J), indicating that high levels of PD-L1 expression in cancer cells desensitizes them to DNA-damaging therapies, independently of immune-mediated responses.
Fig. 4.
Down-regulation or inhibition of CDK5 decreases PD-L1 expression and increases FBXO22 levels in NSCLC cells, enhancing their sensitivity to DNA damage. (A and B) The expression levels of CDK5, PD-L1, and FBXO22 (FBX) were assayed by the Western method after knockdown of CDK5. (C–F) The expression levels of FBXO22 (C and D) and PD-L1 (E and F) were assayed after treatment with Roscovitine (Rosco; 0.1 µM for A549 cells and 5 µM for Calu1 cells) for 24 h. (G and H) The viability of CDK5 knockdown and control cells 7 d after exposure to IR was determined. The fraction of surviving cells was calculated relative to untreated controls. (I and J) The cells were treated with Rosc or Rosc + cisplatin. After 72 h their viability was determined and normalized to dimethylsulfoxide (DMSO) controls. Results are represented by means ± SD of three experiments. *P < 0.05; ***P < 0.001.
Identification of Residues of FBXO22 that Are Phosphorylated in Response to IR.
Phosphorylation of a different member of the F-box family, FBXO31, increases in response to IR, resulting in its stabilization and the subsequent degradation of its substrate (35). Since FBXO22 increases the sensitivity of cells to IR, we analyzed whether FBXO22 is similarly phosphorylated in response to IR. We used mass spectrometry to analyze FBXO22 immunoprecipitated from A549 cells after irradiation. Phosphopeptides were identified that correspond to (157)GIVTPGIVVTPMoGSGSNRPQEIEIGESGF(175) + PO3. The tandem mass spectrometry spectra for the phosphorylated chymotryptic peptide GIVTPGIVVTPMoGSGSNRPQEIEIGESGF + PO3 is shown in SI Appendix, Fig. S5A. The masses of the y7 and y19 ions are consistent with phosphorylation on either S160 or S162. The degree of phosphorylation increased by about 3- or 4.7-fold, respectively, after 6 or 24 h of exposure to IR (SI Appendix, Fig. S5B). The amino acid sequence surrounding these residues in human FBXO22 is highly conserved in several other mammalian species, consistent with the possibility that their phosphorylation might have important functions (SI Appendix, Fig. S5C).
Phosphorylation of FBXO22 Is Required to Decrease PD-L1 Levels and Increase Sensitivity to IR.
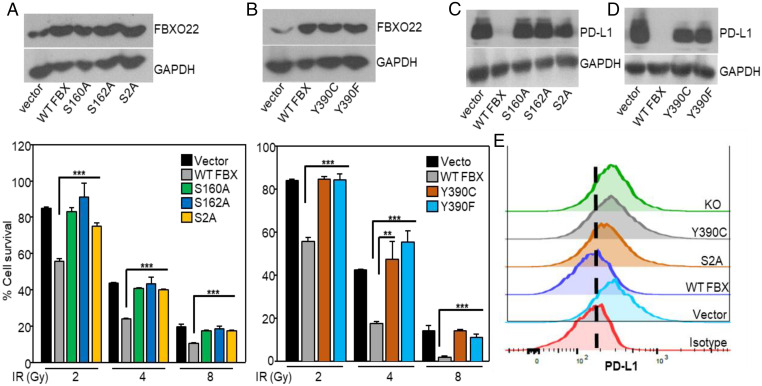
In stable A549 cells overexpressing the single phosphorylation-deficient mutants S160A or S162A, or the double mutant S160/162A (S2A), the increased sensitivity to IR mediated by the wild-type protein was lost (Fig. 5A). To identify cancer-related mutations in FBXO22, we analyzed the The Cancer Genome Atlas database, finding the Y390C mutation in an NSCLC tumor. Furthermore, mutating Y390 to F or C abolished the sensitivity to IR mediated by the wild-type protein (Fig. 5B). FBXO22 mutations also increased the sensitivity to IR in Calu1 cells (SI Appendix, Fig. S6). These results show that phosphorylation and activation of FBXO22 on at least two different residues is necessary to facilitate sensitivity to IR. Consistent with data presented earlier, the decrease in PD-L1 levels observed with increased expression of WT FBXO22 was not seen when the phosphorylation of FBXO22 at S160, S162, or both was prevented by mutation, similar to the effect of FBXO22 depletion (Fig. 5 C–E). In summary, activation of FBXO22 by phosphorylation is required for it to be able to degrade PD-L1 and thus to increase the sensitivity of cells to DNA damage.
