Significance
Extracellular pilus filaments are critical for the virulence and persistence of many bacterial pathogens. A crucial property of these filaments is their ability to dynamically extend and retract from the bacterial surface. A detailed mechanistic understanding of pilus retraction, however, remains lacking in many systems. Here, we reveal that pilus retraction is an inherent property of the pilus filament. These observations are broadly relevant to diverse pilus systems, including those in many bacterial pathogens, and may help inform therapeutic strategies that aim to target pilus dynamic activity.
Keywords: Acinetobacter, Vibrio, type IV filament
Abstract
Type IV pili (T4P) are dynamic surface appendages that promote virulence, biofilm formation, horizontal gene transfer, and motility in diverse bacterial species. Pilus dynamic activity is best characterized in T4P that use distinct ATPase motors for pilus extension and retraction. Many T4P systems, however, lack a dedicated retraction motor, and the mechanism underlying this motor-independent retraction remains a mystery. Using the Vibrio cholerae competence pilus as a model system, we identify mutations in the major pilin gene that enhance motor-independent retraction. These mutants likely diminish pilin–pilin interactions within the filament to produce less-stable pili. One mutation adds a bulky residue to α1C, a universally conserved feature of T4P. We found that inserting a bulky residue into α1C of the retraction motor–dependent Acinetobacter baylyi competence T4P enhances motor-independent retraction. Conversely, removing bulky residues from α1C of the retraction motor–independent, V. cholerae toxin-coregulated T4P stabilizes the filament and diminishes pilus retraction. Furthermore, alignment of pilins from the broader type IV filament (T4F) family indicated that retraction motor–independent T4P, gram-positive Com pili, and type II secretion systems generally encode larger residues within α1C oriented toward the pilus core compared to retraction motor–dependent T4P. Together, our data demonstrate that motor-independent retraction relies, in part, on the inherent instability of the pilus filament, which may be a conserved feature of diverse T4Fs. This provides evidence for a long-standing yet previously untested model in which pili retract in the absence of a motor by spontaneous depolymerization.
Type IV pili (T4P) are ubiquitous, hair-like appendages that dynamically extend and retract from bacterial cells (1–3). These membrane-anchored nanomachines are important for the interactions of bacteria with their environment and facilitate activities like twitching motility, surface attachment, biofilm formation, protein secretion, interbacterial interactions, and horizontal gene transfer by natural transformation (2, 4–10). The dynamic activity of T4P is central to many of these functions. T4P are also essential virulence factors in diverse pathogens (10–13). Thus, studying the regulation of these structures can uncover approaches for the development of therapeutic interventions.
The surface-exposed pilus filament is a multimeric helical structure that is primarily composed of a single repeating subunit called the major pilin. This filament is built on an inner membrane platform protein commonly called PilC (14–16). Extension and retraction of the pilus is thought to occur through the interaction of cytoplasmic hexameric ATPases with the platform, whereby ATP hydrolysis facilitates coordinated conformational changes in the platform that promote polymerization or depolymerization (17, 18).
There are three main categories of T4P systems among bacteria: T4aP, T4bP, and T4cP. All T4P require an extension ATPase motor for pilus polymerization (19). A detailed mechanistic understanding of the factors that promote pilus retraction, however, remains lacking in many T4P. For T4aP, pilus depolymerization is generally mediated by dedicated retraction ATPase motors commonly referred to as PilT and PilU. PilT is the dominant retraction motor, because it is independently sufficient to promote pilus retraction, while PilU is an accessory motor that facilitates retraction only in the presence of PilT (20, 21). However, even in the absence of these retraction motors, numerous T4aP retain the ability to retract (20, 22–24). Many T4bP appear to lack a dedicated retraction ATPase (25–27) yet also retract (25). Furthermore, phylogenetic analysis indicates that T4aP and T4bP most likely evolved from a common ancestor (19, 28). This suggests that diverse T4P may share a conserved mechanism for PilTU-independent retraction. Here, we investigated the mechanism of pilus retraction in the absence of dedicated retraction ATPases and find evidence to support a long-standing yet previously untested model in which pili retract via spontaneous depolymerization.
Results
Vibrio cholerae Competence Pili Exhibit PilTU-Independent Retraction.
To address the mechanism underlying PilTU-independent retraction, we employed the T4aP competence pili of Vibrio cholerae as a model system. These pili dynamically extend into the environment, bind DNA, and then retract to promote DNA uptake into the periplasm during horizontal gene transfer by natural transformation (6, 29, 30). Ingested DNA can then be taken up into the cytoplasm and integrated into the genome via homologous recombination by other (nonpilus) competence machinery (6, 31–33). To study the dynamic activity of V. cholerae competence pili, we can label pili using a technique in which an amino acid residue of the major pilin, PilA, is replaced with a cysteine (pilAS56C) for subsequent labeling with maleimide conjugated fluorescent dyes (5, 20, 34, 35). Using this technique, pili were directly observed by fluorescence microscopy. Functional dynamics of the competence pilus were also assessed through natural transformation assays.
The extension ATPase PilB and the retraction ATPases PilT and PilU drive the dynamic assembly and disassembly of V. cholerae competence pili, respectively. As expected, a ΔpilB mutant lacking the extension ATPase does not assemble pili (Fig. 1A) and is not transformable (Fig. 1B) (6, 20). As shown previously, a mutant strain lacking both retraction motors (ΔpilTU) is hyperpiliated and aggregates (7, 20), yet it is still able to retract pili, albeit with lower retraction speed and frequency, which supports natural transformation at reduced levels (20). We reconfirm these previous findings here (Fig. 1 A and B). Importantly, previous work demonstrated that pilus retraction is required for DNA uptake during natural transformation for the ΔpilTU strain (29). Thus, the PilT and PilU motor ATPases are not absolutely required for pili to retract. Furthermore, the V. cholerae competence pilus is a well-studied system with multiple tools developed to probe both its PilTU-dependent and, albeit artificial, PilTU-independent pilus dynamic activity, making this a well-suited system in which to study pilus retraction.
Fig. 1.
V. cholerae competence pili exhibit PilTU-independent retraction that is not powered by the extension motor PilB. (A) Representative images of surface piliation (Top) and aggregation phenotypes (Bottom) for the indicated strains. (Scale bar, 1 µm.) (B) Natural transformation assays of the indicated strains. Reactions were incubated with 200 ng of transforming DNA (tDNA). All strains, n = 3. Data are shown as the mean ± SD. Asterisk(s) directly above bars denote comparisons to the parent strain. (C) Extension speed (pink) and retraction speed (blue) for the indicated ΔpilTU strains was measured by epifluorescence time-lapse microscopy of AF488-mal-labeled cells. To enhance dynamic activity, pilB alleles were placed under the control of a tightly inducible PBAD promoter in a ΔpilB background, and the expression of pilB was delayed until just before imaging was performed. Data are from three independent biological replicates. All strains, n = 44. Box plots represent the median and the upper and lower quartile, while the whiskers demarcate the range. All strains in A–C are derived from the parent strain, which contains a pilAS56C mutation to allow for pilus labeling. All comparisons were made by one-way ANOVA with Tukey’s post test. LOD, limit of detection; NS, not significant; and *** = P < 0.001.
PilB Does Not Power PilTU-Independent Retraction.
As mentioned above, many T4aP and T4bP are able to retract in the absence of dedicated retraction ATPases, and the mechanism that mediates this PilTU-independent retraction remains unknown. T4cP also lack dedicated retraction motors; however, these pilus systems use a bifunctional motor to power both pilus extension and retraction (36). Therefore, we sought to examine whether PilB powers PilTU-independent retraction of V. cholerae competence pili. Because PilB is required for pilus extension (Fig. 1A), we cannot test this using a ΔpilB mutant. Instead, we sought to determine whether the ATPase activity of PilB was required for PilTU-independent retraction.
In order to test whether PilB powers both extension and retraction via its ATPase activity, we employed the same technique described by Ellison et al. (36) to demonstrate that the ATPase CpaF powers both extension and retraction in Caulobacter crescentus T4cP. For that study, CpaF mutants that exhibit reduced ATPase activity slowed down the speed of both extension and retraction equally, indicating that CpaF likely powers both activities (36). Similarly, if pilB mutants that slow ATPase activity reduce the speed of both extension and PilTU-independent retraction, that would suggest that PilB is also a bifunctional ATPase. Measuring extension and retraction speeds in a ΔpilTU strain, however, is exceedingly difficult, because dynamic pilus events are rare in this hyperpiliated background (20, 37). In order to increase the number of dynamic events, we generated a mutant strain that allowed us to tightly regulate the expression of PilB (ΔpilTU ΔpilB PBAD-pilB) (37). By inducing PilB expression only during imaging, we greatly increase the number of pilus dynamic events observed (Movies S1–S3).
To test whether PilB is a bifunctional ATPase, we used a pilB mutant (pilBM391C) that was previously shown to reduce ATPase activity approximately twofold (38) and to correspondingly reduce the competence pilus extension speed approximately twofold (36). Consistent with these prior studies, ΔpilTU ΔpilB PBAD-pilBM391C exhibits an average extension speed of ∼72 nm/second, which is ∼1.8-fold slower than the ∼128-nm/second extension speed of ΔpilTU ΔpilB PBAD-pilBWT (Fig. 1C) (36). However, retraction speeds in ΔpilTU ΔpilB PBAD-pilBM391C were unaltered compared to ΔpilTU ΔpilB PBAD-pilBWT with both strains exhibiting an average retraction speed of ∼6 nm/second (Fig. 1C), which suggests that PilB is not a bifunctional motor. Importantly, previous work from our laboratory demonstrated that the ATPases required for polymerization of the other two T4P encoded by V. cholerae (MshE and TcpT) do not contribute to PilTU-independent retraction (20). Together, these results indicate that the retraction observed in the absence of PilTU is likely motor independent. However, this does not reveal the mechanism underlying this motor-independent retraction.
A Forward Genetic Selection Reveals a Role for the Major Pilin, PilA, in Motor-Independent Retraction.
In order to identify the mechanism underlying motor-independent retraction, we performed a forward genetic screen. As discussed above, mutants that exhibit enhanced retraction should also exhibit enhanced levels of natural transformation (Fig. 1B) (20). Thus, we reasoned that a suppressor screen selecting for mutants with enhanced levels of natural transformation might uncover gain-of-function mutations to components involved in motor-independent retraction. To that end, we recursively transformed a population of ΔpilT ΔmutS cells to enrich for mutants with enhanced rates of natural transformation. Using a ΔpilT strain, which exhibits reduced retraction efficiency (Fig. 1B) (20), allowed us to select for mutants with enhanced motor-independent retraction. The deletion of mutS in this background increases the spontaneous mutation rate by disrupting the mismatch repair system (39). To maintain selection for each recursive round of natural transformation, different antibiotic resistance markers were swapped at a neutral locus. This selection was carried out in 11 biologically independent lineages. All lineages exhibited an enhanced transformation frequency following four rounds of selection. Single strains were isolated from each lineage, and targeted sequencing of select pilus genes (pilA, pilB, pilC, pilU, and pilM) was performed to identify potentially causative mutations. From the 11 populations, only four unique missense mutations were identified, all in the major pilin PilA (G34R, V74A, G78S, and G80S). When these pilA mutations were introduced into a clean ΔpilTU genetic background, they all increased transformation frequency ∼100-fold (Fig. 2A), confirming that they were sufficient to promote the enhanced rates of natural transformation observed in the original suppressor mutants.
Fig. 2.
A forward genetic screen reveals mutations in PilA that enhance PilTU-independent pilus retraction. (A) Natural transformation assays of the indicated strains. Reactions were incubated with 200 ng of tDNA. All strains, n = 3. (B) Representative images of surface piliation (Top) and aggregation phenotypes (Bottom) for the indicated strains. (Scale bar, 1 µm.) (C) Retraction frequency of the indicated strains measured by epifluorescence time-lapse microscopy of AF488-mal-labeled cells. Data are presented as the percentage of cells within the population that exhibited a retraction event within the 10 min time-lapse. Data are from three independent biological replicates; pilAparent ΔpilTU, n = 1184; pilAG34R ΔpilTU, n = 718; pilAV74A ΔpilTU, n = 709; pilAG78S ΔpilTU, n = 864; and pilAG80S ΔpilTU, n = 674. (D) Retraction speeds of the indicated strains measured by epifluorescence time-lapse microscopy of AF488-mal-labeled cells. Data are from three independent biological replicates; pilAparent ΔpilTU, n = 44; and pilAG78S ΔpilTU, n = 30. (E) Structural model of PilA based on Phyre2 threading of the PilA sequence (NP_232053) onto PDB 1OQW (alignment coverage 80%, confidence 99.9%, and identity 38%). Residues mutated in our suppressor screen are shown in stick representation and colored magenta. The α1N (residues 1 through 27) and α1C (residues 28 through 53) regions of PilA are also annotated. (F) Predicted model of the V. cholerae competence pilus in which the terminal pilin is shown in teal and other pilins in the fiber are shown in gray. The Phyre2-predicted PilA structure was superimposed onto one chain of the P. aeruginosa PAK pilus (PDB 5VXY) and then the helical properties of the PAK pilus were used to generate a structural model for the PilA fiber. The PilA suppressor mutations are shown as magenta stick representations in the terminal pilin to highlight where they are within the fiber. All bar graphs are shown as the mean ± SD. All box plots represent the median and the upper and lower quartile, while the whiskers demarcate the range. Asterisk(s) directly above bars denote comparisons to the parent strain. Comparisons in A and C were made by one-way ANOVA with Tukey’s post test. The comparison in D was made by Student’s t test. ** = P < 0.01 and *** = P < 0.001.
We also assessed the effect of these pilA suppressor mutations on natural transformation in a background where pilTU was intact. This analysis showed that all mutants transformed at levels similar to the parent strain (SI Appendix, Fig. S1A), suggesting that these alterations to the major pilin did not impact pilus biogenesis or motor-dependent retraction. We directly imaged these pili using our pilus labeling approach. All pilA point mutants produced visible external filaments with and without pilTU, although three of the four strains with pilAG34R, pilAV74A, and pilAG80S mutations produced shorter pili than the pilAparent strain (Fig. 2B and SI Appendix, Fig. S1B). Even though the ΔpilTU pilAG34R, ΔpilTU pilAV74A, and ΔpilTU pilAG80S strains produced shorter pili, all of these strains aggregate (Fig. 2B), suggesting that they have surface-exposed pili. Moreover, we further confirmed that the fluorescent foci/filaments observed in the ΔpilTU pilAparent, ΔpilTU pilAG34R, ΔpilTU pilAV74A, ΔpilTU pilAG78S, and ΔpilTU pilAG80S strains (Fig. 2B) are external pilus filaments by introducing a pilQ deletion into each strain. PilQ is the outer-membrane secretin and is required for the assembly of surface-exposed pilus filaments. In the ΔpilQ background, all mutants lacked foci/visible surface structures (SI Appendix, Fig. S2B). This suggests that the foci and filaments observed when pilQ is intact (Fig. 2B and SI Appendix, Fig. S1B) represent bona fide surface-exposed pili. Additionally, in a ΔpilTU background we used bulky maleimide conjugates (biotin-maleimide + neutravidin) to label pili to sterically obstruct pilus retraction (5, 29). This treatment prevented natural transformation, which further indicates that all of these pilA suppressor mutants still produce external pilus fibers and require pilus retraction to facilitate DNA uptake (SI Appendix, Fig. S2). We also assessed whether these pilA point mutants altered PilA expression levels via Western blot analysis. Although PilAG80S expression was slightly reduced, the other point mutants had no effect on PilA expression compared to PilAparent in both the pilTU intact and ΔpilTU backgrounds (SI Appendix, Figs. S1D and S2C). These results indicate that the suppressor mutations in pilA do not induce motor-independent retraction by simply altering PilA expression. Together, these data suggest that the pilA suppressor mutations enhance motor-independent retraction without dramatically altering pilus biogenesis or function.