Fig. 5.
Phosphorylation of FBXO22 is required to increase sensitivity to IR. (A) FBXO22 levels were determined in A549 cells overexpressing WT FBXO22 or the FBXO22 mutants by Western analysis (Upper). The cells were exposed to IR and their viability was determined 7 d later (Lower). (B) FBXO22 levels were determined in A549 cells overexpressing WT FBXO22 (WT FBX) or the FBXO22 mutants (Upper). Cell survival was measured after treatment with IR (Lower). Data shown represent means ± SD of three experiments, in which each measurement was performed in triplicate. (C and D) PD-L1 levels were determined in whole-cell lysates of A549 cells overexpressing WT or mutant FBXO22. (E) PD-L1 membrane protein levels were analyzed by flow cytometry in cells overexpressing WT (WT FBX) or mutant FBXO22 (S2A, Y390C), or in FBXO22-depleted cells (KO). **P < 0.01; ***P < 0.001.
Discussion
In cancer cells, resistance to therapy is often due to the overexpression of a specific protein, often achieved through amplification of the corresponding gene. Previously, we used the VBIM method to show that overexpression of kinesins mediates resistance to docetaxel (3), and that overexpression of SOS1 confers resistance to erlotinib (4). More recently, we reported with others that the VBIM-driven expression of a dominant-negative, truncated form of the histone deacetylase ZIP leads to resistance to tamoxifen in breast cancer cells (36). Here, we used the VBIM method to discover that expression of a dominant-negative truncated form of FBXO22 confers resistance to IR in NSCLC cells, and that overexpression of the full-length protein increases sensitivity to both IR and cisplatin. Some substrates of FBXO22 have already been reported (8, 11). We show here that FBOX22 expression and phosphorylation-dependent activation are necessary to decrease the PD-L1 levels in cancer cells.
Over the past decade, immune checkpoint blockade (ICB) with antibodies targeting PD-L1 or PD-1 has shown remarkable clinical responses in cancers, including NSCLC (37). However, the clinical benefit of ICB has been observed in only a minority of NSCLC patients. In first-line therapy, relatively high PD-L1 expression makes patients eligible for monotherapy with immune checkpoint inhibitors, which is seen in about 20 to 25% of all NSCLC patients (15). Recent studies indicate that, although PD-L1 levels predict a good outcome and improved responses to ICB therapy, elevated PD-L1 levels are paradoxically associated with worse outcomes in NSCLC patients (38, 39). A high level of PD-L1 expression in tumor cells is a major obstacle for ICB therapy since, in addition to its strong immunosuppressive effect on tumor-specific T cells, PD-L1 also delivers intrinsic signals that increase cancer cell proliferation and invasiveness, reducing the efficacy of ICB therapy (17, 40). These observations highlight an underappreciated role of high levels of PD-L1 in increasing the tumorigenic potential of NSCLCs, independently of their immunosuppressive properties. Earlier reports showed that PD-L1 undergoes ubiquitination and degradation catalyzed by some E3 ubiquitin ligases (41, 42), and that PD-L1 degradation effectively promotes immunotherapy in various cancers, including NSCLC (43). We show here that FBXO22 decreases PD-L1 levels by ubiquitination and proteasomal degradation, suggesting that targeting this pathway may be an attractive approach to enhance ICB therapy.