The increased transformation frequencies observed for the suppressor mutations in a ΔpilTU background could result from increased retraction events or from an increased speed of retraction. To distinguish between these possibilities, we directly observed pilus dynamic activity in strains with labeled pili using time-lapse epifluorescence microscopy. The retraction frequency could be assessed in all mutant backgrounds; however, the speed of pilus retraction could only be accurately measured in the pilAparent and pilAG78S strains due to the short length of pilus filaments in the pilAG34R, pilAV74A, and pilAG80S strains. These data revealed that pilus retraction frequency increases ∼10-fold for all of the ΔpilTU pilA suppressor mutants and that ΔpilTU pilAG78S pili retract with an average speed of ∼12 nm/second which is approximately two times faster than the ∼6 nm/second observed in the ΔpilTU pilAparent strain (Fig. 2 C and D). We previously found that altering retraction speed even sevenfold has no discernable effect on transformation (20). Thus, the observed increase in the motor-independent retraction frequency likely explains the increase in transformation rates seen in these pilA suppressor mutants. The discrepancy between the observed 10-fold increase in retraction frequency and the 100-fold increase in transformation frequency may be attributed to the fact that many short, dynamic pilus events occur that are not resolved by our microscopy-based approach.
Interestingly, in a background where the pilTU genes are intact, pilAG78S has no discernable effect on the speed of pilus retraction as both the pilAparent and pilAG78S strains retract with an average ∼220 nm/second (SI Appendix, Fig. S1C). Thus, our data indicate that the pilA suppressor mutations only affect pilus dynamics in the ΔpilTU background and did not affect pilus dynamics when pilTU were intact (Fig. 2 A and C and SI Appendix, Fig. S1 A and C). This suggests that these pilA mutations only affect motor-independent pilus retraction and that their effect on pilus dynamics can be overcome by retraction motors.
The Stability of the Pilus Filament Is a Critical Determinant for Motor-Independent Retraction.
In order to understand how these amino acid changes in PilA might increase the frequency of motor-independent retraction, we sought to understand how they might affect the overall structure of the major pilin and the pilus filament. To that end, we generated a model for PilA and for the competence pilus filament based on existing homologous structures. The predicted PilA structure was generated based on the Pseudomonas aeruginosa major pilin structure (Protein Data Bank [PDB] ID 1OQW) using Phyre2 (40, 41). The competence pilus model was generated by superimposing the PilA model onto one of the subunits in the P. aeruginosa pilus structure (PDB 5VXY) and then imposing helical symmetry to generate the multisubunit filament (42). Additionally, the N-terminal half of the extended α-helix, α1N, of PilA was replaced with that of the P. aeruginosa pilin to reproduce the partial melting that this α-helix undergoes when incorporated into the pilus (42, 43). The model of V. cholerae PilA shows the residue positions of the suppressor mutations: Gly34 is located in α1C, the C-terminal half of the primary N-terminal α-helix (α1), and the other positions Val74, Gly78, and Gly80 are located in the αβ-loop that connects α1 with the central β-sheet of the globular domain (Fig. 2E). The pilus filament model revealed that all four suppressor mutation positions that enhance motor-independent retraction (PilAG34R, PilAV74A, PilAG78S, and PilAG80S) lie at interfaces between pilin subunits (Fig. 2F). When pilins are assembled into a filament, a portion of the α1 of each pilin is melted within a conserved region between residues 14 through 22, presumably to allow α1N to pack within the filament (42, 43). All of the PilA suppressor mutations encode residues that potentially contact α1N of a neighboring pilin subunit including the melted region between residues 14 and 22. Thus, altering pilin contacts in this area might interfere with subunit packing and/or compromise the malleability of the melted region in neighboring subunits, which may destabilize the pilus filament. Additionally, we note that three of the four suppressor mutations in the pilA gene encode substitutions at glycines. Glycine lacks a side chain, which allows greater backbone flexibility and is thus known as a “helix-breaker.” While Val74 is replaced with a smaller alanine, Gly34 is replaced by a bulky positively charged arginine, and Gly78 and Gly80 are both replaced with polar serines. The increase in bulk, polarity, and/or the decrease in flexibility at these sites may disrupt both the backbone conformation at α1C and the αβ-loop as well as the close packing of the subunits, destabilizing the pilus filament.
How might alterations in pilin subunit interactions increase motor-independent retraction in these pilA suppressor mutants? It has previously been suggested that pili can spontaneously depolymerize in the absence of motors (25, 44–46); however, conclusive evidence for this model has been elusive. We hypothesize that a pilus with weaker pilin–pilin interactions would allow newly added subunits at the base of the pilus to diffuse more readily back into the membrane if pilus assembly is stalled, thereby facilitating motor-independent retraction through spontaneous depolymerization (refer to Discussion for a detailed description of this model). A consequence of weaker pilin–pilin interactions may be altered stability of the pilus filament. To test whether the suppressor mutants produce less-stable pili, we purified labeled pili and tested their thermal and chemical stability as well as their protease susceptibility, as previously described (46). Though we were unable to purify the very short PilAG34R, PilAV74A, and PilAG80S filaments in sufficient quantities for these assays, we obtained testable amounts of PilAparent and PilAG78S pili from a ΔpilTU background. We found that the PilAG78S filaments have similar chemical stability to the PilAparent filaments (SI Appendix, Fig. S3) but are less thermally stable and more susceptible to protease degradation (Fig. 3A). This instability may facilitate spontaneous depolymerization, thereby increasing the frequency of motor-independent retraction.
Fig. 3.
The size of residues in α1C may influence motor-independent retraction by altering the stability of the pilus filament. (A) Stability assay comparing thermal stability and protease susceptibility of purified AF488-mal-labeled pilus filaments from ΔpilTU strains expressing the indicated pilA allele. A representative phase-contrast image is shown to highlight that pilus preps are cell free. Fluorescent images show the presence or absence of intact pili under the indicated conditions. (B) Representative images of surface piliation for the indicated ΔpilTU strains after treatment with or without proteinase K. (C) Natural transformation assays of the indicated ΔpilTU strains. Reactions were incubated with 200 ng of tDNA. For ease of mutant generation, the native pilA gene was knocked out, and the indicated pilA allele was expressed at an ectopic location (under the control of an IPTG-inducible Ptac promoter). The amino acid at position 34 is indicated in the bar for each strain. Data are shown as the mean ± SD, and comparisons were made by one-way ANOVA with Tukey’s post test. Asterisk(s) directly above bars denote comparisons to the parent strain. All strains, n = 3. LOD, limit of detection; NS, not significant; and *** = P < 0.001. (D) Representative images of surface piliation for the ΔpilTU strains used in C. All images in panels A, B, and D are representative of three independent biological replicates. (Scale bars, 1 µm.)
Although we could not purify pili from the other pilA point mutants, we sought to test whether the external filaments present on the surface of intact cells are indeed less stable. Since the purified PilAG78S filaments are more susceptible to proteinase K than the PilAparent pili (a phenotypic characteristic of less-stable pili), we hypothesized that the surface-exposed filaments present on all of the ΔpilTU pilA suppressor mutants are also less stable and, therefore, more susceptible to protease degradation compared to the ΔpilTU pilAparent strain. Indeed, when cells with labeled pili were treated with proteinase K, all four pilA suppressor mutants exhibited a complete loss of surface piliation as observed by fluorescence microscopy, while piliation of the pilAparent was unchanged by this treatment (Fig. 3B). These results are consistent with the protease susceptibility profiles of purified PilAparent and PilAG78S filaments and indicate that the pilAG34R, pilAV74A, pilAG78S, and pilAG80S mutations all reduce the stability of pilus filaments in a similar manner. This supports the hypothesis that reducing filament stability enhances motor-independent retraction. The observed reduction in pilus stability among all of the pilA suppressor mutants is also consistent with the hypothesis that these mutations alter pilin–pilin interactions within the filament.
Insertion of Bulky Residues in α1C of the V. cholerae Competence Pilus Enhances Motor-Independent Retraction.
Since these data suggest that altering pilin–pilin interactions reduces filament stability and enhances motor-independent pilus retraction, we sought to understand the molecular mechanism of filament destabilization. We focused on the PilAG34R mutation, as it is located in a region of the major pilin, α1C, that is structurally conserved among all major pilins from the type IV filament (T4F) family (19) despite being variable in sequence (SI Appendix, Fig. S4A). The PilAG34R mutation changes a small, nonpolar amino acid (glycine) to a larger, positively charged amino acid (arginine). To determine whether the size or charge of the arginine side chain is responsible for enhanced motor-independent retraction, we made a panel of mutants with amino acid substitutions at the Gly34 position in PilA. We expected that if the positive charge of the arginine was the critical feature, substitution with lysine but not other amino acids should enhance motor-independent retraction. However, if the size of the arginine is the important feature, substitution with other amino acids that are larger than glycine, like glutamine and phenylalanine, should phenocopy the pilAG34R mutant (SI Appendix, Fig. S4B lists the volume of amino acid R-groups as a correlate of their size) (47). Transformation assays showed that ΔpilTU pilAG34K, ΔpilTU pilAG34Q, and ΔpilTU pilAG34F all phenocopied ΔpilTU pilAG34R, indicating that it is the size of the amino acid at position 34 and not its charge that is critical for motor-independent retraction (Fig. 3C). Importantly, these mutations did not alter PilA expression levels (SI Appendix, Fig. S3B). Since substitutions with glutamine (polar) and phenylalanine (nonpolar) both enhanced transformation similarly, this suggests that residue polarity at the Gly34 position in PilA is also not a critical feature for enhancing motor-independent retraction. Each of these mutants also displayed reduced pilus length compared to ΔpilTU pilAparent, similar to the original ΔpilTU pilAG34R mutant (Fig. 3D). Changing the Gly34 position to a negatively charged residue (pilAG34E) abolished transformation (Fig. 3C), most likely due to disruption of pilus biogenesis, which is supported by a lack of observable pilus filaments in this background (Fig. 3D). Substituting glycine with another small amino acid, alanine (pilAG34A), which is common in α-helices, only had a minor effect on transformation and pilus length (Fig. 3 C and D). Together, these data suggest that amino acids with an R-group volume larger than alanine (SI Appendix, Fig. S4B; henceforth referred to as “bulky” residues) at position 34 in PilA enhance the frequency of motor-independent retraction, which likely occurs through destabilization of the pilus filament.
Residue Bulkiness in α1C Influences Motor-Independent Retraction of the Acinetobacter baylyi Competence Pilus.
We next sought to determine whether the bulkiness at position 34 correlates with the ability of other T4P to retract spontaneously. First, we tested whether bulky residues in α1C promote motor-independent retraction in the naturally retraction motor–dependent competence T4aP of Acinetobacter baylyi. Like V. cholerae PilA, the A. baylyi major pilin ComP has a small amino acid, a serine, at position 34 (SI Appendix, Fig. S4 A and B). A. baylyi competence pili can be studied using the same cysteine knock-in (comPT123C) and maleimide dye labeling approach described above (48), and we can functionally assess competence pilus dynamic activity through natural transformation assays. Unlike V. cholerae, A. baylyi ΔpilTU mutants do not exhibit any residual natural transformation (Fig. 4A) (20), which is consistent with an absence of motor-independent retraction for this T4aP. We hypothesized that introducing a bulky residue at position 34 of ComP would interfere with pilin–pilin interactions, destabilizing the pilus to promote motor-independent retraction. To that end, we substituted Ser34 of the A. baylyi major pilin ComP with arginine (comPS34R). The comPS34R strain produces pilus filaments, indicating that pilus biogenesis is not inhibited (Fig. 4B). Transformation of comPS34R was ∼1 log lower than the parent when pilTU were intact, suggesting an impact of this major pilin mutation on motor-dependent dynamic activity (Fig. 4A). However, strikingly, transformation was readily detectable in a ΔpilTU comPS34R mutant, ∼100-fold above the limit of detection in the assay, which is represented by the ΔpilTU comPparent strain (Fig. 4A). Importantly, this effect was not dependent on the comPT123C mutation required for pilus labeling (SI Appendix, Fig. S5). These data are consistent with the model in which a bulky residue in α1C enhances spontaneous disassembly during motor-independent retraction.
Fig. 4.

Residue bulkiness in α1C influences motor-independent retraction of the A. baylyi competence pilus. (A) Natural transformation assays of the indicated strains. Reactions were incubated with 50 ng of tDNA. All strains, n = 3. Data are shown as the mean ± SD. Asterisk(s) directly above bars denote comparisons to the parent strain. Comparisons were made by one-way ANOVA with Tukey’s post test. LOD, limit of detection; *** = P < 0.001. (B) Representative images of surface piliation for the indicated strains. (Scale bar, 1 µm.)
Reducing Residue Bulkiness in α1C Diminishes Motor-Independent Retraction of the V. cholerae Toxin-Coregulated Pilus.
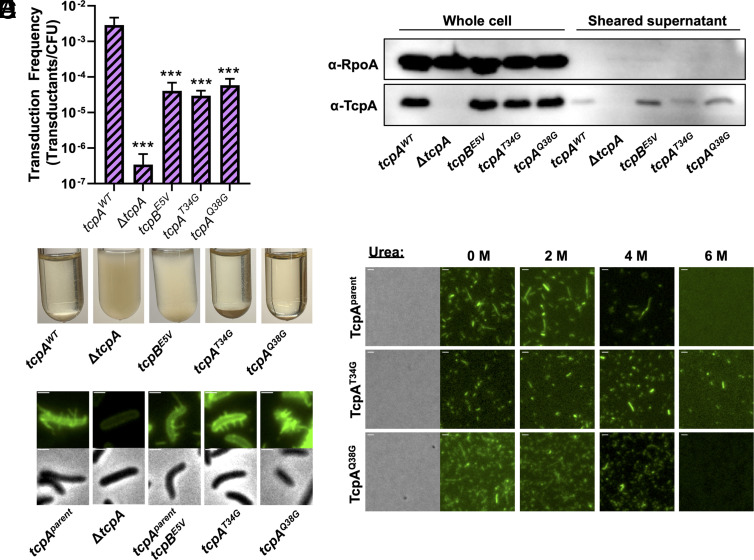
Next, we tested whether reducing residue bulkiness in α1C diminishes the retraction of the naturally motor-independent T4b toxin-coregulated pilus (TCP) of V. cholerae. TCP has no known retraction motors yet is retractile, and retraction was previously proposed to occur via spontaneous depolymerization (25). TCP dynamic pilus activity can be functionally assessed using a transducing phage (CTXΦ-Km) that confers kanamycin resistance (49–51). During transduction, CTXΦ-Km binds to the minor pilin TcpB at the pilus tip (51). TCP retraction is thought to be required for CTXΦ infection, as is the case for many phages that use T4P as receptors (25, 36, 51–54). We formally tested whether CTXΦ-Km transduction requires TCP retraction by using bulky conjugates (biotin-maleimide + neutravidin) to coat cysteine knock-in pili (tcpAT22C), which sterically obstructs pilus retraction as previously described (5, 29). Indeed, sterically obstructing pilus retraction inhibits CTXΦ-Km transduction (SI Appendix, Fig. S6A). Previous work showed that TCP retraction is triggered by incorporation of the minor pilin TcpB into the growing pilus, which presumably stalls pilus extension and initiates TCP retraction (25). All T4P require a glutamate at position 5 of the major pilin for pilus assembly to proceed. This glutamate forms a salt bridge with the N-terminal amine of a neighboring pilin that facilitates subunit docking and neutralizes these charges in the hydrophobic core of the pilus (43). The minor pilin TcpB also requires Glu5 to incorporate into the growing TCP to stall pilus assembly. Thus, a tcpBE5V mutant cannot initiate pilus retraction (25) and was included as an additional control. As expected, we observed that the tcpBE5V mutant exhibits dramatically reduced transduction relative to the parental strain (Fig. 5A), as has been observed previously (25, 55).