To overcome the limitations of using ICB therapy alone, several clinical trials are ongoing in which anti–PD-L1 is combined with standard treatments, including radiotherapy, chemotherapy, or other forms of immunotherapy. Although combined therapies have improved outcomes compared to treatment with anti–PD-L1 alone, resistance still develops. Radiotherapy or chemotherapy up-regulates PD-L1 expression in cancer cells (44, 45), which can also protect cancer cells against DNA damage (19, 20). Combining H1A, a specific anti–PD-L1 antibody that causes PD-L1 degradation, with IR or cisplatin significantly increases antitumor activity (18). Consistently, we found that down-regulating PD-L1 level increases sensitivity to IR or cisplatin in NSCLC cells, suggesting that increased expression of PD-L1 promotes resistance to DNA damage. Furthermore, PD-L1 expression is induced in cancer cells in response to inflammatory cytokines, such as interferon-γ, secreted by immune cells within the tumor microenvironment, which facilitate disease progression (46). In a murine medulloblastoma model, CDK5 regulates interferon-γ–induced PD-L1 expression (21). It is also known that Roscovitine enhances radio sensitivity in NSCLC cells (47). We have now determined that down-regulating CDK5 or inhibiting it with Roscovitine (21) increases FBXO22 levels and decreases PD-L1 levels in NSCLC cells, enhancing their sensitivity to IR or cisplatin. A very recent study showed that CDK5 depletion promotes PD-L1 ubiquitination and degradation through ubiquitin ligase TRIM21 in NSCLC cells (48). In addition to PD-L1, FBXO22 might target other cellular proteins to enhance the sensitivity to IR or cisplatin, a topic for future investigtion. Although Roscovitine inhibits other CDKs (e.g., CDK1, CDK2, CDK7, and CDK9), several studies have shown that it inhibits CDK5 preferentially (49, 50). Roscovitine is the only CDK inhibitor so far that is selective for CDK5 (51). In contrast to CDK5 inhibition, which decreases PD-L1 levels, inhibiting other CDKs, including CDK4/6, increases PD-L1 levels by disrupting the cullin3-SPOP pathway that mediates PD-L1 ubiquitination and degradation (41). Furthermore, CDK4/6 inhibitors effectively enhance ICB therapy (41). These findings suggest that the effect of antitumor drugs can be counteracted by high levels of PD-L1 expression, leading to immune escape. A previous study reported that the E3 ubiquitin ligase FBXW7, a well-characterized tumor suppressor, is also a substrate of CDK5. Activated CDK5 phosphorylates serine residues of FBXW7, thus decreasing its stability (52). Similarly, CDK5-mediated phosphorylation of FBXO22 itself, or of a protein that mediates its stability, decreases the levels of FBXO22 and therefore increases the stability of its substrate PD-L1.