Fig. 5.
Residue bulkiness in α1C helix influences motor-independent retraction in the V. cholerae TCP. (A) Transduction assays of the indicated strains. All strains, n = 3. Data are shown as the mean ± SD. Asterisk(s) directly above bars denote comparisons to the parent strain. Comparisons were made by one-way ANOVA with Tukey’s post test. *** = P < 0.001. (B) Western blot of the indicated strains to detect the major pilin TcpA and RpoA (as a loading and cytoplasmic protein control) in both whole-cell lysates and sheared supernatant samples as indicated. Data are representative of two independent experiments. (C) Representative images of aggregation phenotypes and (D) surface piliation for the indicated strains. (Scale bars in D, 1 µm.) (E) Stability assay comparing chemical stability of purified AF488-mal-labeled pili from the indicated strains. A representative phase-contrast image is shown to highlight that pilus preps are cell free. Fluorescent images show the presence or absence of intact pili under the indicated conditions. All images are representative of three independent biological replicates. (Scale bar, 1 µm.)
To test whether residue bulkiness in the pilus core influences TCP retraction, we targeted residues in TcpA at Thr34 (corresponding to the position of the PilAG34R suppressor) and at Gln38 (a large residue one helical turn away in α1C) (SI Appendix, Figs. S4 A and B and S7A). These bulky residues were changed to the smallest amino acid, glycine (tcpAT34G and tcpAQ38G). We hypothesized that if these bulky residues destabilize the pilus and facilitate retraction, substituting them with smaller residues would reduce TCP retraction frequency and, therefore, reduce phage transduction. Indeed, both the tcpAT34G and tcpAQ38G mutants showed a significant reduction in CTXΦ transduction similar to the tcpBE5V strain (Fig. 5A). A trivial explanation for this phenotype, however, could be that these mutants prevented TCP biogenesis. To test this, we took three approaches to assess the presence of surface-exposed TCP. First, TCP were sheared from the bacterial surface. Western blot analysis for TcpA in sheared-surface fractions indicated that both tcpAT34G and tcpAQ38G produced similar amounts of surface-associated pili as the parent and tcpBE5V mutant (Fig. 5B). Second, surface-associated TCP interact, resulting in aggregation of cells in liquid culture. Aggregation of both tcpAT34G and tcpAQ38G were indistinguishable from the parent (Fig. 5C). Third, TCP can be labeled via the same cysteine knock-in and maleimide dye approach described above (tcpAT22C) (5, 35). We introduced these mutations into the tcpAT22C background to directly observe surface piliation by epifluorescence microscopy. Importantly, both tcpAT34G and tcpAQ38G still exhibit reduced transduction in this background (SI Appendix, Fig. S6B). Consistent with our previous results (Fig. 5 B and C), tcpAT34G and tcpAQ38G produce long, external TCP filaments similar in appearance to the parent strain (Fig. 5D). These results indicate that the reduction in motor-independent retraction observed for the tcpAT34G and tcpAQ38G mutants is not due to reduced pilus biogenesis and are thus consistent with our hypothesis that smaller residues in α1C may allow for more stable subunit packing, which reduces the frequency of spontaneous retraction.
To test this hypothesis, we purified labeled TCP filaments from the parent, tcpAT34G, and tcpAQ38G strains and tested their thermal and chemical stability as well as protease susceptibility as described above. Pilus filaments from all three strains have similar thermal stability and protease susceptibility profiles (SI Appendix, Fig. S6C); however, TcpAT34G filaments are more resistant to denaturation by 6 M urea compared to the parent and TcpAQ38G filaments (Fig. 5E). Threonine is only marginally bulkier than glycine (SI Appendix, Fig. S4B), but the tcpAT34G mutation still enhanced fiber stability and reduced pilus retraction events, which suggests that threonine may be large enough to reduce fiber stability. This is consistent with our hypothesis that small amino acids in α1C of the major pilin allow for stronger pilin–pilin interactions and increase the stability of the pilus, thereby decreasing its propensity to spontaneously retract. In addition to reducing residue bulkiness at these locations, the tcpAT34G and tcpAQ38G mutations also modify the polarity at this position (a change from polar to nonpolar amino acids). Thus, these amino acid changes may stabilize TCP by relieving steric stress or by removing the polar hydroxyl group from an otherwise nonpolar environment. Together, these data further support the idea that bulky residues in α1C oriented into the pilus core promote motor-independent retraction.
The Presence of Bulky Residues in α1C Is a Conserved Property of Retraction Motor–Independent Pilus Systems.
Because we found that bulky residues in α1C are important for promoting motor-independent retraction in three distinct T4P, we next sought to address whether this was a conserved feature of retraction motor-independent systems. Specifically, we hypothesized that retraction motor-independent systems would have bulkier residues in α1C that destabilize filaments, facilitating spontaneous depolymerization, whereas retraction motor–dependent systems may tolerate smaller residues in this region that fit better into the filament core, resulting in more stable pili that reduce the likelihood of spontaneous depolymerization. T4P fit into the broader family of T4Fs, which includes several closely related systems that appear to retract without a dedicated motor, such as the gram-positive Com pili and the type II secretion system (T2SS) (19, 28, 56–58). T2SSs are widely conserved in gram-negative bacteria and work through the action of a putatively dynamic pseudopilus for export of proteins across the outer membrane (59, 60). We predicted that if the T4Fs from these systems also retract by spontaneous depolymerization, as has been previously suggested (25, 44), than the major pilins from these systems might also have bulkier residues in α1C that destabilize the filament.
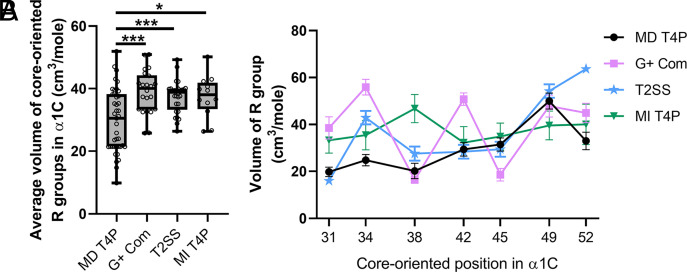
To investigate this, we aligned the α1 region of major pilins from T4aP (further categorized into “MSH [mannose-sensitive hemagglutinin] T4aP” and “other T4aP”), T4bP, gram-positive Com pili, and T2SSs (SI Appendix, Fig. S7 C and D). From each of these categories, phylogenetically distinct systems were selected to represent their broad diversity based on previous analysis (19). The final representative set composed of 110 systems was then categorized as “motor-dependent” or “motor-independent” depending on either (1) the presence of a putative retraction motor ATPase(s) within the genome of the organism from which the system originated or (2) previous studies that demonstrate reliance on a retraction motor. Interestingly, this analysis revealed that some T4aP systems lack a predicted retraction ATPase (SI Appendix, Fig. S7D). The major pilin residues of α1C that are oriented into the pilus core were determined based on structural models of the V. cholerae PilA, A. baylyi ComP, and V. cholerae TcpA major pilins (SI Appendix, Fig. S7B). When we compare the average volume of the R-group of residues in α1C that are core oriented among the different T4F groups, we find that T4P (collectively referring to T4aP and T4bP henceforth), gram-positive Com pili, and T2SSs that are retraction motor independent have significantly larger residues within this region compared to retraction motor–dependent T4P (Fig. 6A). This is consistent with bulkiness in core-oriented α1C residues being a conserved feature among motor-independent T4Fs. Furthermore, we analyzed the difference in R-group volume for each core-oriented α1C position in each of the T4F groups, which revealed that there are trends and distinctions in the location of bulkier residues among different T4F groups. These data indicate that motor-dependent T4P have significantly smaller residues than motor-independent T4F groups at the following α1C core-oriented positions: 31, 34, and 42 for gram-positive Com systems; 34 and 52 for T2SSs; and 38 for motor-independent T4P systems (Fig. 6B). Interestingly, bulkiness at residue 34 in α1C, which is the predominant position we have studied by mutational analysis, was the only position that exhibited a significant difference between more than one group when comparing motor-independent and motor-dependent T4Fs (Fig. 6B), which may suggest that of the core-oriented residues, position 34 is the most critical for determining the propensity for motor-independent retraction. Also, residues 45 and 49 were most similar in size when comparing motor-dependent and motor-independent T4F major pilins, which may suggest that these positions are least important for determining the propensity for motor-independent retraction. Moreover, in TCP, we show that a bulky residue in α1C one helical turn away from position 34 also contributed to motor-independent retraction (TcpAQ38) (Fig. 5), which is consistent with the larger R-group volume at position 38 among motor-independent T4P (Fig. 6B). Thus, it may be that increased bulkiness at one or more of the core-oriented residues in the rigid α1C are critical for destabilizing pilin subunit interactions and promoting motor-independent retraction.