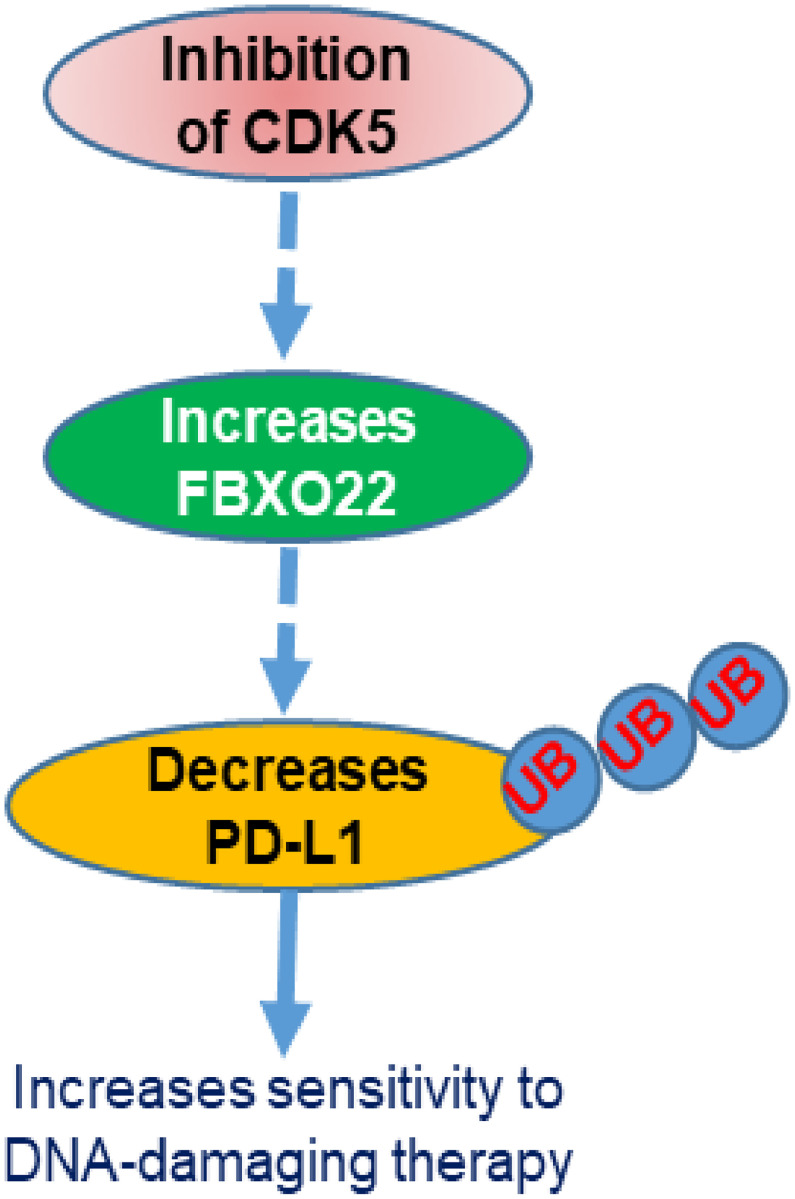
In summary, our study uncovers FBXO22 as a specific E3 ubiquitin ligase that regulates PD-L1 levels through ubiquitination in cancer cells, where PD-L1 has an immune-independent, tumor-intrinsic role. Our findings also provide mechanistic insight into the role of CDK5 in increasing PD-L1 levels by negatively regulating FBXO22 expression in NSCLC cells. We show that CDK5 inhibition increases the level of FBXO22, which in turn causes ubiquitination and degradation of PD-L1, thus decreasing the sensitivity of these cells to DNA-damaging therapies, which are mainstays of treatment for this disease (Fig. 6). Increasing evidence suggests that PD-L1 protein degradation effectively promotes cancer immunotherapy, and that combinations with DNA-damaging therapies significantly enhance this effect. Our study provides the rationale for an approach in which a CDK5 inhibitor is combined not only with anti–PD-L1 alone, but also with standard-of-care DNA-damaging therapies in combination with ICB.
Fig. 6.
Schematic diagram of the CDK5–FBXO22–PD-L1 pathway. Inhibition of CDK5 increases the expression of the ubiquitin ligase FBXO22, which in turn decreases PD-L1 levels by ubiquitination. Degradation of PD-L1 increases sensitivity to chemotherapy or radiation.
Materials and Methods
Cancer cell lines A549, Calu1, H1299 (NSCLC), MDAMB231, MCF7 (breast cancer), HCT116 (colon cancer), and H196 (small cell lung cancer) were obtained from American Type Culture Collection. The PC9 cell line (NSCLC) was a kind gift from Kazuto Nishio (Kinki, University School of Medicine, Osaka, Japan). Noncancerous human mammary epithelial cells, hTERT-HME1, immortalized with human telomerase reverse transcriptase, were purchased from Clontech. VBIM vector constructs were described previously (27). Cell survival assays were done as described previously (3). Western analyses were performed as described previously (4). Ubiquitination assays were performed in cells overexpressing FBXO22 or in FBXO22-depleted cells. Detailed materials and methods are provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Timothy A. Chan, Center for Immunotherapy and Immuno-Oncology, Cleveland Clinic, for his helpful comments on the manuscript. We appreciate the technical support of the Cleveland Clinic Flow Cytometry Core. This work was supported by NIH Grant P01CA062220, to G.R.S. The Fusion Lumos liquid chromatography mass spectrometry instrument was purchased via an NIH shared instrument grant, 1S10OD023436-01.
Footnotes
Reviewers: C.M.H., Northwestern University; and E.K., Roswell Park Comprehensive Cancer Center.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2112674118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Peters S., et al. ; ESMO Guidelines Working Group, Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 23 (suppl. 7), vii56–vii64 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Fennell D. A., et al. , Cisplatin in the modern era: The backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat. Rev. 44, 42–50 (2016). [DOI] [PubMed] [Google Scholar]
- 3.De S., Cipriano R., Jackson M. W., Stark G. R., Overexpression of kinesins mediates docetaxel resistance in breast cancer cells. Cancer Res. 69, 8035–8042 (2009). [DOI] [PubMed] [Google Scholar]
- 4.De S., Dermawan J. K., Stark G. R., EGF receptor uses SOS1 to drive constitutive activation of NFκB in cancer cells. Proc. Natl. Acad. Sci. U.S.A. 111, 11721–11726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johmura Y., et al. , SCF(Fbxo22)-KDM4A targets methylated p53 for degradation and regulates senescence. Nat. Commun. 7, 10574 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skaar J. R., Pagan J. K., Pagano M., Mechanisms and function of substrate recruitment by F-box proteins. Nat. Rev. Mol. Cell Biol. 14, 369–381 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason B., Laman H., The FBXL family of F-box proteins: Variations on a theme. Open Biol. 10, 200319 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai J., et al. , SCFFBXO22 targets HDM2 for degradation and modulates breast cancer cell invasion and metastasis. Proc. Natl. Acad. Sci. U.S.A. 116, 11754–11763 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun R., et al. , FBXO22 possesses both protumorigenic and antimetastatic roles in breast cancer progression. Cancer Res. 78, 5274–5286 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Guo F., et al. , FBXO22 suppresses metastasis in human renal cell carcinoma via inhibiting MMP-9-mediated migration and invasion and VEGF-mediated angiogenesis. Int. J. Biol. Sci. 15, 647–656 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lignitto L., et al. , Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Cell 178, 316–329.e18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge M. K., et al. , FBXO22 degrades nuclear PTEN to promote tumorigenesis. Nat. Commun. 11, 1720 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu X. N., et al. , FBXO22 mediates polyubiquitination and inactivation of LKB1 to promote lung cancer cell growth. Cell Death Dis. 10, 486 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu B., et al. , F-box protein FBXO22 mediates polyubiquitination and degradation of CD147 to reverse cisplatin resistance of tumor cells. Int. J. Mol. Sci. 18, E212 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lantuejoul S., Damotte D., Hofman V., Adam J., Programmed death ligand 1 immunohistochemistry in non-small cell lung carcinoma. J. Thorac. Dis. 11 (suppl. 1), S89–S101 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L., Han X., Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future. J. Clin. Invest. 125, 3384–3391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark C. A., et al. , Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res. 76, 6964–6974 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu X., et al. , PD-L1 (B7-H1) competes with the RNA exosome to regulate the DNA damage response and can be targeted to sensitize to radiation or chemotherapy. Mol. Cell 74, 1215–1226.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz D., et al. , Increased PD-L1 expression in radioresistant HNSCC cell lines after irradiation affects cell proliferation due to inactivation of GSK-3beta. Oncotarget 10, 573–583 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan F., et al. , Elevated cellular PD1/PD-L1 expression confers acquired resistance to cisplatin in small cell lung cancer cells. PLoS One 11, e0162925 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorand R. D., et al. , Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science 353, 399–403 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhavan R., Tsai L. H., A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2, 749–759 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Liu J. L., et al. , Expression of CDK5/p35 in resected patients with non-small cell lung cancer: Relation to prognosis. Med. Oncol. 28, 673–678 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Pozo K., Bibb J. A., The emerging role of Cdk5 in cancer. Trends Cancer 2, 606–618 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrlich S. M., et al. , Targeting cyclin dependent kinase 5 in hepatocellular carcinoma—A novel therapeutic approach. J. Hepatol. 