Fig. 6.
Major pilins from motor-independent T4Fs generally contain bulkier core-oriented residues in α1C. (A) The average volume of R-groups of core-oriented residues in α1C are plotted to compare the major pilins of retraction motor-dependent (MD) T4P, retraction motor-independent (MI) T4P, gram-positive (G+) Com pili, and T2SSs. Refer to SI Appendix, Fig. S7 C and D for alignments. Motor-dependent pilus systems are defined as requiring a retraction motor for normal pilus activity and/or having a predicted retraction ATPase gene. Motor-independent pilus systems are defined as those that lack canonical retraction ATPase genes. Box plots represent the median and the upper and lower quartile, while the whiskers demarcate the range. Statistical comparisons are made one-way ANOVA with Tukey’s post test. NS, not significant; * = P < 0.05, and *** = P < 0.001. (B) The average volume of R-groups for each core-oriented α1C residue is plotted by position for the indicated T4F groups. Data are shown as the mean ± SEM. Statistical comparisons were made by two-way ANOVA with Tukey’s post test and can be found in SI Appendix, Table S5.
Our hypothesis is that bulky residues in α1C destabilize pili and promote spontaneous depolymerization by disrupting interactions between this α1C region and α1Ns of adjacent pilins. However, large residues in α1C could be compensated with small residues in α1N to maintain pilus stability. If true, we expected an inverse trend in residue bulkiness for α1N when comparing motor-dependent and motor-independent T4Fs. Specifically, we assessed residue bulkiness in the α1N region that likely interacts with the α1C of neighboring subunits (residues 2 through 22) (SI Appendix, Fig. S7C). This analysis revealed no difference in the general trend for α1N bulkiness between the motor-dependent T4P and the motor-independent T4P and T2SS (SI Appendix, Fig. S7B). Interestingly, the motor-independent, gram-positive Com pili contain markedly larger residues (as assessed by R-group volume) in α1N compared to motor-dependent T4P (SI Appendix, Fig. S7B), which is the opposite of what would be expected if residues in α1N compensate for the enhanced bulkiness in α1C. Together, these data suggest that retraction motor–independent T4Fs have bulkier residues in α1C as a conserved feature, which may act to destabilize the pilus filament and allow for retraction by spontaneous depolymerization.
Discussion
This study sheds light on the mechanisms underlying pilus retraction by providing evidence to support a long-standing model in which motor-independent retraction occurs via the spontaneous depolymerization of the pilus filament (25, 44, 45). In particular, we reveal that the stability of the pilus filament is likely a critical determinant of motor-independent retraction in diverse T4P, including systems that harbor retraction motors (the competence pili of V. cholerae and A. baylyi) as well as those that lack a retraction motor (the TCP of V. cholerae). Notably, we demonstrate that bulky residues oriented toward the pilus core in α1C of the major pilin, which may destabilize pilin–pilin interactions, are important for regulating motor-independent retraction in these three distinct T4P. Thus, our results uncover a broadly conserved mechanism for T4P retraction that is an inherent property of the pilus filament.
Spontaneous depolymerization is proposed to occur when pilus assembly stalls, either by incorporation of the minor pilin TcpB in the case of V. cholerae TCP (25) or perhaps because of dissociation of the extension ATPase (44). Upon stalling, the terminal pilin subunit at the base of the growing pilus may be in a state of dynamic instability in which it can diffuse back into the inner membrane (Fig. 7) (25, 44). Loss of this terminal pilin may cause the pilus to collapse into the inner membrane, positioning the next subunit into this unstable state and resulting in the iterative spontaneous depolymerization of the pilus filament. This model describes a Brownian ratchet whereby the extended pilus possesses potential energy from the ATPase activity of the assembly motor but defaults to a lower-energy state with release of each subunit back into the membrane after assembly stalls (45). We propose that intersubunit contacts between the terminal pilin and the closest pilin subunits at the base of the filament represent critical contacts that determine whether or not a pilus will depolymerize spontaneously. Strong interactions between subunits may reduce the likelihood of spontaneous depolymerization, stabilizing a nonretracting extended pilus filament (Fig. 7, yellow pilin). On the other hand, weaker pilin–pilin contacts may enhance the likelihood that the terminal pilin will diffuse away from the base of a stalled pilus to trigger spontaneous depolymerization (Fig. 7, red pilin). This may explain the increase in retraction frequency observed in our ΔpilTU pilA suppressor mutants. Furthermore, residues 34, 78, and 80 in PilA all lie near the bottom of the pilin globular domain, which likely contacts the inner membrane and possibly the platform protein PilC. Disrupting the contacts that these residues make with α1N of neighboring subunits may help to release this subunit from the base of the pilus if assembly is stalled (Fig. 7). Thus, pilin–pilin interactions between the terminal pilin and the closest pilin subunits at the base of the filament may represent the most critical determinant for retraction by spontaneous depolymerization. We have characterized one key interface—core-oriented residues in the α1C—that contribute to this process; however, other interaction interfaces within the filament may also play a critical role. In addition to the pilin interactions in the pilus filament, the inner membrane platform protein may also influence the tendency of a subunit to diffuse back into the membrane; subunits in retraction motor–dependent T4P may be stabilized by the platform protein in a motor-dependent manner to prevent spontaneous diffusion back into the membrane (44).
Fig. 7.
Model for motor-independent retraction of T4P. Pilus polymerization involves docking of the major pilin at the pilus base followed by ATP hydrolysis by the assembly motor and outward extrusion of the growing filament. When pilus assembly stalls, the terminal pilin subunit may enter a state of dynamic instability (white pilin with dashes). In retraction motor–dependent pili, pilin–pilin interactions may be strong (yellow pilin), with the terminal pilin being more stably associated with the filament and preventing its diffusion back into the membrane in the absence of retraction motors. In retraction motor–independent pili, pilin–pilin interactions may be sufficiently weak (red pilin) whereby the terminal pilin is less stably associated with the base of the filament, which may favor its diffusion into the membrane. Loss of this terminal pilin may cause the pilus to collapse into the inner membrane, positioning the next subunit into this unstable state. We propose that this iterative diffusion of the terminal pilin from the base of the filament results in motor-independent retraction via spontaneous depolymerization. One factor involved in destabilizing pilin–pilin interactions may be bulkiness of residues in α1C that are oriented into the pilus core. If residues in this region are small (yellow pilin), the resulting pilin–pilin interactions may be strong, and the ability of the filament to retract in the absence of motors is reduced. Conversely, if residues in α1C are large (red pilin), the resulting pilin–pilin interactions may be weaker, and the filaments are more likely to undergo motor-independent retraction.
Differences in the dynamics of the terminal pilin at the base of the pilus may be reflected in the overall stability of the pilus filament. Using mutants of both V. cholerae motor-dependent competence pili and motor-independent TCP, we demonstrate that filament stability correlates with motor-independent retraction in which mutants with reduced filament stability are more likely to retract and mutants with increased filament stability are less likely to retract. Retraction motor–dependent gonococcal pili are more stable than retraction motor–independent TCP, a feature that was attributed, in part, to the presence of several stacking aromatic residues in gonococcal pili that are absent in TCP (46). Here, we find that the retraction motor–dependent competence T4aP in V. cholerae are also more stable than TCP but that stability can be altered with a single amino acid change. Generally, retraction motor–dependent T4P retract with significantly higher forces compared to retraction motor–independent pilus systems (23, 25, 29, 61). The high retraction force of many retraction motor–dependent T4P is likely essential for their functions such as twitching motility and DNA uptake. Thus, it has been proposed that retraction motor–dependent T4P have evolved more stable filaments to withstand the high retraction forces that they experience when attached to a load such as an epithelial cell or DNA (46). Based on our results, it is tempting to speculate that filament instability is an evolutionarily maintained property of naturally retraction motor–independent pilus systems because the nonoptimal pilin–pilin interactions that cause this instability are essential for these pili to retract by spontaneous depolymerization. Moreover, the alignments displayed in SI Appendix, Fig. S7C show that many motor-dependent T4P harbor bulky core-oriented residues in α1C similar to the motor-independent T4Fs. Our data also show that reducing pilus filament stability increases the frequency of motor-independent retraction events without altering motor-dependent retraction speed or natural transformation frequencies in the V. cholerae competence pilus (SI Appendix, Fig. S1). This suggests that retraction motor–dependent pilus filaments do not require small α1C core-oriented residues to function and can tolerate filament instability. Therefore, retraction motor–dependent T4P may have gained the ability to evolve more stable filaments, because the acquisition of retraction motors allowed these systems to overcome the tradeoff that would have prevented these pili from retracting by spontaneous depolymerization (44).
Given that, on average, retraction motor–dependent T4aP harbor more small, core-oriented α1C residues in their major pilins (Fig. 6A and SI Appendix, Fig. S7 C and D), which may yield more stable pili, it is interesting that many T4aP retain the ability to retract in the absence of their respective retraction motors. We hypothesize that the residual retraction observed in these systems in the absence of retraction motors also occurs via spontaneous depolymerization. This propensity to retract via spontaneous depolymerization is, however, increased through the introduction of bulkier or destabilizing residues as seen in our pilA suppressor mutants. Thus, while smaller core-oriented residues may enhance filament stability, spontaneous depolymerization events may still occur. Similarly, reduction of residue bulkiness in α1C of the V. cholerae T4b TCP reduces but does not completely abolish retraction. Therefore, we predict 1) that residue bulkiness in the α1C of major pilins is not the only factor that governs spontaneous depolymerization and 2) that bulky residues in this region are not an “on/off switch” for motor-independent retraction but more of a “dimmer switch” that promote or diminish the likelihood that spontaneous depolymerization will occur. Other factors that contribute to this process are yet to be discovered.
As mentioned above, the T4cP of C. crescentus utilize a bifunctional ATPase motor, CpaF, to power both pilus extension and retraction. Our data indicate that for V. cholerae competence T4aP, the PilB ATPase is not a bifunctional motor (Fig. 1C). T4cP, however, are quite divergent from the T4aP/T4bP and represent a distinct evolutionary branch (5, 19, 28, 46). T4cP have also evolved two distinct platform proteins, a property that is distinct from the T4aP and T4bP, which may be required for the activity of a bifunctional motor (19, 36). Therefore, T4cP and T4aP/T4bP may have evolved distinct mechanisms for retraction. T4aP represent a relatively recent branch of the T4bP, and we imagine that the T4aP that exhibit pilus retraction in the absence of dedicated retraction motors may have maintained the residual ability to spontaneously depolymerize (5, 28). Both gram-positive Com pili and T2SSs are more closely related to the T4aP/T4bP than the T4cP (5, 28). Based on our findings, we hypothesize that motor-independent retraction via spontaneous depolymerization may be a broadly conserved property of T4Fs. However, since the vast majority of these systems are untested, it remains possible that retraction of some T4Fs may be supported by a bifunctional motor or an as-yet-undiscovered mechanism.
Materials and Methods
A detailed description of materials and methods can be found in SI Appendix, Materials and Methods, including the following: bacterial strains and culture conditions, construction of mutant strains, natural transformation assays, pilin labeling, imaging, and quantification, Western blotting, transduction assay, pilus filament stability assays, modeling of the V. cholerae major pilin PilA and the competence pilus, and analysis of α-helix residue size in major pilins from the T4F superfamily and statistics.
Supplementary Material
Acknowledgments
We thank Nicolas Biais and Stefan Kreida for helpful discussions, Kim D. Seed and Zoe Netter for protocols and strains for transduction assays, Courtney K. Ellison for providing protocols for pilus labeling and purification, and Triana N. Dalia for help with strain construction. This work was supported by Grant R35GM128674 from the NIH (to A.B.D.) and Grant RGPIN-2017-05757 from the Natural Sciences and Engineering Research Council (to L.C.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2102780118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Bradley D. E., Evidence for the retraction of Pseudomonas aeruginosa RNA phage pili. Biochem. Biophys. Res. Commun. 47, 142–149 (1972). [DOI] [PubMed] [Google Scholar]
- 2.Merz A. J., So M., Sheetz M. P., Pilus retraction powers bacterial twitching motility. Nature 407, 98–102 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Skerker J. M., Berg H. C., Direct observation of extension and retraction of type IV pili. Proc. Natl. Acad. Sci. U.S.A. 98, 6901–6904 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burrows L. L., Pseudomonas aeruginosa twitching motility: Type IV pili in action. Annu. Rev. Microbiol. 66, 493–520 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Ellison C. K., et al. , Obstruction of pilus retraction stimulates bacterial surface sensing. Science 358, 535–538 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seitz P., Blokesch M., DNA-uptake machinery of naturally competent Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 110, 17987–17992 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams D. W., Stutzmann S., Stoudmann C., Blokesch M., DNA-uptake pili of Vibrio cholerae are required for chitin colonization and capable of kin recognition via sequence-specific self-interaction. Nat. Microbiol. 4, 1545–1557 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirn T. J., Bose N., Taylor R. K., Secretion of a soluble colonization factor by the TCP type 4 pilus biogenesis pathway in Vibrio cholerae. Mol. Microbiol. 49, 81–92 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Watnick P. I., Fullner K. J., Kolter R., A role for the mannose-sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor. J. Bacteriol. 181, 3606–3609 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hélaine S., et al. , PilX, a pilus-associated protein essential for bacterial aggregation, is a key to pilus-facilitated attachment of Neisseria meningitidis to human cells. Mol. Microbiol. 55, 65–77 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Persat A., Inclan Y. F., Engel J. N., Stone H. A., Gitai Z., Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 112, 7563–7568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siryaporn A., Kuchma S. L., O’Toole G. A., Gitai Z., Surface attachment induces Pseudomonas aeruginosa virulence. Proc. Natl. Acad. Sci. U.S.A. 111, 16860–16865 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J., Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. U.S.A. 84, 2833–2837 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolappan S., Craig L., Structure of the cytoplasmic domain of TcpE, the inner membrane core protein required for assembly of the Vibrio cholerae toxin-coregulated pilus. Acta Crystallogr. D Biol. Crystallogr. 