63, 102–113 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Cicenas J., et al. , Roscovitine in cancer and other diseases. Ann. Transl. Med. 3, 135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu T., et al. , Validation-based insertional mutagenesis identifies lysine demethylase FBXL11 as a negative regulator of NFkappaB. Proc. Natl. Acad. Sci. U.S.A. 106, 16339–16344 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garten A., et al. , Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 11, 535–546 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Zerp S. F., Vens C., Floot B., Verheij M., van Triest B., NAD+ depletion by APO866 in combination with radiation in a prostate cancer model, results from an in vitro and in vivo study. Radiother. Oncol. 110, 348–354 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Zheng M., Classification and pathology of lung cancer. Surg. Oncol. Clin. N. Am. 25, 447–468 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Casal R., et al. , Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol. Cancer 12, 94 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu C., et al. , Effects of combined inhibition of STAT3 and VEGFR2 pathways on the radiosensitivity of non-small-cell lung cancer cells. Onco Targets Ther. 12, 933–944 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Escors D., et al. , The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct. Target. Ther. 3, 26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cha J. H., Chan L. C., Li C. W., Hsu J. L., Hung M. C., Mechanisms controlling PD-L1 expression in cancer. Mol. Cell 76, 359–370 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santra M. K., Wajapeyee N., Green M. R., F-box protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest after DNA damage. Nature 459, 722–725 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu N., et al. , Loss of ZIP facilitates JAK2-STAT3 activation in tamoxifen-resistant breast cancer. Proc. Natl. Acad. Sci. U.S.A. 117, 15047–15054 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He J., Hu Y., Hu M., Li B., Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci. Rep. 5, 13110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong T., et al. , CD44 promotes PD-L1 expression and its tumor-intrinsic function in breast and lung cancers. Cancer Res. 80, 444–457 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Chen N., et al. , KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol. Immunother. 66, 1175–1187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren D., et al. , Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol. Cancer 19, 19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J., et al. , Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 553, 91–95 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng L., et al. , Inhibition of mTOR complex 1/p70 S6 kinase signaling elevates PD-L1 levels in human cancer cells through enhancing protein stabilization accompanied with enhanced β-TrCP degradation. Oncogene 38, 6270–6282 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Gou Q., et al. , PD-L1 degradation pathway and immunotherapy for cancer. Cell Death Dis. 11, 955 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong X., et al. , Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J. Thorac. Oncol. 12, 1085–1097 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Lacour M., et al. , Adjuvant chemotherapy increases programmed death-ligand 1 (PD-L1) expression in non-small cell lung cancer recurrence. Clin. Lung Cancer 20, 391–396 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Abiko K., et al. , IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 112, 1501–1509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang F., et al. , Enhancement of radiosensitivity by roscovitine pretreatment in human non-small cell lung cancer A549 cells. J. Radiat. Res. (Tokyo) 49, 541–548 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Gao L., et al. , Knockdown of CDK5 down-regulates PD-L1 via the ubiquitination-proteasome pathway and improves antitumor immunity in lung adenocarcinoma. Transl. Oncol. 14, 101148 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meijer L., et al. , Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243, 527–536 (1997). [DOI] [PubMed] [Google Scholar]
- 50.Sahlgren C. M., et al. , Cdk5 regulates the organization of Nestin and its association with p35. Mol. Cell. Biol. 23, 5090–5106 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huber R. J., O’Day D. H., The cyclin-dependent kinase inhibitor roscovitine inhibits kinase activity, cell proliferation, multicellular development, and Cdk5 nuclear translocation in Dictyostelium discoideum. J. Cell. Biochem. 113, 868–876 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Ko Y. U., et al. , Site-specific phosphorylation of Fbxw7 by Cdk5/p25 and its resulting decreased stability are linked to glutamate-induced excitotoxicity. Cell Death Dis. 10, 579 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.