69, 513–519 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Chang Y. W., et al. , Architecture of the type IVa pilus machine. Science 351, aad2001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takhar H. K., Kemp K., Kim M., Howell P. L., Burrows L. L., The platform protein is essential for type IV pilus biogenesis. J. Biol. Chem. 288, 9721–9728 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCallum M., Tammam S., Khan A., Burrows L. L., Howell P. L., The molecular mechanism of the type IVa pilus motors. Nat. Commun. 8, 15091 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakovljevic V., Leonardy S., Hoppert M., Søgaard-Andersen L., Pil B., PilB and PilT are ATPases acting antagonistically in type IV pilus function in Myxococcus xanthus. J. Bacteriol. 190, 2411–2421 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denise R., Abby S. S., Rocha E. P. C., Diversification of the type IV filament superfamily into machines for adhesion, protein secretion, DNA uptake, and motility. PLoS Biol. 17, e3000390 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chlebek J. L., et al. , PilT and PilU are homohexameric ATPases that coordinate to retract type IVa pili. PLoS Genet. 15, e1008448 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams D. W., Pereira J. M., Stoudmann C., Stutzmann S., Blokesch M., The type IV pilus protein PilU functions as a PilT-dependent retraction ATPase. PLoS Genet. 15, e1008393 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talà L., Fineberg A., Kukura P., Persat A., Pseudomonas aeruginosa orchestrates twitching motility by sequential control of type IV pili movements. Nat. Microbiol. 4, 774–780 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zöllner R., Cronenberg T., Maier B., Motor properties of PilT-independent type 4 pilus retraction in gonococci. J. Bacteriol. 201, e00778-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clausen M., Jakovljevic V., Søgaard-Andersen L., Maier B., High-force generation is a conserved property of type IV pilus systems. J. Bacteriol. 191, 4633–4638 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng D., et al. , The Vibrio cholerae minor pilin TcpB initiates assembly and retraction of the toxin-coregulated pilus. PLoS Pathog. 12, e1006109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazariego-Espinosa K., Cruz A., Ledesma M. A., Ochoa S. A., Xicohtencatl-Cortes J., Longus, a type IV pilus of enterotoxigenic Escherichia coli, is involved in adherence to intestinal epithelial cells. J. Bacteriol. 192, 2791–2800 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuen A. S., Kolappan S., Ng D., Craig L., Structure and secretion of CofJ, a putative colonization factor of enterotoxigenic Escherichia coli. Mol. Microbiol. 90, 898–918 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Sheppard D., et al. , The major subunit of widespread competence pili exhibits a novel and conserved type IV pilin fold. J. Biol. Chem. 295, 6594–6604 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellison C. K., et al. , Retraction of DNA-bound type IV competence pili initiates DNA uptake during natural transformation in Vibrio cholerae. Nat. Microbiol. 3, 773–780 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenz M. G., Wackernagel W., Bacterial gene transfer by natural genetic transformation in the environment. Microbiol. Rev. 58, 563–602 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nero T. M., et al. , ComM is a hexameric helicase that promotes branch migration during natural transformation in diverse Gram-negative species. Nucleic Acids Res. 46, 6099–6111 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortier-Barrière I., et al. , A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA. Cell 130, 824–836 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Draskovic I., Dubnau D., Biogenesis of a putative channel protein, ComEC, required for DNA uptake: Membrane topology, oligomerization and formation of disulphide bonds. Mol. Microbiol. 55, 881–896 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blair K. M., Turner L., Winkelman J. T., Berg H. C., Kearns D. B., A molecular clutch disables flagella in the Bacillus subtilis biofilm. Science 320, 1636–1638 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Ellison C. K., Dalia T. N., Dalia A. B., Brun Y. V., Real-time microscopy and physical perturbation of bacterial pili using maleimide-conjugated molecules. Nat. Protoc. 14, 1803–1819 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellison C. K., et al. , A bifunctional ATPase drives tad pilus extension and retraction. Sci. Adv. 5, eaay2591 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chlebek J. L., Dalia T. N., Biais N., Dalia A. B., Fresh extension of Vibrio cholerae competence type IV pili predisposes them for motor-independent retraction. Appl. Environ. Microbiol. 87, e0047821 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hockenberry A. M., Hutchens D. M., Agellon A., So M., Attenuation of the type IV pilus retraction motor influences Neisseria gonorrhoeae social and infection behavior. MBio 7, e01994-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalia T. N., et al. , Enhancing multiplex genome editing by natural transformation (MuGENT) via inactivation of ssDNA exonucleases. Nucleic Acids Res. 45, 7527–7537 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelley L. A., Mezulis S., Yates C. M., Wass M. N., Sternberg M. J., The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craig L., et al. , Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol. Cell 11, 1139–1150 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Wang F., et al. , Cryoelectron microscopy reconstructions of the Pseudomonas aeruginosa and Neisseria gonorrhoeae type IV pili at sub-nanometer resolution. Structure 25, 1423–1435.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolappan S., et al. , Structure of the Neisseria meningitidis type IV pilus. Nat. Commun. 7, 13015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craig L., Forest K. T., Maier B., Type IV pili: Dynamics, biophysics and functional consequences. Nat. Rev. Microbiol. 17, 429–440 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Merz A. J., Forest K. T., Bacterial surface motility: Slime trails, grappling hooks and nozzles. Curr. Biol. 12, R297–R303 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Li J., Egelman E. H., Craig L., Structure of the Vibrio cholerae Type IVb Pilus and stability comparison with the Neisseria gonorrhoeae type IVa pilus. J. Mol. Biol. 418, 47–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zamyatnin A. A., Amino acid, peptide, and protein volume in solution. Annu. Rev. Biophys. Bioeng. 13, 145–165 (1984). [DOI] [PubMed] [Google Scholar]
- 48.Ellison C. K., et al. , Acinetobacter baylyi regulates type IV pilus synthesis by employing two extension motors and a motor protein inhibitor. Nat. Commun. 12, 3744 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waldor M. K., Mekalanos J. J., Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272, 1910–1914 (1996). [DOI] [PubMed] [Google Scholar]
- 50.Barth Z. K., Netter Z., Angermeyer A., Bhardwaj P., Seed K. D., A family of viral satellites manipulates invading virus gene expression and can affect cholera toxin mobilization. mSystems 5, e00358-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutierrez-Rodarte M., Kolappan S., Burrell B. A., Craig L., The Vibrio cholerae minor pilin TcpB mediates uptake of the cholera toxin phage CTXφ. J. Biol. Chem. 294, 15698–15710 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berry J. L., Pelicic V., Exceptionally widespread nanomachines composed of type IV pilins: The prokaryotic Swiss Army knives. FEMS Microbiol. Rev. 39, 134–154 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradley D. E., Shortening of Pseudomonas aeruginosa pili after RNA-phage adsorption. J. Gen. Microbiol. 72, 303–319 (1972). [DOI] [PubMed] [Google Scholar]
- 54.McCutcheon J. G., Peters D. L., Dennis J. J., Identification and characterization of type IV pili as the cellular receptor of broad host range Stenotrophomonas maltophilia bacteriophages DLP1 and DLP2. Viruses 10, 338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao Y., Hauke C. A., Marles J. M., Taylor R. K., Effects of tcpB mutations on biogenesis and function of the toxin-coregulated pilus, the type IVb pilus of Vibrio cholerae. J. Bacteriol. 198, 2818–2828 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vignon G., et al. , Type IV-like pili formed by the type II secreton: Specificity, composition, bundling, polar localization, and surface presentation of peptides. J. Bacteriol. 185, 3416–3428 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nunn D., Bacterial type II protein export and pilus biogenesis: More than just homologies? Trends Cell Biol. 9, 402–408 (1999). [DOI] [PubMed] [Google Scholar]
- 58.Lam T., et al. , Competence pili in Streptococcus pneumoniae are highly dynamic structures that retract to promote DNA uptake. Mol. Microbiol. 116, 381–396 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Sandkvist M., Type II secretion and pathogenesis. Infect. Immun. 69, 3523–3535 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cianciotto N. P., White R. C., Expanding role of type II secretion in bacterial pathogenesis and beyond. Infect. Immun. 85, e00014-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maier B., et al. , Single pilus motor forces exceed 100 pN. Proc. Natl. Acad. Sci. U.S.A. 99, 16012–16017 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.